- Department of Anaesthesiology, The Second Affiliated Hospital of Inner Mongolia Medical University, Hohhot, China
Postoperative delirium (POD) is a common acute neurocognitive disorder in the perioperative period, significantly increasing the risks of mortality and long-term functional decline. Dexmedetomidine, a highly selective α2-adrenergic receptor agonist, demonstrates potential for POD prevention through multimodal mechanisms, including sympatholytic effects, neuroinflammation attenuation, and physiological sleep preservation. Clinical evidence indicates its efficacy in reducing delirium incidence in cardiac surgery (risk ratio (RR), 0.57) and elderly non-cardiac surgical patients (RR, 0.51), though with notable population heterogeneity and risks of adverse effects such as bradycardia. Current guidelines recommend a dosing range of 0.1–0.7 μg/kg/h, yet monitoring requirements (e.g., electroencephalogram vs. hemodynamics) vary regionally. Future research should focus on precision dosing (e.g., biomarker-guided approaches), next-generation α2-agonists, and optimized multimodal strategies. The clinical application of dexmedetomidine requires careful risk-benefit assessment and integration into individualized perioperative protocols.
1 Introduction
1.1 Clinical significance of postoperative delirium
Postoperative delirium (POD) is an acute neurocognitive disorder marked by inattention, disorientation, and perceptual disturbances (Rudolph and Marcantonio, 2011). Its incidence ranges from 5% to 15% in general surgery to 50% in high-risk groups like elderly and cardiac surgery patients (Li et al., 2023). Major risk factors include preexisting cognitive impairment, polypharmacy, and comorbidities (Shi et al., 2019).
POD significantly worsens clinical outcomes, prolonging hospitalization by 3–5 days (Park and Kim, 2019) and increasing 30-day mortality (odds ratio (OR), 1.9) (Shi et al., 2019). It also predicts long-term functional decline, with persistent impairments in activities of daily living (Aldwikat et al., 2023). Furthermore, 30% of patients develop lasting cognitive dysfunction, accelerating dementia progression (Shi et al., 2019). These findings highlight POD as a critical perioperative challenge requiring targeted prevention strategies.
1.2 Limitations of current treatment strategies
Current approaches to POD management present significant limitations in both pharmacological and non-pharmacological domains. Pharmacological interventions remain controversial, with emerging evidence suggesting that commonly used sedatives may paradoxically increase delirium risk. A recent prospective cohort study in intensive care unit (ICU) patients reported that sedation with midazolam, compared with propofol, was associated with a higher risk of delirium, underscoring the potential role of midazolam in increasing POD incidence in vulnerable population (van Gelder et al., 2024). Similarly, systematic reviews suggest that while benzodiazepines provide effective sedation, their GABAergic mechanism may exacerbate neurocognitive dysfunction (Wang et al., 2023; Makaron et al., 2013). Notably, a meta-analysis by Wang et al. found that benzodiazepines were associated with a higher risk of POD specifically when compared directly to dexmedetomidine (Wang et al., 2023), underscoring a context-dependent risk profile. Other reviews also highlight potential neurocognitive risks (Makaron et al., 2013). Opioid analgesics, though essential for pain management, show dose-dependent associations with delirium development in vulnerable populations (Yoo et al., 2024).
Non-pharmacological strategies, particularly the ABCDEF bundle, face substantial implementation challenges despite strong theoretical foundations. Observational studies reveal adherence rates below 60% in critical care settings, primarily due to staffing limitations and workflow disruptions (DeMellow et al., 2020). A comprehensive meta-analysis confirmed these interventions’ variable effectiveness, noting particular shortcomings in emergency surgical contexts and resource-constrained environments (Sosnowski et al., 2023). This creates a clinical paradox where pharmacological options may inadvertently worsen delirium while behavioral interventions prove difficult to consistently implement.
1.3 Potential value of dexmedetomidine
Dexmedetomidine demonstrates unique pharmacological advantages for POD prevention through its multimodal mechanism of action. As a selective α2-adrenoreceptor agonist, it modulates sympathetic activity by inhibiting norepinephrine release from the locus coeruleus, thereby attenuating the stress response implicated in delirium pathogenesis (Zeng et al., 2019). Its anti-inflammatory properties are particularly relevant, with meta-analyses confirming significant reductions in pro-inflammatory cytokines including interleukin (IL)-6 and tumor necrosis factor-alpha TNF-α when used as an anesthetic adjunct (Li et al., 2015).
Clinically, dexmedetomidine shows superior neurocognitive outcomes compared to traditional sedatives. A randomized controlled trial by Skrobik et al. demonstrated its efficacy in preventing ICU delirium when administered at low nocturnal doses (0.1–0.2 μg/kg/h) (Skrobik et al., 2018). Preclinical studies further support its neuroprotective effects, with Sun et al. identifying specific protection against hippocampal neuron apoptosis through epigenetic regulation of the miR-129/YAP1/JAG1 axis (Sun et al., 2020).
The drug’s ability to preserve natural sleep architecture while avoiding anticholinergic and GABAergic side effects (common with benzodiazepines) represents a significant therapeutic advance (Zeng et al., 2019). These properties collectively position dexmedetomidine as a promising alternative for high-risk surgical populations, though optimal dosing strategies and long-term cognitive benefits require further investigation.
2 Mechanistic insights
2.1 Pathophysiological basis of delirium
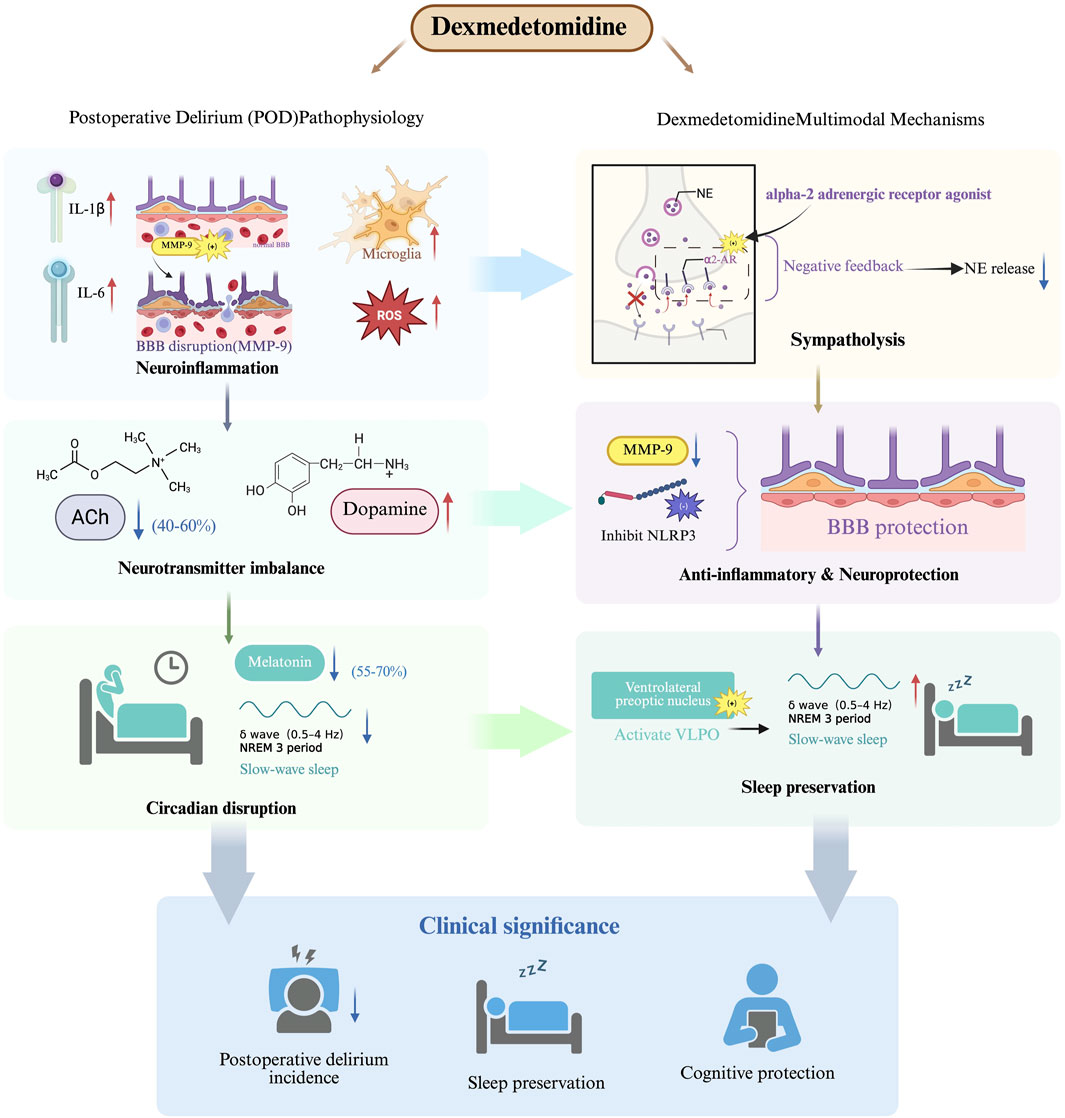
POD arises from three interrelated pathological processes that collectively disrupt neural network function (Figure 1). First, the neuroinflammatory cascade triggered by surgical trauma leads to peripheral cytokine release (IL-1β, IL-6) and subsequent blood-brain barrier dysfunction through matrix metalloproteinase-9 (MMP-9) mediated tight junction degradation (Liu et al., 2022) This inflammatory response activates microglia, generating reactive oxygen species that impair neuronal mitochondrial function (Subramaniyan and Terrando, 2019). Second, neurotransmitter system dysregulation manifests as a characteristic cholinergic deficiency, with cerebrospinal fluid acetylcholine levels decreasing by 40%–60% postoperatively (Hshieh et al., 2008). This occurs concomitantly with dopaminergic hyperactivity in mesolimbic pathways, creating an imbalance that disrupts cortical integration and attentional processing. Third, circadian rhythm disruption emerges through suprachiasmatic nucleus dysfunction, characterized by significant melatonin suppression (55%–70% reduction) and impaired slow-wave sleep generation (Campbell and Figueiro, 2024). These mechanisms form a self-reinforcing cycle of neural instability that clinically manifests as the characteristic symptoms of delirium.
2.2 Multimodal mechanisms of dexmedetomidine in delirium prevention
Dexmedetomidine exhibits a unique multimodal pharmacological profile that addresses several pathophysiological pathways implicated in POD (Figure 1). The drug’s high selectivity for α2-adrenergic receptors (α2:α1 binding ratio >1600:1) enables targeted modulation of sympathetic outflow from the locus coeruleus, with clinical studies demonstrating significant reductions in norepinephrine release during surgical procedures (Kang et al., 2020; Gertler et al., 2001). This sympatholytic effect is particularly relevant given the established association between sympathetic hyperactivity and delirium pathogenesis, while the preservation of partial adrenergic tone maintains cardiovascular stability better than complete sympathetic blockade.
The neuroprotective properties of dexmedetomidine extend beyond sympathetic modulation to include significant anti-inflammatory effects (Figure 1). Experimental evidence indicates the drug attenuates neuroinflammation through multiple mechanisms, including inhibition of NOD-like receptor family, pyrin domain-containing 3 inflammasome assembly (62.4% reduction) and downregulation of MMP-9 activity in hippocampal microvasculature (Hu et al., 2022). These actions help preserve blood-brain barrier integrity and reduce neuronal injury from inflammatory mediators that are strongly correlated with delirium severity.
Unlike conventional sedatives that disrupt normal sleep architecture, dexmedetomidine demonstrates the unique ability to enhance slow-wave sleep duration through selective modulation of ventrolateral preoptic nucleus neurons (Oto et al., 2012). This preservation of physiological sleep patterns is clinically significant given the independent association between slow-wave sleep deficiency and increased delirium risk (OR, 3.2). Comparative clinical trials have demonstrated dexmedetomidine’s superiority over benzodiazepines in reducing acute brain dysfunction, with the MENDS randomized controlled trial showing significant reductions in delirium prevalence and duration (Pandharipande et al., 2007). The drug’s favorable pharmacological profile-including minimal anticholinergic effects and preserved respiratory drive - positions it as an optimal choice for high-risk surgical populations where delirium prevention is paramount.
3 Clinical evidence appraisal: dexmedetomidine for POD prevention
3.1 Supportive clinical trial evidence in POD prevention
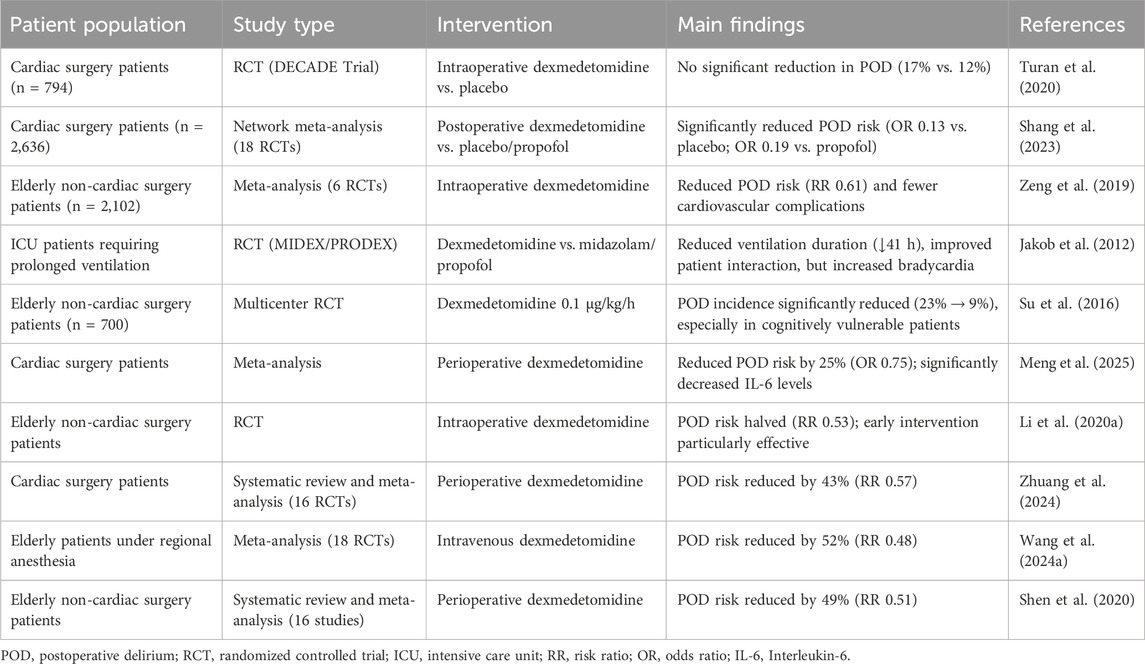
Current evidence demonstrates dexmedetomidine’s variable efficacy in POD prevention across different surgical populations (Table 1). In cardiac surgery, findings remain inconclusive - while the large DECADE trial (n = 794) showed no significant delirium reduction with dexmedetomidine (17% vs. 12% placebo) (Turan et al., 2020), a subsequent network meta-analysis of 18 trials (n = 2,636) revealed postoperative administration significantly lowered delirium risk versus placebo (OR, 0.13; 95% confidence interval (CI), 0.03–0.35) and propofol (OR, 0.19; 95% CI, 0.04–0.66) (Shang et al., 2023). This discrepancy highlights the importance of timing in dexmedetomidine administration for cardiac surgical patients.
For elderly patients undergoing non-cardiac surgery, stronger evidence supports dexmedetomidine’s benefits. A meta-analysis of 6 randomized controlled trials (RCTs) (n = 2,102) demonstrated significant delirium reduction (risk ratio (RR), 0.61; 95% CI, 0.34–0.76) alongside decreased cardiovascular complications (Zeng et al., 2019). The drug’s favorable safety profile in this vulnerable population makes it particularly valuable for non-cardiac procedures where delirium risk remains high but cardiac complications are less prevalent.
In critical care settings, dexmedetomidine shows distinct advantages over traditional sedatives. The MIDEX-PRODEX trials established its superiority in prolonged mechanical ventilation, reducing median ventilation duration by 41 h versus midazolam (123 vs. 164 h, P = 0.03) while improving patient interaction scores (Jakob et al., 2012). These benefits likely stem from dexmedetomidine’s unique ability to maintain physiological sleep patterns and reduce electroencephalographic disruption compared to GABAergic alternatives, though its higher rates of bradycardia and hypotension require careful patient selection and monitoring.
3.2 Controversial findings in dexmedetomidine research
The efficacy of dexmedetomidine for POD prevention remains contested despite promising mechanistic data (Table 1). The multicenter trial by Deiner et al. represents the largest randomized controlled study to date, demonstrating equivalent delirium rates between dexmedetomidine (12.2%) and placebo (11.4%) groups in elderly non-cardiac surgery patients (P = 0.94) (Deiner et al., 2017). This null finding contrasts sharply with positive results from ICU-based studies, suggesting the drug’s effects may be context-dependent, particularly regarding administration timing and surgical population characteristics.
Pharmacokinetic studies provide critical insights into these disparate outcomes. Weerink et al. identified substantial inter-individual variability in drug metabolism, with subtherapeutic plasma concentrations (<0.2 ng/mL) occurring in nearly 40% of patients at standard dosing (Weerink et al., 2017). Furthermore, Ebert et al. characterized dexmedetomidine’s narrow therapeutic window (0.2–0.6 ng/mL) (Ebert et al., 2000), beyond which dose-dependent hypotension and bradycardia may outweigh potential neurological benefits. These pharmacological properties may explain the trial’s neutral results while suggesting opportunities for optimized dosing strategies.
Clinical implementation challenges further complicate dexmedetomidine’s role in delirium prevention. As noted by Vacas and Van de Wiele (2017), the drug’s hemodynamic effects require careful patient selection and monitoring, particularly in elderly populations with cardiovascular comorbidities. These considerations underscore the need for precision medicine approaches that account for individual pharmacokinetic variability, comorbid medication use, and surgical context when considering dexmedetomidine for neuroprotection.
3.3 Population-specific treatment effects
The efficacy of dexmedetomidine for POD prevention exhibits significant variation across patient populations, necessitating careful consideration of clinical context (Table 1). In elderly patients undergoing non-cardiac surgery, the landmark RCT by Su et al. demonstrated particularly robust benefits, with dexmedetomidine infusion (0.1 μg/kg/h) reducing delirium incidence from 23% to 9% (OR, 0.35; 95% CI, 0.22–0.54; P < 0.0001) (Su et al., 2016). This protective effect was most pronounced in patients with preoperative cognitive vulnerability, suggesting particular utility in high-risk geriatric populations.
Cardiac surgery patients represent another responsive subgroup, as evidenced by multiple meta-analyses. Meng et al. reported a 25% relative risk reduction (OR, 0.75; 95% CI, 0.57–0.98) (Meng et al., 2025), while Li et al. found even greater benefits (OR, 0.56; 95% CI, 0.36–0.89) (Li et al., 2021), particularly with postoperative administration. These findings likely reflect dexmedetomidine’s ability to mitigate cardiopulmonary bypass-induced neuroinflammation and sympathetic activation, mechanisms particularly relevant in cardiac surgical settings.
However, clinical benefits appear context-dependent, with notable limitations in certain populations. The multicenter trial by Deiner et al. found no significant delirium reduction with intraoperative dexmedetomidine in major non-cardiac surgery (12.2% vs. 11.4%, P = 0.94) (Deiner et al., 2017), highlighting the importance of administration timing. Furthermore, subgroup analyses suggest limited efficacy in emergency surgeries and patients with advanced dementia, emphasizing the need for careful patient selection and protocol optimization in clinical practice.
3.4 Evidence synthesis and clinical implications
Current evidence demonstrates dexmedetomidine’s efficacy in POD prevention when administered intraoperatively at 0.1–0.5 μg/kg/h. A meta-analysis of cardiac surgery patients showed a 25% reduction in delirium incidence (OR, 0.75) with dexmedetomidine use (Meng et al., 2025). The drug’s anti-inflammatory effects are particularly notable, significantly reducing IL-6 levels by 25.14 pg/mL immediately post-operation (Li et al., 2015). In elderly non-cardiac surgical patients, intraoperative dexmedetomidine administration halved delirium risk (RR, 0.53), highlighting the importance of early intervention (Li CJ. et al., 2020). These findings support dexmedetomidine’s role in multimodal prevention strategies for high-risk surgical populations.
4 Practical recommendations for clinical application
4.1 Target patient populations
Current evidence identifies three high-risk surgical populations that derive significant benefit from dexmedetomidine administration for POD prevention (Table 1). First, cardiac surgery patients undergoing cardiopulmonary bypass demonstrate a 43% relative risk reduction (RR, 0.57; 95% CI, 0.41–0.79) when receiving perioperative dexmedetomidine, as shown in a systematic review of 16 randomized trials (Zhuang et al., 2024). Second, elderly patients (≥60 years) receiving regional anesthesia exhibit a 52% lower delirium incidence (RR, 0.48; 95% CI, 0.37–0.63) with intraoperative dexmedetomidine infusion, according to a meta-analysis of 18 RCTs (Wang et al., 2024a). Third, elderly non-cardiac surgical patients show comparable benefits, with a 49% risk reduction (RR, 0.51; 95% CI, 0.43–0.61) across 16 included trials (Shen et al., 2020).
Clinical application requires careful patient selection due to potential hemodynamic effects. Dexmedetomidine is contraindicated in patients with hemodynamically significant bradycardia, high-grade atrioventricular block, or decompensated shock requiring vasopressor support (Reel and Maani, 2025). Particular caution is warranted in patients with severe hepatic impairment (Child-Pugh C), where reduced dosing may be necessary due to altered pharmacokinetics. These evidence-based recommendations support targeted use of dexmedetomidine while emphasizing the importance of individualized risk-benefit assessment.
4.2 Dosing regimen optimization
Current evidence supports weight-based dexmedetomidine dosing for delirium prevention, with optimal regimens varying by surgical context and patient characteristics. For intraoperative initiation in non-cardiac surgery, a maintenance infusion of 0.1–0.5 μg/kg/h without loading dose provides effective delirium prevention while minimizing hemodynamic instability, as demonstrated in a meta-analysis of 18 randomized trials (Wang et al., 2024b). In cardiac surgery populations, lower infusion rates (0.1–0.5 μg/kg/h) show superior cognitive outcomes compared to higher doses (0.5–0.9 μg/kg/h), with significantly reduced incidence of hypotension (7.3% vs. 31.6%, P = 0.044) (Fang et al., 2023). These findings suggest a therapeutic window exists where adequate α2-adrenoreceptor activation is achieved without excessive sympathetic inhibition.
Postoperative ICU management requires careful dose titration, particularly in elderly patients. A multicenter trial of cardiac surgery patients found overnight dexmedetomidine infusion (8p.m.-8a.m.) failed to reduce delirium incidence (12.6% vs. 12.4%, P = 0.97) while significantly increasing hypotensive events (7.3% vs. 0.6%, P < 0.01) (Huet et al., 2024). Pharmacodynamic studies in elderly patients under spinal anesthesia identified an ED95 of 0.86 μg/kg for adequate sedation, but noted doses exceeding 0.5 μg/kg substantially increase hypotension risk (31.6% vs. 3.6%, P = 0.013) (Ko et al., 2015). Similarly, research in hip replacement surgery demonstrated loading doses ≥0.5 μg/kg cause significant mean arterial pressure reductions at multiple perioperative time points (P < 0.05) (Liu et al., 2023).
Risk stratification is essential for safe administration. A retrospective analysis of 283 ICU patients identified three independent predictors of dexmedetomidine-associated hypotension: baseline mean arterial pressure (OR, 0.97; 95% CI, 0.95–0.99), Acute Physiology and Chronic Health Evaluation II scores (OR, 1.06; 95% CI, 1.01–1.12), and coronary artery disease (OR, 0.48; 95% CI, 0.26–0.90) (Gerlach et al., 2016). These findings support initiating therapy at 0.1–0.3 μg/kg/h in high-risk populations, with gradual titration guided by continuous hemodynamic monitoring. For elderly patients (≥65 years) and those with cardiovascular comorbidities, additional precautions including extended loading infusion duration (30 min) and 25% dose reduction should be considered to balance efficacy and safety.
4.3 Multimodal integration strategies
Effective delirium management necessitates a synergistic approach combining pharmacological and non-pharmacological interventions for optimal delirium prevention. The modified Hospital Elder Life Program demonstrates particular efficacy, reducing delirium incidence by 56% (RR, 0.44; 95% CI, 0.23–0.83) in elderly surgical patients through structured cognitive stimulation, early mobilization, and nutritional support (Chen et al., 2017). Pharmacologically, dexmedetomidine emerges as the preferred sedative, particularly when combined with low-dose ketamine (0.15–0.3 mg/kg/h), which reduces delirium incidence by 43% compared to standard regimens (Perbet et al., 2018). Importantly, benzodiazepines should be avoided in high-risk patients due to their association with prolonged delirium duration (RR, 1.64; 95% CI, 1.27–2.10) (Pisani et al., 2009).
Implementation requires standardized protocols incorporating: (1) preoperative risk assessment, (2) intraoperative dexmedetomidine for high-risk cases, (3) postoperative avoidance of deliriogenic medications, and (4) immediate cognitive/physical rehabilitation. As demonstrated in a stepped-wedge trial, such multimodal programs significantly reduce both delirium incidence (OR, 0.87) and delirium days (5.3% vs. 6.9%) (Deeken et al., 2022). This comprehensive approach addresses multiple pathogenic pathways while minimizing adverse effects, representing the current standard for perioperative delirium prevention.
5 Challenges and future perspectives in dexmedetomidine application for POD
5.1 Critical unresolved clinical questions
Several critical knowledge gaps persist regarding the optimal use of dexmedetomidine for delirium prevention. First, the temporal relationship between administration timing and clinical outcomes remains incompletely characterized, with insufficient comparative data to determine whether preoperative, intraoperative, or postoperative initiation yields superior results. Second, the durability of neuroprotective effects beyond 3 years post-intervention requires clarification, as current evidence demonstrates conflicting results regarding long-term cognitive preservation (Hong et al., 2025; Zhang et al., 2019). Third, the medication’s impact on patients with pre-existing cognitive impairment warrants further investigation, particularly given the established association between delirium and accelerated cognitive decline in vulnerable populations (Gross et al., 2012). Fourth, precise dose-response relationships for both short-term delirium prevention and long-term neurocognitive outcomes have not been adequately defined across different surgical populations and age groups. Finally, population-specific efficacy thresholds need elucidation, including optimal dosing strategies for cardiac versus non-cardiac surgery patients and variations based on baseline risk profiles. These unresolved issues highlight the need for standardized, large-scale longitudinal studies with comprehensive cognitive assessments to establish evidence-based protocols for clinical practice.
5.2 Promising research directions
Several critical research priorities are emerging to advance the clinical application of dexmedetomidine for neuroprotection. First, rigorous validation of predictive biomarkers represents an urgent need, particularly for establishing reliable thresholds of neurofilament light chain and other neuronal injury markers that can guide patient stratification (Hou et al., 2023). Second, the clinical translation of novel delivery systems requires systematic evaluation, including pharmacokinetic studies of the multivalent bioadhesive nanoparticle clusters demonstrated by Jia et al. (2025) and safety assessments of alternative administration routes characterized by Jeong et al. (2023). Third, the development of dynamic dosing algorithms must incorporate multiple data streams including real-time physiological monitoring, individual pharmacokinetic profiles from therapeutic drug monitoring, and comprehensive surgical risk factor analysis. Fourth, pharmacological research should focus on next-generation α2-agonists with improved receptor subtype selectivity to enhance therapeutic effects while minimizing adverse hemodynamic consequences. Finally, the integration of artificial intelligence platforms offers transformative potential for adaptive treatment optimization, though this requires validation through prospective clinical trials comparing algorithm-guided dosing versus standard protocols. These priorities collectively address the key gaps in moving from current empirical approaches to precision medicine paradigms for postoperative neuroprotection, while maintaining rigorous standards of evidence generation and clinical safety evaluation.
5.3 Contemporary guideline landscape
Current guidelines demonstrate regional variations in dexmedetomidine recommendations for delirium prevention. The American Geriatrics Society (AGS) provides conditional support (Grade 2B), favoring ICU use as a GABAergic alternative while prioritizing non-pharmacologic interventions (American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults, 2015; Hebert, 2018). In contrast, Chinese consensus advocates broader perioperative application (Grade B), particularly for elderly surgical patients, reflecting population-specific risk-benefit assessments (Li M. et al., 2020). The recently updated Society of Critical Care Medicine guidelines further delineate its role, reinforcing the integration of dexmedetomidine into multimodal sedation-analgesia protocols to mitigate delirium risk in critically ill adults (Lewis et al., 2025).
Both guidelines agree on dose limits (<0.7 μg/kg/h) but differ in monitoring: AGS emphasizes electroencephalographic for neurocognitive safety, while Asian protocols focus on hemodynamic stability (American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults, 2015; Li M. et al., 2020). These differences highlight how clinical context influences guideline development, with AGS aligning with geriatric deprescribing principles and Asian consensus addressing higher baseline delirium risks. Multidisciplinary care and preoperative risk stratification remain universal recommendations.
6 Summary
Dexmedetomidine demonstrates clinically significant benefits for POD prevention in high-risk surgical populations, particularly elderly cardiac surgery patients. Its multimodal mechanism—combining sympatholytic, anti-inflammatory, and sleep-preserving properties—offers advantages over traditional sedatives. Current evidence supports weight-based dosing (0.1–0.3 μg/kg/h) with hemodynamic monitoring, while contraindications include conduction abnormalities and hemodynamic instability. However, this review acknowledges limitations in analytical depth regarding mechanistic pathways and clinical evidence integration, which may affect the comprehensiveness of the conclusions. Future iterations should strengthen the translational perspective by incorporating more robust biomarker-guided mechanistic analyses and broader clinical validation across diverse surgical settings. Key knowledge gaps persist regarding long-term cognitive outcomes, optimal timing of administration, and population-specific efficacy. Future research should focus on next-generation α2-agonists, biomarker-guided precision dosing, and standardized trials with neuropsychological assessments. Additionally, more comprehensive synthesis of real-world evidence and comparative effectiveness data will be essential to establish standardized, evidence-based clinical guidelines. Clinicians should employ dexmedetomidine judiciously within evidence-based protocols while contributing to outcomes research to refine its role in perioperative neuroprotection.
Author contributions
X-yL: Conceptualization, Data curation, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review and editing. J-yY: Conceptualization, Data curation, Resources, Validation, Visualization, Writing – original draft, Writing – review and editing. YQ: Conceptualization, Resources, Validation, Visualization, Writing – original draft, Writing – review and editing. C-xW: Data curation, Resources, Validation, Visualization, Writing – original draft, Writing – review and editing. X-dW: Conceptualization, Data curation, Investigation, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
POD, postoperative delirium; OR, odds ratio; GABAergic, γ-Aminobutyric Acid-ergic; AGS, American Geriatrics Society; ICU, intensive care unit; IL, interleukin; MMP-9, matrix metalloproteinase-9; CI, confidence interval; RCTs, randomized controlled trials; RR, risk ratio.
References
Aldwikat, R. K., Manias, E., Holmes, A. C., Tomlinson, E., and Nicholson, P. (2023). Associations of postoperative delirium with activities of daily living in older people after major surgery: a prospective cohort study. J. Clin. Nurs. 32 (19-20), 7578–7588. doi:10.1111/jocn.16801
American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults (2015). American geriatrics society abstracted clinical practice guideline for postoperative delirium in older adults. J. Am. Geriatr. Soc. 63 (1), 142–150. doi:10.1111/jgs.13281
Campbell, E., and Figueiro, M. G. (2024). Postoperative cognitive dysfunction: spotlight on light, circadian rhythms, and sleep. Front. Neurosci. 18, 1390216. doi:10.3389/fnins.2024.1390216
Chen, C. C., Li, H. C., Liang, J. T., Lai, I. R., Purnomo, J. D. T., Yang, Y. T., et al. (2017). Effect of a modified hospital Elder Life Program on delirium and length of hospital stay in patients undergoing abdominal surgery: a cluster randomized clinical trial. JAMA Surg. 152 (9), 827–834. doi:10.1001/jamasurg.2017.1083
Deeken, F., Sánchez, A., Rapp, M. A., Denkinger, M., Brefka, S., Spank, J., et al. (2022). Outcomes of a delirium prevention Program in older persons after elective surgery: a stepped-wedge cluster randomized clinical trial. JAMA Surg. 157 (2), e216370. doi:10.1001/jamasurg.2021.6370
Deiner, S., Luo, X., Lin, H. M., Sessler, D. I., Saager, L., Sieber, F. E., et al. (2017). Intraoperative infusion of dexmedetomidine for prevention of postoperative delirium and cognitive dysfunction in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial. JAMA Surg. 152 (8), e171505. doi:10.1001/jamasurg.2017.1505
DeMellow, J. M., Kim, T. Y., Romano, P. S., Drake, C., and Balas, M. C. (2020). Factors associated with ABCDE bundle adherence in critically ill adults requiring mechanical ventilation: an observational design. Intensive Crit. Care Nurs. 60, 102873. doi:10.1016/j.iccn.2020.102873
Ebert, T. J., Hall, J. E., Barney, J. A., Uhrich, T. D., and Colinco, M. D. (2000). The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology 93 (2), 382–394. doi:10.1097/00000542-200008000-00016
Fang, J., Yang, J., Zhai, M., Zhang, Q., Zhang, M., and Xie, Y. (2023). Effects of dexmedetomidine dosage on the short-term cognitive function of elderly patients undergoing cardiac surgery. BMC Anesthesiol. 23 (1), 380. doi:10.1186/s12871-023-02315-6
Gerlach, A. T., Blais, D. M., Jones, G. M., Burcham, P. K., Stawicki, S. P., Cook, C. H., et al. (2016). Predictors of dexmedetomidine-associated hypotension in critically ill patients. Int. J. Crit. Illn. Inj. Sci. 6 (3), 109–114. doi:10.4103/2229-5151.190656
Gertler, R., Brown, H. C., Mitchell, D. H., and Silvius, E. N. (2001). Dexmedetomidine: a novel sedative-analgesic agent. Proc. Bayl Univ. Med. Cent. 14 (1), 13–21. doi:10.1080/08998280.2001.11927725
Gross, A. L., Jones, R. N., Habtemariam, D. A., Fong, T. G., Tommet, D., Quach, L., et al. (2012). Delirium and long-term cognitive trajectory among persons with dementia. Arch. Intern Med. 172 (17), 1324–1331. doi:10.1001/archinternmed.2012.3203
Hebert, C. (2018). Evidence-Based practice in Perianesthesia nursing: application of the American Geriatrics Society Clinical Practice Guideline for postoperative delirium in older adults. J. Perianesth Nurs. 33 (3), 253–264. doi:10.1016/j.jopan.2016.02.011
Hong, H., Li, X., Yang, J., Zhang, Y., Liu, G. Y., Yan, F. X., et al. (2025). Impact of perioperative dexmedetomidine on long-term outcomes in older patients following cardiac surgery: follow-up of a randomized trial. BMC Anesthesiol. 25 (1), 130. doi:10.1186/s12871-025-02963-w
Hou, Y. R., Xu, C. Y., An, M. Z., Li, Z. P., Ni, H. D., Chen, T., et al. (2023). Effect of dexmedetomidine on postoperative plasma neurofilament light chain in elderly patients undergoing thoracoscopic surgery: a prospective, randomized controlled trial. Clin. Interv. Aging 18, 1565–1576. doi:10.2147/CIA.S422560
Hshieh, T. T., Fong, T. G., Marcantonio, E. R., and Inouye, S. K. (2008). Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J. Gerontol. A Biol. Sci. Med. Sci. 63 (7), 764–772. doi:10.1093/gerona/63.7.764
Hu, Y., Zhou, H., Zhang, H., Sui, Y., Zhang, Z., Zou, Y., et al. (2022). The neuroprotective effect of dexmedetomidine and its mechanism. Front. Pharmacol. 13, 965661. doi:10.3389/fphar.2022.965661
Huet, O., Gargadennec, T., Oilleau, J. F., Rozec, B., Nesseler, N., Bouglé, A., et al. (2024). Prevention of post-operative delirium using an overnight infusion of dexmedetomidine in patients undergoing cardiac surgery: a pragmatic, randomized, double-blind, placebo-controlled trial. Crit. Care 28 (1), 64. doi:10.1186/s13054-024-04842-1
Jakob, S. M., Ruokonen, E., Grounds, R. M., Sarapohja, T., Garratt, C., Pocock, S. J., et al. (2012). Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA 307 (11), 1151–1160. doi:10.1001/jama.2012.304
Jeong, S. H., Jang, J. H., and Lee, Y. B. (2023). Drug delivery to the brain via the nasal route of administration: exploration of key targets and major consideration factors. J. Pharm. Investig. 53 (1), 119–152. doi:10.1007/s40005-022-00589-5
Jia, Y., Kong, X., Li, R., Wang, H., Li, C., Cheng, S., et al. (2025). Enhanced nasal-to-brain drug delivery by multivalent bioadhesive nanoparticle clusters for cerebral ischemic reperfusion injury protection. Acta Biomater. 194, 411–427. doi:10.1016/j.actbio.2025.01.036
Kang, R., Jeong, J. S., Ko, J. S., Lee, S. Y., Lee, J. H., Choi, S. J., et al. (2020). Intraoperative dexmedetomidine attenuates norepinephrine levels in patients undergoing transsphenoidal surgery: a randomized, placebo-controlled trial. BMC Anesthesiol. 20 (1), 100. doi:10.1186/s12871-020-01025-7
Ko, K. H., Jun, I. J., Lee, S., Lim, Y., Yoo, B., and Kim, K. M. (2015). Effective dose of dexmedetomidine to induce adequate sedation in elderly patients under spinal anesthesia. Korean J. Anesthesiol. 68 (6), 575–580. doi:10.4097/kjae.2015.68.6.575
Lewis, K., Balas, M. C., Stollings, J. L., McNett, M., Girard, T. D., Chanques, G., et al. (2025). A focused update to the Clinical Practice Guidelines for the prevention and management of pain, anxiety, Agitation/Sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit. Care Med. 53 (3), e711–e727. doi:10.1097/CCM.0000000000006574
Li, B., Li, Y., Tian, S., Wang, H., Wu, H., Zhang, A., et al. (2015). Anti-inflammatory effects of perioperative dexmedetomidine administered as an adjunct to general Anesthesia: a meta-analysis. Sci. Rep. 5, 12342. doi:10.1038/srep12342
Li, C. J., Wang, B. J., Mu, D. L., Hu, J., Guo, C., Li, X. Y., et al. (2020a). Randomized clinical trial of intraoperative dexmedetomidine to prevent delirium in the elderly undergoing major non-cardiac surgery. Br. J. Surg. 107 (2), e123–e132. doi:10.1002/bjs.11354
Li, M., Wang, T. L., and Wang, D. X. (2020b). An overview of Chinese multidisciplinary expert consensus on perioperative brain health in elderly patients. Chin. Med. J. Engl. 134 (1), 5–7. doi:10.1097/CM9.0000000000001213
Li, P., Li, L. X., Zhao, Z. Z., Xie, J., Zhu, C. L., Deng, X. M., et al. (2021). Dexmedetomidine reduces the incidence of postoperative delirium after cardiac surgery: a meta-analysis of randomized controlled trials. BMC Anesthesiol. 21 (1), 153. doi:10.1186/s12871-021-01370-1
Li, S., Li, R., Li, M., Cui, Q., Zhang, X., Ma, T., et al. (2023). Dexmedetomidine administration during brain tumour resection for prevention of postoperative delirium: a randomised trial. Br. J. Anaesth. 130, e307–e316. doi:10.1016/j.bja.2022.10.041
Liu, L. F., Hu, Y., Liu, Y. N., Shi, D. W., Liu, C., Da, X., et al. (2022). Reactive oxygen species contribute to delirium-like behavior by activating CypA/MMP9 signaling and inducing blood-brain barrier impairment in aged mice following anesthesia and surgery. Front. Aging Neurosci. 14, 1021129. doi:10.3389/fnagi.2022.1021129
Liu, H., Gao, M., Zheng, Y., Sun, C., Lu, Q., and Shao, D. (2023). Effects of dexmedetomidine at different dosages on perioperative haemodynamics and postoperative recovery quality in elderly patients undergoing hip replacement surgery under general anaesthesia: a randomized controlled trial. Trials 24 (1), 386. doi:10.1186/s13063-023-07384-z
Makaron, L., Moran, C. A., Namjoshi, O., Rallapalli, S., Cook, J. M., and Rowlett, J. K. (2013). Cognition-impairing effects of benzodiazepine-type drugs: role of GABAA receptor subtypes in an executive function task in rhesus monkeys. Pharmacol. Biochem. Behav. 104, 62–68. doi:10.1016/j.pbb.2012.12.018
Meng, C., Wang, D., Zhao, Y., Sun, J., Miao, G., Chen, L., et al. (2025). Dexmedetomidine for delirium prevention in adult patients following cardiac surgery: a meta-analysis of randomized controlled trials. J. Cardiothorac. Surg. 20 (1), 110. doi:10.1186/s13019-025-03360-7
Oto, J., Yamamoto, K., Koike, S., Onodera, M., Imanaka, H., and Nishimura, M. (2012). Sleep quality of mechanically ventilated patients sedated with dexmedetomidine. Intensive Care Med. 38 (12), 1982–1989. doi:10.1007/s00134-012-2685-y
Pandharipande, P. P., Pun, B. T., Herr, D. L., Maze, M., Girard, T. D., Miller, R. R., et al. (2007). Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 298 (22), 2644–2653. doi:10.1001/jama.298.22.2644
Park, E. A., and Kim, M. Y. (2019). Postoperative delirium is associated with negative outcomes and long-term mortality in elderly Koreans: a retrospective observational Study. Med. Kaunas. 55 (10), 618. doi:10.3390/medicina55100618
Perbet, S., Verdonk, F., Godet, T., Jabaudon, M., Chartier, C., Cayot, S., et al. (2018). Low doses of ketamine reduce delirium but not opiate consumption in mechanically ventilated and sedated ICU patients: a randomised double-blind control trial. Anaesth. Crit. Care Pain Med. 37 (6), 589–595. doi:10.1016/j.accpm.2018.09.006
Pisani, M. A., Murphy, T. E., Araujo, K. L., Slattum, P., Van Ness, P. H., and Inouye, S. K. (2009). Benzodiazepine and opioid use and the duration of intensive care unit delirium in an older population. Crit. Care Med. 37 (1), 177–183. doi:10.1097/CCM.0b013e318192fcf9
Reel, B., and Maani, C. V. (2025). “Dexmedetomidine. 2023 May 1,” in StatPearls. Treasure Island (FL) (Treasure Island: StatPearls Publishing). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK513303/(accessed on April 1, 2025).
Rudolph, J. L., and Marcantonio, E. R. (2011). Review articles: postoperative delirium: acute change with long-term implications. Anesth. Analg. 112 (5), 1202–1211. doi:10.1213/ANE.0b013e3182147f6d
Shang, L., Hou, M., and Guo, F. (2023). Postoperative application of dexmedetomidine is the optimal strategy to reduce the incidence of postoperative delirium after cardiac surgery: a network meta-analysis of randomized controlled trials. Ann. Pharmacother. 57 (3), 221–231. doi:10.1177/10600280221106622
Shen, Q. H., Li, H. F., Zhou, X. Y., and Yuan, X. Z. (2020). Dexmedetomidine in the prevention of postoperative delirium in elderly patients following non-cardiac surgery: a systematic review and meta-analysis. Clin. Exp. Pharmacol. Physiol. 47 (8), 1333–1341. doi:10.1111/1440-1681.13312
Shi, Z., Mei, X., Li, C., Chen, Y., Zheng, H., Wu, Y., et al. (2019). Postoperative delirium is associated with long-term decline in activities of daily living. Anesthesiology 131 (3), 492–500. doi:10.1097/ALN.0000000000002849
Skrobik, Y., Duprey, M. S., Hill, N. S., and Devlin, J. W. (2018). Low-Dose nocturnal dexmedetomidine prevents ICU delirium. A randomized, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 197 (9), 1147–1156. doi:10.1164/rccm.201710-1995OC
Sosnowski, K., Lin, F., Chaboyer, W., Ranse, K., Heffernan, A., and Mitchell, M. (2023). The effect of the ABCDE/ABCDEF bundle on delirium, functional outcomes, and quality of life in critically ill patients: a systematic review and meta-analysis. Int. J. Nurs. Stud. 138, 104410. doi:10.1016/j.ijnurstu.2022.104410
Su, X., Meng, Z. T., Wu, X. H., Cui, F., Li, H. L., Wang, D. X., et al. (2016). Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet 388 (10054), 1893–1902. doi:10.1016/S0140-6736(16)30580-3
Subramaniyan, S., and Terrando, N. (2019). Neuroinflammation and perioperative neurocognitive disorders. Anesth. Analg. 128 (4), 781–788. doi:10.1213/ANE.0000000000004053
Sun, W., Zhao, J., and Li, C. (2020). Dexmedetomidine provides protection against Hippocampal Neuron apoptosis and cognitive impairment in mice with Alzheimer's Disease by mediating the miR-129/YAP1/JAG1 axis. Mol. Neurobiol. 57 (12), 5044–5055. doi:10.1007/s12035-020-02069-z
Turan, A., Duncan, A., Leung, S., Karimi, N., Fang, J., Mao, G., et al. (2020). Dexmedetomidine for reduction of atrial fibrillation and delirium after cardiac surgery (DECADE): a randomised placebo-controlled trial. Lancet 396 (10245), 177–185. doi:10.1016/S0140-6736(20)30631-0
Vacas, S., and Van de Wiele, B. (2017). Designing a pain management protocol for craniotomy: a narrative review and consideration of promising practices. Surg. Neurol. Int. 8, 291. doi:10.4103/sni.sni_301_17
van Gelder, T. G., van Diem-Zaal, I. J., Dijkstra-Kersten, S. M. A., de Mul, N., Lalmohamed, A., and Slooter, A. J. C. (2024). The risk of delirium after sedation with propofol or midazolam in intensive care unit patients. Br. J. Clin. Pharmacol. 90 (6), 1471–1479. doi:10.1111/bcp.16031
Wang, D., Liu, Z., Zhang, W., Zu, G., Tao, H., and Bi, C. (2024a). Intravenous infusion of dexmedetomidine during the surgery to prevent postoperative delirium and postoperative cognitive dysfunction undergoing non-cardiac surgery: a meta-analysis of randomized controlled trials. Eur. J. Med. Res. 29 (1), 239. doi:10.1186/s40001-024-01838-z
Wang, E., Belley-Côté, E. P., Young, J., He, H., Saud, H., D'Aragon, F., et al. (2023). Effect of perioperative benzodiazepine use on intraoperative awareness and postoperative delirium: a systematic review and meta-analysis of randomised controlled trials and observational studies. Br. J. Anaesth. 131 (2), 302–313. doi:10.1016/j.bja.2022.12.001
Wang, D., He, X., Li, Z., Tao, H., and Bi, C. (2024b). The role of dexmedetomidine administered via intravenous infusion as adjunctive therapy to mitigate postoperative delirium and postoperative cognitive dysfunction in elderly patients undergoing regional anesthesia: a meta-analysis of randomized controlled trials. BMC Anesthesiol. 24 (1), 73. doi:10.1186/s12871-024-02453-5
Weerink, M. A. S., Struys, MMRF, Hannivoort, L. N., Barends, C. R. M., Absalom, A. R., and Colin, P. (2017). Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin. Pharmacokinet. 56 (8), 893–913. doi:10.1007/s40262-017-0507-7
Yoo, S. H., Kang, J., Kim, H. J., Lee, S. W., Hong, M., Jung, E. H., et al. (2024). Opioid use and subsequent delirium risk in patients with advanced cancer in palliative care: a multicenter registry study. Sci. Rep. 14 (1), 6004. doi:10.1038/s41598-024-56675-1
Zeng, H., Li, Z., He, J., and Fu, W. (2019). Dexmedetomidine for the prevention of postoperative delirium in elderly patients undergoing noncardiac surgery: a meta-analysis of randomized controlled trials. PLoS One 14 (8), e0218088. doi:10.1371/journal.pone.0218088
Zhang, D. F., Su, X., Meng, Z. T., Li, H. L., Wang, D. X., Li, X. Y., et al. (2019). Impact of dexmedetomidine on long-term outcomes after noncardiac surgery in elderly: 3-year Follow-up of a randomized controlled trial. Ann. Surg. 270 (2), 356–363. doi:10.1097/SLA.0000000000002801
Zhuang, X., Fu, L., Luo, L., Dong, Z., Jiang, Y., Zhao, J., et al. (2024). The effect of perioperative dexmedetomidine on postoperative delirium in adult patients undergoing cardiac surgery with cardiopulmonary bypass: a systematic review and meta-analysis of randomized controlled trials. BMC Anesthesiol. 24 (1), 332. doi:10.1186/s12871-024-02715-2
Keywords: postoperative delirium, dexmedetomidine, clinical application, management, perspective
Citation: Li X-y, Yang J-y, Qiu Y, Wang C-x and Wang X-d (2025) Dexmedetomidine for postoperative delirium in surgical patients: a mini-review of mechanisms, clinical evidence, and practical implementation. Front. Pharmacol. 16:1702862. doi: 10.3389/fphar.2025.1702862
Received: 10 September 2025; Accepted: 02 October 2025;
Published: 10 October 2025.
Edited by:
Tomas Drabek, University of Pittsburgh, United StatesReviewed by:
Hynek Riha, Institute for Clinical and Experimental Medicine (IKEM), CzechiaCopyright © 2025 Li, Yang, Qiu, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-dong Wang, eGlhby1kb25nd2FuZ0Bob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Xiao-yan Li†
Xiao-yan Li† Yi Qiu
Yi Qiu Xiao-dong Wang
Xiao-dong Wang