- 1High Altitude Plant Physiology Research Centre (HAPPRC), HNB. Garhwal University (A Central University), Srinagar Garhwal, Uttarakhand, India
- 2Regional Centre for Organic and Natural Farming, Ghaziabad, Uttar Pradesh, India
- 3Institute for Integrated Natural Sciences, University of Koblenz, Koblenz, Germany
- 4Department of Biotechnology, Kumaun University, Bhimtal, Uttarakhand, India
- 5Laboratory of Neurophysiology, Department of Biology, University of the Balearic Islands, Palma de Mallorca, Spain
- 6Health Research Institute of Balearic Islands (IdISBa), Palma de Mallorca, Spain
- 7CIBER Fisiopatología de la Obesidad y Nutrición (CIBEROBN), Instituto de Salud Carlos III (ISCIII), Madrid, Spain
- 8Research Group in Community Nutrition and Oxidative Stress, University of the Balearic Islands—IUNICS, Palma de Mallorca, Spain
- 9Aromatic Plant Research Center, Lehi, UT, United States
- 10Department of Chemistry, University of Alabama in Huntsville, Huntsville, AL, United States
- 11Department of Food Science, Faculty of Food Science and Technology, Universiti Putra Malaysia, Selangor, Malaysia
- 12Natural Medicines and Products Research Laboratory, Institute of Bioscience, Universiti Putra Malaysia, Selangor, Malaysia
- 13Department of Biochemistry, Faculty of Science, University of Maiduguri, Maiduguri, Nigeria
- 14University of Life Sciences “King Mihai I” From Timisoara, Timis, Romania
- 15Facultad de Medicina, Universidad del Azuay, Cuenca, Ecuador
With the advent of highly effective plant-based medications with few or no side effects, the use of phytomedicines against complex diseases such as cancer is becoming more widespread. The broadly recognized pentacyclic triterpenes known as boswellic acids (BAs) are derived from the oleogum resin, or frankincense, extracted from the plant species of the genus Boswellia. The frankincense mixture contains various BA types, each having a different potential and helping treat certain cancers. This review focuses on details regarding the traits of the BAs, their roles as anti-cancer agents, the mechanism underlying their activities, and the function of their semi-synthetic derivatives in managing and treating certain cancers. The review also explores the biological sources of BAs, how they are conserved, and how biotechnology might help preserve and improve in vitro BA production. The review concludes that the BAs and their semi-synthetic derivatives are effective against a broad spectrum of cancer cell lines. The detailed information in the review can be helpful for researchers to gain more information about BAs and BA-based medications for efficient and cost-effective cancer treatments.
1 Introduction
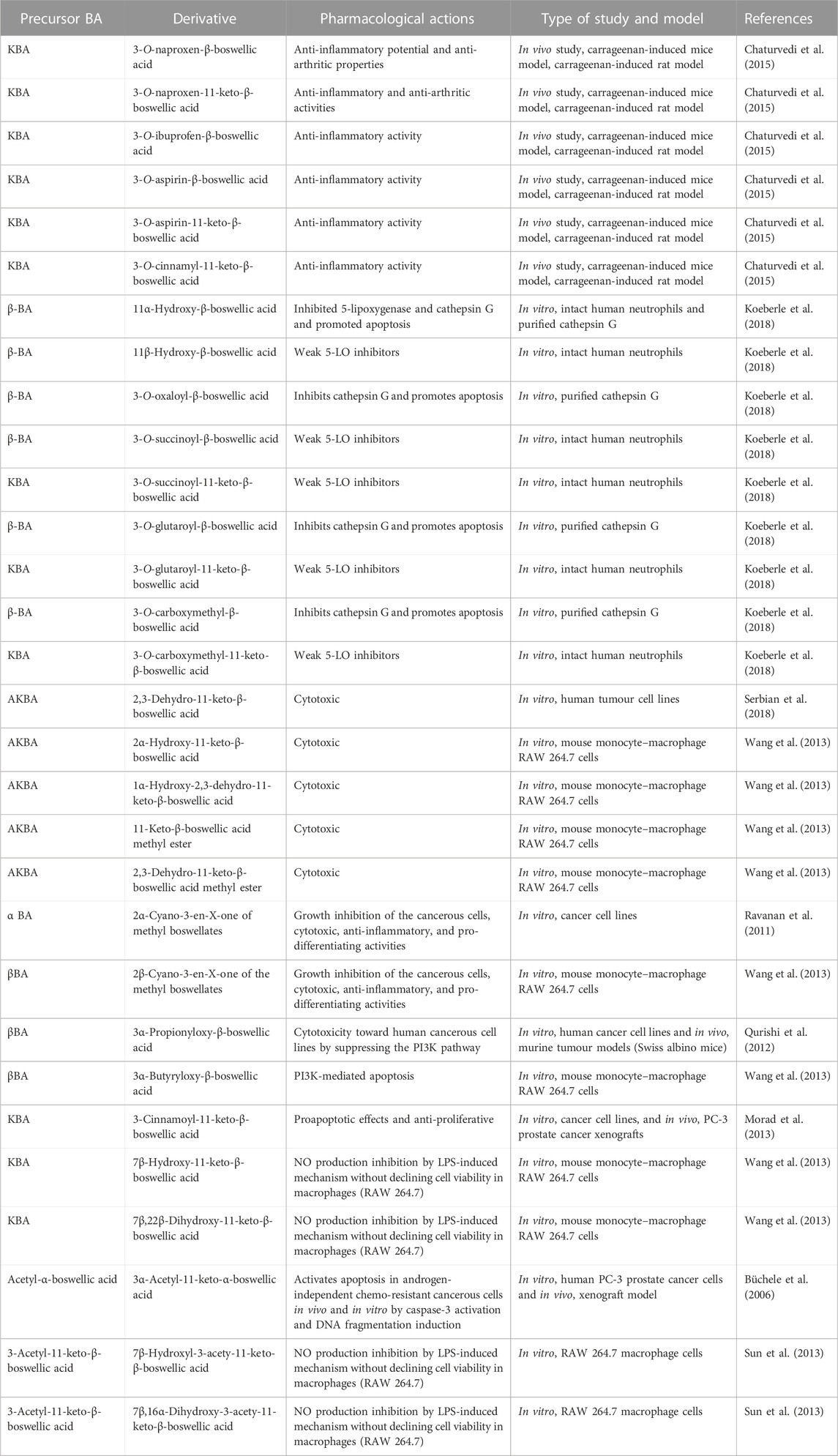
The discovery of several plant-based chemicals with anti-cancer potential reinstated the ancient traditional knowledge of herbal medicines with the support of scientific knowledge. The identification of compounds such as vinblastine, vincristine, and taxol as anti-cancerous agents laid the fundamentals for the discovery of new phytochemical anti-cancerous agents (Dhyani et al., 2022). In recent years, the finding of phytochemicals with potential anti-cancer activities with no or fewer side effects has been accelerated. One such class of compounds, known as boswellic acids, is extracted from the Boswellia genus and is extensively used to treat various other chronic diseases. These include haemolytic, spasmolytic, antiviral, anti-inflammatory, hepatoprotective, gastroprotective, and anti-microbial properties (Sun et al., 2006; Agrawal et al., 2011; Hussain et al., 2017). Boswellic acids (BAs) are pentacyclic triterpenes derived from the frankincense tree. Boswellia serrata, popularly referred to as white guggal, Indian olibanum, Salai Guggal, and dhup, is the main source of BAs (Havel et al., 2002; Qurishi et al., 2012), although B. carteri (Roy et al., 2019), B. sacra, and B. papyrifera are additional sources of BAs (Al-Harrasi et al., 2019). Usually, the gum resins of Boswellia species were employed for various purposes, such as adhesives, cosmetic preparations, coating materials, and incense used in cultural ceremonies and rituals. It is one of the most important and widely used ingredients in traditional Ayurvedic and Unani remedies, which are exceptionally successful in treating a variety of inflammatory, gastrointestinal, hormonal, and microbiological illnesses (Siddiqui, 2011). The BAs are separated from the gum resin frankincense, which is made up of essential oil, mucous, and a lipophilic portion. The grades and content of this resin vary according to the species of Boswellia used to extract it (Saraswati and Agrawal, 2012). The gum resin of Boswellia species contains up to 12 different types of BAs among which four major types of BAs are β-BA, A-β-BA, KBA, and AKBA with different pharmacological properties such as anti-cancer, anti-angiogenic, anti-tumour, apoptosis induction, anti-proliferative, and anti-inflammatory, among others (Liu et al., 2002). Nevertheless, not all BAs have an identical activity or potency (Liu et al., 2002; Yadav et al., 2012). For example, KBA and AKBA are the most effective at suppressing cytokine production and inhibiting the enzymes responsible for inflammatory reactions. As a result, these have been described as effective treatments for a variety of chronic conditions (Siddiqui, 2011; Roy et al., 2019).
BAs have been reported to be beneficial in both the prevention and treatment of various cancers such as breast, bladder, cervix, prostate, colorectal, head and neck, liver, lung, and pancreas (Roy et al., 2019). Several semi-synthetic derivatives of the different BAs, that show chemotherapeutic promise against diverse cancerous human cell lines, were also synthesized further to enhance the BAs’ anti-cancer action (Liu et al., 2002). Apoptosis, reducing angiogenesis of cancerous cells, obstructing blood flow to the tumour tissue, and down-regulating AKT phosphorylation are some of the mechanisms that BAs use to prevent cancer metastasis, depending on the type of cancer cells targeted (Liu et al., 2002; Uthaman et al., 2012).
This review examines BAs concerning their natural sources, conservation status, and in vitro biotechnological production potentials. This review also aims to describe the types of BAs, their chemical properties, semi-synthetic derivatives, and the mechanism of action of these compounds as anti-cancer agents. Scientific evidence supporting their categorization as anti-cancer substances is also presented, along with their modern and traditional applications as valuable drugs. The review will help increase the knowledge about plant-based anti-cancer therapeutics that includes various BAs and their derivatives.
2 General characterization of boswellic acid and its semi-synthetic derivatives
For centuries, frankincense (olibanum) extracted from the Boswellia tree, mainly from B. serrata, has been used as a source of BAs. Other species studied included B. sacra, B. papyrifera, and B. carteri, which are also used as BA sources worldwide (Al-Harrasi et al., 2019; Bongers et al., 2019). The resin of the Boswellia tree is composed of essential oil (5%–9%) and mucilage (6%–20%), the major component of the BAs, which has been quantified around 25%–35% of the resin acid mixture (Ennet, 2000; Al-Harrasi et al., 2021). BAs are a group of bioactive organic acids containing a pentacyclic triterpene and a β-carboxyl group at the C-4 position. BAs are grouped into two groups: the first one is ursane-type (β-BAs), and the second one is oleanane-type (α-BAs). Ursane-type BAs contain ursane triterpene skeleton-type and include β-boswellic acid (BA), 11-keto-β-boswellic acid (KBA), acetyl-β-boswellic acid (ABA), and acetyl-11-keto-β-boswellic acid (AKBA). Oleanane-type boswellic acid consisted oleanane structure and included α-boswellic acid (α-BA), 11-keto-α-boswellic acid (α-KBA), acetyl-α-boswellic acid (α-ABA), and acetyl-11-keto-α-boswellic acid (α-AKBA) (Shah et al., 2009; Al-Harrasi et al., 2019; Al-Harrasi et al., 2021; Schmiech et al., 2021) (Figure 1).
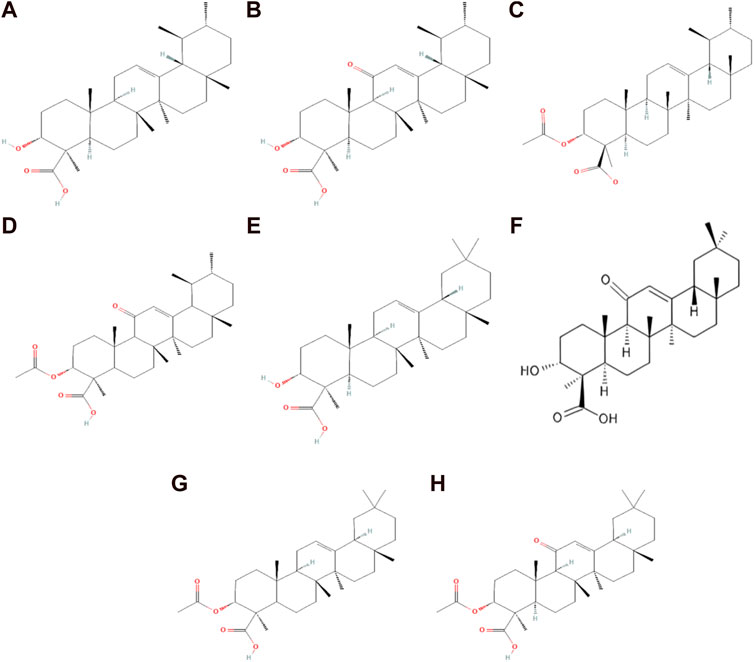
FIGURE 1. Chemical structure of ursane and oleanane-type boswellic acid. (A) β-Boswellic acid; (B)11-keto-β-boswellic acid; (C) acetyl-β-boswellic acid; (D) acetyl-11-keto-β-boswellic acid; (E) α-boswellic acid; (F) 11-keto-α-boswellic acid; (G) acetyl-α-boswellic acid; and (H) acetyl-11-keto-α-boswellic acid.
The resin containing the BAs is isolated from the Boswellia tree through wounding and subsequent tapping. These extraction procedures induced a chain of signaling process in the Boswellia tree involving gene expression and endogenous BAs production in the wounded location of the tree ultimately resulting in the clumpy frankincense (Khan et al., 2018). However, the BA content in the resin of the Boswellia genus varies at inter-species and intra-species levels. Studies showed considerable variation in BAs, their precursor content, and the type of BAs present in the resin of B. serrata, B. sacra, and B. papyrifera (Paul, 2012). For example, in the study of Paul (2012), B. papyrifera showed higher β-AKB concentration but lower concentrations of the other BAs and other secondary metabolites, whereas B. serrata showed lower β-KBA and β-AKBA concentrations and higher concentrations of α-BA and β-BA. Within a species, the BA content is greatly influenced by the micro- and macroclimatic conditions to which the Boswellia trees are subjected (Park et al., 2011). Geographical variations were also observed in various Boswellia spp. populations in the BA content of the resin (Al-Harrasi et al., 2018). Even the different tissues of the same tree showed variations in the BA content and the compositions in the studies; for instance, B. sacra roots were devoid of the BAs (Paul, 2012), whereas the leaves had trace amounts of β-ABA and β-AKBA. The amyrins are the BAs synthesized by the terpenoid biosynthetic pathway (MVA Pathway), the immediate precursor of the boswellic acids. β-Amyrin is the α-boswellic acid precursor, which is an oleanane, and α-amyrin is the β-boswellic acid precursor, which is an ursane. The different BA compounds extracted from the frankincense (resin) of Boswellia are listed in Table 1.
The main problem with most of the BAs is their low bioavailability, particularly for AKBA and KBA, which, in turn, raises questions about the pharmacological relevance of their bioactivities in animal and human research (Du et al., 2015). Synthesis of new derivatives by chemical modification and biotransformation of BAs can be an option (Table 2). BA derivatives have been synthesized for the discovery of new potent drugs, particularly the anti-cancer and tumour suppressors (Meng et al., 2005; Chaturvedi et al., 2015; Koeberle et al., 2018; Serbian et al., 2018; Shamraiz et al., 2020).
3 Mechanism of anti-tumour action of boswellic acid
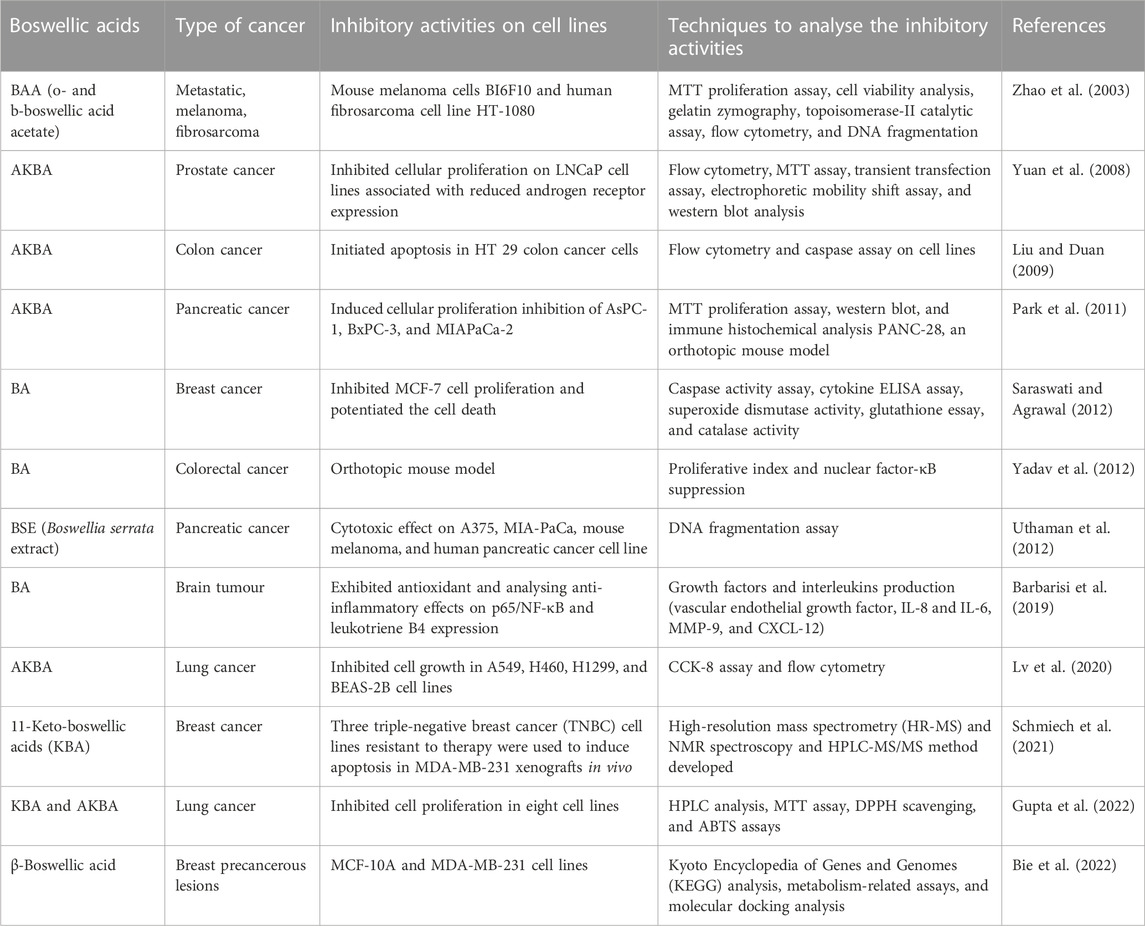
BAs have been investigated for decades and are found to exhibit robust anti-cancer properties in vitro and in vivo (Table 3). Different isomers and extracts from the acids exhibit anti-cancer characteristics with distinct mechanisms in many types of cancer. The mechanisms of activity of BAs comprise a variety of targets, including the enzymes of angiogenesis and others such as topoisomerases, 5-lipoxygenase (5-LO), cytochrome P450, and mitogen-activated protein kinase (MAPK, especially p38) which are either promoted or inhibited by BAs (Iram et al., 2017).
B. serrata gum resin extract (methanolic) showed the occurrence of triterpenoids, β-boswellic acid, and its analogues. Huang et al., 2000 reported that b-BA naturally occurring triterpenoids with their derivatives had been part of traditional medicine for cancer treatment (Huang et al., 2000). Several scientific studies have also shown Boswellia’s pentacyclic triterpenes as one of the most promising anti-cancer agents (Poeckel and Werz, 2006; Yuan et al., 2013; Al-Bahlani et al., 2020). AKBA and KBA are assessed by active inhibition of topoisomerase I and IIa, which restricts the growth of the cells and their proliferation and induces apoptosis through a pathway dependent on caspase-8 in human leukaemia, hepatoma, colon, and in a wide range of cancer cell lines (Xia et al., 2005; Suhail et al., 2011). Moreover, a chemoproteomic study based on mass spectrometry indicated that b-BAs also interact with the ribosomal proteins, inhibit protein synthesis, and thus further modulate cancer progression (Casapullo et al., 2016). Morphological alterations were noticed in treated HL-60 cells with AKBA, which is a signal of apoptosis of the cells. BA, 3-O-acetyl-β-boswellic acid, AKBA, and 3-O-acetyl-11-keto-boswellic acid showed anti-tumour activity and inhibition of DNA, RNA, and proteins synthesis in human leukaemia HL60 cells in a dose-dependent manner (Shao et al., 1998; Hoernlein et al., 1999).
AKBA showed cytotoxic action against three treatment-resistance triple-negative breast cancer cell lines (TNBC) and apoptosis in MDA-MB-231 xenografts in the in vitro study (Schmiech et al., 2021). AKBA diminished the viability of the cell in H460, H1299, A549, and BEAS-2B cell lines. In A549, cells caused cell cycle arrest at the G0/G1 phase, thus suppressing the clone formation and promoting the cellular apoptosis. It also reduced the expression of LC3A/B-I and LC3A/B-II, along with Beclin-1 proteins and inhibition of the signalling pathway of PI3K/Akt. It also suppressed protein expression and autolysosome formation (Lv et al., 2020). The latest β-isomer synthesized and characterized as 11-keto-boswellic acid (KBA) was discovered to have cytotoxic effects against three treatment-resistant triple-negative breast cancer (TNBC) cell lines in vitro and to cause apoptosis in MDA-MB-231 xenografts in vivo (Schmiech et al., 2021).
Recently, β-BA has been shown to inhibit precancerous breast lesions by suppressing the glycolysis pathway and reducing ATP production in MCF-10AT cells without damaging normal MCF-10A. It is also observed to suppress glycolysis which activates the AMPK pathway and inhibits the mTOR pathway to limit MCF-10AT proliferation. In the same study, analysis using molecular docking suggested that the target of β-BA might be GLUT1. The GLUT1 forced expression could rescue the suppression of glycolysis and induce survival checks on MCF-10AT (Bie et al., 2022).
In another study, the effect of β-BA and AKBA has examined in nine human glioma stem-like cells and five glioma-initiating cell lines to analyse the acute growth inhibitory mechanism. The same study includes the anti-clonogenic characteristics along with the application of temozolomide (TMZ) or irradiation. The findings were correlated with previous findings indicating BA cytotoxicity in glioblastoma at low molecular concentrations. A significant synergistic action after application with irradiation and transcranial magnetic stimulation (TMS) was also observed (Schneider and Weller, 2016). These studies have provided insights into the different underlying mechanisms acquired by BAs for their anti-tumour actions. These findings can support the future development of their prospective as anti-inflammatory and anti-cancer drugs. The detailed mechanism of action of BAs as promising anti-cancer agents is depicted in the schematic diagram shown in Figure 2.
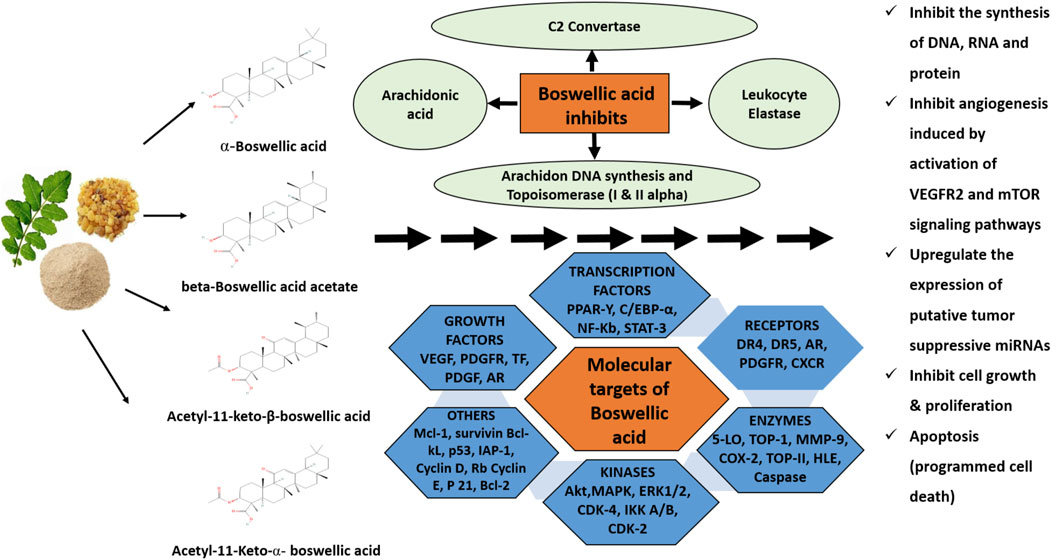
FIGURE 2. Schematic diagram of mechanisms of the boswellic acids extracted from the plant Boswellia serrata. These acids exhibit anti-cancer activities against different cancers by acting on numerous targets like 5-lipoxygenase (5-LO), topoisomerases, angiogenesis, and cytochrome p450 enzymes which finally lead to a reduction in cancer cell growth and apoptosis.
4 Studies that confirmed the anti-cancer properties of boswellic acids
BAs extracted from the plant Boswellia serrata are considered as the essential active constituents to treat many inflammatory diseases, either acute or chronic. Along with their potential as anti-arthritic, anti-asthmatic, anti-rheumatic, anti-diarrhoeal, and anti-hyperlipidemic, actions they also possess anti-microbial, hepatoprotective, analgesic, immunomodulatory actions, and anti-cancer characteristics. These acids have been found to exhibit very effective anti-inflammatory as well as anti-cancer activities in different models both in vitro and in vivo. They showed anti-cancer potential against a range of malignant tumours, and many semi-synthetic BAs illustrate outstanding cytotoxic effects (Hussain et al., 2021).
4.1 Cytotoxic effect of boswellic acid on colorectal cancer
Colorectal cancer is a multifaceted disease with epigenetic and genetic mutations in a wide range of oncogenes and tumour suppressor genes (Parmar and Easwaran, 2022). AKBA shows chemo-preventive characteristics capable of targeting principle oncogenic proteins, including 5-lipoxygenase and nuclear factor-kappa B. These BAs are known to modulate specific microRNA (miRNA) pathways due to their chemo-preventive effects. In the pathways, let-7 and miR-200 are both putative tumour-suppressive miRNAs. AKBA showed significantly upregulated expression of both families in various colorectal cancer cell lines. miRNA knockdown has been shown to inhibit let-7 and enable increased cancer cell propagation, migration, and invasion. AKBA modulates the expression of various downstream targets of the miR-200 and let-7 families (vimentin, CDK6, and E-cadherin). Similar findings of inducing modulation of these downstream genes have been observed in CRC cells orthotopically implanted in nude mice. This study gives novel evidence for the ability of BAs to regulate cellular epigenetic mechanisms that emphasise their anti-cancer characteristics and further highlight their potential in the prevention and treatment of CRC (Takahashi et al., 2012).
4.2 Anti-tumor effect of boswellic acid in human colonic adenocarcinoma
Ranjbarnejad et al. (2017) investigated the methanolic extract of Boswellia serrata for its anti-cancer activity on human colon cancer cells. This study establishes that the methanolic extract decreased the expression of cyclooxygenase-2 gene and its terminal end products, such as microsomal prostaglandin E synthase-1 (mPGES-1), vascular endothelial growth factor (VEGF), C-X-C chemokine receptor type 4 (CXCR4), matrix metalloproteinase-2 (MMP-2), MMP-9, and hypoxia-inducible factor-1 (HIF-1). The study, therefore, suggested that the B. serrata extract can be a potential agent to inhibit the proliferation, angiogenesis, and migration in colorectal cancer. Similarly, another study (Wang et al., 2018) also finds that BA can be used for the growth suppression of HCT-116 colon cancer cells. With an IC50 value of 15 uM, BA altered the Bax/Bcl-2 ratio in the HCT-116 cells. Therefore, a general understanding can be developed of the usage of BA as an anti-cancerous agent for human colon cancer, provided further in vivo detailed studies are performed.
4.3 Role of boswellic acid in growth suppression of human pancreatic tumours and its metastasis
BA acts as a growth suppressor for human pancreatic tumours in a mouse model by interacting with multiple targets and also limiting its metastasis. AKBA activity was studied in an in vitro model of orthotopic nude mice against human PaCa; it revealed that AKBA inhibited the proliferation of four PaCa cell lines. It comprised PANC-28, AsPC-1, and with p53 and K-Ras mutations, BxPC-3 with wild-type K-Ras and p53 mutation were also included. AKBA also inhibited the metastasis of the PaCa in the liver, spleen, and lungs in the same mouse model. The study indicated the potential of AKBA as an anti-tumour agent that exhibited an ability to suppress human pancreatic tumour growth and metastasis with multiple target modulations (Park et al., 2011).
4.4 Inhibitory activity of boswellic acids against human leukaemia
Four BAs of B. serrata: β-boswellic acid, 11-keto-β-boswellic acid, 3-O-acetyl-β-boswellic acid, and 3-O-acetyl-11-keto-β-boswellic acid were evaluated for their anti-cancer properties. They were examined to restrict the DNA, RNA, and protein synthesis in human leukaemia HL-60 cells. 3-O-Acetyl-11-keto-β-boswellic acid significantly inhibited the synthesis of DNA, RNA, and proteins. This compound had an irreversible effect on DNA synthesis, inhibiting the HL-60 cellular growth without affecting cell viability. The result of the study revealed a significant potential of this compound in the regulation of human leukaemia proliferation (Shao et al., 1998).
4.5 Apoptotic effect of boswellic acid in liver cancer cells
Only one study explored the anti-proliferative and apoptotic effect on Hep G2 liver cancer cells of keto-β-boswellic acid and acetyl-keto-β-boswellic acid. Following their application on the cell’s DNA synthesis, apoptosis and cell proliferation were examined. The apoptotic pathway was explored employing specific caspase inhibitors, which revealed the decreased cell viability and thymidine amalgamation and enlarged percentage of sub-G1 in the G1 phase. BAs significantly influenced apoptosis, complemented by the activation of these caspase inhibitors. Hence, the study led to the possibility of using the BAs for anti-cancer and anti-proliferation effects in the liver Hep G2 cells (Liu et al., 2002).
4.6 Apoptosis in prostate cancer cells in vitro and in vivo by boswellic acid
In androgen-independent PC-3 cells, a chemo-resistant prostate cancerous line, acetyl-β-boswellic acid, and acetyl-11-keto-β-boswellic acid inhibited their growth. They promoted the death of the cell in vitro as well as in vivo models (Syrovets et al., 2005). For analysing apoptosis, parameters like DNA fragmentation and mitochondrial cytochrome C release were examined in cultured PC-3 cells. The underlying molecular mechanism involved the inhibition of signalling of the NF-κB (constitutively activated) by IκB kinase (IKK) activity interruption. The IKK inhibition showed specificity because the signalling through the interferon-stimulated response element remained unchanged. The study was further confirmed in nude mice carrying PC-3 tumours, where the systemic application of AKβBA-γ- cyclodextrin reduced tumour growth. This treatment also activated apoptosis without detectable systemic toxicity. AKβBA and related compounds acting on IKK provide a novel approach for treating chemo-resistant human tumours, including androgen-independent human prostate cancers.
5 Conclusion
BAs and their semi-synthetic derivatives are effective against a broad spectrum of cancer cell lines. They have a minimal potential for resistance due to the multiple ways they operate in the cancer cell lines. The ability of BAs to control cellular epigenetic mechanisms highlights their anti-cancer properties, as they promote apoptosis in cancer cells and inhibit the malignant primary metabolic pathways and DNA, RNA, and protein synthesis. BAs are only present in the Boswellia genus, but they display several kinds and contents according to the species. Globally, the survival of the natural sources of frankincense is threatened by over-extraction to obtain BAs and other anthropogenic factors, including climate change. Seed dormancy and slower growth rate make it worse, so more in vitro conservation methods are required to protect these plant species. More research is necessary to develop technology for in vitro production of BAs from Boswellia spp., as well as more clinical trials and scientific studies to validate its anti-cancer potential and obtain novel cancer treatment.
Author contributions
Conceptualization and design were performed by JS-R; investigation, data curation, and writing were performed by VT, RS, PS, and BM; review and editing were performed by PD, ST, AS, WS, AF, MB, and JS-R; validation and supervision were done by JS-R. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BA, boswellic acid; BBA, β-boswellic acid; KBA, 11-keto-β-boswellic acid; ABA, acetyl-β-boswellic acid; AKBA, acetyl-11-keto-β-boswellic acid; AMPK, AMP-activated protein kinase; GLUT1, glucose transporter 1 protein; mTOR, mammalian target of rapamycin; TMS, transcranial magnetic stimulation; EGFR, epidermal growth factor receptor; PVP, polyvinylpyrrolidone.
References
Agrawal, S. S., Saraswati, S., Mathur, R., and Pandey, M. (2011). Antitumor properties of Boswellic acid against Ehrlich ascites cells bearing mouse. Food Chem. Toxicol. 49 (9), 1924–1934. doi:10.1016/j.fct.2011.04.007
Al-Bahlani, S., Burney, I. A., Al-Dhahli, B., Al-Kharusi, S., Al-Kharousi, F., Al-Kalbani, A., et al. (2020). Boswellic acid sensitizes gastric cancer cells to Cisplatin-induced apoptosis via p53-mediated pathway. BMC Pharmacol. Toxicol. 21, 64–10. doi:10.1186/s40360-020-00442-1
Al-Harrasi, A., Hussain, H., Csuk, R., and Khan, H. (2019). Chemistry of boswellic acids and other terpenoids. In (pp. 9–66). doi:10.1016/B978-0-08-102441-6.00002-5
Al-Harrasi, A., Khan, A. L., Asaf, S., and Al-Rawahi, A. (2019). “Frankincense tree physiology and its responses to wounding stress,” in Biology of genus Boswellia Editors A. Al-Harrasi, A. L. Khan, S. Asaf, and A. Al-Rawahi (Berlin, Germany: Springer International Publishing), 53–70. doi:10.1007/978-3-030-16725-7_4
Al-Harrasi, A., Khan, A. L., Rehman, N. U., and Csuk, R. (2021). Biosynthetic diversity in triterpene cyclization within the Boswellia genus. Phytochemistry, 184, 112660. doi:10.1016/j.phytochem.2021.112660
Al-Harrasi, A., Rehman, N. U., Khan, A. L., Al-Broumi, M., Al-Amri, I., Hussain, J., et al. (2018). Chemical, molecular and structural studies of Boswellia species: β-Boswellic aldehyde and 3-epi-11β-Dihydroxy BA as precursors in biosynthesis of boswellic acids. PLoS One 13 (6), e0198666. doi:10.1371/journal.pone.0198666
Barbarisi, M., Barbarisi, A., De Sena, G., Armenia, E., Aurilio, C., Libutti, M., et al. (2019). Boswellic acid has anti-inflammatory effects and enhances the anticancer activities of Temozolomide and Afatinib, an irreversible ErbB family blocker, in human glioblastoma cells. Phytotherapy Res. 33 (6), 1670–1682. doi:10.1002/ptr.6354
Bie, F., Zhang, G., Yan, X., Ma, X., Zhan, S., Qiu, Y., et al. (2022). β-Boswellic acid suppresses breast precancerous lesions via GLUT1 targeting-mediated glycolysis inhibition and AMPK pathway activation. Front. Oncol. 12, 896904. doi:10.3389/fonc.2022.896904
Bongers, F., Groenendijk, P., Bekele, T., Birhane, E., Damtew, A., Decuyper, M., et al. (2019). Frankincense in peril. Nat. Sustain. 2 (7), 602–610. doi:10.1038/s41893-019-0322-2
Büchele, B., Zugmaier, W., Estrada, A., Genze, F., Syrovets, T., Paetz, C., et al. (2006). Characterization of 3alpha-acetyl-11-keto-alpha-boswellic acid, a pentacyclic triterpenoid inducing apoptosis in vitro and in vivo. Planta Med. 72 (14), 1285–1289. doi:10.1055/s-2006-951680
Casapullo, A., Cassiano, C., Capolupo, A., Del Gaudio, F., Esposito, R., Tosco, A., et al. (2016). β-Boswellic acid, a bioactive substance used in food supplements, inhibits protein synthesis by targeting the ribosomal machinery. J. Mass Spectrom. 51 (9), 821–827. doi:10.1002/jms.3819
Chaturvedi, D., Dwivedi, P. K., Chaturvedi, A. K., Mishra, N., Siddiqui, H., and Mishra, V. (2015). Semisynthetic hybrids of boswellic acids: A novel class of potential anti-inflammatory and anti-arthritic agents. Med. Chem. Res. 24 (7), 2799–2812. doi:10.1007/s00044-015-1331-y
Dhyani, P., Quispe, C., Sharma, E., Bahukhandi, A., Sati, P., Chand Attri, D., et al. (2022). Anticancer potential of alkaloids: a key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell International 22, 206. doi:10.1186/s12935-022-02624-9
Du, Z., Liu, Z., Ning, Z., Liu, Y., Song, Z., Wang, C., et al. (2015). Prospects of boswellic acids as potential pharmaceutics. Planta medica. 81 (04), 259–271. doi:10.1055/s-0034-1396313
Ennet, D. (2000). Arzneistoffportrat-Indischer Weihrauch-Pharmazeutische Bewertung der Harzdroge und ihrer Zubereitungen. Dtsch. Apoth. Ztg. 140 (16), 105–114.
Gupta, M., Singh, S., Luqman, S., Saikia, D., Thomas, M., Rout, P. K., et al. (2022). Correlation of boswellic acids with antiproliferative, antioxidant and antimicrobial activities of topographically collected Boswellia serrata oleo-gum-resin. Phytomedicine Plus 2 (3), 100289. doi:10.1016/j.phyplu.2022.100289
Havel, J., Patocka, J., and Bocaz, G. (2002). Determination of physostigmine and pyridostigmine in pharmaceutical formulations by capillary electrophoresis. J. Capill. Electrophor. Microchip Technol. 7 (5-6), 107–112.
Hoernlein, R., Orlikowsky, T., Zehrer, C., Niethammer, D., Sailer, E., Simmet, T., et al. (1999). Acetyl-11-keto-β-boswellic acid induces apoptosis in HL-60 and CCRF-CEM cells and inhibits topoisomerase I. J. Pharmacol. Exp. Ther. 288 (2), 613–619.
Huang, M. T., Badmaev, V., Ding, Y., Liu, Y., Xie, J. G., and Ho, C. T. (2000). Anti-tumor and anti-carcinogenic activities of triterpenoid, β-boswellic acid. Biofactors 13 (1-4), 225–230. doi:10.1002/biof.5520130135
Hussain, H., Al-Harrasi, A., Csuk, R., Shamraiz, U., Green, I. R., Ahmed, I., et al. (2017). Therapeutic potential of boswellic acids: A patent review (1990-2015). Expert Opin. Ther. Pat. 27 (1), 81–90. doi:10.1080/13543776.2017.1235156
Hussain, H., Ali, I., Wang, D., Hakkim, F. L., Westermann, B., Rashan, L., et al. (2021). Boswellic acids: Privileged structures to develop lead compounds for anticancer drug discovery. Expert Opin. Drug Discov. 16 (8), 851–867. doi:10.1080/17460441.2021.1892640
Iram, F., Khan, S. A., and Husain, A. (2017). Phytochemistry and potential therapeutic actions of boswellic acids: A mini-review. Asian Pac. J. Trop. Biomed. 7 (6), 513–523. doi:10.1016/j.apjtb.2017.05.001
Khan, A. L., Mabood, F., Akber, F., Ali, A., Shahzad, R., Al-Harrasi, A., et al. (2018). Endogenous phytohormones of frankincense producing Boswellia sacra tree populations. PLOS ONE 13 (12), e0207910. doi:10.1371/journal.pone.0207910
Koeberle, A., Henkel, A., Verhoff, M., Tausch, L., König, S., Fischer, D., et al. (2018). Triterpene acids from frankincense and semi-synthetic derivatives that inhibit 5-lipoxygenase and cathepsin G. Molecules 23 (2), 506. doi:10.3390/molecules23020506
Liu, J.-J., and Duan, R.-D. (2009). LY294002 enhances boswellic acid-induced apoptosis in colon cancer cells. Anticancer Res. 29 (8), 2987–2991.
Liu, J.-J., Nilsson, A., Oredsson, S., Badmaev, V., and Duan, R.-D. (2002). Keto-and acetyl-keto-boswellic acids inhibit proliferation and induce apoptosis in Hep G2 cells via a caspase-8 dependent pathway. Int. J. Mol. Med. 10 (4), 501–505. doi:10.3892/ijmm.10.4.501
Lv, M., Shao, S., Zhang, Q., Zhuang, X., and Qiao, T. (2020). Acetyl-11-Keto-β-Boswellic acid exerts the anti-cancer effects via cell cycle arrest, apoptosis induction and autophagy suppression in non-small cell lung cancer cells. OncoTargets Ther. 13, 733–744. doi:10.2147/OTT.S236346
Meng, Y., Zhao, L., Wang, Z., Liu, D., and Jing, Y. (2005). Synthesis of boswellic acid derivatives and primary research on their activities. Chin. Chem. Lett. 16.
Morad, S. A., Schmid, M., Büchele, B., Siehl, H.-U., El Gafaary, M., Lunov, O., et al. (2013). A novel semisynthetic inhibitor of the FRB domain of mammalian target of rapamycin blocks proliferation and triggers apoptosis in chemoresistant prostate cancer cells. Mol. Pharmacol. 83 (2), 531–541. doi:10.1124/mol.112.081349
Park, B., Sung, B., Yadav, V. R., Cho, S. G., Liu, M., and Aggarwal, B. B. (2011). Acetyl-11-keto-β-boswellic acid suppresses invasion of pancreatic cancer cells through the downregulation of CXCR4 chemokine receptor expression. Int. J. cancer 129 (1), 23–33. doi:10.1002/ijc.25966
Parmar, S., and Easwaran, H. (2022). Genetic and epigenetic dependencies in colorectal cancer development. Gastroenterol. Rep. 10, goac035. doi:10.1093/gastro/goac035
Paul, M. (2012). Chemotaxonomic investigations on resins of the frankincense species Boswellia papyrifera, Boswellia serrata and Boswellia sacra, respectively, Boswellia carterii: A qualitative and quantitative approach by chromatographic and spectroscopic methodology. Saarbrücken, Saarland, Germany: Saarland University.
Poeckel, D., and Werz, O. (2006). Boswellic acids: Biological actions and molecular targets. Curr. Med. Chem. 13 (28), 3359–3369. doi:10.2174/092986706779010333
Qurishi, Y., Hamid, A., Sharma, P. R., Wani, Z. A., Mondhe, D. M., Singh, S. K., et al. (2012). PARP cleavage and perturbance in mitochondrial membrane potential by 3-α-propionyloxy-β-boswellic acid results in cancer cell death and tumor regression in murine models. Future Oncol. 8 (7), 867–881. doi:10.2217/fon.12.68
Ranjbarnejad, T., Saidijam, M., Moradkhani, S., and Najafi, R. (2017). Methanolic extract of Boswellia serrata exhibits anti-cancer activities by targeting microsomal prostaglandin E synthase-1 in human colon cancer cells. Prostagl. other lipid Mediat. 131, 1–8. doi:10.1016/j.prostaglandins.2017.05.003
Ravanan, P., Singh, S. K., Rao, G., and Kondaiah, P. (2011). Growth inhibitory, apoptotic and anti-inflammatory activities displayed by a novel modified triterpenoid, cyano enone of methyl boswellates. J. Biosci. 36 (2), 297–307. doi:10.1007/s12038-011-9056-7
Roy, N. K., Parama, D., Banik, K., Bordoloi, D., Devi, A. K., Thakur, K. K., et al. (2019). An update on pharmacological potential of boswellic acids against chronic diseases. Int. J. Mol. Sci. 20 (17), 4101. doi:10.3390/ijms20174101
Saraswati, S., and Agrawal, S. (2012). Antiangiogenic and cytotoxic activity of boswellic acid on breast cancer MCF-7 cells. Biomed. Prev. Nutr. 2 (1), 31–37. doi:10.1016/j.bionut.2011.09.006
Schmiech, M., Ulrich, J., Lang, S. J., Büchele, B., Paetz, C., St-Gelais, A., et al. (2021). 11-Keto-α-boswellic acid, a novel triterpenoid from Boswellia spp. with chemotaxonomic potential and antitumor activity against triple-negative breast cancer cells. Molecules 26 (2), 366. doi:10.3390/molecules26020366
Schneider, H., and Weller, M. (2016). Boswellic acid activity against glioblastoma stem-like cells. Oncol. Lett. 11 (6), 4187–4192. doi:10.3892/ol.2016.4516
Serbian, I., Wolfram, R. K., Fischer, L., Al-Harrasi, A., and Csuk, R. (2018). Hydroxylated boswellic and glycyrrhetinic acid derivatives: Synthesis and cytotoxicity. Mediterr. J. Chem. 7 (4), 286–293. doi:10.13171/mjc74181121-csuk
Shah, B. A., Qazi, G. N., and Taneja, S. C. (2009). Boswellic acids: A group of medicinally important compounds. Nat. Prod. Rep. 26 (1), 72–89. doi:10.1039/b809437n
Shamraiz, U., Hussain, H., Ur Rehman, N., Al-Shidhani, S., Saeed, A., Khan, H. Y., et al. (2020). Synthesis of new boswellic acid derivatives as potential antiproliferative agents. Nat. Prod. Res. 34 (13), 1845–1852. doi:10.1080/14786419.2018.1564295
Shao, Y., Ho, C.-T., Chin, C.-K., Badmaev, V., Ma, W., and Huang, M.-T. (1998). Inhibitory activity of boswellic acids from Boswellia serrata against human leukemia HL-60 cells in culture. Planta medica. 64 (04), 328–331. doi:10.1055/s-2006-957444
Siddiqui, M. Z., and Serrata, B. (2003). A potential antiinflammatory agent: an overview. Indian Journal of Pharmaceutical Sciences 73 (3), 255–261. doi:10.4103/0250-474X.93507
Suhail, M. M., Wu, W., Cao, A., Mondalek, F. G., Fung, K.-M., Shih, P.-T., et al. (2011). Boswellia sacra essential oil induces tumor cell-specific apoptosis and suppresses tumor aggressiveness in cultured human breast cancer cells. BMC complementary Altern. Med. 11, 129–214. doi:10.1186/1472-6882-11-129
Sun, H., Fang, W.-S., Wang, W.-Z., and Hu, C. (2006). Structure-activity relationships of oleanane-and ursane-type triterpenoids. Bot. Stud. 47 (4), 339–368.
Sun, Y., Liu, D., Xi, R., Wang, X., Wang, Y., Hou, J., et al. (2013). Microbial transformation of acetyl-11-keto-β-boswellic acid and their inhibitory activity on LPS-induced NO production. Bioorg. Med. Chem. Lett. 23 (5), 1338–1342. doi:10.1016/j.bmcl.2012.12.086
Syrovets, T., Gschwend, J. R. E., Buchele, B., Laumonnier, Y., Zugmaier, W., Genze, F., et al. (2005). Inhibition of IkappaB kinase activity by acetyl-boswellic acids promotes apoptosis in androgen-independent PC-3 prostate cancer cells in vitro and in vivo. J. Biol. Chem. 280 (7), 6170–6180. doi:10.1074/jbc.M409477200
Takahashi, M., Sung, B., Shen, Y., Hur, K., Link, A., Boland, C. R., et al. (2012). Boswellic acid exerts antitumor effects in colorectal cancer cells by modulating expression of the let-7 and miR-200 microRNA family. Carcinogenesis 33 (12), 2441–2449. doi:10.1093/carcin/bgs286
Uthaman, S., Snima, K., Annapoorna, M., Ravindranath, K., Nair, S. V., and Lakshmanan, V.-K. (2012). Novel boswellic acids nanoparticles induces cell death in prostate cancer cells. J. Nat. Prod. 5, 100–108.
Wang, D., Ge, S., Bai, J., and Song, Y. (2018). Boswellic acid exerts potent anticancer effects in HCT-116 human colon cancer cells mediated via induction of apoptosis, cell cycle arrest, cell migration inhibition and inhibition of PI3K/AKT signalling pathway. J. BUON 23 (2), 340–345.
Wang, Y., Sun, Y., Wang, C., Huo, X., Liu, P., Wang, C., et al. (2013). Biotransformation of 11-keto-β-boswellic acid by Cunninghamella blakesleana. Phytochemistry 96, 330–336. doi:10.1016/j.phytochem.2013.07.018
Xia, L., Chen, D., Han, R., Fang, Q., Waxman, S., and Jing, Y. (2005). Boswellic acid acetate induces apoptosis through caspase-mediated pathways in myeloid leukemia cells. Mol. cancer Ther. 4 (3), 381–388. doi:10.1158/1535-7163.MCT-03-0266
Yadav, V. R., Prasad, S., Sung, B., Gelovani, J. G., Guha, S., Krishnan, S., et al. (2012). Boswellic acid inhibits growth and metastasis of human colorectal cancer in orthotopic mouse model by downregulating inflammatory, proliferative, invasive and angiogenic biomarkers. Int. J. cancer 130 (9), 2176–2184. doi:10.1002/ijc.26251
Yuan, H.-Q., Kong, F., Wang, X.-L., Young, C. Y., Hu, X.-Y., and Lou, H.-X. (2008). Inhibitory effect of acetyl-11-keto-β-boswellic acid on androgen receptor by interference of Sp1 binding activity in prostate cancer cells. Biochem. Pharmacol. 75 (11), 2112–2121. doi:10.1016/j.bcp.2008.03.005
Yuan, Y., Cui, S.-X., Wang, Y., Ke, H.-N., Wang, R.-Q., Lou, H.-X., et al. (2013). Acetyl-11-keto-beta-boswellic acid (AKBA) prevents human colonic adenocarcinoma growth through modulation of multiple signaling pathways. Biochim. Biophys. Acta 1830 (10), 4907–4916. doi:10.1016/j.bbagen.2013.06.039
Keywords: Boswellia, cancer, bioactive compounds, apoptosis, triterpenes
Citation: Trivedi VL, Soni R, Dhyani P, Sati P, Tejada S, Sureda A, Setzer WN, Faizal Abdull Razis A, Modu B, Butnariu M and Sharifi-Rad J (2023) Anti-cancer properties of boswellic acids: mechanism of action as anti-cancerous agent. Front. Pharmacol. 14:1187181. doi: 10.3389/fphar.2023.1187181
Received: 15 March 2023; Accepted: 21 July 2023;
Published: 03 August 2023.
Edited by:
Mitesh Patel, Parul University, IndiaReviewed by:
Husain Yar Khan, Wayne State University, United StatesArezoo Rajabian, Mashhad University of Medical Sciences, Iran
Copyright © 2023 Trivedi, Soni, Dhyani, Sati, Tejada, Sureda, Setzer, Faizal Abdull Razis, Modu, Butnariu and Sharifi-Rad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Praveen Dhyani, cHJhdmVlbmRoeWFuaTg2QGdtYWlsLmNvbQ==; Ahmad Faizal Abdull Razis, bWFkZmFpemFsQHVwbS5lZHUubXk=; Monica Butnariu, bW9uaWNhYnV0bmFyaXVAeWFob28uY29t; Javad Sharifi-Rad, amF2YWQuc2hhcmlmaXJhZEBnbWFpbC5jb20=
 Vijay Laxmi Trivedi1
Vijay Laxmi Trivedi1 Praveen Dhyani
Praveen Dhyani Priyanka Sati
Priyanka Sati Silvia Tejada
Silvia Tejada Antoni Sureda
Antoni Sureda William N. Setzer
William N. Setzer Ahmad Faizal Abdull Razis
Ahmad Faizal Abdull Razis Monica Butnariu
Monica Butnariu Javad Sharifi-Rad
Javad Sharifi-Rad