- 1Department of Urology, Kunshan Hospital of Traditional Chinese Medicine, Kunshan, Jiangsu, China
- 2Clinical Laboratory, Kunshan Rehabilitation Hospital, Kunshan, Jiangsu, China
- 3Institute of Basic Theory for Chinese Medicine, China Academy of Chinese Medical Sciences, Beijing, China
Recent advances in bladder cancer immunotherapy have shown promise, particularly in addressing limitations of the current gold standard, Bacillus Calmette-Guérin (BCG). Novel combinations, such as sasanlimab (a PD-1 monoclonal antibody) with BCG, have improved event-free survival in high-risk non-muscle-invasive bladder cancer (NMIBC). Intravesical anti-PD-1/PD-L1 agents like pembrolizumab and nadofaragene firadenovec have demonstrated efficacy and safety in BCG-unresponsive NMIBC, leading to regulatory approval. Additionally, BCG combined with immunostimulatory protein complexes (e.g., N-803) achieved high complete response rates while preserving quality of life. For muscle-invasive bladder cancer (MIBC) patients ineligible for cisplatin, neoadjuvant immunotherapy trials are exploring anti-PD-1/PD-L1 monotherapy or combinations with anti-CTLA-4 antibodies. The Pandore trial highlights the role of mucosal immunity in predicting response to systemic immune checkpoint inhibitors. Promising results have also been observed with intravesical oncolytic immunotherapy combined with systemic anti-PD-1 therapy in cisplatin-ineligible MIBC. These advancements underscore the potential of intravesical and systemic immunotherapies to improve bladder cancer outcomes and warrant further investigation.
Background
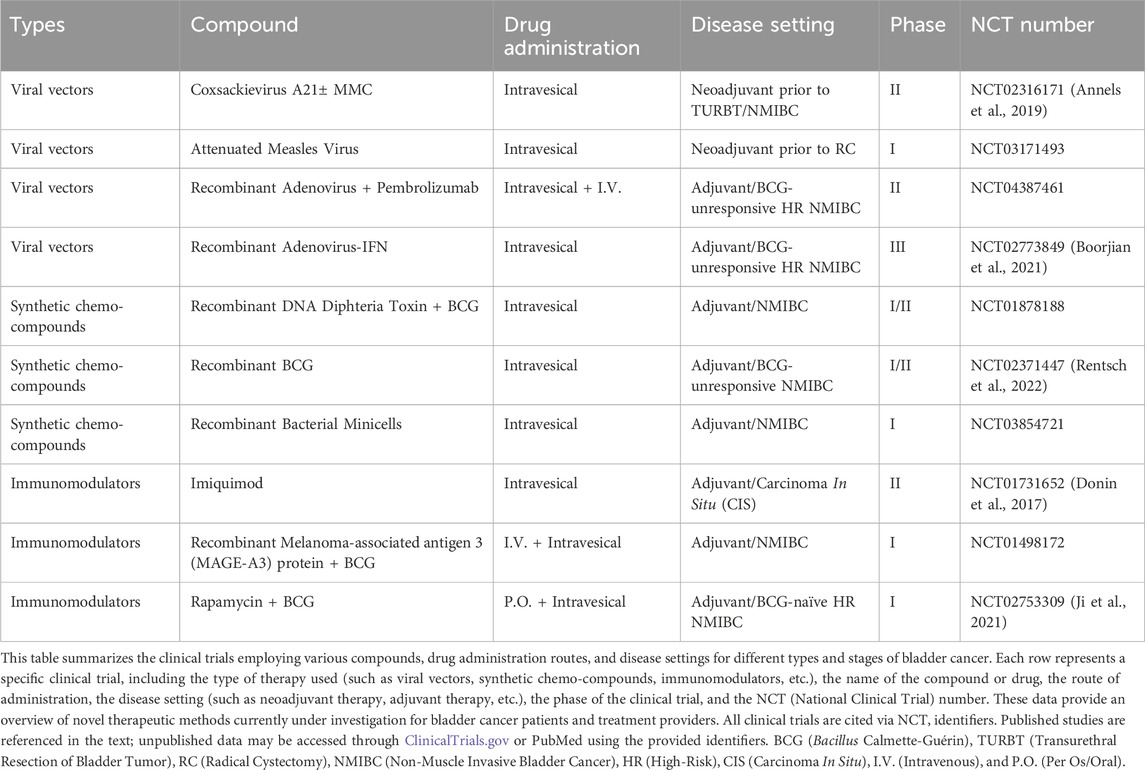
Bladder cancer (BCa), the most common malignancy affecting the urinary tract, stands as one of the most prevalent cancers worldwide. Despite the relative stagnation in clinical approaches to BCa over the years, recent advancements have ushered in a new era of diagnosis and management for this disease. As a distinct disease entity, bladder cancer poses unique challenges and presents promising opportunities for immunotherapy (Kumbham et al., 2025). The bladder, a hollow organ with a direct connection to the external environment through the urethra, offers a favorable setting for the local administration of therapeutic agents directly into the bladder lumen (Lopez-Beltran et al., 2024). This intravesical route of administration offers several advantages, including high local drug concentrations, reduced systemic toxicity, and the potential to stimulate a robust mucosal immune response. Since the publication of a perspective article in 2023 that highlighted bladder cancer as a platform for drug development targeting mucosal immunity, the field has seen significant advancements (Chung et al., 2023). This article provided a comprehensive overview of the current state and future prospects of cancer immunotherapy, particularly the intravesical administration of immunostimulatory agents, laying a solid foundation for subsequent research. Bacillus Calmette-Guérin (BCG), the gold standard therapy for high-risk non-muscle-invasive bladder cancer (NMIBC), while effective, still results in treatment failure within 2 years in 40%–50% of patients (Naselli et al., 2024). Thus, the development of novel therapeutic strategies is of paramount importance. In the ongoing pursuit of innovative treatments, the year 2023 marked a significant milestone in clinical drug development for bladder cancer. Chung’s article revealed 32 clinical drug development projects targeting bladder cancer, with 10 of these projects already completed by early 2025, as detailed in Table 1. These completed projects have not only enhanced our understanding of bladder cancer but have also laid the groundwork for advancements in immunotherapies. Figure 1 provides an overview of integrated immunotherapy strategies in bladder cancer, highlighting the synergy between intravesical priming and systemic immune modulation, along with emerging therapeutic innovations and future directions.
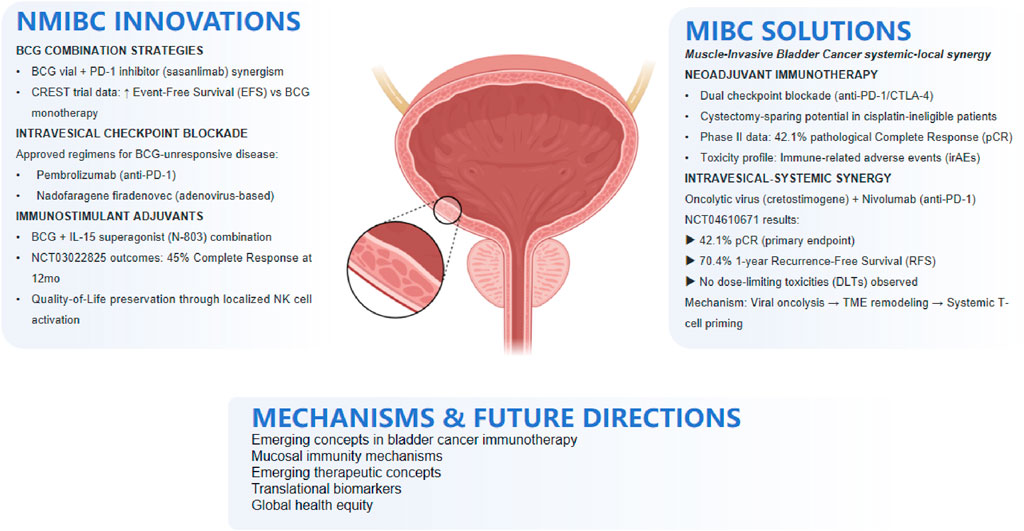
Figure 1. Integrated immunotherapy strategies in bladder cancer: From intravesical priming to systemic response.
Overview of recent advancements
As of early 2025, the progress in bladder cancer immunotherapy is encouraging. Recently, Pfizer announced that its PD-1-targeted monoclonal antibody, sasanlimab, met the primary endpoint in the pivotal Phase 3 CREST trial1. When combined with BCG as induction therapy (with or without maintenance therapy), sasanlimab significantly improved event-free survival (EFS) in BCG-naïve, high-risk NMIBC patients. Data from studies on intravesical administration of anti-PD-1/PD-L1 monoclonal antibodies have demonstrated their efficacy and safety in BCG-unresponsive patients, further confirming their potential as neoadjuvant treatment options (Steinberg et al., 2024). The first-in-human trial validated the feasibility and safety of intravesical pembrolizumab injection for BCG-unresponsive cancer patients (Meghani et al., 2022). Notably, while systemic PD-1 blockade has shown limited efficacy in such patients, intravesical administration elicited a robust immune response at the bladder mucosal surface without significant systemic toxicity. To date, two agents (pembrolizumab and nadofaragene firadenovec) have been approved (Guerrero-Ramos et al., 2024). This strategy not only triggers local anti-tumor immunity but also stimulates a systemic adaptive anti-tumor immune response in preclinical and early clinical studies, highlighting its investigational potential as a neoadjuvant therapy.
Combination therapy with BCG and immunostimulatory protein complexes
In clinical trials, the combination therapy of BCG with immunostimulatory protein complexes, such as N-803, has shown significant efficacy, providing new treatment options for NMIBC patients unresponsive to BCG therapy (Chamie et al., 2024a). The strategy of combining BCG with IL-15-based immunostimulatory protein complexes (like N-803) has made breakthrough progress in addressing the challenge of treating BCG-unresponsive patients. In the multicenter trial NCT03022825 (Chamie et al., 2024b), the combination therapy achieved a complete response rate of up to 45% at 12 months. This synergistic therapeutic effect may be attributed to N-803’s ability to activate natural killer (NK) cells, effectively combating MHC Class I-deficient cancer cells, thereby compensating for BCG’s deficiencies in inducing an immune response and improving patient outcomes and quality of life. Furthermore, the trial focused on patient-reported outcomes (PROs), showing that physical function (PF) and global health (GH) scores remained relatively stable during treatment, further confirming the advantages of this combination therapy in maintaining patient quality of life.
Neoadjuvant immunotherapy for muscle-invasive bladder cancer (MIBC)
Trials on neoadjuvant immunotherapy for muscle-invasive bladder cancer (MIBC) patients, particularly those ineligible for cisplatin, are deepening, aiming to enhance treatment efficacy and reduce toxicity. Although radical cystectomy combined with neoadjuvant cisplatin chemotherapy is the standard treatment, many patients are unsuitable for cisplatin due to high toxicity or underlying comorbidities. Therefore, emerging neoadjuvant trials are exploring the efficacy of anti-PD-1/PD-L1 monotherapy or combination therapy with anti-CTLA-4 monoclonal antibodies before radical cystectomy in cisplatin-ineligible patients.
Recent trials underscore the evolving role of neoadjuvant immunotherapy in cisplatin-ineligible MIBC, though toxicity management remains a priority. Grivas et al. (2021) evaluated neoadjuvant nivolumab (anti-PD-1) monotherapy or combined with lirilumab (anti-KIR) in cisplatin-ineligible patients, reporting dose-limiting toxicities (DLTs) that highlight the need for optimized regimens. Gazzoni et al. (2025) demonstrated that dual PD-1/PD-L1 and CTLA-4 inhibition increased pathological complete response (pCR) rates (42.1% vs. 29.2% for monotherapy), though grade ≥3 adverse events (e.g., colitis, hepatotoxicity) occurred in 35% of patients, limiting broader adoption. Anti-PD-1/PD-L1 monotherapy has shown moderate efficacy in cisplatin-ineligible cohorts. Necchi et al. (2018) reported a 34% pCR rate with pembrolizumab in high-risk MIBC, while Guercio et al. (2022) observed that nivolumab + ipilimumab combinations elevated pCR to 52.1% but incurred a 31.8% rate of severe toxicities, necessitating safer alternatives.
Innovative strategies integrating intravesical and systemic therapies aim to balance efficacy and safety. Li R. et al. (2022) combined CG0070 (GM-CSF-expressing oncolytic adenovirus) with systemic nivolumab, achieving a 42.1% pCR in cisplatin-ineligible MIBC. CG0070 acted as an “in situ vaccine” via tumor lysis and immune activation, synergizing with nivolumab without exacerbating systemic toxicity. Similarly, Cathomas et al. (2024) explored sequential intravesical BCG followed by chemo-immunotherapy, where BCG-induced PD-L1 upregulation enhanced subsequent PD-1/PD-L1 blockade efficacy, reducing chemotherapy reliance. Psutka et al. (2023) reported that TAR-200 (intravesical drug-eluting device) plus cetrelimab (anti-PD-1) achieved localized immune activation with minimal systemic exposure, offering a toxicity-reduction pathway. These data collectively support the rationale for localized immune priming (e.g., oncolytic viruses, BCG) to amplify systemic checkpoint inhibitor responses, thereby improving therapeutic indices in cisplatin-ineligible MIBC.
While these trials report generally favorable efficacy, they also highlight substantial toxicity issues, emphasizing the need to develop less toxic and more effective treatment strategies. Notably, the combination of intravesical delivery of immunotherapy with systemic immune checkpoint inhibition offers a novel and exciting treatment approach for MIBC patients.
Intravesical oncolytic immunotherapy and systemic immune checkpoint inhibition
The Pandore clinical trial indicates a close correlation between mucosal immunity and responsiveness to systemic immune checkpoint inhibitors. Exciting preliminary results have emerged from ongoing trials, such as NCT04610671 (Li et al., 2025), which evaluate the efficacy and safety of intravesical oncolytic viruses or chemotherapy combined with anti-PD-1 monoclonal antibodies in cisplatin-ineligible muscle-invasive bladder cancer (MIBC) patients. This trial assessed the efficacy and safety of intravesical injection of an oncolytic virus (cretostimogene grenadenorepvec, an oncolytic adenovirus type 5 encoding granulocyte-macrophage colony-stimulating factor) combined with systemic use of the anti-PD-1 monoclonal antibody nivolumab. Among the 21 enrolled and treated patients, no dose-limiting toxicity was observed. The combination therapy achieved a pathological complete response rate of 42.1% and a 1-year recurrence-free survival rate of 70.4%. Key efficacy and safety outcomes from the NCT04610671 trial are summarized in Table 2.
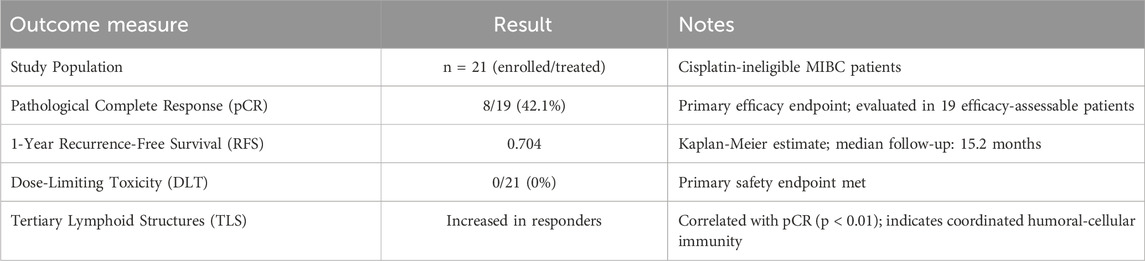
Table 2. Efficacy and safety outcomes of intravesical cretostimogene grenadenorepvec + systemic nivolumab in cisplatin-ineligible MIBC (NCT04610671).
Although intravesical oncolytic immunotherapy broadly induced T-cell infiltration, the formation, expansion, and maturation of tertiary lymphoid structures were particularly associated with complete responses, supporting the importance of a coordinated humoral and cellular immune response. Collectively, these results highlight the potential of this combination therapy to enhance treatment efficacy in cisplatin-intolerant MIBC patients and warrant further investigation as a neoadjuvant treatment option.
Future prospects
The trajectory of bladder cancer immunotherapy is poised for transformative advancement, driven by innovative intravesical-systemic synergies, precision medicine strategies, and global health equity initiatives. Emerging “prime-and-boost” paradigms, exemplified by intravesical oncolytic viruses combined with systemic anti-PD-1 agents, demonstrate enhanced T-cell activation through localized immunogenic cell death and systemic checkpoint blockade (Li X. et al., 2022). Such approaches may be further optimized by coupling intravesical adenoviruses encoding cytokines with bispecific antibodies targeting immune checkpoints, offering potential to overcome immunosuppressive barriers like DC-SIGN + macrophages in the tumor microenvironment (TME).
Precision immunotherapy will be refined by liquid biopsy technologies, such as urinary tumor DNA (utDNA) profiling, which enables real-time monitoring of therapeutic response and early relapse detection (Zhang et al., 2023). Concurrently, biomarkers like CD39 expression and immunoproteasome signatures hold promise for patient stratification, as validated in retrospective analyses of neoadjuvant trials. Prospective integration of these tools into trials could minimize toxicities by excluding non-responders (Cathomas et al., 2024).
Overcoming intrinsic resistance mechanisms remains critical, with emerging strategies targeting TME suppressors: anti-CCR4 antibodies for Treg depletion, CSF-1R inhibitors to counter MDSC activity, and IDO1/adenosine pathway modulators for metabolic reprogramming. For cisplatin-ineligible MIBC, bladder-sparing regimens—such as TAR-200 (intravesical drug-eluting device) plus cetrelimab—may redefine standards of care by balancing local control and quality of life (Williams et al., 2021).
Finally, addressing global disparities in BCG access necessitates scalable innovations, including recombinant BCG strains (e.g., VPM1002BC) and live-attenuated Listeria vectors expressing tumor antigens (Rentsch et al., 2022). Phase II data affirm comparable efficacy to conventional BCG, with logistical advantages for resource-limited settings. Collectively, these advancements promise to reshape bladder cancer care, fostering durable cures while prioritizing equity and patient-centered outcomes.
Conclusion
The field of bladder cancer immunotherapy is experiencing a transformative period, marked by significant advancements in intravesical approaches. The integration of novel agents like sasanlimab with BCG has demonstrated improved outcomes in high-risk NMIBC, while intravesical anti-PD-1/PD-L1 therapies have shown promise in BCG-unresponsive patients, leading to regulatory approvals. The combination of BCG with immunostimulatory protein complexes, such as N-803, has achieved high complete response rates while preserving patient quality of life. For MIBC patients ineligible for cisplatin, neoadjuvant immunotherapy trials are exploring anti-PD-1/PD-L1 monotherapy or combinations with anti-CTLA-4 antibodies, with intriguing results observed in trials combining intravesical oncolytic immunotherapy with systemic anti-PD-1 therapy. These developments underscore the potential of intravesical and systemic immunotherapies to revolutionize bladder cancer treatment paradigms. Future research should focus on refining these therapies to enhance efficacy, reduce toxicity, and expand treatment options for a broader patient population, ultimately improving outcomes and quality of life for those with bladder cancer. The ongoing evolution of immunotherapy in bladder cancer holds great promise and warrants continued investigation to fully realize its potential.
Author contributions
XT: Writing – original draft. TY: Writing – original draft, Writing – review and editing. HT: Conceptualization, Writing – review and editing. YW: Conceptualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project is generously supported by the Youth Fund of Kunshan Hospital of Traditional Chinese Medicine, with project number 2024QNJJ14.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Annels, N. E., Mansfield, D., Arif, M., Ballesteros-Merino, C., Simpson, G. R., Denyer, M., et al. (2019). Phase I trial of an ICAM-1-Targeted immunotherapeutic-coxsackievirus A21 (CVA21) as an oncolytic agent against non muscle-invasive bladder cancer. Clin. Cancer Res. 25 (19), 5818–5831. doi:10.1158/1078-0432.CCR-18-4022
Boorjian, S. A., Alemozaffar, M., Konety, B. R., Shore, N. D., Gomella, L. G., Kamat, A. M., et al. (2021). Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 22 (1), 107–117. doi:10.1016/S1470-2045(20)30540-4
Cathomas, R., Spahn, M., Hayoz, S., Chiquet, S., Schneider, M., Rentsch, C. A., et al. (2024). Intravesical recombinant BCG followed by perioperative chemo-immunotherapy for patients with muscle-invasive bladder cancer (MIBC): a multicenter, single arm phase 2 trial (SAKK 06/19). J. Clin. Oncol. 42 (4_Suppl. l), TPS707. doi:10.1200/jco.2024.42.4_suppl.tps707
Chamie, K., Chang, S. S., Kramolowsky, E. V., Gonzalgo, M. L., Huang, M., Bhar, P., et al. (2024b). Quality of life in the phase 2/3 trial of N-803 plus bacillus calmette-guérin in bacillus Calmette-Guérin‒Unresponsive nonmuscle-invasive bladder cancer. Urol. Pract. 11 (2), 367–375. doi:10.1097/UPJ.0000000000000517
Chamie, K., Chang, S. S., Rosser, C. J., Kramolowski, E., Gonzalgo, M. L., Sexton, W. J., et al. (2024a). N-803 plus BCG treatment for BCG-naïve or -Unresponsive non-muscle invasive bladder cancer: a plain language review. Future Oncol. 20 (31), 2307–2317. doi:10.1080/14796694.2024.2363744
Chung, R., McKiernan, J., Arpaia, N., Marabelle, A., and Rouanne, M. (2023). Neo-adjuvant immunotherapies: bladder cancer as a platform for drug development targeting mucosal immunity. Eur. J. Cancer 187, 58–64. doi:10.1016/j.ejca.2023.03.037
Donin, N. M., Chamie, K., Lenis, A. T., Pantuck, A. J., Reddy, M., Kivlin, D., et al. (2017). A phase 2 study of TMX-101, intravesical imiquimod, for the treatment of carcinoma in situ bladder cancer. Urol. Oncol. 35 (2), 39.e1–39. doi:10.1016/j.urolonc.2016.09.006
Gazzoni, G., Mamede, I., Lopes, G. M. M., and Stecca, C. (2025). Checkpoint blockade as neoadjuvant strategy: a single-arm meta-analysis of dual PD-1/PD-L1 and CTLA-4 inhibition in urothelial cancer. J. Clin. Oncol. 43 (5_Suppl. l), 783. doi:10.1200/jco.2025.43.5_suppl.783
Grivas, P., Yin, J., Koshkin, V. S., Cole, S., Jain, R. K., Dreicer, R., et al. (2021). PrE0807: a phase Ib feasibility trial of neoadjuvant nivolumab (N) without or with lirilumab (L) in cisplatin-ineligible patients (pts) with muscle-invasive bladder cancer (MIBC). J. Clin. Oncol. 39 (15_Suppl. l), 4518. doi:10.1200/jco.2021.39.15_suppl.4518
Guercio, B. J., Pietzak, E. J., Brown, S., Chen, J.-F., Peters, V., Regazzi, A. M., et al. (2022). Neoadjuvant nivolumab (N) +/- ipilimumab (I) in cisplatin-ineligible patients (pts) with muscle-invasive bladder cancer (MIBC). J. Clin. Oncol. 40 (6_Suppl. l), 498. doi:10.1200/jco.2022.40.6_suppl.498
Guerrero-Ramos, F., Boormans, J. L., Daneshmand, S., Gontero, P., Kamat, A. M., Rouprêt, M., et al. (2024). Novel delivery systems and pharmacotherapeutic approaches for the treatment of non-muscle-invasive bladder cancer. Eur. Urol. Oncol. 7 (6), 1267–1279. doi:10.1016/j.euo.2024.05.012
Ji, N., Mukherjee, N., Reyes, R. M., Gelfond, J., Javors, M., Meeks, J. J., et al. (2021). Rapamycin enhances BCG-Specific γδ T cells during intravesical BCG therapy for non-muscle invasive bladder cancer: a randomized, double-blind study. J. Immunother. Cancer 9 (3), e001941. doi:10.1136/jitc-2020-001941
Kumbham, S., Md Mahabubur Rahman, K., Foster, B. A., and You, Y. (2025). A comprehensive review of current approaches in bladder cancer treatment. ACS Pharmacol. Transl. Sci. 8 (2), 286–307. doi:10.1021/acsptsci.4c00663
Li, R., Spiess, P. E., Sexton, W. J., Gilbert, S. M., Poch, M. A., Pow-Sang, J. M., et al. (2022). Preliminary results from phase Ib/II neoadjuvant CG0070 and nivolumab (N) for cisplatin (C)-ineligible muscle invasive bladder cancer (MIBC). J. Clin. Oncol. 40 (16_Suppl. l), 4574. doi:10.1200/jco.2022.40.16_suppl.4574
Li, R., Villa, N. Y., Yu, X., Johnson, J. O., Borjas, G., Dhillon, J., et al. (2025). Oncolytic immunotherapy with nivolumab in muscle-invasive bladder cancer: a phase 1b trial. Nat. Med. 31 (1), 176–188. doi:10.1038/s41591-024-03324-9
Li, X., Lu, M., Yuan, M., Ye, J., Zhang, W., Xu, L., et al. (2022). CXCL10-armed oncolytic adenovirus promotes tumor-infiltrating T-cell chemotaxis to enhance anti-PD-1 therapy. Oncoimmunology 11 (1), 2118210. doi:10.1080/2162402X.2022.2118210
Lopez-Beltran, A., Cookson, M. S., Guercio, B. J., and Cheng, L. (2024). Advances in diagnosis and treatment of bladder cancer. BMJ 384, e076743. doi:10.1136/bmj-2023-076743
Meghani, K., Cooley, L. F., Choy, B., Kocherginsky, M., Swaminathan, S., Munir, S. S., et al. (2022). First-in-human intravesical delivery of pembrolizumab identifies immune activation in bladder cancer unresponsive to bacillus calmette-guérin. Eur. Urol. 82 (6), 602–610. doi:10.1016/j.eururo.2022.08.004
Naselli, A., Pirola, G. M., and Castellani, D. (2024). Bacillus calmette-guérin (BCG) refractory non-muscle-invasive bladder cancer (NMIBC): current guidance and experience from clinical practice. Res. Rep. Urol. 16, 299–305. doi:10.2147/RRU.S464068
Necchi, A., Anichini, A., Raggi, D., Briganti, A., Massa, S., Lucianò, R., et al. (2018). Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. J. Clin. Oncol. 36 (34), 3353–3360. doi:10.1200/jco.18.01148
Psutka, S. P., Cutie, C., Bhanvadia, S. K., Keegan, K. A., Crist, W., Tian, S. K., et al. (2023). SunRISe-4: TAR-200 plus cetrelimab or cetrelimab alone as neoadjuvant therapy in patients with muscle-invasive bladder cancer (MIBC) who are ineligible for or refuse neoadjuvant platinum-based chemotherapy. J. Clin. Oncol. 41 (6_Suppl. l), TPS584. doi:10.1200/jco.2023.41.6_suppl.tps584
Rentsch, C. A., Thalmann, G. N., Lucca, I., Kwiatkowski, M., Wirth, G. J., Strebel, R. T., et al. (2022). A phase 1/2 single-arm clinical trial of recombinant bacillus calmette-guérin (BCG) VPM1002BC immunotherapy in non-muscle-invasive bladder cancer recurrence after conventional BCG therapy: SAKK 06/14. Eur. Urol. Oncol. 5 (2), 195–202. doi:10.1016/j.euo.2021.12.006
Steinberg, G. D., Shore, N. D., Redorta, J. P., Galsky, M. D., Bedke, J., Ku, J. H., et al. (2024). CREST: phase III study of sasanlimab and bacillus calmette-guérin for patients with bacillus calmette-guérin-naïve high-risk non-muscle-invasive bladder cancer. Future Oncol. 20 (14), 891–901. doi:10.2217/fon-2023-0271
Williams, S. B., Cutie, C., Keegan, K. A., Raybold, B., Acharya, M., Zhu, W., et al. (2021). A phase 3, multicenter, randomized study evaluating the efficacy of TAR-200 in combination with cetrelimab versus concurrent chemoradiotherapy in participants with muscle-invasive urothelial carcinoma of the bladder. J. Clin. Oncol. 39 (15_Suppl. l), TPS4586. doi:10.1200/jco.2021.39.15_suppl.tps4586
Keywords: bladder cancer, urinary bladder neoplasms, immunotherapy, immune checkpoint inhibitors, neoadjuvant immunotherapy trials, mucosal immunity, intravesical drug administration
Citation: Tang X, Yu T, Tong H and Wu Y (2025) Advancements in bladder cancer immunotherapy: a focus on intravesical approaches. Front. Pharmacol. 16:1578146. doi: 10.3389/fphar.2025.1578146
Received: 17 February 2025; Accepted: 30 June 2025;
Published: 08 July 2025.
Edited by:
Ajit Prakash, University of North Carolina at Chapel Hill, United StatesReviewed by:
Harpreet Kaur, University Institute of Pharmaceutical Sciences, Panjab University, IndiaCopyright © 2025 Tang, Yu, Tong and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongxuan Tong, dGhvbmd4dWFuMUAxNjMuY29t; Yufan Wu, ZG9jd3V5dWZhbkBuanVtLmVkdS5jbg==
†These authors have contributed equally to this work
 Xia Tang1†
Xia Tang1† Hongxuan Tong
Hongxuan Tong Yufan Wu
Yufan Wu