- 1Graduate School, Heilongjiang University of Chinese Medicine, Harbin, China
- 2The Four Hospital of Heilongjiang University of Chinese Medicine, Heilongjiang University of Chinese Medicine, Harbin, China
Diabetic foot ulcers (DFUs) represent devastating complications associated with high risks of amputation and mortality. The complex pathophysiology of the wound microenvironment and systemic involvement render conventional therapies costly and limited in effectiveness. Consequently, there is an urgent demand for the development of effective and comprehensive therapeutic and management strategies. Herbal medicines and their formulations can transform DFU management from passive debridement to active regeneration through multi-target synergy and holistic intervention, offering low-cost and safe alternatives. Integrating herbal active components with polymeric materials enables the development of novel delivery systems and tissue-engineered scaffolds. This interdisciplinary approach is emerging as a promising therapeutic strategy in regenerative medicine and dermal engineering. This paper compares cellular healing mechanisms in DFUs and normal wounds, elucidates the mechanisms of action of herbal medicines and their formulations in treating DFUs, and synthesizes the applications of novel delivery systems for herbal active components in DFU therapy.
1 Introdution
Diabetes, a formidable global healthcare challenge, without global intervention, over 1.31 billion people are projected to live with diabetes by 2050 (GBD 2021 Diabetes Collaborators, 2023). Among them, DFUs, one of the most dangerous complications of diabetes, remain a major risk factor for disability and death today (GBD, 2021, Diseases and Injuries Collaborators, 2024). Currently, the clinical outcomes of DFUs are unsatisfactory, with approximately one million people worldwide facing amputation each year and poor patient prognosis (Lan et al., 2023). This results in expenditure on DFU wound management far exceeding that on hospitalization (Nussbaum et al., 2018). The global market for trauma treatment products had already reached $12 billion in 2020 and is expected to reach $18.7 billion by 2027, growing at a rate of 40 percent (Sen, 2021).
During DFU wound healing, the hyperglycemic environment causes microcirculatory impairment, while the immunosuppressive state increases infection with multidrug-resistant organisms (MDROs), thus forming an “ischemia-infection-inflammation” vicious cycle. This local immune imbalance ultimately triggers gangrene or even amputation (Barman and Koh, 2020; Nirenjen et al., 2023; Ju et al., 2024a; Zhang H. et al., 2025). Consequently, impaired wound healing in DFUs represents not only a localized tissue defect but also a systemic, life-threatening crisis requiring multidimensional interventions to disrupt this pathological cycle (Kim et al., 2024). Internationally, the standard DFU treatment modalities mainly include glycemic control, infection control, debridement and drainage, decompression therapy, wound dressings, and individualized therapy (Bus et al., 2024b). The International Working Group on the Diabetic Foot (IWGDF) states that the severity of the diabetic foot is largely related to differences in the standard of care for the foot (Bus et al., 2024a). While debridement and negative pressure therapy remain indispensable for severe ulcers, clinical options for DFUs remain limited. Prevention and comprehensive management therefore constitute critical strategies for reducing amputation rates, recurrence, and mortality (Jeffcoate et al., 2024).
Herbal therapies were positioned as both naturopathic and complementary approaches in global medical contexts (Liu et al., 2022; von Schoen-Angerer et al., 2023). Unlike conventional therapies focused on single-target interventions, herbal therapies provide holistic modulation of DFUs. Modern research has transformed herbal therapy from an “empirical medicine” perception to evidence-based, multi-target interventions with clarified mechanisms. Key therapeutic actions include anti-inflammatory effects, oxidative stress inhibition, angiogenesis promotion, and epithelial regeneration (Jarić et al., 2018; Michalak, 2023). These characteristics facilitate a shift from passive debridement to active tissue regeneration in DFUs. Notably, ON101—the world’s first natural drug targeting macrophage polarization—has been approved by the National Medical Products Administration (NMPA). In Phase III multiregional clinical trials (MRCTs) across the U.S. and China, DFU patients treated with ON101 achieved a 60.7% wound healing rate, significantly outperforming the 35.1% rate observed in control groups using absorbent dressings (Huang et al., 2021). Furthermore, active components from Chinese herbal medicines are being integrated with advanced pharmacological technologies to develop novel drug delivery systems. These systems enable targeted delivery, controlled release, reduced dosing frequency, and enhanced patient compliance while optimizing therapeutic efficacy (Kim et al., 2019; Qadir et al., 2021).
This paper initiates a comparative analysis of the dynamic cellular mechanisms underlying normal wound healing versus impaired wound healing in DFUs. From a molecular perspective, we provide a scientific rationale for the multi-target synergistic effects of herbal medicines and their formulations in treating DFUs. Additionally, we summarize a burgeoning research trend: the integration of herbal active components with novel drug delivery systems for DFU therapy.
2 The complex pathophysiology of difficult-to-heal wounds in DFUs
Previous research has primarily focused on the multifactorial etiology of DFUs, including neuropathy, vasculopathy, and infection. However, recent studies highlight the dynamic regulation of DFU wound healing, with cellular-level interventions gaining prominence as a key research frontier. Emerging evidence underscores the critical role of cellular mechanisms and molecular signaling pathways in modulating DFU repair processes.
2.1 The differences in cellular mechanisms between normal wound healing and impaired wound healing in DFUs
The wound healing cascade is a precisely orchestrated and meticulously executed biological process, encompassing numerous biochemical and cellular responses that are regulated by the body’s immune system to restore the integrity of skin and subcutaneous tissues. It is divided into four highly interrelated and partially overlapping phases: hemostasis, inflammation, proliferation, and remodeling (Yuan et al., 2023) In diabetic patients, underlying pathological factors such as neuropathy, peripheral artery disease, and persistent hyperglycemia disrupt the normal wound repair process, leading to stalled healing and increasing vulnerability to infection. Once infection occurs, it combines with oxidative stress and chronic inflammation triggered by excessive reactive oxygen species (ROS), further exacerbating tissue damage, infection severity, and ulcer formation/progression. These interconnected mechanisms form a vicious cycle that drives non-healing wounds to ultimately culminate in DFUs (Dayya et al., 2022; Deng et al., 2023).
The hemostatic phase commences immediately after injury and may persist for several hours, aiming to control bleeding and restrict systemic microbial dissemination. The body initiates primary hemostasis first, during which vasoconstriction occurs instantaneously, and platelets are activated and aggregated to form a provisional platelet plug. Secondary hemostasis begins with the activation of the coagulation cascade, generating thrombin that catalyzes the conversion of fibrinogen to fibrin. The cross-linking of fibrin into a mesh that envelops the platelet aggregate, culminating in the formation of a stable clot, signifies the conclusion of the hemostatic phase (Wilkinson and Hardman, 2020; Singer, 2022). Subsequently, activated platelets release growth factors including platelet-derived growth factor (PDGF) and transforming growth factor-beta (TGF-β), which recruit inflammatory cells such as neutrophils to infiltrate the wound site. This cellular infiltration signifies the commencement of the inflammatory phase of healing (Jarić et al., 2018; Szułdrzyński et al., 2020). In DFUs, healing impairment originates from hemostatic dysfunction (Figure 1A). Hyperglycemia disrupts platelet aggregation, while advanced glycation end-products (AGEs) suppress fibrinogen function, impairing fibrin-platelet cross-linking and reducing clot stability. Concurrently, platelet hyperactivation promotes microthrombosis and hypoperfusion, dysregulating the coagulation cascade (Gawlowski et al., 2007; Brings et al., 2017; Hicks and Selvin, 2019; McDermott et al., 2022).
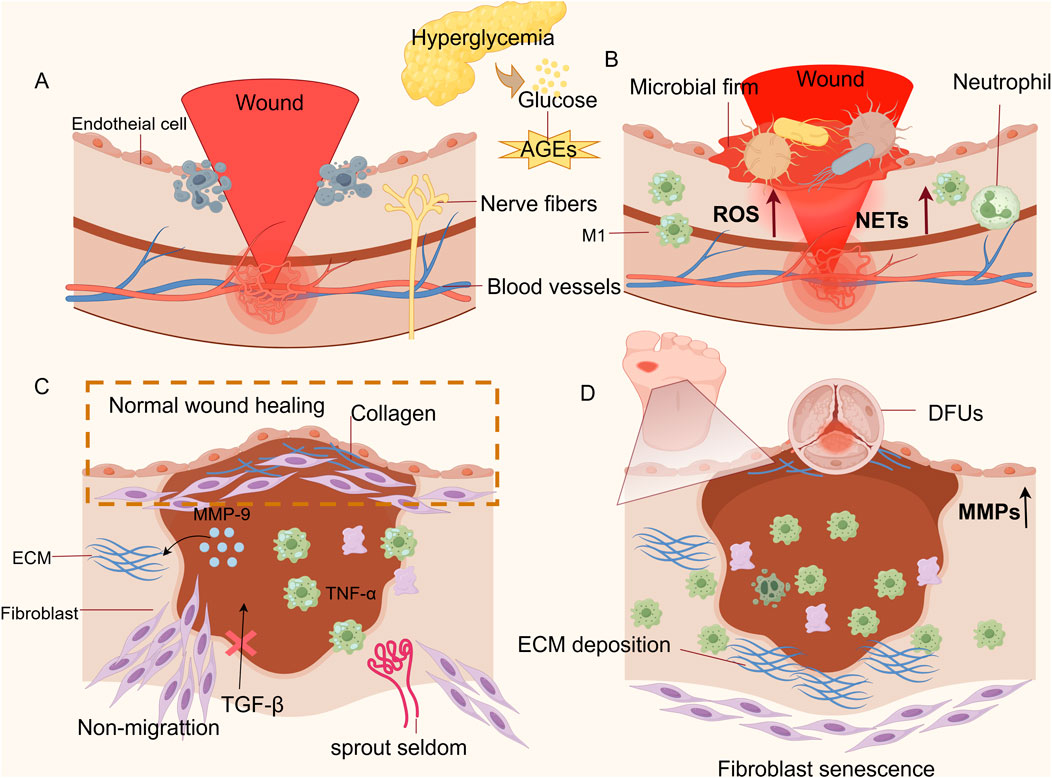
Figure 1. Diagram of the cellular mechanism of non-healing of DFU wounds. (A) Impaired hemostatic phase: There is an impairment in the ability of platelets to aggregate at the wound site, accompanied by their excessive activation, which leads to thrombus formation and an inability to clear necrotic cellular debris from the wound. (B) Prolonged inflammatory phase: The overproduction of ROS and NETs, coupled with the persistent presence of M1 macrophages, establishes a vicious cycle between oxidative stress and inflammation. (C) Shortened proliferative phase: Unlike normal wounds, DFUs fail to achieve epithelial regeneration. Fibroblast migration is impaired, and angiogenesis is reduced. (D) Impaired remodeling phase: Keratinocyte migration is compromised, resulting in abnormal epidermal migration and incomplete wound closure; the sustained high levels of MMPs cause excessive degradation of the ECM, hindering collagen remodeling and leading to difficulties in DFU wounds healing. (Thanks for Fig draw).
The inflammatory phase initiates almost concurrently with hemostasis, typically spanning 1–4 days post-injury. However, its duration may extend to weeks or even years due to underlying pathologies (Tomic-Canic et al., 2020). During this stage, neutrophils may recruit monocytes by secreting several inflammatory factors (IL-1, IL-6, IL-8, IL-10) and the chemokine MCP-1 to regulate the inflammatory response (Singer, 2022; Peña and Martin, 2024). Subsequently, infiltrating monocytes differentiate into M1 macrophages (the predominant cell type during periods of inflammation.) M1 macrophages exert antimicrobial activity primarily by recognizing pathogen-associated modifier proteins (PAMPs) and damage-associated modifier proteins (DAMPs) and by releasing NO, ROS, and pro-inflammatory cytokines to clear pathogens and cellular debris. Ultimately, the sign of inflammation subsiding is the clearance of neutrophils through phagocytosis by macrophages or re-entry into the vascular system (Hassanshahi et al., 2022; Sharifiaghdam et al., 2022). As shown in Figure 1B, whereas the key factor contributing to the delayed healing of DFUs wounds is the persistent overpresence of M1 macrophages and their subsequent inability to convert to the M2 repair phenotype, which amplifies the stimulatory response to cytokines (Aitcheson et al., 2021; Tang et al., 2022). Under hyperglycemic conditions, neutrophils continuously release ROS and stimulate the upregulation of peptidyl arginine deaminase 4 (PAD4), which leads to the overproduction of neutrophil extracellular traps (NETs) and damage to tissue cells (Yang et al., 2023; Zhang Y. et al., 2025). This activates pathways such as NF-κB and promotes the release of inflammatory factors, resulting in local microcirculation disorders that further lead to increased inflammation and vascular and neurological tissue lesions in the ulcerated area (Li et al., 2023; Huang W. et al., 2020; Yang et al., 2020). Important at this stage is an impaired immune response, which will not prevent colonization by pathogenic bacteria, and an excess of AGEs will lead to microbial membrane formation (Liu C. et al., 2020; Xie et al., 2020). In addition, AGEs bind to receptors for advanced glycation end products (RAGE)on various cell types, activating multiple signaling pathways including MAPK/ERK, TGF-β, JNK, and NF-κB. This process increases intracellular oxidative stress, exacerbates local inflammatory responses, and ultimately impedes the transition to subsequent healing phases (Huijberts et al., 2008; Khalid et al., 2022). How to dynamically regulate the timing of the transition from the inflammatory to the proliferative phase is a current difficulty in the treatment of DFUs (Zhou et al., 2022). Concurrently,the disruption of endogenous antioxidant defense mechanisms constitutes a critical pathogenic factor in the development of DFUs (Deng et al., 2021).
After hemorrhage and inflammation are controlled, a proliferative phase, which is longer and can last for several weeks, takes place. The beginning of the proliferative phase is marked by the migration of fibroblasts into the wound, proliferation, and secretion of extracellular matrix (ECM) components to build granulation tissue as a new cellular scaffold (Raziyeva et al., 2021). Angiogenesis is another important process in this phase, where the macrophage phenotype changes from an inflammatory state (type M1) to a healing state (type M2), releasing healing cytokines: PDGF, epidermal growth factor (EGF), and vascular endothelial growth factor (VEGF), which promote deposition of the ECM and repair of broken blood vessels and formation of new blood vessels (Peng et al., 2020). Keratinocytes ultimately achieve epithelial regeneration by secreting local growth factors TGF-α, TGF-β, and keratinocyte growth factor (KGF/FGF 7), as well as by being influenced by mechanical factors (“free edge effect”) (Huang et al., 2023). As shown in Figure 1C, in DFU wounds, the persistence of M1 macrophages causes excessive release of TNF-α and promotes the release of Matrix Metalloproteinase-9 (MMP-9) rom keratinocytes to continuously degrade the ECM, which impairs the migratory ability of keratinocytes and thus prevents the re-epithelialization of the ulcerated wounds (Deng et al., 2024). High accumulation of AGEs leads to fibroblast senescence and is not regulated by the migration stimulator TGF-β, inhibiting their migration into the wound (Deng et al., 2023; Huang et al., 2023). Oxidative stress from hyperglycemia-induced ROS surge elevates oxygen consumption, suppressing hypoxia-inducible factor-1α (HIF-1α) and downstream VEGF expression. This disruption of angiogenic signaling cascades culminates in reduced neovascularization, perpetuating microcirculatory deficits in ulcerated tissues (Catrina and Zheng, 2016).
The remodeling phase is the final stage of wound healing and can last from weeks to years after injury. Fibroblasts upregulate the expression of type I collagen, and macrophages release MMPs into the deposited ECM, causing wound contraction and tissue remodeling through protein hydrolysis and endocytosis. Furthermore, as the ECM subsides, the dense connective tissue is replaced by newborn natural tissue, maintaining normal tissue function of the skin (Sutherland et al., 2023). Finally, as apoptosis of vascular cells and myofibroblasts heralds the end of healing, the conversion of cell-rich granulation tissue into collagen-filled cells results in scarless healing (Eckes et al., 2010; Reinke and Sorg, 2012). As shown in Figure 1D, during the tissue remodeling phase of DFUs, the persistent high-glycemic environment and excessive inflammatory response resulted in high expression of MMPs, leading to excessive degradation of ECM and stagnation of collagen remodeling (Fu et al., 2022). As shown in Figure 1D, persistent ROS and an inflammatory microenvironment induce premature senescence of fibroblasts. On one hand, this significantly inhibits their ability to differentiate into myofibroblasts, preventing the wound from actively contracting. On the other hand, it severely impairs collagen synthesis function, resulting in an insufficient total amount of newly synthesized collagen (Darby et al., 2014). Meanwhile, the accumulation of AGEs disrupts collagen fibers through irreversible covalent cross-linking, causing abnormal cross-linking and excessive degradation of the ECM. This leads to the inability of myofibroblasts to arrange properly at the wound site, ultimately resulting in wound closure failure (Asadipooya and Uy, 2019; Yang Y. et al., 2024).
2.2 Risk factors for difficult wound healing in DFUs
Multiple biological responses affect wound healing in DFUs, including persistent inflammation, oxidative damage, impaired angiogenesis, apoptosis, and aberrant ECM deposition. Among these, the vicious cycle formed by mutual reinforcement of oxidative stress and chronic inflammation is identified as a primary risk factor for delayed wound healing in DFUs (Jeffcoate et al., 2024; Xiao F. et al., 2024; Da Silva et al., 2025). Following organismal injury, a modest inflammatory response is a key defense mechanism to clear pathogens and initiate repair (Huang et al., 2022). However, insufficient inflammation weakens the skin’s antimicrobial barrier and increases the risk of infection. The loss of the natural barrier makes it easier for microorganisms outside the wound to invade the skin tissue and trigger inflammation (Uberoi et al., 2024; Zielińska et al., 2023). Conversely, DFUs often exhibit a hyperinflammatory microenvironment marked by excessive pro-inflammatory cytokine release and ROS overproduction. These factors not only induce oxidative cellular damage but also promote local ischemia and hypoxia, further exacerbating ulcer progression. Importantly, diabetic wounds are frequently prone to infection, which is closely associated with biofilm formation and persistent inflammation. Compared to ordinary chronic wounds, pathogenic microorganisms on DFU wounds exhibit a higher propensity to form biofilms, thereby inducing excessive inflammation. Moreover, patients with DFUs suffer from impaired immune responses, leading to microbial dysbiosis in the wound microenvironment (Jnana et al., 2020a; Mudrik-Zohar et al., 2022). The core mechanism involves biofilm formation by multiple pathogens. The microbial communities in DFUs predominantly consist of Gram-positive and Gram-negative bacteria, including Staphylococcus aureus, Streptococcus spp., and Pseudomonas aeruginosa. Among these, the biofilm structures of S. aureus and P. aeruginosa can physically impede immune cell infiltration. More critically, once microbial biofilms mature, the embedded microorganisms exhibit remarkable resistance and evasion capabilities (evading phagocytosis and clearance by immune cells), posing a fundamental barrier to sustained wound infection and impaired healing (Pouget et al., 2020; Lou et al., 2025).
Currently, the treatment of choice for DFU infections is antibiotic therapy. However, due to the limitations of the antibiotic antimicrobial spectrum, the cost of treatments aimed at killing all microorganisms in the wound is significant and is detrimental to wound healing in DFUs (Jnana et al., 2020b; Liu C. et al., 2020). A recent clinical study supports this notion: metagenomic shotgun sequencing analysis of wound microbiomes from 100 DFU patients revealed that antibiotic therapy struggles to address the complex polymicrobial-multidrug-resistant pathological microenvironment. In contrast, debridement surgery promotes healing by dynamically modulating the wound microbiome to increase the proportion of commensal bacteria (Kalan et al., 2019). Therefore, enhancing healing may be achieved by dynamically regulating the DFU wound microbiome to augment beneficial bacteria while reducing pathogenic bacteria, rather than attempting to eliminate all bacterial species from the wound.
3 Herbal medicines and their formulations for the treatment of DFUs
Herbal medicines and their formulations can exert multi-targeted effects to treat DFUs, mainly including inhibition of inflammatory response, inhibition of oxidative stress, promotion of angiogenesis, epithelial regeneration, and other various effects. The pathways can be summarized as the Wnt-βcatenin, Notch, and NF-κB pathways that regulate inflammation, the Nrf 2/ARE and MAPK pathways that inhibit oxidative stress, and the PI 3K/Akt, TGF-β/Sma, and HIF-1α/VEGF pathways that promote angiogenesis and epithelial regeneration (Zhou et al., 2022). The persistent vicious cycle of oxidative stress and chronic inflammation, coupled with impaired angiogenesis, remains a critical therapeutic target in managing DFUs. Based on dynamic phase-specific regulation of DFU pathogenesis, this study categorizes the multi-target effects of commonly used traditional Chinese medicine (TCM) empirical formulas and classical prescriptions into two synergistic mechanisms: “anti-inflammatory-antioxidative” actions during the inflammatory phase, and “pro-angiogenic-epithelial regenerative” effects during the remodeling phase.
3.1 Anti-inflammatory - inhibits oxidative stress
3.1.1 San Huang Xiao Yan recipe
San Huang Xiao Yan recipe (SHXF), a commonly prescribed TCM empirical formula for DFUs in Chinese hospitals, comprises Rheum palmatum L., Scutellaria baicalensis Georgi, Crataegus pinnatifida Bunge., Phellodendron chinense C.K.Schneid., Isatis tinctoria L., Forsythia suspensa (Thunb.) Vahl, Polygonum bistorta L., Commelina communis L., Glycyrrhiza uralensis Fisch., Coptis chinensis Franch., and Polygonum cuspidatum var. Spectabile Noter.
In a clinical trial involving 86 DFU patients, researchers demonstrated that SHXF combined with silver dressings significantly reduced infections caused by MDROs (Chen et al., 2023). Another trial with 98 DFU patients showed that SHXF combined with yellow horse tincture downregulated matrix metalloproteinase-2 (MMP-2) expression, upregulated tissue inhibitor of metalloproteinases-1 (TIMP-1), and markedly reduced ulcer size (Zhao L. et al., 2021). The remarkable clinical efficacy of SHXF is attributed to its exertion of a multi-target regulatory mechanism for the treatment of DFUs.
Using streptozotocin (STZ)-induced DFU models in C57 mice and Sprague-Dawley (SD) rats, proteomic analysis of skin wounds revealed that SHXF significantly reduced levels of the inflammatory protein high mobility group box 1 (HMGB1), effectively blocking HMGB1-mediated inflammatory responses and decreasing inflammatory cell infiltration at wound sites. Immunofluorescence analysis further showed that SHXF activated AMP-activated protein kinase (AMPK) pathway phosphorylation, promoted nuclear translocation of the transcription factor Nrf2, and enhanced expression of the downstream antioxidant enzyme heme oxygenase-1 (HO-1). This dual anti-inflammatory and antioxidative regulatory mechanism ameliorated oxidative stress injury, reduced the wound’s inflammatory microenvironment, and ultimately facilitated neovascularization and epithelial remodeling (Zhang et al., 2023c). Additionally, a study revealed that SHXF exerts immunomodulatory effects by improving the trauma microenvironment. Animal experiments confirmed that SHXF regulates CD4+ T cells and attenuates immune-inflammatory responses. Simultaneously, SHXF inhibited STAT3 phosphorylation and suppressed Th17 cell activation and interleukin-17 (IL-17) secretion, thereby promoting DFU wound healing through immune modulation and reduction of excessive inflammatory responses. Furthermore, using network pharmacology and molecular docking, the active ingredients in SHXF predicted to be effective against DFUs were identified as β-sitosterol, wogonin, 7-methoxy-2-methyl isoflavone, formononetin, baicalein, isocorypalmine, (S)-canadine, vestitol, shinnerocarpin, and (R)-canadine (Deng et al., 2025).
3.1.2 Ruyi Jinhuang powder
Ruyi Jinhuang powder is a classic formula from the Ming Dynasty in China, included in the Surgical Zhengzong written by Chen Shikong. Its specific medicinal flavor composition is Curcuma longa L., Rheum palmatum L., Phellodendron chinense C.K.Schneid., Atractylodes lancea (Thunb.) DC., Magnolia officinalis Rehder. et Wils., Citrus reticulata Blanco, Glycyrrhiza uralensis Fisch, Arisaema erubescens (Wall.) Schott, Angelica dahurica (Fisch ex Hoffm.) Benth. and Hook. f., and Trichosanthes kirilowii Maxim.
In a single-center clinical trial, combination therapy with moist exposed burn ointment (MEBO) and Ruyi Jinhuang powder achieved up to 92% efficacy in healing DFUs (Zhan et al., 2021). The core mechanism can be summarized as synergistic regulation of the DFU microenvironment through “anti-inflammatory and oxidative stress inhibition.” Ruyi Jinhuang powder reduced the production of ROS in high glucose-treated human skin fibroblasts (HDF-a) and attenuated cellular oxidative stress in a dose-dependent manner. Quantitative PCR (qPCR) results demonstrated significant downregulation of inflammatory cytokines, including IL-1α, IL-1β, and IL-6, with the treated group showing only 50% of the IL-6 levels observed in the control group, indicating robust anti-inflammatory activity. Further in vivo experiments confirmed that Ruyi Jinhuang powder promoted wound angiogenesis by upregulating CD31 and VEGF-A expression, achieving basic wound closure after 14 days of treatment (Wu et al., 2022). Electron microscopy revealed recovery of fibroblasts and neuronal cells, maturation of granulation tissue, and neutrophil infiltration in the treated group of DFU rats. Through molecular docking and KEGG pathway enrichment analysis, it was hypothesized that Ruyi Jinhuang powder alleviates diabetic peripheral neuropathic pain by activating neuroactive ligand-receptor interactions in the PI3K-Akt signaling pathway and inhibiting the FoxO signaling pathway. Further, network pharmacological analysis revealed that the DFU-treating components of Ruyi Jinhuang powder were luteolin, trans-caryophyllene, ar-turmerone, palmitic acid, methyl palmitate, gallic acid, demethoxycurcumin, berberine, and rhein (Li X.-Y. et al., 2022).
3.1.3 Huangbai liniment
Huangbai liniment is a widely used clinical formula in China, composed of Forsythia suspensa (Thunb.) Vahl, P. chinense C.K.Schneid., Lonicera japonica Thunb., Taraxacum sect. Taraxacum F.H.Wigg. , and Scolopendra subspinipes mutilans L. Koch.
In a multicenter clinical trial of 720 patients with DFUs, the overall efficacy of Huangbai liniment treatment was demonstrated to be 96.48% with no adverse events within 4 weeks of treatment (Liu Y. et al., 2020). Another study demonstrated that compared to antimicrobial calcium alginate wound dressings, Huangbai Liniment exhibits superior antimicrobial efficacy while offering greater economic advantages (Yang G. et al., 2024).
Basic studies have elucidated the molecular mechanisms underlying its multi-targeted therapeutic effects on DFUs. The Huangbai liniment upregulates the anti-inflammatory factor TGF-β1 and significantly downregulates downstream pro-inflammatory targets, including IL-6, IL-1β, MMP-9, CXCL-1, and CCL2, while inhibiting the IL-17A pathway to exert anti-inflammatory effects (Zhang et al., 2022). Critically, it ameliorates the oxidative stress microenvironment of DFUs by elevating total antioxidant capacity (T-AOC), superoxide dismutase (SOD) activity, and glutathione (GSH) levels, while reducing oxidative damage markers such as malondialdehyde (MDA) and 8-hydroxydeoxyguanosine (8-OHdG). This is achieved by upregulating the downstream antioxidant factor NQO1 and reversing the nuclear translocation of the transcription factor Nrf2, thereby activating the Nrf2 signaling pathway (Zhang et al., 2020). This dual regulatory mechanism of anti-inflammatory and oxidative stress inhibition enables Huangbai Liniment to achieve favorable therapeutic outcomes in treating DFUs.
3.2 Pro-angiogenic-epithelial regeneration
3.2.1 Sheng-Ji Hua-Yu formula
Sheng-ji Hua-yu formula is an empirically established formula widely utilized in clinical practice in China, consisting of Astragalus membranceus (Fisch) Bunge, Salvia miltiorrhiza Bunge, R. palmatum L., Daemonorops draco (Willd.) Blume, Arnebia euchroma (Royle) I.M. Johnst., A. dahurica (Hoffm.) Benth. and Hook. f.ex Franch. and Sav.,Hyriopsis cumingii (Lea), and Calamine.
Basic experimental studies demonstrated that the Sheng-ji Hua-yu Formula downregulates activin/follistatin levels, inhibits downstream Smad2 phosphorylation and nuclear translocation, and facilitates keratinocyte migration, ultimately achieving wound re-epithelialization. Additionally, reduced pSmad2 levels suppressed TGF-β1-mediated fibrotic signaling and NF-κB nuclear translocation, thereby inhibiting inflammatory cytokine release from keratinocytes and promoting ECM remodeling (Kuai et al., 2018). Another study demonstrated that it promotes the phosphorylation of cyclic adenosine 3′,5′-monophosphate (cAMP) response element-binding protein (CREB), translocates activated protein kinase A (PKA) into the nucleus, upregulates the expression of the downstream angiogenic factor VEGF, and thereby promotes the transformation of pathological blood vessels into healthy neovascularization (Wang G. et al., 2025).
Further biomarker analyses in a DFU mouse model suggested that the Sheng-ji Hua-yu formula synergistically upregulates dopaminergic receptors (DRD1/DRD4) and downregulates cAMP to inhibit Nod-like receptor protein-3 (NLRP3) and CXCR4 expression, thereby modulating inflammation and oxidative stress. Concurrently, it increases angiotensin II type 1 receptor (AGTR1) and δ-opioid receptor (OPRD1) expression, driving dynamic balance between neuropeptides and vasoactive factors through neuroactive ligand-receptor interactions. This dual regulatory mechanism aims to resolve the vascular and neuropathic lesions impeding DFU wound healing (Jiang et al., 2021; Xiang et al., 2021; Ru et al., 2022).
3.2.2 Huiyang Shengji decoction
Huiyang Shengji decoction represents a traditional Chinese medicine formula that has been refined through decades of extensive clinical practice across China. Its composition consists of Sinapis alba L. Astragli Radix, Atractylodis Macrocephalae Rhizoma, Poria, Atractylodis Rhizoma, Cinnamomi Cortex, Aconiti Lateralis Radix Praeparata, Cervi Cornu Degelatinatum, Chaenomelis Fructus, Sinapis Semen, Rehmanniae Radix Praeparata, Angelicae Dahuricae Radix, and Glehniae Radix.
In a clinical trial involving 42 patients with diabetic foot ulcers (DFUs), those treated with Huiyang Shengji decoction combined with Rehabilitation New Liquid achieved an overall efficacy rate of 100% (Huang and Zeng, 2020a). Recent studies on the mechanism of Huiyang Shengji decoction have gradually unveiled the mystery behind its remarkable therapeutic effect—a multi-mechanism co-regulation. On one hand, Huiyang Shengji decoction can synergize the processes of “angiogenesis and epithelial regeneration” to promote wound healing in DFUs. It promotes epithelial regeneration by inducing the secretion and expression of VEGF and increasing the level or activity of epidermal growth factor receptor (EGFR), a key receptor in keratinocytes. This, in turn, activates its downstream the PI3K/AKT signaling pathway (Liu et al., 2021). On the other hand, Huiyang Shengji decoction modulates the immune microenvironment by activating peroxisome proliferator-activated receptor γ (PPARγ), thereby regulating macrophage polarization, ROS production, and controlling NLRP3 inflammasome activation. This inhibits STAT3 phosphorylation and blocks its synergistic interaction with NF-κB, thereby exerting anti-inflammatory effects. Further network pharmacological analysis revealed that the key therapeutic constituents of Huiyang Shengji decoction for treating DFUs are calycosin, calycosin-7-glucoside, hypaconitine, and sinapic acid (Chen et al., 2025; Lin et al., 2025).
3.2.3 Gubu decoction
Gubu decoction is from the famous Qing Dynasty physician Chen Shiduo’s “Record of Discriminating Evidence”, which consists of Scutellaria baicalensis Georgi, Dendrobium nobile Lindl., Angelica sinensis (Oliv.) Diels, L. japonica Thunb., Glycyrrhiza glabra L., Viola philippica var. philippica, Chrysanthemum indicum L., Panax ginseng C.A.Mey, and Achyranthes bidentata Blume.
Gubu decoction downregulates HIF-1α and promotes neovascularization in ulcerated areas, thereby alleviating the hypoxic microenvironment of DFUs. On the other hand, by activating TGF-β1 signaling, it drives Smad3 phosphorylation and nuclear translocation, inhibits matrix metalloproteinase (MMP) overexpression, reduces extracellular matrix degradation, and accelerates granulation tissue proliferation and epithelial regeneration (Wang et al., 2025b). These two mechanisms synergistically form a “vascular reconstruction-epithelial regeneration” dual-effect system, ultimately enabling ulcer repair from microcirculation improvement to parenchymal tissue regeneration throughout the healing process. Another study demonstrated that Gubu Decoction directly promotes fibroblast growth factor 7 (FGF7) release by reducing miR-155 levels, significantly decreasing M1-type markers (iNOS, COX-2) and pro-inflammatory cytokines (IL-6, IL-1β), while enhancing DFU healing (Wang et al., 2025a). This reveals that it can play a synergistic role in regulating macrophage polarization and ameliorating the wound’s inflammatory microenvironment.”
Tables 1, 2 summarize the pharmacological mechanisms and clinical evidence of other herbal medicines and their formulations for DFUs. Additionally, the multi-target mechanisms of action of TCM in treating DFUs are summarized in Figure 2.
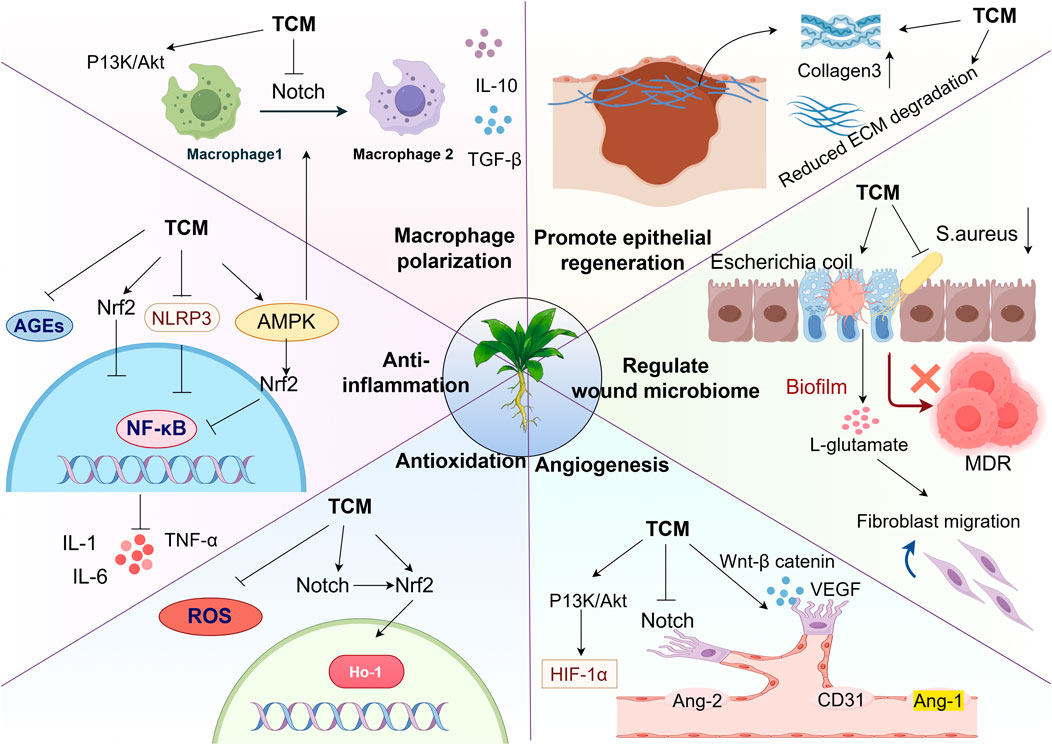
Figure 2. Multi-targeted effects of TCM in the treatment of DFUs: Regulating macrophage polarization: TCM activate the PI3K/Akt, Notch, Nrf2, and AMPK signaling pathways, promoting the transition of macrophages from the M1 to M2 phenotype and releasing pro-healing factors. Suppression of Inflammatory and oxidative cascades: TCM can block the nuclear translocation of NF-κB by inhibiting the accumulation of AGEs and ROS, as well as inhibiting the NLRP3 inflammasome pathway. This reduces the production of pro-inflammatory cytokines (IL-6, IL-10, TNF-α), thereby reducing inflammation and oxidative stress. Promoting epithelial regeneration and angiogenesis: TCM enhances type III collagen synthesis while ECM degradation, thereby accelerating epithelial cell repair. Concurrently, TCM activates the PI3K/Akt and Wnt/β-catenin signaling pathways while inhibiting the Notch pathway to exert pro-angiogenic effects. Modulation of the wound microbiome: Herbal medicines beneficially reduce the abundance of pathogenic bacteria and increase commensal bacteria in wounds without inducing antibiotic resistance, effectively rebalancing the wound microbiome (Thanks for Fig draw).
4 A novel delivery system of herbal active components for the treatment of DFUs
Based on previous studies, it is not difficult to find that the therapeutic effects of single components extracted and isolated from herbal medicines are often inferior to those of chemically synthesized drugs because the latter have enhanced targeted therapeutic properties through chemical modification. It is precisely because many of the active components of herbal medicines have limitations such as poor solubility, poor stability, low bioavailability, short half-life, and lack of targeting. These limitations severely restrict the application of herbal active components to the treatment of DFUs (Alavi et al., 2022). However, recent studies do not support this approach. The combination of herbal active components with novel delivery systems not only has a synergistic healing-promoting effect, but also enhances targeting and reduces toxicity.
4.1 Nanoparticles
Nanoparticles (NPs), as a primary type of modern drug delivery system, are typically based on natural or synthetic materials and form spherical aggregates with drugs through non-covalent interactions. Their proven manufacturing technology and inherent therapeutic properties demonstrate significant potential in DFU treatment. On one hand, nanoparticles are prepared via simple, controllable processes suitable for large-scale production. On the other hand, the functionalities of specific materials—as the antimicrobial properties of silver nanoparticles (Tripathi and Goshisht, 2022), antioxidant effects of cerium oxide nanoparticles (Xu et al., 2023), and broad-spectrum bacteriostatic activity of ZnO nanoparticles (Zhang et al., 2021)—can precisely target the core pathologies of DFU wounds, including inflammation and oxidative stress, thereby achieving a “carrier-treatment” dual advantage.
However, it is not recommended as a stand-alone therapeutic agent due to dose toxicity issues (Lewinski et al., 2008). Recent studies have designed a drug delivery system encapsulating herbal active components that not only retains their therapeutic efficacy but also exerts synergistic effects (Ai et al., 2024). It was found that by encapsulating Resina Draconis extract, which has wound-healing and hemostatic effects, and Rhodiola rosea L. extract, which has antioxidant and anti-inflammatory effects, in novel silver composite nanoparticles (AgNPs) with antimicrobial properties. Compared with the control group, the addition of herbal medicines significantly shortened the inflammatory period by decreasing the levels of inflammatory factors, and promoted angiogenesis by inhibiting LPO and increasing VEGF levels. And the loading of AgNPs was confirmed to significantly promote wound epidermal remodeling and granulation tissue repair compared with different dressing groups. This study reveals that herbal active components based composite nanomaterials can synergistically promote wound healing (Ju et al., 2024b). In another study, aqueous extracts of Tagetes erecta L. and Portulaca oleracea L. were encapsulated in AgNPs and further mixed with a polyherbal gel formulation. This herbal gel achieved 85% wound closure in diabetic rats and further demonstrated that the combination of AgNP-multi-herbal active components enhances antimicrobial activity while promoting DFU repair (Shelar et al., 2025).
4.2 Nanoenzymes
Nanoenzymes, with both nanomaterial properties and natural enzyme catalytic functions, are novel tools for regulating the microenvironment of DFUs. Nanoenzymes can play multiple synergistic roles in dealing with the complex pathological environment of DFUs: nanoenzymes with glucose oxidase (GOx) activity can break down glucose and improve the high-glucose environment (Liao et al., 2025); nanoenzymes mimicking catalase activity clear bacterial infections and inhibit wound microbial film formation (Zhao et al., 2023),Nanoenzymes with SOD- and CAT-like activities can reduce oxidative stress and improve wound ischemia and hypoxia by scavenging ROS (Xiao X. et al., 2024). Notably, the composite system of herbal active components and nano-enzymes can further realize the synergistic and synergistic effects of metabolic regulation and immunomodulation, providing a multidimensional intervention strategy for the treatment of DFUs.
In a recent study, metal-based nano-enzymes (MOFs) were combined with chlorogenic acid (CGA) extracted from Lonicera japonica Thunb and anchored in chitosan hydrogel to obtain a new type of nano-enzymatic hydrogel (MCGC). The results of the study showed that it could play a synergistic role, and the MOFs nano-enzyme and CGA released in the wound could play the catalytic activity of CAT-like enzyme to convert the accumulated H2O2 at the wound site into dissolved oxygen in the wound, alleviate the accumulation of ROS, and increase the oxygenation to promote wound healing. Moreover, CGA can clear the microbial membrane of the wound and reverse the bacterial infection of diabetic wounds (Wei et al., 2024). Another research group developed a novel delivery system for encapsulating astragalus polysaccharide (APS), an active ingredient of traditional Chinese medicine, in borax and iron-modified cerium nanoparticles (Fe/CeNP-PEG). It has been found that it can reduce inflammation by inhibiting the NLRP3/NF-κB signaling pathway, and animal experiments show that the healing rate can reach 97.6% (close to normal tissue). Moreover, the synergistic effect of APS and Fe/CeNP-PEG can dynamically regulate the microenvironment through antioxidant, anti-inflammatory, and pro-angiogenic effects, breaking through the limitations of traditional monotherapy (Zhang X. et al., 2025).
4.3 Exosomes
The latest breakthrough in treating DFUs is exosomes (MSCs-Exo) derived from mesenchymal stem cells (MSCs). One of the reasons for this is that MSCs have the ability to differentiate into a variety of cells that treat DFUs and secrete a variety of cytokines, growth factors, and chemokines that promote the healing of DFUs (Tienda-Vázquez et al., 2023; Wang H. et al., 2025).
Compared with MSC single-cell therapy, the proteins and signaling molecules on the surface of exosomes have strong targeting ability, which can transfer cytokines secreted by MSCs to target cells to regulate cell-to-cell signaling, promote immune response and tissue repair, and show multi-dimensional regulatory effects in wound healing (Bian et al., 2022; Sun et al., 2022). More importantly, adverse reactions such as immune rejection associated with transplantation of MSCs can be avoided (Wang H. et al., 2025). In recent years, studies on the combination of herbal active components and exosomes for wound healing in DFUs have gradually emerged. A recent study confirmed that pretreating mesenchymal stem cells (MSCs) with quercetin—a herbal active ingredient exhibiting anti-inflammatory and antioxidant properties—generated composite exosomes with significantly enhanced capacity to promote cell migration and ECM remodeling compared to untreated exosomes. Moreover, by 16S rRNA sequencing analysis, it was found that it could also play a role in promoting diabetic wound healing by regulating the intestinal microbiota (Wu et al., 2024). A recent study developed a novel vascular-targeting ginseng exosome loaded into a wireless biocompatible thermoelectric hydrogel. By mimicking endogenous electric field stimulation at wound sites, the system precisely controlled the release of pro-angiogenic ginseng exosomes, targeting electrophilic properties in epithelial and fibroblast cells to sustainably reverse endothelial dysfunction. Further experiments confirmed that this approach promoted tissue regeneration and revascularization in DFUs by activating the PPAR signaling pathway (Tan et al., 2025).
Currently, MSCs-Exo for the treatment of DFUs is still in the animal model stage. Several fatal problems still need to be solved for future clinical translation. Firstly, the issue of donor source needs strict regulation, which has failed to form a complete standardization and unification worldwide. Exosomes are under different regulatory models in different countries, in the United States exosomes are classified as drugs; in Europe and Japan, exosomes are classified as biologics (Wang H. et al., 2025). Secondly, as a new type of treatment, exosomes still need to be verified through more clinical trials in the future to see if they are effective in the long term and if there are potential side effects.
4.4 Hydrogels
Advantages of hydrogels for developing tissue scaffolds in DFU healing lie in their structural and functional properties. First, they possess a hydrophilic polymer chain network with three-dimensional cross-linking, closely resembling the natural ECM. This structural mimicry enables effective drug loading and sustained release. Additionally, the hydrogel’s ECM-like environment provides an optimal microenvironment for cellular activities essential to wound repair (Choudhury et al., 2017; Güiza-Argüello et al., 2022). Importantly, the hydrogel is also transparent, allowing real-time monitoring of the complex pathology of DFUs at different stages (Wang X. et al., 2022). Additionally, hydrogels can stop bleeding by adhesion and create a long-term suitably moist local environment for the wound. Also conform to host wound tissue, which prevents excessive inflammatory response and thus protects the wound from infection (Shi et al., 2020; Liang et al., 2021; Gorain et al., 2022).
The combination of herbal active components with hydrogel can extend the action time of them and realize the advantages of “multi-target regulation-intelligent delivery-regulation of micro-environment-synergistic antimicrobial” with the help of advanced material properties of hydrogel. Zhang et al. prepared a composite hydrogel by loading Panax ginseng total saponin (PNS) on hyaluronic acid (HA)/carboxymethyl chitosan (CMCS). By establishing a skin wound model in type II diabetic SD rats, this hydrogel was found to significantly increase the wound healing rate in SD rats. The herbal hydrogel can not only reduce the inflammatory response of the body and reduce the expression of pro-inflammatory factors TNF-α and IL-6 but also enhance the expression of anti-inflammatory factor IL-10. It can also increase the expression of VEGF and play a role in promoting angiogenesis, which synergistically promotes the rapid healing of wounds (Zhang L. et al., 2023). Curcumin, possessing immunomodulatory and angiogenic properties, was formulated into a composite hydrogel. In vivo experiments demonstrated that this system not only scavenges ROS accumulation but also downregulates IL-1β expression while upregulating CD31 expression, thereby promoting angiogenesis and collagen deposition (Fan et al., 2024). In a recent study, a composite hydrogel with antimicrobial and antioxidant properties was made by ligating salvianolic acid B (SAB), which has anti-inflammatory properties and promotes local microcirculation, with Metal-polyphenol nanocomposite hydrogel. Its combined action synergistically promotes the healing of DFUs in an all-encompassing manner. On one hand, SAB and GOx act as anti-infective, microenvironmental modulators and pro-angiogenic agents, and on the other hand, they can also be released to improve mitochondrial energy metabolism through nanoparticles released in response to acidic wounding environments (Gong et al., 2025). Figure 3 summarizes the applications of active components of TCM in combination with novel delivery systems.
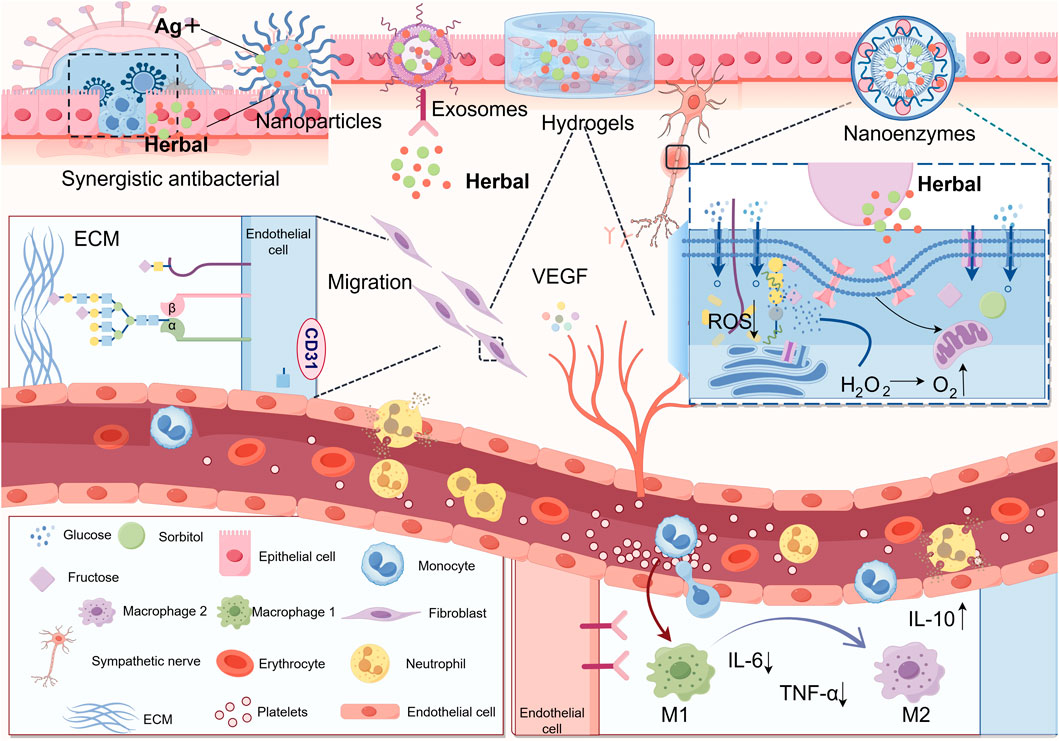
Figure 3. The integration of herbal active components with advanced nanomaterials significantly enhances therapeutic efficacy: (1) Synergistic combination with nanoparticles exhibits potent antibacterial activity by targeting and disrupting bacterial biofilms (2) Conjugation with nanozymes mimicking multienzyme functions enables glucose decomposition to alleviate hyperglycemic conditions, suppresses ROS accumulation to mitigate oxidative stress, and converts hydrogen peroxide into oxygen to improve ischemic-hypoxic wound microenvironments (3) Complexation with exosomes facilitates targeted delivery of herbal constituents, precisely promoting angiogenesis, fibroblast migration, and macrophage polarization regulation at injury sites (4) Incorporation into hydrogels leverages their transparency for real-time monitoring and adjustable release, while providing mechanical support and infection protection for DFU wounds (Thanks for Fig draw).
5 Conclusion and perspectives
This review adopts a molecular biology perspective to compare the mechanisms of normal wound healing versus impaired healing in DFUs. It elucidates the multi-target synergistic effects of herbal medicines and their formulations in DFU management, highlighting a therapeutic paradigm shift from passive debridement to active regeneration. Finally, the paper synthesizes emerging research trends, emphasizing the integration of herbal active components with novel delivery systems. This fusion underscores the promising applications of herbal medicine in interdisciplinary fields such as regenerative medicine and dermal tissue engineering.
Although herbal medicines have demonstrated the therapeutic advantages of multi-target regulation in the treatment of DFUs, which has shifted the healing of DFUs from “passive debridement” to “active regeneration”, there are still many challenges and opportunities in promoting the implementation of Chinese herbal medicine treatment at present. (1) Basic experiments: Existing experimental animal models are insufficient for further clinical translation. The current animal models are only centered around small rodents, which can simulate the pathological process of DFUs but in fact have differences in their human skin structure. In the future, it is necessary to overtake the research towards large animal models such as Ossabaw and Yorkshire pigs, which are highly homologous to human skin, as well as zebrafish juvenile models. In addition, mouse models of MDROs infection should be added, 3D-printed biological models should be developed to simulate DFUs biofilms, and the advantages of herbal medicines over antibiotics should be further evaluated. Future research should continue to explore novel, highly specific biomarkers for DFUs. Leveraging cutting-edge technologies such as single-cell RNA sequencing, multiparameter flow cytometry, and imaging mass cytometry, in-depth analysis of the dynamic DFU microenvironment must be conducted to identify new therapeutic targets and preventive strategies, thereby accelerating the advancement of precision medicine for DFUs. Additionally, ongoing efforts are needed to elucidate whether herbal medicines and their formulations can promote ulcer healing by influencing DFU biomarkers. These collective efforts will facilitate the translation of discoveries from bench to bedside. (2) Clinical trials: Existing clinical trials are mostly small-scale exploratory experiments, and it is recommended that large-scale, multicenter, prospective randomized controlled trials (RCTs) be rigorously designed. To make up for the lack of overall clinical trial data, and to solve the long-term dilemma of the failure of polyherbal therapies to form a widely recognized treatment guideline. In addition, there is a serious gap in long-term efficacy and safety data: currently, most patients are observed after 2–4 weeks of treatment, and it is recommended that systematic and long-term clinical follow-up be included in order to assess the rate of ulcer recurrence and the quality of post-treatment survival, as well as potential toxicity and other risk issues. In the future, a comprehensive assessment of the combined efficacy, safety, and patient prognosis of herbal medicines will provide reliable support for the clinical translation of herbal medicines for DFUs. (3) Quality and control of herbal medicines: Given the inherent complexity of herbal components, variability in extraction processes, and heterogeneity of composite drug delivery systems, we propose a comprehensive quality control framework: For raw herbal materials, standardized cultivation practices should be implemented from the source to eliminate heavy metal and pesticide contamination. Regarding multi-herbal formulations, chromatography and mass spectrometry techniques integrated with Quality Markers (Q-markers) must be adopted to establish quantitative compositional standards. Furthermore, efficacy biomarkers—such as fibroblast migration rate and macrophage polarization ratio—should be incorporated into quality specifications to enhance therapeutic outcomes for DFUs. For mechanistic validation, network pharmacology should be employed to construct “compound-target-disease pathway” models predicting therapeutic mechanisms, followed by rigorous in vitro and in vivo validation to scientifically elucidate the multi-target synergistic mechanisms of herbal formulations, ultimately establishing a standardized quality control system for herbal medicines formulations. (4) The future of novel delivery systems for herbal active components: although the combination of herbal medicines and novel delivery systems can achieve targeted delivery and sustained release deep into the ulcer, given that DFUs wounds are dynamically changing, the future should be designed to be dynamically responsive to the microenvironment of DFUs using PH-responsive, enzyme-responsive, and photo-thermal-responsive composite delivery systems. As well as glucose-sensitive delivery systems targeted at ameliorating peripheral vasculopathy, neuropathy, and smart insulin delivery in patients with DFUs. In recent years, it has been found that modulation of gut flora can affect DFU healing and responsive systems for gut targeting could be designed in the future. In addition, the combined application of 3D printing technology, carbon quantum dot technology, and herbal active ingredients in promoting wound healing for DFUs remains to be widely adopted. (5) Prevention and personalized treatment: Clinically, a stratified prevention system should be established. Type 1 and Type 2 diabetes have heterogeneous pathological foundations, with the latter being the primary conversion type for DFUs due to uncontrolled hyperglycemia. Given the preventable nature of Type 2 diabetes, emphasis should be placed on lifestyle interventions and glycemic control to enable early screening and disease progression interruption. Type 1 diabetes requires lifelong monitoring and complication prevention. The core of ulcer prevention lies in “zero-level prevention”—enhancing patients’ foot self-examination awareness through education and conducting regular systemic complication screenings to facilitate effective early intervention. Furthermore, treatment plans must be individualized: systemic factors (such as the presence of other chronic diseases, vascular disease, neuropathy, and infection), wound characteristics, and lifestyle habits vary among DFUs patients. Adopting a uniform treatment approach is unwise; instead, the key feature of personalized treatment is delivering the right therapy to the patient at the right time, rather than using all possible curative treatments. Integrating artificial intelligence (AI) and wearable devices enables dynamic monitoring and precise stage-based intervention, complemented by the immune-regulating and microcirculation-improving functions of TCM to reconstruct the body’s wound-healing capacity. On this basis, integrating global healthcare systems ensures that patients have no concerns regarding post-treatment care. (6) Comprehensive treatment strategy: In order to cope with DFUs, a chronic disease with extremely complex pathomechanisms and a lengthy course. It is urgent to promote a comprehensive treatment strategy. In a clinical trial of 100 patients with DFUs, treatment with ultrasonic debridement in combination with cortex phellodendri compound fluid showed a significant reduction in the size of the ulcers compared to the control group after 4 weeks of treatment and an overall treatment efficacy of up to 98 percent (Gao et al., 2022). A growing number of basic trials and clinical studies have revealed the therapeutic efficacy and economic advantages of herbal medicines; herbal therapies will be transformed from “empirical medicine” to “multi-targeted therapies with clear mechanisms.” Therefore, in the future, the overall advantages of herbal medicines treatment will be combined with modern medical methods to promote an integrated treatment strategy in order to provide better therapeutic effects and prognostic management for patients with DFUs.
In the future, we should advance the integration of herbal medicines with personalized treatment and precision medicine to accelerate breakthroughs in overcoming the bottlenecks in treating DFU wounds.
Author contributions
BF: Writing – original draft, Writing – review and editing, Investigation, Conceptualization. JD: Writing – original draft, Writing – review and editing. YL: Writing – review and editing, Supervision. YW: Writing – review and editing. CW: Writing – review and editing. XL: Writing – review and editing. YS: Writing – review and editing. XZ: Writing – review and editing, Data curation, Resources, Supervision, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (number 81873161); Heilongjiang Province “Unveiling the Leader” Program (number 2023ZXJ02C02); Heilongjiang Province “Outstanding Young Teachers Basic Research Support Program” Program (number YQJH 2023150); Heilongjiang University of Traditional Chinese Medicine Program (number 2019JC01) and Heilongjiang University of Traditional Chinese Medicine Undergraduate Science and Technology Innovation (number KY 2022-08), National Key Laboratory for the Integration and Innovation of Classical Formulas and Modern Traditional Chinese Medicine (number LSLSKL20240403 and Li Yongji National Heritage Studio of Master Herbalists.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ai, S., Li, Y., Zheng, H., Zhang, M., Tao, J., Liu, W., et al. (2024). Collision of herbal medicine and nanotechnology: a bibliometric analysis of herbal nanoparticles from 2004 to 2023. J. Nanobiotechnology 22, 140. doi:10.1186/s12951-024-02426-3
Aitcheson, S. M., Frentiu, F. D., Hurn, S. E., Edwards, K., and Murray, R. Z. (2021). Skin wound healing: normal macrophage function and macrophage dysfunction in diabetic wounds. Molecules 26, 4917. doi:10.3390/molecules26164917
Alavi, M., Moetasam Zorab, M., Ashengroph, M., Aljelehawy, Q. H. A., and Kahrizi, D. (2022). Antibacterial and wound healing applications of curcumin in micro and nano-scaffolds based on chitosan, cellulose, and collagen. Cell Mol. Biol. (Noisy-le-Grand) 68, 9–14. doi:10.14715/cmb/2022.68.3.2
Asadipooya, K., and Uy, E. M. (2019). Advanced glycation end products (AGEs), receptor for AGEs, diabetes, and bone: review of the literature. J. Endocr. Soc. 3, 1799–1818. doi:10.1210/js.2019-00160
Barman, P. K., and Koh, T. J. (2020). Macrophage dysregulation and impaired skin wound healing in diabetes. Front. Cell Dev. Biol. 8, 528. doi:10.3389/fcell.2020.00528
Bian, D., Wu, Y., Song, G., Azizi, R., and Zamani, A. (2022). The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: a comprehensive review. Stem Cell Res. Ther. 13, 24. doi:10.1186/s13287-021-02697-9
Brings, S., Fleming, T., Freichel, M., Muckenthaler, M. U., Herzig, S., and Nawroth, P. P. (2017). Dicarbonyls and advanced glycation end-products in the development of diabetic complications and targets for intervention. Int. J. Mol. Sci. 18, 984. doi:10.3390/ijms18050984
Bus, S. A., Monteiro-Soares, M., Game, F., van Netten, J. J., Apelqvist, J., Fitridge, R., et al. (2024a). Standards for the development and methodology of the 2023 IWGDF guidelines. Diabetes Metab. Res. Rev. 40, e3656. doi:10.1002/dmrr.3656
Bus, S. A., Sacco, I. C. N., Monteiro-Soares, M., Raspovic, A., Paton, J., Rasmussen, A., et al. (2024b). Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2023 update). Diabetes Metab. Res. Rev. 40, e3651. doi:10.1002/dmrr.3651
Catrina, S.-B., and Zheng, X. (2016). Disturbed hypoxic responses as a pathogenic mechanism of diabetic foot ulcers. Diabetes Metab. Res. Rev. 32 (Suppl. 1), 179–185. doi:10.1002/dmrr.2742
Chen, H., Hongmei, Z., and Wang, J. (2023). Clinical observation of Sanhuang Xiaoyan prescription combined with wet dressing change of silver dressing in treatment of diabetic foot infection (multiple drug resistant bacteria). Liaoning J. Traditional Chin. Med., 82–84. doi:10.13192/j.issn.1000-1719.2023.06.022
Chen, J., Qu, B., Yang, D., Wang, Y., Zhu, H., Wang, Z., et al. (2025). Combined metabolomics and network pharmacology to elucidate the mechanisms of Huiyang Shengji decoction in treating diabetic skin ulcer mice. Phytomedicine 141, 156569. doi:10.1016/j.phymed.2025.156569
Choudhury, H., Gorain, B., Pandey, M., Chatterjee, L. A., Sengupta, P., Das, A., et al. (2017). Recent update on nanoemulgel as topical drug delivery system. J. Pharm. Sci. 106, 1736–1751. doi:10.1016/j.xphs.2017.03.042
Da Silva, J., Figueiredo, A., Tseng, Y.-H., Carvalho, E., and Leal, E. C. (2025). Bone morphogenetic protein 7 improves wound healing in diabetes by decreasing inflammation and promoting M2 macrophage polarization. Int. J. Mol. Sci. 26, 2036. doi:10.3390/ijms26052036
Darby, I. A., Laverdet, B., Bonté, F., and Desmoulière, A. (2014). Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol 7, 301–311. doi:10.2147/CCID.S50046
Dayya, D., O’Neill, O. J., Huedo-Medina, T. B., Habib, N., Moore, J., and Iyer, K. (2022). Debridement of diabetic foot ulcers. Adv. Wound Care 11, 666–686. doi:10.1089/wound.2021.0016
Deng, L., Du, C., Song, P., Chen, T., Rui, S., Armstrong, D. G., et al. (2021). The role of oxidative stress and antioxidants in diabetic wound healing. Oxid. Med. Cell Longev. 2021, 8852759. doi:10.1155/2021/8852759
Deng, P., Liang, H., Wang, S., Hao, R., Han, J., Sun, X., et al. (2022). Combined metabolomics and network pharmacology to elucidate the mechanisms of dracorhodin perchlorate in treating diabetic foot ulcer rats. Front. Pharmacol. 13, 1038656. doi:10.3389/fphar.2022.1038656
Deng, H., Li, B., Shen, Q., Zhang, C., Kuang, L., Chen, R., et al. (2023). Mechanisms of diabetic foot ulceration: a review. J. Diabetes 15, 299–312. doi:10.1111/1753-0407.13372
Deng, L., Wang, G., and Ju, S. (2024). Correlation between inflammatory factors, autophagy protein levels, and infection in granulation tissue of diabetic foot ulcer. Immun. Inflamm. Dis. 12, e1233. doi:10.1002/iid3.1233
Deng, J., Gan, W., Hu, C., Liu, Z., Chen, N., Jia, C., et al. (2025). San Huang Xiao Yan recipe promoted wound healing in diabetic ulcer mice by inhibiting Th17 cell differentiation. J. Ethnopharmacol. 341, 119243. doi:10.1016/j.jep.2024.119243
Ding, X., Yang, C., Li, Y., He, T., Xu, Y., Cheng, X., et al. (2024). Reshaped commensal wound microbiome via topical application of Calvatia gigantea extract contributes to faster diabetic wound healing. Burns Trauma 12, tkae037. doi:10.1093/burnst/tkae037
Eckes, B., Nischt, R., and Krieg, T. (2010). Cell-matrix interactions in dermal repair and scarring. Fibrogenesis Tissue Repair 3, 4. doi:10.1186/1755-1536-3-4
Fan, X., Huang, J., Zhang, W., Su, Z., Li, J., Wu, Z., et al. (2024). A multifunctional, tough, stretchable, and transparent curcumin hydrogel with potent antimicrobial, antioxidative, anti-inflammatory, and angiogenesis capabilities for diabetic wound healing. ACS Appl. Mater. Interfaces 16, 9749–9767. doi:10.1021/acsami.3c16837
Fu, K., Zheng, X., Chen, Y., Wu, L., Yang, Z., Chen, X., et al. (2022). Role of matrix metalloproteinases in diabetic foot ulcers: potential therapeutic targets. Front. Pharmacol. 13, 1050630. doi:10.3389/fphar.2022.1050630
Gao, H., Chen, J., Zhao, Z., and Wang, G. (2022). A combination of ultrasonic debridement and topical cortex phellodendri compound fluid in patients with diabetic foot ulcers. Medicine 101, e29604. doi:10.1097/md.0000000000029604
Gawlowski, T., Stratmann, B., Stirban, A. O., Negrean, M., and Tschoepe, D. (2007). AGEs and methylglyoxal induce apoptosis and expression of Mac-1 on neutrophils resulting in platelet-neutrophil aggregation. Thromb. Res. 121, 117–126. doi:10.1016/j.thromres.2007.03.002
GBD 2021 Diabetes Collaborators (2023). Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the global Burden of Disease Study 2021. Lancet 402, 203–234. doi:10.1016/S0140-6736(23)01301-6
GBD 2021 Diseases and Injuries Collaborators (2024). Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the global Burden of Disease Study 2021. Lancet 403, 2133–2161. doi:10.1016/S0140-6736(24)00757-8
Gong, H., Yang, L., Li, Y., Zhang, X., Zheng, C., Gan, T., et al. (2025). Metal-polyphenol nanocomposite hybrid hydrogel: a multifunctional platform for treating diabetic foot ulcers through metabolic microenvironment reprogramming. Biomaterials 322, 123414. doi:10.1016/j.biomaterials.2025.123414
Gorain, B., Pandey, M., Leng, N. H., Yan, C. W., Nie, K. W., Kaur, S. J., et al. (2022). Advanced drug delivery systems containing herbal components for wound healing. Int. J. Pharm. 617, 121617. doi:10.1016/j.ijpharm.2022.121617
Güiza-Argüello, V. R., Solarte-David, V. A., Pinzón-Mora, A. V., Ávila-Quiroga, J. E., and Becerra-Bayona, S. M. (2022). Current advances in the development of hydrogel-based wound dressings for diabetic foot ulcer treatment. Polym. (Basel) 14, 2764. doi:10.3390/polym14142764
Hassanshahi, A., Moradzad, M., Ghalamkari, S., Fadaei, M., Cowin, A. J., and Hassanshahi, M. (2022). Macrophage-mediated inflammation in skin wound healing. Cells 11, 2953. doi:10.3390/cells11192953
Hicks, C. W., and Selvin, E. (2019). Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr. Diabetes Rep. 19, 86. doi:10.1007/s11892-019-1212-8
Huang, L., and Zeng, J. (2020a). Effect of Huiyang Shengji decoction combined with Kangfuxin liquid on postoperative wound healing in patients with anal fistula and type 2 diabetes. Hunan J. Traditional Chin. Med., 10–12. doi:10.16808/j.cnki.issn1003-7705.2020.05.004
Huang, W., Jiao, J., Liu, J., Huang, M., Hu, Y., Ran, W., et al. (2020b). MFG-E8 accelerates wound healing in diabetes by regulating “NLRP3 inflammasome-neutrophil extracellular traps” axis. Cell Death Discov. 6, 84. doi:10.1038/s41420-020-00318-7
Huang, Y., Wang, J.-S., Yang, L., Yue, L., Zhang, L., Zhang, Y.-H., et al. (2020c). Paeoniflorin ameliorates glycemic variability-induced oxidative stress and platelet activation in HUVECs and DM rats. RSC Adv. 10, 42605–42612. doi:10.1039/d0ra02036b
Huang, Y.-Y., Lin, C.-W., Cheng, N.-C., Cazzell, S. M., Chen, H.-H., Huang, K.-F., et al. (2021). Effect of a novel macrophage-regulating drug on wound healing in patients with diabetic foot ulcers: a randomized clinical trial. JAMA Netw. Open 4, e2122607. doi:10.1001/jamanetworkopen.2021.22607
Huang, C., Dong, L., Zhao, B., Lu, Y., Huang, S., Yuan, Z., et al. (2022). Anti-inflammatory hydrogel dressings and skin wound healing. Clin. Transl. Med 12, e1094. doi:10.1002/ctm2.1094
Huang, F., Lu, X., Yang, Y., Yang, Y., Li, Y., Kuai, L., et al. (2023). Microenvironment-based diabetic foot ulcer nanomedicine. Adv. Sci. (Weinh) 10, e2203308. doi:10.1002/advs.202203308
Huijberts, M. S. P., Schaper, N. C., and Schalkwijk, C. G. (2008). Advanced glycation end products and diabetic foot disease. Diabetes/Metabolism Res. Rev. 24, S19–S24. doi:10.1002/dmrr.861
Jarić, S., Kostić, O., Mataruga, Z., Pavlović, D., Pavlović, M., Mitrović, M., et al. (2018). Traditional wound-healing plants used in the Balkan region (Southeast Europe). J. Ethnopharmacol. 211, 311–328. doi:10.1016/j.jep.2017.09.018
Jeffcoate, W., Boyko, E. J., Game, F., Cowled, P., Senneville, E., and Fitridge, R. (2024). Causes, prevention, and management of diabetes-related foot ulcers. Lancet Diabetes Endocrinol. 12, 472–482. doi:10.1016/S2213-8587(24)00110-4
Jiang, J.-S., Zhang, Y., Luo, Y., Ru, Y., Luo, Y., Fei, X.-Y., et al. (2021). The identification of the biomarkers of Sheng-Ji Hua-Yu formula treated diabetic wound healing using modular pharmacology. Front. Pharmacol. 12, 726158. doi:10.3389/fphar.2021.726158
Jnana, A., Muthuraman, V., Varghese, V. K., Chakrabarty, S., Murali, T. S., Ramachandra, L., et al. (2020a). Microbial community distribution and core microbiome in successive wound grades of individuals with diabetic foot ulcers. Appl. Environ. Microbiol. 86, e02608-19. doi:10.1128/AEM.02608-19
Jnana, A., Muthuraman, V., Varghese, V. K., Chakrabarty, S., Murali, T. S., Ramachandra, L., et al. (2020b). Microbial community distribution and core microbiome in successive wound grades of individuals with diabetic foot ulcers. Appl. Environ. Microbiol. 86, e02608-19. doi:10.1128/aem.02608-19
Ju, Y., Luo, Y., Li, R., Zhang, W., Ge, Y., and Tang, J. (2024a). Multifunctional combined drug-loaded nanofibrous dressings with anti-inflammatory, antioxidant stress and microenvironment improvement for diabetic wounds. RSC Adv. 14, 29606–29623. doi:10.1039/D4RA04860A
Ju, Y., Luo, Y., Li, R., Zhang, W., Ge, Y., and Tang, J. (2024b). Multifunctional combined drug-loaded nanofibrous dressings with anti-inflammatory, antioxidant stress and microenvironment improvement for diabetic wounds. RSC Adv. 14, 29606–29623. doi:10.1039/d4ra04860a
Kalan, L. R., Meisel, J. S., Loesche, M. A., Horwinski, J., Soaita, I., Chen, X., et al. (2019). Strain- and species-level variation in the microbiome of diabetic wounds is associated with clinical outcomes and therapeutic efficacy. Cell Host Microbe 25, 641–655. doi:10.1016/j.chom.2019.03.006
Khalid, M., Petroianu, G., and Adem, A. (2022). Advanced glycation end products and diabetes mellitus: mechanisms and perspectives. Biomolecules 12, 542. doi:10.3390/biom12040542
Kim, H. S., Sun, X., Lee, J.-H., Kim, H.-W., Fu, X., and Leong, K. W. (2019). Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 146, 209–239. doi:10.1016/j.addr.2018.12.014
Kim, J., Hurh, K., Han, S., Kim, H., Park, E.-C., and Jang, S.-Y. (2024). Association between antidepressants and the risk of diabetic foot ulcers and amputation in antidepressant-naïve type 2 diabetes mellitus patients: a nested case-control study. Diabetes Res. Clin. Pract. 209, 111591. doi:10.1016/j.diabres.2024.111591
Kuai, L., Zhang, J., Deng, Y., Xu, S., Xu, X., Wu, M., et al. (2018). Sheng-Ji Hua-Yu formula promotes diabetic wound healing of re-epithelization via activin/follistatin regulation. BMC Complement. Altern. Med. 18, 32. doi:10.1186/s12906-017-2074-8
Lan, T., Li, Z., and Chen, J. (2023). FusionSegNet: fusing global foot features and local wound features to diagnose diabetic foot. Comput. Biol. Med. 152, 106456. doi:10.1016/j.compbiomed.2022.106456
Lewinski, N., Colvin, V., and Drezek, R. (2008). Cytotoxicity of nanoparticles. Small 4, 26–49. doi:10.1002/smll.200700595
Li, S., Yang, P., Ding, X., Zhang, H., Ding, Y., and Tan, Q. (2022a). Puerarin improves diabetic wound healing via regulation of macrophage M2 polarization phenotype. Burns Trauma 10, tkac046. doi:10.1093/burnst/tkac046
Li, X.-Y., Zhang, X.-T., Jiao, Y.-C., Chi, H., Xiong, T.-T., Zhang, W.-J., et al. (2022b). In vivo evaluation and mechanism prediction of anti-diabetic foot ulcer based on component analysis of Ruyi Jinhuang powder. World J. Diabetes 13, 622–642. doi:10.4239/wjd.v13.i8.622
Li, S., Ding, X., Yan, X., Qian, J., and Tan, Q. (2023). ceAF ameliorates diabetic wound healing by alleviating inflammation and oxidative stress via TLR4/NF-κB and Nrf2 pathways. J. Diabetes Res. 2023, 2422303–2422312. doi:10.1155/2023/2422303
Liang, Y., He, J., and Guo, B. (2021). Functional hydrogels as wound dressing to enhance wound healing. ACS Nano 15, 12687–12722. doi:10.1021/acsnano.1c04206
Liao, Y., Zhang, Z., Zhao, Y., Zhang, S., Zha, K., Ouyang, L., et al. (2025). Glucose oxidase: an emerging multidimensional treatment option for diabetic wound healing. Bioact. Mater 44, 131–151. doi:10.1016/j.bioactmat.2024.10.006
Lin, Z., Li, L.-Y., Chen, L., Jin, C., Li, Y., Yang, L., et al. (2024). Lonicerin promotes wound healing in diabetic rats by enhancing blood vessel regeneration through Sirt1-mediated autophagy. Acta Pharmacol. Sin. 45, 815–830. doi:10.1038/s41401-023-01193-5
Lin, L., Yu, F., Tang, X., Cai, W., Wang, Y., Hong, Y., et al. (2025). Huiyang Shengji decoction promotes healing of diabetic skin ulcers via the NF-κB/STAT3/NLRP3 signaling pathway: a multi-omics analysis. Phytomedicine 143, 156695. doi:10.1016/j.phymed.2025.156695
Liu, C., Ponsero, A. J., Armstrong, D. G., Lipsky, B. A., and Hurwitz, B. L. (2020). The dynamic wound microbiome. BMC Med. 18, 358. doi:10.1186/s12916-020-01820-6
Liu, Y., Li, Y., Du, Y., Huang, T., and Zhu, C. (2020). Multicenter clinical trials analyzing efficacy and safety of topical cortex phellodendri compound fluid in treatment of diabetic foot ulcers. Med. Sci. Monit. 26, e923424. doi:10.12659/MSM.923424
Liu, Q., Zhang, J., Han, X., Chen, J., Zhai, Y., Lin, Y., et al. (2021). Huiyang Shengji decoction promotes wound healing in diabetic mice by activating the EGFR/PI3K/ATK pathway. Chin. Med. 16, 111. doi:10.1186/s13020-021-00497-0
Liu, F.-S., Li, Y., Guo, X.-S., Liu, R.-C., Zhang, H.-Y., and Li, Z. (2022). Advances in traditional Chinese medicine as adjuvant therapy for diabetic foot. World J. Diabetes 13, 851–860. doi:10.4239/wjd.v13.i10.851
Lou, J., Xiang, Z., Zhu, X., Li, J., Jin, G., Cui, S., et al. (2025). Skin microbiota and diabetic foot ulcers. Front. Microbiol. 16, 1575081. doi:10.3389/fmicb.2025.1575081
Mao, X., Li, Z., Li, B., and Wang, H. (2021). Baicalin regulates mRNA expression of VEGF-c, Ang-1/Tie2, TGF-β and Smad2/3 to inhibit wound healing in streptozotocin-induced diabetic foot ulcer rats. J. Biochem. Mol. Toxicol. 35, e22893. doi:10.1002/jbt.22893
McDermott, K., Fang, M., Boulton, A. J. M., Selvin, E., and Hicks, C. W. (2022). Etiology, epidemiology, and disparities in the burden of diabetic foot ulcers. Diabetes Care 46, 209–221. doi:10.2337/dci22-0043
Michalak, M. (2023). Plant extracts as skin care and therapeutic agents. Int. J. Mol. Sci. 24, 15444. doi:10.3390/ijms242015444
Mudrik-Zohar, H., Carasso, S., Gefen, T., Zalmanovich, A., Katzir, M., Cohen, Y., et al. (2022). Microbiome characterization of infected diabetic foot ulcers in association with clinical outcomes: traditional cultures versus molecular sequencing methods. Front. Cell Infect. Microbiol. 12, 836699. doi:10.3389/fcimb.2022.836699
Nirenjen, S., Narayanan, J., Tamilanban, T., Subramaniyan, V., Chitra, V., Fuloria, N. K., et al. (2023). Exploring the contribution of pro-inflammatory cytokines to impaired wound healing in diabetes. Front. Immunol. 14, 1216321. doi:10.3389/fimmu.2023.1216321
Nussbaum, S. R., Carter, M. J., Fife, C. E., DaVanzo, J., Haught, R., Nusgart, M., et al. (2018). An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health J. Int. Soc. Pharmacoeconomics Outcomes Res. 21, 27–32. doi:10.1016/j.jval.2017.07.007
Peña, O. A., and Martin, P. (2024). Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 25, 599–616. doi:10.1038/s41580-024-00715-1
Peng, H., Xian, D., Liu, J., Pan, S., Tang, R., and Zhong, J. (2020). Regulating the polarization of macrophages: a promising approach to vascular dermatosis. J. Immunol. Res. 2020, 8148272. doi:10.1155/2020/8148272
Pouget, C., Dunyach-Remy, C., Pantel, A., Schuldiner, S., Sotto, A., and Lavigne, J.-P. (2020). Biofilms in diabetic foot ulcers: significance and clinical relevance. Microorganisms 8, 1580. doi:10.3390/microorganisms8101580
Qadir, A., Jahan, S., Aqil, M., Warsi, M. H., Alhakamy, N. A., Alfaleh, M. A., et al. (2021). Phytochemical-based nano-pharmacotherapeutics for management of burn wound healing. Gels 7, 209. doi:10.3390/gels7040209
Qiu, F., Fan, S., Diao, Y., Liu, J., Li, B., Li, K., et al. (2024). The mechanism of Chebulae Fructus Immaturus promote diabetic wound healing based on network pharmacology and experimental verification. J. Ethnopharmacol. 322, 117579. doi:10.1016/j.jep.2023.117579
Raziyeva, K., Kim, Y., Zharkinbekov, Z., Kassymbek, K., Jimi, S., and Saparov, A. (2021). Immunology of acute and chronic wound healing. Biomolecules 11, 700. doi:10.3390/biom11050700
Reinke, J. M., and Sorg, H. (2012). Wound repair and regeneration. Eur. Surg. Res. Eur. Chir. Forschung. Recherches Chir. Eur. 49, 35–43. doi:10.1159/000339613
Ru, Y., Zhang, Y., Xiang, Y., Luo, Y., Luo, Y., Jiang, J., et al. (2022). Gene set enrichment analysis and ingenuity pathway analysis to identify biomarkers in Sheng-ji Hua-yu formula treated diabetic ulcers. J. Ethnopharmacol. 285, 114845. doi:10.1016/j.jep.2021.114845
Sen, C. K. (2021). Human wound and its burden: updated 2020 compendium of estimates. Adv. Wound Care 10, 281–292. doi:10.1089/wound.2021.0026
Sharifiaghdam, M., Shaabani, E., Faridi-Majidi, R., De Smedt, S. C., Braeckmans, K., and Fraire, J. C. (2022). Macrophages as a therapeutic target to promote diabetic wound healing. Mol. Ther. 30, 2891–2908. doi:10.1016/j.ymthe.2022.07.016
Shelar, K., Salve, P. S., Qutub, M., Tammewar, S., Tatode, A. A., and Hussain, U. M. (2025). Advanced bioinspired silver nanoparticles integrated into polyherbal gel for enhanced diabetic foot ulcer regeneration. Biol. Trace Elem. Res. doi:10.1007/s12011-025-04666-2
Shi, C., Wang, C., Liu, H., Li, Q., Li, R., Zhang, Y., et al. (2020). Selection of appropriate wound dressing for various wounds. Front. Bioeng. Biotechnol. 8, 182. doi:10.3389/fbioe.2020.00182
Singer, A. J. (2022). Healing mechanisms in cutaneous wounds: tipping the balance. Tissue Eng. Part B Rev. 28, 1151–1167. doi:10.1089/ten.teb.2021.0114
Sun, X., Wang, X., Zhao, Z., Chen, J., Li, C., and Zhao, G. (2021). Paeoniflorin inhibited nod-like receptor protein-3 inflammasome and NF-κB-mediated inflammatory reactions in diabetic foot ulcer by inhibiting the chemokine receptor CXCR2. Drug Dev. Res. 82, 404–411. doi:10.1002/ddr.21763
Sun, B., Wu, F., Wang, X., Song, Q., Ye, Z., Mohammadniaei, M., et al. (2022). An optimally designed engineering exosome-reductive COF integrated nanoagent for synergistically enhanced diabetic fester wound healing. Small 18, e2200895. doi:10.1002/smll.202200895
Sun, J., You, Y., Fan, W., Yuan, M., Wang, H., and Liu, G. (2025). Mechanism of inhibition of PAD4-mediated NETs formation by Zi Zhu ointment to promote wound healing in diabetic mice. J. Hainan Med. Uni. 1, 18. doi:10.13210/j.cnki.jhmu.20250429.001
Sutherland, T. E., Dyer, D. P., and Allen, J. E. (2023). The extracellular matrix and the immune system: a mutually dependent relationship. Sci. (New York, N.Y.) 379, eabp8964. doi:10.1126/science.abp8964
Szułdrzyński, K., Jankowski, M., Potaczek, D. P., and Undas, A. (2020). Plasma fibrin clot properties as determinants of bleeding time in human subjects: association with histidine-rich glycoprotein. Dis. Markers 2020, 7190828. doi:10.1155/2020/7190828
Tan, M., Liu, Y., Wang, Y., Li, Y., Wu, C., Jiang, Z., et al. (2025). Wireless thermoelectric hydrogel recreates biomimetic electric field and angiogenic signal accelerating diabetic ulcer repair. Adv. Funct. Mater. 35, 2425610. doi:10.1002/adfm.202425610
Tang, Y.-B., Uwimana, M. M. P., Zhu, S.-Q., Zhang, L.-X., Wu, Q., and Liang, Z.-X. (2022). Non-coding RNAs: role in diabetic foot and wound healing. World J. Diabetes 13, 1001–1013. doi:10.4239/wjd.v13.i12.1001
Tang, G., Wang, Y., Deng, P., Wu, J., Lu, Z., Zhu, R., et al. (2025). Mechanism of dracorhodin in accelerating diabetic foot ulcer healing via the Nrf2 pathway, a network pharmacology, molecular docking and experimental validation. Sci. Rep. 15, 12492. doi:10.1038/s41598-025-97831-5
Tienda-Vázquez, M. A., Hanel, J. M., Márquez-Arteaga, E. M., Salgado-Álvarez, A. P., Scheckhuber, C. Q., Alanis-Gómez, J. R., et al. (2023). Exosomes: a promising strategy for repair, regeneration and treatment of skin disorders. Cells 12, 1625. doi:10.3390/cells12121625
Tripathi, N., and Goshisht, M. K. (2022). Recent advances and mechanistic insights into antibacterial activity, antibiofilm activity, and cytotoxicity of silver nanoparticles. ACS Appl. Bio. Mater 5 (4), 1391–1463. doi:10.1021/acsabm.2c00014
Tomic-Canic, M., Burgess, J. L., O’Neill, K. E., Strbo, N., and Pastar, I. (2020). Skin microbiota and its interplay with wound healing. Am. J. Clin. Dermatol. 21, 36–43. doi:10.1007/s40257-020-00536-w
Uberoi, A., McCready-Vangi, A., and Grice, E. A. (2024). The wound microbiota: microbial mechanisms of impaired wound healing and infection. Nat. Rev. Microbiol. 22, 507–521. doi:10.1038/s41579-024-01035-z
von Schoen-Angerer, T., Manchanda, R. K., Lloyd, I., Wardle, J., Szöke, J., Benevides, I., et al. (2023). Traditional, complementary and integrative healthcare: global stakeholder perspective on WHO’s current and future strategy. BMJ Glob. Health 8, e013150. doi:10.1136/bmjgh-2023-013150
Wang, J., Wang, Y., Huang, R., Li, W., Fan, W., Hu, X., et al. (2022). Uncovering the pharmacological mechanisms of Zizhu ointment against diabetic ulcer by integrating network analysis and experimental evaluation in vivo and in vitro. Front. Pharmacol. 13, 1027677. doi:10.3389/fphar.2022.1027677
Wang, X., Yuan, c.-X., Xu, B., and Yu, Z. (2022). Diabetic foot ulcers: classification, risk factors and management. World J. Diabetes 13, 1049–1065. doi:10.4239/wjd.v13.i12.1049
Wang, G., Hu, S., Shen, F., Wang, Q., Ye, B., Wang, X., et al. (2025). Sheng-ji Hua-yu Formula promotes diabetic ulcer healing via regulating the cAMP/PKA/CREB signaling pathway. J. Ethnopharmacol. 351, 120105. doi:10.1016/j.jep.2025.120105
Wang, H., Wu, S., Bai, X., Pan, D., Ning, Y., Wang, C., et al. (2025). Mesenchymal stem cell-derived exosomes hold promise in the treatment of diabetic foot ulcers. Int. J. Nanomedicine 20, 5837–5857. doi:10.2147/IJN.S516533
Wang, R., Wang, Q., Liu, M., Xiao, H., Zhang, G., Yao, J., et al. (2025). Jingfang granules for diabetic wound healing: insights from network pharmacology and experimental validation. Drug Des. Dev. Ther. 19, 4835–4860. doi:10.2147/dddt.s516298
Wang, J., Yang, X., Zhou, T., Yan, S., Wang, S., Xia, L., et al. (2025a). Exploration of the molecular mechanism of Gubu decoction in regulating miR-155/FGF7 pathway mediated macrophage polarization to treat diabetic foot ulcers. J. Hainan Med. Univ. 1, 17. doi:10.13210/j.cnki.jhmu.20250327.006
Wang, J., Yang, X., Zhou, T., Yan, S., Wang, S., Xia, L., et al. (2025b). Exploring the mechanism of Gubu Decoction in treating diabetes foot ulcer based on HIF-1α/TGF-β1/Smad3 pathway. J. Hainan Med. Univ. 1, 16. doi:10.13210/j.cnki.jhmu.20250331.003
Wei, Y.-J., Chen, H., Zhou, Z.-W., Liu, C.-X., Cai, C.-X., Li, J., et al. (2024). Kill two birds with one stone: Dual-Metal MOF-Nanozyme-Decorated hydrogels with ROS-Scavenging, Oxygen-generating, and antibacterial abilities for accelerating infected diabetic wound healing. Small 20, e2403679. doi:10.1002/smll.202403679
Wilkinson, H. N., and Hardman, M. J. (2020). Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 10, 200223. doi:10.1098/rsob.200223
Wu, M., Huang, J., Shi, J., Shi, L., Zeng, Q., and Wang, H. (2022). Ruyi Jinhuang powder accelerated diabetic ulcer wound healing by regulating Wnt/β-catenin signaling pathway of fibroblasts in vivo and in vitro. J. Ethnopharmacol. 293, 115321. doi:10.1016/j.jep.2022.115321
Wu, S., Zhou, Z., Li, Y., Wu, R., and Jiang, J. (2024). Pretreatment of human umbilical cord mesenchymal stem cell-derived exosomes with quercetin enhances the healing of diabetic skin wounds by modulating host-microbiota interactions. Int. J. Nanomedicine 19, 12557–12581. doi:10.2147/IJN.S491471
Xiang, Y., Kuai, L., Ru, Y., Jiang, J., Li, X., Li, F., et al. (2021). Transcriptional profiling and circRNA-miRNA-mRNA network analysis identify the biomarkers in Sheng-ji Hua-yu formula treated diabetic wound healing. J. Ethnopharmacol. 268, 113643. doi:10.1016/j.jep.2020.113643
Xiao, F., Rui, S., Zhang, X., Ma, Y., Wu, X., Hao, W., et al. (2024a). Accelerating diabetic wound healing with Ramulus Mori (Sangzhi) alkaloids via NRF2/HO-1/eNOS pathway. Phytomedicine 134, 155990. doi:10.1016/j.phymed.2024.155990
Xiao, X., Zhao, F., DuBois, D. B., Liu, Q., Zhang, Y. L., Yao, Q., et al. (2024b). Nanozymes for the therapeutic treatment of diabetic foot ulcers. ACS Biomater. Sci. Eng. 10, 4195–4226. doi:10.1021/acsbiomaterials.4c00470
Xie, X., Liu, X., Li, Y., Luo, L., Yuan, W., Chen, B., et al. (2020). Advanced glycation end products enhance biofilm formation by promoting extracellular DNA release through sigB upregulation in Staphylococcus aureus. Front. Microbiol. 11, 1479. doi:10.3389/fmicb.2020.01479
Xu, H., Li, S., Ma, X., Xue, T., Shen, F., Ru, Y., et al. (2023). Cerium oxide nanoparticles in diabetic foot ulcer management: advances, limitations, and future directions. Colloids Surf. B Biointerfaces 231, 113535. doi:10.1016/j.colsurfb.2023.113535
Yang, S., Gu, Z., Lu, C., Zhang, T., Guo, X., Xue, G., et al. (2020). Neutrophil extracellular traps are markers of wound healing impairment in patients with diabetic foot ulcers treated in a multidisciplinary setting. Adv. Wound Care (New Rochelle) 9, 16–27. doi:10.1089/wound.2019.0943
Yang, S., Wang, S., Chen, L., Wang, Z., Chen, J., Ni, Q., et al. (2023). Neutrophil extracellular traps delay diabetic wound healing by inducing endothelial-to-mesenchymal transition via the Hippo pathway. Int. J. Biol. Sci. 19, 347–361. doi:10.7150/ijbs.78046
Yang, G., Wang, G., Li, Z., Deng, L., Wang, N., Wang, X., et al. (2024a). Efficacy and pharmacoeconomic advantages of Fufang Huangbai Fluid hydropathic compress in diabetic foot infections: a comparative clinical study with antimicrobial calcium alginate wound dressing. Front. Pharmacol. 15, 1285946. doi:10.3389/fphar.2024.1285946
Yang, Y., Huang, S., Ma, Q., Li, N., Li, R., Wang, Y., et al. (2024b). Combined therapeutic strategy based on blocking the deleterious effects of AGEs for accelerating diabetic wound healing. Regen. Biomater. 11, rbae062. doi:10.1093/rb/rbae062
Yuan, N., Shao, K., Huang, S., and Chen, C. (2023). Chitosan, alginate, hyaluronic acid and other novel multifunctional hydrogel dressings for wound healing: a review. Int. J. Biol. Macromol. 240, 124321. doi:10.1016/j.ijbiomac.2023.124321
Zhan, H.-B., Sun, Q.-Q., Yan, L., and Cai, J. (2021). Clinical study of MEBO combined with Jinhuang powder for diabetic foot with infection. Evid. Based Complement. Altern. Med. 2021, 5531988. doi:10.1155/2021/5531988
Zhang, J., Zhou, R., Xiang, C., Jia, Q., Wu, H., and Yang, H. (2020). Huangbai liniment accelerated wound healing by activating Nrf2 signaling in diabetes. Oxid. Med. Cell Longev. 2020, 4951820. doi:10.1155/2020/4951820
Zhang, J., Hu, K., Di, L., Wang, P., Liu, Z., Zhang, J., et al. (2021). Traditional herbal medicine and nanomedicine: converging disciplines to improve therapeutic efficacy and human health. Adv. Drug Deliv. Rev. 178, 113964. doi:10.1016/j.addr.2021.113964
Zhang, J. J., Zhou, R., Deng, L. J., Cao, G. Z., Zhang, Y., Xu, H., et al. (2022). Huangbai liniment and berberine promoted wound healing in high-fat diet/streptozotocin-induced diabetic rats. Biomed. Pharmacother. 150, 112948. doi:10.1016/j.biopha.2022.112948
Zhang, L., Tan, W., Zhang, M., Ma, Z., Zhao, T., and Zhang, Y. (2023a). Preparation and characterization of Panax notoginseng saponins loaded hyaluronic acid/carboxymethyl chitosan hydrogel for type o diabetic wound healing. Mater. Today Commun. 34, 105284. doi:10.1016/j.mtcomm.2022.105284
Zhang, Z., Chen, T., Liu, W., Xiong, J., Jiang, L., and Liu, M. (2023b). Paeonol accelerates skin wound healing by regulating macrophage polarization and inflammation in diabetic rats. Korean J. Physiol. Pharmacol. 27, 437–448. doi:10.4196/kjpp.2023.27.5.437
Zhang, Z., Zheng, Y., Chen, N., Xu, C., Deng, J., Feng, X., et al. (2023c). San Huang Xiao Yan recipe modulates the HMGB1-mediated abnormal inflammatory microenvironment and ameliorates diabetic foot by activating the AMPK/Nrf2 signalling pathway. Phytomedicine 118, 154931. doi:10.1016/j.phymed.2023.154931
Zhang, H., Dong, X., Liu, Y., Duan, P., Liu, C., Liu, K., et al. (2025a). An injectable and adaptable system for the sustained release of hydrogen sulfide for targeted diabetic wound therapy by improving the microenvironment of inflammation regulation and angiogenesis. Acta Biomater. 196, 364–379. doi:10.1016/j.actbio.2025.02.048
Zhang, J., Shi, Y., Wang, J., Gao, M., Zhong, S., Chen, Y., et al. (2025b). Mechanisms of Huhuang decoction in treating diabetic wounds: a network pharmacological and experimental study. Int. J. Med. Sci. 22, 1811–1824. doi:10.7150/ijms.108187
Zhang, Q., Li, J., Wang, L., Zhang, Q.-Y., and Guan, J. (2025c). Exploring the molecular mechanism of Si-Miao-Yong-An Decoction in treating diabetic foot using network pharmacological analysis and molecular docking technique. Medicine 104, e42629. doi:10.1097/md.0000000000042629
Zhang, S., Shao, Y., Jin, R., and Ma, B. (2025d). Combining network pharmacology, molecular docking, molecular dynamics simulation, and experimental validation to uncover the efficacy and mechanisms of Si-Miao-Yong-An decoction in diabetic wound healing. J. Inflamm. Res. 18, 4087–4101. doi:10.2147/jir.s506739
Zhang, X., Wen, S., Liu, Q., Cai, W., Ning, K., Liu, H., et al. (2025e). Multi-functional nanozyme-integrated astragalus polysaccharide hydrogel for targeted phased therapy in diabetic wound healing. Nano Today 62, 102739. doi:10.1016/j.nantod.2025.102739
Zhang, Y., Lin, S., Xu, X., Yao, Y., Feng, S., Jiang, S., et al. (2025f). Programmable hierarchical hydrogel dressing for sequential release of growth factor and DNase to accelerate diabetic wound healing. J. Control Release 383, 113825. doi:10.1016/j.jconrel.2025.113825
Zhao, Y., Wang, Q., Yan, S., Zhou, J., Huang, L., Zhu, H., et al. (2021). Bletilla striata polysaccharide promotes diabetic wound healing through inhibition of the NLRP3 inflammasome. Front. Pharmacol. 12, 659215. doi:10.3389/fphar.2021.659215
Zhao, L., Zhou, S., Leng, J., and Yu, X. (2021). Analysis of effect of huangma tincture combined with sanhuang xiaoyan formula on diabetic foot ulcer. Chin. Arch. Tradit. Chin. 159–162. doi:10.13193/j.issn.1673-7717.2021.10.038
Zhao, Y., Wang, D., Qian, T., Zhang, J., Li, Z., Gong, Q., et al. (2023). Biomimetic nanozyme-decorated hydrogels with H2O2-Activated oxygenation for modulating immune microenvironment in diabetic wound. ACS Nano 17, 16854–16869. doi:10.1021/acsnano.3c03761
Zhou, R., Xiang, C., Cao, G., Xu, H., Zhang, Y., Yang, H., et al. (2021). Berberine accelerated wound healing by restoring TrxR1/JNK in diabetes. Clin. Sci. (Lond) 135, 613–627. doi:10.1042/CS20201145
Zhou, X., Guo, Y., Yang, K., Liu, P., and Wang, J. (2022). The signaling pathways of traditional Chinese medicine in promoting diabetic wound healing. J. Ethnopharmacol. 282, 114662. doi:10.1016/j.jep.2021.114662
Keywords: diabetic foot ulcers, polyherbal formulations, herbal medicines, novel drug delivery systems, regenerative medicine, herbal active components
Citation: Feng B, Dou J, Li Y, Wang Y, Wu C, Lan X, Su Y and Zhang X (2025) Management of diabetic foot ulcers: polyherbal formulations and novel delivery systems for herbal active components. Front. Pharmacol. 16:1648540. doi: 10.3389/fphar.2025.1648540
Received: 17 June 2025; Accepted: 05 September 2025;
Published: 03 October 2025.
Edited by:
Vinod Kumar Yata, Malla Reddy University, IndiaReviewed by:
Prafulla Kumar, Sardar Vallabhbhai Patel University of Agriculture and Technology, IndiaAnjaneyulu Musini, Jawaharlal Nehru Technological University, Hyderabad, India
Copyright © 2025 Feng, Dou, Li, Wang, Wu, Lan, Su and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiwu Zhang, MTQ5NzcyMTA1QHFxLmNvbQ==
†These authors have contributed equally to this work
 Bowen Feng1†
Bowen Feng1† Jinjin Dou
Jinjin Dou Yihan Wang
Yihan Wang Xiwu Zhang
Xiwu Zhang