- Postgraduate Training Base Alliance of Wenzhou Medical University (Wenzhou People’s Hospital), Wenzhou, China
Background: Baclofen, a centrally acting muscle relaxant, is widely utilized for the management of muscle spasms and alcohol use disorders associated with conditions. However, its neurological safety and tolerability in a large population remain limited. This study aimed to assess the neurological safety and potential risks of baclofen in the real world.
Methods: Data covering the period from the first quarter of 2004 to the third quarter of 2024 were collected from the Food and Drug Administration Adverse Event Reporting System (FAERS). Four disproportionality analysis methods were employed: the Reporting Odds Ratio, the Proportional Reporting Ratio, Bayesian Confidence Propagation Neural Network, and the Multi-item Gamma Poisson Shrinkage (MGPS). These methods were used to detect and evaluate adverse events Adverse drug events associated with baclofen. Additionally, the time to onset analysis was conducted.
Results: A total of 432 neurological-related preferred terms (PTs) were identified. The number of PT that were positive for all four algorithms was 40, and the top 5 PT were Hypotonia, Encephalopathy, Coma, Unresponsive to stimuli, and Cerebrospinal fluid leakage. The top 5 PTs for ROR values are Intracranial hypotension [ROR 66.24 (55.45–79.13)], Cerebrospinal fluid leakage [ROR 51.34 (45.84–57.51)], Autonomic dysreflexia [ROR 47.4 (32.27–69.63)], Basal ganglion degeneration [ROR 33.03 (18.54–58.84)], Sciatic nerve palsy [ROR 21.6 (11.14–41.87)]. The median onset time for baclofen -related ADEs was 27 days. Most cases (n = 241, 55.5%) occurred within the first month of baclofen administration. In an analysis of severe vs. non-severe ADEs, the study found that the incidence of severe cases was higher than that of non-severe cases, with no gender-related differences observed.
Conclusion: This study identified clinically significant PTs using four different algorithms and performed gender subgroup analysis. The TTO analysis indicated that the onset of most ADEs occurred within 27 days. Furthermore, the frequency of severe ADEs was higher than that of non-severe ones. Clinicians should closely monitor for neurological adverse effects caused by baclofen, particularly severe ADEs, and consider individualized dosing strategies. Further research based on real-world data is needed to validate these findings.
1 Introduction
Baclofen is a centrally acting muscle relaxant that diminishes neuronal excitability by selectively activating GABA-B receptors within the central nervous system, thereby effectively alleviating muscle spasms and stiffness (Finnimore et al., 1995; Creamer et al., 2018a). Given its substantial clinical efficacy, baclofen has been extensively utilized in managing spasticity associated with conditions such as multiple sclerosis, stroke, and spinal cord injury (Kofler et al., 2009; Creamer et al., 2018a; De Sousa et al., 2022; Shkodina et al., 2024). Furthermore, baclofen serves as an adjunctive therapeutic option for alcohol dependence, effectively alleviating alcohol cravings and reducing the risk of relapse (Garbutt et al., 2021; Agabio et al., 2023). Baclofen can be administered via oral ingestion or intrathecal injection. Oral administration is typically suitable for patients with mild to moderate spasticity, whereas intrathecal injection is indicated for those with severe symptoms or inadequate responses to oral therapy (Bonouvrié et al., 2019).
However, the mechanism of action of baclofen also renders it closely associated with various neurological adverse reactions. Studies have demonstrated that baclofen may induce severe adverse effects, including somnolence, dizziness, confusion, seizures, and even coma, particularly when administered at high dose (Caron et al., 2014; Farhat et al., 2020; Ghannoum et al., 2021). Adverse drug events (ADEs) not only compromise patients’ quality of life but may also result in serious clinical outcomes and impose an additional healthcare burden (Patton and Borshoff, 2018). While the common adverse effects of baclofen have been extensively documented, there remains a paucity of studies addressing rare reactions, events not specified in the drug’s package insert, and their associated risks across diverse patient populations. Therefore, a systematic evaluation of baclofen’s adverse reactions is crucial for optimizing clinical medication strategies and enhancing patient safety.
The FAERS database is one of the largest global platforms for monitoring adverse drug reactions, encompassing millions of patient medication records and associated adverse event reports (Yang et al., 2024). It collects standardized real-world data to support the FDA’s safety monitoring programs for drugs through spontaneous reports submitted by consumers, healthcare professionals, drug manufacturers, and other nonmedical individuals (Ding et al., 2025; Zhu et al., 2025). FAERS offers real-time updates, encompassing an extensive array of drugs and patient populations, while maintaining high levels of data transparency and accessibility (Gong et al., 2024; Li et al., 2025). Its robust signal detection capacity renders it a crucial instrument in pharmacovigilance studies, particularly in the discovery of drug-related adverse events and the evaluation of drug safety. Disproportionality analysis is a powerful tool for pharmacovigilance and drug safety. When disproportionality analysis is applied to the FAERS databases, it helps identify signals for rare adverse events by comparing the observed number of reports for a specific drug-adverse event pair with the expected number (Bate and Evans, 2009).
Given that the adverse reactions of baclofen have not been fully clarified, this study, based on real-world data from the FAERS database, uses disproportionality analysis to identify risk signals in adverse reaction reports related to baclofen, with a focus on analyzing the neurological adverse reactions caused by baclofen. We applied the signal detection method to identify under-reported rare adverse reactions, studied the characteristics of the time to onset (TTO), and analyzed the risk differences between different genders. Our study is intended to offer elaborate information regarding the adverse reactions of baclofen, thereby compensating for the existing research deficiencies and facilitating the provision of valuable guidance for clinicians in the utilization of baclofen and the promotion of patient safety.
2 Materials and methods
2.1 Study design and data source
The FAERS database is a widely accessible database for postmarketing safety surveillance that collects ADEs from health professionals, drug manufacturers, and patients. As a global database, FAERS covers information on adverse drug events from all over the world, providing an important basis for the safety assessment and regulation of drugs (Li B. et al., 2024). The FAERS database encompasses seven sub-datasets: DEMO (Demographic information), which includes patient age, gender, weight, and other relevant details; DRUG (Drug information), detailing drug names and their roles (primary, secondary, or concomitant drugs); REAC (Adverse event information); OUTC (Patient outcome information), documenting outcomes such as hospitalization and death; RPSR (Report source), identifying the origin of the report (e.g., healthcare professionals or consumers); THER (Treatment dates), specifying the start and end dates of medication; and INDI (Indication for use), indicating the therapeutic purpose of the drug (Wan et al., 2024). ADEs were coded in the FAERS database with the use of the Medical Dictionary for Regulatory Activities (MedDRA), version 27.0 (Zhou et al., 2024). The terminology system of MedDRA is divided into five levels: System-organ Class (SOC), high-level group term (HLGT), high-level term (HLT), preferred term (PT), and lowest level term (LLT). Drugs in the FAERS database are classified into four types: PS (major suspicion), SS (minor suspicion), C (concomitant medication), and I (drug interaction). The primary outcomes of patients included death (DE), life-threatening events (LT), hospitalization (including primary or prolonged hospitalization) (HO), disability (DS), congenital anomalies (CA), and other important medical events (OT). We defined serious outcomes as death, life-threatening, hospitalization, and disability, and the rest as non-serious outcomes.
2.2 Data mining and cleaning
During the data mining and cleansing process, we implemented the following systematic steps to enhance data quality and analytical accuracy. Given that FAERS data may contain duplicate reports, we cleaned the data according to FDA-recommended standards (Huang et al., 2024). Specifically, if CASEID was identical, the most recent FDA_DT (report date) was retained. If both CASEID and FDA_DT were identical, the higher PRIMARYID (report identifier) was kept. This process ensured the uniqueness and reliability of the data. In this study, we selected the reports in which baclofen was identified as the PS. Both brand names (Lioresal) and generic names (Baclofen) are utilized to identify records associated with baclofen. All adverse effect terms were standardized using MedDRA version 27.0, and analyses were stratified accordingly. These cleansing procedures not only enhance the reliability of the data but also safeguard the integrity of subsequent signal mining outcomes. Ultimately, we acquired a refined and high-quality baclofen ADEs dataset suitable for in-depth statistical analysis. A schematic diagram of the flow of data screening was shown in Figure 1. All procedures were implemented using R software (version 4.3.1).
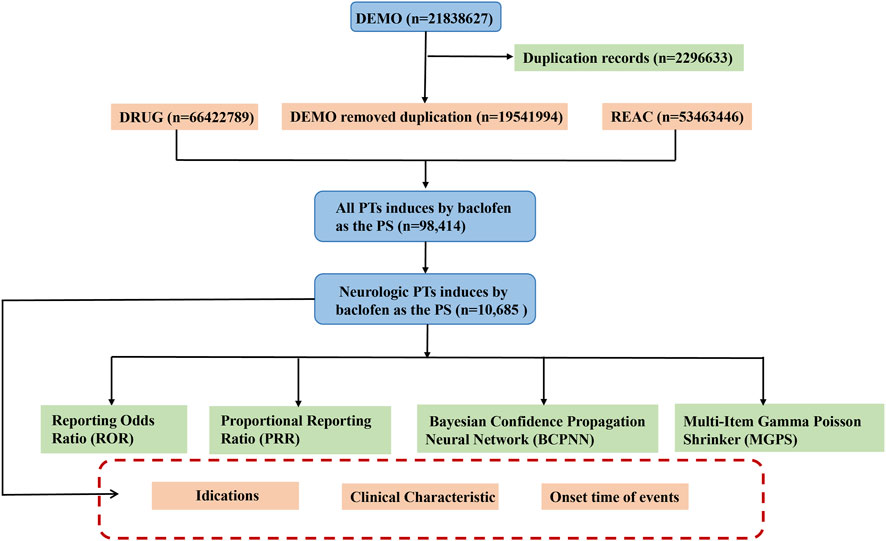
Figure 1. The schematic flowchart of data extraction, processing, and analysis. Footnotes: Abbreviation: DEMO, Demographic information; DRUG, Drug information; REAC, Adverse event information; PT, preferred terms; PS, major suspicion.
2.3 Statistical analysis
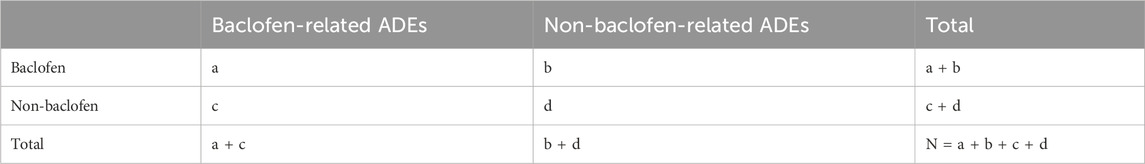
In the statistical analysis, we used a combination of frequency and signal intensity analyses to explore the relationship between baclofen and adverse effects. Disproportionality analysis is an important tool in pharmacovigilance analysis to identify potential safety signals by analyzing the reported frequency of drug-related adverse events compared with the event frequency of other drugs in the overall database (Ding et al., 2025). In this study, multiple disproportionality analysis methods, including frequency method and Bayesian method, were used to comprehensively mine the signals of drug-related ADEs. Signal strength analysis was performed using the following method: Reporting Odds Ratio (ROR): The strength of association between baclofen and adverse effects was calculated. The ROR is a widely utilized statistical method for conducting disequilibrium analysis. It is derived from the principles of a 2 × 2 contingency table (Table 1). The criterion was that the lower limit of the 95% confidence interval of the ROR value was >1. Proportional Reporting Ratio (PRR): A measure of the relative risk of baclofen based on the proportion of adverse reactions reported in the database as a whole. The screening criteria were PRR ≥ 2 and χ2 value ≥ 4. Bayesian methods: including Bayesian Confidence Propagation Neural Network (BCPNN) and Multi-Item Gamma Poisson Shrinker (MGPS) were used to evaluate the association strength of rare events (Chen et al., 2024; Shu et al., 2024). Detailed calculation methods are provided in Table 2. ROR can effectively minimize the bias introduced by low-frequency reported events, whereas PRR, owing to its higher specificity, demonstrates superior performance in signal detection during the screening process (Scosyrev et al., 2025). On the other hand, when data is scarce or has missing values, BCPNN can still perform effective signal detection, and as the number of reports increases, its results will tend to stabilize. While MGPS possesses a distinctive advantage in recognizing rare adverse reaction signals. This study integrated the four aforementioned methods, thereby broadening the scope of signal detection and validation. Simultaneously, it effectively minimized false positives via cross-validation, ultimately enhancing the capability to detect rare adverse reactions (Li et al., 2025; Zhu et al., 2025).
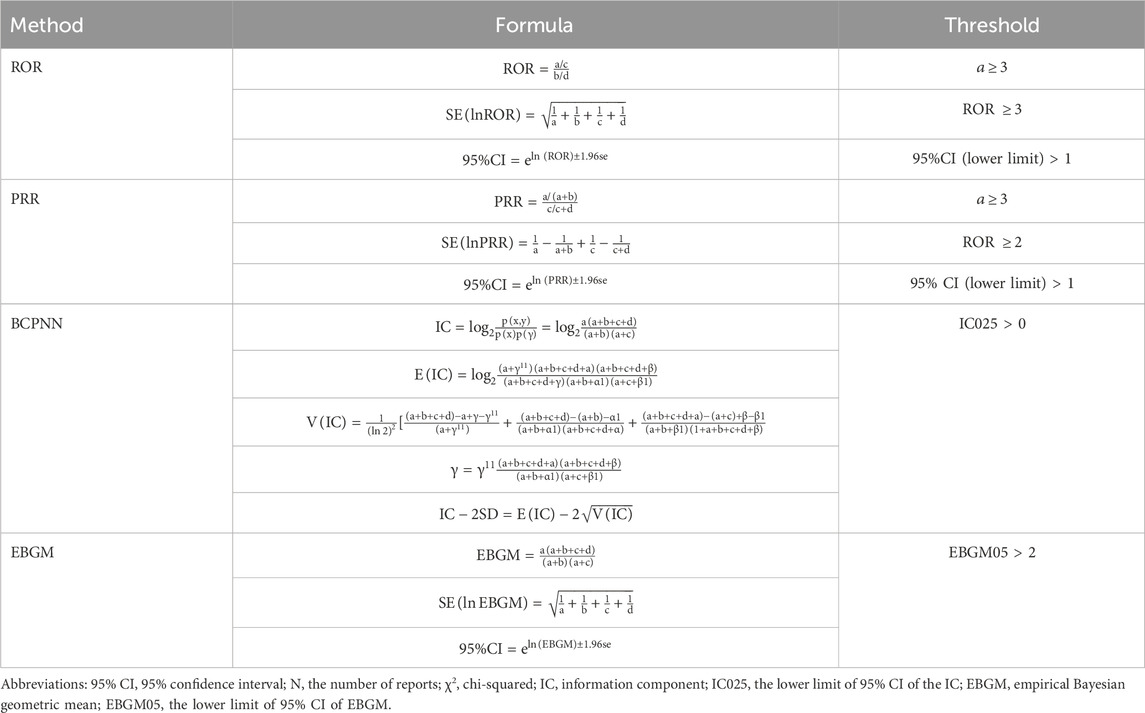
Table 2. Overview of the ROR, PRR, BCPNN, and EBGM methods, including their formulas and threshold values.
2.4 Time-to-onset analysis
The TTO of baclofen-related adverse events was defined as the time difference between the date of the adverse event (EVENT_DT in the DEMO file) and the date of drug initiation (START_DT in the THER file). Cases with inaccurate or missing dates, as well as those with an adverse event occurring prior to the initiation of baclofen treatment, were excluded from the analysis. To ensure data integrity, reports containing erroneous date entries adverse events reported before the treatment start date or incomplete date information was also excluded.
3 Results
3.1 General characteristics
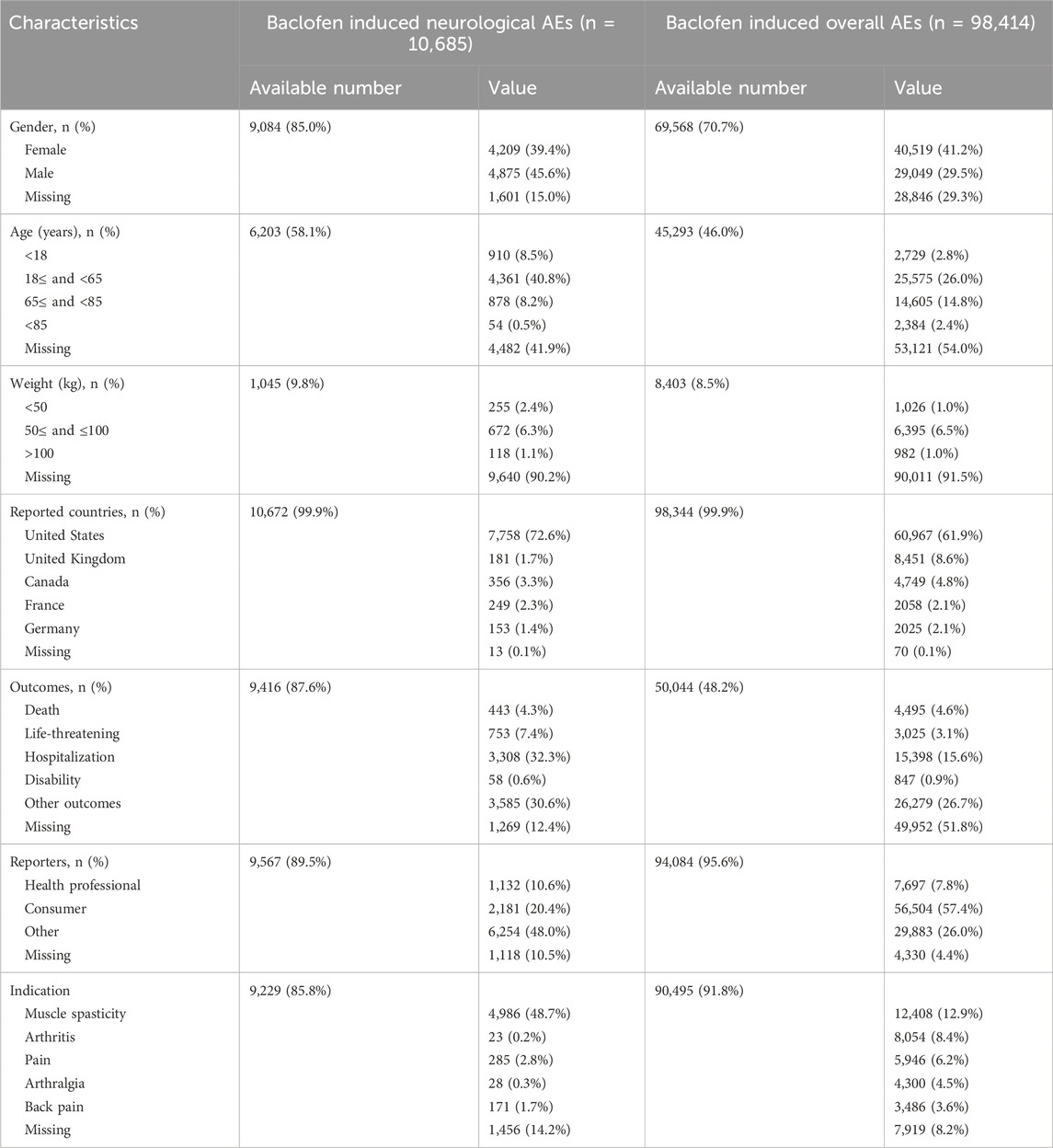
A total of 19,541,994 adverse event reports were available from the FAERS database for the period from the first quarter of 2004 through the third quarter of 2024, after removing duplications. Among these, there were 98,414 baclofen-related adverse events, with 10,685 specifically associated with neurologic adverse events. For all adverse event reports, the gender distribution was as follows: 29,049 (29.5%) were submitted by men and 40,519 (41.2%) by women. Among the 9,084 reports of adverse neurological events, 4,875 (45.6%) involved males, and 4,209 (39.4%) involved females (Figure 2A). The total number of ADEs containing age-specific information was 45,293. Among these, 2,729 (2.8%) were individuals younger than 18 years old, 25,575 (26.0%) were between 18 and 64 years old, and 16,989 (27.2%) were older than 64 years old. For nervous system adverse reactions with age-specific information, the total count was 6,203. Specifically, 910 (8.5%) were younger than 18 years old, 4,361 (40.8%) were between 18 and 64 years old, and 878 (8.7%) were older than 64 years old (Figure 2B). Among the overall adverse events recorded, the countries reporting the highest number of ADEs were the United States (61.9%), followed by the United Kingdom (8.6%), Canada (4.8%), France (2.1%), and Germany (2.1%) (Figure 2C). A similar distribution was observed for the reporting of nervous system adverse events (Figure 2D). The total number of adverse reaction reports for baclofen has exhibited an upward trend, with a peak of 15,688 cases reported in 2021. The number of reported adverse events related to the nervous system associated with baclofen has presented an overall stable trend (Figure 2E). Detailed information is provided in Table 3.
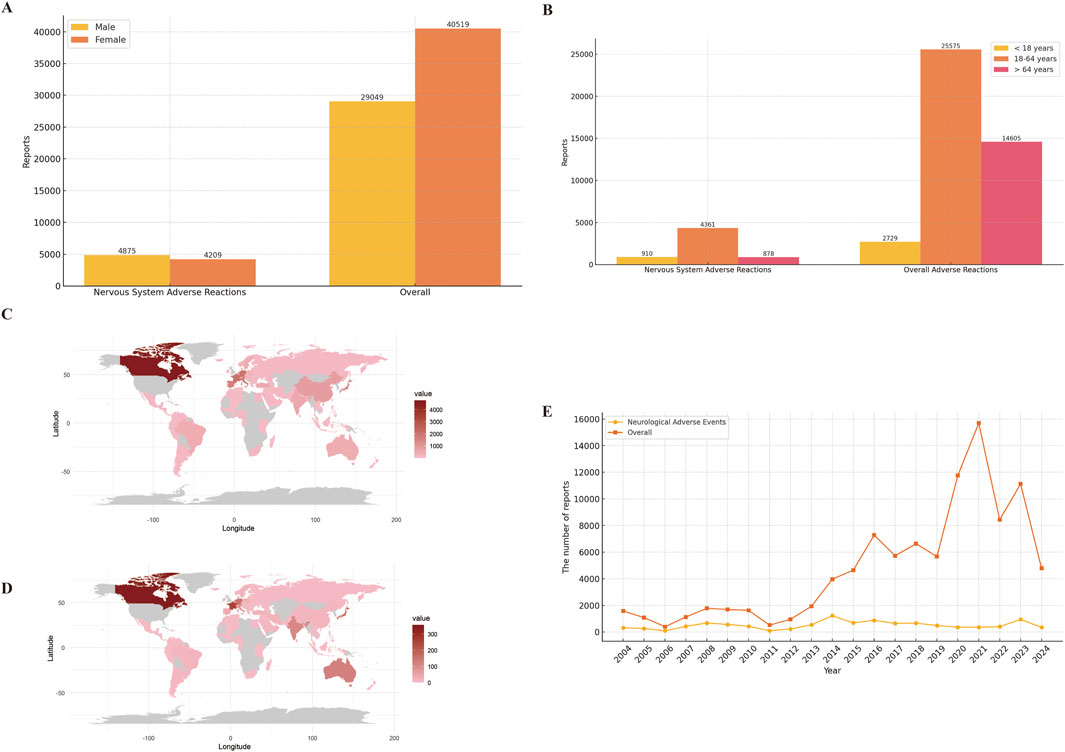
Figure 2. Clinical characteristics of baclofen associated reports from the FAERS database. Footnotes: (A) Gender; (B) Age; (C) Overall adverse drug reactions were reported by country; (D) Neurological adverse reactions were reported by country; (E) Year distribution of baclofen overall and nervous system adverse reactions reported.
3.2 Signal detection at the PT level
In this study, we systematically screened a total of 432 neurologically relevant PTs. To minimize the false positive rates, four signal detection methods were employed, and the pertinent PT was included in the analysis solely when all methods yielded positive results. Ultimately, according to this screening criterion, we identified 40 neurologically relevant PTs associated with baclofen. The Venn diagram in Figure 3 visually illustrated the PTs that met the positive threshold of all four algorithms at the PT level. Table 4 presents the top 20 ADEs associated with baclofen at the PT levels. To enhance the clarity of the data presentation, we visualized the PT signals using a forest plot. The top 5 PTs in terms of the number of adverse events reported were Hypotonia (n = 864), Encephalopathy (n = 796), Coma (n = 736), Unresponsive to stimuli (n = 537), Cerebrospinal fluid leakage (n = 399) (Figure 4A). The top 5 PTs for ROR values are Intracranial hypotension [ROR 66.24 (55.45–79.13)], Cerebrospinal fluid leakage [ROR 51.34 (45.84–57.51)], Autonomic dysreflexia [ROR 47.4 (32.27–69.63)],Basal ganglion degeneration [ROR 33.03 (18.54–58.84)], Sciatic nerve palsy [ROR 21.6 (11.14–41.87)] (Figure 4B).
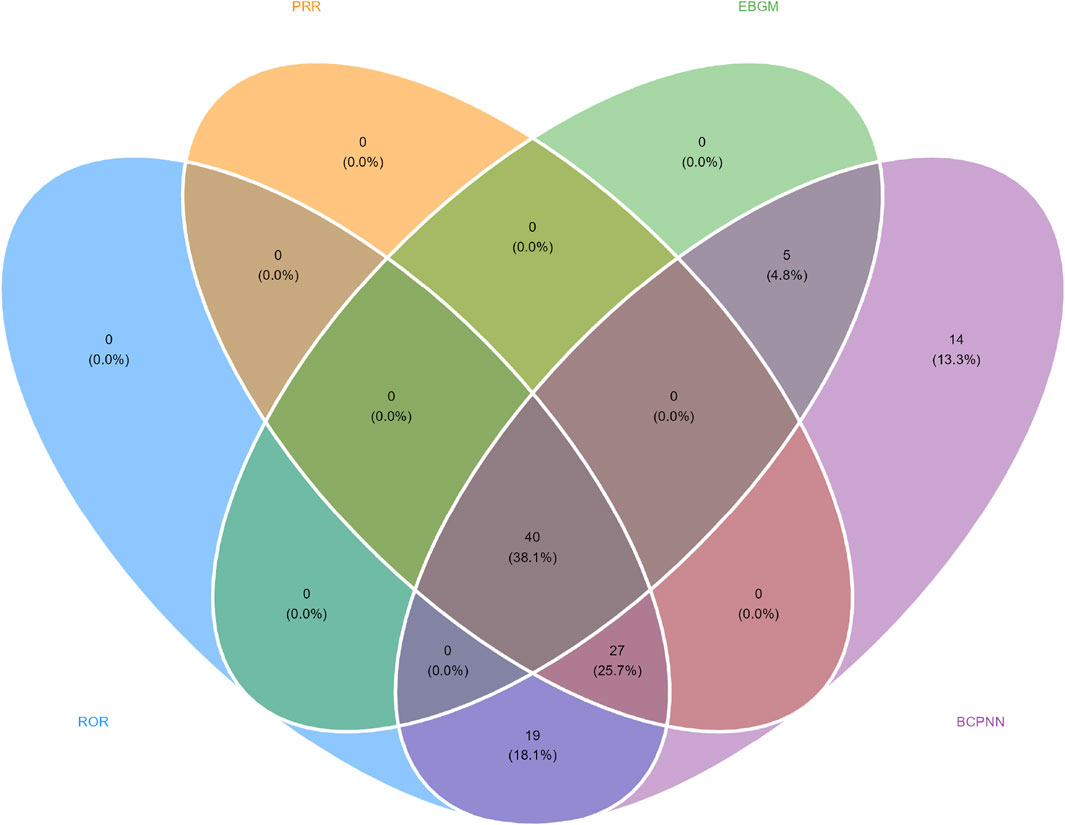
Figure 3. Venn diagram representation of baclofen-Induced nervous system adverse reaction signal values.
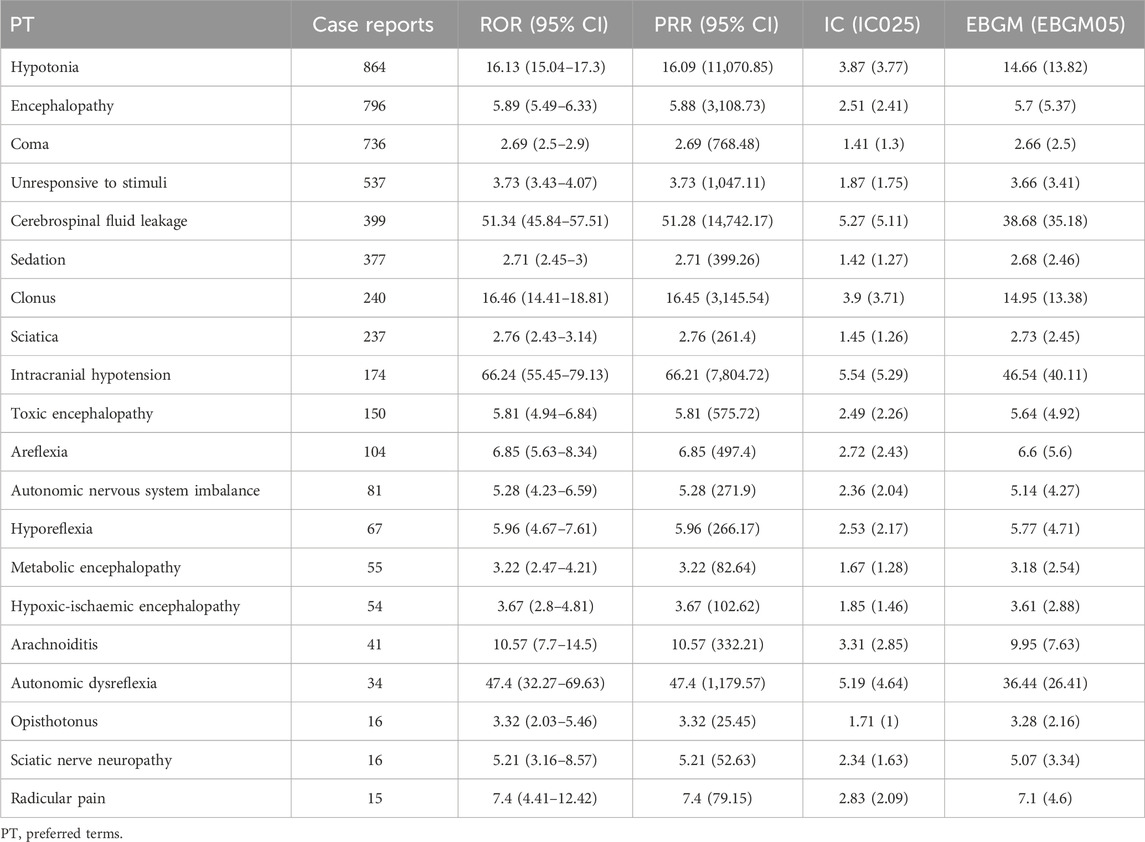
Table 4. Signal strength of neurological adverse drug events induced by baclofen at the PT level in the FAERS database.
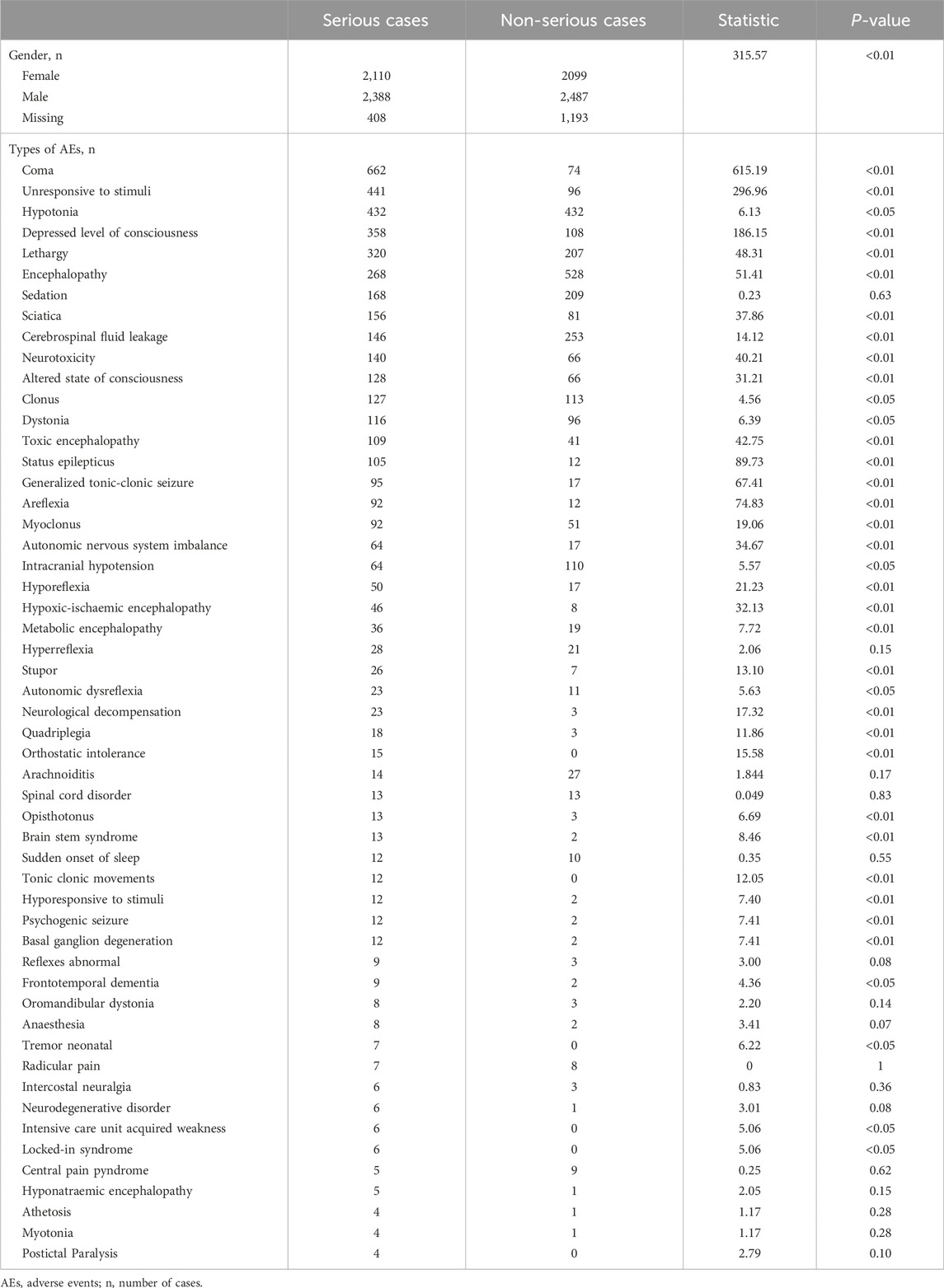
Table 5. Comparative analysis of clinical characteristics between serious and non-serious adverse event reports.
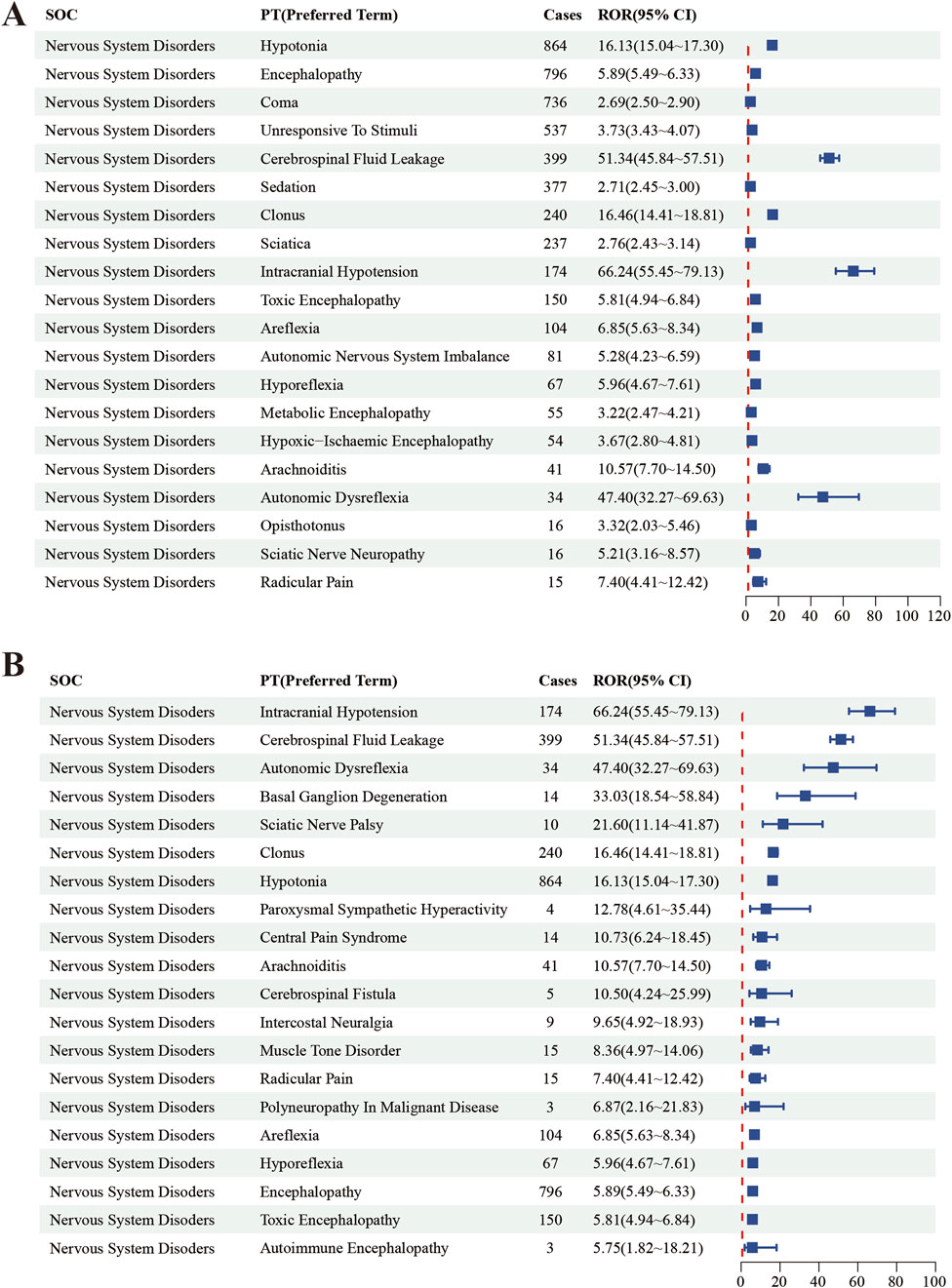
Figure 4. The top 20 PT results for baclofen positivity in ROR. Footnotes: (A) The highest signal frequency; (B) The highest signal intensity.
3.3 Analysis of gender-specific differences in baclofen risk signals
To investigate the potential influence of gender on baclofen-induced adverse drug reactions in neurological disorders, we performed an in-depth analysis of various neurology-related ADEs reported in female and male patients. In male patients, the top five most prevalent PTs linked with baclofen use were respectively Hypotonia (n = 422), Coma (n = 318), Lethargy (n = 242), Unresponsive to stimuli (n = 238), Depressed level of consciousness (n = 200) (Figure 5A). The top five adverse reactions in women were Coma (n = 363), Hypotonia (n = 361), Unresponsive to stimuli (n = 268), Cerebrospinal fluid leakage (n = 180), Sciatica (n = 163) (Figure 5C). In male patients, the top five major factors related to baclofen that met all four calculation methods were Low Intracranial pressure [ROR 108.61 (83.66–141.01)], Abnormal autonomic reflex [ROR 93.91 (58.1–151.78)], Cerebrospinal fluid leakage [ROR 73.16 (60.8–88.04)], Intercostal neuralgia [ROR 36.64 (17.18–78.16)], and Clonus [ROR 31.23 (26.07–37.39)] (Figure 5B). In female patients, the top five major PT signals of the nervous system associated with baclofen that met all four calculation methods were Basal ganglion degeneration [52.49 (28.13–97.96)], Low intracranial pressure [ROR 48.58 (36.92–63.91)], Cerebrospinal fluid leakage [ROR 45.82 (38.84–54.04)], Sciatic nerve paralysis [ROR 22.83 (8.03–64.95)], and Abnormal autonomic reflex [ROR 18.81 (7.46–47.44)] (Figure 5D).

Figure 5. The distribution of ROR signal values and PT factors for baclofen-Induced nervous system adverse reactions by gender. Footnotes: (A) The highest signal frequency in males; (B) The highest signal intensity in males; (C) The highest signal frequency in females; (D) The highest signal intensity in females; (E) Relative distribution of PT by gender for baclofen-induced nervous system adverse reactions.
This study provides a detailed analysis of the proportions of male and female patients experiencing baclofen-related nervous system adverse reactions, along with their gender-based differences. The names of each PT, the corresponding female-to-male patient-reported number ratio (female/male), the ROR, and the 95% confidence interval are presented in forest plots (Figure 5E). Blue squares represent the ROR values for each adverse reaction, horizontal lines denote the 95% confidence intervals, and a red dashed line serves as the baseline for ROR = 1, indicating an equal reporting ratio between genders. The results indicate that the confidence intervals for many adverse reactions lie entirely to the left of ROR = 1, suggesting a significantly lower proportion of adverse event reports from women compared to men. For instance, Coma (ROR = 0.6, 95% CI: 0.52–0.7), Hypotonia (ROR = 0.45,95% CI: 0.39–0.52), Unresponsive to Stimuli (ROR = 0.6,95% CI:0.50–0.71), Cerebrospinal fluid leakage (ROR = 0.61,95% CI: 0.49–0.76), Encephalopathy (ROR = 0.56,95% CI: 0.45–0.70), demonstrating that the reporting proportion of females is conspicuously lower than that of males. Additionally, the confidence intervals of some adverse reactions traverse ROR = 1, such as Hyporeflexia (ROR = 0.82, 95% CI: 0.5–1.34), indicating that there is no marked difference in the reporting of these adverse reactions between males and females. Through such data and graphical analyses, the distribution characteristics of adverse reactions reported by male and female patients are lucidly disclosed.
3.4 Time-to-onset analysis
After removing reports with inaccuracies, missing information, or unknown details, 434 ADEs were included, with a median TTO of 27 days. The majority of cases occurred within the first 0–30 days of baclofen use (n = 241, 55.5%). There were 35 cases (8.1%) within 30–60 days, 21 cases (4.8%) within 60–90 days, 14 cases (3.2%) within 90–120 days, 7 cases (1.6%) within 120–150 days, 13 cases (3%) within 150–180 days, and 20 cases (4.6%) within 181–360 days. Notably, ADEs were still observed in 83 cases (19.1%) after 1 year of baclofen treatment.
3.5 Serious vs. non-serious cases: baclofen neurological adverse events
There was a statistically significant difference in the gender ratio between severe and non-severe cases (p < 0.01). Among severe cases, 2,388 were male (48.7%), which was higher than non-severe cases (n = 2,487, 43.1%). In the same way, the proportion of females was slightly higher in severe cases (n = 2,110, 44.1%) compared to non-severe cases (n = 2,099, 43.1%). In severe cases, the top five neurological adverse reactions identified consistently by all four signal detection methods were: Coma (n = 662), Unresponsive to stimuli (n = 441), Hypotonia (n = 432), Depressed level of consciousness (n = 358), and Lethargy (n = 320). Among non-severe adverse reactions, the top five neurological adverse reactions confirmed by all four signal detection methods included Encephalopathy (n = 528), Hypotonia (n = 432), Cerebrospinal fluid leakage (n = 253), Somnolence (n = 225), and Sedation (n = 209). Differences in clinical characteristics of serious and non-serious reports were shown in (Table 5).
4 Discussion
To our knowledge, this study is the first to employ the FAERS database to disclose the distribution of neurological adverse reactions associated with baclofen, offering a significant reference for pharmacovigilance and clinical risk management. In this research, we placed emphasis on the clinical characteristics of baclofen in the nervous system, gender disparities, TTO, and the distinctions between severe and non-severe cases. The occurrence of adverse reactions in the nervous system can have a significant impact on patients’ health, underscoring the critical need for healthcare professionals to increase their awareness and improve related diagnostic and management skills.
The results of our analysis demonstrated a significant upward trend in baclofen-related ADEs, with the total number of reported incidents peaking at 15,688 in 2021, accounting for 15.9% of all reported ADEs. This growth trend may be closely associated with the expansion of the approved indication range for baclofen and its widespread adoption in clinical practice (Creamer et al., 2018a; Bonouvrié et al., 2019; Romito et al., 2021). However, it is notable that although the overall quantity of ADEs has increased, the number of ADEs associated with neurological adverse reactions has not presented a significant changing trend. This phenomenon could be ascribed to the elevated awareness of medication safety among patients and the continuous refinement of the clinical medication monitoring system. Although no significant increase in ADEs related to the nervous system has been observed with baclofen, it can still cause a variety of serious neurological adverse reactions, including coma, and may even lead to death. Therefore, in clinical practice, high attention should still be paid to the adverse neurological reactions of baclofen.
Our real-world data reveal that among the neurological adverse reactions, the top five most frequently reported PTs meeting all four algorithms were Hypotonia (n = 864), Encephalopathy (n = 796), Coma (n = 736), Unresponsive to stimuli (n = 537), Cerebrospinal fluid leakage (n = 399). This is in accordance with previous research findings. For example, in a real-world cohort study, the use of baclofen was associated with a higher risk of encephalopathy compared with the use of tizanidine or cyclobenzaprine (Hwang et al., 2023). The kidney plays a crucial role in baclofen elimination, accounting for approximately 70% of its clearance (Miller, 2017). Consequently, renal impairment may result in elevated serum concentrations of baclofen (Meillier et al., 2015). Physicians should take into account the potential toxicity of baclofen in patients with impaired renal function and altered mental status following its administration. For instance, a case report described a 57-year-old patient with end-stage renal disease who experienced progressive confusion and generalized hypotonia following the administration of baclofen (Marquez, 2015). Furthermore, in a population-based cohort study involving 360 elderly patients undergoing maintenance dialysis, 24 patients developed encephalopathy as a complication following baclofen administration and subsequently required hospitalization (Chauvin et al., 2020). Baclofen is a muscle relaxant that acts primarily in the spinal cord through the activation of inhibitory GABA-B receptors. Due to the moderate lipophilicity of baclofen, its ability to penetrate the blood-brain barrier is somewhat limited, so a higher oral dose is required to achieve the desired therapeutic effect (Romito et al., 2021). However, excessive intake of baclofen may lead to adverse reactions in the nervous system, such as drowsiness and even coma. In a retrospective analysis of 37 patients, the common clinical manifestations of baclofen poisoning included encephalopathy, coma as well as respiratory depression, hyporeflexia, and autonomic dysfunction (Creamer et al., 2018b). This is in line with our research findings. Furthermore, our analysis unexpectedly revealed several central nervous system adverse reactions, including Cerebrospinal fluid leakage [n = 399, ROR 51.34 (45.84–57.51)] and Intracranial hypotension [n = 174, ROR 66.24 (55.45–79.13)]. Some studies have shown that intrathecal baclofen (ITB) can lead to Cerebrospinal fluid leakage (Bonouvrié et al., 2022). In a 7-year retrospective study, among all patients who received intrathecal baclofen therapy, 21% developed cerebrospinal fluid leakage (Imerci et al., 2020b). When cerebrospinal fluid leakage occurs, patients have more perioperative adverse events and a higher 90-day readmission rate compared with those without these complication (Imerci et al., 2020b). Excessive cerebrospinal fluid leakage may also lead to intracranial hypotension (Wilson et al., 2023). A blood patch can be performed to prevent Cerebrospinal fluid leakage, and indeed, some surgical teams do this systematically (Imerci et al., 2020a). Epidural blood patches (EBPs) are also frequently performed in children with cerebral palsy (CP) to manage post-dural puncture headache (PDPH) due to cerebrospinal fluid leaks after ITB placement (Imerci et al., 2019). Additionally, we observed an increased number of reports for conditions such as Encephalopathy, Toxic encephalopathy, Metabolic encephalopathy, Hypoxic-ischemic encephalopathy, and Arachnoiditis. We identified several previously underreported adverse events, including Sciatica (n = 32), Hyporeflexia (n = 67), Autonomic nervous system dysfunction (n = 81), Opisthotonus (n = 16), and Radicular pain (n = 16). These findings were infrequently documented in both animal experiments and clinical trials (Karol et al., 2011).
Analysis of gender differences reveals that among all ADEs associated with baclofen, the proportion of female patients (41.20%) is significantly higher than that of male patients (29.50%). This discrepancy could potentially be associated with alcohol use disorder, as well as the primary indications for baclofen, including muscle spasms and neuralgia. As a GABA-B receptor agonist, baclofen has been proposed as a potential therapeutic option for the treatment of alcohol use disorders (Garbutt et al., 2021; Agabio et al., 2023). The significant increase in female alcohol dependence cases in recent years may be one of the important reasons for the gender difference in the reporting of adverse effects of baclofen (Khemiri et al., 2016). In addition, the main indications for baclofen, such as muscle spasms (Limerick et al., 2023) and multiple sclerosis (McGinley et al., 2021), are generally more common in women, which may also be another contributing factor. Our study further indicates that, in comparison with females, males constitute a marginally higher proportion of the neurological adverse reactions related to baclofen. However, the number of relevant studies is limited, and additional research is warranted to elucidate the specific mechanisms underlying these gender differences. In addition, the FAERS data may be susceptible to reporting bias, particularly in the context of sex-specific data. Since FAERS data collection is based on voluntary reporting by healthcare professionals and the general public, disparities in reporting frequency between genders could exist, which might influence the accuracy and comparability of the data (Guan et al., 2024).
According to the statistical analysis, the TTO of baclofen-induced neurological adverse reactions exhibited a significant temporal clustering. The majority of these adverse effects were observed in the early stages of treatment, particularly within the first 30 days. Furthermore, the TTO distribution for specific adverse reactions demonstrated notable variations (Heetla et al., 2014). For rapidly induced reactions, such as encephalopathy and Coma, approximately 75% of cases occurred within the first 2 weeks of medication. Rapidly induced adverse reactions may be related to the current administration methods of baclofen pumps or intrathecal injections (Shlobin et al., 2023; Cespedes et al., 2024; AlShaqaq et al., 2025). For baclofen pumps, mechanical drug delivery systems provide distinct therapeutic advantages; however, they are also susceptible to human error or equipment malfunctions. Both scenarios can result in drug overdose, insufficient dosage, or abrupt drug withdrawal, which may lead to severe health consequences (Caron et al., 2014; Romito et al., 2021; Fu and Perloff, 2022). Muscle spasticity, on the other hand, is a delayed adverse reaction with a significantly prolonged median induction time of 151 days. The data suggests that such reactions may be related to long-term medication and require continuous monitoring throughout the treatment period. Additionally, a few cases have shown significant long-term delayed effects; for instance, case reports indicate that some neurological adverse reactions may still occur even after 1 year of medication.
Our study found that baclofen use was associated with a higher number of serious adverse events, with a significantly higher proportion of serious adverse events than nonserious adverse events in both men and women. In a cohort study involving approximately 47,000 patients treated with baclofen for alcohol use disorder (AUD), which utilized a French health insurance claims database, the use of baclofen was associated with a significantly higher risk of hospitalization and mortality compared to other approved medications (Chaignot et al., 2018). In addition, a study conducted in Australia examining 102 baclofen-related fatalities revealed that the mean age at death was below 50 years and that intentional poisoning constituted the most prevalent cause of death (Zahra et al., 2024). Multiple studies have shown that people with alcohol use disorders are at higher risk when using baclofen, especially those individuals with a history of self-harm, suicide attempts, or substance use disorder (Holla et al., 2015; Beraha et al., 2016; Auffret et al., 2017). Therefore, healthcare providers should pay special attention to these high-risk groups and conduct a comprehensive assessment before starting baclofen treatment. Baclofen withdrawal syndrome is one of the most concerned complications during baclofen treatment, which can progress rapidly and has a high morbidity and mortality. Abrupt and drastic discontinuation or reduction of medication may trigger withdrawal symptoms, including changes in mental state, increased convulsions, fever, nausea, fatigue, etc. In severe cases, it may even lead to coma (Lad et al., 2008; Caron et al., 2014). Baclofen causes slow synaptic depression by activating G protein-coupled GABAB receptors, increasing potassium conductance in neuronal membranes, and reducing calcium influx at presynaptic terminal (Marquez, 2015). This mechanism results in a slowing of the hyperpolarization of neurons and a reduction in the release of excitatory neurotransmitters, which in turn reduces the number and amplitude of excitatory postsynaptic potentials along the dendrites of motor neurons (Salim et al., 2018). However, abrupt withdrawal of the drug may trigger rebound excitation at all levels of the neural axis, leading to a series of serious side effects (Pierce et al., 2018). For baclofen withdrawal syndrome, emergency resumption of medication should be carried out promptly, and the route of administration and dosage should remain the same as before the interruption (Romito et al., 2021).
This study conducted a systematic analysis of neurological adverse reactions of baclofen based on the FAERS database. First, we identified potential neurologic adverse events that may have been triggered by baclofen. Second, adverse events with significant signals were explored in depth. This study not only provides valuable insights into the neurological adverse effects of baclofen but also provides a new theoretical basis for evaluating the safety of this widely used class of drugs. In addition, this study points the way for future pharmacovigilance analyses and clinical trials that can help to further validate our findings and elucidate the mechanisms of associated neurological adverse effects.
Nevertheless, this study possesses limitations. The FAERS database bears inherent defects, including under-reporting of adverse events and over-reporting of events unassociated with drugs (Xia et al., 2024). The quality and accuracy of the reports hinge upon the expertise of the reporters Furthermore, the majority of the data results stem from countries such as the United States and those in the West, and the results might not be applicable to Asian regions like China (Li R. et al., 2024). Ultimately, the restricted and regionally concentrated data demand more extensive and long-term studies to validate these discoveries.
5 Conclusion
In conclusion, this study performed an in-depth analysis of baclofen-associated neurological adverse events using real-world data from the FAERS database. Moreover, the major adverse effects identified in this study align with those listed on the baclofen product label; however, several potential central nervous system adverse effects, such as Cerebrospinal fluid leakage and Intracranial hypotension, which have not been widely recognized, were also detected. Our results enhance the understanding of baclofen-related neurotoxicity and provide valuable insights for healthcare professionals, aiding them in reducing the risk of adverse events more effectively through postmarketing safety evaluation.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
YuZ: Conceptualization, Data curation, Formal Analysis, Writing – original draft, Writing – review and editing, Methodology. HH: Data curation, Methodology, Writing – original draft, Writing – review and editing, Formal Analysis. JM: Conceptualization, Data curation, Visualization, Writing – review and editing. JH: Formal Analysis, Writing – review and editing. YiZ: Visualization, Writing – review and editing. XY: Conceptualization, Data curation, Formal Analysis, Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to express our gratitude to FAERS for providing and permitting the utilization of the data analyzed in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agabio, R., Saulle, R., Rösner, S., and Minozzi, S. (2023). Baclofen for alcohol use disorder. Cochrane Db. Syst. Rev. 2023, CD012557. doi:10.1002/14651858.CD012557.pub3
AlShaqaq, A., Al Sahlawi, M., Amir, A., Ahmed, M., and Alkhunaizi, A. (2025). Baclofinding solutions: baclofen-induced encephalopathy in a peritoneal dialysis patient. Perit. Dial. Int., 08968608241311717. doi:10.1177/08968608241311717
Auffret, M., Labreuche, J., Duhamel, A., Deheul, S., Cottencin, O., Bordet, R., et al. (2017). Proactive regional pharmacovigilance system versus national spontaneous reporting for collecting safety data on concerning off-label prescribing practices: an example with baclofen and alcohol dependence in France. Drug Saf. 40, 257–262. doi:10.1007/s40264-016-0489-7
Bate, A., and Evans, S. J. W. (2009). Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidem. Dr. S. 18, 427–436. doi:10.1002/pds.1742
Beraha, E. M., Salemink, E., Goudriaan, A. E., Bakker, A., de Jong, D., Smits, N., et al. (2016). Efficacy and safety of high-dose baclofen for the treatment of alcohol dependence: a multicentre, randomised, double-blind controlled trial. Eur. Neuropsychopharmacol. 26, 1950–1959. doi:10.1016/j.euroneuro.2016.10.006
Bonouvrié, L. A., Becher, J. G., Vles, J. S. H., Vermeulen, R. J., and Buizer, A. I.IDYS Study Group (2019). The effect of intrathecal baclofen in dyskinetic cerebral palsy: the IDYS trial. Ann. Neurol. 86, 79–90. doi:10.1002/ana.25498
Bonouvrié, L. A., Lagendijk, K. E., Beckerman, H., Slot, K. M., van de Pol, L. A., and Buizer, A. I. (2022). Surgical complications of intrathecal baclofen in children: a single centre, 20-year retrospective cohort study. Eur. J. Paediatr. Neurol. 37, 94–97. doi:10.1016/j.ejpn.2022.02.002
Caron, E., Morgan, R., and Wheless, J. W. (2014). An unusual cause of flaccid paralysis and coma: baclofen overdose. J. Child. Neurol. 29, 555–559. doi:10.1177/0883073813479668
Cespedes, J., Escobar Vidarte, O. A., Uparela, M. J., Osorio-Fonseca, E., and Alvernia, J. E. (2024). History and evolution of surgical treatment for spasticity: a journey from neurotomy to selective dorsal rhizotomy. Neurosurg. Focus 56, E2. doi:10.3171/2024.3.FOCUS2452
Chaignot, C., Zureik, M., Rey, G., Dray-Spira, R., Coste, J., and Weill, A. (2018). Risk of hospitalisation and death related to baclofen for alcohol use disorders: comparison with nalmefene, acamprosate, and naltrexone in a cohort study of 165 334 patients between 2009 and 2015 in France. Pharmacoepidem. Dr. S. 27, 1239–1248. doi:10.1002/pds.4635
Chauvin, K. J., Blake, P. G., Garg, A. X., Weir, M. A., Bathini, L., Dixon, S. N., et al. (2020). Baclofen has a risk of encephalopathy in older adults receiving dialysis. Kidney Int. 98, 979–988. doi:10.1016/j.kint.2020.04.047
Chen, H., He, G., Huang, J., Hu, L., and Ma, J. (2024). Ocular adverse events associated with antibody-drug conjugates: a comprehensive pharmacovigilance analysis. Front. Immunol. 15, 1495137. doi:10.3389/fimmu.2024.1495137
Creamer, M., Cloud, G., Kossmehl, P., Yochelson, M., Francisco, G. E., Ward, A. B., et al. (2018a). Effect of intrathecal baclofen on pain and quality of life in poststroke spasticity. Stroke 49, 2129–2137. doi:10.1161/STROKEAHA.118.022255
Creamer, M., Cloud, G., Kossmehl, P., Yochelson, M., Francisco, G. E., Ward, A. B., et al. (2018b). Intrathecal baclofen therapy versus conventional medical management for severe poststroke spasticity: results from a multicentre, randomised, controlled, open-label trial (SISTERS). J. Neurol. Neurosurg. Psychiatry 89, 642–650. doi:10.1136/jnnp-2017-317021
De Sousa, N., Santos, D., Monteiro, S., Silva, N., Barreiro-Iglesias, A., and Salgado, A. J. (2022). Role of baclofen in modulating spasticity and neuroprotection in spinal cord injury. J. Neurotrauma 39, 249–258. doi:10.1089/neu.2020.7591
Ding, P., Luo, Q., and Cao, L. (2025). Drug-induced kidney stones: a real-world pharmacovigilance study using the FDA adverse event reporting system database. Front. Pharmacol. 16, 1511115. doi:10.3389/fphar.2025.1511115
Farhat, S., El Halabi, T., Makki, A., Atweh, S. F., Nasreddine, W., and Beydoun, A. (2020). Coma with absent brainstem reflexes and a burst suppression on EEG secondary to baclofen toxicity. Front. Neurol. 11, 404. doi:10.3389/fneur.2020.00404
Finnimore, A. J., Roebuck, M., Sajkov, D., and McEvoy, R. D. (1995). The effects of the GABA agonist, baclofen, on sleep and breathing. Eur. Respir. J. 8, 230–234. doi:10.1183/09031936.95.08020230
Fu, J. L., and Perloff, M. D. (2022). Pharmacotherapy for spine-related pain in older adults. Drug. Aging 39, 523–550. doi:10.1007/s40266-022-00946-x
Garbutt, J. C., Kampov-Polevoy, A. B., Pedersen, C., Stansbury, M., Jordan, R., Willing, L., et al. (2021). Efficacy and tolerability of baclofen in a U.S. community population with alcohol use disorder: a dose-response, randomized, controlled trial. Neuropsychopharmacology 46, 2250–2256. doi:10.1038/s41386-021-01055-w
Ghannoum, M., Berling, I., Lavergne, V., Roberts, D. M., Galvao, T., Hoffman, R. S., et al. (2021). Recommendations from the EXTRIP workgroup on extracorporeal treatment for baclofen poisoning. Kidney Int. 100, 720–736. doi:10.1016/j.kint.2021.07.014
Gong, Z., Umoru, G., Monge, J., Shah, N., Mohyuddin, G. R., Radhakrishnan, S. V., et al. (2024). Adverse effects and non-relapse mortality of BCMA directed T cell therapies in multiple myeloma: an FAERS database study. Blood Cancer J. 14, 36. doi:10.1038/s41408-024-01023-9
Guan, X., Yang, Y., Li, X., Feng, Y., Li, J., and Li, X. (2024). Analysis of eplerenone in the FDA adverse event reporting system (FAERS) database: a focus on overall patient population and gender-specific subgroups. Front. Pharmacol. 15, 1417951. doi:10.3389/fphar.2024.1417951
Heetla, H. W., Staal, M. J., Proost, J. H., and van Laar, T. (2014). Clinical relevance of pharmacological and physiological data in intrathecal baclofen therapy. Arch. Phys. Med. Rehabil. 95, 2199–2206. doi:10.1016/j.apmr.2014.04.030
Holla, B., Gowda, G. S., Prabhu, L., Baby, S., Viswanath, B., Chand, P., et al. (2015). High doses of Baclofen as suicide attempt in patients with alcohol use disorders - a serious concern. Asian J. Psychiatry 17, 99–100. doi:10.1016/j.ajp.2015.06.015
Huang, S., Dong, H., Luo, D., Jiang, J., Liu, M., Wu, J., et al. (2024). Adverse events associated with carbamazepine: a pharmacovigilance study using the FDA Adverse Event Reporting System. Expert Opin. Drug Saf., 1–13. doi:10.1080/14740338.2024.2416926
Hwang, Y. J., Chang, A. R., Brotman, D. J., Inker, L. A., Grams, M. E., and Shin, J.-I. (2023). Baclofen and the risk of encephalopathy: a real-world, active-comparator cohort study. Mayo Clin. Proc. 98, 676–688. doi:10.1016/j.mayocp.2022.11.004
Imerci, A., Rogers, K., Dixit, D., McManus, M., Miller, F., and Sees, J. P. (2020a). The effectiveness of epidural blood patch in patients with cerebral palsy treated with intrathecal baclofen implantation. Pediatr. Anesth. 30, 153–160. doi:10.1111/pan.13791
Imerci, A., Rogers, K. J., Miller, F., and Sees, J. P. (2020b). Evaluation of risk factors for cerebrospinal leakage in pediatric patients with cerebral palsy treated with intrathecal baclofen. J. Pediatr. Orthop. 40, e522–e526. doi:10.1097/BPO.0000000000001472
Imerci, A., Rogers, K. J., Pargas, C., Sees, J. P., and Miller, F. (2019). Identification of complications in paediatric cerebral palsy treated with intrathecal baclofen pump: a descriptive analysis of 15 years at one institution. J. Child. Orthop. 13, 529–535. doi:10.1302/1863-2548.13.190112
Karol, D. E., Muzyk, A. J., and Preud’homme, X. A. (2011). A case of delirium, motor disturbances, and autonomic dysfunction due to baclofen and tizanidine withdrawal: a review of the literature. Gen. Hosp. Psychiatry 33, 84.e1–84.e2. doi:10.1016/j.genhosppsych.2010.10.003
Khemiri, L., Kuja-Halkola, R., Larsson, H., and Jayaram-Lindström, N. (2016). Genetic overlap between impulsivity and alcohol dependence: a large-scale national twin study. Psychol. Med. 46, 1091–1102. doi:10.1017/S0033291715002652
Kofler, M., Quirbach, E., Schauer, R., Singer, M., and Saltuari, L. (2009). Limitations of intrathecal baclofen for spastic hemiparesis following stroke. Neurorehabil Neural Repair 23, 26–31. doi:10.1177/1545968308317700
Lad, S. P., Li, G., Lin, S.-C., and Henderson, J. M. (2008). Intracranial hypotension from intrathecal baclofen pump insertion. A case report and review of the literature. Stereotact. Funct. Neurosurg. 86, 75–79. doi:10.1159/000112427
Li, B., Hu, X., and Yue, Z. (2024). Drug-induced hearing disorders: a disproportionality analysis of the FAERS database. Front. Pharmacol. 15, 1480994. doi:10.3389/fphar.2024.1480994
Li, R., Yuan, X., Chen, X., Ou, Y., and Chen, J. (2024). Analysis of adverse drug reactions of Denosumab (Prolia) in osteoporosis based on FDA adverse event reporting system (FAERS). Front. Pharmacol. 15, 1358592. doi:10.3389/fphar.2024.1358592
Li, Y., Zhang, J., Yang, G., Jiao, S., and Guan, M. (2025). Identifying cardiovascular toxicity associated with sphingosine 1-phosphate receptor modulators: a case-control study based on the FDA adverse event reporting system. Int. Immunopharmacol. 153, 114520. doi:10.1016/j.intimp.2025.114520
Limerick, G., Christo, D. K., Tram, J., Moheimani, R., Manor, J., Chakravarthy, K., et al. (2023). Complex regional pain syndrome: evidence-based advances in concepts and treatments. Curr. Pain Headache Rep. 27, 269–298. doi:10.1007/s11916-023-01130-5
Marquez, J. G., Tariq, H., and Kashif, M. (2015). Encephalopathy and hypotonia due to baclofen toxicity in a patient with end-stage renal disease. Am. J. Case Rep. 16, 232–235. doi:10.12659/AJCR.893222
McGinley, M. P., Goldschmidt, C. H., and Rae-Grant, A. D. (2021). Diagnosis and treatment of multiple sclerosis: a review. JAMA 325, 765–779. doi:10.1001/jama.2020.26858
Meillier, A., Heller, C., and Patel, S. (2015). Baclofen-induced encephalopathy in end stage renal disease. Case Rep. Med. 2015, 203936. doi:10.1155/2015/203936
Miller, J. J. (2017). Baclofen overdose mimicking anoxic encephalopathy: a case report and review of the literature. Ther. Adv. Drug Saf. 8, 165–167. doi:10.1177/2042098617693571
Patton, K., and Borshoff, D. C. (2018). Adverse drug reactions. Anaesthesia 73 (Suppl. 1), 76–84. doi:10.1111/anae.14143
Pierce, M., Sutterland, A., Beraha, E. M., Morley, K., and Van Den Brink, W. (2018). Efficacy, tolerability, and safety of low-dose and high-dose baclofen in the treatment of alcohol dependence: a systematic review and meta-analysis. Eur. Neuropsychopharmacol. 28, 795–806. doi:10.1016/j.euroneuro.2018.03.017
Romito, J. W., Turner, E. R., Rosener, J. A., Coldiron, L., Udipi, A., Nohrn, L., et al. (2021). Baclofen therapeutics, toxicity, and withdrawal: a narrative review. SAGE Open Med. 9, 20503121211022197. doi:10.1177/20503121211022197
Salim, S. A., Thomas, L., Achanti, A., Gööz, M. B., Castaneda, J., Arany, I., et al. (2018). Baclofen-induced neurotoxicity in patients with compromised renal function: review. Int. J. Clin. Pharmacol. Ther. 56, 467–475. doi:10.5414/CP203243
Scosyrev, E., Behr, S., Jain, D., Ponnuru, A., and Michel, C. (2025). Disproportionality analysis and causal inference in drug safety. Pharm. Med. 39, 97–107. doi:10.1007/s40290-024-00549-4
Shkodina, A. D., Bardhan, M., Chopra, H., Anyagwa, O. E., Pinchuk, V. A., Hryn, K. V., et al. (2024). Pharmacological and non-pharmacological approaches for the management of neuropathic pain in multiple sclerosis. Cns Drugs 38, 205–224. doi:10.1007/s40263-024-01072-5
Shlobin, N. A., Hofmann, K., Keating, R. F., and Oluigbo, C. O. (2023). Deep brain stimulation and intrathecal/intraventricular baclofen for glutaric aciduria type 1: a scoping review, individual patient data analysis, and clinical trials review. J. Inherit. Metab. Dis. 46, 543–553. doi:10.1002/jimd.12638
Shu, Y., Wang, Y., Liu, J., Hu, L., Tong, S., Wu, G., et al. (2024). Safety assessment of deutetrabenazine: real-world adverse event analysis from the FAERS database. Front. Pharmacol. 15, 1498215. doi:10.3389/fphar.2024.1498215
Wan, H., Xu, X., Yi, D., and Shuai, K. (2024). Real-world safety evaluation of atorvastatin: insights from the US FDA adverse event reporting system (FAERS). Expert Opin. Drug Saf. 24, 305–314. doi:10.1080/14740338.2024.2424438
Wilson, C., Linczer, J., Newman, S., Weyhenmeyer, J., Roper, A., Miller, J., et al. (2023). Intrathecal baclofen and opioid therapy: cerebrospinal fluid leak and infection incidence, risk factors, and outcomes. World Neurosurg. 171, e456–e463. doi:10.1016/j.wneu.2022.12.039
Xia, S., Li, Y.-F., Raschi, E., Zhang, B.-K., Noguchi, Y., Sarangdhar, M., et al. (2024). Disproportional signal of pericarditis with biological diseasemodifying antirheumatic drugs (bDMARDs) in patients with ankylosing spondylitis: a disproportionality analysis in the FAERS database. Front. Pharmacol. 15, 1275814. doi:10.3389/fphar.2024.1275814
Yang, Y., Wei, S., Tian, H., Cheng, J., Zhong, Y., Zhong, X., et al. (2024). Adverse event profile of memantine and donepezil combination therapy: a real-world pharmacovigilance analysis based on FDA adverse event reporting system (FAERS) data from 2004 to 2023. Front. Pharmacol. 15, 1439115. doi:10.3389/fphar.2024.1439115
Zahra, E., Darke, S., Lappin, J., Duflou, J., and Farrell, M. (2024). Baclofen-related deaths in Australia 2000-2022. Forensic Sci. Int. 365, 112281. doi:10.1016/j.forsciint.2024.112281
Zhou, Y., Jia, P., Fang, Y., Zhu, W., Gong, Y., Fan, T., et al. (2024). Comprehensive understanding of the adverse effects associated with temozolomide: a disproportionate analysis based on the FAERS database. Front. Pharmacol. 15, 1437436. doi:10.3389/fphar.2024.1437436
Keywords: adverse events, drug safety, FAERS, baclofen, disproportionality analysis, pharmacovigilance, TTO
Citation: Zhao Y, Hu H, Mao J, He J, Zhang Y and Yang X (2025) Neurological adverse events associated with baclofen: a pharmacovigilance study based on FDA adverse event reporting system. Front. Pharmacol. 16:1569602. doi: 10.3389/fphar.2025.1569602
Received: 01 February 2025; Accepted: 30 April 2025;
Published: 14 May 2025.
Edited by:
Claudio Rivera, Aix-Marseille Université, FranceReviewed by:
Eli Ehrenpreis, Advocate Lutheran General Hospital, United StatesChiara Pennisi, Kore University of Enna, Italy
Copyright © 2025 Zhao, Hu, Mao, He, Zhang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaokai Yang, eWFrZXdvcmxkQHdtdS5lZHUuY24=
†These authors have contributed equally to this work
 Yunhan Zhao
Yunhan Zhao Haoxiang Hu
Haoxiang Hu Jiesheng Mao
Jiesheng Mao Jianghai He
Jianghai He Xiaokai Yang
Xiaokai Yang