- 1Institute of Molecular Physiology and Genetics, Centre of Biosciences, Slovak Academy of Sciences, Bratislava, Slovakia
- 2Institute of Experimental Endocrinology, Biomedical Research Center, Slovak Academy of Sciences, Bratislava, Slovakia
Background: There is growing evidence that the treatment of several mental disorders can potentially benefit from activation of delta-opioid receptors. In the future, delta-agonists with a safe pharmacological profile can be used for the treatment of mood disorders in pregnant women. However, the data on prenatal exposure to delta-opioid agonists are missing. The present study is aimed to test the hypothesis that the activation of delta-opioid receptors during gravidity has positive effects on the behaviour accompanied by changes in glutamate and monoamine neurotransmission.
Methods: Gestating Wistar rats were chronically treated with a selective delta-agonist SNC80 or vehicle. Adult male and female offspring underwent novel object recognition (for the assessment of cognition) and open field (for the assessment of anxiety and habituation) tests, followed by in vivo electrophysiological examination of the activity of hippocampal glutamate and midbrain serotonin (5-HT) and dopamine neurons.
Results: We found that the maternal treatment with SNC80 did not affect the offspring’s anxiety, habituation, and 5-HT neuronal firing activity. Female offspring of SNC80-treated dams exhibited improved novelty recognition associated with decreased firing rate and burst activity of glutamate and dopamine neurons.
Conclusion: Maternal treatment with delta-opioid agonists during gestation may have a pro-cognitive effect on offspring without any negative effects on anxiety and habituation. The putative pro-cognitive effect might be mediated via mechanism(s) involving the firing activity of hippocampal glutamate and mesolimbic dopamine neurons.
1 Introduction
For a long time, psychopharmacotherapy during pregnancy has been a contradictory issue. It is hardly possible to get full evidence to decide, whether the treatment with psychotropic drugs represents a lower risk than the mental disease itself. This is particularly important for patients with depressive and anxiety disorders. American College of Obstetricians and Gynaecologists recommends using selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors in pregnancy, as first-line pharmacotherapy, due to their low rates of malformations (ACOG Practice Bulletin, 2008). Prescribed antidepressants cross the placenta, and they may increase the levels of monoamines in the brain (Nordeng et al., 2001; Laine et al., 2003). These can affect the functional development of the brain and the behaviour of the child. On the other hand, untreated depression can adversely affect maternal health and may lead to maternal-child relationship disturbances (Dubovicky et al., 2017; Idunkova et al., 2023). Therefore, an unmet need of pharmaco-psychiatry is the development of antidepressant and anxiolytic drugs with a safe profile.
There is growing evidence that the treatment of several mental disorders can potentially benefit from affecting opioid receptors. The endogenous opioid system may be dysregulated in depressive disorders and drugs targeting mu-, delta-, and kappa-opioid receptors have antidepressant potential. Of interest are the delta-opioid ligands because they have a different pharmacological profile than mu- and kappa-opioid agonists (Jelen et al., 2022). Indeed, preclinical studies demonstrate the ability of delta-opioid receptor agonists to induce positive effects on anxiety and depression-like behaviour (Saitoh et al., 2004; Jutkiewicz et al., 2005). We have shown in rats that chronic treatment with the delta-opioid agonist SNC80 induced mild anxiolytic effects and increased locomotion. The behavioural changes were accompanied by alterations in central neurotransmission as revealed by in vivo electrophysiology (Dremencov et al., 2023).
Moreover, the preclinical studies also suggest the ability of opioid receptor ligands to modulate cognitive processes (Jacobson et al., 2018). The mechanisms involved in the cognitive and other behavioural effects can be related to monoamines and other central neurotransmitters, neurogenesis, neuroplasticity, and neurotrophic factors (Jelen et al., 2023). It has been shown that delta-opioid agonists can increase the proliferation of hippocampal progenitor cells (Persson et al., 2003a), while the treatment with delta antagonists induced an opposite effect (Persson et al., 2003b). The positive effects of delta-opioid agonists on gene expression of brain-derived neurotrophic factor (BDNF), known to be important for processes of learning and memory, have also been described (Torregrossa et al., 2006).
Based on current preclinical and early clinical studies, new agonistic molecules acting at delta-opioid receptors are being developed with satisfactory antidepressant/anxiolytic effects without the risk of seizures and other significant adverse events (Jelen et al., 2022). In the future, such delta-agonists with a safe pharmacological profile can be used for the treatment of mood disorders in pregnant women. However, the data on prenatal exposure to delta-opioid agonists are missing. The present study is aimed to test the hypotheses that (i) the activation of delta-opioid receptors during gravidity has positive effects on the behaviour and (ii) behavioural changes are associated with alterations in glutamate and monoamine neurotransmission. We have focussed in glutamate neurons of the hippocampus, serotonin (5-HT) neurons of the dorsal raphe nucleus (DRN), and dopamine neurons of the ventral tegmental area (VTA) because of the key roles played by these neurons and brain areas in both cognitive and emotional behaviour (Lyon et al., 2012).
2 Methods
2.1 Drugs and chemicals
The SNC80 was purchased from Bio-Techne Ltd., Abingdon, United Kingdom, and other drugs and chemicals from Merck Life Science s. r.o, Bratislava, Slovakia. Chloral hydrate and urethane were dissolved in saline (0.9% sodium chloride). SNC80 was dissolved in 1M hydrochloric acid. The SNC80 solution was next diluted with saline (1:100) and adjusted to pH 7 with sodium hydroxide.
2.2 Animals
Nulliparous Wistar rats, weighting 200–220 g, were obtained from the Breeding Facility of the Centre of Experimental Medicine of the Slovak Academy of Sciences Dobra Voda, Slovakia). Animals were kept under standard conditions with a 12:12 h light/dark cycle (lights on at 7 a.m.), temperature (22°C ± 2°C), and humidity (55% ± 10%). All experimental procedures were approved by the Animal Health and Animal Welfare Division of the State Veterinary and Food Administration of the Slovak Republic (Permit number Ro 4103/18-221/3) and conformed to the Directive 2010/63/EU of the European Parliament.
2.3 Mating and SNC80 treatment
Female rats were mated with males in a ratio of 3:1. The presence of spermatozoa in vaginal smears was considered day zero of gestation. SNC80 (2.5 mg/kg/day) or vehicle (1M hydrochloric acid diluted with saline 1:100, pH ≈ 7) were injected subcutaneously, every day between days 11th and 19th of the gestation. There were 11 dams in each treatment group. The dose of SNC80 has been selected according to our previous dose-response study (Grinchii et al., 2023). Similarly, the period of gestation was chosen according to previous experiments with antidepressant drug treatments during gestation (Viñas-Noguera et al., 2022; Grinchii et al., 2024).
2.4 Offspring study design
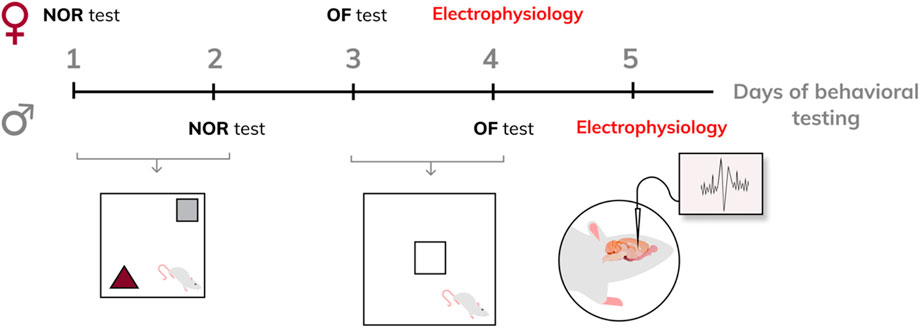
The offspring were weaned on postpartum day 21 and housed in litter groups of the same sex (four animals per cage). Rats reaching adulthood first underwent the novel object recognition (NOR) test. Forty-8 hours after the NOR test, the same animals were subjected to the open field (OF) test. Twenty-4 hours after the OF test, the same rats were used for in vivo electrophysiological recordings. At the end of electrophysiological measurements, the animals were euthanized by an overdose of urethane or chloralhydrate. A scheme of behavioral testing is provided in Figure 1.
2.5 Novel object recognition (NOR) test
To investigate learning and memory, adult offspring were subjected to the NOR test, as previously described (Hlavacova et al., 2015). The black plastic apparatus (Ekoplast, Telc, Czech Republic) consisted of a 50 cm × 50 cm square-shaped arena with 40 cm high walls was used. The arena was illuminated by dim light with an intensity of 50 lx. The two identical objects were placed in the arena before the test. Each animal was gently put in the corner of the arena and allowed to explore the objects for 5 min. After that, the rat was placed in a separate cage for 3 min while the apparatus and objects were cleaned with 20% ethanol and water. One of the familiar objects was replaced by a new one and the animal was placed back in the arena for another 5 min. The last 5 min of the test were evaluated in the computer program H77, Budapest, Hungary. The time spent exploring each object was recorded. Based on these values, the ratio of the time of recognition of a new object to the sum of the time of recognition of a new and an old object was calculated (recognition index).
2.6 The open field (OF) tests
The anxiety behaviour, locomotor activity and habituation processes were evaluated in adult offspring. The OF apparatus (Ekoplast, Telc, Czech Republic) which consisted of a rubber square area of 100 × 100 cm surrounded by a plastic 52 cm high walls was used for the performance of the OF tests. The illumination intensity was 40–45 lx in the central zone and 15–25 lx in the peripheral area of the apparatus. To begin the test, each rat was placed in the corner of the OF. The OF test lasted 15 min. The movement of the rat was continuously recorded using a video camera. The records were analyzed using the H77 program (Institute of Experimental Medicine, Budapest, Hungary).
The number of entries and time spent in the central zone of the OF were evaluated as measures of anxiety behavior. Four paws entering the central zone were defined as a central zone entry.
To assess the total locomotion activity and habituation processes, the OF arena was divided into 36 squares grid. The number of crossed squares was recorded. Average values of squares crossed in the first, second, and third 5-min periods of the test were calculated according to the approach published in our previous studies. Linear regression was used to evaluate the habituation course of locomotor activity. The individual rate of habituation (k-value) expresses the rapidity of habituation (Dubovicky et al., 1999; Dubovicky and Jezova 2004; Dremencov et al., 2023).
2.7 Electrophysiology in vivo
In vivo electrophysiological experiments described previously (Dremencov et al., 2022; Grinchii et al., 2022; Dremencov et al., 2023; Grinchii et al., 2023) were performed in male and female adult offspring of the SNC80- and vehicle-treated dams 24 h following behavioral testing. Urethane (1.25 g/kg, intraperitoneally: i. p.) or chloral hydrate (0.4 g/kg, i. p) anesthesia was used in experiments aiming to assess the excitability of hippocampal glutamate and brainstem monoamine neurons, respectively. The rats were mounted in the stereotaxic frame (David Kopf Instruments, Tujunga, CA), their scalp was opened, and a 3 mm hole was drilled in the skull for insertion of electrodes. Glass electrodes pulled with a DMZ-Universal Puller (Zeitz-Instruments GmbH, Martinsried, Germany) to a fine tip of ∼1 µM and filled with 2M sodium chloride (NaCl) The impedance of the electrodes was 4–6 MΩ. The electrodes were lowered through the Cornu Ammonis 1/3 (CA1/3) area of the hippocampus (3.9–4.2 mm posterior to bregma, 2.2–2.8 mm lateral to the midline, and 1.9–3.5 mm ventral to brain surface), DRN (7.8–8.3 mm posterior to bregma and 4.5–7.0 mm ventral to brain surface), or VTA (4.5–5.5 mm posterior to bregma, 0.6–0.8 mm lateral to the midline, and 7.0–8.5 mm ventral to the brain surface; Paxinos and Watson, 2014), using the hydraulic micro-positioner (David Kopf Instruments, Tujunga, CA). The action potentials generated by the neurons were recorded using the AD Instruments Extracellular Recording System (Dunedin, New Zealand). Glutamate neurons of the CA1/3, 5-HT neurons of the DRN, and dopamine neurons of the VTA were identified according to the waveform of their action potentials and the pattern of their generation (Figure 2), as explained in our previous works (Dremencov et al., 2022; Grinchii et al., 2022; Dremencov et al., 2023; Grinchii et al., 2023). During the experiment, the rats’ body temperature was maintained at 37°C with a heating pad (Gaymor Instruments, Orchard Park, NY, United States).
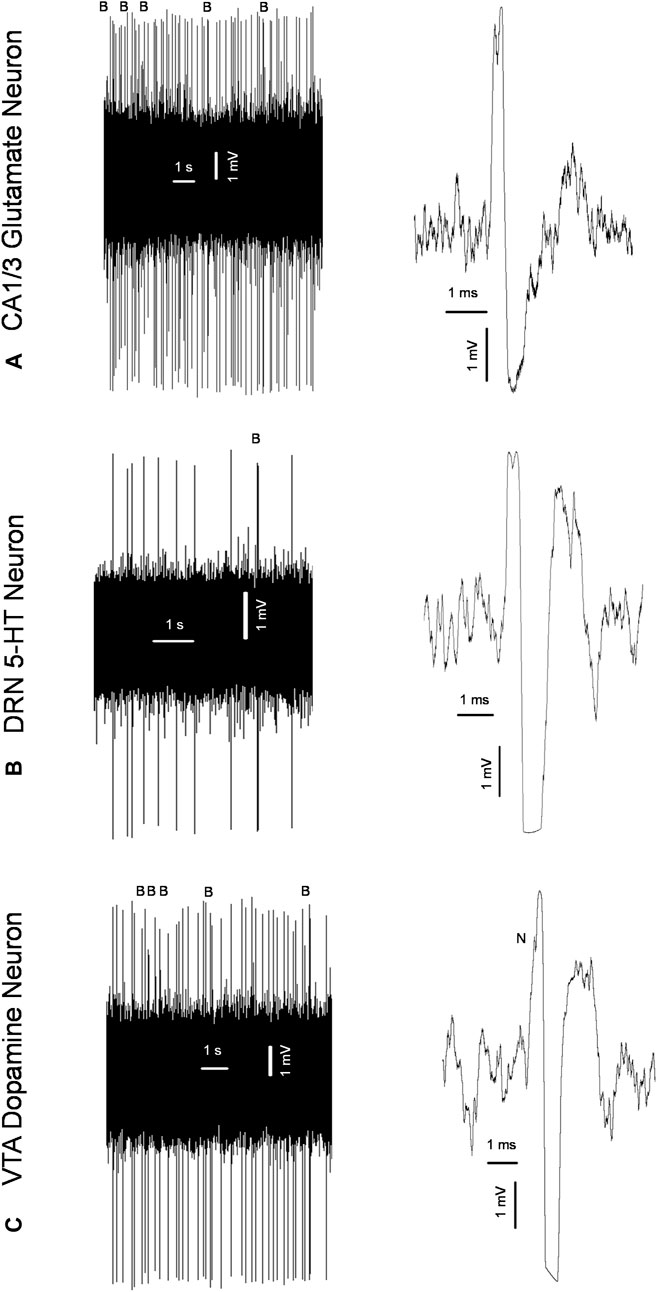
Figure 2. Original representative recordings from a glutamate neuron of the cornu ammonis-1/3 (CA1/3); (A) serotonin (5-HT) neuron of the dorsal raphe nucleus (DRN); (B) and dopamine neuron of the ventral tegmental area [VTA; (C)] from the present study, showing characteristic firing pattern (left) and waveform of the action potential (right) used for the identification of these neurons; B: bursts; N: “notch” during the rising phase of the action potential characteristic for dopamine neurons.
2.8 Data analysis
Action potentials (spikes) were detected using the spike sorting algorithm, with the version 6.02 of Spike2 software (Cambridge Electronic Design, Cambridge, United Kingdom). The neuronal firing rate and burst activity characteristics were calculated using the burstiDAtor software (www.github.com/nno/burstidator). The onset of the burst mode of firing and its termination were detected as previously described (Hajós et al., 2007; Csatlosova et al., 2021; Dremencov et al., 2022; Grinchii et al., 2022). Statistical assessments were performed using SigmaPlot 12.5 software (Systat Software Inc., Chicago, IL, United States). Two-way analysis of variance (ANOVA), for the factors sex (male versus female) and treatment (prenatal vehicle versus prenatal SNC80), followed by Bonferroni post hoc test, was used to assess the effects of the maternal SNC80 treatment and sex of the offspring on the excitability characteristics of the neurons and scores obtained in behavioural tests. Since behavior is heavily dependent on the perinatal environment, we performed the analysis by pooling offspring data from each litter and analyzing the average of each litter, to prevent confounding litter effects. For comparison, the results of statistical analyses using the values of individual animals are shown in Supplementary Material. In accordance with Wei and colleagues’ study (Wei et al., 2012), post hoc analysis was performed only when the ANOVA main effect of the corresponding factor or analysis or interaction between the factors were significant. Repeated measure ANOVA was used to evaluate the habituation process. The probability of p ≤ 0.05 was considered significant.
3 Results
The recognition index calculated in the offspring of SNC80-treated dams was higher than that calculated in the offspring of vehicle-treated controls in females but not males. Two-way ANOVA revealed a significant main effect of Treatment (F1,42 = 6.19, p = 0.02; data pooled per litters/dams), but no effect of sex and no sex × treatment interaction (Figure 3A). Bonferroni post hoc test revealed significant difference between prenatally SNC80- and vehicle-treated females (p = 0.03). Data based on individual animals used as the values for statistical analysis are shown in the Supplementary Figure S1A.
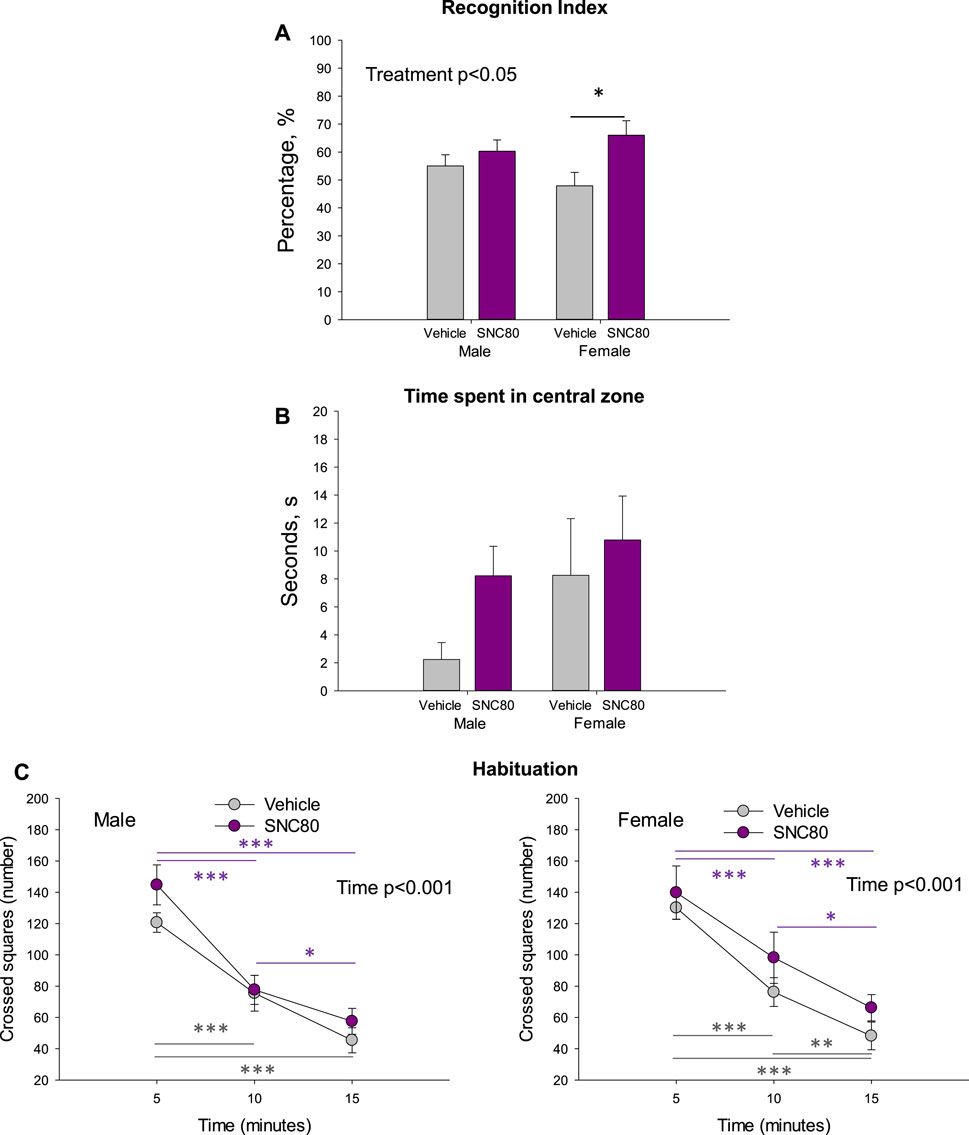
Figure 3. Novel object recognition index (A), time spend in central zone of the open field (B), and habituation behavior of the male and female offspring of the vehicle- or delta opioid agonist SNC80-treated dams (C); *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001, Bonferroni post hoc test. Were used as individual data points for statistical analysis. Are shown in the Supplementary Figure S1A.
Prenatal treatment with SNC80 failed to significantly modify anxiety behaviour as measured in the OF test (time spent in the central zone). When data was pooled per litters/dams, no effects of sex, treatment, or sex × treatment interaction were observed (Figure 3B). When the individual animals were used as data points for statistical analysis, two-way ANOVA showed a marginally significant main effect of sex (F1,36 = 3.991, p = 0.05), but still no effect of treatment and sex × treatment interaction (Supplementary Figure S1B). Female offspring regardless of treatment spent a longer time in the central zone of the OF than males.
Prentatal exposure to maternal SNC80 treatment failed to modify the rapidity of habituation (k-values) in the open field in the offspring (3C). By evaluating the number of squares crossed in the consecutive three 5-min time periods in the open field, two-way ANOVA for repeated measures (data polled per litters/dams) revealed a significant main effect of time (males: F1,44 = 83.13, p < 0.001; females: F1,29 = 88.12, p < 0.001) but no effect of treatment and or sex × treatment interaction. In all groups, the number of squares crossed decreased in time. Data based on individual animals used as the values for statistical analysis are shown in the Supplementary Figure S1C.
Figure 4 illustrates the excitability of glutamate neurons of the CA1/3 in male and female rats prenatally exposed to the vehicle or SNC80. Female (n = 36 neurons from five rats), but not male (n = 93 neurons from eight rats) offspring of SNC80-treated dams exhibited lower mean basal firing rate of CA1/3 glutamate neurons compared to that in offspring of the vehicle-treated controls (n = 64 neurons from six male and 100 neurons from 10 female rats; A). Two-way ANOVA showed significant effect of the treatment (F1,292 = 8.23, p = 0.004), significant sex × treatment interaction (F1,292 = 5.18, p = 0.02), but no effect of sex. Bonferroni post hoc test revealed significant difference between prenatally SNC80- and vehicle-treated females (p < 0.001) and between prenatally vehicle-treated males and females (p = 0.03) with higher values in female rats.
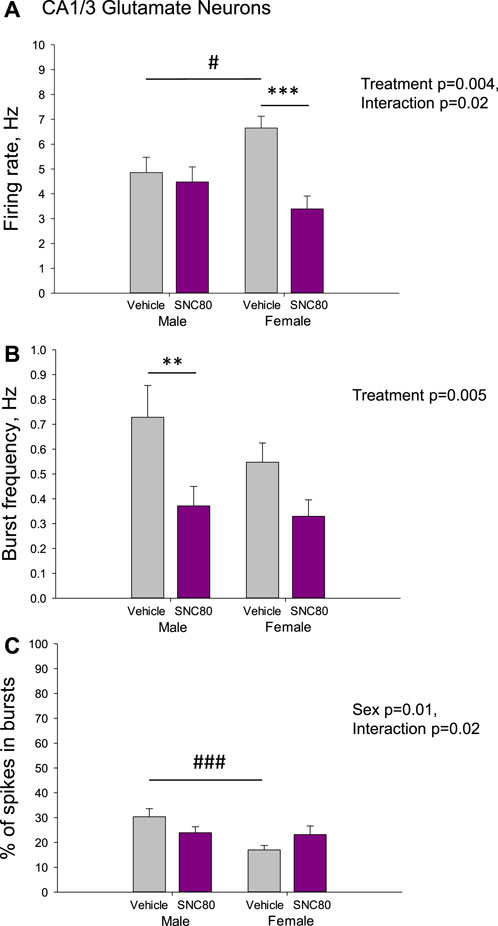
Figure 4. Firing rate (A) and characteristics of the burst firing (B, C) of glutamate neurons of the cornu ammonis-1/3 (CA1/3) in the male and female offspring of the vehicle- or delta opioid agonist SNC80-treated dams; **p ≤ 0.01 and ***p ≤ 0.001 in comparison with the same-sex offspring of the vehicle-treated dams; #p ≤ 0.05 and ###p ≤ 0.001 in comparison with the males of the same treatment group, Bonferroni post hoc test.
The frequency of the bursts was lower in offspring of SNC80-treated dams compared to that in offspring of vehicle-treated controls, but in male rats only (B). Two-way ANOVA showed significant main effect treatment (F1,249 = 8.17, p = 0.005), but no effect of sex or sex × treatment interaction. Bonferroni post hoc test revealed significant difference between prenatally SNC80- and vehicle-treated males (p = 0.007).
With respect to the percentage of spikes occurring in the bursts, this parameter was lower in female than in male offspring of the control dams and this sex difference disappeared in offspring of the SNC80-treated dams (C). Two-way ANOVA showed significant effect of sex (F1,249 = 6.59, p = 0.01) and sex × treatment interaction (F1,249 = 5.23, p = 0.02), but no effects of treatment. Bonferroni post hoc test revealed significant difference between prenatally vehicle-treated males and females (p < 0.001).
Figure 5 illustrates the excitability of dopamine neurons of the VTA in male and female rats prenatally exposed to vehicle or SNC80. Male (n = 77 neurons from six rats) and female (n = 40 neurons from five rats) offspring of SNC80-treated dams exhibited lower mean basal firing rate of VTA dopamine neurons, comparing to that in offspring of the vehicle-treated controls (n = 52 neurons from five male and 93 neurons from female 12 rats; A). Two-way ANOVA showed significant main effect of treatment (F1,261 = 15.58, p < 0.001), but no effect of sex and no sex × treatment interaction. Bonferroni post hoc test revealed significant difference between prenatally SNC80- and vehicle-treated males (p = 0.05) and between prenatally SNC80- and vehicle-treated females (p < 0.001).
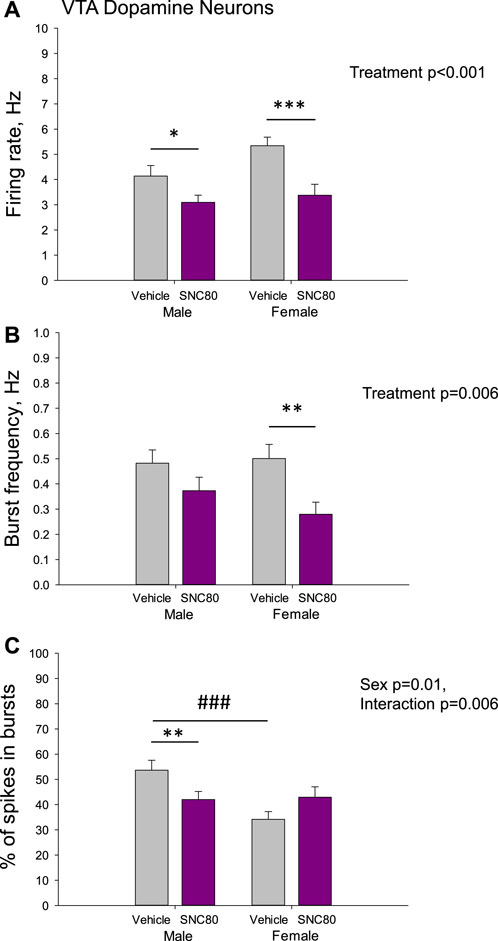
Figure 5. Firing rate (A) and characteristics of the burst firing (B, C) of dopamine neurons of the ventral tegmental area (VTA) in the male and female offspring of the vehicle- or delta opioid agonist SNC80-treated dams; *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001 in comparison with the same-sex offspring of the vehicle-treated dams; ###p ≤ 0.001 in comparison with the males of the same treatment group, Bonferroni post hoc test.
With respect to the burst frequency, it was lower in female, but not in male offspring of SNC80-treated dams, comparing to that in offspring of the vehicle-treated controls (B). Two-way ANOVA showed significant main effect of the treatment (F1,261 = 7.61, p = 0.006), but no effect of sex and no sex × treatment interaction. Bonferroni post hoc test revealed significant difference between prenatally SNC80- and vehicle-treated females (p = 0.01).
The percentage of spikes occurring in the burst was lower in the male, but not female offspring of SNC80-treated dams, comparing to that in offspring of the vehicle-treated controls(C). Two-way ANOVA showed significant effect of sex (F1,261 = 6.29, p = 0.01) and sex × treatment interaction (F1,261 = 7.58, p = 0.006), but no effect of treatment. Bonferroni post hoc test revealed significant difference between prenatally SNC80- and vehicle-treated males (p = 0.01), as well as between prenatally vehicle-treated males and females (p < 0.001) with higher values in males.
There were no differences in the mean basal firing rate of 5-HT neurons of the DRN between males and females or between the offspring of SNC80 (n = 71 neurons from 7 to 50 neurons from six rats, respectively)- and vehicle (n = 59 neurons from 9 to 36 neurons from four rats, respectively)-treated dams. The characteristics of the burst firing of 5-HT neurons, such as frequency of the bursts and percent of spikes occurring in the bursts, were not different between the sexes and between the treatment groups as well (Figure 6).
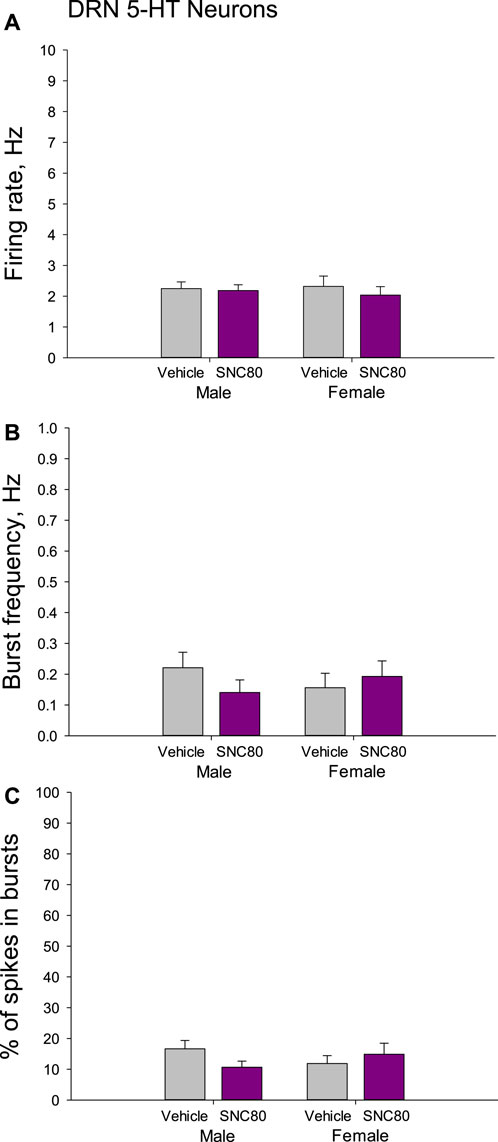
Figure 6. Firing rate (A) and characteristics of the burst firing (B, C) of serotonin (5-HT) neurons of the dorsal raphe nucleus (DRN) in the male and female offspring of the vehicle- or delta opioid agonist SNC80-treated dams.
4 Discussion
The present results demonstrate that the treatment with a delta-opioid agonist during gestation does not induce negative effects on anxiety, cognitive performance or habituation processes in coping with new environment in the offspring. Moreover, female rats prenatally exposed to maternal treatment with a delta-opioid agonist showed a better cognitive performance in a NOR test. Consistently, the decrease in firing of glutamate and dopamine neurons as well as the burst frequency of dopamine neurons observed in female offspring prenatally exposed to maternal treatment with a delta-opioid agonist was less pronounced or absent in male ones.
Offspring of dams treated with the delta-opioid agonist during gravidity did not show any negative changes in the behavioral tests used in present experiments. In existing studies on prenatal opioid exposure, the authors examined mainly the effects of heroin, methadone or buprenorphine showing many negative neurodevelopmental alterations in the offspring (Conradt et al., 2019; Lee et al., 2023). The treatment with the delta-opioid agonist SNC80 in the present study failed to negatively modify the offspring behavior. To our best knowledge, there are no other studies exploring the consequences of maternal treatment with selective delta-opioid agonists on offspring behavior.
Interestingly, female offspring of SNC80-treated dams showed even a better cognitive performance compared to that in female offspring of vehicle-treated dams. Though the data on effects of prenatal exposure are missing, the present data are consistent with positive effects of delta-opioid agonists on cognitive performance in adults. As mentioned in the introduction, delta-opioid agonists can positively modulate several processes of brain plasticity, such as cell proliferation or BDNF expression (Persson et al., 2003a; Torregrossa et al., 2006). It was also shown that the activation of delta-opioid receptors in adult rats experienced hypoxia improve spatial cognition, and hippocampal neurogenesis and synaptic transmission (Wang et al., 2016; Zhang et al., 2021). Treatment of adult rats with delta-opioid agonists appears to induce mild anxiolytic effects (Saitoh et al., 2004; Dremencov et al., 2023). However, as shown in the present study, such effects were not transferred to the offspring. Electrophysiological measurements in glutamate neurons have shown that the treatment with SNC80, a delta-opioid agonist, during gestation resulted in decreased firing rate in female and suppressed burst activity in male offspring. A clear inhibitory effect of prenatal exposure to maternal SNC80 treatment on both firing rate and burst frequency of dopamine neurons were observed in female offspring. In male offspring of dams treated with SNC80, the firing rate and the percentage of spikes in bursts were lower compared to corresponding values in control offspring. Chronic treatment with the same delta-opioid agonist during adulthood in our previous study (Dremencov et al., 2023) led to an opposite effect, namely, increased firing rate of glutamate and dopamine neurons in male rats. In the mentioned study, the SNC80 effects in female rats were however not investigated. The present data cannot be discussed based on previous studies on prenatal selective delta-opioid agonists, as such studies could not be found in the literature available.
It is known that the burst firing of glutamate (Wozny et al., 2008) and dopamine (Cooper, 2002) neurons enhances the nerve terminal neurotransmitter release, in comparison with the same amount of action potentials fired in a single-spike mode. Suppression of the burst firing of glutamate and dopamine neurons in the offspring of dams treated with SNC80-treated dams may thus result in a further decrease in corresponding neurotransmission.
The activation of maternal delta-opioid receptors during gestation had no effect on characteristics of the firing activity of 5-HT neurons of the DRN in their offspring. Apparently, serotoninergic neurotransmission is not affected by prenatal exposure to delta-opioid agonist treatment.
Improved NOR performance in female offspring of delta opioid agonist-treated rats might be associated with decreased firing rate of hippocampal glutamate and mesolimbic dopamine neurons. The present findings are supported by the results of a previous study showing a link between elevated hippocampal glutamate neurotransmission and impaired NOR performance (Gómez-Galán et al., 2013). Furthermore, a recent study describes that enhanced cognitive performance induced by exposure of rodents to multiple NOR sessions was accompanied by decreased activity of VTA dopamine neurons, measured indirectly by c-Fos immunohistochemistry (Fleury et al., 2023).
With respect to the sex differences, the time spent in the central zone measured in the open field test (anxiety behavior) was marginally longer in female than in male offspring. That agrees with the known high exploratory activity in the open field in females (Dubovický et al., 1999).
Interestingly, the firing rate of both glutamate and dopamine neurons was higher and the percentage of spikes occurring in burst was lower in males compared to females. Observed sex differences in the firing activity of dopamine neurons are in agreement with a similar tendency found in our previous experiments (Csatlosova et al., 2021). A recent study from a different laboratory (Zhu and Grace, 2023), however, reported no differences in the firing activity of hippocampal glutamate and VTA dopamine neurons between males and females under basal conditions. The mentioned authors used however a different strain of rats. In agreement with our previous results (Csatlosova et al., 2021) there were no sex differences in the firing activity of 5-HT neurons. The characteristics of the firing activity of hippocampal glutamate and brainstem 5-HT and dopamine neurons in offspring of the control dams were similar to those observed in control animals in our previous studies without any behavioral testing (Grinchii et al., 2022; Grinchii et al., 2023). Even though in the present study behavioral tests were performed on the same animals before electrophysiological recordings, they are unlikely to affect the firing activity of the neurons.
Maternal treatment with delta-opioid agonists can alter the excitability of the hippocampal glutamate and VTA dopamine neurons via several possible mechanisms. First, these drugs are likely to pass the placenta and to affect the embryonal brain directly. Their ability to alter the excitability of hippocampal glutamate and VTA dopamine neurons was shown in our previous studies (Dremencov et al., 2023). Second, activation of delta-opioid receptors may affect the expression of certain maternal neurotrophic factors, such as BDNF (Torregrossa et al., 2006). Maternal neurotrophins are in turn suggested to interact with the embryonal ones and to affect developmental and functioning of the embryonal brain (Singh et al., 2022). Finally, delta agonists can affect the maternal care behavior that may influence offspring behavior and neurophysiology (Gemmel et al., 2018). Further studies should be performed to assess the mechanism(s) interconnecting maternal treatment with delta-opioid agonists and the activity of the hippocampal glutamate and VTA dopamine neurons in offspring.
There are a few limitations of the present study. As the results were evaluated using post hoc analyses even when the interaction between the main factors was not statistically significant, the conclusions were formulated with caution.
In conclusion, the original findings of the present study suggest that the maternal treatment with delta-opioid agonists during the gestation may have pro-cognitive effect in female offspring. Observed pro-cognitive effect induced might be mediated via mechanism(s) involving the firing activity of hippocampal glutamate and mesolimbic dopamine neurons.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Animal Health and Animal Welfare Division of the State Veterinary and Food Administration of the Slovak Republic. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
ED: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. HO: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing–original draft, Writing–review and editing. DG: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Writing–original draft, Writing–review and editing. ZR: Data curation, Investigation, Methodology, Validation, Visualization, Writing–original draft, Writing–review and editing. RD: Data curation, Investigation, Methodology, Writing–original draft, Writing–review and editing. LL: Conceptualization, Funding acquisition, Writing–original draft, Writing–review and editing. DJ: Conceptualization, Formal Analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Slovak Research and Development Agency (Grant Nos APVV-19-0435 and APVV-20-0202) and Research Grant Agency and Research Grant Agency of the Slovak Academy of Sciences and Ministry of Education, Science, Research and Sport of the Slovak Republic (grant No VEGA 2/0057/22).
Acknowledgments
The authors thank Doctors Lucia Moravčíková and Eszter Bögi for their excellent technical assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1357575/full#supplementary-material
References
ACOG Practice Bulletin (2008). ACOG Practice Bulletin: clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet. Gynecol. 111, 1001–1020. doi:10.1097/AOG.0b013e31816fd910
Conradt, E., Flannery, T., Aschner, J. L., Annett, R. D., Croen, L. A., Duarte, C. S., et al. (2019). Prenatal opioid exposure: neurodevelopmental consequences and future research priorities. Pediatrics 144, e20190128. doi:10.1542/peds.2019-0128
Cooper, D. C. (2002). The significance of action potential bursting in the brain reward circuit. Neurochem. Int. 41, 333–340. doi:10.1016/s0197-0186(02)00068-2
Csatlosova, K., Bogi, E., Durisova, B., Grinchii, D., Paliokha, R., Moravcikova, L., et al. (2021). Maternal immune activation in rats attenuates the excitability of monoamine-secreting neurons in adult offspring in a sex-specific way. Eur. Neuropsychopharmacol. 43, 82–91. doi:10.1016/j.euroneuro.2020.12.002
Dremencov, E., Grinchii, D., Hrivikova, K., Lapshin, M., Komelkova, M., Graban, J., et al. (2022). Exposure to chronic stressor upsurges the excitability of serotoninergic neurons and diminishes concentrations of circulating corticosteroids in rats two weeks thereafter. Pharmacol. Rep. 74, 451–460. doi:10.1007/s43440-022-00366-z
Dremencov, E., Grinchii, D., Romanova, Z., Chomanic, P., Lacinova, L., and Jezova, D. (2023). Effects of chronic delta-opioid receptor agonist on the excitability of hippocampal glutamate and brainstem monoamine neurons, anxiety, locomotion, and habituation in rats. Pharmacol. Rep. 75, 585–595. doi:10.1007/s43440-023-00485-1
Dubovicky, M., Belovicova, K., Csatlosova, K., and Bogi, E. (2017). Risks of using SSRI/SNRI antidepressants during pregnancy and lactation. Interdiscip. Toxicol. 10, 30–34. doi:10.1515/intox-2017-0004
Dubovický, M., Skultétyová, I., and Jezová, D. (1999). Neonatal stress alters habituation of exploratory behavior in adult male but not female rats. Pharmacol. Biochem. Behav. 64, 681–686. doi:10.1016/s0091-3057(99)00166-5
Fleury, S., Kolaric, R., Espera, J., Ha, Q., Tomaio, J., Gether, U., et al. (2023). Role of dopamine neurons in familiarity. bioRxiv. doi:10.1101/2023.10.25.564006
Gemmel, M., Bogi, E., Ragan, C., Hazlett, M., Dubovicky, M., van den Hove, D. L., et al. (2018). Perinatal selective serotonin reuptake inhibitor medication (SSRI) effects on social behaviors, neurodevelopment and the epigenome. Neurosci. Biobehav Rev. 85, 102–116. doi:10.1016/j.neubiorev.2017.04.023
Gómez-Galán, M., De Bundel, D., Van Eeckhaut, A., Smolders, I., and Lindskog, M. (2013). Dysfunctional astrocytic regulation of glutamate transmission in a rat model of depression. Mol. Psychiatry 18, 582–594. doi:10.1038/mp.2012.10
Grinchii, D., Hoener, M. C., Khoury, T., Dekhtiarenko, R., Nejati Bervanlou, R., Jezova, D., et al. (2022). Effects of acute and chronic administration of trace amine-associated receptor 1 (TAAR1) ligands on in vivo excitability of central monoamine-secreting neurons in rats. Mol. Psychiatry 27, 4861–4868. doi:10.1038/s41380-022-01739-9
Grinchii, D., Janáková Csatlósová, K., Viñas-Noguera, M., Dekhtiarenko, R., Paliokha, R., Lacinová, Ľ., et al. (2024). Effects of pre-gestational exposure to the stressors and perinatal bupropion administration on the firing activity of serotonergic neurons and anxiety-like behavior in rats. Behav. Brain Res. 459, 114796. doi:10.1016/j.bbr.2023.114796
Grinchii, D., Lacinova, L., and Dremencov, E. (2023). Effects of the acute administration of delta-opioid receptor ligands on the excitability of rat hippocampal glutamate and brainstem monoamine neurons in vivo. Gen. Physiol. Biophys. 42, 273–283. doi:10.4149/gpb_2023010
Hajós, M., Allers, K. A., Jennings, K., Sharp, T., Charette, G., Sík, A., et al. (2007). Neurochemical identification of stereotypic burst-firing neurons in the rat dorsal raphe nucleus using juxtacellular labelling methods. Eur. J. Neurosci. 25, 119–126. doi:10.1111/j.1460-9568.2006.05276.x
Hlavacova, N., Chmelova, M., Danevova, V., Csanova, A., and Jezova, D. (2015). Inhibition of fatty-acid amide hydrolyse (FAAH) exerts cognitive improvements in male but not female rats. Endocr. Regul. 49, 131–136. doi:10.4149/endo_2015_03_131
Idunkova, A., Lacinová, Ľ., and Dubiel-Hoppanova, L. (2023). Impact of depression on first and second generation: from biochemistry to electrophysiology. Focus hippocampus. Gen. Physiol. Biophys. 42, 107–122. doi:10.4149/gpb_2023001
Jacobson, M. L., Wulf, H. A., Browne, C. A., and Lucki, I. (2018). Opioid modulation of cognitive impairment in depression. Prog. Brain Res. 239, 1–48. doi:10.1016/bs.pbr.2018.07.007
Jelen, L. A., Stone, J. M., Young, A. H., and Mehta, M. A. (2022). The opioid system in depression. Neurosci. Biobehav Rev. 140, 104800. doi:10.1016/j.neubiorev.2022.104800
Jelen, L. A., Young, A. H., and Mehta, M. A. (2023). Opioid mechanisms and the treatment of depression. Curr. Top. Behav. Neurosci. doi:10.1007/7854_2023_448
Jutkiewicz, E. M., Rice, K. C., Traynor, J. R., and Woods, J. H. J. P. (2005). Separation of the convulsions and antidepressant-like effects produced by the delta-opioid agonist SNC80 in rats. Psychopharmacol. Berl. 182, 588–596. doi:10.1007/s00213-005-0138-9
Laine, K., Heikkinen, T., Ekblad, U., and Kero, P. (2003). Effects of exposure to selective serotonin reuptake inhibitors during pregnancy on serotonergic symptoms in newborns and cord blood monoamine and prolactin concentrations. Arch. Gen. Psychiatry 60, 720–726. doi:10.1001/archpsyc.60.7.720
Lee, J. J., Saraiya, N., and Kuzniewicz, M. W. (2023). Prenatal opioid exposure and neurodevelopmental outcomes. J. Neurosurg. Anesthesiol. 35, 142–146. doi:10.1097/ANA.0000000000000876
Lyon, L., Saksida, L. M., and Bussey, T. J. (2012). Spontaneous object recognition and its relevance to schizophrenia: a review of findings from pharmacological, genetic, lesion and developmental rodent models. Psychopharmacol. Berl. 220, 647–672. doi:10.1007/s00213-011-2536-5
Nordeng, H., Lindemann, R., Perminov, K. V., and Reikvam, A. (2001). Neonatal withdrawal syndrome after in utero exposure to selective serotonin reuptake inhibitors. Acta Paediatr. 90, 288–291. doi:10.1111/j.1651-2227.2001.tb00306.x
Paxinos, G., and Watson, C. (2014). Paxino's and Watson's the rat brain in stereotaxic coordinates. Amsterdam ; Boston: Elsevier/AP, Academic Press is an imprint of Elsevier.
Persson, A. I., Thorlin, T., Bull, C., and Eriksson, P. S. (2003a). Opioid-induced proliferation through the MAPK pathway in cultures of adult hippocampal progenitors. Mol. Cell Neurosci. 23, 360–372. doi:10.1016/s1044-7431(03)00061-7
Persson, A. I., Thorlin, T., Bull, C., Zarnegar, P., Ekman, R., Terenius, L., et al. (2003b). Mu- and delta-opioid receptor antagonists decrease proliferation and increase neurogenesis in cultures of rat adult hippocampal progenitors. Eur. J. Neurosci. 17, 1159–1172. doi:10.1046/j.1460-9568.2003.02538.x
Saitoh, A., Kimura, Y., Suzuki, T., Kawai, K., Nagase, H., and Kamei, J. (2004). Potential anxiolytic and antidepressant-like activities of SNC80, a selective delta-opioid agonist, in behavioral models in rodents. J. Pharmacol. Sci. 95, 374–380. doi:10.1254/jphs.fpj04014x
Singh, S., Fereshetyan, K., Shorter, S., Paliokha, R., Dremencov, E., Yenkoyan, K., et al. (2022). Brain-derived neurotrophic factor (BDNF) in perinatal depression: side show or pivotal factor? Drug Discov. Today 28, 103467. doi:10.1016/j.drudis.2022.103467
Torregrossa, M. M., Jutkiewicz, E. M., Mosberg, H. I., Balboni, G., Watson, S. J., and Woods, J. H. (2006). Peptidic delta opioid receptor agonists produce antidepressant-like effects in the forced swim test and regulate BDNF mRNA expression in rats. Brain Res. 1069, 172–181. doi:10.1016/j.brainres.2005.11.005
Viñas-Noguera, M., Csatlósová, K., Šimončičová, E., Bögi, E., Ujházy, E., Dubovický, M., et al. (2022). Sex- and age-dependent effect of pre-gestational chronic stress and mirtazapine treatment on neurobehavioral development of Wistar rat offspring. PLoS One 17, e0255546. doi:10.1371/journal.pone.0255546
Wang, S. Y., Duan, Y. L., Zhao, B., Wang, X. R., Zhao, Z., and Zhang, G. M. (2016). Effect of delta opioid receptor activation on spatial cognition and neurogenesis in cerebral ischemic rats. Neurosci. Lett. 620, 20–26. doi:10.1016/j.neulet.2016.03.035
Wei, J., Carroll, R. J., Harden, K. K., and Wu, G. (2012). Comparisons of treatment means when factors do not interact in two-factorial studies. Amino Acids 42, 2031–2035. doi:10.1007/s00726-011-0924-0
Wozny, C., Maier, N., Fidzinski, P., Breustedt, J., Behr, J., and Schmitz, D. (2008). Differential cAMP signaling at hippocampal output synapses. J. Neurosci. 28, 14358–14362. doi:10.1523/JNEUROSCI.4973-08.2008
Zhang, G., Lai, Z., Gu, L., Xu, K., Wang, Z., Duan, Y., et al. (2021). Delta opioid receptor activation with delta opioid peptide [d-Ala2, d-leu5] enkephalin contributes to synaptic improvement in rat Hippocampus against global ischemia. Cell Transpl. 30, 9636897211041585. doi:10.1177/09636897211041585
Keywords: delta opioid receptor (DOR), prenatal treatment, dopamine, glutamate, hippocampus, electrophysiology, novel object recognition (NOR)
Citation: Dremencov E, Oravcova H, Grinchii D, Romanova Z, Dekhtiarenko R, Lacinova L and Jezova D (2024) Maternal treatment with a selective delta-opioid receptor agonist during gestation has a sex-specific pro-cognitive action in offspring: mechanisms involved. Front. Pharmacol. 15:1357575. doi: 10.3389/fphar.2024.1357575
Received: 18 December 2023; Accepted: 28 March 2024;
Published: 16 April 2024.
Edited by:
Harry Pantazopoulos, University of Mississippi Medical Center, United StatesReviewed by:
Caroline A. Browne, Uniformed Services University of the Health Sciences, United StatesAntonia Artacho-Cordón, University of Granada, Spain
Daniela Rueedi-Bettschen, University of Mississippi Medical Center, United States
Copyright © 2024 Dremencov, Oravcova, Grinchii, Romanova, Dekhtiarenko, Lacinova and Jezova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eliyahu Dremencov, ZWxpeWFodS5kcmVtZW5jb3ZAc2F2YmEuc2s=
†These authors have contributed equally to this work
 Eliyahu Dremencov
Eliyahu Dremencov Henrieta Oravcova
Henrieta Oravcova Daniil Grinchii
Daniil Grinchii Zuzana Romanova
Zuzana Romanova Roman Dekhtiarenko
Roman Dekhtiarenko Lubica Lacinova
Lubica Lacinova Daniela Jezova
Daniela Jezova