- 1Department of Integrated Traditional Chinese and Western Medicine, Shandong Public Health Clinical Center, Shandong University, Jinan, China
- 2Department of Oncology, Zibo Central Hospital, Zibo, China
- 3Department of Respiratory Medicine, Shandong Public Health Clinical Center, Shandong University, Jinan, China
The combination of chemotherapy and immune checkpoint inhibitors (ICIs) represents a promising strategy for enhancing the efficacy of tumor immunotherapy. This review elaborates on its mechanisms and clinical significances. Chemotherapy-induced immunogenic cell death (ICD) serves as the foundation of this therapeutic synergy, involving the release of damage-associated molecular patterns (DAMPs) such as calreticulin, ATP, and HMGB1, which enhance immune activation in the presence of ICIs. Clinical trials have demonstrated that this combination approach markedly improves clinical outcomes across multiple tumor types, including non-small cell lung cancer, melanoma, bladder cancer, and triple-negative breast cancer. In clinical practice, this combination is increasingly adopted as a first-line or advanced-stage treatment, often guided by personalized medicine approaches. However, several challenges persist, including the management of treatment-related toxicity, high costs, and the identification of predictive biomarkers.
1 Introduction
Tumor immunotherapy has fundamentally transformed the therapeutic landscape of cancer treatment (Yasinjan et al., 2023; Rui et al., 2023; da Silva et al., 2019). Immune checkpoint inhibitors (ICIs), including anti-PD-1 and anti-PD-L1 antibodies, have shown substantial effectiveness in certain patient populations (Dall’Olio et al., 2022; Naimi et al., 2022). These agents function by blocking inhibitory signals on T cells, thereby enhancing the immune system’s ability to target and eliminate tumor cells. However, not all patients respond favorably, and resistance to ICIs continues to pose a significant clinical challenge (Sun and Xu, 2020; Sharma and Allison, 2015; Hughes et al., 2016).
Chemotherapy, a longstanding cornerstone in cancer treatment, utilizes various drugs that primarily target rapidly proliferating cells, including cancer cells (Knezevic and Clarke, 2020; Jiang et al., 2024). These agents exert their effects by inducing DNA damage (Bai et al., 2024), arresting cell cycle progression (Sun et al., 2021a), and triggering cell death. Specifically, chemotherapy-induced ICD serves as the pivotal process enabling the immune system to recognize and attack tumor cells more effectively when combined with ICIs. ICD is marked by the exposure and release of immunostimulatory signals—particularly calreticulin, ATP, and HMGB1—which collectively enhance T cell-mediated immune responses (Bian et al., 2022; Obeid et al., 2007; Kroemer et al., 2013).
The synergistic potential of combining chemotherapy and ICIs is promising. This dual approach can amplify the immune system’s antitumor response (Qian et al., 2022; Galluzzi et al., 2020). Chemotherapy triggers ICD, leading to tumor antigen release and TME modulation, which subsequently activates antigen-presenting cells and promotes T cells recruitment to the tumor site. ICIs can block T cells’ inhibitory signals, further boosting their antitumor efficiency (Zouein et al., 2022). This combinatorialstrategy helps overcome the limitations of monotherapies, providing a more comprehensive and potent anticancer approach. It holds the promise of improved patient outcomes, particularly for those who do not respond adequately to either ICIs or chemotherapy alone (Roskoski, 2024; Li et al., 2022).
In addition to inducing immunogenic cell death, chemotherapy exerts multiple immunomodulatory effects within the tumor microenvironment. For instance, chemotherapeutic agents like cyclophosphamide and gemcitabine have been demonstrated to selectively deplete immunosuppressive cells, including regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs). This depletion alleviats local immune suppression and promoting effector T-cell infiltration and activity. Moreover, chemotherapy can enhance antigen presentation by upregulating major histocompatibility complex (MHC) class I molecules on tumor cells, thus improving tumor recognition by cytotoxic T lymphocytes (CTLs) (Galluzzi et al., 2015). These multifaceted immunological alterations, when combined with ICIs, foster a more permissive immune landscape that significantly enhances antitumor efficacy relative to monotherapy (Pfirschke et al., 2016).
2 Clinical trial outcomes of the combination of chemotherapy and ICIs in tumor immunotherapy
2.1 Melanoma
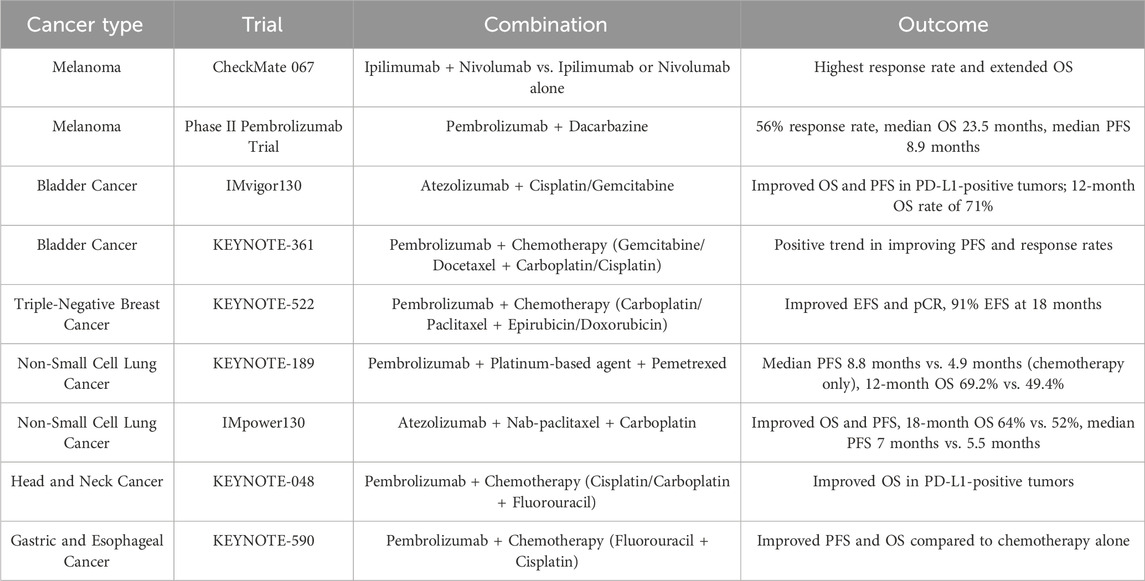
In the treatment of melanoma, combining chemotherapy with ICIs has emerged as a promising strategy. The CheckMate 067 trial compared the effects of ipilimumab and nivolumab combination therapy to ipilimumab or nivolumab alone in patients with melanoma (Wan et al., 2021; Choueiri Toni et al., 2023; Owonikoko et al., 2021). The results were noteworthy, with the combination regimen yielding the highest response rate and the longest OS observed. The median OS didn’t achieve the combination group, while patients receiving ipilimumab alone had a median OS of 19.9 months and 36.9 months in the nivolumab-alone group. These findings indicate that the combination of ipilimumab and nivolumab exerts a synergistic effect, intensifying the antitumor immune response and substantially improving survival outcomes.
In addition, pembrolizumab combined with chemotherapy has been tested in melanoma patients. A phase II trial examined the use of pembrolizumab alongside dacarbazine in metastatic melanoma patients. The outcomes suggest that this combination achieved a 56% response rate, with a median OS of 23.5 months and a median PFS of 8.9 months. These results suggest that integratingchemotherapy with ICIs may represent an effective therapeutic approach for melanoma, capable of improving both response rates and survival.
2.2 Bladder cancer
In the treatment of bladder cancer, combining chemotherapy with ICIs has shown notable advantages. The IMvigor130 trial investigated the addition of atezolizumab to chemotherapy (cisplatin and gemcitabine) vs. chemotherapy alone in patients with metastatic or locally advanced urothelial carcinoma (Funt et al., 2022; Galsky et al., 2024; Balar et al., 2017). The results showed a notable improvement in OS and FPS among patients with PD-L1-positive tumors. The combination therapy group experienced a median PFS of 8.2 months, while the chemotherapy-only group had a median PFS of 6.3 months. Additionally, the 12-month OS rate was 71% in the combination group, compared to 62% in those treated with chemotherapy alone.
The KEYNOTE-361 trial trial assessed the efficacy of pembrolizumab combined with chemotherapy (gemcitabine or docetaxel plus carboplatin or cisplatin) compared to chemotherapy alone in patients with urothelial carcinoma. While the combination therapy did not meet its primary endpoint for OS, it showed a positive trend toward improvoved PFS and response rates (Suzuki et al., 2023; Kelley et al., 2023; Sharma et al., 2024; Nakamura et al., 2023).
2.3 Triple-negative breast cancer
In triple-negative breast cancer (TNBC), the combination of chemotherapy and ICIs has produced encouraging results. For instance, the KEYNOTE-522 trial assessed the use of pembrolizumab combined with chemotherapy (carboplatin and paclitaxel followed by epirubicin or doxorubicin and cyclophosphamide) vs. chemotherapy alone in early-stage TNBC patients (Rizzo et al., 2022; Pusztai et al., 2024; Dent et al., 2024; Zhao et al., 2023). The trial demonstrated significant improvements in both event-free survival (EFS) and pathological complete response (pCR) for the combination treatment. Specifically, patients receiving the combination therapy achieved an 18-month event-free survival (EFS) rate of 91%, significantly higher than the 85% observed in those treated with chemotherapy alone. Additionally, the combination group achieved a pCR rate of 65%, which was higher than the 51% observed with chemotherapy alone. These results suggest that incorporating ICIs into chemotherapy could enhance outcomes for patients with TNBC, potentially lowering recurrence rates and boosting survival. This combination approach may offer a promising strategy for patients with this aggressive breast cancer subtype. A summary of key clinical trials is presented in Table 1.
2.4 Non-small cell lung cancer
In non-small cell lung cancer (NSCLC), combining chemotherapy with ICIs has led to significant improvements in patient outcomes. The KEYNOTE-189 trial evaluated pembrolizumab in combination with chemotherapy (a platinum-based agent and pemetrexed) versus chemotherapy alone in patients with metastatic nonsquamous NSCLC (Gandhi et al., 2018; Wu et al., 2022; Yang et al., 2022; Huang et al., 2024). The findings were compelling, showing a marked improvement in progression-free survival (PFS) among patients receiving the combination therapy. In the combination group, the median PFS in the combination group was 8.8 months, significantly longer than the 4.9 months observed in the chemotherapy-alone group. This data highlights a significant delay in disease progression and offering patients more time with stable disease and improved quality of life. Additionally, the overall survival (OS) benefit was impressive, with 12-month OS rates of 69.2% in the combination group vs. 49.4% in the chemotherapy-alone group. These results indicate not only delayed disease progression but also a clinically meaningful extension in overall survival.
The IMpower130 trial further confirmed the efficacy of this combination in NSCLC. This trial compared atezolizumab combined with chemotherapy (nab-paclitaxel and carboplatin) to chemotherapy alone for the treatment of advanced nonsquamous NSCLC (Zhang et al., 2023; Felip et al., 2021; Horn et al., 2018; Park et al., 2023). The oresults confirmed that the combination therapy significantly enhanced OS and PFS. The 18-month OS rate was 64% in the combination set vs. 52% in the chemotherapy-alone set, while the median PFS was 7 months in the combination set, vs. 5.5 months in the chemotherapy-only set, while.
2.5 Other tumor types
The combination of ICIs and chemotherapy has also been investigated in several other malignancies, including head and neck, gastric, and esophageal cancers. In head and neck cancer, the KEYNOTE-048 trial assessed the effect of pembrolizumab combined with chemotherapy (cisplatin or carboplatin plus fluorouracil) in comparison to chemotherapy alone or with cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma (Burtness et al., 2022; Harrington et al., 2022; Burtness et al., 2019). The study demonstrated that the addition of pembrolizumab to chemotherapy significantly improved OS in patients with PD-L1-positive tumors compared to chemotherapy alone.
Similarly, the KEYNOTE-590 trial evaluated pembrolizumab combined with chemotherapy (fluorouracil and cisplatin) versus chemotherapy alone in patients with gastroesophageal junction or esophageal cancer (Kojima et al., 2022; Sun et al., 2021b; Kato et al., 2019). This combination treatment significantly enhanced both PFS and OS compared to chemotherapy alone.
2.6 Real-world applications
For certain malignancies, such as NSCLC, the combination of pembrolizumab and chemotherapy has been established as a standard first-line treatment. This regimen has demonstrated superior PFS and OS compared to chemotherapy alone (Gandhi et al., 2018). Similarly, in melanoma, the combination of chemotherapy with ICIs such as ipilimumab and nivolumab may also be incorporated into treatment regimen (Larkin et al., 2015). For patients with advanced-stage cancers, particularly those with limited treatment options and poor prognoses after prior therapies, combining chemotherapy with ICIs may improve quality of life and delay disease progression. For instance, in TNBC and bladder cancer, the addition of pembrolizumab or atezolizumab to chemotherapy has shown promising benefits for patients (Emens et al., 2021).
In clinical practice, there is an increasing emphasis on personalized medicine approach. Physicians increasingly tailor treatment strategies according to tumor-specific characteristics, including biomarker expression profiles. Tumors with high PD-L1 expression may respond better to the combination therapy, while those with lower expression may require alternative strategies. Genomic profiling plays a crucial role in identifying mutations or alterations that may be more responsive to this combined treatment, thereby allowing for a more individualized and potentially more effective therapeutic approach (Xu et al., 2024; Malone et al., 2020).
2.7 Selected failed or negative trials
Although numerous clinical trials have demonstrated the efficacy of chemotherapy combined with ICIs, several studies have reported limited or negligible benefits. For instance, the KEYNOTE-361 trial evaluated pembrolizumab in combination with chemotherapy versus chemotherapy alone in advanced urothelial carcinoma. Despite showing a trend toward improved PFS, it failed to meet the primary endpoints for OS or PFS statistically (Powles et al., 2021). Potential reasons for this failure include a heterogeneous patient population with variable PD-L1 expression, suboptimal selection of chemotherapy agents for immune synergy, and insufficient biomarker-based stratification.
Another example is the IMvigor211 study in metastatic urothelial cancer, where atezolizumab failed to demonstrate OS superiority compared to chemotherapy in patients with high PD-L1 expression (Powles et al., 2018). Although early-phase trials yielded promising results, phase III studies failed to replicate these benefits, underscoring the variability of immune responses and the critical need for improved patient selection strategies. These failed trials highlight the importance of biomarker-guided patient selection, appropriate chemotherapy pairing, and understanding of tumor immunobiology to enhance future trial success. It is worth noting that the chemotherapeutic agents used in these successful combinations are recognized for their ability to robustly induce ICD, significantly contributing to the observed clinical benefits.
3 Analysis of clinical trial success factors
A comparative analysis of clinical trials highlights notable similarities and differences in treatment outcomes but alsoreveals several critical factors underlying the varying degrees of success observed when combining chemotherapy with immune checkpoint inhibitors (ICIs).
3.1 Commonalities and individualities across tumor types
Clinical trials spanning diverse cancer types (e.g., NSCLC, melanoma, bladder cancer, TNBC) consistently demonstrate that high PD-L1 expression is positively associated with superior clinical outcomes, as evidenced by studies such as KEYNOTE-189 and IMvigor130. Nevertheless, tumor-intrinsic characteristics significantly influence the therapeutic benefit. Melanoma and NSCLC typically exhibit more robust responses to chemotherapy-ICI combinations, This heightened responsiveness is likely attributable to their relatively higher tumor mutational burdens (TMB) and intrinsic immunogenicity, which enhance the potential for immune recognition and attack. In sharp contrast, bladder cancer demonstrates variable responses to such combinations. This variability indicates that the complexity and heterogeneity of the tumor microenvironment may profoundly impact the tumor’s responsiveness to chemotherapy-ICI regimens, as illustrated by the findings of KEYNOTE-361 (Reck et al., 2016; Galsky et al., 2024).
3.2 Why some treatments worked and others did not
Successful clinical trials, including KEYNOTE-189 (NSCLC) and KEYNOTE-522 (TNBC) typically employed regimens integrating chemotherapy agents with proven immunogenic potential (e.g., platinum compounds, taxanes). These regimens were highly effective in enhancing antigen presentation, depleting immunosuppressive populations (e.g., Tregs, MDSCs), and robustly inducing ICD, thereby potentiating the effect of ICIs. Conversely, trials with limited success or failures—such as KEYNOTE-361 in bladder cancer—commonly exhibited inadequate patient stratification, suboptimal chemotherapy drug selection, or less favorable immune modulation. These included insufficient patient stratification, suboptimal selection of chemotherapy drugs, and ineffective immune modulation. Such deficiencies impeded the creation of an optimal immune microenvironment, which is essential for the successful activity of ICIs (Gandhi et al., 2018; Felip et al., 2021; Pusztai et al., 2024).
3.3 Factors influencing superior outcomes
The selection of chemotherapy selection plays a pivotal role in determining treatment efficacy. Platinum-based chemotherapy regimens (KEYNOTE-189, IMpower130) consistently yield improved clinical outcomes due to their potent immunomodulatory effects, including robust ICD induction and enhanced CTL infiltration into tumors. Timing and dosing are equally critical: administering chemotherapy concurrently with or shortly before ICIs maximizes immune priming and antigen release, fosteringa favorable immune environment that significantly enhances therapeutic efficacy. A key determinant of success in chemotherapy-ICI combinations is the ability of chemotherapeutic agents to induce ICD. Agents such as anthracyclines, oxaliplatin, and cyclophosphamide are well documented to elicit strong ICD responses (Zitvogel et al., 2010). The combination of pembrolizumab and certain chemotherapy drugs (KEYNOTE-361) in bladder cancer illustrates such a scenario, indicating that not all chemotherapy agents synergize equally well with ICIs (Galluzzi et al., 2015; Pfirschke et al., 2016).
4 Mechanisms of action
The synergistic effect of chemotherapy and ICIs relies on a multifaceted mechanism network that collectively boost the antitumor immune response. As a cornerstone of cancer treatment, chemotherapy influences the immune system through various pathways. A pivotal mechanism is the induction of immunogenic cell death: chemotherapy-induced damage to cancer cells leads to the release of tumor antigens and DAMPs (Galluzzi et al., 2017; Kroemer et al., 2022). Tumor antigens are captured and processed by antigen-presenting cells (APCs), while DAMPs act as “danger signals” that alert the immune system. This dual activation primes APCs to mature and migrate to lymph nodes, where they initiate a cascade of immune responses—including the activation of CTLs—to recognize and eliminate tumor cells. Moreover, chemotherapy can modify the TME, which is inherentlyimmunosuppressive, filled with factors that dampen immune cell activity. A core fundamental mechanism driving the efficacy of chemotherapy in this combination is its ability to induce ICD, characterized by the release of DAMPs—including calreticulin, ATP, and HMGB1. Calreticulin promotes dendritic cell phagocytosis of dying cancer cells, ATP serves as a chemoattractant and immunomodulator for dendritic cells, and HMGB1 enhances antigen presentation and T-cell priming (Szulc and Woźniak, 2024; Li et al., 2024; Zhou et al., 2019; Kepp et al., 2014). By reducing immunosuppressive elements and releasing these immunostimulatory signals, chemotherapy transforms the TME into a more “inflammatory” state, thereby enhancing the responsiveness of ICIs and enabling a robust antitumor immune response.
ICIs are pivotal in amplifying the antitumor immune response. T cells express inhibitory receptorslike PD-1 on their surface, while tumor cells in the TME (tumor microenvironment) often overexpress ligands such as PD-L1 (Wei et al., 2018; Kuzume et al., 2020). When PD-L1 on tumor cells interacts with PD-1 on T cells, it transmitsan inhibitory signal that suppresses T cell activity, suppressing effective tumor attack. ICIs, such as anti-PD-L1 or anti-PD-1 antibodies, block this interaction, essentially releasing the inhibitory “brakes” on T cells, enabling them to restore their antitumor activity.
When used in combination, ICIs and chemotherapy work synergistically (Hodi et al., 2010; Peggs and Quezada, 2010). Chemotherapy initiates a cascade by inducing ICD and alters the TME, thereby creating favorable conditions for immune activation. The activation of APCs and the release of tumor antigens prime naïve T cells and recruit them to the tumor site. Concurrently, ICIs prevent these T cells from being inhibited by the immune evasion strategies employed by tumors, allowing them to mount a stronger attack against tumor cells. This integrated approach offers a promising strategy to overcome the limitations of each treatment on its own and enhance the overall effectiveness of cancer therapy (Brahmer et al., 2015; Horn et al., 2017). As illustrated in Figure 1, the synergistic mechanism of chemotherapy-induced ICD in combination with ICIs. By promoting the exposure and release of damage-associated molecular patterns (DAMPs)—such as calreticulin, ATP, and HMGB1—chemotherapy enhances dendritic cell (DC) activation and T cell priming. This process creates a highly immunogenic TME that, when paired with ICIs, amplifies the anti-tumor immune response through sustained T cell-mediated tumor elimination.
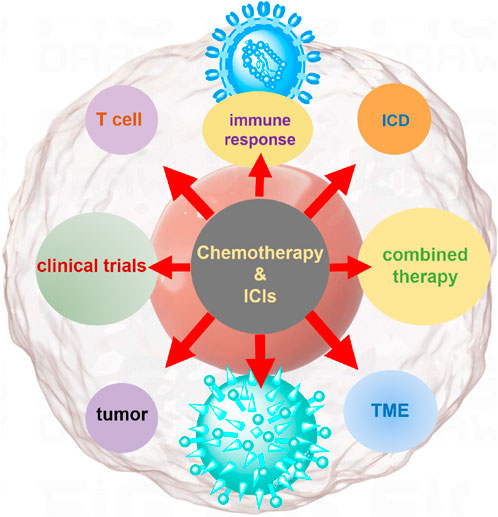
Figure 1. Mechanistic illustration of chemotherapy in conjunction with ICIs for tumor treatment. Chemotherapy induces ICD, leading to the release of tumor-associated antigens and DAMPs, such as ATP, calreticulin, and HMGB1. These signals activate dendritic cells (DCs), which process and present tumor antigens to CD8+ T cells. Chemotherapy also modulates the tumor microenvironment by reducing immunosuppressive populations such as Tregs and MDSCs. Meanwhile, immune checkpoint inhibitors (e.g., anti-PD-1, anti-PD-L1) block inhibitory signals between tumor cells and T cells, restoring T cell cytotoxic function. The combination of chemotherapy and ICIs enhances T cell activation, tumor infiltration, and tumor cell killing, providing a synergistic anti-tumor immune response.
5 Conclusion
In conclusion, the combination of chemotherapy and ICIs represents a highly promising strategy in tumor treatment. Mechanistically, chemotherapy induces immunogenic cell death and reprograms the tumor microenvironment, while ICIs block inhibitory signals on T cells, working synergistically to enhance the antitumor immune response. Clinical trials across diverse tumor types, including NSCLC, melanoma, bladder cancer, and TNBC, have demonstrated improved patient outcomes such as enhanced progression - free survival and overall survival. In clinical practice, this combination is increasingly utilized as a first-line or advanced treatment option, with a growing emphasis on personalized medicine. However, several challenges warrant attention, including toxicity management, cost considerations, and the identification of predictive biomarkers to guide patient selection. Future research should prioritize optimizing treatment protocols to further enhance the efficacy and safety of this combination, ultimately providing better treatment options for cancer patients. The analysis of unsuccessful trials underscore the necessity for meticulous trial design, including patient selection based on predictive biomarkers, appropriate chemotherapy regimens that promote ICD, and strategic treatment sequencing. These elements are indispensable for optimizing the therapeutic potential of chemotherapy and ICI combinations.
Author contributions
CL: Data curation, Writing – original draft, Writing – review and editing. XQ: Validation, Writing – review and editing. MY: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bai, B., Ma, Y., Liu, D., Zhang, Y., Zhang, W., Shi, R., et al. (2024). DNA damage caused by chemotherapy has duality, and traditional Chinese medicine may be a better choice to reduce its toxicity. Front. Pharmacol. 15, 1483160. doi:10.3389/fphar.2024.1483160
Balar, A. V., Galsky, M. D., Rosenberg, J. E., Powles, T., Petrylak, D. P., Bellmunt, J., et al. (2017). Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 389 (10064), 67–76. doi:10.1016/S0140-6736(16)32455-2
Bian, X., Jiang, H., Meng, Y., Li, Y.-p., Fang, J., and Lu, Z. (2022). Regulation of gene expression by glycolytic and gluconeogenic enzymes. Trends Cell Biol. 32 (9), 786–799. doi:10.1016/j.tcb.2022.02.003
Brahmer, J., Reckamp Karen, L., Baas, P., Crinò, L., Eberhardt Wilfried, E. E., Poddubskaya, E., et al. (2015). Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N. Engl. J. Med. 373 (2), 123–135. doi:10.1056/NEJMoa1504627
Burtness, B., Harrington, K. J., Greil, R., Soulières, D., Tahara, M., de Castro, G., et al. (2019). Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394 (10212), 1915–1928. doi:10.1016/S0140-6736(19)32591-7
Burtness, B., Rischin, D., Greil, R., Soulières, D., Tahara, M., de Castro, Jr G., et al. (2022). Pembrolizumab alone or with chemotherapy for recurrent/metastatic head and neck squamous cell carcinoma in KEYNOTE-048: subgroup analysis by programmed death ligand-1 combined positive score. J. Clin. Oncol. 40 (21), 2321–2332. doi:10.1200/JCO.21.02198
Choueiri Toni, K., Powles, T., Albiges, L., Burotto, M., Szczylik, C., Zurawski, B., et al. (2023). Cabozantinib plus nivolumab and ipilimumab in renal-cell carcinoma. N. Engl. J. Med. 388 (19), 1767–1778. doi:10.1056/NEJMoa2212851
Dall’Olio, F. G., Marabelle, A., Caramella, C., Garcia, C., Aldea, M., Chaput, N., et al. (2022). Tumour burden and efficacy of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 19 (2), 75–90. doi:10.1038/s41571-021-00564-3
da Silva, J. L., Dos Santos, A. L. S., Nunes, N. C. C., de Moraes Lino da Silva, F., Ferreira, C. G. M., and de Melo, A. C. (2019). Cancer immunotherapy: the art of targeting the tumor immune microenvironment. Cancer Chemother. Pharmacol. 84 (2), 227–240. doi:10.1007/s00280-019-03894-3
Dent, R., Cortés, J., Pusztai, L., McArthur, H., Kümmel, S., Bergh, J., et al. (2024). Neoadjuvant pembrolizumab plus chemotherapy/adjuvant pembrolizumab for early-stage triple-negative breast cancer: quality-of-life results from the randomized KEYNOTE-522 study. JNCI J. Natl. Cancer Inst. 116 (10), 1654–1663. doi:10.1093/jnci/djae129
Emens, L. A., Molinero, L., Loi, S., Rugo, H. S., Schneeweiss, A., Diéras, V., et al. (2021). Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer: biomarker evaluation of the IMpassion130 study. JNCI J. Natl. Cancer Inst. 113 (8), 1005–1016. doi:10.1093/jnci/djab004
Felip, E., Altorki, N., Zhou, C., Csőszi, T., Vynnychenko, I., Goloborodko, O., et al. (2021). Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 398 (10308), 1344–1357. doi:10.1016/S0140-6736(21)02098-5
Funt, S. A., Lattanzi, M., Whiting, K., Al-Ahmadie, H., Quinlan, C., Teo, M. Y., et al. (2022). Neoadjuvant atezolizumab with gemcitabine and cisplatin in patients with muscle-invasive bladder cancer: a multicenter, single-arm, phase II trial. J. Clin. Oncol. 40 (12), 1312–1322. doi:10.1200/JCO.21.01485
Galluzzi, L., Buqué, A., Kepp, O., Zitvogel, L., and Kroemer, G. (2017). Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 17 (2), 97–111. doi:10.1038/nri.2016.107
Galluzzi, L., Buquأ, A., Kepp, O., Zitvogel, L., and Kroemer, G. (2015). Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 28 (6), 690–714. doi:10.1016/j.ccell.2015.10.012
Galluzzi, L., Humeau, J., Buqué, A., Zitvogel, L., and Kroemer, G. (2020). Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 17 (12), 725–741. doi:10.1038/s41571-020-0413-z
Galsky, M. D., Guan, X., Rishipathak, D., Rapaport, A. S., Shehata, H. M., Banchereau, R., et al. (2024). Immunomodulatory effects and improved outcomes with cisplatin-versus carboplatin-based chemotherapy plus atezolizumab in urothelial cancer. Cell Rep. Med. 5 (2), 101393. doi:10.1016/j.xcrm.2024.101393
Gandhi, L., Rodríguez-Abreu, D., Gadgeel, S., Esteban, E., Felip, E., De Angelis, F., et al. (2018). Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N. Engl. J. Med. 378 (22), 2078–2092. doi:10.1056/nejmoa1801005
Harrington, K. J., Burtness, B., Greil, R., Soulières, D., Tahara, M., de Castro, G., et al. (2022). Pembrolizumab with or without chemotherapy in recurrent or metastatic head and neck squamous cell carcinoma: updated results of the phase III KEYNOTE-048 study. J. Clin. Oncol. 41 (4), 790–802. doi:10.1200/JCO.21.02508
Hodi, F. S., O'Day Steven, J., McDermott David, F., Weber, R. W., Sosman Jeffrey, A., Haanen John, B., et al. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363 (8), 711–723.
Horn, L., Mansfield, A. S., Szczęsna, A., Havel, L., Krzakowski, M., Hochmair Maximilian, J., et al. (2018). First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 379 (23), 2220–2229. doi:10.1056/NEJMoa1809064
Horn, L., Spigel, D. R., Vokes, E. E., Holgado, E., Ready, N., Steins, M., et al. (2017). Nivolumab versus docetaxel in previously treated patients with advanced non–small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J. Clin. Oncol. 35 (35), 3924–3933. doi:10.1200/JCO.2017.74.3062
Huang, S., Huang, Z., Huang, X., Luo, R., Liang, W., and Qin, T. (2024). Comparative long-term outcomes of pembrolizumab plus chemotherapy versus pembrolizumab monotherapy as first-line therapy for metastatic non-small-cell lung cancer: a systematic review and network meta-analysis. Front. Immunol. 15, 1375136. doi:10.3389/fimmu.2024.1375136
Hughes, P. E., Caenepeel, S., and Wu, L. C. (2016). Targeted therapy and checkpoint immunotherapy combinations for the treatment of cancer. Trends Immunol. 37 (7), 462–476. doi:10.1016/j.it.2016.04.010
Jiang, H., Gong, Q., Zhang, R., and Yuan, H. (2024). Tetrazine-based metal-organic frameworks. Coord. Chem. Rev. 499, 215501. doi:10.1016/j.ccr.2023.215501
Kato, K., Shah, M. A., Enzinger, P., Bennouna, J., Shen, L., Adenis, A., et al. (2019). KEYNOTE-590: phase III study of first-line chemotherapy with or without pembrolizumab for advanced esophageal cancer. Future Oncol. 15 (10), 1057–1066. doi:10.2217/fon-2018-0609
Kelley, R. K., Ueno, M., Yoo, C., Finn, R. S., Furuse, J., Ren, Z., et al. (2023). Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 401 (10391), 1853–1865. doi:10.1016/S0140-6736(23)00727-4
Kepp, O., Laura, S., Ilio, V., Erika, V., Sandy, A., Patrizia, A., et al. (2014). Consensus guidelines for the detection of immunogenic cell death. OncoImmunology 3 (9), e955691. doi:10.4161/21624011.2014.955691
Knezevic, C. E., and Clarke, W. (2020). Cancer chemotherapy: the case for therapeutic drug monitoring. Ther. Drug Monit. 42 (1), 6–19. doi:10.1097/FTD.0000000000000701
Kojima, T., Hara, H., Tsuji, A., Yasui, H., Muro, K., Satoh, T., et al. (2022). First-line pembrolizumab + chemotherapy in Japanese patients with advanced/metastatic esophageal cancer from KEYNOTE-590. Esophagus 19 (4), 683–692. doi:10.1007/s10388-022-00920-x
Kroemer, G., Galassi, C., Zitvogel, L., and Galluzzi, L. (2022). Immunogenic cell stress and death. Nat. Immunol. 23 (4), 487–500. doi:10.1038/s41590-022-01132-2
Kroemer, G., Galluzzi, L., Kepp, O., and Zitvogel, L. (2013). Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 31, 51–72. doi:10.1146/annurev-immunol-032712-100008
Kuzume, A., Chi, S., Yamauchi, N., and Minami, Y. (2020). Immune-checkpoint blockade therapy in lymphoma. Int. J. Mol. Sci. 21, 5456. doi:10.3390/ijms21155456
Larkin, J., Chiarion-Sileni, V., Gonzalez, R., Grob Jean, J., Cowey, C. L., Lao Christopher, D., et al. (2015). Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373 (1), 23–34. doi:10.1056/NEJMoa1504030
Li, C., Kaur, A., Pavlidaki, A., Spenlé, C., Rajnpreht, I., Donnadieu, E., et al. (2024). Targeting the MAtrix REgulating MOtif abolishes several hallmarks of cancer, triggering antitumor immunity. Proc. Natl. Acad. Sci. U. S. A. 121, e2404485121. doi:10.1073/pnas.2404485121
Li, K., Yu, H., Bao, Z., Xu, L., Zhang, H., Wang, T., et al. (2022). Combination of photosensitizer and immune checkpoint inhibitors for improving the efficacy of tumor immunotherapy. Int. J. Pharm. 629, 122384. doi:10.1016/j.ijpharm.2022.122384
Malone, E. R., Oliva, M., Sabatini, P. J. B., Stockley, T. L., and Siu, L. L. (2020). Molecular profiling for precision cancer therapies. Genome Med. 12 (1), 8. doi:10.1186/s13073-019-0703-1
Naimi, A., Mohammed, R. N., Raji, A., Chupradit, S., Yumashev, A. V., Suksatan, W., et al. (2022). Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun. Signal. 20 (1), 44. doi:10.1186/s12964-022-00854-y
Nakamura, R., Hasegawa, G., Ohashi, K., Hashimoto, T., Ikeda, Y., Hara, N., et al. (2023). Primary lung cancer treatable with radical resection after complete remission with pembrolizumab therapy following gemcitabine and carboplatin chemotherapy for multiple metastases of bladder cancer. IJU Case Rep. 6 (1), 85–88. doi:10.1002/iju5.12550
Obeid, M., Tesniere, A., Ghiringhelli, F., Fimia, G. M., Apetoh, L., Perfettini, J.-L., et al. (2007). Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 13 (1), 54–61. doi:10.1038/nm1523
Owonikoko, T., Park, K., Govindan, R., Ready, N., Reck, M., Peters, S., et al. (2021). Nivolumab and ipilimumab as maintenance therapy in extensive-disease small-cell lung cancer: CheckMate 451. J. Clin. Oncol. 39, 1349–1359. JCO.20.02212. doi:10.1200/JCO.20.02212
Park, S., Kim, T. M., Han, J.-Y., Lee, G.-W., Shim, B. Y., Lee, Y.-G., et al. (2023). Phase III, randomized study of atezolizumab plus bevacizumab and chemotherapy in patients with EGFR- or ALK-mutated non-small-cell lung cancer (ATTLAS, KCSG-LU19-04). J. Clin. Oncol. 42 (11), 1241–1251. doi:10.1200/JCO.23.01891
Peggs, K., and Quezada, S. (2010). Ipilimumab: attenuation of an inhibitory immune checkpoint improves survival in metastatic melanoma. Expert Rev. Anticancer Ther. 10, 1697–1701. doi:10.1586/era.10.144
Pfirschke, C., Engblom, C., Rickelt, S., Cortez-Retamozo, V., Garris, C., Pucci, F., et al. (2016). Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity 44 (2), 343–354. doi:10.1016/j.immuni.2015.11.024
Powles, T., Csإëszi, T., أñzgأroؤülu, M., Matsubara, N., Gأczi, L., Cheng, S. Y. S., et al. (2021). Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 22 (7), 931–945. doi:10.1016/S1470-2045(21)00152-2
Powles, T., Durأn, I., van der Heijden, M. S., Loriot, Y., Vogelzang, N. J., De Giorgi, U., et al. (2018). Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 391 (10122), 748–757. doi:10.1016/S0140-6736(17)33297-X
Pusztai, L., Denkert, C., O’Shaughnessy, J., Cortes, J., Dent, R., McArthur, H., et al. (2024). Event-free survival by residual cancer burden with pembrolizumab in early-stage TNBC: exploratory analysis from KEYNOTE-522☆. Ann. Oncol. 35 (5), 429–436. doi:10.1016/j.annonc.2024.02.002
Qian, X., Hu, W., and Yan, J. (2022). Nano-Chemotherapy synergize with immune checkpoint inhibitor- A better option? Front. Immunol. 13, 963533. doi:10.3389/fimmu.2022.963533
Reck, M., Rodrأguez-Abreu, D., Robinson Andrew, G., Hui, R., Csإëszi, T., Fأlأ٦p, A., et al. (2016). Pembrolizumab versus chemotherapy for PD-L1قÄ…Positive NonقÄ…Small-cell lung cancer. N. Engl. J. Med. 375 (19), 1823–1833. doi:10.1056/nejmoa1606774
Rizzo, A., Cusmai, A., Acquafredda, S., Giovannelli, F., Rinaldi, L., Misino, A., et al. (2022). KEYNOTE-522, IMpassion031 and GeparNUEVO: changing the paradigm of neoadjuvant immune checkpoint inhibitors in early triple-negative breast cancer. Future Oncol. 18 (18), 2301–2309. doi:10.2217/fon-2021-1647
Roskoski, R. (2024). Combination immune checkpoint and targeted protein kinase inhibitors for the treatment of renal cell carcinomas. Pharmacol. Res. 203, 107181. doi:10.1016/j.phrs.2024.107181
Rui, R., Zhou, L., and He, S. (2023). Cancer immunotherapies: advances and bottlenecks. Front. Immunol. 14, 1212476. doi:10.3389/fimmu.2023.1212476
Sharma, P., and Allison, J. (2015). Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161, 205–214. doi:10.1016/j.cell.2015.03.030
Sharma, P., Stecklein, S. R., Yoder, R., Staley, J. M., Schwensen, K., O’Dea, A., et al. (2024). Clinical and biomarker findings of neoadjuvant pembrolizumab and carboplatin plus docetaxel in triple-negative breast cancer: NeoPACT phase 2 clinical trial. JAMA Oncol. 10 (2), 227–235. doi:10.1001/jamaoncol.2023.5033
Sun, C., and Xu, S. (2020). Advances in personalized neoantigen vaccines for cancer immunotherapy. Biosci. Trends 14 (5), 349–353. doi:10.5582/bst.2020.03267
Sun, J.-M., Shen, L., Shah, M. A., Enzinger, P., Adenis, A., Doi, T., et al. (2021b). Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 398 (10302), 759–771. doi:10.1016/S0140-6736(21)01234-4
Sun, Y., Liu, Y., Ma, X., and Hu, H. (2021a). The influence of cell cycle regulation on chemotherapy. Int. J. Mol. Sci. 22, 6923. doi:10.3390/ijms22136923
Suzuki, R., Hamada, K., Ohkuma, R., Homma, M., Tsurui, T., Iriguchi, N., et al. (2023). Case Report: combined pembrolizumab, 5-fluorouracil, and cisplatin therapy were remarkably effective in p16-positive squamous cell carcinoma of unknown primary. Front. Oncol. 13, 1231986. doi:10.3389/fonc.2023.1231986
Szulc, A., and Woźniak, M. (2024). Targeting pivotal hallmarks of cancer for enhanced therapeutic strategies in triple-negative breast cancer treatment—in vitro, in vivo and clinical trials literature review. Cancers 16, 1483. doi:10.3390/cancers16081483
Wan, X., Zeng, X., Peng, L., Peng, Y., Liu, Q., Yi, L., et al. (2021). Cost-effectiveness analysis of nivolumab plus ipilimumab for advanced non-small-cell lung cancer. Front. Pharmacol. 12. doi:10.3389/fphar.2021.580459
Wei, S. C., Duffy, C. R., and Allison, J. P. (2018). Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8 (9), 1069–1086. doi:10.1158/2159-8290.CD-18-0367
Wu, M., Qin, S., Wang, L., Tan, C., Peng, Y., Zeng, X., et al. (2022). Cost-effectiveness of pembrolizumab plus chemotherapy as first-line therapy for advanced oesophageal cancer. Front. Pharmacol. 13, 881787. doi:10.3389/fphar.2022.881787
Xu, W., Jia, A., Lei, Z., Wang, J., Jiang, H., Wang, S., et al. (2024). Stimuli-responsive prodrugs with self-immolative linker for improved cancer therapy. Eur. J. Med. Chem. 279, 116928. doi:10.1016/j.ejmech.2024.116928
Yang, Z., Chen, Y., Wang, Y., Hu, M., Qian, F., Zhang, Y., et al. (2022). Pembrolizumab plus chemotherapy versus chemotherapy monotherapy as a first-line treatment in elderly patients (≥75 Years old) with non-small-cell lung cancer. Front. Immunol. 13, 807575. doi:10.3389/fimmu.2022.807575
Yasinjan, F., Xing, Y., Geng, H., Guo, R., Yang, L., Liu, Z., et al. (2023). Immunotherapy: a promising approach for glioma treatment. Front. Immunol. 14, 1255611. doi:10.3389/fimmu.2023.1255611
Zhang, C., Liu, Y., Tan, J., Tian, P., and Li, W. (2023). Cost-effectiveness evaluation based on two models of first-line atezolizumab monotherapy and chemotherapy for advanced non-small cell lung cancer with high-PDL1 expression. Front. Oncol. 13, 1093469. doi:10.3389/fonc.2023.1093469
Zhao, H., Yu, J., Zhang, R., Chen, P., Jiang, H., and Yu, W. (2023). Doxorubicin prodrug-based nanomedicines for the treatment of cancer. Eur. J. Med. Chem. 258, 115612. doi:10.1016/j.ejmech.2023.115612
Zhou, J., Wang, G., Chen, Y., Wang, H., Hua, Y., and Cai, Z. (2019). Immunogenic cell death in cancer therapy: present and emerging inducers. J. Cell. Mol. Med. 23 (8), 4854–4865. doi:10.1111/jcmm.14356
Zitvogel, L., Kepp, O., Senovilla, L., Menger, L., Chaput, N., and Kroemer, G. (2010). Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin. Cancer Res. 16 (12), 3100–3104. doi:10.1158/1078-0432.CCR-09-2891
Keywords: cancer therapy, ICIS, chemotherapy, immunotherapy, clinical applications
Citation: Li C, Qi X and Yan M (2025) Chemotherapy-induced immunogenic cell death in combination with ICIs: a brief review of mechanisms, clinical insights, and therapeutic implications. Front. Pharmacol. 16:1572195. doi: 10.3389/fphar.2025.1572195
Received: 06 February 2025; Accepted: 23 May 2025;
Published: 05 June 2025.
Edited by:
Xinyu Wang, Philadelphia College of Osteopathic Medicine (PCOM), United StatesReviewed by:
Dianzheng Zhang, Philadelphia College of Osteopathic Medicine (PCOM), United StatesCopyright © 2025 Li, Qi and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyan Qi, Njc0MzM4NDM2QHFxLmNvbQ==; Min Yan, eW12aXA3Nzc4QDE2My5jb20=
 Chengwei Li
Chengwei Li Xiaoyan Qi2*
Xiaoyan Qi2*