- 1Unit of Global Health, Department of Health Sciences, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
- 2Faculty of Pharmacy, Bhakti Kencana University, Bandung, Indonesia
- 3Department of Pharmacy, Faculty of Mathematics and Natural Sciences, Universitas Islam Indonesia, Yogyakarta, Indonesia
- 4Department of Clinical Pharmacy and Pharmacology, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
- 5Groningen Research Institute for Asthma and COPD (GRIAC), University Medical Center Groningen, University of Groningen, Groningen, Netherlands
- 6Centre for Medicine Use and Safety, Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia
- 7Department of Economics, Econometrics, and Finance, Faculty of Economics and Business, University of Groningen, Groningen, Netherlands
- 8Department of Pharmacology and Therapy, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
- 9Center of Excellence in Higher Education for Pharmaceutical Care Innovation, Universitas Padjadjaran, Bandung, Indonesia
Objective: This study aims to systematically review the content and potential effects of clinical pharmacy services in tuberculosis (TB) care management.
Methods: Searches were performed in PubMed, Embase, Cochrane, Scopus, and Web of Science databases following Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. Study characteristics and outcomes were extracted, and clinical pharmacy service components were characterized using the Descriptive Elements of Pharmacist Intervention Characterization Tool.
Results: Twenty articles were included for full-text assessment, of which 10 fulfilled inclusion criteria, comprising 1,168 patients (N = 39 to 258 per study). These articles included five prospective cohort studies, two case–control studies, two quasi-experimental studies, and one cross-sectional study. Intervention foci within clinical pharmacy services were medication adherence (50%), medication safety (40%), education to patients/caregivers regarding needs/beliefs (30%), optimizing medication/therapy effectiveness (30%), emphasizing HRQoL (10%), and drug selections (10%). The three most frequently applied interventions were drug information/patient counseling (80%), adverse drug reaction monitoring (50%), and drug use evaluation (20%). Based on the World Health Organization (WHO) outcome classification, treatment success ranged from 72% to 93%, with higher cure outcomes (53%–86%) than treatment completion (7%–19%). Other outcomes, including isoniazid metabolites, medication counts, sputum conversion, adherence/compliance, knowledge, and quality of life, were better in the intervention group than those in comparator groups, and/or they improved over time. Risk of bias analysis indicated that the included studies were not comparable to a randomized clinical trial.
Conclusion: Clinical pharmacy services as single or composite interventions potentially improve TB outcomes, but its evidence is still inconsistent and limited due to the lack of randomized controlled studies using the WHO outcome classification.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=199028, identifier CRD42020199028.
Introduction
Tuberculosis (TB), as one of the leading causes of death from a single infectious agent, is still a disease of major global concern (World Health Organization, 2014). In 2020, 10 million people developed TB globally, followed by an 18% decline in the number of people newly diagnosed with TB between 2019 and 2020 (World Health Organization, 2021a). Steps have been taken to reduce the number of TB cases and deaths through improvements in the financing, research, innovation, and development of a multi-sectoral accountability framework (World Health Organization, 2019a; World Health Organization, 2019b).
Between 2000 and 2020, TB treatment averted 66 million deaths (World Health Organization, 2021a) as effective and timely TB treatment affects patients’ survival and potentially decreases M. tuberculosis transmission indirectly (Schoenbaechler et al., 2021; Tierney et al., 2021). Additionally, supervised treatment increases the treatment success probability of new TB cases (Wen et al., 2018), avoiding retreatment, which has a similar failure likelihood when the initial treatment fails (Cohen et al., 2018).
In 2019, there was an 86% treatment success rate for people treated for TB with first-line regimens, accompanied by increasing rifampicin resistance, from 61% in 2019 to 71% in 2020 (World Health Organization, 2021a). Hence, initial treatment warrants particular concerns because failing increases resistance probability (van der Werf et al., 2012; DiPiro et al., 2017) and affects treatment outcomes (Chaves Torres et al., 2019). When resistance develops, the drug of choice being based on a susceptibility test is a significant treatment outcome predictor (Gegia et al., 2017; Ahmad et al., 2018) because using more potent or newer drugs or fixed-dose combinations (FDCs) does not guarantee favorable outcomes in the potential absence of adequate susceptibility (Albanna et al., 2013; Lee et al., 2016; Xu et al., 2017). In the case of resistance, drug effectiveness and adverse drug events are of major concern; for that matter, appropriate monitoring by involving a pharmacist could better optimize the therapy and adverse event management (Khan et al., 2022).
In general, pharmacist services positively affect patient outcomes (Shrestha et al., 2022), especially when targeting specific conditions using well-defined interventions and outcome measures (Rotta et al., 2015); therefore, with the current significant TB burden, pharmacists’ active involvement in treating and managing TB cases could be a valuable resource for further improving pharmacological treatment outcomes.
Pharmaceutical, patient-centered care delivered as clinical pharmacy services (CPS) by pharmacists aims to support patients in achieving optimal drug outcomes within a multi-disciplinary healthcare team (Franklin and van Mil, 2005; Whittlesea and Hodson, 2019; Negaard et al., 2020). Conceptually, pharmaceutical care and CPS are often overlapping. Pharmaceutical care is about cooperative systems and relationship ethics, not pharmacists or technical functions per se, which drive CPS activities (Dreischulte and Fernandez-Llimos, 2016). Pharmaceutical care is an extension of CPS (Hepler, 2004), while CPS itself is more of a scientific discipline of rational medication use, empowering pharmacists to provide patient-centered interventions (Hepler, 2004; American College of Clinical Pharmacy, 2008; Holdford and Brown, 2010; Dreischulte and Fernandez-Llimos, 2016). CPS can be seen as a professional practice of pharmacists, which is a building block of pharmaceutical care that requires adequate knowledge and skill in pharmacists for delivering the intervention or providing treatment. Having a clear picture of the contribution of clinical pharmacy services to broader pharmaceutical care services in TB care is essential in improving TB care further. Hence, this study aims to systematically review the content and potential effects of clinical pharmacy services in TB care management.
Methods
Study design
A systematic review was performed and reported according to the “Preferred Reporting Items for Systematic Reviews and Meta-Analysis” (PRISMA) statement (Page et al., 2021). The study has been registered at the International Prospective Register of Systematic Reviews (PROSPERO) with the number CRD42020199028.
Eligibility criteria
Peer-reviewed publications meeting the following inclusion and exclusion criteria were eligible:
Inclusion criteria: published studies in English, either observational or intervention studies of clinical pharmacy services in TB care management in the hospital setting in adult patients (≥18 years) with confirmed drug-susceptible TB or (extended or multi) drug-resistant TB by microbiological verification, were included.
Exclusion criteria: we excluded non-full-text studies, conference reports, reviews, editorials, comments, or letters. Studies focused on TB patients with specific comorbidities, like diabetes or HIV, were also excluded.
It should be noted that post-protocol registration in the PROSPERO, we decided to restrict our inclusion to drug-susceptible TB studies only, excluding drug-resistant TB studies. This was carried out to reduce heterogeneity and enhance result interpretation, given that the initially included studies encompassed a very broad variety of interventions and outcomes.
Information sources
The systematic literature search was performed on 20 September 2021, in PubMed, Embase, Cochrane, Scopus, and Web of Science databases.
Search strategy
The search was carried out using Medical Subject Headings (MeSHs) and other relevant subject headings and text words. The main categories were “tuberculosis” and “pharmacy service,” which were combined by the Boolean operator “AND.” The “OR” operator combined each category’s subject headings and text words. A detailed overview of the search strategy is provided (see Supplementary Appendix, Search Strategy).
Selection process
The article screening process was performed using Rayyan (https://rayyan.ai/). Rayyan is an electronic tool that provides consistent exclusion reasoning corresponding to the study criteria of language, publication type, study design, intervention, subject, medication, and outcomes. Specific requirements were established from each study criteria, and articles not fulfilling these criteria were excluded with an assigned exclusion reason label (see Supplementary Appendix Table S1). To assist the screening process, article screening criteria were complemented with an article screening flowchart, according to the Rayyan workflow (see Supplementary Appendix Figure S1). In addition, those articles’ important text terms and metadata were analyzed using VOSviewer (https://www.vosviewer.com/) to describe and visualize the bibliometric network.
Two authors (DI and FDA) independently screened the titles and abstracts of all studies identified by the search strategy based on inclusion and exclusion criteria, guided by the article screening criteria and flowchart. First, duplicated articles across databases were removed; then, the title and abstract of the articles were screened to find relevant and potential articles for further full-text reviews. Any disagreement was resolved through discussion until consensus was achieved.
Data collection process
Two authors (DI and FDA) used an electronically pre-designed form created using REDCap (https://redcap.umcg.nl/redcap/) to collect and extract data from each included study independently. The extraction forms captured information related to the study characteristics, clinical pharmacy services, outcomes, and risk of bias, as further specified in Data items.
Data items
The following data were extracted: 1) study characteristics, including first author and year of study publication, study design, duration, setting, number of participants, and location; 2) clinical pharmacy service components, consisting of clinical data sources, interventions (focus and activities), and supporting materials; 3) outcomes, including TB care management treatment outcomes as per the World Health Organization (WHO) classifications (cured, treatment completed, treatment failed, defaulted, and transfer) (World Health Organization, 2010) and other outcomes that include process outcomes, such as patient satisfaction and medication adherence; and 4) risk of bias, including the bias due to confounding, participant selection, intervention classification, intervention deviation, missing data, outcomes, and reporting. The latter is further described in the “Risk of bias assessment”.
A taxonomy tool was used to identify and classify the study design when the included studies were not clearly stating it (see Supplementary Appendix Table S2). For extracting clinical pharmacy service information, the Descriptive Elements of Pharmacist Intervention Characterization Tool (DEPICT) was used (see Supplementary Appendix Table S3). The DEPICT provides consistent and structured information, avoiding variability in the naming or nomenclature of common pharmacy service conceptual frameworks and constructs (Correr et al., 2013).
Intervention focus refers to the parameters pharmacists evaluated, while intervention activities are actions carried out by pharmacists to address the identified problem.
Risk of bias assessment
When there was no limitation to the study design, all retrieved studies included in the review were non-randomized (see Result). The Risk of Bias in Non-randomized Studies—of Intervention (ROBINS-I) was used to appraise the potential bias in studies without randomization (Sterne et al., 2016). The main feature of this assessment is to compare whether the study systematically differs from a well-designed and performed randomized trial. The bias is low when the study is comparable, moderate when it has sound evidence but is not comparable, serious when it has problematic aspects of its design and performance, and critical when it is unlikely to provide valuable evidence. The following dimensions were assessed individually before concluding about its overall bias: bias due to confounding, selection of participants, classification of the intervention, deviation from the intended intervention, missing data, measurements of outcomes, and selection of the reported result. Two reviewers independently assessed the articles based on the table and guidelines provided by the development group for ROBINS-I (Development Group for ROBINS-I, 2016). A joint review and consensus between reviewers resolved any disagreement. The result was visualized using R statistics version 4.1.3 (https://www.r-project.org/) based on the robvis package (McGuinness and Higgins, 2020).
Results
Study selection
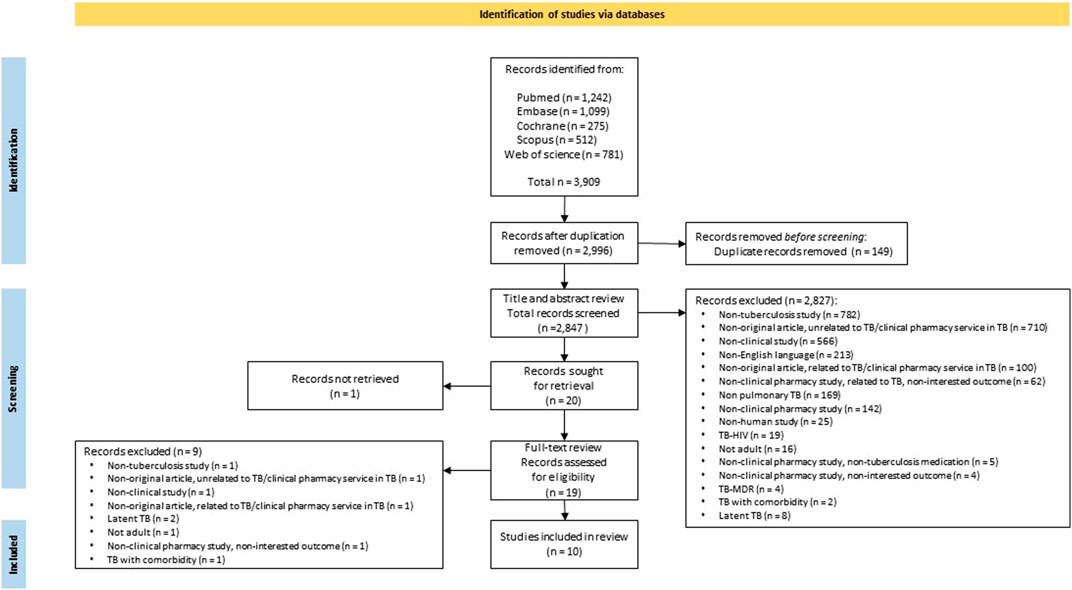
The study selection flow is presented in Figure 1. In total, after applying the search strategy, 3,909 publications were retrieved. For the 2,996 articles obtained after duplicate removal, a bibliometric network analysis based on important text terms was carried out, resulting in five clusters of articles. One cluster related to the keywords of pharmacist, health, and service was identified. This cluster represents pharmacist health services in TB care management (see Supplementary Appendix Figure S2). Subsequently, 2,847 articles were screened after further manual checking and removing duplicates. No unique focus on TB was the main exclusion reason. Other predominant reasons for exclusion were non-original articles, no clinical pharmacy services, and no clinical study.
Twenty articles were included for full-text retrieval upon the consensus of the two authors. Finally, 10 articles were included in the review based on the eligibility assessment and consensus between the two authors.
Table 1 provides an overview of the included studies. Six studies originated from high-TB-burden countries, including four from India (Bhardwaja et al., 2012; Venkatapraveen et al., 2012; Thomas et al., 2018; Narayana et al., 2020), one from China (Tang et al., 2018), and one from Indonesia (Karuniawati et al., 2019). Others were from different regions: Thailand (Tanvejsilp et al., 2018), Ivory Coast (Abrogoua et al., 2016), Türkiye (Clark et al., 2007), and Brazil (Lopes et al., 2017). The earliest included study was published in 2007. Notably, among the 10 studies, no randomized controlled studies were identified. Study designs were heterogeneous and included five prospective cohort studies (50%) (Bhardwaja et al., 2012; Venkatapraveen et al., 2012; Lopes et al., 2017; Tanvejsilp et al., 2018; Thomas et al., 2018), two case–control studies (20%) (Clark et al., 2007; Tang et al., 2018), two quasi-experimental studies (with and without control) (Karuniawati et al., 2019; Narayana et al., 2020), and a cross-sectional study (Abrogoua et al., 2016). Most studies were prospective; for only one study, this was unknown (Abrogoua et al., 2016). Most study settings were in the tertiary care hospital, except for one, which was in a secondary care hospital (Narayana et al., 2020). The study duration ranged from 5 to 35 months, and patient numbers varied between 39 and 258.

TABLE 1. Characteristics of the included studies on clinical pharmacy services for TB care management.
Clinical pharmacy services
The clinical pharmacy service component comprised clinical data sources, intervention focus, intervention activities, and supporting materials identified using the DEPICT.
Of the 10 studies included in the review, clinical data sources used for clinical pharmacy services were varied; seven studies used adherence measuring tools (70%) (Clark et al., 2007; Bhardwaja et al., 2012; Venkatapraveen et al., 2012; Tang et al., 2018; Thomas et al., 2018; Karuniawati et al., 2019; Narayana et al., 2020), six used medical records (60%) (Clark et al., 2007; Bhardwaja et al., 2012; Abrogoua et al., 2016; Tang et al., 2018; Tanvejsilp et al., 2018; Thomas et al., 2018), five used patient interviews (50%) (Clark et al., 2007; Bhardwaja et al., 2012; Lopes et al., 2017; Tanvejsilp et al., 2018; Thomas et al., 2018), and four used laboratory testing/therapeutic drug monitoring (40%) (Clark et al., 2007; Venkatapraveen et al., 2012; Tang et al., 2018; Thomas et al., 2018), amongst others. The intervention focus provided within the clinical pharmacy services was medication adherence, defined as the voluntary cooperation of the patient in taking the drugs or medicine as prescribed (DEPICT, 2013), in five studies (50%) (Clark et al., 2007; Bhardwaja et al., 2012; Tang et al., 2018; Thomas et al., 2018; Karuniawati et al., 2019); medication safety in four studies (40%) (Clark et al., 2007; Abrogoua et al., 2016; Lopes et al., 2017; Tang et al., 2018); education of patients/caregivers regarding the needs/beliefs in three studies (30%) (Venkatapraveen et al., 2012; Thomas et al., 2018; Narayana et al., 2020); optimizing the medication/therapy effectiveness in three studies (30%) (Venkatapraveen et al., 2012; Tang et al., 2018; Tanvejsilp et al., 2018); and lastly, emphasis on HRQoL (10%) (Tanvejsilp et al., 2018) and drug selections (10%) (Abrogoua et al., 2016) in one study (see Supplementary Appendix Figures S3, S4).
In all studies, intervention activities that corresponded to intervention focus within clinical pharmacy services for TB management could include single (Bhardwaja et al., 2012; Venkatapraveen et al., 2012; Lopes et al., 2017; Thomas et al., 2018; Karuniawati et al., 2019; Narayana et al., 2020) or composite (Clark et al., 2007; Abrogoua et al., 2016; Tang et al., 2018; Tanvejsilp et al., 2018) activities (see Supplementary Appendix Table S4).
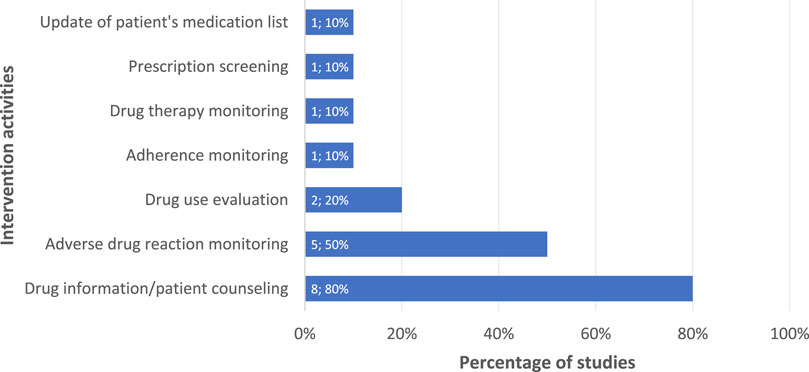
The three most frequently applied intervention activities identified from 10 included studies were drug information/patient counseling (Clark et al., 2007; Bhardwaja et al., 2012; Venkatapraveen et al., 2012; Tang et al., 2018; Tanvejsilp et al., 2018; Thomas et al., 2018; Karuniawati et al., 2019; Narayana et al., 2020), adverse drug reaction monitoring (Clark et al., 2007; Abrogoua et al., 2016; Lopes et al., 2017; Tang et al., 2018; Tanvejsilp et al., 2018), and drug use evaluation (Abrogoua et al., 2016; Tang et al., 2018), as presented in Figure 2. Further details on the type of intervention activities based on their corresponding intervention focus are shown in the Supplementary Appendix Table S5. Aligned with intervention activities, the mainly used supporting materials for interventions were educational materials/leaflets/written action plans, used in seven studies (70%) (Clark et al., 2007; Bhardwaja et al., 2012; Venkatapraveen et al., 2012; Tang et al., 2018; Thomas et al., 2018; Karuniawati et al., 2019; Narayana et al., 2020); patient data collection forms in four studies (40%) (Bhardwaja et al., 2012; Venkatapraveen et al., 2012; Tanvejsilp et al., 2018; Thomas et al., 2018); and validated adherence questionnaires in three studies (30%) (Venkatapraveen et al., 2012; Karuniawati et al., 2019; Narayana et al., 2020), amongst others (see Supplementary Appendix Figure S5).
Outcomes
The outcomes of clinical pharmacy services for TB care management varied as per the study. An overview is provided in Table 2.
We classified identified outcomes of interest as WHO outcomes and other outcomes. WHO outcomes are standardized treatment outcomes of TB patients recorded at the treatment course’s end. It includes cure, treatment completion, default, death, treatment success, not completed, transfer out, and failure (World Health Organization, 2010; World Health Organization, 2021b). The rest were classified as other outcomes. Of all the outcomes measured, we reported timings, follow-up, and group comparisons, if reported in the studies.
WHO outcomes
Two studies (Tang et al., 2018; Tanvejsilp et al., 2018) reported intervention outcomes between different groups as per the WHO classification at the end of the intensive (2 months) and continuation (4 months) phase of TB care, although there were slight differences in the measured outcomes. Percentage values represent the proportion of patients within the group having an outcome as per the WHO classification (Table 3). One study compared a pharmaceutical care group with two different intervention groups (Tanvejsilp et al., 2018), and others compared it to one group, usual care (Tang et al., 2018). The value of “Treatment success” in one study (Tanvejsilp et al., 2018) was calculated from the sum of “Treatment completion” and “Cure” outcomes since it was not readily available.
Treatment outcomes from the study by Tang et al. (2018) for the pharmaceutical care group showed an improvement compared to usual care, except for “Failure” outcomes. However, no statistical significance was found in all the outcomes measured between groups. On the contrary, a comparison between groups in the study by Tanvejsilp et al. (2018) showed that outcome differences were statistically significant when considered as a composite of outcomes, whereas the pharmaceutical care group was not always providing better outcomes.
In Tang et al. (2018), the “Treatment success” in the pharmaceutical care group was higher than that in another group, which was usual care; furthermore, in the study by Tanvejsilp et al. (2018), it was higher than that in the SAT group and lower than the home visit group.
The “Treatment completion” in the pharmaceutical care group from Tang et al. (2018) was higher than that in the usual care group care, yet the study by Tanvejsilp et al. (2018) showed that the pharmaceutical care group had lower values than the home visit and SAT group. For the “Cure” outcome, studies consistently showed that the pharmaceutical care group scored higher than other groups.
For “Default” and “Death” outcomes, the pharmaceutical care group scored lower across studies or at least the same as the other groups (Tang et al., 2018). However, the “Not completed” outcome was higher in the pharmaceutical care group than in the home visit and SAT group (Tanvejsilp et al., 2018). Lastly, the “Failure” outcome in the pharmaceutical care group was higher than the usual care group, and the “Transfer out” outcome was lower (Tang et al., 2018).
Other outcomes
Unlike WHO outcomes, other outcomes were primarily process outcomes related to the intervention given by the pharmacist and were reported at the end or at a specific follow-up time interval within the intensive and continuation phase. Table 4 presents outcomes measured at the end of the intensive and continuation phase identified in four studies (Clark et al., 2007; Venkatapraveen et al., 2012; Tang et al., 2018; Karuniawati et al., 2019), and Table 5 shows outcomes measured at a specific follow-up time interval within the intensive and continuation phase in three studies (Venkatapraveen et al., 2012; Tanvejsilp et al., 2018; Karuniawati et al., 2019). One study measured both outcomes at the end or at a specific follow-up time interval within intensive and continuation phases (Venkatapraveen et al., 2012).

TABLE 4. Other outcomes of clinical pharmacy services for TB care management measured at the end of the intensive and continuation phases.

TABLE 5. Other outcomes of clinical pharmacy services for TB care management measured at a follow-up time interval within the intensive and continuation phases.
Percentage values of attendance outcomes referred to the proportion of patients who adhered to a predetermined visit/monitoring schedule. For isoniazid metabolites, this included the proportion of patients with detected isoniazid metabolites in their urine samples. For medication counting, this was the proportion of medications the patient took. Sputum conversion identifies the result of a sputum test from positive to negative for M. tuberculosis, reported as a time interval or as the proportion of patients having a negative result.
Of the three studies included, the outcomes were specific for each study. For “Knowledge and adherence score” and “Compliance” outcomes, interventions of Education (Venkatapraveen et al., 2012) and counseling and leaflet (Karuniawati et al., 2019) showed statistically significant improvements over time. There was a significant improvement in the quality of life measures within the pharmaceutical care group; however, it was lower than the SAT and home visit group (Tanvejsilp et al., 2018). The percentage of compliance in taking medicines exhibited a proportion of patients who adhere to the medication schedule.
Risk of bias assessment
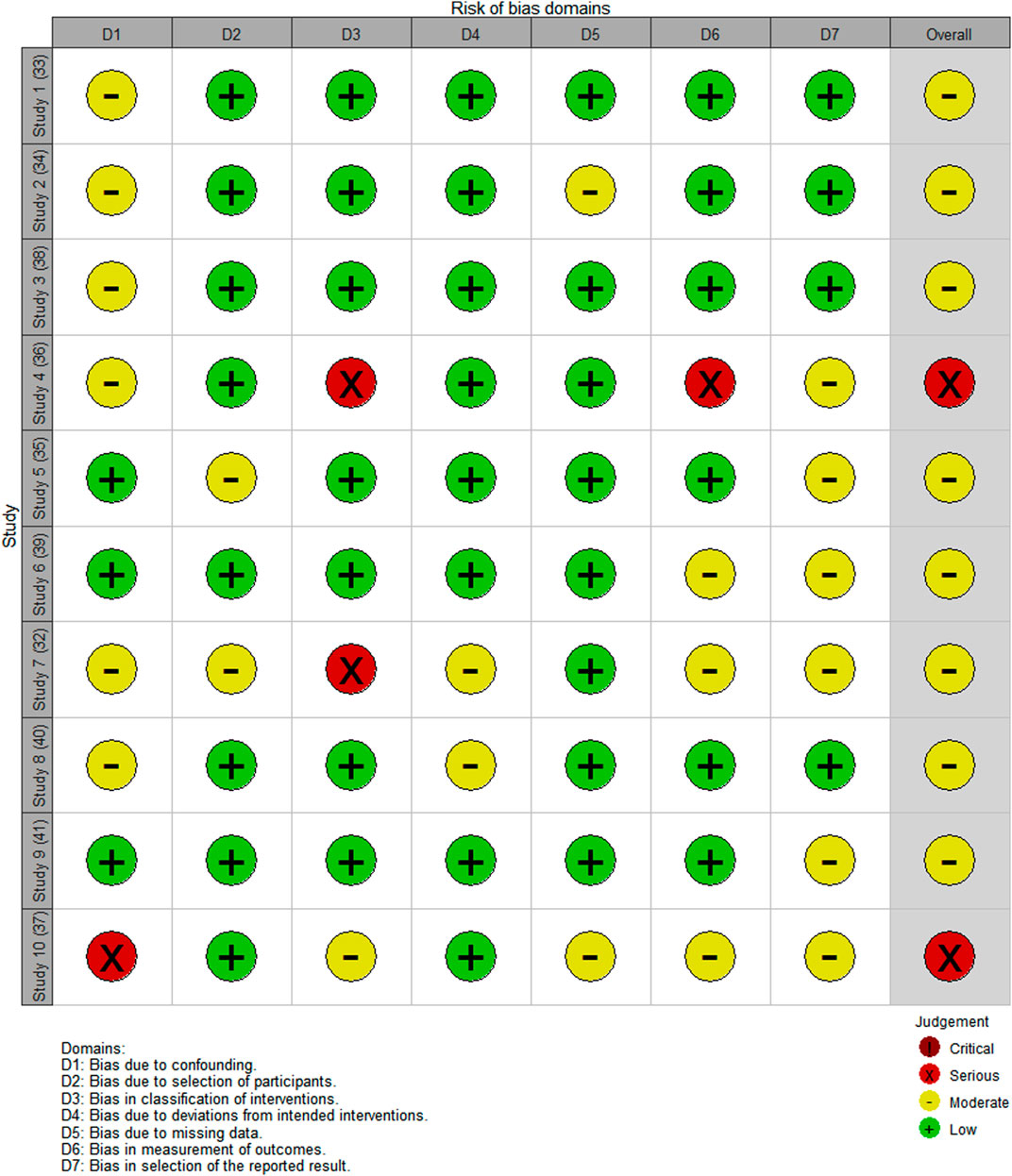
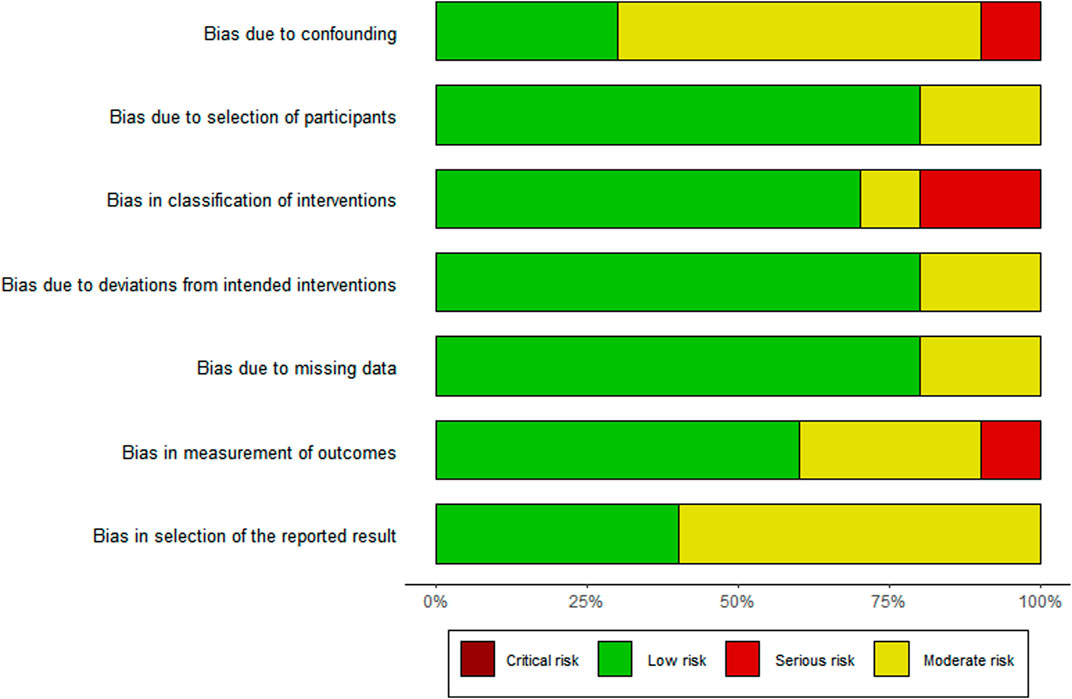
The risk of bias assessment is visually presented overall per study and per domain in Figures 3, 4.
Of the 10 studies assessed, the overall bias for eight studies was moderate (Clark et al., 2007; Bhardwaja et al., 2012; Venkatapraveen et al., 2012; Abrogoua et al., 2016; Tang et al., 2018; Tanvejsilp et al., 2018; Thomas et al., 2018; Karuniawati et al., 2019) and in two, it was serious (Lopes et al., 2017; Narayana et al., 2020). Commonly identified factors contributing to moderate bias occurrence were no group comparisons in the study (Bhardwaja et al., 2012; Venkatapraveen et al., 2012; Abrogoua et al., 2016; Lopes et al., 2017; Thomas et al., 2018; Narayana et al., 2020), patient selection (Tanvejsilp et al., 2018), intervention on the baseline patient assessment (Clark et al., 2007; Bhardwaja et al., 2012; Abrogoua et al., 2016; Lopes et al., 2017; Tang et al., 2018), and the use of a convenience sampling method (Lopes et al., 2017; Karuniawati et al., 2019).
Discussion
Main findings
The number of studies evaluating clinical pharmacy services in TB management is relatively scarce. Of note, none were randomized controlled trials, i.e., most were prospective studies with a non-randomized controlled design. In providing clinical pharmacy services, adherence measuring tools, medical records, patient interviews, and laboratory tests/therapeutic drug monitoring were frequently used as the clinical data source. Medication adherence and safety were the two intervention foci mostly provided in clinical pharmacy services for TB, followed by patient/caregiver educational needs/beliefs and medication/therapy effectiveness. Commonly conducted intervention activities were drug information/patient counseling, adverse drug reaction monitoring, drug use evaluation, and adherence monitoring. Educational materials/leaflets/written action plans, patient data collection forms, validated adherence questionnaires, and medical records were typically used to support the intervention activities.
The measured clinical pharmacy service outcomes were commonly process outcomes, i.e., not using the WHO outcome classification, making it wide-ranging and complex to make quantitative interpretations and comparisons between studies and outcomes. Only two studies used the WHO outcome classification. Most of the outcomes showed an improvement.
Of the 10 studies included, the risk of bias per domain was mainly low due to the limitations to the non-randomized study design. Therefore, despite the promising potential, convincing evidence for pharmacy services in TB management is still lacking.
Interpretation
Our search strategy retrieved relevant studies of pulmonary TB conducted primarily in high-burden countries; however, studies focusing on clinical pharmacy services for TB in other countries were limited. We found this in line with the trend of increasing TB research in high-burden countries with more context-specific research to meet its own national strategic plan goals toward TB elimination, where fundamental and epidemiology research is mainly conducted (Cazabon et al., 2017; World Health Organization, 2017; Nafade et al., 2018; Morishita et al., 2020), making studies on clinical pharmacy services in TB relatively uncommon. A review also highlighted this by stating that clinical pharmacy services primarily target specific medical conditions, but TB was not mentioned (Rotta et al., 2017).
In the studies we reviewed, clinical pharmacy services were given as a supplementary service, focusing on its utilization to enhance an existing TB care program, supported by multiple relevant patient-related data sources to provide the service appropriately (World Health Organization, 2017; World Health Organization, 2019c; Mahmoud, 2019). This practical type of study falls into an operational research category to maximize the effectiveness and efficiency of interventions that can contribute to achieving the desired TB outcome (Kumar et al., 2020; Morishita et al., 2020). However, the absence of sound randomized studies of clinical pharmacy services as part of existing TB care indicated that accurate assessments of the effect of clinical pharmacy services within TB care are difficult.
Most clinical pharmacy service studies did not always follow the consistent characterization of their components, which means that the content or terminology describing the detailed activities of the services tends to vary across different studies (Bonetti et al., 2020). However, as a part of broader multi-disciplinary services, pharmacists’ actions through clinical pharmacy services have a noticeable benefit, for example, in curbing microbial resistance (Padayatchi et al., 2016; Kunoor et al., 2021), especially in TB care. Using the DEPICT (Correr et al., 2013), we characterized the components of clinical pharmacy services and their outcomes in TB care. The two most common interventions in our review were focused on enhancing medication adherence and safety.
Medication adherence interventions, such as patient education, counseling, patient support (material or psychological), patient scheduling, and digital technology, improve TB treatment outcomes (Alipanah et al., 2018). Drug information or patient counseling was the most frequently conducted activity within clinical pharmacy services to influence and alter patients’ knowledge of TB (Venkatapraveen et al., 2012; Thomas et al., 2018; Karuniawati et al., 2019; Narayana et al., 2020), and we found that this knowledge was improving. The improvement was in agreement with a previous study showing that structured counseling improved patient/caregiver educational needs/beliefs on TB care-related knowledge (Sajjad et al., 2020). However, sometimes it was hindered by the different perceptions of information adequacy and reception between patients and health services (Moodley et al., 2020). In addition, comparing the improvement between studies is challenging due to different settings and assessment frameworks. Improved knowledge does not warrant better TB care outcomes. Nevertheless, it could be a means to build better communication with patients and can lead to medication adherence.
Regarding medication safety interventions, most of the studies we reviewed focused on resolving pharmaceutical care needs/issues raised during treatment (Clark et al., 2007; Lopes et al., 2017; Tang et al., 2018). With 66%–87% of issues resolved, this is comparable to a study mentioning that 74.1% of pharmaceutical care needs/issues were completely solved by pharmacist interventions (Garin et al., 2021). Only one study identified the prevalence of adverse drug reactions, and with the result of 24.5% (Abrogoua et al., 2016), it is within the range of 8%–85%, as identified by Singh et al. (2015). Our review showed that medication safety issues were well-handled by the pharmacist, as also highlighted by Phansalkar et al. on the prominent contribution of pharmacists to medication safety (Phansalkar et al., 2007).
Regarding TB outcomes of the interventions identified, the WHO outcome classification (Tang et al., 2018; Tanvejsilp et al., 2018) and sputum conversion (Venkatapraveen et al., 2012; Tang et al., 2018) were generally improved through drug information or patient counseling activities. This was in agreement with studies stating that health education improves TB prognosis (Westerlund et al., 2015) and treatment completion (Alipanah et al., 2018), leading to a reduction in the treatment default rate (Müller et al., 2018).
Other than the WHO outcomes, adherence-related outcomes were among the most used. Measures for adherence included isoniazid metabolite presence (Clark et al., 2007), attendance (Clark et al., 2007; Tang et al., 2018), adherence level (Karuniawati et al., 2019), compliance (Karuniawati et al., 2019), medication counting (Clark et al., 2007; Tang et al., 2018), and the Morisky Adherence Questionnaire Scale (Bhardwaja et al., 2012; Thomas et al., 2018). Although the methods were diverse and adherence is only seen as an intermediate outcome, a study providing drug information and patient counseling activities did improve treatment outcomes (Alipanah et al., 2018), disregarding the method of measuring adherence (Valencia et al., 2017). HRQoL outcomes were also used (Tanvejsilp et al., 2018), yet conflicting results were observed, as also noted by a study about their negative impact on the various domains of the quality of life during long-term TB care (Salehitali et al., 2019). We found that quality of life improvements through clinical pharmacy services are still scarce; only one very recent study provided RCT evidence (Khan et al., 2023), making it difficult to draw any general conclusions on the impact of clinical pharmacy services on patients’ quality of life.
Most studies in this review were adequately carried out despite their non-randomized design and could provide preliminary evidence on the benefit of clinical pharmacy service interventions, especially single interventions. Still, due to the nature of observational studies, prone to undetected confounding and bias, their results are not comparable to a well-designed randomized trial.
Pharmacy services generally consist of composite interventions, making it challenging to directly meta-analyze their overall effect (Teoh et al., 2019; Bonetti et al., 2020). The association between drug information and patient counseling with TB-related knowledge, medication adherence, medication effectiveness, and quality of life in improving TB care-related outcomes is complex. We anticipate that improving knowledge through structured education will lead to better adherence, improved medication effectiveness, and achieving TB care treatment goals, which will benefit the quality of life of patients with TB in the long run.
Strengths and limitations
The strength of this review is the use of a standardized framework for identifying and assessing clinical pharmacy services as single or composite intervention activities in TB management, resulting in a consistent reporting of clinical pharmacy service component identification. A limitation is focusing on English language publications only and, therefore, retrieving studies from a limited number of countries, potentially missing relevant studies in high-burden countries that use their national language. In addition, the analysis was restricted to the effect of clinical pharmacy services in patients with drug-susceptible TB only. Even while this resulted in more focus, due to the heterogeneity of the outcomes identified, a quantitative analysis of the effectiveness of clinical pharmacy services was not possible.
Recommendations
Identification and prioritization of the provision for clinical pharmacy services for TB management need to be established by increasing operational research activities and, at the same time, providing high-quality effectiveness evidence of clinical pharmacy services in TB management through randomized controlled trials, aside from process outcomes, which directly measure clinical pharmacy service process accomplishments; WHO outcomes should be included as the main outcome of interest in evaluating the clinical pharmacy service impact. The integration of clinical pharmacy services in our daily routine for TB care management is necessary to support patient-centered care practices and to improve TB care management outcomes.
Conclusion
Although clinical pharmacy services as single or composite interventions potentially improve TB outcomes, its evidence is still inconsistent and limited due to the lack of randomized controlled studies using the WHO outcome classification. Furthermore, well-designed RCTs are required for supporting larger-scale implementations.
Registration and protocol
In accordance with the guidelines, our systematic review protocol was registered with the PROSPERO on 15 August 2020 and was last updated on 18 January 2021 (registration number CRD42020199028).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
DI, JB, and MP conceptualized the study. DI and FS conducted the data curation and formal analysis. DI contributed to software, visualization, project administration, and writing the original draft. DI and FS have access to and verified the underlying data for analysis. DI, FS, JB, and MP contributed to the methodology, investigation, review, and editing of the manuscript. DI, MP, and JB contributed to funding acquisition, resources, and validation. JB and MP supervised the research. All authors contributed to the article and approved the submitted version.
Funding
This research is part of a doctoral study under the scholarship financed by the Directorate General of Higher Education, Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia. The funders had no role in the design, analysis, write-up, or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1186905/full#supplementary-material
References
Abrogoua, D. P., Kamenan, B. A. T., Ahui, B. J. M., and Doffou, E. (2016). Pharmaceutical interventions in the management of tuberculosis in a pneumophtisiology department, Ivory Coast. Ivory Coast. Ther. Clin. risk Manag. 12, 1749–1756. doi:10.2147/TCRM.S118442
Ahmad, N., Ahuja, S. D., Akkerman, O. W., Alffenaar, J-W. C., Anderson, L. F., Baghaei, P., et al. (2018). Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: An individual patient data meta-analysis. Lancet 392 (10150), 821–834. doi:10.1016/S0140-6736(18)31644-1
Albanna, A. S., Smith, B. M., Cowan, D., and Menzies, D. (2013). Fixed-dose combination antituberculosis therapy: A systematic review and meta-analysis. Eur. Respir. J. 42 (3), 721–732. doi:10.1183/09031936.00180612
Alipanah, N., Jarlsberg, L., Miller, C., Linh, N. N., Falzon, D., Jaramillo, E., et al. (2018). Adherence interventions and outcomes of tuberculosis treatment: A systematic review and meta-analysis of trials and observational studies. PLoS Med. 15 (7), 1002595. doi:10.1371/journal.pmed.1002595
American College of Clinical Pharmacy (2008). The definition of clinical pharmacy. Pharmacotherapy 28 (6), 816–817. doi:10.1592/phco.28.6.816
Bhardwaja, A., Kumar, R., Dabas, V., and Alam, N. (2012). Assessment and enhancing adherence to treatment regimen in tuberculosis out patients. Int. J. Pharmcy Pharm. Sci. 4 (3), 517–522. Available At: https://www.researchgate.net/publication/288714958_Assessment_and_enhancing_adherence_to_treatment_regimen_in_tuberculosis_out_patients.
Bonetti, A. F., Della Rocca, A. M., Lucchetta, R. C., Tonin, F. S., Fernandez-Llimos, F., and Pontarolo, R. (2020). Mapping the characteristics of meta-analyses of pharmacy services: A systematic review. Int. J. Clin. Pharm. 42 (5), 1252–1260. doi:10.1007/s11096-020-01058-5
Cazabon, D., Alsdurf, H., Satyanarayana, S., Nathavitharana, R., Subbaraman, R., Daftary, A., et al. (2017). Quality of tuberculosis care in high burden countries: The urgent need to address gaps in the care cascade. Int. J. Infect. Dis. 56, 111–116. doi:10.1016/j.ijid.2016.10.016
Chaves Torres, N. M., Quijano Rodríguez, J. J., Porras Andrade, P. S., Arriaga, M. B., and Netto, E. M. (2019). Factors predictive of the success of tuberculosis treatment: A systematic review with meta-analysis. PLoS ONE 14 (12), 0226507. doi:10.1371/journal.pone.0226507
Clark, P. M., Karagoz, T., Apikoglu-Rabus, S., and Izzettin, F. V. (2007). Effect of pharmacist-led patient education on adherence to tuberculosis treatment. Am. J. health-system Pharm. AJHP official J. Am. Soc. Health-System Pharm. 64 (5), 497–505. doi:10.2146/ajhp050543
Cohen, D. B., Meghji, J., and Squire, S. B. (2018). A systematic review of clinical outcomes on the WHO Category II retreatment regimen for tuberculosis. Int. J. Tuberc. Lung Dis. 22 (10), 1127–1134. doi:10.5588/ijtld.17.0705
Correr, C. J., Melchiors, A. C., Souza, T. T. de, Rotta, I., Salgado, T. M., and Fernandez-Llimos, F. (2013). A tool to characterize the components of pharmacist interventions in clinical pharmacy services: The DEPICT project. Ann. Pharmacother. 47 (7-8), 946–952. doi:10.1345/aph.1S006
DEPICT (2013). Descriptive Elements of pharmacist intervention characterization tool: DEPICT manual of instructions. Available At: http://depictproject.org/downloads_files/Depict_Version_2_manual.pdf.
Development Group for ROBINS-I (2016). Risk of bias in non-randomized studies of interventions (ROBINS-I): Detailed guidance. Available At: www.riskofbias.info.
DiPiro, J. T. (2017). “Pharmacotherapy,” in A pathophysiologic approach. Editors J. T. DiPiro, R. L. Talbert, G. C. Yee, G. R. Matzke, B. G. Wells, L. Michael Poseyet al. (New York: McGraw-Hill Education). Available At: https://accesspharmacy.mhmedical.com/book.aspx?bookid=1861.
Dreischulte, T., and Fernandez-Llimos, F. (2016). Current perceptions of the term clinical pharmacy and its relationship to pharmaceutical care: A survey of members of the European society of clinical pharmacy. Int. J. Clin. Pharm. 38 (6), 1445–1456. doi:10.1007/s11096-016-0385-3
Franklin, B. D., and van Mil, J. W. F. (2005). Defining clinical pharmacy and pharmaceutical care. Pharm. World Sci. 27 (3), 137. doi:10.1007/s11096-005-7060-4
Garin, N., Sole, N., Lucas, B., Matas, L., Moras, D., Rodrigo-Troyano, A., et al. (2021). Drug related problems in clinical practice: A cross-sectional study on their prevalence, risk factors and associated pharmaceutical interventions. Sci. Rep. 11 (1), 883. doi:10.1038/s41598-020-80560-2
Gegia, M., Winters, N., Benedetti, A., van Soolingen, D., and Menzies, D. (2017). Treatment of isoniazid-resistant tuberculosis with first-line drugs: A systematic review and meta-analysis. Lancet Infect. Dis. 17 (2), 223–234. doi:10.1016/S1473-3099(16)30407-8
Hepler, C. D. (2004). Clinical pharmacy, pharmaceutical care, and the quality of drug therapy. Pharmacotherapy 24 (11), 1491–1498. doi:10.1592/phco.24.16.1491.50950
Holdford, D. A., and Brown, T. R. (2010). Introduction to hospital & health-system pharmacy practice. Bethesda MD: American Society of Health-System Pharmacists.
Karuniawati, H., Putra, O. N., and Wikantyasning, E. R. (2019). Impact of pharmacist counseling and leaflet on the adherence of pulmonary tuberculosis patients in lungs hospital in Indonesia. Indian J. Tuberc. 66 (3), 364–369. doi:10.1016/j.ijtb.2019.02.015
Khan, F. U., Khan, A., Khan, F. U., Hayat, K., Rehman, A. U., Chang, J., et al. (2022). Assessment of adverse drug events, their risk factors, and management among patients treated for multidrug-resistant TB: A prospective cohort study from Pakistan. Front. Pharmacol. 13, 876955. doi:10.3389/fphar.2022.876955
Khan, F. U., Khan, F. U., Aqeel, M. T., Hayat, K., Chang, J., Rehman, A. U., et al. (2023). A randomized controlled trial to evaluate the impact of pharmacist-led clinical interventions on the health-related quality of life among TB patients. Front. Pharmacol. 14, 1171985. doi:10.3389/fphar.2023.1171985
Kumar, A. M., Harries, A. D., Satyanarayana, S., Thekkur, P., Shewade, H. D., and Zachariah, R. (2020). What is operational research and how can national tuberculosis programmes in low- and middle-income countries use it to end TB? Indian J. Tuberc. 67 (4), S23–S32. doi:10.1016/j.ijtb.2020.11.009
Kunoor, A., Prabhu, B., Menon, V. P., Kumar, A. S., Shameen, F., Chandra, S., et al. (2021). Impact of implementing a novel anti tuberculosis. Kerala, India: Treatment Stewardship Program from a Tertiary Care Centre. doi:10.2139/ssrn.3797584
Lee, H. W., Lee, J. K., Kim, E., Yim, J-J., and Lee, C-H. (2016). The effectiveness and safety of fluoroquinolone-containing regimen as a first-line treatment for drug-sensitive pulmonary tuberculosis: A systematic review and meta-analysis. PLoS ONE 11 (7), 0159827. doi:10.1371/journal.pone.0159827
Lopes, A. R. V., Miranda, S. S. de, Ceccato, M. D. G. B., Silveira, M. R., Resende, N. H. de, and Carvalho, W. S. (2017). Evaluation of the impact of pharmaceutical care for tuberculosis patients in a secondary referral outpatient clinic, minas gerais, Brazil. An. Acad. Bras. Ciencias 89 (4), 2911–2919. doi:10.1590/0001-3765201720170301
Mahmoud, S. H. (2019). Patient assessment in clinical pharmacy. Cham: Springer International Publishing.
McGuinness, L. A., and Higgins, J. P. T. (2020). Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Syn. Meth 12, 55–61. doi:10.1002/jrsm.1411
Moodley, N., Saimen, A., Zakhura, N., Motau, D., Setswe, G., Charalambous, S., et al. (2020). They are inconveniencing us' - exploring how gaps in patient education and patient centred approaches interfere with TB treatment adherence: Perspectives from patients and clinicians in the free state province, South Africa. BMC Public Health 20 (1), 454. doi:10.1186/s12889-020-08562-3
Morishita, F., Yamanaka, T., and Islam, T. (2020). Intensified research on tuberculosis in the western pacific region: A bibliometric analysis, 2000-2019. West. Pac Surveill. Response J. 11 (4), 24–31. doi:10.5365/wpsar.2020.11.3.003
Müller, A. M., Osório, C. S., Silva, D. R., Sbruzzi, G., Tarso, P. de, and Dalcin, R. (2018). Interventions to improve adherence to tuberculosis treatment: Systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 22 (7), 731–740. doi:10.5588/ijtld.17.0596
Nafade, V., Nash, M., Huddart, S., Pande, T., Gebreselassie, N., Lienhardt, C., et al. (2018). A bibliometric analysis of tuberculosis research, 2007-2016. PLoS ONE 13 (6), 0199706. doi:10.1371/journal.pone.0199706
Narayana, G., Jyoshna, K., Kishore, M., Pradeepkumar, B., Bogireddy, S., Smg, I., et al. (2020). Impact of pharmacist counselling on knowledge, and medication adherence in tuberculosis patients: A quasi-experimental design. Int. J. Pharma Bio Sci. 10, 5. doi:10.22376/ijpbs/lpr.2020.10.5.p6-10
Negaard, B. J., Lyons, K. P., Nichol, C. L., and Polgreen, L. A. (2020). What does a pharmacist do? A time and motion study. Res. Soc. Adm. Pharm. 16 (9), 1314–1317. doi:10.1016/j.sapharm.2019.03.007
Padayatchi, N., Mahomed, S., Loveday, M., and Naidoo, K. (2016). Antibiotic stewardship for drug resistant tuberculosis. Expert Opin. Pharmacother. 17 (15), 1981–1983. doi:10.1080/14656566.2016.1225724
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, 790–799. doi:10.1016/j.rec.2021.07.010
Phansalkar, S., Hoffman, J. M., Nebeker, J. R., and Hurdle, J. F. (2007). Pharmacists versus nonpharmacists in adverse drug event detection: A meta-analysis and systematic review. Am. J. health-system Pharm. AJHP official J. Am. Soc. Health-System Pharm. 64 (8), 842–849. doi:10.2146/ajhp060335
Rotta, I., Salgado, T. M., Silva, M. L., Correr, C. J., and Fernandez-Llimos, F. (2015). Effectiveness of clinical pharmacy services: An overview of systematic reviews (2000-2010). Int. J. Clin. Pharm. 37 (5), 687–697. doi:10.1007/s11096-015-0137-9
Rotta, I., Souza, T. T., Salgado, T. M., Correr, C. J., and Fernandez-Llimos, F. (2017). Characterization of published randomized controlled trials assessing clinical pharmacy services around the world. Res. Soc. Adm. Pharm. 13 (1), 201–208. doi:10.1016/j.sapharm.2016.01.003
Sajjad, S. S., Sajid, N., Fatimi, A., Maqbool, N., Baig-Ansari, N., and Amanullah, F. (2020). The impact of structured counselling on patient knowledge at a private TB program in Karachi. Pak J. Med. Sci. 36 (1), S49–S54. doi:10.12669/pjms.36.ICON-Suppl.1713
Salehitali, S., Noorian, K., Hafizi, M., and Dehkordi, A. H. (2019). Quality of life and its effective factors in tuberculosis patients receiving directly observed treatment short-course (DOTS). J. Clin. Tuberc. Other Mycobact. Dis. 15, 100093. doi:10.1016/j.jctube.2019.100093
Schoenbaechler, V., Guilavogui, Y., Onivogui, S., Hébélamou, J., Mugglin, C., Furrer, H., et al. (2021). Rate of treatment success and associated factors in the program for drug-susceptible tuberculosis in the forest region, republic of Guinea, 2010-2017: A real-world retrospective observational cohort study. Int. J. Infect. Dis. 110, 6–14. doi:10.1016/j.ijid.2021.06.014
Shrestha, S., Shrestha, R., Ahmed, A., Sapkota, B., Khatiwada, A. P., Christopher, C. M., et al. (2022). Impact of pharmacist services on economic, clinical, and humanistic outcome (ECHO) of South asian patients: A systematic review. J. Pharm. Policy Pract. 15 (1), 37. doi:10.1186/s40545-022-00431-1
Singh, A., Prasad, R., Balasubramanian, V., Gupta, N., and Gupta, P. (2015). Prevalence of adverse drug reaction with first-line drugs among patients treated for pulmonary tuberculosis. Clin. Epidemiol. Glob. Health 3, S80–S90. doi:10.1016/j.cegh.2015.10.005
Sterne, J. A., Hernán, M. A., Reeves, B. C., Savović, J., Berkman, N. D., Viswanathan, M., et al. (2016). ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355, i4919. doi:10.1136/bmj.i4919
Tang, Z-Q., Jiang, R-H., and Xu, H-B. (2018). Effectiveness of pharmaceutical care on treatment outcomes for patients with first-time pulmonary tuberculosis in China. J. Clin. Pharm. Ther. 43 (6), 888–894. doi:10.1111/jcpt.12746
Tanvejsilp, P., Loeb, M., Dushoff, J., and Xie, F. (2018). Out-of-Pocket expenditures, indirect costs and health-related quality of life of patients with pulmonary tuberculosis in Thailand. PharmacoEconomics 2 (3), 281–296. doi:10.1007/s41669-017-0057-9
Teoh, K. W., Khan, T. M., Chaiyakunapruk, N., and Lee, S. W. H. (2019). Examining the use of network meta-analysis in pharmacy services research: A systematic review. J. Am. Pharm. Assoc. 59 (6), 787–791. doi:10.1016/j.japh.2019.06.015
Thomas, A., Joy, J., and Kurian, A. (2018). Socio-epidemiological evaluation of tuberculosis and impact of pharmaceutical care on medication adherence among tuberculosis patients. Asian J. Pharm. Clin. Res. 11 (2), 265. doi:10.22159/ajpcr.2018.v11i2.20503
Tierney, D. B., Orvis, E., Nathavitharana, R. R., Hurwitz, S., Tintaya, K., Vargas, D., et al. (2021). FAST tuberculosis transmission control strategy speeds the start of tuberculosis treatment at a general hospital in Lima, Peru. Infect. Control Hosp. Epidemiol. 43, 1459–1465. doi:10.1017/ice.2021.422
Valencia, S., León, M., Losada, I., Sequera, V. G., Fernández Quevedo, M., and García-Basteiro, A. L. (2017). How do we measure adherence to anti-tuberculosis treatment? Expert Rev. Anti Infect. Ther. 15 (2), 157–165. doi:10.1080/14787210.2017.1264270
van der Werf, M. J., Langendam, M. W., Huitric, E., and Manissero, D. (2012). Multidrug resistance after inappropriate tuberculosis treatment: A meta-analysis. Eur. Respir. J. 39 (6), 1511–1519. doi:10.1183/09031936.00125711
Venkatapraveen, A., Rampure, M. V., Patil, N., Hinchageri, S. S., and Lakshmi, D. P. (2012). Assessment of clinical pharmacist intervention to improve compliance and health care outcomes of tuberculosis patients. Der Pharm. Lett. 4 (3), 931–937. Available At: https://www.scholarsresearchlibrary.com/articles/assessment-of-clinical-pharmacist-intervention-to-improve-compliance-and-health-care-outcomes-of-tuberculosis-patients.pdf.
Wen, Y., Zhang, Z., Li, X., Xia, D., Ma, J., Dong, Y., et al. (2018). Treatment outcomes and factors affecting unsuccessful outcome among new pulmonary smear positive and negative tuberculosis patients in anqing, China: A retrospective study. BMC Infect. Dis. 18 (1), 104. doi:10.1186/s12879-018-3019-7
Westerlund, E. E., Tovar, M. A., Lönnermark, E., Montoya, R., and Evans, C. A. (2015). Tuberculosis-related knowledge is associated with patient outcomes in shantytown residents; results from a cohort study, Peru. J. Infect. 71 (3), 347–357. doi:10.1016/j.jinf.2015.05.010
Whittlesea, C., and Hodson, K. (2019). Clinical Pharmacy and Therapeutics. Sixth edition. International ed. Oxford: Elsevier.
World Health Organization (2017). Global investments in tuberculosis research and development: Past, present, and future. Geneva: World Health Organization. Available from: http://apps.who.int/iris/bitstream/10665/259412/1/9789241513326-eng.pdf?ua=1.
World Health Organization (2021a). Global tuberculosis report 2021. Geneva: World Health Organization.
World Health Organization (2021b). Meeting report of the WHO expert consultation on drug-resistant tuberculosis treatment outcome definitions: 17–19 november 2020. Geneva: WHO.
World Health Organization (2019b). Multisectoral accountability framework: To accelerate progress to end tuberculosis by 2030. Geneva: World Health Organization.
World Health Organization (2019c). People-centred framework for tuberculosis programme planning and prioritization: User guide. Geneva: World Health Organization.
World Health Organization (2014). The End TB Strategy: Global strategy and targets for tuberculosis prevention, care and control after 2015. Geneva: World Health Organization.
World Health Organization (2010). Treatment of tuberculosis: Guidelines. 4th ed. Geneva: World Health Organization.
World Health Organization (2019a). WHO guidelines on tuberculosis infection prevention and control: 2019 update 2019 update. Geneva: World Health Organization.
Keywords: clinical pharmacy, pharmaceutical care, pharmacist intervention, treatment outcomes, tuberculosis
Citation: Iskandar D, Suryanegara FDA, van Boven JFM and Postma MJ (2023) Clinical pharmacy services for tuberculosis management: a systematic review. Front. Pharmacol. 14:1186905. doi: 10.3389/fphar.2023.1186905
Received: 15 March 2023; Accepted: 26 June 2023;
Published: 07 July 2023.
Edited by:
Tshokey Tshokey, Jigme Dorji Wangchuck National Referral Hospital (JDWNRH), BhutanReviewed by:
Tandin Zangpo, Ministry of Health, BhutanBernd Rosenkranz, Fundisa African Academy of Medicines Development, South Africa
Copyright © 2023 Iskandar, Suryanegara, van Boven and Postma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: D. Iskandar, ZC5pc2thbmRhckBydWcubmw=
 D. Iskandar
D. Iskandar F. D. A. Suryanegara
F. D. A. Suryanegara J. F. M. van Boven
J. F. M. van Boven M. J. Postma
M. J. Postma