Abstract
Non-adherence to prescribed treatments remains a major challenge facing the healthcare system. Despite decades of research, interventions to improve adherence typically have not shown large or sustained effects on adherence and are rarely implemented. Digital technologies provide a potential platform to increase the reach and cost-effectiveness of adherence interventions, allowing them to be widely rolled out. Current evidence suggests that digital interventions can increase adherence, but results are mixed with many interventions failing to improve adherence. This is likely because whilst the included interventions all utilise digital platforms, they vary significantly in their design, content and delivery. Many interventions are not theory or evidence based, do not include patient or healthcare practitioner involvement or focus simply on providing reminders. Evidence suggests that well-designed interventions which are evidence-based, are personalised and maximise interactivity are more likely to be successful. These well-designed interventions hold promise for improving adherence at scale. This narrative review discusses the current challenges facing digital adherence interventions and describes barriers to implementation or adoption which need to be resolved. These include considering reach, accessibility, and acceptability, to avoid increasing existing health inequalities. It is also critical to consider the quality, safety and regulation of available apps and other digital tools, as well as investigating ways to enhance engagement and retention. Finally, some digital tools may require integration into existing systems or may necessitate training of relevant staff. Overall, digital interventions appear to be a promising tool for improving medication adherence, but further work is needed to optimise these tools.
1 Introduction
The World Health Organization has classified treatment adherence as a major global problem (Burkhart and Sabaté, 2003). It is estimated that 20%–50% of patients do not take their medication as prescribed (Bosworth et al., 2016). The reasons patients do not take their medication correctly can be either unintentional, such as confusion or simple forgetfulness (Mira et al., 2015), or intentional, where the patient makes a deliberate decision not to take their treatment (Horne et al., 2019). Support to improve adherence therefore needs to foster both motivation and ability to adhere (Horne et al., 2019).
Digital technologies are increasingly being used to deliver these interventions, due to the proliferation of smart phones and other technology developments globally. Internet access continues to grow, with an estimated 5.44 billion internet users worldwide in 2024, accounting for two-thirds of the global population (Statista, 2024b). 69% of the global population have access to a smart phone (Statista, 2024c), with very high levels of penetration in the United Kingdom (84% (Statistics, 2020)) and US (90% (Center, 2024)). Engagement with and implementation of digital healthcare has also been rising over the past 10 years, particularly since the COVID-19 pandemic in 2020 (Mahajan et al., 2021; Mosnaim et al., 2020).
These figures highlight the potential for delivering health programs such as adherence interventions through a mobile phone, computer or similar device. These technologies are often very cost-effective and can reach large numbers of patients with little effort, as well as enhancing the potential for personalisation and automation of interventions.
This narrative review will first provide an overview the current evidence base for digital interventions to improve adherence, followed by an outline of issues in the field and factors associated with the success of digital health adherence tools.
2 Current evidence base for digital interventions
2.1 Text messaging
Several systematic reviews of studies including both randomised and non-randomised designs have shown that Short Message Service (SMS) text interventions can improve medication adherence in patients with diabetes, hypertension and/or dyslipidemia (Belete et al., 2023; Bingham et al., 2021). However, other systematic reviews including just Randomised Controlled Trials (RCTs) with a greater number of studies show more mixed results (Bond et al., 2021; Redfern et al., 2024). For example, a recent Cochrane review of RCTs evaluating text messaging for medication adherence in secondary prevention of cardiovascular disease concluded that the evidence was very uncertain, with only 10 out of 18 studies showing a beneficial effect on adherence compared to usual care (Redfern et al., 2024). Furthermore, any effects tend to be very short term, or do not persist past the end of the intervention (Mulawa et al., 2018; Gautier et al., 2021), and issues have been raised with study quality, such as issues with blinding and selective reporting (Palmer et al., 2021; Adler et al., 2018). Despite these mixed effects, one review found that of the studies (13 of 18) who reported user feedback, satisfaction and interest was very high (Bond et al., 2021).
Some studies have attempted to identify the best ways of optimising text message interventions. Whilst no studies have directly compared tailored vs generic messaging to enhance medication adherence, text message interventions which go beyond simple reminders and are tailored to the individual’s beliefs have shown success in improving adherence (Petrie et al., 2012; Riaz and Jones Nielsen, 2019) and tailored messages have been shown to be more effective in changing other health behaviours (Head et al., 2013). Interventions lasting 6 months or longer were more effective than those that are shorter term (Belete et al., 2023), and tapering for an additional 3 months has also shown to be useful in maintaining adherence after the initial intervention (Belzer et al., 2025).
2.2 Apps and web-based programs
Many health behaviour change interventions are now being delivered through mobile applications (apps) or other web-based programs. These allow for the delivery of content direct to the user, along with enhanced options for personalisation, interaction and reminders. A review in 2015 found 681 available adherence apps on the Apple App Store or Google Play Store (Ahmed et al., 2018). The number of adherence apps has grown since then, with a 2017 review estimating 800 medication management apps available (Dayer et al., 2017), and a 2024 review finding 53 available health apps in asthma alone (Robinson et al., 2024). However, despite this proliferation of available apps, there is little consistent evidence for their efficacy (Chong et al., 2023; Cao et al., 2024). Clinical studies evaluating the impact of apps on improving adherence have shown mixed results (Aungst, 2021). A 2020 review of 9 trials of mobile medication adherence apps across a range of health conditions found a significant pooled effect, but five of the nine included studies did not report a significant effect on adherence (Armitage et al., 2020), potentially due to variability in adherence measurement, techniques used and the extent of the tailoring within the included apps.
This lack of consistent evidence for apps is likely due to the significant variation in design, content and delivery (Ng et al., 2020). Few of the publicly available apps meet relevant criteria for quality, content or functionality (Masterson Creber et al., 2016) and most lack a sufficient evidence base. Reviews of trials suggest those that include more interactive features, such as interaction with medical providers, social networking and gamification features, tended to be more effective (Unni et al., 2018; Cazeau, 2021), yet these features are often lacking from publicly available apps (Wang et al., 2024). Similarly, it has been suggested that tailoring intervention content to the individual user will also be associated with positive effects on adherence (Armitage et al., 2020; Goradia et al., 2021; Stewart et al., 2023). For example, several digital interventions which tailor the content to the individual’s medication beliefs have shown success in improving adherence (Lakshminarayana et al., 2017; Chapman et al., 2020; Hughes et al., 2022). In the 2020 review mentioned above, the authors highlight examples of successful highly tailored interventions and state their results may support the hypothesis that level of tailoring is associated with the effectiveness of adherence apps (Armitage et al., 2020).
Recent innovations include the use of gamification in medication adherence apps, including features such as social connectivity, avatars, alternate realities, leaderboards, points and badges. These features are proposed to enhance medication adherence as well as adherence to the app itself (Ahonkhai et al., 2021). A review of five studies using gamification features (e.g., leaderboards, levelling up, quests) to improve medication adherence across a range of conditions, found that three of the five studies showed significant improvements in adherence (Tran et al., 2022). Overall, the evidence base for adherence apps is mixed with many poor quality studies, making it difficult to draw conclusions on their effectiveness (Ng et al., 2020). Reported issues with quality include small sample sizes, self-presentation bias, potential conflicts of interest, lack of appropriate control arms and self-reporting of adherence outcomes (Ng et al., 2020).
2.3 Monitoring and smart products
Over the last decade, the popularity of digital medication adherence systems has surged, with both healthcare providers and patients acknowledging their role in enhancing adherence and overall health results. A recent review by Mason et al. (2022) identified a variety of technology applications for monitoring medication adherence, including electronic pill bottles or boxes, ingestible sensors, video-based technology, and motion sensor technology. The common expectation is that these technologies accurately monitor medication adherence and can easily be adopted in patients’ daily lives owing to their unobtrusiveness and convenience of use.
Sensor technologies have been increasingly used to track the medication-taking behaviours of patients. For example, the Medication Event Monitoring System (MEMS) can record every time the patient opens the pill bottle via a sensor embedded in the pill cap. These medication monitors are increasingly used as part of strategies to improve adherence. Despite this, there is limited consensus on how to determine or select the appropriate medication adherence monitoring technology for use. There is a growing need for technology assessment criteria to guide the development and selection of appropriate technologies for monitoring medication adherence to improve patient outcomes (Basu et al., 2019).
Some recent studies have shown promising findings for the use of smart technologies to improve medication adherence. A systematic review by Chan et al. (2022) found that patients receiving an electronic adherence monitoring (EAM) intervention (most commonly devices which record pillbox being opened and sent reminders) had significantly better adherence than those who did not. In this review, data from 27 studies (n = 2,584) were extracted for the adherence outcome, Most studies were conducted on adults (87%) and the most common conditions were in asthma (21%) or human immunodeficiency virus (HIV) (19%), or hypertension (13%). The authors concluded that improved adherence did not consistently translate into clinical benefits (Chan et al., 2022). Acceptability data were mixed, with perceptions of the device being negative in nearly half of the included studies. Issues with acceptability included the reminder beeps, the size of the device and concerns about disclosure. Feedback on the intervention itself was more positive, with patients looking forward to receiving their adherence data. The authors conclude that further research is required to assess patient acceptability and explore effects on clinical outcomes and. A study by van de Hei et al. (2023) found that digital inhaler–based interventions can yield long term cost-savings by optimising medication adherence and inhaler technique and reducing additional biologic prescriptions in patients with difficult-to-treat asthma (van de Hei et al., 2023).
Stakeholders’ expectations regarding the use of health information technology for monitoring medication adherence can also vary. From a clinical practice perspective, a user-friendly interface and the accurate monitoring of adherence are considered when selecting appropriate monitoring technologies. From the technological development perspective, although system accuracy and data fidelity remain high priorities, developers also need to consider the feasibility of technical engineering of the system, such as energy consumption and battery lifetime (Aldeer et al., 2018). Human interactions with these technologies can be complicated owing to the comprehensive medical and pharmacological contexts, as well as multidimensional patient medication adherence behaviours.
2.4 Artificial Intelligence and adaptive interventions
Artificial Intelligence (AI)-powered mobile applications are those that apply logical algorithms which are capable of learning from data and making autonomous decisions based on generalizable rules (Zavaleta-Monestel et al., 2025). These have proven to be valuable tools in monitoring and improving medication adherence (Zavaleta-Monestel et al., 2025). In a study conducted by Labovitz et al. (2017), an AI-based smartphone app was developed for stroke patients on direct oral anticoagulant therapy. The app used a neural network to identify the patient and the prescribed drug, confirm ingestion through the phone’s camera, and provide medication reminders. The study found a 100% adherence rate among patients using the app compared to 50% in the control group, and identified positive patient feedback. However, the study was small (n = 28), and therefore further research with larger sample sizes is needed to determine long-term effectiveness. Similarly, Bain and colleagues (Bain et al., 2017) used an AI platform incorporating facial recognition and drug verification for real-time monitoring of schizophrenia patients in a 24-week clinical trial. The study demonstrated 17.9% higher adherence in the AI-monitored group compared to a control group receiving modified direct observation therapy. Another clinical trial used a voice-based conversational AI application to support type 2 diabetes patients (Nayak et al., 2023). The results showed that insulin adherence rates were 32.7% higher in the AI-voice application compared to the standard care group.
AI-driven reminder systems have been developed to encourage medication adherence by sending timely reminders to patients. Brar Prayaga et al. (2018) explored the use of “mPulse Mobile,” an SMS-based AI reminder system in older patients with non-communicable diseases. They observed significantly higher medication refill rates in the group that received AI-generated SMS reminders compared to a control group that did not receive any reminders. A study by Chaix et al. (2019) found that AI can also play an important role in indirectly improving adherence by empowering patients (Chaix et al., 2019). In their study they used “Vik,” a chatbot designed for breast cancer patients to provide personalised health information, including medication reminders. The study showed that patients who engaged more with Vik were observant when using a treatment reminder function, and that medication adherence improved by more than 20% in this group.
AI-assisted technology could also be used to optimise prescriptions by prioritising medications that match the insurance/preferred pharmacy of the patients and check drug–drug interactions. AI has already been shown to be useful for medication reconciliation, which is a procedure often used to reduce medication errors (Long et al., 2016). One of the major contributions that AI-assisted technologies has had in recent years in disease management is through machine learning (ML) and big data analytics. For example, Koesmahargyo and colleagues (2020) used ML to predict medication non-adherence based on real-time dosing data collected from smartphone videos of patients taking their medications (Koesmahargyo et al., 2020). This approach provided highly accurate predictions of adherence across both the trial period and subsequent days or weeks. A systematic review of literature on AI highlighted that machine learning is currently the most commonly used AI technology in healthcare (Guo et al., 2020). In general, however, this field is still in its infancy; there are currently 100 FDA-approved AI/machine learning-based medical devices and algorithms, which are constantly updated on an online database (Medicalfuturist, 2025). A recent review examined the use of AI tools for patient support to enhance medication adherence, with results showing that although the evidence supporting AI tools to assist patients is weak, smart systems using AI tools are promising in helping patients use prescribed medications (Reis et al., 2025). Based on current evidence, AI-powered, personalised approaches are best suited to complex behavioral barriers to intentional adherence, whereas basic digital tools can serve as reminders and educational aids to improve unintentional adherence by providing real-time feedback and tracking.
3 Factors associated with success of digital health adherence tools
As described above, evidence on the effectiveness of digital adherence interventions is mixed, with many interventions failing to improve adherence. In order to develop interventions which will successfully engage participants and improve adherence, the following factors need to be considered (see Figure 1 for a summary).
FIGURE 1
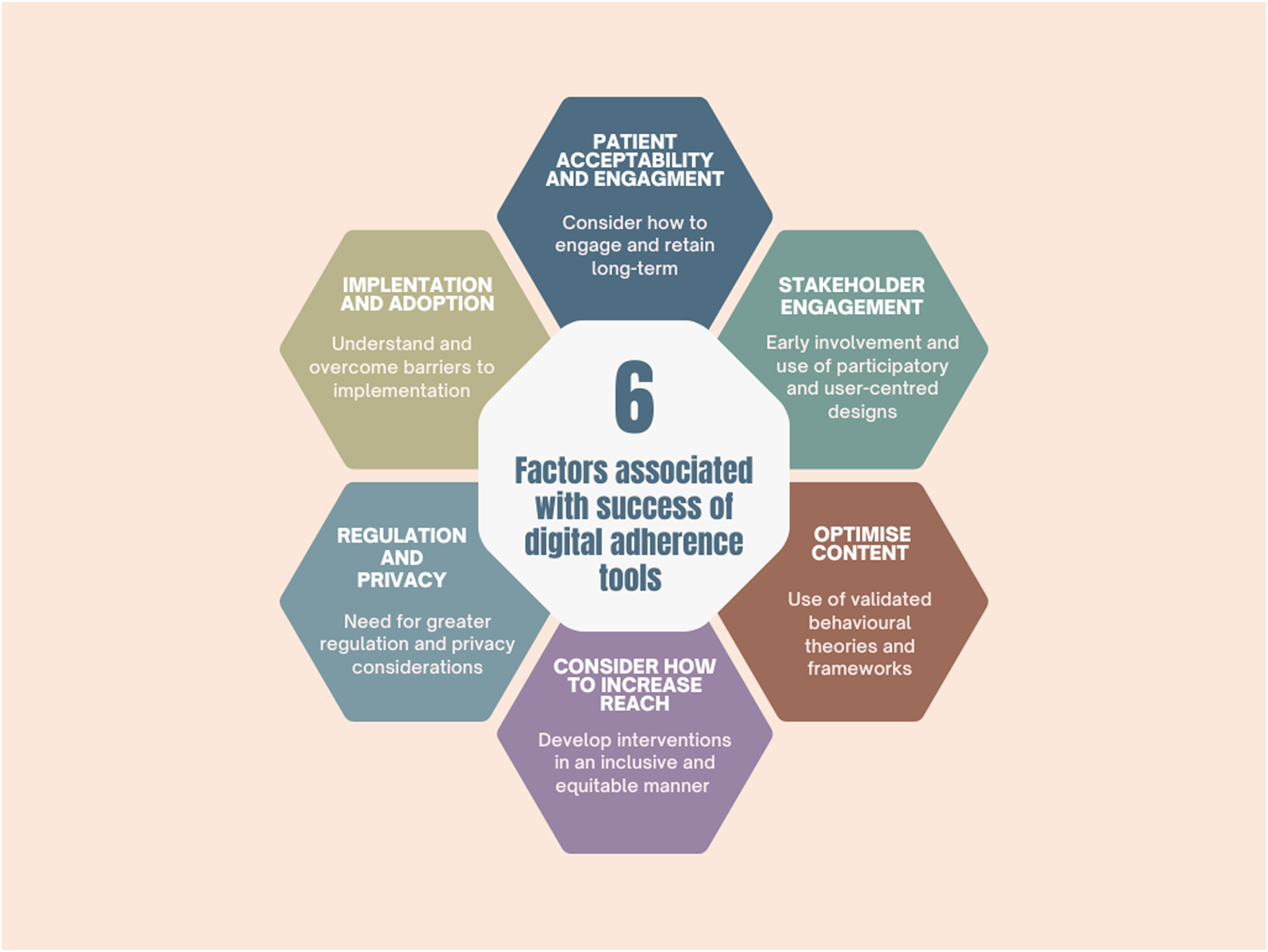
Factors associated with the success of digital adherence tools.
3.1 Patient acceptability and engagement
Engaging patients in an intervention and retaining them throughout is one of the biggest challenges facing any e-health intervention (Eysenbach, 2005), including adherence interventions, with many participants declining, dropping out of or not fully engaging with adherence interventions (Habib et al., 2021; Ping et al., 2022; Côté et al., 2020). For example, a web-based intervention to support medication adherence in people with HIV found that only 69% accessed the intervention, and of these only 36% completed more than one session. Only four of the initial 45 participants reached session four (Côté et al., 2020).
Reasons for this lack of engagement are complex and multi-faceted. Barriers to e-health in general include concerns about privacy and confidentiality, limited access to the relevant device or the internet, and lack of perceived need for digital support (Moecke et al., 2024; Morrissey et al., 2018). With regards to adherence interventions, concerns around privacy and data ownership are particularly relevant in interventions involving monitoring or any form of AI, with participants reporting concerns that this data could be used against them (e.g., with insurance companies) (Klugman et al., 2018). Technical issues or lack of user-friendly designs may also impede engagement to digital interventions (Ping et al., 2022; Grindrod et al., 2014). Older adults may face physical difficulties such as issues using small buttons on smartphones, reading small fonts or hearing notifications (van Acker et al., 2023). To overcome these issues, it is essential to involve the target population in intervention development and identify barriers relating to engagement and trust. For example, a survey of medication reminder app users in Singapore suggested that highlighting how apps protect personal data or offering anonymous usage should increase app usage (Ping et al., 2022). Another study found that people were more likely to agree to use an app if a clinical staff member would help them (Morano et al., 2019).
Usability testing and stakeholder feedback can help to develop interventions which are easy to understand and use for the target population (Grindrod et al., 2014; Hosszú et al., 2024). For example, Blixen et al. (2018) collected user feedback as part of the development of a text messaging intervention to improve adherence in people with bipolar disorder and hypertension (Blixen et al., 2018). Results highlighted key areas to increase patient acceptability, such as customising messages, writing out in full instead of abbreviated text speak and a focus on positive rather than negative messages. Similarly, user testing of a web-based diabetes adherence intervention identified several errors and provided recommendations on how to improve the site’s user interface (Nelson et al., 2016). Applying user experience (UX) principles, such as clear instructions and user-friendly error messages is also essential in developing apps which are intuitive and that people do not get frustrated with and stop using (Omaghomi et al., 2024). Gamification features such as rewards systems, points and leaderboards may also increase engagement (Omaghomi et al., 2024), as does the overall aesthetic and appearance of the app (Michie et al., 2017). Users also report engaging more with apps that appear to be credible, that are personalised and that allow communication with other users or HCPs (Michie et al., 2017). For example, a personalised smartphone based tracker app in Parkinsons disease showed significant improvements in adherence (Lakshminarayana et al., 2017). Analysis of usage found that 72% of participants in the intervention group continued to use and engage with the application across the 16 week period, with most using the app almost every day. However engagement with apps does not always lead to improved adherence. For example, a gaming app to improve medication in rheumatoid artritis found no significant improvements in adherence, despite the fact that 79% installed the game and 65% of these were active for at least 30 days out of 90 (Pouls et al., 2022).
3.2 Stakeholder engagement
Engaging end-users and wider stakeholders in the early design of interventions is essential to ensure participant acceptability, engagement and retention. Participatory approaches to digital health research have received increasing attention over the past 2 decades, particularly with regards to their role in developing effective digital interventions to promote medication adherence. Public and patient involvement (PPI) refers to the process of involving members of the public or patient groups in the research or design process. This involvement can occur at different stages of the research or design process. One of the driving motivations behind participatory approaches such as PPI is the idea that, in the case of public health research, members of the public have a right to input into designs and decisions in the context of research which may affect them (Bagley et al., 2016). Involving stakeholders who will interact directly or indirectly with the outcomes of research, tool design, or interventions, serves to ensure that the research is relevant, conducted in an ethical and acceptable manner, and that the research is designed in a participant-friendly or user-friendly manner.
In the past, mHealth tools have commonly been designed with consideration only given to existing healthcare systems and protocols, with little or no involvement of the end-users (Schnall et al., 2016). Increasingly however it is recognised that, in order for mHealth tools and applications to be effective, careful consideration needs to be given to the needs, requirements, and capacities of the end-users. Some reported barriers and enablers such as the importance of data privacy and security appear to be unique to PPI in digital innovation and these need to be addressed as part of this process (Baines et al., 2022).
A strong emphasis on participatory research and user-centred design, are said to play a key role in overcoming the uptake and retention issues described previously (Morton et al., 2020). While PPI focuses primarily on the involvement of patients in research and design, participatory approaches may involve engagement with stakeholders across various levels of healthcare delivery, depending on the purpose of the research design. Involving stakeholders, such as community health workers, nurses, administrators, and data managers in the design of mHealth tools allows for the gathering of valuable input in relation to various factors in effective design, including relevance, usability, and acceptability (de Beurs et al., 2017; Brewer et al., 2020). Effective eHealth interventions for self-management involve multidisciplinary teams harnessing diverse expertise. Systematic frameworks for intervention design and evidence-based user-centred methods, such as the person-based approach and Public and Patient Involvement (PPI) (Baines et al., 2022) facilitate this teamwork.
The WHO underscores involving end users in initial design phases to inform critical elements like perceived benefits and barriers to behaviour change, aligning interventions with community characteristics (WHO, 2021). Recognising this, increased emphasis has been placed on early involvement of users and stakeholders. The person-based approach leverages in-depth qualitative research to define guiding principles and key intervention features, essential across development stages, including planning, testing, and clinical evaluation. It aligns with in-depth approaches from information systems and human-computer interaction, emphasising understanding user knowledge, behaviour, motivations, and cultural contexts. Traditional user-testing focuses on utility and engagement, aiming to enhance technology usage. In contrast, the person-based approach, rooted in health psychology, targets behaviour change techniques and their implementation to boost participant engagement, driving intended outcomes.
3.3 Optimised content–use of behavioural theories/frameworks
Behaviour change theories can be used to aid the development of interventions to address relevant barriers to adherence and identify solutions for improving adherence. There are many long-standing, influential theories, including the Theory of Planned Behaviour (Ajzen, 1991), Goal-Setting Theory (Locke and Latham, 2015), the Health Belief Model (Janz and Becker, 1984), and Bandura’s (1986) Self-Efficacy Theory (Bandura, 1982). The Perceptions and Practicalities Approach (PaPA (Horne et al., 2019)) is a behaviour change theory developed specifically to understand non-adherence. The United Kingdom National Institute for Health and Care Excellence (NICE) guidelines for Supporting Adherence (Nunes, 2009) recommend the application of the PaPA, suggesting that any adherence support needs to consider the perceptual factors (e.g., beliefs about illness and treatment) that influence motivation to take a prescribed treatment, as well as the practical factors influencing ability to take the treatment (Horne et al., 2019). Evidence suggests that interventions which address both perceptual and practical factors influencing adherence are more likely to succeed. For example, a review of interventions to improve adherence to antiretroviral therapy found that interventions which addressed individuals’ specific perceptual and practical barriers to adherence were more effective than those that just addressed practical barriers like forgetting (Zoe Moon et al., 2023).
Another approach is the Behaviour Change Wheel (Michie et al., 2011) developed by synthesising 19 different frameworks of behaviour change. The Behaviour Change Wheel provides a useful way of linking a model of behaviour to common functions of interventions to change that behaviour (e.g., education, persuasion, coercion, incentivisation), and in turn, linking these intervention functions to policy categories (e.g., service provision, guidelines) that facilitate behaviour change. In addition, the Behaviour Change Technique Ontology (BCTO) (Marques et al., 2023) promotes the use of Behaviour Change Techniques (BCTs), defined as the observable, replicable components of behaviour change interventions. The BCTO provides a standard terminology and comprehensive classification system for the content of behaviour change interventions that can be reliably used to describe interventions. The techniques included in the ontology have been synthesised from related constructs drawn from theories and frameworks across clinical and health psychology research and practice. Using the BCT ontology to design effective interventions is therefore not inconsistent with other theoretical approaches.
Kahwati and colleagues used the BCT Taxonomy to conduct a qualitative comparative analysis of a systematic review of 60 complex interventions to identify combinations of BCTs that were most effective for improving medication adherence in outpatients with chronic conditions. Improvement in adherence was reported in more than half of the studies (57%). Of these studies, there were seven different configurations of BCTs that increased adherence. However, the most common and efficacious combination of techniques was ‘increasing knowledge’ coupled with ‘increasing self-efficacy’ (Kahwati et al., 2016). A content analysis of the BCTs present in 166 available apps reported that 12 of a possible 96 BCTs were present across these apps, and that 96% of the apps included the BCTs of ‘action-planning’, and ‘prompting/cues’. More than one-third of the apps that were reviewed featured the BCTs ‘self-monitoring’ and ‘feedback on behaviour’ (Morrissey et al., 2016).
3.4 Reach and inequalities in access
It has been suggested that digital technologies hold great potential for offsetting health inequalities, by increasing access and reaching those who may not traditionally receive support (Sharma and Patten, 2022; van de Vijver et al., 2023). However, there is also the potential for digital technologies to widen existing health inequalities, causing a “digital divide”, should they not have equitable reach or effectiveness (Latulippe et al., 2017). For example, digital health literacy and internet access are reported to be lower in underprivileged populations such as immigrants and individuals with lower socioeconomic status or less formal education (Estrela et al., 2023). This is of concern as these are groups who are already facing health inequalities.
Research has highlighted differences in terms of who has access to relevant digital devices. A survey of 2009 women with breast cancer in the United Kingdom found that 20% did not have access to a Tablet or Smartphone, and that the women without access were more likely to be older, have less formal education and be from a more deprived area (Moon et al., 2022). In the US, whilst 97% of college graduates own a smartphone, this falls to 83% in people with no college education (Center, 2024). In the United Kingdom, 96% of the highest socioeconomic group are smartphone users compared to 84% of the lowest socioeconomic status group (Statista, 2024a). Across the world, a UN report cited in the least developed countries only 27% of the populations are Internet users (Nations, 2023).
However, access to the internet, smartphones or other devices is only one part of the picture. It is also important to consider whether there are any factors influencing willingness to engage with digital health interventions. For example, studies report that older adults, those who are less highly educated and people from minority ethnic groups are less likely to be users of mobile health apps or to seek health information online (Bol et al., 2018; Fareed et al., 2021). Receptivity towards mobile phone text messages as a healthcare intervention also reduces with increasing age, and lower education and income levels (Serrano et al., 2016). Specifically with regards to digital adherence interventions, a US study showed that people with diabetes with lower health literacy and who were not of white ethnicity were less likely to participate in the intervention (Nelson et al., 2016). However, engagement did not differ by age, gender, education, income or health literacy, suggesting fairly wide reach. In another study, people with HIV who had less formal education were less willing to adopt mobile phone technology to improve their adherence (Morano et al., 2019).
Taken together, these studies support the idea that digital health interventions may be less likely to be accessed or used by people from lower socioeconomic status backgrounds, which is of concern given the existing health inequalities in these groups. However, some other studies have shown that patients in diverse or low-income communities show greater interest in mHealth apps than those from white or high-income communities (Humble et al., 2016; Ramirez et al., 2016).
Research has also highlighted potential differences in how effective digital behaviour change interventions are for different groups of people. Whilst this has not been explored in adherence specifically, a systematic review and meta-analysis of digital behaviour change interventions for physical activity found that the interventions were effective in those with high socioeconomic status but not in people of low socioeconomic status (Western et al., 2021). Therefore, attention may need to be paid to understanding whether the benefits of adherence interventions are equitable across all participants.
More research is warranted to fully understand whether adherence digital interventions will further the “digital divide” or help to close existing gaps. However, regardless, intervention developers need to be mindful of developing interventions in an inclusive and equitable manner. Several guidelines have been developed to assist with this (Latulippe et al., 2017; Miller et al., 2023), as well as the Carnegie United Kingdom Trust 12 recommendations for eliminating digital exclusion (Georgina Bowyer, 2020). Key elements of these guidelines include ensuring universal access to the tool, co-creating with a diverse and relevant stakeholder groups, accounting for varying levels of health literacy, and collecting quality data to monitor access and engagement.
3.5 Regulation and privacy
Rapid developments in digital technology has far outpaced regulatory bodies’ capacity to address issues around quality, data regulation and privacy. A study by Backes et al. (2020) investigated whether healthcare providers could safely recommend mobile health apps to their patients to promote medication adherence (Backes et al., 2020). In their study they evaluated eligible apps and concluded that none of the apps had undergone a process for certification, little information was provided on security and data protection and that more clinical studies with chronic patients are necessary to measure long-term app impacts. The authors suggest that some of these shortcomings might be corrected through the introduction of General Data Protection Regulation (GDPR) in the European Economic Area (EEA) and more scrutiny through regulatory bodies in the EU/EEA and the United States. They further concluded that none of the applications should be recommended by healthcare providers.
Research by Grundy et al. (2019) found that the accuracy and quality of information provided within medical and health apps cannot easily be ascertained and these factors are likely to affect medication adherence and, more importantly, patient health outcomes (Grundy et al., 2019). They found that apps often involve communicating patient-specific data over the internet which raises the issue of patient privacy. They further reported that up to 80% of mobile health apps transmit user-related information to online services and 66% of apps sent unencrypted identifying information over the Internet. They conclude that the benefits of secure communication of information between health providers and patients cannot be ignored.
Magrabi et al. (2019) examined the challenges around regulation of apps to promote medication adherence and concluded that an evidence-based approach that is informed by the current landscape of health apps is required (Farah et al., 2019). They suggest that operational oversight and surveillance could be considered at a national and regional level using common frameworks so that it is possible to compare patterns over time and between settings, and to develop and prioritise preventive and corrective strategies. A professional foundation for regulation of such technologies would permit more widespread use of evidence-based apps to promote medication adherence. Finally, the role of citizen developers should also be considered within this digital health ecosystem.
3.6 Implementation and adoption
A final issue with digital adherence interventions is that they are often under-utilised and few are implemented at scale (Kardas et al., 2022). Trials of adherence interventions tend to fail to consider factors relevant to implementation into real-world settings (Kostalova et al., 2022), and reviews have concluded that the long-term sustainability and feasibility of digital adherence interventions remains to be determined (Chan et al., 2022; Griffee et al., 2022). Barriers to the successful implementation of eHealth interventions in general include cost, increased workloads, lack of healthcare professional motivation, issues with interoperability, and lack of suitable infrastructure, training and support (Kardas et al., 2022; Ahmed et al., 2019; Granja et al., 2018; Chimweta et al., 2025). Particularly in the developing world, issues with local telecommunication networks may act as a barrier to intervention implementation (O'Connor et al., 2022). Across all contexts, acquiring the funding for ongoing maintenance and hosting can be a barrier to implementation and utilisation (Ahmed et al., 2019). Implementation science can provide useful insights and should be considered from the start of any project to ensure that the digital adherence interventions developed have a chance of being implemented (Kostalova et al., 2022; Zullig et al., 2019). Issues around reimbursement are also a barrier to implementation, and more data on long-term clinical effects and cost-effectiveness may be needed to overcome this (Kardas et al., 2022; Borah et al., 2025).
4 Conclusion
Digital technologies have emerged as a promising tool for addressing the significant global issue of medication non-adherence. However, the evidence supporting the effectiveness of these digital interventions is mixed, with many studies showing inconsistent or short-term improvements in adherence. This variability is largely due to the diverse designs, content, and delivery methods used in digital tools, many of which lack a strong evidence base or user-centered design. While digital interventions have the potential to reduce healthcare costs and improve medication adherence, careful attention must be paid to ensure these technologies do not inadvertently widen existing health inequalities. Addressing the “digital divide” by ensuring equitable access, usability, and acceptability across diverse populations is essential to prevent exacerbating disparities in healthcare access and outcomes.
Future interventions aimed at improving medication adherence should emphasise personalised approaches that consider individual patient needs, beliefs, and preferences. Leveraging AI and machine learning algorithms can enhance engagement and effectiveness by tailoring content, reminders, and feedback. Incorporating interactive elements, such as communication with healthcare providers and peer support networks, can further boost adherence. Rigorous evaluation and the establishment of quality standards are essential, with a focus on long-term outcomes, patient engagement, and clinical benefits through well-designed clinical trials.
Statements
Author contributions
ZM: Conceptualization, Investigation, Writing – original draft, Writing – review and editing. JW: Conceptualization, Investigation, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The journal publication fee for this article was paid by Abbot Pharmaceutical Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Adler A. J. Casas J. P. Martin N. Free C. Perel P. (2018). Cochrane corner: text messaging to improve adherence to drugs for secondary prevention of cardiovascular disease. Heart104 (22), 1814–1816. 10.1136/heartjnl-2017-312888
2
Ahmed I. Ahmad N. S. Ali S. Ali S. George A. Saleem Danish H. et al (2018). Medication adherence apps: review and content analysis. JMIR Mhealth Uhealth6 (3), e62. 10.2196/mhealth.6432
3
Ahmed B. Dannhauser T. Philip N. (2019). A systematic review of reviews to identify key research opportunities within the field of eHealth implementation. J. Telemed. Telecare25 (5), 276–285. 10.1177/1357633X18768601
4
Ahonkhai A. A. Pierce L. J. Mbugua S. Wasula B. Owino S. Nmoh A. et al (2021). PEERNaija: a gamified mHealth behavioral intervention to improve adherence to antiretroviral treatment among adolescents and young adults in Nigeria. Front. Reprod. Health3, 656507. 10.3389/frph.2021.656507
5
Ajzen I. (1991). The Theory of planned behavior. Organ. Behav. Hum. Decis. Process.50, 179–211. 10.1016/0749-5978(91)90020-t
6
Aldeer M. Javanmard M. Martin R. P. (2018). A review of medication adherence monitoring technologies. Appl. Syst. Innov.1 (2), 14. 10.3390/asi1020014
7
Armitage L. C. Kassavou A. Sutton S. (2020). Do mobile device apps designed to support medication adherence demonstrate efficacy? A systematic review of randomised controlled trials, with meta-analysis. BMJ Open10 (1), e032045. 10.1136/bmjopen-2019-032045
8
Aungst T. D. (2021). Reevaluating medication adherence in the era of digital health. Expert Rev. Med. Devices18 (Suppl. 1), 25–35. 10.1080/17434440.2021.2019012
9
Backes C. Moyano C. Rimaud C. Bienvenu C. Schneider M. P. (2020). Digital medication adherence support: could healthcare providers recommend mobile health apps?Front. Med. Technol.2, 616242. 10.3389/fmedt.2020.616242
10
Bagley H. J. Short H. Harman N. L. Hickey H. R. Gamble C. L. Woolfall K. et al (2016). A patient and public involvement (PPI) toolkit for meaningful and flexible involvement in clinical trials–a work in progress. Res. Involv. Engagem.2, 15–14. 10.1186/s40900-016-0029-8
11
Bain E. E. Shafner L. Walling D. P. Othman A. A. Chuang-Stein C. Hinkle J. et al (2017). Use of a novel artificial intelligence platform on mobile devices to assess dosing compliance in a phase 2 clinical trial in subjects with schizophrenia. JMIR mHealth uHealth5 (2), e18. 10.2196/mhealth.7030
12
Baines R. Bradwell H. Edwards K. Stevens S. Prime S. Tredinnick-Rowe J. et al (2022). Meaningful patient and public involvement in digital health innovation, implementation and evaluation: a systematic review. Health Expect.25 (4), 1232–1245. 10.1111/hex.13506
13
Bandura A. (1982). Self-efficacy mechanism in human agency. Am. Psychol.37 (2), 122–147. 10.1037//0003-066x.37.2.122
14
Basu S. Garg S. Sharma N. Singh M. M. (2019). Improving the assessment of medication adherence: challenges and considerations with a focus on low-resource settings. Tzu Chi Med. J.31 (2), 73–80. 10.4103/tcmj.tcmj_177_18
15
Belete A. M. Gemeda B. N. Akalu T. Y. Aynalem Y. A. Shiferaw W. S. (2023). What is the effect of mobile phone text message reminders on medication adherence among adult type 2 diabetes mellitus patients: a systematic review and meta-analysis of randomized controlled trials. BMC Endocr. Disord.23 (1), 18. 10.1186/s12902-023-01268-8
16
Belzer M. E. MacDonell K. Cain D. Ghosh S. Zhao R. McAvoy-Banerjea J. et al (2025). An adaptive antiretroviral therapy adherence intervention for youth with HIV through text message and cell phone support with and without incentives: a sequential multiple assignment randomized trial (smart). AIDS Behav.29 (3), 769–780. 10.1007/s10461-024-04558-x
17
Bingham J. M. Black M. Anderson E. J. Li. Y. Toselli N. Fox S. et al (2021). Impact of telehealth interventions on medication adherence for patients with type 2 diabetes, hypertension, and/or dyslipidemia: a systematic review. Ann. Pharmacother.55 (5), 637–649. 10.1177/1060028020950726
18
Blixen C. Sajatovic M. Moore D. J. Depp C. Cushman C. Cage J. et al (2018). Patient participation in the development of a customized m-Health intervention to improve medication adherence in poorly adherent individuals with bipolar disorder (BD) and hypertension (HTN). Int. J. Healthc.4 (1), 25–35. 10.5430/ijh.v4n1p25
19
Bol N. Helberger N. Weert J. C. M. (2018). Differences in mobile health app use: a source of new digital inequalities?Inf. Soc.34 (3), 183–193. 10.1080/01972243.2018.1438550
20
Bond Z. Scanlon T. Judah G. (2021). Systematic review of RCTs assessing the effectiveness of mHealth interventions to improve statin medication adherence: using the behaviour-change technique Taxonomy to identify the techniques that improve adherence. Healthc. (Basel)9 (10), 1282. 10.3390/healthcare9101282
21
Borah B. J. Pieretti L. J. Balch A. J. Wani R. J. Daly C. J. Dawoud D. et al (2025). Stakeholders’ perspectives on medication adherence enhancing interventions. Value Health28 (5), 676–679. 10.1016/j.jval.2025.01.022
22
Bosworth H. B. Zullig L. L. Mendys P. Ho M. Trygstad T. Granger C. et al (2016). Health information technology: meaningful use and next steps to improving electronic facilitation of medication adherence. JMIR Med. Inf.4 (1), e9. 10.2196/medinform.4326
23
Brar Prayaga R. Jeong E. W. Feger E. Noble H. K. Kmiec M. Prayaga R. S. (2018). Improving refill adherence in medicare patients with tailored and interactive mobile text messaging: pilot study. JMIR mHealth uHealth6 (1), e8930. 10.2196/mhealth.8930
24
Brewer L. C. Fortuna K. L. Jones C. Walker R. Hayes S. N. Patten C. A. et al (2020). Back to the future: achieving health equity through health informatics and digital health. JMIR Mhealth Uhealth8 (1), e14512. 10.2196/14512
25
Burkhart P. V. Sabaté E. (2003). Adherence to long-term therapies: evidence for action. J. Nurs. Scholarsh.35 (3), 207. 10.1111/j.1547-5069.2003.tb00001.x
26
Cao W. Kadir A. A. Tang W. Wang J. Yuan J. Hassan I. I. (2024). Effectiveness of mobile application interventions for stroke survivors: systematic review and meta-analysis. BMC Med. Inf. Decis. Mak.24 (1), 6. 10.1186/s12911-023-02391-1
27
Cazeau N. (2021). Mobile health interventions: examining medication adherence outcomes among patients with cancer. Clin. J. Oncol. Nurs.25 (4), 431–438. 10.1188/21.CJON.431-438
28
Center P. R. (2024). Mobile fact sheet. Available online at: https://www.pewresearch.org/internet/fact-sheet/mobile/.
29
Chaix B. Bibault J. E. Pienkowski A. Delamon G. Guillemassé A. Nectoux P. et al (2019). When chatbots meet patients: one-year prospective study of conversations between patients with breast cancer and a chatbot. JMIR Cancer5 (1), e12856. 10.2196/12856
30
Chan A. H. Y. Foot H. Pearce C. J. Horne R. Foster J. M. Harrison J. (2022). Effect of electronic adherence monitoring on adherence and outcomes in chronic conditions: a systematic review and meta-analysis. PLoS One17 (3), e0265715. 10.1371/journal.pone.0265715
31
Chapman S. Sibelli A. St-Clair Jones A. Forbes A. Chater A. Horne R. (2020). Personalised adherence support for maintenance treatment of inflammatory bowel disease: a tailored digital intervention to change adherence-related beliefs and barriers. J. Crohn's Colitis14 (10), 1394–1404. 10.1093/ecco-jcc/jjz034
32
Chimweta I. C. Foster N. Bahukudumbi S. Mohamed M. S. Zary M. Kafie C. et al (2025). Implementation outcomes of tuberculosis digital adherence technologies: a scoping review using the RE-AIM framework. BMJ Glob. Health10 (2), e016535. 10.1136/bmjgh-2024-016535
33
Chong C. J. Bakry M. M. Hatah E. Mohd Tahir N. A. Mustafa N. (2023). Effects of mobile apps intervention on medication adherence and type 2 diabetes mellitus control: a systematic review and meta-analysis. J. Telemed. Telecare, 1357633X231174933. 10.1177/1357633X231174933
34
Côté J. Rouleau G. Ramirez-Garcia M. P. Auger P. Thomas R. Leblanc J. (2020). Effectiveness of a web-based intervention to support medication adherence among people living with HIV: web-based randomized controlled trial. JMIR Public Health Surveillance6 (2), e17733. 10.2196/17733
35
Dayer L. E. Shilling R. Van Valkenburg M. Martin B. C. Gubbins P. O. Hadden K. et al (2017). Assessing the medication adherence app marketplace from the health professional and consumer vantage points. JMIR mHealth uHealth5 (4), e45. 10.2196/mhealth.6582
36
de Beurs D. van Bruinessen I. Noordman J. Friele R. van Dulmen S. (2017). Active involvement of end users when developing web-based mental health interventions. Front. Psychiatry8, 72. 10.3389/fpsyt.2017.00072
37
Estrela M. Semedo G. Roque F. Ferreira P. L. Herdeiro M. T. (2023). Sociodemographic determinants of digital health literacy: a systematic review and meta-analysis. Int. J. Med. Inf.177, 105124. 10.1016/j.ijmedinf.2023.105124
38
Eysenbach G. (2005). The law of attrition. J. Med. Internet Res.7 (1), e11. 10.2196/jmir.7.1.e11
39
Farah M. Habli I. Sujan M. Wong D. Thimbleby H. Baker M. et al (2019). Why is it so difficult to govern mobile apps in healthcare?BMJ Health and Care Inf.26 (1), e100006. 10.1136/bmjhci-2019-100006
40
Fareed N. Swoboda C. M. Jonnalagadda P. Huerta T. R. (2021). Persistent digital divide in health-related internet use among cancer survivors: findings from the Health Information National Trends Survey, 2003–2018. J. Cancer Surviv.15, 87–98. 10.1007/s11764-020-00913-8
41
Gautier J.-F. Boitard C. Michiels Y. Raymond G. Vergez G. Guedon G. (2021). Impact of personalized text messages from pharmacists on medication adherence in type 2 diabetes in France: a real-world, randomized, comparative study. Patient Educ. Couns.104 (9), 2250–2258. 10.1016/j.pec.2021.02.022
42
Georgina Bowyer A. G. D. W. (2020). Learning from lockdown: 12 steps to eliminate digital exclusion Editor UKC.
43
Goradia S. Holland R. Alexander S. Greenbaum D. Chen T. Aslani P. (2021). A new age intervention to support medication adherence. Res. Soc. Adm. Pharm.17 (6), 1204–1207. 10.1016/j.sapharm.2020.07.038
44
Granja C. Janssen W. Johansen M. A. (2018). Factors determining the success and failure of eHealth interventions: systematic review of the literature. J. Med. Internet Res.20 (5), e10235. 10.2196/10235
45
Griffee K. Martin R. Chory A. Vreeman R. (2022). A systematic review of digital interventions to improve ART adherence among youth living with HIV in sub-saharan africa. AIDS Res. Treat.2022, 9886306. 10.1155/2022/9886306
46
Grindrod K. A. Li M. Gates A. (2014). Evaluating user perceptions of mobile medication management applications with older adults: a usability study. JMIR Mhealth Uhealth2 (1), e11. 10.2196/mhealth.3048
47
Grundy Q. Chiu K. Held F. Continella A. Bero L. Holz R. (2019). Data sharing practices of medicines related apps and the mobile ecosystem: traffic, content, and network analysis. BMJ, 364. 10.1136/bmj.l920
48
Guo Y. Hao Z. Zhao S. Gong J. Yang F. (2020). Artificial intelligence in health care: bibliometric analysis. J. Med. Internet Res.22 (7), e18228. 10.2196/18228
49
Habib B. Buckeridge D. Bustillo M. Marquez S. N. Thakur M. Tran T. et al (2021). Smart about Meds (SAM): a pilot randomized controlled trial of a mobile application to improve medication adherence following hospital discharge. JAMIA Open4 (3), ooab050. 10.1093/jamiaopen/ooab050
50
Head K. J. Noar S. M. Iannarino N. T. Grant Harrington N. (2013). Efficacy of text messaging-based interventions for health promotion: a meta-analysis. Soc. Sci. and Med.97, 41–48. 10.1016/j.socscimed.2013.08.003
51
Horne R. Cooper V. Wileman V. Chan A. (2019). Supporting adherence to medicines for long-term conditions. Eur. Psychol.24, 82–96. 10.1027/1016-9040/a000353
52
Hosszú D. Dima A. L. Fernández F. L. Schneider M. P. van Dijk L. Tóth K. et al (2024). Engagement of medication users in the development and implementation of digital medication adherence technologies: a multi-stakeholder study. Expert Rev. Pharmacoeconomics and Outcomes Res.24, 853–860. 10.1080/14737167.2024.2373184
53
Hughes L. Moss-Morris R. Hunter M. Norton S. Moon Z. (2022). “Effectiveness of an mHealth intervention targeting treatment adherence in breast cancer: a randomized controlled trial,” in European health psychology society conference (Bratislava).
54
Humble J. R. Tolley E. A. Krukowski R. A. Womack C. R. Motley T. S. Bailey J. E. (2016). Use of and interest in mobile health for diabetes self-care in vulnerable populations. J. Telemed. Telecare22 (1), 32–38. 10.1177/1357633X15586641
55
Janz N. K. Becker M. H. (1984). The health belief model: a decade later. Health Educ. Q.11 (1), 1–47. 10.1177/109019818401100101
56
Kahwati L. Viswanathan M. Golin C. E. Kane H. Lewis M. Jacobs S. (2016). Identifying configurations of behavior change techniques in effective medication adherence interventions: a qualitative comparative analysis. Syst. Rev.5, 83–89. 10.1186/s13643-016-0255-z
57
Kardas P. Bago M. Barnestein-Fonseca P. Garuolienė K. Granas A. G. Gregório J. et al (2022). Reimbursed medication adherence enhancing interventions in 12 european countries: current state of the art and future challenges. Front. Pharmacol., 13–2022. 10.3389/fphar.2022.944829
58
Klugman C. M. Dunn L. B. Schwartz J. Cohen I. G. (2018). The ethics of smart pills and self-acting devices: autonomy, truth-telling, and trust at the dawn of digital medicine. Am. J. Bioeth.18 (9), 38–47. 10.1080/15265161.2018.1498933
59
Koesmahargyo V. Abbas A. Zhang L. Guan L. Feng S. Yadav V. et al (2020). Accuracy of machine learning-based prediction of medication adherence in clinical research. Psychiatry Res.294, 113558. 10.1016/j.psychres.2020.113558
60
Kostalova B. Ribaut J. Dobbels F. Gerull S. Mala-Ladova K. Zullig L. L. et al (2022). Medication adherence interventions in transplantation lack information on how to implement findings from randomized controlled trials in real-world settings: a systematic review. Transpl. Rev. Orl.36 (1), 100671. 10.1016/j.trre.2021.100671
61
Labovitz D. L. Shafner L. Reyes Gil M. Virmani D. Hanina A. (2017). Using artificial intelligence to reduce the risk of nonadherence in patients on anticoagulation therapy. Stroke48 (5), 1416–1419. 10.1161/STROKEAHA.116.016281
62
Lakshminarayana R. Wang D. Burn D. Chaudhuri K. R. Galtrey C. Guzman N. V. et al (2017). Using a smartphone-based self-management platform to support medication adherence and clinical consultation in Parkinson's disease. NPJ Park. Dis.3, 2. 10.1038/s41531-016-0003-z
63
Latulippe K. Hamel C. Giroux D. (2017). Social health inequalities and eHealth: a literature review with qualitative synthesis of theoretical and empirical studies. J. Med. Internet Res.19 (4), e136. 10.2196/jmir.6731
64
Locke E. Latham G. (2015). “Goal-setting theory,” in Organizational behavior 1 (London: Routledge), 159–183.
65
Long J. Yuan M. J. Poonawala R. (2016). An observational study to evaluate the usability and intent to adopt an artificial intelligence–powered medication reconciliation tool. Interact. J. Med. Res.5 (2), e14. 10.2196/ijmr.5462
66
Mahajan S. Lu Y. Spatz E. S. Nasir K. Krumholz H. M. (2021). Trends and predictors of use of digital health technology in the United States. Am. J. Med.134 (1), 129–134. 10.1016/j.amjmed.2020.06.033
67
Marques M. M. Wright A. J. Corker E. Johnston M. West R. Hastings J. et al (2023). The behaviour change technique ontology: transforming the behaviour change technique Taxonomy v1. Wellcome Open Res.8, 308. 10.12688/wellcomeopenres.19363.2
68
Masterson Creber R. M. Maurer M. S. Reading M. Hiraldo G. Hickey K. T. Iribarren S. (20216). Review and analysis of existing mobile phone apps to support heart failure symptom monitoring and self-care management using the Mobile Application Rating Scale (MARS). JMIR mHealth uHealth4 (2), e74. 10.2196/mhealth.5882
69
Medicalfuturist (2025). T.M.F. FDA-Approved A.I.-Based Algorithms. Available online at: https://medicalfuturist.com/fda-approved-ai-based-algorithms/.
70
Michie S. Van Stralen M. M. West R. (2011). The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement. Sci.6, 42–12. 10.1186/1748-5908-6-42
71
Michie S. Yardley L. West R. Patrick K. Greaves F. (2017). Developing and evaluating digital interventions to promote behavior change in health and health care: recommendations resulting from an international workshop. J. Med. Internet Res.19 (6), e232. 10.2196/jmir.7126
72
Miller S. J. Sly J. R. Alcaraz K. I. Ashing K. Christy S. M. Gonzalez B. et al (2023). Equity and behavioral digital health interventions: strategies to improve benefit and reach. Transl. Behav. Med.13 (6), 400–405. 10.1093/tbm/ibad010
73
Mira J. J. Lorenzo S. Guilabert M. Navarro I. Pérez-Jover V. (2015). A systematic review of patient medication error on self-administering medication at home. Expert Opin. drug Saf.14 (6), 815–838. 10.1517/14740338.2015.1026326
74
Moecke D. P. Holyk T. Beckett M. Chopra S. Petlitsyna P. Girt M. et al (2024). Scoping review of telehealth use by Indigenous populations from Australia, Canada, New Zealand, and the United States. J. Telemed. Telecare30 (9), 1398–1416. 10.1177/1357633X231158835
75
Moon Z. Zuchowski M. Moss-Morris R. Hunter M. S. Norton S. Hughes L. D. (2022). Disparities in access to mobile devices and e-health literacy among breast cancer survivors. Support. Care Cancer30, 117–126. 10.1007/s00520-021-06407-2
76
Morano J. P. Clauson K. Zhou Z. Escobar-Viera C. G. Lieb S. Chen I. K. et al (2019). Attitudes, beliefs, and willingness toward the use of mHealth tools for medication adherence in the Florida mHealth adherence project for people living with HIV (FL-mAPP): pilot questionnaire study. JMIR Mhealth Uhealth7 (7), e12900. 10.2196/12900
77
Morrissey E. C. Corbett T. K. Walsh J. C. Molloy G. J. (2016). Behavior change techniques in apps for medication adherence: a content analysis. Am. J. Prev. Med.50 (5), e143–e146. 10.1016/j.amepre.2015.09.034
78
Morrissey E. C. Casey M. Glynn L. G. Walsh J. C. Molloy G. J. (2018). Smartphone apps for improving medication adherence in hypertension: patients' perspectives. Patient Prefer Adherence12, 813–822. 10.2147/PPA.S145647
79
Morton E. Barnes S. J. Michalak E. E. (2020). Participatory digital health research: a new paradigm for mHealth tool development. Gen. Hosp. Psychiatry66, 67–69. 10.1016/j.genhosppsych.2020.07.005
80
Mosnaim G. S. Stempel H. Van Sickle D. Stempel D. A. (2020). The adoption and implementation of digital health care in the post-COVID-19 era. J. Allergy Clin. Immunol. Pract.8 (8), 2484–2486. 10.1016/j.jaip.2020.06.006
81
Mulawa M. I. LeGrand S. Hightow-Weidman L. B. (2018). eHealth to enhance treatment adherence among youth living with HIV. Curr. HIV/AIDS Rep.15 (4), 336–349. 10.1007/s11904-018-0407-y
82
Nations U. (2023). Widening digital gap between developed, developing states threatening to exclude world’s poorest from next industrial revolution, speakers tell second committee. Available online at: https://press.un.org/en/2023/gaef3587.doc.htm.
83
Nayak A. Vakili S. Nayak K. Nikolov M. Chiu M. Sosseinheimer P. et al (2023). Use of voice-based conversational artificial intelligence for basal insulin prescription management among patients with type 2 diabetes: a randomized clinical trial. JAMA Netw. Open6 (12), e2340232. 10.1001/jamanetworkopen.2023.40232
84
Nelson L. A. Mulvaney S. A. Gebretsadik T. Ho Y. X. Johnson K. B. Osborn C. Y. (2016). Disparities in the use of a mHealth medication adherence promotion intervention for low-income adults with type 2 diabetes. J. Am. Med. Inf. Assoc.23 (1), 12–18. 10.1093/jamia/ocv082
85
Ng R. Carter S. R. El-Den S. (2020). The impact of mobile applications on medication adherence: a systematic review. Transl. Behav. Med.10 (6), 1419–1435. 10.1093/tbm/ibz125
86
Nunes V. (2009). Medicines adherence: involving patients in decisions about prescribed medicines and supporting adherence.
87
O'Connor C. Leyritana K. Doyle A. M. Birdthistle I. Lewis J. J. Gill R. et al (2022). Delivering an mHealth adherence support intervention for patients with HIV: mixed methods process evaluation of the Philippines connect for life study. JMIR Form. Res.6 (8), e37163. 10.2196/37163
88
Omaghomi T. T. Elufioye E. A. Akomolafe O. Anyanwu E. C. Daraojimba A. I. (2024). Health apps and patient engagement: a review of effectiveness and user experience. World J. Adv. Res. Rev.21 (2), 432–440. 10.30574/wjarr.2024.21.2.0476
89
Palmer M. J. Barnard S. Perel P. Free C. (2021). Mobile phone-based interventions for improving adherence to medication prescribed for the primary prevention of cardiovascular disease in adults. Cochrane Database Syst. Rev.3 (3), CD012675. 10.1002/14651858.CD012675.pub2
90
Petrie K. J. Perry K. Broadbent E. Weinman J. (2012). A text message programme designed to modify patients’ illness and treatment beliefs improves self‐reported adherence to asthma preventer medication. Br. J. health Psychol.17 (1), 74–84. 10.1111/j.2044-8287.2011.02033.x
91
Ping Y. Visaria A. Suppiah S. D. Tan Y. W. Malhotra R. (2022). Prevalence and correlates of medication reminder app 'use and use intention' among older adults. Explor Res. Clin. Soc. Pharm.6, 100150. 10.1016/j.rcsop.2022.100150
92
Pouls B. P. H. Bekker C. L. Gundogan F. Hebing R. C. van Onzenoort H. A. van de Ven L. I. et al (2022). Gaming for Adherence to Medication using Ehealth in Rheumatoid arthritis (GAMER) study: a randomised controlled trial. RMD Open8 (2), e002616. 10.1136/rmdopen-2022-002616
93
Ramirez V. Johnson E. Gonzalez C. Rubino B. Rossetti G. (2016). Assessing the use of mobile health technology by patients: an observational study in primary care clinics. JMIR mHealth uHealth4 (2), e41. 10.2196/mhealth.4928
94
Redfern J. Tu Q. Hyun K. Hollings M. A. Hafiz N. Zwack C. et al (2024). Mobile phone text messaging for medication adherence in secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev.3 (3), Cd011851. 10.1002/14651858.CD011851.pub3
95
Reis Z. S. N. Pereira G. M. V. Dias C. D. S. Lage E. M. de Oliveira I. J. R. Pagano A. S. (2025). Artificial intelligence-based tools for patient support to enhance medication adherence: a focused review. Front. Digital Health7, 1523070. 10.3389/fdgth.2025.1523070
96
Riaz S. Jones Nielsen J. D. (2019). A pilot study of a text messaging intervention to modify illness and medication beliefs among patients diagnosed with inflammatory bowel disease. J. Technol. Behav. Sci.4, 42–52. 10.1007/s41347-018-0083-1
97
Robinson B. Proimos E. Zou D. Gong E. Oldenburg B. See K. (2024). Functionality and quality of asthma mHealth apps and their consistency with international guidelines: structured search and evaluation. JMIR Mhealth Uhealth12, e47295. 10.2196/47295
98
Schnall R. Rojas M. Bakken S. Brown W. Carballo-Dieguez A. Carry M. et al (2016). A user-centered model for designing consumer mobile health (mHealth) applications (apps). J. Biomed. Inf.60, 243–251. 10.1016/j.jbi.2016.02.002
99
Serrano K. J. Yu M. Riley W. T. Patel V. Hughes P. Marchesini K. et al (2016). Willingness to exchange health information via mobile devices: findings from a population-based survey. Ann. Fam. Med.14 (1), 34–40. 10.1370/afm.1888
100
Sharma P. Patten C. A. (2022). A need for digitally inclusive health care service in the United States: recommendations for clinicians and health care systems. Perm. J.26 (3), 149–153. 10.7812/TPP/21.156
101
Statista (2024a). Share of smartphone users in the United Kingdom (UK) from 2012 to 2023, by socio-economic group. Available online at: https://www.statista.com/statistics/300421/smartphone-usage-in-the-uk-by-socio-economic-group/#:∼:text=In%202012%2C%2055%20percent%20of,reporting%20to%20use%20a%20smartphone.
102
Statista (2024b). Internet usage worldwide. Available online at: https://www.statista.com/topics/1145/internet-usage-worldwide/.
103
Statista (2024c). Global smartphone penetration rate as share of population from 2016 to 2023. Available online at: https://www.statista.com/statistics/203734/global-smartphone-penetration-per-capita-since-2005/#:∼:text=The%20global%20smartphone%20penetration%20rate,population%20of%20around%207.4%20billion.
104
Statistics O. F. N. (2020). Internet access - households and individuals. Available online at: https://www.ons.gov.uk/peoplepopulationandcommunity/householdcharacteristics/homeinternetandsocialmediausage/datasets/internetaccesshouseholdsandindividualsreferencetables.
105
Stewart S.-J. F. Moon Z. Horne R. (2023). Medication nonadherence: health impact, prevalence, correlates and interventions. Psychol. and health38 (6), 726–765. 10.1080/08870446.2022.2144923
106
Tran S. Smith L. El-Den S. Carter S. (2022). The use of gamification and incentives in mobile health apps to improve medication adherence: scoping review. JMIR Mhealth Uhealth10 (2), e30671. 10.2196/30671
107
Unni E. Gabriel S. Ariely R. (2018). A review of the use and effectiveness of digital health technologies in patients with asthma. Ann. Allergy Asthma Immunol.121 (6), 680–691. 10.1016/j.anai.2018.10.016
108
van Acker J. Maenhout L. Compernolle S. (2023). Older adults' user engagement with mobile health: a systematic review of qualitative and mixed-methods studies. Innov. Aging7 (2), igad007. 10.1093/geroni/igad007
109
van de Hei S. J. Kim C. H. Honkoop P. J. Sont J. K. Schermer T. R. J. MacHale E. et al (2023). Long-term cost-effectiveness of digital inhaler adherence technologies in difficult-to-treat asthma. J. Allergy Clin. Immunol. Pract.11 (10), 3064–3073. 10.1016/j.jaip.2023.06.051
110
van de Vijver S. Tensen P. Asiki G. Requena-Méndez A. Heidenrijk M. Stronks K. et al (2023). Digital health for all: how digital health could reduce inequality and increase universal health coverage. Digit. health9, 20552076231185434. 10.1177/20552076231185434
111
Wang T. Huang Y.-M. Chan H.-Y. (2024). Exploration of features of mobile applications for medication adherence in asia: narrative review. J. Med. Internet Res.26, e60787. 10.2196/60787
112
Western M. J. Armstrong M. E. G. Islam I. Morgan K. Jones U. F. Kelson M. J. (2021). The effectiveness of digital interventions for increasing physical activity in individuals of low socioeconomic status: a systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act.18 (1), 148. 10.1186/s12966-021-01218-4
113
Who W. H. O. (2021). Global strategy on digital health 2020-2025.
114
Zavaleta-Monestel E. Monge Bogantes L. C. Chavarría-Rodríguez S. Arguedas-Chacón S. Bastos-Soto N. Villalobos-Madriz J. (2025). Artificial intelligence tools that improve medication adherence in patients with chronic noncommunicable diseases: an updated review. Cureus17 (4), e83132. 10.7759/cureus.83132
115
Zoe Moon A. A. Campbell L. Michie S. Ogden D. Bondaronek P. King K. et al (2023). “ESPACOMP-23-OP3.5: interventions to increase adherence to antiretroviral therapy in people living with HIV: a systematic review and meta-analysis,” in 27th annual Meeting of ESPACOMP, the international Society for medication adherence. 2023 (Budapest, Hungary).
116
Zullig L. L. Deschodt M. Liska J. Bosworth H. B. De Geest S. (2019). Moving from the trial to the real world: improving medication adherence using insights of implementation science. Annu. Rev. Pharmacol. Toxicol.59 (1), 423–445. 10.1146/annurev-pharmtox-010818-021348
Summary
Keywords
adherence, interventions, digital, eHealth, mHealth
Citation
Moon Z and Walsh J (2025) Digital interventions in medication adherence: a narrative review of current evidence and challenges. Front. Pharmacol. 16:1632474. doi: 10.3389/fphar.2025.1632474
Received
21 May 2025
Accepted
22 September 2025
Published
10 October 2025
Volume
16 - 2025
Edited by
Ad Kaptein, Leiden University Medical Center (LUMC), Netherlands
Reviewed by
Daisy Volmer, University of Tartu, Estonia
Sofia Georgopoulou, King’s College London, United Kingdom
Updates
Copyright
© 2025 Moon and Walsh.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jane Walsh, jane.walsh@universityofgalway.ie
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.