- 1Key Laboratory of Pathobiology, Ministry of Education, Jilin University, Changchun, Jilin, China
- 2Department of Urology, The First Hospital of Jilin University, Changchun, Jilin, China
Background: Mesalazine, a preparation of 5-aminosalicylic acid, is a medication widely used in clinical practice as a first-line therapy in the treatment of mild and moderate inflammatory bowel disease. However, the long-term safety of mesalazine in large sample population was unknown. The current study was to assess mesalazine -related adverse events of real-world through data mining of the US Food and Drug Administration Adverse Event Reporting System (FAERS).
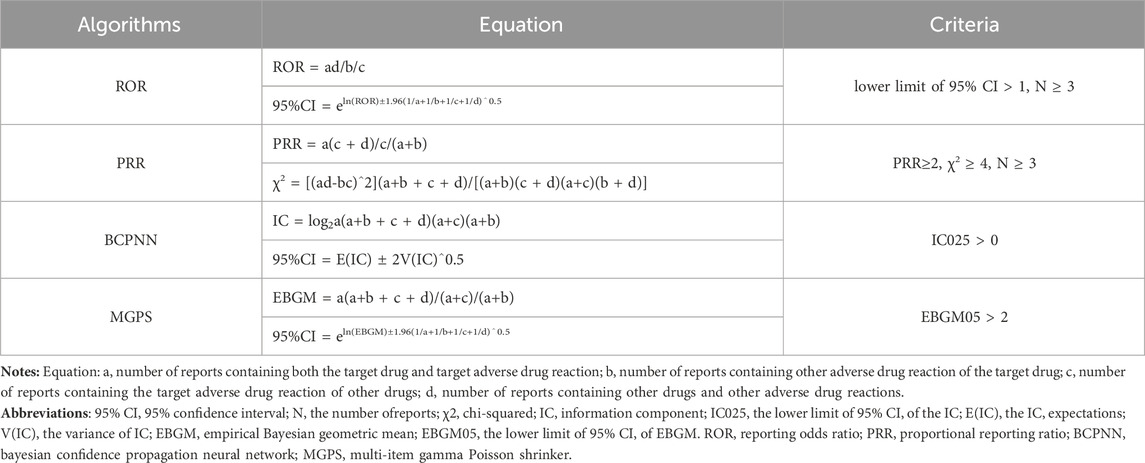
Methods: Disproportionality analyses, including the reporting odds ratio (ROR), the proportional reporting ratio the Bayesian confidence propagation neural network and the multi-item gamma Poisson shrinker (MGPS) algorithms were employed to quantify the signals of mesalazine -associated AEs.
Results: Out of 14,149,980 reports collected from the FDA Adverse Event Reporting System database, 24,284 reports of mesalazine -associated AEs were identified. A total of 170 significant disproportionality preferred terms conforming to the four algorithms simultaneously were retained. The most common AEs included colitis ulcerative, diarrhoea, condition aggravated, crohn’s disease, fatigue, abdominal pain, nausea, haematochezia, which were corresponding to those reported in the specification and clinical trials. Unexpected significant AEs as dizziness, drug ineffective, drug hypersensitivity, infection, off label use, weight decreased, decreased appetite, arthralgia, rash might also occur. The median onset time of mesalazine -related AEs was 1,127 days (interquartile range [IQR] 1,127–1,674 days), and most of the cases occurred 2 years later (n = 610, 70.93%) and within the first 1 month (n = 89, 10.35%) after mesalazine initiation.
Conclusion: Results of our study were consistent with clinical observations. We also found potential new and unexpected AEs signals for mesalazine, suggesting prospective clinical studies were needed to confirm these results and illustrate their relationship. Our results could provide valuable evidence for further safety studies of mesalazine.
1 Introduction
The prevalence of inflammatory bowel disease (IBD), encompassing ulcerative colitis (UC) and Crohn’s disease (CD), is increasing worldwide, and new treatments for IBD (including biologics) have emerged in recent years (Ford et al., 2011a). Among the first-line treatments for IBD are 5-aminosalicylic acid derivatives (5-ASAs) especially mesalazine, which is effective in inducing and maintaining remission in most cases of UC with mild or moderate activity (Veloso et al., 2021; Suzuki et al., 2022).
Mesalazine (mesalamine), also known chemically as 5-aminosalicylic acid (5-ASA), is commonly used in the treatment of UC (Ford et al., 2012). Mesalazine exerts an anti-inflammatory effect on the intestinal wall after taken orally (Ford et al., 2011b). Because of the anti-ulcer and antioxidant efficacy, mesalazine is not only used in the treatment of UC, but also in other diseases (Beiranvand and Bahramikia, 2020; Li et al., 2022). Some studies have shown that mesalazine also improve mucosal healing and reduced risk of colorectal cancer, and that it halves the risk of colitis-associated cancer or dysplasia in patients with UC (Bonovas et al., 2017). Mesalazine, a non-steroidal anti-inflammatory drug (NSAIDs), has an anti-inflammatory effect that has not been fully explained; however, the available data suggest that it antagonises pro-inflammatory mediators such as NF-κB, γ-IFN, IL-8 and TNF-α. It also inhibits the cyclo-oxygenase (COX) and lipoxygenase (LOX) pathways, which inhibit the release of the inflammation-associated prostaglandins E2 and leukotriene. There is also an anti-inflammatory mechanism of action suggesting that mesalazine increases PPARγ expression in gastrointestinal epithelial cells. The available data suggest that mesalazine may also inhibit the proliferation of tumor cells through a number of pathways (Słoka et al., 2023). Mesalazine is the pharmacologically active ingredient in sulfasalazine, which was the first compound used in the treatment of ulcerative colitis. Sulfasalazine, on the other hand, is the inactive ingredient in sulfasalazine and can cause serious side effects. For example, it sometimes produces symptoms such as fever, diarrhoea and bloody stools (Matsumoto and Mashima, 2020).
The FDA Adverse Event Reporting System (FAERS) database is a publicly accessible spontaneous reporting system, which covers tens of millions of case reports of adverse drug events (ADEs) submitted by physicians, pharmacists, manufacturers, and others (Cohen et al., 2000; Rahimi et al., 2009). The FAERS is currently the world’s largest pharmacovigilance database and is an effective tool for detecting ADRs associated with drug exposure (Shu et al., 2022a; Shu et al., 2022b). In the present study, we retrospectively analyzed the AEs reported from January 2018 to December 2022 with mesalazine through data mining of FAERS.
2 Materials and methods
2.1 Study design and data sources
FAERS, as a well-known publicly available post-marketing safety surveillance database, which researchers collect AEs reports by health professionals, pharmaceutical manufacturers, individual patients and others. The FAERS data files contained seven types of datasets: patient demographic and administrative information (DEMO), drug information (DRUG), coded for the adverse events (REAC), patient outcomes (OUTC), report sources (RPSR), therapy start dates and end dates for reported drugs (THER), and indications for drug administration (INDI), and deleted cases. All the data downloaded from the U.S. FDA website were imported into MySQL 8.0 for further analysis. This research based on FAERS database, extracting data from January 2018 to December 2022.
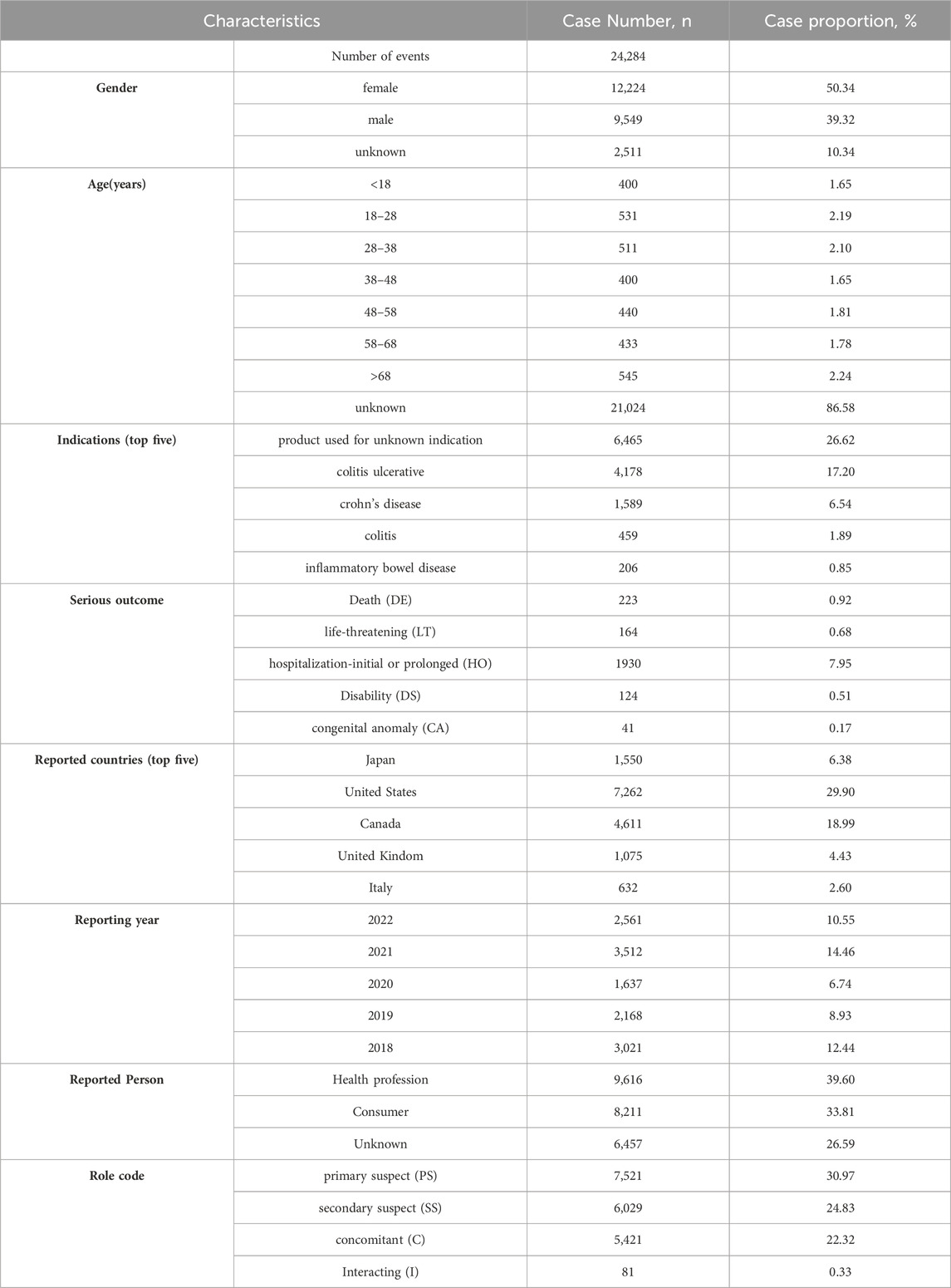
AEs in the FAERS database were coded by Medical Dictionary for Regulatory Activities 24.0 (MedDRA). The structural hierarchy of the MedDRA terminology was divided into five levels: system organ class (SOC), high-level group term (HLGT), high-level term (HLT), preferred term (PT), and lowest-level term (LLT) (Burr et al., 2019). All AEs of mesalazine reports taken from the REAC files in the FAERS database were identified to describe the frequency and intensity based on MedDRA at SOC and PT levels in our study (Cammà et al., 1997). The reported drugs in FAERS were categorized into four patterns: PS (primary suspect), SS (secondary suspect), C (concomitant), and I (interacting). Serious patient outcomes were defined as death (DE), life-threatening (LT), hospitalization-initial or prolonged (HO), disability (DS), congenital anomaly (CA) or other important medical event (OT) (Steinhart et al., 1994). Clinical characteristics including gender, age, reporting area, reporter, reporting time and outcomes of patients with mesalazine -related AEs were collected (Louis et al., 2022) (Figure 1).
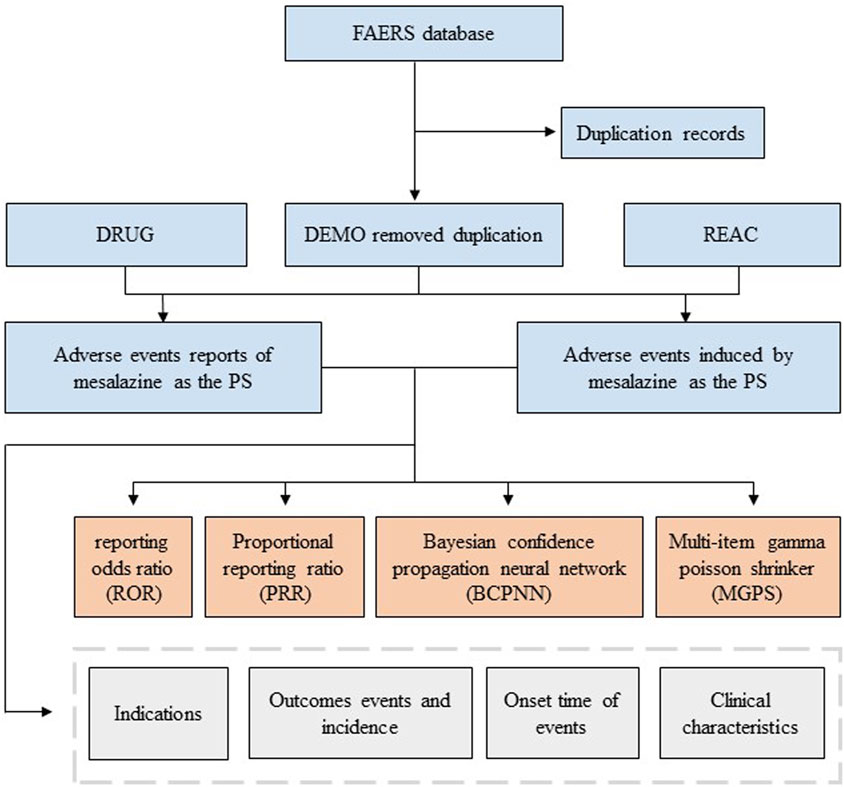
FIGURE 1. Flowchart of identifying adverse event cases of mesalazine and statins from the FAERS database.
2.2 Statistical analysis
Descriptive analysis was used to show the characteristics of all AE reports regarding to mesalazine. Disproportionality analysis, which is widely used in pharmacovigilance study, was performed to identify potential signals between mesalazine and all AEs in our investigation. Reporting odds ratio (ROR), the proportional reporting ratio (PRR), the Bayesian confidence propagation neural network (BCPNN), and the multi-item gamma Poisson shrinker (MGPS) are four major specific indices that were calculated using standard formulas to assess potential associations between mesalazine and AEs as presented in Table 1. Only those signals with at least three target AE records to target drugs were calculated in our study. At least one of the four algorithms meets the criteria should be considered as a positive signal of drug-associated AEs (lower limit of 95% CI > 1, N ≥ 3; PRR≥2, χ2 ≥ 4, N ≥ 3; IC025 > 0 or EBGM05 > 2). In this study, we selected AE signals that simultaneously met all of the above four algorithm standards for research (Shu et al., 2022a; Shu et al., 2022b; Hou et al., 2022).
The time to onset and serious outcome probability of AEs were calculated. The onset time is defined as the interval between EVENT_DT (date of AE occurrence) and START_DT (start date for mesalazine use). In addition, reports with input errors (EVENT_DT earlier than START_DT), inaccurate date entries and missing specific data were excluded. Severe outcomes mainly included life threatening events or those causing hospitalization, disability, or death. Moreover, reports with serious outcome events attributed to drug toxicity were counted, and the proportion was calculated as dividing the number of serious outcomes by the total number of reported events. All data processing and statistical analyses were performed using MYSQL 8.0, Navicat Premium 15, Microsoft EXCEL 2019 and the GraphPad Prism 8 (GraphPad Software, CA, United States) (Li et al., 2021; Shu et al., 2022a; Shu et al., 2022b).
3 Results
3.1 General characteristics
From January 2018 to December 2022, a total of 14,149,980 AE reports submitted to FAERS database, among which 24,284 reports on mesalazine were reported. The characteristics of AE reports submitted for mesalazine are described in Table 2. The number of reported AEs was variable from 2018 to 2022, the most reported year was 2021 (14.46%), followed by 2018 (12.44%). Among all reports, females (50.34%) accounted for a larger proportion than males (39.32%). The largest percentages of reports (2.24%) were in elderly individuals (aged >68 years) and patients aged 18–28 years also accounted for a high proportion with 2.19% (n = 531), followed by aged 28–38 years (2.10%). Colitis ulcerative was the most reported indication (17.20%), followed by crohn’s disease (6.54%), colitis (1.89%), inflammatory bowel disease (0.85%). Most of reports were came from United States (29.90%), followed by Canada (18.99%), Japan (6.38%), United Kindom (4.43%), and Italy (2.60%), mainly submitted by health professionals (39.60%) and Consumers (33.81%). Mesalazine was the primary suspect (30.97%) in most reports. Hospitalization-initial or prolonged (HO) (7.95%) was the most frequently reported serious outcome, and death or life-threatening events were reported in 223 (0.92%) and 164 (0.68%) cases respectively.
3.2 Signal detection
A total of 170 significant PTs of interest conforming to all of the four algorithms simultaneously are described in Table 3. In this study, anaemia (PT: 10002034), colitis ulcerative, diarrhoea, crohn’s disease, abdominal pain, nausea (PT: 10028813), infection, maternal exposure during pregnancy, foetal exposure during pregnancy were present, which consistent with the instructions and medication warnings. Of note, unexpected significant AEs, including Dizziness, Drug hypersensitivity, Infection, Weight decreased, Decreased appetite, Arthralgia, Headache, Dyspnoea, Rash and so on, were uncovered in the label.
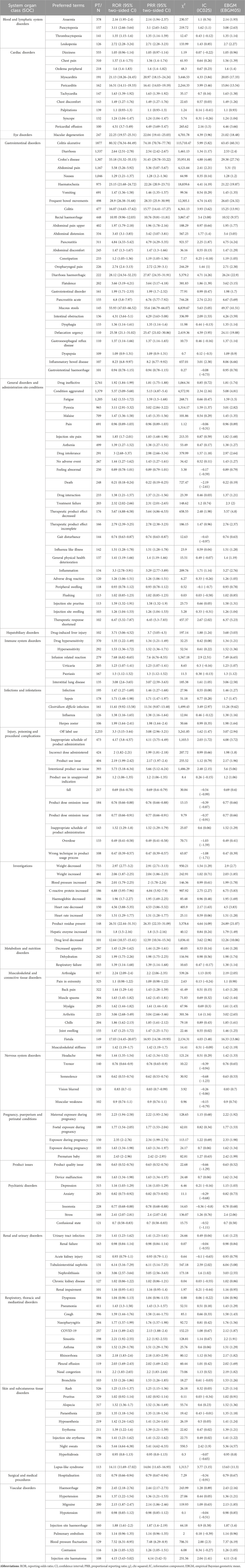
TABLE 3. Signal Strength of AEs of mesalazine at the System Organ Class (SOC) Level in FAERS Database.
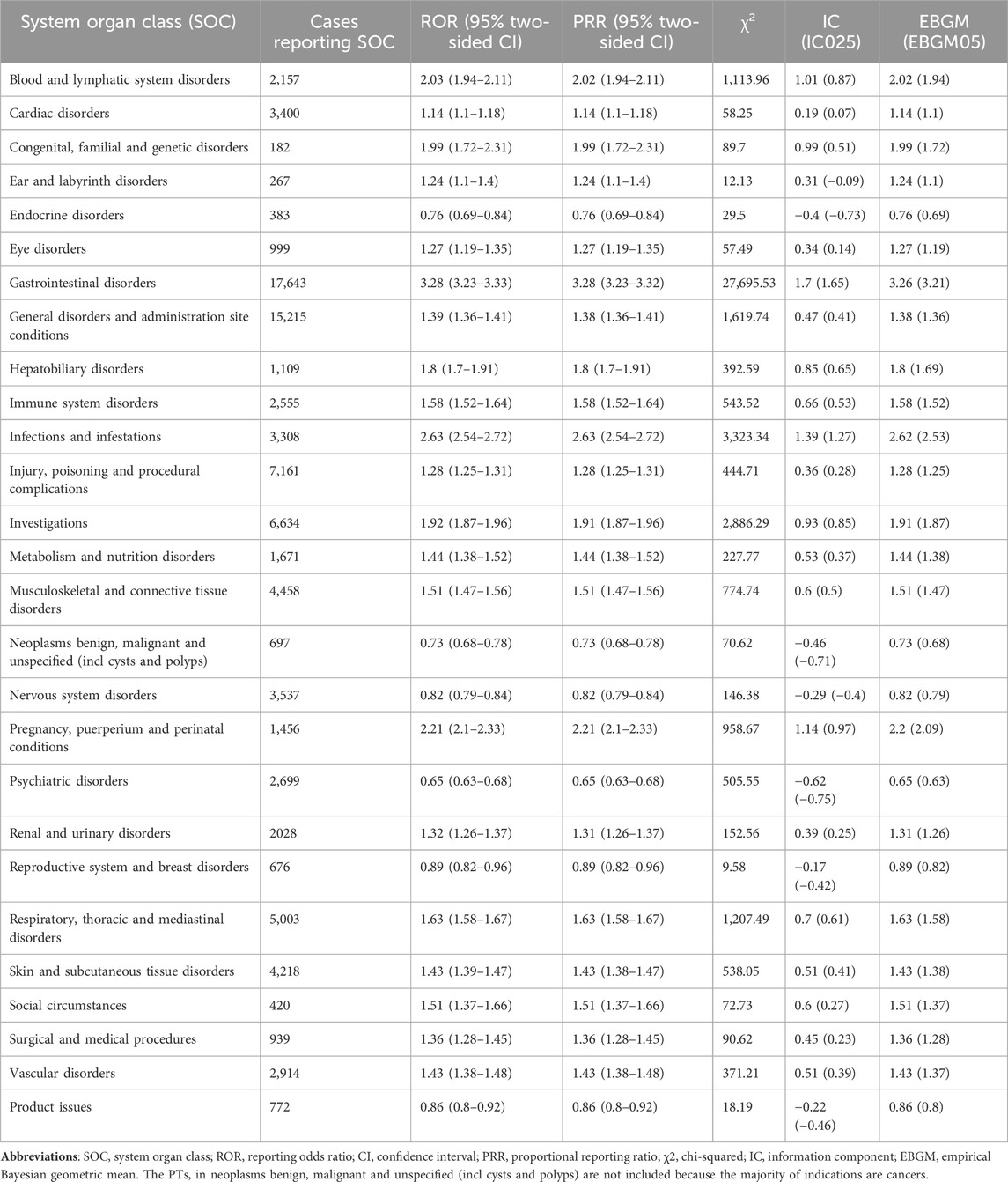
Signal strengths and reports of mesalazine at the SOC level (PT > 100) were described in Table 4. Statistically, we found that mesalazine -induced AEs occurrence targeted 27 SOCs. The significant SOCs were “Gastrointestinal disorders (SOC: 10017947)”, “blood and lymphatic system disorders (SOC: 10005329)”, “Infections and infestations (SOC: 10021881)” and “Pregnancy, puerperium and perinatal conditions (SOC: 10036585)”.
3.3 Time to onset of mesalazine-associated adverse events
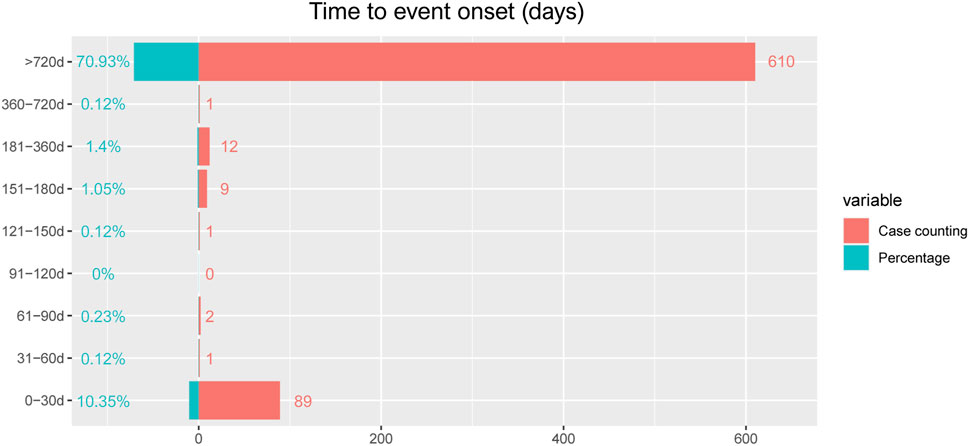
The onset times of mesalazine-associated AEs were extracted from the database. Excluding inaccurate, missing or unknown onset time reports, a total of 725 mesalazine -associated AEs reported onset time and the median onset time was 1,127 days (interquartile range [IQR] 1,127–1,164 days). As Figure 2 illustrated, results indicated that most of the AE cases occurred 2 years later (n = 610, 70.93%) and within the first 1 month (n = 89, 10.35%) after mesalazine initiation.
4 Conclusion
In conclusion, the present study using pharmacovigilance analysis of FAERS database scientifically and systematically quantified the potential risks, time to AE onsets and the safety signal spectrum with mesalazine treatment. Unexpected and new significant AEs as Dizziness, Drug ineffective, Drug hypersensitivity, Infection, Off label use, Weight decreased, Decreased appetite, Arthralgia, Headache, Dyspnoea, Rash AEs are frequent AEs for which patients should be monitored. Our study could provide valuable evidence for further studies and clinical practice of mesalazine.
5 Discussion
To the best of our knowledge, this is the first most comprehensive and systematic pharmacovigilance study on mesalazine-associated AEs by post-marketing based on the FAERS database. We presented a more accurate and detailed description and characterization of mesalazine-associated AEs to date. However, because results of the low response rate in active ulcerative colitis and in Crohn’s disease treatment, some AEs such as gastrointestinal disorders and infections and infestations might be considered as the signs of ineffectiveness rather than real adverse events. In the cases with mesalazine-associated AEs, the proportion of female is 50.34% slightly higher than that of males (39.32%). However, based on the unknown cases (10.34%), we cannot compare credible gender ratios in these cases. Almost half of the reports (49.94%) were submitted by health professionals, which might be considered a more reliable source of reporting. Reports of AEs since 2018 have continued to increase due to the widespread use and increased awareness of healthcare professionals, strongly underling the need for constant epidemiological surveillance (Singh et al., 2015). In our study, mesalazine demonstrated a slightly higher AEs proportion in patients aged in elderly individuals (aged >68 years) and patients aged 18–28 years, although the difference among all different ages is so small. Besides, a large number of cases with unknown ages also results in the difficulty in the discovery of the ages of patients which is most easily to occur mesalazine-associated AEs.
According to the disproportionality analysis, the most common and significant SOCs such as “Gastrointestinal disorders”, “blood and lymphatic system disorders”, “Infections and infestations”, and “Pregnancy, puerperium and perinatal conditions” were consistent with the safety data in the label and clinical trials. Among the SOC of Gastrointestinal disorders, AEs with the highest number of reports were colitis ulcerative, diarrhoea, crohn’s disease, abdominal pain, nausea. Blood and lymphatic system disorders includes anaemia, pancytopenia, thrombocytopenia, leukopenia.
Notably, long-term use of mesalazine is associated with a risk of skin AEs, and the most common is rash. However, early study provided experimental evidence that oral mesalazine treatment is effective to prevent cutaneous fibrosis development in genetically predisposed mice. Because lossing of PLK2 function induces spontaneous fibrotic remodeling of the skin due to aberrant myofibroblast activation and collagen accumulation (Newe et al., 2021; Xie et al., 2022).
The mesalazine are generally well tolerated; headache, nausea, diarrhea and abdominal pain are the most common, still very rare side effects (Zhang et al., 2019). Nephrotoxicity is extremely rare in patients on 5-ASA medications, with a mean incidence of 0.3% per person-year (Bonovas et al., 2019; Castro Tejera et al., 2022). In most cases, renal failure is caused by an acute or chronic interstitial nephritis, which is idiosyncratic and unrelated to 5-ASA formulation and dose (Gisbert et al., 2007; Yang et al., 2014). Despite its rarity, nephrotoxicity has to be taken into account in all patients treated with mesalazine, and scheduled controls of renal function are suggested in IBD treatment guidelines (Nardelli et al., 2017; Nguyen et al., 2018).
Results of this study indicated that the median onset time was 1,127 days, and most of the cases occurred 2 years later (n = 610, 70.93%) and within the first 1 month (n = 89, 10.35%) after mesalazine initiation. This may be due to the existence of a latency period of the drug, or the patient’s body resistance, slow metabolism, and prolonged duration required for cumulative drug concentration build-up, the body is unable to fully absorb the drug, so most people’s adverse drug reaction appears later. Therefore, a longer follow-up period is needed to observe the ADRs of mesalazine in future clinical studies.
Despite the advantages of real-world large-sample research and the data mining techniques in this study, there are still some limitations that warrant discussion (Nguyen et al., 2012; Moja et al., 2015). First, FAERS is a spontaneous reporting system with incomplete and incorrect information collected from different countries and professionals, thus the quality might be variable, which may lead to bias in the analysis. Second, multiple unmeasured confounders such as potential drug-drug interactions, comorbidities and drug combinations, which might affect AEs, were not included in the data analysis. Third, despite having access to thousands of case reports, the safety reports do not provide detailed information of patients exposed to the drug without AEs. Moreover, since mesalazine is marketed in some country as a generic drug and this might modify the rate of absorption and thus the safety profile of the drug. Therefore, the true incidence of AEs cannot be determined from FAERS data. Fourth, it was unable to infer an exact causal relationship. The disproportionality analysis neither quantified risk nor existed causality, but only provided an estimation of the signal strength, which was only statistically significant. Prospective clinical studies are still needed to confirm the causal relationship between them. Despite these limitations, our results would provide a valuable reference for healthcare professionals to closely follow-up patients and monitor the associated adverse reactions of mesalazine (Law et al., 2020; Shu et al., 2022a; Shu et al., 2022b).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
ML: Conceptualization, Funding acquisition, Writing–original draft, Writing–review and editing. LG: Conceptualization, Writing–review and editing. YZ: Formal Analysis, Writing–original draft, Writing–review and editing. HZ: Supervision, Writing–review and editing. YW: Funding acquisition, Supervision, Writing–review and editing. Z-XX: Funding acquisition, Supervision, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (82020108024 and 81772924), Health Commission of Jilin Province (2020J033), and Graduate Innovation Fund of Jilin University (101832020CX260).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Beiranvand, M., and Bahramikia, S. (2020). Ameliorating and protective effects mesalazine on ethanol-induced gastric ulcers in experimental rats. Eur. J. Pharmacol. 888, 173573. doi:10.1016/j.ejphar.2020.173573
Bonovas, S., Fiorino, G., Lytras, T., Nikolopoulos, G., Peyrin-Biroulet, L., and Danese, S. (2017). Systematic review with meta-analysis: use of 5-aminosalicylates and risk of colorectal neoplasia in patients with inflammatory bowel disease. Alimentary Pharmacol. Ther. 45, 1179–1192. doi:10.1111/apt.14023
Bonovas, S., Nikolopoulos, G., Piovani, D., González-Lorenzo, M., Pantavou, K., Lytras, T., et al. (2019). Comparative assessment of budesonide-MMX and mesalamine in active, mild-to-moderate ulcerative colitis: a systematic review and network meta-analysis. Br. J. Clin. Pharmacol. 85, 2244–2254. doi:10.1111/bcp.14051
Burr, N., Hall, B., Hamlin, P., Selinger, C., Ford, A., and O'Connor, A. (2019). Systematic review and network meta-analysis of medical therapies to prevent recurrence of post-operative crohn's disease. J. Crohn's colitis 13, 693–701. doi:10.1093/ecco-jcc/jjy216
Cammà, C., Giunta, M., Rosselli, M., and Cottone, M. (1997). Mesalamine in the maintenance treatment of Crohn's disease: a meta-analysis adjusted for confounding variables. Gastroenterology 113, 1465–1473. doi:10.1053/gast.1997.v113.pm9352848
Castro Tejera, V., Öhman, L., Aabakken, L., Fellström, B., Hausken, T., Hovde, Ø., et al. (2022). Randomised clinical trial and meta-analysis: mesalazine treatment in irritable bowel syndrome-effects on gastrointestinal symptoms and rectal biomarkers of immune activity. Alimentary Pharmacol. Ther. 56, 968–979. doi:10.1111/apt.17182
Cohen, R., Woseth, D., Thisted, R., and Hanauer, S. (2000). A meta-analysis and overview of the literature on treatment options for left-sided ulcerative colitis and ulcerative proctitis. Am. J. gastroenterology 95, 1263–1276. doi:10.1111/j.1572-0241.2000.01940.x
Ford, A., Kane, S., Khan, K., Achkar, J., Talley, N., Marshall, J., et al. (2011a). Efficacy of 5-aminosalicylates in Crohn's disease: systematic review and meta-analysis. Am. J. gastroenterology 106, 617–629. doi:10.1038/ajg.2011.71
Ford, A., Khan, K., Achkar, J., and Moayyedi, P. (2012). Efficacy of oral vs. topical, or combined oral and topical 5-aminosalicylates, in Ulcerative Colitis: systematic review and meta-analysis. Am. J. gastroenterology 107, 167–176. doi:10.1038/ajg.2011.410
Ford, A., Khan, K., Talley, N., and Moayyedi, P. (2011b). 5-aminosalicylates prevent relapse of Crohn's disease after surgically induced remission: systematic review and meta-analysis. Am. J. gastroenterology 106, 413–420. doi:10.1038/ajg.2010.317
Gisbert, J. P., Gonzalez-Lama, Y., and Mate, J. (2007). 5-Aminosalicylates and renal function in inflammatory bowel disease: a systematic review. Inflamm. Bowel Dis. 13, 629–638. doi:10.1002/ibd.20099
Hou, W. B., Sun, W. J., Zhang, X. W., Li, Y. X., Zheng, Y. Y., Sun, Y. X., et al. (2022). Five-flavor Sophora flavescens enteric-coated capsules for ulcerative colitis: a systematic review and meta-analysis of randomized clinical trials. Evid. Based Complement. Altern. Med. 2022, 9633048. doi:10.1155/2022/9633048
Law, C., Koh, D., Bao, Y., Jairath, V., and Narula, N. (2020). Risk of postoperative infectious complications from medical therapies in inflammatory bowel disease: a systematic review and meta-analysis. Inflamm. bowel Dis. 26, 1796–1807. doi:10.1093/ibd/izaa020
Li, J., Ling, F., Guo, D., Zhao, J., Cheng, L., Chen, Y., et al. (2022). The efficacy of mesalazine on nonspecific terminal ileal ulcers: a randomized controlled trial. Front. Pharmacol. 13, 989654. doi:10.3389/fphar.2022.989654
Li, S., Yin, Y., Xiao, D., and Zou, Y. (2021). Supplemental bifid triple viable capsule treatment improves inflammatory response and T cell frequency in ulcerative colitis patients. BMC Gastroenterol. 21, 314. doi:10.1186/s12876-021-01887-2
Louis, E., Paridaens, K., Al Awadhi, S., Begun, J., Cheon, J. H., Dignass, A. U., et al. (2022). Modelling the benefits of an optimised treatment strategy for 5-ASA in mild-to-moderate ulcerative colitis. BMJ Open Gastroenterol. 9, e000853. doi:10.1136/bmjgast-2021-000853
Matsumoto, S., and Mashima, H. (2020). Mesalazine allergy and an attempt at desensitization therapy in patients with inflammatory bowel disease. Sci. Rep. 10, 22176. doi:10.1038/s41598-020-79207-z
Moja, L., Danese, S., Fiorino, G., Del Giovane, C., and Bonovas, S. (2015). Systematic review with network meta-analysis: comparative efficacy and safety of budesonide and mesalazine (mesalamine) for Crohn's disease. Alimentary Pharmacol. Ther. 41, 1055–1065. doi:10.1111/apt.13190
Nardelli, S., Pisani, L. F., Tontini, G. E., Vecchi, M., and Pastorelli, L. (2017). MMX® technology and its applications in gastrointestinal diseases. Ther. Adv. Gastroenterol. 10, 545–552. doi:10.1177/1756283X17709974
Newe, M., Kant, T. A., Hoffmann, M., Rausch, J. S. E., Winter, L., Kunzel, K., et al. (2021). Systemic mesalazine treatment prevents spontaneous skin fibrosis in PLK2-deficient mice. Naunyn Schmiedeb. Arch. Pharmacol. 394, 2233–2244. doi:10.1007/s00210-021-02135-w
Nguyen, G., Gulamhusein, A., and Bernstein, C. (2012). 5-aminosalicylic acid is not protective against colorectal cancer in inflammatory bowel disease: a meta-analysis of non-referral populations. Am. J. gastroenterology 107, 1298–1304. doi:10.1038/ajg.2012.198
Nguyen, N. H., Fumery, M., Dulai, P. S., Prokop, L. J., Sandborn, W. J., Murad, M. H., et al. (2018). Comparative efficacy and tolerability of pharmacological agents for management of mild to moderate ulcerative colitis: a systematic review and network meta-analyses. Lancet Gastroenterol. Hepatol. 3, 742–753. doi:10.1016/S2468-1253(18)30231-0
Rahimi, R., Nikfar, S., Rezaie, A., and Abdollahi, M. (2009). Comparison of mesalazine and balsalazide in induction and maintenance of remission in patients with ulcerative colitis: a meta-analysis. Dig. Dis. Sci. 54, 712–721. doi:10.1007/s10620-008-0428-2
Shu, Y., Ding, Y., Liu, Y., Wu, P., He, X., and Zhang, Q. (2022a). Post-marketing safety concerns with secukinumab: a disproportionality analysis of the FDA adverse event reporting system. Front. Pharmacol. 13, 862508. doi:10.3389/fphar.2022.862508
Shu, Y., He, X., Liu, Y., Wu, P., and Zhang, Q. (2022b). A real-world disproportionality analysis of olaparib: data mining of the public version of FDA adverse event reporting system. Clin. Epidemiol. 14, 789–802. doi:10.2147/CLEP.S365513
Singh, S., Garg, S., Pardi, D., Wang, Z., Murad, M., and Loftus, E. (2015). Comparative efficacy of pharmacologic interventions in preventing relapse of Crohn's disease after surgery: a systematic review and network meta-analysis. Gastroenterology 148, 64–76. e2; quiz e14. doi:10.1053/j.gastro.2014.09.031
Słoka, J., Madej, M., and Strzalka-Mrozik, B. (2023). Molecular mechanisms of the antitumor effects of mesalazine and its preventive potential in colorectal cancer. Molecules 28, 5081. doi:10.3390/molecules28135081
Steinhart, A., Hemphill, D., and Greenberg, G. (1994). Sulfasalazine and mesalazine for the maintenance therapy of Crohn's disease: a meta-analysis. Am. J. gastroenterology 89, 2116–2124.
Suzuki, K., Kakuta, Y., Naito, T., Takagawa, T., Hanai, H., Araki, H., et al. (2022). Genetic background of mesalamine-induced fever and diarrhea in Japanese patients with inflammatory bowel disease. Inflamm. Bowel Dis. 28, 21–31. doi:10.1093/ibd/izab004
Veloso, P. M., Machado, R., and Nobre, C. (2021). Mesalazine and inflammatory bowel disease - from well-established therapies to progress beyond the state of the art. Eur. J. Pharm. Biopharm. 167, 89–103. doi:10.1016/j.ejpb.2021.07.014
Xie, F., Li, S., Fan, Y., Li, W., Lv, Q., Sun, X., et al. (2022). Efficacy and safety of bifidobacterium quadruple viable bacteria combined with mesalamine against UC management: a systematic review and meta-analysis. Oxidative Med. Cell. Longev. 2022, 8272371. doi:10.1155/2022/8272371
Yang, Z., Ye, X., Wu, Q., Wu, K., and Fan, D. (2014). A network meta-analysis on the efficacy of 5-aminosalicylates, immunomodulators and biologics for the prevention of postoperative recurrence in Crohn's disease. Int. J. Surg. Lond. Engl. 12, 516–522. doi:10.1016/j.ijsu.2014.02.010
Keywords: mesalazine, adverse event, FAERS, pharmacovigilance, data mining
Citation: Liu M, Gu L, Zhang Y, Zhou H, Wang Y and Xu Z-X (2024) A real-world disproportionality analysis of mesalazine data mining of the public version of FDA adverse event reporting system. Front. Pharmacol. 15:1290975. doi: 10.3389/fphar.2024.1290975
Received: 08 September 2023; Accepted: 18 January 2024;
Published: 31 January 2024.
Edited by:
Tin Wui Wong, Universiti Teknologi MARA Puncak Alam, MalaysiaReviewed by:
Linling Que, Wuxi People’s Hospital, ChinaMara Medeiros, Federico Gómez Children’s Hospital, Mexico
Copyright © 2024 Liu, Gu, Zhang, Zhou, Wang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Honglan Zhou, aGx6aG91QGpsdS5lZHUuY24=; Yishu Wang, d2FuZ3lzQGpsdS5lZHUuZW4=; Zhi-Xiang Xu, emhpeGlhbmd4dUBqbHUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Mingdi Liu
Mingdi Liu Liting Gu
Liting Gu Yuning Zhang
Yuning Zhang Honglan Zhou
Honglan Zhou Yishu Wang
Yishu Wang Zhi-Xiang Xu
Zhi-Xiang Xu