A Corrigendum on
BI 1015550 is a PDE4B inhibitor and a clinical drug candidate for the oral treatment of idiopathic pulmonary fibrosis
by Herrmann FE, Hesslinger C, Wollin L and Nickolaus P (2022). Front. Pharmacol. 13:838449. doi: 10.3389/fphar.2022.838449
In the original article, there was an error in Table 1 as published. The n numbers given in the legend to Table 1 were only applicable to the first row of the table. The corrected Table 1 and its caption appear below.
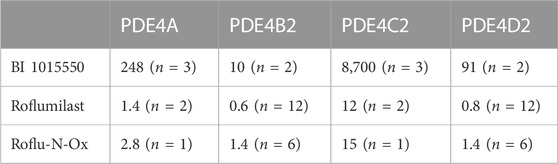
TABLE 1. Inhibition of human recombinant PDE4 subtypes by BI 1015550 in comparison to roflumilast and its active metabolite Roflu-N-Ox. IC50 values (nmol/L) are given as means from n independent experiments. Cell extracts containing the active site fragments mediated the conversion of 10 µL [3H]cAMP (0.05 µCi in H2O) to AMP resulting in the binding of this radiolabeled molecule to the yttrium silicate SPA beads and the subsequent generation of scintillation events determined using a Wallac Microbeta counter. AMP, adenosine monophosphate; IC50, inhibitory concentration (nM) for half-maximal inhibition; PDE, phosphodiesterase; SPA, scintillation proximity assay.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: PDE4B, IPF, lung fibrosis, phosphodiesterase, cAMP, ILDs
Citation: Herrmann FE, Hesslinger C, Wollin L and Nickolaus P (2023) Corrigendum: BI 1015550 is a PDE4B inhibitor and a clinical drug candidate for the oral treatment of idiopathic pulmonary fibrosis. Front. Pharmacol. 14:1219760. doi: 10.3389/fphar.2023.1219760
Received: 09 May 2023; Accepted: 22 May 2023;
Published: 30 May 2023.
Edited by:
Stefan Brocke, University of Connecticut Health Center, United StatesReviewed by:
Paul Mark Epstein, UCONN Health, United StatesRobert B. Nelson, MindImmune Therapeutics, Inc., United States
Copyright © 2023 Herrmann, Hesslinger, Wollin and Nickolaus. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter Nickolaus, cGV0ZXIubmlja29sYXVzQGJvZWhyaW5nZXItaW5nZWxoZWltLmNvbQ==
 Franziska Elena Herrmann
Franziska Elena Herrmann Christian Hesslinger
Christian Hesslinger Lutz Wollin
Lutz Wollin Peter Nickolaus
Peter Nickolaus