- 1Department of Biology, University of Rome Tor Vergata, Rome, Italy
- 2Laboratory of Virology, National Institute for Infectious Diseases “Lazzaro Spallanzani” (IRCCS), Rome, Italy
- 3Department of Biology and Biotechnology “Charles Darwin”, Sapienza University of Rome, Rome, Italy
Mpox, caused by the monkeypox virus (MPXV), is a zoonotic disease that has gained global relevance after the 2022–2024 outbreak. MPXV exists in two distinct clades: clade I, associated with higher virulence and mortality, and clade II, which demonstrated increased human-to-human transmission and adaptation. Clinically, Mpox presents with rash, fever, and lymphadenopathy, with severe complications in immunocompromised individuals. Genomic surveillance has revealed rapid evolution, partly driven by APOBEC3-mediated mutagenesis, especially within immune-modulating regions. Although smallpox vaccines like MVA-BN and ACAM2000 provide cross-protection against Mpox, the MVA-BN vaccine showed a more favourable safety profile, but variable effectiveness compared to replicating vaccines. Antiviral agents such as tecovirimat and cidofovir have been used off-label, but emerging resistance and limited clinical efficacy highlight an urgent need for MPXV-specific therapeutics. The current epidemiological scenario emphasizes the importance of novel antiviral development and optimized prophylactic strategies to improve clinical outcomes and global preparedness. This review aims to provide a comprehensive overview of the molecular biology, clinical features, the current drugs used to treat Mpox infection and the vaccines used to prevent the infection. It also discussing the limitations of these therapeutic tools and the improvements needed to enhance their efficacy.
Highlights
• Current antiviral options for monkeypox, including tecovirimat, brincidofovir, and cidofovir, show promising preclinical efficacy but are limited by insufficient clinical data, potential toxicity, and the risk of resistance. Future efforts should prioritize well-designed clinical trials, resistance monitoring, and the development of safer, more effective agents or combination regimens.
• Like other OPXVs, MPXV possess a high capacity for genetic recombination and can incorporate genes from other viruses, making it a candidate for gain-of function (GOF) or research of concern (GOFROC) experiments.
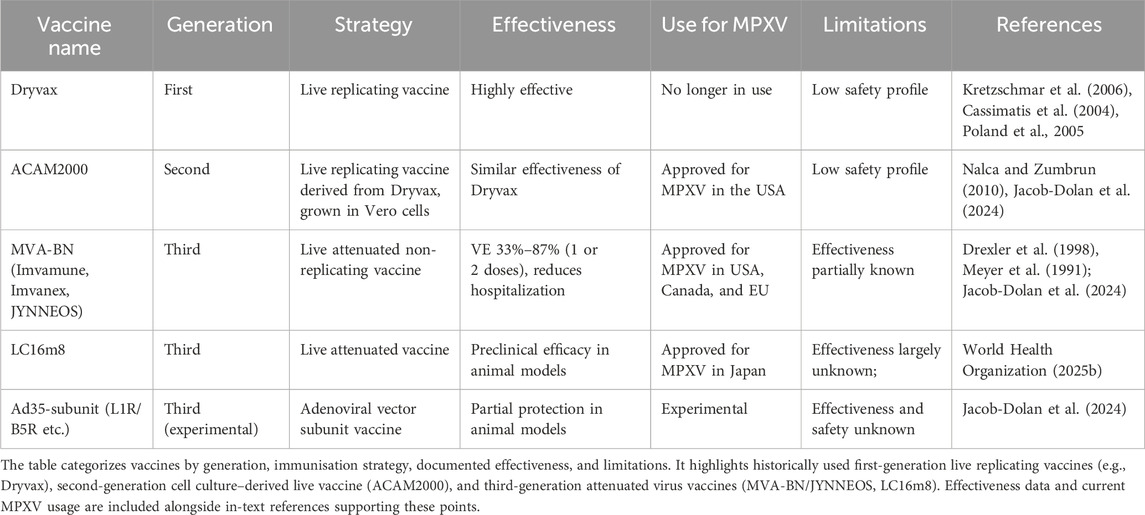
• Second and third generation smallpox vaccines can be used to protect people at high risk of MPXV exposure. The currently available vaccines differ in safety profile and efficacy. Further studies are needed to establish the role of humoral and cellular immune responses in vaccine induced protection.
1 Introduction
The monkeypox virus (MPXV) is a double-stranded DNA (dsDNA) virus, belonging to the Orthopoxvirus (OPXV) genus within the Poxviridae family. This group also includes other notable viruses such as variola virus (VARV, smallpoxvirus), vaccinia virus (VACV), cowpox, camelpox virus, Taterapox virus, and ectromelia virus (World Health Organization, 2024a). The high homology among these viruses (more than 95.5% with smallpox) leads to an immunological cross-reactivity within the genus.
MPXV is the causative agent of Mpox, formerly known as monkeypox, a zoonotic disease characterized by skin lesions, fever, and lymphadenopathy, which can range from mild to severe and may lead to complications, particularly in immunocompromised individuals. The first case of Mpox was identified in 1959 during vaccine-related studies involving a laboratory monkey in Copenhagen, Denmark. However, the first confirmed human case of Mpox was observed in 1970 in a child living in the Basankusu territory of Democratic Republic of Congo (DRC) (Ladnyj et al., 1972).
Genomic analyses have identified two distinct MPXV clades: clade I and clade II.
Prior to the 1980s, the spread of MPXV was limited due to the cross-protection provided by the smallpox vaccination. However, since Mpox is a zoonosis, sporadic cases have been observed in rural areas, where zoonotic transmission from infected animals remains a key route of infection. Natural reservoirs include various African rodents and non-human primates such as apes and monkeys (Li et al., 2025; Sun et al., 2024). From 1970 to 1999, over 500 Mpox cases had been reported in Africa, but most of them (98.7%) occurred in DRC (Brown and Leggat, 2016; Jezek et al., 1987). The first Mpox outbreak outside of the African continent was reported in the United States in 2003. It was caused by the importation of rodents from Ghana (Anderson et al., 2003). Between 2018 and 2021, international travel-related cases were described in countries such as USA, Israel, the United Kingdom, and Singapore with secondary cases among healthcare workers (Vaughan et al., 2018). In May 2022, the greatest outbreak outside endemic area occurred, leading the WHO to declare the event a public health emergency of international concern on July 23, 2022 (Adegboye et al., 2022; Thornhill et al., 2022). As of July 31, 2025, a total of 34386 confirmed Mpox cases and 138 deaths had been reported across 84 countries (World Health Organization, 2025b). The first wave of outbreak was caused by clade II strains, but in September 2024 several cases, which were linked to clade I, were reported in DRC (The Johns Hopkins University, 2024a). This review will explore the general aspects of Mpox infection, including clinical manifestations and its classification as a select agent requiring stringent biosafety indications. We will examine the genome structure, emerging patterns of antiviral resistance to drugs used for the treatment of Mpox, such as Tecovirimat and Cidofovir and vaccination. Moreover, an overview is done on limitations of these therapeutic tools and the improvements needed to enhance their efficacy.
2 MPXV genome
The MPXV genome is a linear, double-stranded DNA (dsDNA) of approximately 197 kbp, characterized by covalently closed hairpin termini that lack free 3′ or 5′ ends (Monzón et al., 2024). It encodes over 190 proteins, including polymerases. There are ∼6.4 kb inverted terminal repeats (ITRs) at both extremities of the genome. The genome is divided into three regions: a central core and two flanking arms (left and right), each containing an ITR. The conserved core region genes are essential for viral replication, transcription, and virion assembly (Karagoz et al., 2023).
In contrast, the terminal arms are more variable and encode proteins that modulate host range and pathogenicity (Young et al., 2024). In addition to coding regions, the MPXV genome contains several noncoding regions that play essential roles in viral replication and gene regulation. These include promoter regions, which regulate viral gene transcription by serving as binding sites for viral RNA polymerase and associated transcription factors, and replication origin, which are specific DNA sequences that initiate and control viral genome replication, ensuring accurate and efficient genome duplication during the viral replication cycle (Cheng et al., 2025).
Gene density is high, with most genes arranged compactly and intergenic regions rarely exceeding 100 bp (Karagoz et al., 2023). MPXV, like other members of the Poxviridae family, exhibits a relatively low mutation rate, estimated at approximately 10-5 to 10-6 substitutions per nucleotide per replication cycle. However, this rate is more than three orders of magnitude (i.e., over 1000-fold) higher than that of host cellular DNA polymerases (Karagoz et al., 2023; Mohanto et al., 2023). The Central coding region is a highly conserved segment comprising approximately 138 kbp (Chen et al., 2005), that encodes essential proteins for viral replication, transcription, virion assembly, and host cell interactions (Yu et al., 2024).
This region included core genes encoding viral RNA and DNA polymerases (such as F8), characteristic of a DNA virus with cytoplasmic transcription and replication. Viral DNA polymerase is a complex comprising processivity factor A22, DNA glycosylase E4, and phosphoprotein H5 (Wang et al., 2023; Xie et al., 2025). Other proteins are involved in DNA replication, such as helicase-primase (E5), single-strand binding proteins (I3), and topoisomerases, which facilitate the unwinding and stabilization of the viral genome during replication (Wang et al., 2023). Moreover, core genes encode several virion core proteins, including the mRNA capping enzyme and structural proteins that contribute to the formation of the viral core and facilitate the assembly of new virions.
This region of the MPXV genome also encodes proteins essential for viral entry and virion assembly: A27L, B5R, and F13L are membrane-associated proteins that play critical roles in viral entry, fusion, and budding (Hughes et al., 2014; Sagdat et al., 2024). The B5R glycoprotein is the MPXV VAP (Viral Attachment Protein) involved in receptor binding, which allows the virus to enter host cells by interacting with glycosaminoglycans (GAGs) on the target cell surface (Sagdat et al., 2024; Tang et al., 2023). Adjacent to central coding region, the MPXV genome has the ends of variable regions containing inverted terminal repeats (ITRs). These regions contain genes involved in immune modulation, pathogenicity, and host range determination, collectively influencing virulence and rapidly enabling the virus to adapt to new hosts (Karagoz et al., 2023).
At least four open reading frames (ORFs) have been identified in ITR (Kugelman et al., 2014).
In particular, the ITRs represent hotspots of genetic variability within the variable regions of OPXV genomes and are essential for the replication and packaging of the viral genome, promoting circularization that facilitates efficient cytoplasmic replication and the formation of new virions (Ji et al., 2024).
MPXV employs sophisticated strategies to evade the host’s immune system, contributing to its capacity for persistent infection. One of the mechanisms of immune response evasion involves the production of cytokine-binding proteins that sequester and neutralize host cytokines, thereby dampening immune activation, including cytokine response modifier B (CrmB), viral chemokine inhibitor (vCCI) and A41 (Jiang et al., 2024; Jones et al., 2008). Furthermore, MPXV encodes IL-18 binding protein (IL-18BP) and effectively inhibits IL-18-induced pro-inflammatory NF-κB activation, reducing IFN-γ production and altering NK cell and T cell activation (Yi et al., 2024). Unlike vaccinia virus (VAXV), MPXV produces truncated, inactive orthologs of the interferon antagonist proteins E3L and K3L that interfere with host interferon signaling pathways; hence, antiviral defences and viral persistence are promoted mainly by expression of the soluble type I interferon binding protein (IFNα/βBP) (Guan et al., 2023) (Figure 1).
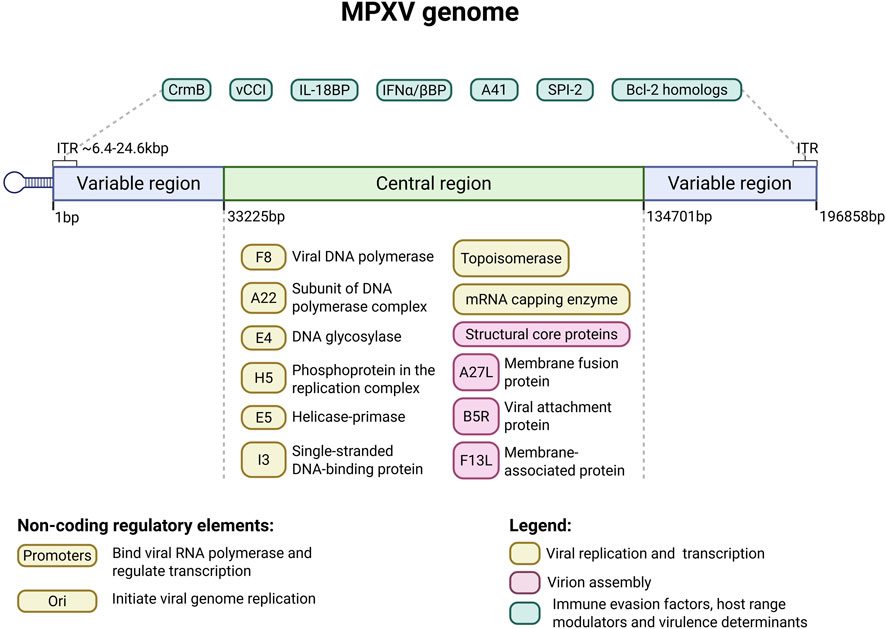
Figure 1. Schematic representation of the monkeypox virus (MPXV) genome. The MPXV genome is a linear, double-stranded DNA molecule of approximately 197 kilobase pairs (kbp), flanked by inverted terminal repeats (ITRs) at both ends. It is divided into a highly conserved central core region (∼138 kbp; shown in light green) and two variable terminal arms (shown in blue). The central region encodes essential genes for viral replication, transcription, and virion assembly, including DNA and RNA polymerases (e.g., F8), helicase-primase (E5), single-stranded DNA-binding protein (I3), topoisomerases, and membrane-associated proteins (A27L, B5R, F13L) involved in viral entry and morphogenesis. The terminal arms, including the ITRs (∼6.4–24.6 kbp), contain genes involved in host range determination, immune evasion (e.g., CrmB, vCCI, IL-18BP, IFNα/βBP, A41), and anti-apoptotic functions (e.g., SPI-2 and Bcl-2 homologs: A47R, B13R, C6L, D11L, and P1L). Notably, the ITRs contain genes that can be deleted or duplicated through genomic rearrangements—such as those observed during the 2022 outbreak—that affect both the left and right terminal arms, contributing to viral adaptation and plasticity (Otieno et al., 2025). Created on Biorender.
Additionally, MPXV expresses anti-apoptotic factors, including the Bcl-2 homologs A47R, B13R, C6L, D11L, and P1L, and the caspase-1 and caspase-8 inhibitor protein SPI-2, which suppress programmed cell death in infected cells, prolonging their viability and promoting viral replication (Alakunle et al., 2024; Lu et al., 2023; Lum et al., 2022). MPXV encodes proteins that enable binding to host cell receptors like CD8 and CD4 on T-cells, influencing the virus’s host range (Hammarlund et al., 2008). Genes involved in immune evasion contribute to strain-specific virulence.
Comparative analyses across the OPXV genus reveal that ITRs are present in most species (over 90%), with only minor sequence differences observed among homologous segments. Specifically, analyses of 825 MPXV genomes identified ITRs in 823 samples, highlighting their conserved and indispensable role in the virus’s life cycle (Yu et al., 2024). The ITRs have significant genomic plasticity, characterized by dynamic events such as gene duplications, deletions, and the proliferation of tandem repeat sequences. Brinkmann and colleagues demonstrated that significant structural variations were observed during the 2022 Mpox outbreak, including extensive duplications and deletions of genetic material. These genomic rearrangements contributed to an expansion of the ITR regions from approximately 6.4 kbp to nearly 24.6 kbp. Specifically, duplications spanned up to 18.2 kilobases, while deletions reached lengths of up to 16.9 kilobases. Such modifications predominantly impacted genes implicated in immune evasion and host range adaptation, underscoring the evolutionary plasticity of the viral genome (Brinkmann et al., 2023). Unique 16-nucleotide tandem repeats have been identified within the ITRs of the MPXV genome. These repeat elements exhibit clade-specific variation in copy number and appear exclusive to MPXV, with no homologous sequences detected in other Poxviruses or human or rodent genomes (Desingu et al., 2023). This clade-dependent polymorphism suggests a possible role for these repeats in MPXV genomic plasticity and evolution, with potential implications for understanding viral diversity and informing targeted vaccine design (Desingu et al., 2023).
3 Strain variability
Since the first diagnosed human case in the Democratic Republic of Congo in 1970, MPXV has become endemic in Central and Western Africa (Kantele et al., 2016). Genetic characterization divides MPXV into two major clades with distinct geographic distributions and pathogenic profiles: clade I (previously known as the Congo Basin or Central African clade) and clade II (formerly the West African clade) (Otieno et al., 2025; Yu et al., 2024).
Clade I viruses, including the ZAI-96 strain, are linked to increased virulence and mortality rates that can reach 10% (1.4%–10% range), potentially due to unique genetic elements involved in apoptotic pathways that enhance the pathogenicity (Alakunle et al., 2024; Lu et al., 2023; The Johns Hopkins University, 2024b). Conversely, clade II strains, such as SL-V70, COP-58, and WRAIR-6, cause less severe disease and exhibit mortality rates up to 3.6% (0.1%–3.6% range) (Monzón et al., 2024; The Johns Hopkins University, 2024b; Zhang et al., 2024). Clade II is further subdivided into subclades IIa and IIb, the latter was responsible for the outbreak from 2022 to 2024, originated in Nigeria (Cevik et al., 2024; The Johns Hopkins University, 2024b). Both clade I and subclade IIa primarily circulate endemically within animal reservoirs, which remain incompletely characterized but likely include rodents and nonhuman primates.
Notably, the 2022 Mpox outbreak revealed a significant shift in epidemiology with the emergence of a novel grouping designated clade IIb that exhibit ongoing adaptive evolution (Isidro et al., 2022). This clade, particularly the B.1 lineage, has become the dominant circulating strain globally, demonstrating enhanced human-to-human transmissibility outside endemic regions (Yu et al., 2024). Genomic comparisons demonstrate that MPXV clades differ primarily in genes involved in host interaction, including viral entry, immune evasion, and antigenic diversity (Molteni et al., 2023). The 2022 outbreak strains are distinguished from ancestral MPXV isolates by approximately 46 single-nucleotide polymorphisms (SNPs), underscoring substantial molecular evolution (Yu et al., 2024). Notably, the B.1 lineage displays an elevated substitution rate estimated to be six to twelve-fold greater than the mutation rate observed in isolates from 2018 to 2019 suggesting accelerated viral evolution (Lu et al., 2023).
Comparative analysis of the genomic sequences of MPXV strains from the Congo Basin and West Africa revealed approximately 99% nucleotide identity within each geographic cluster, but only about 95% identity when comparing strains across these regions (Rabaan et al., 2023).
The global outbreak of Mpox in 2022 prompted a rapid and extensive virological response, including the large-scale sequencing of viral genomes from numerous cases across different regions (Brinkmann et al., 2024; Isidro et al., 2022). These genomic investigations uncovered a range of mutations, particularly within genomic regions associated with viral replication and immune evasion. Mutational changes in these regions have been hypothesized to influence several virological and epidemiological parameters, including host range, vaccine responsiveness, and virulence (Young et al., 2024). Specifically, alterations in the genome may affect zoonotic transmission dynamics, enabling the virus to infect a broader range of species or enhance human-to-human transmissibility (Pan et al., 2023).
The ITRs are especially susceptible to editing by host-derived cytidine deaminases, such as members of the APOBEC3 (apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3) family (Brinkmann et al., 2023; O’Toole et al., 2023). APOBEC3 enzymes preferentially introduce G to A and C to T mutations within single-stranded DNA, particularly targeting cruciform DNA structures that can form in palindromic or repetitive regions such as ITRs (Van Norden et al., 2024). In the 2022 MPXV outbreak, a substantial percentage of observed SNPs were located within these ITR domains, suggesting that APOBEC3-mediated editing represents a significant factor in the evolution of MPXV during human infection (Fouad and Elsaid, 2023). Concerning this, recent studies have identified mutations in the APOBEC-3 gene, suggesting an adaptation of the virus to humans (Fouad and Elsaid, 2023). Further support for APOBEC3 activity in MPXV evolution comes from mutational signatures identified in recent genomic studies. The elevated frequency of TC>TT and the corresponding GA>AA substitutions has been particularly pronounced in MPXV clade IIb, which has demonstrated an increased substitution rate relative to other OPXV (Alakunle et al., 2024; Otieno et al., 2025; Yu et al., 2023). Despite the adaptive pressures exerted by the human immune system, MPXV continues to exhibit considerable genetic diversity, reflecting complex transmission dynamics and long-term maintenance in animal reservoirs. This is particularly evident when comparing viral genomes from different geographical clades.
Otieno and colleagues found that clade I is believed to be divergent from a common ancestor around 1917, and was detected in regions such as Sudan, indicating sustained circulation in animal reservoirs (Otieno et al., 2025). Notably, genomic analysis of the Sudan isolates revealed approximately 10.5 kbp of duplication and a TC>TT transition mutation rate, consistent with that observed in animal hosts and much lower than that expected from sustained human transmission, suggesting that this lineage has predominantly evolved within a nonhuman host for several decades, reinforcing the importance of zoonotic reservoirs in the persistence and re-emergence of Mpox (Otieno et al., 2025).
The geographic and temporal distribution of MPXV clades further illustrates the complex epidemiology of the virus, and the persistence of genetically distinct clades in zoonotic reservoirs highlights the persistent risk of spillover events (Otieno et al., 2025).
4 Clinical manifestation
Mpox, unlike smallpox, is a zoonotic disease that can be transmitted to humans through different animals. These include rodents (tree squirrels, dormice, African squirrel, Gambian Kangaroos), rabbits, prairie dogs (Bunge et al., 2022; Hutson et al., 2009), apes, and monkeys, even though the natural reservoir remains unknown. Studies in animal models, such as mice and monkeys, have demonstrated the clade I strains are more virulent than those of clade II, consistent with observed clinical outcomes in humans (Falendysz et al., 2023). The clinical presentation of both clades is broadly similar, with severity being the main distinguishing factor (Kipkorir et al., 2022). The animal-to-human transmission generally results from direct contact with skin lesions of infected animals or through the consumption of undercooked meat of infected animals (Angelo et al., 2019; Grant et al., 2020). Human-to-human transmission occurs through direct contact with virus particles in lesion secretion and body fluids (Grant et al., 2020). MPXV can also be transmitted via respiratory droplets during prolonged close contact, and through fomites such as contaminated clothing, bedding, and medical instruments (e.g., needles). Given that the virus is present in oropharyngeal secretions from the onset of clinical symptoms, extended exposure to respiratory droplets represents an additional route of transmission. Exudative lesions, blood, and other body fluids serve as sources of infection in both zoonotic and interhuman transmission. Secondary human-to-human transmission has been well-documented, such as during the 1996–1997 Central African outbreak, where 73% (65/89) of patients reported close contact with a confirmed case 7–21 days before symptoms onset (Centers for Disease Control and Prevention, 1997). Nosocomial transmissions were also documented in African outbreak (Nakoune et al., 2017) and in the United Kingdom, where healthcare workers were exposed while handling contaminated bedding without appropriate personal protective equipment (PPE) (Vaughan et al., 2020). In Human primates, infection can be initiated by intrabronchial administration of 5 × 104 plaque forming units (PFU) (Johnson et al., 2011). MPXV can be isolated from various types of clinical samples, each typically containing at least 106 viral copies (Rheinbaben et al., 2007).
The infectious dose of MPXV differs between clade I and clade II and depends on the route of exposure and the animal model used. Experimental studies have demonstrated that clade I strains are generally more virulent than clade II strains. For example, in prairie dogs infected intranasally, clade I viruses showed greater pathogenicity compared to clade II viruses with the same viral doses administrated (Hutson et al., 2009).
In a separate study, the infectivity and pathogenesis of clade I, clade IIa, and clade IIb MPXV strains were compared using the CAST/EiJ mouse model. Animals were infected via intranasal and intraperitoneal routes with viral doses ranging from 102 to 105 plaque-forming units (PFU), depending on the administration route. Results demonstrated that clade I virus exhibited significantly higher virulence compared to clade IIa and IIb strains, as evidenced by increased morbidity, mortality, and viral dissemination within infected tissues. These findings highlight the differential pathogenic potential among monkeypox virus clades (Americo et al., 2023).
Although these findings underscore clade-dependent differences in virulence, it is important to note that the minimum infectious dose (ID50) in humans remains undetermined. No studies to date have established a definitive human infectious dose for either clade I or clade II.
In humans the incubation period ranges from 5 days to 21 days, with a median of 6–13 days (Guzzetta et al., 2022; Miura et al., 2022). However, infected individuals may be contagious 1–4 days before the onset of symptoms (Sun et al., 2024). The symptomatology is consistent between clade I and clade II, with severity being the primary differentiator (Kipkorir et al., 2022). Initial symptoms include muscle aches, headaches, fatigue, and fever. The characteristic rash and mucosal involvement usually emerge within 1–3 days following the onset of fever, typically affecting the face, back, soles of the feet, and palms (Falendysz et al., 2017). During the 2022 outbreak in non-endemic geographical areas, most of Mpox cases occurred among men have sex with men (MSM), where the sexual contacts represented the main route of transmission. In these patients, atypical manifestations such as genital ulcers and perianal lesions were frequently observed, along with a higher prevalence of inguinal lymphadenopathy compared to axillary or cervical lymph node involvement (Antinori et al., 2022; Bragazzi et al., 2023; Ferré et al., 2022; Mitjà et al., 2023b; Ogoina, 2022).
The virus penetrates the body through skin lesion or mucous membranes and replicate in keratinocytes, fibroblasts, and endothelial cells (Mitjà et al., 2023b). From the initial infection site, MPXV can spread to draining lymph nodes, where the virus can replicate and subsequently reach spleen and liver. Some patients also present oropharyngeal involvement, including oral ulcers and tonsillitis, and ocular symptoms such as conjunctivitis and blepharitis (Damon, 2011). Serious complications may include bronchopneumonia and sepsis (Petersen et al., 2019). Although rare, cardiovascular complications, such as myocarditis, viral pericarditis, heart failure, and arrhythmias, have been reported. MPXV can access the central nervous system (CNS) via the olfactory epithelium or infected circulating monocytes/macrophages, potentially resulting in encephalitis (Sepehrinezhad et al., 2023). Additional complications may include conjunctivitis, diarrhea, and vomiting (McCollum and Damon, 2014). Moreover, new complications in 2022 outbreak had been described like perineal lesions and lymphadenopathy (Damon, 2011; Liu et al., 2023), paraphimosis, whitlow, and proctitis (Cherfan et al., 2023; Grau Echevarría et al., 2023; Pinnetti et al., 2024).
In most cases, Mpox is a self-limiting illness with a favourable prognosis, resolving within 2–4 weeks. However, the disease may take a more severe course in young children, pregnant individuals, and those with compromised immune systems (Liu et al., 2022). Moreover, some different clinical manifestations had been observed between different MPXV clades and lineage. For example, clade Ia, IIa and IIb lineage A show a generalized lymphadenopathy (Pittman et al., 2023), whereas in clade IIb lineage B.1 cases more frequently lymphadenopathy is localized in specific areas such as genital, anal, and oral region (Mitjà et al., 2023b; Thornhill et al., 2022). Clade IIb generally does not lead to severe complications except in immune compromised patients (Mitjà et al., 2023a). The higher severity of clade I infection could be correlated with high capacity of clade I to induce marked dysregulation of host cytokines, causing a strong inflammatory response and tissue damage (Huang et al., 2024). Furthermore, clade I replicates more efficiently in human cells. This allows to virus to replicates faster in comparison to clade II and to have a wide dissemination in short time (Americo et al., 2023).
5 MPXV as select agent
MPXV is classified in risk group 3 (Government of Canada, 2023). The recommendations for packing, shipping, inactivation methods, and managing of waste contaminated with MPXV differ depending on the viral clade. The clade I is classified as a Select Agent (SA), whereas specimens infected with clade II are not subject to SA regulations (Centers for Disease Control and Prevention, 2025a). Specimens containing MPXV clade II can be packed and shipped as Category B infectious substances (UN3373, Biological substances, Category B) and do not require a DOT (DOT Transporting Infectious substance safety) special permit under the DOT guidelines for transporting infectious substances (Centers for Disease Control and Prevention, 2024a). Routine diagnostic activities on specimens suspected to contain MPXV should be conducted in Biosafety Level 2 (BSL2) Laboratory. The recommended precautions that should be included are: eye protection, NIOSH-approved respirator with N95 filters or higher, double gloves, limiting the number of laboratory personnel involved in specimen manipulation, and working in laboratory with directional air flow (Meechan and Potts, 2020a; 2020b).
The wastewater samples suspected to contain MPXV should be pasteurized (60 °C for 1 h) before processing. However, all culture-based testing for monkeypox virus must be carried out in BSL3 Laboratory (i.e., seroneutralization assays) (Centers for Disease Control and Prevention, 2024b).
Although the case fatality rate of Mpox (up to 10% for clade I) is lower than that of smallpox (up to 30%), several factors could contribute to MPXV emerging as a serious viral pathogen representing a potential public health risk:
• Eradication of Mpox is challenging due to its zoonotic nature;
• A large portion of the global population under 40 years of age has not been vaccinated against smallpox and is therefore susceptible to MPXV infection;
• Currently circulating MPXV strains had been adapted to human (Zhang et al., 2023);
• The ongoing Mpox outbreak has facilitated the global diffusion of MPXV strains, that could potentially be manipulated to engineer more virulent strains using genetic biotechniques.
OPXVs are known to have a great capacity to accommodate foreign DNA. As early as 1993, a team of Russian researchers created a recombinant vaccinia virus containing an inserted DNA copy of 26S RNA from Venezuelan equine encephalomyelitis (VEE) virus. The VEE RNA was inserted into the thymidine kinase gene of the vaccinia virus (Sviatchenko et al., 1993). In 1980, a recombinant poxvirus was constructed by inserting hemagglutinin gene from influenza virus (Panicali et al., 1983). One of the main objectives of engineering poxviruses has been the development of new vaccines. For instance, a copy of the VP24 gene from Ebola virus or a gene encoding the envelope (E) protein of Japanese encephalitis virus was inserted into the vaccinia virus genome (Cheshenko et al., 1997; Shchelkunov et al., 1993). The resulting recombinant viruses were also very stable. These experiments also revealed that inactivation of the E7R gene did not affect the known biological properties of the virus but could contribute to the development of attenuated viral strains. Furthermore, murine interleukin-4 (IL-4) was inserted into the genome of ectromelia virus (mousepox virus) and expressed during infection. Expression of IL-4 by a thymidine kinase-positive strain of ectromelia virus suppressed both natural killer (NK) cell and cytotoxic T lymphocyte (CTL) cytolytic responses, as well as the expression of the interferon-gamma. The mice infected with this IL-4 expressing virus developed symptoms of acute mousepox accompanied by high mortality (Jackson et al., 2001). Even though MPXV has not met the definition of Potential Pandemic Pathogen (PPP), because it does not spread easily, like other OPXVs, MPXV possesses a high capacity for genetic recombination and can incorporate genes from other viruses, making it a candidate for gain-of-function (GoF) or research of concern (GOFROC) experiments. In 2015, Dr. Moss, one of the leading experts in the study of OPXVs, conducted experiments of transferring different genes from a clade II strain of MPXV into the genome of a more virulent clade I strain to assess whether the genes of clade II could reduce virulence (Kaiser, 2022; Kupferschmidt, 2022; Moffit and Howell, 2024). The resulting recombinant virus retained the same level of virulence, indicating that the clade II genes did not attenuate the pathogenicity of clade I. In the second step of experiments, Moss proposed to transfer clade I genes into the genome of a clade II strain to evaluate whether increased virulence could be conferred to the more transmissible clade II strain. Although this experiment was approved by the NIH, it is not clear whether it was ever performed (The United States House of Representatives, 2024). If conducted, this research could result in the creation of a clade II strain with both higher transmissibility (R0>1) and enhanced virulence, characteristics which would elevate it to the status of PPP, representing a great danger for the community. For these reasons, the Federal Government banned these experiments in May 2024.
These findings highlight the need for implementing infection programme prevention by enforcing biosecurity measures to prevent both new variants spreading and to speed the detection of genetically modified MPXV strains. The genomic sequencing is a key tool in the detection of exiting or emerging variants and should be improved with rapid and reliable instrument such as MinION, that is already used in genome surveillance where the resources are limited.
6 Antiviral therapy: current limitations, pharmacoresistant mutants, and new drugs
Currently, there are no FDA-approved therapies for Mpox specifically. However, several FDA-approved smallpox antivirals such as cidofovir, brincidofovir, and tecovirimat have been used in Mpox cases (as off label use). Although their efficacy against MPXV in humans has yet to be confirmed, these antivirals are used for immunocompromised individuals, children, pregnant or lactating women, and patients with lesions in sensitive areas such as the mouth, eyes, or genitals (Karagoz et al., 2023).
Cidofovir (CDV) is a non-cyclic nucleoside analogue of deoxycytidine monophosphate with broad-spectrum activity against DNA viruses, including MPXV. It is phosphorylated by host kinases to its active diphosphate form (CDV-PP), which inhibits viral DNA polymerase by competing with dCTP, leading to incorporation into viral DNA and chain termination (Andrei et al., 2006). Unlike other antivirals like acyclovir, CDV does not require viral thymidine kinase, maintaining efficacy against resistant strains (Paintsil and Cheng, 2009). In vitro and preclinical studies confirm its activity against MPXV, although clinical use is limited. Due to its nephrotoxicity, it must be co-administered with probenecid and adequate hydration (De Clercq, 2002). In patients with renal insufficiency, it may lead to nephrotoxicity (Lu et al., 2023). In vivo studies (rats, rabbits, primates) showed CDV is mainly limited by dose-dependent nephrotoxicity, which affects the proximal tubules. Oral coadministration of probenecid protects against CDV-induced nephrotoxicity (Lacy et al., 1998). It has also shown ocular toxicity (hypotonia, retinal damage) in guinea pigs or rabbits and carcinogenicity (mammary gland) in rats (European Medicines Agency, 2025; Taskintuna et al., 1997). Reproductive toxicity includes testicular atrophy and embryotoxicity in rodents.
During the outbreak of MPXV in 2022, it was rapidly repurposed for off-label use, particularly in severely immunocompromised individuals (Lu et al., 2023; Siegrist and Sassine, 2023). As with human papillomavirus lesions, CDV has also been used topically in MPXV lesions; as well as for cytomegalovirus infections in AIDS patients, CDV has also been used systemically for MPXV (Mertens et al., 2016; Titanji et al., 2024; Yin et al., 2022). In patients with renal insufficiency, it may lead to nephrotoxicity (Lu et al., 2023). CDV remains a therapeutic option in severe or refractory MPXV infections when other antivirals are unavailable or unsuitable. Furthermore, CDV resistance in vaccinia virus was associated with A314T and A684V mutations on the viral DNA polymerase at the exonuclease and palm subdomain levels, respectively (Andrei et al., 2006). The alanine residues at positions 314 and 684 are also conserved in the amino acid sequence of the DNA polymerase of MPXV, suggesting that resistance to this antiviral in MPXV could potentially emerge through analogous mutational mechanisms (Kannan et al., 2022).
Brincidofovir (BDV), also known as CMX001 or Tembex, is an oral lipid prodrug of cidofovir, specifically developed for higher bioavailability and lower nephrotoxicity. After absorption, the lipid portion facilitates cellular uptake and is cleaved intracellularly to release CDV, which is then phosphorylated to its active diphosphate form. Like CDV, BDV inhibits viral DNA polymerase by acting as a competitive analogue of deoxycytidine triphosphate (dCTP), leading to chain termination during viral DNA replication. Brincidofovir therapy offers partial protection and reduces viral load in lethal challenge with MPXV clade II a (Prévost et al., 2024). Compared to CDV, BDV has reduced renal toxicity but has been associated with gastrointestinal side effects, particularly diarrhoea and hepatotoxicity with increased liver enzymes, which may limit tolerability in some patients. Despite limited clinical data in MPXV, CDV remains a potential oral treatment option, particularly when intravenous administration of CDV or tecovirimat is not feasible (Centers for Disease Control and Prevention, 2025b; Lu et al., 2023). Carcinogenicity (e.g., squamous cell carcinoma) was observed in animal studies at sub-therapeutic exposures (Elsevier Health, 2025).
Among the guanosine analogues, ribavirin, a known inhibitor of viral replication of both DNA and RNA viruses, and KAY-2-41 were tested in vitro on MPXV or other OPXV; both were effective against resistant CDV mutants (Duraffour et al., 2014; Graci and Cameron, 2006; Lu et al., 2023).
Kannan and colleagues identified ten mutations in the replication complex (RC) present in almost all 2022 genomes, including two in F8L (catalytic subunit of the RC) and two in G9R (processivity factor) (Kannan et al., 2022). Dose-limiting toxicity is related to hemolytic anemia, occurring in 10%–30% of patients, especially during prolonged treatment or in combination with interferon (Russmann et al., 2006).
Tecovirimat (ST-246, TPOXX) is an antiviral agent approved by the FDA for the treatment of smallpox and authorized for MPXV treatment (Russo et al., 2021; U.S. Food and Drug Administration, 2024). It targets the highly conserved VP37 protein, encoded by the F13L gene, which is essential for the envelopment of intracellular mature virions by trans-Golgi-derived membranes to form enveloped virions released from host cell (Blasco and Moss, 1991; Karagoz et al., 2023; Lu et al., 2023). By inhibiting VP37, tecovirimat disrupts the maturation and release of intracellular mature virions without affecting viral replication, thereby limiting intercellular spread (Lu et al., 2023). Preclinical and clinical data have demonstrated potent in vitro and in vivo activity against MPXV, with observational studies and case reports, particularly in immunocompromised individuals, indicating favourable outcomes and good tolerability (Centers for Disease Control and Prevention, 2025b; Frenois-Veyrat et al., 2022; Postal et al., 2025; Warner et al., 2022). In phase III clinical trials in healthy volunteers, no serious adverse events related to treatment were reported. Tecovirimat is a weak enzyme modulator (CYP3A4, CYP2C8, CYP2C19), with a low risk of clinically relevant interactions (Li et al., 2025). Tecovirimat is metabolized via amide hydrolysis and glucuronidation by uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) and uridine diphosphate glucuronosyltransferase 1A4 (UGT1A4), reaching steady state in 4–6 days. Its half-life is about 21 h after 600 mg oral dosing and 19 h after 200 mg intravenous (IV) dosing (Mishra et al., 2025).
However, recent randomized controlled trial results in the DRC showed no significant reduction in lesion duration in clade I MPXV infections (PALM007 Writing Group et al., 2025; Van Dijck et al., 2024). Resistance, primarily due to mutations in the F13L gene, has emerged in patients receiving prolonged treatment, and cases of transmitted resistance have been documented (Smith et al., 2023). In fact, reported single nucleotide variations such as H238Q, P243S, N267D, A288P, A290V, A295E, and I372N alter key hydrophobic pockets or binding regions of the VP37 protein, thereby reducing the ability of tecovirimat to inhibit viral replication (Chenchula et al., 2025). However, the results regarding the efficacy of tecovirimat are conflicting, as reported in several clinical trials (Cohen, 2025; Postal et al., 2025).
In addition, NIOCH-14, a prodrug of tecovirimat, showed antiviral effects against OPXV in in vitro and in vivo studies (Lu et al., 2023; Mazurkov et al., 2016) (Table 1).
During MPXV replication, crescent-shaped membranes form and mature into immature virions (IVs) within viral factories (Barreto-Vieira et al., 2025; Hong et al., 2024). Several antiviral agents disrupt virus assembly, maturation, and release (Lu et al., 2023). PA104 and drugs like imatinib mesylate (STI-571, Gleevec) inhibit actin tail formation, blocking viral egress and showing anti-OPXVs activity in vitro (Lu et al., 2023; Priyamvada et al., 2020; Reeves et al., 2005, 2011). EGFR inhibitor gefitinib (Iressa) and MEK inhibitors also impaired OPXV spread (Brayman et al., 1990; Lu et al., 2023; Sliva and Schnierle, 2007).
The viral protein A36R is essential for intercellular transmission and EEV release (Lu et al., 2023). Miah and colleagues identified by virtual screening three peptides that bind A36R with high affinity and potentially effective against MPXV (Lu et al., 2023; Miah et al., 2022).
Overall, current antivirals such as tecovirimat and brincidofovir show efficacy both in vitro and in vivo, but robust clinical data in MPXV patients are limited. Tecovirimat is usually well tolerated with mild side effects, while brincidofovir is limited by gastrointestinal and hepatic toxicity. The nephrotoxicity of cidofovir limits its routine use. Resistance mutations have been documented in vitro, especially to tecovirimat, which targets the viral F13L gene, making resistance monitoring necessary.
Future research should focus on large clinical trials, combination therapies to reduce resistance and toxicity, new antivirals with improved safety, pharmacokinetic studies, and genomic surveillance of resistance.
Combination therapies may offer synergistic effects, enabling comparable efficacy at lower doses while reducing the frequency and severity of adverse events. Furthermore, they represent a promising strategy to prevent the emergence of drug resistance, which has already become a critical concern for tecovirimat in Mpox patients and may also affect cidofovir and brincidofovir (Smee et al., 2002). Because some immunocompromised patients may experience persistent MPXV infection (Contag et al., 2023; Rousseau et al., 2023), long-term use of a single antiviral agent could heighten the likelihood of resistance, highlighting the potential benefits of combination therapy for these individuals.
However, while combination therapies can overcome resistance to single agents, they may also increase the risk of toxicity compared with monotherapy. For these reasons, further studies and research are needed to develop new, specific antivirals for the treatment of Mpox.
7 Smallpox and Mpox vaccinia virus vaccines
Protection against Mpox disease is achieved by vaccination with vaccinia viruses that are protective also against smallpox disease. Vaccinia virus was initially used to vaccinate against smallpox disease at same time during the 19th century, when it replaced the cowpox virus for smallpox vaccination and became the preferred virus for it (Fenner et al., 1988; Parrino and Graham, 2006).
For about one century, until 1965 smallpox vaccination was unregulated and non-standardized and different strains of vaccinia virus and methods of storage and application were used. The vaccinia virus (VACV) strains New York City Board of Health (NYCBH), EM-63, and Lister/Elstree, were predominantly used during the early smallpox eradication campaign (Fenner et al., 1988).
In 1968, the WHO recommended the use of either the NYCBH strain or the Lister strain in the worldwide eradication campaign. From 1968 to 1971 the Lister strain became the most widely used vaccine worldwide (Rosenthal et al., 2001).
The vaccine used for eradication was grown in the skin of calves or other animals including sheep, buffaloes and rabbits (Committee for Proprietary Medicinal Products, 2002). After the eradication of smallpox, declared by the Health World Organization in 1980, the remaining licensed vaccines using the NYCBH strain were the lyophilized Dryavx (Wyeth), and liquid Wetvax (Aventis Pasteur). Dryvax was used in 2002 in two United States vaccination programs. Both programs reported similar rates of expected serious adverse events and in addition, abnormally high rates of cardiac related adverse events (Cassimatis et al., 2004; Poland et al., 2005).
The first-generation smallpox vaccine was reactogenic. Up to 40% of vaccinees experienced systemic symptoms including fever, myalgia, malaise and headaches. Moderate to severe complications that included generalized vaccinia, eczema vaccinatum, progressive vaccinia, post-vaccination encephalitis or encephalopathy and death were estimated to be 1–250 cases per million primary vaccinations, although the rates varied depending on the strain used, age groups (Jacobs et al., 2009). In general, among the vaccinia strains used for worldwide eradication, Dryvax®, was considered the safest, having the fewest adverse events (Kretzschmar et al., 2006).
In summary first generation smallpox vaccines were highly efficient as demonstrated during the Smallpox eradication campaign but they were relatively high reactogenic with a wide range of contraindications (Wiser et al., 2007).
The production of vaccine in live animals had important limitations due to contaminations by bacteria and adventitious agents, and to the presence of potentially allergenic animal proteins (Murphy and Osburn, 2007).
To overcome these limitations a second-generation vaccinia virus vaccine (VACV), named ACAM2000 (Novartis, formerly ACAMbis) was generated. It was a replication competent vaccine developed from a single clonal strain of Dryvax, that was propagated in Vero cell culture (Nalca and Zumbrun, 2010). ACAM2000 was approved by the FDA in 2007. Preclinical and clinical trials reported in 2008 demonstrated that ACAM2000™ had comparable immunogenicity to that of Dryvax® and caused a similar frequency of adverse events.
To increase the safety of smallpox vaccine, third-generation attenuated VACVs were developed. Attenuated VACVs were obtained through sequential passages of the virus in tissue culture cells from alternative hosts (McCurdy et al., 2004). Modified Vaccinia Ankara (MVA) is an attenuated replication deficient poxvirus generated by more than 500 serial passages of vaccinia virus in chicken embryo fibroblast. Following the acquisition of multiple deletions and mutations, it lost the capacity to replicate in human cells (Drexler et al., 1998; Meyer et al., 1991). MVA-BN, that is derived from the MVA strain, is a further attenuated MVA strain. Due to the lack of replication competence in mammalian cells MVA-BN has a favorable safety profile for individuals including those with atopic dermatitis and immunodeficiency.
No signal for inflammatory cardiac disorders was identified throughout the MVA-BN development program. This is in sharp contrast to the older, replicating vaccinia smallpox vaccines, which have a known risk for myocarditis and/or pericarditis in up to 1 in 200 vaccinees (Volkmann et al., 2021).
8 Effectiveness and immunogenicity of smallpox vaccines against Mpox
Data from the active surveillance program for Mpox supported by the World Health Organization during 1980–1986 in the Democratic Republic of Congo provided the first evidence that smallpox vaccines protected against MPXV infection. This program, started after the official discontinuation of smallpox vaccination in 1980 and aimed to predict the future of Mpox dynamics in the absence of mass vaccination, showed a vaccine effectiveness of about 85% (Fine et al., 1988). The assessment of the risk of MPXV infection in the same endemic areas, performed about 25 years later demonstrated that people who were vaccinated with smallpox vaccine had a 5.2-fold lower risk of Mpox than the unvaccinated individuals confirming the protective role of smallpox vaccination. In addition, this and other studies reported that vaccine induced immunity was long lasting (Hammarlund et al., 2005; Rimoin et al., 2010).
Although mass vaccination with smallpox vaccine is not recommended during Mpox outbreak, during the multi-country Mpox outbreak in 2022, primary preventive vaccination was recommended for individuals at high-risk of exposure. They included gay, bisexual or MSM individuals with multiple casual sexual partners, sex workers; health workers and laboratory personnel working with orthopoxviruses. In addition, post-exposure preventive vaccination (PEPV) was recommended for contacts of cases, ideally within 4 days of first exposure (and up to 14 days in the absence of symptoms). Vaccination was used to interrupt human to human transmission, protect vulnerable individuals and minimize zoonotic transmission (World Health Organization, 2025a).
The vaccines considered in the response to the outbreak were, ACAM2000, MVA-BN, and LC16.
All of them, were developed against smallpox and while ACAM2000 was a second-generation vaccine, MVA-BN and LC16 were third-generation vaccines. MVA-BN was licensed as a vaccine against Mpox in humans in Canada (known as Imvamune) and the United States (known as JYNNEOS) and was approved by the European Medicines Agency under special circumstances (known as Imvanex), while LC16m8, a strain derived from the Lister strain of vaccinia virus, is approved for the prevention of Mpox in Japan. The efficacy of these vaccines could not be tested in randomized placebo- controlled clinical studies because of the infrequency of outbreaks. Evaluation of the protective effect of ACAM2000 and MVA-BN in a monkeypox model of infection in cynomolgus macaques showed that animals receiving either a prime and boost of Imvamune or a single immunization with ACAM2000 were protected completely from severe and/or lethal infection. Both antibody and cell-mediated immune responses were stimulated, and similar high titers of neutralizing antibodies were detected following the second dose of MVA-BN and one dose of ACAM2000 (Hatch et al., 2013) (Table 2).
More recently the comparison of the immunogenicity and protective efficacy of MVA-BN, ACAM2000 and Ad35 vector-based subunit (L1R/B5R or L1R/B5R/A27L/A33R) vaccine in a Rhesus macaque model of MPXV infection demonstrated that all vaccines provided robust protection against a high dose of intravenous MPXV challenge with the 2022–2023 outbreak strain (Jacob-Dolan et al., 2024). However, while a single dose of ACAM2000 provided a near complete protection, incomplete protection was achieved by the MVA-BN and the Ad35-subunit vaccines. During the clade IIb global spread, clinical studies have evaluated the MVA-BN vaccine effectiveness (VE) for Mpox, providing different estimates. The studies were performed in different countries and used various designs. The adjusted VE estimates against Mpox disease ≥14 days after pre-exposure vaccination with a single dose of MVA-BN were between 35% and 86% (Brousseau et al., 2024; Dalton et al., 2023; Deputy et al., 2023; Mason et al., 2024; Navarro et al., 2023; Payne et al., 2022; Ramchandani et al., 2023; Rosenberg et al., 2023; Wolff Sagy et al., 2023). For two doses of pre-exposure vaccination the VE estimates ranged from 66% to 90% (Dalton et al., 2023; Deputy et al., 2023; Payne et al., 2022). Crude VE estimates ranged from 33% to 84% and 57%–87% for one or two doses of MVA-BN respectively. Additionally, persons with Mpox who had received one or two doses of MVA-BN had a lower risk of hospitalization and decreased severity of Mpox clinical manifestation compared to unvaccinated individuals (Granskog et al., 2025; Guagliardo et al., 2024; Hazra et al., 2024; Schildhauer et al., 2023).
Data on LC16m8 vaccine effectiveness against Mpox are limited. A single immunization of LC16m8 induces robust cellular immune responses and broad neutralizing antibodies in immunocompetent and HIV infected individuals (Okumura et al., 2025). At present, based on vaccine effectiveness and safety profile MVA-BN represents the most promising candidate for broad use compared to ACAM2000 and LC16m8.
The Rhesus macaque model of monkeypox infection was also adopted to characterize the protective immune responses induced by vaccinia virus vaccine (Edghill-Smith et al., 2005). In this model, animals were vaccinated with the first-generation smallpox vaccine Dryvax by scarification and challenged 4 weeks later with monkeypox virus. The contribution of humoral or cell mediated immune response was assessed by antibody depletion of B cells or CD8 T cells in groups of animals. Results of this study showed that depletion of B cells, but not CD8+ T, affected the production of neutralizing antibodies and abrogated the vaccine induced protection from a lethal intravenous challenge with monkeypox virus demonstrating that the antibodies induced by smallpox vaccine are necessary and sufficient for protection against monkeypox virus (Edghill-Smith et al., 2005). Although there are not data on the same cohort, by combining the available immunogenicity data of MVA-BN to the corresponding vaccine effectiveness, a weak but significant association between antibody titers and vaccine effectiveness against Mpox could be demonstrated providing further support to the assumption that antibody levels may represent a correlate of protection (Berry et al., 2024). However, the recent assessment of MPXV neutralizing antibodies following two-shot MVA-BN immunization in non-primed individuals revealed that such antibodies were detected only in 63% of sera 4 weeks after the second MVA-BN vaccine dose and that in general, neutralizing antibodies showed a little increase after the second dose (Zaeck et al., 2023). At variance evaluation of the T cell memory response in convalescent and MVA-BN vaccinated individuals, using vaccinia virus infected cells or MPXV specific peptides, demonstrated that both individuals were able to mount a significant anti-MPXV T cell response with similar magnitude of CD4+ and CD8+ virus specific T cell responses, although T cells elicited by MPXV infection showed increased cytotoxicity and activation, a more expanded TCR repertoire and high potential to migrate to the site of infection compared to vaccine induced T cells suggesting that cellular immunity could be relevant (Chen et al., 2025).
9 Discussion
The large-scale outbreak of MPXV prompted the WHO to declare a public health emergency of international concern in July 2022, which is still in effect today, emphasising the need for continued surveillance of emerging pathogens (World Health Organization, 2024b), and underscores the importance of understanding the etiopathogenetic mechanisms and genomic evolution underlying the rapid spread of the MPXV.
Data sharing from MPXV strains isolated in various geographic regions has revealed an increased mutation rate, likely driven by APOBEC3-mediated mutagenesis, with a high concentration of single nucleotide polymorphisms (SNPs) found in the inverted terminal repeat (ITR) regions (Brinkmann et al., 2023; O’Toole et al., 2023). Although the phenotypic effects of recent MPXV mutations are not yet fully understood, the observed genomic changes likely underlie the virus’s increased transmissibility, atypical clinical features, and expanded geographic spread. Monitoring these evolutionary trends is essential for anticipating future outbreaks and informing public health responses.
The 2022 outbreak also highlighted the high rate of sexual transmission of Mpox: in non-endemic countries, an estimated 94% of MSM (Li et al., 2023). This epidemiological evidence supports the recommendation of targeted vaccination strategies for this population, as implemented in several countries (United States, Democratic Republic of the Congo and several neighbouring countries in Central and Eastern Africa, EU/EEA countries) (Centers for Disease Control and Prevention, 2025c; European Centre for Disease Prevention and Control, 2025; UNICEF, 2024).
Another critical aspect that emerged during the outbreaks starting in 2022 is the lack of specific effective antiviral therapies for Mpox. All drugs used in clinical settings have been FDA-approved for the treatment of smallpox, such as cidofovir and tecovirimat, or for other double-stranded DNA viruses. Unfortunately, clinical trials of tecovirimat conducted in the Democratic Republic of the Congo showed only limited efficacy in reducing Mpox-related lesions (PALM007 Writing Group et al., 2025; Van Dijck et al., 2024).
Cidofovir has been employed both systemically and topically, especially in severe cases, although concerns remain regarding its nephrotoxicity. Additionally, chemical compounds, such as the tecovirimat prodrug NIOCH-14, have demonstrated antiviral activity in vitro, and may offer potential future therapeutic options (Mazurkov et al., 2016).
Although some studies have identified peptides with potential antiviral activity against Mpox (Miah et al., 2022), these findings remain preliminary, and no clinical trials have been initiated to date. Recently, the viral resolvase enzyme has emerged as a promising therapeutic target. Mahoney and colleagues identified small molecules capable of inhibiting the resolvase of both vaccinia virus and MPXV in vitro, supporting its potential as an antiviral target against orthopoxviruses (Mahoney et al., 2025).
The ongoing epidemic should serve as an impetus for the development of MPXV-specific antiviral drugs with greater efficacy, as well as for the optimisation of more effective prophylactic strategies in terms of protective immunity.
Although tecovirimat is an approved and clinically effective antiviral, the range of available therapeutics targeting MPXV-encoded proteins remains limited, and the potential for the emergence of drug-resistant viral strains is a significant concern. These considerations underscore the urgent need to develop alternative and complementary therapeutic strategies. Among these, host-targeted antivirals (HTAs) have emerged as a promising and complementary therapeutic approach. Unlike direct-acting antivirals (DAAs), HTAs modulate host cell pathways involved in the viral life cycle or immune escape. This approach offers several advantages, including a lower risk of resistant mutants emerging due to the evolutionary stability of host targets; broad-spectrum antiviral potential; and additive or synergistic activity in combination with DAAs. Host-targeted strategies, successfully applied to other viruses, could also be exploited against MPXV; however, their clinical translation is still hindered by challenges such as cellular toxicity, the need for high selectivity to minimize off-target effects, pharmacokinetic optimization, and the lack of robust animal models that accurately reproduce human MPXV infection.
To date, vaccination remains the most effective form of prevention against Mpox. Among the available options, the non-replicating attenuated vaccine MVA-BN is currently the preferred one due to its safety profile and effectiveness.
Despite the availability of effective preventive vaccines, the absence of FDA-approved antivirals specifically targeting MPXV underscores the critical need for the development of dedicated therapeutic agents to improve clinical management and outbreak preparedness.
The Mpox outbreaks were caused by different virus strains, the first one by MPXV clade IIb, was characterized mainly by sexual transmission and it occurred in countries where the MPXV infection is not endemic. The second outbreak started in the Democratic Republic of Congo and rapidly spread to surrounding countries such as Uganda, Zimbabwe, the Central African Republic, Burundi, Rwanda, Kenya, underlining the importance of a rapid concerted response from various states to achieve concrete results. The management of the COVID-19 pandemic has taught us a lot in improving strategies and preparedness in health emergency situations: 1) an effective surveillance systems is based on a quickly identification of pathogens, 2) adequate information can improve adherence or preventive measures, 3) technical assistance and training for healthcare resource workers is indispensable in low-resource setting, 4) equitable distribution of vaccines support limit the diffusion of diseases and deaths (Adebiyi, 2025). Importantly, COVID-19 has also reshaped paradigms of vaccine deployment and pharmacological response, offering valuable insights for Mpox. In particular, integrating genomic surveillance with clinical and pharmacological data can reveal how viral evolution influences vaccine performance, drug response, and resistance development. Applying this approach to Mpox could support the optimization of therapeutic strategies, early detection of resistance, and the development of novel antivirals, ultimately improving both clinical management and public health interventions.
10 Conclusions
Recent Mpox outbreaks have highlighted how new variants can lead to different clinical manifestations, with varying severity and adaptation to humans, while also unveiling the limitations of currently used treatments. Although the genomic features of these lineages do not appear to affect treatment efficacy, monitoring the evolutionary dynamics within lineages remains essential for the development, evaluation, and efficacy of new antiviral compounds against Mpox. Furthermore, vaccination remains the most effective form of prevention against Mpox. Among the available options, the non-replicating attenuated vaccine MVA-BN is currently the preferred one due to its safety profile and effectiveness. However, even for MVA-BN, issues related to the high reactogenicity, the incomplete immunity, the uncertainty of cross protection and of the durability of vaccination exist. The solution of these issues drives the research aimed to improve or develop new Mpox vaccine strategies.
Author contributions
SL: Investigation, Writing – original draft, Writing – review and editing. AG: Conceptualization, Funding acquisition, Investigation, Writing – original draft, Writing – review and editing. SP: Investigation, Visualization, Writing – review and editing. VZ: Investigation, Visualization, Writing – review and editing. PD: Conceptualization, Investigation, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by EVORA project, from the European Union’s Horizon Europe research and innovation program under grant agreement number 101131959.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adebiyi, B. O. (2025). Mpox alert: COVID-19’s hard-earned lessons put to the test. One Health 20, 101003. doi:10.1016/j.onehlt.2025.101003
Adegboye, O. A., Eugenia Castellanos, M., Alele, F. O., Pak, A., Ezechukwu, H. C., Hou, K., et al. (2022). Travel-related monkeypox outbreaks in the era of COVID-19 pandemic: are we prepared? Viruses 14, 1283. doi:10.3390/v14061283
Alakunle, E., Kolawole, D., Diaz-Cánova, D., Alele, F., Adegboye, O., Moens, U., et al. (2024). A comprehensive review of monkeypox virus and mpox characteristics. Front. Cell Infect. Microbiol. 14, 1360586. doi:10.3389/fcimb.2024.1360586
Americo, J. L., Earl, P. L., and Moss, B. (2023). Virulence differences of mpox (monkeypox) virus clades I, IIa, and IIb.1 in a small animal model. Proc. Natl. Acad. Sci. U. S. A. 120, e2220415120. doi:10.1073/pnas.2220415120
Anderson, M. G., Frenkel, L. D., Homann, S., and Guffey, J. (2003). A case of severe monkeypox virus disease in an American child: emerging infections and changing professional values. Pediatr. Infect. Dis. J. 22, 1093–1098. doi:10.1097/01.inf.0000101821.61387.a5
Andrei, G., Gammon, D. B., Fiten, P., De Clercq, E., Opdenakker, G., Snoeck, R., et al. (2006). Cidofovir resistance in vaccinia virus is linked to diminished virulence in mice. J. Virol. 80, 9391–9401. doi:10.1128/JVI.00605-06
Angelo, K. M., Petersen, B. W., Hamer, D. H., Schwartz, E., and Brunette, G. (2019). Monkeypox transmission among international travellers-serious monkey business? J. Travel Med. 26, taz002. doi:10.1093/jtm/taz002
Antinori, A., Mazzotta, V., Vita, S., Carletti, F., Tacconi, D., Lapini, L. E., et al. (2022). Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill. 27, 2200421. doi:10.2807/1560-7917.ES.2022.27.22.2200421
Barreto-Vieira, D. F., Miranda, M. D., da Silva, M. A. N., de Almeida, A. S., de Almeida, A. L. T., Bandeira, D. M., et al. (2025). MPXV: update on morphological and morphogenesis aspects through transmission and scanning electron microscopies and 3D reconstruction. J. Med. Virol. 97, e70180. doi:10.1002/jmv.70180
Berry, M. T., Khan, S. R., Schlub, T. E., Notaras, A., Kunasekaran, M., Grulich, A. E., et al. (2024). Predicting vaccine effectiveness for mpox. Nat. Commun. 15, 3856. doi:10.1038/s41467-024-48180-w
Blasco, R., and Moss, B. (1991). Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-Dalton outer envelope protein. J. Virol. 65, 5910–5920. doi:10.1128/JVI.65.11.5910-5920.1991
Bragazzi, N. L., Kong, J. D., Mahroum, N., Tsigalou, C., Khamisy-Farah, R., Converti, M., et al. (2023). Epidemiological trends and clinical features of the ongoing monkeypox epidemic: a preliminary pooled data analysis and literature review. J. Med. Virol. 95, e27931. doi:10.1002/jmv.27931
Brayman, K. L., Naji, A., and Barker, C. F. (1990). Studies of host immunomodulation and prevention of pancreatic beta cell destruction. Transpl. Proc. 22, 851–852.
Brinkmann, A., Kohl, C., Pape, K., Bourquain, D., Thürmer, A., Michel, J., et al. (2023). Extensive ITR expansion of the 2022 Mpox virus genome through gene duplication and gene loss. Virus Genes 59, 532–540. doi:10.1007/s11262-023-02002-1
Brinkmann, A., Pape, K., Uddin, S., Woelk, N., Förster, S., Jessen, H., et al. (2024). Genome sequencing of the mpox virus 2022 outbreak with amplicon-based Oxford nanopore MinION sequencing. J. Virol. Methods 325, 114888. doi:10.1016/j.jviromet.2024.114888
Brousseau, N., Carazo, S., Febriani, Y., Padet, L., Hegg-Deloye, S., Cadieux, G., et al. (2024). Single-dose effectiveness of mpox vaccine in Quebec, Canada: test-negative design with and without adjustment for self-reported exposure risk. Clin. Infect. Dis. 78, 461–469. doi:10.1093/cid/ciad584
Brown, K., and Leggat, P. A. (2016). Human monkeypox: current state of knowledge and implications for the future. Trop. Med. Infect. Dis. 1, 8. doi:10.3390/tropicalmed1010008
Bunge, E. M., Hoet, B., Chen, L., Lienert, F., Weidenthaler, H., Baer, L. R., et al. (2022). The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl. Trop. Dis. 16, e0010141. doi:10.1371/journal.pntd.0010141
Cassimatis, D. C., Atwood, J. E., Engler, R. M., Linz, P. E., Grabenstein, J. D., and Vernalis, M. N. (2004). Smallpox vaccination and myopericarditis: a clinical review. J. Am. Coll. Cardiol. 43, 1503–1510. doi:10.1016/j.jacc.2003.11.053
Centers for Disease Control and Prevention (1997). Human monkeypox--Kasai oriental, Zaire, 1996-1997. MMWR Morb. Mortal. Wkly. Rep. 46, 304–307.
Centers for Disease Control and Prevention (2024a). Biosafety laboratory guidance for handling and processing mpox specimens. Available online at: https://www.cdc.gov/mpox/hcp/laboratories/biosafety.html (Accessed June 6, 25).
Centers for Disease Control and Prevention (2024b). Biosafety laboratory guidance for handling and processing mpox specimens - select agent regulations. Available online at: https://www.cdc.gov/mpox/hcp/laboratories/biosafety.html#cdc_generic_section_3-select-agent-regulations (Accessed June 6, 25).
Centers for Disease Control and Prevention (2025a). Select agents and toxins list. Available online at: https://www.selectagents.gov/sat/list.htm (Accessed October 7, 25).
Centers for Disease Control and Prevention (2025b). Clinical treatment of mpox. Available online at: https://www.cdc.gov/mpox/hcp/clinical-care/index.html (Accessed March 7, 25).
Centers for Disease Control and Prevention (2025c). Interim clinical considerations for use of vaccine for mpox prevention in the United States. Available online at: https://www.cdc.gov/mpox/hcp/vaccine-considerations/vaccination-overview.html (Accessed October 7, 25).
Cevik, M., Tomori, O., Mbala, P., Scagliarini, A., Petersen, E., Low, N., et al. (2024). The 2023 - 2024 multi-source mpox outbreaks of clade I MPXV in Sub-Saharan Africa: alarm bell for Africa and the world. IJID Reg. 12, 100397. doi:10.1016/j.ijregi.2024.100397
Chen, N., Li, G., Liszewski, M. K., Atkinson, J. P., Jahrling, P. B., Feng, Z., et al. (2005). Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology 340, 46–63. doi:10.1016/j.virol.2005.05.030
Chen, J.-L., Wang, B., Lu, Y., Antoun, E., Bird, O., Drennan, P. G., et al. (2025). T cell memory response to MPXV infection exhibits greater effector function and migratory potential compared to MVA-BN vaccination. Nat. Commun. 16, 4362. doi:10.1038/s41467-025-59370-5
Chenchula, S., Atal, S., Ghanta, M. K., Uppugunduri, C. R., Karunakaran, S., Amerneni, K. C., et al. (2025). Emerging variants of Mpox virus and tecovirimat resistance: genomic insights and implications for treatment strategies. Virology 608, 110532. doi:10.1016/j.virol.2025.110532
Cheng, Y., Han, P., Peng, Q., Liu, R., Liu, H., Yuan, B., et al. (2025). Assembly and breakage of head-to-head double hexamer reveals mpox virus E5-catalyzed DNA unwinding initiation. Nat. Commun. 16, 5176. doi:10.1038/s41467-025-60539-1
Cherfan, P., Massaad, E., and Hui, V. W. (2023). Anorectal manifestations of treatment-refractory monkeypox requiring surgical intervention. Am. Surg. 89, 6370–6373. doi:10.1177/00031348231177931
Cheshenko, N. V., Petrov, V. S., Protopopova, E. V., Netesova, N. A., Konovalova, S. N., Belavin, P. A., et al. (1997). Recombinant vaccinia virus expressing Japanese encephalitis virus protein E. Mol. Gen. Mikrobiol. Virusol., 24–27.
Cohen, J. (2025). Mpox drug wins approval in Japan—But it doesn’t work. Science 387, 349–350. doi:10.1126/science.adw1657
Committee for Proprietary Medicinal Products (2002). Note for guidance on the development of vaccinia virus based vaccines against smallpox.
Contag, C. A., Mische, L., Fong, I., Karan, A., Vaidya, A., McCormick, D. W., et al. (2023). Treatment of mpox with suspected tecovirimat resistance in immunocompromised patient, United States, 2022. Emerg. Infect. Dis. 29, 2520–2523. doi:10.3201/eid2912.230849
Dalton, A. F., Diallo, A. O., Chard, A. N., Moulia, D. L., Deputy, N. P., Fothergill, A., et al. (2023). Estimated effectiveness of JYNNEOS vaccine in preventing Mpox: a multijurisdictional case-control study — united States, August 19, 2022–March 31, 2023. MMWR Morb. Mortal. Wkly. Rep. 72, 553–558. doi:10.15585/mmwr.mm7220a3
Damon, I. K. (2011). Status of human monkeypox: clinical disease, epidemiology and research. Vaccine 29 (Suppl. 4), D54–D59. doi:10.1016/j.vaccine.2011.04.014
De Clercq, E. (2002). Cidofovir in the treatment of poxvirus infections. Antivir. Res. 55, 1–13. doi:10.1016/s0166-3542(02)00008-6
Deputy, N. P., Deckert, J., Chard, A. N., Sandberg, N., Moulia, D. L., Barkley, E., et al. (2023). Vaccine effectiveness of JYNNEOS against mpox disease in the United States. N. Engl. J. Med. 388, 2434–2443. doi:10.1056/nejmoa2215201
Desingu, P. A., Nagarajan, K., and Sundaresan, N. R. (2023). Unique tandem repeats in the inverted terminal repeat regions of monkeypox viruses. Microbiol. Spectr. 11, e0319922. doi:10.1128/spectrum.03199-22
Drexler, I., Heller, K., Wahren, B., Erfle, V., and Sutter, G. (1998). Highly attenuated modified vaccinia virus Ankara replicates in baby hamster kidney cells, a potential host for virus propagation, but not in various human transformed and primary cells. J. Gen. Virol. 79 (Pt 2), 347–352. doi:10.1099/0022-1317-79-2-347
Duraffour, S., Drillien, R., Haraguchi, K., Balzarini, J., Topalis, D., van den Oord, J. J., et al. (2014). KAY-2-41, a novel nucleoside analogue inhibitor of orthopoxviruses in vitro and in vivo. Antimicrob. Agents Chemother. 58, 27–37. doi:10.1128/AAC.01601-13
Edghill-Smith, Y., Golding, H., Manischewitz, J., King, L. R., Scott, D., Bray, M., et al. (2005). Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 11, 740–747. doi:10.1038/nm1261
Elsevier Health (2025). Brincidofovir. Available online at: https://elsevier.health/en-US/preview/brincidofovir (Accessed September 09, 25).
European Centre for Disease Prevention and Control (2025). Rapid scientific advice on public health measures for mpox (2024-2025). Available online at: https://www.ecdc.europa.eu/en/infectious-disease-topics/mpox/rapid-scientific-advice-public-health-measures-mpox-2024-2025 (Accessed October 7, 25).
European Medicines Agency (EMA) (2025). Scientific discussion. Available online at: https://www.ema.europa.eu/en/documents/scientific-discussion/vistide-epar-scientific-discussion_en.pdf (Accessed September 09, 25).
Falendysz, E. A., Lopera, J. G., Doty, J. B., Nakazawa, Y., Crill, C., Lorenzsonn, F., et al. (2017). Characterization of monkeypox virus infection in African rope squirrels (Funisciurus sp.). PLoS Negl. Trop. Dis. 11, e0005809. doi:10.1371/journal.pntd.0005809
Falendysz, E. A., Lopera, J. G., Rocke, T. E., and Osorio, J. E. (2023). Monkeypox virus in animals: current knowledge of viral transmission and pathogenesis in wild animal reservoirs and captive animal models. Viruses 15, 905. doi:10.3390/v15040905
F. Fenner, D. A. Henderson, I. Arita, Z. Jezek, and I. D. Ladnyj (1988). Smallpox and its eradication, history of international public health (Geneva: World Health Organization).
Ferré, V. M., Bachelard, A., Zaidi, M., Armand-Lefevre, L., Descamps, D., Charpentier, C., et al. (2022). Detection of monkeypox virus in anorectal swabs from asymptomatic men who have sex with men in a sexually transmitted infection screening program in Paris, France. Ann. Intern Med. 175, 1491–1492. doi:10.7326/M22-2183
Fine, P. E., Jezek, Z., Grab, B., and Dixon, H. (1988). The transmission potential of monkeypox virus in human populations. Int. J. Epidemiol. 17, 643–650. doi:10.1093/ije/17.3.643
Fouad, F. M., and Elsaid, M. (2023). Mutations of the monkeypox virus responsible for the 2022 outbreak. Gene Rep. 33, 101828. doi:10.1016/j.genrep.2023.101828
Frenois-Veyrat, G., Gallardo, F., Gorgé, O., Marcheteau, E., Ferraris, O., Baidaliuk, A., et al. (2022). Tecovirimat is effective against human monkeypox virus in vitro at nanomolar concentrations. Nat. Microbiol. 7, 1951–1955. doi:10.1038/s41564-022-01269-8
Government of Canada (2023). Monkeypox virus: infectious substances pathogen safety data sheet. Available online at: https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/monkeypox-virus.html (Accessed April 6, 25).
Graci, J. D., and Cameron, C. E. (2006). Mechanisms of action of ribavirin against distinct viruses. Rev. Med. Virol. 16, 37–48. doi:10.1002/rmv.483
Granskog, L., Saadeh, K., Lorenz, K., Quint, J., Salih, T., Lo, T., et al. (2025). Effect of JYNNEOS vaccination on mpox clinical progression: a case–control study. Lancet Infect. Dis. doi:10.1016/s1473-3099(25)00180-x
Grant, R., Nguyen, L.-B. L., and Breban, R. (2020). Modelling human-to-human transmission of monkeypox. Bull. World Health Organ 98, 638–640. doi:10.2471/BLT.19.242347
Grau Echevarría, A., Peñuelas Leal, R., Labrandero Hoyos, C., Casanova Esquembre, A., Magdaleno Tapial, J., Pérez Ferriols, A., et al. (2023). Monkeypox whitlow: a novel presentation. Clin. Exp. Dermatol 48, 781–784. doi:10.1093/ced/llad103
Guagliardo, S. A. J., Kracalik, I., Carter, R. J., Braden, C., Free, R., Hamal, M., et al. (2024). Monkeypox virus infections after 2 preexposure doses of JYNNEOS vaccine — united States, May 2022–May 2024. MMWR Morb. Mortal. Wkly. Rep. 73, 460–466. doi:10.15585/mmwr.mm7320a3
Guan, H., Gul, I., Xiao, C., Ma, S., Liang, Y., Yu, D., et al. (2023). Emergence, phylogeography, and adaptive evolution of mpox virus. New Microbes New Infect. 52, 101102. doi:10.1016/j.nmni.2023.101102
Guzzetta, G., Mammone, A., Ferraro, F., Caraglia, A., Rapiti, A., Marziano, V., et al. (2022). Early estimates of monkeypox incubation period, generation time, and reproduction number, Italy, May-June 2022. Emerg. Infect. Dis. 28, 2078–2081. doi:10.3201/eid2810.221126
Hammarlund, E., Lewis, M. W., Carter, S. V., Amanna, I., Hansen, S. G., Strelow, L. I., et al. (2005). Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat. Med. 11, 1005–1011. doi:10.1038/nm1273
Hammarlund, E., Dasgupta, A., Pinilla, C., Norori, P., Früh, K., and Slifka, M. K. (2008). Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation. Proc. Natl. Acad. Sci. U. S. A. 105, 14567–14572. doi:10.1073/pnas.0800589105
Hatch, G. J., Graham, V. A., Bewley, K. R., Tree, J. A., Dennis, M., Taylor, I., et al. (2013). Assessment of the protective effect of imvamune and Acam2000 vaccines against aerosolized monkeypox virus in cynomolgus macaques. J. Virol. 87, 7805–7815. doi:10.1128/jvi.03481-12
Hazra, A., Zucker, J., Bell, E., Flores, J., Gordon, L., Mitjà, O., et al. (2024). Mpox in people with past infection or a complete vaccination course: a global case series. Lancet Infect. Dis. 24, 57–64. doi:10.1016/S1473-3099(23)00492-9
Hong, Y., Huang, B., Zhang, J., Peng, C., Kong, W., Tan, W., et al. (2024). Molecular architecture of monkeypox mature virus. Cell Discov. 10, 108. doi:10.1038/s41421-024-00741-5
Huang, Y., Bergant, V., Grass, V., Emslander, Q., Hamad, M. S., Hubel, P., et al. (2024). Multi-omics characterization of the monkeypox virus infection. Nat. Commun. 15, 6778. doi:10.1038/s41467-024-51074-6
Hughes, L. J., Goldstein, J., Pohl, J., Hooper, J. W., Lee Pitts, R., Townsend, M. B., et al. (2014). A highly specific monoclonal antibody against monkeypox virus detects the heparin binding domain of A27. Virology 464–465, 264–273. doi:10.1016/j.virol.2014.06.039
Hutson, C. L., Olson, V. A., Carroll, D. S., Abel, J. A., Hughes, C. M., Braden, Z. H., et al. (2009). A prairie dog animal model of systemic orthopoxvirus disease using West African and Congo Basin strains of monkeypox virus. J. Gen. Virol. 90, 323–333. doi:10.1099/vir.0.005108-0
Isidro, J., Borges, V., Pinto, M., Sobral, D., Santos, J. D., Nunes, A., et al. (2022). Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat. Med. 28, 1569–1572. doi:10.1038/s41591-022-01907-y
Jackson, R. J., Ramsay, A. J., Christensen, C. D., Beaton, S., Hall, D. F., and Ramshaw, I. A. (2001). Expression of mouse interleukin-4 by a recombinant ectromelia virus suppresses cytolytic lymphocyte responses and overcomes genetic resistance to mousepox. J. Virol. 75, 1205–1210. doi:10.1128/JVI.75.3.1205-1210.2001
Jacob-Dolan, C., Ty, D., Hope, D., McMahan, K., Liu, J., Powers, O. C., et al. (2024). Comparison of the immunogenicity and protective efficacy of ACAM2000, MVA, and vectored subunit vaccines for Mpox in rhesus macaques. Sci. Transl. Med. 16, eadl4317. doi:10.1126/scitranslmed.adl4317
Jacobs, B. L., Langland, J. O., Kibler, K. V., Denzler, K. L., White, S. D., Holechek, S. A., et al. (2009). Vaccinia virus vaccines: past, present and future. Antivir. Res. 84, 1–13. doi:10.1016/j.antiviral.2009.06.006
Jezek, Z., Szczeniowski, M., Paluku, K. M., and Mutombo, M. (1987). Human monkeypox: clinical features of 282 patients. J. Infect. Dis. 156, 293–298. doi:10.1093/infdis/156.2.293
Ji, X., Liang, R., Bao, C., Cai, X., Chen, S., Chen, L., et al. (2024). Evolutionary variation of the monkeypox virus detected for the first time in Nantong, Jiangsu. Jiangsu. Virol. J. 21, 334. doi:10.1186/s12985-024-02616-3
Jiang, H., Li, J., Jian, Y., Yang, T., Zhang, J., and Li, J. (2024). Expression, purification, and crystal structure of mpox virus A41 protein. Protein Expr. Purif. 219, 106480. doi:10.1016/j.pep.2024.106480
Johnson, R. F., Dyall, J., Ragland, D. R., Huzella, L., Byrum, R., Jett, C., et al. (2011). Comparative analysis of monkeypox virus infection of cynomolgus macaques by the intravenous or intrabronchial inoculation route. J. Virol. 85, 2112–2125. doi:10.1128/JVI.01931-10
Jones, J. M., Messauodi, I., Estep, R. D., Orzechowska, B., and Wong, S. W. (2008). Monkeypox virus viral chemokine inhibitor (MPV vCCI), a potent inhibitor of rhesus macrophage inflammatory protein-1. Cytokine 43, 220–228. doi:10.1016/j.cyto.2008.05.016
Kannan, S. R., Sachdev, S., Reddy, A. S., Kandasamy, S. L., Byrareddy, S. N., Lorson, C. L., et al. (2022). Mutations in the monkeypox virus replication complex: potential contributing factors to the 2022 outbreak. J. Autoimmun. 133, 102928. doi:10.1016/j.jaut.2022.102928
Kantele, A., Chickering, K., Vapalahti, O., and Rimoin, A. W. (2016). Emerging diseases-the monkeypox epidemic in the democratic Republic of the Congo. Clin. Microbiol. Infect. 22, 658–659. doi:10.1016/j.cmi.2016.07.004
Karagoz, A., Tombuloglu, H., Alsaeed, M., Tombuloglu, G., AlRubaish, A. A., Mahmoud, A., et al. (2023). Monkeypox (mpox) virus: classification, origin, transmission, genome organization, antiviral drugs, and molecular diagnosis. J. Infect. Public Health 16, 531–541. doi:10.1016/j.jiph.2023.02.003
Kipkorir, V., Dhali, A., Srichawla, B., Kutikuppala, S., Cox, M., Ochieng, D., et al. (2022). The re-emerging monkeypox disease. Trop. Med. Int. Health 27, 961–969. doi:10.1111/tmi.13821
Kretzschmar, M., Wallinga, J., Teunis, P., Xing, S., and Mikolajczyk, R. (2006). Frequency of adverse events after vaccination with different vaccinia strains. PLoS Med. 3, e272. doi:10.1371/journal.pmed.0030272
Kugelman, J. R., Johnston, S. C., Mulembakani, P. M., Kisalu, N., Lee, M. S., Koroleva, G., et al. (2014). Genomic variability of monkeypox virus among humans, democratic Republic of the Congo. Emerg. Infect. Dis. 20, 232–239. doi:10.3201/eid2002.130118
Lacy, S. A., Hitchcock, M. J., Lee, W. A., Tellier, P., and Cundy, K. C. (1998). Effect of oral probenecid coadministration on the chronic toxicity and pharmacokinetics of intravenous cidofovir in cynomolgus monkeys. Toxicol. Sci. 44, 97–106. doi:10.1006/toxs.1998.2481
Ladnyj, I. D., Ziegler, P., and Kima, E. (1972). A human infection caused by monkeypox virus in basankusu territory, democratic Republic of the Congo. Bull. World Health Organ 46, 593–597.
Li, P., Li, J., Ayada, I., Avan, A., Zheng, Q., Peppelenbosch, M. P., et al. (2023). Clinical features, antiviral treatment, and patient outcomes: a systematic review and comparative analysis of the previous and the 2022 mpox outbreaks. J. Infect. Dis. 228, 391–401. doi:10.1093/infdis/jiad034
Li, Y., Wang, L., and Chen, S. (2025). An overview of the progress made in research into the Mpox virus. Med. Res. Rev. 45, 788–812. doi:10.1002/med.22085
Liu, X., Zhu, Z., He, Y., Lim, J. W., Lane, B., Wang, H., et al. (2022). Monkeypox claims new victims: the outbreak in men who have sex with men. Infect. Dis. Poverty 11, 84. doi:10.1186/s40249-022-01007-6
Liu, Q., Fu, L., Wang, B., Sun, Y., Wu, X., Peng, X., et al. (2023). Clinical characteristics of human mpox (Monkeypox) in 2022: a systematic review and meta-analysis. Pathogens 12, 146. doi:10.3390/pathogens12010146
Lu, J., Xing, H., Wang, C., Tang, M., Wu, C., Ye, F., et al. (2023). Mpox (formerly monkeypox): pathogenesis, prevention, and treatment. Signal Transduct. Target Ther. 8, 458. doi:10.1038/s41392-023-01675-2
Lum, F.-M., Torres-Ruesta, A., Tay, M. Z., Lin, R. T. P., Lye, D. C., Rénia, L., et al. (2022). Monkeypox: disease epidemiology, host immunity and clinical interventions. Nat. Rev. Immunol. 22, 597–613. doi:10.1038/s41577-022-00775-4
Mahoney, J. P., Offei, S., Pant, A., Wang, Z., Weiner, B., Yin, L., et al. (2025). Discovery of small-molecule orthopoxvirus resolvase inhibitors with antiviral activity. J. Med. Chem. 68, 13358–13375. doi:10.1021/acs.jmedchem.5c00019
Mason, L. M. K., Betancur, E., Riera-Montes, M., Lienert, F., and Scheele, S. (2024). MVA-BN vaccine effectiveness: a systematic review of real-world evidence in outbreak settings. Vaccine 42, 126409. doi:10.1016/j.vaccine.2024.126409
Mazurkov, O. Y., Kabanov, A. S., Shishkina, L. N., Sergeev, A. A., Skarnovich, M. O., Bormotov, N. I., et al. (2016). New effective chemically synthesized anti-smallpox compound NIOCH-14. J. Gen. Virol. 97, 1229–1239. doi:10.1099/jgv.0.000422
McCollum, A. M., and Damon, I. K. (2014). Human monkeypox. Clin. Infect. Dis. 58, 260–267. doi:10.1093/cid/cit703
McCurdy, L. H., Larkin, B. D., Martin, J. E., and Graham, B. S. (2004). Modified vaccinia Ankara: potential as an alternative smallpox vaccine. Clin. Infect. Dis. 38, 1749–1753. doi:10.1086/421266
P. J. Meechan, and J. Potts (2020a). “Section IV—Laboratory biosafety level criteria,” Biosafety in microbiological and biomedical laboratories, birkhäuser advances in infectious diseases, 37–43. doi:10.1007/978-3-7643-7557-7_19
P. J. Meechan, and J. Potts (2020b). “Appendix N—clinical laboratories,” Biosafety in microbiological and biomedical laboratories, birkhäuser advances in infectious diseases, 529–541.
Mertens, B., Nogueira, T., Stranska, R., Naesens, L., Andrei, G., and Snoeck, R. (2016). Cidofovir is active against human papillomavirus positive and negative head and neck and cervical tumor cells by causing DNA damage as one of its working mechanisms. Oncotarget 7, 47302–47318. doi:10.18632/oncotarget.10100
Meyer, H., Sutter, G., and Mayr, A. (1991). Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J. General Virology 72, 1031–1038. doi:10.1099/0022-1317-72-5-1031
Miah, M. M., Tabassum, N., Afroj Zinnia, M., and Islam, A. B. M. M. K. (2022). Drug and anti-viral peptide design to inhibit the monkeypox virus by restricting A36R protein. Bioinform Biol. Insights 16, 11779322221141164. doi:10.1177/11779322221141164
Mishra, P., Singh, R., and Patil, A. (2025). Epidemiology, pathogenesis, and treatment options of monkeypox: a narrative review. Cureus 17, e77892. doi:10.7759/cureus.77892
Mitjà, O., Alemany, A., Marks, M., Lezama Mora, J. I., Rodríguez-Aldama, J. C., Torres Silva, M. S., et al. (2023a). Mpox in people with advanced HIV infection: a global case series. Lancet 401, 939–949. doi:10.1016/S0140-6736(23)00273-8
Mitjà, O., Ogoina, D., Titanji, B. K., Galvan, C., Muyembe, J.-J., Marks, M., et al. (2023b). Monkeypox. Lancet 401, 60–74. doi:10.1016/S0140-6736(22)02075-X
Miura, F., van Ewijk, C. E., Backer, J. A., Xiridou, M., Franz, E., Op de Coul, E., et al. (2022). Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill. 27, 2200448. doi:10.2807/1560-7917.ES.2022.27.24.2200448
Moffit, R., and Howell, M. (2024). Monkeypox: House investigators find NIH hiding risky viral gain-of-function research. Available online at: https://www.dailysignal.com/2024/07/01/house-investigators-find-nih-hiding-risky-viral-gain-function-research (Accessed June 5, 25).
Mohanto, S., Faiyazuddin, M., Dilip Gholap, A., Jc, D., Bhunia, A., Subbaram, K., et al. (2023). Addressing the resurgence of global monkeypox (Mpox) through advanced drug delivery platforms. Travel Med. Infect. Dis. 56, 102636. doi:10.1016/j.tmaid.2023.102636
Molteni, C., Forni, D., Cagliani, R., Arrigoni, F., Pozzoli, U., De Gioia, L., et al. (2023). Selective events at individual sites underlie the evolution of monkeypox virus clades. Virus Evol. 9, vead031. doi:10.1093/ve/vead031
Monzón, S., Varona, S., Negredo, A., Vidal-Freire, S., Patiño-Galindo, J. A., Ferressini-Gerpe, N., et al. (2024). Monkeypox virus genomic accordion strategies. Nat. Commun. 15, 3059. doi:10.1038/s41467-024-46949-7
Murphy, F. A., and Osburn, B. I. (2007). Adventitious agents and smallpox vaccine in strategic national stockpile. Emerg. Infect. Dis. 11, 1086–1089. doi:10.3201/eid1107.050277
Nakoune, E., Lampaert, E., Ndjapou, S. G., Janssens, C., Zuniga, I., Van Herp, M., et al. (2017). A nosocomial outbreak of human monkeypox in the Central African Republic. Open Forum Infect. Dis. 4, ofx168. doi:10.1093/ofid/ofx168
Nalca, A., and Zumbrun, E. E. (2010). ACAM2000: the new smallpox vaccine for United States strategic national stockpile. Drug Des. Devel Ther. 4, 71–79. doi:10.2147/dddt.s3687
Navarro, C., Lau, C., Buchan, S. A., Burchell, A. N., Nasreen, S., Friedman, L., et al. (2023). Effectiveness of one dose of MVA-BN vaccine against mpox infection in males in Ontario, Canada: a target trial emulation. doi:10.1101/2023.10.04.23296566
Ogoina, D. (2022). Sexual behaviours and clinical course of human monkeypox in Spain. Lancet 400, 636–637. doi:10.1016/S0140-6736(22)01497-0
Okumura, N., Morino, E., Nomoto, H., Yanagi, M., Takahashi, K., Iwasaki, H., et al. (2025). LC16m8 for pre-exposure prophylaxis against mpox in a high-risk population: an open-label randomized trial. Clin. Infect. Dis. ciaf074, ciaf074. doi:10.1093/cid/ciaf074
Otieno, J. R., Ruis, C., Onoja, A. B., Kuppalli, K., Hoxha, A., Nitsche, A., et al. (2025). Global genomic surveillance of monkeypox virus. Nat. Med. 31, 342–350. doi:10.1038/s41591-024-03370-3
O’Toole, Á., Neher, R. A., Ndodo, N., Borges, V., Gannon, B., Gomes, J. P., et al. (2023). APOBEC3 deaminase editing in mpox virus as evidence for sustained human transmission since at least 2016. Science 382, 595–600. doi:10.1126/science.adg8116
Paintsil, E., and Cheng, Y.-C. (2009). “Antiviral agents,” in Encyclopedia of microbiology (Elsevier), 223–257. doi:10.1016/B978-012373944-5.00178-4
PALM007 Writing Group Ali, R., Alonga, J., Biampata, J.-L., Kombozi Basika, M., Maljkovic Berry, I., et al. (2025). Tecovirimat for clade I MPXV infection in the democratic republic of Congo. N. Engl. J. Med. 392, 1484–1496. doi:10.1056/NEJMoa2412439
Pan, D., Nazareth, J., Sze, S., Martin, C. A., Decker, J., Fletcher, E., et al. (2023). Transmission of monkeypox/mpox virus: a narrative review of environmental, viral, host, and population factors in relation to the 2022 international outbreak. J. Med. Virol. 95, e28534. doi:10.1002/jmv.28534
Panicali, D., Davis, S. W., Weinberg, R. L., and Paoletti, E. (1983). Construction of live vaccines by using genetically engineered poxviruses: biological activity of recombinant vaccinia virus expressing influenza virus hemagglutinin. Proc. Natl. Acad. Sci. U. S. A. 80, 5364–5368. doi:10.1073/pnas.80.17.5364
Parrino, J., and Graham, B. (2006). Smallpox vaccines: past, present, and future. J. Allergy Clin. Immunol. 118, 1320–1326. doi:10.1016/j.jaci.2006.09.037
Payne, A. B., Ray, L. C., Cole, M. M., Canning, M., Houck, K., Shah, H. J., et al. (2022). Reduced risk for mpox after receipt of 1 or 2 doses of JYNNEOS vaccine compared with risk among unvaccinated persons - 43 U.S. jurisdictions, July 31-October 1, 2022. MMWR Morb. Mortal. Wkly. Rep. 71, 1560–1564. doi:10.15585/mmwr.mm7149a5
Petersen, E., Kantele, A., Koopmans, M., Asogun, D., Yinka-Ogunleye, A., Ihekweazu, C., et al. (2019). Human monkeypox: Epidemiologic and clinical characteristics, diagnosis, and prevention. Infect. Dis. Clin. North Am. 33, 1027–1043. doi:10.1016/j.idc.2019.03.001
Pinnetti, C., Mondi, A., Mazzotta, V., Vita, S., Carletti, F., Aguglia, C., et al. (2024). Pharyngo-tonsillar involvement of Mpox in a cohort of men who have sex with men (MSM): a serious risk of missing diagnosis. J. Infect. Public Health 17, 130–136. doi:10.1016/j.jiph.2023.11.015
Pittman, P. R., Martin, J. W., Kingebeni, P. M., Tamfum, J.-J. M., Mwema, G., Wan, Q., et al. (2023). Clinical characterization and placental pathology of mpox infection in hospitalized patients in the Democratic Republic of the Congo. PLoS Negl. Trop. Dis. 17, e0010384. doi:10.1371/journal.pntd.0010384
Poland, G., Grabenstein, J., and Neff, J. (2005). The US smallpox vaccination program: a review of a large modern era smallpox vaccination implementation program. Vaccine 23, 2078–2081. doi:10.1016/j.vaccine.2005.01.012
Postal, J., Guivel-Benhassine, F., Porrot, F., Grassin, Q., Crook, J. M., Vernuccio, R., et al. (2025). Antiviral activity of tecovirimat against monkeypox virus clades 1a, 1b, 2a, and 2b. Lancet Infect. Dis. 25, e126–e127. doi:10.1016/S1473-3099(25)00014-3
Prévost, J., Sloan, A., Deschambault, Y., Tailor, N., Tierney, K., Azaransky, K., et al. (2024). Treatment efficacy of cidofovir and brincidofovir against clade II monkeypox virus isolates. Antivir. Res. 231, 105995. doi:10.1016/j.antiviral.2024.105995
Priyamvada, L., Alabi, P., Leon, A., Kumar, A., Sambhara, S., Olson, V. A., et al. (2020). Discovery of Retro-1 analogs exhibiting enhanced anti-vaccinia virus activity. Front. Microbiol. 11, 603. doi:10.3389/fmicb.2020.00603
Rabaan, A. A., Alasiri, N. A., Aljeldah, M., Alshukairiis, A. N., AlMusa, Z., Alfouzan, W. A., et al. (2023). An updated review on monkeypox viral disease: emphasis on genomic diversity. Biomedicines 11, 1832. doi:10.3390/biomedicines11071832
Ramchandani, M. S., Berzkalns, A., Cannon, C. A., Dombrowski, J. C., Brown, E., Chow, E. J., et al. (2023). Effectiveness of the modified vaccinia Ankara vaccine against mpox in men who have sex with men: a retrospective cohort analysis, Seattle, Washington. Open Forum Infect. Dis. 10, ofad528. doi:10.1093/ofid/ofad528
Reeves, P. M., Bommarius, B., Lebeis, S., McNulty, S., Christensen, J., Swimm, A., et al. (2005). Disabling poxvirus pathogenesis by inhibition of Abl-family tyrosine kinases. Nat. Med. 11, 731–739. doi:10.1038/nm1265
Reeves, P. M., Smith, S. K., Olson, V. A., Thorne, S. H., Bornmann, W., Damon, I. K., et al. (2011). Variola and monkeypox viruses utilize conserved mechanisms of virion motility and release that depend on abl and SRC family tyrosine kinases. J. Virol. 85, 21–31. doi:10.1128/JVI.01814-10
Rheinbaben, F. V., Gebel, J., Exner, M., and Schmidt, A. (2007). “Environmental resistance, disinfection, and sterilization of poxviruses,” in Poxviruses, birkhäuser advances in infectious diseases. Editors A. A. Mercer, A. Schmidt, and O. Weber (Basel: Birkhäuser Basel), 397–405.
Rimoin, A. W., Mulembakani, P. M., Johnston, S. C., Lloyd Smith, J. O., Kisalu, N. K., Kinkela, T. L., et al. (2010). Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the democratic Republic of Congo. Proc. Natl. Acad. Sci. U.S.A. 107, 16262–16267. doi:10.1073/pnas.1005769107
Rosenberg, E. S., Dorabawila, V., Hart-Malloy, R., Anderson, B. J., Miranda, W., O’Donnell, T., et al. (2023). Effectiveness of JYNNEOS vaccine against diagnosed mpox infection - new York, 2022. MMWR Morb. Mortal. Wkly. Rep. 72, 559–563. doi:10.15585/mmwr.mm7220a4
Rosenthal, S. R., Merchlinsky, M., Kleppinger, C., and Goldenthal, K. L. (2001). Developing new smallpox vaccines. Emerg. Infect. Dis. 7, 920–926. doi:10.3201/eid0706.010602
Rousseau, A., Ferrier, A., Stabler, S., Vuotto, F., Massip, E., Ouafi, M., et al. (2023). Absence of association between persistent skin lesion and virological replication in severe disseminated monkeypox infection in solid organ transplant recipient. Infect. Dis. Now. 53, 104749. doi:10.1016/j.idnow.2023.104749
Russmann, S., Grattagliano, I., Portincasa, P., Palmieri, V. O., and Palasciano, G. (2006). Ribavirin-induced anemia: mechanisms, risk factors and related targets for future research. Curr. Med. Chem. 13, 3351–3357. doi:10.2174/092986706778773059
Russo, A. T., Grosenbach, D. W., Chinsangaram, J., Honeychurch, K. M., Long, P. G., Lovejoy, C., et al. (2021). An overview of tecovirimat for smallpox treatment and expanded anti-orthopoxvirus applications. Expert Rev. Anti Infect. Ther. 19, 331–344. doi:10.1080/14787210.2020.1819791
Sagdat, K., Batyrkhan, A., and Kanayeva, D. (2024). Exploring monkeypox virus proteins and rapid detection techniques. Front. Cell Infect. Microbiol. 14, 1414224. doi:10.3389/fcimb.2024.1414224
Schildhauer, S., Saadeh, K., Vance, J., Quint, J., Salih, T., Lo, T., et al. (2023). Reduced odds of Mpox-Associated hospitalization among persons who received JYNNEOS vaccine - california, May 2022-May 2023. MMWR Morb. Mortal. Wkly. Rep. 72, 992–996. doi:10.15585/mmwr.mm7236a4
Sepehrinezhad, A., Ashayeri Ahmadabad, R., and Sahab-Negah, S. (2023). Monkeypox virus from neurological complications to neuroinvasive properties: current status and future perspectives. J. Neurol. 270, 101–108. doi:10.1007/s00415-022-11339-w
Shchelkunov, S. N., Blinov, V. M., and Sandakhchiev, L. S. (1993). Genes of variola and vaccinia viruses necessary to overcome the host protective mechanisms. FEBS Lett. 319, 80–83. doi:10.1016/0014-5793(93)80041-r
Siegrist, E. A., and Sassine, J. (2023). Antivirals with activity against mpox: a clinically oriented review. Clin. Infect. Dis. 76, 155–164. doi:10.1093/cid/ciac622
Sliva, K., and Schnierle, B. (2007). From actually toxic to highly specific--novel drugs against poxviruses. Virol. J. 4, 8. doi:10.1186/1743-422X-4-8
Smee, D. F., Sidwell, R. W., Kefauver, D., Bray, M., and Huggins, J. W. (2002). Characterization of wild-type and Cidofovir-Resistant strains of camelpox, cowpox, monkeypox, and vaccinia viruses. Antimicrob. Agents Chemother. 46, 1329–1335. doi:10.1128/AAC.46.5.1329-1335.2002
Smith, T. G., Gigante, C. M., Wynn, N. T., Matheny, A., Davidson, W., Yang, Y., et al. (2023). Tecovirimat resistance in mpox patients, United States, 2022-2023. Emerg. Infect. Dis. 29, 2426–2432. doi:10.3201/eid2912.231146
Sun, Y., Nie, W., Tian, D., and Ye, Q. (2024). Human monkeypox virus: epidemiologic review and research progress in diagnosis and treatment. J. Clin. Virol. 171, 105662. doi:10.1016/j.jcv.2024.105662
Sviatchenko, V. A., Agapov, E. V., Urmanov, I. K., Serpinskiĭ, O. I., Frolov, I. V., Kolykhalov, A. A., et al. (1993). The immunogenic properties of a recombinant vaccinia virus with an incorporated DNA copy of the 26S RNA of the Venezuelan equine encephalomyelitis virus. Vopr. Virusol. 38, 222–226.
Tang, D., Liu, X., Lu, J., Fan, H., Xu, X., Sun, K., et al. (2023). Recombinant proteins A29L, M1R, A35R, and B6R vaccination protects mice from mpox virus challenge. Front. Immunol. 14, 1203410. doi:10.3389/fimmu.2023.1203410
Taskintuna, I., Banker, A. S., Rao, N. A., Wiley, C. A., Flores-Aguilar, M., Munguia, D., et al. (1997). An animal model for cidofovir (HPMPC) toxicity: intraocular pressure and histopathologic effects. Exp. Eye Res. 64, 795–806. doi:10.1006/exer.1996.0273
The Johns Hopkins University (2024a). Mpox virus: clade I and clade II. Available online at: https://publichealth.jhu.edu/sites/default/files/2024-09/mpox-su-91624.pdf (Accessed September 7, 25).
The Johns Hopkins University (2024b). Mpox virus: clade I and clade II. Available online at: https://publichealth.jhu.edu/sites/default/files/2024-12/Mpox-SU-12.10.24.pdf (Accessed February 7, 25).
The United States House of Representatives (2024). Interim staff report on investigation into risky MPXV experiment at the national institute of allergy and infectious diseases. Available online at: https://d1dth6e84htgma.cloudfront.net/MPVX_Interim_Staff_Report_and_Appendices_final_844c87e06f.pdf.
Thornhill, J. P., Barkati, S., Walmsley, S., Rockstroh, J., Antinori, A., Harrison, L. B., et al. (2022). Monkeypox virus infection in humans across 16 countries - april-june 2022. N. Engl. J. Med. 387, 679–691. doi:10.1056/NEJMoa2207323
Titanji, B. K., Hazra, A., and Zucker, J. (2024). Mpox clinical presentation, diagnostic approaches, and treatment strategies: a review. JAMA 332, 1652–1662. doi:10.1001/jama.2024.21091
UNICEF (2024). Vaccine doses allocated to nine African countries hardest hit by mpox surge. Available online at: https://www.unicef.org/press-releases/vaccine-doses-allocated-nine-african-countries-hardest-hit-mpox-surge#main-content (Accessed September 7, 25).
U.S. Food and Drug Administration (2024). Mpox. FDA’s role in mpox preparedness and response, and information about mpox (formerly referred to as monkeypox). Available online at: https://www.fda.gov/emergency-preparedness-and-response/mcm-issues/mpox (Accessed February 7, 25).
Van Dijck, C., Crozier, I., Vercauteren, K., Brosius, I., Mbala-Kingebeni, P., Dodd, L., et al. (2024). Beware of drug resistance: let’s not lose tecovirimat against mpox. Clin. Microbiol. Infect. 30, 276–278. doi:10.1016/j.cmi.2023.09.014
Van Norden, M., Falls, Z., Mandloi, S., Segal, B. H., Baysal, B. E., Samudrala, R., et al. (2024). The implications of APOBEC3-mediated C-to-U RNA editing for human disease. Commun. Biol. 7, 529. doi:10.1038/s42003-024-06239-w
Vaughan, A., Aarons, E., Astbury, J., Balasegaram, S., Beadsworth, M., Beck, C. R., et al. (2018). Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill. 23, 1800509. doi:10.2807/1560-7917.ES.2018.23.38.1800509
Vaughan, A., Aarons, E., Astbury, J., Brooks, T., Chand, M., Flegg, P., et al. (2020). Human-to-Human transmission of monkeypox virus, United Kingdom, October 2018. Emerg. Infect. Dis. 26, 782–785. doi:10.3201/eid2604.191164
Volkmann, A., Williamson, A.-L., Weidenthaler, H., Meyer, T. P. H., Robertson, J. S., Excler, J.-L., et al. (2021). The brighton collaboration standardized template for collection of key information for risk/benefit assessment of a modified vaccinia Ankara (MVA) vaccine platform. Vaccine 39, 3067–3080. doi:10.1016/j.vaccine.2020.08.050
Wang, X., Ma, L., Li, N., and Gao, N. (2023). Structural insights into the assembly and mechanism of mpox virus DNA polymerase complex F8-A22-E4-H5. Mol. Cell 83, 4398–4412.e4. doi:10.1016/j.molcel.2023.10.038
Warner, B. M., Klassen, L., Sloan, A., Deschambault, Y., Soule, G., Banadyga, L., et al. (2022). In vitro and in vivo efficacy of tecovirimat against a recently emerged 2022 monkeypox virus isolate. Sci. Transl. Med. 14, eade7646. doi:10.1126/scitranslmed.ade7646
Wiser, I., Balicer, R. D., and Cohen, D. (2007). An update on smallpox vaccine candidates and their role in bioterrorism related vaccination strategies. Vaccine 25, 976–984. doi:10.1016/j.vaccine.2006.09.046
Wolff Sagy, Y., Zucker, R., Hammerman, A., Markovits, H., Arieh, N. G., Abu Ahmad, W., et al. (2023). Real-world effectiveness of a single dose of mpox vaccine in males. Nat. Med. 29, 748–752. doi:10.1038/s41591-023-02229-3
World Health Organization (2024a). Mpox. Available online at: https://www.who.int/news-room/fact-sheets/detail/mpox (Accessed July 10, 25).
World Health Organization (2024b). WHO director-general declares mpox outbreak a public health emergency of international concern. Available online at: https://www.who.int/news/item/14-08-2024-who-director-general-declares-mpox-outbreak-a-public-health-emergency-of-international-concern (Accessed July 10, 25).
World Health Organization (2025a). Public advice on mpox vaccination. Available online at: https://www.who.int/news-room/public-advice/mpox-vaccination (Accessed July 10, 25).
World Health Organization (WHO) (2025b). Mpox: multi-country external situation report no.57. Available online at: https://cdn.who.int/media/docs/default-source/documents/emergencies/multi-country-outbreak-of-mpox--external-situation-report--57.pdf?sfvrsn=420b121c_5&download=true (Accessed July 10, 25).
Xie, Y., Kuai, L., Peng, Q., Wang, Q., Wang, H., Li, X., et al. (2025). Structural basis of DNA replication fidelity of the Mpox virus. Proc. Natl. Acad. Sci. U. S. A. 122, e2411686122. doi:10.1073/pnas.2411686122
Yi, X.-M., Lei, Y.-L., Li, M., Zhong, L., and Li, S. (2024). The monkeypox virus-host interplays. Cell Insight 3, 100185. doi:10.1016/j.cellin.2024.100185
Yin, Z., Sun, J., Yang, Y., Xu, N., Jiang, L., Fan, Z., et al. (2022). Cidofovir, a choice for salvage treatment of cytomegalovirus infection in patients with haploidentical hematopoietic stem cell transplantation. Transpl. Infect. Dis. 24, e13776. doi:10.1111/tid.13776
Young, B., Seifert, S. N., Lawson, C., and Koehler, H. (2024). Exploring the genomic basis of Mpox virus-host transmission and pathogenesis. mSphere 9, e0057624. doi:10.1128/msphere.00576-24
Yu, J., Zhang, X., Liu, J., Xiang, L., Huang, S., Xie, X., et al. (2023). Phylogeny and molecular evolution of the first local monkeypox virus cluster in Guangdong Province, China. Nat. Commun. 14, 8241. doi:10.1038/s41467-023-44092-3
Yu, Z., Zou, X., Deng, Z., Zhao, M., Gu, C., Fu, L., et al. (2024). Genome analysis of the mpox (formerly monkeypox) virus and characterization of core/variable regions. Genomics 116, 110763. doi:10.1016/j.ygeno.2023.110763
Zaeck, L. M., Lamers, M. M., Verstrepen, B. E., Bestebroer, T. M., Van Royen, M. E., Götz, H., et al. (2023). Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat. Med. 29, 270–278. doi:10.1038/s41591-022-02090-w
Zhang, Y., Zhou, Y., Pei, R., Chen, X., and Wang, Y. (2023). Potential threat of human pathogenic orthopoxviruses to public health and control strategies. J. Biosaf. Biosecur 5, 1–7. doi:10.1016/j.jobb.2022.12.004
Keywords: Mpox, genome, pathogen characteristics, transmission, vaccines
Citation: La Frazia S, Garbuglia AR, Pauciullo S, Zulian V and Del Porto P (2025) Genomic variability and immunological aspects involved in response to MPXV infection. Front. Pharmacol. 16:1665830. doi: 10.3389/fphar.2025.1665830
Received: 14 July 2025; Accepted: 15 September 2025;
Published: 24 September 2025.
Edited by:
Alfonso J. Rodriguez-Morales, Fundacion Universitaria Autónoma de las Américas, ColombiaReviewed by:
Nourhan Naga, Alexandria University, EgyptAysel Karagoz, Independent Researcher, Ank, Türkiye
Copyright © 2025 La Frazia, Garbuglia, Pauciullo, Zulian and Del Porto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Rosa Garbuglia, YW5uYXJvc2EuZ2FyYnVnbGlhQGlubWkuaXQ=, YXJnYXJidWdsaWFAaW9sLml0
†These authors have contributed equally to this work
 Simone La Frazia
Simone La Frazia Anna Rosa Garbuglia
Anna Rosa Garbuglia Silvia Pauciullo
Silvia Pauciullo Verdiana Zulian
Verdiana Zulian Paola Del Porto
Paola Del Porto