- 1Department of Neurosurgery, First Hospital of Shanxi Medical University, Taiyuan, Shanxi, China
- 2Graduate School, Shanxi Medical University, Taiyuan, Shanxi, China
- 3Department of Neurology, Second Hospital of Shanxi Medical University, Taiyuan, Shanxi, China
- 4Department of Neurosurgery, Second Hospital of Shanxi Medical University, Taiyuan, Shanxi, China
Objective: Alkylating agents and bevacizumab are both first-line chemotherapeutic options for the treatment of glioblastoma; however, their mechanisms of action differ substantially. This study aimed to compare the safety profiles of these two drug classes in the treatment of glioblastoma to inform clinical decision-making.
Methods: Adverse events reported between the first quarter of 2004 and the fourth quarter of 2023 were analyzed using data from the FDA Adverse Event Reporting System (FAERS) database. Disproportionality analysis was employed to assess and compare the AE signals associated with bevacizumab and alkylating agents.
Results: In the context of glioblastoma treatment, 3,323 adverse reports were associated with bevacizumab, 5,283 with temozolomide, and 427 with lomustine. The most frequently reported AEs for bevacizumab were fatigue (n = 276), hypertension (n = 220), and headache (n = 199). Compared to temozolomide, bevacizumab was more strongly associated with “vascular disorders,” “renal and urinary disorders,” and “hypertension.” Notably, bevacizumab appeared to offer a potential safety advantage with respect to hematological adverse events.
Conclusion: Our analysis indicates that bevacizumab exhibits a distinct safety profile compared to alkylating agents, particularly demonstrating a lower incidence of hematological adverse events. Further prospective studies are warranted to validate these findings and to elucidate the underlying mechanisms responsible for the observed adverse events.
1 Introduction
Glioblastoma is the most aggressive and prevalent type of primary brain tumor (Dolecek et al., 2012). For over a decade, the standard treatment protocol for glioblastoma has involved maximal surgical resection, followed by concurrent radiotherapy and temozolomide chemotherapy, and subsequent adjuvant temozolomide therapy for 6–12 months (Stupp et al., 2017).
The pathology of glioblastoma is characterized by the overexpression of vascular endothelial growth factor-A (VEGF-A) (Chinot et al., 2011; Hicklin and Ellis, 2005), prompting the development of therapies targeting the angiogenic pathway (Batchelor et al., 2014). Bevacizumab, a humanized monoclonal antibody, specifically binds to VEGF and inhibits its activity (Yang et al., 2003). In 2009, bevacizumab was approved by the U.S. Food and Drug Administration (FDA) for the treatment of recurrent glioblastoma, demonstrating an improvement in progression-free survival among patients with disease progression (Friedman et al., 2009; Kreisl et al., 2009). The introduction of bevacizumab heralded a new class of anti-cancer therapies, and its immunomodulatory properties have expanded therapeutic options for glioblastoma (Garcia et al., 2020).
Although the therapeutic efficacy of both alkylating agents and bevacizumab is well established, their associated adverse effects warrant careful consideration. Temozolomide has been linked to risks such as anemia, thrombocytopenia, hepatotoxicity, nausea, and vomiting (Zhou et al., 2024). In contrast, adverse events related to bevacizumab include hypertension, embolism, hemorrhage, neutropenia, and gastrointestinal perforation (Tang et al., 2024). These adverse effects can not only compromise patient compliance but also pose significant challenges to clinicians in balancing efficacy with safety.
The FDA Adverse Event Reporting System (FAERS) is a valuable database for detecting potential safety signals associated with pharmaceutical products (Yu et al., 2021). Both alkylating agents and bevacizumab serve as first-line treatments for glioblastoma but exert their effects through distinct mechanisms. Leveraging the FAERS database and data mining techniques, this study aims to identify and compare the safety signals associated with these two therapies, ultimately providing clinicians with critical information to support informed decision-making in the management of glioblastoma.
2 Method
2.1 Study design and data acquisition
This study was designed as a retrospective analysis. The FAERS collects hundreds of millions of adverse event reports submitted by healthcare professionals, consumers, and manufacturers worldwide. We selected the FAERS database as the data source for this study to identify and compare the adverse events associated with bevacizumab and alkylating agents in the treatment of glioblastoma. The study was approved by the Ethics Committee of the First Hospital of Shanxi Medical University.
2.2 Data processing
The FAERS database consists of several key components, including patient demographics (DEMO), drug information (DRUG), reported adverse events (REAC), patient outcomes (OUTC), report sources (RPSR), treatment start and end dates (THER), and drug indications (INDI).
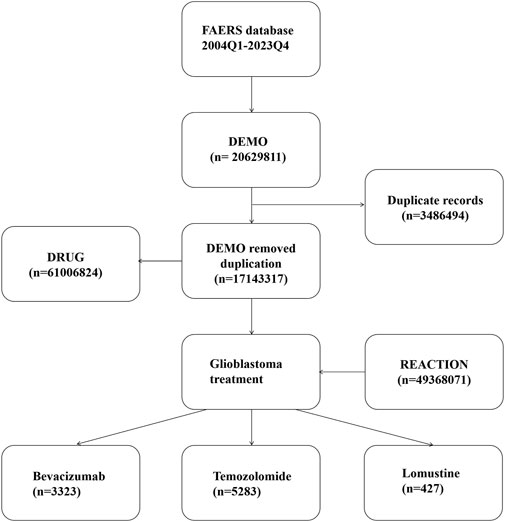
Since the database is updated quarterly, we implemented a de-duplication process in accordance with FDA guidelines: for identical CASEIDs, the most recent FDA_DT was retained; if both CASEID and FDA_DT were identical, the record with the larger PRIMARYID was selected. Additionally, to ensure data completeness and accuracy, we excluded entries listed in the ‘deleted cases’ files (Services USD, 2024). The de-duplication process is illustrated in Figure 1.
The study population consisted of patients whose drug indication was recorded as glioblastoma. The drugs of interest were bevacizumab, temozolomide, and lomustine. Synonyms were considered during data retrieval:
Bevacizumab: bevacizumab, anti-VEGF monoclonal antibody, bevacizumab-awwb.
Temozolomide: methazolastone, temozolodida, temozolomid, temozolomida, temozolomide, temozolomide, temozolomidum.
Lomustine: lomustina, lomustine, lomustinum; Drug identification in the DRUG table was performed through fuzzy matching of the ‘prod_ai’ and ‘drugname’ fields. To ensure that adverse events were drug-related, only reports where the “role_cod” was coded as “Primary Suspect” or “Secondary Suspect” were included.
2.3 Signal detection and statistical analysis
In this study, signal detection was conducted using the reporting odds ratio (ROR) method. A 2 × 2 contingency table was constructed based on the number of reported adverse events for the target drug and other drugs. The ROR and its 95% confidence interval (CI) were calculated as follows:
where:
a = number of reports of the target event with the target drug;
b = number of reports of other events with the target drug;
c = number of reports of the target event with other drugs;
d = number of reports of other events with other drugs.
Although the ROR method remains applicable when comparing a target drug to a control drug, a positive ROR result in this context should not be interpreted as a confirmed safety signal. Instead, it reflects the relative risk of specific adverse events between drugs at different classification levels (SOC and PT).
To facilitate visualization and interpretation, temozolomide was chosen as the control drug. Given the relatively small number of reports for lomustine compared with bevacizumab and temozolomide, lomustine was only included in descriptive analyses and excluded from comparative analyses.
2.4 Adverse event coding
Adverse events were coded according to the System Organ Class (SOC) and Preferred Term (PT) categories based on the Medical Dictionary for Regulatory Activities (MedDRA) version 25.0. MedDRA provides a hierarchical structure consisting of five levels: Lowest Level Term (LLT), Preferred Term (PT), High Level Term (HLT), High Level Group Term (HLGT), and System Organ Class (SOC). In our study, adverse events associated with bevacizumab were identified at both the SOC and PT levels to characterize its safety profile. Subsequently, the safety profiles of bevacizumab and temozolomide were compared at both levels, using temozolomide as a reference. Specifically, an ROR value greater than 1 indicated a stronger association between the adverse event and bevacizumab relative to temozolomide, whereas an ROR value less than 1 indicated a weaker association.
3 Results
3.1 Descriptive analysis
From Q1 2004 to Q4 2023, a total of 49,368,071 adverse event reports were extracted from the FAERS database, including 13,116 reports for bevacizumab, 15,621 reports for temozolomide, and 1,595 reports for lomustine (Figure 2). Data on adverse events related to the treatment of glioblastoma with these three drugs are shown in Figure 2.
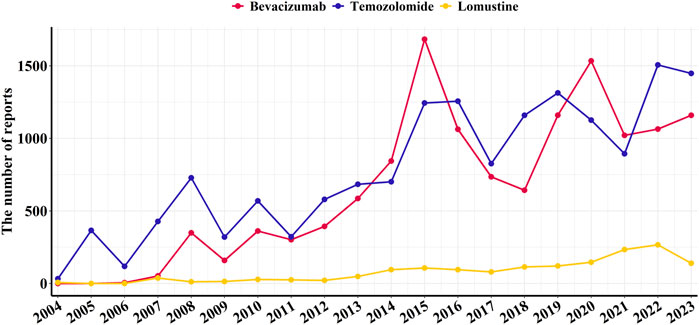
Figure 2. Annual number of reported adverse events associated with bevacizumab and alkylating agents from Q1 2004 to Q4 2023.
It is noteworthy that bevacizumab has been used for glioblastoma treatment primarily within the past decade; therefore, adverse event reports for bevacizumab were not available for every year. The temporal distribution of adverse event reports peaked in 2015 and 2017, with 332 and 341 reports, respectively (Table 1).
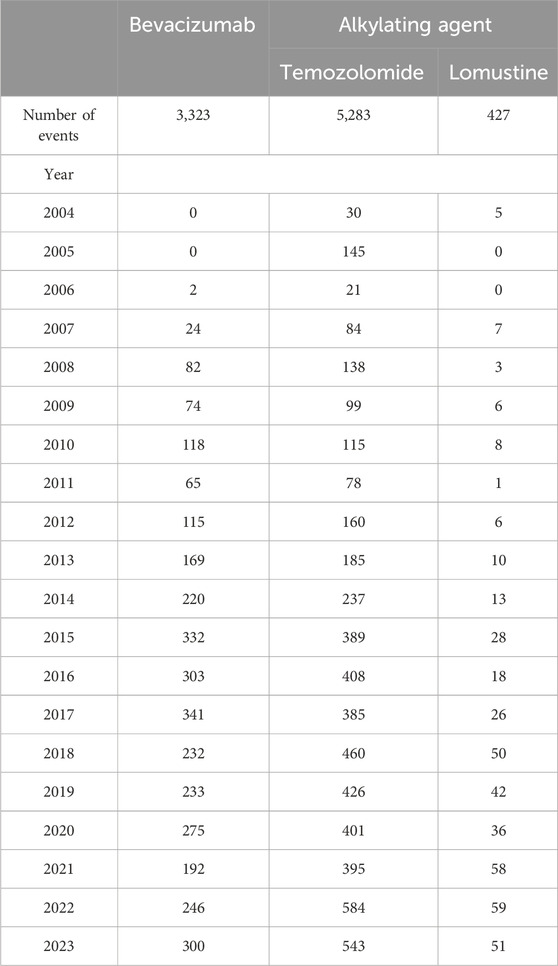
Table 1. Summary of adverse event reports related to bevacizumab and alkylating agents from Q1 2004 to Q4 2023.
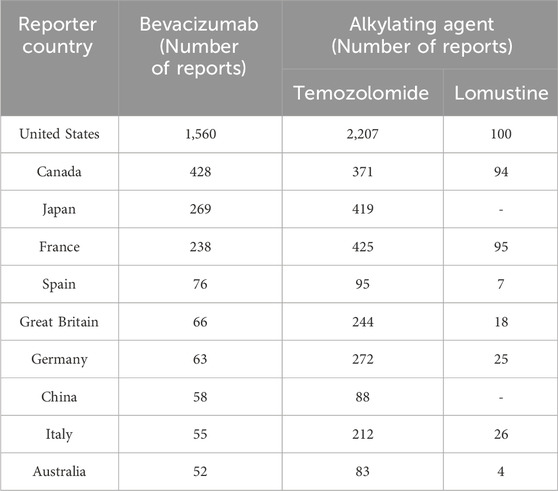
Table 2 presents the demographic characteristics of glioblastoma patients reporting adverse events. A slight predominance of male patients was observed, accounting for 47.2%, 45.1%, and 46.4% of the reports for bevacizumab, temozolomide, and lomustine, respectively. Most patients were aged between 18 and 75 years. Physicians were the primary reporters for all three drugs, with the majority of reports originating from the United States, followed by Canada (Table 3).
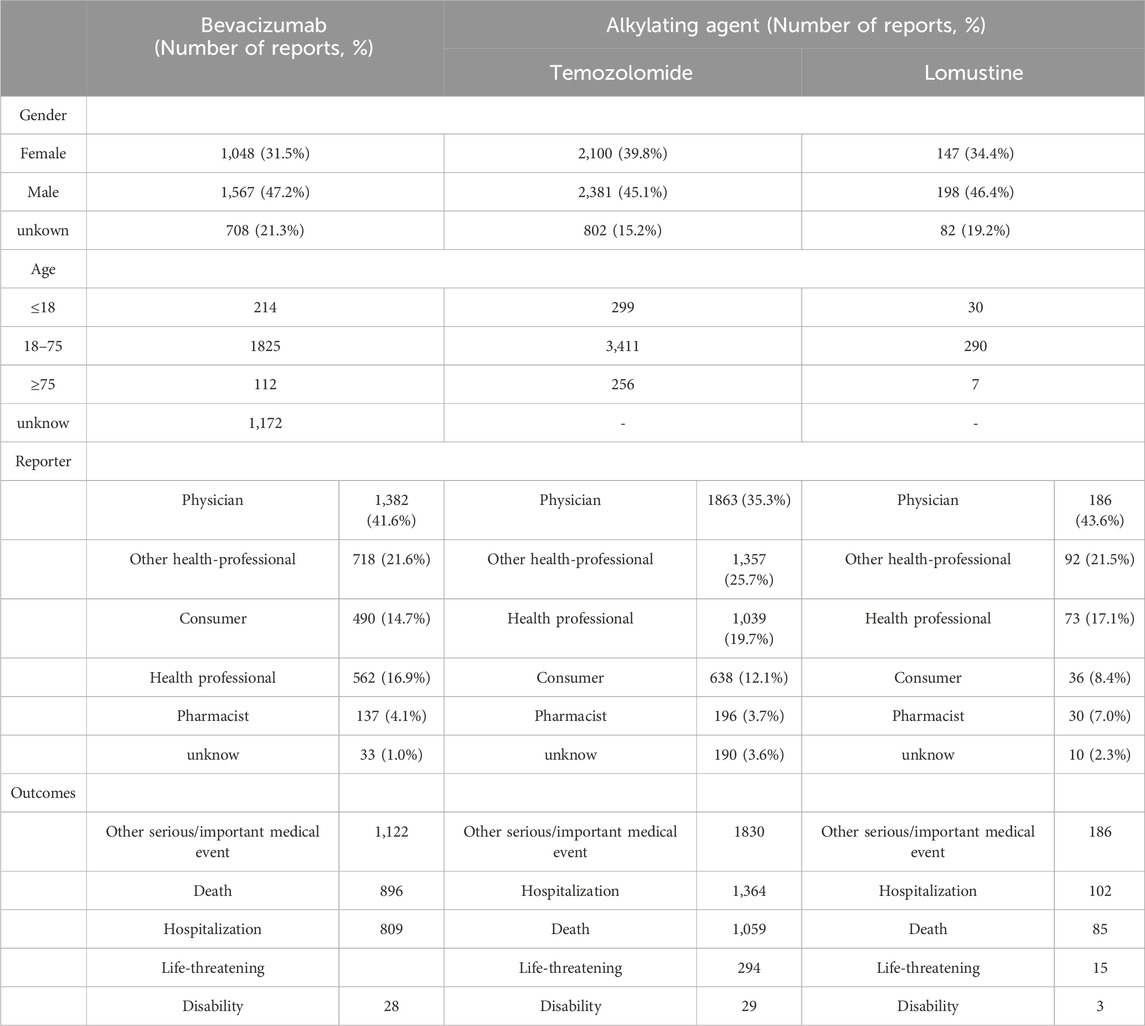
Table 2. Demographic characteristics of patients associated with bevacizumab and alkylating agents from Q1 2004 to Q4 2023.
The adverse event profiles of the three drugs, summarized in Table 4, exhibited distinct patterns. The most frequently reported adverse events for bevacizumab were fatigue (n = 276), hypertension (n = 220), and headache (n = 199). For temozolomide, thrombocytopenia (n = 581), disease progression (n = 475), and drug ineffectiveness (n = 356) were most commonly reported. In the case of lomustine, the most frequently reported events were thrombocytopenia (n = 67), progression of malignant neoplasm (n = 38), and fatigue (n = 36).
Table 5 details the distribution of adverse event onset times. Both bevacizumab and lomustine were associated with adverse events occurring predominantly within 2 months of treatment initiation. The median time to onset was 56 days (IQR: 21–138) for bevacizumab and 54 days (IQR: 18–175) for lomustine. Temozolomide, however, demonstrated a trend toward earlier onset, with a significant increase in adverse event reports occurring within 30 days after administration, and a median time to onset of 37 days (IQR: 19–92).
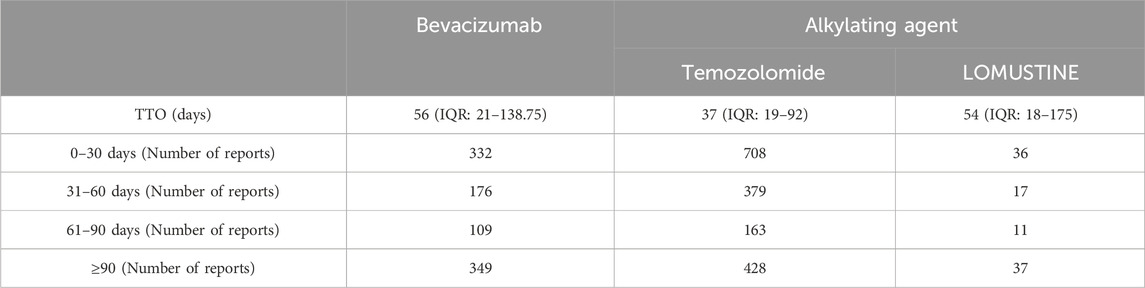
Table 5. Time-to-onset (TTO) analysis of adverse events associated with bevacizumab and alkylating agents.
3.2 Distribution of safety signals in glioblastoma patients treated with bevacizumab
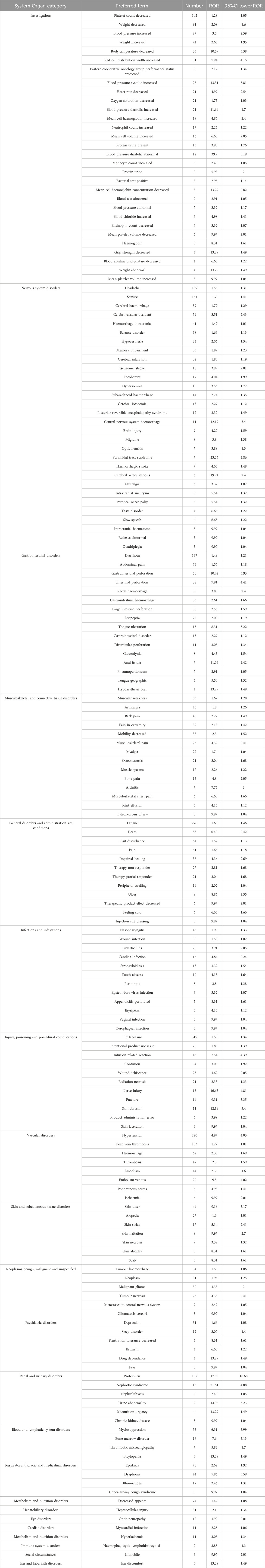
Table 6 shows that a total of 174 safety signals (at the PT level) were identified across 22 SOCs for bevacizumab. The SOCs with the highest numbers of positive signals were “Investigations” (30 PTs, 17.24%), “Nervous system disorders” (30 PTs, 17.24%), and “Gastrointestinal disorders” (16 PTs, 9.19%). Notably, no safety signals related to neutropenia, febrile neutropenia, or hematotoxicity were observed among all PTs associated with bevacizumab, compared to the two alkylating agents (Table 7).
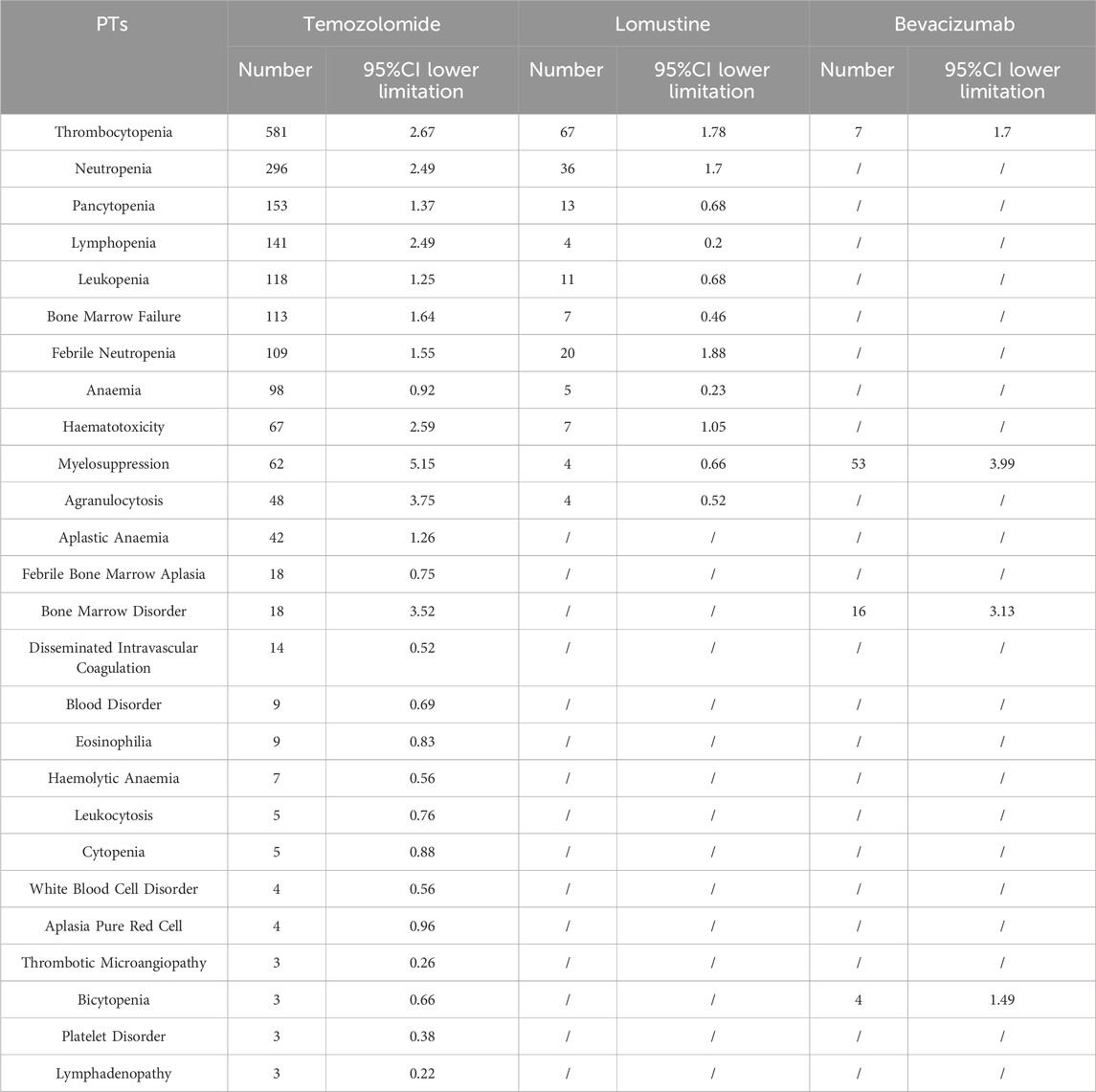
Table 7. Comparative analysis of safety signal distribution between bevacizumab and alkylating agents.
3.3 Comparative safety analysis of bevacizumab versus temozolomide at the SOC level
Bevacizumab was compared with temozolomide at the SOC level, using temozolomide as the reference drug. Several SOCs demonstrated positive signals in glioblastoma patients treated with bevacizumab, including “Musculoskeletal and connective tissue disorders” (ROR = 2.77, 95% CI: 2.46–3.12), “Vascular disorders” (ROR = 2.17, 95% CI: 1.98–2.39), “Renal and urinary disorders” (ROR = 1.63, 95% CI: 1.43–1.87), “Nervous system disorders” (ROR = 1.46, 95% CI: 1.38–1.53), and “Investigations” (ROR = 1.36, 95% CI: 1.29–1.43).
Conversely, bevacizumab was associated with lower odds of adverse events in SOCs such as “Pregnancy, puerperium and perinatal conditions” (ROR = 0.27, 95% CI: 0.11–0.67), “Product issues” (ROR = 0.37, 95% CI: 0.18–0.76), “Surgical and medical procedures” (ROR = 0.42, 95% CI: 0.32–0.54), “Blood and lymphatic system disorders” (ROR = 0.39, 95% CI: 0.37–0.42), and “Ear and labyrinth disorders” (ROR = 0.61, 95% CI: 0.39–0.95) (Figure 3).
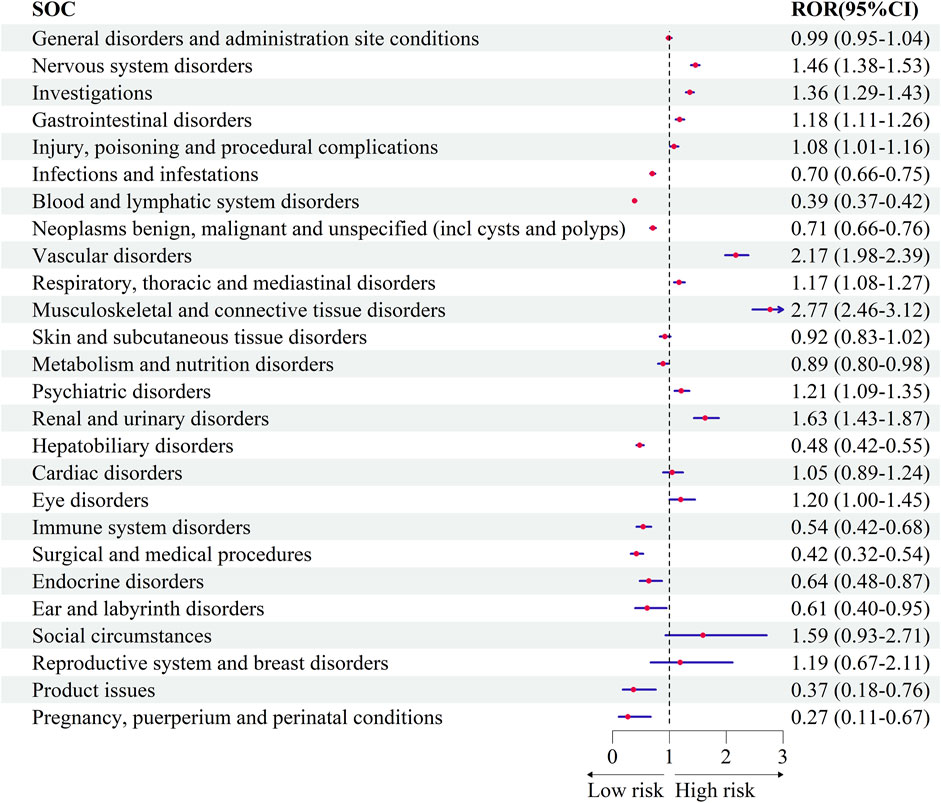
Figure 3. Comparative safety profiles across System Organ Classes (SOCs) for bevacizumab versus temozolomide.
3.4 Comparison of safety signals at the PT level between bevacizumab and temozolomide
To further explore differences in safety signals between bevacizumab and temozolomide, we analyzed signal intensities at the PT level. Figure 4 shows that bevacizumab was strongly associated with certain adverse events compared to temozolomide, including dysphonia (Respiratory, thoracic and mediastinal disorders) (ROR = 26.28, 95% CI: 9.55–72.34), perfusion-related reactions (Injury, poisoning and procedural complications) (ROR = 25.68, 95% CI: 9.33–70.23), and increased erythrocyte distribution width (Investigations) (ROR = 37.01, 95% CI: 8.92–153.48).
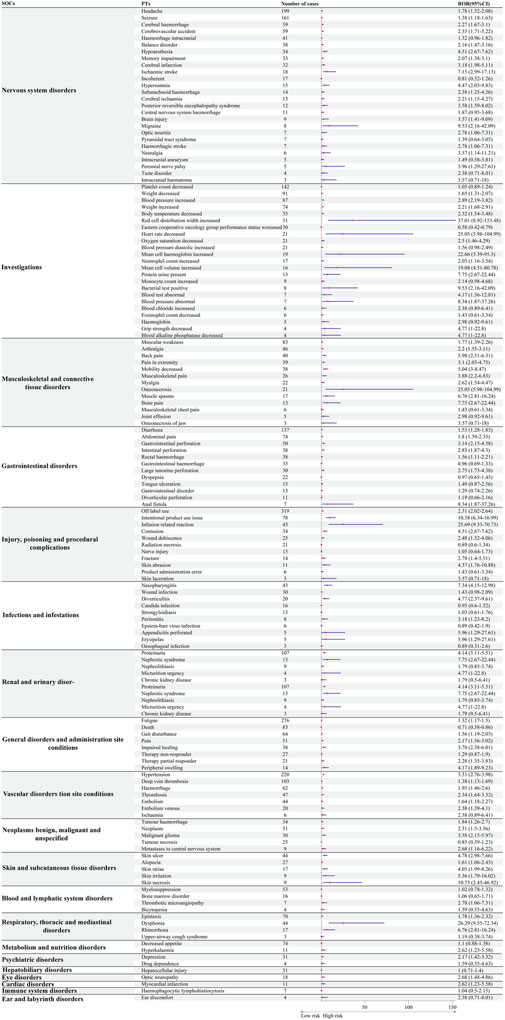
Figure 4. Comparative safety profiles at the Preferred Term (PT) level: safety signals of bevacizumab compared with temozolomide.
4 Discussion
Glioblastoma is the most common and aggressive primary malignant brain tumour (Stupp et al., 2007). Temozolomide and lomustine are DNA-alkylating agents, while bevacizumab is a humanised monoclonal antibody against vascular endothelial growth factor that inhibits angiogenesis. Both alkylating agents and bevacizumab are currently first-line treatments for glioblastoma (Pombo Antunes et al., 2020). The increasing number of adverse event reports associated with alkylating agents and bevacizumab between 2004 and 2023 highlights the complexity of glioblastoma treatment. By 2023, the annual number of reports had nearly tripled, underscoring the critical need for rigorous post-marketing surveillance to safeguard patient health.
In this study, temozolomide was reported significantly more frequently than bevacizumab, suggesting wider use in glioblastoma treatment (Lee, 2016). Bevacizumab was approved by the U.S. Food and Drug Administration (FDA) in 2009 for glioblastoma and is also included in the European Association of Neuro-Oncology (EANO) guidelines (Fu et al., 2023). Reports of adverse events related to bevacizumab peaked in 2015 and 2017, with 332 and 341 cases, respectively, possibly reflecting its increasing global uptake after approval. The United States led in the number of bevacizumab-related reports, potentially due to earlier market approval, broader expansion of indications, and greater patient engagement in reporting adverse events. In contrast, bevacizumab was approved several years later in other countries (Funakoshi et al., 2020; Australian Government Department of Health and Aged Care, 2019).
Our findings are consistent with, and extend, previous clinical trials on bevacizumab. Specifically, the highest number of significant safety signals for bevacizumab were observed in the “nervous system disorders” category (30 events, 17.24%). The NRG Oncology RTOG 0825 study by Wefel et al. showed that adding bevacizumab to standard chemotherapy did not improve survival endpoints but did significantly worsen neurocognitive function over time (Wefel et al., 2021). Similarly, Rodríguez et al. reported lower neurocognitive test scores among glioblastoma patients treated with bevacizumab compared to placebo (Rodriguez-Camacho et al., 2022). Hypertension, proteinuria, bone marrow failure, and leukopenia are among the most common adverse events listed on the bevacizumab label. In our study, adverse events such as elevated blood pressure, increased systolic and diastolic pressure were notably prominent, in line with previous reports estimating hypertension affects about 40% of patients treated with bevacizumab (Carvalho et al., 2020; Khan et al., 2022). A randomized controlled trial also reported hypertension and headache in over 10% of glioblastoma patients receiving bevacizumab (Cloughesy et al., 2020). Both the EMA and FDA have identified hypertension, fatigue, and malaise as the most frequent adverse events (European Medicines Agency, 2017; United States Food and Drug Administration (U.S. FDA)), underscoring the importance of continuous blood pressure monitoring during treatment. Additionally, significant positive signals were detected for thrombotic events such as deep vein thrombosis, thrombosis, and thrombotic microangiopathy, aligning with earlier studies observing vascular adverse events associated with bevacizumab (Oki et al., 2018; Perry, 2012). Ranpura et al. further reported an increased risk of venous thromboembolism in bevacizumab-treated patients (Ranpura et al., 2011). Interestingly, we also identified safety signals not mentioned in the official prescribing information, including psychiatric-related adverse events such as depression, sleep disturbances, and decreased frustration tolerance. These findings suggest the need for healthcare providers to remain vigilant for emerging safety signals to optimize prescribing practices and improve patient management.
Our results indicate that glioblastoma patients treated with bevacizumab may have a higher risk of adverse events in specific System Organ Classes (SOCs) compared with those treated with temozolomide, particularly “musculoskeletal and connective tissue disorders” (ROR = 2.77, 95% CI: 2.46–3.12), “vascular disorders” (ROR = 2.17, 95% CI: 1.98–2.39), and “renal and urinary disorders” (ROR = 1.63, 95% CI: 1.43–1.87). These findings do not suggest that bevacizumab should be avoided, but rather that further research is warranted to better understand these risks. Chinot et al. reported that adding bevacizumab to radiotherapy and temozolomide did not improve survival in glioblastoma patients, while the incidence of adverse events was higher in the bevacizumab group, particularly arterial thromboembolic events, bleeding, wound healing complications, gastrointestinal perforation, and congestive heart failure (Chinot et al., 2014). Notably, bevacizumab demonstrated a lower signal for certain SOCs compared to temozolomide, including “pregnancy, puerperium and perinatal conditions” (ROR = 0.27, 95% CI: 0.11–0.67), “blood and lymphatic system disorders” (ROR = 0.39, 95% CI: 0.37–0.42), and “ear and labyrinth disorders” (ROR = 0.61, 95% CI: 0.39–0.95). Moreover, bevacizumab was not associated with positive safety signals for neutropenia, febrile neutropenia, or hematological toxicity, suggesting a potentially lower risk of hematological disorders compared to temozolomide. If confirmed in future studies, these findings could represent a meaningful therapeutic advantage for glioblastoma patients.
Given the urgent need for novel therapeutic approaches, promising new avenues are being explored. SurVaxM, a survivin-targeted peptide vaccine, has demonstrated good tolerability in combination with temozolomide, with the most common adverse event being injection site inflammation, which was self-limited (Ahluwalia et al., 2023). Similarly, the CheckMate 498 study reported fatigue as the most common adverse event during treatment with nivolumab and radiotherapy, with a neurological adverse event rate of 16.5% (Omuro et al., 2023). These advances highlight the importance of keeping abreast of emerging therapies in glioblastoma management.
In our study, male patients accounted for 47.2%, 45.1%, and 46.4% of adverse event reports related to bevacizumab, temozolomide, and lomustine, respectively. This aligns with epidemiological data showing glioblastoma incidence is 1.58 times higher in men (Weller and Le Rhun, 2020; Tan et al., 2020). Therefore, special attention should be given to male patients when prescribing these therapies.
We also observed that adverse events associated with bevacizumab occurred over a longer duration compared with temozolomide. Temozolomide was associated with a shorter time to adverse event onset, typically peaking around 30 days post-treatment initiation, consistent with previous reports (Villano et al., 2012). Meanwhile, adverse events related to bevacizumab generally emerged within 50 days of starting treatment (Gerriets and Kasi, 2025). These findings suggest a need for heightened vigilance early in temozolomide therapy.
Despite the insights provided, this study has several limitations. First, the FAERS database is a spontaneous reporting system susceptible to reporting bias, duplication, and inaccuracies, potentially skewing safety assessments. Second, FAERS data only reflect a snapshot in time, with ongoing changes in report numbers. Third, the lack of detailed comorbidity and treatment information, including precise medical histories, limits interpretability. Fourth, the actual incidence rates cannot be calculated due to the absence of denominator data (i.e., total number of drug users). Most importantly, FAERS does not provide severity ratings based on the Common Terminology Criteria for Adverse Events (CTCAE), restricting clinical severity comparisons. Nevertheless, our findings provide valuable guidance for future research and clinical practice.
The distinct safety profiles of alkylating agents and bevacizumab carry important clinical implications. For instance, bevacizumab’s lower incidence of hematological adverse events may make it a preferable choice for patients with pre-existing hematological conditions. Additionally, the delayed onset of bevacizumab-related adverse events may favor its use in patients with complex comorbidities who cannot tolerate early toxicities. The novelty of this study lies in its extensive use of the FAERS database to systematically compare the safety profiles of alkylating agents and bevacizumab. Our results emphasize the urgent need for continuous post-marketing surveillance and evaluation. Future research should incorporate prospective designs and integrate genetic and biomarker data to personalize glioblastoma therapy and elucidate the mechanisms behind observed adverse events.
Over the 20-year period from 2004 to 2023, bevacizumab demonstrated fewer overall adverse events than temozolomide for glioblastoma treatment. While bevacizumab may have certain safety advantages, particularly regarding hematological disorders, vigilance regarding hypertension and vascular events remains essential. Ultimately, our findings aim to support therapeutic decision-making, although further follow-up studies, treatment interruption analyses, and pharmacokinetic evaluations are required to confirm causality.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
ZQ: Conceptualization, Data curation, Formal Analysis, Methodology, Visualization, Writing – original draft, Writing – review and editing, Investigation, Resources, Software. JZ: Data curation, Formal Analysis, Investigation, Software, Writing – review and editing. LY: Conceptualization, Data curation, Software, Writing – review and editing. YF: Investigation, Methodology, Software, Writing – review and editing. RB: Investigation, Software, Writing – review and editing. JL: Data curation, Formal Analysis, Methodology, Writing – review and editing. HW: Funding acquisition, Visualization, Writing – review and editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Capacity Building for National Clinical Key Specialties, (grant number 231616).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CI, Confidence Interval; EMA, European Medicines Agency; FAERS, FDA Adverse Event Reporting System; HLT, High level term; HLGT, High group level term; LLT, Lowest level term; MedDRA, Medical Dictionary for Regulatory Activities; PT, Preferred Terminology; ROR, Ratio of reported ratios; SOC, System Organ Class; VEGF, Vascular epithelial growth factor.
References
Ahluwalia, M. S., Reardon, D. A., Abad, A. P., Curry, W. T., Wong, E. T., Figel, S. A., et al. (2023). Phase IIa study of SurVaxM plus adjuvant temozolomide for newly diagnosed glioblastoma. JAMA Oncol. 41 (7), 1453–1465. doi:10.1200/JCO.22.00996
Australian Government Department of Health and Aged Care (2019). Public summary document – may 2019 PBAC meeting. Available online at: https://www.pbs.gov.au/pbs/industry/listing/elements/pbac-meetings/psd/pbac-public-summary-documents-may-2019 (Accessed June 21, 2023).
Batchelor, T. T., Reardon, D. A., de Groot, J. F., Wick, W., and Weller, M. (2014). Antiangiogenic therapy for glioblastoma: current status and future prospects. Clin. Cancer Res. 20 (22), 5612–5619. doi:10.1158/1078-0432.CCR-14-0834
Carvalho, B., Lopes, R. G., Linhares, P., Costa, A., Caeiro, C., Fernandes, A. C., et al. (2020). Hypertension and proteinuria as clinical biomarkers of response to bevacizumab in glioblastoma patients. J. Neurooncol 147 (1), 109–116. doi:10.1007/s11060-020-03404-z
Chinot, O. L., de La Motte Rouge, T., Moore, N., Zeaiter, A., Das, A., Phillips, H., et al. (2011). AVAglio: phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiforme. Adv. Ther. 28, 334–340. doi:10.1007/s12325-011-0007-3
Chinot, O. L., Wick, W., Mason, W., Henriksson, R., Saran, F., Nishikawa, R., et al. (2014). Bevacizumab plus radiotherapy–temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 370 (8), 709–722. doi:10.1056/NEJMoa1308345
Cloughesy, T. F., Brenner, A., de Groot, J. F., Butowski, N. A., Zach, L., Campian, J. L., et al. (2020). A randomized controlled phase III study of VB-111 combined with bevacizumab vs bevacizumab monotherapy in patients with recurrent glioblastoma (GLOBE). Neuro Oncol. 22 (5), 705–717. doi:10.1093/neuonc/noz232
Dolecek, T. A., Propp, J. M., Stroup, N. E., and Kruchko, C. (2012). CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol. 14 (Suppl. 5), v1–v49. doi:10.1093/neuonc/nos218
Friedman, H. S., Prados, M. D., Wen, P. Y., Mikkelsen, T., Schiff, D., Abrey, L. E., et al. (2009). Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J. Clin. Oncol. 27 (28), 4733–4740. doi:10.1200/JCO.2008.19.8721
Fu, M., Zhou, Z., Huang, X., Chen, Z., Zhang, L., Zhang, J., et al. (2023). Use of Bevacizumab in recurrent glioblastoma: a scoping review and evidence map. BMC Cancer 23 (1), 544. doi:10.1186/s12885-023-11043-6
Funakoshi, Y., Hata, N., Kuga, D., Hatae, R., Sangatsuda, Y., Fujioka, Y., et al. (2020). Update on chemotherapeutic approaches and management of bevacizumab usage for glioblastoma. Pharm. (Basel) 13 (12), 470. doi:10.3390/ph13120470
Garcia, J., Hurwitz, H. I., Sandler, A. B., Miles, D., Coleman, R. L., Deurloo, R., et al. (2020). Bevacizumab (Avastin®) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 86, 102017. doi:10.1016/j.ctrv.2020.102017
Gerriets, V., and Kasi, A. (2025). “Bevacizumab,” in StatPearls (Treasure Island, FL: StatPearls Publishing).
Hicklin, D. J., and Ellis, L. M. (2005). Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 23, 1011–1027. doi:10.1200/JCO.2005.06.081
Khan, K. I., Ramesh, P., Kanagalingam, S., Zargham Ul Haq, F., Victory Srinivasan, N., Khan, A. I., et al. (2022). Bevacizumab-induced hypertension as a potential physiological clinical biomarker for improved outcomes in patients with recurrent glioblastoma multiforme: a systematic review. Cureus 14 (9), e29269. doi:10.7759/cureus.29269
Kreisl, T. N., Kim, L., Moore, K., Duic, P., Royce, C., Stroud, I., et al. (2009). Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J. Clin. Oncol. 27 (5), 740–745. doi:10.1200/JCO.2008.16.3055
Lee, S. Y. (2016). Temozolomide resistance in glioblastoma multiforme. Genes Dis. 3 (3), 198–210. doi:10.1016/j.gendis.2016.04.007
Oki, E., Kato, T., Bando, H., Yoshino, T., Muro, K., Taniguchi, H., et al. (2018). A multicenter clinical phase II study of FOLFOXIRI plus bevacizumab as first-line therapy in patients with metastatic colorectal cancer: QUATTRO study. Clin. Colorectal Cancer 17 (2), 147–155. doi:10.1016/j.clcc.2018.01.011
Omuro, A., Brandes, A. A., Carpentier, A. F., Idbaih, A, Reardon, D. A., Cloughesy, T., et al. (2023). Radiotherapy combined with nivolumab or temozolomide for newly diagnosed glioblastoma with unmethylated MGMT promoter: an international randomized phase III trial. Neuro Oncol. 25 (1), 123–134. doi:10.1093/neuonc/noac099
Perry, J. R. (2012). Thromboembolic disease in patients with high-grade glioma. Neuro Oncol. 14 (Suppl. 4), iv73–iv80. doi:10.1093/neuonc/nos197
Pombo Antunes, A. R., Scheyltjens, I., Duerinck, J., Neyns, B., Movahedi, K., and Van Ginderachter, J. A. (2020). Understanding the immunosuppressive microenvironment of glioma: mechanistic insights and therapeutic opportunities. Nat. Rev. Cancer. 20 (12), 701–718. doi:10.7554/eLife.52176
Ranpura, V., Hapani, S., and Wu, S. (2011). Treatment-related mortality with bevacizumab in cancer patients: a meta-analysis. JAMA 305 (5), 487–494. doi:10.1001/jama.2011.51
Rodriguez-Camacho, A., Flores-Vázquez, J. G., Moscardini-Martelli, J., Torres-Ríos, J. A., Olmos-Guzmán, A., Ortiz-Arce, C. S., et al. (2022). Glioblastoma treatment: state-of-the-art and future perspectives. Int. J. Mol. Sci. 23 (13), 7207. doi:10.3390/ijms23137207
Services USD (2024). Available online at: https://fis.fda.gov/extensions/fpdwidgets/2e01da82-13fe-40e0-8c38-4da505737e36.html#_Toc493751926.
Stupp, R., Hegi, M. E., Gilbert, M. R., and Chakravarti, A. (2007). Chemoradiotherapy in malignant glioma: standard of care and future directions. J. Clin. Oncol. 25 (26), 4127–4136. doi:10.1200/JCO.2007.11.8554
Stupp, R., Taillibert, S., Kanner, A. A., Read, W., Steinberg, D., Lhermitte, B., et al. (2017). Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 318 (23), 2306–2316. doi:10.1001/jama.2017.18718
Tan, A. C., Ashley, D. M., López, G. Y., Malinzak, M., Friedman, H. S., and Khasraw, M. (2020). Management of glioblastoma: state of the art and future directions. CA Cancer J. Clin. 70 (4), 299–312. doi:10.3322/caac.21613
Tang, L., Ding, C., Li, H., Yin, G., Zhang, H., Liu, W. S., et al. (2024). A pharmacovigilance study of adverse event profiles and haemorrhagic safety of bevacizumab based on the FAERS database. Expert Opin. Drug Saf. 23 (2), 213–220. doi:10.1080/14740338.2023.2248876
Villano, J. L., Letarte, N., Yu, J. M., Abdur, S., and Bressler, L. R. (2012). Hematologic adverse events associated with temozolomide. Cancer Chemother. Pharmacol. 69 (1), 107–113. doi:10.1007/s00280-011-1679-8
Wefel, J. S., Armstrong, T. S., Pugh, S. L., Gilbert, M. R., Wendland, M. M., Brachman, D. G., et al. (2021). Neurocognitive, symptom, and health-related quality of life outcomes of a randomized trial of bevacizumab for newly diagnosed glioblastoma (NRG Oncology/RTOG 0825). Neuro Oncol. 23 (7), 1125–1138. doi:10.1093/neuonc/noab011
Weller, M., and Le Rhun, E. (2020). How did lomustine become standard of care in recurrent glioblastoma? Cancer Treat. Rev. 87, 102029. doi:10.1016/j.ctrv.2020.102029
Yang, J. C., Haworth, L., Sherry, R. M., Hwu, P., Schwartzentruber, D. J., Topalian, S. L., et al. (2003). A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N. Engl. J. Med. 349 (5), 427–434. doi:10.1056/NEJMoa021491
Yu, R. J., Krantz, M. S., Phillips, E. J., and Stone, C. A. (2021). Emerging causes of drug-induced anaphylaxis: a review of anaphylaxis-associated reports in the FDA adverse event reporting system (FAERS). J. Allergy Clin. Immunol. Pract. 9 (2), 819–829.e2. doi:10.1016/j.jaip.2020.09.021
Keywords: temozolomide, bevacizumab, alkylating agent, glioblastoma, FAERS, safety profile
Citation: Qu Z, Zhao J, Yang L, Fu Y, Bai R, Li J and Wang H (2025) Comparative safety analysis of bevacizumab and alkylating agent in glioblastoma management – What have we learned recently?. Front. Pharmacol. 16:1595642. doi: 10.3389/fphar.2025.1595642
Received: 18 March 2025; Accepted: 30 April 2025;
Published: 15 May 2025.
Edited by:
Zhenyu Gong, Technical University of Munich, GermanyReviewed by:
Hao Wu, The second affiliated hospital of Xi’an Medical University, ChinaPaola Palumbo, University of L’Aquila, Italy
Alejandro Rodríguez Camacho, Departamento de Radioneurocirugía del Instituto Nacional de Neurología y Neurocirugía Manuel Velasco Suárez, Mexico
Copyright © 2025 Qu, Zhao, Yang, Fu, Bai, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongqin Wang, d2hxMTk2OGhxQDE2My5jb20=
 Zhizhao Qu
Zhizhao Qu Jiajia Zhao3
Jiajia Zhao3 Hongqin Wang
Hongqin Wang