Abstract
IKKε (inhibitor of nuclear factor kappa-B kinase ε) is a member of the noncanonical NF-κB pathway. It participates in the inflammatory response and innate immunity against bacteria. In recent decades, IKKε has been closely associated with metabolic regulation. Inhibition of the IKKε pathway can improve fat deposition in the liver, reduce subcutaneous fat inflammation, and improve liver gluconeogenesis in obesity. IKKε is expected to be a new therapeutic target for metabolic diseases such as nonalcoholic fatty liver disease, diabetes, and obesity. Herein, we summarize the structural characterization, physiological function, and pathological role of IKKε in metabolic diseases and small molecule inhibitors of IKKε.
1 Introduction
IKKε (inhibitor of nuclear factor kappa-B kinase ε), also named IKK-inducible kinase, IKKe, IKBKE, and IKK-i, belongs to the noncanonical IKK family which consists of IKKε and TANK binding kinase 1 (TBK1). In 1999, Shimada et al. (1999) discovered IKKε from the mouse macrophage (RAW 264.7 cell), which was induced by lipopolysaccharides (LPS) and then phosphorylated serine residues (Ser32 and Ser36) of IκB-α, resulting in NF-κB activation. Recent studies have shown that energy adjustment disorders are closely related to widespread and low inflammation involving the classical NF-κB pathway (Yuan et al., 2001; Cai et al., 2005).
The role of IKKε in energy regulation was not revealed until 2009, and the expression of IKKε in the liver, adipocytes, and macrophages in the adipose tissue was induced by a high-fat diet in the mice. IKKε−/− mice were resistant to HFD-induced obesity and chronic inflammation in the adipose tissue and improved insulin sensitivity (Chiang et al., 2009). Recently, IKKε has been associated with obesity, diabetes, and nonalcoholic fatty liver disease (NAFLD), and IKKε inhibition suppresses inflammation and increases energy expenditure and thermogenesis. This review focuses on the biochemical structure, physiological function, regulation, and pathological role of IKKε and its inhibitors function.
2 Biochemical Structure of IKKε
2.1 Structure and Function of IKKε
The human IKKε gene is located in the 32.1 region of the long arm of chromosome 1, encoding gene KIAA0151, which is a 3.2-kb DNA fragment (
Peters et al., 2000). IKKε gene contains 22 exons and possesses three different isoforms (IKKε v1, IKKε v2, and IKKε v3, as shown in
Figure 1) (
Chang et al., 2021). IKKε v1 is a full-length coding DNA sequence, while IKKε v2 and IKKε v3 lack exon 20 and exon 3, respectively, due to RNA alternative splicing and editing (
Chang et al., 2021). The IKKε v2 lacking the helical domain and missing 59 amino acids after amino acid position 644 still maintains its kinase activity (
Chang et al., 2021). The kinase domain of IKKε v3 is defective, and the kinase activity is lost due to the loss of amino acids at positions 1–85 (
Chang et al., 2021). Mature IKKε is located in the cytoplasm and contains 716 amino acids (IKKε v1). Specifically including the following domains:
1) A total of 9–300 amino acids form the kinase structure domain (kinase, Figure 1), which can phosphorylate the serine (Ser36, Ser32) of the IκB-α, which will remove the inhibitory effect of the IκB-α on the NF-κB pathway (Shimada et al., 1999). The 38th amino acid (lysine, K38) contributed to the phosphorylation of IκB at the amino acid residues (Ser36). If Lys38 is mutated to alanine (K38A), the IKKε will lose the kinase activity which is critical to the DNA damage–inducible translocation of IKKε to the nuclear bodies (Renner et al., 2010). In addition, the Ser172 of the IKKε is located in the mitogen-activated protein kinase kinase (MAPKK) activation loop, which is an important phosphorylation site for MAPKK, while Ser172 phosphorylation of IKKε is the active form of this kinase (Kishore et al., 2002). Interestingly, there is autophosphorylation of Ser172 once IKKε phosphorylates downstream IkB-α (Shimada et al., 1999).
2) Amino acids at positions 350–383 constitute the ubiquitin-like domain (ULD), which is short but essential for the kinase activity of IKKε. IKKε loses its kinase activity when this region is lost, or when both Leu353 and Phe354 are simultaneously mutated to alanine (Ikeda et al., 2007). It should be noted that the mutation of Leu353 or Phe354 to alanine alone does not affect kinase activity. It is suggested that IKKε could have ULD binding to its kinase domain via the surface containing the hydrophobic patch at Leu353 and Phe354 in the ULD. This intramolecular folding is a distinctive characteristic of IKKε (Ikeda et al., 2007). Another function of ULD is to bind IRF3, which is phosphorylated by the kinase domain of IKKε. When IRF3 is phosphorylated, ULD loses its ability to bind IRF3 and the phosphorylated IRF3 enters the nucleus and promotes the transcriptional IFNβ gene expression (Ikeda et al., 2007).
3) Amino acids at positions 383–647 form a domain that interacts with DEAD-box protein 3 (DDX3), which promotes autophosphorylation of IKKε at Ser172 (Kishore et al., 2002). Phosphorylated IKKε could phosphorylate the Ser102 residue of DDX3, which is critical for the recruitment of IRF3 to DDX3 (Gu et al., 2013). DDX3 enhances IKKε phosphorylation, thereby promoting IRF3 phosphorylation and ultimately promoting IFN-β promoter activation, initiating the innate immune response to viruses. DDX3 plays the role of a scaffolding protein (Gu et al., 2013). Notably, certain IKKε inhibitors, such as BX795, inhibit Ser172 phosphorylation of IKKε and inhibit its activation when IKKε is overexpressed. However, this inhibitory effect does not prevent LPS, TNF-α, Poly(I:C), and IL-1α–induced endogenous IKKε phosphorylation of Ser172 (Clark et al., 2009).
4) The leucine zipper (LZ) structure of IKKε is composed of amino acids at positions 500–527, which is present in all members of the IKK family. IFN-β-induced Thr501 phosphorylation of IKKɛ can directly phosphorylate STAT1(Ser708) which could promote transcription of cytokines, contributing to innate immunity (Tenoever et al., 2007; Rajsbaum et al., 2014). This process requires K48-linked polyubiquitination of IKKε (Rajsbaum et al., 2014).
5) IKKε 578–619 amino acids constitute a helix–loop–helix (HLH) structure, but the function of this structure remains unclear (Shen and Hahn, 2010).
6) C-terminal (617–716) of IKKε is crucial for inducing the production of type I interferon. Amino acid deficiency at positions 686–705 of the IKKε C-terminal significantly decreases IFN-β promoter activation (Nakatsu et al., 2014).
FIGURE 1
Until now, the crystal structure of IKKε has been not elucidated. There is no report on dimerization of IKKε. The function of the leucine zipper and HLH structure had been fully revealed. The structure of IKKε also can be modified by ubiquitination. Certain functions of IKKε require polyubiquitination (e.g., K48-linked and K63-linked polyubiquitination). IKKε can be observed as an oncogene in about 30% of breast cancer patients (Zhou et al., 2013). This is closely related to the regulation of the NF-κB pathway after K63 polyubiquitin modification at the Lys30 and Lys401 positions (Zhou et al., 2013). IKKε polyubiquitinated expression is observed in LPS-treated RAW 264.7 macrophages. Further research has shown that cIAP1/cIAP2/TRAF2 E3 ubiquitin ligase complex contributed to K63-linked polyubiquitination by ubiquitination of IKKε at Lys30, Lys401, and Met416 (Zhou et al., 2013). Polyubiquitinated IKKε recruits and activates the downstream signaling pathway of the NF-κB pathway. If Lys30 or Lys401 (not Lys416) residue of IKKɛ was mutated to alanine, ubiquitination and kinase activity of IKKɛ are decreased (Zhou et al., 2013).
In addition, other studies have shown that SUMOylation modification on Lys231 of IKKε was a process dependent on topoisomerase I–binding arginine/serine-rich protein (TOPORS) which is an E3 ubiquitin ligase (Renner et al., 2010). SUMOylation of Lys231 at IKKε can prevent DNA damage–induced apoptosis (Renner et al., 2010). SUMO-ubiquitination helps locate IKKε in the nucleus. After nucleation, IKKε regulates downstream pathways, phosphorylates nucleosomes to aggregate, and inhibits DNA damage–induced apoptosis (Renner et al., 2010). It is worth noting that reporter gene assays comparing the SUMO-modified IKKε-K231R with the wild-type IKKε found that the SUMO-modified IKKε-K231R still induced interferon-β transcription but lost the ability for entry of IKKε into the nucleus (Renner et al., 2010).
2.2 IKKs and IKKε
2.2.1 IKK Family Constitution
IKK family members are classified into canonical family members (namely, IKKα and IKKβ) and noncanonical family members (namely, IKKε and TBK1) (Karin and Delhase, 2000). The members of the IKK family with kinase activity show sequential homology (seen in Figure 2). The N-terminal region of IKKs has a comparable kinase domain, allowing them to trigger the phosphorylation of downstream molecules (Shen and Hahn, 2010). They also have LZ and HLH (Shen and Hahn, 2010). ULD is only discovered in the structure of IKKβ, TBK1, and IKKε. Furthermore, IKKα and IKKβ both have a distinctive domain called the NEMO-binding domain (NBD) (Courtois and Israel, 2011).
FIGURE 2
IKKα and IKKβ have 51% homologous sequences such as kinase domain, HLH, and LZ (Mercurio et al., 1997; Courtois and Israel, 2011). IKKε and TBK1 also have high sequence homology (Pomerantz and Baltimore, 1999). In addition, the kinase sequence of IKKε shares 27% of the homologous sequence with that of IKKα, but only 24% with that of IKKβ (May et al., 2004; Hiscott et al., 2006; Perkins, 2007; Robinson et al., 2017). Sequence Homology of TBK1 and IKKɛ are 65% in amino acid full-sequences, 65% in ATP-binding region (Reilly et al., 2013), 65% in ubiquitin-like domain (ULD) in mice (Ikeda et al., 2007). In addition, IKKα can also exist in dimer form (noncanonical IKK complexes) (Sun, 2017).
2.2.2 Functional Differentiation of IKKs
IKK members can phosphorylate IκB in the NF-κB pathway, thereby initiating the NF-κB pathway in innate inflammation and tumor. IKKε/TBK1 phosphorylates the Ser36 of the IκB-α subunit, which promotes IκB degradation (Peters et al., 2000). By contrast, IKKβ phosphorylates IκB-α on Ser36 and Ser32, trigging IκB-α degradation (Clément et al., 2008; Zhang et al., 2017). And Ser36 residue is more preferentially phosphorylated than Ser32 by IKKɛ (Shimada et al., 1999).
IKKα and IKKβ both phosphorylate insulin receptor substrate-1 (IRS-1) on Ser312 and inhibit protein kinase B (Akt) (Gao et al., 2002). Targeted disruption of IKKβ in the liver would reverse diet-induced insulin resistance, while systemic insulin resistance results from hepatic overexpression of IKKβ and NF-κB (Yuan et al., 2001; Cai et al., 2005). Overexpression of IKKε or TBK1 also induces a significant increase in Akt phosphorylation at both T308 and S473 and leads to Akt activation (Xie et al., 2011). However, they also play different roles in gene expression, cell growth, and apoptosis (Antonia et al., 2021). IKKε and TBK1 can also form a complex which includes three types: NAP1/IKKε/TBK1 (Pomerantz and Baltimore, 1999; Nomura et al., 2000), TANK/IKKε/TBK1 (Fujita et al., 2003), and SINTBAD/IKKε/TBK1 (Ryzhakov and Randow, 2007), according to different scaffold proteins which they could connect with. IKKε/TBK1 mainly promotes interferon-β transcription (antivirus) and regulates energy metabolism (seen in Figure 3).
FIGURE 3
2.2.3 Phenotype of IKKs Knockout Mice
There were differential phenotypes in IKKs knockout mice. IKKα−/− mice mostly miscarried during the perinatal period and presented with extensive developmental disorders, such as craniofacial bone malformation and shorter tailbone at E12.5 (12.5 days of gestational age) (Li et al., 1999a). A few IKKα−/− mice survived for 1 month after birth, presenting with thickened epidermis and indistinguishable skin structures (Li et al., 1999a). IKKβ−/− mice showed extensive liver degeneration and apoptosis at E12.5–E14.5 (12.5–14.5 days of gestational age), resulting in embryonic and fetal death (Li et al., 1999b; Tanaka et al., 1999). TBK1−/− mouse embryos showed mild liver degeneration at E13.5, extensive phenotype at E14.5, and prenatal death (Bonnard et al., 2000). IKKε−/− mice were not affected in their life span, but infection-related mortality was significantly higher than was found in wild-type mice, and IKKε−/− mice also showed resistance to HFD-induced obesity (Tenoever et al., 2007; Chiang et al., 2009).
2.3 Physiological Functions of IKKε
In a physiological state, IKKε mRNA is expressed the highest in the spleen, human aortic smooth muscle cells (HAOSMC), thymus, peripheral blood white blood cells, placenta, and pancreas (Shimada et al., 1999). In addition, a small amount of IKKε mRNA expression was detected in the lungs, kidneys, prostate, ovaries, colon, and vascular endothelial cells (Shimada et al., 1999; Zhu et al., 2021). There was low expression in the heart, brain, small intestine while little expression was detected in the skeletal muscles and testes (Shimada et al., 1999; Gravel and Servant, 2005). In the liver, IKKε was specifically expressed in stellate cells and not in hepatocytes in the physiological condition but could also be induced to express in hepatocytes by LPS and a high-fat diet (He et al., 2019). In the adipose tissue, under physiological conditions, IKKε was specifically expressed in adipose tissue macrophages (ATMs) and not in the adipocytes (Chiang et al., 2009). Mature IKKε is ubiquitously distributed in the cytoplasm and phosphorylates substrates such as IκBα (Shimada et al., 1999), IRF3 (Sharma et al., 2003), IRF7 (Sharma et al., 2003), PDE-3B (Mowers et al., 2013), DDX3 (Gu et al., 2013), Akt (Zhu et al., 2021), p65 (also known as RelA) (Mattioli et al., 2006), CYLD (Hutti et al., 2009), and YAP (Wang et al., 2017). IKKɛ could also shuttle from cytoplasm into nucleus and phosphorylate nucleosomes while the detailed mechanism is still unclear. (Renner et al., 2010; Rajsbaum et al., 2014). Although IKKε is a member of the IKK family, the NF-κB pathway of the IKKε−/− model is not affected (Hemmi et al., 2004). This suggests that IKKε is not required for the activation of the NF-κB pathway (Shin and Choi, 2019). IKKε deficiency does not affect the classical NF-κB pathway but inhibits LPS-induced C/EBP-δ (CCAAA/enhancer-binding protein-δ) activation and C/EBP-NF-κB–targeted gene transcription (Schwamborn et al., 2003). In addition, IKKε negatively inhibits the NF-κB pathway. In the human IL-17–mediated NF-κB pathway, IKKε phosphorylates nuclear factor kappa-B activator 1 at Ser162 and Ser220 in the signaling complex IL-17R-ACT1-TRAF6, which inhibits the downstream regulation of Act1 and interferes with the downstream NF-κB signaling pathway (Qu et al., 2012). IKKε also phosphorylates p65 (Ser468) which is subsequently shuttled to the nucleus (Mattioli et al., 2006).
2.4 Regulation of IKKε Expression
Some common pro-inflammatory factors, such as TNFα, IL-1α, IL-1β, IL-6, IFN-γ, LPS, and peptidoglycan (PGN), can upregulate IKKε gene expression in a variety of cell lines (such as NK cell line and mature B cell line) (Patel et al., 2015). It is noteworthy that the interleukin (IL) family members have different effects on IKKε. IL-4 inhibits the expression of IKKε, while IL-10 does not regulate the expression of IKKε, and IL-17 also promotes the expression and activation of IKKε (Lee et al., 2017), with increased mRNA levels of TBK1, IKKε, IFN-γ, IL-1β, and IL-6 (Lee et al., 2017). TNFα, IL-1β, IFN-γ, and IL-6 stimulated the expression of IKKε in macrophages, but the expression levels of IKKα and IKKβ remained unchanged in vivo. TNFα-treated and IL-1β–treated mononuclear macrophages resulted in a three-fold increase in IKKε transcription from baseline (Wang et al., 2005). Increasing the TNFα concentration induced elevation in IKKε expression, which is regulated in a dose-dependent manner (Reilly et al., 2013). T-cell receptor (TCR) is also involved in the regulation of IKKε. When TCR is stimulated, IKKε is activated and further phosphorylates the serine residues (Ser117, Ser151, Ser161, and Ser324) in the N-terminal regulatory domain of nuclear factors of activated T cells (NFATs) to inhibit CD8+ T-cell activation, inhibiting T cells by negative feedback (Zhang et al., 2016). In total, IKKε serves as a bridge between pro-inflammatory factors and downstream phosphorylated substrate.
3 IKKε in Pathological State
In the pathological state, IKKε can be induced to express in more organs under stimulus factors (virus, LPS, and TNF-α). It is highly expressed in synovial cells of rheumatoid arthritis (Sweeney et al., 2005) and malignant tumor cells, such as glioma (Li et al., 2012), esophageal squamous cell carcinoma (Kang et al., 2009), pancreatic ductal adenocarcinoma (Zubair et al., 2016), lung squamous cell carcinoma (Li et al., 2015). It is highly expressed in the adipose tissue of obese patients, islet tissues, and nonalcoholic fatty liver. The following are examples of the important roles of IKKε in metabolic diseases (Figures 3, 4).
FIGURE 4
3.1 Obesity
In the past two decades, a large number of studies have shown that metabolic diseases are related to aberrant activation of the natural immune system, and the activated immune cells contribute to mild inflammation in the adipose tissue, pancreas, and liver, leading to a series of metabolic syndromes (Saltiel and Olefsky, 2017). In the physiological state, the adipocytes do not express IKKε. However, the adipose tissue macrophages (ATMs) commonly reside in the adipose tissues. These ATMs are essential for maintaining the sensitivity of adipocytes to hormones such as insulin. ATMs could induce low inflammation in the adipose tissue of obese animals. When ATMs interact with adipocytes, the mRNA content of IKKε in the adipocytes increase significantly (Sanada et al., 2014). Moreover, this increase was found related to the number or density of the ATMs (Sanada et al., 2014). The number of ATMs in the obese mouse model increased rapidly, and F4/80+ CD11c+ ATMs were present but not found in the adipose tissue of nonobese mice (Lumeng et al., 2007). The macrophages in adiposity were in two polarization states (M1 and M2), which alternately maintained the sensitivity of the adipocytes to insulin (Saltiel and Olefsky, 2017). In obese mice, M2-polarized macrophages (anti-inflammatory macrophages) were transformed into M1 macrophages (pro-inflammatory macrophages), which released inflammatory factors (such as TNF-α) to induce inflammatory responses (Lumeng et al., 2007). The increased proportion of M1/M2 macrophages is a biomarker of adipose tissue inflammation and is related to insulin resistance and the development of metabolic diseases caused by obesity (Baker et al., 2011).
In addition, adipocytes are involved in inflammatory regulation. A recent study showed that TNF-α–upregulated IKKε expression in adipocytes through microRNA let-7a and protein Lin-28 homolog B (Lin28B) led to resistance to catecholamine-stimulated lipolysis in adipocytes (Li et al., 2019). In obese mice, IKKε/TBK1 could activate and phosphorylate phosphodiesterase 3B (PDE-3B) at Ser318 (Mowers et al., 2013), decreasing the cAMP level and catecholamine-stimulated lipolysis. Also, activated PDE-3B inhibited uncoupling protein-1 (UCP1) gene transcription and reduced fat oxidation (Mowers et al., 2013; Reilly et al., 2015). UCP1 can promote uncoupling of oxidative phosphorylation in the mitochondria and accelerate heat production. Inhibition of UCP1 leads to a decrease in thermogenic response and energy expenditure, which in turn promotes fat deposition in mice and humans (Mowers et al., 2013). Amlexanox (AM) inhibition of IKKε/TBK1 increased cAMP content in adipocytes and promoted IL-6 secretion by adipocytes and preadipocytes in the dorsal subcutaneous and groin of mice through the cAMP/p38-dependent pathway. Then, IL-6 stimulated hepatic STAT3 phosphorylation to inhibit the gene transcription involved in gluconeogenesis and decreased blood glucose (Reilly et al., 2015). However, there is still no evidence that AM could directly affect the hepatic IKKε/TBK1 pathway.
Notably, the regulation of IKKε on energy balance does not occur in mice fed with a chow diet (Chiang et al., 2009). There was no significant difference in bodyweight between systemic IKKε deletion (IKKε−/−) mice and wild-type mice when they were fed a chow diet, although IKKε−/− mice showed lower triglycerides and higher fasting insulin levels than the control (Chiang et al., 2009). This may be due to the low level of inflammatory responses in the adipose tissue on the chow diet, and therefore no phenotype differences of adiposity between IKKε−/− mice and wild-type mice. In addition, different strains of IKKε−/− mice (C57BL/6 and 129 background) showed different changes in bodyweight and insulin sensitivity in response to different feeding strategies (Yuan et al., 2001; Scheja et al., 2011). These results perhaps were due to other unknown genes regulated by IKKε.
Interestingly, some other studies suggested that IKKε limited meta-inflammation response to overnutrition. Meta-inflammation was termed metabolically triggered inflammation; this chronic state of inflammation is mediated by macrophages located within the colon, liver, muscles, and adipose tissue. Nod-like receptor thermal protein domain–associated protein 3 (NLRP3) inflammasome transcription expression was more significantly increased in M1-activated macrophages from ApoE−/−/IKKε−/− than ApoE−/−/IKKε+/+ with IL-1/LPS stimulation (Patel et al., 2015). ApoE−/−/IKKε−/− mice were protected from diet-induced obesity but developed meta-inflammation in the adipose tissue, liver steatosis, and hypercholesterolemia and readily developed atherosclerotic plaques. Moreover, macrophages in the ApoE−/−/IKKε−/− mice can be primed for NLRP3 activity following HFD. The transplantation of ApoE−/−/IKKε+/+ bone marrow to ApoE−/−/IKKε−/− mice prevented double knockout mice from developing HFD-induced obesity, and the inflammasome and inflammatory response in the adipose tissue were reduced (Patel et al., 2015). Furthermore, ApoE−/−/IKKε+/+ bone marrow transplantation was associated with decreased expression of inflammatory factors (NLRP3 and IL-1β) in the liver (Patel et al., 2015). Another study suggested that IKKε-deficient M1 macrophages showed a stronger inflammatory response (NLRP3 pathway activation) to inflammatory cytokines than wild-type cells (from C57BL/6 mice) (Fischer et al., 2021). In general, IKKε is identified as a pro-inflammatory gene from cell signaling transduction. However, the anti-inflammatory function of IKKε in macrophages has been validated. The possible explanation is that IKKɛ may play a different role in various tissues or cell types, or it simply compensates the inflammatory injury, not initiating the inflammation pathway.
3.2 Nonalcoholic Fatty Liver Disease
Nonalcoholic fatty liver disease (NAFLD) is a term for a series of diseases, namely, nonalcoholic steatohepatitis, cirrhosis, liver fibrosis, and liver cancer (Tilg et al., 2017). LPS increased IKKε expression in the liver of the LPS+HFD–induced NAFLD mice model, in an LPS dose-independent manner. Long-term low-dose LPS+HFD–induced mice (18 weeks, 125 μg/kg−1 day−1) were more likely to develop lipid-deposition–induced steatohepatitis and had a more prominent NAFLD phenotype than HFD mice and high-dose LPS+HFD–induced mice (18 weeks, 250 μg/kg−1 day−1). In addition, this induction can be mitigated by amlexanox (AM) (He et al., 2020). Interestingly, hepatic IKKε expression existed in hepatic stellate cells (HSCs). AM enhanced the expression of insulin-IRS-1-Akt by inhibiting the inflammatory response of HSCs (IKKε-NF-κB-TNF-α/IL-1α) (He et al., 2019). Furthermore, AM promoted the release of IL-6 from the adipose tissues, which phosphorylated STAT3, thereby inhibiting liver gluconeogenesis and reducing blood glucose (Reilly et al., 2015).
In terms of liver fibrosis, mice were treated with a 0.1% diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet for four consecutive weeks to establish a liver fibrosis model. The results showed that phosphorylated IKKε/TBK1 was increased in HSCs (Zhou et al., 2020b). After treatment with AM, mice with hepatobiliary fibrosis showed significantly improved liver function, lower serum AST, and ALT levels and reduced inflammation of liver Kupffer cells (KCs) (Zhou et al., 2020b). During this process, AM inhibited the phosphorylation of IKKε/TBK1 in hepatic Kupffer cells, which may affect the phosphorylation of downstream STAT3. STAT3 phosphorylation and α-SMA expression were decreased when AM was co-incubated with HSCs and TGF-β–activated LX-2 cell lines (hepatic stellate cell) (Zhou et al., 2020b). STAT3 was specifically found in fibroblasts and HSCs, and not in hepatocytes. Therefore, AM inhibited KCs activation and liver fibrosis through IKKε/TBK1. Previous studies have shown that palmitic acid (PA) promoted the transformation of KCs into M1 macrophages (Luo et al., 2017). The expression of arginase 1 (Arg1) and IL-10 in KCs cells treated with PA was increased by AM. Arg1 and IL-10 are markers of polarization of M2 macrophages, indicating that KCs are transformed into M2 macrophages under AM intervention (Zhou et al., 2020a). In addition, the therapeutic effect of AM on NAFLD also depended on the activation of M1. The content of activator protein-1 (AP-1) in subcutaneous fat of patients with obesity and type 2 diabetes was higher (Oral et al., 2017). AP-1 is a transcription regulatory factor of M1 and is involved in obesity-related adipose tissue inflammation. AP-1 and inflammatory factors transcription were decreased following AM treatment, suggesting that AM reduced the M1/M2 ratio in adipose cells and inhibited the inflammatory response in adipose tissue (Oral et al., 2017).
3.3 Diabetes
IKKε is involved in pancreatic β-cell regeneration in animal models of type 1 diabetes (T1D). Xu et al. (2018) found that cinnamic acid derivative (E)-3-(3-phenylbenzo[c]isoxazol-5-yl)acrylic acid (abbreviated as PIAA) inhibited IKKε/TBK1 and stimulated cAMP-dependent protein kinase A (PKA). Mitosis of islet β cells was subsequently promoted through the cAMP/PKA-mTORC1 signaling pathway. The role of IKKε in the pathologic progression and treatment of T1D has also been demonstrated in some clinical trials. One trial found that in newly onset T1D treated by α-1 antitrypsin (AAT), IKKε expression in whole blood cells was inhibited by 50% (Weir et al., 2018). In addition, transcriptome and interactome analysis of pancreatic β cells and peripheral monocytes in T1D also showed that IKKε played an important role in T1D (Safari-Alighiarloo et al., 2020).
From the perspective of a clinical trial, IKKε inhibitors (such as AM) show favorable effects on diabetes. For example, patients with type 2 diabetes (T2D) combined with NAFLD were given AM orally (25 mg three times a day for 2 weeks), titrated to 50 mg three times a day for 10 weeks, and observed for 4 weeks after treatment to ensure patient safety). The expression of UCP1 in subcutaneous fat of patients with type 2 diabetes was increased, and the β 3-adrenergic receptor ADRB3 gene was also highly expressed in the adipose tissue after treatment (Oral et al., 2017). It is worth noting that the hypoglycemic effect of AM seems to be more dependent on the inflammatory response of patients (Oral et al., 2017). A 12-week randomized, double-blind controlled trial was conducted in 42 patients (obese with type 2 diabetes) divided into two groups. In the experimental group, only seven patients (responders) had a decrease of more than 0.5% in A1c (HbA1c). When the gene expressions in the adipose tissue of responders and nonresponders were compared, it showed that the responders had higher expressions of inflammatory factors before treatment (such as FOSB, FOSL1, and AP-1) (Oral et al., 2017). These results indicated that AM played a therapeutic role in diabetes by inhibiting IKKε/TBK1. Notably, there was no significant difference in bodyweight between the placebo group and the experimental group (namely, responder and nonresponder) (Oral et al., 2017). Body fat percentage, limb fat percentage, and muscle percentage did not change, but liver fat content was decreased while serum HDL content was increased in the experimental group (Oral et al., 2017).
4 IKKε Inhibitors
In 2006, Bamborough et al. found a compound that specifically inhibits IKKε/TBK1 (5-(1H-benzimidazol-1-yl)-3-alkoxy-2-thiophenecarbonitriles). This inhibitor inhibits the ATP binding site of IKKε (Bamborough et al., 2006). In 2014, Li et al. found three kinds of scaffolds (SR8185, 200A, and 200B) based on 2-amino-4-(30-Cyano-40-pyrrolidine) phenyl pyrimidine in the screening of JNK candidate inhibition (Li et al., 2014). These compounds specifically inhibited the Ser172 phosphorylation of IKKε and showed a significant inhibitory effect on the tumor-bearing mouse model. In addition, the number of novel inhibitors developed based on benzimidazole is also increasing (Johannes et al., 2014; Lefranc et al., 2019). In 2019, Lefranc et al. analyzed 3,050,000 compounds and found that BAY-985 specifically inhibited IKKε/TBK1. Bay-985 is a benzimidazole derivative that competitively inhibits the binding of ATP to the Lys38 of IKKε (Lefranc et al., 2019). It is worth noting that although a variety of inhibitors have been developed (see Table 1), few other inhibitors have been used in clinical trials in addition to AM.
TABLE 1
| Name | Action site | IC50 (nM) | Specificity on inhibiting IKKε | Structural formula | Reference |
|---|---|---|---|---|---|
| BX795 | Inhibition specifically on Ser172 phosphorylation of IKKε | 41 ± 1 | IKKε/TBK1 | 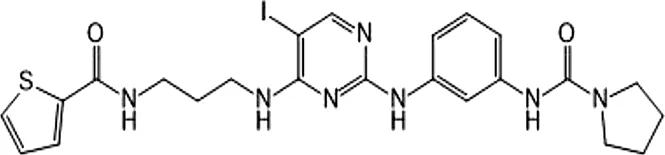 | Clark et al. (2009) |
| Amlexanox | Competitive inhibition of ATP binding sites of IKKε (Lys38) | 1,000–2,000 | IKKε/TBK1 | 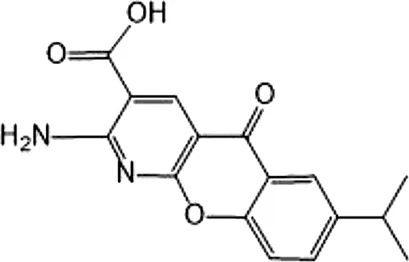 | Reilly et al. (2013) |
| (E)-3 (3-phenylbenzo[c]isoxazol-5-yl)acrylic acid (PIAA) | * | 1,070 | IKKε/TBK1 | 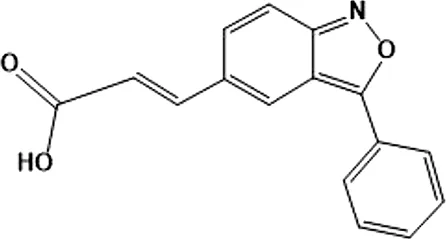 | Xu et al. (2018) |
| SR8185, 200A, 200B | Specifically inhibit phosphorylation of IKKε (Ser172) | * | IKKε/TBK1 | 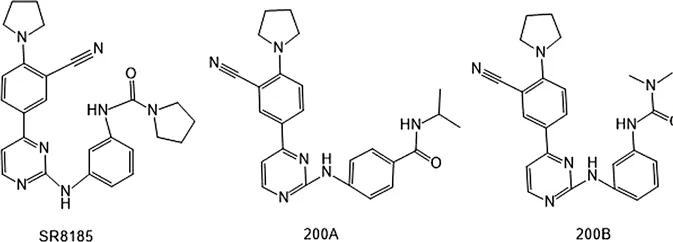 | Li et al. (2014) |
| GSK319347A | * | 469 | IKKβ/IKKε/TBK1 | 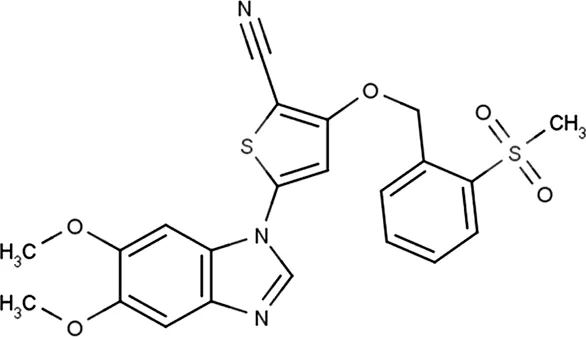 | Johannes et al. (2014) |
| BAY-985 | Competitive inhibition of ATP binding sites of IKKε (Lys38) | 2 | IKKε/TBK1 | 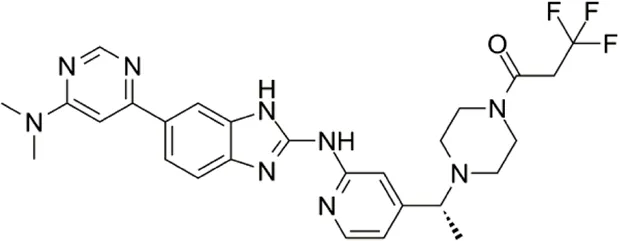 | Lefranc et al. (2019) |
| MRT67307 | * | 160 | IKKε/TBK1 | 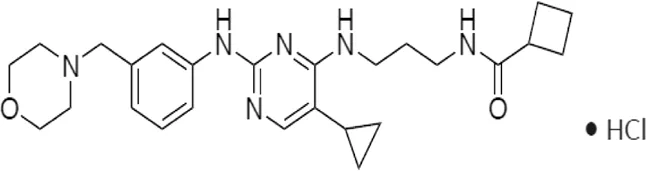 | Clark et al. (2011) |
| CYT387 | Decreased IKKε expression at the protein level rather than at the mRNA level | 17,680 ± 2,940** | IKKε# | 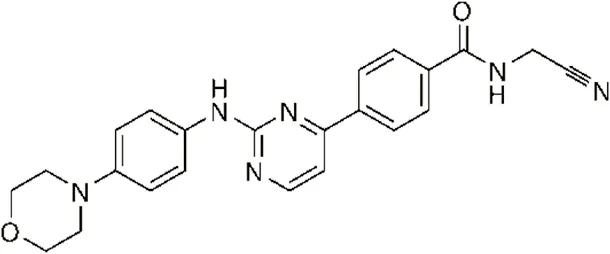 | Wang et al. (2021) |
| TBK1/IKKε-IN-2 | * | 3.9 | IKKε/TBK1 | 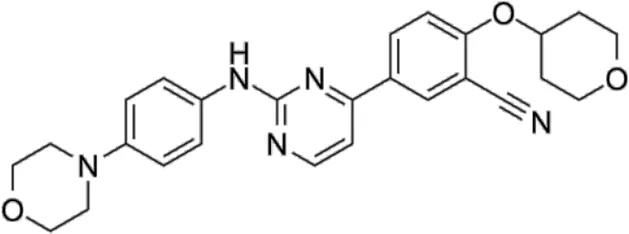 | Muvaffak et al. (2014) |
| TBK1/IKKε-IN-4 | * | 59 | IKKε/TBK1 | 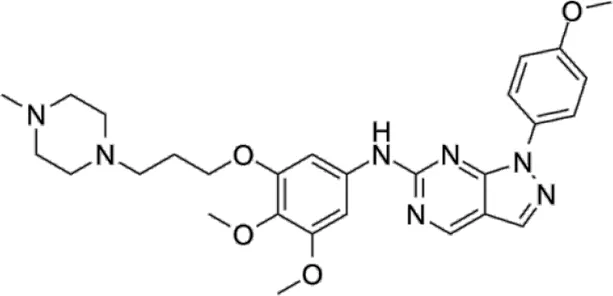 | Ou et al. (2011) |
| TBK1/IKKε-IN-5 | * | 5.6 | IKKε/TBK1 | 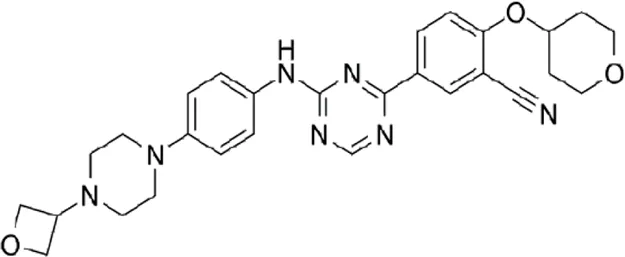 | Jenkins et al. (2018) |
| HPN-01 | * | <4.8 | IKKα/IKKβ/IKKε | 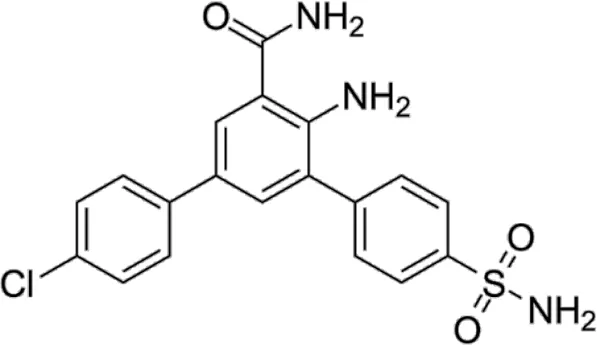 | Christopher et al. (2007) |
| Azabenzimidazole derivatives | * | *** | IKKε/TBK1 | 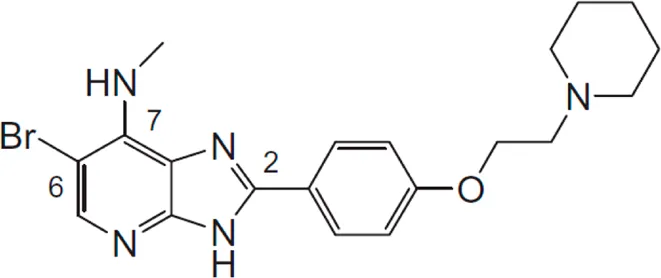 | Wang et al. (2012) |
Properties and action sites of IKKε inhibitors.
*No data; **U87-MG cells for 48 h; *** It has many derivatives possessing different IC50; IC50, maximum half inhibitory concentration. # No evidence can prove its inhibition effect to TBK1
In 2013, Reilly et al. screened 150,000 compounds and found that AM could specifically inhibit IKKε/TBK1 and lead to IKKε protein suppression (Reilly et al., 2013). AM was first applied clinically in Japan as an anti-asthma drug. In the United States and China, AM was made into an oral patch and used for the treatment of recurrent intractable oral ulcers, but it has been discontinued in clinics in the United States since 2017 (Fu et al., 2012; Uma Maheswari and Shanmugasundaram, 2013; Dosanjh and Won, 2020). Previous studies have shown that AM could be given orally to patients (50 mg three times daily, lasting for 12 weeks) in the treatment of obese type 2 diabetes, showing weight loss, lower blood glucose, and safety (Oral et al., 2017). Notably, the active part of AM lies in its carboxylic acid moiety, and its carboxylic acid derivatives also show strong inhibition of IKKε/TBK1(Beyett et al., 2018a; Beyett et al., 2018b). The carboxyl competition of AM inhibited the activity of IKKε/TBK1 by inhibiting the ATP-binding site of IKKε (Thr156). AM reduced the contents of triglyceride and liver glycogen and inhibited the inflammatory response of the liver and adipose tissue in obese mice. It also decreased serum TNF-α, IL-1α, and MIP-1α and increased the content of the anti-inflammatory factor IL-10 (Reilly et al., 2013). Studies have shown that the liver triglyceride, blood triglyceride, and blood cholesterol contents were decreased significantly in HFD-fed AM-treated mice when compared with the control group (He et al., 2019). AM also increased the expression of adipocyte enrichment proteins (SLC2A4 and PPARγ) and increased insulin sensitivity in mice. AM inhibited the phosphorylation of S6K and S6 in the mammalian target of rapamycin complex 1 (mTORC1) pathway induced by HFD. Correspondingly, insulin-induced phosphorylation of S6 was significantly decreased in IKKε−/−3T3-L1 adipocytes. In addition, AM treatment increased the expression of hormone-sensitive lipase (HSL) and UCP1 in brown adipose tissue (Reilly et al., 2013).
The maximum 50% inhibitory concentration (IC50) of AM is about 1–2 μM. HFD-induced C57BL/6 mice were administered orally by gavage 25 mg/kg or 100 mg/kg AM (serum concentration was 5 μM) and the results showed that AM prevented HFD-induced obesity within 4 weeks, independent of the dietary intake (Reilly et al., 2013). Interestingly, there was no further weight loss after 4 weeks, even when the AM dose was increased and dietary intake was not changed. The body temperature of mice treated with AM increased by about 1°C when compared with the vehicle (Reilly et al., 2013). However, the weight loss due to AM was reversible, and it lost its inhibitory effect after drug withdrawal (Reilly et al., 2013). Most of the IKKε inhibitors could simultaneously inhibit TBK1. Although the effects of TBK1 and IKKε are similar in structure, there are differences in physiological functions. The loss of IKKε led to increased thermogenesis and insulin sensitivity in animal models (Chiang et al., 2009; Shin and Choi, 2019). Specific ablation of TBK1 in adipocytes reduced HFD-induced obesity but showed glucose intolerance and insulin resistance (Zhao et al., 2018; Shin and Choi, 2019), so specific inhibitors on IKKε should be necessary to avoid the side effects.
Although certain IKKε inhibitors have been discovered, there is no adequate information on IKKε inhibitors on their side effects in clinical practice or mice models. As mentioned in the pathological role of IKKε, IKKε is the key regulator of the IFN-β transcription (Tenoever et al., 2007). Thus, IKKε inhibitors might inhibit the activation of the IFN-β signaling pathway. A previous study showed that although IKKε knockout mice could produce a normal level of IFN-β, they were still hypersusceptible to viral infection because of lacking the IFN-β signaling pathway (Tenoever et al., 2007). Therefore, it could be proposed that patients taking IKKε inhibitors might have undermined immunity and the risk of viral infection. However, AM used as an oral paste of recurrent aphthous ulcers did not show any serious side effects in long-term research and clinical application for decades (Khandwala et al., 1997); it is of great necessity for us to notice its inhibition effect on innate immunity.
5 Summary and Prospect
In the past two decades, IKKε has been identified to a certain homology sequence with other IKK family numbers. The physiological function and substrates of IKKε are still not fully revealed. IKKε has been linked with the occurrence and development of obesity, diabetes, and NAFLD. The inhibition of IKKε limited the inflammatory response in vivo and improved insulin sensitivity and glucose/lipid metabolism in patients with obesity and diabetes, which proposed a potential therapeutic approach. However, most of the current IKKε inhibitors have low specificity (e.g., combined inhibition of TBK1). Therefore, the development of specific IKKε inhibitors is a challenge and priority for future studies. Furthermore, current studies have shown that IKKε is involved in interferon production and antiviral effects, and systemic overwhelming inhibition on IKKε may lead to susceptibility to viral infection. The development of organ-specific (for adiposity, liver) targeted IKKε inhibitors may help to reduce the side effects of drugs. In conclusion, IKKε plays a pivotal role as a potential therapeutic target in many diseases, especially metabolic diseases, and deserves further investigations in the future.
Statements
Author contributions
QX reviewed all the literature, collected data, and drafted the manuscript. QH drafted partly and made important suggestions for the amendments. XX conceived the review and drafted partly. JZ drafted partly and reviewed the manuscript. LL, YS, and YC contributed substantially by giving insightful comments and suggestions during the creation of the manuscript. XX and QH were responsible for funding acquisition. All authors have read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 82100473) and Hubei Natural Science Foundation (2015CFB316, 2019CFB549).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- α-SMA
α-smooth muscle actin
- aa
amino acid
- Akt
protein kinase B
- ALT
alanine aminotransferase
- AM
amlexanox
- AP-1
activator protein-1
- Arg1
arginase 1
- AST
aspartate aminotransferase
- ATMs
adipose tissue macrophages
- CC1
coiled-coil domain 1
- CC2
coiled-coil domain 2
- C/EBP-δ
CCAAA/enhancer-binding protein-δ
- CIA
collagen II–induced rheumatoid arthritis
- CYLD
cylindromatosis
- DDC
diethoxycarbonyl-1,4-dihydrocollidine
- DDX3
DEAD-box protein 3
- HFD
high-fat diet
- HLH
helix–loop–helix
- HSCs
hepatic stellate cells
- HSL
hormone-sensitive lipase
- IFN-γ
interferon-γ
- IFNA 4
interferon alpha-4
- IL-1α
interleukin-1α
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- IL-10
interleukin-10
- IL-17
interleukin 17
- IL-17R
interleukin 17 receptor
- IKK
inhibitor of nuclear factor kappa-B kinase
- IκBα
inhibitor of nuclear factor kappa-B kinase α
- IKKβ
inhibitor of nuclear factor kappa-B kinase β
- IKKε
inhibitor of nuclear factor kappa-B kinase ε
- IRF3
interferon regulatory Factor 3
- IRF7
interferon regulatory Factor 7
- IRS-1
insulin receptor substrate-1
- KCs
Kupffer cells
- Lin28B
protein lin-28 homolog B
- LPS
lipopolysaccharide
- Lys
lysine
- LZ
leucine zipper
- MAPKK
mitogen-activated protein kinase kinase
- Met
methionine
- mTORC1
mammalian target of Rapamycin Complex 1
- NAFLD
nonalcoholic fatty liver disease
- NAP1
NF-κB essential modifier
- NBD
NEMO-binding domain
- NFATs
nuclear factors of activated T cells
- NF-κB
nuclear factor kappa-B
- NLRP3
NOD-like receptor thermal protein domain–associated protein 3
- PA
palmitic acid
- PDE-3B
phosphodiesterase 3B
- PIAA
(E)-3-(3-phenylbenzo[c]isoxazol-5-yl)acrylic acid
- PPARγ
peroxisome proliferators-activated receptor γ
- SINTBAD
similar to NAP1 TBK1 adapter
- STAT1
signal transducer and activator of transcription 1
- STAT3
signal transducer and activator of transcription 3
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- TBK1
TANK-binding kinase 1
- TCR
T-cell receptor
- Thr
threonine
- TNF-α
tumor necrosis factor α
- TOPORS
topoisomerase I–binding arginine serine-rich protein
- TRAF6
TNF receptor–associated factor 6
- Ub
ubiquitination
- UCP1
uncoupling protein-1
- ULD
ubiquitin-like domain
- YAP
Yes‐associated protein
- Zn
zinc-finger
References
1
AntoniaR. J.HaganR. S.BaldwinA. S. (2021). Expanding the View of IKK: New Substrates and New Biology. Trends Cel Biol31, 166–178. 10.1016/j.tcb.2020.12.003
2
BakerR. G.HaydenM. S.GhoshS. (2011). NF-κB, Inflammation, and Metabolic Disease. Cell Metab13, 11–22. 10.1016/j.cmet.2010.12.008
3
BamboroughP.ChristopherJ. A.CutlerG. J.DicksonM. C.MellorG. W.MoreyJ. V.et al (2006). 5-(1H-Benzimidazol-1-yl)-3-alkoxy-2-thiophenecarbonitriles as Potent, Selective, Inhibitors of IKK-Epsilon Kinase. Bioorg. Med. Chem. Lett.16, 6236–6240. 10.1016/j.bmcl.2006.09.018
4
BeyettT. S.GanX.ReillyS. M.ChangL.GomezA. V.SaltielA. R.et al (2018a). Carboxylic Acid Derivatives of Amlexanox Display Enhanced Potency toward TBK1 and IKKε and Reveal Mechanisms for Selective Inhibition. Mol. Pharmacol.94, 1210–1219. 10.1124/mol.118.112185
5
BeyettT. S.GanX.ReillyS. M.GomezA. V.ChangL.TesmerJ. J. G.et al (2018b). Design, Synthesis, and Biological Activity of Substituted 2-Amino-5-Oxo-5h-Chromeno[2,3-B]pyridine-3-Carboxylic Acid Derivatives as Inhibitors of the Inflammatory Kinases TBK1 and IKKε for the Treatment of Obesity. Bioorg. Med. Chem.26, 5443–5461. 10.1016/j.bmc.2018.09.020
6
BonnardM.MirtsosC.SuzukiS.GrahamK.HuangJ.NgM.et al (2000). Deficiency of T2K Leads to Apoptotic Liver Degeneration and Impaired NF-kappaB-dependent Gene Transcription. EMBO J.19, 4976–4985. 10.1093/emboj/19.18.4976
7
CaiD.YuanM.FrantzD. F.MelendezP. A.HansenL.LeeJ.et al (2005). Local and Systemic Insulin Resistance Resulting from Hepatic Activation of IKK-Beta and NF-kappaB. Nat. Med.11, 183–190. 10.1038/nm1166
8
ChangY. L.LiaoY. W.ChenM. H.ChangS. Y.HuangY. T.HoB. C.et al (2021). IKKε Isoform Switching Governs the Immune Response against EV71 Infection. Commun. Biol.4, 663. 10.1038/s42003-021-02187-x
9
ChiangS. H.BazuineM.LumengC. N.GeletkaL. M.MowersJ.WhiteN. M.et al (2009). The Protein Kinase IKKepsilon Regulates Energy Balance in Obese Mice. Cell138, 961–975. 10.1016/j.cell.2009.06.046
10
ChristopherJ. A.AvitabileB. G.BamboroughP.ChampignyA. C.CutlerG. J.DyosS. L.et al (2007). The Discovery of 2-Amino-3,5-Diarylbenzamide Inhibitors of IKK-Alpha and IKK-Beta Kinases. Bioorg. Med. Chem. Lett.17, 3972–3977. 10.1016/j.bmcl.2007.04.088
11
ClarkK.PeggieM.PlaterL.SorcekR. J.YoungE. R.MadwedJ. B.et al (2011). Novel Cross-Talk within the IKK Family Controls Innate Immunity. Biochem. J.434, 93–104. 10.1042/BJ20101701
12
ClarkK.PlaterL.PeggieM.CohenP. (2009). Use of the Pharmacological Inhibitor BX795 to Study the Regulation and Physiological Roles of TBK1 and IkappaB Kinase Epsilon: a Distinct Upstream Kinase Mediates Ser-172 Phosphorylation and Activation. J. Biol. Chem.284, 14136–14146. 10.1074/jbc.M109.000414
13
ClémentJ.-F.MelocheS.ServantM. J. (2008). The IKK-Related Kinases: from Innate Immunity to Oncogenesis. Cel Res.18, 889. 10.1038/cr.2008.273
14
CourtoisG.IsraëlA. (2011). IKK Regulation and Human Genetics. Curr. Top. Microbiol. Immunol.349, 73–95. 10.1007/82_2010_98
15
DosanjhA.WonC. Y. (2020). Amlexanox: A Novel Therapeutic for Atopic, Metabolic, and Inflammatory Disease. Yale J. Biol. Med.93, 759–763.
16
FischerF. A.MiesL. F. M.NizamiS.PantaziE.DanielliS.DemarcoB.et al (2021). TBK1 and IKKε Act like an OFF Switch to Limit NLRP3 Inflammasome Pathway Activation. Proc. Natl. Acad. Sci. U S A.118. 10.1073/pnas.2009309118
17
FuJ.ZhuX.DanH.ZhouY.LiuC.WangF.et al (2012). Amlexanox Is as Effective as Dexamethasone in Topical Treatment of Erosive Oral Lichen Planus: a Short-Term Pilot Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol.113, 638–643. 10.1016/j.oooo.2011.10.013
18
FujitaF.TaniguchiY.KatoT.NaritaY.FuruyaA.OgawaT.et al (2003). Identification of NAP1, a Regulatory Subunit of IkappaB Kinase-Related Kinases that Potentiates NF-kappaB Signaling. Mol. Cel Biol23, 7780–7793. 10.1128/mcb.23.21.7780-7793.2003
19
GaoZ.HwangD.BatailleF.LefevreM.YorkD.QuonM. J.et al (2002). Serine Phosphorylation of Insulin Receptor Substrate 1 by Inhibitor Kappa B Kinase Complex. J. Biol. Chem.277, 48115–48121. 10.1074/jbc.M209459200
20
GravelS. P.ServantM. J. (2005). Roles of an IkappaB Kinase-Related Pathway in Human Cytomegalovirus-Infected Vascular Smooth Muscle Cells: a Molecular Link in Pathogen-Induced Proatherosclerotic Conditions. J. Biol. Chem.280, 7477–7486. 10.1074/jbc.M410392200
21
GuL.FullamA.BrennanR.SchröderM. (2013). Human DEAD Box Helicase 3 Couples IκB Kinase ε to Interferon Regulatory Factor 3 Activation. Mol. Cel Biol33, 2004–2015. 10.1128/MCB.01603-12
22
HeQ.XiaX.YaoK.ZengJ.WangW.WuQ.et al (2019). Amlexanox Reversed Non-alcoholic Fatty Liver Disease through IKKε Inhibition of Hepatic Stellate Cell. Life Sci.239, 117010. 10.1016/j.lfs.2019.117010
23
HeQ.ZengJ.YaoK.WangW.WuQ.TangR.et al (2020). Long-term Subcutaneous Injection of Lipopolysaccharides and High-Fat Diet Induced Non-alcoholic Fatty Liver Disease through IKKε/NF-Κb Signaling. Biochem. Biophys. Res. Commun.532, 362–369. 10.1016/j.bbrc.2020.08.036
24
HemmiH.TakeuchiO.SatoS.YamamotoM.KaishoT.SanjoH.et al (2004). The Roles of Two IkappaB Kinase-Related Kinases in Lipopolysaccharide and Double Stranded RNA Signaling and Viral Infection. J. Exp. Med.199, 1641–1650. 10.1084/jem.20040520
25
HiscottJ.NguyenT. L.ArguelloM.NakhaeiP.PazS. (2006). Manipulation of the Nuclear Factor-kappaB Pathway and the Innate Immune Response by Viruses. Oncogene25, 6844–6867. 10.1038/sj.onc.1209941
26
HuttiJ. E.ShenR. R.AbbottD. W.ZhouA. Y.SprottK. M.AsaraJ. M.et al (2009). Phosphorylation of the Tumor Suppressor CYLD by the Breast Cancer Oncogene IKKepsilon Promotes Cell Transformation. Mol. Cel34, 461–472. 10.1016/j.molcel.2009.04.031
27
IkedaF.HeckerC. M.RozenknopA.NordmeierR. D.RogovV.HofmannK.et al (2007). Involvement of the Ubiquitin-like Domain of TBK1/IKK-I Kinases in Regulation of IFN-Inducible Genes. EMBO J.26, 3451–3462. 10.1038/sj.emboj.7601773
28
JenkinsR. W.ArefA. R.LizotteP. H.IvanovaE.StinsonS.ZhouC. W.et al (2018). Ex Vivo Profiling of PD-1 Blockade Using Organotypic Tumor Spheroids. Cancer Discov.8, 196–215. 10.1158/2159-8290.CD-17-0833
29
JohannesJ. W.ChuaquiC.CowenS.DevereauxE.GingipalliL.MolinaA.et al (2014). Discovery of 6-Aryl-Azabenzimidazoles that Inhibit the TBK1/IKK-ε Kinases. Bioorg. Med. Chem. Lett.24, 1138–1143. 10.1016/j.bmcl.2013.12.123
30
KangM. R.KimM. S.KimS. S.AhnC. H.YooN. J.LeeS. H. (2009). NF-kappaB Signalling Proteins P50/p105, P52/p100, RelA, and IKKepsilon Are Over-expressed in Oesophageal Squamous Cell Carcinomas. Pathology41, 622–625. 10.3109/00313020903257756
31
KarinM.DelhaseM. (2000). The I Kappa B Kinase (IKK) and NF-Kappa B: Key Elements of Proinflammatory Signalling. Semin. Immunol.12, 85–98. 10.1006/smim.2000.0210
32
KhandwalaA.Van InwegenR. G.CharneyM. R.AlfanoM. C. (1997). 5% Amlexanox Oral Paste, a New Treatment for Recurrent Minor Aphthous Ulcers: II. Pharmacokinetics and Demonstration of Clinical Safety. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod.83, 231–238. 10.1016/s1079-2104(97)90010-x
33
KishoreN.HuynhQ. K.MathialaganS.HallT.RouwS.CreelyD.et al (2002). IKK-i and TBK-1 Are Enzymatically Distinct from the Homologous Enzyme IKK-2: Comparative Analysis of Recombinant Human IKK-I, TBK-1, and IKK-2. J. Biol. Chem.277, 13840–13847. 10.1074/jbc.M110474200
34
KravchenkoV. V.MathisonJ. C.SchwambornK.MercurioF.UlevitchR. J. (2003). IKKi/IKKepsilon Plays a Key Role in Integrating Signals Induced by Pro-inflammatory Stimuli. J. Biol. Chem.278, 26612–26619. 10.1074/jbc.M303001200
35
LeeS. H.JhunJ.ByunJ. K.KimE. K.JungK.LeeJ. E.et al (2017). IL-17 axis Accelerates the Inflammatory Progression of Obese in Mice via TBK1 and IKBKE Pathway. Immunol. Lett.184, 67–75. 10.1016/j.imlet.2017.02.004
36
LefrancJ.SchulzeV. K.HilligR. C.BriemH.PrinzF.MengelA.et al (2019). Discovery of BAY-985, a Highly Selective TBK1/IKKε Inhibitor. J. Med. Chem.63, 601–612. 10.1021/acs.jmedchem.9b01460
37
LiH.ChenL.ZhangA.WangG.HanL.YuK.et al (2012). Silencing of IKKε Using siRNA Inhibits Proliferation and Invasion of Glioma Cells In Vitro and In Vivo. Int. J. Oncol.41, 169–178. 10.3892/ijo.2012.1452
38
LiJ.HuangJ.JeongJ. H.ParkS. J.WeiR.PengJ.et al (2014). Selective TBK1/IKKi Dual Inhibitors with Anticancer Potency. Int. J. Cancer134, 1972–1980. 10.1002/ijc.28507
39
LiM.WangM.LiuY.HuangS.YiX.YinC.et al (2019). TNF-α Upregulates IKKε Expression via the Lin28B/let-7a Pathway to Induce Catecholamine Resistance in Adipocytes. Obesity (Silver Spring)27, 767–776. 10.1002/oby.22434
40
LiQ.LuQ.HwangJ. Y.BüscherD.LeeK. F.Izpisua-BelmonteJ. C.et al (1999a). IKK1-deficient Mice Exhibit Abnormal Development of Skin and Skeleton. Genes Dev.13, 1322–1328. 10.1101/gad.13.10.1322
41
LiQ.Van AntwerpD.MercurioF.LeeK. F.VermaI. M. (1999b). Severe Liver Degeneration in Mice Lacking the IkappaB Kinase 2 Gene. Science284, 321–325. 10.1126/science.284.5412.321
42
LiW.ChenY.ZhangJ.HongL.YuanN.WangX.et al (2015). IKBKE Upregulation Is Positively Associated with Squamous Cell Carcinoma of the Lung In Vivo and Malignant Transformation of Human Bronchial Epithelial Cells In Vitro. Med. Sci. Monit.21, 1577–1586. 10.12659/MSM.893815
43
LumengC. N.BodzinJ. L.SaltielA. R. (2007). Obesity Induces a Phenotypic Switch in Adipose Tissue Macrophage Polarization. J. Clin. Invest.117, 175–184. 10.1172/JCI29881
44
LuoW.XuQ.WangQ.WuH.HuaJ. (2017). Effect of Modulation of PPAR-γ Activity on Kupffer Cells M1/M2 Polarization in the Development of Non-alcoholic Fatty Liver Disease. Sci. Rep.7, 44612. 10.1038/srep44612
45
MattioliI.GengH.SebaldA.HodelM.BucherC.KrachtM.et al (2006). Inducible Phosphorylation of NF-Kappa B P65 at Serine 468 by T Cell Costimulation Is Mediated by IKK Epsilon. J. Biol. Chem.281, 6175–6183. 10.1074/jbc.M508045200
46
MayM. J.LarsenS. E.ShimJ. H.MadgeL. A.GhoshS. (2004). A Novel Ubiquitin-like Domain in IkappaB Kinase Beta Is Required for Functional Activity of the Kinase. J. Biol. Chem.279, 45528–45539. 10.1074/jbc.M408579200
47
MercurioF.ZhuH.MurrayB. W.ShevchenkoA.BennettB. L.LiJ.et al (1997). IKK-1 and IKK-2: Cytokine-Activated IkappaB Kinases Essential for NF-kappaB Activation. Science278, 860–866. 10.1126/science.278.5339.860
48
MowersJ.UhmM.ReillyS. M.SimonJ.LetoD.ChiangS. H.et al (2013). Inflammation Produces Catecholamine Resistance in Obesity via Activation of PDE3B by the Protein Kinases IKKε and TBK1. Elife2, e01119. 10.7554/eLife.01119
49
MuvaffakA.PanQ.YanH.FernandezR.LimJ.DolinskiB.et al (2014). Evaluating TBK1 as a Therapeutic Target in Cancers with Activated IRF3. Mol. Cancer Res.12, 1055–1066. 10.1158/1541-7786.MCR-13-0642
50
NakatsuY.MatsuokaM.ChangT. H.OtsukiN.NodaM.KimuraH.et al (2014). Functionally Distinct Effects of the C-Terminal Regions of IKKε and TBK1 on Type I IFN Production. PLoS One9, e94999. 10.1371/journal.pone.0094999
51
NomuraF.KawaiT.NakanishiK.AkiraS. (2000). NF-kappaB Activation through IKK-i-dependent I-TRAF/TANK Phosphorylation. Genes Cells5, 191–202. 10.1046/j.1365-2443.2000.00315.x
52
OralE. A.ReillyS. M.GomezA. V.MeralR.ButzL.AjluniN.et al (2017). Inhibition of IKKɛ and TBK1 Improves Glucose Control in a Subset of Patients with Type 2 Diabetes. Cel Metab26, 157–e7. e157. 10.1016/j.cmet.2017.06.006
53
OuY. H.TorresM.RamR.FormstecherE.RolandC.ChengT.et al (2011). TBK1 Directly Engages Akt/PKB Survival Signaling to Support Oncogenic Transformation. Mol. Cel41, 458–470. 10.1016/j.molcel.2011.01.019
54
PatelM. N.BernardW. G.MilevN. B.CawthornW. P.FiggN.HartD.et al (2015). Hematopoietic IKBKE Limits the Chronicity of Inflammasome Priming and Metaflammation. Proc. Natl. Acad. Sci. U S A.112, 506–511. 10.1073/pnas.1414536112
55
PerkinsN. D. (2007). Integrating Cell-Signalling Pathways with NF-kappaB and IKK Function. Nat. Rev. Mol. Cel Biol8, 49–62. 10.1038/nrm2083
56
PetersR. T.LiaoS.-M.ManiatisT. (2000). IKKε Is Part of a Novel PMA-Inducible IκB Kinase Complex. Mol. Cel5, 513–522. 10.1016/s1097-2765(00)80445-1
57
PomerantzJ. L.BaltimoreD. (1999). NF-kappaB Activation by a Signaling Complex Containing TRAF2, TANK and TBK1, a Novel IKK-Related Kinase. EMBO J.18, 6694–6704. 10.1093/emboj/18.23.6694
58
QuF.GaoH.ZhuS.ShiP.ZhangY.LiuY.et al (2012). TRAF6-dependent Act1 Phosphorylation by the IκB Kinase-Related Kinases Suppresses Interleukin-17-Induced NF-Κb Activation. Mol. Cel Biol32, 3925–3937. 10.1128/MCB.00268-12
59
RajsbaumR.VersteegG. A.SchmidS.MaestreA. M.Belicha-VillanuevaA.Martínez-RomeroC.et al (2014). Unanchored K48-Linked Polyubiquitin Synthesized by the E3-Ubiquitin Ligase TRIM6 Stimulates the Interferon-Ikkε Kinase-Mediated Antiviral Response. Immunity40, 880–895. 10.1016/j.immuni.2014.04.018
60
ReillyS. M.AhmadianM.ZamarronB. F.ChangL.UhmM.PoirierB.et al (2015). A Subcutaneous Adipose Tissue-Liver Signalling axis Controls Hepatic Gluconeogenesis. Nat. Commun.6, 6047. 10.1038/ncomms7047
61
ReillyS. M.ChiangS. H.DeckerS. J.ChangL.UhmM.LarsenM. J.et al (2013). An Inhibitor of the Protein Kinases TBK1 and IKK-Ɛ Improves Obesity-Related Metabolic Dysfunctions in Mice. Nat. Med.19, 313–321. 10.1038/nm.3082
62
RennerF.MorenoR.SchmitzM. L. (2010). SUMOylation-Dependent Localization of IKKepsilon in PML Nuclear Bodies Is Essential for protection against DNA-Damage-Triggered Cell Death. Mol. Cel37, 503–515. 10.1016/j.molcel.2010.01.018
63
RobinsonD. R.WuY. M.LonigroR. J.VatsP.CobainE.EverettJ.et al (2017). Integrative Clinical Genomics of Metastatic Cancer. Nature548, 297–303. 10.1038/nature23306
64
RyzhakovG.RandowF. (2007). SINTBAD, a Novel Component of Innate Antiviral Immunity, Shares a TBK1-Binding Domain with NAP1 and TANK. EMBO J.26, 3180–3190. 10.1038/sj.emboj.7601743
65
Safari-AlighiarlooN.TaghizadehM.Mohammad TabatabaeiS.NamakiS.Rezaei-TaviraniM. (2020). Identification of Common Key Genes and Pathways between Type 1 Diabetes and Multiple Sclerosis Using Transcriptome and Interactome Analysis. Endocrine68, 81–92. 10.1007/s12020-019-02181-8
66
SaltielA. R.OlefskyJ. M. (2017). Inflammatory Mechanisms Linking Obesity and Metabolic Disease. J. Clin. Invest.127, 1–4. 10.1172/JCI92035
67
SanadaY.KumotoT.SuehiroH.YamamotoT.NishimuraF.KatoN.et al (2014). IκB Kinase Epsilon Expression in Adipocytes Is Upregulated by Interaction with Macrophages. Biosci. Biotechnol. Biochem.78, 1357–1362. 10.1080/09168451.2014.925776
68
SchejaL.HeeseB.SeedorfK. (2011). Beneficial Effects of IKKε-Deficiency on Body Weight and Insulin Sensitivity Are Lost in High Fat Diet-Induced Obesity in Mice. Biochem. Biophys. Res. Commun.407, 288–294. 10.1016/j.bbrc.2011.02.137
69
SharmaS.TenoeverB. R.GrandvauxN.ZhouG. P.LinR.HiscottJ. (2003). Triggering the Interferon Antiviral Response through an IKK-Related Pathway. Science300, 1148–1151. 10.1126/science.1081315
70
ShenR. R.HahnW. C. (2010). Emerging Roles for the Non-canonical IKKs in Cancer. Oncogene30, 631–641. 10.1038/onc.2010.493
71
ShimadaT.KawaiT.TakedaK.MatsumotoM.InoueJ.TatsumiY.et al (1999). IKK-i, a Novel Lipopolysaccharide-Inducible Kinase that Is Related to IkappaB Kinases. Int. Immunol.11, 1357–1362. 10.1093/intimm/11.8.1357
72
ShinC.ChoiD.-S. (2019). Essential Roles for the Non-canonical IκB Kinases in Linking Inflammation to Cancer, Obesity, and Diabetes. Cells8, 20178. 10.3390/cells8020178
73
SunS. C. (2017). The Non-canonical NF-Κb Pathway in Immunity and Inflammation. Nat. Rev. Immunol.17, 545–558. 10.1038/nri.2017.52
74
SweeneyS. E.HammakerD.BoyleD. L.FiresteinG. S. (2005). Regulation of C-Jun Phosphorylation by the I Kappa B Kinase-Epsilon Complex in Fibroblast-like Synoviocytes. J. Immunol.174, 6424–6430. 10.4049/jimmunol.174.10.6424
75
TanakaM.FuentesM. E.YamaguchiK.DurninM. H.DalrympleS. A.HardyK. L.et al (1999). Embryonic Lethality, Liver Degeneration, and Impaired NF-Kappa B Activation in IKK-Beta-Deficient Mice. Immunity10, 421–429. 10.1016/s1074-7613(00)80042-4
76
TenoeverB. R.NgS. L.ChuaM. A.McwhirterS. M.García-SastreA.ManiatisT. (2007). Multiple Functions of the IKK-Related Kinase IKKepsilon in Interferon-Mediated Antiviral Immunity. Science315, 1274–1278. 10.1126/science.1136567
77
TilgH.MoschenA. R.RodenM. (2017). NAFLD and Diabetes Mellitus. Nat. Rev. Gastroenterol. Hepatol.14, 32–42. 10.1038/nrgastro.2016.147
78
Uma MaheswariT. N.ShanmugasundaramP. (2013). Amlexanox in Treatment of Aphthous Ulcers: A Systematic Review. J. Pharm. Res.6, 214–217. 10.1016/j.jopr.2012.11.033
79
WangN.AhmedS.HaqqiT. M. (2005). Genomic Structure and Functional Characterization of the Promoter Region of Human IkappaB Kinase-Related Kinase IKKi/IKKvarepsilon Gene. Gene353, 118–133. 10.1016/j.gene.2005.04.013
80
WangS.XieF.ChuF.ZhangZ.YangB.DaiT.et al (2017). YAP Antagonizes Innate Antiviral Immunity and Is Targeted for Lysosomal Degradation through IKKɛ-Mediated Phosphorylation. Nat. Immunol.18, 733–743. 10.1038/ni.3744
81
WangT.BlockM. A.CowenS.DaviesA. M.DevereauxE.GingipalliL.et al (2012). Discovery of Azabenzimidazole Derivatives as Potent, Selective Inhibitors of TBK1/IKKε Kinases. Bioorg. Med. Chem. Lett.22, 2063–2069. 10.1016/j.bmcl.2012.01.018
82
WangX.LuJ.LiJ.LiuY.GuoG.HuangQ. (2021). CYT387, a Potent IKBKE Inhibitor, Suppresses Human Glioblastoma Progression by Activating the Hippo Pathway. J. Transl Med.19, 396. 10.1186/s12967-021-03070-3
83
WeirG. C.EhlersM. R.HarrisK. M.KanaparthiS.LongA.PhippardD.et al (2018). Alpha-1 Antitrypsin Treatment of New-Onset Type 1 Diabetes: An Open-Label, Phase I Clinical Trial (RETAIN) to Assess Safety and Pharmacokinetics. Pediatr. Diabetes19, 945–954. 10.1111/pedi.12660
84
XieX.ZhangD.ZhaoB.LuM. K.YouM.CondorelliG.et al (2011). IkappaB Kinase Epsilon and TANK-Binding Kinase 1 Activate AKT by Direct Phosphorylation. Proc. Natl. Acad. Sci. U S A.108, 6474–6479. 10.1073/pnas.1016132108
85
XuJ.JiaY.-F.TapadarS.WeaverJ. D.RajiI. O.PithadiaD. J.et al (2018). Inhibition of TBK1/IKKε Promotes Regeneration of Pancreatic β-cells. Sci. Rep.8, 15587. 10.1038/s41598-018-33875-0
86
YuanM.KonstantopoulosN.LeeJ.HansenL.LiZ. W.KarinM.et al (2001). Reversal of Obesity- and Diet-Induced Insulin Resistance with Salicylates or Targeted Disruption of Ikkbeta. Science293, 1673–1677. 10.1126/science.1061620
87
ZhangJ.FengH.ZhaoJ.FeldmanE. R.ChenS. Y.YuanW.et al (2016). IκB Kinase ε Is an NFATc1 Kinase that Inhibits T Cell Immune Response. Cell Rep16, 405–418. 10.1016/j.celrep.2016.05.083
88
ZhangQ.LenardoM. J.BaltimoreD. (2017). 30 Years of NF-Κb: A Blossoming of Relevance to Human Pathobiology. Cell168, 37–57. 10.1016/j.cell.2016.12.012
89
ZhaoP.WongK. I.SunX.ReillyS. M.UhmM.LiaoZ.et al (2018). TBK1 at the Crossroads of Inflammation and Energy Homeostasis in Adipose Tissue. Cell172, 731–e12. e712. 10.1016/j.cell.2018.01.007
90
ZhouA. Y.ShenR. R.KimE.LockY. J.XuM.ChenZ. J.et al (2013). Ikkε-Mediated Tumorigenesis Requires K63-Linked Polyubiquitination by a cIAP1/cIAP2/TRAF2 E3 Ubiquitin Ligase Complex. Cel Rep3, 724–733. 10.1016/j.celrep.2013.01.031
91
ZhouZ.QiJ.LimC. W.KimJ. W.KimB. (2020a). Dual TBK1/IKKε Inhibitor Amlexanox Mitigates Palmitic Acid-Induced Hepatotoxicity and Lipoapoptosis In Vitro. Toxicology444, 152579. 10.1016/j.tox.2020.152579
92
ZhouZ.QiJ.ZhaoJ.LimC. W.KimJ. W.KimB. (2020b). Dual TBK1/IKKɛ Inhibitor Amlexanox Attenuates the Severity of Hepatotoxin-Induced Liver Fibrosis and Biliary Fibrosis in Mice. J. Cel Mol Med24, 1383–1398. 10.1111/jcmm.14817
93
ZhuL.YangH.ChaoY.GuY.ZhangJ.WangF.et al (2021). Akt Phosphorylation Regulated by IKKε in Response to Low Shear Stress Leads to Endothelial Inflammation via Activating IRF3. Cell Signal80, 109900. 10.1016/j.cellsig.2020.109900
94
ZubairH.AzimS.SrivastavaS. K.AhmadA.BhardwajA.KhanM. A.et al (2016). Glucose Metabolism Reprogrammed by Overexpression of IKKε Promotes Pancreatic Tumor Growth. Cancer Res.76, 7254–7264. 10.1158/0008-5472.CAN-16-1666
Summary
Keywords
inhibitor of nuclear factor kappa-B kinase ε, nonalcoholic fatty liver disease, diabetes, obesity, metabolic diseases, amlexanox
Citation
Xiao Q-A, He Q, Li L, Song Y, Chen Y, Zeng J and Xia X (2022) Role of IKKε in the Metabolic Diseases: Physiology, Pathophysiology, and Pharmacology. Front. Pharmacol. 13:888588. doi: 10.3389/fphar.2022.888588
Received
03 March 2022
Accepted
19 April 2022
Published
19 May 2022
Volume
13 - 2022
Edited by
Emanuela Ricciotti, University of Pennsylvania, United States
Reviewed by
Chunjiong Wang, Tianjin Medical University, China
Tom Huxford, San Diego State University, United States
Updates
Copyright
© 2022 Xiao, He, Li, Song, Chen, Zeng and Xia.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Zeng, junzeng@live.com; Xuan Xia, xiaxuan_2004@163.com
†These authors have contributed equally to this work
This article was submitted to Inflammation Pharmacology, a section of the journal Frontiers in Pharmacology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.