Abstract
Background: It is still uncertain whether Nanoparticle albumin-bound paclitaxel (nab-paclitaxel) and programmed cell death protein 1 (PD-1) inhibitor have synergistic effects on metastatic soft tissue sarcomas (STSs). The purpose of this study was to evaluate the safety and activity of nab-paclitaxel plus camrelizumab (a PD-1 inhibitor) in patients with advanced STS who had previously failed chemotherapy.
Methods: In this single-center, open-label, single-arm phase II clinical trial, patients with advanced (unresectable or metastatic) STS who had previously failed chemotherapy received up to six cycles of nab-paclitaxel plus camrelizumab, whereas camrelizumab treatment was continued for up to 1 year. The median progression-free survival (PFS), objective response rate (ORR) and safety were collected and evaluated.
Results: This trial included 40 patients (28 men and 12 women). The overall ORR was 22.5%, and the median PFS was 1.65 months (95% confidence interval [CI], 1.3–2.0 months). Patients with epithelioid sarcoma demonstrated a longer PFS compared with those with other histological subtypes (2.3 months vs. 1.5 months, respectively); however, this difference was not significant. Patients who had received only one line of previous chemotherapy had a significantly longer PFS compared with those who had undergone two or more lines of previous chemotherapy (2.8 months vs. 1.3 months, respectively, p = 0.046). In terms of safety, the toxicity of this combination therapy is mild and no serious adverse events have occurred.
Conclusion: Nab-paclitaxel plus camrelizumab exhibited modest activity and mild toxicity in treating epithelioid sarcoma, angiosarcoma, and fibrosarcoma. The overall effectiveness of this treatment regimen for advanced STS is relatively low. Further research on combining nab-paclitaxel with effective drugs, including chemotherapy and targeted agents, for these specific STS subtypes is needed.
1 Introduction
Soft tissue sarcomas (STSs) are malignancies with a low incidence rate (approximately four per 100000 people) and high heterogeneity (>70 subtypes) (Yang et al., 2019; WHO Classification of Tumours, 2020; Buja et al., 2023). Moreover, approximately 50% of STS cases eventually progress to late stages. Traditionally, the main treatment method for advanced STS is chemotherapy, with first-line and second-line chemotherapy regimens including doxorubicin and docetaxel plus gemcitabine (Tian and Yao, 2023; von Mehren et al., 2020; George, 2019). However, the objective response rate (ORR) for this regimen is approximately 20%, and the median overall survival for patients with advanced STS is approximately 12 months (Tian and Yao, 2023). Therefore, effective treatment strategies are needed.
Nanoparticle albumin-bound paclitaxel (nab-paclitaxel) is a anticancer drug of taxane family (Yared and Tkaczuk, 2012; Kudlowitz and Muggia, 2014). It is a nano-sized paclitaxel, and has higher water solubility and bioavailability, lower toxicity, and improved antitumor efficacy compared with the two main taxanes (traditional paclitaxel and docetaxel) (Tian and Yao, 2022a; Mercatali et al., 2022). Nab-paclitaxel has been used to treat many types of cancer. In addition, recent reports have shown that it is effective in treating STSs (Tian et al., 2022a; Tian and Yao, 2022a).
Programmed cell death protein 1 (PD-1) inhibitors are the most widely used immunotherapy drugs in anticancer therapy, and they also have been used as novel antitumor therapies in the treatment and research of STS (Saerens et al., 2021). Despite recent evidence suggesting the low efficacy of PD-1 inhibitor monotherapy in STSs, there are promising reports of its effectiveness in some histological subtypes of sarcoma (Baldi et al., 2022; Kerrison et al., 2022). In addition, in order to improve the efficacy of PD-1 inhibitor, combination chemotherapy has been proven to be a promising method for treating malignancies (including STS) (Tian and Yao, 2022b).
Nab-paclitaxel plus PD-1 inhibitor has achieved promising results in the treatment of various types of cancer (Li et al., 2021; An et al., 2023; Sonoda et al., 2023; Yin et al., 2023; Zhang et al., 2023). However, clinical trials evaluating the efficacy and safety of this combination for STS treatment have not been reported. We have carry out a single-center, open-label, single-arm phase II clinical trial that used nab-paclitaxel plus camrelizumab (a PD-1 inhibitor) as a second-line treatment for metastatic or locally unresectable STS. We report the results of this trial here, and hoped to provide references for the treatment and clinical research for patients with STS.
2 Materials and methods
2.1 Patients
In this trial, the effects of nab-paclitaxel plus camrelizumab as a second or subsequent line of therapy for advanced STS were assessed. All patients received nab-paclitaxel plus camrelizumab between January 2022 and March 2023. The main eligibility criteria included: 1) age ≥18 years and <70 years, 2) histologically proven STS [include undifferentiated pleomorphic sarcoma (UPS), leiomyosarcoma, angiosarcoma, synovial sarcoma, clear cell sarcoma, epithelioid sarcoma, fibrosarcoma, malignant peripheral nerve sheath tumor (MPNST) and undifferentiated or poorly differentiated liposarcoma], 3) an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, 4) locally unresectable or multiple metastatic disease, 5) failure of previous chemotherapy, 6) acceptable hematological, renal and hepatic functions, 7) measurable lesions according to the response evaluation criteria in solid tumors (RECIST; version 1.1).
This study was approved by the Ethics Committee of the Henan Cancer Hospital and registered at ClinicalTrials.gov (NCT05189483). Written informed consent was obtained from all patients. This trial was performed in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki.
2.2 Treatment protocol
All patients received 260 mg/m2 of nab-paclitaxel (Hengrui Pharmaceutical, Lianyungang, China), via a 30-min intravenous infusion on day 1, and repeated every 3 weeks for up to six cycles or until the occurrence of progressive disease (PD) or unacceptable adverse events (AEs). All patients received 200 mg of camrelizumab (Hengrui Pharmaceutical, Lianyungang, China) via a 30-min intravenous infusion on day 1, and repeated every 3 weeks for up to 1 year unless there was PD or unacceptable AEs. Treatment could be delayed for a maximum of 2 weeks in the case of grade 3 or 4 AEs.
2.3 Evaluations
Patient demographics and characteristics were recorded. The RECIST (version 1.1) was used to assessed Activity. During the first 16 cycles, tumor imaging assessments were conducted every two cycles or immediately after obtaining a clear evidence of PD; Afterwards, tumor imaging assessments will be conducted every four cycles. Safety was evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). The main indicators included AEs and immune-related AEs (irAEs). The safe follow-up period for the subjects starts from the last dose and follows up every 30 days until 90 days after the last dose.
2.4 Statistical analysis
Perform all statistical analysis using SPSS (version 21.0; SPSS Inc., Chicago, Illinois, United States of America). The corresponding figures were drawn using Graph Prism 5.0 (GraphPad Software, San Diego, CA, United States of America). Two-tailed tests were performed at a significance level of α = 0.05, with p < 0.05 indicative of statistical significance. Subgroup comparisons of count date were performed using the chi-square test or Fisher’s exact test. The relationship between the variables and survival was assessed using Kaplan-Meier curves and the log-rank test’s subgroup differences in survival were assessed. The follow-up period was extended to 30 September 2023.
3 Results
3.1 Patient characteristics
From January 2022 to March 2023, a total of 40 patients with unresectable or metastatic STS were enrolled in this study, with 28 men and 12 women. The average age of the patients was 49.28 ± 14.17 years (Table 1). All patients had an ECOG performance status of 0 or 1. The histological subtypes included UPS (n = 10), epithelioid sarcoma (n = 8), fibrosarcoma (n = 7), angiosarcoma (n = 5), myxofibrosarcoma (n = 3), MPNST (n = 2), leiomyosarcoma (n = 2), synovial sarcoma (n = 2), and differentiated liposarcoma (n = 1). The primary tumor sites were predominantly the limbs, followed by the trunk. Most patients experienced lung metastases, with a small number (12.5%) of them being locally unresectable. All patients had previously received at least one line of chemotherapy (Table 1).
TABLE 1
| Characteristics | No. (%) (n = 40) |
|---|---|
| Gender | |
| Female | 12 (30.0%) |
| Male | 28 (70.0%) |
| Age | 49.28 ± 14.17 |
| ECOG PS | |
| 0 | 20 (50.0%) |
| 1 | 20 (50.0%) |
| Histology | |
| UPS | 10 (25.0%) |
| Epithelioid sarcoma | 8 (20.0%) |
| Fibrosarcoma | 7 (17.5%) |
| Angiosarcoma | 5 (12.5%) |
| Myxofibrosarcoma | 3 (7.5%) |
| MPNST | 2 (5%) |
| Leiomyosarcoma | 2 (5%) |
| Synovial sarcoma | 2 (5%) |
| Dedifferentiated liposarcoma | 1 (2.5%) |
| Primary site | |
| Extremities | 26 (65.0%) |
| Trunk | 13 (32.5%) |
| Head | 1 (2.5%) |
| Stage | |
| IV | 35 (87.5%) |
| Locally unresectable | 5 (12.5%) |
| Metastatic site | |
| lungs | 32 (80.0%) |
| other | 3 (7.5%) |
| Lines of previous systemic therapy | |
| 1 | 11 (27.5%) |
| 2 | 15 (37.5%) |
| 3 | 14 (35.5%) |
Clinical characteristics of patients in this study.
Abbreviations: ECOG PS, eastern cooperative oncology group performance status; MPNST, malignant peripheral nerve sheath tumor; UPS, undifferentiated pleomorphic sarcoma.
3.2 Activity of treatment
Among the 40 patients, 1 case of CR (UPS) and 8 cases of PR (3 epithelioid sarcomas, two fibrosarcomas, two angiosarcomas, and one dedifferentiated liposarcoma) were identified (Table 2; Figure 1). The ORR, DCR, median PFS, and 4-month PFS rates were 22.5%, 50%, 1.65 months, and 7.5%, respectively (Table 3 and Figure 1).
TABLE 2
| Histology | Number of patients | |||
|---|---|---|---|---|
| CR | PR | SD | PD | |
| UPS (n = 10) | 1 | 0 | 3 | 6 |
| Epithelioid sarcoma (n = 8) | 0 | 3 | 2 | 3 |
| Fibrosarcoma (n = 7) | 0 | 2 | 2 | 3 |
| Angiosarcoma (n = 5) | 0 | 2 | 1 | 2 |
| Myxofibrosarcoma (n = 3) | 0 | 0 | 1 | 2 |
| MPNST (n = 2) | 0 | 0 | 1 | 1 |
| Leiomyosarcoma (n = 2) | 0 | 0 | 1 | 1 |
| Synovial sarcoma (n = 1) | 0 | 0 | 0 | 2 |
| Dedifferentiated liposarcoma (n = 1) | 0 | 1 | 0 | 0 |
| Total | 1 | 8 | 11 | 20 |
Efficacy of various histological subtypes.
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; MPNST, malignant peripheral nerve sheath tumor; UPS, undifferentiated pleomorphic sarcoma.
FIGURE 1

(A) Waterfall plot illustrating the maximum reduction in target lesion size from baseline, evaluated according to the response evaluation criteria for solid tumors (version 1.1). (B) Line plot showing the duration of response of target lesions from baseline. Abbreviations: MPNST, malignant peripheral nerve sheath tumor; UPS, undifferentiated pleomorphic sarcoma.
TABLE 3
| Characteristics | Data |
|---|---|
| ORR (%) | 22.50 (95% CI: 10.84–38.45) |
| DCR (%) | 50.00 (95% CI: 33.80–66.20) |
| Median-PFS (months) | 1.65 (95%CI: 1.30–2.00) |
| 4-month PFS rate (%) | 7.50 (95%CI: 1.94–18.24) |
| 6-month PFS rate (%) | 2.50 (95%CI: 0.20–11.27) |
Efficacy of all patients to the treatment.
Abbreviations: ORR, objective response rate; DCR, disease control rate; PFS, progression-free survival; CI, confidence interval.
Patients with epithelioid sarcoma had a longer PFS than those with other histological subtypes (2.3 months vs. 1.5 months, respectively); however, this difference was not significant (Figure 2). Patients who had received only one line of previous chemotherapy had a significantly longer PFS compared with those who had undergone two or more lines of previous chemotherapy (2.8 months vs. 1.3 months, respectively, p = 0.046) (Figure 2).
FIGURE 2
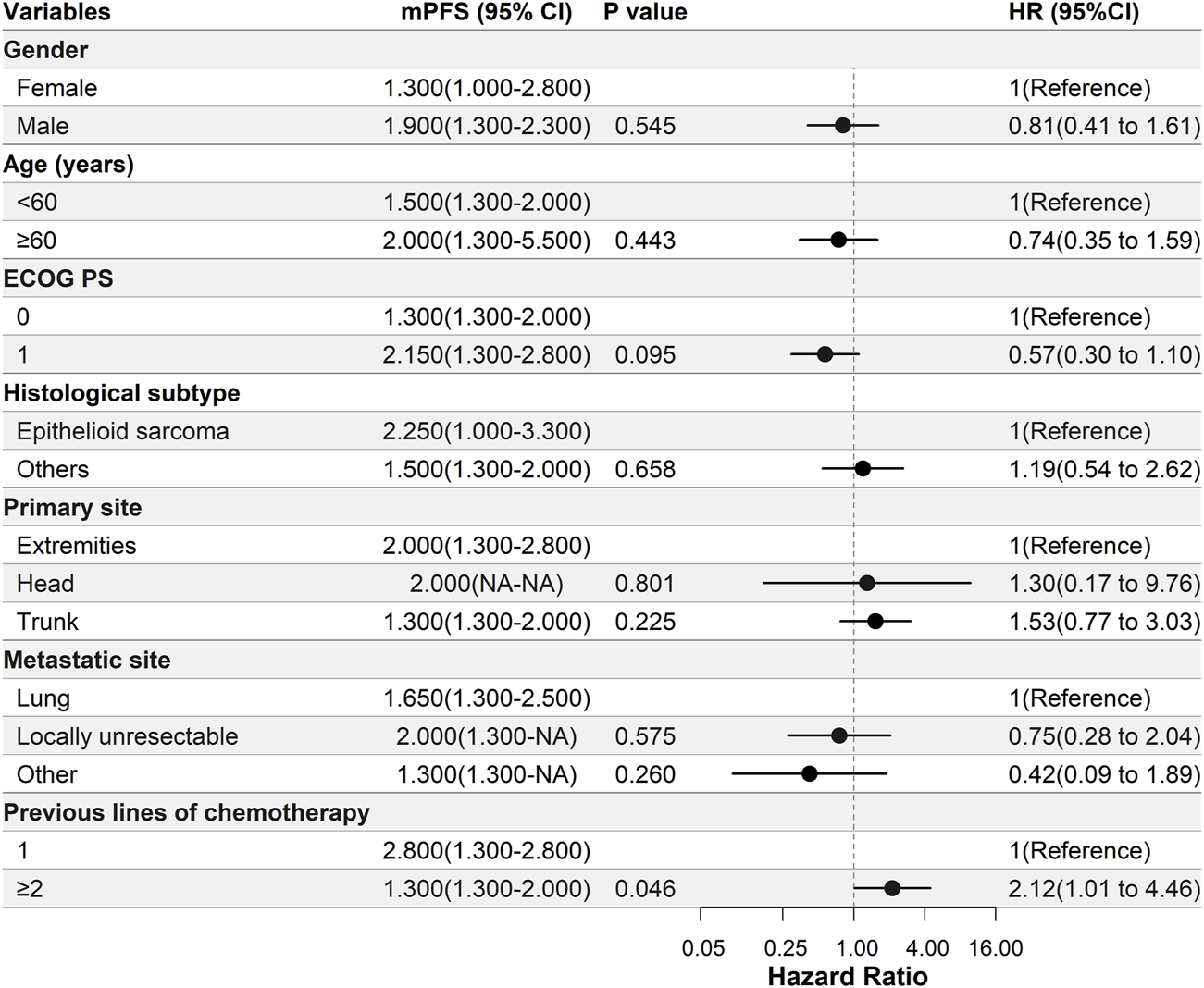
Univariate Cox regression analysis of the relationship between clinical parameters and progression-free survival (PFS). In this study, patients with epithelioid sarcoma had a longer PFS than those with other histological subtypes; however, there was no significant difference. Patients who underwent one line of previous chemotherapy had a significantly longer PFS compared with those who had undergone two or more lines of previous chemotherapy. Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; mPFS, median progression-free survival.
3.3 Toxicity and safety
In general, nab-paclitaxel plus camdelizumab was well-tolerated (Table 4). Most AEs were associated with nab-paclitaxel treatment. The most common grade 1–2 AEs were alopecia (89.3%, 25/28), lymphocytopenia (75.0%, 30/40), leukopenia (55.0%, 22/40), anemia (32.5%, 13/40), and nausea (22.5%, 9/40). The most common grade 3 AEs were lymphocytopenia (22.5%; 9/40) and leucopenia (15.0%, 6/40). No grade 4 AEs were observed. IrAEs were mild and were of only two types: hyperthyroidism (15%, 6/40) and rash (5.0%, 2/40). No patient needed to reduce the dosage of nab-paclitaxel or PD-1 inhibitor due to AEs, and there were no treatment-related deaths.
TABLE 4
| Adverse events | All grades | Grade 3–4 |
|---|---|---|
| Alopecia | 90.0% (36/40) | |
| Lymphocytopenia | 75.0% (30/40) | 22.5% (9/40) |
| Leucopenia | 55.0% (22/40) | 15.0% (6/40) |
| Anemia | 32.5% (13/40) | 2.5% (1/40) |
| Nausea | 22.5% (9/40) | |
| Numbness of limbs | 20.0% (8/40) | 2.5% (1/40) |
| Pain | 20.0% (8/40) | 2.5% (1/40) |
| Thrombocytopenia | 17.5% (7/40) | 2.5% (1/40) |
| Fatigue | 17.5% (7/40) | |
| Transaminase increase | 17.5% (7/40) | |
| Anorexia | 15.0% (6/40) | |
| Hypothyroidism | 12.5% (5/40) | 2.5% (1/40) |
| Diarrhea | 12.5% (5/40) | |
| Fever | 7.5% (3/40) | |
| Rash | 5.0% (2/40) |
Adverse events.
4 Discussion
The study aimed to assess the activity and safety of nab-paclitaxel in combination with a PD-1 inhibitor, camrelizumab, as a second or subsequent line of therapy for advanced STS. Our results revealed noteworthy findings regarding this treatment approach.
Evidence suggests that chemotherapy can enhance the anti-tumor effects of PD-1 inhibitor by reducing the number of tumor cells, promoting immunogenic tumor cell death, consuming immunosuppressive cells, increasing the number and activity of anti-tumor immune effector cells, and enhancing the secretion of cytokines that promote immune cell proliferation (Principe et al., 2022; Zhu et al., 2022). Nab-paclitaxel is an immunogenic cell death inducer that has been shown to enhance the efficacy of PD-1 inhibitors by regulating various immune functions (Li et al., 2021; Yoneshima et al., 2021). Currently, chemotherapy combined with PD-1 inhibitor has been approved for the treatment of gastroesophageal, lung and breast cancers (Tian and Yao, 2022b; Principe et al., 2022).
In this study, although an ORR of 22.5%, comparable to that achieved with doxorubicin plus PD-1 inhibitors, was achieved (Pollack et al., 2020; Livingston et al., 2021; Tian et al., 2022b), the median PFS was only 1.65 months. This indicates that the treatment only elicited therapeutic effects for a brief initial period, and that there is no synergistic effect. Notably, a CR was observed in a patient with UPS. This response may be due to the well-documented sensitivity of UPS to PD-1 inhibitors, as indicated in previous studies (Moreno Tellez et al., 2022). The efficacy in the other histological subtypes can be attributed to nab-paclitaxel. Although the overall treatment effect is not satisfactory, this regimen exhibited therapeutic effects against epithelioid sarcoma, angiosarcoma, and fibrosarcoma, consistent with previous studies (Tian et al., 2022a). Owing to the observed short PFS for these STS histological subtypes, the use of nab-paclitaxel alone or in combination with a PD-1 inhibitor is not recommended for advanced STS treatment. Instead, consideration should be given to exploring combinations involving nab-paclitaxel with other effective drugs, such as chemotherapeutic and targeted drugs, for these subtypes.
In terms of safety, our findings revealed a relatively low incidence of AE, with rare occurrences of grade 3–4 AEs. This indicates that the combination of nab-paclitaxel and a PD-1 inhibitor is safe, consistent with previous studies (Hao et al., 2023), and notably better than first-line chemotherapy for STS (Gronchi et al., 2017; Seddon et al., 2017). This safety profile can greatly enhance patient satisfaction and quality of life during treatment. Given the lower incidence of AEs, considering the addition of other drugs, such as targeted drugs or chemotherapy drugs, to the protocol used in this study may improve treatment effectiveness.
Our study has certain limitations, including the absence of a control group, a relatively small number of patients, and a short follow-up period. Despite these limitations, this study demonstrated the limited efficacy of nab-paclitaxel plus camrelizumab in treating STSs. Non-etheless, nab-paclitaxel shows promise in treating epithelioid sarcoma, angiosarcoma, and fibrosarcoma. Therefore, further research investigating the use of nab-paclitaxel in combination with other effective drugs, such as chemotherapy and targeted drugs, for these specific subtypes of STSs is warranted.
5 Conclusion
Nab-paclitaxel plus camrelizumab exhibited modest activity and mild toxicity in treating epithelioid sarcoma, angiosarcoma, and fibrosarcoma. The overall effectiveness of this treatment regimen for advanced STS is relatively low. Further research on combining nab-paclitaxel with effective drugs, including chemotherapy and targeted agents, for these STS subtypes is needed.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Henan Cancer Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
ZT: Conceptualization, Investigation, Methodology, Writing–original draft, Writing–review and editing. YF: Conceptualization, Methodology, Software, Writing–review and editing. YaY: Data curation, Formal Analysis, Methodology, Software, Writing–review and editing. XL: Investigation, Writing–original draft. GQ: Investigation, Writing–review and editing. YoY: Methodology, Resources, Writing–review and editing. XW: Investigation, Resources, Writing–review and editing. JW: Investigation, Resources, Writing–review and editing. PZ: Investigation, Resources, Writing–review and editing. WY: Conceptualization, Data curation, Investigation, Methodology, Resources, Software, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Jiangsu HengRui Medicine Co. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Nomenclature
| PD-1 | programmed cell death protein 1 |
| STS | soft tissue sarcoma |
| UPS | undifferentiated pleomorphic sarcoma |
| MPNST | malignant peripheral nerve sheath tumor |
| ECOG | Eastern Cooperative Oncology Group |
| RECIST | response evaluation criteria in solid tumors |
| AEs | adverse events |
References
1
An J. Li X. Wang J. Zhu L. An R. Jiang K. et al (2023). Efficacy and safety of serplulimab plus nab-paclitaxel in previously treated patients with PD-L1-positive advanced cervical cancer: a phase II, single-arm study. Front. Immunol.14, 1142256. 10.3389/fimmu.2023.1142256
2
Baldi G. G. Gronchi A. Tazzari M. Stacchiotti S. (2022). Immunotherapy in soft tissue sarcoma: current evidence and future perspectives in a variegated family of different tumor. Expert Rev. Anticancer Ther.22 (5), 491–503. 10.1080/14737140.2022.2065986
3
Buja A. Rugge M. Tropea S. Cozzolino C. Formaro C. M. Grotto G. et al (2023). Sex differences in soft tissue sarcoma: incidence, clinicopathological profile, survival, and costs. J. Womens Health (Larchmt)32, 1257–1264. 10.1089/jwh.2023.0019
4
George S. (2019). Developments in systemic therapy for soft tissue and bone sarcomas. J. Natl. Compr. Canc Netw.17 (5.5), 625–628. 10.6004/jnccn.2019.5020
5
Gronchi A. Ferrari S. Quagliuolo V. Broto J. M. Pousa A. L. Grignani G. et al (2017). Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol.18 (6), 812–822. 10.1016/S1470-2045(17)30334-0
6
Hao W. Zhang J. Wang Y. Fang B. Jin S. Yuan J. et al (2023). Immune-related adverse events associated with nab-paclitaxel/paclitaxel combined with immune checkpoint inhibitors: a systematic review and network meta-analysis. Front. Immunol.14, 1175809. 10.3389/fimmu.2023.1175809
7
Kerrison W. G. J. Lee A. T. J. Thway K. Jones R. L. Huang P. H. (2022). Current status and future directions of immunotherapies in soft tissue sarcomas. Biomedicines10 (3), 573. 10.3390/biomedicines10030573
8
Kudlowitz D. Muggia F. (2014). Nanoparticle albumin-bound paclitaxel (nab-paclitaxel): extending its indications. Expert Opin. Drug Saf.13 (6), 681–685. 10.1517/14740338.2014.910193
9
Li J. J. Wang J. H. Dingv Y. Li D. D. Wen X. Z. Zhao J. J. et al (2021). Efficacy and safety of anti-PD-1 inhibitor combined with nab-paclitaxel in Chinese patients with refractory melanoma. J. Cancer Res. Clin. Oncol.148, 1159–1169. 10.1007/s00432-021-03700-9
10
Livingston M. B. Jagosky M. H. Robinson M. M. Ahrens W. A. Benbow J. H. Farhangfar C. J. et al (2021). Phase II study of pembrolizumab in combination with doxorubicin in metastatic and unresectable soft-tissue sarcoma. Clin. Cancer Res.27 (23), 6424–6431. 10.1158/1078-0432.CCR-21-2001
11
Mercatali L. Vanni S. Miserocchi G. Liverani C. Spadazzi C. Cocchi C. et al (2022). The emerging role of cancer nanotechnology in the panorama of sarcoma. Front. Bioeng. Biotechnol.10 (null), 953555. 10.3389/fbioe.2022.953555
12
Moreno Tellez C. Leyfman Y. D'Angelo S. P. Wilky B. A. Dufresne A. (2022). Immunotherapy in sarcoma: where do things stand?Surg. Oncol. Clin. N. Am.31 (3), 381–397. 10.1016/j.soc.2022.03.004
13
Pollack S. M. Redman M. W. Baker K. K. Wagner M. J. Schroeder B. A. Loggers E. T. et al (2020). Assessment of doxorubicin and pembrolizumab in patients with advanced anthracycline-naive sarcoma: a phase 1/2 nonrandomized clinical trial. JAMA Oncol.6 (11), 1778–1782. 10.1001/jamaoncol.2020.3689
14
Principe D. R. Kamath S. D. Korc M. Munshi H. G. (2022). The immune modifying effects of chemotherapy and advances in chemo-immunotherapy. Pharmacol. Ther.236, 108111. 10.1016/j.pharmthera.2022.108111
15
Saerens M. Brusselaers N. Rottey S. Decruyenaere A. Creytens D. Lapeire L. (2021). Immune checkpoint inhibitors in treatment of soft-tissue sarcoma: a systematic review and meta-analysis. Eur. J. Cancer152, 165–182. 10.1016/j.ejca.2021.04.034
16
Seddon B. Strauss S. J. Whelan J. Leahy M. Woll P. J. Cowie F. et al (2017). Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol.18 (10), 1397–1410. 10.1016/S1470-2045(17)30622-8
17
Sonoda T. Umeda Y. Demura Y. Tada T. Nakashima K. Anzai M. et al (2023). Efficacy and safety of nanoparticle albumin-bound paclitaxel monotherapy after immune checkpoint inhibitor administration for advanced non-small cell lung cancer: a multicenter Phase 2 clinical trial. Cancer Med.12 (12), 13041–13053. 10.1002/cam4.5978
18
Tian Z. Dong S. Yang Y. Gao S. Yang Y. Yang J. et al (2022a). Nanoparticle albumin-bound paclitaxel and PD-1 inhibitor (sintilimab) combination therapy for soft tissue sarcoma: a retrospective study. BMC Cancer22 (1), 56. 10.1186/s12885-022-09176-1
19
Tian Z. Dong S. Zuo W. Li P. Zhang F. Gao S. et al (2022b). Efficacy and safety of sintilimab plus doxorubicin in advanced soft tissue sarcoma: a single-arm, phase II trial. Front. Pharmacol.13, 987569. 10.3389/fphar.2022.987569
20
Tian Z. Yao W. (2022a). Albumin-bound paclitaxel: worthy of further study in sarcomas. Front. Oncol.12, 815900. 10.3389/fonc.2022.815900
21
Tian Z. Yao W. (2022b). PD-1/L1 inhibitor plus chemotherapy in the treatment of sarcomas. Front. Immunol.13, 898255. 10.3389/fimmu.2022.898255
22
Tian Z. Yao W. (2023). Chemotherapeutic drugs for soft tissue sarcomas: a review. Front. Pharmacol.14, 1199292. 10.3389/fphar.2023.1199292
23
von Mehren M. Kane J. M. Bui M. M. Choy E. Connelly M. Dry S. et al (2020). NCCN guidelines insights: soft tissue sarcoma, version 1.2021. J. Natl. Compr. Canc Netw.18 (12), 1604–1612. 10.6004/jnccn.2020.0058
24
WHO Classification of Tumours (2020). Soft tissue and bone. 5th ed, 3. Lyon, France: IARC Press, 368.
25
Yang Z. Zheng R. Zhang S. Zeng H. Li H. Chen W. (2019). Incidence, distribution of histological subtypes and primary sites of soft tissue sarcoma in China. Cancer Biol. Med.16 (3), 565–574. 10.20892/j.issn.2095-3941.2019.0041
26
Yared J. A. Tkaczuk K. H. (2012). Update on taxane development: new analogs and new formulations. Drug Des. Devel Ther.6, 371–384. 10.2147/DDDT.S28997
27
Yin C. Zou G. R. He Y. Li J. Yan H. W. Su Z. et al (2023). Efficiency and toxicity of nab-paclitaxel and camrelizumab in the second or above line treatment of advanced non-small cell lung cancer: a retrospective cohort study. J. Thorac. Dis.15 (4), 1838–1847. 10.21037/jtd-23-387
28
Yoneshima Y. Morita S. Ando M. Nakamura A. Iwasawa S. Yoshioka H. et al (2021). Phase 3 trial comparing nanoparticle albumin-bound paclitaxel with docetaxel for previously treated advanced NSCLC. J. Thorac. Oncol.16 (9), 1523–1532. 10.1016/j.jtho.2021.03.027
29
Zhang G. Yuan J. Pan C. Xu Q. Cui X. Zhang J. et al (2023). Multi-omics analysis uncovers tumor ecosystem dynamics during neoadjuvant toripalimab plus nab-paclitaxel and S-1 for esophageal squamous cell carcinoma: a single-center, open-label, single-arm phase 2 trial. EBioMedicine90, 104515. 10.1016/j.ebiom.2023.104515
30
Zhu C. Shi Y. Li Q. Luo L. Li X. Luo Z. et al (2022). Rational administration sequencing of immunochemotherapy elicits powerful anti-tumor effect. J. Control Release341, 769–781. 10.1016/j.jconrel.2021.12.022
Summary
Keywords
nab-paclitaxel, camrelizumab, PD-1 inhibitor, sarcoma, immunochemotherapy
Citation
Tian Z, Feng Y, Yang Y, Liu X, Qu G, Yang Y, Wang X, Wang J, Zhang P and Yao W (2024) Combining nanoparticle albumin-bound paclitaxel with camrelizumab in advanced soft tissue sarcoma: activity, safety, and future perspectives. Front. Pharmacol. 15:1335054. doi: 10.3389/fphar.2024.1335054
Received
08 November 2023
Accepted
15 January 2024
Published
01 February 2024
Volume
15 - 2024
Edited by
Hong Duan, Sichuan University, China
Reviewed by
Milena Villarroel, Hospital Luis Calvo Mackenna, Chile
Alessandro De Vita, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), Italy
Updates
Copyright
© 2024 Tian, Feng, Yang, Liu, Qu, Yang, Wang, Wang, Zhang and Yao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weitao Yao, ywt00001@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.