Abstract
Hydrogels, owing to their outstanding physicochemical properties and excellent biocompatibility, have emerged as a focal point of research and clinical application in andrological diseases. This review systematically summarizes cutting-edge progress in hydrogel applications for Prostate Cancer (PC), Bladder Cancer (BC), Erectile Dysfunction (ED), Male Reproductive Medicine (MRM), and urinary tract tissue engineering. Current studies indicate that hydrogels can serve as protective spacers in PC radiotherapy to mitigate radiation-induced rectal toxicity and as precise drug-delivery vehicles to enhance antitumor efficacy. Moreover, hydrogels demonstrate unique and broad potential in neurovascular repair, immunomodulation, sperm selection, in vitro spermatogenesis modeling, and tissue regeneration. Future advancements in hydrogel technology—through intelligent responsive design, integration of bioactive molecules, incorporation of advanced manufacturing processes, and rigorous translational research—are expected to significantly elevate treatment standards for andrological diseases and improve patient quality of life.
Graphical Abstract
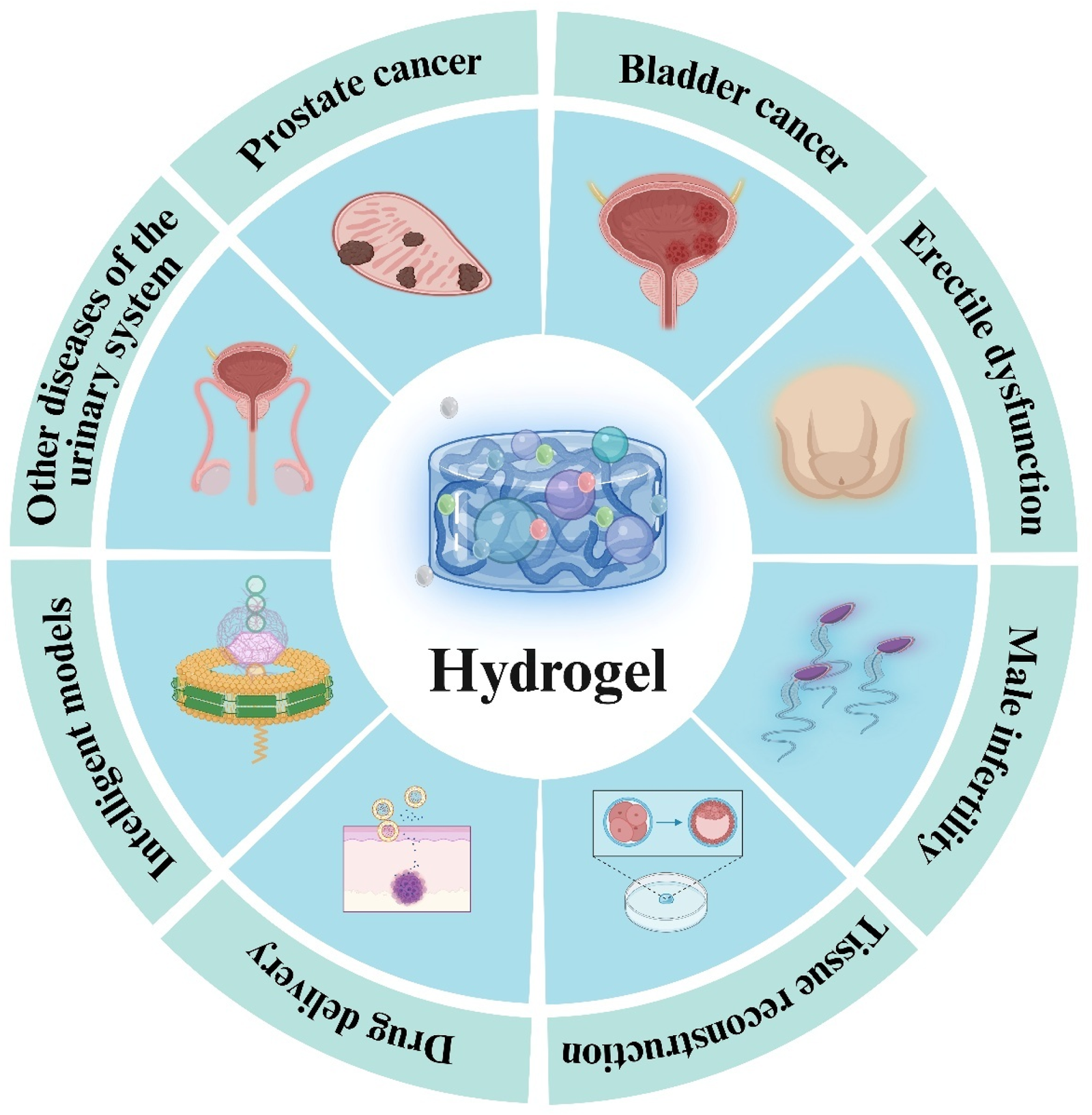
Applications of hydrogel in andrology.
1 Introduction
As global populations age, the incidence and mortality of andrological diseases have continued to increase (Schafer et al., 2025). Although conventional therapies have achieved substantial clinical success, significant challenges persist in managing andrological malignancies, male infertility, Erectile Dysfunction (ED), and urethral reconstruction. For example, in 2022, approximately 1,460,000 new Prostate Cancer (PC) cases were diagnosed worldwide, resulting in over 390,000 deaths; in 2020, Bladder Cancer (BC) accounted for more than 500,000 new cases—both demonstrating year-on-year increases, According to projections, the prevalence of PC is expected to reach roughly 2.4 million confirmed cases and about 712,000 deaths by 2040 (Sung et al., 2021). The steep rise in patient numbers will not only profoundly diminish patients’ quality of life but also impose immense pressure on healthcare systems worldwide (Bray et al., 2024). Despite the widespread adoption of existing treatment paradigms, the non-targeted nature of current drug-delivery systems often leads to suboptimal therapeutic outcomes and increased patient discomfort; precise control over drug release remains a critical unmet need (Londhe et al., 2025; Choi et al., 2024). In urethral tissue engineering, reconstruction is particularly hindered by mechanical deformation stresses, the harsh Urinary Microenvironment (UME), and the absence of a Regenerative Microenvironment (RME), factors that collectively inhibit repair and may result in irreversible urethral scarring (Jin et al., 2025).
Hydrogels, three-dimensional (3D) networks of crosslinked hydrophilic polymers characterized by high water content and excellent biocompatibility, mimic the natural Extracellular Matrix (ECM) (Liang Y. et al., 2022). Due to their ECM-mimetic architecture, hydrogels replicate the hydrated state of biological tissues in vivo, providing an ideal platform for drug delivery, regenerative medicine, and tissue repair, thereby offering new perspectives and potential solutions to the challenges faced by traditional therapies in andrology (Ho et al., 2022; Yang Y. et al., 2022; Pablos et al., 2024). In andrological oncology, hydrogels have been employed as drug-delivery systems to achieve precise locoregional release of chemotherapeutic agents, thereby elevating intratumoral drug concentrations, reducing systemic exposure, minimizing the risk of drug resistance and off-target toxicity, and enhancing antitumor efficacy (Jiang et al., 2024). For male infertility, hydrogels have been utilized as biomaterial carriers for cryopreservation of testicular tissue or cells, preserving viability with minimal damage, and supporting in vitro spermatogenesis modeling, which offers a potential route to fertility restoration (Tan et al., 2024). In urethral tissue engineering, hydrogel-based scaffolds have been shown to stabilize the defect site, provide a supportive cellular environment, and reconstitute the RME (Jin et al., 2025; Fischer-Valuck et al., 2017). Moreover, continued advances in nanotechnology and bioprinting are expected to enable the integration of hydrogels with intelligent and customizable therapeutic platforms (Zhao et al., 2023).
Owing to their unique physicochemical properties and excellent biocompatibility, hydrogels have demonstrated significant potential for the treatment of andrological diseases. As fabrication techniques and biomaterials science advance, hydrogels are anticipated to facilitate innovative therapeutic solutions for andrological disorders. This review systematically summarizes research progress in hydrogel applications in andrology, focusing on their roles in andrological oncology, reproductive health, and tissue engineering, and forecasts future development trends to provide a theoretical framework for related investigations (Mittal et al., 2024a).
2 Advantages of hydrogel characteristics in modern biomedicine
Hydrogels are formed by hydrophilic polymers through specific chemical or physical crosslinking, which enables them to absorb large volumes of water while maintaining the integrity of their 3D network structure, thereby providing a stable, continuous matrix (Tang et al., 2025). To date, hydrogels have been widely applied in biomedical engineering fields such as drug delivery, antimicrobial agents, biosensors, tissue engineering, and wound healing (Pablos et al., 2024; Li J. et al., 2024; Karmakar et al., 2024). Their principal properties include high hydration and hydrophilicity, distinctive microporous architecture, excellent biocompatibility, and tunable physicochemical characteristics (Mittal et al., 2024b).
2.1 High hydration, hydrophilicity, and unique microporous architecture
Hydrogels are distinguished by their high hydration capacity and hydrophilicity (Figure 1). These properties arise from hydrophilic macromolecular networks formed by water-soluble polymers via chemical or physical crosslinking (Patel and Mequanint, 2011). Such networks can absorb up to 1,000-fold their dry weight in water, swelling in aqueous environments without dissolving (Chai et al., 2017). This creates an internal aqueous microenvironment that protects encapsulated cells and sensitive drug molecules, making hydrogels ideal for cell culture and drug encapsulation (Saludas et al., 2017).
FIGURE 1
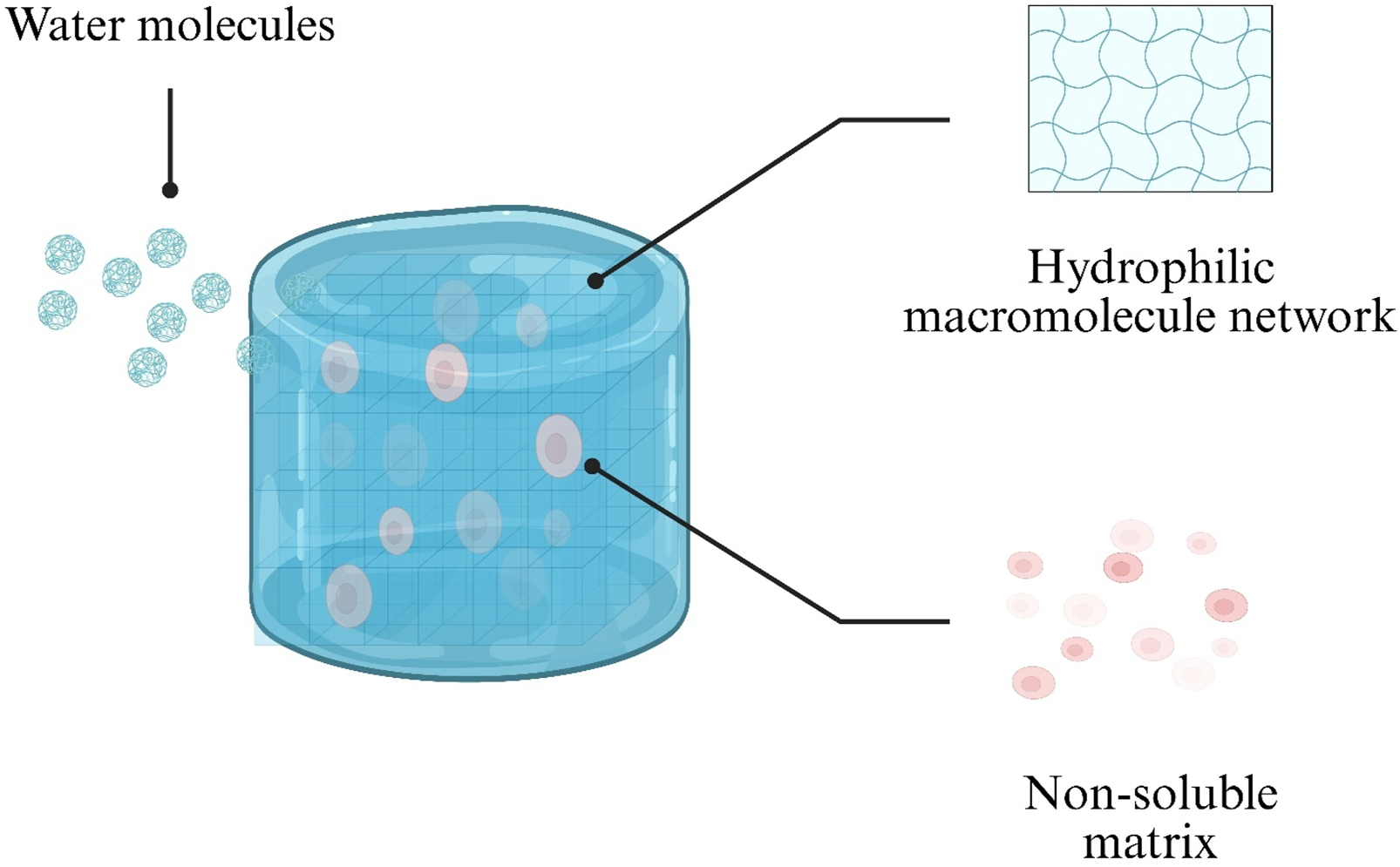
Schematic illustration of water uptake by a hydrogel. Water molecules diffuse into the hydrophilic polymer network (blue), while the non-soluble matrix (pink) maintains structural integrity.
The unique microporous architecture of hydrogels confers high permeability. Pore size and distribution can be precisely controlled through specific fabrication methods to achieve programmable release of loaded agents (Fazli et al., 2024). For example, incorporation of zinc oxide nanoparticles increases hydrogel porosity, water uptake capacity, thermal stability, and mechanical strength, thereby modulating the release kinetics of nettle extract and providing sustained therapeutic effects (Fazli et al., 2024). Consequently, hydrogel systems with these characteristics have been extensively utilized for drug delivery applications in andrology (Pishavar et al., 2021).
2.2 Biocompatibility
Biocompatibility of hydrogels is primarily determined by their constituent materials. Based on material origin, hydrogels are classified as synthetic, natural, or hybrid. Synthetic hydrogels offer precise tunability and robust mechanical properties; examples include acrylamide and its derivatives, Polyvinyl Alcohol (PVA), Polyacrylic Acid (PAA), and polyurethane-based hydrogels (Figure 2A) (Sennakesavan et al., 2020; Xia and Chen, 2022; Mohammad et al., 2024; Luthfianti et al., 2024; Elliott et al., 2004). Natural hydrogels derive from biomolecules and include Gelatin (GEL), agarose, collagen, polypeptides, pullulan, Chitosan (CS), and Hyaluronic Acid (HA) (Figure 2B) (Huang et al., 2020; Su et al., 2021; Yoo et al., 2020; Wollenberg et al., 2018; Pan et al., 2021; Xu et al., 2019; Wang et al., 2022). Hybrid hydrogels integrate two or more polymer types, combining the mechanical strength of synthetic materials with the intrinsic biocompatibility of natural materials to overcome limitations such as insufficient mechanical integrity of natural hydrogels and lack of bioactivity in purely synthetic systems (Xia and Chen, 2022; Saroia et al., 2018).
FIGURE 2
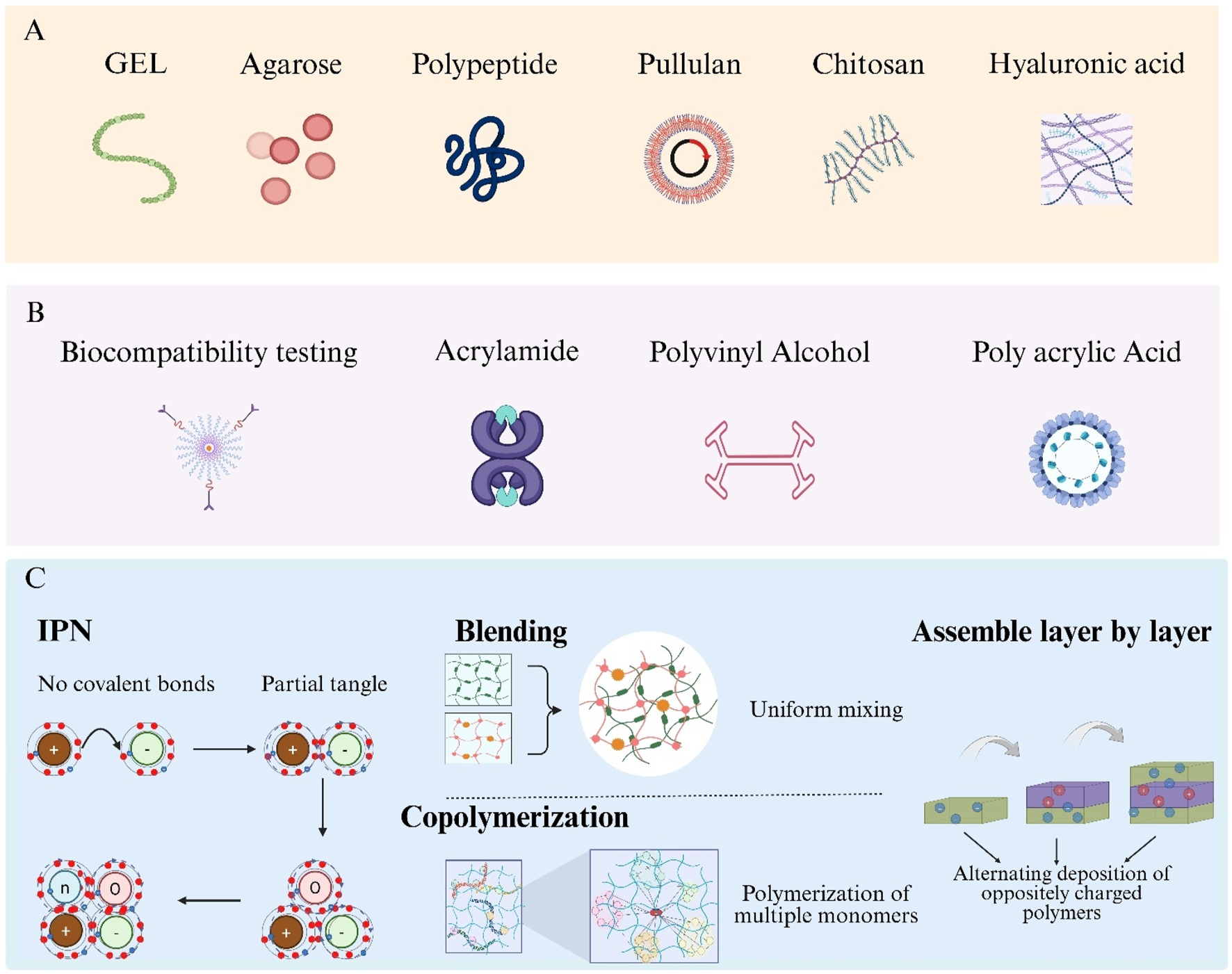
Hydrogel categories and hybrid-fabrication strategies. (A) Natural hydrogel formers: gelatin, agarose, polypeptide, pullulan, chitosan and hyaluronic acid. (B) Typical synthetic precursors: acrylamide, Polyvinyl Alcohol (PVA) and Polyacrylic Acid (PAA). (C) Four routes to build hybrid hydrogels—Interpenetrating Polymer Networks (IPNs), polymer blending, copolymerisation and layer-by-layer assembly.
Formation of hybrid hydrogels is achieved via four primary strategies: Interpenetrating Polymer Networks (IPNs), blending, copolymerization, and layer-by-layer assembly (Figure 2C) (Vasile et al., 2020; Cai et al., 2021; Shymborska et al., 2023; Park et al., 2018). IPNs consist of two or more polymer networks interlaced at the molecular scale without covalent bonds, enhancing mechanical strength and stimuli responsiveness. Blending involves uniform mixing of distinct hydrogel polymers to balance their individual advantages. Copolymerization employs multiple monomers in a single polymerization reaction, allowing precise control over network composition at the molecular level. Layer-by-layer assembly is performed by alternately depositing oppositely charged polymers to build multilayered composite structures.
Because many hydrogel materials are composed of ECM components, they inherently exhibit high biocompatibility. Moreover, by mimicking the native tissue microenvironment during fabrication, both compatibility and structural stability are further enhanced, facilitating applications in tissue engineering and regenerative medicine (Naahidi et al., 2017). In andrology, this biocompatibility has been widely harnessed for drug-delivery systems and tissue-repair scaffolds (Ren Y. et al., 2023; Moon et al., 2023).
2.3 Tunable physicochemical properties
Hydrogels, as highly tunable smart materials, have physicochemical properties that can be precisely adjusted through variations in polymer composition, network architecture, and fabrication techniques, allowing customization for diverse biomedical applications (Wang Y. et al., 2025). Controllable parameters include swelling ratio, porosity, pH-responsiveness, temperature-responsiveness, photoresponsiveness, and electrical and magnetic properties, which confer distinct advantages over conventional materials as Figure 3 (Wu et al., 2024; Maiti et al., 2024; Amirthalingam et al., 2023). These physicochemical traits not only govern the hydrogel’s physical and chemical behavior but also directly influence its functional performance in drug delivery and tissue engineering contexts (De Nitto et al., 2024; Luo et al., 2023).
FIGURE 3
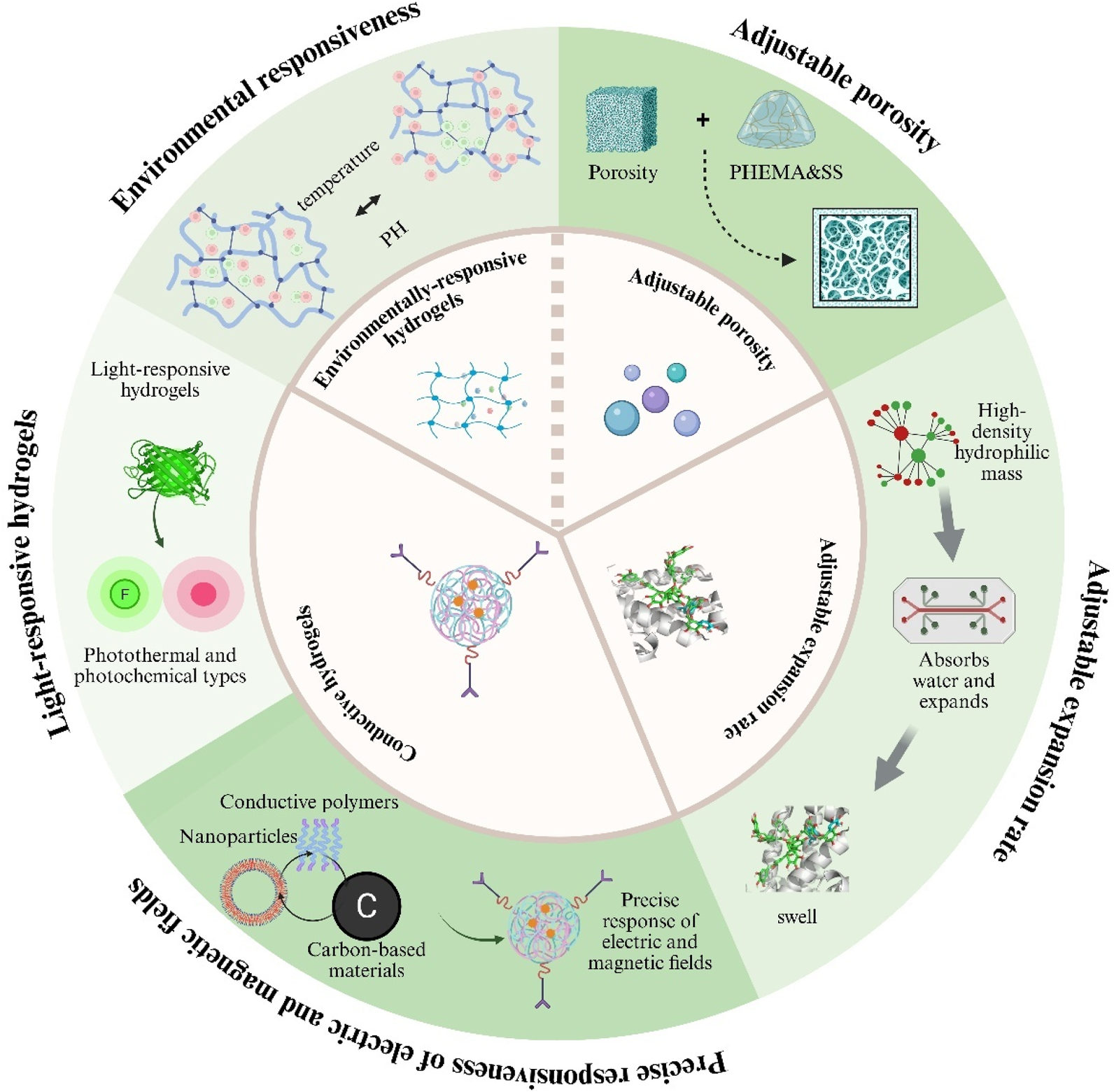
Key tunable properties of hydrogels for biomedical use. Illustrated are five adjustable features: (i) environmental responsiveness to pH or temperature; (ii) controllable porosity; (iii) variable swelling (expansion) rates; (iv) light-responsive behaviour (photothermal or photochemical); and (v) electrical/magnetic responsiveness achieved with conductive fillers.
2.3.1 Swelling ratio
The swelling behaviour of hydrogels is jointly governed by surface pore size, network architecture and overall hydrophilicity/hydrophobicity. A high density of hydrophilic functional groups together with a macroporous structure markedly boosts swelling capacity and drug-loading efficiency, better satisfying the demands of drug delivery in andrology (Feng and Wang, 2023). However, pronounced swelling is often accompanied by macroscopic deformation and diminished mechanical strength. To overcome this, Zhou et al. designed highly cross-linked, hydrophobic hydrogels that exhibit controllable swelling in aqueous environments, showing excellent potential as bio-adhesive sealants and antibacterial agents for suture-free wound-closure systems (Zhou et al., 2019).
2.3.2 Porosity
Hydrogels offer finely tunable porosity: their characteristic porous networks emulate native tissue and, under dynamic loading, deliver high mass-transport efficiency alongside excellent mechanical performance (Foudazi et al., 2023). Such architecture also accelerates in-vivo cell adhesion and proliferation, giving hydrogels a natural advantage in andrological applications—for example, corpus cavernosum repair for Erectile Dysfunction (ED) (Martin et al., 2021). However, excessive porosity dramatically lowers the material’s elastic modulus, resulting in greater deformation and a noticeable loss of load-bearing capacity (Argun et al., 2014). To mitigate this, researchers have shown that increasing the silk-fibroin content within a poly(2-hydroxyethyl methacrylate) matrix effectively reduces pore size, thereby optimising the hydrogel’s overall properties (Tuancharoensri et al., 2023).
2.3.3 Stimuli-responsive properties and functional applications
Hydrogels can be chemically modified or supplemented with additives to endow them with reversible responses to external stimuli (Koetting et al., 2015; Liao et al., 2025). pH-responsive hydrogels, which contain acidic or basic groups, ionise and swell as environmental pH changes and are widely used for targeted drug release (Xiong et al., 2024). Thermoresponsive hydrogels take advantage of the fact that tumour sites are warmer than normal tissue, finding applications in cancer therapy and wound healing (Bordat et al., 2019). Photo-responsive hydrogels—classified as photothermal or photochemical—offer high precision, non-invasiveness and reversibility, and are primarily used in disease modelling and smart wearable devices (Homma et al., 2021).
Conductive hydrogels achieve precise responsiveness to electric and magnetic fields by incorporating conductive fillers such as metal nanoparticles, conductive polymers or carbon-based materials (Lo et al., 2021). These composites couple the intrinsic softness of hydrogels with the electron-transport capability of conductive materials. Although their conductivity is lower than that of conventional metal conductors, their unique “aqueous soft-material” architecture imparts excellent biocompatibility and tunable mechanics, giving them distinctive advantages and broad prospects in cutting-edge areas such as smart drug-delivery systems (Chen et al., 2023).
2.4 Performance differences among hydrogel materials
Natural hydrogels, distinguished by their unique architecture and high water content, are widely employed in andrological disease management (drug delivery) and male infertility therapy (tissue preservation, regeneration and repair) because of their excellent controlled-release performance, biocompatibility and tissue non-toxicity (Tan et al., 2024; Ahmed, 2015). Their clinical utility, however, is often constrained by weak mechanical strength and limited diffusion rates (Calvert, 2009). Synthetic hydrogels, produced through tailored fabrication techniques, can offset these shortcomings. Studies have demonstrated that double-network hydrogels—with two interpenetrating, highly cross-linked networks—exhibit orders-of-magnitude increases in strength, stiffness and toughness, thereby markedly improving the mechanical limitations of natural hydrogels (Zhang and Khademhosseini, 2017). In addition, topological design strategies that interlock polymer chains with figure-eight cross-linkers yield highly elastic hydrogels; the system developed by Wenz et al. achieved notably higher swelling capacity and fracture strain than conventional types, with a swelling ratio of up to forty-fold (Kali et al., 2016). Such high-strength synthetic hydrogels show great promise for repairing tissues exposed to harsh physiological environments, such as the urethra and bladder (Xiao et al., 2021). Nevertheless, despite advances in mechanical tuning, synthetic hydrogels often exhibit poor fatigue resistance, as irreversible breakage of intra- or intermolecular covalent bonds can occur within the network (Zhang and Khademhosseini, 2017). Given the respective limitations of natural and synthetic hydrogels, hybrid hydrogels—created by integrating multiple materials through sophisticated fabrication approaches—have emerged to provide better drug-release profiles and bioavailability (Omidian et al., 2025). This strategy introduces design complexity: different material combinations influence interfacial affinity, solubility, stability and release kinetics, thereby modulating drug-release behaviour. If processing conditions are not carefully controlled, the sustained-release potential may be diminished (Table 1) (Cirri et al., 2022).
TABLE 1
| Category | Advantages | Shortcomings | Preferred andrological applications |
|---|---|---|---|
| Natural hydrogels | Excellent biocompatibility and drug-delivery capability | Low mechanical strength | Drug delivery male infertility (Tan et al., 2024; Ahmed, 2015) |
| Synthetic hydrogels | High mechanical strength | Poor fatigue resistance | Bladder and urethral reconstruction (Xiao et al., 2021) |
| Hybrid hydrogels | Improved drug release and bioavailability | Difficult-to-control release behaviour and complex fabrication | Drug delivery (Tao et al., 2019) male infertility (Rahbar et al., 2024) ED (Wang et al., 2024) andrological-disease diagnostics (Kim et al., 2021) |
Advantages and disadvantages of hydrogel materials and their preferential applications in andrological diseases.
3 Therapeutic applications of hydrogels in andrological diseases
3.1 Applications of hydrogels in prostate cancer
PC treatment strategies aim for curative outcomes, a key challenge is the reduction of therapy-related toxicities. Owing to their outstanding biocompatibility, injectability for in-situ formation and tunable physicochemical responsiveness, hydrogels show unique promise in PC radiotherapy for lowering rectal toxicity, minimising side effects and enabling more precise irradiation.
3.1.1 Hydrogel spacers in prostate radiotherapy
Radical prostatectomy remains the primary treatment modality for PC, yet postoperative microcirculatory changes and alterations in the immune microenvironment due to residual tumor tissue can lead to local recurrence and metastasis (Fan et al., 2022). Adjuvant External-Beam Radiotherapy (EBRT), Stereotactic Body Radiotherapy (SBRT), and focal laser ablation are employed to prevent recurrence (Zhong et al., 2021; Fujihara and Ukimura, 2022). Clinical trials have demonstrated that dose-escalated EBRT prolongs survival in PC patients (Klein and Silverman, 2008). However, because the prostate lies immediately anterior to the rectum, radiation-induced rectal toxicity is a common adverse effect (Karsh et al., 2018). Although Image-Guided Radiotherapy (IGRT) with daily imaging can mitigate these toxicities, higher radiation doses continue to pose risks (Wang SL. et al., 2023; Dearnaley et al., 2014; Gharbieh et al., 2025). Placement of a hydrogel spacer into the perirectal fat plane to increase the distance between the prostate and rectum has been developed as a strategy to reduce rectal exposure (Icht et al., 2024). When inserted, the spacer physically displaces the rectum away from the prostate, thereby lowering the rectal radiation dose and reducing toxicity risk (Ohira et al., 2024).
SpaceOAR, an injectable Polyethylene Glycol (PEG) hydrogel, creates this separation and permits SBRT delivery within 24 h of injection, shortening the overall treatment timeline (Saito et al., 2022). In a prospective study of 70 patients treated with SpaceOAR and dose escalation up to 82 Gy, rates of grade 3 gastrointestinal and genitourinary adverse events were 0% and 2.9%, respectively—significantly lower than in prior dose-escalation trials—indicating that hydrogel spacers facilitate safe dose escalation while minimizing rectal toxicity (See et al., 2022). Additional studies have reported superior Planning Target Volume (PTV) coverage in hydrogel-spacer cohorts compared with controls, confirming that spacers allow for higher EBRT doses without compromising rectal sparing (Takayesu et al., 2023; Morita et al., 2024; Kawaguchi et al., 2024). In focal laser ablation, thermal insults to surrounding tissues remain a concern (Alzahrani and Abbas, 2021). Hydrogel spacers, owing to their low thermal conductivity, act as insulating barriers; they have been shown to limit rectal temperatures from 101.1°C to below 42°C during laser ablation, thereby protecting the rectal wall (Namakshenas and Mojra, 2021).
3.1.2 Impact on rectal toxicity and optimization strategies
Hydrogel spacer–induced separation between the prostate and rectum has been shown to correlate inversely with rectal radiation dose and subsequent toxicity. A minimum prostate–rectum distance of 7.5 mm has been identified as the threshold for SpaceOAR efficacy, with greater separation distances yielding reduced rectal exposure (Narukawa et al., 2023). Spacer symmetry has also been demonstrated to positively correlate with both the prostate–PTV distance and the magnitude of rectal dose reduction, thereby enhancing spacer performance (Ohira et al., 2024). however, even asymmetrical spacer distributions significantly decrease rectal dose toxicity (Fischer-Valuck et al., 2017). Spacer thickness varies by anatomical level. One study reported an average hydrogel thickness of 7.1 mm at the prostate apex—significantly less than the 9.4 mm observed at the mid-gland level—resulting in lower mid-gland rectal toxicity (Song et al., 2013). In contrast, Fukumitsu et al. observed greater thickness at the apex with corresponding reductions in rectal radiation exposure (Fukumitsu et al., 2022). These discrepancies are likely attributable to technical variations in injection technique, as the liquid PEG hydrogel distribution is operator-dependent.
In addition, optimization of hydrogel spacer implantation is critical for minimizing rectal toxicity. A retrospective analysis proposed a pacer Quality Score (SQS) system to evaluate hydrogel placement efficacy. Computed Tomography (CT) scans of patients undergoing prostate SBRT demonstrated significant differences in SQS between the anterior and posterior perirectal spaces, with higher total SQS in the anterior gap at the midgland and apex levels; moreover, SQS correlated inversely with rectal dose metrics (Giacometti et al., 2024). A subsequent study employing SQS measurements at the rectal midline and 1 cm lateral to midline confirmed a significant inverse relationship between SQS and late rectal toxicity (Grossman et al., 2023). By applying SQS intraoperatively, implantation quality can be assessed in real time and spacer position adjusted to further reduce rectal exposure. Optimal spacer positioning has been achieved by injecting into the caudal and lateral aspects of the prostate, which further decreases rectal dose (Fukumitsu et al., 2022), and by targeting the midgland plane along the midline (Hwang et al., 2018). Injection technique also influences outcomes: compared with the conventional fixed-needle approach, an apex-directed expansion method yielded greater rectal sparing (Kobayashi et al., 2023). A novel continuous-needle advancement technique—from midgland to apex levels during injection—resulted in significantly increased apex–rectum separation without elevating adverse event rates (Narukawa et al., 2022). Because hydrogel implantation is performed via a perineal approach with limited needle visualization, a disposable, probe-mounted puncture frame has been developed to secure the hydrogel delivery apparatus and enhance real-time visualization of spacer placement around the rectum (Meyer et al., 2021). These advances underscore the importance of refining implantation methods to mitigate radiotherapy-induced rectal toxicity.
3.1.3 Long-term safety and potential adverse effects of hydrogel spacers in PC radiotherapy
The toxicity-reducing benefit of hydrogel spacers during prostate-cancer radiotherapy is well established, but their long-term in-body effects remain uncertain—an issue that may influence some patients’ willingness to choose this adjunctive approach. Extended clinical follow-up is therefore needed to clarify their ultimate value. A 5-year quality-of-life review of PC patients who received hydrogel spacers showed not only durable therapeutic performance but also measurable improvements in overall wellbeing, suggesting a favourable cost–benefit profile (Pinkawa et al., 2017). Nevertheless, hydrogel spacers can still elicit rare adverse events. Hoe et al. reported a case of abscess formation potentially attributable to spacer implantation, and other studies have documented grade-3 complications such as rectal ulceration or fistula (Hoe et al., 2019). Continuous long-term monitoring is thus essential to fully assess the safety profile of hydrogel spacers in clinical practice.
3.1.4 Precision radiotherapy and additional effects
Hydrogel technology offers multifaceted innovations in PC treatment, with its implantable nature enabling unique roles in precision radiotherapy. Under standard departmental practice, patients without contraindications undergo transperineal placement of three Gold Fiducial Markers (GFMs) under local anesthesia to optimize IGRT. However, repeated invasive procedures increase both patient discomfort and economic burden. It has been demonstrated that hydrogels closely mimic the positional stability of intraprostatic GFMs and can serve as real-time anatomical surrogates for IGRT, thereby obviating multiple marker-placement procedures and reducing costs (Icht et al., 2024). To further enhance radiotherapy precision and rectal sparing, an improved polyethylene glycol hydrogel spacer—SpaceOAR Vue™—has been developed. This radiopaque spacer is compatible with Magnetic Resonance Imaging (MRI), thus improving localization accuracy in IGRT while reducing rectal toxicity (Gross et al., 2021).
In moderate hypofractionation protocols, standard hydrogels protect the rectum from radiation injury but degrade slowly over approximately 3 months, with complete absorption possibly extending to 6 months; this prolonged presence may elevate the risk of rare late-onset adverse events (Hamstra et al., 2017). A novel hydrogel formulation has been reported that undergoes rapid degradation following radiotherapy while maintaining excellent imaging conspicuity and biocompatibility throughout treatment (Yu et al., 2023).
Radiation-induced injury to the periprostatic Neurovascular Bundles (NVB) during SBRT is implicated in ED in about 50% of men (Kundu et al., 2004). Hydrogel spacers have been shown to mitigate NVB displacement toward high-dose regions, effectively reducing NVB radiation exposure (Hwang et al., 2021). Although hydrogel spacers lower radiation-induced rectal toxicity, their impact on post-radiotherapy ED remains controversial, potentially reflecting individual variability and limited clinical data (Seymour et al., 2023; Nakai et al., 2024). Further studies are warranted to substantiate these findings.
3.2 Applications of hydrogels in bladder cancer immunomodulatory combination therapies
BC is one of the most common malignancies worldwide and presents significant challenges due to high recurrence rates and substantial burdens on healthcare systems (Lopez-Beltran et al., 2024). BC is classified into non–muscle-invasive BC and muscle-invasive BC. Standard treatments—transurethral resection of bladder tumor and radical cystectomy—are often accompanied by complications such as urinary incontinence and bladder dysfunction, which markedly impair patient quality of life (Alfred et al., 2024). Recently, immunotherapy has emerged as a promising approach for BC treatment. Immune Checkpoint Inhibitors (ICIs), such as programmed cell death protein-1 (PD-1)/programmed death-ligand 1 (PD-L1) antagonists, provide bladder-preserving options by restoring tumor-specific T-cell responses through PD-1/PD-L1 blockade and have demonstrated significant antitumor efficacy (Liu et al., 2024; Rhea et al., 2021). However, ICI monotherapy is limited by high dosage requirements, development of immune tolerance, and immune-related toxicities (Liu et al., 2024). To address these challenges, a Shikonin–Ferric Ion (Fe3+) Nanocomposite Hydrogel System (SHFe) was developed to induce ferroptosis-mediated immunogenic cell death. Shikonin encapsulation within SHFe stabilizes the molecule, enhances bioavailability, and enables sustained locoregional delivery. When SHFe is formulated with Tragacanth Gum (SHFe@TG), intravesical drug retention is extended to 72 h. In combination with a PD-1 inhibitor, SHFe@TG synergistically inhibits the Phosphoinositide 3-Kinase–Protein Kinase B (PI3K–AKT) signaling pathway, thereby suppressing BC cell proliferation (Zhao et al., 2025). Furthermore, a Recombinant Oncolytic Adenovirus–Loaded Hydrogel (Adv-CRB3@gel) was engineered to co-deliver an edited CRB3 gene and Granulocyte–Macrophage Colony-Stimulating Factor (GM-CSF). This system not only inhibits BC tumor cells and recruits T cells to remodel the tumor immune microenvironment and enhance tumor antigen–specific immune responses, but it also synergizes with PD-L1 blockade to directly kill BC cells, resulting in marked suppression of tumor proliferation, migration, and invasion (Figure 4) (Zhang WQ. et al., 2024).
FIGURE 4
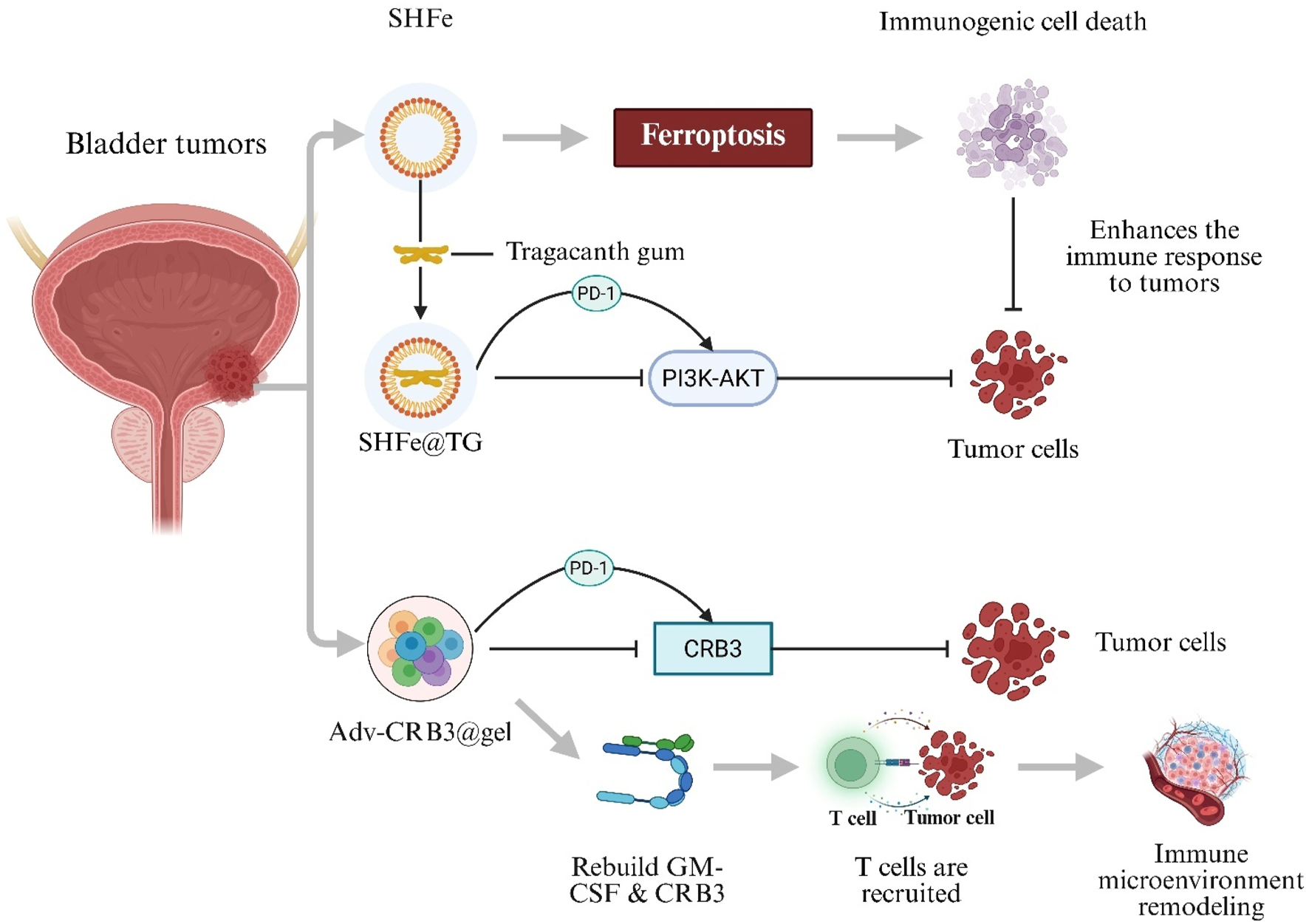
Hydrogel-mediated combination immunotherapy for bladder cancer. Shikonin–Ferric Ion Hydrogel in Tragacanth Gum (SHFe@TG) triggers ferroptosis-induced immunogenic cell death and enhances programmed cell-death protein-1 (PD-1) blockade by inhibiting the PI3K–AKT pathway. Recombinant Oncolytic Adenovirus Hydrogel (Adv-CRB3@gel) Co-Delivers Crumbs-3 (CRB3) and Granulocyte–Macrophage Colony-Stimulating Factor (GM-CSF), recruits T cells and remodels the tumour immune micro-environment.
3.3 Applications of hydrogels in neurovascular repair for erectile dysfunction
ED affects over 150,000,000 men worldwide (Wang CM. et al., 2023). Current ED therapies include oral pharmacotherapy, shockwave therapy, hormone replacement therapy, vacuum erection devices, intracavernosal injections, vascular surgery, and penile prosthesis implantation (Ren Y. et al., 2023). However, these approaches are limited by low bioavailability, systemic adverse effects, poor patient compliance, invasiveness, and narrow applicability (Salonia et al., 2021; Hatzimouratidis et al., 2016; Atipairin et al., 2020). In contrast, hydrogels offer multiple advantages for ED treatment.
Neurogenic ED results from impaired neural signaling to the corpora cavernosa (Yang et al., 2024). Although phosphodiesterase type 5 inhibitors are first-line agents for ED, they are ineffective in neuronally induced ED (Salonia et al., 2021). Hydrogels have been investigated as biomaterial carriers to promote nerve regeneration and repair nervous system injury associated with cavernosal neuropathy (Bellotti et al., 2021; Nih et al., 2016). A Sonic Hedgehog (SHH)-loaded peptide amphiphile nanofiber hydrogel (SHH-PA) delivers SHH protein and, via a caspase-9-dependent mechanism, inhibits intrinsic caspase-9 and extrinsic caspase-8 signaling, reducing smooth muscle apoptosis and collagen deposition to improve erectile function (Figure 5A) (Martin et al., 2021). Similarly, a sprayable conductive adhesive hydrogel (GACM) with high adhesion and anti-swelling properties conforms to injured nerves to form an electrical bridge, facilitating nerve regeneration and suppressing macrophage-mediated inflammation to restore cavernosal innervation and erectile function (Figure 5B) (Wang et al., 2024).
FIGURE 5
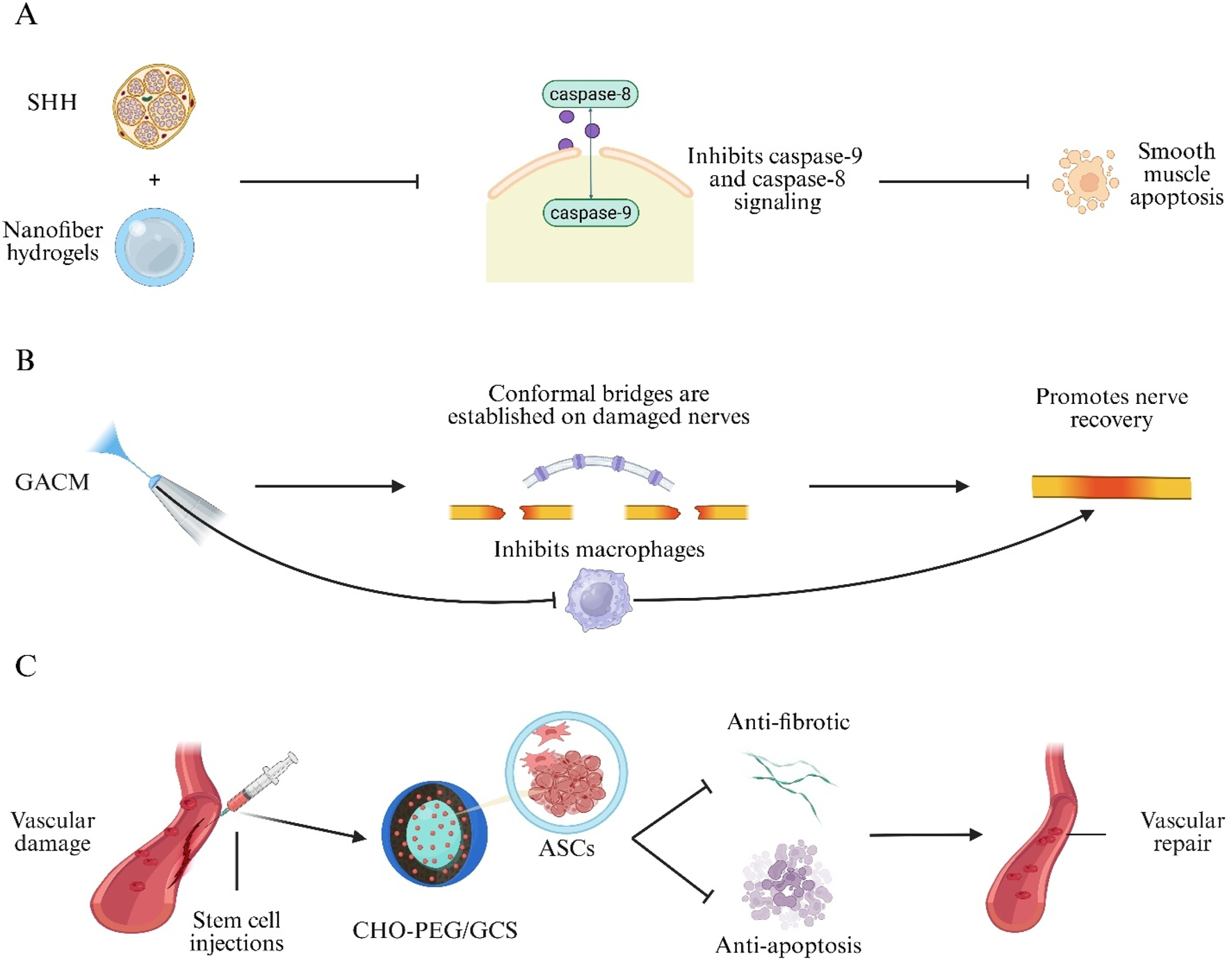
Hydrogel approaches for neurovascular repair in Erectile Dysfunction (ED). (A) Sonic-hedgehog peptide-amphiphile nanofibre hydrogel (SHH-PA) blocks caspase-9/-8 signalling, reducing smooth-muscle apoptosis. (B) Sprayable Gelatin–Alginate Conductive Matrix (GACM) forms a conformal bridge on damaged cavernous nerves, limits macrophage activity and promotes regeneration. (C) Self-healing CHO-terminated poly(ethylene glycol)/glycol-chitosan (CHO-PEG/GCS) hydrogel delivers Adipose-Derived Stem Cells (ASCs) to injured vessels, providing anti-fibrotic and anti-apoptotic cues for vascular repair.
Erectile Dysfunction in Type 1 Diabetes Mellitus (T1D-ED) is a common complication of diabetes mellitus (Wessells et al., 2018). Stem cell therapies have been explored for T1D-ED but are hindered by hemodynamic washout of cells into cavernosal venules, especially in the flaccid state (Aziz et al., 2010; Huang et al., 2010). To address this, a self-healing hydrogel composed of benzaldehyde-terminated PEG and Glycol Chitosan (GCS) (CHO-PEG/GCS) was engineered to encapsulate Adipose-Derived Stem Cells (ASCs), prolong cellular retention, and synergize angiogenic, antifibrotic, and antiapoptotic effects to restore erectile function in diabetic ED models (Figure 5C) (Yan et al., 2020). These studies demonstrate the broad potential of hydrogels for neurovascular repair in ED.
3.4 Applications of hydrogels in male reproductive medicine
In recent years, hydrogel technologies have demonstrated significant potential in male infertility treatment and reproductive tissue engineering due to their biomimetic properties and tunable physicochemical characteristics, particularly in sperm selection and release and in testicular tissue transplantation and regeneration. Male-factor infertility accounts for 30%–50% of infertility cases and has increased globally (Eisenberg et al., 2023). Assisted reproductive technologies, including Intracytoplasmic Sperm Injection (ICSI), have markedly improved pregnancy rates (Esteves et al., 2018). However, ICSI bypasses the natural sperm selection mechanisms of the female reproductive tract and the oocyte’s zona pellucida barrier (Sánchez-Calabuig et al., 2014), Defective sperm can lower fertilisation rates, impair embryo quality and markedly raise the risk of miscarriage, thereby greatly increasing the likelihood of ART failure and potentially posing unknown long-term health risks to offspring (Li et al., 2023). Consequently, selecting high-quality sperm has become a pivotal challenge.
HA, a natural polymer synthesized by various cell types in reproductive and nonreproductive tissues, has been shown to selectively bind sperm with high motility and intact acrosomes, thereby improving ICSI outcomes (Casillas et al., 2018; Canepa et al., 2019). A semi-interpenetrating polymer network hydrogel composed of poly(N-isopropylacrylamide-co-hydroxyethyl methacrylate) (PNIPAM-HMA) and HA was developed to achieve charge-mediated sperm capture. In bovine models, this hydrogel achieved 50% specific attachment of bull spermatozoa. Following hyaluronidase treatment, 47% of bound sperm were released; these released sperm exhibited 70% progressive motility and maintained high plasma-membrane integrity (Figure 6) (Blois et al., 2021).
FIGURE 6
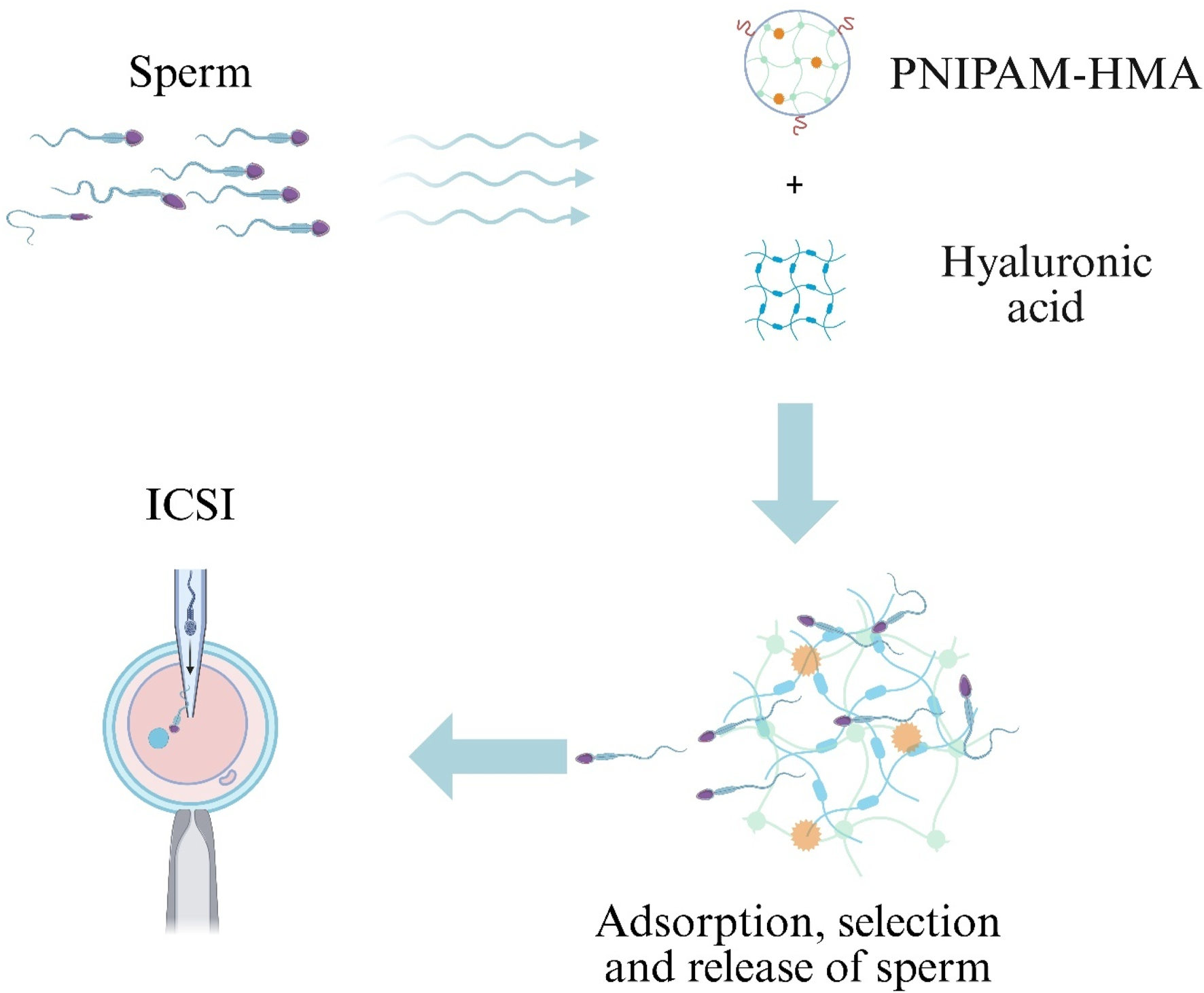
Hydrogel-assisted sperm selection for Intracytoplasmic Sperm Injection (ICSI).
Testicular tissue autotransplantation and in vitro maturation techniques have garnered significant attention, particularly for prepubertal pediatric oncology patients who do not produce sperm and whose future fertility may be irreversibly compromised by chemotherapy and radiotherapy (Wyns et al., 2011; Wyns et al., 2015). However, limitations in current cryopreservation protocols predispose grafts to ischemic injury during revascularization, substantially reducing spermatogonial survival and viability posttransplantation (Poels et al., 2013; Van Saen et al., 2013). The spatiotemporal delivery of Vascular Endothelial Growth Factor (VEGF) is a critical determinant of graft outcome (Cao et al., 2005). In murine models, alginate hydrogels loaded with testicular tissue and VEGF were histologically analyzed posttransplantation; VEGF-laden grafts exhibited significantly increased microvascular density and reduced undifferentiated spermatogonial counts, demonstrating that alginate hydrogels effectively preserve spermatogonia and enhance posttransplant recovery of testicular architecture (Poels et al., 2016).
Spermatogenesis—the proliferation and differentiation of Spermatogonial Stem Cells (SSCs) within seminiferous tubules—requires efficient culture systems for fertility preservation and assisted reproductive technology applications (Stukenborg et al., 2008; Zhang J-Y. et al., 2024). Conventional SSC culture relies on feeder-layer cells, resulting in inefficiencies and contamination risks (Yang et al., 2020). Decellularized Testicular Matrix (DTM) hydrogels have emerged as promising scaffolds for generating testicular organoids (Saldin et al., 2017). A three-dimensional (3D) culture system utilizing DTM hydrogel was developed and seeded with murine SSCs. Compared with feeder-layer methods, SSC proliferation rates were markedly increased, and gene-expression profiles matched those of native SSCs, offering a feeder-free approach to more efficient in vitro SSC expansion and differentiation (Yang et al., 2020). Furthermore, the incorporation of epididymal cells and glial cell line–derived neurotrophic factor into the DTM hydrogel system was shown to enhance postthaw SSC proliferation. This composite system significantly reduced caspase-3 expression and upregulated miR-21, resulting in increased spermatogenic cell numbers, indicative of antiapoptotic effects and enhanced SSC regenerative capacity (Rahbar et al., 2024).
A semi-interpenetrating poly(N-isopropylacrylamide-co-hydroxyethyl methacrylate)/hyaluronic-acid (PNIPAM-HMA/HA) network captures motile, acrosome-intact sperm via charge interactions; brief hyaluronidase treatment then releases the enriched sperm population for ICSI.
3.5 Applications of hydrogels in urinary tract reconstruction
Hydrogels formulated with biomaterials such as alginate, Silk Fibroin (SF), and Bladder Acellular Matrix (BAM) exhibit exceptional biocompatibility, tunable physicochemical characteristics, and cell‐adhesive and–differentiative capacities, conferring high stability even in hostile environments. Consequently, they hold significant promise for bladder and urethral repair and reconstruction.
3.5.1 Applications in bladder tissue reconstruction
The incidence of bladder disorders—including BC, bladder contracture, congenital malformations, and trauma—has increased, leading to an increased need for surgical bladder repair and reconstruction (Shi et al., 2017). Current reconstruction methods are limited by risks of infection, metabolic disturbances, and secondary malignancies (Lam Van Ba et al., 2015). Effective bladder reconstruction requires scaffolds with adequate mechanical strength and a biomimetic microenvironment (Xiao et al., 2017). Hydrogels have been adopted as key materials in bladder tissue engineering due to their favorable properties.
BAM–based scaffolds combine robust mechanical performance with endogenous growth factors such as Vascular Endothelial Growth Factor (VEGF) and Patelet-Derived Growth Factor-BB (PDGF-BB). SF enhances mechanical integrity and provides a biomimetic surface for cell adhesion and differentiation (Seth et al., 2013; Jiang et al., 2017). In one study, an SF solution was processed into a bilayer silk film–sponge scaffold and combined with pepsin-digested BAM to form a hydrogel composite. This system exhibited excellent retention and stability of VEGF and PDGF-BB and promoted smooth muscle regeneration and angiogenesis, highlighting its potential for bladder reconstruction (Xiao et al., 2021).
Although natural hydrogels offer favorable biological properties, their mechanical strength may be insufficient for bladder tissue regeneration. Synthetic hydrogels can be modified to enhance mechanical properties but often lack protein- and cell-adhesive sites (Fu et al., 2023). To address this, a composite hydrogel composed of acrylic ester polymers, extracellular matrix components, and bladder smooth muscle cells was developed. This hydrogel significantly improved mechanical strength and elastic modulus while maintaining strong capacity for bladder tissue repair and regeneration (Sivaraman et al., 2015).
3.5.2 Applications in urethral reconstruction
Severe urethral strictures commonly arise following major trauma, inflammation, malignancy, or congenital anomalies (Xu et al., 2009). Strictures can lead to ED, painful erections, urethral fistulae, and stone formation (Chapple et al., 2014). However, limited availability of autologous graft tissue, the harsh UME, and deficient scarless‐repair mechanisms often result in repair failure (Mangir et al., 2019). In tissue‐engineering approaches, ECM–based scaffolds such as BAM and SF have shown promise. In a New Zealand rabbit model of urethral defect, a bilayer hydrogel scaffold composed of porous BAM hydrogel and dense SF was implanted. The scaffold markedly increased neovascularization and maintained a patent urethral caliber. Histological analysis revealed formation of a continuous, multilayered urothelial lining and aligned smooth‐muscle regeneration, demonstrating support for both angiogenesis and tissue regeneration (Cao et al., 2019).
To overcome the UME barrier—which impedes materials, promotes fibrosis, and fails to direct progenitor differentiation—an UME‐responsive composite hydrogel (SA-nHA-SiQD) was fabricated using layer-by-layer three-dimensional bioprinting. This construct combines a sodium alginate backbone with Silicon Quantum Dots (SiQDs) to enable structural remodeling under UME conditions, inhibit Transforming Growth Factor-β/smad (TGF-β/Smad) signaling, and activate Matrix Metalloproteinase-1 (MMP-1) and Collagen Type III Alpha One Chain (COL3A1). These effects promote epithelial proliferation and differentiation, modulate collagen deposition, reduce fibrosis, and generate Reactive Oxygen Species (ROS) to enhance scaffold strength—thereby enabling scarless, “memory” repair of the urethra (Mao et al., 2020; Yang M. et al., 2022). Additionally, electrospun Polyurethane-Urea (PUU) fiber membranes coated with collagen (cPUU) were developed as stretchable hydrogel scaffolds. These cPUU scaffolds exhibited a tensile elongation at break of 404% ± 40% and supported adhesion and proliferation of bladder smooth‐muscle cells in vitro. In vivo evaluation demonstrated that cPUU hydrogels significantly promoted re‐epithelialization of urethral defects and facilitated effective urethral repair (Wang et al., 2019).
3.6 Applications of hydrogels in male urogenital tract infections
Chronic inflammation of the bladder and prostate remains inadequately managed by current therapies. Novel hydrogel systems, formulated via diverse crosslinking strategies, offer sustained drug release, enhanced inflammatory repair, and improved barrier penetration, providing efficient treatment avenues for chronic urogenital inflammation.
Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS) is characterized by pelvic pain, lower urinary tract symptoms, and bladder hypersensitivity (Payne et al., 2007). Intravesical injection of OnabotulinumtoxinA (BTX-A) is an established therapy; however, the injection procedure can be extremely painful for IC/BPS patients and is associated with bleeding, infection, and urinary retention (Rappaport et al., 2018). Intravesical instillation has emerged as a less invasive alternative but is hampered by rapid drug washout and potential instillation-related adverse events (Krhut and Zvara, 2011). A temperature-responsive TC-3 hydrogel system (TC-3) was developed to deliver BTX-A via bladder instillation. Following treatment, patients exhibited significant reductions in pain visual analog scale scores, inflammatory index, problem index, and nocturia frequency at week 6. These findings indicate that the TC-3 hydrogel enables continuous intravesical BTX-A release while mitigating instillation-related side effects (Rappaport et al., 2018).
Hemorrhagic Cystitis (HC) is a debilitating inflammatory condition of the bladder characterized by hemorrhage and ulceration, often accompanied by severe pain, hematuria, and dysuria, which profoundly impair patient quality of life (D'Amico et al., 2022). HC can be classified into subtypes, with chemical HC typically induced by chemotherapeutic agents such as cyclophosphamide (Matz and Hsieh, 2017). Current treatments—including pharmacotherapy and surgical intervention—are limited by systemic side effects and risks of infection, anesthesia‐related complications, and prolonged recovery, respectively (Payne et al., 2013). A major challenge is maintaining robust adhesion and mechanical integrity of intravesical therapies in the bladder’s dynamic fluid environment under cyclic mechanical stresses. To address these obstacles, a Silk Fibroin Methacryloyl (SFMA)/tea polyphenol composite hydrogel was engineered via photopolymerization. This hydrogel exhibits strong mucoadhesive strength and excellent compressive resilience, enabling it to withstand cyclic bladder distension. In a cyclophosphamide‐induced rat HC model, SFMA/tea polyphenol hydrogels significantly reduced hematuria, suppressed interleukin-1β (IL-1β) expression, and upregulated CD31, CD63, and α-smooth muscle actin (α-SMA), thereby enhancing neovascularization and smooth muscle regeneration and accelerating tissue repair (Chen et al., 2024). Similarly, a Chitosan Methacryloyl (CHMA)/SFMA hydrogel system—produced via photoinitiated crosslinking and methacrylation—demonstrated high adhesive strength and rapid hemostasis on injured porcine bladder tissue. In vitro, the CHMA/SFMA hydrogel promoted microaggregate formation of ureteral epithelial cells and modulated macrophage polarization, facilitating inflammation resolution, accelerating angiogenesis and myogenesis, and improving HC-associated bladder damage (Yao et al., 2024).
Chronic Prostatitis (CP) is a syndrome characterized by pelvic pain and lower urinary tract symptoms (Rees et al., 2015). More than 90% of CP cases are classified as Chronic Nonbacterial Prostatitis (CNP) (Cyril et al., 2022). Current treatments for CNP include antibiotics, alpha-adrenergic blockers, and nonsteroidal anti-inflammatory drugs (Franco et al., 2019). Emodin (EMO), a natural anthraquinone derivative, exhibits anti-inflammatory, antioxidant, and antifibrotic properties; however, its therapeutic efficacy is limited by poor permeability across the Blood–Prostate Barrier (BPB) (Xia et al., 2021). To overcome this barrier, a triple-targeted, thermosensitive rectal hydrogel nanodelivery system was engineered by dialysis and electrostatic adsorption to encapsulate EMO. This system targets lactoferrin receptors on intestinal epithelial NCM460 cells and prostate epithelial RWPE-1 cells, as well as CD44 receptors on macrophages, thereby significantly enhancing cellular uptake and inhibiting Toll-Like Receptor 4/Nuclear Factor-κB (TLR4/NF-κB) signaling. Furthermore, the hydrogel penetrates the BPB and accumulates in the prostatic acini, resulting in marked reductions in inflammatory cytokines, oxidative stress markers, and fibrosis in a CNP rat model, all without significant systemic toxicity (Ye et al., 2024).
3.7 Applications of hydrogels in other andrological disorders
Hydrogel materials have been extensively investigated across major andrological fields—PC, BC, ED, reproductive medicine, urinary tract reconstruction, and genitourinary infections—and have also shown potential in treating Overactive Bladder (OAB).
OAB, which is characterized by detrusor smooth muscle overactivity leading to increased urinary frequency, urgency, and occasional incontinence, is conventionally managed with anticholinergic agents (Wein, 2003). Oxybutynin Hydrochloride (Oxy) reduces bladder contractility by blocking postganglionic muscarinic receptors, thereby lowering intravesical pressure and raising the micturition threshold; however, long-term oral administration is limited by systemic side effects (Escobar et al., 2021). To address these limitations, a composite hydrogel was engineered via dynamic Schiff-base crosslinking between aldehyde-functionalized oxidized dextran and carboxymethyl chitosan to encapsulate Oxy, enabling localized sustained release. In OAB rat models, intravesical administration of the Oxy-loaded hydrogel was found to significantly reduce systemic adverse effects, downregulate Orai1/STIM1 calcium-channel proteins, and improve urodynamic parameters, demonstrating a promising targeted drug-delivery strategy for OAB therapy (Sun et al., 2022).
4 Applications of hydrogels in andrological disease diagnostics
Hydrogel‐based diagnostic platforms have been investigated primarily for PC and BC, reflecting the high mortality rates and profound quality-of-life impact of these malignancies, as well as limitations in current diagnostic modalities (Phookum et al., 2024). Research has focused on leveraging hydrogel technologies to develop more streamlined, sensitive, and efficient diagnostic assays.
4.1 Applications in prostate cancer diagnostics
PC 5-year and 10-year survival rates are estimated at 65%–90% for patients diagnosed at an early stage, making early detection and accurate identification of PC critical for selecting optimal treatment strategies (Damborska et al., 2017). Although diagnostic methods such as Enzyme-Linked Immunosorbent Assay (ELISA), Surface Plasmon Resonance Imaging (SPRI), and PSA testing are available, they remain invasive, time-consuming, costly, and procedurally complex (Liu et al., 2016). Consequently, a noninvasive assay is urgently needed to replace these traditional approaches. Elevated urinary sarcosine levels have emerged as a novel biomarker of PC cell growth (Sreekumar et al., 2009). A 3D hydrogel was fabricated by borax cross-linking regenerated cellulose (sourced from waste paper) with PVA, Carboxymethyl Cellulose (CMC), Graphene Oxide (GO), and 2-naphthoquinone-4-sulfonic acid sodium salt (NQS). This PVA/CMC/GO/NQS hydrogel exhibits a linear colorimetric response to sarcosine over a 0–100 µM range, shows negligible interference from urea or uric acid, and produces urinary assay results that correlate closely with mass spectrometry analyses, demonstrating its potential for noninvasive PC screening (Figure 7A) (Phookum et al., 2024).
FIGURE 7
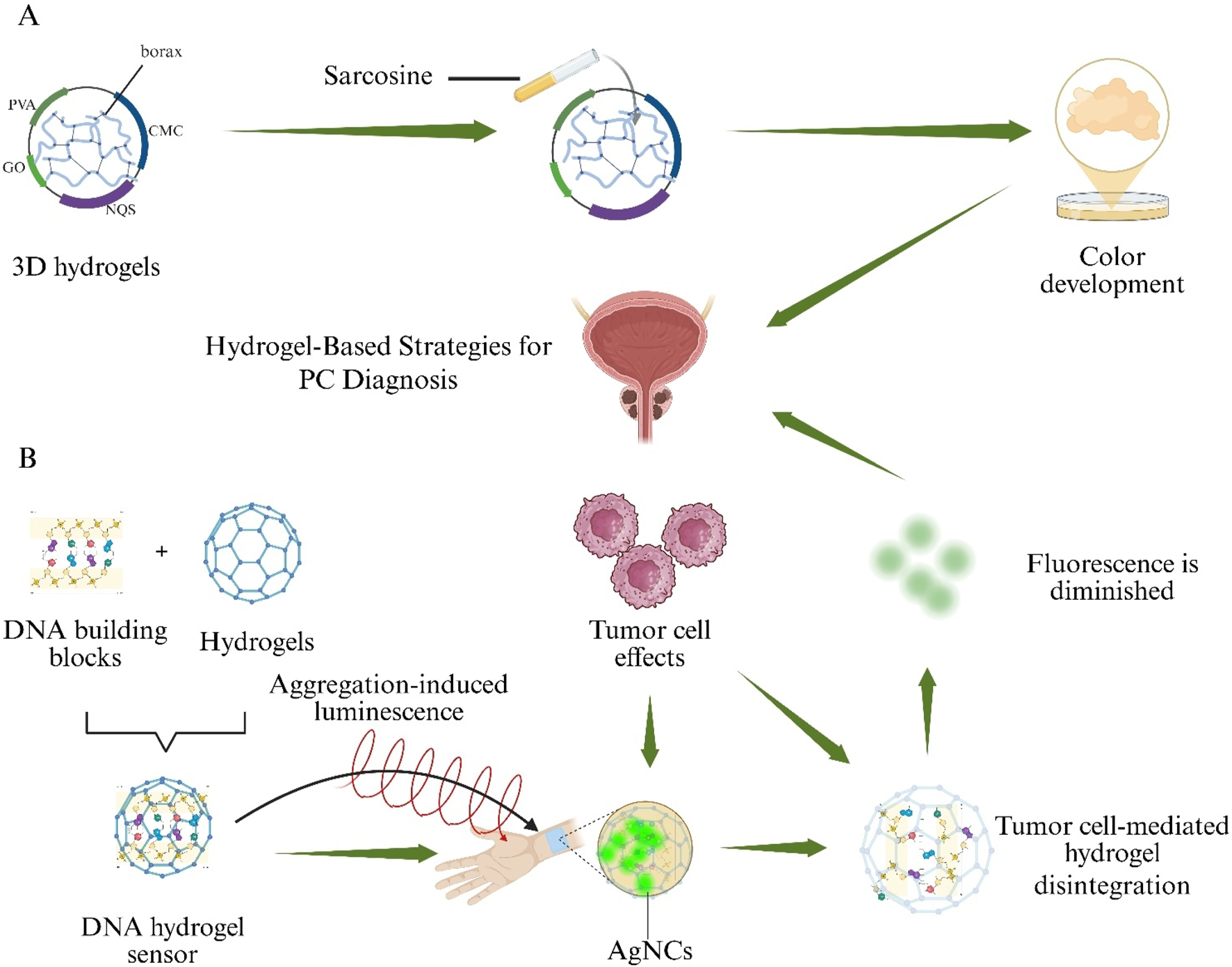
Hydrogel-based sensing systems for non-invasive Prostate Cancer (PC) detection. (A) A borax-cross-linked PVA/CMC/GO/NQS hydrogel produces a visible colour change in the presence of urinary sarcosine. (B) A DNA hydrogel aptasensor loaded with aggregation-induced-emission silver nanoclusters (AIE-AgNCs) collapses upon binding Prostate-Specific Antigen (PSA), resulting in fluorescence quenching.
A DNA-based hydrogel aptasensor employing Silver Nanoclusters with Aggregation-Induced Emission (AIE–AgNCs) was prepared to detect Prostate-Specific Antigen (PSA) without antibodies. Upon PSA binding, hydrogel collapse occurred and fluorescence intensity decreased proportionally, enabling quantification down to 4.4 pg mL−1. The sensor exhibited minimal cross-reactivity with Immunoglobulin G (IgG) and provided a rapid, highly sensitive alternative to conventional immunoassays (Figure 7B) (Ahmadi-Sangachin et al., 2023).
PSA is a single-chain glycoprotein secreted by normal and malignant prostatic cells, and its serum levels are increased in PC (Meyer and Gorin, 2019). Although serum PSA concentration is widely utilized as a diagnostic biomarker for PC, it may lead to overdiagnosis and unnecessary prostate biopsies (Barry, 2001). Therefore, improved noninvasive and highly specific diagnostic methods are required. Detection of PC-associated biomarkers in bodily fluids such as urine is an attractive approach (Alix-Panabier̀es and Pantel, 2013). Exosomal microRNAs (miRNAs) have emerged as promising biomarkers for PC (Gao et al., 2019). A hydrogel-based HCR assay was developed for the detection of urinary exosomal miRNAs and validated clinically, demonstrating high sensitivity and specificity. This method quantified two urinary exosomal miRNAs—hsa-miR-6090 and hsa-miR-3665—with approximately 35-fold signal amplification and attomole-level limits of detection, indicating its potential as an adjunctive diagnostic tool for PC (Kim et al., 2021).
4.2 Applications in bladder cancer diagnostics
BC affects approximately 550,000 new cases annually and ranks among the top ten most common malignancies worldwide, imposing a severe burden on patient quality of life (Richters et al., 2020). Early detection and treatment of BC have been shown to achieve high cure rates (Kamat et al., 2016). Cystoscopy, CT, and MRI are the principal methods for early BC diagnosis; however, their invasiveness and ionizing‐radiation exposure limit routine use (Karaoglu et al., 2014).
Plasma‐based proteomic biomarker discovery is hindered by masking effects of high‐abundance proteins, and current depletion strategies are costly and time‐consuming (Anderson and Anderson, 2002). To address this, a protein‐equalization technique using Dithiothreitol (DTT) was coupled with Two‐Dimensional Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (2D‐SDS‐PAGE). Analysis of BC patient sera revealed overexpression of six proteins—albumin, gelsolin, fibrinogen γ chain, immunoglobulin α-1 chain C region, immunoglobulin α-2 chain C region, and haptoglobin—which were proposed as BC-associated biomarkers, offering a rapid, low-cost marker‐discovery strategy (Figure 8) (Araújo et al., 2018).
FIGURE 8
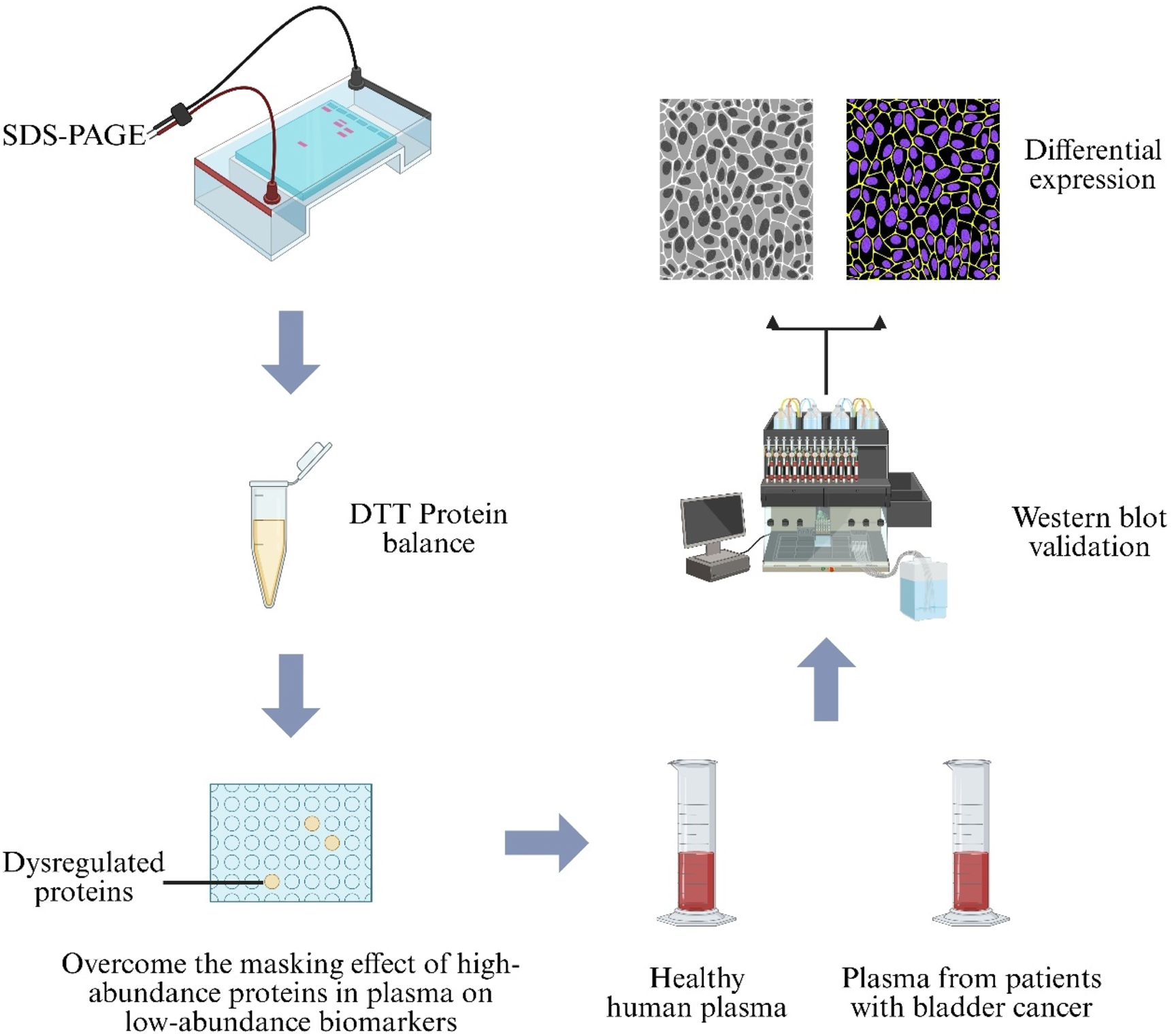
Proteomic workflow for bladder-cancer biomarker discovery. Plasma from healthy donors and Bladder Cancer (BC) patients is first treated with Dithiothreitol (DTT) to balance protein abundance, then separated by Sodium-Dodecyl-Sulfate Polyacrylamide-Gel Electrophoresis (SDS-PAGE). Differentially expressed bands are confirmed by Western blot analysis.
Optical fluorescence imaging within the second near-infrared window (NIR-II; 1,000–1,700 nm) has improved spatial resolution and tissue penetration for early tumor detection (Liu et al., 2022). However, NIR-II diagnostics require sufficient nanoprobe accumulation, and short bladder retention times limit effectiveness (Yoon et al., 2020). To overcome these challenges, an ultraminiature AuPd–Poly(Amidoamine)–Folic Acid (AuPd-P-FA) nanoprobe was incorporated into a temperature-responsive poly(amidoamine) hydrogel for bidirectional intravesical infusion. Folic acid targeting enhanced tumor-specific binding, shifted fluorescence emission to 1,250 nm, and increased quantum yield, thereby prolonging bladder residence and enriching signal in BC lesions—offering a novel noninvasive diagnostic strategy for BC (Ren SL. et al., 2023).
5 Applications of hydrogels in andrological drug delivery
Hydrogel systems have been shown to hold considerable promise for drug delivery in andrological applications. By leveraging their intrinsic properties and optimizing fabrication processes, these systems can prolong drug residence time, increase local drug concentration, and—through thermosensitivity and other stimuli-responsive features—provide more effective therapeutic options for andrological diseases.
5.1 PC drug delivery
In drug-delivery contexts, smart responsive hydrogels address limitations of conventional chemotherapy (Table 2). DTX is a chemotherapeutic agent that induces apoptosis via phosphorylation of B-cell lymphoma 2 (Bcl-2) to inhibit tumour growth; however, its high lipophilicity and low aqueous solubility complicate its delivery (Shekari et al., 2025). Similarly, Doxorubicin (DOX) suffers from poor water solubility and systemic toxicity (Harris et al., 2002). To overcome these challenges, a star-shaped peptide supramolecular hydrogel responsive to zinc ions was developed. In situ self-assembly is triggered by the high zinc-ion concentration in prostatic tissue, enabling targeted DTX delivery and sustained release, and proliferation of DU-145 and PC-3 cells was inhibited in a dose-dependent manner (Tao et al., 2019). Silveira et al. formulated a composite thermosensitive hydrogel embedding DOX-loaded Mesoporous Silica Nanoparticles (MSNs). Using Pluronic F127s body-temperature–induced gelation, this intraperitoneal depot selectively adsorbs DOX within its nanopores and releases it via electrostatic interactions, resulting in a significant reduction in invasive PC incidence and decreased myocardial uptake of DOX (Silveira et al., 2016). These studies demonstrate the potential of hydrogel platforms to optimize PC therapy through targeted delivery and local controlled release.
TABLE 2
| Category | Drug Loaded | Limitations of Free Drug | Formulation and Features | Function | Target Disease | References |
|---|---|---|---|---|---|---|
| Supramolecular Hydrogel | DTX | High lipophilicity and low solubility | Zinc‐ion–responsive star‐shaped peptide hydrogel self‐assembled in situ by prostatic Zn2+ triggers | Targeted DTX delivery and sustained release; dose‐dependent inhibition of DU‐145 and PC-3 cell growth | PC | Tao et al. (2019) |
| DOX | Poor water solubility; systemic toxicity | Semi‐interpenetrating CCA hydrogel (chitosan + SBE-β-CD + CD) | Prolonged intravesical DOX retention | Urothelial carcinoma | Zhang et al. (2024c) | |
| EPO | Inefficient in vivo transport | Chitosan/alginate hydrogel for local EPO encapsulation | ↑ Sperm motility; ↓ testicular malondialdehyde levels | Male infertility | Rasekh et al. (2025) | |
| Thermosensitive Hydrogel | DOX | Poor water solubility; systemic toxicity | DOX‐loaded mesoporous silica nanoparticles + Pluronic F127 gel depot | Extended DOX release; ↓ invasive PC incidence; ↓ myocardial DOX uptake | PC | Silveira et al. (2016) |
| Que | Poor aqueous solubility | P407 thermosensitive in situ gel with Que‐loaded lipid vehicles | Extended Que retention ex vivo; ↓ T-24 cell viability | Urothelial carcinoma | Shawky et al. (2022) | |
| RAP | Poor solubility, permeability, and uptake | RAP‐loaded liposomes (±FA‐modification) dispersed in P407 thermogel | 2× ↑ RAP cellular uptake; 60% ↑ tumor inhibition | Urothelial carcinoma | Yoon et al. (2019) | |
| ASC-Exo | Rapid clearance from corpora cavernosa | In situ–polymerized polydopamine nanoparticle–PELA thermogel; photoacoustic‐guided injection | Precise, sustained ASC-Exo release to tunica albuginea | ED | Liang et al. (2022b) | |
| atRA | Poor solubility, instability, rapid metabolism | Thermosensitive CS/β-glycerophosphate hydrogel scaffold | Prevents atRA degradation; promotes spermatogonial differentiation, meiosis, sperm release; ↑ in vivo atRA dose | Male infertility | Zarei et al. (2025) | |
| In Situ Gel–Liposome (LP-Gel) | Paclitaxel | Rapid urinary clearance; poor urothelial penetration/adhesion | Fluidic liposomes embedded in gellan‐gum hydrogel; ion‐triggered cross‐linking | ↑ urothelial permeability; prolonged paclitaxel bladder retention | Urothelial carcinoma | GuhaSarkar et al. (2017) |
| Microemulsion Hydrogel | Sildenafil citrate | Low bioavailability; systemic side effects; delayed onset | Non‐microemulsion hydrogel with isopropyl myristate and water | 1.97× ↑ membrane permeation; ↓ metabolites by 50%; faster onset | ED | Atipairin et al. (2020) |
| Bigels | Que | High‐dose oxidative toxicity | Cottonseed/hempseed oil‐based bigel with acacia gum | ↓ Que oxidative toxicity; ↓ sperm abnormalities and DNA fragmentation; ↑ serum testosterone | Male infertility | Mousavi et al. (2021) |
Applications of hydrogel systems in drug delivery.
5.2 Drug delivery in urothelial carcinoma
Urothelial carcinoma ranks among the ten most common malignancies worldwide, with BC as the predominant subtype (Sung et al., 2021). Although therapeutic advances have markedly reduced mortality, postoperative recurrence remains a major clinical challenge (Rouprêt et al., 2023). Intravesical chemotherapy is recognized as an effective strategy to lower recurrence rates; however, its efficacy is hampered by the urothelial barrier, short drug residence time, and suboptimal targeting (GuhaSarkar et al., 2017). To address these limitations, a biodegradable in situ Gel–Liposome Hydrogel (LP-Gel) system was developed for bladder instillation. In this system, fluidic liposomes are embedded within a gellan gum hydrogel matrix and ion-triggered to form a cross-linked network, thereby enhancing permeability across the urothelial barrier and adhesion to the mucin layer. As a result, paclitaxel retention in the bladder was significantly prolonged (GuhaSarkar et al., 2017). Additionally, a polysaccharide-based supramolecular injectable hydrogel, termed Chitosan–Cyclodextrin Amphiphilic (CCA), was engineered via one-step co-encapsulation of CS, Sulfobutyl ether-β-Cyclodextrin (SBE-β-CD), and β-Cyclodextrin (β-CD) with DOX. In a murine BC model, this CCA hydrogel extended intravesical DOX retention and markedly enhanced its inhibitory effect on bladder tumour cells (Zhang C. et al., 2024).
Quercetin (Que), a natural polyphenolic flavonoid with potent anticancer activity and low toxicity, has been widely investigated for BC therapy. However, its poor aqueous solubility substantially reduces intravesical residence time (Pralhad and Rajendrakumar, 2004). To overcome this limitation, carrier systems were employed to enhance delivery and retention of Que. Shawky et al. evaluated various lipid vehicles for Que solubilization and used Poloxamer 407 (P407) as a thermosensitive in situ gel to create a mucoadhesive depot. This P407 formulation achieved Que penetration depths of up to 350 µm in ex vivo bladder tissue, extended retention to 24 h, and significantly inhibited T-24 cell viability (Shawky et al., 2022). Similarly, Rapamycin (RAP) was incorporated into both unmodified and folic acid–modified liposomes via thin-film hydration and preloading, and these liposomes were dispersed in a P407 thermosensitive hydrogel. This system doubled RAP cellular uptake and, through erosion-controlled release, increased tumor-inhibition rates by 60% in BC models (Yoon et al., 2019).
Intravesical instillation remains a cornerstone of BC treatment, yet most instilled drug is expelled during the first void, and the procedure can provoke urinary tract obstruction (Collado et al., 2000). To address these challenges, a floating hydrogel system was developed by generating microbubbles within the gel to reduce its density, allowing it to float atop urine. When hydrogel density falls below that of urine, buoyancy is maintained, thereby markedly reducing the risk of urinary tract blockage (Lin et al., 2016).
5.3 Drug delivery in ED
Sildenafil citrate is widely used to treat mild‐to‐moderate ED; however, it exhibits low oral bioavailability, frequent systemic adverse effects, and delayed onset of action (Elnaggar et al., 2011). A non-microemulsion hydrogel was formulated by incorporating isopropyl myristate and water with sildenafil citrate, resulting in a membrane permeation rate 1.97-fold higher than that of the pure drug solution, a 50% reduction in metabolite formation, and a shortened onset time (Atipairin et al., 2020). Intramuscular administration of Adipose-Derived Stem Cell Exosomes (ASC-Exo) has emerged as a promising ED therapy; however, the high vascularization of the corpora cavernosa limits ASC-Exo retention (Liang L. et al., 2022). To overcome this, an in situ–Polymerized Polydopamine Nanoparticle–Poly(Ethylene Glycol)–Poly(Lactic Acid) Thermosensitive Hydrogel (PDNPs–PELA) was developed and, when combined with photoacoustic imaging guidance, enabled precise, sustained delivery of ASC-Exo to the tunica albuginea, providing a novel approach to ED treatment (Liang L. et al., 2022).
5.4 Drug delivery in male infertility
All-Trans Retinoic Acid (atRA), an active metabolite of vitamin A, supports blood–testis barrier formation and promotes spermatogonial differentiation (Punab et al., 2017); however, its poor solubility, instability, and rapid metabolism limit its clinical application (Zheng et al., 2022). A thermosensitive hydrogel scaffold was engineered to encapsulate atRA, preventing its degradation and enabling sustained high-dose delivery in vivo, thereby enhancing spermatogonial differentiation, completion of meiosis, and release of mature sperm—a novel tissue-engineering approach for male infertility (Zarei et al., 2025).
Nonalcoholic Fatty Liver Disease (NAFLD) contributes to infertility. Although high-dose Quercetin (Que; ≥10 mg/kg/day) exerts anti-inflammatory, anti-apoptotic, and antioxidant effects in NAFLD (Khaki et al., 2010), pro-oxidant activity at higher doses may impair fertility (Ranawat et al., 2013). To address this, a bigel hydrogel composed of cottonseed oil, hempseed oil, and acacia gum was formulated for controlled Que release, attenuating its oxidative toxicity. In NAFLD rat models, this Que-loaded bigel significantly reduced sperm abnormalities and DNA fragmentation rates, restored serum testosterone levels, and enhanced spermatogenesis (Mousavi et al., 2021).
SCI induces oxidative damage mediated by Reactive Oxygen Species (ROS), which elevates sperm DNA fragmentation and impairs fertility (Gosálvez et al., 2022). Erythropoietin (EPO) demonstrates anti-apoptotic, antioxidant, and anti-inflammatory effects via activation of antioxidant enzymes and inhibition of lipid peroxidation but suffers from inefficient in vivo delivery (Zhang et al., 2022). A chitosan/alginate hydrogel was developed for local EPO delivery following SCI. In treated rats, sperm motility was significantly improved and testicular malondialdehyde levels were decreased, indicating that the EPO-loaded hydrogel enhances therapeutic outcomes for SCI-related infertility (Rasekh et al., 2025).
6 Applications of hydrogels in intelligent models
For complex challenges in treating urogenital diseases, innovations in biomedical engineering have driven the development of intelligent in vitro models with diverse applications. Hydrogel technologies overcome limitations of traditional systems, enabling mechanistic studies, functional reconstruction, defect repair, and reproductive tissue engineering in urogenital disorders.
A high postoperative recurrence rate is observed in BC, with non–muscle-invasive BC exhibiting recurrence rates of up to 90% after transurethral resection (Aldousari and Kassouf, 2010). Emerging evidence implicates chemotherapy-evasive dormant cancer cells in this process (Kottke et al., 2013; Páez et al., 2012). Conventional two-dimensional and spheroid cultures fail to replicate the dynamic dormancy microenvironment in vivo, including cell-cycle arrest, drug resistance, and reactivation phases (Aguirre-Ghiso, 2007). Moreover, standard chemotherapies target proliferating cells and are ineffective against G0/G1-arrested dormant cells (Wells et al., 2013). Thus, advanced in vitro systems are required to study dormancy mechanisms and to facilitate targeted therapy development (Knuchel et al., 1989). To address this need, a 3D Tumor Microenvironment Model (3D-DTM) was established using an amikacin-based hydrogel (“Amikagel”), in which amikacin was cross-linked with Polyethylene Glycol Diglycidyl Ether (PEGDGE) at controlled densities. This system faithfully mimicked BC cell dormancy, escape, and reactivation in vitro, providing a platform for anticancer drug screening, enhancing drug sensitivity, and eradicating dormant tumor cells (Figure 9A) (Pavan Grandhi et al., 2017).
FIGURE 9
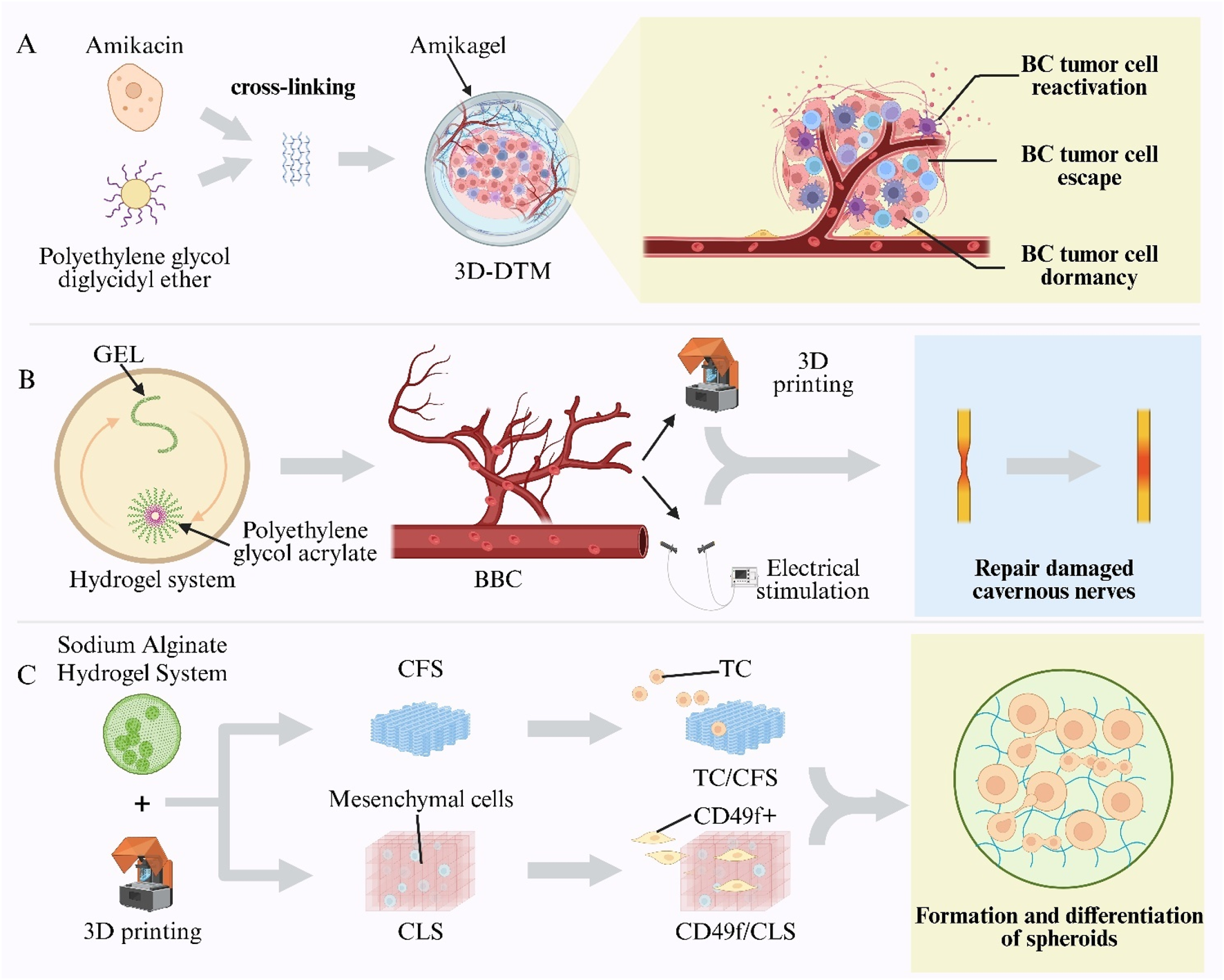
Hydrogel-enabled intelligent models in urogenital research. (A) Amikagel—amikacin cross-linked with Poly(Ethylene Glycol) Diglycidyl Ether (PEGDGE)—creates a Three-Dimensional Dormant-Tumour Micro-Environment (3D-DTM) for bladder-cancer studies. (B) Biomimetic Cavernosum Construct (BBC) printed from gelatin/Poly(Ethylene Glycol) Diacrylate (PEGDA) hydrogel replicates corporal biomechanics and aids nerve repair. (C) Sodium-alginate scaffolds printed as Cell-Free Scaffolds (CFS) or Cell-Laden Scaffolds (CLS) constructs support Testicular-Cell (TC) culture and spermatogonial differentiation.
Vascular ED is a leading cause of organic ED, often resulting from impaired cavernosal arterial inflow and inadequate venous occlusion (Montorsi et al., 2005; Shamloul and Ghanem, 2013). However, no physiologically relevant in vitro model of the corpora cavernosa has existed, hindering studies of cavernosal biomechanics, pathology, and repair. To overcome this gap, a BBC was fabricated via digital light processing 3D printing using Gelatin (GEL) and Polyethylene Glycol Diacrylate (PEGDA) hydrogels. The biomimetic cavernosal model (BBC) exhibited an elastic modulus and tensile strength comparable to native tissue, with >90% recovery after cyclic stretching. By simulating venous occlusion, the model validated key hemodynamic principles of erection and achieved in vitro rigidity. Furthermore, BBC scaffolds seeded with cavernous nerve-like constructs and subjected to electrical stimulation were implanted into rabbit and porcine cavernous defects, resulting in restored erectile function and enabling spontaneous mating (Figure 9B) (Wang Z. et al., 2025).
3D bioprinting has been applied to reproductive tissue engineering to replicate spermatogenesis in vitro (Martinovitch, 1937). Sodium alginate hydrogels serve as natural polymers for 3D bioprinting (Yokonishi et al., 2013; Jørgensen et al., 2014). Using an alginate-based hydrogel system, Cell-Free Scaffolds (CFS) and Cell-Laden Scaffolds (CLS) were fabricated (Aljohani et al., 2018). Unsorted prepubertal Testicular Cells (TCs) were seeded into the macropores of CFS to create single-cell compartments (TC/CFS), while CD49f+ epithelial cells, enriched by magnetic sorting, were seeded into CLS pores to form dual-cell compartments (CD49f+/CLS). Both scaffold types supported spheroid formation and germ-cell differentiation within their pores: 66% of TC/CFS constructs and 100% of CD49f+/CLS constructs contained postmeiotic germ cells, and elongated spermatids were detected in some constructs. These results validate the feasibility of 3D bioprinting for reproductive tissue engineering and provide a programmable platform for constructing biomimetic testicular microenvironments and studying cell–cell interactions (Figure 9C) (Baert et al., 2019).
7 Challenges and limitations
Hydrogels, owing to their distinctive properties and drug-loading capabilities, have demonstrated a broad spectrum of potential applications in the diagnosis and treatment of male urological diseases. Nonetheless, a review of current investigations reveals that their practical application encounters multiple challenges and limitations (Sánchez-Cid et al., 2022; Perez-Valle et al., 2020). These include an incongruity between the intrinsic properties of existing hydrogel systems and the complex therapeutic requirements of urological pathologies, restricted functionality of hydrogel-based systems within specific pathological microenvironments, unresolved issues related to the multi-targeted, synergistic control of drug delivery, as well as a paucity of long-term clinical data and standardized fabrication protocols.
Presently, the utilization of hydrogels in certain male reproductive disorders remains in the exploratory phase, with limited research particularly in the context of rare male malignancies such as penile and testicular cancers. In the realm of tissue engineering, current natural hydrogel materials exhibit significant deficiencies in maintaining morphological stability and structural integrity within the highly dynamic settings of urethral, penile, or bladder reconstruction. Conversely, synthetic hydrogels often face biocompatibility challenges (Hu et al., 2024). Although some studies have attempted to combine the advantageous features of both to engineer hydrogels with desirable elastic moduli suitable for tissue regeneration, such composite strategies are yet to be standardized, and their long-term stability remains inadequately validated. Moreover, current hydrogel-based tissue-engineering technologies still confront multi-faceted, complex hurdles on the path toward human clinical translation (Forouzandegan et al., 2025). The primary barrier is the considerable discrepancy between the human pathological microenvironment and the conditions recreated in existing experimental models. Animal models rarely reproduce the inflammation, fibrosis and latent injuries that may arise in vivo, making it difficult to guarantee ideal clinical outcomes (Kumari and Goyal, 2024). Compounding this problem is the severe paucity of data on inter-individual variability and long-term safety.
Furthermore, the bioresponsive properties of most hydrogel systems are heavily dependent on their microenvironment. For instance, in Erectile Dysfunction (ED) therapy, conductive hydrogels (e.g., GACM) are designed to preserve adhesive, conductive, and anti-inflammatory functions, which are notably influenced by cellular responses in the perilesional neural tissue. However, in male urological pathologies, the microenvironment tends to be highly complex, characterized by ischemia, hyperosmotic urine, or tumor heterogeneity. Whether such microenvironmental factors precipitate functional impairments of hydrogels warrants further investigation.
In the domain of drug delivery, current strategies predominantly rely on monolithic targeted approaches controlling the release of specific agents—such as thermosensitive release of Doxorubicin (DOX) or rapamycin, or zinc-triggered self-assembly for Docetaxel (DTX) delivery. However, in clinical practice, particularly in combined modalities involving immunotherapy, chemotherapy, and molecular targeting, there is a demand for multi-temporal, multi-site, and multi-pathway coordinated release systems. Currently, no hydrogel platform has been developed that precisely meets these multifaceted therapeutic requirements.
Regarding safety profiles, while existing research indicates that hydrogels possess high reliability, the majority of investigations in the context of male reproductive health remain confined to animal models, with limited progression into extensive clinical trials. At present, the only well-established clinical use centres on hydrogel spacers in prostate-cancer radiotherapy, and the scarcity of large-scale trials likely reflects the immaturity of hydrogel technology, technical limitations and low clinical acceptance. Moreover, scaling up hydrogel production encounters technical obstacles that hinder widespread clinical application and quality control. Because these systems require precise synthetic methods and advanced polymer engineering, the absence of standardised evaluation criteria and harmonised regulatory frameworks has slowed approval processes, introduced scalability issues, and—together with batch-to-batch variability and inconsistent fabrication performance—undermined industrial feasibility and reproducibility (Li S. et al., 2024). In addition, thermosensitive and supramolecular hydrogels demand strict temperature control during formulation and storage, leaving long-term preservation an unresolved obstacle (Klaewklod et al., 2015). Finally, Critical concerns pertaining to in vivo biocompatibility, especially long-term effects of degradation products on the male reproductive system, have yet to be thoroughly evaluated, and long-term follow-up data are lacking.
8 Conclusions and outlook
This review systematically summarizes research advances and application prospects of hydrogel technologies in andrology. As biomaterials characterized by high water content, excellent biocompatibility, and tunable physicochemical properties, hydrogels have demonstrated multidimensional utility in managing andrological disorders. In PC therapy, hydrogel spacers have been shown to physically separate the prostate and rectum, significantly reducing radiation-induced rectal toxicity during EBRT, while hydrogel-based drug-delivery systems enable precise locoregional release of chemotherapeutic agents. In BC, hydrogels combined with ICIs have established synergistic immunomodulatory regimens. For ED, hydrogel carriers have facilitated neurovascular repair and reconstruction. In male reproductive medicine, hydrogels have supported innovative sperm-selection platforms and in vitro spermatogenesis models. In urinary tract reconstruction, hydrogel scaffolds have been successfully applied to bladder and urethral repair, overcoming challenges posed by the urinary microenvironment. Moreover, hydrogels have enhanced diagnostic assays and optimized drug-delivery strategies across andrological diseases.
Future developments in hydrogel applications for andrology are expected to follow several key trends. First, smart, stimuli-responsive hydrogels will be engineered for multimodal responsiveness, integrating and reacting selectively to multiple physiological cues and disease-specific microenvironmental biomarkers. Second, integration with advanced manufacturing techniques—particularly three-dimensional (3-D) bioprinting—will enable customization of hydrogel implants to patient-specific anatomy and pathology. Moreover, the rapid advance of artificial intelligence is ushering in revolutionary breakthroughs in hydrogel research. Data-driven analytics, predictive modelling and optimisation algorithms are effectively tackling the core challenge of fine-tuning hydrogel properties and performance. In biomedical design, hydrogels must simultaneously balance multiple complex parameters—biocompatibility, mechanical robustness, degradation kinetics and drug-release behaviour. Traditional research relies on laborious trial-and-error experiments that are time-consuming and inefficient. AI, by swiftly processing vast datasets and accurately mapping structure–property relationships, enables reliable prediction of hydrogel behaviour and markedly accelerates the development of optimal systems. With AI’s continued empowerment, hydrogel technology is poised to transcend current constraints and drive a systemic revolution in precision medicine for andrology (Negut and Bita, 2023).
Third, hierarchical incorporation of bioactive molecules (e.g., growth factors, small-molecule mediators, non-coding RNAs) into hydrogel networks will facilitate spatiotemporally controlled release profiles. Finally, deep integration of hydrogels with stem-cell technologies is poised to drive transformative advances in andrological regenerative medicine, with future research focusing on designing hydrogel niches that direct stem-cell differentiation toward specialized male reproductive cell phenotypes.
Hydrogel technology in andrology is poised for clinical translation. Established hydrogel products—such as the SpaceOAR spacer—are being optimized for broader applications. As research advances, hydrogel platforms in male reproductive health are expected to transition from laboratory studies to clinical trials. In the long term, hydrogels are anticipated to serve as a primary modality for tissue engineering and regenerative medicine in andrology. Advancement of hydrogel technology will require interdisciplinary collaboration, with integrated innovation across materials science, biomedical engineering, andrology, and molecular biology. Rigorous application of translational medicine principles will facilitate the progression of hydrogel systems from bench to bedside, while partnerships with the medical-device and pharmaceutical industries will support industrialization and expand patient access.
In summary, owing to their distinctive physicochemical properties and superior biocompatibility, hydrogels offer expansive potential and growth prospects in andrology. Continued progress in materials science and fabrication methods is expected to enable hydrogels to provide novel solutions for the diagnosis, treatment, and prevention of andrological diseases, thereby improving patient quality of life and clinical outcomes.
Statements
Author contributions
XG: Writing – original draft, Conceptualization. JZ: Writing – original draft. YGu: Writing – original draft. XT: Writing – original draft. YGo: Writing – original draft. YX: Writing – original draft. FY: Writing – review and editing. DC: Supervision, Writing – review and editing. XY: Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was Supported by (National Natural Science Foundation of China) grant number (82274534) (Sichuan Science and Technology Program) grant number (2024NSFSC0684) and (Xinglin Scholar Research Premotion Project of Chengdu University of TCM) grant number (QNTD2024004).
Acknowledgments
All figures in this manuscript were created using Biorender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- ASCs
Adipose-Derived Stem Cells
- ASC-Exo
Adipose-Derived Stem Cell Exosomes
- atRA
All-Trans Retinoic Acid
- BC
Bladder Cancer
- BAM
Bladder Acellular Matrix
- BTX-A
OnabotulinumtoxinA
- CCA
Chitosan–Cyclodextrin Amphiphilic
- CNP
Chronic Nonbacterial Prostatitis
- CP
Chronic Prostatitis
- CS
Chitosan
- CHMA
Chitosan Methacryloyl
- CMC
Carboxymethyl Cellulose
- CFS
Cell-Free Scaffolds
- DTM
Decellularized Testicular Matrix
- DTX
Docetaxel
- DOX
Doxorubicin
- ECM
Extracellular Matrix
- ED
Erectile Dysfunction
- EBRT
External-Beam Radiotherapy
- EMO
Emodin
- EPO
Erythropoietin
- GEL
Gelatin
- GFMs
Gold Fiducial Markers
- GCS
Glycol Chitosan
- HA
Hyaluronic Acid
- HC
Hemorrhagic Cystitis
- ICIs
Immune Checkpoint Inhibitors
- ICSI
Intracytoplasmic Sperm Injection
- IGRT
Image-Guided Radiotherapy
- IPN
Interpenetrating Polymer Networks
- MRI
Magnetic Resonance Imaging
- MRM
Male Reproductive Medicine
- NAFLD
Nonalcoholic Fatty Liver Disease
- NVB
Neurovascular Bundles
- OAB
Overactive Bladder
- Oxy
Oxybutynin Hydrochloride
- PAA
Polyacrylic Acid
- PC
Prostate Cancer
- PEG
Polyethylene Glycol
- PTV
Planning Target Volume
- PVA
Polyvinyl Alcohol
- PDGF-BB
Patelet-Derived Growth Factor-BB
- PSA
Prostate-Specific Antigen
- PUU
Polyurethane-Urea
- RAP
Rapamycin
- RME
Regenerative Microenvironment
- ROS
Reactive Oxygen Species
- SBRT
Stereotactic Body Radiotherapy
- SFMA
Silk Fibroin Methacryloyl
- SSCs
Spermatogonial Stem Cells
- SQS
Spacer Quality Score
- SHFe
Shikonin–Ferric Ion (Fe3+) Nanocomposite Hydrogel System
- SHH
Sonic Hedgehog
- SF
Silk Fibroin
- T1D-ED
Erectile Dysfunction in Type 1 Diabetes Mellitus
- UME
Urinary Microenvironment
- VEGF
Vascular Endothelial Growth Factor
References
1
Aguirre-GhisoJ. A. (2007). Models, mechanisms and clinical evidence for cancer dormancy. Nat. Rev. Cancer7 (11), 834–846. 10.1038/nrc2256
2
Ahmadi-SangachinE.MohammadnejadJ.HosseiniM. (2023). Fluorescence self-assembled DNA hydrogel for the determination of prostate specific antigen by aggregation induced emission. Spectrochimica Acta Part A Mol. Biomol. Spectrosc.303, 123234. 10.1016/j.saa.2023.123234
3
AhmedE. M. (2015). Hydrogel: Preparation, characterization, and applications: a review. J. Adv. Res.6 (2), 105–121. 10.1016/j.jare.2013.07.006
4
AldousariS.KassoufW. (2010). Update on the management of non-muscle invasive bladder cancer. J. Can. Urological Assoc.4 (1), 56–64. 10.5489/cuaj.777
5
AlfredW. J.Max BruinsH.CarriónA.CathomasR.CompératE.EfstathiouJ. A.et al (2024). European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2023 guidelines. Eur. Urol.85 (1), 17–31. 10.1016/j.eururo.2023.08.016
6
Alix-Panabier̀esC.PantelK. (2013). Circulating tumor cells: liquid biopsy of cancer. Clin. Chem.59 (1), 110–118. 10.1373/clinchem.2012.194258
7
AljohaniW.UllahM. W.ZhangX.YangG. (2018). Bioprinting and its applications in tissue engineering and regenerative medicine. Int. J. Biol. Macromol.107, 261–275. 10.1016/j.ijbiomac.2017.08.171
8
AlzahraniF. S.AbbasI. A. (2021). Analytical solutions of thermal damage in living tissues due to laser irradiation. Waves Random Complex Media31 (6), 1443–1456. 10.1080/17455030.2019.1676934
9
AmirthalingamS.RajendranA. K.MoonY. G.HwangN. S. (2023). Stimuli-responsive dynamic hydrogels: design, properties and tissue engineering applications. Mater. Horizons10 (9), 3325–3350. 10.1039/d3mh00399j
10
AndersonN. L.AndersonN. G. (2002). The human plasma proteome: history, character, and diagnostic prospects. Mol. Cell. Proteomics1 (11), 845–867. 10.1074/mcp.r200007-mcp200
11
AraújoJ. E.López-FernándezH.DinizM. S.BaltazarP. M.PinheiroL. C.da SilvaF. C.et al (2018). Dithiothreitol-based protein equalization technology to unravel biomarkers for bladder cancer. Talanta180, 36–46. 10.1016/j.talanta.2017.11.063
12
ArgunA.CanV.AltunU.OkayO. (2014). Nonionic double and triple network hydrogels of high mechanical strength. Macromolecules47 (18), 6430–6440. 10.1021/ma5014176
13
AtipairinA.ChunhachaichanaC.NakphengT.ChangsanN.SrichanaT.SawatdeeS. (2020). Development of a sildenafil citrate microemulsion-loaded hydrogel as a potential system for drug delivery to the penis and its cellular metabolic mechanism. Pharmaceutics12 (11), 1055. 10.3390/pharmaceutics12111055
14
AzizM. T. A.El-HaggarS.MostafaT.AttaH.FouadH.MahfouzS.et al (2010). Effect of mesenchymal stem cell penile transplantation on erectile signaling of aged rats. Andrologia42 (3), 187–192. 10.1111/j.1439-0272.2009.00977.x
15
BaertY.Dvorakova-HortovaK.MargaryanH.GoossensE. (2019). Mouse in vitro spermatogenesis on alginate-based 3D bioprinted scaffolds. Biofabrication11 (3), 035011. 10.1088/1758-5090/ab1452
16
BarryM. J. (2001). Clinical practice. Prostate-specific-antigen testing for early diagnosis of prostate cancer. N. Engl. J. Med.344 (18), 1373–1377. 10.1056/NEJM200105033441806
17
BellottiE.SchillingA. L.LittleS. R.DecuzziP. (2021). Injectable thermoresponsive hydrogels as drug delivery system for the treatment of central nervous system disorders: a review. J. Control Release329, 16–35. 10.1016/j.jconrel.2020.11.049
18
BloisD. A.LiaudatA. C.CapellaV.MorillaG.RiveroR. E.BrogliaM. F.et al (2021). Interaction between hyaluronic acid semi-interpenetrated hydrogel with bull spermatozoa: studies of sperm attachment–release and sperm quality. Adv. Mater. Interfaces8 (21), 2101155. 10.1002/admi.202101155
19
BordatA.BoissenotT.NicolasJ.TsapisN. (2019). Thermoresponsive polymer nanocarriers for biomedical applications. Adv. Drug Deliv. Rev.138, 167–192. 10.1016/j.addr.2018.10.005
20
BrayF.LaversanneM.SungH.FerlayJ.SiegelR. L.SoerjomataramI.et al (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.74 (3), 229–263. 10.3322/caac.21834
21
CaiM.-H.ChenX.-Y.FuL.-Q.DuW.-L.YangX.MouX.-Z.et al (2021). Design and development of hybrid hydrogels for biomedical applications: recent trends in anticancer drug delivery and tissue engineering. Front. Bioeng. Biotechnol.9, 630943. 10.3389/fbioe.2021.630943
22
CalvertP. (2009). Hydrogels for soft machines. Adv. Mater.21 (7), 743–756. 10.1002/adma.200800534
23
CanepaP.CascianoI.De LeoC.MassarottiC.AnseriniP.RemorgidaV.et al (2019). A successful healthy childbirth and an ongoing evolutive pregnancy in a case of partial globozoospermia by hyaluronic acid sperm selection. Andrologia51 (2), e13178. 10.1111/and.13178
24
CaoN.SongL.LiuW.FanS.JiangD.MuJ.et al (2019). Prevascularized bladder acellular matrix hydrogel/silk fibroin composite scaffolds promote the regeneration of urethra in a rabbit model. Biomed. Mater.14 (1), 015002. 10.1088/1748-605X/aae5e2
25
CaoY.HongA.SchultenH.PostM. J. (2005). Update on therapeutic neovascularization. Cardiovasc. Res.65 (3), 639–648. 10.1016/j.cardiores.2004.11.020
26
CasillasF.BetancourtM.CuelloC.DucolombY.LópezA.Juárez-RojasL.et al (2018). An efficiency comparison of different in vitro fertilization methods: IVF, ICSI, and PICSI for embryo development to the blastocyst stage from vitrified porcine immature oocytes. Porc. Health Manag.4, 16. 10.1186/s40813-018-0093-6
27
ChaiQ.JiaoY.YuX. (2017). Hydrogels for biomedical applications: their characteristics and the mechanisms behind them. Gels (Basel, Switz.)3 (1), 6. 10.3390/gels3010006
28
ChappleC.AndrichD.AtalaA.BarbagliG.CavalcantiA.KulkarniS.et al (2014). SIU/ICUD consultation on urethral strictures: the management of anterior urethral stricture disease using substitution urethroplasty. Urology83 (3), S31–S47. 10.1016/j.urology.2013.09.012
29
ChenY.CaoX.YaoJ.HuZ.LuoY.LiG.et al (2024). Enhancing under-urine adhesion and bladder adaptation of silk fibroin hydrogels with tea polyphenols for hemorrhagic cystitis. Int. J. Biol. Macromol.283, 137487. 10.1016/j.ijbiomac.2024.137487
30
ChenY.WangX.TaoS.WangQ.MaP.-Q.LiZ.-B.et al (2023). Research advances in smart responsive-hydrogel dressings with potential clinical diabetic wound healing properties. Mil. Med. Res.10 (1), 37. 10.1186/s40779-023-00473-9
31
ChoiH.JeongS. H.SimóC.BakeneckerA.LiopJ.LeeH. S.et al (2024). Urease-powered nanomotor containing STING agonist for bladder cancer immunotherapy. Nat. Commun.15 (1), 9934. 10.1038/s41467-024-54293-z
32
CirriM.NerliG.MenniniN.MaestrelliF.MuraP. (2022). Development and characterization of cyclodextrin-based nanogels as a new ibuprofen cutaneous delivery system. Pharmaceutics14 (12), 2567. 10.3390/pharmaceutics14122567
33
ColladoA.ChéchileG. E.SalvadorJ.VicenteJ. (2000). Early complications of endoscopic treatment for superficial bladder tumors. J. Urol.164 (5), 1529–1532. 10.1016/s0022-5347(05)67021-8
34
CyrilA. C.JanR. K.RadhakrishnanR. (2022). Pain in chronic prostatitis and the role of ion channels: a brief overview. Br. J. Pain16 (1), 50–59. 10.1177/20494637211015265
35
DamborskaD.BertokT.DosekovaE.HolazovaA.LorencovaL.KasakP.et al (2017). Nanomaterial-based biosensors for detection of prostate specific antigen. Microchim. Acta184 (9), 3049–3067. 10.1007/s00604-017-2410-1
36
D'AmicoM. J.FossH.UhrA.RudnickB.KlonieckeE.GomellaL. G. (2022). Hemorrhagic cystitis: a review of the literature and treatment options. Can. J. Urology29 (5), 11276–11283.
37
DearnaleyD. P.JovicG.SyndikusI.KhooV.CowanR. A.GrahamJ. D.et al (2014). Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol.15 (4), 464–473. 10.1016/S1470-2045(14)70040-3
38
De NittoS.SerafinA.KaradimouA.SchmalenbergerA.MulvihillJ. J. E.CollinsM. N. (2024). Development and characterization of 3D-printed electroconductive pHEMA-co-MAA NP-laden hydrogels for tissue engineering. Bio-Design Manuf.7 (3), 262–276. 10.1007/s42242-024-00272-8
39
EisenbergM. L.EstevesS. C.LambD. J.HotalingJ. M.GiwercmanA.HwangK.et al (2023). Male infertility. Nat. Rev. Dis. Prim.9 (1), 49. 10.1038/s41572-023-00459-w
40
ElliottJ. E.MacdonaldM.NieJ.BowmanC. N. (2004). Structure and swelling of poly(acrylic acid) hydrogels: effect of pH, ionic strength, and dilution on the crosslinked polymer structure. Polymer45 (5), 1503–1510. 10.1016/s0032-3861(03)01191-1
41
ElnaggarY. S.El-MassikM. A.AbdallahO. Y. (2011). Fabrication, appraisal, and transdermal permeation of sildenafil citrate-loaded nanostructured lipid carriers versus solid lipid nanoparticles. Int. J. Nanomed.6, 3195–3205. 10.2147/IJN.S25825
42
EscobarC. M.FalkK. N.MehtaS.HallE. F.MenhajiK.SappenfieldE. C.et al (2021). Rethinking second-line therapy for overactive bladder to improve patient access to treatment options. Obstetrics Gynecol.137 (3), 454–460. 10.1097/AOG.0000000000004279
43
EstevesS. C.RoqueM.BedoschiG.HaahrT.HumaidanP. (2018). Intracytoplasmic sperm injection for Male infertility and consequences for offspring. Nat. Rev. Urol.15 (9), 535–562. 10.1038/s41585-018-0051-8
44
FanR. R.ChengY.WangR. R.ZhangT.ZhangH.LiJ. C.et al (2022). Thermosensitive hydrogels and advances in their application in disease therapy. Polymers14 (12), 2379. 10.3390/polym14122379
45
FazliS.HezariS.OladA. (2024). Preparation of hydrogels based on okra pods/chia seeds mucilage for drug delivery application. Polym. Bull.81 (13), 11539–11562. 10.1007/s00289-024-05221-0
46
FengW.WangZ. (2023). Tailoring the swelling-shrinkable behavior of hydrogels for biomedical applications. Adv. Sci.10 (28), e2303326. 10.1002/advs.202303326
47
Fischer-ValuckB. W.ChunduryA.GayH.BoschW.MichalskiJ. (2017). Hydrogel spacer distribution within the perirectal space in patients undergoing radiotherapy for prostate cancer: impact of spacer symmetry on rectal dose reduction and the clinical consequences of hydrogel infiltration into the rectal wall. Pract. Radiat. Oncol.7 (3), 195–202. 10.1016/j.prro.2016.10.004
48
ForouzandeganM.SadeghmousaviS.HeidariA.KhaboushanA. S.KajbafzadehA.-M.ZolbinM. M. (2025). Harnessing the potential of tissue engineering to target Male infertility: insights into testicular regeneration. Tissue Cell93, 102658. 10.1016/j.tice.2024.102658
49
FoudaziR.ZowadaR.Manas-ZloczowerI.FekeD. L. (2023). Porous hydrogels: present challenges and future opportunities. Langmuir39 (6), 2092–2111. 10.1021/acs.langmuir.2c02253
50
FrancoJ. V.TurkT.JungJ. H.XiaoY. T.IakhnoS.TirapeguiF. I.et al (2019). Pharmacological interventions for treating chronic prostatitis/chronic pelvic pain syndrome. Cochrane Database Syst. Rev.10 (10), Cd012552. 10.1002/14651858.CD012552.pub2
51
FuZ.XiaoS.WangP.ZhaoJ.LingZ.AnZ.et al (2023). Injectable, stretchable, toughened, bioadhesive composite hydrogel for bladder injury repair. RSC Adv.13 (16), 10903–10913. 10.1039/d3ra00402c
52
FujiharaA.UkimuraO. (2022). Focal therapy of localized prostate cancer. Int. J. Urol.29 (11), 1254–1263. 10.1111/iju.14991
53
FukumitsuN.MimaM.DemizuY.SuzukiT.IshidaT.MatsushitaK.et al (2022). Separation effect and development of implantation technique of hydrogel spacer for prostate cancers. Pract. Radiat. Oncol.12 (3), 226–235. 10.1016/j.prro.2021.10.010
54
GaoX.LiS.DingF.FanH.ShiL.ZhuL.et al (2019). Rapid detection of exosomal MicroRNAs using virus-mimicking fusogenic vesicles. Angew. Chem. - Int. Ed.58 (26), 8719–8723. 10.1002/anie.201901997
55
GharbiehS.MullinJ.JafferA.ChiaD.ChallacombeB. (2025). Epidemiology, diagnosis and treatment of anterior prostate cancer. Nat. Rev. Urol.22, 439–446. 10.1038/s41585-024-00992-7
56
GiacomettiV.McLaughlinO.ComiskeyP.MarshallH.HoulihanO. A.WhittenG.et al (2024). Validation of a quality metric score to assess the placement of hydrogel rectal spacer in patients treated with prostate stereotactic radiation therapy. Adv. Radiat. Oncol.9 (3), 101396. 10.1016/j.adro.2023.101396
57
GosálvezJ.Vargas-BaqueroE.López-FernándezC.Bartolomé-NebredaJ.FernándezJ. L.JohnstonS. (2022). Sperm DNA damage in men with spinal cord injury: the relative incidence of single- and double-strand DNA breaks. Andrology10 (7), 1292–1301. 10.1111/andr.13210
58
GrossA.YuanJ. K.SprattD.FredmanE. (2021). Case report: role of an iodinated rectal hydrogel spacer, SpaceOAR vue™, in the context of low-dose-rate prostate brachytherapy, for enhanced post-operative contouring to aid in accurate implant evaluation and dosimetry. Front. Oncol.11, 810955. 10.3389/fonc.2021.810955
59
GrossmanC. E.FolkertM. R.LobaughS.DesaiN. B.KollmeierM. A.GorovetsD.et al (2023). Quality metric to assess adequacy of hydrogel rectal spacer placement for prostate radiation therapy and association of metric score with rectal toxicity outcomes. Adv. Radiat. Oncol.8 (4), 101070. 10.1016/j.adro.2022.101070
60
GuhaSarkarS.MoreP.BanerjeeR. (2017). Urothelium-adherent, ion-triggered liposome-in-gel system as a platform for intravesical drug delivery. J. Control. Release245, 147–156. 10.1016/j.jconrel.2016.11.031
61
HamstraD. A.MariadosN.SylvesterJ.ShahD.KarshL.HudesR.et al (2017). Continued benefit to rectal separation for prostate radiation therapy: final results of a phase III trial. Int. J. Radiat. Oncol. Biol. Phys.97 (5), 976–985. 10.1016/j.ijrobp.2016.12.024
62
HarrisL.BatistG.BeltR.RoviraD.NavariR.AzarniaN.et al (2002). Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer94 (1), 25–36. 10.1002/cncr.10201
63
HatzimouratidisK.SaloniaA.AdaikanG.BuvatJ.CarrierS.El-MeliegyA.et al (2016). Pharmacotherapy for erectile dysfunction: recommendations from the fourth international consultation for sexual medicine (ICSM 2015). J. Sex. Med.13 (4), 465–488. 10.1016/j.jsxm.2016.01.016
64
HoT. C.ChangC. C.ChanH. P.ChungT. W.ShuC. W.ChuangK. P.et al (2022). Hydrogels: properties and applications in biomedicine. Molecules27 (9), 2902. 10.3390/molecules27092902
65
HoeV.YaoH. H.-I.HuangJ. G.GuerrieriM. (2019). Abscess formation following hydrogel spacer for prostate cancer radiotherapy: a rare complication. BMJ case Rep.12 (10), e229143. 10.1136/bcr-2018-229143
66
HommaK.ChangA. C.YamamotoS.TamateR.UekiT.NakanishiJ. (2021). Design of azobenzene-bearing hydrogel with photoswitchable mechanics driven by photo-induced phase transition for in vitro disease modeling. Acta Biomater.132, 103–113. 10.1016/j.actbio.2021.03.028
67
HuS. F.ZengC.JiangY. C.KongW. Q.ZhuM. F. (2024). Macrostructure and microenvironment biomimetic hydrogel: design, properties, and tissue engineering application. Chem. Mater.36 (3), 1054–1087. 10.1021/acs.chemmater.3c02098
68
HuangY.ZhaoX.ZhangZ. Y.LiangY. P.YinZ. H.ChenB. J.et al (2020). Degradable gelatin-based IPN cryogel hemostat for rapidly stopping deep noncompressible hemorrhage and simultaneously improving wound healing. Chem. Mater.32 (15), 6595–6610. 10.1021/acs.chemmater.0c02030
69
HuangY.-C.NingH.ShindelA. W.FandelT. M.LinG.HarrazA. M.et al (2010). The effect of intracavernous injection of adipose tissue-derived stem cells on hyperlipidemia-associated erectile dysfunction in a rat model. J. Sex. Med.7 (4), 1391–1400. 10.1111/j.1743-6109.2009.01697.x
70
HwangM. E.BlackP. J.EllistonC. D.WolthuisB. A.SmithD. R.WuC. C.et al (2018). A novel model to correlate hydrogel spacer placement, perirectal space creation, and rectum dosimetry in prostate stereotactic body radiotherapy. Radiat. Oncol.13 (1), 192. 10.1186/s13014-018-1135-6
71
HwangM. E.MayedaM.ShaishH.EllistonC. D.SpinaC. S.WenskeS.et al (2021). Dosimetric feasibility of neurovascular bundle-sparing stereotactic body radiotherapy with periprostatic hydrogel spacer for localized prostate cancer to preserve erectile function. Br. J. Radiol.94 (1119), 20200433. 10.1259/bjr.20200433
72
IchtO.SchlosserS.Weinstock-SabbahM.RephaelM.BragilovskiD.MooreA.et al (2024). The role of a radiopaque peri-rectal hydrogel spacer in aiding accurate daily image-guidance for prostate stereotactic radiotherapy. Front. Oncol.14, 1386058. 10.3389/fonc.2024.1386058
73
JiangD.HuangJ.ShaoH.HuX.SongL.ZhangY. (2017). Characterization of bladder acellular matrix hydrogel with inherent bioactive factors. Mater. Sci. Eng. C77, 184–189. 10.1016/j.msec.2017.03.222
74
JiangZ. M.FuY. Z.ShenH. X. (2024). Development of intratumoral drug delivery based strategies for antitumor therapy. Drug Des. Dev. Ther.18, 2189–2202. 10.2147/DDDT.S467835
75
JinY.WangY.YangR.FangW.ZhangK.LiuM.et al (2025). Multilayered hydrogel scaffold construct with native tissue matched elastic modulus: a regenerative microenvironment for urethral scar-free healing. Biomaterials312, 122711. 10.1016/j.biomaterials.2024.122711
76
JørgensenA.YoungJ.NielsenJ. E.JoensenU. N.ToftB. G.Rajpert-De MeytsE.et al (2014). Hanging drop cultures of human testis and testis cancer samples: a model used to investigate activin treatment effects in a preserved niche. Br. J. Cancer110 (10), 2604–2614. 10.1038/bjc.2014.160
77
KaliG.EisenbarthH.WenzG. (2016). One pot synthesis of a polyisoprene polyrotaxane and conversion to a slide-ring gel. Macromol. Rapid Commun.37 (1), 67–72. 10.1002/marc.201500548
78
KamatA. M.HahnN. M.EfstathiouJ. A.LernerS. P.MalmströmP. U.ChoiW.et al (2016). Bladder cancer. Lancet.388 (10061), 2796–2810. 10.1016/S0140-6736(16)30512-8
79
KaraogluI.van der HeijdenA. G.WitjesJ. A. (2014). The role of urine markers, white light cystoscopy and fluorescence cystoscopy in recurrence, progression and follow-up of non-muscle invasive bladder cancer. World J. Urol.32 (3), 651–659. 10.1007/s00345-013-1035-1
80
KarmakarP. D.VeluK.KumarC. M. V.PalA. (2024). Advances in injectable hydrogel: design, functional regulation, and biomedical applications. Polym. Adv. Technol.35 (1). 10.1002/pat.6193
81
KarshL. I.GrossE. T.PieczonkaC. M.AliottaP. J.SkomraC. J.PonskyL. E.et al (2018). Absorbable hydrogel spacer use in prostate radiotherapy: a comprehensive review of phase 3 clinical trial published data. Urology115, 39–44. 10.1016/j.urology.2017.11.016
82
KawaguchiG.IshidaK.NishiyamaH.IkedaY.HaraN.NishiyamaT. (2024). Rectal toxicity of 3-dimensional conformal radiation therapy following hydrogel spacer (space OAR) injection for men with prostate cancer. SAGE Open Med.12, 20503121241287086. 10.1177/20503121241287086
83
KhakiA.FathiazadF.NouriM.KhakiA.MalekiN. A.KhamneiH. J.et al (2010). Beneficial effects of quercetin on sperm parameters in streptozotocin-induced diabetic Male rats. Phytotherapy Res.24 (9), 1285–1291. 10.1002/ptr.3100
84
KimJ.ShimJ. S.HanB. H.KimH. J.ParkJ.ChoI.-J.et al (2021). Hydrogel-based hybridization chain reaction (HCR) for detection of urinary exosomal miRNAs as a diagnostic tool of prostate cancer. Biosens. Bioelectron.192, 113504. 10.1016/j.bios.2021.113504
85
KlaewklodA.TantishaiyakulV.HirunN.SangfaiT.LiL. (2015). Characterization of supramolecular gels based on β-cyclodextrin and polyethyleneglycol and their potential use for topical drug delivery. Mater. Sci. Eng. C50, 242–250. 10.1016/j.msec.2015.02.018
86
KleinE. A.SilvermanR. (2008). Inflammation, infection, and prostate cancer. Curr. Opin. Urology18 (3), 315–319. 10.1097/MOU.0b013e3282f9b3b7
87
KnuchelR.HofstadterF.JenkinsW. E. A.MastersJ. R. W. (1989). Sensitivities of monolayers and spheroids of the human bladder cancer cell line MGH-U1 to the drugs used for intravesical chemotherapy. Cancer Res.49 (6), 1397–1401.
88
KobayashiH.EriguchiT.TanakaT.OgataT.OsakiN.SuzukiH.et al (2023). An optimal method of hydrogel spacer insertion for stereotactic body radiation therapy of prostate cancer. Jpn. J. Radiol.42, 406–414. 10.1007/s11604-023-01506-y
89
KoettingM. C.PetersJ. T.SteichenS. D.PeppasN. A. (2015). Stimulus-responsive hydrogels: theory, modern advances, and applications. Mater. Sci. Eng. R Rep.93, 1–49. 10.1016/j.mser.2015.04.001
90
KottkeT.BoisgeraultN.DiazR. M.DonnellyO.Rommelfanger-KonkolD.PulidoJ.et al (2013). Detecting and targeting tumor relapse by its resistance to innate effectors at early recurrence. Nat. Med.19 (12), 1625–1631. 10.1038/nm.3397
91
KrhutJ.ZvaraP. (2011). Intravesical instillation of botulinum toxin A: an in vivo murine study and pilot clinical trial. Int. Urol. Nephrol.43 (2), 337–343. 10.1007/s11255-010-9790-z
92
KumariP.GoyalA. K. (2024). Challenges and opportunities in intravesical drug delivery approaches for the treatment of lower urinary diseases. J. Drug Deliv. Sci. Technol.100, 106110. 10.1016/j.jddst.2024.106110
93
KunduS. D.RoehlK. A.EggenerS. E.AntenorJ. A.HanM.CatalonaW. J. (2004). Potency, continence and complications in 3,477 consecutive radical retropubic prostatectomies. J. Urol.172 (6 Pt 1), 2227–2231. 10.1097/01.ju.0000145222.94455.73
94
Lam Van BaO.AharonyS.LoutochinO.CorcosJ. (2015). Bladder tissue engineering: a literature review. Adv. Drug Deliv. Rev.82-83, 31–37. 10.1016/j.addr.2014.11.013
95
LiJ.YangF.DongL.ChangD.YuX. (2023). Seminal plasma biomarkers for predicting successful sperm retrieval in patients with nonobstructive azoospermia: a narrative review of human studies. Basic Clin. Androl.33 (1), 9. 10.1186/s12610-023-00184-0
96
LiJ.ZhaiY. N.XuJ. P.ZhuX. Y.YangH. R.CheH. J.et al (2024a). An injectable collagen peptide-based hydrogel with desirable antibacterial, self-healing and wound-healing properties based on multiple-dynamic crosslinking. Int. J. Biol. Macromol.259, 129006. 10.1016/j.ijbiomac.2023.129006
97
LiS.LongM.LiJ.ZhangY.FengN.ZhangZ. (2024b). Improved topical delivery of curcumin by hydrogels formed by composite carriers integrated with Cyclodextrin metal-organic frameworks and Cyclodextrin nanosponges. Int. J. Pharm. X8, 100310. 10.1016/j.ijpx.2024.100310
98
LiangL.ShenY.DongZ.GuX. (2022b). Photoacoustic image-guided corpus cavernosum intratunical injection of adipose stem cell-derived exosomes loaded polydopamine thermosensitive hydrogel for erectile dysfunction treatment. Bioact. Mater.9, 147–156. 10.1016/j.bioactmat.2021.07.024
99
LiangY.XuH.LiZ.ZhangjiA.GuoB. (2022a). Bioinspired injectable self-healing hydrogel sealant with fault-tolerant and repeated thermo-responsive adhesion for sutureless post-wound-closure and wound healing. Nano-Micro Lett.14 (1), 185. 10.1007/s40820-022-00928-z
100
LiaoZ.LiuT.YaoZ.HuT.JiX.YaoB. (2025). Harnessing stimuli-responsive biomaterials for advanced biomedical applications. Exploration5 (1), 20230133. 10.1002/EXP.20230133
101
LinT. S.ZhaoX. Z.ZhangY. F.LianH. B.ZhuangJ. L.ZhangQ.et al (2016). Floating hydrogel with self-generating micro-bubbles for intravesical instillation. Materials9 (12), 1005. 10.3390/ma9121005
102
LiuA.ZhaoF.ZhaoY.ShangguanL.LiuS. (2016). A portable chemiluminescence imaging immunoassay for simultaneous detection of different isoforms of prostate specific antigen in serum. Biosens. Bioelectron.81, 97–102. 10.1016/j.bios.2016.02.049
103
LiuQ.GuanY.LiS. (2024). Programmed death receptor (PD-)1/PD-ligand (L)1 in urological cancers: the “all-around warrior” in immunotherapy. Mol. Cancer23, 183. 10.1186/s12943-024-02095-8
104
LiuY.LiY.KooS.SunY.LiuY.LiuX.et al (2022). Versatile types of inorganic/organic NIR-IIa/IIb fluorophores: from strategic design toward molecular imaging and theranostics. Chem. Rev.122 (1), 209–268. 10.1021/acs.chemrev.1c00553
105
LoC.-Y.ZhaoY.KimC.AlsaidY.KhodambashiR.PeetM.et al (2021). Highly stretchable self-sensing actuator based on conductive photothermally-responsive hydrogel. Mater. Today50, 35–43. 10.1016/j.mattod.2021.05.008
106
LondheP. V.LondheM. V.SalunkheA. B.LahaS. S.MeffordO. T.ThoratN. D.et al (2025). Magnetic hydrogel (MagGel): an evolutionary pedestal for anticancer therapy. Coord. Chem. Rev.522, 216228. 10.1016/j.ccr.2024.216228
107
Lopez-BeltranA.CooksonM. S.GuercioB. J.ChengL. (2024). Advances in diagnosis and treatment of bladder cancer. Bmj-British Med. J.384, e076743. 10.1136/bmj-2023-076743
108
LuoJ. L.ZhaoX.GuoB. L.HanY. (2023). Preparation, thermal response mechanisms and biomedical applications of thermosensitive hydrogels for drug delivery. Expert Opin. Drug Deliv.20 (5), 641–672. 10.1080/17425247.2023.2217377
109
LuthfiantiH. R.WaresindoW. X.EdikresnhaD.HapidinD. A.NoorF. A.ElfahmiE.et al (2024). Bioactive compounds-loaded polyvinyl alcohol hydrogels: advancements in smart delivery media for biomedical applications. Mater. Res. Express11 (6), 062002. 10.1088/2053-1591/ad4fdd
110
MaitiS.MajiB.YadavH. (2024). Progress on green crosslinking of polysaccharide hydrogels for drug delivery and tissue engineering applications. Carbohydr. Polym.326, 121584. 10.1016/j.carbpol.2023.121584
111
MangirN.WilsonK. J.OsmanN. I.ChappleC. R. (2019). Current state of urethral tissue engineering. Curr. Opin. Urol.29 (4), 385–393. 10.1097/MOU.0000000000000637
112
MaoL.LiuL.ZhangT.WuX.ZhangT.XuY. (2020). MKL1 mediates TGF-β-induced CTGF transcription to promote renal fibrosis. J. Cell Physiol.235 (5), 4790–4803. 10.1002/jcp.29356
113
MartinS.HarringtonD. A.OhlanderS.StuppS. I.McVaryK. T.PodlasekC. A. (2021). Peptide amphiphile nanofiber hydrogel delivery of sonic hedgehog protein to the penis and cavernous nerve suppresses intrinsic and extrinsic apoptotic signaling mechanisms, which are an underlying cause of erectile dysfunction. Nanomedicine-Nanotechnology Biol. Med.37, 102444. 10.1016/j.nano.2021.102444
114
MartinovitchP. N. (1937). Development in vitro of the mammalian gonad. Nature139 (3514), 413. 10.1038/139413a0
115
MatzE. L.HsiehM. H. (2017). Review of advances in uroprotective agents for cyclophosphamide- and ifosfamide-induced hemorrhagic cystitis. Urology100, 16–19. 10.1016/j.urology.2016.07.030
116
MeyerA. R.DharmarajD.HarbR.PavlovichC. P.AllafM. E.GorinM. A. (2021). Perirectal hydrogel spacer placement prior to prostate radiation therapy using a probe-mounted needle guide. Clin. Transl. Radiat. Oncol.29, 102–105. 10.1016/j.ctro.2021.05.003
117
MeyerA. R.GorinM. A. (2019). First point-of-care PSA test for prostate cancer detection. Nat. Rev. Urol.16 (6), 331–332. 10.1038/s41585-019-0179-1
118
MittalR. K.KrishnaG.MishraR.UddinR.SharmaV. (2024a). From synthesis to solutions: hydrogels' impact on the biomedical landscape. Curr. Pharm. Biotechnol.26, 1634–1651. 10.2174/0113892010294727240502051954
119
MittalR. K.MishraR.UddinR.SharmaV. (2024b). Hydrogel breakthroughs in biomedicine: recent advances and implications. Curr. Pharm. Biotechnol.25 (11), 1436–1451. 10.2174/0113892010281021231229100228
120
MohammadM. N.KumarH.KimK.SundararajU.ShorR. J.NataleG. (2024). Manipulating mechanical properties of PEG-based hydrogel nanocomposite: a potential versatile bio-adhesive for the suture-less repair of tissue. J. Mech. Behav. Biomed. Mater.150, 106285. 10.1016/j.jmbbm.2023.106285
121
MontorsiP.RavagnaniP. M.GalliS.RotatoriF.BrigantiA.SaloniaA.et al (2005). The artery size hypothesis: a macrovascular link between erectile dysfunction and coronary artery disease. Am. J. Cardiol.96 (12, Suppl. 2), 19M–23M. 10.1016/j.amjcard.2005.07.006
122
MoonH. W.KimI. G.KimM. Y.JungA. R.ParkK.LeeJ. Y. (2023). Erectile dysfunction treatment using stem cell delivery patch in a cavernous nerve injury rat model. Bioengineering-Basel.10 (6), 635. 10.3390/bioengineering10060635
123
MoritaM.HiramatsuA.NishimuraK.YanagidaW.NakamuraS.YamatoyaJ.et al (2024). Radiation proctitis after iodine-125 low-dose-rate prostate brachytherapy utilizing SpaceOAR hydrogel. Int. J. Urol.31 (9), 1001–1008. 10.1111/iju.15504
124
MousaviS. N.HosseiniE.DorrajiM. S. S.MohammadiS. S.PourmansouriZ.RasoulifardM. H.et al (2021). Synthesis of a green bigel using cottonseed oil/cannabis oil/alginate/ferula gum for quercetin release: synergistic effects for treating infertility in rats. Int. J. Biol. Macromol.177, 157–165. 10.1016/j.ijbiomac.2021.02.121
125
NaahidiS.JafariM.LoganM.WangY.YuanY.BaeH.et al (2017). Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv.35 (5), 530–544. 10.1016/j.biotechadv.2017.05.006
126
NakaiY.TanakaN.AsakawaI.OhnishiK.MiyakeM.YamakiK.et al (2024). Efficacy of a hydrogel spacer for improving quality of life in patients with prostate cancer undergoing low-dose-rate brachytherapy alone or in combination with intensity-modulated radiotherapy: an observational study using propensity score matching. Prostate84 (12), 1104–1111. 10.1002/pros.24744
127
NamakshenasP.MojraA. (2021). Optimization of polyethylene glycol-based hydrogel rectal spacer for focal laser ablation of prostate peripheral zone tumor. Phys. Medica Eur. J. Med. Phys.89, 104–113. 10.1016/j.ejmp.2021.07.034
128
NarukawaT.AibeN.TsujimotoM.ShiraishiT.KimotoT.SuzukiG.et al (2023). Increasing rectum-prostate distance using a hydrogel spacer to reduce radiation exposure during proton beam therapy for prostate cancer. Sci. Rep.13 (1), 18319. 10.1038/s41598-023-45557-7
129
NarukawaT.ShiraishiT.AibeN.FujiharaA.HongoF.YamazakiH.et al (2022). New modified technique of hydrogel spacer implantation for prostate cancer: a novel method for separation at the prostate apex level under real-time ultrasound guidance. J. Med. Ultrason.49 (4), 751–752. 10.1007/s10396-022-01254-y
130
NegutI.BitaB. (2023). Exploring the potential of artificial intelligence for hydrogel development-A short review. Gels9 (11), 845. 10.3390/gels9110845
131
NihL. R.CarmichaelS. T.SeguraT. (2016). Hydrogels for brain repair after stroke: an emerging treatment option. Curr. Opin. Biotechnol.40, 155–163. 10.1016/j.copbio.2016.04.021
132
OhiraS.YamashitaH.MinamitaniM.SawayanagiS.OgitaM.ImaeT.et al (2024). Relationship between hydrogel spacer distribution and dosimetric parameters in linear-accelerator-based stereotactic body radiotherapy for prostate cancer. J. Appl. Clin. Med. Phys.25 (6), e14294. 10.1002/acm2.14294
133
OmidianH.AkhzarmehrA.GillE. J. (2025). Cyclodextrin-hydrogel hybrids in advanced drug delivery. Gels11 (3), 177. 10.3390/gels11030177
134
PablosJ. L.LozanoD.ManzanoM.Vallet-RegiM. (2024). Regenerative medicine: hydrogels and mesoporous silica nanoparticles. Mater. Today Bio29, 101342. 10.1016/j.mtbio.2024.101342
135
PáezD.LabonteM. J.BohanesP.ZhangW.BenhanimL.NingY.et al (2012). Cancer dormancy: a model of early dissemination and late cancer recurrence. Clin. Cancer Res.18 (3), 645–653. 10.1158/1078-0432.CCR-11-2186
136
PanX.ChengS.ZhangC.JiaoY.LinX.DongW.et al (2021). Mussel-inspired magnetic pullulan hydrogels for enhancing catalytic degradation of antibiotics from biomedical wastewater. Chem. Eng. J.409, 128203. 10.1016/j.cej.2020.128203
137
ParkS.HanU.ChoiD.HongJ. (2018). Layer-by-layer assembled polymeric thin films as prospective drug delivery carriers: design and applications. Biomaterials Res.22, 29. 10.1186/s40824-018-0139-5
138
PatelA.MequanintK. (2011). Hydrogel biomaterials, in Biomedical Engineering - Frontiers and Challenges. Editor Fazel-RezaiR. (New York: InTech), 275–296.
139
Pavan GrandhiT. S.PottaT.NitiyanandanR.DeshpandeI.RegeK. (2017). Chemomechanically engineered 3D organotypic platforms of bladder cancer dormancy and reactivation. Biomaterials142, 171–185. 10.1016/j.biomaterials.2017.07.008
140
PayneC. K.JoyceG. F.WiseM.ClemensJ. Q. (2007). Interstitial cystitis and painful bladder syndrome. J. Urol.177 (6), 2042–2049. 10.1016/j.juro.2007.01.124
141
PayneH.AdamsonA.BahlA.BorwellJ.DoddsD.HeathC.et al (2013). Chemical- and radiation-induced haemorrhagic cystitis: current treatments and challenges. BJU Int.112 (7), 885–897. 10.1111/bju.12291
142
Perez-ValleA.Del AmoC.AndiaI. (2020). Overview of current advances in extrusion bioprinting for skin applications. Int. J. Mol. Sci.21 (18), 6679. 10.3390/ijms21186679
143
PhookumT.BoobphahomS.SiripongpredaT.UmmartyotinS.RodthongkumN. (2024). Recycled cellulose/PVA/CMC-GO/NQS hydrogel as a noninvasive sarcosine sensor for prostate cancer screening. Cellulose31 (14), 8555–8568. 10.1007/s10570-024-06093-3
144
PinkawaM.BernekingV.SchlenterM.KrenkelB.EbleM. J. (2017). Quality of life after radiation therapy for prostate cancer with a hydrogel spacer: 5-year results. Int. J. Radiat. Oncol. Biol. Phys.99 (2), 374–377. 10.1016/j.ijrobp.2017.05.035
145
PishavarE.KhosraviF.NaserifarM.GhomiE. R.LuoH. R.ZavanB.et al (2021). Multifunctional and self-healable intelligent hydrogels for cancer drug delivery and promoting tissue regeneration in vivo. Polymers13 (16), 2680. 10.3390/polym13162680
146
PoelsJ.Abou-GhannamG.DecampsA.LeymanM.RieuxA.WynsC. (2016). Transplantation of testicular tissue in alginate hydrogel loaded with VEGF nanoparticles improves spermatogonial recovery. J. Control Release234, 79–89. 10.1016/j.jconrel.2016.05.037
147
PoelsJ.Van LangendoncktA.ManyM. C.WeseF. X.WynsC. (2013). Vitrification preserves proliferation capacity in human spermatogonia. Hum. Reprod.28 (3), 578–589. 10.1093/humrep/des455
148
PralhadT.RajendrakumarK. (2004). Study of freeze-dried quercetin–cyclodextrin binary systems by DSC, FT-IR, X-ray diffraction and SEM analysis. J. Pharm. Biomed. Analysis34 (2), 333–339. 10.1016/S0731-7085(03)00529-6
149
PunabM.PoolametsO.PajuP.VihljajevV.PommK.LadvaR.et al (2017). Causes of Male infertility: a 9-year prospective monocentre study on 1737 patients with reduced total sperm counts. Hum. Reprod.32 (1), 18–31. 10.1093/humrep/dew284
150
RahbarM.AsadpourR.MazaheriZ. (2024). The effect of epididymosomes on the development of frozen-thawed mouse spermatogonial stem cells after culture in a decellularized testicular scaffold and transplantation into azoospermic mice. J. Assisted Reprod. Genet.41 (8), 2079–2098. 10.1007/s10815-024-03157-y
151
RanawatP.KaushikG.SaikiaU. N.PathakC. M.KhandujaK. L. (2013). Quercetin impairs the reproductive potential of Male mice. Andrologia45 (1), 56–65. 10.1111/j.1439-0272.2012.01311.x
152
RappaportY. H.ZismanA.Jeshurun-GutshtatM.GerassiT.HakimG.VinshtokY.et al (2018). Safety and feasibility of intravesical instillation of botulinum Toxin-A in hydrogel-based slow-release delivery system in patients with interstitial cystitis-bladder pain syndrome: A pilot study. Urology.114, 60–65. 10.1016/j.urology.2017.12.028
153
RasekhM.GilanpourH.SadeghinezhadJ.MortazaviP.AkbariG. (2025). Therapeutic effects of erythropoietin-alginate/chitosan hydrogel on impaired Male rat fertility after spinal cord injury. Mol. Biol. Rep.52 (1), 137. 10.1007/s11033-025-10224-9
154
ReesJ.AbrahamsM.DobleA.CooperA.Prostatitis Expert Reference Group PERG (2015). Diagnosis and treatment of chronic bacterial prostatitis and chronic prostatitis/chronic pelvic pain syndrome: a consensus guideline. BJU Int.116 (4), 509–525. 10.1111/bju.13101
155
RenS. L.DaiR.ZhengZ. L.LiuQ.ZhengX. C.LiJ.et al (2023b). A novel bidirectional perfusion-like administered system for NIR-II fluorescence imaging precision diagnosis of bladder cancer. Nanomedicine-Nanotechnology Biol. Med.49, 102661. 10.1016/j.nano.2023.102661
156
RenY.YuanJ.XueY.ZhangY.LiS.LiuC.et al (2023a). Advanced hydrogels: new expectation for the repair of organic erectile dysfunction. Mater. Today Bio19, 100588. 10.1016/j.mtbio.2023.100588
157
RheaL. P.Mendez-MartiS.KimD.Aragon-ChingJ. B. (2021). Role of immunotherapy in bladder cancer. Cancer Treat. Res. Commun.26, 100296. 10.1016/j.ctarc.2020.100296
158
RichtersA.AbenK. K. H.KiemeneyLALM (2020). The global burden of urinary bladder cancer: an update. World J. Urol.38 (8), 1895–1904. 10.1007/s00345-019-02984-4
159
RouprêtM.SeisenT.BirtleA. J.CapounO.CompératE. M.Dominguez-EscrigJ. L.et al (2023). European association of urology guidelines on upper urinary tract urothelial carcinoma: 2023 update. Eur. Urol.84 (1), 49–64. 10.1016/j.eururo.2023.03.013
160
SaitoM.SuzukiT.SuzukiH.KomiyamaT.MarinoK.AokiS.et al (2022). Minimum required interval between hydrogel spacer injection and treatment planning for stereotactic body radiation therapy for prostate cancer. Pract. Radiat. Oncol.12 (6), e556–e559. 10.1016/j.prro.2022.01.004
161
SaldinL. T.CramerM. C.VelankarS. S.WhiteL. J.BadylakS. F. (2017). Extracellular matrix hydrogels from decellularized tissues: structure and function. Acta Biomater.49, 1–15. 10.1016/j.actbio.2016.11.068
162
SaloniaA.BettocchiC.BoeriL.CapogrossoP.CarvalhoJ.CilesizN. C.et al (2021). European association of urology guidelines on sexual and reproductive Health-2021 update: male sexual dysfunction. Eur. Urol.80 (3), 333–357. 10.1016/j.eururo.2021.06.007
163
SaludasL.Pascual-GilS.ProsperF.GarbayoE.Blanco-PrietoM. (2017). Hydrogel based approaches for cardiac tissue engineering. Int. J. Pharm.523 (2), 454–475. 10.1016/j.ijpharm.2016.10.061
164
Sánchez-CalabuigM. J.López-CardonaA. P.Fernández-GonzálezR.Ramos-IbeasP.Fonseca BalvísN.Laguna-BarrazaR.et al (2014). Potential health risks associated to ICSI: insights from animal models and strategies for a safe procedure. Front. Public Health2, 241. 10.3389/fpubh.2014.00241
165
Sánchez-CidP.Jiménez-RosadoM.RomeroA.Pérez-PuyanaV. (2022). Novel trends in hydrogel development for biomedical applications: a review. Polymers14 (15), 3023. 10.3390/polym14153023
166
SaroiaJ.WangY.WeiQ.ZhangK.LuT.ZhangB. (2018). A review on biocompatibility nature of hydrogels with 3D printing techniques, tissue engineering application and its future prospective. Bio-Design Manuf.1 (4), 265–279. 10.1007/s42242-018-0029-7
167
SchaferE. J.LaversanneM.SungH.SoerjomataramI.BrigantiA.DahutW.et al (2025). Recent patterns and trends in global prostate cancer incidence and mortality: an update. Eur. Urol.87 (3), 302–313. 10.1016/j.eururo.2024.11.013
168
SeeA. W.BowdenP.WellsG.AppuS.LawrentschukN.LiodakisP.et al (2022). Dose-escalated radiotherapy to 82 Gy for prostate cancer following insertion of a peri-rectal hydrogel spacer: 3-year outcomes from a phase II trial. Radiat. Oncol.17 (1), 131. 10.1186/s13014-022-02103-5
169
SennakesavanG.MostakhdeminM.DkharL. K.SeyfoddinA.FatihhiS. J. (2020). Acrylic acid/acrylamide based hydrogels and its properties - a review. Polym. Degrad. Stab.180, 109308. 10.1016/j.polymdegradstab.2020.109308
170
SethA.ChungY. G.GilE. S.TuD.FranckD.Di VizioD.et al (2013). The performance of silk scaffolds in a rat model of augmentation cystoplasty. Biomaterials34 (20), 4758–4765. 10.1016/j.biomaterials.2013.03.038
171
SeymourZ. A.PinkawaM.Daignault-NewtonS.BoschW.MichalskiJ. M.GayH.et al (2023). A pooled long-term follow-up after radiotherapy for prostate cancer with and without a rectal hydrogel spacer: impact of hydrogel on decline in sexual quality of life. Front. Oncol.13, 1239104. 10.3389/fonc.2023.1239104
172
ShamloulR.GhanemH. (2013). Erectile dysfunction. Lancet381 (9861), 153–165. 10.1016/S0140-6736(12)60520-0
173
ShawkyS.MakledS.AwaadA.BoraieN. (2022). Quercetin loaded cationic solid lipid nanoparticles in a mucoadhesive in situ Gel-A novel intravesical therapy tackling bladder cancer. Pharmaceutics14 (11), 2527. 10.3390/pharmaceutics14112527
174
ShekariM. R.JafarianV.BahariA.GhajariY.FardoodS. T. (2025). Evaluation of side effects and efficacy of ferromagnetic alginate-chitosan nanoparticles encapsulating docetaxel on PBMCs and MCF-7 breast cancer cells. J. Biochem. Mol. Toxicol.39 (3), e70178. 10.1002/jbt.70178
175
ShiC.ChenW.ChenB.ShanT.JiaW.HouX.et al (2017). Bladder regeneration in a canine model using a bladder acellular matrix loaded with a collagen-binding bFGF. Biomater. Sci.5 (12), 2427–2436. 10.1039/c7bm00806f
176
ShymborskaY.StetsyshynY.AwsiukK.RaczkowskaJ.BernasikA.JaniszewskaN.et al (2023). Temperature- and pH-responsive schizophrenic copolymer brush coatings with enhanced temperature response in pure water. ACS Appl. Mater Interfaces15 (6), 8676–8690. 10.1021/acsami.2c20395
177
SilveiraC. P.ApolinarioL. M.FavaroW. J.PaulaA. J.DuranN. (2016). Doxorubicin-functionalized silica nanoparticles incorporated into a thermoreversible hydrogel and intraperitoneally administered result in high prostate antitumor activity and reduced cardiotoxicity of doxorubicin. Acs Biomaterials Sci. Eng.2 (7), 1190–1199. 10.1021/acsbiomaterials.6b00241
178
SivaramanS.OstendorffR.FleishmanB.NagatomiJ. (2015). Tetronic®-based composite hydrogel scaffolds seeded with rat bladder smooth muscle cells for urinary bladder tissue engineering applications. J. Biomater. Sci-Polym Ed.26 (3), 196–210. 10.1080/09205063.2014.989482
179
SongD. Y.HerfarthK. K.UhlM.EbleM. J.PinkawaM.van TriestB.et al (2013). A multi-institutional clinical trial of rectal dose reduction via injected polyethylene-glycol hydrogel during intensity modulated radiation therapy for prostate cancer: analysis of dosimetric outcomes. Int. J. Radiat. Oncol. Biol. Phys.87 (1), 81–87. 10.1016/j.ijrobp.2012.12.019
180
SreekumarA.PoissonL. M.RajendiranT. M.KhanA. P.CaoQ.YuJ.et al (2009). Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature457 (7231), 910–914. 10.1038/nature07762
181
StukenborgJ.-B.WistubaJ.LuetjensC. M.Abu ElhijaM.HuleihelM.LunenfeldE.et al (2008). Coculture of spermatogonia with somatic cells in a novel three-dimensional soft-agar-culture-system. J. Androl.29 (3), 312–329. 10.2164/jandrol.107.002857
182
SuT.ZhangM.ZengQ.PanW.HuangY.QianY.et al (2021). Mussel-inspired agarose hydrogel scaffolds for skin tissue engineering. Bioact. Mater6 (3), 579–588. 10.1016/j.bioactmat.2020.09.004
183
SunP.WangZ.WuT.ZuoS. S.HuangX. Y.CuiZ. L.et al (2022). Injectable, adhesive, and self-healing composite hydrogels loaded with oxybutynin hydrochloride for the treatment of overactive bladder in rats. Front. Bioeng. Biotechnol.10, 906835. 10.3389/fbioe.2022.906835
184
SungH.FerlayJ.SiegelR. L.LaversanneM.SoerjomataramI.JemalA.et al (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71 (3), 209–249. 10.3322/caac.21660
185
TakayesuJ. S. K.HeckmanP.BsE. S.HurleyP.NarayanaV.McLaughlinP. W. (2023). Quality rectal hydrogel placement allows for gel-enabled dose-escalated EBRT (GEDE-EBRT) without rectal interference in prostate cancer. Med. Dosim.48 (4), 286–292. 10.1016/j.meddos.2023.07.004
186
TanJ.JiaS. Q.XuQ.LinC. Y.CaoY. K.ShenJ.et al (2024). Hydrogel encapsulation facilitates a low-concentration cryoprotectant for cryopreservation of mouse testicular tissue. Colloids Surfaces B-Biointerfaces242, 114096. 10.1016/j.colsurfb.2024.114096
187
TangS. X.FengK. R.YangR.ChengY.ChenM. Y.ZhangH.et al (2025). Multifunctional adhesive hydrogels: from design to biomedical applications. Adv. Healthc. Mater14 (2). 10.1002/adhm.202403734
188
TaoM.HeS.LiuJ.LiH.MeiL.WuC.et al (2019). The conjugates of forky peptides and nonsteroidal anti-inflammatory drugs (NSAID) self-assemble into supramolecular hydrogels for prostate cancer-specific drug delivery. J. Mater. Chem. B7 (3), 469–476. 10.1039/c8tb02307g
189
TuancharoensriN.SonjanS.PromkrainitS.DaengmankhongJ.PhimnuanP.MahasaranonS.et al (2023). Porous Poly(2-hydroxyethyl methacrylate) hydrogel scaffolds for tissue engineering: influence of crosslinking systems and silk sericin concentration on scaffold properties. Polymers15 (20), 4052. 10.3390/polym15204052
190
Van SaenD.GoossensE.HaentjensP.BaertY.TournayeH. (2013). Exogenous administration of recombinant human FSH does not improve germ cell survival in human prepubertal xenografts. Reprod. Biomed. Online26 (3), 286–298. 10.1016/j.rbmo.2012.11.013
191
VasileC.PamfilD.StoleruE.BaicanM. (2020). New developments in medical applications of hybrid hydrogels containing natural polymers. Molecules25 (7), 1539. 10.3390/molecules25071539
192
WangC. M.WuB. R.XiangP.XiaoJ.HuX. C. (2023b). Management of Male erectile dysfunction: from the past to the future. Front. Endocrinol. (Lausanne)14, 1148834. 10.3389/fendo.2023.1148834
193
WangC. Y.ChenC. Y.GuoM. Y.LiB.HanF. X.ChenW. G. (2019). Stretchable collagen-coated polyurethane-urea hydrogel seeded with bladder smooth muscle cells for urethral defect repair in a rabbit model. J. Mater. Science-Materials Med.30 (12), 135. 10.1007/s10856-019-6342-7
194
WangM.DengZ.GuoY.XuP. (2022). Engineering functional natural polymer-based nanocomposite hydrogels for wound healing. Nanoscale Adv.5 (1), 27–45. 10.1039/d2na00700b
195
WangS. L.TangW.LuoH. L.JinF.WangY. (2023a). The role of image-guided radiotherapy in prostate cancer: a systematic review and meta-analysis. Clin. Transl. Radiat. Oncol.38, 81–89. 10.1016/j.ctro.2022.11.001
196
WangS. T.WangZ. Q.YangW.XuZ.DaiH.HeF. P.et al (2024). In situ-sprayed bioinspired adhesive conductive hydrogels for cavernous nerve repair. Adv. Mater36 (19), e2311264. 10.1002/adma.202311264
197
WangY.YeL. J.YanR. K.TangC.ZhouH. B.ZhaoG. J. (2025a). Development of high-strength, 3D-printable, and biocompatible gelatin/κ-carrageenan dual-network hydrogels for wound healing. Int. J. Biol. Macromol.301, 140380. 10.1016/j.ijbiomac.2025.140380
198
WangZ.LiuX.YeT.ZhaiZ.WuK.KuangY.et al (2025b). 3D-printed perfused models of the penis for the study of penile physiology and for restoring erectile function in rabbits and pigs. Nat. Biomed. Eng.10.1038/s41551-025-01367-y
199
WeinA. J. (2003). Diagnosis and treatment of the overactive bladder. Urology62 (5B), 20–27. 10.1016/j.urology.2003.09.008
200
WellsA.GriffithL.WellsJ. Z.TaylorD. P. (2013). The dormancy dilemma: quiescence versus balanced proliferation. Cancer Res.73 (13), 3811–3816. 10.1158/0008-5472.CAN-13-0356
201
WessellsH.BraffettB. H.HoltS. K.JacobsonA. M.KusekJ. W.CowieC.et al (2018). Burden of urological Complications in Men and Women with long-standing type 1 diabetes in the diabetes control and complications Trial/epidemiology of diabetes interventions and complications cohort. Diabetes Care41 (10), 2170–2177. 10.2337/dc18-0255
202
WollenbergA. L.O'SheaT. M.KimJ. H.CzechanskiA.ReinholdtL. G.SofroniewM. V.et al (2018). Injectable polypeptide hydrogels via methionine modification for neural stem cell delivery. Biomaterials178, 527–545. 10.1016/j.biomaterials.2018.03.057
203
WuJ. P.YunZ. H.SongW. L.YuT.XueW.LiuQ. Y.et al (2024). Highly oriented hydrogels for tissue regeneration: design strategies, cellular mechanisms, and biomedical applications. Theranostics14 (5), 1982–2035. 10.7150/thno.89493
204
WynsC.CollienneC.ShenfieldF.RobertA.LaurentP.RoegiersL.et al (2015). Fertility preservation in the Male pediatric population: factors influencing the decision of parents and children. Hum. Reprod.30 (9), 2022–2030. 10.1093/humrep/dev161
205
WynsC.CurabaM.PetitS.VanabelleB.LaurentP.WeseJ. F. X.et al (2011). Management of fertility preservation in prepubertal patients: 5 years’ experience at the catholic university of louvain. Hum. Reprod.26 (4), 737–747. 10.1093/humrep/deq387
206
XiaB.ChenG. (2022). Research progress of natural tissue-derived hydrogels for tissue repair and reconstruction. Int. J. Biol. Macromol.214, 480–491. 10.1016/j.ijbiomac.2022.06.137
207
XiaH.YangD.HeW.ZhuX.YanY.LiuZ.et al (2021). Ultrasound-mediated microbubbles cavitation enhanced chemotherapy of advanced prostate cancer by increasing the permeability of blood-prostate barrier. Transl. Oncol.14 (10), 101177. 10.1016/j.tranon.2021.101177
208
XiaoD.YanH.WangQ.LvX.ZhangM.ZhaoY.et al (2017). Trilayer three-dimensional hydrogel composite scaffold containing encapsulated adipose-derived stem cells promotes bladder reconstruction via SDF-1α/CXCR4 pathway. ACS Appl. Mater Interfaces9 (44), 38230–38241. 10.1021/acsami.7b10630
209
XiaoS.WangP.ZhaoJ.LingZ.AnZ.FuZ.et al (2021). Bi-layer silk fibroin skeleton and bladder acellular matrix hydrogel encapsulating adipose-derived stem cells for bladder reconstruction. Biomaterials Sci.9 (18), 6169–6182. 10.1039/d1bm00761k
210
XiongM.ChenY.HuH.-J.ChengH.LiW.-X.TangS.et al (2024). Multifunctional pH-responsive hydrogel dressings based on carboxymethyl chitosan: synthesis, characterization fostering the wound healing. Carbohydr. Polym.341, 122348. 10.1016/j.carbpol.2024.122348
211
XuQ.JiY.SunQ.FuY.XuY.JinL. (2019). Fabrication of cellulose nanocrystal/chitosan hydrogel for controlled drug release. Nanomater. (Basel)9 (2), 253. 10.3390/nano9020253
212
XuY.-M.QiaoY.SaY.-L.ZhangJ.FuQ.SongL.-J. (2009). Urethral reconstruction using colonic mucosa graft for complex strictures. J. Urol.182 (3), 1040–1043. 10.1016/j.juro.2009.05.030
213
YanH.RongL.XiaoD.ZhangM.SheikhS. P.SuiX.et al (2020). Injectable and self-healing hydrogel as a stem cells carrier for treatment of diabetic erectile dysfunction. Mater. Sci. Eng. C116, 111214. 10.1016/j.msec.2020.111214
214
YangM.ZhangY.FangC.SongL.WangY.LuL.et al (2022b). Urine-microenvironment-initiated composite hydrogel patch reconfiguration propels scarless memory repair and reinvigoration of the urethra. Adv. Mater34 (14), 2109522. 10.1002/adma.202109522
215
YangW. D.FangJ. F.ZhaiJ. C.QiuC.LiangZ. K.LiuQ. H.et al (2024). IL-17A exacerbates corpus cavernosum fibrosis and neurogenic erectile dysfunction by inducing CSMC senescence via the mTORC2-ACACA pathway. Bmc Med.22 (1), 376. 10.1186/s12916-024-03609-3
216
YangY.LiangY.ChenJ.DuanX.GuoB. (2022a). Mussel-inspired adhesive antioxidant antibacterial hemostatic composite hydrogel wound dressing via photo-polymerization for infected skin wound healing. Bioact. Mater8, 341–354. 10.1016/j.bioactmat.2021.06.014
217
YangY.LinQ. L.ZhouC. X.LiQ.LiZ. Y.CaoZ.et al (2020). A testis-derived hydrogel as an efficient feeder-free culture platform to promote mouse spermatogonial stem cell proliferation and differentiation. Front. Cell Dev. Biol.8, 250. 10.3389/fcell.2020.00250
218
YaoJ.ChenY.ZhangX.ChenJ.ZhouC.JiangJ.et al (2024). Slightly photo-crosslinked chitosan/silk fibroin hydrogel adhesives with hemostasis and anti-inflammation for pro-healing cyclophosphamide-induced hemorrhagic cystitis. Mater Today Bio25, 100947. 10.1016/j.mtbio.2024.100947
219
YeY.ZhongW. Z.LuoR. F.WenH. Z.MaZ. Y.QiS. S.et al (2024). Thermosensitive hydrogel with emodin-loaded triple-targeted nanoparticles for a rectal drug delivery system in the treatment of chronic non-bacterial prostatitis. J. Nanobiotechnol.22 (1), 33. 10.1186/s12951-023-02282-7
220
YokonishiT.SatoT.KatagiriK.KomeyaM.KubotaY.OgawaT. (2013). In vitro reconstruction of mouse seminiferous tubules supporting germ cell Differentiation1. Biol. Reprod.89 (1), 1–6. 10.1095/biolreprod.113.108613
221
YooJ.ParkJ. H.KwonY. W.ChungJ. J.ChoiI. C.NamJ. J.et al (2020). Augmented peripheral nerve regeneration through elastic nerve guidance conduits prepared using a porous PLCL membrane with a 3D printed collagen hydrogel. Biomater. Sci.8 (22), 6261–6271. 10.1039/d0bm00847h
222
YoonH. Y.ChangI. H.GooY. T.KimC. H.KangT. H.KimS.-Y.et al (2019). Intravesical delivery of rapamycin via folate-modified liposomes dispersed in thermo-reversible hydrogel. Int. J. Nanomedicine14, 6249–6268. 10.2147/IJN.S216432
223
YoonH. Y.YangH. M.KimC. H.GooY. T.KangM. J.LeeS.et al (2020). Current status of the development of intravesical drug delivery systems for the treatment of bladder cancer. Expert Opin. Drug Deliv.17 (11), 1555–1572. 10.1080/17425247.2020.1810016
224
YuH.WangC.WuL. Y.ZhouZ. Y.WangY. Q.LiW. X.et al (2023). A novel hydrogel orthotopic injection model in moderately hypofractionated radiation therapy for prostate cancer: adaptive degradation and durable imaging. Front. Oncol.12, 1077900. 10.3389/fonc.2022.1077900
225
ZareiH.MovahedinM.GanjiF.GhiaseddinA. (2025). Retinoic acid-releasing scaffold based on chitosan hydrogel and testis decellular plates. Bioimpacts15, 30007. 10.34172/bi.30007
226
ZhangC.NiuJ.LiJ.ZhangH.YuQ.ChenY.et al (2024c). Polysaccharide based supramolecular injectable hydrogels for in situ treatment of bladder cancer. Chin. Chem. Lett.35 (1), 108556. 10.1016/j.cclet.2023.108556
227
ZhangJ.-Y.YuX.-J.LiJ.-J.XiaoY.LiG.-S.YangF.et al (2024b). Cuproptosis mediates copper-induced testicular spermatogenic cell death. Asian J. Androl.26 (3), 295–301. 10.4103/aja202383
228
ZhangW. Q.ZhangJ. Q.ZhangJ. W.ChuJ.ZhangZ. X. (2024a). Novel combination therapy using recombinant oncolytic adenovirus silk hydrogel and PD-L1 inhibitor for bladder cancer treatment. J. Nanobiotechnol22 (1), 638. 10.1186/s12951-024-02903-9
229
ZhangY. S.KhademhosseiniA. (2017). Advances in engineering hydrogels. Science.356 (6337), eaaf3627. 10.1126/science.aaf3627
230
ZhangY. Y.YaoM.ZhuK.XueR. R.XuJ. H.CuiX. J.et al (2022). Neurological recovery and antioxidant effect of erythropoietin for spinal cord injury: a systematic review and meta-analysis. Front. Neurol.13, 925696. 10.3389/fneur.2022.925696
231
ZhaoK. K.HuJ. L.ZhangH. L.ChenT. Y.YangS. Y.WangH.et al (2025). A Shikonin-Iron-Tragacanth gum nanocomplex for synergistic treatment of bladder cancer. Chem. Eng. J.505, 159202. 10.1016/j.cej.2024.159202
232
ZhaoL. L.ZhouY. F.ZhangJ. Y.LiangH. Z.ChenX. W.TanH. (2023). Natural polymer-based hydrogels: from polymer to biomedical applications. Pharmaceutics15 (10), 2514. 10.3390/pharmaceutics15102514
233
ZhengA. J.XieF.ShiS. Y.LiuS. N.LongJ. F.XuY. H. (2022). Sustained drug release from liposomes for the remodeling of systemic immune homeostasis and the tumor microenvironment. Front. Immunol.13, 829391. 10.3389/fimmu.2022.829391
234
ZhongJ.SlevinF.ScarsbrookA. F.SerraM.ChoudhuryA.HoskinP. J.et al (2021). Salvage reirradiation options for locally recurrent prostate cancer: a systematic review. Front. Oncol.11, 681448. 10.3389/fonc.2021.681448
235
ZhouQ.YangK. X.HeJ. Q.YangH. Y.ZhangX. Y. (2019). A novel 3D-printable hydrogel with high mechanical strength and shape memory properties. J. Mater. Chem. C7 (47), 14913–14922. 10.1039/c9tc04945b
Summary
Keywords
hydrogel, andrological diseases, drug delivery, tissue engineering, tumor treatment, reproductive medicine
Citation
Guo X, Zhang J, Guan Y, Tian X, Gong Y, Xiao Y, Yang F, Chang D and Yu X (2025) Hydrogel technologies in andrology: advances in research and prospective applications. Front. Pharmacol. 16:1635007. doi: 10.3389/fphar.2025.1635007
Received
25 May 2025
Accepted
10 July 2025
Published
28 July 2025
Volume
16 - 2025
Edited by
Jianxiang Zhang, Army Medical University, China
Reviewed by
Devesh U Kapoor, Gujarat Technological University, India
Himanshu Paliwal, Prince of Songkla University, Thailand
Ravi Kumar Mittal, Galgotias College of Pharmacy, India
Updates
Copyright
© 2025 Guo, Zhang, Guan, Tian, Gong, Xiao, Yang, Chang and Yu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Degui Chang, changdg@cdutcm.edu.cn; Xujun Yu, 20639179@qq.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.