- Molecular Neuropsychiatry Research Branch, DHHS/NIH/NIDA Intramural Research Program, Baltimore, MD, United States
Background: The number of individuals diagnosed with opioid use disorder (OUD) has risen steeply because of increased prescribing of opioid drugs including oxycodone for chronic pain relief. When rats given extended access to oxycodone only a subset of animals self-administers more drug over time. Identifying the molecular mechanism associated with this behavior can introduce novel ways to combat OUD. Herein, we sought to identify the alteration in the expression of voltage gated and calcium activated potassium channels after extended access to oxycodone self-administration.
Methods: We used male Sprague-Dawley rats that self-administered oxycodone for 20 days according to short-access (ShA, 3 h per day) and long-access (LgA, 9 h per day) paradigms.
Results: LgA rats escalated their oxycodone intake and developed into two phenotypes, named long-access high (LgA-H, escalated intake) and long-access low (LgA-L, non-escalated intake) rats, based on the quantities of oxycodone intake during the self-administration experiment. ShA rats maintained similar oxycodone intake throughout 20 days of self-administration. Rats were euthanized 2 h after the last self-administration session and their prefrontal cortex (PFC), nucleus accumbens (NAc), and hippocampus (HIP) were dissected out for gene expression analysis. Given the relationship between potassium channels and substance use disorder we performed gene expression analysis for voltage and calcium activated potassium channels. The expression of potassium channels in oxycodone self-administered rats was found to be brain region dependent. Specifically, LgA-H rats displayed increased expression of Kcnd2, Kcnd3, Kcng2 and Kcnt1 in their NAc. In the PFC, LgA-L group showed higher mRNA levels for Kcna3, Kcna4, Kcnd3, Kcnq4, Kcnq5, Kcnma1 and Kcnn2. Finally, Kcna5, Kcna10, Kcng1, Kcnn1 and Kcnn2 found to be upregulated in the HIP of ShA rats.
Conclusion: Our observation is of significant translational importance providing further support that targeting potassium channel can lead to development of better therapeutic approaches against OUD in humans.
1 Introduction
The opioid epidemic remains a major public health crisis (Bergeria and Strain, 2022; Boscarino et al., 2010), despite efforts to reduce the overprescription of pain medications like oxycodone (King et al., 2011; Marie and Noble, 2023). Repeated oxycodone use often leads to tolerance and dependence among patients (Ellis et al., 2019; Kibaly et al., 2021), which can escalate to the misuse of more potent opioids, resulting in neuropsychiatric and neuropathological complications (Blackwood and Cadet, 2021; Cadet et al., 2014; Chamakalayil et al., 2024), and fatal overdoses (Sahebi-Fakhrabad et al., 2024; Tsang and Rodda, 2024). Oxycodone use disorder (OUD) is a biopsychosocial disorder in which someone loses control of drug taking even after the presence of adverse consequences (APA, 2024).
In 2024, the United States experienced a decline in overdose deaths according to data from the Centers for Disease Control and Prevention (CDC) which indicates a 14% decrease from the previous year (Ahmad FB et al., 2025). It is important to note that policy shifts that allowed over-the-counter naloxone sales and broadened Good Samaritan protections led to higher bystander intervention rates, played a measurable role in curbing opioid-related deaths. Meaning the public is likely to be abusing opioids at similar rates (Bohler et al., 2023). This decline represents the first substantial reduction in overdose fatalities in several years, validating the effectiveness of current research methods and offering hope for a potential reversal of the opioid epidemic’s trajectory. Ongoing efforts are essential to sustain and further this trend in reducing overdose fatalities.
Pharmacological treatments for OUD have traditionally focused on opioid receptor-related systems (Ali et al., 2024; Grande et al., 2023; Hayes et al., 2024; Olson et al., 2017). Advancing these treatments requires a deeper understanding of how repeated oxycodone use affects the human brain. Previously, we investigated this by utilizing an animal model that simulates key aspects of OUD and explore the molecular pathways impacted by oxycodone use (Blackwood and Cadet, 2021; Blackwood et al., 2019a; Salisbury et al., 2020). These studies from the past have confirmed a significant correlated relationship between potassium channels and substance use disorder (Cadet et al., 2017; Jayanthi et al., 2020). Potassium channels are important due to their roles in maintaining membrane potential, generating action potentials (Bean, 2007; Jan & January, 2012; Trimmer, 2015), facilitating neurotransmitter release (Klein et al., 1980), and supporting rhythmic neuronal firing (Noble, 1976; Solessio et al., 2000).
This current study focused on two specific subcategories of potassium channels, voltage gated, and calcium activated potassium channels. These potassium channels play crucial roles in regulating neuronal excitability and neurotransmitter release (Agarwal et al., 2025). Voltage-gated potassium channels (Kv) are essential for action potential repolarization, thus influencing neuronal firing patterns (Shah and Aizenman, 2014). By facilitating the return of neurons to their resting state, Kv channels regulate synaptic activity, a critical role in the brains reward pathways (Ramirez-Navarro et al., 2024). There are eleven families in the Kv group, which are organized by their subunit composition, location in the cell and voltage threshold (Alfaro-Ruíz et al., 2019). Calcium-activated potassium channels (KCa) are activated by intracellular calcium levels and contribute to modulating synaptic plasticity, a key process underlying learning, memory, and habit formation (Kuiper et al., 2012). There are three groups of KCa: small conductance like Kcnn1, Kcnn2 and Kcnn3, Large conductance like Kcnma1, and sodium activated like Kcnt1 (Agarwal et al., 2025; McCoy et al., 2021). Small conductance KCa play an important role in synaptic plasticity and brain rhythmic activity (Sun et al., 2020).
Irregularities in either type of potassium channel can disrupt normal neuronal signaling, enhancing reward-related behaviors and increasing vulnerability to substance abuse (Alam et al., 2023). Previous studies suggest that impaired potassium channel function may alter dopamine release, which in turn alters excitability within the brain’s reward circuitry, contributing to the development and persistence of addictive behaviors (Blackwood et al., 2019b; Daiwile et al., 2022; Pignatelli and Bonci, 2015; Shah and Aizenman, 2014). To further understand the relationship between potassium channels and OUD, we examined transcriptional changes in the prefrontal cortex (PFC), nucleus accumbens (NAc) and the hippocampus (Hip) in rats that self-administered small or large amounts of oxycodone over a 20-day period.
2 Materials and methods
2.1 Subjects
Male Sprague Dawley rats, weighing between 350 and 400 g were procured from Charles River, Kingston, NY, United States. The rats were housed in a controlled setting with a reversed 12-h light/dark cycle with free access to food and water. All self-administration sessions began (∼9:00 a.m. everyday) at the start of the dark phase of the light/dark cycle. All experimental procedures adhered to the guidelines outlined in the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the NIDA (National Institute of Drug Abuse) Animal Care and Use Committee at the Intramural Research Program (IRP).
2.2 Intravenous surgery
Rats were first anesthetized using a combination of ketamine (50 mg/kg) and xylazine (5 mg/kg). A polyurethane catheter was surgically inserted into the right jugular vein while the external end was mounted to the back of the rat (Blackwood et al., 2019a). The rats were then given a recovery period of 7 days before beginning self-administration training.
2.3 Oxycodone self-administration
Following an established protocol, drug-naive rats were trained to self-administer oxycodone (0.1 mg/kg/infusion) or saline within a sound-attenuated chamber using a FR1 schedule (Blackwood et al., 2019b). A total of 36 rats were divided into three groups: Saline (Sal) (n = 8), Short-access (ShA) (n = 10), or Long-access (LgA) (n = 18). Short-access rats were allowed to self-administer oxycodone for only one 3-h session for the entirety of the study (days 1–20). Long-access and saline rats were scheduled to self-administer for three sessions using, one 3-h session during days 1–5, followed by two 3-h sessions during days 6–10 and then three 3-h sessions for the rest of the study (days 11–20). The 20-day timeframe was selected based on previous paradigms in the literature that reliably produce escalation and allow for the emergence of compulsive-like drug intake behaviors (Blackwood and Cadet, 2021; Blackwood et al., 2019a; Blackwood et al., 2019b).
We gradually increased access to oxycodone over 4 weeks to prevent any adverse effects of oxycodone intake. There was a 20 s timeout between each infusion. Each 3-h session for LgA and Sal from day 6 to day 20 was separated by a 30-min timeout. This 30-min break was implemented to prevent overdoses as there was no limit to the number of infusions a rat could take during a session. We also included a 48h weekend abstinence period between every 5 days of SA to prevent significant weight loss that might have led to the elimination of some rats from the study. Catheter patency was tested thought the experiment. Rats were euthanized 2 hours after the first session of the last day. Saline animals underwent similar surgical procedures as oxycodone rats, were placed in the identical operant chambers, and experienced similar cue presentations during SA sessions.
2.4 RNA extraction and cDNA conversion
Two hours after the final self-administration session, rats were euthanized via rapid decapitation using a guillotine. PFC, NAc and HIP tissues were dissected using precise neuroanatomical coordinates using the Atlas (Paxinos and Watson, 2006) and then immediately snap-frozen on dry ice before being stored at −80 °C (Blackwood et al., 2019b). Total RNA was extracted from tissue using Qiagen RNeasy Mini kit (Qiagen, Valencia, CA, United States). A half microgram (0.5 μg) of total RNA was reverse-transcribed to cDNA with oligo dT primers using Advantage RT-for-PCR kit (Clontech, Mountain View, CA, United States).
2.5 Quantitative RT-PCR
qRT-PCR was carried out using a Roche LightCycler 480 II (Roche Diagnostics, Indianapolis, IN) with Luna Universal qPCR SYBR GREEN (NEB Inc, Ipswich, MA) following the manufacturer’s protocol. We purchased gene-specific primers from Integrated DNA Technologies (IDT) (Coralville, IA, United States). These primers were designed using Thermo Fisher Scientific (OligoPrefect Primer Designer software). We normalized mRNA using beta-2 microglobulin (B2M), Clathrin, and ornithine decarboxylase antizyme (OAZ1) as reference genes and the mRNA expression of target genes were reported as fold changes. The primer sequences used for PCR are listed in Supplementary Table S1.
2.6 Statistical analyses
Behavioral data were analyzed with the statistical program GraphPad Prism 10 using factorial ANOVA with repeated measures. Independent variables were the rat reward types (Sal, ShA, LgA-L, LgA-H), within-subject factor SA day (training days 1–20). Oxycodone intake served as the dependent variable. A second-degree polynomial regression model was used to identify potential non-linear patterns in oxycodone intake over 20 days of SA for individual animals, to segregate the rats into LgA-H and LgA-L subgroups. The rats which escalated their intake were termed as LgA-H where those who did not were termed as LgA-L. Biochemical data were analyzed using one-way ANOVA followed by the Tukey’s multiple comparisons test if the main effect was significant. The slopes of all the regression lines were calculated using one-way ANOVA. Statistical significance for all hypothesis tests was set at p < 0.05.
3 Results
3.1 Some rats exposed to LgA oxycodone self-administration escalated their drug intake
As shown previously (Blackwood et al., 2019a) we analyzed the behavioral data using repeated measures two-way ANOVA with groups (ShA vs. LgA) and training weeks as factors. We observed significant effects for group [F(1, 49) = 37.6, p < 0.0001], training week [F(1.994, 97.71) = 26.56, p < 0.0001], and group × training week [F(3, 147) = 23.51, p < 0.0001]. Post-hoc test showed LgA rats had greater total oxycodone intake than ShA and Sal rats (Figure 1A).
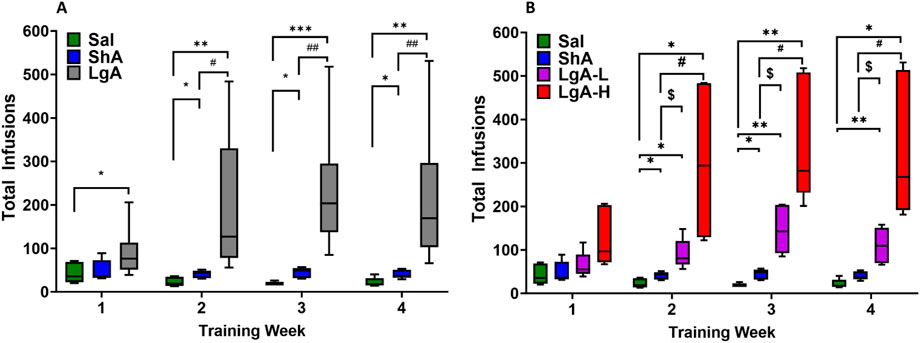
Figure 1. Insights into Oxycodone Self-Administration: Behavioral Data. (A) Total infusions by Sal (8), LgA (18) and ShA (10) groups. (B) LgA rats show two distinct intake phenotypes, high (LgA-H) (n = 11) and low (LgA-L) (n = 7) based on their drug intake. Key to statistics: *, **, *** = p < 0.05, 0.01, 0.001, LgA-H, LgA-L, or ShA in comparison to saline rats; #, ##, = p < 0.05, 0.01 when comparing LgA-H/LgA rats to ShA rats; $, = p < 0.05, when comparing LgA-L rats to ShA rats.
Interestingly, not all LgA rats self-administered oxycodone to the same degree. We performed regression analyses to compare oxycodone acquisition and rate of change of oxycodone intake over time (Blackwood et al., 2019b). We found that some LgA rats significantly escalated their oxycodone intake over the period of 20 days, whereas others did not escalate their intake. Animals that escalated their oxycodone intake over 20 days of SA were called Long-access High (LgA-H), whereas those that did not escalate were named Long-access Low (LgA-L) (Figure 1B). We reanalyzed the behavioral data with four phenotypes LgA-H, LgA-L, ShA, and Sal. Two-way ANOVA showed significant effects for groups [F(2, 48) = 86.30, p < 0.0001], oxycodone intake [F(2.594, 124.5) = 82.67, p < 0.0001], and group × oxycodone intake interaction [F(6, 144) = 34.17, p < 0.0001] (Figure 1B).
Previous studies reasoned that the differences at the behavioral level may be due to different drug-induced molecular neuroadaptations in potassium channel expression between the different phenotypes (McCoy et al., 2021). Building on these findings, we sought to investigate the molecular mechanisms underlying potassium channel differences to better understand their role in OUD vulnerability.
3.2 Prefrontal cortex (PFC)
The ANOVA for voltage gated potassium channels (Kv) in the PFC showed significant effects of treatment group on Kcna1 [F(3, 25) = 3.612, p = 0.0271], Kcna3 [F(3, 26) = 3.097, p = 0.0442], Kcna4 [F(3, 26) = 4.735, p = 0.0091], Kcnb1 [F(3, 27) = 3.344, p = 0.0338], Kcnb2 [F(3, 23) = 4.037, p = 0.0192], Kcnd2 [F(3, 27) = 4.028, p = 0.0172], Kcnd3 [F(3, 29) = 22.43, p < 0.0001], Kcnq1 [F(3, 28) = 14.87, p < 0.0001], Kcnq2 [F(3, 26) = 3.615, p = 0.0264], Kcnq3 [F(3, 28) = 6.843, p = 0.0013], Kcnq4 [F(3, 27) = 5.265, p = 0.0054], and Kcnq5 [F(3, 26) = 5.099, p = 0.0066] (Figures 2A–L). For Kcna1 and Kcnb1, LgA-H rats revealed decreased mRNA expression compared to Sal (Figures 2A,D). LgA-L rats showed elevated mRNA levels for Kcna3 and Kcna4 when compared to LgA-H (Figures 2B,C). Likewise LgA-L rats displayed increased expression of Kcnq4, and Kcnq5 when compared to Sal and LgA-H (Figures 2K,L). LgA-L rats and ShA rats exhibited higher expression of Kcnb2 compared to the LgA-H phenotype (Figure 2E). Only ShA animals unveiled increased Kcnd2 expression when compared to Sal and LgA-H rats (Figure 2F), while for Kcnq3 this increase was only if compared to the Sal phenotype (Figure 2J). Kcnd3’s mRNA level was found to be decreased among LgA-H and ShA rats when compared to Sal, whereas LgA-L rats showed increased expression compared to Sal, LgA-H and ShA (Figure 2G). The mRNA level of Kcnq1 was found to be decreased among LgA-H, LgA-L and ShA, in addition there was a significant difference between the magnitude of LgA-L’s downregulation and that of LgA-H and ShA (Figure 2H). We also observed increased expression of Kcnq2 in the PFC of ShA rats than Sal (Figure 2I). No significance was found for Kcna2, Kcna5, Kcna6, Kcnd1, Kcng1, Kcng2, Kcng3, and Kcng4 (Supplementary Figure S1A–H).
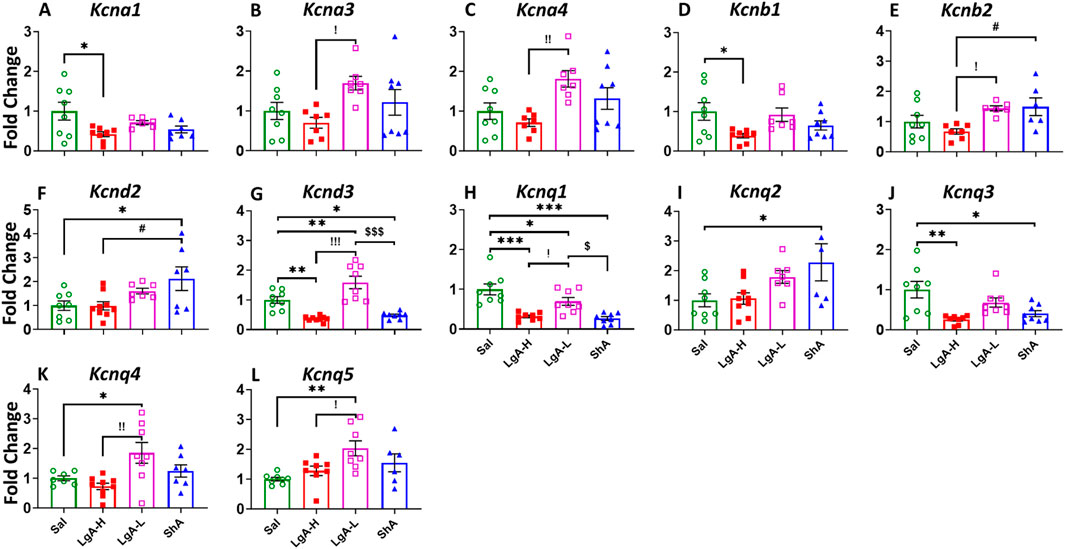
Figure 2. Voltage gated potassium channels showed increased expression in Prefrontal Cortex of LgA-L rats. (A) Kcna1, (B) Kcna3, (C) Kcna4, (D) Kcnb1, (E) Kcnb2, (F) Kcnd2, (G) Kcnd3, (H) Kcnq1, (I) Kcnq2, (J) Kcnq3, (K) Kcnq4, and (L) Kcnq5. Key to statistics: *, *** = p < 0.05, 0.001, LgA-H, LgA-L, or ShA in comparison to saline rats; #, ##, ### = p < 0.05, 0.01, 0.001, when comparing LgA-H rats to ShA rats; $, $$ = p < 0.05, 0.01, when comparing LgA-L rats to ShA rats. !, !!, !!! = p < 0.05, 0.01, 0.001, when comparing LgA-H rats to LgA-L rats.
We also analyzed the results for calcium-activated potassium channels (KCa), ANOVA revealed significant effects for Kcnma1 [F(3, 28) = 34.98, p < 0.0001], Kcnn1 [F(3, 28) = 3.923, p = 0.0186], Kcnn2 [F(3, 26) = 20.96, p < 0.0001], Kcnt1 [F(3, 28) = 3.65, p = 0.0244], and Kcnt2 [F(3, 28) = 4.493, p = 0.0107] (Figures 3A–E). LgA-L revealed increased mRNA expression for Kcnma1 and Kcnn2 in their PFC when compared to Sal, LgA-H and ShA rats (Figures 3A,C). LgA-L also displayed higher expression then LgA-H for Kcnt2 (Figure 3E). Interestingly ShA showed decreased mRNA level for Kcnn1 when compared with LgA-L (Figure 3B) and increased expression for Kcnt1 when compared to LgA-H rats (Figure 3D).
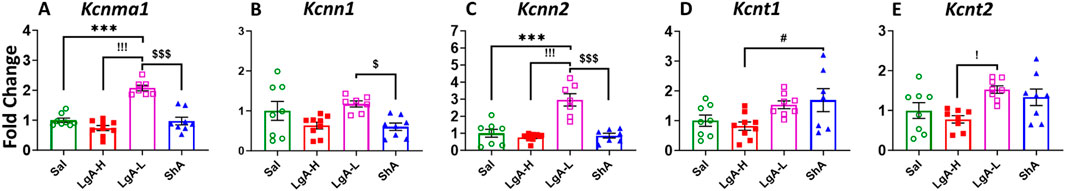
Figure 3. Calcium activated potassium channels showed increased expression in Prefrontal Cortex of LgA-L. (A) Kcnma1, (B) Kcnn1, (C) Kcnn2, (D) Kcnt1, and (E) Kcnt2. Key to statistics: *, *** = p < 0.05, 0.001, LgA-H, LgA-L, or ShA in comparison to saline rats; #, ##, ### = p < 0.05, 0.01, 0.001, when comparing LgA-H rats to ShA rats; $, $$ = p < 0.05, 0.01, when comparing LgA-L rats to ShA rats. !, !!, !!! = p < 0.05, 0.01, 0.001, when comparing LgA-H rats to LgA-L rats.
3.3 Nucleus accumbens (NAc)
We observed a significant one-way ANOVA results in the rats NAc for Kcnb1 [F(3, 27) = 3.268, p = 0.0365], Kcnb2 [F(3, 27) = 3.785, p = 0.0218], Kcnd1 [F(3, 25) = 5.122, p = 0.0067], Kcnd2 [F(3, 28) = 4.043, p = 0.0166], Kcnd3 [F(3, 26) = 7.580, p = 0.0008], Kcng2 [F(3, 29) = 4.037, p = 0.0163], Kcnq2 [F(3, 27) = 4.868, p = 0.0078], and Kcnq3 [F(3, 25) = 3.841, p = 0.0217] (Figures 4A–H). Moreover, LgA-H rats revealed a significant increase for Kcnb1, Kcnd1, Kcnq2, and Kcnq3 when compared to LgA-L (Figures 4A,C,G,H) and exhibited higher Kcnb2 expression when compared with ShA (Figure 4B). Higher mRNA level for Kcnd2 and Kcng2 was also seen in LgA-H rats than LgA-L and ShA rats (Figures 4D,F). Likewise, only LgA-H rats had higher expression of Kcnd3 than Sal, LgA-L and ShA rats (Figure 4E). No significance changes were found for Kcna1, Kcna2, Kcna3, Kcna4, Kcna5, Kcna6, Kcna10, Kcng1, Kcng3, Kcng4, Kcnq1, Kcnq4, and Kcnq5 (Supplementary Figure S2A–M).
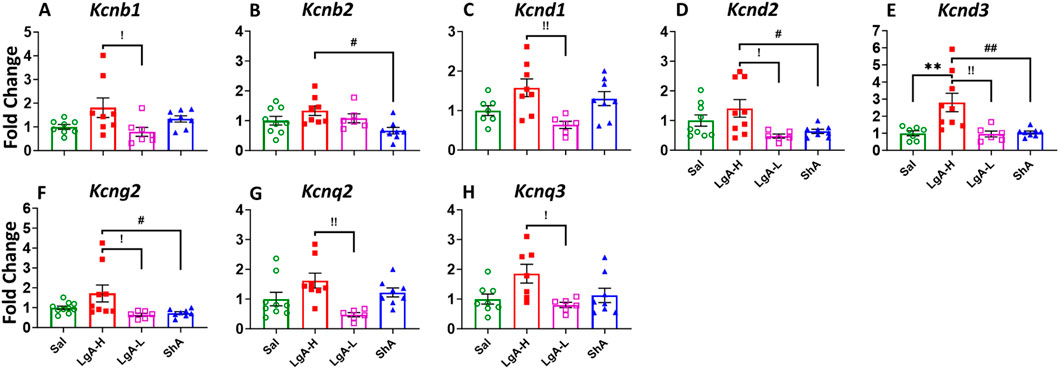
Figure 4. Voltage gated potassium channels showed increased expression in Nucleus Accumbens of LgA-H rats. (A) Kcnb1, (B) Kcnb2, (C) Kcnd1, (D) Kcnd2, (E) Kcnd3, (F) Kcng2, (G) Kcnq2, and (H) Kcnq3. Key to statistics: *, *** = p < 0.05, 0.001, LgA-H, LgA-L, or ShA in comparison to saline rats; #, ##, ### = p < 0.05, 0.01, 0.001, when comparing LgA-H rats to ShA rats; $, $$ = p < 0.05, 0.01, when comparing LgA-L rats to ShA rats. !, !!, !!! = p < 0.05, 0.01, 0.001, when comparing LgA-H rats to LgA-L rats.
Like that of Kv, KCa revealed significant effects of treatment group on Kcnn2 [F(3, 29) = 3.398, p = 0.0309], Kcnt1 [F(3, 27) = 6.787, p = 0.0015] and Kcnt2 [F(2, 25) = 3.499, p = 0.0302] (Figures 5A–C). mRNA expression levels of Kcnn2 and Kcnt2 was higher in LgA-H animals when compared to Sal (Figures 5A,C). LgA-H rats also revealed a higher level for Kcnt1 when compared with LgA-L and ShA rats (Figure 5C). No changes were seen for Kcnn1 (Supplementary Figure S3A).
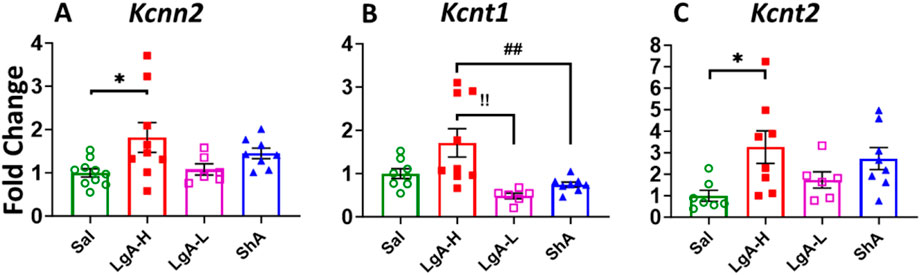
Figure 5. Calcium activated potassium channels showed increased expression in Nucleus Accumbens of LgA-H rats. (A) Kcnn2, (B) Kcnt1, and (C) Kcnt2. Key to statistics: *, *** = p < 0.05, 0.001, LgA-H, LgA-L, or ShA in comparison to saline rats; #, ##, ### = p < 0.05, 0.01, 0.001, when comparing LgA-H rats to ShA rats; $, $$ = p < 0.05, 0.01, when comparing LgA-L rats to ShA rats. !, !!, !!! = p < 0.05, 0.01, 0.001, when comparing LgA-H rats to LgA-L rats.
3.4 Hippocampus (HIP)
When looking at Kv in the HIP we found a significant ANOVA effect of treatment group on Kcna2 [F(3, 29) = 10.08, p = 0.0001], Kcna5 [F(3, 28) = 5.066, p = 0.0063], Kcna10 [F(3, 27) = 3.778, p = 0.0220], Kcng1 [F(3, 29) = 3.342, p = 0.0328], Kcng2 [F(3, 30) = 3.132, p = 0.0401], and Kcnq3 [F(3, 30) = 3.834, p = 0.0195] (Figures 6A–F). Further LgA-H rats displayed significant increase in the expression of Kcna2 compared to Sal, LgA-L and ShA (Figure 6A) and for Kcnq3 then ShA (Figure 5F). Whereas ShA animals revealed higher mRNA level for Kcna5 and Kcna10 than LgA-H and LgA-L (Figures 5B,C) and for Kcng1 only when compared to LgA-L (Figure 5D). While LgA-L phenotype revealed elevated mRNA level for Kcng1 when compared with LgA-H rats (Figure 5E). No significance difference was found for Kcna1, Kcna3, Kcna6, Kcnb1, Kcnb2, Kcnd1, Kcngd2, Kcnd3, Kcng3, Kcng4, Kcnq1, Kcnq2, Kcnq4, and Kcnq5 (Supplementary Figure S4A–N).
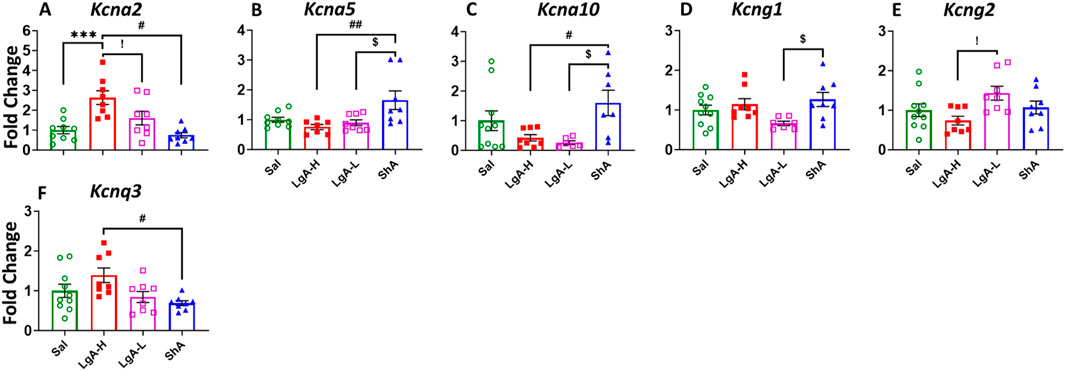
Figure 6. Voltage gated potassium channels showed increased expression in Hippocampus of ShA rats. (A) Kcna2, (B) Kcna5, (C) Kcna10, (D) Kcng1, (E) Kcng2, and (F) Kcnq3. Key to statistics: *, *** = p < 0.05, 0.001, LgA-H, LgA-L, or ShA in comparison to saline rats; #, ##, ### = p < 0.05, 0.01, 0.001, when comparing LgA-H rats to ShA rats; $, $$ = p < 0.05, 0.01, when comparing LgA-L rats to ShA rats. !, !!, !!! = p < 0.05, 0.01, 0.001, when comparing LgA-H rats to LgA-L rats.
Moreover, we also saw a significant effect for Kcnma1 [F(3, 30) = 5.024, p = 0.0061], Kcnn1 [F(3, 29) = 4.851, p = 0.0074], and Kcnn2 [F(3, 29) = 5.134, p = 0.0057] (Figures 7A–C). ShA rats displayed significant higher mRNA levels for Kcnn1 and Kcnn2 than LgA-H rats (Figures 7B,C). Interestingly, expression of Kcnma1 mRNA found to be significantly increased in the LgA-H group compared to Sal, LgA-L and ShA (Figure 7A). No significance was found for Kcnt1, Kcnt2 and Kcnn3 (Supplementary Figure S5A–C).
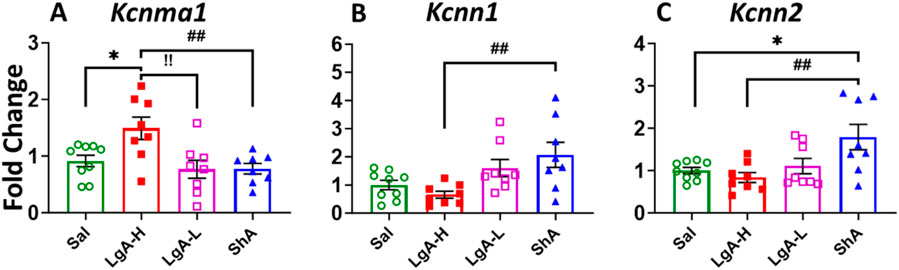
Figure 7. Calcium activated potassium channels showed increased expression in Hippocampus of ShA rats. (A) Kcna1, (B) Kcnn1, and (C) Kcnn2. Key to statistics: *, *** = p < 0.05, 0.001, LgA-H, LgA-L, or ShA in comparison to saline rats; #, ##, ### = p < 0.05, 0.01, 0.001, when comparing LgA-H rats to ShA rats; $, $$ = p < 0.05, 0.01, when comparing LgA-L rats to ShA rats. !, !!, !!! = p < 0.05, 0.01, 0.001, when comparing LgA-H rats to LgA-L rats.
In the present study, we observed significant differences among the experimental groups, including: (1) LgA-H compared to LgA-L and/or ShA; (2) LgA-L compared to LgA-H and/or ShA; and (3) ShA compared to LgA-H and/or LgA-L. These differences may reflect inherent phenotypic variability or adaptive processes not directly attributable to the self-administration (SA) procedure. Rather than representing direct drug-induced molecular changes, such variability could result from pre-existing individual differences or secondary effects of prolonged drug exposure and behavioral stratification. Therefore, we propose that future studies should not limit comparisons to control groups alone but also include contrasts among drug-exposed groups to better capture the contribution of intrinsic variability and adaptive mechanisms to substance use disorder.
4 Discussion
The present study assessed potential changes in the expression of voltage-gated and calcium-activated potassium channels in the mesocorticolimbic projection areas of rats who were allowed to self-administer oxycodone for 20 days. We observed a difference in drug intake behavior among rats with long access (LgA) to oxycodone. LgA rats took more infusions than ShA rats and post facto divided into two oxycodone self-administering phenotypes: LgA-H and LgA-L. Our current behavior observations of high and low self-administration by LgA rats align with previously published studies for methamphetamine (METH) (Daiwile et al., 2021; Daiwile et al., 2019; Daiwile et al., 2022), cocaine (de Guglielmo et al., 2024) and oxycodone (Blackwood and Cadet, 2021; Blackwood et al., 2019a; Blackwood et al., 2019b). Moreover, repeated oxycodone use has been reported in tolerance development among patients (Ellis et al., 2019; Kibaly et al., 2021) resulting in higher drug use to achieve comparable effects. Likewise in the present study, LgA-H rats may have developed tolerance earlier than LgA-L rats, potentially contributing to their higher oxycodone intake. Evidence reviewed by McCoy et al. (2021) implicates the involvement of potassium channels in substance use disorders including METH (Cadet et al., 2017), alcohol (Rinker et al., 2017) and cocaine (McCall et al., 2017). Prior studies, using RNA sequencing also identified that oxycodone exposure can alter potassium channel expression and, be associated with different behavioral patterns (McCall et al., 2017). We believed that the observed behavior is due to alterations in the expression of potassium channels in the PFC, NAc and Hip. Moreover, these brain structures receive projections from the VTA which plays an important role in reward processing (Khayat and Yaka, 2024). Our study identified a brain region dependent difference in the expression of potassium channels in oxycodone self-administered rats. Rats that self-administered the most oxycodone (LgA-H) revealed a significant increase in the expression of both voltage gated and calcium activated potassium channels in their NAc. In contrast, LgA-L phenotypes increased in both voltage gated and calcium activated potassium channels in their PFC. We also identified a significant increase of potassium channels in the hippocampus of ShA rats.
4.1 Activation of potassium channels in the NAc of LgA-H rats
Rats that took the highest levels of oxycodone, LgA-H, were suspected to be the most vulnerable to OUD. They showed a very interesting expression pattern for potassium channels in their NAc. The Nucleus Accumbens is a key hub for reward circuitry involved in drug-taking behaviors (Kuhn et al., 2014), and can enhance the reinforcing effects of opioids (Cornish et al., 1999). It also plays a significant role in processing pleasurable experiences like eating, drug use, and social interactions (Bassareo and Di Chiara, 1999; Carelli, 2002; Olsen, 2011). Potassium channels in the NAc regulate reward behavior by influencing synaptic plasticity (Fernández-Fernández and Lamas, 2021; Kim and Hoffman, 2008). Potassium channels help control inhibitory signaling within the brain (Qiu et al., 2025), which is crucial for suppressing impulsive behaviors. Dysfunction in potassium channel activity can lead to hyperexcitability of neurons, heightening drug-cue reactivity and impairing self-regulation (Humphries and Dart, 2015; Wu et al., 2024). The symptoms you can expect to see because of this dysregulation are irregular heartbeats (Fanoe et al., 2009; Meents et al., 2018) respiratory depression (Montandon et al., 2016; Wei and Ramirez, 2019) and antinociceptive affects (Nakamura et al., 2014). Dysregulation of potassium channels can also lead to seizures (Gross et al., 2016; Zhang et al., 2021), autism (Liu et al., 2022), and ataxia (Pollini et al., 2020).
In the NAc, LgA-H rats revealed consistent upregulation of potassium channels, when compared to LgA-L rats, supporting the link between potassium channel expression and increased vulnerability to OUD. There was higher level of Kcnd2, Kcnd3, Kcng2 and Kcnt1 in NAc of LgA-H rats compared to ShA and LgA-L and expression of Kcnn2 and Kcnt2 was different then Sal. Moreover, administration of a potassium channel inhibitor in the NAc of rats was found to attenuate cocaine seeking behaviors in them (Xia et al., 2024), further supporting their role in addiction-related neuroadaptations. Additionally, increased potassium channels in the NAc would cause a hyperexcitable reward circuit making it easier to succumb to oxycodone abuse (Yuferov et al., 2018). These findings suggest that reducing the frequency of potassium channels in the NAc, could serve as a treatment for OUD.
4.2 Activation of potassium channels in the PFC of LgA-L rats
We thought studying molecular neuroadaptations in LgA-L rats might be interesting because they failed to increase their oxycodone intake despite having the same extended access as the LgA-H group. LgA-L phenotypes displayed higher mRNA levels for Kcna3, Kcna4, Kcnd3, Kcnq4, Kcnq5, Kcnma1 and Kcnn2 in their PFC. The PFC is a brain region of significant interest for its role in decision making, cognitive function (Bausch et al., 2015; Yu et al., 2016; Yu et al., 2019), and impulse control (Arnsten, 2009; Ramnani and Owen, 2004). The PFC, along with subcortical circuits, also plays a crucial role in self-control, and social behavior, influencing both drug-cue reactivity and the regulation of craving and drug-seeking in substance use disorders (Daiwile et al., 2022; Jasinska et al., 2015). Potassium channels control the flow of potassium ions across cell membranes, which is essential for maintaining the resting membrane potential. This means they can play a significant role in regulating self-control and decision-making, by influencing the excitability of neurons (Greene and Hoshi, 2017; Humphries and Dart, 2015). By stabilizing neuronal excitability, potassium channels play a vital role in maintaining balanced neural activity, necessary for recovery in individuals with substance use disorders.
The results we found in the PFC contrasts the pattern we saw in the NAc, where upregulated potassium channel expression was associated with LgA-H rats. The connection between high potassium channel expression in the PFC and low intake behavior suggest the presence of a compensatory mechanism that can reduce a subject’s susceptibility to OUD. Previous studies have shown that enhanced potassium channel activity in the PFC is linked to reduced compulsive drug use (Buchta and Riegel, 2015). Studies on the alcohol consumption in drosophila (Cavaliere et al., 2012), rodents (Padula et al., 2015), and humans (Clarke et al., 2011) have found KCa and Kv levels to be decreased in compulsive animals (Cannady et al., 2018), meaning increasing their levels may decrease OUD susceptibility. Other studies show that acute or chronic drug exposure can decrease potassium channel expression in the PFC (Dong et al., 2005). Taken together, these results show that increased expression of potassium channels in the PFC might lead to a decreased oxycodone intake in LgA-L rats. More importantly, these discoveries highlight the PFC as another fundamental brain region where potassium channel modulation plays a role in the neurobiology of OUD (Padula et al., 2015) and suggest that targeting potassium channels may be an advantageous therapeutic approach.
4.3 Activation of potassium channels in the hip of ShA rats
Lastly, we investigated neuro-molecular changes in the brains of ShA rats, as this group can give us insights into molecular adaptations that are due to the mere exposure of oxycodone. The results show multiple Kv (Kcna5, Kcna10, Kcng1) and KCa (Kcnn1, Kcnn2) to be upregulated in the Hip of ShA rats. The hippocampus is responsible for the rats’ abilities in learning and memory (O'Dell et al., 2015), functions that we know are impacted by oxycodone intake (Cherrier et al., 2009). Specifically, the VTA to hippocampus neuronal projection plays a vital role in the primary reward circuit and is even more essential for memory formation (Atweh and Kuhar, 1977; Bird and Burgess, 2008). Potassium channels contribute to these functions by maintaining the balance between excitation and inhibition in this circuit (Tsuboi et al., 2024). By maintaining proper neuronal excitability, they ensure that signals associated with reward experiences are accurately processed. Dysfunction in this system will contribute to impaired reward learning (Faulkner et al., 2024), potentially influencing behaviors seen in substance use disorder. This result is important to note as these same genes were not seen to be impacted in other brain regions by any behavioral group, indicating that their activation is linked to a protective neural state. This implies a connection between these genes and an early neurobiological response that reduces vulnerability to compulsive drug-seeking behavior. Moreover, the absence of these changes in LgA-H rats implies that the loss of this protective mechanism contributes to addiction progression.
5 Conclusion
In conclusion, we observed two phenotypes among LgA rats which are LgA-H and LgA-L, based on their oxycodone intake. We also found brain region-specific changes in the mRNA expression of voltage-gated and calcium-activated potassium channels in the PFC, NAc, and HIP. We suggest that the activation of Kv and KCa channel in the NAc of LgA-H rats might result in reduced excitability of neurons involved in reward circuit, thus influencing oxycodone taking behaviors. Alternatively, these changes might serve compensatory functions in that circuit. Unexpectedly, whereas LgA-L rats showed increased expression of potassium channel in their PFC, changes in the HIP were found in the ShA phenotypes. Together, these observations suggest potential important relationships between potassium channels in mesocorticolimbic systems and behavioral responses associated with oxycodone intake. Our results further support the notion that more efforts need to be spent to identify potential roles that brain regional differences might play in the clinical manifestations of oxycodone use disorder. Our findings also have important implications for potential treatment strategies, as they further support the therapeutic potential of potassium channel inhibitors or agonists. Although potassium channel represents a promising target, brain region-specific modulation within functionally diverse circuits poses significant challenges and need to take in account when developing therapeutic application. Future studies should focus on subregional and circuit-level analyses using imaging, genetic, and pharmacological approaches to clarify the role of potassium channels in drug use and relapse for improved translational relevance.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by National Institute of Drug Abuse Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
AW: Validation, Writing – review and editing, Formal Analysis, Writing – original draft, Methodology, Software, Data curation, Visualization, Investigation. NA: Validation, Writing – review and editing, Investigation, Software, Methodology, Formal Analysis, Visualization, Resources, Data curation. BL: Supervision, Data curation, Writing – review and editing, Methodology, Software, Visualization, Validation, Formal Analysis, Resources. JC: Supervision, Project administration, Writing – review and editing, Conceptualization, Funding acquisition. AD: Formal Analysis, Supervision, Writing – review and editing, Investigation, Project administration, Methodology, Software, Data curation, Resources, Validation, Conceptualization, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was supported by the Intramural Research Program of the National Institute on Drug Abuse (NIDA), NIH, and DHHS [grant # DA000552 (2021)].
Acknowledgments
This research was supported [in part] by the Intramural Research Program of the National Institutes of Health (NIH)/National Institute on Drug Abuse (NIDA) Baltimore, MD, United States. The contributions of the NIH author(s) were made as part of their official duties as NIH federal employees, are in compliance with agency policy requirements, and are considered Works of the United States Government. However, the findings and conclusions presented in this paper are those of the author(s) and do not necessarily reflect the views of the NIH or the U.S. Department of Health and Human Services.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1653356/full#supplementary-material
References
Agarwal, S., Kim, E. D., Lee, S., Simon, A., Accardi, A., and Nimigean, C. M. (2025). Ball-and-chain inactivation of a human large conductance calcium-activated potassium channel. Nat. Commun. 16 (1), 1769. doi:10.1038/s41467-025-56844-4
Ahmad Fb, C. J., Rossen, L. M., and Sutton, P. (2025). Provisional drug overdose death counts. United States: CDC. doi:10.15620/cdc/20250305008
Alam, K. A., Svalastoga, P., Martinez, A., Glennon, J. C., and Haavik, J. (2023). Potassium channels in behavioral brain disorders. Molecular mechanisms and therapeutic potential: a narrative review. Neurosci. Biobehav Rev. 152, 105301. doi:10.1016/j.neubiorev.2023.105301
Alfaro-Ruíz, R., Aguado, C., Martín-Belmonte, A., Moreno-Martínez, A. E., and Luján, R. (2019). Expression, cellular and subcellular localisation of Kv4.2 and Kv4.3 channels in the rodent hippocampus. Int. J. Mol. Sci. 20 (2), 246. doi:10.3390/ijms20020246
Ali, M. M., Chen, J., and Novak, P. J. (2024). Utilization of buprenorphine for opioid use disorder after the practitioner waiver removal. Am. J. Prev. Med. 68, 207–209. doi:10.1016/j.amepre.2024.09.013
APA (2024). APA diagnostic and statistical manual of mental disorders. American Psychiatric Publishing. Available online at: https://www.psychiatry.org/psychiatrists/practice/dsm.
Arnsten, A. F. (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 10 (6), 410–422. doi:10.1038/nrn2648
Atweh, S. F., and Kuhar, M. J. (1977). Autoradiographic localization of opiate receptors in rat brain. III. The telencephalon. Brain Res. 134 (3), 393–405. doi:10.1016/0006-8993(77)90817-4
Bassareo, V., and Di Chiara, G. (1999). Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience 89 (3), 637–641. doi:10.1016/s0306-4522(98)00583-1
Bausch, A. E., Dieter, R., Nann, Y., Hausmann, M., Meyerdierks, N., Kaczmarek, L. K., et al. (2015). The sodium-activated potassium channel slack is required for optimal cognitive flexibility in mice. Learn Mem. 22 (7), 323–335. doi:10.1101/lm.037820.114
Bean, B. P. (2007). The action potential in Mammalian central neurons. Nat. Rev. Neurosci. 8 (6), 451–465. doi:10.1038/nrn2148
Bergeria, C. L., and Strain, E. C. (2022). Opioid use disorder: pernicious and persistent. Am. J. Psychiatry 179 (10), 708–714. doi:10.1176/appi.ajp.20220699
Bird, C. M., and Burgess, N. (2008). The hippocampus and memory: insights from spatial processing. Nat. Rev. Neurosci. 9 (3), 182–194. doi:10.1038/nrn2335
Blackwood, C. A., and Cadet, J. L. (2021). The molecular neurobiology and neuropathology of opioid use disorder. Curr. Res. Neurobiol. 2, 100023. doi:10.1016/j.crneur.2021.100023
Blackwood, C. A., Hoerle, R., Leary, M., Schroeder, J., Job, M. O., McCoy, M. T., et al. (2019a). Molecular adaptations in the rat dorsal striatum and hippocampus following abstinence-induced incubation of drug seeking after escalated oxycodone self-administration. Mol. Neurobiol. 56 (5), 3603–3615. doi:10.1007/s12035-018-1318-z
Blackwood, C. A., McCoy, M. T., Ladenheim, B., and Cadet, J. L. (2019b). Escalated oxycodone self-administration and punishment: differential expression of opioid receptors and immediate early genes in the rat dorsal striatum and prefrontal cortex. Front. Neurosci. 13, 1392. doi:10.3389/fnins.2019.01392
Bohler, R. M., Freeman, P. R., Villani, J., Hunt, T., Linas, B. S., Walley, A. Y., et al. (2023). The policy landscape for naloxone distribution in four states highly impacted by fatal opioid overdoses. Drug Alcohol Depend. Rep. 6, 100126. doi:10.1016/j.dadr.2022.100126
Boscarino, J. A., Rukstalis, M., Hoffman, S. N., Han, J. J., Erlich, P. M., Gerhard, G. S., et al. (2010). Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction 105 (10), 1776–1782. doi:10.1111/j.1360-0443.2010.03052.x
Buchta, W. C., and Riegel, A. C. (2015). Chronic cocaine disrupts mesocortical learning mechanisms. Brain Res. 1628 (Pt A), 88–103. doi:10.1016/j.brainres.2015.02.003
Cadet, J. L., Bisagno, V., and Milroy, C. M. (2014). Neuropathology of substance use disorders. Acta Neuropathol. 127 (1), 91–107. doi:10.1007/s00401-013-1221-7
Cadet, J. L., Brannock, C., Krasnova, I. N., Jayanthi, S., Ladenheim, B., McCoy, M. T., et al. (2017). Genome-wide DNA hydroxymethylation identifies potassium channels in the nucleus accumbens as discriminators of methamphetamine addiction and abstinence. Mol. Psychiatry 22 (8), 1196–1204. doi:10.1038/mp.2016.48
Cannady, R., Rinker, J. A., Nimitvilai, S., Woodward, J. J., and Mulholland, P. J. (2018). Chronic alcohol, intrinsic excitability, and potassium channels: neuroadaptations and drinking behavior. Handb. Exp. Pharmacol. 248, 311–343. doi:10.1007/164_2017_90
Carelli, R. M. (2002). Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs. 'natural' reinforcement. Physiol. Behav. 76 (3), 379–387. doi:10.1016/s0031-9384(02)00760-6
Cavaliere, S., Gillespie, J. M., and Hodge, J. J. (2012). KCNQ channels show conserved ethanol block and function in ethanol behaviour. PLoS One 7 (11), e50279. doi:10.1371/journal.pone.0050279
Chamakalayil, S., Stohler, R., Moldovanyi, A., Gerber, M., Brand, S., and Dürsteler, K. M. (2024). Neurocognitive performance of patients undergoing intravenous versus oral opioid agonist treatment: a prospective multicenter study on three-month treatment effects. Front. Psychiatry 15, 1375895. doi:10.3389/fpsyt.2024.1375895
Cherrier, M. M., Amory, J. K., Ersek, M., Risler, L., and Shen, D. D. (2009). Comparative cognitive and subjective side effects of immediate-release oxycodone in healthy middle-aged and older adults. J. Pain 10 (10), 1038–1050. doi:10.1016/j.jpain.2009.03.017
Clarke, T. K., Laucht, M., Ridinger, M., Wodarz, N., Rietschel, M., Maier, W., et al. (2011). KCNJ6 is associated with adult alcohol dependence and involved in gene × early life stress interactions in adolescent alcohol drinking. Neuropsychopharmacology 36 (6), 1142–1148. doi:10.1038/npp.2010.247
Cornish, J. L., Duffy, P., and Kalivas, P. W. (1999). A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience 93 (4), 1359–1367. doi:10.1016/s0306-4522(99)00214-6
Daiwile, A. P., Jayanthi, S., Ladenheim, B., McCoy, M. T., Brannock, C., Schroeder, J., et al. (2019). Sex differences in escalated methamphetamine self-administration and altered gene expression associated with incubation of methamphetamine seeking. Int. J. Neuropsychopharmacol. 22 (11), 710–723. doi:10.1093/ijnp/pyz050
Daiwile, A. P., Jayanthi, S., and Cadet, J. L. (2021). Sex- and brain region-specific changes in gene expression in Male and female rats as consequences of methamphetamine self-administration and abstinence. Neuroscience 452, 265–279. doi:10.1016/j.neuroscience.2020.11.025
Daiwile, A. P., Sullivan, P., Jayanthi, S., Goldstein, D. S., and Cadet, J. L. (2022). Sex-specific alterations in dopamine metabolism in the brain after methamphetamine self-administration. Int. J. Mol. Sci. 23 (8), 4353. doi:10.3390/ijms23084353
de Guglielmo, G., Carrette, L., Kallupi, M., Brennan, M., Boomhower, B., Maturin, L., et al. (2024). Large-scale characterization of cocaine addiction-like behaviors reveals that escalation of intake, aversion-resistant responding, and breaking-points are highly correlated measures of the same construct. Elife 12. doi:10.7554/eLife.90422
Dong, Y., Nasif, F. J., Tsui, J. J., Ju, W. Y., Cooper, D. C., Hu, X. T., et al. (2005). Cocaine-induced plasticity of intrinsic membrane properties in prefrontal cortex pyramidal neurons: adaptations in potassium currents. J. Neurosci. 25 (4), 936–940. doi:10.1523/jneurosci.4715-04.2005
Ellis, M. S., Cicero, T. J., Dart, R. C., and Green, J. L. (2019). Understanding multi-pill ingestion of prescription opioids: prevalence, characteristics, and motivation. Pharmacoepidemiol Drug Saf. 28 (1), 117–121. doi:10.1002/pds.4687
Fanoe, S., Jensen, G. B., Sjøgren, P., Korsgaard, M. P., and Grunnet, M. (2009). Oxycodone is associated with dose-dependent QTc prolongation in patients and low-affinity inhibiting of hERG activity in vitro. Br. J. Clin. Pharmacol. 67 (2), 172–179. doi:10.1111/j.1365-2125.2008.03327.x
Faulkner, I. E., Pajak, R. Z., Harte, M. K., Glazier, J. D., and Hager, R. (2024). Voltage-gated potassium channels as a potential therapeutic target for the treatment of neurological and psychiatric disorders. Front. Cell Neurosci. 18, 1449151. doi:10.3389/fncel.2024.1449151
Fernández-Fernández, D., and Lamas, J. A. (2021). Metabotropic modulation of potassium channels during synaptic plasticity. Neuroscience 456, 4–16. doi:10.1016/j.neuroscience.2020.02.025
Grande, L. A., Cundiff, D., Greenwald, M. K., Murray, M., Wright, T. E., and Martin, S. A. (2023). Evidence on buprenorphine dose limits: a review. J. Addict. Med. 17 (5), 509–516. doi:10.1097/adm.0000000000001189
Greene, D. L., and Hoshi, N. (2017). Modulation of Kv7 channels and excitability in the brain. Cell Mol. Life Sci. 74 (3), 495–508. doi:10.1007/s00018-016-2359-y
Gross, C., Yao, X., Engel, T., Tiwari, D., Xing, L., Rowley, S., et al. (2016). MicroRNA-Mediated downregulation of the potassium channel Kv4.2 contributes to seizure onset. Cell Rep. 17 (1), 37–45. doi:10.1016/j.celrep.2016.08.074
Hayes, C. J., Raciborski, R. A., Nowak, M., Acharya, M., Nunes, E. V., and Winhusen, T. J. (2024). Medications for opioid use disorder: predictors of early discontinuation and reduction of overdose risk in US military veterans by medication type. Addiction 120, 138–151. doi:10.1111/add.16659
Humphries, E. S., and Dart, C. (2015). Neuronal and cardiovascular potassium channels as therapeutic drug targets: promise and pitfalls. J. Biomol. Screen 20 (9), 1055–1073. doi:10.1177/1087057115601677
Jan, L. Y., and Jan, Y. N. (2012). Voltage-gated potassium channels and the diversity of electrical signalling. J. Physiol. 590 (11), 2591–2599. doi:10.1113/jphysiol.2011.224212
Jasinska, A. J., Chen, B. T., Bonci, A., and Stein, E. A. (2015). Dorsal medial prefrontal cortex (MPFC) circuitry in rodent models of cocaine use: implications for drug addiction therapies. Addict. Biol. 20 (2), 215–226. doi:10.1111/adb.12132
Jayanthi, S., Torres, O. V., Ladenheim, B., and Cadet, J. L. (2020). A single prior injection of methamphetamine enhances methamphetamine self-administration (SA) and blocks SA-Induced changes in DNA methylation and mRNA expression of potassium channels in the rat nucleus accumbens. Mol. Neurobiol. 57 (3), 1459–1472. doi:10.1007/s12035-019-01830-3
Khayat, A., and Yaka, R. (2024). Activation of nucleus accumbens projections to the ventral tegmental area alters molecular signaling and neurotransmission in the reward system. Front. Mol. Neurosci. 17, 1271654. doi:10.3389/fnmol.2024.1271654
Kibaly, C., Alderete, J. A., Liu, S. H., Nasef, H. S., Law, P. Y., Evans, C. J., et al. (2021). Oxycodone in the opioid epidemic: high 'Liking', 'Wanting', and abuse liability. Cell Mol. Neurobiol. 41 (5), 899–926. doi:10.1007/s10571-020-01013-y
Kim, J., and Hoffman, D. A. (2008). Potassium channels: newly found players in synaptic plasticity. Neuroscientist 14 (3), 276–286. doi:10.1177/1073858408315041
King, S. J., Reid, C., Forbes, K., and Hanks, G. (2011). A systematic review of oxycodone in the management of cancer pain. Palliat. Med. 25 (5), 454–470. doi:10.1177/0269216311401948
Klein, M., Shapiro, E., and Kandel, E. R. (1980). Synaptic plasticity and the modulation of the Ca2+ current. J. Exp. Biol. 89, 117–157. doi:10.1242/jeb.89.1.117
Kuhn, J., Möller, M., Treppmann, J. F., Bartsch, C., Lenartz, D., Gruendler, T. O., et al. (2014). Deep brain stimulation of the nucleus accumbens and its usefulness in severe opioid addiction. Mol Psychiatry 19 (2), 145–146.
Kuiper, E. F., Nelemans, A., Luiten, P., Nijholt, I., Dolga, A., and Eisel, U. (2012). K(Ca)2 and k(ca)3 channels in learning and memory processes, and neurodegeneration. Front. Pharmacol. 3, 107. doi:10.3389/fphar.2012.00107
Liu, Z., Yang, X., Guo, P., Wang, F., Xia, W., Chen, Y., et al. (2022). The association between gene polymorphisms in voltage-gated potassium channels Kv2.1 and Kv4.2 and susceptibility to autism spectrum disorder. Front. Psychiatry 13, 994166. doi:10.3389/fpsyt.2022.994166
Marie, N., and Noble, F. (2023). Oxycodone, an opioid like the others? Front. Psychiatry 14, 1229439. doi:10.3389/fpsyt.2023.1229439
McCall, N. M., Kotecki, L., Dominguez-Lopez, S., Marron Fernandez de Velasco, E., Carlblom, N., Sharpe, A. L., et al. (2017). Selective ablation of GIRK channels in dopamine neurons alters behavioral effects of cocaine in mice. Neuropsychopharmacology 42 (3), 707–715. doi:10.1038/npp.2016.138
McCoy, M. T., Jayanthi, S., and Cadet, J. L. (2021). Potassium channels and their potential roles in substance use disorders. Int. J. Mol. Sci. 22 (3), 1249. doi:10.3390/ijms22031249
Meents, J. E., Juhasz, K., Stölzle-Feix, S., Peuckmann-Post, V., Rolke, R., and Lampert, A. (2018). The opioid oxycodone use-dependently inhibits the cardiac sodium channel Na(V) 1.5. Br. J. Pharmacol. 175 (14), 3007–3020. doi:10.1111/bph.14348
Montandon, G., Ren, J., Victoria, N. C., Liu, H., Wickman, K., Greer, J. J., et al. (2016). G-protein-gated inwardly rectifying potassium channels modulate respiratory depression by opioids. Anesthesiology 124 (3), 641–650. doi:10.1097/aln.0000000000000984
Nakamura, A., Fujita, M., Ono, H., Hongo, Y., Kanbara, T., Ogawa, K., et al. (2014). G protein-gated inwardly rectifying potassium (KIR3) channels play a primary role in the antinociceptive effect of oxycodone, but not morphine, at supraspinal sites. Br. J. Pharmacol. 171 (1), 253–264. doi:10.1111/bph.12441
Noble, S. J. (1976). Potassium accumulation and depletion in frog atrial muscle. J. Physiol. 258 (3), 579–613. doi:10.1113/jphysiol.1976.sp011436
O'Dell, T. J., Connor, S. A., Guglietta, R., and Nguyen, P. V. (2015). β-Adrenergic receptor signaling and modulation of long-term potentiation in the Mammalian hippocampus. Learn Mem. 22 (9), 461–471. doi:10.1101/lm.031088.113
Olsen, C. M. (2011). Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology 61 (7), 1109–1122. doi:10.1016/j.neuropharm.2011.03.010
Olson, K. M., Lei, W., Keresztes, A., LaVigne, J., and Streicher, J. M. (2017). Novel molecular strategies and targets for opioid drug discovery for the treatment of chronic pain. Yale J. Biol. Med. 90 (1), 97–110.
Padula, A. E., Griffin, W. C., Lopez, M. F., Nimitvilai, S., Cannady, R., McGuier, N. S., et al. (2015). KCNN genes that encode small-conductance Ca2+-Activated K+ channels influence alcohol and drug addiction. Neuropsychopharmacology 40 (8), 1928–1939. doi:10.1038/npp.2015.42
Paxinos, G., and Watson, C. (2006). The rat brain in stereotaxic coordinates: hard cover edition. Elsevier.
Pignatelli, M., and Bonci, A. (2015). Role of dopamine neurons in reward and aversion: a synaptic plasticity perspective. Neuron 86 (5), 1145–1157. doi:10.1016/j.neuron.2015.04.015
Pollini, L., Galosi, S., Tolve, M., Caputi, C., Carducci, C., Angeloni, A., et al. (2020). KCND3-Related neurological disorders: from old to emerging clinical phenotypes. Int. J. Mol. Sci. 21 (16), 5802. doi:10.3390/ijms21165802
Qiu, Q., Yang, M., Gong, D., Liang, H., and Chen, T. (2025). Potassium and calcium channels in different nerve cells act as therapeutic targets in neurological disorders. Neural Regen. Res. 20 (5), 1258–1276. doi:10.4103/nrr.Nrr-d-23-01766
Ramirez-Navarro, A., Lima-Silveira, L., Glazebrook, P. A., Dantzler, H. A., Kline, D. D., and Kunze, D. L. (2024). Kv2 channels contribute to neuronal activity within the vagal afferent-nTS reflex arc. Am. J. Physiol. Cell Physiol. 326 (1), C74–c88. doi:10.1152/ajpcell.00366.2023
Ramnani, N., and Owen, A. M. (2004). Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat. Rev. Neurosci. 5 (3), 184–194. doi:10.1038/nrn1343
Rinker, J. A., Fulmer, D. B., Trantham-Davidson, H., Smith, M. L., Williams, R. W., Lopez, M. F., et al. (2017). Differential potassium channel gene regulation in BXD mice reveals novel targets for pharmacogenetic therapies to reduce heavy alcohol drinking. Alcohol 58, 33–45. doi:10.1016/j.alcohol.2016.05.007
Sahebi-Fakhrabad, A., Sadeghi, A. H., Kemahlioglu-Ziya, E., and Handfield, R. (2024). Exploring opioid prescription patterns and overdose rates in South Carolina (2017-2021): insights into rising deaths in high-risk areas. Healthc. (Basel) 12 (13), 1268. doi:10.3390/healthcare12131268
Salisbury, A. J., Blackwood, C. A., and Cadet, J. L. (2020). Prolonged withdrawal from escalated oxycodone is associated with increased expression of glutamate receptors in the rat hippocampus. Front. Neurosci. 14, 617973. doi:10.3389/fnins.2020.617973
Shah, N. H., and Aizenman, E. (2014). Voltage-gated potassium channels at the crossroads of neuronal function, ischemic tolerance, and neurodegeneration. Transl. Stroke Res. 5 (1), 38–58. doi:10.1007/s12975-013-0297-7
Solessio, E., Linn, D. M., Perlman, I., and Lasater, E. M. (2000). Characterization with barium of potassium currents in turtle retinal müller cells. J. Neurophysiol. 83 (1), 418–430. doi:10.1152/jn.2000.83.1.418
Sun, J., Liu, Y., Baudry, M., and Bi, X. (2020). SK2 channel regulation of neuronal excitability, synaptic transmission, and brain rhythmic activity in health and diseases. Biochim. Biophys. Acta Mol. Cell Res. 1867 (12), 118834. doi:10.1016/j.bbamcr.2020.118834
Trimmer, J. S. (2015). Subcellular localization of K+ channels in Mammalian brain neurons: remarkable precision in the midst of extraordinary complexity. Neuron 85 (2), 238–256. doi:10.1016/j.neuron.2014.12.042
Tsang, A., and Rodda, L. N. (2024). Assessment of postmortem urine fentanyl detection by autopsy dipstick testing in accidental overdose deaths. J. Anal. Toxicol. 48, 667–671. doi:10.1093/jat/bkae072
Tsuboi, D., Nagai, T., Yoshimoto, J., and Kaibuchi, K. (2024). Neuromodulator regulation and emotions: insights from the crosstalk of cell signaling. Front. Mol. Neurosci. 17, 1376762. doi:10.3389/fnmol.2024.1376762
Wei, A. D., and Ramirez, J. M. (2019). Presynaptic mechanisms and KCNQ potassium channels modulate opioid depression of respiratory drive. Front. Physiol. 10, 1407. doi:10.3389/fphys.2019.01407
Wu, J., Quraishi, I. H., Zhang, Y., Bromwich, M., and Kaczmarek, L. K. (2024). Disease-causing slack potassium channel mutations produce opposite effects on excitability of excitatory and inhibitory neurons. Cell Rep. 43 (3), 113904. doi:10.1016/j.celrep.2024.113904
Xia, M., Anderson, T. L., Prantzalos, E. R., Hawkinson, T. R., Clarke, H. A., Keohane, S. B., et al. (2024). Voltage-gated potassium channels control extended access cocaine seeking: a role for nucleus accumbens astrocytes. Neuropsychopharmacology 49 (3), 551–560. doi:10.1038/s41386-023-01718-w
Yu, W., Parakramaweera, R., Teng, S., Gowda, M., Sharad, Y., Thakker-Varia, S., et al. (2016). Oxidation of KCNB1 potassium channels causes neurotoxicity and cognitive impairment in a mouse model of traumatic brain injury. J. Neurosci. 36 (43), 11084–11096. doi:10.1523/jneurosci.2273-16.2016
Yu, W., Zhang, H., Shin, M. R., and Sesti, F. (2019). Oxidation of KCNB1 potassium channels in the murine brain during aging is associated with cognitive impairment. Biochem. Biophys. Res. Commun. 512 (4), 665–669. doi:10.1016/j.bbrc.2019.03.130
Yuferov, V., Zhang, Y., Liang, Y., Zhao, C., Randesi, M., and Kreek, M. J. (2018). Oxycodone self-administration induces alterations in expression of integrin, semaphorin and ephrin genes in the mouse striatum. Front. Psychiatry 9, 257. doi:10.3389/fpsyt.2018.00257
Keywords: oxycodone, potassium channels, mRNA, prefrontal cortex, nucleus accumbens, hippocampus, self-administration
Citation: Wabreha AY, Adjei N, Ladenheim B, Cadet JL and Daiwile AP (2025) Escalated oxycodone self-administration is associated with expression of voltage gated and calcium activated potassium channels in the mesocorticolimbic system in rats. Front. Pharmacol. 16:1653356. doi: 10.3389/fphar.2025.1653356
Received: 24 June 2025; Accepted: 30 July 2025;
Published: 11 August 2025.
Edited by:
Stephen Lewis, Case Western Reserve University, United StatesReviewed by:
Kabirullah Lutfy, Western University of Health Sciences, United StatesJoanne Mathiasen, College of Medicine, Drexel University, United States
Copyright © 2025 Wabreha, Adjei, Ladenheim, Cadet and Daiwile. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Atul P. Daiwile, YXR1bC5kYWl3aWxlQG5paC5nb3Y=
†ORCID: Ammanuel Y. Wabreha, orcid.org/0009-0003-9391-8004; Nasser Adjei, orcid.org/0009-0003-8684-5767; Bruce Ladenheim, orcid.org/0000-0001-9124-6994; Jean Lud Cadet, orcid.org/0000-0001-5635-3524; Atul P. Daiwile, orcid.org/0000-0002-5494-0044
 Ammanuel Y. Wabreha†
Ammanuel Y. Wabreha† Bruce Ladenheim
Bruce Ladenheim Jean Lud Cadet
Jean Lud Cadet Atul P. Daiwile
Atul P. Daiwile