Abstract
Background: In last 2 decades, there have been substantial changes in the utilization patterns of antihypertensive medicines following new clinical trials and the introduction of new treatment guidelines. The aim of this study was to analyze utilization and prescribing patterns regarding antihypertensive medicines in the Republic of Srpska, Bosnia and Herzegovina during an 11-years follow-up according to national and European treatment guidelines.
Methods: In this retrospective, observational study, medicine utilization data were analyzed between 2009–2019 period using the ATC/DDD methodology and expressed as the number of DDD/1,000 inhabitants/day (DID/TID). The medicine utilization 90% (DU90%) method was used for determine the quality of prescribing.
Results: During the observed period, the use of antihypertensive medicines increased more than 3-times (125.97 DDD/TID in 2009 vs 414.95 DDD/TID in 2019), corresponding to a rise in the prevalence of hypertensive patients from 91.7/1,000 to 186.3/1,000 in the same period. This was mainly driven by increased use of angiotensin converting enzyme inhibitors with 241.69%, beta blockers with 146.87%, calcium channel blockers with 251.55%, and diuretics with 178.95%. Angiotensin receptor blockers were the fastest growing group of antihypertensive medicines in this period and their utilization increased nearly 40 times.
Conclusions: The overall antihypertensive medicines utilization was largely influenced by national and ESH/ESC guidelines and strongly corresponded to the positive medicine list of the national health insurance fund. Antihypertensive medicines utilization is comparable with medicine utilization trends in other countries.
Introduction
Hypertension is the most common chronic disease of modern era and one of the leading causes of cardiovascular morbidity and mortality accounting for 10.4 million deaths globally per year (Global Health Metrics, 2018; Stanaway et al., 2018). In 2010, an estimated 1.39 billion people globally had hypertension (Mills et al., 2020). More recently, it is estimated that 1.28 billion people aged between 30 and 79 years have hypertension globally, greater in men than women, with an appreciable number unaware of their condition (NCD Risk Factor Collaboration (NCD-RisC), 2021; World Health Organisation, 2021a). Morbidity trends showed a clear shift away from high-income countries (HIC) to low and middle-income countries (LMIC) (NCD Risk Factor Collaboration (NCD-RisC), 2021; World Health Organisation, 2021a), with over a billion people with hypertension now living in LMICs (Mills et al., 2020). The most recent estimates suggest that, across both the European Union and non-European Union countries, the prevalence of hypertension is around 40% and tends to be higher in Central and Eastern European countries than other European countries (NCD Risk Factor Collaboration (NCD-RisC), 2021). This is reflected in the aged-standardized prevalence of hypertension among European countries varying for instance from 20.8% in Spain, 24.4% in France, 24.6% in Sweden, 25.0% in Germany, 26.1% in Belgium, and 26.2% in Greece to 38.9% in Latvia, 41.3% in Bosnia and Herzegovina, 42.7% in Poland and 45.3% in Croatia (NCD Risk Factor Collaboration (NCD-RisC), 2021; World Health Organization, 2019).
Several factors impact on prevalence rates. These include smoking rates, current lifestyles, income and education with some of these captured as part of human development indices (Tirapani and Fernandes, 2019; Zeng et al., 2020). Due to the growing global burden of cardiovascular diseases, international professional bodies, as well as the national health authorities in many countries, have developed and disseminated treatment guidelines in order to improve the management of hypertensive patients. This includes the World Health Organization (WHO), the International Society of Hypertension and those from European societies (Williams et al., 2018; Unger et al., 2020; Verdecchia et al., 2020; World Health Organisation, 2021b). The trends in the utilization of different medicines for the management of hypertension can provide important insight into how fast the therapeutic recommendations are being adopted in clinical practice and the impact of different therapeutic options on treatment outcomes. However, in both LMICs and HICs the ambiguities of the latest guidelines can be met with confusion among healthcare professionals, anxiety among patients, and the fear among healthcare providers that new treatments will additionally increase treatment costs (Rehan et al., 2017; Messerli and Bangalore, 2018; Poulter et al., 2018). This includes issues whether diuretics, calcium channel blockers (CCBs), diuretics combined with angiotensin-converting enzyme inhibitors (ACEIs) or with CCBs should be first line, and whether treatment approaches need to be adapted bases on age and ethnicity (Kaiser et al., 2014; Mbui et al., 2017; Benetos et al., 2019; Mashozhera et al., 2021). However, costs of medicines to treat hypertension and a number of other cardiovascular diseases are now less of an issue across Europe with these typically available as low-cost generics (Markovic-Pekovic et al., 2012), e.g., the prices of generic losartan were already 88% lower shortly after their availability in Scotland with similar low prices in Sweden, and prices of generic statins have been as low as 2%–4% of pre-patent loss prices among European countries (Woerkom et al., 2012; Bennie et al., 2013; Godman et al., 2013; Leporowski et al., 2018). The effectiveness of major antihypertensive treatment options including diuretics, CCBs, ACEIs, ARBs, and beta blockers (BBs) are well documented (Neal et al., 2000; Psaty et al., 2003; Mancia et al., 2013).
However, we are aware that the treatment of hypertension differs among European countries and between Europe and other geographic regions despite international and Pan-European guidance. Comparative data suggests that the use of antihypertensive treatment is lower in Europe (England 25%, Germany 26%, Spain 27%, and Italy 32%) than in the United States (53%) and Canada (36%) (Wolf-Maier et al., 2004). As mentioned, despite improvements in the availability of different antihypertensive medications with different side-effect profiles, an appreciable number of patients with arterial hypertension remain uncontrolled while on treatment (NCD Risk Factor Collaboration (NCD-RisC), 2021; World Health Organisation, 2021a). The European Study on Cardiovascular Risk Prevention and Management in Usual Daily Practice (EURIKA) published in 2016 documented a 51.6% prevalence of uncontrolled hypertension in Europe, ranging from 38.6% in Greece to 59.7% in Turkey (Borghi et al., 2016).
In 2003, the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC) jointly published guidelines for hypertension treatment that replaced the previous guidelines issued by the WHO and the International Society of Hypertension (ISH) (Zanchetti et al., 1993; Zanchetti, 20032003), which have subsequently been updated. A year later, the Cardiology Society of the Republic of Srpska published the first national guideline for hypertension in collaboration with the Ministry of Health and Social Welfare, and it became the leading guideline for hypertension used in daily practice among family physicians (Guidelines Committee, 2004; Pilipovic-Broceta et al., 2018). Furthermore, these guidelines were updated in 2009 and 2015 (Guidelines Committee, 2009; Ministry of Health and Social Welfare of Republic of Srpska, 2015), and together with the guidelines for other cardiovascular diseases, have significantly influenced medicine utilization patterns (Markovic-Pekovic et al., 2009; Marković-Peković et al., 2016).
Consequently, the aim of this study was firstly, to examine the 11-year trends in the prescribing of antihypertensive medicine in the Republic of Srpska. Secondly, to analyze the most prescribed classes of hypertensive medicines and the trends in their usage. Thirdly, the compatibility of the usage patterns seen versus national and European treatment guidelines. This is because adherence to guidelines is seen as a robust quality indicator with increased adherence to guidelines improving patient outcomes (Asmar et al., 2007; Mbui et al., 2017; NICE, 2017; Kamath et al., 2020; Mashozhera et al., 2021). We have shown in an earlier publication when researching medicines for hypertension prescribed among polypharmacy patients that prescribing appeared to compliment guideline recommendations; however, this was not formalized (Marković-Peković et al., 2016). Consequently, there was a need for this separate study. The findings can help to inform future activities in the Republic of Srpska and wider.
Materials and Methods
A retrospective, observational study was conducted to analyze the utilization of antihypertensive medicines of Republic of Srpska during the period 2009–2019. The Republic of Srpska is one of two constitutive entities of Bosnia & Herzegovina with a total population of 1.2 million (Republic of Srpska Institute of Statistics, 2020). It has executive and legislative functional responsibilities covering healthcare policies. The Ministry of Health and Social Welfare is responsible for planning, regulation and management functions, while the Health Insurance Fund (HIF) provides compulsory health insurance coverage for the entire population based on solidarity and mutuality (Markovic-Pekovic et al., 2012). All inhabitants in the Republic have equal and free access to universal health care, including the services at primary, secondary and tertiary health care levels provided in all health institutions, which have a contract with the HIF. Most of the services, including prescribed medicines, are reimbursed by the HIF. The list of reimbursed medicines, the so-called “positive list”, is based on Anatomical Therapeutic Chemical (ATC) classification and comprises medicine listed by international non-proprietary names (INN-ATC level 5) recommended by the guidelines of clinical practice (Markovic-Pekovic et al., 2012). The positive list is updated regularly, at least once a year, and published in the Official Gazette. The vast majority of antihypertensive medicines are included in the positive medicines list and prescribed by doctors at the primary healthcare level. These prescribed medicines are dispensed in community pharmacies as these are prescription medicines only. Medicines proposed by physicians at discharge from hospitals or at ambulatory care visits are also dispensed from community pharmacies and consequently are included in primary healthcare sector sales (Health Insurance Fund of Republic of Srpska, 2021a).
The medicines utilization data were retrieved from Public Health Institute (PHI) of the Republic of Srpska. The PHI is responsible for collecting medicines utilization data from all health institutions and preparing an annual report (Markovic-Pekovic et al., 2012). Medicines utilization analysis was undertaken using the ATC/DDD (Defined Daily Dose) methodology (World Health Organization, 2003), which is the internationally accepted methodology for measuring medicines utilization within and across populations (Godman et al., 2014a; Godman et al., 2014b; Moon et al., 2014). DDDs are defined as the amount of medicines most commonly used for the most common indication in adults. It is a suitable measure to describe and compare medicines utilization patterns between different geographical regions and health facilities (Godman et al., 2014a; Godman et al., 2014b; Moon et al., 2014; Marković-Peković et al., 2016). Data on outpatient medicines utilization are typically expressed in DDDs/1,000 inhabitants/day (DIDs/TID) for comparative purposes (World Health Organization, 2003; Vončina et al., 2011; Godman et al., 2014a). For assessing the quality of antihypertensive prescribing, the medicines utilization 90% (DU90%) parameter was used. DU90% reflects the number of medicines that account for 90% of medicine prescriptions and serve as a general quality indicator (Bergman et al., 1998; Gustafsson et al., 2011; Dhananjay et al., 2016). Changes in prescribing patterns are measured with the index of change, a statistical measure of changes in a representative group of individual data points.
The antihypertensive treatment recommendations were followed in line with the updated National Guidelines (2009 and 2015 Guidelines for arterial hypertension of Republic of Srpska (Guidelines Committee, 2009; Ministry of Health and Social Welfare of Republic of Srpska, 2015)), the Guidelines of the ESC (2018 Practice Guidelines for the Management of Arterial Hypertension of the ESH and the ESC (Williams et al., 2018)), and the actual prescription patterns were compared to similar data from published sources.
The number of patients with hypertension (I10-I25 code according to the WHO International Classification of Diseases–ICD-revision 10) was obtained from the HIF of the Republic of Srpska data information system. The data extraction was carried out anonymously, with patients fully de-identified and thus this investigation was exempt from review by the institutional review board. This is in line with previous publications for the Republic of Srpska (Markovic-Pekovic et al., 2009; Markovic-Pekovic et al., 2012; Marković-Peković et al., 2016).
Changes in prescribing patterns were presented with the index of change, a statistical measure of changes in a representative group reflecting the changes in prescribing in DDDs/1,000 in 2019 compared with 2009.
Results
During the 11-year period, there was a continual increase in the number of hypertensive patients in the Republic of Srpska. In 2009, the number of these patients was 108,051 with a prevalence of 91.7/1,000 people and in 2019 that number almost doubled with 212,874 patients and a prevalence of 186.3/1,000 people (Table 1).
TABLE 1
| Years | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Percent (%) of patients ≥65 years | 51.43 | 51.02 | 52.52 | 51.46 | 51.85 | 52.03 | 53.14 | 53.51 | 54.77 | 55.6 | 57.36 |
| Total number of hypertensive patients | 108051 | 115615 | 123708 | 132367 | 141633 | 151548 | 171146 | 184168 | 193910 | 201412 | 212874 |
| Prevalence of hypertension (‰) | 91.7 | 98.3 | 105.3 | 112.8 | 120.9 | 129.9 | 147.3 | 159.1 | 168.2 | 175.5 | 186.3 |
Percent of patients over 65 years, and total number and prevalence of patients with hypertension in the Republic of Srpska during the period 2009–2019.
Total medicine consumption also significantly increased during this period from 448.16 DDDs/TID in 2009 to 1,157.7 DDDs/TID in 2019. Out of this, the cardiovascular medicines (group C) were the most prescribed group of medicines accounting for 36.6%–42.04% of all medicines dispensed during this period. Among these, antihypertensive medicine was the most prescribed, with their total utilization increasing more than 3-fold from 125.98 DDDs/TID in 2009 to 414.95 DDDs/TID in 2019 (Table 2; Figures 1, 2).
TABLE 2
| Year | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall medicine use in DDD/TID | 448.16 | 622.31 | 746.57 | 731.55 | 764.28 | 841.68 | 861.23 | 969.54 | 1,036.31 | 1,225.86 | 1,157.70 |
| Group C DDD/TID | 163.81 | 242.89 | 294.4 | 285.39 | 300.85 | 338.72 | 348.74 | 422.86 | 460.17 | 502.43 | 486.93 |
| % | 36.55 | 39.03 | 39.43 | 39.01 | 39.36 | 40.24 | 40.49 | 43.61 | 44.40 | 41.00 | 42.10 |
| AHD DDD/TID | 125.98 | 188.65 | 233.42 | 228.79 | 247.97 | 280.88 | 296.38 | 361.54 | 397.46 | 428.36 | 414.95 |
| % | 28.11 | 30.31 | 31.27 | 31.27 | 32.44 | 33.37 | 34.41 | 37.29 | 38.35 | 34.94 | 35.84 |
The utilization of cardiovascular medicines (group C) and antihypertensive medicines expressed in DDD/TID and as a share (%) of the total medicine utilization in the Republic of Srpska during the period 2009–2019.
DDD, Defined Daily Dose; TID, Thousand Inhabitants per Day; AHD, Antihypertensive medicines according to ATC, classification.
FIGURE 1

The timeline of utilization of antihypertensive medicines in the Republic of Srpska and publication years of the most relevant international and national therapeutic guidelines (DDD–Defined Daily Dose; TID–Thousand Inhabitant per Day; ESC–the European Society of Cardiology ESH–the European Society of Hypertension).
FIGURE 2

The utilization of major antihypertensive medicine groups in the Republic of Srpska expressed as DDD/TID during the period 2009–2019. DDD–Defined Daily Dose; TID–Thousand Inhabitant per Day.
The most prescribed antihypertensive medicine class during this period belonged to ACEIs (C09), with their utilization increasing from 69.78 DDDs/TID in 2009 to 238.43 DDDs/TID in 2019, equivalent to an increase of 241.69%. This was followed by CCBs (C08), where their utilization continuously increased from 30.51 DDDs/TID in 2009, to 75.32 DDDs/TID in 2019, equivalent to 146.87%. The utilization of BBs (C07) increased from 11.93 DDDs/TID in 2009 to 41.94 DDDs/TID in 2019, an increase of 251.55%. The fastest growing subgroup of antihypertensive medicines were ARBs with an approximately 40-fold increase over the study period. This compared with the subgroup antihypertensives (C02), which comprises different types of antihypertensives including central and peripheral acting antiadrenergic agents, agents acting on arterial smooth muscles, and their combinations with diuretics and alkaloids, which showed a decrease of –33.53% (Table 2; Figure 2).
The ACEIs as monotherapy (C09A) were the leading most-prescribed subgroup with 46.67 DDDs/TID in 2009 rising to 165.12 DDDs/TID in 2019. The utilization of ACEIs in combination with diuretics, mainly with hydrochlorothiazide (HCTZ), increased by 217.22% in the observed period (Table 3; Figure 3A,B). Among the agents acting on the renin-angiotensin system (C09), the highest growth rates were in the ARB subgroup (C09D); from 0.28 DDDs/TID in 2009 to 12.23 DDDs/TID in 2019 (Table 3; Figure 4).
TABLE 3
| ATC | Pharmacological Groups and Subgroups | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DDDs/TID | ||||||||||||
| C09A | ACEI | 46.67 | 70.89 | 93.68 | 91.55 | 96.76 | 109.28 | 118.74 | 147.16 | 154.80 | 167.65 | 165.12 |
| C09B | ACEI + HCTZ | 23.11 | 33.49 | 41.13 | 39.18 | 45.09 | 48.82 | 56.94 | 64.73 | 78.17 | 79.16 | 73.31 |
| C08 | CCB | 30.51 | 42.48 | 50.29 | 48.42 | 53.85 | 64.21 | 56.55 | 69.95 | 72.41 | 79.06 | 75.32 |
| C07 | BB | 11.93 | 16.35 | 21.26 | 20.61 | 22.79 | 25.54 | 26.88 | 34.07 | 40.18 | 42.70 | 41.94 |
| C03 | DU | 11.40 | 20.97 | 20.50 | 21.70 | 20.34 | 21.97 | 24.36 | 27.04 | 28.86 | 32.39 | 31.80 |
| C09C | ARB | 0.37 | 1.04 | 1.98 | 2.62 | 3.44 | 4.82 | 6.08 | 8.66 | 10.94 | 13.33 | 14.10 |
| C09D | ARB + HCTZ | 0.28 | 0.95 | 1.94 | 2.44 | 3.35 | 4.85 | 5.61 | 8.68 | 10.93 | 12.78 | 12.23 |
| C02 | AH | 1.70 | 2.48 | 2.63 | 2.27 | 2.35 | 1.37 | 1.22 | 1.25 | 1.17 | 1.29 | 1.13 |
| Total DDDs/TID | 125.97 | 188.65 | 233.41 | 228.79 | 247.97 | 280.86 | 296.38 | 361.54 | 397.46 | 428.36 | 414.95 | |
The utilization of antihypertensive medicine groups and subgroups expressed as DDD/TID in the Republic of Srpska during the period 2009–2019.
ACEI, Angiotensin Converting Enzyme Inhibitors; CCB, Calcium Channel Blockers; BB, Beta Blockers; DU, Diuretics; ARB, Angiotensin Receptor Blockers; HCTZ, hydrochlorothiazide; AH, antihypertensives.
FIGURE 3

The utilization of ACEI as monotherapy (A) and ACEI in combination with diuretics (B), expressed as DDD/TID in the Republic of Srpska from 2009 to 2019 (ACEI–Angiotensin Converting Enzyme Inhibitros; DDD–Defined Daily Dose; TID–Thousand Inhabitant per Day).
FIGURE 4
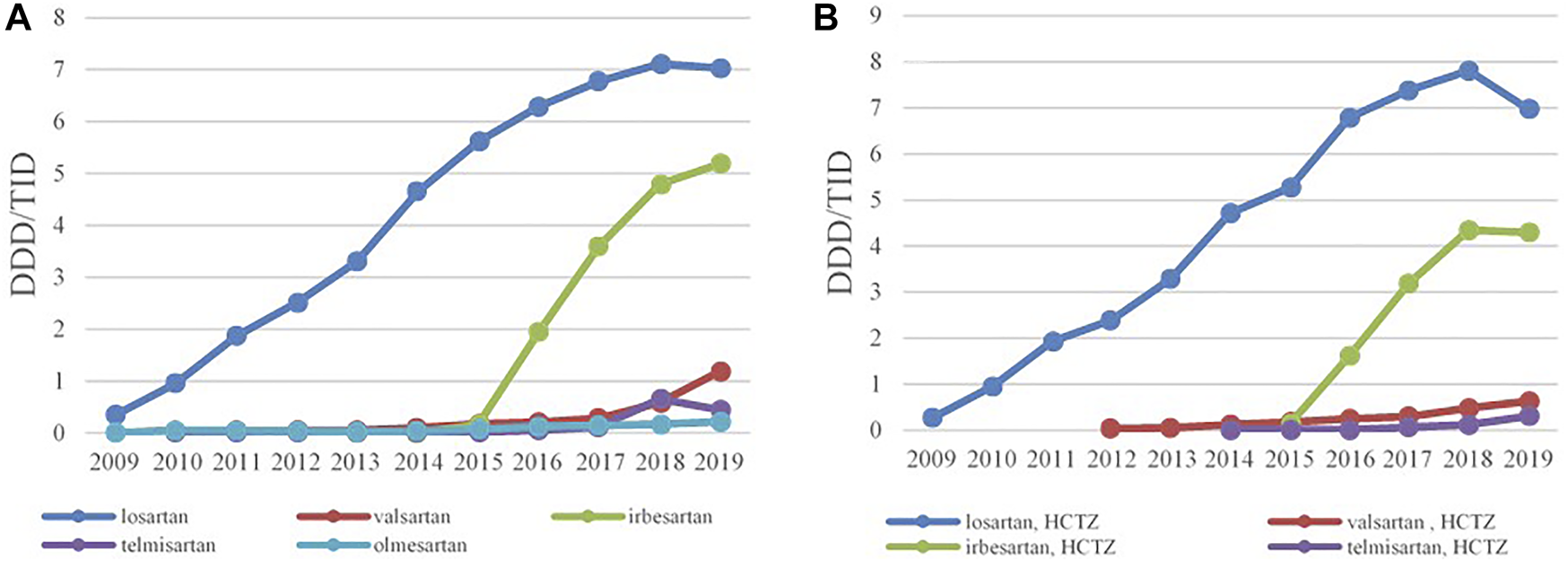
The utilization of ARBs as monotherapy (A) and ARBs in combination with HCTZ (B), expressed as DDD/TID in the Republic of Srpska from 2009 to 2019 (ARB–Angiotensin Receptor Blockers; HCTZ–Hydrochlorothiazide; DDD–Defined Daily Dose; TID–Thousand Inhabitant per Day).
Overall, enalapril was the most-prescribed ACEI during the 11-year follow-up period with 34.27 DDDs/TID in 2009, reaching the 90.37 DDDs/TID in 2019, an increase of 163.7%. Ramipril was the second most prescribed medicine in this subgroup with its utilization increasing more than 13 times, from 3.11 DDDs/TID in 2009 to 41.44 DDDs/TID in 2019 (Figure 3A), while lisinopril was the third most prescribed ACE inhibitor in 2019 with 15.34 DDDs/TID. Among the fixed combination of ACEI and diuretics, the greatest utilization belonged to the enalapril-HCTZ combination, with 15.52 DDDs/TID in 2009 and 43.20 DDDs/TID in 2019, showing the increase rate of 178.35%, followed by the lisinopril-HCTZ combination with 10.98 DDDs/TID in 2019 (Figure 3B).
Losartan was the leading ARB, both as monocomponent medicine as well as in combination with HCTZ, and its utilization has increased constantly from 0.35 DDDs/TID and 0.28 DDDs/TID in 2009 to 7.03 DDDs/TID and 6.98 DDDs/TID in 2019, respectively. Since 2015, irbesartan has become the second most prescribed ARB, both as monotherapy and in combination with HCTZ, followed by valsartan and telmisartan (Figure 4A,B).
Amlodipine was the most prescribed CCBs with 25.21 DDDs/TID in 2009 and 51.92 DDDs/TID in 2019, with the increase rate of 105.95%. The second most prescribed CCB was lercanidipine whose consumption has started to increase significantly from 2013 (Figure 5A). The prescribing pattern of BBs have appreciably changed during the study period. Until 2017, metoprolol was the most-prescribed BBs reaching 17.3 DDDs/TID, but in a following 2 years the utilization of metoprolol started to decline, while bisoprolol became the leading BBs with 15.74 DDDs/TID in 2019, followed by nebivolol with 5.63 DDDs/TID (Figure 5B).
FIGURE 5
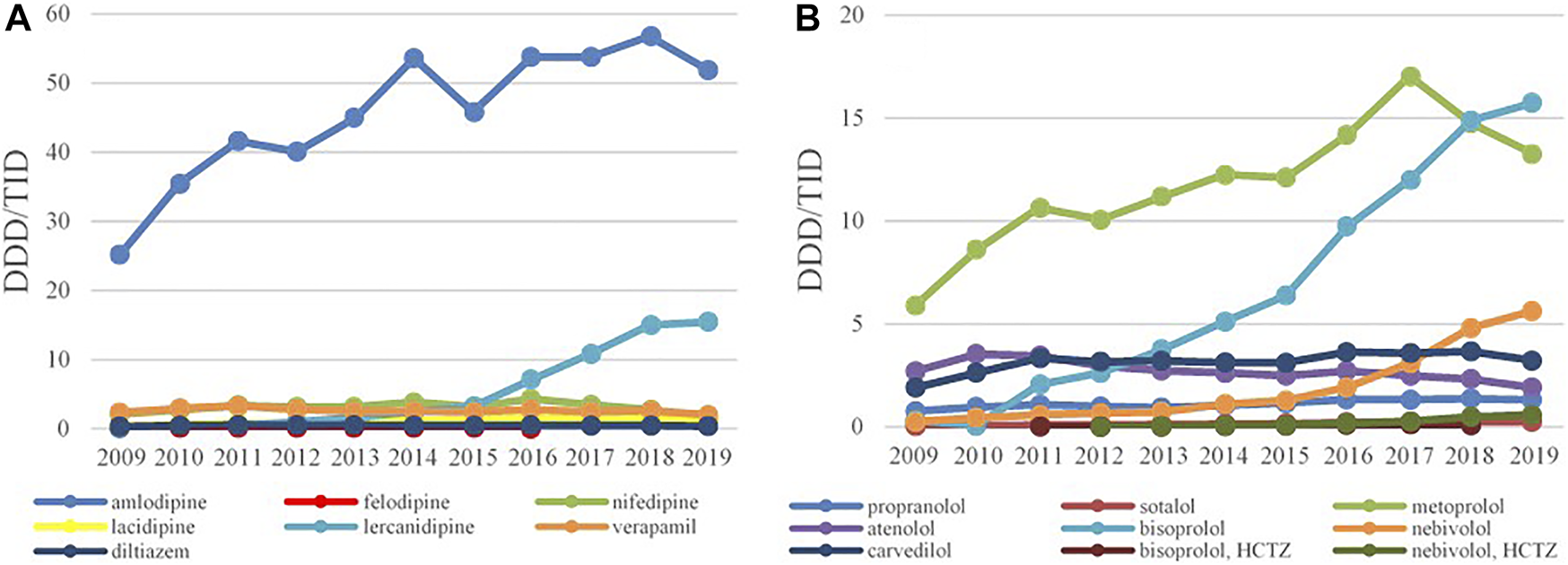
The utilization of CCB (A) and BB (B), expressed as DDD/TID in the Republic of Srpska from 2009 to 2019 (CCB–Calcium Channel Blockers; BB–Beta Blockers; DDD–Defined Daily Dose; TID–Thousand Inhabitant per Day).
Among the diuretics, furosemide had the biggest growth rate of 267.1%; its utilization increased from 5.53 DDDs/TID in 2009 to 20.30 DDDs/TID in 2019 (Figure 6A). The use of other diuretics such as HCTZ, spironolactone, indapamide and torasemide increased modestly over time reaching 3.09, 3.0, 3.40 and 0.69 DDDs/TID in 2019, respectively.
FIGURE 6

The utilization of diuretics (A) and ‘antihypertensives’ (B), expressed as DDD/TID in the Republic of Srpska from 2009 to 2019 (DDD–Defined Daily Dose; TID–Thousand Inhabitant per Day).
In the subgroup of ‘antihypertensives’ doxazosin and methyldopa were the leading medicines although their utilization was very low, usually less than one and 0.5 DDDs/TID, respectively, followed by methyldopa with the use less than 0.5 DDDs/TID (Figure 6B). Due to unproven therapeutic effect and increased potential for side effects he fixed dose combination containing reserpine and clopamide with dihydroegocristine was completely withdrawn from the positive medicine list in 2013.
Despite the fact that the total prescribing of all classes of antihypertensive medicines increased during the investigated period, the utilization share between the subgroups did not appreciably change. ACEIs as mono therapy or in combination with a diuretic were the leading antihypertensive medicines dispensed during the study period with a 47% share of prescriptions dispensed on a DDDs basis. The CCBs were the second most commonly dispensed although their relative contribution decreased from 24.2% to 18.2%. This was followed by BBs with 10.1% and diuretics with 7.6% of total utilization. The ARBs, both as monotherapy and in combination with HCTZ, remained constant during the period of time, reaching 3.2% of the annual share in 2019 (Figure 7).
FIGURE 7
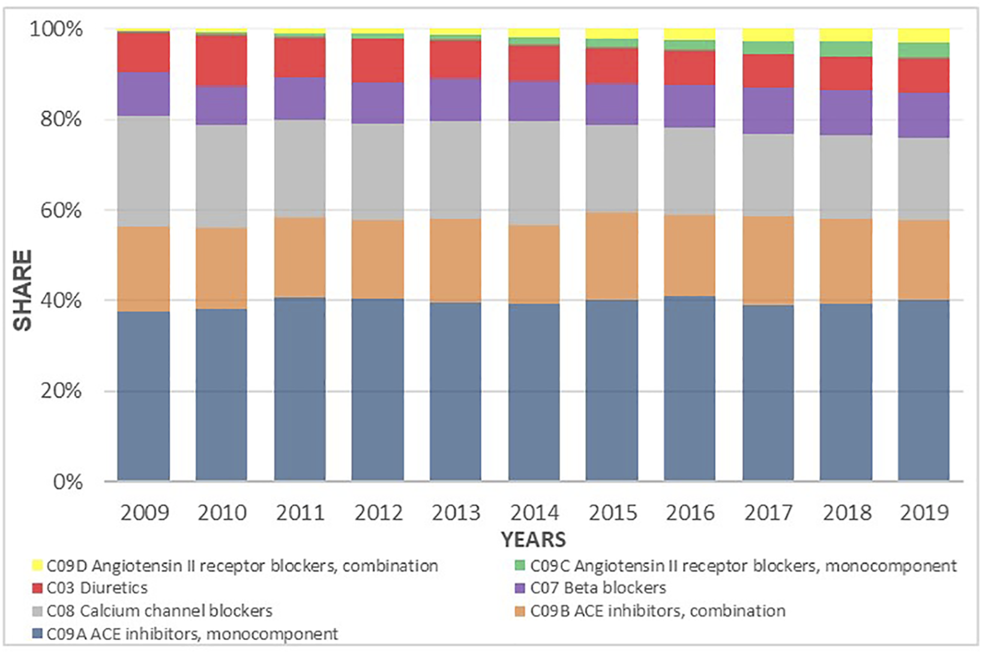
Utilization share of antihypertensive medicines groups and subgroups from 2009 to 2019 expressed in percentages.
The number of antihypertensive medicines in the DU90% segment varied from 16 in 2009 to 18 in 2015 and 19 in 2019. The DU90% segment in 2009 included all antihypertensive subgroups except ARBs (C09C, C09D), and antihypertensives (C02), while in 2015 and 2019 it included all except antihypertensives (C02). The number of fixed dose combinations of ACEI with HCTZ in the DU90% segment increased from two in 2009 to four in 2015 and 2019. No fixed dose combinations of ARB with HCTZ were seen within DU90% segment in 2009, but in 2019 two medicine combinations of this type were present (Table 4).
TABLE 4
| No | 2009 | 2015 | 2019 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ATC | % | DDD/TID | ATC | % | DDD/TID | ATC | % | DDD/TID | |
| 1 | C09AA02 | 27.2 | 34.27 | C09AA02 | 27.11 | 80.31 | C09AA02 | 21.99 | 90.37 |
| 2 | C08CA01 | 20.03 | 25.21 | C08CA01 | 15.48 | 45.86 | C08CA01 | 12.63 | 51.92 |
| 3 | C09BA02 | 12.33 | 15.52 | C09BA02 | 13.2 | 39.09 | C09BA02 | 10.51 | 43.2 |
| 4 | C07AB02 | 4.68 | 5.9 | C09AA05 | 5.94 | 17.59 | C09AA05 | 10.08 | 41.44 |
| 5 | C03CA01 | 4.39 | 5.53 | C03CA01 | 4.57 | 13.53 | C03CA01 | 4.94 | 20.3 |
| 6 | C09BA06 | 4.03 | 5.08 | C07AB02 | 4.1 | 12.12 | C07AB07 | 3.83 | 15.74 |
| 7 | C09AA05 | 2.47 | 3.11 | C09AA03 | 2.72 | 8.07 | C08CA13 | 3.77 | 15.5 |
| 8 | C09AA06 | 2.32 | 2.92 | C09BA03 | 2.66 | 7.89 | C09AA03 | 3.73 | 15.34 |
| 9 | C07AB03 | 2.15 | 2.71 | C07AB07 | 2.15 | 6.38 | C07AB02 | 3.23 | 13.26 |
| 10 | C08DA01 | 1.87 | 2.36 | C09BA06 | 1.94 | 5.76 | C09BA03 | 2.67 | 10.98 |
| 11 | C09AA03 | 1.71 | 2.15 | C09CA01 | 1.9 | 5.62 | C09AA06 | 1.71 | 7.04 |
| 12 | C09AA01 | 1.69 | 2.13 | C09DA01 | 1.78 | 5.27 | C09CA01 | 1.71 | 7.03 |
| 13 | C08CA05 | 1.69 | 2.13 | C09AA06 | 1.54 | 4.55 | C09DA01 | 1.7 | 6.98 |
| 14 | C07AG02 | 1.52 | 1.91 | C09AA01 | 1.32 | 3.9 | C09BA06 | 1.68 | 6.91 |
| 15 | C03AA03 | 1.18 | 1.49 | C09AA09 | 1.26 | 3.74 | C07AB12 | 1.37 | 5.63 |
| 16 | C03EA01 | 1.14 | 1.44 | C09BA05 | 1.2 | 3.54 | C09BA05 | 1.61 | 6.61 |
| 17 | — | — | — | C03AA03 | 1.08 | 3.21 | C09CA04 | 1.26 | 5.19 |
| 18 | — | — | — | C08CA13 | 1.08 | 3.2 | C09AA09 | 1.2 | 4.95 |
| 19 | — | — | — | — | — | — | C09DA04 | 1.05 | 4.3 |
| DU90% 1–16 (average) | — | 90.4 | 113.86 | — | 91.03 | 269.63 | — | 90.67 | 372.69 |
| Others 17–55 (average) | — | 9.6 | 12.14 | — | 8.97 | 26.6 | — | 9.33 | 38.3 |
| Total 1–55 | — | 100 | 126 | — | 100 | 296.23 | — | 100 | 410.99 |
Antihypertensive medicines utilization expressed in DDDs/TID as DU90% segment in the Republic of Srpska during 3 years, 2009, 2015 and 2019.
HCTZ, hydrochlorothiazide; C09AA02-enalapril; C08CA01-amlodipine; C09BA02-enalapril, HCTZ; C09AA05-ramipril; C03CA01-furosemide; C07AB07-bisoprolol; C08CA13-lercanidipine; C09AA03-lisinopril; C07AB02-metoprolol; C09BA03-lisinopril, HCTZ; C09AA06-quinapril; C09CA01-losartan; C09DA01-losartan, HCTZ; C09BA06-quinapril, HCTZ; C07AB12-nebivolol; C09BA05-ramipril, HCTZ; C09CA04-irbesartan; C09AA09-fosinopril; C09DA04-irbesartan, HCTZ; C07AB03-atenolol; C08DA01-verapamil; C09AA01-captopril; C08CA05-nifedipine; C07AG02-carvedilol; C03AA03-HCTZ; C03EA01-amilorid, HCTZ.
Discussion
The results of this study showed that during the past 11 years, the utilization of antihypertensive medicine in the Republic of Srpska increased more than three times with similar trend already seen in our earlier study (Markovic-Pekovic et al., 2009). At the same time, the number of hypertensive patients increased more than two-fold. The explanation for this appreciable increase in prevalence rates in such a short period could be partly explained by improved preventive measures advocating the prescribing of antihypertensives and a better data collecting system. However, the potential increase in the number of hypertensive patients cannot be neglected especially with growing elderly populations. This reflects a general increase in the number of patients with hypertension in LMICs in recent years.
The additional reason for the increased use of antihypertensive medicines in this period could be explained by better identification and implementation of national and ESC/ESH guidelines for the treatment of hypertension and coronary heart disease in the Republic of Srpska. It is clearly stated in these guidelines that all antihypertensive medicines are suitable and available for the treatment of hypertension, either as mono- or combination therapy, and that treatment should be initiated at the early stage of the disease (Mancia et al., 2013). Based on that recommendations, all hypertensive patients should be treated with low doses of thiazides or thiazide-like diuretics, and that ACEIs, ARBs, CCBs or BBs should be prescribing taking into consideration the patient’s health status and the presence of any comorbidities (Mancia et al., 2013; Ministry of Health and Social Welfare of Republic of Srpska, 2015). It is important to mention that recommendations in the national guidelines for treatment options and combination therapy in the Republic of Srpska have been fully harmonized with the ESH/ESC guidelines (Zanchetti, 20032003; Guidelines Committee, 2004; Guidelines Committee, 2009; Perk et al., 2012; Ministry of Health and Social Welfare of Republic of Srpska, 2015; Williams et al., 2018).
The trends of antihypertensive medicines use are also influenced by registration status and national drug policy presented by HIF positive medicine lists. Based on the HIF rules, the family practitioners as exclusive prescribers at the primary healthcare level are obliged to prescribe medicines listed on positive medicine list according to INN (Health Insurance Fund of Republic of Srpska, 2021a; Health Insurance Fund of Republic of Srpska, 2021b). The appreciable increase in the utilization of cardiovascular medicines from 163.81 DDD/TID in 2009 to 486.93 DDD/TID in 2019 is similar to the previously published studies form neighboring countries including Croatia and Serbia, as well as other European countries (Tomas et al., 2016; Lisauskienė et al., 2017; Sundbøll et al., 2017; Kucan et al., 2018). The significant usage of ACEIs seen in this study, rising more than three-fold during the study period, was also seen in the DU90% profile–out of 16 medicines within DU90% in 2009, seven were ACEIs and their combinations with diuretics. The number of these antihypertensives within the DU90% was ten of 18 in 2015 and nine of 19 in 2019. However, diuretics, which are recommended as first line therapy are ranked as fourth in total antihypertensive medicine utilization (National Institute for Health and Care Excellence, 2011; Borghi et al., 2020).
We believe this appreciable increase in the prescribing of ACEIs during the study period was fuelled by the HOPE (Yusuf et al., 2000) and EUROPA (Fox et al., 2003) trials, and confirmed by ESH/ESC guidelines (Mancia et al., 20072007) that were adopted by National Cardiology Society of the Republic of Srpska in the national Guidelines for Arterial Hypertension, Second Update from 2009 (Guidelines Committee, 2009). These medicines are primarily used to treat hypertension and congestive heart failure as monotherapy or in combination with diuretics. The fixed-dose combinations also offer the potential to lower blood pressure more quickly with decreased adverse effects (Frank, 2008). It has been clearly emphasized and confirmed that low-dose combinations of antihypertensive agents in general are more effective in blood pressure lowering and with fewer side effects than high-dose monotherapies (Atkins and Chow, 2020).
During 2009 to 2019, the use of ARBs also significantly increased. This was mainly due to the sustained increase in losartan prescribing and further reinforced by adding irbesartan, valsartan and telmisartan on the HIF positive medicine lists following national Guidelines on Secondary Prevention of Coronary Heart Disease from 2011 (Vulić, 2013) and Guidelines for Arterial Hypertension from 2015 (Ministry of Health and Social Welfare of Republic of Srpska, 2015). The ARBs were not present on DU90% profile in 2009, but in 2015 and 2019 this group was presented with two and four INNs, respectively, due to generic availability of these medicines in the country. We believe this steady increase in the prescribing of ARBs was based on favorable results of clinical trials demonstrating beneficial effects of valsartan (Cohn and Tognoni, 2001), losartan (Dahlöf et al., 2002) and candesartan (Young et al., 2004) on various outcomes in patients with hypertension or hearth failure, as well as with better tolerability and less side effects compared to ACEIs.
CCBs were the second most prescribed group of antihypertensive medicines with a 3.5-fold increase in 2019 compared to 2009, with amlodipine accounting for more than 80% of all CCBs in 2009 and 62% in 2019. The favorable effects of amlodipine in reducing major cardiovascular events in patients with hypertension shown in clinical trials, including VALUE (Julius et al., 2004), ACCOT-BPLA (Dahlöf et al., 2005) and ACCOMPLISH (Jamerson et al., 2008), may well have enhanced its use. Moreover, amlodipine as a long-acting medicine, has shown more acceptable safety profile with better patient compliance than previously used nifedipine. The use of lercanidipine significantly increased after it was introduced in the national guidelines and the HIF positive medicine list in 2011 due to its lower incidence of side effects and its availability as a generic medicine.
It has become obvious that most patients in the Republic of Srpska and globally require two or more antihypertensive medicines to achieve blood pressure control. Fixed-dose combinations (FDCs) of ACEIs or ARBs with diuretics or CCBs can help with improved patient compliance compared with multiple medicine administration and improve outcomes (Kawalec et al., 2018; Verma et al., 2018; Webster et al., 2018; Benjamin et al., 2019; Hassanein, 2020). These medicines, mainly ARBs and their combinations with diuretics are widely used in Norway, Finland, Denmark, with well-developed pharmacotherapeutic practices [ (NSDUH, 2014; Norwegian Institute for Public Health, 2014; Sundbøll et al., 2017). Similar trends were observed in studies from Croatia and Serbia (Tomas et al., 2016; Kucan et al., 2018). However, there can be concerns with an increase in medication errors with FDCs in patients with hypertension (Moriarty et al., 2019).
The reason for the increasing use of bisoprolol could be partly explained by the results of CIBIS-II (Dargie and Lechat, 1999) trial which demonstrated a relative risk reduction in all-cause mortality for patients with heart failure. The significant increase in furosemide prescribing during the study period could be due to an increase in the number of patients with comorbidities, particularly heart failure and those with poorly controlled and/or resistant hypertension. The prescribing of indapamide increased more than 8 times during the study period supported by HYVET trial, which provided evidence that antihypertensive treatment with indapamide reduces all-cause mortality in older patients (Kostis, 2008). Both, national and international guidelines clearly stated the superiority and effectiveness of the thiazide diuretics in the treatment of hypertension in the elderly, but their consumption in the Republic of Srpska was unreasonably low (National Institute for Health and Care Excellence, 2011; Williams et al., 2018). This may be due to their well-known side effects and concerns with adherence in this patient population leading to increased prescribing of FDCs. We will be exploring this further in future studies.
Based on our findings, it can be seen that the implementation of guidelines has influenced the prescribing patterns of antihypertensive medicines, especially ACEIs and ARBs with thiazides, during the study period. The main changes in the utilization patterns seen strongly correlated with the recommendations proposed by national and international guidelines for hypertension, for instance in 2015, where CCBs, ACEIs and ARBs as monotherapy were switched to combination therapy with diuretics. However, the findings regarding the utilization of diuretics showed that some factors, other than clinical guidelines, including personal or previous experiences have influenced physicians prescribing habits. Alongside this medicine promotion, media coverage, market competition and purchasing contracts may influence physician prescribing practices; however, medicines outside the positive list are subject to high patient co-payment levels impacting on their influence in practice. It is clear that treatment guidelines has improved doctors prescribing practices, not only in having the better control of patients with hypertension, but also in providing them with most appropriate treatment options according to the specific patient’s needs and comorbidities. However, in this study we did not deal with treatment outcomes, and at this moment it would be difficult to prove that implementation of clinical guidelines has been associated with significant changes in major adverse cardiovascular events.
In addition to guidelines on treating hypertension that are widely available, it is becoming more important to perform pharmacoepidemilogic analyses that might reveal the trends of the most frequently used medicine groups as initial therapy. Subsequently, based on the findings, it would be reasonable to redefine treatment strategies in order to improve prescribing habits especially if this revolves around new national robust treatment guidelines.
Strengths and Limitations
Pharmacoepidemiological analyses serve as an important tool for health professionals to track down medicinal expenditures over the period of time and to improve their prescribing skills, which is important on this occasion to help reach therapeutic goals in hypertension treatment.
This study has several limitations. First, it was unable to link the medicine utilization database to patient’s diagnoses including the number of patients with comorbidities that could influence the medicine utilization figures. Second, this study cannot account for the actual use of antihypertensive medications since treatment adherence has always been a problem in the management of hypertension. However, it is unlikely that these factors could significantly influence the overall figures of utilization of antihypertensive medicines in observed period.
Conclusion
The present study showed an appreciable increase in antihypertensive medicine utilization in the Republic of Srpska between 2009 and 2019. The overall increase was driven by increased prescribing of ACEIs (enalapril, ramipril, lisinopril, both as monotherapy and in combination with thiazides), BBs (metoprolol, bisoprolol, nebivolol), CCBs (amlodipine, lercanidipine), ARBs (losartan, irbesartan, both as monotherapy and in combination with thiazides) and diuretics (furosemide). These results are comparable with results from other countries. Except for diuretics, the overall antihypertensive medicine utilization was largely influenced with national and ESH/ESC guidelines for hypertension.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
AsmarR.AchoubaA.BrunelP.El FeghaliR.DenolleT.VaisseB. (2007). A Specific Training on Hypertension Guidelines Improves Blood Pressure Control by More Than 10% in Hypertensive Patients: the VALNORM Study. J. Am. Soc. Hypertens.1, 278–285. 10.1016/j.jash.2007.04.005
2
AtkinsE. R.ChowC. K. (2020). Low-Dose Combination Therapy for Initial Treatment of Hypertension. Curr. Hypertens. Rep.22, 65. 10.1007/s11906-020-01069-7
3
BenetosA.StrandbergT.PetrovicM. (2019). Response by Benetos et al to Letter Regarding Article, "Hypertension Management in Older and Frail Older Patients". Circ. Res.125, e3–e4. 10.1161/CIRCRESAHA.119.315270
4
BenjaminI. J.KreutzR.OlsenM. H.SchutteA. E.Lopez-JaramilloP.FriedenT. R.et al (2019). Fixed-dose Combination Antihypertensive Medications. Lancet394, 637–638. 10.1016/S0140-6736(19)31629-0
5
BennieM.BishopI.GodmanB.CampbellS.MirandaJ.FinlaysonA. E.et al (2013). Are Prescribing Initiatives Readily Transferable across Classes: the Case of Generic Losartan in Scotland?Qual. Prim. Care21, 7–15.
6
BergmanU.PopaC.TomsonY.WettermarkB.EinarsonT. R.AbergH.et al (1998). Drug Utilization 90%--a Simple Method for Assessing the Quality of Drug Prescribing. Eur. J. Clin. Pharmacol.54, 113–118. 10.1007/s002280050431
7
BorghiC.TubachF.de BackerG.DallongevilleJ.GuallarE.MedinaJ.et al (2016). Lack of Control of Hypertension in Primary Cardiovascular Disease Prevention in Europe: Results from the EURIKA Study. Int. J. Cardiol.218, 83–88. 10.1016/j.ijcard.2016.05.044
8
BorghiC.SoldatiM.BragagniA.CiceroA. F. G. (2020). Safety Implications of Combining ACE Inhibitors with Thiazides for the Treatment of Hypertensive Patients. Expert Opin. Drug Saf.19, 1577–1583. 10.1080/14740338.2020.1836151
9
CohnJ. N.TognoniG. (2001). A Randomized Trial of the Angiotensin-Receptor Blocker Valsartan in Chronic Heart Failure. N. Engl. J. Med.345, 1667–1675. 10.1056/NEJMoa010713
10
DahlöfB.DevereuxR. B.KjeldsenS. E.JuliusS.BeeversG.de FaireU.et al (2002). Cardiovascular Morbidity and Mortality in the Losartan Intervention for Endpoint Reduction in Hypertension Study (LIFE): A Randomised Trial against Atenolol. Lancet359, 995–1003. 10.1016/S0140-6736(02)08090-X
11
DahlöfB.SeverP. S.PoulterN. R.WedelH.BeeversD. G.CaulfieldM.et al (2005). Prevention of Cardiovascular Events with an Antihypertensive Regimen of Amlodipine Adding Perindopril as Required versus Atenolol Adding Bendroflumethiazide as Required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): A Multicentre Randomised Controlled Trial. Lancet366, 895–906. 10.1016/S0140-6736(05)67185-1
12
DargieH. J.LechatP. (1999). The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): A Randomised Trial. Lancet353, 9–13.
13
DhananjayK.KokilaB.SahuS.KumarG. (2016). Drug Utilization 90%: an Innovative Method in Assessing Quality of Drug Prescription with Specific Reference to Non-steroidal Anti-inflammatory Drugs Prescription. Int. J. Basic Clin. Pharmacol.5, 1746–1751. 10.18203/2319-2003.ijbcp20162893
14
FoxK. M.BertrandM.FerrariR.RemmeW. J.SimoonsM. L.SimoonsM.et al (2003). Efficacy of Perindopril in Reduction of Cardiovascular Events Among Patients with Stable Coronary Artery Disease: Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial (The EUROPA Study). Lancet362, 782–788. 10.1016/s0140-6736(03)14286-9
15
FrankJ. (2008). Managing Hypertension Using Combination Therapy. Am. Fam. Physician77, 1279–1286.
16
Global Health Metrics (2018). Global, Regional, and National Age-sex-specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980-2017: a Systematic Analysis for the Global Burden of Disease Study 2017. Lancet392, 1736–1788. 10.1016/S0140-6736(18)32203-7
17
GodmanB.WettermarkB.MirandaJ.BennieM.MartinA.MalmströmR. E. (2013). Influence of Multiple Initiatives in Sweden to Enhance ARB Prescribing Efficiency Following Generic Losartan; Findings and Implications for Other Countries. Int. J. Clin. Pract.67, 853–862. 10.1111/ijcp.12130
18
GodmanB.WettermarkB.van WoerkomM.FraeymanJ.Alvarez-MadrazoS.BergC.et al (2014). Multiple Policies to Enhance Prescribing Efficiency for Established Medicines in Europe with a Particular Focus on Demand-Side Measures: Findings and Future Implications. Front. Pharmacol.5, 106. 10.3389/fphar.2014.00106
19
GodmanB.PetzoldM.BennettK.BennieM.BucsicsA.FinlaysonA. E.et al (2014). Can Authorities Appreciably Enhance the Prescribing of Oral Generic Risperidone to Conserve Resources? Findings from across Europe and Their Implications. BMC Med.12, 98. 10.1186/1741-7015-12-98
20
Guidelines Committee (2004). Hypertension, Guideline for Clinical Practice. Banja Luka: Ministry of Health and Social Welfare of the Republic of Srpska (Accessed December 14, 2021).
21
Guidelines Committee (2009). Hypertension, Guideline for Clinical Practice. Banja Luka: Ministry of health and social welfare of the Republic of Srpska (Accessed December 14, 2021).
22
GustafssonL. L.WettermarkB.GodmanB.Andersén-KarlssonE.BergmanU.HasselströmJ.et al (2011). The 'wise List'- a Comprehensive Concept to Select, Communicate and Achieve Adherence to Recommendations of Essential Drugs in Ambulatory Care in Stockholm. Basic Clin. Pharmacol. Toxicol.108, 224–233. 10.1111/j.1742-7843.2011.00682.x
23
HassaneinM. (2020). Adherence to Antihypertensive Fixed-Dose Combination Among Egyptian Patients Presenting with Essential Hypertension. Egypt Heart J.72, 10. 10.1186/s43044-020-00044-6
24
Health Insurance Fund of Republic of Srpska (2021). The Rights of the Insured. Available at: https://www.zdravstvosrpske.org/files/regulativa/osig/Pravilnik%20o%20son%20ostvarivanja%20prava%20na%20zz.PDF (Accessed December 12, 2021).
25
Health Insurance Fund of Republic of Srpska (2021). Drug Lists. Available at: https://www.zdravstvo-srpske.org/files/cjenovnici/lijekovi.pdf (Accessed December 12, 2021).
26
JamersonK.WeberM. A.BakrisG. L.DahlöfB.PittB.ShiV.et al (2008). Benazepril Plus Amlodipine or Hydrochlorothiazide for Hypertension in High-Risk Patients. N. Engl. J. Med.359, 2417–2428. 10.1056/NEJMoa0806182
27
JuliusS.KjeldsenS. E.WeberM.BrunnerH. R.EkmanS.HanssonL.et al (2004). Outcomes in Hypertensive Patients at High Cardiovascular Risk Treated with Regimens Based on Valsartan or Amlodipine: The VALUE Randomised Trial. Lancet363, 2022–2031. 10.1016/S0140-6736(04)16451-9
28
KaiserE. A.LotzeU.SchäferH. H. (2014). Increasing Complexity: Which Drug Class to Choose for Treatment of Hypertension in the Elderly?Clin. Interv. Aging9, 459–475. 10.2147/CIA.S40154
29
KamathC. C.DoblerC. C.McCoyR. G.LampmanM. A.PajouhiA.ErwinP. J.et al (2020). Improving Blood Pressure Management in Primary Care Patients with Chronic Kidney Disease: a Systematic Review of Interventions and Implementation Strategies. J. Gen. Intern Med.35, 849–869. 10.1007/s11606-020-06103-7
30
KawalecP.HolkoP.GawinM.PilcA. (2018). Effectiveness of Fixed-Dose Combination Therapy in Hypertension: Systematic Review and Meta-Analysis. Arch. Med. Sci.14, 1125–1136. 10.5114/aoms.2018.77561
31
KostisJ. B. (2008). Treating Hypertension in the Very Old. N. Engl. J. Med.358, 1958–1960. 10.1056/NEJMe0801709
32
KucanM.Mrsic-PelcicJ.VitezicD. (2018). Antihypertensive Drugs in Croatia: What Changes the Drug Usage Patterns?Clin. Ther.40, 1159–1169. 10.1016/j.clinthera.2018.05.018
33
LeporowskiA.GodmanB.KurdiA.MacBride-StewartS.RyanM.HurdingS.et al (2018). Ongoing Activities to Optimize the Quality and Efficiency of Lipid-Lowering Agents in the Scottish National Health Service: Influence and Implications. Expert Rev. Pharmacoecon Outcomes Res.18, 655–666. 10.1080/14737167.2018.1501558
34
LisauskienėI.GaruolienėK.GulbinovičJ. (2017). Utilization of Cardiovascular Medicines and Cardiovascular Mortality in Lithuania, Sweden and Norway in 2003-2012. Med. Kaunas.53, 259–267. 10.1016/j.medici.2017.07.004
35
ManciaG.de BackerG.DominiczakA.CifkovaR.FagardR.GermanoG.et al (2007). 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J.28, 1462–1536. 10.1097/HJH.0b013e3281fc975a
36
ManciaG.FagardR.NarkiewiczK.RedonJ.ZanchettiA.BöhmM.et al (2013). 2013 ESH/ESC Guidelines for the Management of Arterial Hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J.34, 2159–2219. 10.1093/eurheartj/eht151
37
Markovic-PekovicV.Stoisavljevic-SataraS.SkrbicR. (2009). Utilisation of Cardiovascular Medicines in Republic of Srpska, Bosnia and Herzegovina, 5 Years Study. Pharmacoepidemiol Drug Saf.18, 320–326. 10.1002/pds.1704
38
Markovic-PekovicV.SkrbićR.GodmanB.GustafssonL. L. (2012). Ongoing Initiatives in the Republic of Srpska to Enhance Prescribing Efficiency: Influence and Future Directions. Expert Rev. Pharmacoecon Outcomes Res.12, 661–671. 10.1586/erp.12.48
39
Marković-PekovićV.ŠkrbićR.PetrovićA.Vlahović-PalčevskiV.MrakJ.BennieM.et al (2016). Polypharmacy Among the Elderly in the Republic of Srpska: Extent and Implications for the Future. Expert Rev. Pharmacoecon Outcomes Res.16, 609–618. 10.1586/14737167.2016.1115347
40
MashozheraS.BamitaleS. K.GodmanB.KibuuleD. (2021). Compliance to Hypertensive Prescribing Guidelines and Blood Pressure Control in Elderly Patients in Namibia: Findings and Implications. J. Pharm. Health Serv. Res.12, 53–60. 10.1093/jphsr/rmaa017
41
MbuiJ. M.OlukaM. N.GuantaiE. M.SineiK. A.AchiengL.BakerA.et al (2017). Prescription Patterns and Adequacy of Blood Pressure Control Among Adult Hypertensive Patients in Kenya; Findings and Implications. Expert Rev. Clin. Pharmacol.10, 1263–1271. 10.1080/17512433.2017.1371590
42
MesserliF. H.BangaloreS. (2018). The Blood Pressure Landscape: Schism Among Guidelines, Confusion Among Physicians, and Anxiety Among Patients. J. Am. Coll. Cardiol.72, 1313–1316. 10.1016/j.jacc.2018.07.026
43
MillsK. T.StefanescuA.HeJ. (2020). The Global Epidemiology of Hypertension. Nat. Rev. Nephrol.16, 223–237. 10.1038/s41581-019-0244-2
44
Ministry of Health and Social Welfare of Republic of Srpska (2015). Guideline for Clinical Practice: Arterial Hipertension. Available at: https://www.vladars.net/sr-SPCyrl/Vlada/Ministarstva/MZSZ/Documents/13.%204.%20Arterijska%20hipertenzija.pdf (Accessed December 12, 2021).
45
MoonJ. C.GodmanB.PetzoldM.Alvarez-MadrazoS.BennettK.BishopI.et al (2014). Different Initiatives across Europe to Enhance Losartan Utilization Post Generics: Impact and Implications. Front. Pharmacol.5, 219. 10.3389/fphar.2014.00219
46
MoriartyF.BennettK.FaheyT. (2019). Fixed-dose Combination Antihypertensives and Risk of Medication Errors. Heart105, 204–209. 10.1136/heartjnl-2018-313492
47
National Institute for Health and Care Excellence (2011). Hypertension: Clinical Management of Primary Hypertension in Adults (NICE Clinical Guideline 127). NICE. Available at: http://www.nice.org.uk/nicemedia/live/13561/56008/56008.pdf. (Accessed December 18, 2021).
48
NCD Risk Factor Collaboration (NCD-RisC) (2021). Worldwide Trends in Hypertension Prevalence and Progress in Treatment and Control from 1990 to 2019: a Pooled Analysis of 1201 Population-Representative Studies with 104 Million Participants. Lancet398, 957–980. 10.1016/S0140-6736(21)01330-1
49
NealB.MacMahonS.ChapmanN.CutlerJ.FagardR.WheltonP.et al (2000). Effects of ACE Inhibitors, Calcium Antagonists, and Other Blood-Pressure-Lowering Drugs: Results of Prospectively Designed Overviews of Randomised Trials. Blood Pressure Lowering Treatment Trialists' Collaboration. Lancet356, 1955–1964. 10.1016/s0140-6736(00)03307-9
50
NICE. Hypertension: Improving Blood Pressure Management in Primary Care (2017). Available at: https://www.nice.org.uk/sharedlearning/hypertension-improving-blood-pressure-management-in-primary-care [Accessed December 12, 2021].
51
Norwegian Institute for Public Health (2014). Drug Consumption in Norway 2004–2008. Available at: http://www.legemiddelforbruk.no/english (Accessed December 12, 2021).
52
NSDUH. (2014). National Agency for Medicines F. Drug Consumption Statistics. NSDUH. Available at: http://raportit.nam.fi/raportit/kulutus/laakekulutus_e.pdf. [Accessed December 12, 2021].
53
PerkJ.de BackerG.GohlkeH.GrahamI.ReinerZ.VerschurenM.et al (2012). European Guidelines on Cardiovascular Disease Prevention in Clinical Practice (Version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by Representatives of Nine Societies and by Invited Experts). Eur. Heart J.33, 1635–1701. 10.1093/eurheartj/ehs092
54
Pilipovic-BrocetaN.VasiljevicN.MarinkovicJ.TodorovicN.JankovicJ.OstricI.et al (2018). Assessment of Hypertension Chronic Care Model: Pacic Application in Bosnia and Herzegovina. PLoS ONE13, e0202250. 10.1371/journal.pone.0202250
55
PoulterN. R.CastilloR.CharcharF. J.SchlaichM. P.SchutteA. E.TomaszewskiM.et al (2018). Are the American Heart Association/American College of Cardiology High Blood Pressure Guidelines Fit for Global Purpose?: Thoughts from the International Society of Hypertension. Hypertension72, 260–262. 10.1161/HYPERTENSIONAHA.118.11452
56
PsatyB. M.LumleyT.FurbergC. D.SchellenbaumG.PahorM.AldermanM. H.et al (2003). Health Outcomes Associated with Various Antihypertensive Therapies Used as First-Line Agents: A Network Meta-Analysis. JAMA289, 2534–2544. 10.1001/jama.289.19.2534
57
RehanH. S.GroverA.HunginA. P. (2017). Ambiguities in the Guidelines for the Management of Arterial Hypertension: Indian Perspective with a Call for Global Harmonization. Curr. Hypertens. Rep.19, 17. 10.1007/s11906-017-0715-4
58
Republic of Srpska Institute of Statistics (2020). Demographic Statistics. Statistical Yearbook 2020. Available at: http://www.rzs.rs.ba/. (Accessed December 12, 2021).
59
StanawayJ. D.AfshinA.GakidouE.LimS. S.AbateD.AbateK. H.et al (2018). Global, Regional, and National Comparative Risk Assessment of 84 Behavioural, Environmental and Occupational, and Metabolic Risks or Clusters of Risks for 195 Countries and Territories, 1990-2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet392, 1923–1994. 10.1016/S0140-6736(18)32225-6
60
SundbøllJ.AdelborgK.MansfieldK. E.TomlinsonL. A.SchmidtM. (2017). Seventeen-Year Nationwide Trends in Antihypertensive Drug Use in Denmark. Am. J. Cardiol.120, 2193–2200. 10.1016/j.amjcard.2017.08.042
61
TirapaniL. D. S.FernandesN. M. D. S. (2019). A Narrative Review of the Impacts of Income, Education, and Ethnicity on Arterial Hypertension, Diabetes Mellitus, and Chronic Kidney Disease in the World. Saudi J. Kidney Dis. Transpl.30, 1084–1096. 10.4103/1319-2442.270264
62
TomasA.TomićZ.MilijasevićB.BanM.HorvatO.VukmirovićS.et al (2016). Patterns of Prescription Antihypertensive Drug Utilization and Adherence to Treatment Guidelines in the City of Novi Sad. Vojnosanit. Pregl.73, 531–537. 10.2298/VSP141119047T
63
UngerT.BorghiC.CharcharF.KhanN. A.PoulterN. R.PrabhakaranD.et al (2020). 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension75, 1334–1357. 10.1161/HYPERTENSIONAHA.120.15026
64
VerdecchiaP.ReboldiG.AngeliF. (2020). The 2020 International Society of Hypertension Global Hypertension Practice Guidelines - Key Messages and Clinical Considerations. Eur. J. Intern Med.82, 1–6. 10.1016/j.ejim.2020.09.001
65
VermaA. A.KhuuW.TadrousM.GomesT.MamdaniM. M. (2018). Fixed-dose Combination Antihypertensive Medications, Adherence, and Clinical Outcomes: A Population-Based Retrospective Cohort Study. PLoS Med.15, e1002584. 10.1371/journal.pmed.1002584
66
VončinaL.StrizrepT.GodmanB.BennieM.BishopI.CampbellS.et al (2011). Influence of Demand-Side Measures to Enhance Renin-Angiotensin Prescribing Efficiency in Europe: Implications for the Future. Expert Rev. Pharmacoecon Outcomes Res.11, 469–479. 10.1586/erp.11.42
67
VulićD. (2013). Taking Measures of Secondary Prevention of Coronary Heart Disease in Clinical Practice. Srce i krvni sudovi32, 205–207. 10.5937/siks1303205V
68
WebsterR.SalamA.de SilvaH. A.SelakV.StepienS.RajapakseS.et al (2018). Fixed Low-Dose Triple Combination Antihypertensive Medication vs Usual Care for Blood Pressure Control in Patients with Mild to Moderate Hypertension in Sri Lanka: A Randomized Clinical Trial. JAMA320, 566–579. 10.1001/jama.2018.10359
69
WilliamsB.ManciaG.SpieringW.Agabiti RoseiE.AziziM.BurnierM.et al (2018). 2018 Practice Guidelines for the Management of Arterial Hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Press27, 314–340. 10.1080/08037051.2018.1527177
70
WoerkomMv.PiepenbrinkH.GodmanB.MetzJd.CampbellS.BennieM.et al (2012). Ongoing Measures to Enhance the Efficiency of Prescribing of Proton Pump Inhibitors and Statins in The Netherlands: Influence and Future Implications. J. Comp. Eff. Res.1, 527–538. 10.2217/cer.12.52
71
Wolf-MaierK.CooperR. S.KramerH.BanegasJ. R.GiampaoliS.JoffresM. R.et al (2004). Hypertension Treatment and Control in Five European Countries, Canada, and the United States. Hypertension43, 10–17. 10.1161/01.HYP.0000103630.72812.10
72
World Health Organisation (2021). Hypertension: Key Facts. Available at: https://www.who.int/news/item/25-08-2021-more-than-700-million-people-with-untreated-hypertension (Accessed December 12, 2021).
73
World Health Organisation (2021). Guideline for the Pharmacological Treatment of Hypertension in Adults. Available at: https://apps.who.int/iris/bitstream/handle/10665/344424/9789240033986-eng.pdf (Accessed December 12, 2021).
74
World Health Organization (2003). Introduction to Drug Utilization Research. WHO International Working Group for Drug Statistics Methodology, WHO Collaborating Centre for Drug Statistics Methodology, WHO Collaborating Centre for Drug Utilization Research and Clinical Pharmacological Services. Available at: https://apps.who.int/iris/bitstream/handle/10665/42627/924156234X.pdf (Accessed December 12, 2021).
75
World Health Organization (2019). European Health for All Database. Available at: http://www.euro.who.int/hfadb (Accessed December 12, 2021).
76
YoungJ. B.DunlapM. E.PfefferM. A.ProbstfieldJ. L.Cohen-SolalA.DietzR.et al (2004). Mortality and Morbidity Reduction with Candesartan in Patients with Chronic Heart Failure and Left Ventricular Systolic Dysfunction: Results of the CHARM Low-Left Ventricular Ejection Fraction Trials. Circulation110, 2618–2626. 10.1161/01.CIR.0000146819.43235.A9
77
YusufS.SleightP.DagenaisG.MontagueT.BoschJ.PogueJ. (2000). Correction: Effects of an Angiotensin-Converting-Enzyme Inhibitor, Ramipril, on Cardiovascular Events in High-Risk Patients. N. Engl. J. Med.342, 748–753. 10.1056/NEJM200001203420301
78
ZanchettiA.ChalmersJ. P.ArakawaK.GyarfasI.HametP.HanssonL.et al (1993). The 1993 Guidelines for the Management of Mild Hypertension: Memorandum from a WHO/ISH Meeting. Blood Press2, 86–100. 10.3109/08037059309077535
79
ZanchettiA. (20032003). 2003 European Society of Hypertension-European Society of Cardiology Guidelines for the Management of Arterial Hypertension. J. Hypertens.21, 1011–1053. 10.1097/00004872-200306000-00001
80
ZengZ.ChenJ.XiaoC.ChenW. (2020). A Global View on Prevalence of Hypertension and Human Develop Index. Ann. Glob. Health86, 67. 10.5334/aogh.2591
Summary
Keywords
antihypertensive medicines, medicine utilisation, ATC/DDD analyses, national guidelines, prescribing practices
Citation
Kalinić D, Škrbić R, Vulić D, Stojaković N, Stoisavljević-Šatara S, Stojiljković MP, Marković-Peković V, Golić Jelić A, Pilipović-Broćeta N, Wong ND and Godman B (2022) Trends in Antihypertensive Medicine Utilization in the Republic of Srpska, Bosnia and Herzegovina: An Eleven-Year Follow-Up. Front. Pharmacol. 13:889047. doi: 10.3389/fphar.2022.889047
Received
03 March 2022
Accepted
30 May 2022
Published
15 June 2022
Volume
13 - 2022
Edited by
Chuenjid Kongkaew, Naresuan University, Thailand
Reviewed by
Evelyn Flahavan, Roche, United Kingdom
Jerzy Chudek, Medical University of Silesia, Poland
Updates
Copyright
© 2022 Kalinić, Škrbić, Vulić, Stojaković, Stoisavljević-Šatara, Stojiljković, Marković-Peković, Golić Jelić, Pilipović-Broćeta, Wong and Godman.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dragan Kalinić, dragan.kalinic@med.unibl.org
This article was submitted to Pharmacoepidemiology, a section of the journal Frontiers in Pharmacology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.