- Institute of Special Animal and Plant Sciences, Chinese Academy of Agricultural Sciences, Changchun, China
Coronary heart disease (CHD) is the most common clinical manifestation of cardiovascular disease. It is characterized by myocardial ischemia, which is caused by coronary atherosclerosis. CHD is a significant global health problem with increasing prevalence every year because of significant changes in the lifestyles and diets. Ginseng is a traditional Chinese medicinal herb that has been used in food preparations and traditional medicine for several centuries. Several studies have demonstrated that ginseng improved cardiac function by normalizing blood glucose levels and decreasing blood pressure, oxidative stress, platelet aggregation, and lipid dysregulation in vivo. This review describes the current understanding of the mechanisms by which ginseng alleviates CHD, and provides a reference for the clinical development and application of ginseng as an alternative therapy for CHD.
1 Introduction
Coronary heart disease (CHD) is a cardiovascular disease caused by gradual narrowing of the coronary artery because of plaque buildup or atherosclerosis. Eventually, significant blockage of the vascular lumen causes myocardial ischemia, hypoxia, and necrosis of the cardiomyocytes. CHD is manifested in the form of heart failure, arrhythmia, and sudden death in severe cases (Bruetsch, 1959). The main symptoms of CHD are chest pain, chest tightness, or myocardial infarction (Lu et al., 2022).
Several types of drugs are available for the clinical treatment of CHD. These include statins (Lee et al., 2019; Yao et al., 2022), aspirin (Morimoto et al., 2007), antiplatelet drugs (Fuentes et al., 2019), and calcium channel blockers (Thomas et al., 2018). These drugs significantly reduce the risk of cardiovascular events, relieve disease symptoms, and improve the quality of life. The pathophysiological mechanisms of CHD and the mechanisms of action of different drugs used for treating CHD is constantly being updated because of robust advances in clinical research. This has led to the discovery of new drugs, including PCSK9 inhibitors and SGLT2 inhibitors, which reduce the risk of cardiovascular events by lowering blood lipid levels and blood pressure, respectively (Sabouret et al., 2022).
Ginseng is a commonly used Chinese medicinal herb with a variety of pharmacological effects, including management of blood sugar levels and cardiovascular protection by lowering cholesterol levels and blood pressure (Aminifard et al., 2021). In the last 20 years, several clinical trials have been conducted regarding the clinical efficacy of ginseng in the treatment of metabolic, cardiovascular, cognitive, and pulmonary diseases. Among these, 23.5% of the clinical trials have focused on the clinical efficacy of ginseng in the treatment of cardiovascular diseases (Fan et al., 2020). Ginseng is a natural product obtained from the herb plant, Panax ginseng. The clinical safety of ginseng is higher than several chemically synthesized drugs. Therefore, ginseng is a promising candidate drug for the treatment of CHD.
2 Methods
A search of the literature was performed based on the methodology of the preferred reporting items for systematic review and meta-analysis (PRISMA) (Page et al., 2021) in Web of Science and PubMed. The search terms were ginseng; coronary heart disease; ginseng and “anti-coronary heart disease”; ginseng and “blood pressure”; ginseng and “cardiac function”; ginseng and antioxidant; ginseng and “antiplatelet coagulation”; ginseng and “lipotropic effects”; ginseng and “intestinal flora”; ginseng and “adverse reactions.” All articles generated through bibliographic searches that met the inclusion criteria (covering the years 1965–2023) were considered. The first database dealt with the effects of coronary heart disease and the mechanism of action of ginseng in the treatment of coronary heart disease, and the second database dealt with adverse reactions to ginseng.
3 Plant phytochemical composition
To date, more than 200 ginsenoside and non-saponin components have been isolated and characterized from ginseng (Chen et al., 2015), including ginsenosides, polysaccharides, alkaloids, Polyacetylene, volatile oils, lignans and flavonoids (Wang et al., 2012). Ginsenosides are widely recognized as the main bioactive compounds of ginseng. The most important non-ginsenoside bioactive components of ginseng are biophenols and polysaccharides (Kim et al., 2007). According to the previous summary, ginseng has better cardiovascular protection due to its pharmacologically active components, but the relationship between the cardiovascular protection provided by ginseng and its main active components still needs to be further investigated in order to draw accurate conclusions (Liu et al., 2020).
4 Cardioprotective mechanisms of ginseng for the treatment of CHD
4.1 Ginseng improves cardiac functions
Coronary heart disease is caused by stenosis or obstruction of the coronary artery. Patients with CHD demonstrate abnormal cardiac function because of myocardial ischemia, hypoxia, and necrosis (Liao et al., 2017). Myocardial ischemia causes decreased myocardial oxygenation and local accumulation of metabolic waste products because of insufficient coronary blood flow (Sarhene et al., 2021). Therefore, deficient oxygen and nutrient conditions adversely affect the metabolism and function of the cardiomyocytes. Subsequently, under these conditions, cardiomyocytes undergo programmed or necrotic cell death and cause myocardial ischemic injury, which is presented as angina pectoris, myocardial infarction, and/or myocarditis (Del Rio-Pertuz et al., 2022). Myocardial ischemia is life-threatening because of impaired cardiac function, myocardial necrosis, and arrhythmias in patients with CHD and requires immediate treatment.
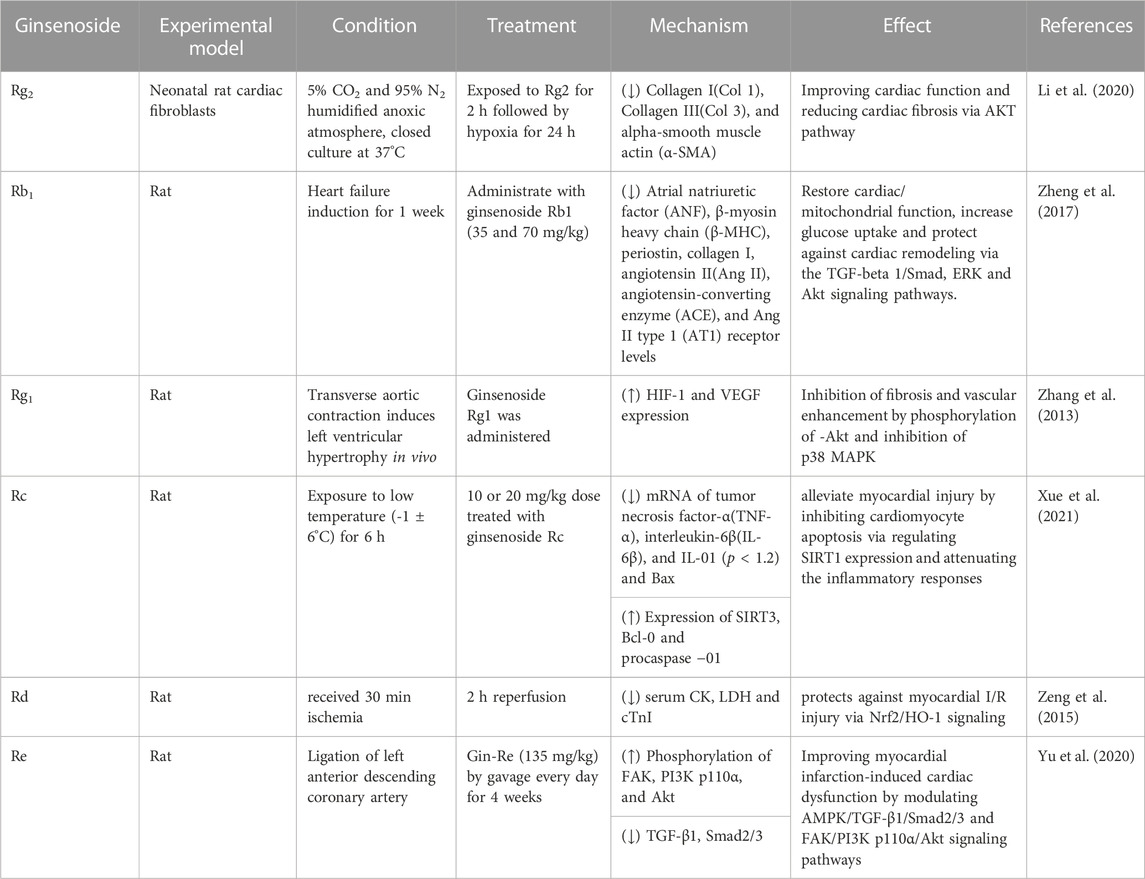
Ginseng is a natural product that is commonly used in foods and traditional herbal medicine in China. The clinical safety of ginseng is well established. Ginseng is associated with cardioprotective, anti-oxidative, anti-inflammatory, and anticoagulation properties (Hyun et al., 2022). Therefore, there is immense potential for the clinical use of ginseng in the treatment of cardiovascular diseases. In a rat model, ginseng increased cardiac contractility without altering the heartbeat rate through activation of PPARδ and elevated levels of intracellular calcium and cardiac troponin I phosphorylation (Lin et al., 2014). This demonstrated that ginseng may improve cardiac functions without causing adverse effects such as arrhythmias. Ginsenosides are the main bioactive ingredients in the extracts of Panax ginseng. They improved myocardial blood supply and pumping action of the heart by significantly increasing myocardial contractility, myocardial vasodilatation, and the myocardial blood flow (Chang et al., 2020). The antiapoptotic and anti-inflammatory activities of ginsenoside Rg3 significantly alleviated myocardial I/R-induced cardiac dysfunction (Zhang et al., 2016). Ginsenoside Rh2 is the pharmacologically active compound in red ginseng and it significantly improves cardiac function by alleviating cardiac fibrosis (Liu et al., 2022). The anti-inflammatory properties of ginsenoside Rb3 significantly alleviate inflammation-induced ventricular systolic dysfunction (Shao et al., 2022). Ginsenoside Rb2 significantly improved cardiac function by decreasing infarct size in the in vivo animal model of myocardial ischemia/reperfusion (MI/R) injury; it also decreased in vitro H2O2-induced stress in the H9c2 cardiomyocytes (Fu et al., 2016). Currently, there is abundant literature and experimental data to show that ginsenosides are the main pharmacologically active components in ginseng that improve cardiac function through multiple mechanisms (Table 1). Therefore, ginseng is a promising candidate for the prevention and treatment of CHD.
4.2 Ginseng decreases blood pressure
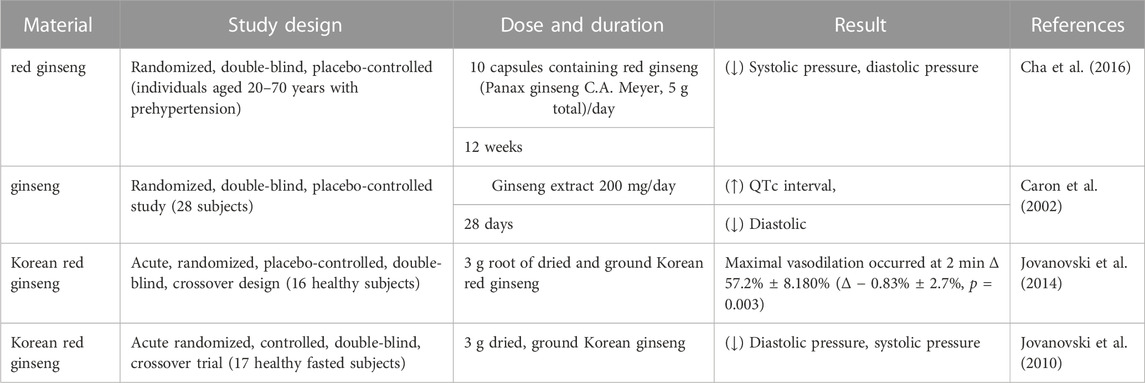
Hypertension is one of the main risk factors for CHD because it increases the cardiac load and increases the oxygen demand for the cardiac muscles (Kannel et al., 1965). Hypertension also decreases blood supply to the heart by narrowing the arteries because Therefore, hypertension contributes to the occurrence of CHD. In CHD, cardiac lesions decrease the blood flow to the heart by reducing the pumping function and adversely affecting the blood pressure regulation (Figure 1). Blood pressure changes are also common in patients with CHD because of drug therapy, surgical treatments, and other reasons. Therefore, treatment with antihypertensive drugs for regulating blood pressure is very critical for patients with CHD because it can reduce the cardiac load and alleviate the occurrence and progression of cardiac lesions. Administration of ginseng for 8–12 weeks significantly reduced the systolic and diastolic blood pressure in a dose dependent manner (Ha and Chun, 2016). The antihypertensive effects of the bioactive compounds in ginseng reduce blood pressure by increasing the dilation of the blood vessels, which subsequently increases the vascular lumen, reduces the vascular resistance, and improves the circulation of blood (Moon et al., 2019). Korean red ginseng shows promising therapeutic effects in patients with CHD by promoting the dilation of blood vessels and improving the endothelial function (Jovanovski et al., 2014). Ginsenoside Rb1 improved arterial blood pressure and survival rate of the septic shock model rats by down-regulating the levels of Toll-like receptor 4 (TLR4) transcripts and TNF-α protein (Li et al., 2014b). Red ginseng is obtained by washing, steaming, and drying Panax ginseng C. A. Meyer, a perennial herb that belongs to the Araliaceae family (Hyung, 2007). Korean red ginseng administration decreased blood pressure in rats (Joo et al., 2008). Red ginseng is rich in hypotensive compounds such as ginsenoside Rg3 and arginine fructose (Arg-Fru), which decrease arterial blood pressure by mediating the release of NO from the vascular endothelial cells (Lee et al., 2016). Several clinical studies have verified the blood pressure lowering effects of ginseng and its products (Table 2). Systematic review and meta-analysis of randomized controlled clinical trials showed that ginseng was associated with neutral vascular effects and improved blood pressure in patients with risk factors associated with cardiovascular diseases, such as diabetes, metabolic syndrome, and obesity (Komishon et al., 2016).
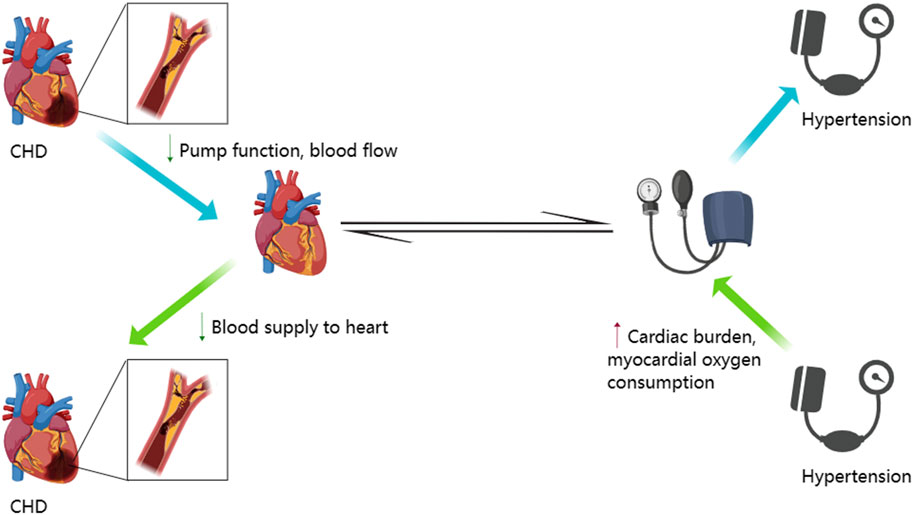
FIGURE 1. Interactive mechanism between hypertension and coronary heart disease. This figure was generated with MedPeer (www.medpeer.cn).
4.3 Antioxidant effects of ginseng
Oxidative stress is caused by excessive production of reactive free radicals and other oxidative molecules, which cause oxidative damage to functional biomolecules such as lipids, proteins, and nucleic acids. Oxidative damage is implicated as the main causative factor of human aging and several diseases, including cardiovascular diseases and metabolic disorders (Richardson and Schadt, 2014; Zeliger, 2016). Conversely, antioxidants are molecules that scavenge the free radicals in the body, thereby reducing oxidative stress and protecting cardiovascular health. Oxidative stress promotes development of cardiovascular diseases by inducing cardiovascular tissue damage and inflammatory response (Venkataraman et al., 2013). The intake of antioxidants through natural foods such as fruits, vegetables, nuts, fish, and other natural food resources, or antioxidant supplements is critical in protecting cardiovascular health (Zitnanova et al., 2006). Ginseng contains several antioxidant compounds that can protect the cardiovascular system by scavenging the free radicals and reducing oxidative damage (Jiang et al., 2012; Han et al., 2015). For example, ginsenosides Rb1, Rg1, and Rg2 protect against coronary heart diseases and atherosclerosis by inhibiting oxidative stress (Chang et al., 2020). A protein-protein interaction (PPI) network was constructed between the ginseng drug targets and disease targets based on the integrated network analysis of the Chinese Medicine Database. The results showed that ginseng regulated target genes that were related to the pathogenesis of coronary heart disease. For example, C-X-C Motif Chemokine Receptor 1 (CXCR1) modulated interleukin-8 (IL-8) via coupled G receptor proteins and activated the phosphatidylinositol signaling pathway, which plays a key role in mediating inflammation and maintaining neutrophil homeostasis (Li et al., 2018). The combined use of exercise and Korean red ginseng (KRG) supplementation is an effective anti-inflammatory therapy against atherosclerosis through reduction of the expression levels of C-reactive protein (CRP) and pro-inflammatory proteins in the aortic serum and concomitant increase in the levels of NO and eNOS (Lee et al., 2014).
4.4 Antiplatelet coagulation effects of ginseng
Anti-platelet and anti-coagulant drugs reduce the development and progression of cardiovascular diseases by inhibiting the formation of blood clots (Carlin et al., 2022). Many cardiac and vascular diseases, including myocardial infarction, CHD, and cerebrovascular disease are caused by vascular intima damage or abnormal blood coagulation. Blood platelets form thrombi at the sites of damaged blood vessels. Subsequently, these thrombi obstruct blood flow and promote the development of cardiovascular disease (Sang et al., 2021). The daily intake of the water extract of Korean red ginseng (KRG-WE) reduces the risk of thrombotic diseases by inhibiting platelet aggregation and thrombosis (Figure 2) (Hwang et al., 2008). Dihydroginsenoside Rg3, a stabilized chemical derivative of ginsenoside Rg3, stimulated the expression of matrix metalloproteinase (MMP) by increasing intracellular cAMP levels in a concentration-dependent manner and subsequently reduced platelet coagulation (Lee et al., 2008).
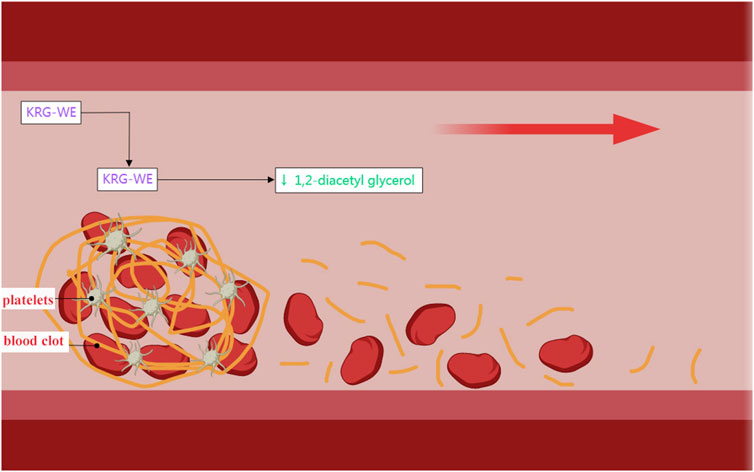
FIGURE 2. Antiplatelet coagulation effects of KRG-WE. Generated with MedPeer (www.medpeer.cn).
4.5 Lipid-modifying effects of ginseng
Dyslipidemia is closely related with the pathogenesis of CHD (May et al., 2016) and is characterized by hypercholesterolemia (Eisenberg, 1998; Poulsen et al., 2015), hypertriglyceridemia (Fontbonne et al., 1989; Wang et al., 2019), and elevated low-density lipoprotein cholesterol (LDL-C) levels (Li et al., 2014a). Dyslipidemia plays a significant role in the dysfunction of the vascular endothelial cells and promotes erosion of the vascular intima by inducing inflammation that results in the formation of vascular plaques (Bentzon et al., 2014). The accumulation of platelets at the site of plaque rupture also promotes formation of thrombi, which obstruct the coronary artery and cause myocardial infarction, angina pectoris, and/or other cardiovascular diseases. Hypercholesterolemia is one of the major risk factors for CHD (Archbold and Timmis, 1998; Saleheen et al., 2015). Cholesterol is a type of lipid in the human body and is present in the form of low-density lipoprotein cholesterol (LDL-C) or high-density lipoprotein cholesterol (HDL-C). LDL-C is a major carrier of cholesterol and is a significant risk factor for CHD (Gidding and Allen, 2019). Hypercholesterolemia increases the risk of CHD because of increased deposition of LDL-C in the walls of the blood vessels and atherosclerosis (Oster et al., 1999). Hypertriglyceridemia is also a risk factor for CHD, especially in patients with obesity and diabetes. Hypertriglyceridemia causes dysfunction of the vascular endothelial cells and increases the risk of platelet aggregation and thrombosis. Therefore, low lipid levels in blood are important for preventing CHD (Pignone et al., 2000). Ginseng-derived ginsenosides reduce intravascular lipid deposition and alleviate dyslipidemia by decreasing the levels of cholesterol and triacylglycerol in blood (Ziaei et al., 2020).
4.6 Regulation of intestinal flora by ginseng
Several studies have demonstrated that dysregulation of gut microbiota is involved in the development of atherosclerosis and coronary heart diseases (Battson et al., 2018). The host provides an optimal environment and essential nutrients for the growth and maintenance of the intestinal flora, which in turn are involved in the regulation of various body functions. Furthermore, several studies have shown that some types of gut bacteria promote development and progression of atherosclerosis, whereas other types of gut bacteria prevent the formation of atherosclerotic plaques (Jin et al., 2019). Ginsenoside Rc was the most abundant ginsenoside in various ginseng samples that were isolated from Korea and all over the world (Gu et al., 2019). Administration of an high-fat diet (HFD)-fed apolipoprotein E-deficient (ApoE−/−) mice with ginsenoside Rc through tube feeding significantly reduced the abundance of Bacteroides thicketi and Bacteroides mimosus, thereby partially restoring balance of the intestinal flora and reversing the effects of HFD by altering the bacterial flora composition at the genus level (Xie et al., 2022).
5 Ginseng and adverse reactions
In Asian countries, ginseng has been used in foods and therapeutic supplements for more than 2000 years. A low incidence of toxicity has been observed in human studies in ginseng preparations. However, ginseng is rarely associated with toxic side effects, adverse events, or interactions with prescription drugs (Gao et al., 2011). Moreover, adverse events are mostly caused by high doses and long-term use of ginseng. A review of literature by Paik et al., showed that misuse of ginseng was associated with affective disorders, allergies, cardiovascular and nephrotoxicity, genital hemorrhage, gynecomastia, hepatotoxicity, hypertension, reproductive toxicity, and anticoagulant-ginseng interactions (Paik and Lee, 2015). However, randomized controlled trials showed that ginseng was not associated with any significant adverse effects in the treatment of cardiovascular diseases (Sarhene et al., 2021).
6 Conclusion
CHD is the leading cause of death in most developed and developing countries (Kannel, 1998). Clinical complications of CHD are associated with severe disability and are a major source of rising healthcare costs (Assmann et al., 1999). Angina pectoris is one of the most early symptoms of CHD (Oram et al., 1972), wherein patients experience precordial chest discomfort or pain. CHD significantly affects the quality of life. Patients with CHD are prone to arrhythmias characterized by rapid, slow, or irregular heartbeats (Liu et al., 2023). Severe cases of arrhythmias lead to cardiac arrest (Lin et al., 2018). Another serious consequence of CHD is myocardial infarction, which is caused by blockage of the coronary artery that restricts the supply of oxygen and nutrients to the heart muscle. This causes necrosis of the heart muscle tissues and is a life-threatening condition. Therefore, angina pectoris, myocardial infarction, heart failure, arrhythmia, sudden death, and other cardiovascular diseases represent a serious life-threatening condition for the patients. Henceforth, it is very important to prevent the occurrence of CHD or adequately treat the condition when diagnosed.
Chinese herbal medicine is a traditional and mild treatment for CHD and has multiple advantages. Firstly, compared with western medicine, treatment with Chinese herbal medicine is milder with fewer side effects. Secondly, treatment with Chinese herbal medicine is multifaceted and involves disease maintenance or alleviation, improvement in the overall health status, and enhanced immunity. Furthermore, Chinese herbal medicine can eliminate cardiac lesions, relieve symptoms, delay disease progression, and effectively prevent recurrence of the disease. Finally, Chinese herbal medicine can treat a variety of diseases, including CHD, hepatitis, diabetes, and hypertension. In conclusion, treatment with Chinese herbal medicine is mild, personalized, and comprehensive, and can prevent disease recurrence and improve the overall health status of the patient (Chen, 2020). However, treatment with Chinese herbal medicine should be performed under the guidance of a professional physician because it may be associated with side effects and adverse reactions (Valli and Giardina, 2002; Chan et al., 2015).
Author contributions
M-MT: Writing–original draft. S-TZ: Writing–review and editing. R-QL: Writing–review and editing. WH: Writing–review and editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by funds from the Natural Science Foundation of Jilin Province (Grant No. YDZJ202201ZYTS190) and Central Public interest Scientific Institution Basal Research Fund (Grant No. 1610342023011).
Acknowledgments
The authors sincerely thank Zheng-Yi Qu for participating in the revision of the article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CHD, Coronary heart disease; MI/R, myocardial ischemia/reperfusion; TLR4, Toll-like receptor 4; Arg-Fru, arginine-fructose; CXCR1, C-X-C Motif Chemokine Receptor 1; IL-8, interleukin-8; KRG, Korean red ginseng; KRG-WE, KRG-water extract; MMP, matrix metalloproteinase; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; ApoE−/−, apolipoprotein E-deficient; HFD, high-fat diet; Col 1, Collagen I; Col 3, Collagen III; α-SMA, alpha-smooth muscle actin; ANF, Atrial natriuretic factor; β-MHC, β-myosin heavy chain; Ang II, angiotensin II; ACE, angiotensin-converting enzyme; AT1, Ang II type 1; TNF-α, tumor necrosis factor- α; IL-6β, interleukin-6β.
References
Aminifard, T., Razavi, B. M., and Hosseinzadeh, H. (2021). The effects of ginseng on the metabolic syndrome: An updated review. Food Sci. Nutr. 9, 5293–5311. doi:10.1002/fsn3.2475
Archbold, R. A., and Timmis, A. D. (1998). Cholesterol lowering and coronary artery disease: Mechanisms of risk reduction. Heart 80, 543–547. doi:10.1136/hrt.80.6.543
Assmann, G., Cullen, P., Jossa, F., Lewis, B., and Mancini, M. (1999). Coronary heart disease: Reducing the risk: The scientific background to primary and secondary prevention of coronary heart disease. A worldwide view. International task force for the prevention of coronary heart disease. Arteriosclerosis, Thrombosis, Vasc. Biol. 19, 1819–1824. doi:10.1161/01.ATV.19.8.1819
Battson, M. L., Lee, D. M., Weir, T. L., and Gentile, C. L. (2018). The gut microbiota as a novel regulator of cardiovascular function and disease. J. Nutr. Biochem. 56, 1–15. doi:10.1016/j.jnutbio.2017.12.010
Bentzon, J. F., Otsuka, F., Virmani, R., and Falk, E. (2014). Mechanisms of plaque formation and rupture. Circ. Res. 114, 1852–1866. doi:10.1161/CIRCRESAHA.114.302721
Bruetsch, W. L. (1959). The earliest record of sudden death possibly due to atherosclerotic coronary occlusion. Circulation 20, 438–441. doi:10.1161/01.CIR.20.3.438
Carlin, S., de Vries, T. A. C., Budaj, A., and Eikelboom, J. (2022). Dual pathway inhibition for atherosclerotic cardiovascular disease: Recent advances. Kardiol. Pol. 80, 1200–1210. doi:10.33963/KP.a2022.0283
Caron, M. F., Hotsko, A. L., Robertson, S., Mandybur, L., Kluger, J., and White, C. M. (2002). Electrocardiographic and hemodynamic effects of Panax ginseng. Ann. Pharmacother. 36, 758–763. doi:10.1345/aph.1A411
Cha, T. W., Kim, M., Kim, M., Chae, J. S., and Lee, J. H. (2016). Blood pressure-lowering effect of Korean red ginseng associated with decreased circulating Lp-PLA2 activity and lysophosphatidylcholines and increased dihydrobiopterin level in prehypertensive subjects. Hypertens. Res. 39, 449–456. doi:10.1038/hr.2016.7
Chan, K., Zhang, H., and Lin, Z.-X. (2015). An overview on adverse drug reactions to traditional Chinese medicines. Br. J. Clin. Pharmacol. 80, 834–843. doi:10.1111/bcp.12598
Chang, X., Zhang, T., Zhang, W., Zhao, Z., and Sun, J. (2020). Natural drugs as a treatment strategy for cardiovascular disease through the regulation of oxidative stress. Oxidative Med. Cell. Longev. 2020, e5430407. doi:10.1155/2020/5430407
Chen, K.-X. (2020). Academician kai-xian chen talks about the development of traditional Chinese medicine and global medicine. World J. Traditional Chin. Med. 6, 1. doi:10.4103/wjtcm.wjtcm_30_19
Chen, X., Lin, Y., Hu, Y., Liu, C., Lan, K., and Jia, W. (2015). Phytochemistry, metabolism, and metabolomics of ginseng. Chin. Herb. Med. 7, 98–108. doi:10.1016/S1674-6384(15)60026-0
Del Rio-Pertuz, G., Morataya, C., Iskandir, M., and Argueta-Sosa, E. (2022). Acute myocardial infarction associated with a mobile left ventricular thrombi. J. Invest. Med. High. Impact Case Rep. 10, 23247096221078704. doi:10.1177/23247096221078704
Eisenberg, D. (1998). The importance of lowering cholesterol in patients with coronary heart disease. Clin. Cardiol. 21, 81–84. doi:10.1002/clc.4960210204
Fan, S., Zhang, Z., Su, H., Xu, P., Qi, H., Zhao, D., et al. (2020). Panax ginseng clinical trials: Current status and future perspectives. Biomed. Pharmacother. 132, 110832. doi:10.1016/j.biopha.2020.110832
Fontbonne, A., Eschwege, E., Cambien, F., Richard, J. L., Ducimetiere, P., Thibult, N., et al. (1989). Hypertriglyceridaemia as a risk factor of coronary heart disease mortality in subjects with impaired glucose tolerance or diabetes. Results from the 11-year follow-up of the Paris Prospective Study. Diabetologia 32, 300–304. doi:10.1007/BF00265546
Fu, W., Yu, X., Lu, Z., Sun, F., Wang, Y., Zhang, Y., et al. (2016). Protective effects of ginsenoside Rb2 on myocardial ischemia in vivo and in vitro. Int. J. Clin. Exp. Med. 9, 9843–9855.
Fuentes, E., Moore-Carrasco, R., de Andrade Paes, A. M., and Trostchansky, A. (2019). Role of platelet activation and oxidative stress in the evolution of myocardial infarction. J. Cardiovasc. Pharmacol. Ther. 24, 509–520. doi:10.1177/1074248419861437
Gao, Y. L., Liu, Z. F., Li, C. M., Shen, J. Y., Yin, H. X., and Li, G. S. (2011). Subchronic toxicity studies with ginsenoside compound K delivered to dogs via intravenous administration. Food Chem. Toxicol. 49, 1857–1862. doi:10.1016/j.fct.2011.05.003
Gidding, S. S., and Allen, N. B. (2019). Cholesterol and atherosclerotic cardiovascular disease: A lifelong problem. J. Am. Heart Assoc. 8, e012924. doi:10.1161/JAHA.119.012924
Gu, L. D., Lee, J. S., Kim, K. T., Kim, H. Y., and Lee, S. (2019). Analysis of major ginsenosides in various ginseng samples. J. Appl. Biol. Chem. 62, 87–91. doi:10.3839/jabc.2019.013
Ha, H. M., and Chun, P. (2016). Effect of ginseng on blood pressure: A systematic review and meta-analysis. Korean J. Clin. Pharm. 26, 163–171.
Han, Y., Xu, Q., Hu, J., Han, X., Li, W., and Zhao, L. (2015). Maltol, a food flavoring agent, attenuates acute alcohol-induced oxidative damage in mice. Nutrients 7, 682–696. doi:10.3390/nu7010682
Hwang, S. Y., Son, D. J., Kim, I. W., Kim, D. M., Sohn, S. H., Lee, J. J., et al. (2008). Korean red ginseng attenuates hypercholesterolemia-enhanced platelet aggregation through suppression of diacylglycerol liberation in high-cholesterol-diet-fed rabbits. Phytotherapy Res. 22, 778–783. doi:10.1002/ptr.2363
Hyun, S. H., Bhilare, K. D. G., Park, C. K., and Kim, J. H. (2022). Effects of Panax ginseng and ginsenosides on oxidative stress and cardiovascular diseases: Pharmacological and therapeutic roles. J. Ginseng Res. 46, 33–38. doi:10.1016/j.jgr.2021.07.007
Hyung, R. G. (2007). Recent trend in red ginseng manufacturing process and characteristics of extruded red ginseng. Food Eng. Prog. 11, 1–10.
Jiang, Z., Wang, Y., Zhang, X., Peng, T., Li, Y., and Zhang, Y. (2012). Protective effect of ginsenoside R0 on anoxic and oxidative damage in vitro. Biomol. Ther. 20, 544–549. doi:10.4062/biomolther.2012.20.6.544
Jin, M., Qian, Z., Yin, J., Xu, W., and Zhou, X. (2019). The role of intestinal microbiota in cardiovascular disease. J. Cell. Mol. Med. 23, 2343–2350. doi:10.1111/jcmm.14195
Joo, I., Park, J. M., Park, K. D., and Hwan, L. J. (2008). Effect of Korean red ginseng on blood pressure and aortic vascular(endothelial) histological changes in rats. J. Ginseng Res. 32, 324–331.
Jovanovski, E., Jenkins, A., Dias, A. G., Peeva, V., Sievenpiper, J., Arnason, J. T., et al. (2010). Effects of Korean red ginseng (Panax ginseng C.A. Mayer) and its isolated ginsenosides and polysaccharides on arterial stiffness in healthy individuals. Am. J. Hypertens. 23, 469–472. doi:10.1038/ajh.2010.5
Jovanovski, E., Peeva, V., Sievenpiper, J. L., Jenkins, A. L., Desouza, L., Rahelic, D., et al. (2014). Modulation of endothelial function by Korean red ginseng (Panax ginseng C.A. Meyer) and its components in healthy individuals: A randomized controlled trial. Cardiovasc. Ther. 32, 163–169. doi:10.1111/1755-5922.12077
Kannel, W. B., Dawber, T. R., Thomas, H. E. J., and Mcnamara, P. M. (1965). Comparison of serum lipids in the prediction of coronary heart disease. Framingham study indicates that cholesterol level and blood pressure are major factors in coronary heart disease; effect of obesity and cigarette smoking also noted. R. I. Med. J. 48, 243–250.
Kannel, W. B. (1998). Overview of atherosclerosis. Clin. Ther. 20, B2–B17. doi:10.1016/S0149-2918(98)80027-1
Kim, S. J., Murthy, H. N., Hahn, E. J., Lee, H. L., and Paek, K. Y. (2007). Parameters affecting the extraction of ginsenosides from the adventitious roots of ginseng (Panax ginseng CA Meyer). Sep. Purif. Technol. 56, 401–406. doi:10.1016/j.seppur.2007.06.014
Komishon, A. M., Shishtar, E., Ha, V., Sievenpiper, J. L., de Souza, R. J., Jovanovski, E., et al. (2016). The effect of ginseng (genus Panax) on blood pressure: A systematic review and meta-analysis of randomized controlled clinical trials. J. Hum. Hypertens. 30, 619–626. doi:10.1038/jhh.2016.18
Lee, J., Cho, J.-Y., and Kim, W. K. (2014). Anti-inflammation effect of Exercise and Korean red ginseng in aging model rats with diet-induced atherosclerosis. Nutr. Res. Pract. 8, 284–291. doi:10.4162/nrp.2014.8.3.284
Lee, K. H., Bae, I. Y., Park, S. I., Park, J.-D., and Lee, H. G. (2016). Antihypertensive effect of Korean Red Ginseng by enrichment of ginsenoside Rg3 and arginine–fructose. J. Ginseng Res. 40, 237–244. doi:10.1016/j.jgr.2015.08.002
Lee, M. M. Y., Sattar, N., McMurray, J. J. V., and Packard, C. J. (2019). Statins in the prevention and treatment of heart failure: A review of the evidence. Curr. Atheroscler. Rep. 21, 41. doi:10.1007/s11883-019-0800-z
Lee, W. M., Kim, S. D., Park, M. H., Cho, J. Y., Park, H. J., Seo, G. S., et al. (2008). Inhibitory mechanisms of dihydroginsenoside Rg3 in platelet aggregation: Critical roles of ERK2 and cAMP. J. Pharm. Pharmacol. 60, 1531–1536. doi:10.1211/jpp/60.11.0015
Li, J., Zhang, L., Xie, N. Z., Deng, B., Lv, L. X., and Zheng, L. Q. (2014a). Relationship between the cholesterol ester transfer protein TaqIB polymorphism and the lipid-lowering effect of atorvastatin in patients with coronary atherosclerotic heart disease. Genet. Mol. Res. 13, 2140–2148. doi:10.4238/2014.March.24.21
Li, S., Tang, S. H., Liu, J. L., Su, J., and He, F. Y. (2018). Ginseng prescription rules and molecular mechanism in treating coronary heart disease based on data mining and integrative pharmacology. Zhongguo Zhong Yao Za Zhi 43, 1303–1309. doi:10.19540/j.cnki.cjcmm.20180115.006
Li, W. L., Hui, J. B., Jian, S. U. N., Xi, C. J., Ying, L. Z., and Yuan, L. I. U. (2014b). Protective effects of ginsenoside Rb1 on septic rats and its mechanism. BES 27, 300–303. doi:10.3967/bes2014.053
Li, X., Xiang, N., and Wang, Z. (2020). Ginsenoside Rg2 attenuates myocardial fibrosis and improves cardiac function after myocardial infarction via AKT signaling pathway. Biosci. Biotechnol. Biochem. 84, 2199–2206. doi:10.1080/09168451.2020.1793292
Liao, J., Huang, W., and Liu, G. (2017). Animal models of coronary heart disease. J. Biomed. Res. 31, 3–10. doi:10.7555/JBR.30.20150051
Lin, J. W., Cherng, Y. G., Chen, L. J., Niu, H. S., Chang, C. K., and Niu, C. S. (2014). Ginseng is useful to enhance cardiac contractility in animals. BioMed Res. Int. 2014, e723084. doi:10.1155/2014/723084
Lin, Y., Tsai, S. H., Yang, C. S., Wu, C. H., Huang, C. H., Lin, F. H., et al. (2018). Improved survival of hospitalized patients with cardiac arrest due to coronary heart disease after implementation of post-cardiac arrest care: A population-based study. Medicine 97, e12382. doi:10.1097/MD.0000000000012382
Liu, F., Liu, Y., Li, Z., Yu, L., Li, L., Ma, M., et al. (2023). Association between sensitivity to thyroid hormones and risk of arrhythmia in patients with coronary heart disease: A RCSCD-TCM study in China. Endocrine 79, 349–357. doi:10.1007/s12020-022-03223-4
Liu, H., Lu, X., Hu, Y., and Fan, X. (2020). Chemical constituents of Panax ginseng and Panax notoginseng explain why they differ in therapeutic efficacy. Pharmacol. Res. 161, 105263. doi:10.1016/j.phrs.2020.105263
Liu, Y., Deng, Y., Wang, F., Liu, X., Wang, J., Xiao, J., et al. (2022). A new mechanism for ginsenoside Rb1 to promote glucose uptake, regulating riboflavin metabolism and redox homeostasis. Metabolites 12, 1011. doi:10.3390/metabo12111011
Lu, H., Yao, Y., Wang, L., Yan, J., Tu, S., Xie, Y., et al. (2022). Research progress of machine learning and deep learning in intelligent diagnosis of the coronary atherosclerotic heart disease. Comput. Math. Methods Med. 2022, e3016532. doi:10.1155/2022/3016532
May, H. T., Nelson, J. R., Lirette, S. T., Kulkarni, K. R., Anderson, J. L., Griswold, M. E., et al. (2016). The utility of the apolipoprotein A1 remnant ratio in predicting incidence coronary heart disease in a primary prevention cohort: The Jackson Heart Study. Eur. J. Prev. Cardiol. 23, 769–776. doi:10.1177/2047487315612733
Moon, J.-N., Kim, J.-K., Lee, S., and Kwon, J.-H. (2019). Antihypertensive effects of Korean wild simulated ginseng (Sanyangsam) extracts in spontaneously hypertensive rats. Food Sci. Biotechnol. 28, 1563–1569. doi:10.1007/s10068-019-00617-5
Morimoto, T., Nakayama, M., Saito, Y., and Ogawa, H. (2007). Aspirin for primary prevention of atherosclerotic disease in Japan. J. Atheroscler. Thromb. 14, 159–166. doi:10.5551/jat.E482
Oram, S., Kidner, P., and Livesley, B. (1972). Early symptoms of coronary heart disease. BMJ 2, 767. doi:10.1136/bmj.2.5816.767-d
Oster, G., Thompson, D., Edelsberg, J., Bird, A. P., and Colditz, G. A. (1999). Lifetime health and economic benefits of weight loss among obese persons. Am. J. Public Health 89, 1536–1542. doi:10.2105/AJPH.89.10.1536
Page, M. J., Moher, D., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ-British Med. J. 372, n160. doi:10.1136/bmj.n160
Paik, D. J., and Lee, C. H. (2015). Review of cases of patient risk associated with ginseng abuse and misuse. J. Ginseng Res. 39, 89–93. doi:10.1016/j.jgr.2014.11.005
Pignone, M., Phillips, C., and Mulrow, C. (2000). Use of lipid lowering drugs for primary prevention of coronary heart disease: meta-analysis of randomised trials. BMJ 321, 983–986. doi:10.1136/bmj.321.7267.983
Poulsen, C. B., Pedrigi, R. M., Mehta, V. V., Post, A., Pareek, N., Holm, N. R., et al. (2015). Induction of perturbed shear stress leads to focal advanced atherosclerotic plaque formation in transgenic minipigs with hypercholesterolemia. J. Am. Coll. Cardiol. 65, A1927. doi:10.1016/S0735-1097(15)61927-1
Richardson, A. G., and Schadt, E. E. (2014). The role of macromolecular damage in aging and age-related disease. Journals Gerontology Ser. A 69, S28–S32. doi:10.1093/gerona/glu056
Sabouret, P., Angoulvant, D., Pathak, A., Fysekidis, M., Laterra, G., Costa, F., et al. (2022). How to fill the GAPS-I in secondary prevention: Application of a strategy based on GLP1 analogues, antithrombotic agents, PCSK9 inhibitors, SGLT2 inhibitors and immunomodulators. Panminerva Medica 64, 265–273. doi:10.23736/S0031-0808.21.04284-1
Saleheen, D., Scott, R., Javad, S., Zhao, W., Rodrigues, A., Picataggi, A., et al. (2015). Association of HDL cholesterol efflux capacity with incident coronary heart disease events: A prospective case-control study. Lancet Diabetes and Endocrinol. 3, 507–513. doi:10.1016/S2213-8587(15)00126-6
Sang, Y., Roest, M., De Laat, B., De Groot, P. G., and Huskens, D. (2021). Interplay between platelets and coagulation. Blood Rev. 46, 100733. doi:10.1016/j.blre.2020.100733
Sarhene, M., Ni, J. Y., Duncan, E. S., Liu, Z., Li, S., Zhang, J., et al. (2021). Ginsenosides for cardiovascular diseases; update on pre-clinical and clinical evidence, pharmacological effects and the mechanisms of action. Pharmacol. Res. 166, 105481. doi:10.1016/j.phrs.2021.105481
Shao, M., Gao, P., Cheng, W., Ma, L., Yang, Y., Lu, L., et al. (2022). Ginsenoside Rb3 upregulates sarcoplasmic reticulum Ca2+-ATPase expression and improves the contractility of cardiomyocytes by inhibiting the NF-?B pathway. Biomed. Pharmacother. 154, 113661. doi:10.1016/j.biopha.2022.113661
Thomas, I. C., Forbang, N. I., and Criqui, M. H. (2018). The evolving view of coronary artery calcium and cardiovascular disease risk. Clin. Cardiol. 41, 144–150. doi:10.1002/clc.22842
Valli, G., and Giardina, E.-G. V. (2002). Benefits, adverse effects and drug interactionsof herbal therapies with cardiovascular effects. J. Am. Coll. Cardiol. 39, 1083–1095. doi:10.1016/S0735-1097(02)01749-7
Venkataraman, K., Khurana, S., and Tai, T. C. (2013). Oxidative stress in aging-matters of the heart and mind. Int. J. Mol. Sci. 14, 17897–17925. doi:10.3390/ijms140917897
Wang, J., Gao, W.-Y., Zhang, J., Zuo, B.-M., Zhang, L.-M., and Huang, L.-Q. (2012). Advances in study of ginsenoside biosynthesis pathway in Panax ginseng C. A. Meyer. Acta Physiol. Plant 34, 397–403. doi:10.1007/s11738-011-0844-3
Wang, Y., Shen, L., and Xu, D. (2019). Aerobic exercise reduces triglycerides by targeting apolipoprotein C3 in patients with coronary heart disease. Clin. Cardiol. 42, 56–61. doi:10.1002/clc.23104
Xie, B., Zu, X., Wang, Z., Xu, X., Liu, G., and Liu, R. (2022). Ginsenoside Rc ameliorated atherosclerosis via regulating gut microbiota and fecal metabolites. Front. Pharmacol. 13, 990476. doi:10.3389/fphar.2022.990476
Xue, Y., Yu, X., Zhang, X., Yu, P., Li, Y., Fu, W., et al. (2021). Protective effects of ginsenoside Rc against acute cold exposure-induced myocardial injury in rats. J. Food Sci. 86, 3252–3264. doi:10.1111/1750-3841.15757
Yao, T., Lu, W., Ke, J., Zhang, H., Zhao, X., Song, B., et al. (2022). Residual risk of coronary atherosclerotic heart disease and severity of coronary atherosclerosis assessed by ApoB and ldl-C in participants with statin treatment: A retrospective cohort study. Front. Endocrinol. 13, 865863. doi:10.3389/fendo.2022.865863
Yu, Y., Sun, J., Liu, J., Wang, P., and Wang, C. (2020). Ginsenoside Re preserves cardiac function and ameliorates left ventricular remodeling in a rat model of myocardial infarction. J. Cardiovasc. Pharmacol. 75, 91–97. doi:10.1097/FJC.0000000000000752
Zeliger, H. I. (2016). Predicting disease onset in clinically healthy people. Interdiscip. Toxicol. 9, 39–54. doi:10.1515/intox-2016-0006
Zeng, X., Li, J., and Li, Z. (2015). Ginsenoside Rd mitigates myocardial ischemia-reperfusion injury via Nrf2/HO-1 signaling pathway. Int. J. Clin. Exp. Med. 8, 14497–14504.
Zhang, L., Jiang, Y., Yu, X., Xu, H., Li, M., Zhao, X., et al. (2016). Ginsenoside Rg3 improves cardiac function after myocardial ischemia/reperfusion via attenuating apoptosis and inflammation. Evidence-Based Complementary Altern. Med. 2016, e6967853. doi:10.1155/2016/6967853
Zhang, Y. J., Zhang, X. L., Li, M. H., Iqbal, J., Bourantas, C. V., Li, J. J., et al. (2013). The ginsenoside Rg1 prevents transverse aortic constriction–induced left ventricular hypertrophy and cardiac dysfunction by inhibiting fibrosis and enhancing angiogenesis. J. Cardiovasc. Pharmacol. 62, 50–57. doi:10.1097/FJC.0b013e31828f8d45
Zheng, X., Wang, S., Zou, X., Jing, Y., Yang, R., Li, S., et al. (2017). Ginsenoside Rb1 improves cardiac function and remodeling in heart failure. Exp. Anim. 66, 217–228. doi:10.1538/expanim.16-0121
Ziaei, R., Ghavami, A., Ghaedi, E., Hadi, A., Javadian, P., and Clark, C. C. T. (2020). The efficacy of ginseng supplementation on plasma lipid concentration in adults: A systematic review and meta-analysis. Complementary Ther. Med. 48, 102239. doi:10.1016/j.ctim.2019.102239
Keywords: ginseng, coronary heart disease (CHD), atherosclerosis, heart, blood pressure, antioxidation, lipid, platelets
Citation: Tang M-M, Zhao S-T, Li R-Q and Hou W (2023) Therapeutic mechanisms of ginseng in coronary heart disease. Front. Pharmacol. 14:1271029. doi: 10.3389/fphar.2023.1271029
Received: 01 August 2023; Accepted: 22 September 2023;
Published: 03 October 2023.
Edited by:
Adolfo Andrade-Cetto, National Autonomous University of Mexico, MexicoReviewed by:
Syed Anees Ahmed, East Carolina University, United StatesCopyright © 2023 Tang, Zhao, Li and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Hou, amlsaW5ob3V3ZWlAMTYzLmNvbQ==
 Miao-Miao Tang
Miao-Miao Tang Shu-Ting Zhao
Shu-Ting Zhao Wei Hou
Wei Hou