- 1Brain Science and Advanced Technology Institute, Hubei Province Key Laboratory of Occupational Hazard Identification and Control, School of Medicine, Wuhan University of Science and Technology, Wuhan, Hubei, China
- 2School of Medicine, Wuhan University of Science and Technology, Wuhan, Hubei, China
- 3School of Life Sciences, Westlake University, Hangzhou, China
Post-traumatic stress disorder (PTSD) is a severe mental disorder that occurs after experiencing or witnessing a traumatic event. Not only does this disorder severely impair the quality of life and emotional wellbeing of patients, but in recent years the global rate of PTSD diagnoses has increased to 1.5–2 times, and the prevalence of PTSD associated with COVID-19 events in particular has surged to 10%–25%, underscoring the urgency of developing effective treatments. The lifetime prevalence of PTSD in the general population is estimated to be approximately 3.9%, while in high-risk populations, such as war veterans, it can be as high as 30%. As a key pathway connecting the central nervous system to peripheral organs, the gut-brain axis has received increasing attention for its role in PTSD. Although the gut-brain axis has been shown to be associated with several psychiatric disorders, especially depression, its specific role in PTSD remains undercharacterized. Existing studies suggest that specific strains of Lactobacillus (e.g., Lactobacillus reuteri) may alleviate inflammatory responses and improve PTSD-like behaviors by down-regulating the expression of pro-inflammatory factors (IL-6 and TNF-α). In this study, we used a narrative review approach to sort out the research progress of gut microbiota alteration in PTSD, and compared the characteristics of changes in specific microbial taxa (e.g., Bacteroides, Lactobacillus, etc.), the index of microbiota diversity (α/β diversity), and the levels of inflammatory markers (e.g., IL-6, TNF-α) between the animal model and the human patients, respectively, in order to We further explored the potential pathogenic mechanisms mediated by microorganisms, such as influencing the vagal pathway, hypothalamic-pituitary-adrenal (HPA) axis function, immune system and other processes involved in the pathology of PTSD, and summarized the intervention strategies targeting gut microecology, such as probiotic supplementation, dietary interventions and fecal bacteria transplantation.
1 Introduction
Post-traumatic stress disorder (PTSD) is a severe mental disorder characterized by re-experiencing of trauma, avoidance of trauma reminders, and hyperarousal symptoms that result in adverse emotional, cognitive, and physiological health responses (Compean and Hamner, 2019). Individuals with PTSD not only experience personal dysfunction (e.g., decreased occupational performance and broken family relationships), but also exhibit symptoms associated with depression, anxiety disorders, substance use disorders, and suicidal high levels of co-morbidity with ideation, resulting in a socioeconomic burden that far exceeds the direct medical burden (Davis et al., 2022; Kalisch et al., 2024). Currently, the conventional treatment of post-traumatic stress disorder (PTSD) consists mainly of pharmacologic and psychological interventions. In terms of medications, selective 5-hydroxytryptamine reuptake inhibitors (SSRIs) and norepinephrine reuptake inhibitors (SNRIs) are the first-line medications, but their efficacy is often limited, and some patients do not respond well to the medications, with problems such as a high relapse rate and pronounced side effects (Krediet et al., 2020). Psychological treatments such as cognitive behavioral therapy (CBT), although widely used, are also difficult to cover all patients due to treatment resources, compliance, and individual differences. Therefore, it is particularly urgent to explore alternative or complementary treatment strategies. In recent years, the gut-brain axis, as an important pathway regulating neuropsychiatric states, has gradually gained attention, providing new ideas for understanding the pathogenesis of PTSD and developing novel interventions.
Since Leclercq et al. suggested in 2016 that an early imbalanced gut microbiota may have long-lasting immune and other physiological effects that make individuals more susceptible to PTSD after traumatic events, attention has been focused on the role of the microbiota in PTSD and attempts have been made to manipulate certain gut bacterial communities to target PTSD (Chang et al., 2022; Leclercq et al., 2016; Petakh et al., 2024).
The gut microbiota, as a symbiotic system of trillions of microorganisms in the digestive tract, critically regulates central nervous system (CNS) function through the microbial-gut-brain axis (Cryan et al., 2019), and gut strains synthesize biologically active molecules such as neurotransmitters that directly intervene in brain activity and behavioral patterns, and whose metabolites enable individual and synergistic modulation of the immune response (Bremner, 2006; Dicks, 2022).
In this review, we first analyze the heterogeneity of gut microbiota and their common associations between animal models of PTSD and clinical patients, respectively, to reveal the ecological dysregulation patterns specific to this disease. Subsequently, the latest evidence on the influence of gut microbiota on the progression of PTSD is highlighted in terms of the classical pathological pathways of PTSD (e.g., dysregulation of the HPA axis, dysregulation of neurotransmitter signaling, dysregulation of the immune system, etc.). Finally, based on the theory of gut microbiota-PTSD interactions, innovative therapeutic strategies targeting microbiota modulation (e.g., probiotic interventions, fecal bacterial transplantation, and natural medicines targeting the gut-brain axis) are proposed.
2 Changes in the composition of gut microbiota in PTSD
2.1 Gut microbiota dysbiosis in people with PTSD
There was a significant difference in gut microbial diversity between people with PTSD and without PTSD (Table 1) (Núñez-Ríos et al., 2024).
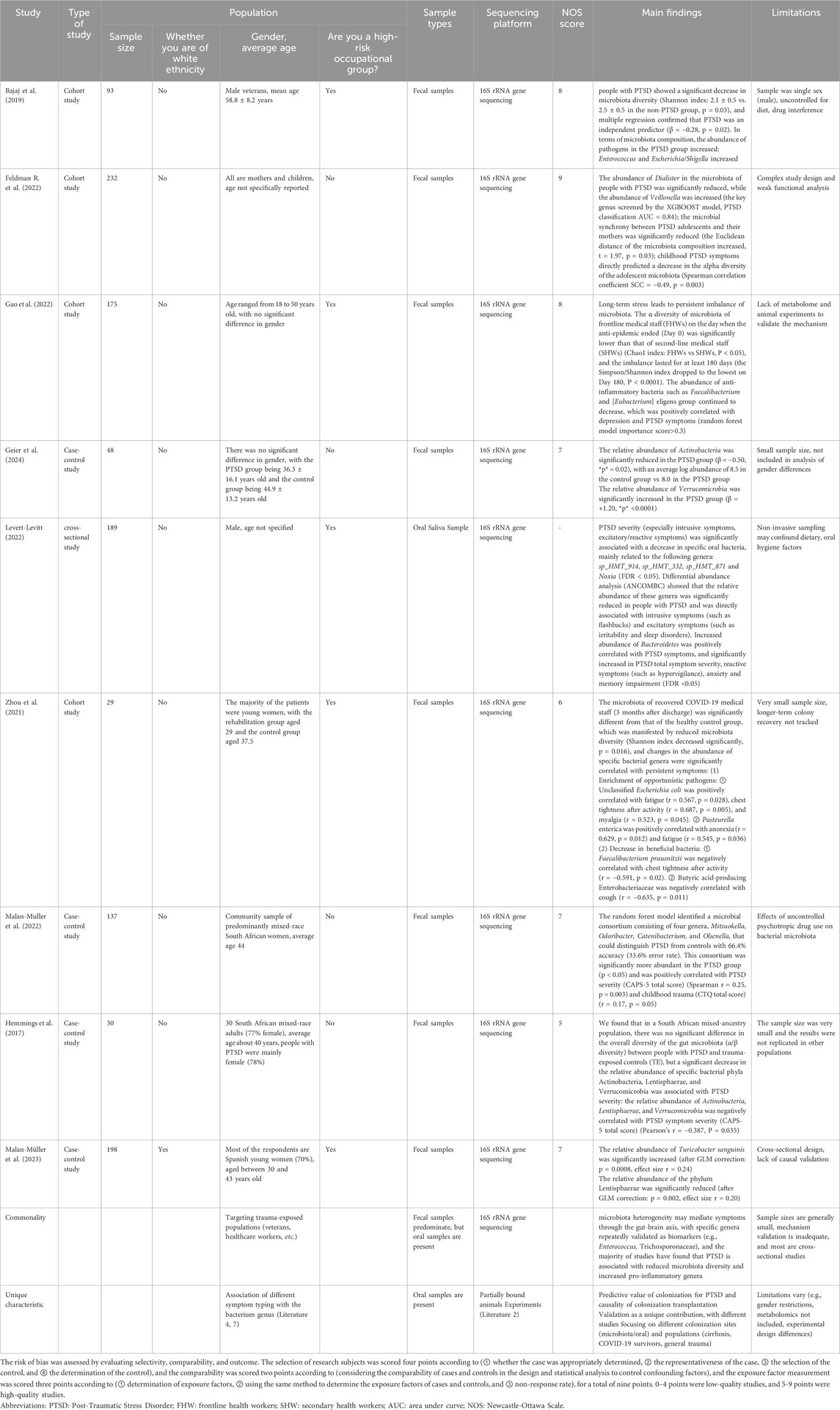
Hemmings et al. used the DSM-5 PTSD scale to diagnose PTSD, extracted microbial DNA from stool samples of PTSD individuals and 12 TE control participants, generated bacterial 16S ribosomal RNA gene V3/V4 amplicons, and sequenced them, and although randomized forest analyses indicated that the total abundance (The total relative abundance of specific taxonomic units detected in a given sample, which equals to relative abundance in this paper) Actinobacteria, Microbacterium verticillioides, and Lentisphaerae was significantly negatively correlated with PTSD symptoms, alpha diversity and beta diversity of microorganisms of this genus were found to be significantly higher in people with PTSD and without PTSD (Hemmings et al., 2017). Although Random Forest analysis showed a significant negative correlation between the total abundance of Actinobacteria, Micrococcus verticillioides, and Lentisphaerae and PTSD symptoms, suggesting a detrimental effect of this genus of microorganisms on the PTSD population, the alpha and beta diversity of the microorganisms revealed that the overall microbial diversity (in the original language, the correct term should be “relative abundance”) measurements were similar between the PTSD and TE controls, a discrepancy which may be due to the very small sample size. A methodologically similar study was conducted by Malan et al. who also examined the association between gut microbiota composition and PTSD outcomes using a randomized forest model among participants, selecting and identifying four genera that were able to differentiate between the PTSD and TEC groups, i.e., Mitsuokella, Odoribacter, Catenoidea, and C. elegans, as well as the PTSD and TEC groups. Odoribacter, Catenibacterium, and Olsenella, and the relative abundance of these genera was higher in people with PTSD compared with controls and positively correlated with CAPS-5 scores (Malan-Muller et al., 2022), suggesting a pathogenic role of these four genera in PTSD. In addition, another Mendelian randomization study found a significant negative correlation between Micrococcus luteus and the risk of post-traumatic stress-related depression (PTMDD), suggesting a protective effect of Micrococcus wartyi against PTMDD (S. Liu et al., 2024). At the level of microbiota categorization, it has been shown that individuals with PTSD generally exhibit reduced levels of butyric acid-producing anti-inflammatory strains (e.g., Enterococcus faecalis) and significant enrichment of pro-inflammatory strains (e.g., Eggerthella). This bacterial imbalance may be closely related to the impaired anti-inflammatory function and activation of the inflammatory cascade response, which will be mentioned later, and constitutes an important pathological basis for the pathogenesis of PTSD (Nikolova et al., 2021). Future studies need to further clarify the potential mechanisms of damage by pro-inflammatory microbiota on the pathologic state of PTSD in order to reveal its complex role in disease development.
In addition to sample size, selection of study population, source of biological samples are important factors in determining the results of microbiota studies. Bajaj et al. (2019); Feldman E. L. et al. (2022) explored the association between PTSD and gut microbiota, with the former using adult male veterans with cirrhosis of the liver, and found that people with PTSD had decreased gut diversity and accompanied by an increase in pathogenic bacteria. In contrast, Feldman et al. used a broader population (including mothers and infants) to validate the causal relationship between gut microbiota and anxiety behaviors through a colony transplantation experiment, which strengthens the biological explanatory power of the study. For biological samples, most studies used fecal samples (e.g., Hemmings, Geier, Malan-Muller) (Geier et al., 2024; Hemmings et al., 2017; Malan-Muller et al., 2022), which are convenient to reflect the overall structure of the gut microbiota. Levert-Levitt et al. (2022) used oral saliva samples, and found that specific oral bacterial genera were correlated with the severity of PTSD, but because oral microbiota is more susceptible to diet, hygiene, and smoking, their conclusion that oral microbiota characteristics (decrease in HMT_914 and increase in Mycobacterium avium phylum) correlate with PTSD severity remains to be tested. Exposure to chronic stress-induced PTSD is also influenced by the role of the gut-brain axis, and a study on the relationship between gut microbial ecology and secondary PTSD in frontline healthcare workers in the New Crown epidemic reported that chronic exposure to stressful and anxious frontline work environments resulted in significantly lower individual counts of each species of gut microbiota among frontline workers than among secondary healthcare workers, and demonstrated sustained 6-month follow up changes (Zhang et al., 2023). 16S rRNA gene sequencing longitudinal analysis revealed that high abundance of Ehrlichia anomalies was an important determinant of the recurrence of post-traumatic stress symptoms in frontline healthcare workers, and that a series of stressful events fighting COVID-19 induced characteristic longitudinal changes in the gut microbiota, which underlie the changes in the dynamic mental state (F. Gao et al., 2022). In addition, the gut microbiota of COVID-19 convalescent patients 3 months after discharge from the hospital differs from that of healthy individuals, with increases in unclassified Escherichia coli, Enterobacter pasteurianus, and Enteromonas acidophilus butyric acidophilus associated with persistent postdischarge symptoms (Zhou et al., 2021). These findings suggest that future PTSD and microbiota-related studies need to continue to advance in terms of more rigorous study design, more scientific sample selection, and more in-depth biological mechanisms, with a view to establishing more robust microecological intervention strategies to serve the treatment of precision psychiatric disorders.
2.2 Gut microbiota dysbiosis in PTSD animal models
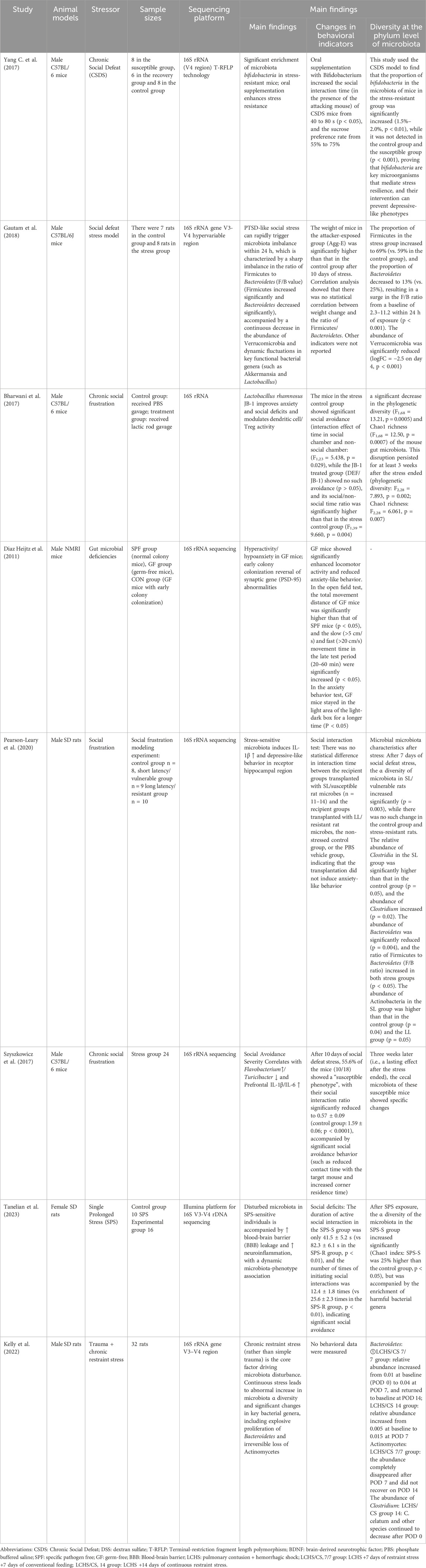
Animal models are a valuable addition to human studies compared with populations, and most studies on the microbiota composition of animals have explored genus-level changes in dexposed mice infected with the gut pathogen Citrobacter rodentium to acute stress showed significant memory dysfunction (Gareau et al., 2011), and Yang et al. found in a model of chronic social defeat stress (CSDS) that stress-resistant mice had a significant enrichment of Bifidobacterium bifidum in their intestines (Yang W. T. et al., 2017). Bifidobacterium were significantly enriched in the intestine, as well as Actinobacteria, suggesting that the rise in gut microbiota may affect memory, behavior after acute and chronic stress.
Szyszkowicz et al. reported that the genus Turicibacter showed a decreasing trend in a model of chronic social frustration, whereas the genus Flavonifractor was significantly elevated (Szyszkowicz et al., 2017). These genera all belong to the phylum Firmicutes, suggesting that there is also structural remodeling within this phylum within the microbiota under stress. In the same vein, a novel animal model of PTSD based on arousal-based individual screening (AIS) demonstrated an increase in the genera Tuzzerella and Lachnospiraceae NK4A13 in the family Lachnospiraceae, but Tyzzerella, Lachnospiraceae UCG 010, and Lachnospiraceae FCS020 groups decreased (Laudani et al., 2023), also suggesting structural remodeling within the clade under stress. Meanwhile, studies using the classical animal model of PTSD with single-time prolonged stress (SPS) showed that the abundance of Roseburia, Oscillibacter and Trichoderma unspecified in the PTSD group was also significantly lower than that in the control group (Tanelian et al., 2023), all suggesting that stress affects the decline in the level of gut microbiota in the animals. Ipth, allowing us to assign animal controls to trauma-exposed groups and assess the gut microbiota before and after trauma (Ke et al., 2023). For example, studies that en addition, more and more studies have confirmed that the synthesis of key mediators of the gut-brain axis can be induced by the use of probiotics (Bifidobacterium longum, Lactobacillus mucosus) to regulate the occurrence of stress behaviors, whose mechanisms will be introduced in the subsequent content (Bercik et al., 2011a; Bercik et al., 2011b; Han and Kim, 2019) (Table 2).
3 Interaction between PTSD pathogenesis and gut microbiota
The pathogenesis of PTSD involves a complex interplay of biological, psychological and environmental factors (Settanni et al., 2021). Its classical pathogenesis is mainly related to vagal dysfunction, neurotransmitter imbalance (Shin and Liberzon, 2010), hypothalamic-pituitary-adrenal axis (HPA axis) dysregulation and immune system dysfunction (Bandelow et al., 2017; Schumacher et al., 2019) (Figure 1). The gut microbiota affects the nervous system through the above regulatory mechanisms (Rieder et al., 2017). At the same time, gut microbiota can also regulate stress, providing new therapeutic targets for the treatment of PTSD (Leclercq et al., 2016) (Figure 2).
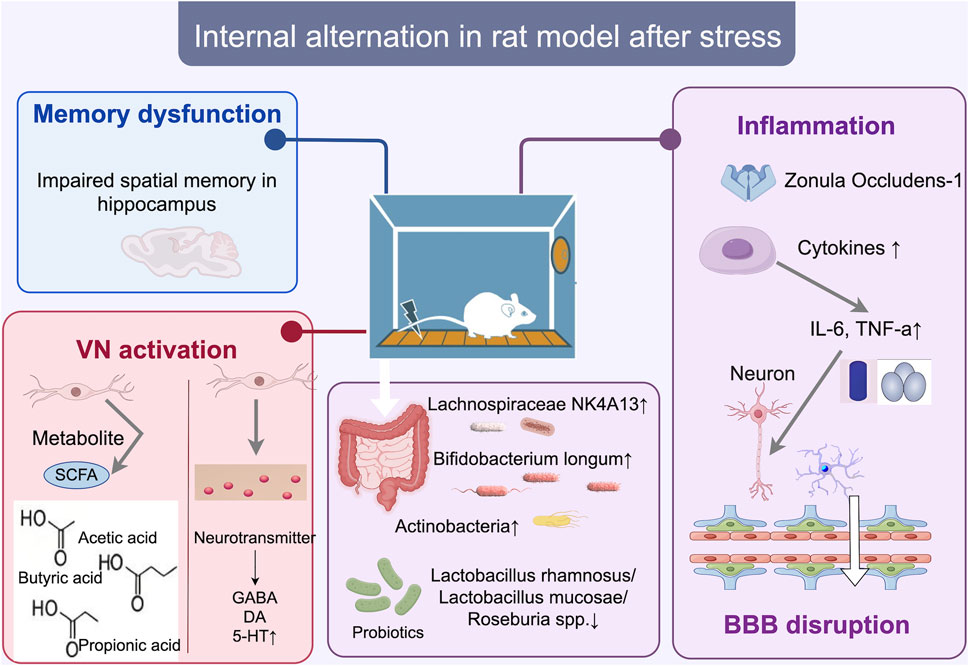
Figure 1. Internal changes in the SD rat model of stress disorder. After conditioned fear training, bacterial microbiota such as Lachnospiraceae NK4A131, Bifidobacterium longum, and Actinobacteria increased, triggering a systemic inflammatory response (increase in IL-6, TNF-a), which may directly affect brain regions such as the hippocampus, where memory deficits occur, and inducing ZO-1 proteins and cytokine Disrupts the blood-brain barrier (BBB) and neurons or microglia, and activates vagal pathways, increases colony metabolites SCFA (acetic acid, butyric acid, propionic acid), and induces neurotransmitter changes (increase in 5-HT and decrease in GABA). bbb, blood-brain barrier; zo-1, Zonula Occludens-1 (Zonula Occludens-1).
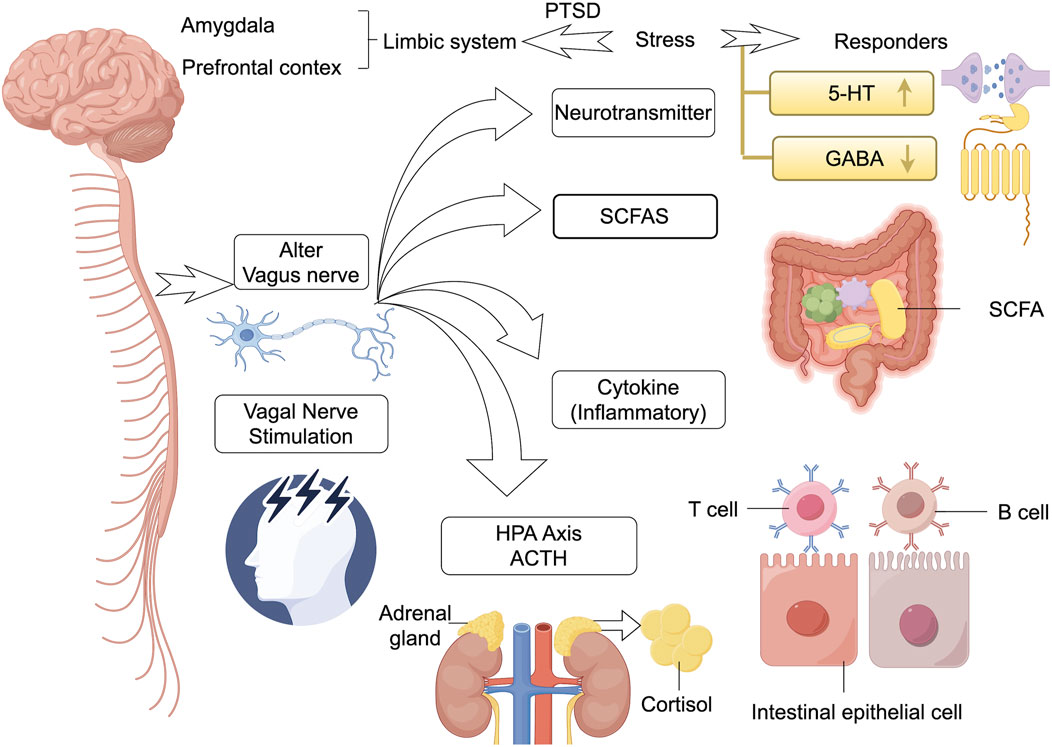
Figure 2. Interplay and connections inside brain-gut microbiot. The gut microbiota and its metabolites (e.g., SCFAs) communicate bi-directionally with key brain regions (e.g., prefrontal cortex, amygdala, limbic system) through multiple pathways (including the vagus nerve, the HPA axis, the immune system, and neurotransmitters). Stimulation of the microbiota can lead to hyperactivation of the HPA axis (involving ACTH, cortisol), dysbiosis levels of neurotransmitters (e.g., 5-HT, GABA), T/Bcell activation, and changes in the function of gut epithelial cells, which collectively contribute to the stress dysregulation and pathology of PTSD. In addition, vagus nerve stimulation has been shown to be effective in reversing such brain-gut reactions. HPA, hypothalamic-pituitary-adrenal; SCFA, chain fatty acids; 5-HT, 5-hydroxytryptamine; GABA, gamma-aminobutyric acid; ↑ represents the increased levels of this neurotransmitter.
3.1 Vagal pathways
The gut microbiota is able to act on the vagus nerve and transmit this information to the CNS, which directly activates neurons in the brain (Eisenstein, 2016; Fülling et al., 2019), thereby altering host behavioral outcomes. It was found that when mice were infected with subclinical doses of Campylobacter jejuni, by activating neurons within the solitary tract nucleus of the vagus nerve, the mice began to exhibit more anxiety-like behaviors (Goehler et al., 2005). Conversely, ingestion of Lactobacillus rhamnosus can modulate anxiety via the vagus nerve thereby treating PTSD (Bravo et al., 2011).
The vagus nerve, as the main neural pathway connecting the brain and visceral organs, regulates the activity of the autonomic nervous system in people with PTSD and modulates the stress response (Q. Zhou et al., 2020). Specifically, the vagus nerve acts to restore homeostasis in the body after stress (Costantini et al., 2010). It reduces heart rate and blood pressure and lowers cortisol levels, thereby reducing the effects of trauma (Almeida et al., 2021). In patients with PTSD, both vagal tone and heart rate variability (HRV, an indicator of parasympathetic activity) are significantly reduced, and increased vagal activity is associated with a reduced risk of developing PTSD (L. J. Noble et al., 2019). Non-invasive percutaneous auricular vagus nerve stimulation is increasingly recognized as a promising approach for the treatment of PTSD. For example, electrical stimulation of vagal afferent fibers alters neurotransmitter levels in the brain (Schumacher et al., 2019). This improves heart rate variability and attenuates symptoms of persistent hypervigilance in people with PTSD, thereby significantly reducing their emotional and somatic symptoms (N. C. Noble et al., 2023).
In addition, Liu et al. showed that a lack of vagal integrity also interrupts the immune component of the microbiota-gut-brain axis, which inhibits the effects of L. rhamnosus on behavioral and cortisol stress responses (Y. Liu et al., 2021). Animal models of PTSD have shown that elevated vagal tone is also accompanied by dysregulation of the HPA axis, alterations in the neurotransmitter system, and immune dysregulation (Cohen and Zohar, 2004). During stress, the vagus nerve inhibits m1-type pro-inflammatory macrophages, whose anti-inflammatory effects alter gut permeability and gut microbiota (Bonaz et al., 2017). Impairment of vagal tone reduces the body’s ability to suppress the hyperinflammatory response that is a key factor in the chronic stress response in PTSD (Dabrowska, 2023). This inflammatory imbalance may further lead to neurobiological changes that reinforce adverse fear responses and emotional susceptibility after stress (Breit et al., 2018). In summary, the vagus nerve plays a central role in the bidirectional neuroimmunoendocrine pathway of the microbiota-gut-brain axis.
3.2 HPA axis dysregulation
Changes in the composition of the gut microbiota may lead to dysregulation of the HPA axis, which in turn affects the neuroendocrine system of the brain (Sudo et al., 2004). GF mice have significantly higher elevations of plasma adrenocorticotropic hormone and corticosterone in response to inhibitory stress than mice without specific pathogens, and this HPA stress response can, in turn, be reversed by reconstituting bifidobacteria at a young age (Huo et al., 2017). Prebiotic and probiotic interventions have emerged as potential strategies for PTSD treatment. Animal experiments have shown that Lactobacillus farciminis attenuates acute psychological stress in rats by modulating hypothalamic adrenocorticotropin-releasing hormone gene expression, decreasing adrenocorticotropic hormone and cortisol secretion, preventing gut barrier damage, and decreasing circulating LPS levels (Ait-Belgnaoui et al., 2012). The GF animal model has shown that the microbiota influences the stress and stress response in rats by modulating the baseline of HPA activity and by lowering adrenocorticotropic hormone and cortisol levels to influence stress and anxiety-like behaviors (Hendrickson and Raskind, 2016). Probiotics have been shown to be effective in the treatment of PTSD with cortisol levels (Pivac et al., 2023).
Animal and population studies have confirmed the presence of HPA axis dysfunction in patients with PTSD (Bremner, 2006), with elevated levels of adrenocorticotropin-releasing hormone and significantly lower basal cortisol levels, leading to abnormal stress responses (Dunlop and Wong, 2019; van der Kolk et al., 1985). Low cortisol levels not only make individuals more susceptible to PTSD after traumatic events (Leclercq et al., 2016), but also alter gut barrier function: increasing gut permeability (“leaky gut”), facilitating the entry of bacteria and their products into the mucosal layer, and triggering inflammation (Rudzki et al., 2017). Thus, gut microbiota not only regulates the stress response of the HPA axis, but is also inversely regulated by the HPA axis (Bailey and Coe, 1999), and glucocorticoids, mainly cortisol, are also immunosuppressive and may disrupt gut immune homeostasis (Van Wyngene et al., 2021). Dysregulation of the neuroendocrine system has been shown to be an important feature of patients with PTSD (Foster et al., 2017).
The HPA axis influences immune and inflammatory responses not only because cortisol is a major inhibitor of inflammation, but also because inflammatory cytokines can inversely activate the HPA axis and reduce levels of anti-inflammatory cytokines (e.g., IL-4) downstream of the HPA axis, suggesting that microbiota regulation produces the immune hyperactivation and inflammatory responses present in PTSD (Doney et al., 2022).
3.3 Immune system
Dysbiosis of the gut microbiota leads to increased gut permeability (Myint et al., 2009) allowing bacterial metabolites and antigens to enter the circulation (Förstermann and Sessa, 2012). These substances trigger immune activation via pattern recognition receptors, such as toll-like receptors on the surface of immune cells (Torreilles et al., 1999), inducing the release of pro-inflammatory cytokines such as IL-6, TNF-α and IL-1β. These cytokines contribute to the chronic inflammatory state (Alpert et al., 2021) and exacerbate PTSD symptoms by disrupting the gut-brain axis, as can be observed, for example, by the fact that another downstream acute-phase protein, c-reactive protein, secreted by the inflammatory cytokine IL-6, is significantly elevated in people with PTSD (Miller et al., 2018). Changes in mRNA expression of interleukin IL-1β and IL-6 within the prefrontal cortex have been associated with increased abundance of Flavobacterium and decreased abundance of Pineobacterium, which also correlates strongly with the severity of social avoidance (Szyszkowicz et al., 2017). Repeated social defeat stress induces an inflammatory bowel environment by altering mucosal barrier integrity and gut microbiota homeostasis (Yadav et al., 2023). It has also been found that gut microbiota dysbiosis directly contributes to increased blood-brain barrier permeability (Braniste et al., 2014; Diaz Heijtz et al., 2011), and gut microbiota imbalances occurring early in life may similarly increase the susceptibility of mother-isolated individuals to PTSD following a traumatic event, which participates in the progression of the disease course by persistently influencing immune system and neurophysiological functioning, making the individual more susceptible to PTSD following a traumatic event, and contributing to the disease (Usui et al., 2021). Gut microbiota have also been shown to affect the response of microglia to signals from the CNS region, thereby affecting the response to pain and inflammation in people with PTSD (Nathalie et al., 2021). Their chronic activation also leads to the release of proinflammatory mediators and reactive oxygen species (Merz et al., 2021), exacerbating neuronal damage and impairing synaptic plasticity, both of which are associated with PTSD symptoms.
A growing body of evidence emphasizes the involvement of the immune system in the pathogenesis of PTSD (Bekhbat et al., 2017; Elder et al., 2019; Vasileva et al., 2019). Patients with PTSD have significantly elevated levels of proinflammatory cytokines (Doney et al., 2022) which, in turn, disrupt neural circuits responsible for emotion regulation, memory consolidation (Yirmiya and Goshen, 2011), and fear-abatement learning (Doney et al., 2022) through systemic and neuroinflammatory responses. At the same time, proinflammatory cytokines affect brain function through multiple pathways. They can activate microglia in the CNS by crossing the blood-brain barrier or by signaling through the vagus nerve (Cai et al., 2023; Jones et al., 2018). This activation rapidly triggers an inflammatory microenvironment that destroys brain regions (e.g., amygdala, hippocampus, and prefrontal cortex) that are closely associated with PTSD. Thus, correspondingly, the immune system shapes the composition and activity of the microbiota (Belkaid and Hand, 2014).
Cytokines can also alter neurotransmitter systems associated with PTSD: proinflammatory cytokines drive the kynurenine pathway (Stefano et al., 2018), which depletes tryptophan required for serotonin synthesis, leading to mood disorders and anxiety (M.-T. Liu et al., 2009), as well as decreasing dopamine production by depleting tetrahydrobiopterin (BH4), a key cofactor in dopamine synthesis (McVey Neufeld et al., 2015). In addition, elevated levels of quinolinic acid, a neurotoxic metabolite of the kynurenine pathway, potentiate the excitotoxicity of glutamate (De Vadder et al., 2018), which may exacerbate cognitive deficits and mood dysregulation in people with PTSD.
3.4 Neurotransmitter
Bacteria within the GM have been shown to produce different neurotransmitters. Some of the major neurotransmitters include GABA (Lactobacillus and Bifidobacterium), norepinephrine (Escherichia, Bacillus, and Saccharomyces), dopamine (Bacillus), acetylcholine (Lactobacillus), and 5-hydroxytryptophan (Escherichia, Enterococcus, Candida, and Streptococcus) (De Vadder et al., 2018; Hendrickson and Raskind, 2016; Lyte, 2013). The metabolic process products of the microbiota also contain a variety of neurotransmitters, including 5-HT and GABA. 5-HT is a classic stress-related neurotransmitter, 80% of which is synthesized by gut microbiota (e.g., Escherichia, Halobacterium, Pseudomonas, Streptococcus, Bifidobacterium, Lactococcus, Morgellus, Klebsiella, Propionibacterium, Fusobacterium, Rossella and Prevotella) Synthesis (Stefano et al., 2018).
Neurotransmitter imbalance is a central mechanism in the pathophysiology of PTSD (Bonaz et al., 2017). Hanscom et al. proposed that reduced 5-HT activity (particularly via 5-HT1A receptor dysregulation) in people with PTSD following traumatic brain injury is strongly associated with impaired mood disorders, anxiety, and fear extinction (Hanscom et al., 2021). McVey et al. argued that neuronal dysfunction in GF mice can be colonization reversal, further supporting a critical role for gut-derived 5-HT in neural development and function (McVey Neufeld et al., 2015). Three studies by De Vadder et al. confirmed that the microbiota regulates 5-HT synthesis (De Vadder et al., 2018), suggesting an important role for 5-HT in the pathogenesis of PTSD (Bonnin and Levitt, 2011; Chan et al., 2024; Myint et al., 2009). Interestingly, neurons in GF mice are unable to synthesize 5-HT, and supplementation with a 5-HT4 receptor agonist significantly improves their enteric neurodevelopment (PTSD manifests itself in mice as a state of generalized fear stemming from a glutamate-to-GABA transmitter switch in dorsal lateral flanking neurons of the middle suture bundle, and a similar shift has been observed in postmortem brain tissue of people with PTSD (Li et al., 2024). In a rat model of PTSD/alcohol use disorder co-morbidity, Borgonetti et al. concluded that there are sex differences in IL-18 regulation of GABA synapses (Vaiva et al., 2004), and thus inhibition of amygdala GABA neurons is critical for precise regulation of the consolidation, expression, and extinction of fear memories. GABA is an inhibitory neurotransmitter, and classical studies have shown that microbiota downregulation of low plasma GABA levels as a predictor of acute PTSD (Vaiva et al., 2004). The GABA system is involved in the pathophysiological processes of PTSD when functioning is significantly reduced in patients (Huang et al., 2023), and Meyerhoff et al. demonstrated that changes in GABA concentrations in patients with PTSD were consistent with the results of panic disorder and social anxiety disorder (Meyerhoff et al., 2014).
Reduced 5-HT transmission is influenced not only by emotional stability, a characteristic manifestation of PTSD (Liu W.-Z. et al., 2018), but also by the release of short-chain fatty acids (SCFAs) from gut chromaffin cells. SCFAs, stimulate 5-HT receptors located on sensory fibers of the vagus nerve (Fukumoto et al., 2003).
3.5 SCFAs
Short-chain fatty acids including butyrate, acetate, lactate and propionate, which are mainly produced by Bifidobacterium, Lactobacillus, Rhizobium bradyrhizogenes, Kolbachia, Roseobacter, and E. faecalis in the colon, have significant neurological effects by modulating neurotransmitter synthesis, inhibiting neuroinflammation, and enhancing the integrity of the blood-brain barrier through the gut-brain axis. Among the aforementioned Gram-positive anaerobes, butyrate-producing bacteria, namely, E. faecalis in the Clostridium globosum group and Enterobacter cloacae/Rose bacillus in the C. globosum group, are widely distributed (Mokhtari et al., 2017).
Butyrate is a double-edged sword (Liu H. et al., 2018), and its sodium salt is involved in psychiatric mechanisms through the production of neurotrophic factors (Varela et al., 2015) and the conversion of subthreshold learning events into long-term memories through brain-derived neurotrophic factor-dependent mechanisms (Intlekofer et al., 2013). These findings offer hope for inhibiting the recurrence of traumatic memories in PTSD. An abnormal gut environment (e.g., gut barrier dysfunction, SCFAs concentrations, and a variety of microbial metabolites) also characterizes the pathology of PTSD (Mellon et al., 2019) Veterans with PTSD have a proinflammatory gut environment that includes higher levels of metabolites of microbial origin, such as acetic, lactic, and succinic acids, and gut barrier dysfunction [lipopolysaccharides (LPS) and lipopolysaccharide-binding proteins,] increased HMGB1, along with an increased number of extracellular vesicles of gut epithelial cell origin (Voigt et al., 2022).
3.6 Other mechanisms
Initially, it was thought that the gut microbiota might be a viable target for the direct treatment of disorders associated with amygdala dysregulation, including visceral pain, post-traumatic stress disorder, and depression (Cowan et al., 2018; Hoban et al., 2017; Seo and Anderson, 2019; Stilling et al., 2015). Hoban et al. demonstrated for the first time, by means of a genome-wide transcriptome analysis approach, that the presence of the host microbiota is essential for amygdala-dependent memory retention during appropriate Behavioral responses are critical because microbiota-driven modulation of neuronal function can directly lead to fear suppression and dysbiosis learning. It also induces neuronal changes in the amygdala region of the brain, thereby alleviating PTSD symptoms (Hoban et al., 2018). Transmembrane two-photon imaging performed by Chu et al. showed that ablative learning deficits following gut microbiota manipulation in adult mice were associated with deficits in synaptic dendritic spine remodeling and reduced activity of cue-encoding neurons in the medial prefrontal cortex (Chu et al., 2019). Although these important observations highlight the relevance of the gut-brain axis and fear circuits, the mechanisms by which the gut-brain axis directly influences fear and stress circuits remain largely unknown.
PTSD is more common in women than in men (Tolin and Foa, 2006), and previous studies in human and animal models suggest that differences between the ways in which the gonadal hormones testosterone or estrogen interact with the HPA axis or regulate hippocampal function may have contributed to this (Briscione et al., 2017; Fenchel et al., 2015; Lilly et al., 2009; Maeng and Milad, 2015; Richter-Levin et al., 2019) report. Gender is also one of the important host factors influencing the human microbiota (Dominianni et al., 2015; Kim et al., 2020) and the animal microbiota (Elderman et al., 2018; Org et al., 2016). Gender-related differences, such as sex hormones, may interact with gut microbiota, potentially influencing the gut-brain axis and contributing to the development of PTSD.
Interestingly, medical dogs of veterans with PTSD may also influence human health by altering the microbiota of the population, thus potentially providing additional mechanisms to influence human health (Hoisington et al., 2018).
4 Intervention strategies for PTSD based on the gut-brain axis
4.1 Probiotics
Sudo et al. have shown that in GF mice, exposure to stress induces hyperactivation of the HPA axis, which can be completely reversed by reconstitution with Bifidobacterium infantis. These data further open the possibility that supplementation with diet and/or beneficial bacteria may ultimately influence disordered behavior (Sudo et al., 2004).
Targeting gut microbiota, a potential key modulator of the immune and nervous systems, could lead to greater improvement in mood symptoms in patients with depression or anxiety. The composition and function of the gut bacterial community can be improved through dietary interventions or the use of beneficial bacteria such as probiotics. Although, in this regard, clinical trials have not been adequate in terms of design or number of subjects involved. However, studies that have been conducted have shown that administration of different species of Lactobacillus and Bifidobacterium is associated with improved mood and reduced anxiety (Benton et al., 2007; Rao et al., 2009). This is especially true in subjects with low cortisol levels (Messaoudi et al., 2011). In addition, taking fermented dairy products containing probiotics affects activity in brain regions that control central processing of emotions in women (Tillisch et al., 2013). In addition, two intervention studies for PTSD examined the reported effects of microbiota-targeted supplements on PTSD symptoms in veterans (Brenner et al., 2020; Gocan et al., 2012). The first study (Gocan et al., 2012), of 10 veterans with PTSD, found that regular consumption of a fermented soy preparation (FSWW 08) for 6 months reduced anxiety and panic. However, causal inferences from this study were limited by the lack of a control group as well as small sample size, selection factors, and reporting bias. In the second study (Brenner et al., 2020), participants were randomly assigned to either the intervention group or the placebo group (once daily for 8 weeks ± 2 weeks) in a 1:1 ratio, stratified according to irritable bowel syndrome status. Plasma C-reactive protein (CRP) concentrations tended to decrease in the probiotic-supplemented group compared with the placebo group, and the stress response was more pronounced in the placebo group. Brenner et al. also conducted a systematic review to evaluate existing studies on prebiotic and probiotic interventions in patients with traumatic brain injury and PTSD (Brenner et al., 2017).
4.2 Dietary interventions
Because dietary interventions can be effective in ameliorating the effects of chronic inflammation, medical associations have identified them as first-line and/or adjunctive tools for many neurodegenerative diseases (Parkinson et al., 2023), which is also an important avenue for psychiatric disorders, particularly PTSD (Lee et al., 2022). In addition, dietary interventions may promote positive neuroplasticity (Sugden et al., 2024), strengthen the gut-brain axis system (Campaniello et al., 2022), reduce the effects of neuroinflammation, and increase the window of tolerance. Leclercq et al. have proposed that it is possible to target abnormalities in these systems through manipulation of certain gut bacterial communities, either directly through supplementation or indirectly through dietary and other novel approaches (Leclercq et al., 2016). Two recent feasibility studies have shown that it improves PTSD symptoms. Herbert et al. selected 10 U.S. veterans with PTSD and chronic pain and gave them a plant-based diet high in dietary fiber for 2 weeks, followed by 2 weeks of a regular diet. The veterans reported improvements in both chronic pain and PTSD symptoms (Herbert et al., 2023). Arcan et al. studied responders to the World Trade Center disaster. Responders either received nutritional counseling or help from the Mediterranean diet. People on the Mediterranean diet showed greater changes in the Posttraumatic Checklist-dsm -5 (PCL-5) (Arcan et al., 2024).
People with PTSD also need to be aware of the intake of fast food as well as other ultra-processed foods, as ultra-processed foods increase inflammatory processes in the gut (Tristan Asensi et al., 2023) and may also promote neuroinflammation (Firth et al., 2019), and a key component of ultra-processed foods is the lack of dietary fiber. Conversely, diets rich in dietary fiber promote certain types of healthy bacteria (i.e., Bifidobacterium, Lactobacillus, Tricholobacteriaceae, Cyanobacteria, Coccidioides faecalis, Roseobacteria and E. faecalis) that are able to break down complex carbohydrates into short-chain fatty acids through fermentation (So et al., 2018), potentially helping to alleviate PTSD.
4.3 Natural medicines targeting the gut microbiota for PTSD
In the treatment of PTSD, the only drugs that target the treatment of people with PTSD and animal models via the gut-brain axis are the natural medicines cannabis, yellow essence, and jiawei xiaoyaoshan, with more discussion focusing on the broad neuroprotective effects of natural medicines (including marine natural products and plant natural products) (Fakhri et al., 2021; Shimizu et al., 2015), prevention of apoptosis, and the effects of oxidative stress (Baek and Kim, 2020; Xing et al., 2020).
Cannabinoids alter the gut microbiota of people with PTSD by modulating fear memory and influence n-3 polyunsaturated fatty acid metabolic pathways (Okubo et al., 2018). A few years ago, McLaughlin et al. suggested that endogenous cannabinoid signaling systems in the medial frontal cortex, a key brain region for fear memory, may be attractive targets for the treatment of stress-related disorders (McLaughlin et al., 2014). In recent years, it has been found that such drugs also modulate GABA receptor activity and cortisol levels via the gut-brain axis (Fraguas-Sánchez and Torres-Suárez, 2018) to enhance psychological resilience (B. Gao et al., 2023). Research suggests that cannabinoids may play a potential therapeutic role in PTSD-related symptoms by modulating gut microbiota and the gut-brain axis. In contrast, treatment of Chronic Unpredictable Mild Stress (CUMS)-induced mice with flavonoid polysaccharides increased the relative abundance of Muribaculaceae, Dubosiella, and Lactobacillus, and decreased the relative abundance of Akkermansia, Helicobacter, and Clostridium methylpentosum relative abundance (Shen et al., 2022), which may regulate oxidative stress and nlrp3-mediated inflammation in a Nrf2/HO-1 signaling pathway-dependent manner, thereby preventing sps-induced ptsd-like behavior and synaptic damage (Xie et al., 2024). In addition, the herbal medicine jiaweixiaoyaosan, which acts as a complex of natural medicines that can influence the composition of the gut microbiota, has also been reported to alleviate ptsd-related symptomatic aspects by regulating the improvement of the HPA axis and hormonal disorders, increasing neurotransmitter content, neurogenesis, and modulating the synthesis of related enzymes (Xie et al., 2023).
5 Perspective
This review explores the potential role of gut microbiota in the development of post-traumatic stress disorder (PTSD) from several perspectives, and remains wary of the limitations of extrapolating its conclusions to human studies in terms of animal modeling. On the one hand, there are essential differences in the composition of gut microbiota between species, resulting in the abundance and function of certain bacteria not being consistent between humans and animals; on the other hand, acute, single stress stimuli (e.g., restraints, electric shocks, etc.) are often used to simulate PTSD in animal models, whereas actual clinical traumatic events are often complex, diverse, and involve long-term psychological and socio-environmental factors, making it difficult to replicate them exactly. In addition, most animal experiments use homozygous strain individuals, ignoring genetic background and lifestyle differences in human populations. Therefore, animal studies on PTSD and gut microbiota should remain cautious in the interpretation of results and extrapolation of mechanisms. With regard to population studies, little is still known about the longitudinal dynamics of gut microbiota in people with PTSD. Most of the studies used cross-sectional design, which makes it difficult to reveal the temporal evolution of the microbiota at different stages before, during, and after trauma and its potential causal links with changes in clinical symptoms. In the future, there is an urgent need to conduct longitudinal follow-up studies based on large samples, combined with gender stratification and multi-omics technology, to comprehensively depict the key nodes and mechanisms of gut microbiota in the occurrence, development and recovery of PTSD, so as to provide theoretical support for individualized treatment.
Currently, the neurobiological mechanisms of PTSD are regulated by gut microbiota, and future research could be further expanded in the following directions: to develop therapeutic strategies that precisely target the gut-brain axis. Currently, the main pharmacological treatment for PTSD is based on selective 5-hydroxytryptamine reuptake inhibitors (e.g., sertraline, paroxetine), and there is still a lack of research exploring whether gut microbiota can enhance its efficacy or serve as an adjunctive therapeutic target. Promoting clinical research on probiotics and fecal transplants: Preliminary studies have suggested that probiotics and Fecal Microbial Transplantation (FMT) may have therapeutic potential in mood disorders, but their long-term efficacy, strain-specific selection, timing of interventions, and individualized response mechanisms in PTSD need to be systematically validated in large clinical cohorts. Standardization of probiotic clinical application and scientific education: Currently, there is excessive expectation or misuse of probiotic efficacy in the general public and in some clinical practices, with vague intervention goals, random strain selection, and a lack of unified regulatory standards, which limits its scientific promotion. In the future, we should strengthen the research on the joint intervention mechanism of diet and probiotics, clarify its indications and mechanism, promote the formulation of regulatory standards at the policy level, and strengthen public health education.
In addition, attention needs to be paid to the potential of traditional medicine and natural medicines: natural medicines may affect the gut-brain axis by modulating the inflammatory response, maintaining neurotransmitter homeostasis, and improving the gut barrier function. Although it has been reported in the literature that drugs such as Cannabis sativa, Rhizoma Polygoni Multiflori and Jiawei Yiwu San have some interventional effects on PTSD, there is a lack of systematic research to elucidate their mechanisms of action and targets of intervention. In the future, we should explore the precise pathways of natural drugs to regulate the gut microbiota and central nervous system function.
This review still has some limitations. There is a large heterogeneity in the literature included in this paper in terms of study population, gut microbiota testing methods, and PTSD modeling approaches (e.g., population studies vs animal models), which limits cross-sectional comparisons and comprehensive interpretation of results. Most studies were cross-sectional in design, making it difficult to clarify the causal relationship between gut microbiota and PTSD. In addition, the lack of uniform quantitative indicators and intervention evaluation systems also prevented meta-analysis in this review, affecting the systematic summary of the strength of evidence.
6 Conclusion
In recent years, with the deepening of gut-brain axis research, more and more evidence suggests that gut microecology plays an important role in the occurrence and development of post-traumatic stress disorder (PTSD). In this review, we systematically reviewed the current research progress on the association between gut microbiota and PTSD, and summarized the potential mechanisms by which the gut-brain axis may affect PTSD in terms of inflammatory response, neurotransmitter metabolism, HPA axis regulation and immune pathways. Meanwhile, we summarize the current major microecological intervention strategies, including probiotics, prebiotics, dietary modification, and FMT, and discuss their potential value in alleviating PTSD symptoms.
Author contributions
JP: Conceptualization, Writing – original draft. SL: Conceptualization, Writing – review and editing. QQ: Writing – original draft. SF: Writing – original draft. XL: Funding acquisition, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This review was supported by grants from National College Students Innovation and Entrepreneurship Training Program (202410488037 to PJ).
Acknowledgments
Thanks to the researchers who published relevant papers. We would like to thank the academic editor and reviewers for their important contributions that improved the quality of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ait-Belgnaoui, A., Durand, H., Cartier, C., Chaumaz, G., Eutamene, H., Ferrier, L., et al. (2012). Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 37 (11), 1885–1895. doi:10.1016/j.psyneuen.2012.03.024
Almeida, F. B., Pinna, G., and Barros, H. M. T. (2021). The role of HPA axis and allopregnanolone on the neurobiology of major depressive disorders and PTSD. Int. J. Mol. Sci. 22 (11), 5495. doi:10.3390/ijms22115495
Alpert, O., Begun, L., Issac, T., and Solhkhah, R. (2021). The brain-gut axis in gastrointestinal cancers. J. Gastrointest. Oncol. 12 (Suppl. 2), S301–S310. doi:10.21037/jgo-2019-gi-04
Arcan, C., Hou, W., Hoffman, K., Reichardt, A., Yang, X., Clouston, S. A. P., et al. (2024). Mediterranean diet intervention among world trade center responders with post-traumatic stress disorder: feasibility and outcomes of a pilot randomized controlled trial. Obes. Sci. Pract. 10 (1), e725. doi:10.1002/osp4.725
Baek, S. Y., and Kim, M. R. (2020). Neuroprotective effect of carotenoid-rich Enteromorpha prolifera extract via TrkB/Akt pathway against oxidative stress in hippocampal neuronal cells. Mar. Drugs 18 (7), 372. doi:10.3390/md18070372
Bailey, M. T., and Coe, C. L. (1999). Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev. Psychobiol. 35 (2), 146–155. doi:10.1002/(sici)1098-2302(199909)35:2<146::aid-dev7>3.0.co;2-g
Bajaj, J. S., Sikaroodi, M., Fagan, A., Heuman, D., Gilles, H., Gavis, E. A., et al. (2019). Posttraumatic stress disorder is associated with altered gut microbiota that modulates cognitive performance in veterans with cirrhosis. Am. J. Physiol. Gastrointest. Liver Physiol. 317 (5), G661–G669. doi:10.1152/ajpgi.00194.2019
Bandelow, B., Baldwin, D., Abelli, M., Bolea-Alamanac, B., Bourin, M., Chamberlain, S. R., et al. (2017). Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J. Biol. Psychiatry 18 (3), 162–214. doi:10.1080/15622975.2016.1190867
Bekhbat, M., Rowson, S. A., and Neigh, G. N. (2017). Checks and balances: the glucocorticoid receptor and NFĸB in good times and bad. Front. Neuroendocrinol. 46, 15–31. doi:10.1016/j.yfrne.2017.05.001
Belkaid, Y., and Hand, T. W. (2014). Role of the microbiota in immunity and inflammation. Cell 157 (1), 121–141. doi:10.1016/j.cell.2014.03.011
Benton, D., Williams, C., and Brown, A. (2007). Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 61 (3), 355–361. doi:10.1038/sj.ejcn.1602546
Bercik, P., Denou, E., Collins, J., Jackson, W., Lu, J., Jury, J., et al. (2011a). The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141 (2), 599–609.e6093. doi:10.1053/j.gastro.2011.04.052
Bercik, P., Park, A. J., Sinclair, D., Khoshdel, A., Lu, J., Huang, X., et al. (2011b). The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 23 (12), 1132–1139. doi:10.1111/j.1365-2982.2011.01796.x
Bharwani, A., Mian, M. F., Surette, M. G., Bienenstock, J., and Forsythe, P. (2017). Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Med. 15 (1), 7. doi:10.1186/s12916-016-0771-7
Bonaz, B., Sinniger, V., and Pellissier, S. (2017). Vagus nerve stimulation: a new promising therapeutic tool in inflammatory bowel disease. J. Intern. Med. 282 (1), 46–63. doi:10.1111/joim.12611
Bonnin, A., and Levitt, P. (2011). Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience 197, 1–7. doi:10.1016/j.neuroscience.2011.10.005
Braniste, V., Al-Asmakh, M., Kowal, C., Anuar, F., Abbaspour, A., Tóth, M., et al. (2014). The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 6 (263), 263ra158. doi:10.1126/scitranslmed.3009759
Bravo, J. A., Forsythe, P., Chew, M. V., Escaravage, E., Savignac, H. M., Dinan, T. G., et al. (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U. S. A. 108 (38), 16050–16055. doi:10.1073/pnas.1102999108
Breit, S., Kupferberg, A., Rogler, G., and Hasler, G. (2018). Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front. Psychiatry 9, 44. doi:10.3389/fpsyt.2018.00044
Bremner, J. D. (2006). Traumatic stress: effects on the brain. Dialogues Clin. Neurosci. 8 (4), 445–461. doi:10.31887/DCNS.2006.8.4/jbremner
Brenner, L. A., Forster, J. E., Stearns-Yoder, K. A., Stamper, C. E., Hoisington, A. J., Brostow, D. P., et al. (2020). Evaluation of an immunomodulatory probiotic intervention for veterans with co-occurring mild traumatic brain injury and posttraumatic stress disorder: a pilot study. Front. Neurol. 11, 1015. doi:10.3389/fneur.2020.01015
Brenner, L. A., Stearns-Yoder, K. A., Hoffberg, A. S., Penzenik, M. E., Starosta, A. J., Hernández, T. D., et al. (2017). Growing literature but limited evidence: a systematic review regarding prebiotic and probiotic interventions for those with traumatic brain injury And/Or posttraumatic stress disorder. Brain, Behav. Immun. 65, 57–67. doi:10.1016/j.bbi.2017.06.003
Briscione, M. A., Michopoulos, V., Jovanovic, T., and Norrholm, S. D. (2017). Neuroendocrine underpinnings of increased risk for posttraumatic stress disorder in women. Vitamins Hormones 103, 53–83. doi:10.1016/bs.vh.2016.08.003
Cai, M., Park, H. R., and Yang, E. J. (2023). Electroacupuncture modulates glutamate neurotransmission to alleviate PTSD-Like behaviors in a PTSD animal model. Transl. Psychiatry 13 (1), 357. doi:10.1038/s41398-023-02663-4
Campaniello, D., Corbo, M. R., Sinigaglia, M., Speranza, B., Racioppo, A., Altieri, C., et al. (2022). How diet and physical activity modulate gut microbiota: evidence, and perspectives. Nutrients 14 (12), 2456. doi:10.3390/nu14122456
Chan, J. C., Alenina, N., Cunningham, A. M., Ramakrishnan, A., Shen, L., Bader, M., et al. (2024). Serotonin transporter-dependent histone serotonylation in placenta contributes to the neurodevelopmental transcriptome. J. Mol. Biol. 436 (7), 168454. doi:10.1016/j.jmb.2024.168454
Chang, L., Wei, Y., and Hashimoto, K. (2022). Brain-gut-microbiota axis in depression: a historical overview and future directions. Brain Res. Bull. 182, 44–56. doi:10.1016/j.brainresbull.2022.02.004
Chu, C., Murdock, M. H., Jing, D., Won, T. H., Chung, H., Kressel, A. M., et al. (2019). The microbiota regulate neuronal function and fear extinction learning. Nature 574 (7779), 543–548. doi:10.1038/s41586-019-1644-y
Cohen, H., and Zohar, J. (2004). An animal model of posttraumatic stress disorder: the use of cut-off behavioral criteria. Ann. N. Y. Acad. Sci. 1032, 167–178. doi:10.1196/annals.1314.014
Compean, E., and Hamner, M. (2019). Posttraumatic stress disorder with secondary psychotic features (PTSD-SP): diagnostic and treatment challenges. Prog. Neuro-Psychopharmacology Biol. Psychiatry 88, 265–275. doi:10.1016/j.pnpbp.2018.08.001
Costantini, T. W., Bansal, V., Krzyzaniak, M., Putnam, J. G., Peterson, C. Y., Loomis, W. H., et al. (2010). Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells. Am. J. Physiol. Gastrointest. Liver Physiol. 299 (6), G1308–G1318. doi:10.1152/ajpgi.00156.2010
Cowan, C. S. M., Hoban, A. E., Ventura-Silva, A. P., Dinan, T. G., Clarke, G., and Cryan, J. F. (2018). Gutsy moves: the amygdala as a critical node in microbiota to brain signaling. BioEssays News Rev. Mol. Cell. Dev. Biol. 40 (1). doi:10.1002/bies.201700172
Cryan, J. F., O’Riordan, K. J., Cowan, C. S. M., Sandhu, K. V., Bastiaanssen, T. F. S., Boehme, M., et al. (2019). The microbiota-gut-brain axis. Physiol. Rev. 99 (4), 1877–2013. doi:10.1152/physrev.00018.2018
Dabrowska, J. (2023). From recent advances in underlying neurocircuitry of fear and anxiety to promising pharmacotherapies for PTSD: the Saga of heart, sex and the developing brain. Neuropharmacology 232, 109529. doi:10.1016/j.neuropharm.2023.109529
Davis, L. L., Schein, J., Cloutier, M., Gagnon-Sanschagrin, P., Maitland, J., Urganus, A., et al. (2022). The economic burden of posttraumatic stress disorder in the United States from a societal perspective. J. Clin. Psychiatry 83 (3), 21m14116. doi:10.4088/JCP.21m14116
De Vadder, F., Grasset, E., Mannerås Holm, L., Karsenty, G., Macpherson, A. J., Olofsson, L. E., et al. (2018). Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc. Natl. Acad. Sci. U. S. A. 115 (25), 6458–6463. doi:10.1073/pnas.1720017115
Diaz Heijtz, R., Wang, S., Anuar, F., Qian, Y., Björkholm, B., Samuelsson, A., et al. (2011). Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. U. S. A. 108 (7), 3047–3052. doi:10.1073/pnas.1010529108
Dicks, L. M. T. (2022). Gut bacteria and neurotransmitters. Microorganisms 10 (9), 1838. doi:10.3390/microorganisms10091838
Dominianni, C., Sinha, R., Goedert, J. J., Pei, Z., Yang, L., Hayes, R. B., et al. (2015). Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS One 10 (4), e0124599. doi:10.1371/journal.pone.0124599
Doney, E., Cadoret, A., Dion-Albert, L., Lebel, M., and Menard, C. (2022). Inflammation-driven brain and gut barrier dysfunction in stress and mood disorders. Eur. J. Neurosci. 55 (9–10), 2851–2894. doi:10.1111/ejn.15239
Dunlop, B. W., and Wong, A. (2019). The hypothalamic-pituitary-adrenal axis in PTSD: pathophysiology and treatment interventions. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 89, 361–379. doi:10.1016/j.pnpbp.2018.10.010
Eisenstein, M. (2016). Microbiome: bacterial broadband. Nature 533 (7603), S104–S106. doi:10.1038/533S104a
Elder, G. A., Ehrlich, M. E., and Gandy, S. (2019). Relationship of traumatic brain injury to chronic mental health problems and dementia in military veterans. Neurosci. Lett. 707, 134294. doi:10.1016/j.neulet.2019.134294
Elderman, M., Hugenholtz, F., Belzer, C., Boekschoten, M., van Beek, A., de Haan, B., et al. (2018). Sex and strain dependent differences in mucosal immunology and microbiota composition in mice. Biol. Sex Differ. 9 (1), 26. doi:10.1186/s13293-018-0186-6
Fakhri, S., Yarmohammadi, A., Yarmohammadi, M., Farzaei, M. H., and Echeverria, J. (2021). Marine natural products: promising candidates in the modulation of gut-brain axis towards neuroprotection. Mar. Drugs 19 (3), 165. doi:10.3390/md19030165
Feldman, E. L., Goutman, S. A., Petri, S., Mazzini, L., Savelieff, M. G., Shaw, P. J., et al. (2022). Amyotrophic lateral sclerosis. Lancet 400 (10360), 1363–1380. doi:10.1016/S0140-6736(22)01272-7
Feldman, R., Yirmiya, K., Turjeman, S., Shtossel, O., Zagoory-Sharon, O., Moadi, L., et al. (2022). Microbiome mediates development of PTSD and resilience. Review. doi:10.21203/rs.3.rs-1940296/v1
Fenchel, D., Levkovitz, Y., Vainer, E., Kaplan, Z., Zohar, J., and Cohen, H. (2015). Beyond the HPA-axis: the role of the gonadal steroid hormone receptors in modulating stress-related responses in an animal model of PTSD. Eur. Neuropsychopharmacol. 25 (6), 944–957. doi:10.1016/j.euroneuro.2015.02.004
Firth, J., Marx, W., Dash, S., Carney, R., Teasdale, S. B., Solmi, M., et al. (2019). The effects of dietary improvement on symptoms of depression and anxiety: a meta-analysis of randomized controlled trials. Psychosom. Med. 81 (3), 265–280. doi:10.1097/PSY.0000000000000673
Förstermann, U., and Sessa, W. C. (2012). Nitric oxide synthases: regulation and function. Eur. Heart J. 33 (7), 829–837. 837a–837d. doi:10.1093/eurheartj/ehr304
Foster, J. A., Rinaman, L., and Cryan, J. F. (2017). Stress and the gut-brain axis: regulation by the microbiome. Neurobiol. Stress 7, 124–136. doi:10.1016/j.ynstr.2017.03.001
Fraguas-Sánchez, A. I., and Torres-Suárez, A. I. (2018). Medical use of cannabinoids. Drugs 78 (16), 1665–1703. doi:10.1007/s40265-018-0996-1
Fukumoto, S., Tatewaki, M., Yamada, T., Fujimiya, M., Mantyh, C., Voss, M., et al. (2003). Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am. J. Physiology. Regul. Integr. Comp. Physiol. 284 (5), R1269–R1276. doi:10.1152/ajpregu.00442.2002
Fülling, C., Dinan, T. G., and Cryan, J. F. (2019). Gut microbe to brain signaling: what happens in vagus. Neuron 101 (6), 998–1002. doi:10.1016/j.neuron.2019.02.008
Gao, B., Qu, Y.-C., Cai, M.-Y., Zhang, Y.-Y., Lu, H.-T., Li, H.-X., et al. (2023). Phytochemical interventions for post-traumatic stress disorder: a cluster co-occurrence network analysis using CiteSpace. J. Integr. Med. 21 (4), 385–396. doi:10.1016/j.joim.2023.06.006
Gao, F., Guo, R., Ma, Q., Li, Y., Wang, W., Fan, Y., et al. (2022). Stressful events induce long-term gut microbiota dysbiosis and associated post-traumatic stress symptoms in healthcare workers fighting against COVID-19. J. Affect. Disord. 303, 187–195. doi:10.1016/j.jad.2022.02.024
Gareau, M. G., Wine, E., Rodrigues, D. M., Cho, J. H., Whary, M. T., Philpott, D. J., et al. (2011). Bacterial infection causes stress-induced memory dysfunction in mice. Gut 60 (3), 307–317. doi:10.1136/gut.2009.202515
Gautam, A., Kumar, R., Chakraborty, N., Muhie, S., Hoke, A., Hammamieh, R., et al. (2018). Altered fecal microbiota composition in all Male aggressor-exposed rodent model simulating features of post-traumatic stress disorder. J. Neurosci. Res. 96 (7), 1311–1323. doi:10.1002/jnr.24229
Geier, T. J., Atkinson, S. N., Pan, A. Y., Mantz-Wichman, M., Jazinski-Chambers, K., Hillard, C. J., et al. (2024). Differences in intestinal bacteria in traumatic injury survivors with and without probable posttraumatic stress disorder. J. Affect. Disord. 361, 528–535. doi:10.1016/j.jad.2024.06.075
Gocan, A. G., Bachg, D., Schindler, A. E., and Rohr, U. D. (2012). Balancing steroidal hormone Cascade in treatment-resistant veteran soldiers with PTSD using a fermented soy product (FSWW08): a pilot study. Hormone Mol. Biol. Clin. Invest. 10 (3), 301–314. doi:10.1515/hmbci-2011-0135
Goehler, L. E., Gaykema, R. P. A., Opitz, N., Reddaway, R., Badr, N., and Lyte, M. (2005). Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain, Behav. Immun. 19 (4), 334–344. doi:10.1016/j.bbi.2004.09.002
Han, S.-K., and Kim, D. H. (2019). Lactobacillus mucosae and Bifidobacterium longum synergistically alleviate immobilization stress-induced anxiety/depression in mice by suppressing gut dysbiosis. J. Microbiol. Biotechnol. 29 (9), 1369–1374. doi:10.4014/jmb.1907.07044
Hanscom, M., Loane, D. J., and Shea-Donohue, T. (2021). Brain-gut axis dysfunction in the pathogenesis of traumatic brain injury. J. Clin. Investigation 131 (12), e143777. doi:10.1172/JCI143777
Hemmings, S. M. J., Malan-Müller, S., van den Heuvel, L. L., Demmitt, B. A., Stanislawski, M. A., Smith, D. G., et al. (2017). The microbiome in posttraumatic stress disorder and trauma-exposed controls: an exploratory study. Psychosom. Med. 79 (8), 936–946. doi:10.1097/PSY.0000000000000512
Hendrickson, R. C., and Raskind, M. A. (2016). Noradrenergic dysregulation in the pathophysiology of PTSD. Exp. Neurol. 284 (Pt B), 181–195. doi:10.1016/j.expneurol.2016.05.014
Herbert, M. S., McLean, C. L., Chu, G. M., Lerman, I., Baker, D. G., and Lang, A. J. (2023). High fiber plant-based diet for chronic pain and posttraumatic stress disorder: a feasibility study. Pain Med. (Malden, Mass.) 24 (7), 900–902. doi:10.1093/pm/pnac200
Hoban, A. E., Stilling, R. M., M Moloney, G., Moloney, R. D., Shanahan, F., Dinan, T. G., et al. (2017). Microbial regulation of microRNA expression in the amygdala and prefrontal cortex. Microbiome 5 (1), 102. doi:10.1186/s40168-017-0321-3
Hoban, A. E., Stilling, R. M., Moloney, G., Shanahan, F., Dinan, T. G., Clarke, G., et al. (2018). The microbiome regulates amygdala-dependent fear recall. Mol. Psychiatry 23 (5), 1134–1144. doi:10.1038/mp.2017.100
Hoisington, A. J., Billera, D. M., Bates, K. L., Stamper, C. E., Stearns-Yoder, K. A., Lowry, C. A., et al. (2018). Exploring service dogs for rehabilitation of veterans with PTSD: a microbiome perspective. Rehabil. Psychol. 63 (4), 575–587. doi:10.1037/rep0000237
Huang, J., Xu, F., Yang, L., Tuolihong, L., Wang, X., Du, Z., et al. (2023). Involvement of the GABAergic system in PTSD and its therapeutic significance. Front. Mol. Neurosci. 16, 1052288. doi:10.3389/fnmol.2023.1052288
Huo, R., Zeng, B., Zeng, L., Cheng, K., Li, B., Luo, Y., et al. (2017). Microbiota modulate anxiety-like behavior and endocrine abnormalities in hypothalamic-pituitary-adrenal axis. Front. Cell. Infect. Microbiol. 7, 489. doi:10.3389/fcimb.2017.00489
Intlekofer, K. A., Berchtold, N. C., Malvaez, M., Carlos, A. J., McQuown, S. C., Cunningham, M. J., et al. (2013). Exercise and sodium butyrate transform a subthreshold learning event into long-term memory via a brain-derived neurotrophic factor-dependent mechanism. Neuropsychopharmacol 38 (10), 2027–2034. doi:10.1038/npp.2013.104
Jones, M. E., Lebonville, C. L., Paniccia, J. E., Balentine, M. E., Reissner, K. J., and Lysle, D. T. (2018). Hippocampal interleukin-1 mediates stress-enhanced fear learning: a potential role for astrocyte-derived interleukin-1β. Brain, Behav. Immun. 67, 355–363. doi:10.1016/j.bbi.2017.09.016
Kalisch, R., Russo, S. J., and Müller, M. B. (2024). Neurobiology and systems biology of stress resilience. Physiol. Rev. 104 (3), 1205–1263. doi:10.1152/physrev.00042.2023
Ke, S., Hartmann, J., Ressler, K. J., Liu, Y.-Y., and Koenen, K. C. (2023). The emerging role of the gut microbiome in posttraumatic stress disorder. Brain, Behav. Immun. 114, 360–370. doi:10.1016/j.bbi.2023.09.005
Kelly, J. R., Clarke, G., Harkin, A., Corr, S. C., Galvin, S., Pradeep, V., et al. (2022). Seeking the psilocybiome: psychedelics meet the microbiota-gut-brain axis. Int. J. Clin. Health Psychol. 23 (2), 100349. doi:10.1016/j.ijchp.2022.100349
Kim, Y. S., Unno, T., Kim, B. Y., and Park, M. S. (2020). Sex differences in gut microbiota. World J. Men’s Health 38 (1), 48–60. doi:10.5534/wjmh.190009
Krediet, E., Bostoen, T., Breeksema, J., van Schagen, A., Passie, T., and Vermetten, E. (2020). Reviewing the potential of psychedelics for the treatment of PTSD. Int. J. Neuropsychopharmacol. 23 (6), 385–400. doi:10.1093/ijnp/pyaa018
Laudani, S., Torrisi, S. A., Alboni, S., Bastiaanssen, T. F. S., Benatti, C., Rivi, V., et al. (2023). Gut microbiota alterations promote traumatic stress susceptibility associated with p-cresol-induced dopaminergic dysfunctions. Brain, Behav. Immun. 107, 385–396. doi:10.1016/j.bbi.2022.11.004
Leclercq, S., Forsythe, P., and Bienenstock, J. (2016). Posttraumatic stress disorder: does the gut microbiome hold the key? Can. J. Psychiatry. Revue Can. De Psychiatrie 61 (4), 204–213. doi:10.1177/0706743716635535
Lee, D.-H., Lee, J.-Y., Hong, D.-Y., Lee, E.-C., Park, S.-W., Lee, M.-R., et al. (2022). Neuroinflammation in post-traumatic stress disorder. Biomedicines 10 (5), 953. doi:10.3390/biomedicines10050953
Levert-Levitt, E., Shapira, G., Sragovich, S., Shomron, N., Lam, J. C. K., Li, V. O. K., et al. (2022). Oral microbiota signatures in post-traumatic stress disorder (PTSD) veterans. Mol. Psychiatry 27, 4590–4598. doi:10.1038/s41380-022-01704-6
Li, H.-Q., Jiang, W., Ling, L., Pratelli, M., Chen, C., Gupta, V., et al. (2024). Generalized fear after acute stress is caused by change in neuronal cotransmitter identity. Sci. (New York, N.Y.) 383 (6688), 1252–1259. doi:10.1126/science.adj5996
Lilly, M. M., Pole, N., Best, S. R., Metzler, T., and Marmar, C. R. (2009). Gender and PTSD: what can we learn from female police officers? J. Anxiety Disord. 23 (6), 767–774. doi:10.1016/j.janxdis.2009.02.015
Liu, H., Wang, J., He, T., Becker, S., Zhang, G., Li, D., et al. (2018). Butyrate: a double-edged sword for health? Adv. Nutr. (Bethesda, Md) 9 (1), 21–29. doi:10.1093/advances/nmx009
Liu, M.-T., Kuan, Y.-H., Wang, J., Hen, R., and Gershon, M. D. (2009). 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J. Neurosci. 29 (31), 9683–9699. doi:10.1523/JNEUROSCI.1145-09.2009
Liu, S., Wang, Y., Zhang, Y., Zeng, L., Ling, L., Luo, Y., et al. (2024). The gut microbiota and post-traumatic major depression disorder: insights from bidirectional two-sample Mendelian randomization. Front. Psychiatry 15, 1383664. doi:10.3389/fpsyt.2024.1383664
Liu, W.-Z., Huang, B.-W., You, W.-J., Hu, P., Wang, X.-H., Zhang, J.-Y., et al. (2018). Harmine enhances GABAergic transmission onto basoamygdala projection neurons in mice. Brain Res. Bull. 137, 294–300. doi:10.1016/j.brainresbull.2018.01.004
Liu, Y., Sanderson, D., Mian, M. F., McVey Neufeld, K.-A., and Forsythe, P. (2021). Loss of vagal integrity disrupts immune components of the microbiota-gut-brain axis and inhibits the effect of Lactobacillus rhamnosus on behavior and the corticosterone stress response. Neuropharmacology 195, 108682. doi:10.1016/j.neuropharm.2021.108682
Lyte, M. (2013). Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 9 (11), e1003726. doi:10.1371/journal.ppat.1003726
Maeng, L. Y., and Milad, M. R. (2015). Sex differences in anxiety disorders: interactions between fear, stress, and gonadal hormones. Hormones Behav. 76, 106–117. doi:10.1016/j.yhbeh.2015.04.002
Malan-Muller, S., Valles-Colomer, M., Foxx, C. L., Vieira-Silva, S., Van Den Heuvel, L. L., Raes, J., et al. (2022). Exploring the relationship between the gut microbiome and mental health outcomes in a posttraumatic stress disorder cohort relative to trauma-exposed controls. Eur. Neuropsychopharmacol. 56, 24–38. doi:10.1016/j.euroneuro.2021.11.009
Malan-Müller, S., Valles-Colomer, M., Palomo, T., and Leza, J. C. (2023). The gut-microbiota-brain axis in a Spanish population in the aftermath of the COVID-19 pandemic: microbiota composition linked to anxiety, trauma, and depression profiles. Gut Microbes 15 (1), 2162306. doi:10.1080/19490976.2022.2162306
McLaughlin, R. J., Hill, M. N., and Gorzalka, B. B. (2014). A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emotional behavior. Neurosci. Biobehav. Rev. 42, 116–131. doi:10.1016/j.neubiorev.2014.02.006
McVey Neufeld, K. A., Perez-Burgos, A., Mao, Y. K., Bienenstock, J., and Kunze, W. A. (2015). The gut microbiome restores intrinsic and extrinsic nerve function in germ-free mice accompanied by changes in Calbindin. Neurogastroenterol. Motil. 27 (5), 627–636. doi:10.1111/nmo.12534
Mellon, S. H., Bersani, F. S., Lindqvist, D., Hammamieh, R., Donohue, D., Dean, K., et al. (2019). Metabolomic analysis of Male combat veterans with post traumatic stress disorder. PloS One 14 (3), e0213839. doi:10.1371/journal.pone.0213839
Merz, T., McCook, O., Denoix, N., Radermacher, P., Waller, C., and Kapapa, T. (2021). Biological connection of psychological stress and polytrauma under intensive care: the role of oxytocin and hydrogen sulfide. Int. J. Mol. Sci. 22 (17), 9192. doi:10.3390/ijms22179192
Messaoudi, M., Violle, N., Bisson, J.-F., Desor, D., Javelot, H., and Rougeot, C. (2011). Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2 (4), 256–261. doi:10.4161/gmic.2.4.16108
Meyerhoff, D. J., Mon, A., Metzler, T., and Neylan, T. C. (2014). Cortical gamma-aminobutyric acid and glutamate in posttraumatic stress disorder and their relationships to self-reported sleep quality. Sleep 37 (5), 893–900. doi:10.5665/sleep.3654
Miller, M. W., Maniates, H., Wolf, E. J., Logue, M. W., Schichman, S. A., Stone, A., et al. (2018). CRP polymorphisms and DNA methylation of the AIM2 gene influence associations between trauma exposure, PTSD, and C-reactive protein. Brain, Behav. Immun. 67, 194–202. doi:10.1016/j.bbi.2017.08.022
Mokhtari, Z., Gibson, D. L., and Hekmatdoost, A. (2017). Nonalcoholic fatty liver disease, the gut microbiome, and diet. Adv. Nutr. (Bethesda, Md) 8 (2), 240–252. doi:10.3945/an.116.013151
Myint, A. M., Schwarz, M. J., Steinbusch, H. W. M., and Leonard, B. E. (2009). Neuropsychiatric disorders related to interferon and interleukins treatment. Metab. Brain Dis. 24 (1), 55–68. doi:10.1007/s11011-008-9114-5
Nathalie, M., Polineni, S. P., Chin, C. N., Fawcett, D., Clervius, H., Maria, Q. S. L., et al. (2021). Targeting microglial polarization to improve TBI outcomes. CNS Neurological Disord. Drug Targets 20 (3), 216–227. doi:10.2174/1871527319666200918145903
Nikolova, V. L., Smith, M. R. B., Hall, L. J., Cleare, A. J., Stone, J. M., and Young, A. H. (2021). Perturbations in gut microbiota composition in psychiatric disorders: a review and meta-analysis. JAMA Psychiatry 78 (12), 1343–1354. doi:10.1001/jamapsychiatry.2021.2573
Noble, L. J., Souza, R. R., and McIntyre, C. K. (2019). Vagus nerve stimulation as a tool for enhancing extinction in exposure-based therapies. Psychopharmacology 236 (1), 355–367. doi:10.1007/s00213-018-4994-5
Noble, N. C., Merker, J. B., Webber, T. K., Ressler, K. J., and Seligowski, A. V. (2023). PTSD and depression severity are associated with cardiovascular disease symptoms in trauma-exposed women. Eur. J. Psychotraumatol. 14 (2), 2234810. doi:10.1080/20008066.2023.2234810
Núñez-Ríos, D. L., Nagamatsu, S. T., Martínez-Magaña, J. J., Hurd, Y., Rompala, G., Krystal, J. H., et al. (2024). Mapping the epigenomic landscape of post-traumatic stress disorder in human cortical neurons. medRxiv Prepr. Serv. Health Sci. 10.11, 24315258. doi:10.1101/2024.10.11.24315258
Okubo, R., Chen, C., Sekiguchi, M., Hamazaki, K., and Matsuoka, Y. J. (2018). Mechanisms underlying the effects of n-3 polyunsaturated fatty acids on fear memory processing and their hypothetical effects on fear of cancer recurrence in cancer survivors. Prostagl. Leukot. Essent. Fat. Acids 131, 14–23. doi:10.1016/j.plefa.2018.03.006
Org, E., Mehrabian, M., Parks, B. W., Shipkova, P., Liu, X., Drake, T. A., et al. (2016). Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 7 (4), 313–322. doi:10.1080/19490976.2016.1203502
Parkinson, M. D., Stout, R., and Dysinger, W. (2023). Lifestyle medicine: prevention, treatment, and reversal of disease. Med. Clin. N. Am. 107 (6), 1109–1120. doi:10.1016/j.mcna.2023.06.007
Pearson-Leary, J., Zhao, C., Bittinger, K., Eacret, D., Luz, S., Vigderman, A. S., et al. (2020). The gut microbiome regulates the increases in depressive-type behaviors and in inflammatory processes in the ventral hippocampus of stress vulnerable rats. Mol. Psychiatry 25 (5), 1068–1079. doi:10.1038/s41380-019-0380-x
Petakh, P., Oksenych, V., Kamyshna, I., Boisak, I., Lyubomirskaya, K., and Kamyshnyi, O. (2024). Exploring the interplay between posttraumatic stress disorder, gut microbiota, and inflammatory biomarkers: a comprehensive meta-analysis. Front. Immunol. 15, 1349883. doi:10.3389/fimmu.2024.1349883
Pivac, N., Vuic, B., Sagud, M., Nedic Erjavec, G., Nikolac Perkovic, M., Konjevod, M., et al. (2023). PTSD, immune system, and inflammation. Adv. Exp. Med. Biol. 1411, 225–262. doi:10.1007/978-981-19-7376-5_11
Rao, A. V., Bested, A. C., Beaulne, T. M., Katzman, M. A., Iorio, C., Berardi, J. M., et al. (2009). A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 1 (1), 6. doi:10.1186/1757-4749-1-6
Richter-Levin, G., Stork, O., and Schmidt, M. V. (2019). Animal models of PTSD: a challenge to be met. Mol. Psychiatry 24 (8), 1135–1156. doi:10.1038/s41380-018-0272-5
Rieder, R., Wisniewski, P. J., Alderman, B. L., and Campbell, S. C. (2017). Microbes and mental health: a review. Brain, Behav. Immun. 66, 9–17. doi:10.1016/j.bbi.2017.01.016
Rudzki, L., Pawlak, D., Pawlak, K., Waszkiewicz, N., Małus, A., Konarzewska, B., et al. (2017). Immune suppression of IgG response against dairy proteins in major depression. BMC Psychiatry 17 (1), 268. doi:10.1186/s12888-017-1431-y
Schumacher, S., Niemeyer, H., Engel, S., Cwik, J. C., Laufer, S., Klusmann, H., et al. (2019). HPA axis regulation in posttraumatic stress disorder: a meta-analysis focusing on potential moderators. Neurosci. Biobehav. Rev. 100, 35–57. doi:10.1016/j.neubiorev.2019.02.005
Seo, M., and Anderson, G. (2019). Gut-amygdala interactions in autism spectrum disorders: developmental roles via regulating mitochondria, exosomes, immunity and microRNAs. Curr. Pharm. Des. 25 (41), 4344–4356. doi:10.2174/1381612825666191105102545
Settanni, C. R., Ianiro, G., Bibbò, S., Cammarota, G., and Gasbarrini, A. (2021). Gut microbiota alteration and modulation in psychiatric disorders: current evidence on fecal microbiota transplantation. Prog. Neuro-Psychopharmacology Biol. Psychiatry 109, 110258. doi:10.1016/j.pnpbp.2021.110258
Shen, F., Xie, P., Li, C., Bian, Z., Wang, X., Peng, D., et al. (2022). Polysaccharides from Polygonatum cyrtonema hua reduce depression-like behavior in mice by inhibiting oxidative Stress-Calpain-1-NLRP3 signaling axis. Oxidative Med. Cell. Longev. 2022, 2566917. doi:10.1155/2022/2566917
Shimizu, H., Koyama, T., Yamada, S., Lipton, S. A., and Satoh, T. (2015). Zonarol, a sesquiterpene from the brown algae Dictyopteris undulata, provides neuroprotection by activating the Nrf2/ARE pathway. Biochem. Biophysical Res. Commun. 457 (4), 718–722. doi:10.1016/j.bbrc.2015.01.059
Shin, L. M., and Liberzon, I. (2010). The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacol. 35 (1), 169–191. doi:10.1038/npp.2009.83
So, D., Whelan, K., Rossi, M., Morrison, M., Holtmann, G., Kelly, J. T., et al. (2018). Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. Am. J. Clin. Nutr. 107 (6), 965–983. doi:10.1093/ajcn/nqy041
Stefano, G. B., Pilonis, N., Ptacek, R., Raboch, J., Vnukova, M., and Kream, R. M. (2018). Gut, microbiome, and brain regulatory axis: relevance to neurodegenerative and psychiatric disorders. Cell. Mol. Neurobiol. 38 (6), 1197–1206. doi:10.1007/s10571-018-0589-2
Stilling, R. M., Ryan, F. J., Hoban, A. E., Shanahan, F., Clarke, G., Claesson, M. J., et al. (2015). Microbes and neurodevelopment—Absence of microbiota during early life increases activity-related transcriptional pathways in the amygdala. Brain, Behav. Immun. 50, 209–220. doi:10.1016/j.bbi.2015.07.009
Sudo, N., Chida, Y., Aiba, Y., Sonoda, J., Oyama, N., Yu, X.-N., et al. (2004). Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 558 (Pt 1), 263–275. doi:10.1113/jphysiol.2004.063388
Sugden, S. G., Merlo, G., and Manger, S. (2024). Can lifestyle medicine improve global mental health? Acad. Ment. Health Well-Being 1 (1). doi:10.20935/mhealthwellb6224
Szyszkowicz, J. K., Wong, A., Anisman, H., Merali, Z., and Audet, M.-C. (2017). Implications of the gut microbiota in vulnerability to the social avoidance effects of chronic social defeat in Male mice. Brain, Behav. Immun. 66, 45–55. doi:10.1016/j.bbi.2017.06.009
Tanelian, A., Nankova, B., Cheriyan, A., Arens, C., Hu, F., and Sabban, E. L. (2023). Differences in gut microbiota associated with stress resilience and susceptibility to single prolonged stress in female rodents. Neurobiol. Stress 24, 100533. doi:10.1016/j.ynstr.2023.100533
Tillisch, K., Labus, J., Kilpatrick, L., Jiang, Z., Stains, J., Ebrat, B., et al. (2013). Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 144 (7), 1394–1401.e14014. doi:10.1053/j.gastro.2013.02.043
Tolin, D. F., and Foa, E. B. (2006). Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol. Bull. 132 (6), 959–992. doi:10.1037/0033-2909.132.6.959
Torreilles, F., Salman-Tabcheh, S., Guérin, M., and Torreilles, J. (1999). Neurodegenerative disorders: the role of peroxynitrite. Brain Res. Brain Res. Rev. 30 (2), 153–163. doi:10.1016/s0165-0173(99)00014-4
Tristan Asensi, M., Napoletano, A., Sofi, F., and Dinu, M. (2023). Low-grade inflammation and ultra-processed foods consumption: a review. Nutrients 15 (6), 1546. doi:10.3390/nu15061546
Usui, N., Matsuzaki, H., and Shimada, S. (2021). Characterization of early life stress-affected gut microbiota. Brain Sci. 11 (7), 913. doi:10.3390/brainsci11070913
Vaiva, G., Thomas, P., Ducrocq, F., Fontaine, M., Boss, V., Devos, P., et al. (2004). Low posttrauma GABA plasma levels as a predictive factor in the development of acute posttraumatic stress disorder. Biol. Psychiatry 55 (3), 250–254. doi:10.1016/j.biopsych.2003.08.009
van der Kolk, B., Greenberg, M., Boyd, H., and Krystal, J. (1985). Inescapable shock, neurotransmitters, and addiction to trauma: toward a psychobiology of post traumatic stress. Biol. Psychiatry 20 (3), 314–325. doi:10.1016/0006-3223(85)90061-7
Van Wyngene, L., Vanderhaeghen, T., Petta, I., Timmermans, S., Corbeels, K., Van der Schueren, B., et al. (2021). ZBTB32 performs crosstalk with the glucocorticoid receptor and is crucial in glucocorticoid responses to starvation. iScience 24 (7), 102790. doi:10.1016/j.isci.2021.102790
Varela, R. B., Valvassori, S. S., Lopes-Borges, J., Mariot, E., Dal-Pont, G. C., Amboni, R. T., et al. (2015). Sodium butyrate and mood stabilizers block ouabain-induced hyperlocomotion and increase BDNF, NGF and GDNF levels in brain of wistar rats. J. Psychiatric Res. 61, 114–121. doi:10.1016/j.jpsychires.2014.11.003
Vasileva, L. V., Ivanovska, M. V., Murdjeva, M. A., Saracheva, K. E., and Georgiev, M. I. (2019). Immunoregulatory natural compounds in stress-induced depression: an alternative or an adjunct to conventional antidepressant therapy? Food Chem. Toxicol. 127, 81–88. doi:10.1016/j.fct.2019.03.004
Voigt, R. M., Zalta, A. K., Raeisi, S., Zhang, L., Brown, J. M., Forsyth, C. B., et al. (2022). Abnormal intestinal milieu in posttraumatic stress disorder is not impacted by treatment that improves symptoms. Am. J. Physiol. Gastrointest. Liver Physiol. 323 (2), G61–G70. doi:10.1152/ajpgi.00066.2022
Xie, J., Xu, D., Wang, C., and Huang, J. (2023). Jiawei xiaoyao san in treatment of anxiety disorder and anxiety: a review. Chin. Herb. Med. 15 (2), 214–221. doi:10.1016/j.chmed.2022.12.007
Xie, P., Chen, L., Wang, J., Wang, X., Yang, S., and Zhu, G. (2024). Polysaccharides from Polygonatum cyrtonema hua prevent post-traumatic stress disorder behaviors in mice: mechanisms from the perspective of synaptic injury, oxidative stress, and neuroinflammation. J. Ethnopharmacol. 319 (Pt 1), 117165. doi:10.1016/j.jep.2023.117165
Xing, M., Cao, Q., Wang, Y., Xiao, H., Zhao, J., Zhang, Q., et al. (2020). Advances in research on the bioactivity of alginate oligosaccharides. Mar. Drugs 18 (3), 144. doi:10.3390/md18030144
Yadav, S. K., Ahmad, R., Moshfegh, C. M., Sankarasubramanian, J., Joshi, V., Elkhatib, S. K., et al. (2023). Repeated social defeat stress induces an inflammatory gut milieu by altering the mucosal barrier integrity and gut microbiota homeostasis. Biol. Psychiatry Glob. Open Sci. 3 (4), 824–836. doi:10.1016/j.bpsgos.2023.03.005
YangC., , Fujita, Y., Ren, Q., Ma, M., Dong, C., and Hashimoto, K. (2017). Bifidobacterium in the gut microbiota confer resilience to chronic social defeat stress in mice. Sci. Rep. 7 (1), 45942. doi:10.1038/srep45942
YangW. T., , Zheng, X. W., Chen, S., Shan, C. S., Xu, Q. Q., Zhu, J. Z., et al. (2017). Chinese herbal medicine for Alzheimer’s disease: clinical evidence and possible mechanism of neurogenesis. Biochem. Pharmacol. 141, 143–155. doi:10.1016/j.bcp.2017.07.002
Yirmiya, R., and Goshen, I. (2011). Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain, Behav. Immun. 25 (2), 181–213. doi:10.1016/j.bbi.2010.10.015
Zhang, D., Zhou, Y., Ma, Y., Chen, P., Tang, J., Yang, B., et al. (2023). Gut microbiota dysbiosis correlates with long COVID-19 at one-year after discharge. J. Korean Med. Sci. 38 (15), e120. doi:10.3346/jkms.2023.38.e120
Zhou, Q., Sun, T., Wu, F., Li, F., Liu, Y., Li, W., et al. (2020). Correlation of gut microbiota and neurotransmitters in a rat model of post-traumatic stress disorder. J. Traditional Chin. Med. Sci. 7 (4), 375–385. doi:10.1016/j.jtcms.2020.10.005
Keywords: posttraumatic stress disorder, gut microbiome, gut-microbiota-brain axis, vagus nerve, HPA axis, natural medicines
Citation: Pan J, Lin S, Qian Q, Fu S and Liu X (2025) Gut-brain axis in post-traumatic stress disorder: microbial - mediated mechanisms and new therapeutic approaches - A narrative review. Front. Pharmacol. 16:1621678. doi: 10.3389/fphar.2025.1621678
Received: 01 May 2025; Accepted: 16 June 2025;
Published: 25 June 2025.
Edited by:
Tamara Timic Stamenic, University of Colorado Denver, United StatesReviewed by:
Ana Pamela Gómez-García, National Institute of Cancerology (INCAN), MexicoAmeer Luqman, Chongqing University, China
Copyright © 2025 Pan, Lin, Qian, Fu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoliu Liu, bGl1eGlhb2xpdUB3dXN0LmVkdS5jbg==
†These authors have contributed equally to this work
 Jiayue Pan
Jiayue Pan Shuairong Lin
Shuairong Lin Qiuling Qian3
Qiuling Qian3