- 1Department of Biology, The Polytechnic, Ibadan, Nigeria
- 2Department of Microbiology, University of Ibadan, Ibadan, Nigeria
- 3Department of Food Science and Technology, Obafemi Awolowo University, Ile-Ife, Nigeria
Though not a known producer of alpha-amylase inhibitor, the potential of Streptomyces xinghaiensis AAI-2 to produce this important metabolite was assessed and the process optimised in solid substrate using response surface methodology. The isolate was grown in an inoculum medium, inoculated into wheat bran and supplemented with a basal medium for production of alpha amylase inhibitor. Optimum conditions were determined by Response Surface Methodology. The extract was recovered using sodium phosphate buffer at refrigerated temperature and assay for the presence of alpha-amylase inhibitor was carried out by Dinitrosalicylic acid method. Based on the results of the experimental trials and iteration with those values, it was predicted that optimal pH for alpha-amylase inhibitor production using S. xinghaiensis in solid culture of wheat bran was pH 6.4–6.9 while optimal moisture content and incubation time were predicted as 71%–73% and 9–12 days respectively.
Introduction
Alpha-amylase inhibitors are glycoproteins that can inhibit starch hydrolysis by alpha-amylase. This hydrolysis yields simpler, less complex subunits by hydrolysing the α-1, 4 bonds of starch at its non-reducing end. Due to the presence and role of this enzyme in the digestive tract of some animals including man, alpha-amylase inhibitors (αAI) have been proposed to be useful in the regulation of the rate of digestion of starch. From its discovery by Marshall and Lauda (1975) as a source of an amylase inhibitor, white kidney bean (Phaseolus vulgaris) is arguably the most widely studied source of the alpha amylase inhibitor. Related research has involved purification and the elucidation of characteristics, application and clinical trials, expression of gene in competent microorganisms, uses as nutraceutical agent among others, including a more recent publication on the incorporation of P. vulgaris in yoghurt for the control of blood glucose in hyperglycemic mice via the modulation of microorganisms in the gastrointestinal tract (Wang et al., 2021). While numerous plant sources exist and have been identified, synthetic types including miglitol, acarbose, voglibose and indazoles also exist (DiNicolantonio et al., 2015; Garg et al., 2022). Submerged fermentation of filamentous bacteria of the genus Streptomyces has also yielded alpha-amylase inhibitors which have been variously characterized and applied (Manivasagan et al., 2015). The advantages associated with solid state fermentation include secretion of metabolites that are better adapted to numerous environmental conditions and applications (Leite et al., 2021). Furthermore, the process is considered cheaper, since the carbon and energy sources for microbial use are mostly agricultural wastes. Since the need for water is less, there is a consequent low effluent produced and a reduced need to use water treatment processes when done on an industrial scale (Holker and Lenz, 2005). The low water activity is thought to discourage the growth of contaminating yeast and bacteria thereby minimising the strict need for sterilisation, hence energy. The optimal conditions for production of αAI in solid production medium using actinomycetes was investigated.
Materials and methods
Isolation and identification
Soil samples collected from 10 cm depth of an area (7.44°N, 3.89°E) within the premises of University of Ibadan, Nigeria having active plant degradation was pre-treated by air drying for 24 h. Further pre-treatment was done by subjecting a suspension of the pre-treated soil to heat at 70°C for 15 min and subsequently adding 1.5% phenol (v/v) to the soil suspension (Seong et al., 2001; Tiwari and Gupta, 2012). A ten-fold serial dilution of the soil suspension was done and 0.1 mL of the diluted sample was inoculated in sterile starch casein nitrate agar (g/L): [starch (10), casein (0.3), KNO3 (2.0), NaCl (2.0), K2HPO4 (2.0), CaCO3 (0.02), MgSO4.7H2O (0.05), FeSO4.7H2O (0.01), agar (15)] and supplemented with nystatin (50 μg/mL) and nalidixic acid (20 μg/mL) as antifungal and antibacterial agents respectively. The culture was incubated for 10 days at 28°C.
Isolates were characterised by observation of morphological characteristics, biochemical tests (Nonomura, 1974) and molecular identification.
Production of alpha-amylase inhibitor
Wheat bran was air-dried to constant weight and sterile basal medium (cornsteep liquor (0.4% v/v), glucose (1% w/v), (NH4)2HPO4 (0.8% w/v), soyflour (0.4% w/v), peptone (1% w/v) at pH 8.3) was introduced and the set up was sterilized. Streptomyces inoculum was prepared in the following medium: [glucose (1%), soy flour (1%) and NaCl (0.25%)] (Oeding et al., 1980). The inoculum was introduced into sterile solid substrate and incubated at 28°C ± 2°C for production of αAI.
Optimisation of production conditions
Optimum conditions for the production of αAI were determined by the Response Surface Methodology (RSM). Three independent parameters; moisture content (%), pH and period of incubation (days) were varied at three levels (−1, 0, +1), using the Box–Behnken design. The conditions were varied as follows: moisture content (65%, 70%, 75%), pH (6.0, 7.0, 8.0) and incubation period (5 days, 10 days, 15 days). The variables were set up for 17 experimental runs (Table 1).
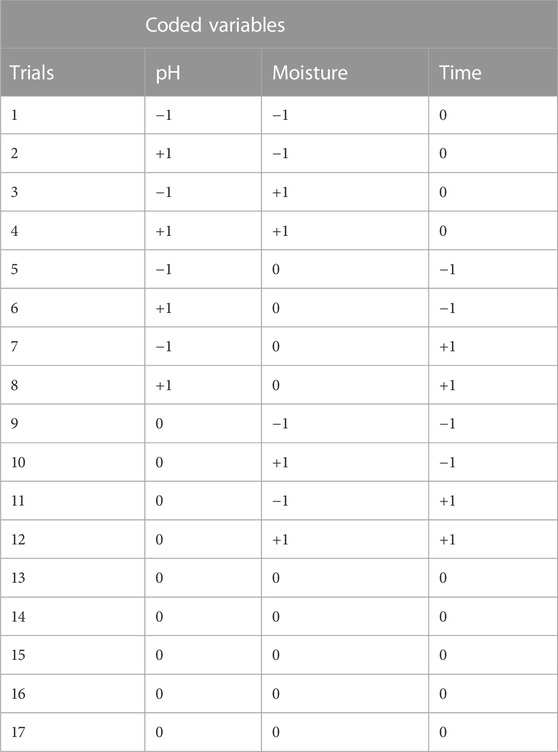
TABLE 1. Box—Behnken design matrix for optimisation of pH, moisture content and incubation time for alpha–amylase inhibitor production by actinomycetes.
Extraction and purification
Recovery of the αAI from the Streptomyces culture on wheat bran was carried out according to the method of Mulimani and Supriya (1993) with modifications. At refrigerated temperature, using sodium phosphate buffer (20mM; pH 6.9), the extract was recovered in a centrifuge at 10,000×g for 20 min at 4°C. The supernatant was decanted and stored as crude alpha-amylase inhibitor extract. Further purification was done by ammonium sulphate precipitation, overnight dialysis against extraction buffer at 4°C and column chromatography using Sephadex G-100.
Assay
The presence of αAI in the extract was determined by the Dinitrosalicylic acid (DNSA) assay method described by Bernfeld (1955). Readings were taken at 540 nm using a UV-Visible Spectrophotometer.
Results
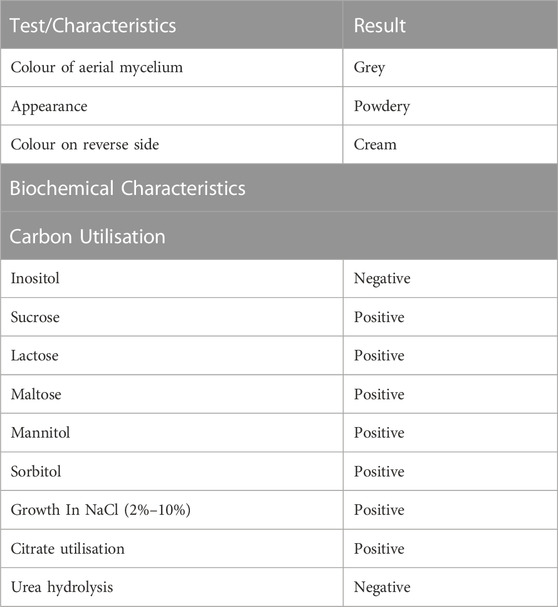
The isolate obtained was identified with a grey aerial mycelium, which appeared powdery with a firm adhesion to the agar surface. It could utilise all Carbon sources tested except inositol and could grow in the presence of NaCl up to 10%. Moreover, the isolate could grow in the presence of citrate but not in the presence of urea (Table 2). Molecular identification using the partial sequence of the 16S rRNA identified the isolate as Streptomyces xinghaiensis (GenBank accession number—KY858944). The isolate was observed to fall into the same sub-clade with S. rubrus and S. tendae which have 98% and 97% similarity respectively with the isolate (Figure 1).
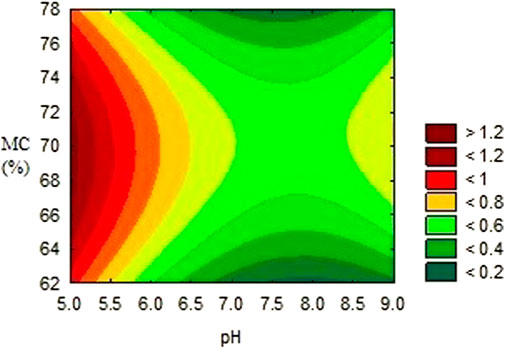
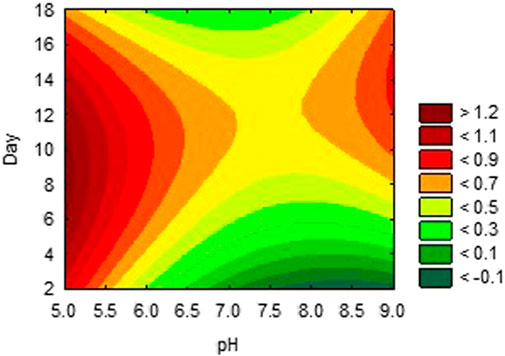
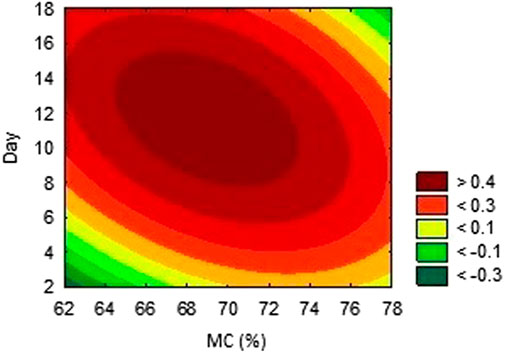
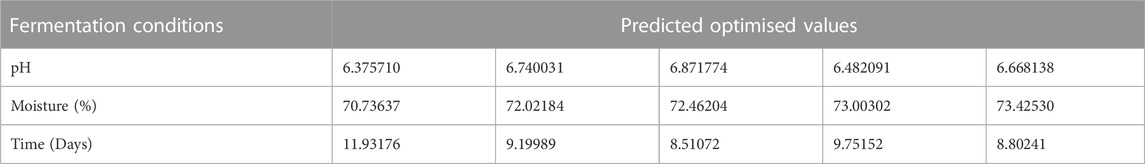
The activities obtained from each experimental trial were analysed, in order to obtain the optimum conditions of each environmental condition. The results are presented in Figures 2, 3, 4. These results were further iterated and the optimum values were predicted as shown in Table 3. The results show that optimum production conditions were obtained at 6.38–6.87 pH, 70.7%–73.4% moisture content and 8.5–12 days of incubation.
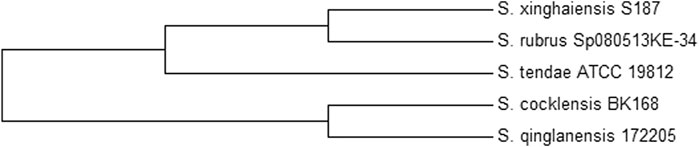
FIGURE 3. Phylogenetic tree showing the position of S. xinghaiensis among some closely related species.
Discussion
Streptomyces xinghaiensis AAI2 isolated from soil was positive for starch, citrate and casein hydrolysis as well as the utilisation of many carbon sources for growth except inositol. These group of organisms are known to be able to degrade various kinds of substrates usually due to their ability to secrete a wide range of hydrolytic enzymes. This isolate was obtained from an environment characterized by active degradation of plant material; a factor that is capable of contributing to its ability to synthesize hydrolytic enzymes which are required for growth and absorption of nutrients. Citrate hydrolysis was not initially reported in this specie (Zhao et al., 2009; Kumar et al., 2012) as observed in this study. The partial sequence of the isolate bore 97% similarity to the complete genome of a known producer of alpha amylase inhibitor, S. tendae which is responsible for the production of the alpha amylase inhibitor named tendamistat (Vertesy et al., 1984). This similarity, in addition to information from the phylogenetic tree may infer relatedness in evolution and the presence of genes responsible for αAI production. Though S. xinghaiensis has never been identified as a producer of alpha amylase inhibitor, its metabolic potential tend towards being prolific, which is a characteristic of the genus. It has been shown from the draft genome sequence that this species can produce many new metabolic products. Of the 6,654 coding sequences contained in the genome sequence of S. xinghaiensis, 1,091 coding sequences are for proteins which do not match any proteins in the COG reference databases (Zhao and Yang, 2011). Furthermore, a study conducted between 2013 and 2017 discovered 167 new bioactive metabolites from 58 rare actinomycetes; confirming the metabolic dexterity of actinomycetes (Subramani and Sikpema, 2019).
Using the starch hydrolysis test, the isolate was putatively presented as amylase-producing. This ability formed the basis of its selection for αAI production since it has been opined that enzyme inhibitors may be found in the presence of the enzymes that they inhibit (Umezawa, 1973). αAI was produced by the isolate, using solid substrates which acted both as support for growth of the actinomycetes as well as the source of Carbon. The production of enzyme inhibitors in fermentation media is affected by factors including nature of the substrate, its moisture content, nutrient and mineral composition of the medium. Moreover, the importance of trace elements such as Manganese and Magnesium in the fermentation medium for enzyme inhibitor production has been emphasized. Wheat bran has been used as a support as well as nutrient source for the growth of many microorganisms and its success is attributed to the components of wheat bran which includes starch, protein and minerals (Rao et al., 2005; Verni et al., 2019). The ability of Streptomyces sp. to secrete extracellular enzymes such as hydrolases which are used to utilise both simple and complex molecules as nutrients makes them able to effectively utilise wheat bran as a solid substrate (Kapur et al., 2018). Wheat bran and the basal medium also contain nitrogen sources which have been found to have significant effects in the secondary metabolism of Streptomyces (Dilipkumar et al., 2013). In fact, cornsteep liquor and soybean flour were identified as Nitrogen sources which are necessary in the synthesis of inhibitor by Streptomyces (Vertesy et al., 1984). Tan et al. (2020) also supported the notion that the inclusion of soybean meal (which contains 49% crude protein, many important amino acids and microelements) in synthetic media has an advantage of enhancing metabolism in Streptomyces. Other important factors that determine product formation also include the pH, moisture content and duration of incubation.
The simplex search method of optimising conditions, using the data obtained from laboratory trials predicted the optimum production of αAI, as occurring between 9 and 12 days of incubation. This claim is corroborated by the fact that the production of metabolites occurs after the exponential phase, which lasts for about 3–7 days in actinomycetes, depending on the environmental conditions and the species being used. Metabolite production which is initiated at this stage, is usually triggered by hyphal differentiation, stress or depletion of nutrients in the environment (Yague, et al., 2013; Manteca and Yagüe, 2018). The αAI AI-3688 was formed in culture solution after a maximum of 10 days. Research has reported other secondary metabolites produced by Streptomyces are produced in similar duration.
The importance of moisture in SSF and its effect on utilization of substrate for secondary metabolites formation is attributed to interference with physical properties of the solid particles (Nema et al., 2019). In fermentation, wheat bran absorbs liquid, leading to a swelling of the substrate particles, release and dissolution of the nutrients embedded in the substrate, resulting in better utilisation of the substrate by the microorganism (Manan and Webb, 2019). While increase in moisture beyond the optimum may reduce porosity of the substrate and limit oxygen and mass transfer, low moisture content however, causes reduction in nutrient solubility and a low degree of swelling (Pandey, 2003). In similar research optimising metabolite production by Streptomyces, Kar et al. (2010) found the optimum production of secondary metabolite using Streptomyces at 70% moisture content. Tylosin production by a strain of S. fradiae and oxytetracycline by S. rimosus and S. vendagensis in solid substrate were reported to be optimum at moisture content of about 70% (Okorie and Asagbra, 2008; Khaliq et al., 2009).
An initial pH of 6.4–6.7 was predicted as optimum for the production of αAI using RSM. pH of the medium plays an important part in the physiology of the microorganism as it can affect the morphology of the organism as well as production of secondary metabolites (Bajaj and Singh, 2010). The results obtained favourably compares with the results of Vertesy et al. (1984) that discovered the production of an alpha–amylase inhibitor at the pH values that favour the growth of Streptomyces.
Conclusion
In addition to the suitability of solid substrate (wheat bran) culture medium and other conditions for production of alpha amylase inhibitor by S. xinghaiensis AAI2, this novel producer of alpha amylase inhibitor within the Streptomyces genus, had optimum production conditions for pH, moisture content and incubation period at 6.4–6.9, 71%–73% and 9–12 days respectively.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/nuccore/, KY858944.
Author contributions
AO conceptualized the research, supervised laboratory experiments, validated procedures and results obtained, corrected the initial draft and reviewed the final draft of the manuscript OF carried out laboratory experiments including choice of resources and software, wrote and corrected initial draft and involved in editing of the final draft YE was involved in project administration, assisted in the preparation and vetting of the manuscript CA advised on choice of computer software, supervised its utilisation and vetted the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bajaj, B. J., and Singh, N. P. (2010). Production of xylanase from an alkalitolerant Streptomyces sp.7b under solid state fermentation, its purification and characterization. Appl. Biochem. Biotechnol. 162 (6), 1804–1818. doi:10.1007/s12010-010-8960-x
Dilipkumar, M., Rajasimman, M., and Rajamohan, N. (2013). Enhanced inulinase production by Streptomyces sp in solid state fermentation through statistical designs. Biotechnology 3, 509–515. doi:10.1007/s13205-012-0112-2
DiNicolantonio, J. J., Bhutani, J., and O'Keefe, J. H. (2015). Acarbose: Safe and effective for lowering postprandial hyperglycaemia and improving cardiovascular outcomes. Open Heart 2, 1–13. doi:10.1136/openhrt-2015-000327
Garg, P., Rawat, R. S., Bhatt, H., Kumar, S., and Reddy, S. R. (2022). Recent developments in the synthesis of N-heterocyclic compounds as α-amylase inhibitors via in-vitro and in-silico analysis: Future drugs for treating diabetes. Chem. Sel. 7 (28), 202201706. doi:10.1002/slct.202201706
Holker, U., and Lenz, J. (2005). Solid State Fermentation – are there any biotechnological advantages? Curr. Opin. Microbiol. 8, 301–306. doi:10.1016/j.mib.2005.04.006
Kapur, M. K., Das, P., Kumar, P., Kumar, M., and Solanki, R. (2018). Role of microbial extracellular enzymes in the biodegradation of wastes. J. Pharm. Chem. Biol. Sci. 6 (3), 237–249.
Kar, S., Datta, T. K., and Ray, R. C. (2010). Optimization of thermostable α-amylase production by Streptomyces erumpens MTCC 7317 in solid state fermentation using cassava fibrous residue. Braz. Archives Biol. Technol. 53 (2), 301–309. doi:10.1590/s1516-89132010000200008
Khaliq, S., Rashid, N., Akhtar, K., and Ghauri, A. (2009). Production of tylosin in solid-state fermentation by Streptomyces fradiae NRRL-2702 and its gamma-irradiated mutant (gamma-1). Lett. Appl. Microbiol. 49, 635–640. doi:10.1111/j.1472-765X.2009.02720.x
Kumar, K. S., Anuradha, S., Sarma, G. R., Venkateshwarlu, Y., and Kishan, V. (2012). Screening, isolation, taxonomy and fermentation of an antibiotic producer Streptomyces xinghaiensis from soil capable of acting against linezolid resistant strains. Indian J. Exp. Biol. 50, 718–728.
Leite, P., Sousa, D., Fernandes, H., Ferreira, M., Costa, A. R., Filipe, D., et al. (2021). Recent advances in production of lignocellulolytic enzymes by solid-state fermentation of agro-industrial wastes. Curr. Opin. Green Sustain. Chem. 27, 100407. doi:10.1016/j.cogsc.2020.100407
Manan, M. A., and Webb, C. (2019). Insights into physical characterization of solid state fermentation: From preliminary knowledge to practical application. J. Biotech. Res. 10, 271–282.
Manivasagan, P., Venkatesan, J., Sivakumar, K., and Kim, S. (2015). Actinobacterial enzyme inhibitors – a review. Crit. Rev. Microbiol. 41 (2), 261–272. doi:10.3109/1040841X.2013.837425
Manteca, Á., and Yagüe, P. (2018). Streptomyces differentiation in liquid cultures as a trigger of secondary metabolism. Antibiotics 7 (2), 41. doi:10.3390/antibiotics7020041
Marshall, J. J., and Lauda, C. M. (1975). Purification and properties of Phaseolamin, an inhibitor of alpha-amylase, from the kidney bean, Phaseolus vulgaris. J. Biol. Chem. 25020, 8030–8037. doi:10.1016/s0021-9258(19)40811-9
Mulimani, V. H., and Supriya, D. (1993). Alpha amylase inhibitors in sorghum (Sorghum bicolor). Plant Foods Hum. Nutr. 44, 261–266. doi:10.1007/BF01088321
Nema, A., Patnala, S. H., Mandari, V., Kota, S., and Devarai, S. K. (2019). Production and optimization of lipase using Aspergillus niger MTCC 872 by solid-state fermentation. Bull. Natl. Res. Cent. 43, 82. doi:10.1186/s42269-019-0125-7
Nonomura, H. (1974). Key for classification and identification of 458 species of the streptomycetes included in the ISP. J. Ferment Technol. 52, 78–92.
Oeding, V., Pfaff, W., Vertesy, L., and Weidenmuller, H. (1980). Alpha-amylase inhibitor from a Streptomycete and process for its preparation. CA1105404 A.
Okorie, P. C., and Asagbra, A. E. (2008). Oxytetracycline production by mix culture of Streptomyces rimosus and Streptomyces vendagensis in solid state fermentation of cassava peels. J. Ind. Res. Technol. 2, 43–47.
Pandey, A. (2003). Solid state fermentation. Biochem. Eng. J. 13, 81–84. doi:10.1016/s1369-703x(02)00121-3
Rao, K. C. S., Karanth, N. G., and Sattur, A. P. (2005). Production of nigerloxin, an enzyme inhibitor, and a free radical scavenger, by Aspergillus niger using solid state fermentation. Process Biochem. 40 (7), 2517–2522. doi:10.1016/j.procbio.2004.10.008
Subramani, R., and Sipkema, D. (2019). Marine rare actinomycetes: A promising source of structurally diverse and unique novel natural products. Mar. Drugs 17 (5), 249. doi:10.3390/md17050249
Tan, L. T. H., Lee, L. H., and Goh, B. H. (2020). Critical review of fermentation and extraction of anti-Vibrio compounds from Streptomyces. Prog. Mircobes Mol. Bio1. 3 (1), a0000051. doi:10.36877/pmmb.a0000051
Tiwari, K., and Gupta, R. K. (2012). Diversity and isolation of rare actinomycetes: An overview. Crit. Rev. Microbiol. 39 (3), 256–294. doi:10.3109/1040841X.2012.709819
Umezawa, H. (1973). Chemistry of enzyme inhibitors of microbial origin. Pure Appl. Chem. 331, 129–144. doi:10.1351/pac197333010129
Verni, M., Rizzello, C. G., and Coda, R. (2019). Fermentation biotechnology applied to cereal industry by-products: Nutritional and functional insights. Front. Nutr. 6, 42. doi:10.3389/fnut.2019.00042
Vertesy, L., Tripier, D., and Ritzel, H. (1984). Novel polypeptides with an α-amylase inhibiting action, a process for their preparation, their use and pharmaceutical products. US4623714A.
Wang, S., Guo, C., Xing, Z., Li, M., Yang, H., Zhang, Y., et al. (2021). Dietary intervention with α-amylase inhibitor in white kidney beans added yogurt modulated gut microbiota to adjust blood glucose in mice. Front. Nutr. 8, 664976. doi:10.3389/fnut.2021.664976
Yague, P., Lopes-Garcia, M. T., Rioseras, B., Sanchez, J., and Manteca, A. (2013). Pre-sporulation stages of Streptomyces differentiation: State-of-the-art and future perspectives. Microbiol. Lett. 342 (2), 79–88. doi:10.1111/1574-6968.12128
Zhao, X. Q., Li, W. J., Jiao, W. C., Li, Y., Yuan, W. J., Zhang, Y. Q., et al. (2009). Streptomyces xinghaiensis sp. nov., isolated from marine sediment. Int. J. Syst. Evol. Microbiol. 59, 2870–2874. doi:10.1099/ijs.0.009878-0
Keywords: alpha-amylase inhibitor, optimisation, RSM, Streptomyces, solid state fermentation
Citation: Fatoki OA, Onilude AA, Ekanola YA and Akanbi CT (2023) Optimisation of alpha-amylase inhibitor production in solid state fermentation. Front. Pharmacol. 14:1073754. doi: 10.3389/fphar.2023.1073754
Received: 18 October 2022; Accepted: 14 February 2023;
Published: 22 March 2023.
Edited by:
Teodorico Castro Ramalho, Universidade Federal de Lavras, BrazilReviewed by:
Sadia Sultan, Universiti Teknologi MARA Puncak Alam, MalaysiaAlexandre Alves De Castro, Universidade Federal de Lavras, Brazil
Copyright © 2023 Fatoki, Onilude, Ekanola and Akanbi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: O. A. Fatoki, ZmF0b2tpLm9sYWRvdHVuQHBvbHlpYmFkYW4uZWR1Lm5n
 O. A. Fatoki
O. A. Fatoki A. A. Onilude2
A. A. Onilude2