- Department of Clinical Pharmacy, Xiangtan Central Hospital (The Affiliated Hospital of Hunan University), Xiangtan, China
Pramipexole, a novel dopamine receptor agonist, is widely used in the treatment of Parkinson’s disease and related syndromes. While studies have demonstrated that pramipexole can cause various adverse reactions, primarily involving the central nervous system or gastrointestinal symptoms, there remains limited reporting on pramipexole-induced syndrome of inappropriate antidiuretic hormone secretion (SIADH). This article analyzes and discusses a case of pramipexole-induced SIADH that occurred in our hospital. At the same time, we also summarize and discuss all current cases of pramipexole-induced SIADH. We anticipate these findings will serve as a valuable clinical reference to guide appropriate medication use.
Introduction
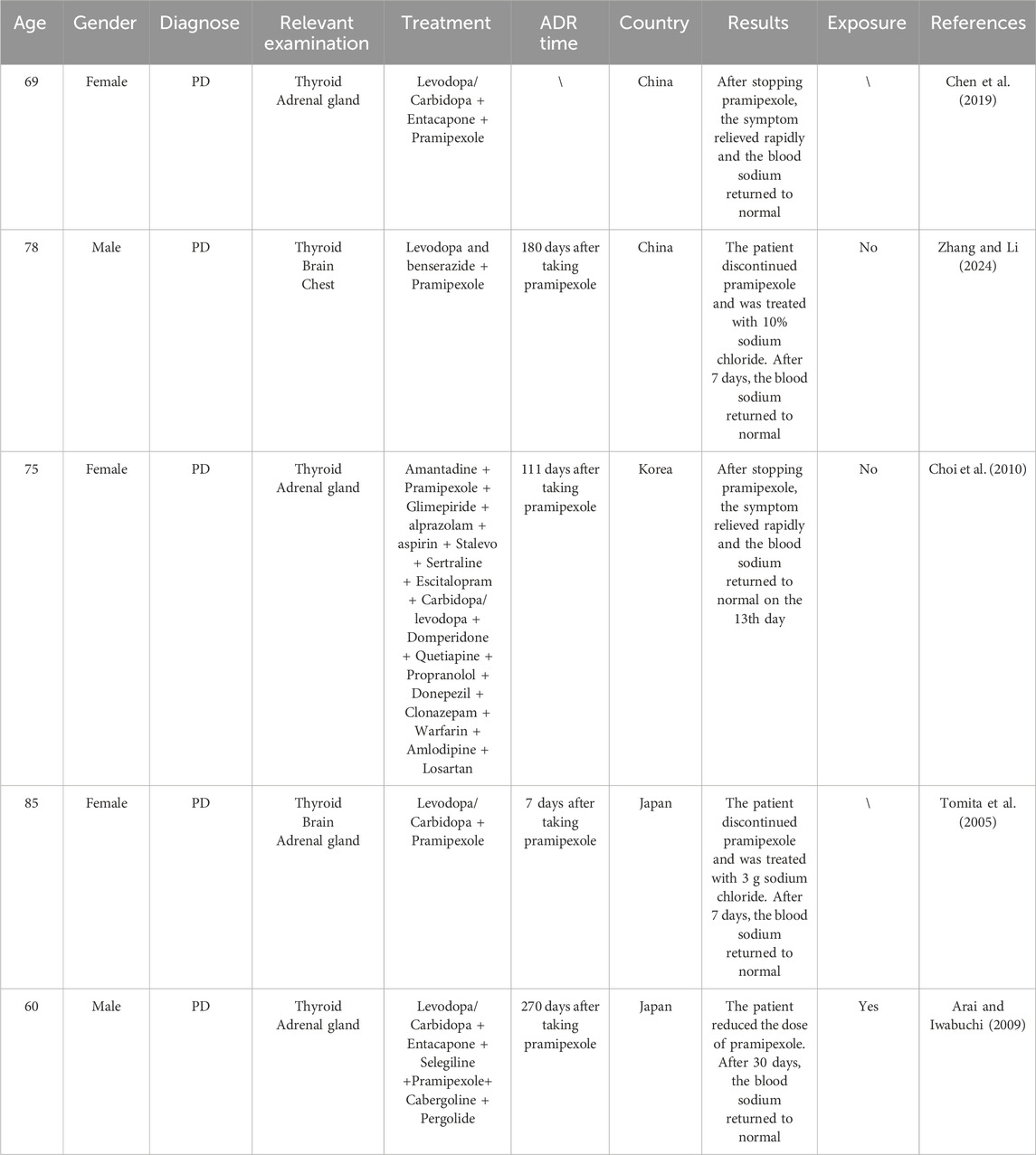
Parkinson’s disease (PD) is a neurological disorder with evolving layers of complexity. It has long been characterised by the classical motor features of parkinsonism associated with Lewy bodies and loss of dopaminergic neurons in the substantia nigra. However, the symptomatology of PD is now recognised as heterogeneous, with clinically significant non-motor features. Similarly, its pathology involves extensive regions of the nervous system, various neurotransmitters, and protein aggregates other than just Lewy bodies (Kalia and Lang, 2015). Pramipexole is a dopamine receptor agonist and has a high affinity for D2, D3, and D4 receptors. On the one hand, pramipexole is primarily used for the treatment of early-onset PD. Meanwhile, it is also used in the treatment of treatment-resistant depression, the treatment of anhedonia, or bipolar depression (Lemke et al., 2006). On the other hand, pramipexole can be used alone or in combination with levodopa to treat advanced PD. While studies have demonstrated that pramipexole can cause various adverse reactions, primarily involving the central nervous system or gastrointestinal symptoms (Xu et al., 2012), there remains limited reporting on pramipexole-induced syndrome of inappropriate antidiuretic hormone secretion (SIADH). SIADH is a syndrome characterized by clinical manifestations such as water retention in the body, increased urinary sodium excretion, and hyponatremia. The essential diagnostic criteria for hyponatremia secondary to SIADH include Serum sodium<135 mmol/L, Decreased measured plasma osmolality<275 mOsm/kg, Urinary osmolality >100 mOsm/kg during hypotonicity, Clinical euvolemia (No clinical signs of contraction of extracellular fluid, No clinical signs of expansion extracellular fluid), Increased urinary sodium excretion >30 mmol/L with normal dietary salt and water intake, Normal thyroid and adrenal function determined by both clinical and laboratory assessment, No recent use of diuretic agents. The supporting diagnostic criteria for hyponatremia secondary to SIADH include Plasma uric acid <4 mg/dL (<0.24 mmol/L), Blood urea nitrogen <10 mg/dL (<3.6 mmol/L), Fractional sodium excretion >1%, fractional urea excretion >55%, Failure to improve hyponatremia after 0.9% saline infusion, Improvement of hyponatremia with fluid restriction. The typical symptoms of hyponatremia include weakness, fatigue, nausea, vomiting, confusion and muscle spasms (Zhou and Wang, 2014). It is mainly caused by various reasons such as malignant tumors, lung diseases, central nervous system diseases, and drugs (such as SSRI, SNRI, proton pump inhibitors, thiazide and loop diuretics, carbamazepine, etc.), resulting in an abnormal increase of endogenous antidiuretic hormone (ADH) secretion (Mo et al., 2025). Meanwhile, a very important risk factor for hyponatremia in the course of pharmacotherapy is also old age (Pisa and Del Cerro, 2019). This article analyzes and discusses a case of pramipexole-induced SIADH that occurred in our hospital. At the same time, we also summarize and discuss all current cases of pramipexole-induced SIADH (Chen et al., 2019; Zhang and Li, 2024; Choi et al., 2010; Tomita et al., 2005; Arai and Iwabuchi, 2009) (Table 1). We anticipate these findings will serve as a valuable clinical reference to guide appropriate medication use.
Clinical presentation
An 84-year-old woman visited our neurology outpatient clinic duo to dizziness, fatigue, and limb tremors. Upon neurological examination, she had bradykinesia and intermittent limb tremors. This patient has a history of percutaneous coronary intervention (PCI) surgery for coronary heart disease, pituitary tumor surgery, type 2 diabetes with peripheral neuropathy and retinopathy, and hypertension. Her current medications include isophane protamine human insulin (21U sc qd), metformin hydrochloride extended-release tablets (0.5 g po qd), and indapamide tablets (2.5 mg po qd) for blood pressure and blood sugar control. The patient denies a history of infectious diseases such as hepatitis, typhoid fever, and tuberculosis. Additionally, this patient has a known allergy to ceftazidime.
The patient was hospitalized on July 28th (Day 1). Cranial CT revealed senile brain atrophy, white matter degeneration, and multiple lacunar cerebral infarctions. Biochemical studies showed the following electrolyte levels: Serum sodium: 139 mmol/L, Serum potassium: 4.04 mmol/L, and Serum chloride: 99 mmol/L. On July 30th (Day 3), the patient was diagnosed with Parkinson’s syndrome. Electromyography detected a 4.0 Hz tremor in both upper limbs at rest. The patient exhibited Parkinson’s motor symptoms, including bradykinesia, muscle rigidity, and tremor, as well as non-motor symptoms such as dizziness, constipation, sleep disorders, anxiety, and depression. Treatment was initiated with oral dopashydrazine tablets (125 mg po tid) and pramipexole tablets (0.125 mg po tid). On August 5th (Day 5), the patient developed dizziness, drowsiness, and vomiting. Biochemical studies revealed significant electrolyte imbalances: Serum sodium: 114.2 mmol/L, Serum potassium: 3.35 mmol/L, Serum chloride: 74.3 mmol/L, Effective plasma osmotic pressure: 249.8 mmol/L (<275 mmol/L). Cortisol and adrenocorticotropic hormone levels were within normal ranges. The diagnosis was hypotonic hyponatremia, which was attributed to pramipexole-induced SIADH after consultation with the clinical pharmacy and endocrinology departments. The patient’s blood volume status was euvolemia, with oliguria and high urinary osmolarity. Brain and chest CT scans showed no abnormalities, and pituitary, adrenal, and thyroid functions (corticotropin: 8.16 pg/mL, thyrotropin: 2.34 µIU/mL) were normal. SIADH caused by underlying conditions (malignancies, lung diseases, central nervous system diseases) was ruled out. Pramipexole was discontinued, and the patient was treated with 10% sodium chloride injection (20 mL po bid) and potassium chloride sustained-release tablets (1 g po bid). On August 7th (Day 11), biochemical studies showed the following electrolyte levels: Serum sodium: 137 mmol/L, Serum potassium: 3.9 mmol/L, Serum chloride: 104 mmol/L. On August 11th (Day 15), biochemical studies showed the following electrolyte levels: Serum sodium: 136 mmol/L, Serum potassium: 4.39 mmol/L, Serum chloride: 103 mmol/L.
Literature review
This study utilized the CNKI, Web of Science, and PubMed databases to search for case reports related to “pramipexole” and “antidiuretic hormone” published between 2000 and 2024. Through systematic screening, we identified five reported cases of pramipexole-induced SIADH. Including the present case, a total of six cases (China: 3 cases, South Korea: 1 case, Japan: 2 cases) have been documented worldwide. The study included 6 patients (4 females and 2 males) aged 60–85 years. The onset of SIADH typically occurred between 2 and 270 days after initiating pramipexole treatment. Symptom resolution was achieved through pramipexole dose reduction or discontinuation, fluid restriction, and hypertonic saline administration. Serum sodium levels returned to normal within 30 days of dose reduction or within 7–13 days after complete discontinuation. Among the 6 patients, 3 patients had no re-exposure to pramipexole, 2 patients lacked documented re-exposure status, and 1 patient maintained treatment at a reduced dose without SIADH recurrence.
Discussion
According to national adverse drug reaction monitoring principles, this case was analyzed as follows: Both the package insert and literature suggest that pramipexole can cause SIADH. The patient’s electrolyte levels were normal prior to medication but developed hyponatremia and hypochloremia following pramipexole initiation, accompanied by anorexia, vomiting, and mental status deterioration. After discontinuing pramipexole and administering hypertonic saline treatment, electrolyte levels normalized within 2 days. Subsequent monitoring showed no recurrence of electrolyte abnormalities without pramipexole rechallenge. Brain and chest CT scans showed no abnormalities, and pituitary, adrenal, and thyroid functions were normal. SIADH caused by underlying conditions (malignancies, lung diseases, central nervous system diseases) was ruled out. These findings establish a “very likely” causal relationship between pramipexole and SIADH in this case.
The mechanism of pramipexole-induced SIADH is as follows:Pramipexole is a dopamine receptor agonist and has a high affinity for D2, D3, and D4 receptors (Azdad et al., 2003). ADH, synthesized by neurons in the hypothalamic supraoptic and paraventricular nuclei, is regulated by γ-aminobutyric acid (GABA). The D4 receptors are located at the GABA terminal within the supraoptic nucleus. By activating D4 receptors, pramipexole reduces GABA release, which in turn promotes ADH secretion (Kvernmo et al., 2006). This neuroendocrine disruption leads to increased water reabsorption, ultimately resulting in SIADH.
SIADH may present with symptoms including nausea, vomiting, weakness, drowsiness, and mental confusion. The therapeutic approach involves: correcting underlying etiologies, implementing fluid restriction, administering intravenous sodium chloride solution, and utilizing ADH receptor antagonists or diuretics. Treatment plan should be tailored to individual patient conditions. Drug-induced SIADH require immediate drug withdrawal, and low serum sodium levels can be corrected after drug withdrawal (Cuesta et al., 2016). Therefore, PD patients initiating pramipexole therapy require regular serum sodium monitoring to facilitate early detection and management of drug-induced SIADH.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
K-QC: Writing – original draft. XL: Writing – review and editing. H-BL: Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Arai, M., and Iwabuchi, M. (2009). Pramipexole as a possible cause of the syndrome of inappropriate antidiuresis. BMJ Case Rep. 2009, bcr01.2009.1484. doi:10.1136/bcr.01.2009.1484
Azdad, K., Piet, R., Poulain, D. A., and Oliet, S. H. (2003). Dopamine D4 receptor-mediated presynaptic inhibition of GABAergic transmission in the rat supraoptic nucleus. J. Neurophysiol. 90 (2), 559–565. doi:10.1152/jn.00226.2003
Chen, Q. M., Tang, K. K., Yang, T. M., and Mao, Y. S. (2019). One case of Pramipexole-induced vasopressin secretion disorder syndrome. China J. Mod. Med. 29 (17), 125–126.
Choi, Y., Park, J. J., Ryoo, N. Y., Kim, S. H., Song, C., Han, I. T., et al. (2010). Syndrome of inappropriate antidiuretic hormone secretion associated with pramipexole in a patient with parkinson's disease. J. Mov. Disord. 3 (2), 54–56. doi:10.14802/jmd.10015
Cuesta, M., Garrahy, A., and Thompson, C. J. (2016). SIAD: practical recommendations for diagnosis and management. J. Endocrinol. Invest. 39 (9), 991–1001. doi:10.1007/s40618-016-0463-3
Kalia, L. V., and Lang, A. E. (2015). Parkinson's disease. Lancet 386 (9996), 896–912. doi:10.1016/S0140-6736(14)61393-3
Kvernmo, T., Härtter, S., and Burger, E. (2006). A review of the receptor-binding and pharmacokinetic properties of dopamine agonists. Clin. Ther. 28 (8), 1065–1078. doi:10.1016/j.clinthera.2006.08.004
Lemke, M. R., Brecht, H. M., Koester, J., and Reichmann, H. (2006). Effects of the dopamine agonist pramipexole on depression, anhedonia and motor functioning in parkinson's disease. J. Neurol. Sci. 248 (1-2), 266–270. doi:10.1016/j.jns.2006.05.024
Mo, H., Channa, Y., Ferrara, T. M., Waxse, B. J., Schlueter, D. J., Tran, T. C., et al. (2025). Hyponatremia associated with the use of common antidepressants in the all of us research program. Clin. Pharmacol. Ther. 117 (2), 534–543. doi:10.1002/cpt.3484
Tomita, M., Otsuka, Y., Iida, R., Kobayashi, S., Kuriyama, S., and Hosoya, T. (2005). Syndrome of inappropriate ADH secretion (SIADH) induced by pramipexole in a patient with parkinson's disease. Nihon Jinzo Gakkai Shi 47 (5), 531–535.
Xu, J. J., Xu, D., and Xia, S. (2012). Data analysis of adverse drug reactions caused by pramipexole. World Clin. Drug 33 (11), 681–683.
Zhang, Y., and Li, Y. L. (2024). One case of Pramipexole-induced vasopressin secretion disorder syndrome. Her. Med. 43 (9), 1500–1501.
Keywords: pramipexole, syndrome of inappropriate antidiuretic hormone secretion, adverse drug reactions, ADH, SIADH
Citation: Chen K-Q, Liu X and Lei H-B (2025) Case Report: Syndrome of inappropriate antidiuretic hormone secretion induced by pramipexole: a case report and literature review. Front. Pharmacol. 16:1627245. doi: 10.3389/fphar.2025.1627245
Received: 12 May 2025; Accepted: 29 July 2025;
Published: 15 August 2025.
Edited by:
Heike Wulff, University of California, Davis, United StatesReviewed by:
Marcin Siwek, Jagiellonian University, Krakow, PolandAnna Arecco, University of Genova, Genova, Italy
Copyright © 2025 Chen, Liu and Lei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang Liu, TFgxOTg5MEAxNjMuY29t; Hai-Bo Lei, Mjg2MjAwNTcxQHFxLmNvbQ==
 Ke-Qian Chen
Ke-Qian Chen Xiang Liu*
Xiang Liu*