- 1Laboratory of Behavioral Neuroscience, Department of Psychology, School of Social Sciences, University of Crete, Rethymno, Greece
- 2Laboratory of Behavioural Neuroscience, School of Nursing and Human Sciences, Faculty of Science and Health, Dublin City University, Dublin, Ireland
Over the last decades, the endocannabinoid system has been implicated in a large variety of functions, including a crucial modulation of brain-reward circuits and the regulation of motivational processes. Importantly, behavioral studies have shown that cannabinoid compounds activate brain reward mechanisms and circuits in a similar manner to other drugs of abuse, such as nicotine, alcohol, cocaine, and heroin, although the conditions under which cannabinoids exert their rewarding effects may be more limited. Furthermore, there is evidence on the involvement of the endocannabinoid system in the regulation of cue- and drug-induced relapsing phenomena in animal models. The aim of this review is to briefly present the available data obtained using diverse behavioral experimental approaches in experimental animals, namely, the intracranial self-stimulation paradigm, the self-administration procedure, the conditioned place preference procedure, and the reinstatement of drug-seeking behavior procedure, to provide a comprehensive picture of the current status of what is known about the endocannabinoid system mechanisms that underlie modification of brain-reward processes. Emphasis is placed on the effects of cannabinoid 1 (CB1) receptor agonists, antagonists, and endocannabinoid modulators. Further, the role of CB1 receptors in reward processes is investigated through presentation of respective genetic ablation studies in mice. The vast majority of studies in the existing literature suggest that the endocannabinoid system plays a major role in modulating motivation and reward processes. However, much remains to be done before we fully understand these interactions. Further research in the future will shed more light on these processes and, thus, could lead to the development of potential pharmacotherapies designed to treat reward-dysfunction-related disorders.
Introduction
Cannabis is considered as one of the oldest and most widely used recreational drugs in the world. Its consumption has increased dramatically in recent decades along with questions of its categorization as an illegal substance (1–4). The attraction of cannabis and the many issues surrounding its illegality stem from its effects on sensory processing, euphoric sensations, and its relaxing inferences. These effects are mainly attributed to the key psychoactive ingredient of marijuana, Δ9-tetrahydrocannabinol (Δ9-THC) (5–8). The effects of this psychoactive component lead to drug-seeking behavior and drug abuse in humans (1, 4). Conversely, investigation of the rewarding effects of Δ9-THC and other synthetic cannabinoids in animal models of drug abuse and dependence has provided us with valuable information on the biphasic effects of these compounds through contradictory findings (9–11). The discovery of the endogenous cannabinoid system has fueled the progressing amount of cannabinoid research in recent years, with particular emphasis on the effects of endogenous and synthetic cannabinoid compounds on cannabinoid 1 receptors (CB1 receptors) found in different areas of the brain. This system is thought to modulate the motivational processes and reward-seeking behaviors associated with the use of cannabis. Hence, the present review summarizes recent animal studies that investigate the function of the endocannabinoid system and its involvement in brain-reward systems, with particular emphasis on the role of CB1 receptors.
Endogenous Cannabinoid System
Definition
The endogenous cannabinoid or endocannabinoid system was first identified in the early 1990s when researchers were trying to shed light on the mechanisms of action of Δ9-THC (12–15). For the past couple of decades, with the contribution of various research groups, it has been discovered that the endocannabinoid system is composed of cannabinoid 1 and 2 receptors (CB1, CB2, respectively, and possibly others), endogenous ligands for these receptors and enzymes responsible for the synthesis, reuptake and degradation of these endogenous ligands (14, 16, 17). Genetic, pharmacological, and behavioral methods have all been utilized in order to elucidate the function and mechanisms of this system.
Cannabinoid Receptors
The discovery of Δ9-THC has resulted in a wealth of research surrounding cannabinoid receptors. Further, the discovery of synthetic cannabinoid agonists with the ability to simulate the effects of Δ9-THC suggested the existence of specific cannabinoid receptors (18) and increased our understanding of the mechanisms of action of Δ9-THC and the function of cannabinoid receptors. Two cannabinoid receptors have so far been identified, CB1 (19, 20) and CB2 receptors (21), both of which are metabotropic receptors coupled to Gi/o proteins. CB1 receptors are observed throughout the central and peripheral nervous system, but with higher concentrations in the brain and spinal cord (22). This convergence of CB1 receptors in the central nervous system (CNS) is consistent with the studied behavioral and physiological effects of cannabinoids (23). High levels of these receptors are found in brain areas such as the hippocampus, which may explain the memory deficits associated with the use of cannabis. Similarly, a high concentration of these receptors is also observed in brain areas, such as the basal ganglia and cerebellum, associated mainly with motor function and coordination (24, 25). The mesocorticolimbic dopaminergic pathway of the brain similarly features a high amount of CB1 receptors. Brain areas that are part of the mesocorticolimbic dopaminergic pathway include the prefrontal cortex, the hippocampus, the olfactory bulb, and the nucleus accumbens, all of which are implicated in motivational and reward processes, which have also been found to be altered by cannabinoid compounds (26, 27). CB1 receptors are also thought to inhibit release of glutamate, GABA, and other neurotransmitters, such as dopamine (28). More recent evidence suggests that CB2 receptors are also implicated in the moderation of cannabinoids in the CNS (28). Further, a number of behavioral and pharmacological effects of cannabinoid compounds cannot be explained by their action specifically on CB1 and CB2 receptors, proposing the existence of additional cannabinoid receptors, further to be identified and characterized (29, 30).
Endocannabinoid Ligands and Their Metabolizing Enzymes
The discovery of cannabinoid receptors alludes to the existence of endogenous ligands that bind and impact the function of these receptors. The two most widely studied endocannabinoids are N-arachidonoylethanolamide (AEA), also called anandamide (31) and 2-arachidonoylglycerol (2-AG) (32, 33), which were first discovered in the early 1990s. Endocannabinoids are synthesized on demand, mainly postsynaptically and act as retrograde messengers regulating the presynaptic release of neurotransmitters (34). This occurs in response to physiological and pathological stimulus resulting after an increase of the intracellular concentration of Ca2+ (35). Different pathways are involved in the synthesis of AEA and 2-AG. AEA is formed by transacylation of phosphatidylinositol and subsequent degradation by the phospholipid precursor N-acetyl-phosphatidylethanolamine (NAPE), as well as via a pathway involving the phospholipase C (PLC)-catalyzed cleavage of NAPE to generate a lipid, phosphoanandamide, which is subsequently dephosphorylated by phosphatases (36, 37). Although several pathways have been proposed for 2-AG synthesis, the one which dominates in the CNS involves the production of 2-AG via a two-step process: degradation of arachidonate-containing phospholipids to diacylglycerol (DAG) by PLC followed by DAG lipase-catalyzed degradation to 2-AG (38). AEA and 2-AG activate both CB1 and CB2 receptors. These endogenous ligands emulate many behavioral and biochemical properties of cannabinoids (36, 39). In the case of AEA, activation of the transient receptor potential vanilloid type 1 (TRPV1) receptor has also been noted (40). In recent years, more endocannabinoid ligands have been discovered such as N-arachidonoyldopamine, virodhamine, and noladine ether (41, 42). However, the physiological effects of these endocannabinoids are yet to be revealed. Thus, the focus of our review will be on effects of AEA and 2-AG, as these are the first two endocannabinoids discovered and mostly studied. Endocannabinoids are present in the mesolimbocortical dopaminergic system of the brain (24) suggesting an association with motivation and reward (31). The control of rewarding processes seems to be mainly moderated by CB1 receptors. Endocannabinoids can passively diffuse through lipid membranes, but a highly affinity transporter, which is not yet identified, seems to accelerate this process. Finally, two types of metabolizing enzymes seem to play a role in endocannabinoid deactivation, a fatty acid amide hydrolase (FAAH) is the main hydrolase for AEA, whereas 2-AG inactivation is mainly degraded by two other enzymes, called monoacyl-glycerol lipases (MAGLs) (34).
Pharmacological Modulation of the Endocannabinoid System
The discovery of the endocannabinoid system has led to the synthesis of agonists and antagonists that have proven useful in the investigation of CB1 and CB2 receptors and their functions. Such pharmacological modulation of the endocannabinoid system has led to recent advances in behavioral and pharmacological research (43, 44). There are currently five classes of cannabinoid analogs that have been classified based on their structure (45–47). These classes are classical, non-classical, aminoalkylindoles, eicosanoids, and biarylpyrazoles.
Classical cannabinoids are tricyclic terpenoid derivatives. This group includes the main psychoactive component of cannabis Δ9-THC, the phytocannabinoid Δ8-THC, and other synthetic equivalents. Levonantradol and AMG-3 are two examples of cannabinoid compounds belonging to this class (43, 44, 48–50).
Non-classical cannabinoids incorporate bicyclic and tricyclic analogs of Δ9-THC. These include, among others, the potent non-selective CB1/CB2 receptor agonists CP-55,940, CP-47,497, and CP-55,244 (43, 51).
The eicosanoid group consists of CB1 and CB2 receptor agonists that have markedly different structures not only from aminoalkylindoles but also from classical and non-classical cannabinoids. Notable members of this group are the endocannabinoids AEA and 2-AG (33, 52).
Aminoalkylindoles have a completely different chemical structure from other classes of cannabinoids. They are less lipophilic and differ in the way they interact with cannabinoid receptors (32). The highly studied WIN 55,212-2 is a member of this class of cannabinoids. It has high stereoselectivity, but low affinity for the CB2 receptor (48).
There are many compounds that selectively activate CB1 receptors more effectively than CB2 receptors. Many of these are synthetic analogs of AEA, which include R-(+)-methanandamide, arachidonyl-2′-chloroethylamide (ACEA), and arachidonylcyclopropylamide (ACPA) (48, 53). Although this is not the focus of this review, compounds with a selective affinity for CB2 receptors have also been developed and feature cannabinoids such as JWH-133, L-759633, and L-759656, and the non-classical cannabinoid HU-308. Other selective CB2 receptor agonists are the aminoalkylindoles JWH-015 and AM1241 (43, 44, 48).
Many selective compounds have been used extensively in research as CB1 receptor competitive antagonists. Two of the most well-known members of this group are SR141716A (rimonabant), AM-251, as well as AM-281 and LY320135 (47, 54, 55). These cannabinoids have greater affinity for CB1 receptors than for CB2 receptors and can also inhibit agonist-induced activation of CB1 receptors. In some cases, however, these can act as inverse agonists (47). Recently, compounds have been developed that act as CB1 receptor antagonists, yet they do not induce signs of inverse agonism at these receptors. Cannabinoids such as NESS O327, O-2050, and AM4113 show such effects (47, 56). In addition, the compounds AM630 and SR144528 are stronger in blocking CB2 than CB1 receptor activation (57, 58). However, both are considered to be CB2 receptor inverse agonists, due to the fact that, when administered alone, they can cause inverse cannabimimetic effects in CB2 receptor-expressing tissues (59).
Other cannabinoids that show an affinity for CB1 and/or CB2 receptors are the phytocannabinoids cannabinol, cannabidiol, and cannabigerol. Cannabinol acts as a CB1 receptor partial agonist, yet there is evidence to suggest that it can also serve as a CB2 receptor agonist/inverse agonist (60). Cannabidiol and cannabigerol have been shown to act as CB1 receptor antagonists/inverse agonists. Furthermore, cannabidiol has been found to have considerable potency as a CB2 receptor antagonist/inverse agonist (61). Recent research has indicated that the actions of AEA and 2-AG are halted by cellular uptake and intracellular enzymatic hydrolysis. This has been highlighted by the synthesis of several drugs that inhibit these actions (62–64). The use of these drugs as tools in animal experiments has elucidated the pathophysiological actions of endocannabinoids. Significant members of this group include the FAAH inhibitors/indirect agonists PMSF, palmitylsulphonyl fluoride (AM374), stearylsulphonyl fluoride (AM381), O-1887, OL-135, URB-532, URB-597, and URB-602 (65–67). In the last few years, selective pharmacological tools that disrupt the activity of MAGL in vivo have also become available. MAGL activity is sensitive to general serine hydrolase inhibitors, such as PMSF. However, as such compounds also inhibit FAAH, they are not suitable to distinguish the function of these enzymes. More selective compounds include URB602, NAM, OMDM169, JZL184, and KML29 (68).
There is some pharmacological evidence that points toward the existence of the reuptake transporter of endocannabinoids through the use of specific reuptake inhibitors. Amongst these reuptake inhibitors, AM-404 is the most widely investigated. However, this compound not selective, as it also halts the action of FAAH and binds to CB1 receptors (67).
Genetic Modulation of the Endocannabinoid System
Transgenic mice have been used in recent research to understand the pharmacological and behavioral actions of cannabinoids [for details on genetic modulation of the endocannabinoid system, please see Ref. (69–71)]. These mice lack CB1, CB2, or both CB1 and CB2 receptors. They have proven useful tools to elucidate whether responses to cannabinoid compounds are attributed to CB1 receptors and/or CB2 receptors as well as the physiological roles of these receptors (70, 71). FAAH- and MAGL-deficient mice are also useful in understanding the physiological role of these endocannabinoid components in various functions and disorders, including brain reward and drug addiction (68, 72). However, several adaptive changes in CB1 receptor function have been reported in MAGL knockout mice, limiting the use of these mutants in behavioral studies. Recently, a novel line of transgenic mice that overexpress MAGL in the forebrain has been generated. Since these mice do not express adaptive changes in other endocannabinoid components, this opens the possibility to expand the study of the physiological role of 2-AG in brain reward processes and drug addiction (73).
Cannabinoid Effects on Brain Reward Processes
Cannabinoid Effects on Brain-Stimulation Reward
Intracranial self-stimulation (ICSS) is an operant behavioral paradigm in which animals would work to obtain intracranial stimulation through electrodes implanted into discrete brain areas (often referred as brain reward areas/circuit) (74, 75). This observation is based on the original discovery by Olds and Milner (76) that rats will repeatedly press a lever to stimulate components of their brain reward circuit. Historically, ICSS has been utilized in rodents to study how pharmacological or molecular manipulations affect brain reward function (77). More importantly, manipulations that increase reward and manipulations that decrease reward produce opposite outputs in self-stimulation behavior. Accordingly, most drugs of abuse are able to lower ICSS threshold (i.e., increase the rewarding efficacy of intracranial stimulation), which support the notion that they activate the same substrate with electrical stimulation in a synergistic manner (78–80). Thus, ICSS can be considered as a model to study the reward-facilitating effects of various drugs of abuse with addictive properties in humans.
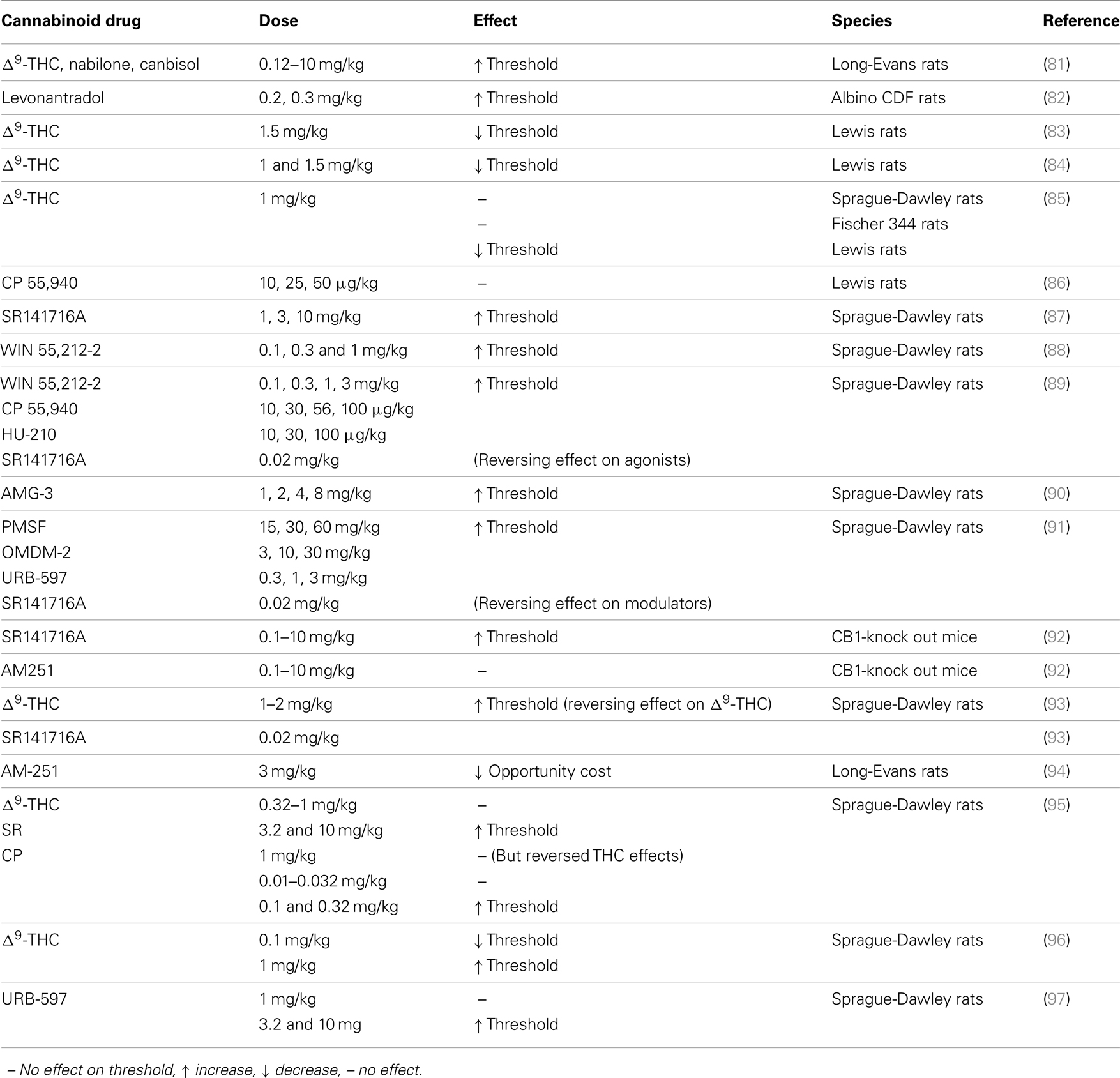
Over the last years, a considerable amount of literature has been published on the effects of cannabinoids in the ICSS paradigm (see Table 1). Importantly, different effects have been observed after the administration of Δ9-THC or other CB1 receptor agonists and endocannabinoid modulators. Overall, the corresponding findings appear to be dispersed and dependent on various methodological variables (i.e., strain of the animal, cannabinoid compound, and dose).
A number of studies have been conducted on the effects of Δ9-THC in the ICSS paradigm. Gardner and colleagues were among the first who studied the effects of Δ9-THC on ICSS. In their experiments, 1 and 1.5 mg/kg of Δ9-THC decreased ICSS thresholds in Lewis rats, but not in Fisher 344 rats, whereas in Sprague-Dawley rats the effect was only marginal (83, 85). In contrast, other studies failed to show an enhancement of brain-stimulation reward with Δ9-THC in the dose range from 0.5 to 10 mg/kg in Sprague-Dawley rats under baseline conditions (93, 95, 96) or in animals pre-exposed to stress (98). Similar results have been reported in Long-Evans rats with p.o administration of 10/mg/kg Δ9-THC and various doses of three synthetic analogs structurally related to Δ9-THC, namely levonantradol, nabilone, and canbisol (81, 82). Interestingly, however, in a recent study from our research group, we showed that Δ9-THC can induce both rewarding and anhedonic effects in the ICSS paradigm in Sprague-Dawley rats, depending on the dose used (96). Thus, a low dose of 0.1 mg/kg, decreased ICSS thresholds and caused clear parallel leftward shifts in the rate-frequency function, whereas a higher dose of 1 mg/kg increased ICSS thresholds, producing rightward shifts. These effects were long-lasting, since they remained for 2 h post-injection and the reward-facilitating effect that we observed with 0.1 mg/kg of Δ9-THC was more pronounced after 1 h. Both the rewarding and the anhedonic effects of Δ9-THC observed in our studies are specifically mediated by cannabinoid CB1 receptors, since they have been reversed by a low dose of SR141716A. Comparing findings from the above studies, it can, thus, be suggested that Lewis rats may have a differential sensitivity to Δ9-THC, compared to Sprague-Dawley and Fisher 344 rats and that the dose–response function of Δ9-THC on brain-stimulation reward is not linear, but rather biphasic.
Only a few studies have examined the effects of various synthetic cannabinoid agonists on brain-stimulation reward. Arnold and colleagues have reported that the potent synthetic CB1 receptor agonist CP55,940 did not affect the reinforcing efficacy of medial forebrain bundle (MFB) stimulation (86). In the same way, other studies have shown that the synthetic CB1 receptor agonists WIN55,212-2, CP55,940, HU-210, and AMG-3 either do not affect or increase ICSS threshold, depending on the dose used (88–90, 95). Similarly, in a series of studies from our laboratory we have shown that the indirect cannabinoid agonists (endocannabinoid modulators) PMSF, AM-404, OMDM-2, and URB-597 in low doses do not affect ICSS thresholds, while in high, and possibly non-selective doses, decrease the reinforcing efficacy of brain stimulation (91, 99). Similar results have been reported very recently with the FAAH inhibitor URB-597 (97).
Several studies have examined the effects of CB1 receptor antagonists on ICSS. Low doses of the CB1 receptor antagonists SR141716A and AM-251 did not affect ICSS thresholds (89–92, 95), while higher doses of SR141716A have been reported to increase ICSS thresholds (86, 87, 92) However, in such high doses it is possible that SR141716A acts as a partial or inverse agonist at cannabinoid receptors, as it has been observed in other studies (100, 101). Indeed, this could be a plausible explanation for its anhedonic effects observed with high doses on brain-stimulation reward. Shizgal’s group (94) utilizing a novel method for measuring reward have shown that AM-251 decreased performance for MFB self-stimulation. Indeed, AM-251 produced leftward shifts of the function that relates operant performance to the opportunity cost of the reward, but did not affect the function that relates operant performance to the stimulation strength. The authors suggest that this shift may be related to a decrease in the reward signal gain or an increase in the subjective reward cost.
In summary, although most drugs abused by humans are able to increase the rewarding efficacy of brain stimulation over a wide range of doses, results with Δ9-THC and other synthetic cannabinoid agonists have not always been consistent. In the studies by Gardner’s group, the most robust reward-facilitating effect of Δ9-THC in the ICSS paradigm was found in rats of the Lewis strain. Thus, it is possible to hypothesize that the reward-facilitating effect of Δ9-THC may preferentially be obtained in certain strains of rat, suggesting an important genetic component in this action. One major finding was that Δ9-THC induces biphasic effects, i.e., is able to induce both rewarding and anhedonic effects, in the ICSS paradigm in Sprague-Dawley rats, depending on the dose used. On the other hand, studies using the ICSS paradigm failed to show any reward-facilitating effects for direct and indirect (i.e., endocannabinoid modulators) synthetic cannabinoid agonists, or to the contrary, they present data for anhedonic actions of these compounds. Thus, it is possible that cannabinoids have negative or dysphoric effects in animals that mask their reward-facilitating effects in the ICSS paradigm and that these effects are suppressed under a limited dose range.
Cannabinoids Effects on Conditioned Place Preference
Conditioned place preference (CPP) is a non-operant procedure for assessing the reinforcing properties of drugs using a Pavlovian conditioning. The reinforcing properties of abused drugs are easily associated with environmental stimuli, such as an environment or context in which the drugs are administered. Through multiple pairings, these environmental (contextual) cues acquire conditioned reinforcing properties. The CPP paradigm is based on the assumption that animals learn to approach stimuli paired with rewards and to avoid stimuli paired with aversive agents. Thus, it can be used to evaluate whether the repeated pairing of one specific environment with a drug produces a preference for that environment (102). Indeed, in this procedure, the animal develops an association between the subjective state produced by the drug (e.g., a heightened feeling of euphoria comparable to pleasure in humans) and the environmental cues present during the drug state. Most drugs abused by humans produce place preference in experimental animals (103). Although CPP provides a less direct evaluation of the rewarding effects of drugs, it presents several advantages: (1) it can be sensitive even to low doses of the drug studied, (2) it can be also used to assess the aversive or dysphoric properties of a drug (in this case, the animal will avoid staying in a compartment previously associated with a drug), (3) the animals are tested in a drug-free state, (4) it can be used to study non-drug stimuli, such as food, sucrose, or sex.
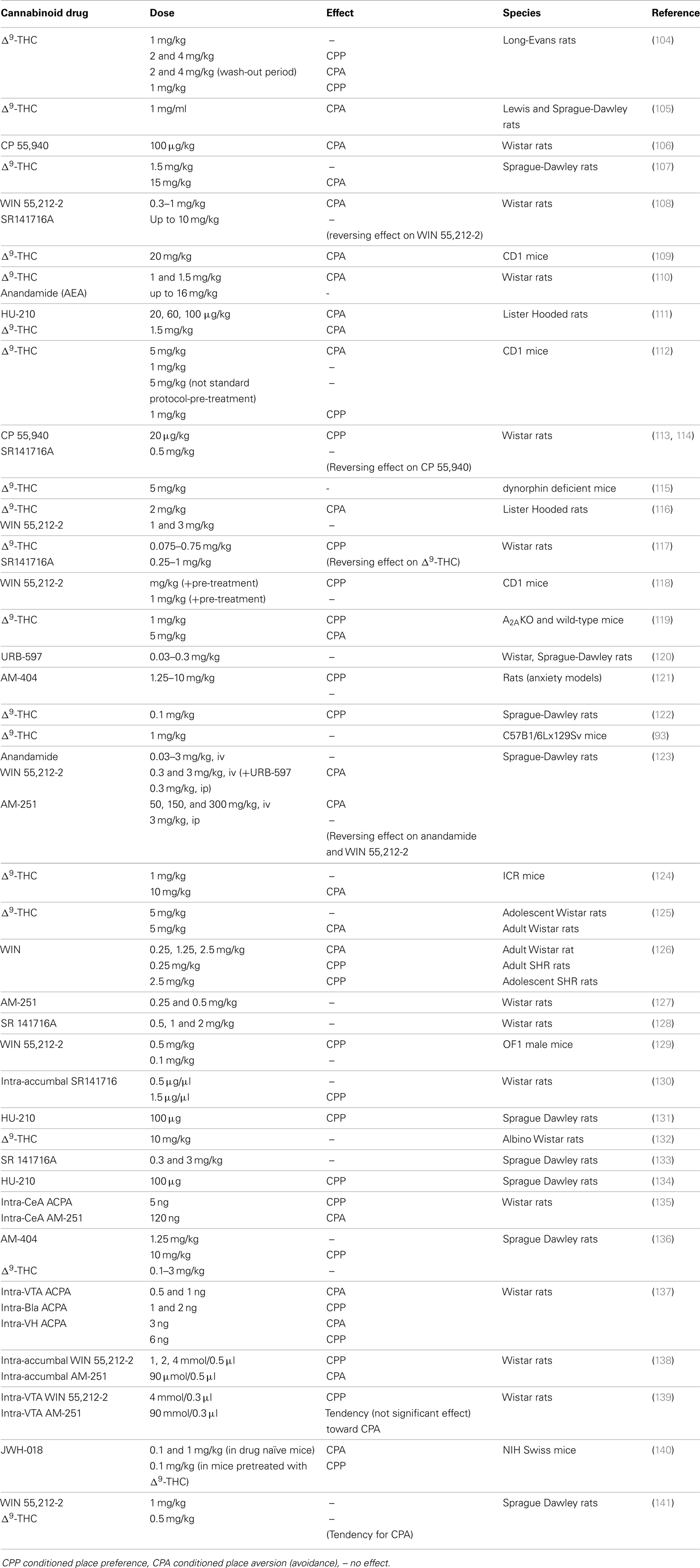
Studies using the CPP paradigm have shown that Δ9-THC and other synthetic cannabinoid agonists can induce both appetitive and aversive effects under various experimental conditions (see Table 2). Notably, in the studies reporting place preference of cannabinoids, these effects are usually dependent upon the particular dose used and the preference is connected to a single dose. Furthermore, other factors, such as the administration of a priming injection and the timing between injections have been suggested to be important in determining whether cannabinoids produce preference or aversion.
In the first study, Δ9-THC-produced CPP was published in 1995 by the Gardner research group (104). In their experiments, 2–4 mg/kg Δ9-THC produced CPP in Long-Evans rats, when the CPP pairing interval was 24 h, while the dose of 1 mg/kg of Δ9-THC did not produce any preference. However, when the schedule of daily injections was changed, allowing a longer wash-out interval between injections (48 h), Δ9-THC produced a clear place aversion in the dose range of 2–4 mg/kg, but place preference in the dose of 1 mg/kg. In other words, Δ9-THC-induced CPP was dependent upon the dose and the injection schedule. Similar results have been reported by Braida and colleagues (117) and Le Foll and colleagues (122). In the first study, Δ9-THC produced CPP in Wistar rats in a dose range between 0.075 and 0.75 mg/kg, whereas higher doses produced aversive effects. In the latter study, a low 0.1 mg/kg dose of Δ9-THC produced CPP, while doses lower or higher than this did not produce any preference. Two other studies in male Lister-Hooded rats not only failed to find any rewarding effects of Δ9-THC (1.5 and 2 mg/kg) in the CPP paradigm, but reported aversive effects (111, 116). Two more recent studies did not find any rewarding or aversive effects of Δ9-THC in the CPP paradigm in Sprague-Dawley rats and in the dose range of 0.1–3 mg/kg (136, 141). A few studies have examined whether adolescent rats respond differently (are more vulnerable) to Δ9-THC than adult rats. According to a study by the McGregor group (125), Δ9-THC (5 mg/kg) produced CPA in adult rats, whereas in adolescent rats there was only a trend toward aversion, which was not significant. Interestingly, the aversive effect reported in adult rats was long-lasting, since the animals still avoided Δ9-THC-paired environment 16 days following the last drug exposure. Surprisingly, in a more recent study in adolescent Wistar rats, although Δ9-THC (10 mg/kg) did not induce CPP when administered alone, it tended to produce a preference when administered in combination with cannabidiol (132).
Studies in mice have also shown controversial results. Valjent and Maldonado reported Δ9-THC-induced CPP bypassing the dysphoric/aversive effects of Δ9-THC that has been reported in naïve animals with a priming injection 24 h before the first conditioning session (112). However, Vlachou and colleagues did not observe CPP with the 1 mg dose of Δ9-THC using the same experimental manipulation (93). These differences can be explained in part by the different strain of animals used, the number of pairings or the periods of conditioning, and administration of the drugs. Although the Maldonado group replicated their findings in a subsequent study (119), it is worth noting that they also report conditioned place aversion (CPA) with the dose of 5 mg/kg of Δ9-THC. Finally, a number of studies have found that Δ9-THC produces CPA and not CPP in rats or mice (105, 107, 109, 110, 124).
Bidirectional and/or conflicting effects have been also reported in the literature for synthetic cannabinoid agonists. In a very recent study, the synthetic cannabinoid agonist JWH-018 produced CPA in naïve mice, but CPP in mice pre-treated with Δ9-THC (140). Thus, we could speculate that Δ9-THC pre-exposure may reveal the appetitive effects of other cannabinoid agonists. Braida and colleagues using the potent synthetic CB1 receptor agonist CP55,940 in Wistar rats reported CPP only at the dose of 20 μg/kg, but not in lower or higher doses (113). Another study using CP55,940 reported CPA in the dose of 10 μg/kg, as well as in a higher dose of 100 μg/kg (106). In the same study, the dose of 100 μg/kg of CP55,940 was also aversive in the conditioned taste aversion paradigm. The literature on the reinforcing effects of WIN55,212-2 and HU-210 in the CPP paradigm is also controversial. According to Castané and colleagues, WIN55,212-2 produced CPP in mice pre-exposed to a priming injection of the drug (118). Similar results have also been reported with HU-210 (100 μg/kg) in Sprague-Dawley rats pre-exposed to a priming phase consisting of four daily home injections of the drug (131, 134). Notably, CPP with WIN55,212-2 has also been reported in OF1 mice without utilizing a pre-exposure protocol (129). However, two other studies reported CPA after systemic administration of WIN55,212-2 (108) and HU-210 (111). Adding to this complexity are studies that failed to reveal either a preference or an aversive effect with WIN55,212-2 in a dose range between 0.1 and 3 mg/kg (116, 142). Contrasting effects of WIN55,212-2 in Wistar and spontaneously hypertensive rats, a validated animal model of attention deficit/hyperactivity disorder, have been reported in the literature (126). Thus, WIN55,212-2 produced CPA only in adult, but not adolescent Wistar rats. In contrast, WIN55,212-2 produced CPP in both adolescent and adult spontaneously hypertensive rats.
A limited number of studies have also examined the effect of intracranial injections of CB1 receptor agonists and antagonists in the CPP paradigm. Data from two recent studies have shown that intra-accumbal (138) and intra-VTA (139) injection of WIN55,212-2 produces CPP. In contrast, intra-accumbal (138) or intra-central amygdala (135) injection of the CB1 receptor antagonist AM-251 produces CPA, while intra-VTA injection of AM-251 produces a tendency toward CPA (139). Similarly, intra-central amygdala (135) and intra-basolateral amygdala injection of the cannabinoid agonist ACPA produces CPP, whereas intra-VTA injection of ACPA produces CPA (137). Interestingly, biphasic effects of intra-ventral hippocampus injection of ACPA have also been reported in the literature in the CPP test, with lower doses producing CPP, while higher doses CPA (137).
Although most of the studies have used CB1 receptor antagonists to test for CB1-receptor selectivity of cannabinoid compounds on brain reward, there are a few studies that have tested the effects of CB1 receptor antagonists on reward per se. Cheer and colleagues found that the CB1 receptor antagonist/inverse agonist SR141716A produced a clear CPP (111), indicating the possibility that an endogenous cannabinoid tone might be present in the brain, as a physiological system to suppress reward or induce aversion. Importantly, in a major study, intra-accumbens injection of SR141716A also produces CPP, although in vivo silencing of accumbal CB1 receptors induced CPA to cocaine (130). Based on these results, the authors suggest that SR141716A acts as an inverse agonist on the CPP test. However, in other studies SR141716A or AM-251 failed to produce either CPP or CPA (108, 113, 127, 128, 133).
A limited number of studies have examined the effects of endogenous cannabinoids or compounds increasing their levels in the brain on CPP. The first report that the administration of the endogenous cannabinoid anandamide did not produce any significant effects in place conditioning was published by Mallet and Beninger (110). As anandamide is quickly degraded, its physiological roles can be best studied by blocking the mechanisms of its degradation and, thus, prolonging its actions. As previously described, anandamide degradation is mainly mediated by the enzyme FAAH. Accordingly, inhibition of FAAH by drugs, such as URB-597, can be used as a pharmacological tool to study the role of anandamide in brain reward. In a study investigating the antidepressant properties of URB-597, Gobbi and colleagues (120) did not find any rewarding effects in the CPP paradigm. In another major study (123), intravenous administration of anandamide did not produce CPP or CPA. However, when rats were pretreated with the FAAH inhibitor, URB597 anandamide produced dose-related CPA (123).
As mentioned previously, termination of endocannabinoid signaling is also mediated by cellular uptake. Inhibition of endocannabinoid transport by drugs, such as AM-404, is an additional pharmacological tool to study the role of endocannabinoids on brain reward. CPP by AM-404 was first demonstrated experimentally by Bortolato and colleagues (121) in rats housed under enriched conditions, but not in rats kept in standard cages. However, it is worth noting that AM-404 induced CPP at a dose that did not increase tissue levels of anandamide or 2-AG in the brain areas investigated (121). Thus, the involvement of the endocannabinoid system in AM-404-induced CPP remains questionable. In a more recent study, Scherma and colleagues examined different doses (1.25–10 mg/kg) of AM-404 in the CPP test and found that only the high dose of 10 mg/kg was able to produce a clear CPP in Sprague-Dawley rats (136).
In summary, while almost all drugs abused by humans are able to produce a clear and reliable place preference (i.e., increase the time spent in the drug paired compartment) over a range of doses, results with Δ9-THC and other cannabinoids have not always been consistent. The studies reporting a CPP associated with administration of a cannabinoid have either used a particular experimental methodology or the preference occurred at only a single dose. In addition, although endocannabinoids are able to regulate reward-related processes, they do not produce CPP and do not seem to have reinforcing properties that have been associated with Δ9-THC and other cannabinoid receptor agonists. It is possible, therefore, that the rewarding properties of cannabinoids in the CPP procedure may be masked by aversive or dysphoric effects, under particular circumstances. Thus, we highlight the difficulty of drawing general conclusions on whether Δ9-THC and other cannabinoids have reinforcing properties in the CPP paradigm.
Cannabinoid Effects on Self-Administration Studies
Human subjects and laboratory animals will self-administer addictive drugs by a variety of routes, including oral, intragastric, intraperitoneal, and intracranial routes. Intravenous drug self-administration has been one of the most direct approaches to study the rewarding properties of drugs of abuse in experimental animals, such as rodents or primates. In this behavioral paradigm, based on operant conditioning, animals learn to make an operant response, such as pressing a lever in an operant chamber or inserting their nose into a hole, to self-administer a reinforcer (e.g., a drug of abuse) after the completion of the reinforcement schedule requirement. A reinforcer is an event that follows a response and increases the probability of a response to reoccur (143–147). Reinforcing effects of a drug assessed by intravenous self-administration procedures in experimental animals are considered as one of the most reliable predictors of abuse potential in human subjects. The main schedules of reinforcement used in the self-administration procedure to resemble the human condition are the fixed-ratio, the progressive-ratio, and the discrete-trials schedules of reinforcement.
Briefly, under the fixed-ratio schedule, the reinforcer is delivered every time a predetermined number of responses is completed, and the delivery of a reinforcer is usually followed by a timeout period in self-administration studies to prevent the subjects from overdosing (e.g., FR1 or continuous reinforcement schedule, FR2, FR4, FR5, etc.). Data obtained from a fixed-ratio schedule provide a measure of drug intake and reinforcement efficacy. Further, under the progressive-ratio schedule, the response requirements are progressively increased after the delivery of each reinforcer, according to a predetermined progression. For example, the number of responses required to earn a nicotine infusion or food pellet on the progressive-ratio can be determined by the exponential progression [5e(0.25 × (infusion number + 3)) − 5] with the first two values replaced by 5 and 10, so that the response requirements for successive reinforcers are 5, 10, 17, 24, 32, 42, 56, 73, 95, 124, 161, 208, etc. Breakpoints in this schedule are typically defined as the highest response rate achieved to obtain a single reinforcer before an animal fails to complete the next ratio requirement within a predetermined time period (e.g., 60 min). Data obtained from a progressive-ratio schedule provide a measure of the motivation (i.e., incentive value) to obtain a reinforcer. Finally, in the discrete-trial schedule of reinforcement procedure, only a single injection of the drug is delivered during individual trials. The intertrial interval (ITI) can be adjusted to manipulate the influence of one injection on subsequent trials. When short ITIs are used, animals continuously self-administer a drug for long periods of time (hours or even days). When long ITIs are used, a regular circadian pattern of self-administration occurs (i.e., periods of abstinence during the light phase of the cycle alternate with periods of self-administration during the dark phase). Data obtained from a discrete-trials schedule provide a measure of the motivation to initiate drug-taking behavior. Thus, all three schedules can reliably predict abuse potential in human subjects (147).
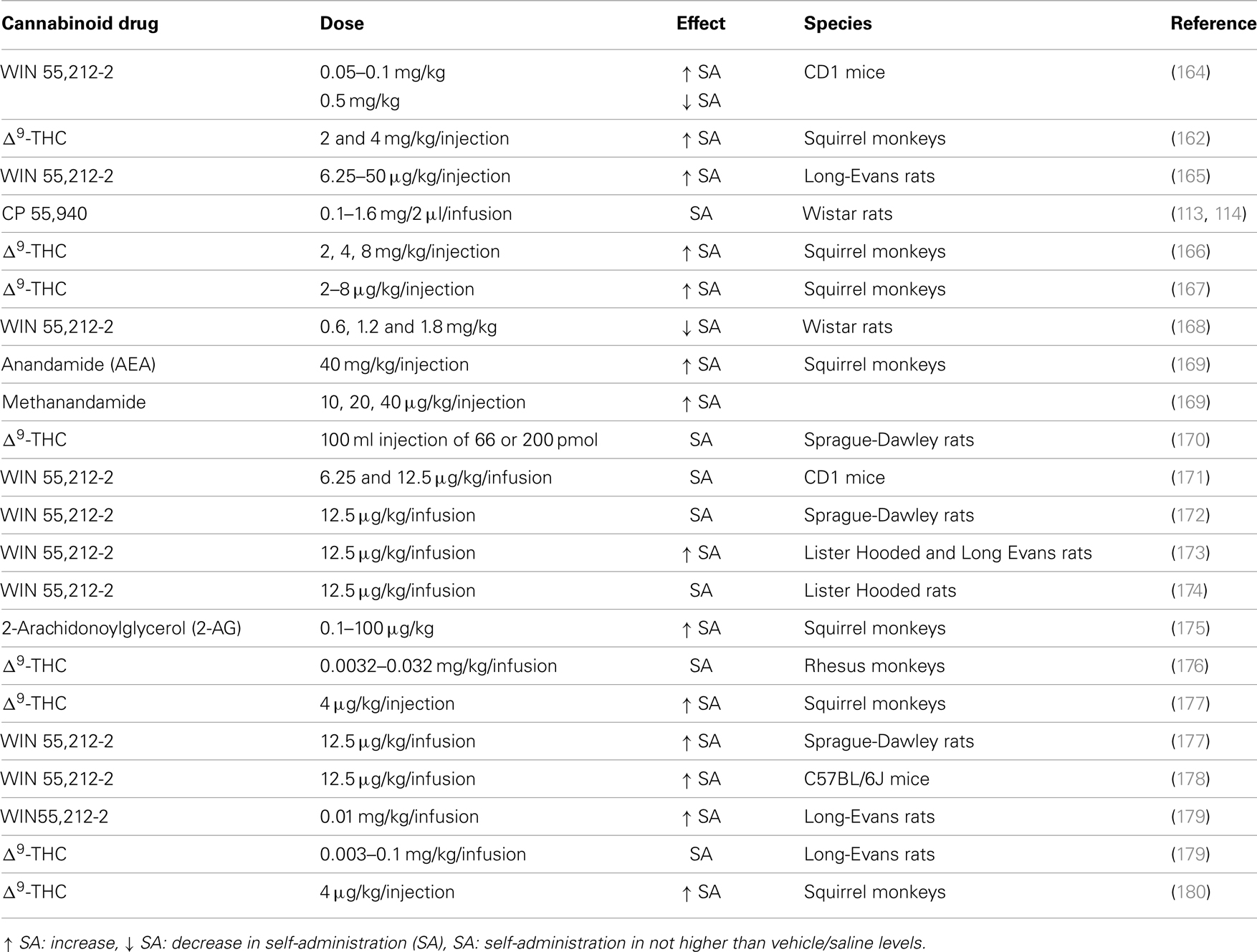
Most drugs abused by humans, including psychostimulants, opiates, ethanol, and nicotine, support reliable and persistent self-administration behavior in drug-naïve experimental animals (148). However, for many years, it has been rather difficult to show self-administration of cannabis, Δ9-THC or other cannabinoid compounds in experimental animals (149–155), with the first studies showing either no effect of Δ9-THC (156, 157), self-administration of Δ9-THC only in food- or water-deprived animals (117, 158–160), or in animals that were previously pre-exposed to or trained to self-administer other drugs of abuse, such as phencyclidine, cocaine, amphetamine, ethanol, or pentobarbital (150, 155, 161–163), with not a robust effect (i.e., relatively low rates of responding). Interestingly, in the past few years, different research groups have successfully varied the parameters of self-administration procedure in order to demonstrate reliable and persistent self-administration of Δ9-THC or other synthetic cannabinoids in rodents or primates (see Table 3).
The first self-administration of cannabis, with a low success rate, was reported by Deneau and Kaymakcalan (156) and Kaymakcalan (157), who demonstrated acquisition of Δ9-THC self-administration behavior in two monkeys out of six studied, but only after withdrawal from forced automatic i.v. injections of Δ9-THC, when signs of physical dependence on Δ9-THC occurred. Naïve monkeys did not self-administer Δ9-THC, while one monkey exhibited Δ9-THC self-administration behavior following cocaine self-administration. Furthermore, in a study by Pickens and colleagues (161) where animals had been pre-exposed to phencyclidine before Δ9-THC self-administration, rates of responding were relative low and there was no clear evidence that responding for Δ9-THC could persist above vehicle control levels over repeated daily sessions. The functional state as well as the motivational state in naïve animals compared with animals that self-administer other drugs of abuse could be different, and therefore, their corresponding response could also vary accordingly (154, 181). Similarly to the above study, food deprivation was also used to initiate and subsequently maintain Δ9-THC self-administration. Takahashi and Singer (158, 159) reported Δ9-THC self-administration above placebo levels in diet-restricted rats maintained at 80% of normal body weight, under conditions where a food pellet was automatically delivered every minute. Interestingly, self-administration immediately decreased to placebo levels when food restriction was discontinued. This manipulation may also alter the motivational state of the animal, which per se is an inherent limitation, as it has been repeatedly shown that food restriction (or deprivation) can facilitate the initiation and maintenance of drug self-administration (160, 182–185).
Interestingly, initiation and maintenance of high rates of intravenous self-administration of low doses of Δ9-THC in drug-naïve squirrel monkeys was only accomplished in the past few years (166, 180, 186). In the first of these studies, low doses of Δ9-THC initiated and sustained high rates of intravenous self-administration in drug-naïve squirrel monkeys. Three drug naïve squirrel monkeys were used and low doses of Δ9-THC (1–8 μg/kg/injection) that, according to the authors, were several times lower than doses generally used in previous attempts to demonstrate Δ9-THC self-administration in monkeys and comparable to those delivered by an average marijuana cigarette. Furthermore, Δ9-THC was dissolved in a Tween-80 vehicle resulting in a clear solution that was rapidly delivered (0.2 ml injection delivered in 200 ms) in the drug-naïve animals. The self-administration behavior was rapidly extinguished either by substituting vehicle injections for Δ9-THC injections or by administering the CB1 receptor antagonist SR141716A before the session, demonstrating that this effect was mediated by direct stimulation of the CB1 receptors. Most recently, in a study by a different research group (176), rhesus monkeys could self-administer Δ9-THC alone (0.0032–0.032 mg/kg/infusion), although Δ9-THC alone did not maintain responding above that obtained with saline.
Importantly, Braida and colleagues also showed intracerebroventricular self-administration of Δ9-THC in rats under water-deprived conditions (117), while in a latter study Zangen and colleagues (170) identified the posterior ventral tegmental area and the shell of the nucleus accumbens, but not the anterior ventral tegmental area, the region dorsal to this, or the core of the nucleus accumbens, as possible brain sites for the rewarding effects of the reported intracerebral self-administration of Δ9-THC (170).
Synthetic cannabinoid analogs have also been used in the self-administration procedure. The most commonly used synthetic cannabinoid analog is the potent non-selective CB1/CB2 receptor agonist WIN55,212-2. Fattore and colleagues (165) showed that rats could self-administer intravenously several doses of WIN55,212-2 under food restriction. This effect was blocked by the CB1 receptor antagonist SR141716A, indicating that the self-administration of WIN55,212-2 was mediated by activation of the CB1 receptors. This finding was replicated in more recent studies by the same group using the same experimental design (173, 174). Further, Lecca and colleagues (172) reported self-administration of WIN55,212-2 in rats following a different experimental protocol from that of the above mentioned studies. In their study, rats were not food-restricted, but they were maintained on a daily ratio of 20 g of food, made available at the end of each self-administration session.
In a most recent study (179), male Long-Evans rats were trained to self-administer WIN55,212-2 (0.01 mg/kg/infusion) on a fixed ratio 3 schedule. Dose–effect curves for WIN55,212-2 were determined, followed by vehicle substitution and a dose–effect curve with Δ9-THC. WIN55,212-2 self-administration was acquired; however, substitution with Δ9-THC did not maintain responding above vehicle levels. WIN55,212-2’s reinforcing effects were CB1 receptor-mediated, as they were dose-dependently attenuated by SR141716A. As authors indicated, the lack of substitution with Δ9-THC seen in this study is problematic and may suggest that WIN55,212-2 self-administration may be of limited usefulness as a screening tool for detection of the reinforcing effects of potential cannabinoid medications (179).
Importantly, Martellotta and colleagues showed intravenous self-administration of WIN55,212-2 in mice in a dose-dependent manner (164). This effect was also blocked by pre-treatment with the CB1 receptor antagonist SR141716A, indicating the direct involvement of the CB1 receptors. Self-administration of WIN55,212-2 in mice under a fixed- and a progressive-ratio schedule of reinforcement was also shown recently (178), an effect that was blocked by systemic administration of the hypocretin receptor-1 (Hcrtr-1) antagonist SB334867. This role of Hcrtr-1 in the reinforcing and motivational properties of WIN55,212-2 was confirmed in Hcrtr-1 knockout mice (178).
The same experimental protocol as Martellota and colleagues (164) was also used by another research group (187) to study the reinforcing effects of WIN55,212-2 in CB1 knockout mice. The genetically modified mice did not self-administer WIN55,212-2. In another study, drug-naïve mice self-administered the synthetic CB1 receptor agonist WIN55,212-2 and the Δ9-THC derivative HU-210 (188). However, it should be emphasized that these studies have an important inherent limitation as 1-day experimental tests were used and the animals were severely restrained. Thus, validity of these data is questionable and difficult to correlate with drug addiction in humans, which is a chronic state or even compare with chronic self-administration procedures in animals under baseline conditions (i.e., no restraint). Furthermore, since the animals were severely restrained, the reported self-administration may be affected by analgesic or anxiolytic effects resulting in a reduction of pain or stress produced by the restrain.
Interestingly, both AEA (as well as its metabolically stable synthetic analog methanandamide) (169) and 2-AG (175) are intravenously self-administered by squirrel monkeys, although four out of six squirrel monkeys used in the first study (169) had a history of Δ9-THC or methohexital self-administration. Similarly, in the more recent study indicating 2-AG self-administration, the researchers used monkeys with either a history of AEA self-administration or a history of nicotine self-administration (175). Interestingly, however, the reinforcing effects of AEA and 2-AG appear to be mediated by cannabinoid CB1 receptors, since daily pre-treatment with SR141716A resulted in complete blockade of AEA or 2-AG self-administration behavior. It is also noteworthy that in both studies, the authors report rates of responding comparable with those maintained under the same conditions by cocaine or Δ9-THC. More importantly, there is also evidence that treatment with the FAAH inhibitor URB597 shifts the AEA self-administration dose–response curve to the left, indicating that AEA has rewarding effects even in lower doses (189).
Further, only a few studies have focused on the intracranial self-administration of Δ9-THC or other cannabinoid analogs by experimental animals. Intracerebral administration of the potent non-selective CB1/CB2 receptor agonist CP-55,940 was shown in rats in a free-choice procedure (114). This effect was antagonized by the CB1 receptor antagonist SR141716A, indicating that it was specifically mediated by CB1 receptors. However, one limitation of this study is that the animals were water-deprived and water was concurrently delivered with each infusion. This may have altered the motivational state of the animals, provoking the self-administration response. In a previous study, CP-55,940 was not self-administered by rhesus monkeys (155).
In summary, most attempts to obtain a robust self-administration of Δ9-THC or other synthetic cannabinoids, under regular experimental conditions (i.e., drug-naïve unrestrained animals, and not food deprived), have been unsuccessful or partly successful. Only a limited number of studies report a robust procedure for cannabinoid self-administration either in a limited number of squirrel monkeys or intracerebrally in rodents. This is in accordance with other behavioral studies on rewarding and reinforcing effects of cannabinoids (i.e., ICSS, CPP) and illustrates the differential status of cannabinoids as atypical drugs of abuse.
Cannabinoid Effects on Reinstatement Procedures
A procedure used to study cue-, context-, drug-, or stress-induced reinstatement of drug seeking is hypothesized to be a putative model of relapse to drug seeking in humans. Animals learn to self-administer a drug for a period of time, in the same manner as during the self-administration procedure. Drug-reinforced lever responding is then extinguished, and reinstatement of drug-seeking behavior is subsequently triggered by a priming injection of a compound (drug-induced), a cue (or context) previously associated with the self-administration of the drug (cue- or context-induced), or a stressor (stress-induced reinstatement) (147). The reinstatement model of relapse to drug-seeking behavior is uniquely responsive to drugs with addictive properties. Only drugs which support drug-seeking and drug-taking behaviors can initiate or trigger relapse in the reinstatement model. Especially, compelling is the fact that cross-priming (from one class of addictive drug to another) is seen in this model (190).
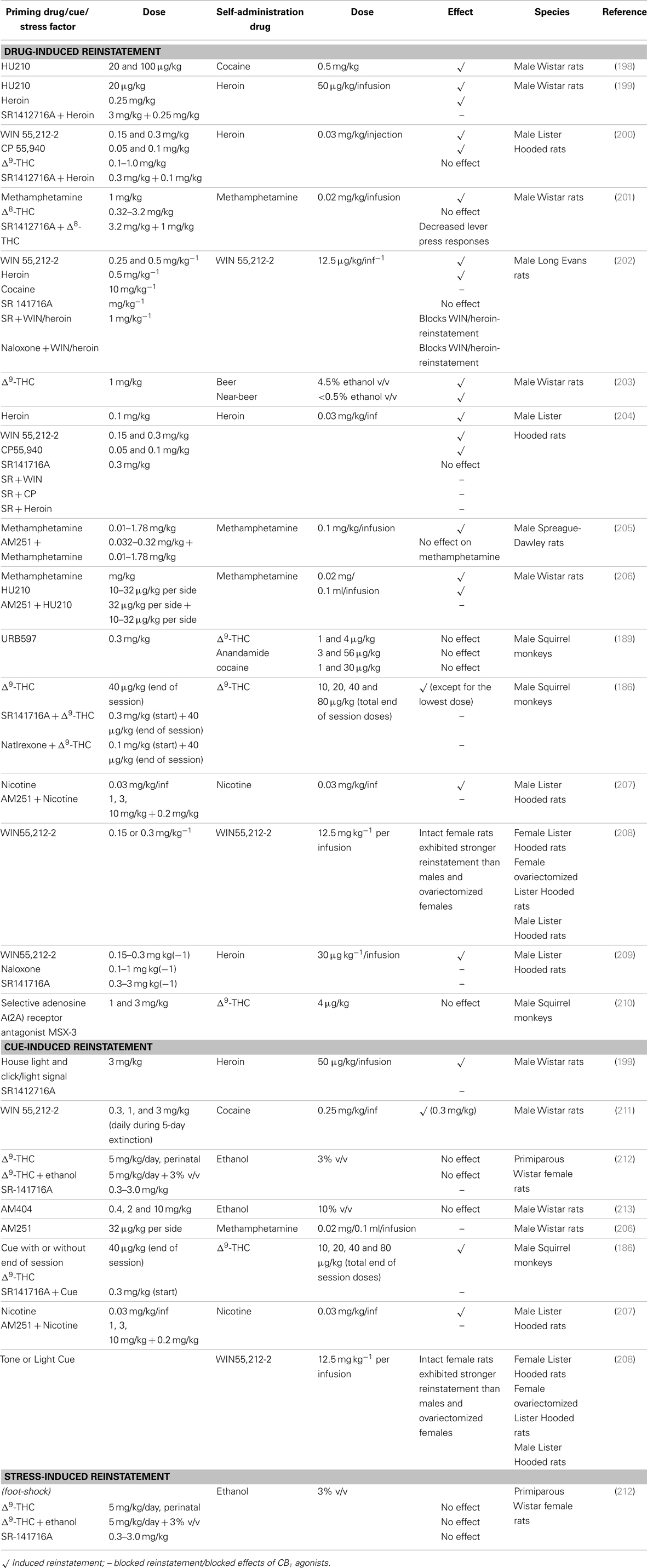
Little work has been done with cannabinoids per se in this model. However, existing literature in cannabinoid research indicates that most of the reinstatement studies conducted with cannabinoid compounds test for the cross-priming effect (i.e., the interactions between cannabinoid compounds and other drugs of abuse in inducing reinstatement of drug seeking) and is suggestive of cannabinoids fitting the same pattern as other addictive drugs in these procedures [for reviews, see Ref. (191–196)]. In many cases, cannabinoids trigger reinstatement of drug-seeking behavior in animals behaviorally extinguished from intravenous drug self-administration behavior and, thus, pharmacologically detoxified from their self-administered drug. Thus, in most cases, either different drug of abuse has been used before extinction (e.g., cocaine, heroin, morphine) or the drug-induced reinstatement is triggered by cannabinoids or vice versa (186, 197) (please see Table 4).
CB1 receptors have been found to play a critical role in mediating reinstatement of previously extinguished drug-seeking behavior upon re-exposure to the drug or drug-associated cues. The neuroanatomical bases as well as the neuronal mechanisms of the relapse-promoting effects of CB1 receptor agonists or the relapse-attenuating effects of CB1 receptor antagonists are still poorly understood, although interactions of the endogenous cannabinoid system with afferent glutamatergic and possibly dopaminergic projections to the nucleus accumbens are most likely involved (214).
Systemic injections of the potent CB1 receptor agonist HU-210 dose-dependently reinstate cocaine-seeking behavior in laboratory rats behaviorally extinguished from intravenous cocaine self-administration (198). Systemic injections of HU-210 also reinstate heroin-seeking behavior in laboratory rats behaviorally extinguished from intravenous heroin self-administration (199). Interestingly, however, the same research group found that the CB1 cannabinoid receptor antagonist SR-141716A blocked reinstatement to drug-seeking behavior triggered by cocaine, heroin, or cocaine-associated environmental cues, but not reinstatement induced by exposure to stress, suggesting a potential role for cannabinoid antagonists in the treatment of addiction. Cue-induced reinstatement to cocaine seeking has also been found when rats were administered with different doses of WIN55,212-2 (0.3, 1, and 3 mg/kg) during a 5-day extinction period. In this case, the lowest dose of WIN55,212-2 (0.3 mg/kg) induced the highest resistance to extinction and reinstatement (i.e., the highest responding at the active lever during conditioned-reinstatement) (211).
Interestingly, however, squirrel monkeys did not self-administer the FAAH inhibitor URB597, and the drug did not promote reinstatement of extinguished drug-seeking behavior previously maintained by Δ9-THC, anandamide, or cocaine (189). Further, reinstatement to Δ9-THC-seeking behavior does not seem to be affected by striatal adenosine receptors, as the selective adenosine A(2A) receptor antagonist MSX-3 (1 mg/kg) neither promoted reinstatement of extinguished drug-seeking behavior nor altered reinstatement of drug-seeking behavior by non-contingent priming injections of Δ9-THC (210).
In another study using psychostimulants (201), following 12 days of self-administration of methamphetamine (METH), under extinction conditions, METH-priming or re-exposure to cues previously paired with METH infusion triggered reinstatement of METH seeking. The cannabinoid CB1 receptor antagonist SR141716A blocked this effect, while administration of the cannabinoid agonist, Δ8-tetrahydrocannabinol (Δ8-THC), had no effect by itself, and co-administration of the Δ8-THC and METH at small doses reinstated the drug-seeking behavior. Interestingly, Δ8-THC attenuated the effects of the reinstatement-inducing dose of METH, but enhanced the effect of cues. Either given repeatedly during the extinction or singly, 24 h before the first METH-priming or cues challenge, Δ8-THC suppressed the reinstatement (201). These results suggest that the endocannabinoid system may be involved in the reinstating effects of METH-priming and cues. A follow-up study by the same group examined whether the reinstatement involves interactions between CB1 and nicotinic acetylcholine receptors (nAChRs) in the reinstatement of METH-seeking behavior (206). Systemic and intracranial administration of the potent CB1 receptor agonist HU210 into the nucleus accumbens core and prelimbic cortex reinstated METH-seeking behavior. The reinstatement caused by the systemic HU210 treatment was attenuated by intracranial administration of the CB1 receptor antagonist AM251 into the regions mentioned above, while reinstatement induced by the METH-associated cues and METH-priming injection was also attenuated by intracranial administration of AM251 in each region. Interestingly, in these regions, the attenuating effects of AM251 on the reinstatement induced by each stimulus were blocked by the intracranial administration of mecamylamine, a non-selective nAChR antagonist, but not by scopolamine, a muscarinic ACh receptor (mAChR) antagonist. Moreover, the intracranial administration of DHβE, an α4β2 nAChR antagonist, but not MLA, an α7 nAChR antagonist, into each region blocked the AM251-induced attenuation of the reinstatement. These findings suggest that reinstatement (or relapse in humans) to MAP-seeking behavior may be due to two steps: inhibition of ACh transmission by the activation of cannabinoid CB1 receptors and inactivation of α4β2 nAChRs (206). On the contrary, another study using AM251 did not modify METH-induced reinstatement of METH-seeking behavior (205).
The effects of the selective CB1 receptor antagonist AM251 have also been tested in nicotine-seeking behavior (207), where it has been found to dose-dependently (1–10 mg/kg) attenuate the reinstatement effects produced by both a nicotine priming dose (0.2 mg/kg) and its contingently presented cues.
Similarly to the studies presented above, Fattore and colleagues (200) showed that intraperitoneal priming injections of the potent non-selective CB1/CB2 receptor agonists WIN 55,212-2 (0.15 and 0.3 mg/kg) and CP 55,940 (0.05 and 0.1 mg/kg), but not Δ9-THC (0.1–1.0 mg/kg), effectively restored heroin-seeking behavior. In the same study, intraperitoneal priming injection of the CB1 receptor antagonist SR141716A (0.3 mg/kg) did not reinstate responding, but completely prevented heroin-induced reinstatement of drug-seeking behavior. Moreover, heroin-seeking behavior was still present for a few days following cannabinoid primings, indicating a long-lasting effect of cannabinoids on responding for heroin. These findings indicate that relapse to heroin after an extended drug-free period is triggered by cannabinoid agonists and that SR 141716A prevents drug-seeking behavior, suggesting that the use of the cannabinoid antagonists could have some therapeutic benefits in heroin-induced relapse (200). A follow-up study also presented similar findings (204). In continuation of the above study, a very interesting study from the same group showed that rats previously trained to intravenously self-administer the CB1 receptor agonist WIN 55,212-2 (12.5 μg/kg/inf) showed reinstatement in WIN 55,212-2-seeking behavior after priming injections of either the previously self-administered CB1 agonist (0.25 and 0.5 mg/kg) or heroin (0.5 mg/kg), but not cocaine (10 mg/kg), following 3 weeks of extinction. The selective CB1 receptor antagonist SR 141716A (0.3 mg/kg) did not reinstate responding when given alone, but completely prevented the cannabinoid-seeking behavior triggered by WIN 55,212-2 or heroin primings. Further, the non-selective opioid antagonist naloxone (1 mg/kg) had no effect on operant behavior per se, but significantly blocked cannabinoid- and heroin-induced reinstatement of cannabinoid-seeking behavior (202).
Most recently, WIN55,212-2-induced reinstatement of heroin-seeking behavior was significantly attenuated by naloxone (1 mg/kg) and rimonabant (3 mg/kg) and fully blocked by co-administration of sub-threshold doses of the two CB1 receptor antagonists. Moreover, contrary to immediate (1 day) or delayed (90 days) drug substitution, rats readily self-administered WIN when access was given after 7, 14, or 21 days of extinction from heroin, and showed a response rate that was positively correlated with the extinction period (209). Taken together, this set of data suggests some strong interactions between the cannabinoid and opioid systems in relapse mechanisms.
In relation to ethanol/alcohol-seeking behavior, Δ9-THC (1 mg/kg) significantly reinstated responding, previously reinforced with beer or near-beer (low alcohol beer) (203), while the anandamide transport inhibitor AM404 did not affect cue-induced reinstatement of alcohol-seeking behavior (213). On the other hand, perinatal administration of Δ9-THC (5 mg/kg, daily) either alone or in combination with ethanol (3% v/v) did not affect alcohol self-administration or alcohol seeking in any of the rat groups, while SR141716A (0.3–3.0 mg/kg) significantly reduced lever pressing for ethanol and blocked conditioned reinstatement of alcohol seeking, although the same doses of SR141716A failed to block foot-shock stress-induced reinstatement of alcohol seeking (212).
Finally, sex differences and ovarian hormones also appear to play a role in modulating cannabinoid-seeking behavior after exposure to drug priming or drug-asociated cues. In the study by Fattore and colleagues (208), after a priming dose of 0.15 or 0.3 mg/kg WIN55,212-2, intact female rats exhibited stronger reinstatement than males and ovariectomized females. Responses of intact female rats were higher than those of male and ovariectomized rats even after priming with a drug-associated visual or auditory cue, or a WIN55,212-2 + Cue combination (208).
In summary, the majority of the studies presented show that CB1 receptor agonists or endocannabinoid enhancers tend to promote either drug-, or cue-, or stress-induced reinstatement of drug-seeking behavior either to cannabinoid compounds or to other drugs of abuse. Overall, the above findings indicate that the endocannabinoid system, and in particular the CB1 receptors, play an important role in the processes underlying reinstatement to different drugs of abuse, such as psychostimulants (e.g., cocaine, methamphetamine, and nicotine), opioids (e.g., heroin) and alcohol. Further research will help clarify the mechanisms underlying these drug interactions and cross-priming effects in reinstatement processes.
Conclusion
Although the euphorigenic properties of cannabis preparations have been appreciated by humans for centuries, only the last years we have acquired the experimental tools to evaluate cannabinoid reward and abuse liability in experimental animals. It is now clear that cannabinoids exert emotional and motivational effects in experimental animals and can activate the same reward circuits in the brain and produce drug reinforcement/drug-seeking behavior, although under more limited conditions. The rewarding properties of Δ9-THC are clearly shown by a decrease in brain-stimulation reward thresholds and self-administration behavior. However, CB1 receptor agonists and endocannabinoid modulators (indirect agonists) do not affect the reinforcing efficacy of brain stimulation and are self-administered basically under particular experimental conditions. Moreover, contrasting findings have been shown in the CPP paradigm, where cannabinoids produce both positive (rewarding) and negative (aversive) effects, depending on the specific experimental procedures followed. Beyond any doubt, cannabinoids and the endocannabinoid system appear to be involved in reinstatement of extinguished self-administration of several drugs of abuse. Much remains to be done before we fully understand the actions of cannabinoids in critical areas of the reward circuit that mediate both rewarding and aversive phenomena and relapse mechanisms. Furthermore, since new cannabinoid-related medications are being developed, there will be a need to assess their potential rewarding actions and abuse liability, using the animal models and experimental procedures described here. The fact, for example, that enhancement of endocannabinoid neurotransmission does not increase brain reward, neither produces reward-related behaviors makes the drugs that directly affect endocannabinoid levels promising therapeutics, with less unwanted side-effects and minimal abuse potential.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by funding by the School of Nursing and Human Sciences, Dublin City University, Dublin, Ireland to Dr. Styliani Vlachou.
References
1. Budney AJ, Hughes JR. The cannabis withdrawal syndrome. Curr Opin Psychiatry (2006) 19:233–8. doi: 10.1097/01.yco.0000218592.00689.e5
2. Schulden JD, Thomas YF, Compton WM. Substance abuse in the United States: findings from recent epidemiologic studies. Curr Psychiatry Rep (2009) 11:353–9. doi:10.1007/s11920-009-0053-6
3. Ramo DE, Liu H, Prochaska JJ. Reliability and validity of young adults’ anonymous online reports of marijuana use and thoughts about use. Psychol Addict Behav (2012) 26(4):801–11. doi:10.1037/a0026201
4. Crippa JA, Hallak JE, Zuardi AW. Marijuana, feijoada and the debate on drug legalization. Front Psychiatry (2013) 4:7. doi:10.3389/fpsyt.2013.00007
5. Mechoulam R, Braun P, Gaoni Y. A stereospecific synthesis of (-)-delta 1- and (-)-delta 1(6)-tetrahydrocannabinols. J Am Chem Soc (1967) 89:4552–4. doi:10.1021/ja00993a072
6. Mechoulam R, Gaoni Y. The absolute configuration of delta-1-tetrahydrocannabinol, the major active constituent of hashish. Tetrahedron Lett (1967) 12:1109–11. doi:10.1016/S0040-4039(00)90646-4
7. Gaoni Y, Mechoulam R. The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. J Am Chem Soc (1971) 93:217–24.
8. Cooper ZD, Haney M. Actions of delta-9-tetrahydrocannabinol in cannabis: relation to use, abuse, dependence. Int Rev Psychiatry (2009) 21:104–12. doi:10.1080/09540260902782752
9. Solinas M, Yasar S, Goldberg SR. Endocannabinoid system involvement in brain reward processes related to drug abuse. Pharmacol Res (2007) 56:393–405. doi:10.1016/j.phrs.2007.09.005
10. Panagis G, Vlachou S, Nomikos GG. Behavioral pharmacology of cannabinoids with a focus on preclinical models for studying reinforcing and dependence-producing properties. Curr Drug Abuse Rev (2008) 1:350–74. doi:10.2174/1874473710801030350
11. Vlachou S, Panagis G. Regulation of brain reward by the endocannabinoid system: a critical review of behavioral studies in animals. Curr Pharm Des (2014) 20:2072–88. doi:10.2174/13816128113199990433
12. Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci (2003) 4:873–84. doi:10.1038/nrn1247
13. De Petrocellis L, Cascio MG, Di Marzo V. The endocannabinoid system: a general view and latest additions. Br J Pharmacol (2004) 141:765–74. doi:10.1038/sj.bjp.0705666
14. Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov (2004) 3(9):771–84. doi:10.1038/nrd1495
15. Fonseca BM, Costa MA, Almada M, Correia-Da-Silva G, Teixeira NA. Endogenous cannabinoids revisited: a biochemistry perspective. Prostaglandins Other Lipid Mediat (2013) 102-103:13–30. doi:10.1016/j.prostaglandins.2013.02.002
16. Jonsson KO, Holt S, Fowler CJ. The endocannabinoid system: current pharmacological research and therapeutic possibilities. Basic Clin Pharmacol Toxicol (2006) 98:124–34. doi:10.1111/j.1742-7843.2006.pto_376.x
17. Mouslech Z, Valla V. Endocannabinoid system: an overview of its potential in current medical practice. Neuro Endocrinol Lett (2009) 30(2):153–79.
18. Howlett AC, Bidaut-Russell M, Devane WA, Melvin LS, Johnson MR, Herkenham M. The cannabinoid receptor: biochemical, anatomical and behavioral characterization. Trends Neurosci (1990) 13:420–3. doi:10.1016/0166-2236(90)90124-S
19. Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature (1990) 346:561–4. doi:10.1038/346561a0
20. Herkenham M, Lynn AB, Johnson MR, Melvin LS, De Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci (1991) 11:563–83.
21. Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature (1993) 365:61–5. doi:10.1038/365061a0
22. Howlett AC. The cannabinoid receptors. Prostaglandins Other Lipid Mediat (2002) 68-69:619–31. doi:10.1016/S0090-6980(02)00060-6
23. Ameri A. The effects of cannabinoids on the brain. Prog Neurobiol (1999) 58:315–48. doi:10.1016/S0301-0082(98)00087-2
24. Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, De Costa BR, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A (1990) 87:1932–6. doi:10.1073/pnas.87.5.1932
25. Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol (2013) 64:21–47. doi:10.1146/annurev-psych-113011-143739
26. Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience (1998) 83:393–411.
27. Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci (1999) 11:4213–25. doi:10.1046/j.1460-9568.1999.00847.x
28. Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science (2002) 296:678–82. doi:10.1126/science.1063545
29. Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol (2001) 60(1):155–63. doi:10.1124/mol.60.1.155
30. Begg M, Pacher P, Batkai S, Osei-Hyiaman D, Offertaler L, Mo FM, et al. Evidence for novel cannabinoid receptors. Pharmacol Ther (2005) 106:133–45. doi:10.1016/j.pharmthera.2004.11.005
31. Melis M, Muntoni AL, Pistis M. Endocannabinoids and the processing of value-related signals. Front Pharmacol (2012) 3:7. doi:10.3389/fphar.2012.00007
32. Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol (1995) 50:83–90. doi:10.1016/0006-2952(95)00109-D
33. Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun (1995) 215:89–97. doi:10.1006/bbrc.1995.2437
34. Castillo PE, Younts TJ, Chavez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron (2012) 76:70–81. doi:10.1016/j.neuron.2012.09.020
35. Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature (2001) 410(6828):588–92. doi:10.1038/35069076
36. Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature (1994) 372:686–91. doi:10.1038/372686a0
37. Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, et al. A biosynthetic pathway for anandamide. Proc Natl Acad Sci U S A (2006) 103:13345–50. doi:10.1073/pnas.0601832103
38. Murataeva N, Straiker A, Mackie K. Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. Br J Pharmacol (2014) 171:1379–91. doi:10.1111/bph.12411
39. Di Marzo V, Melck D, Bisogno T, De Petrocellis L. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci (1998) 21:521–8. doi:10.1016/S0166-2236(98)01283-1
40. Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature (1999) 400:452–7.
41. Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, et al. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci U S A (2001) 98:3662–5. doi:10.1073/pnas.061029898
42. Porter AC, Sauer JM, Knierman MD, Becker GW, Berna MJ, Bao J, et al. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J Pharmacol Exp Ther (2002) 301:1020–4. doi:10.1124/jpet.301.3.1020
43. Pertwee RG. Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict Biol (2008) 13:147–59. doi:10.1111/j.1369-1600.2008.00108.x
44. Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2). Pharmacol Rev (2010) 62:588–631. doi:10.1124/pr.110.003004
45. Goutopoulos A, Makriyannis A. From cannabis to cannabinergics: new therapeutic opportunities. Pharmacol Ther (2002) 95:103–17. doi:10.1016/S0163-7258(02)00250-4
46. Makriyannis A, Mechoulam R, Piomelli D. Therapeutic opportunities through modulation of the endocannabinoid system. Neuropharmacology (2005) 48:1068–71. doi:10.1016/j.neuropharm.2005.03.012
47. Pertwee RG. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci (2005) 76:1307–24. doi:10.1016/j.lfs.2004.10.025
48. Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev (2002) 54:161–202. doi:10.1124/pr.54.2.161
49. Mavromoustakos T, Theodoropoulou E, Zervou M, Kourouli T, Papahatjis D. Structure elucidation and conformational properties of synthetic cannabinoids (-)-2-(6a,7,10,10a-tetrahydro-6,6,9-trimethyl-1-hydroxy-6H-dibe nzo [b,d]pyranyl)-2-hexyl-1,3-dithiolane and its methylated analog. J Pharm Biomed Anal (1999) 18:947–56. doi:10.1016/S0731-7085(98)00100-9
50. Pertwee RG. The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obes (Lond) (2006) 30(Suppl 1):S13–8. doi:10.1038/sj.ijo.0803272
51. Gatley SJ, Lan R, Pyatt B, Gifford AN, Volkow ND, Makriyannis A. Binding of the non-classical cannabinoid CP 55,940, and the diarylpyrazole AM251 to rodent brain cannabinoid receptors. Life Sci (1997) 61:191–7. doi:10.1016/S0024-3205(97)00690-5
52. Devane WA, Breuer A, Sheskin T, Jarbe TU, Eisen MS, Mechoulam R. A novel probe for the cannabinoid receptor. J Med Chem (1992) 35:2065–9. doi:10.1021/jm00089a018
53. Khanolkar AD, Abadji V, Lin S, Hill WA, Taha G, Abouzid K, et al. Head group analogs of arachidonylethanolamide, the endogenous cannabinoid ligand. J Med Chem (1996) 39:4515–9. doi:10.1021/jm960152y
54. Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett (1994) 350:240–4. doi:10.1016/0014-5793(94)00773-X
55. Gatley SJ, Gifford AN, Volkow ND, Lan R, Makriyannis A. 123I-labeled AM251: a radioiodinated ligand which binds in vivo to mouse brain cannabinoid CB1 receptors. Eur J Pharmacol (1996) 307:331–8. doi:10.1016/0014-2999(96)00279-8
56. Ruiu S, Pinna GA, Marchese G, Mussinu JM, Saba P, Tambaro S, et al. Synthesis and characterization of NESS 0327: a novel putative antagonist of the CB1 cannabinoid receptor. J Pharmacol Exp Ther (2003) 306:363–70. doi:10.1124/jpet.103.049924
57. Hosohata K, Quock RM, Hosohata Y, Burkey TH, Makriyannis A, Consroe P, et al. AM630 is a competitive cannabinoid receptor antagonist in the guinea pig brain. Life Sci (1997) 61:L115–8. doi:10.1016/S0024-3205(97)00596-1
58. Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, et al. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther (1998) 284:644–50.
59. Ross RA, Brockie HC, Stevenson LA, Murphy VL, Templeton F, Makriyannis A, et al. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. Br J Pharmacol (1999) 126:665–72. doi:10.1038/sj.bjp.0702351
61. Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol (2007) 150:613–23. doi:10.1038/sj.bjp.0707133
62. Cravatt BF, Lichtman AH. Fatty acid amide hydrolase: an emerging therapeutic target in the endocannabinoid system. Curr Opin Chem Biol (2003) 7:469–75. doi:10.1016/S1367-5931(03)00079-6
63. Di Marzo V, De Petrocellis L, Bisogno T. The biosynthesis, fate and pharmacological properties of endocannabinoids. Handb Exp Pharmacol (2005) 168:147–85. doi:10.1007/3-540-26573-2_5
64. Ortega-Gutierrez S. Therapeutic perspectives of inhibitors of endocannabinoid degradation. Curr Drug Targets CNS Neurol Disord (2005) 4:697–707. doi:10.2174/156800705774933032
65. Deutsch DG, Lin S, Hill WA, Morse KL, Salehani D, Arreaza G, et al. Fatty acid sulfonyl fluorides inhibit anandamide metabolism and bind to the cannabinoid receptor. Biochem Biophys Res Commun (1997) 231:217–21. doi:10.1006/bbrc.1997.6072
66. Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med (2003) 9:76–81. doi:10.1038/nm803
67. Fowler CJ, Tiger G, Ligresti A, Lopez-Rodriguez ML, Di Marzo V. Selective inhibition of anandamide cellular uptake versus enzymatic hydrolysis – a difficult issue to handle. Eur J Pharmacol (2004) 492:1–11. doi:10.1016/j.ejphar.2004.03.048
68. Blankman JL, Cravatt BF. Chemical probes of endocannabinoid metabolism. Pharmacol Rev (2013) 65:849–71. doi:10.1124/pr.112.006387
69. Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A (2001) 98:9371–6. doi:10.1073/pnas.161191698
70. Kunos G, Batkai S. Novel physiologic functions of endocannabinoids as revealed through the use of mutant mice. Neurochem Res (2001) 26:1015–21. doi:10.1023/A:1012301021419
71. Valverde O, Karsak M, Zimmer A. Analysis of the endocannabinoid system by using CB1 cannabinoid receptor knockout mice. Handb Exp Pharmacol (2005) 168:117–45. doi:10.1007/3-540-26573-2_4
72. Maldonado R, Robledo P, Berrendero F. Endocannabinoid system and drug addiction: new insights from mutant mice approaches. Curr Opin Neurobiol (2013) 23:480–6. doi:10.1016/j.conb.2013.02.004
73. Jung KM, Clapper JR, Fu J, D’Agostino G, Guijarro A, Thongkham D, et al. 2-arachidonoylglycerol signaling in forebrain regulates systemic energy metabolism. Cell Metab (2012) 15:299–310. doi:10.1016/j.cmet.2012.01.021
74. Carlezon WA Jr., Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc (2007) 2:2987–95. doi:10.1038/nprot.2007.441
75. Vlachou S, Markou A. The use of the intracranial self-stimulation in drug abuse research. In: Olmstead MC, editor. Animal Models of Drug Addiction. Neuromethods. (Vol. 53), New York: Humana Press and Springer Science (2011). p. 3–56.
76. Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol (1954) 47:419–27. doi:10.1037/h0058775
77. Bozarth MA, Gerber GJ, Wise RA. Intracranial self-stimulation as a technique to study the reward properties of drugs of abuse. Pharmacol Biochem Behav (1980) 13(Suppl 1):245–7. doi:10.1016/S0091-3057(80)80037-2
78. Wise RA. Action of drugs of abuse on brain reward systems. Pharmacol Biochem Behav (1980) 13(Suppl 1):213–23.
79. Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci (1996) 19:319–40. doi:10.1146/annurev.ne.19.030196.001535
80. Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depend (1998) 51:13–22. doi:10.1016/S0376-8716(98)00063-5
82. Kucharski LT, Williams JE, Kornetsky C. The effects of levonantradol on rewarding brain stimulation thresholds in the rat. Pharmacol Biochem Behav (1983) 19:149–51. doi:10.1016/0091-3057(83)90324-6
83. Gardner EL, Paredes W, Smith D, Donner A, Milling C, Cohen D, et al. Facilitation of brain stimulation reward by delta 9-tetrahydrocannabinol. Psychopharmacology (Berl) (1988) 96:142–4.
84. Gardner EL, Paredes W, Smith D, Zukin RS. Facilitation of brain stimulation reward by delta-9-tetrahydrocannabinol is mediated by an endogenous opioid mechanism. In: Cros J, Meunier JC, Hamon M, editors. Progress in Opioid Research (Series title: Advances in the Biosciences, Vol. 75). Oxford: Pergamon Press (1989). p. 671–4.
85. Lepore M, Liu X, Savage V, Matalon D, Gardner EL. Genetic differences in delta 9-tetrahydrocannabinol-induced facilitation of brain stimulation reward as measured by a rate-frequency curve-shift electrical brain stimulation paradigm in three different rat strains. Life Sci (1996) 58:L365–72. doi:10.1016/0024-3205(96)00237-8
86. Arnold JC, Hunt GE, Mcgregor IS. Effects of the cannabinoid receptor agonist CP 55,940 and the cannabinoid receptor antagonist SR 141716 on intracranial self-stimulation in Lewis rats. Life Sci (2001) 70:97–108. doi:10.1016/S0024-3205(01)01366-2
87. Deroche-Gamonet V, Le Moal M, Piazza PV, Soubrie P. SR141716, a CB1 receptor antagonist, decreases the sensitivity to the reinforcing effects of electrical brain stimulation in rats. Psychopharmacology (Berl) (2001) 157:254–9. doi:10.1007/s002130100804
88. Vlachou S, Nomikos GG, Panagis G. WIN 55,212-2 decreases the reinforcing actions of cocaine through CB1 cannabinoid receptor stimulation. Behav Brain Res (2003) 141:215–22. doi:10.1016/S0166-4328(02)00370-4
89. Vlachou S, Nomikos GG, Panagis G. CB1 cannabinoid receptor agonists increase intracranial self-stimulation thresholds in the rat. Psychopharmacology (Berl) (2005) 179:498–508. doi:10.1007/s00213-004-2050-0
90. Antoniou K, Galanopoulos A, Vlachou S, Kourouli T, Nahmias V, Thermos K, et al. Behavioral pharmacological properties of a novel cannabinoid 1′,1′-dithiolane delta8-THC analog, AMG-3. Behav Pharmacol (2005) 16:499–510. doi:10.1097/00008877-200509000-00024
91. Vlachou S, Nomikos GG, Panagis G. Effects of endocannabinoid neurotransmission modulators on brain stimulation reward. Psychopharmacology (Berl) (2006) 188:293–305. doi:10.1007/s00213-006-0506-0
92. Xi ZX, Spiller K, Pak AC, Gilbert J, Dillon C, Li X, et al. Cannabinoid CB1 receptor antagonists attenuate cocaine’s rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropsychopharmacology (2008) 33:1735–45. doi:10.1038/sj.npp.1301552
93. Vlachou S, Nomikos GG, Stephens DN, Panagis G. Lack of evidence for appetitive effects of Delta 9-tetrahydrocannabinol in the intracranial self-stimulation and conditioned place preference procedures in rodents. Behav Pharmacol (2007) 18(4):311–9. doi:10.1097/FBP.0b013e3282186cf2
94. Trujillo-Pisanty I, Hernandez G, Moreau-Debord I, Cossette MP, Conover K, Cheer JF, et al. Cannabinoid receptor blockade reduces the opportunity cost at which rats maintain operant performance for rewarding brain stimulation. J Neurosci (2011) 31:5426–35. doi:10.1523/JNEUROSCI.0079-11.2011
95. Kwilasz AJ, Negus SS. Dissociable effects of the cannabinoid receptor agonists Delta9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats. J Pharmacol Exp Ther (2012) 343:389–400. doi:10.1124/jpet.112.197780
96. Katsidoni V, Kastellakis A, Panagis G. Biphasic effects of Delta9-tetrahydrocannabinol on brain stimulation reward and motor activity. Int J Neuropsychopharmacol (2013) 16:2273–84. doi:10.1017/S1461145713000709
97. Kwilasz AJ, Abdullah RA, Poklis JL, Lichtman AH, Negus SS. Effects of the fatty acid amide hydrolase inhibitor URB597 on pain-stimulated and pain-depressed behavior in rats. Behav Pharmacol (2014) 25:119–29. doi:10.1097/FBP.0000000000000023
98. Fokos S, Panagis G. Effects of delta9-tetrahydrocannabinol on reward and anxiety in rats exposed to chronic unpredictable stress. J Psychopharmacol (2010) 24:767–77. doi:10.1177/0269881109104904
99. Vlachou S, Stamatopoulou F, Nomikos GG, Panagis G. Enhancement of endocannabinoid neurotransmission through CB1 cannabinoid receptors counteracts the reinforcing and psychostimulant effects of cocaine. Int J Neuropsychopharmacol (2008) 11:905–23. doi:10.1017/S1461145708008717
100. Landsman RS, Burkey TH, Consroe P, Roeske WR, Yamamura HI. SR141716A is an inverse agonist at the human cannabinoid CB1 receptor. Eur J Pharmacol (1997) 334:R1–2. doi:10.1016/S0014-2999(97)01160-6
101. De Vry J, Jentzsch KR. Intrinsic activity estimation of cannabinoid CB1 receptor ligands in a drug discrimination paradigm. Behav Pharmacol (2003) 14(5–6):471–6. doi:10.1097/01.fbp.0000087739.21047.d8
102. Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) (2000) 153:31–43. doi:10.1007/s002130000569
103. Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol (2006) 11(1):2–38. doi:10.1111/j.1369-1600.2006.00012.x
104. Lepore M, Vorel SR, Lowinson J, Gardner EL. Conditioned place preference induced by delta 9-tetrahydrocannabinol: comparison with cocaine, morphine, and food reward. Life Sci (1995) 56:2073–80. doi:10.1016/0024-3205(95)00191-8
105. Parker LA, Gillies T. THC-induced place and taste aversions in Lewis and Sprague-Dawley rats. Behav Neurosci (1995) 109:71–8. doi:10.1037/0735-7044.109.1.71
106. McGregor IS, Issakidis CN, Prior G. Aversive effects of the synthetic cannabinoid CP 55,940 in rats. Pharmacol Biochem Behav (1996) 53:657–64. doi:10.1016/0091-3057(95)02066-7
107. Sanudo-Pena MC, Tsou K, Delay ER, Hohman AG, Force M, Walker JM. Endogenous cannabinoids as an aversive or counter-rewarding system in the rat. Neurosci Lett (1997) 223:125–8. doi:10.1016/S0304-3940(97)13424-3
108. Chaperon F, Soubrie P, Puech AJ, Thiebot MH. Involvement of central cannabinoid (CB1) receptors in the establishment of place conditioning in rats. Psychopharmacology (Berl) (1998) 135:324–32. doi:10.1007/s002130050518
109. Hutcheson DM, Tzavara ET, Smadja C, Valjent E, Roques BP, Hanoune J, et al. Behavioural and biochemical evidence for signs of abstinence in mice chronically treated with delta-9-tetrahydrocannabinol. Br J Pharmacol (1998) 125:1567–77. doi:10.1038/sj.bjp.0702228
110. Mallet PE, Beninger RJ. Delta9-tetrahydrocannabinol, but not the endogenous cannabinoid receptor ligand anandamide, produces conditioned place avoidance. Life Sci (1998) 62:2431–9. doi:10.1016/S0024-3205(98)00226-4
111. Cheer JF, Kendall DA, Marsden CA. Cannabinoid receptors and reward in the rat: a conditioned place preference study. Psychopharmacology (Berl) (2000) 151(1):25–30. doi:10.1007/s002130000481
112. Valjent E, Maldonado R. A behavioural model to reveal place preference to delta 9-tetrahydrocannabinol in mice. Psychopharmacology (Berl) (2000) 147:436–8. doi:10.1007/s002130050013
113. Braida D, Pozzi M, Cavallini R, Sala M. Conditioned place preference induced by the cannabinoid agonist CP 55,940: interaction with the opioid system. Neuroscience (2001) 104:923–6. doi:10.1016/S0306-4522(01)00210-X
114. Braida D, Pozzi M, Parolaro D, Sala M. Intracerebral self-administration of the cannabinoid receptor agonist CP 55,940 in the rat: interaction with the opioid system. Eur J Pharmacol (2001) 413:227–34. doi:10.1016/S0014-2999(01)00766-X
115. Zimmer A, Valjent E, Konig M, Zimmer AM, Robledo P, Hahn H, et al. Absence of delta -9-tetrahydrocannabinol dysphoric effects in dynorphin-deficient mice. J Neurosci (2001) 21:9499–505.
116. Robinson L, Hinder L, Pertwee RG, Riedel G. Effects of delta9-THC and WIN-55,212-2 on place preference in the water maze in rats. Psychopharmacology (Berl) (2003) 166(1):40–50. doi:10.1007/s00213-002-1302-0
117. Braida D, Iosue S, Pegorini S, Sala M. Delta9-tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. Eur J Pharmacol (2004) 506:63–9. doi:10.1016/j.ejphar.2004.10.043
118. Castane A, Maldonado R, Valverde O. Role of different brain structures in the behavioural expression of WIN 55,212-2 withdrawal in mice. Br J Pharmacol (2004) 142:1309–17. doi:10.1038/sj.bjp.0705882
119. Soria G, Castane A, Berrendero F, Ledent C, Parmentier M, Maldonado R, et al. Adenosine A2A receptors are involved in physical dependence and place conditioning induced by THC. Eur J Neurosci (2004) 20:2203–13. doi:10.1111/j.1460-9568.2004.03682.x
120. Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A (2005) 102:18620–5. doi:10.1073/pnas.0509591102
121. Bortolato M, Campolongo P, Mangieri RA, Scattoni ML, Frau R, Trezza V, et al. Anxiolytic-like properties of the anandamide transport inhibitor AM404. Neuropsychopharmacology (2006) 31:2652–9. doi:10.1038/sj.npp.1301061
122. Le Foll B, Wiggins M, Goldberg SR. Nicotine pre-exposure does not potentiate the locomotor or rewarding effects of Delta-9-tetrahydrocannabinol in rats. Behav Pharmacol (2006) 17:195–9. doi:10.1097/01.fbp.0000197460.16516.81
123. Scherma M, Medalie J, Fratta W, Vadivel SK, Makriyannis A, Piomelli D, et al. The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology (2008) 54:129–40. doi:10.1016/j.neuropharm.2007.08.011
124. Vann RE, Gamage TF, Warner JA, Marshall EM, Taylor NL, Martin BR, et al. Divergent effects of cannabidiol on the discriminative stimulus and place conditioning effects of Delta(9)-tetrahydrocannabinol. Drug Alcohol Depend (2008) 94:191–8. doi:10.1016/j.drugalcdep.2007.11.017
125. Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaran N, et al. Adolescent rats find repeated Delta(9)-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology (2008) 33:1113–26. doi:10.1038/sj.npp.1301475
126. Pandolfo P, Vendruscolo LF, Sordi R, Takahashi RN. Cannabinoid-induced conditioned place preference in the spontaneously hypertensive rat-an animal model of attention deficit hyperactivity disorder. Psychopharmacology (Berl) (2009) 205:319–26. doi:10.1007/s00213-009-1542-3
127. Budzynska B, Kruk M, Biala G. Effects of the cannabinoid CB1 receptor antagonist AM 251 on the reinstatement of nicotine-conditioned place preference by drug priming in rats. Pharmacol Rep (2009) 61:304–10. doi:10.1016/S1734-1140(09)70036-2
128. Biala G, Budzynska B, Staniak N. Effects of rimonabant on the reinstatement of nicotine-conditioned place preference by drug priming in rats. Behav Brain Res (2009) 202:260–5. doi:10.1016/j.bbr.2009.03.042
129. Manzanedo C, Rodriguez-Arias M, Daza-Losada M, Maldonado C, Aguilar MA, Minarro J. Effect of the CB1 cannabinoid agonist WIN 55212-2 on the acquisition and reinstatement of MDMA-induced conditioned place preference in mice. Behav Brain Funct (2010) 6:19. doi:10.1186/1744-9081-6-19
130. Ramiro-Fuentes S, Ortiz O, Moratalla R, Fernandez-Espejo E. Intra-accumbens rimonabant is rewarding but induces aversion to cocaine in cocaine-treated rats, as does in vivo accumbal cannabinoid CB1 receptor silencing: critical role for glutamate receptors. Neuroscience (2010) 167:205–15. doi:10.1016/j.neuroscience.2010.02.019
131. Liu Z, Han J, Jia L, Maillet JC, Bai G, Xu L, et al. Synaptic neurotransmission depression in ventral tegmental dopamine neurons and cannabinoid-associated addictive learning. PLoS One (2010) 5:e15634. doi:10.1371/journal.pone.0015634
132. Klein C, Karanges E, Spiro A, Wong A, Spencer J, Huynh T, et al. Cannabidiol potentiates Delta(9)-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology (Berl) (2011) 218:443–57. doi:10.1007/s00213-011-2342-0
133. Fang Q, Li FQ, Li YQ, Xue YX, He YY, Liu JF, et al. Cannabinoid CB1 receptor antagonist rimonabant disrupts nicotine reward-associated memory in rats. Pharmacol Biochem Behav (2011) 99:738–42. doi:10.1016/j.pbb.2011.06.019
134. Han J, Liu Z, Ren W, Zhang X. Counteractive effects of cannabinoid and nicotine-addictive behavior. Neuroreport (2011) 22:181–4. doi:10.1097/WNR.0b013e328343f5cb
135. Rezayof A, Sardari M, Zarrindast MR, Nayer-Nouri T. Functional interaction between morphine and central amygdala cannabinoid CB1 receptors in the acquisition and expression of conditioned place preference. Behav Brain Res (2011) 220:1–8. doi:10.1016/j.bbr.2011.01.023
136. Scherma M, Justinova Z, Zanettini C, Panlilio LV, Mascia P, Fadda P, et al. The anandamide transport inhibitor AM404 reduces the rewarding effects of nicotine and nicotine-induced dopamine elevations in the nucleus accumbens shell in rats. Br J Pharmacol (2012) 165:2539–48. doi:10.1111/j.1476-5381.2011.01467.x
137. Rezayof A, Ghandipour M, Nazari-Serenjeh F. Effect of co-injection of arachydonilcyclopropylamide and ethanol on conditioned place preference in rats. Physiol Behav (2012) 107:301–8. doi:10.1016/j.physbeh.2012.08.009
138. Karimi S, Azizi P, Shamsizadeh A, Haghparast A. Role of intra-accumbal cannabinoid CB1 receptors in the potentiation, acquisition and expression of morphine-induced conditioned place preference. Behav Brain Res (2013) 247:125–31. doi:10.1016/j.bbr.2013.03.022
139. Rashidy-Pour A, Pahlevani P, Vaziri A, Shaigani P, Zarepour L, Vafaei AA, et al. Involvement of CB1 receptors in the ventral tegmental area in the potentiation of morphine rewarding properties in acquisition but not expression in the conditioned place preference model. Behav Brain Res (2013) 247:259–67. doi:10.1016/j.bbr.2013.03.015
140. Hyatt WS, Fantegrossi WE. Delta9-THC exposure attenuates aversive effects and reveals appetitive effects of K2/‘Spice’ constituent JWH-018 in mice. Behav Pharmacol (2014) 25:253–7. doi:10.1097/FBP.0000000000000034
141. Gallo A, Bouchard C, Rompre PP. Animals with a schizophrenia-like phenotype are differentially sensitive to the motivational effects of cannabinoid agonists in conditioned place preference. Behav Brain Res (2014) 268:202–12. doi:10.1016/j.bbr.2014.04.020
142. Polissidis A, Chouliara O, Galanopoulos A, Marselos M, Papadopoulou-Daifoti Z, Antoniou K. Behavioural and dopaminergic alterations induced by a low dose of WIN 55,212-2 in a conditioned place preference procedure. Life Sci (2009) 85:248–54. doi:10.1016/j.lfs.2009.05.015
143. Schuster CR, Thompson T. Self administration of and behavioral dependence on drugs. Annu Rev Pharmacol (1969) 9:483–502. doi:10.1146/annurev.pa.09.040169.002411
144. Griffiths RR. Common factors in human and infrahuman drug self-administration. Psychopharmacol Bull (1980) 16:45–7.
145. Henningfield JE, Cohen C, Heishman SJ. Drug self-administration methods in abuse liability evaluation. Br J Addict (1991) 86:1571–7. doi:10.1111/j.1360-0443.1991.tb01750.x
146. Gardner EL. What we have learned about addiction from animal models of drug self-administration. Am J Addict (2000) 9:285–313. doi:10.1080/105504900750047355
147. Vlachou S, Markou A. GABAB receptors in reward processes. Adv Pharmacol (2010) 58:315–71. doi:10.1016/S1054-3589(10)58013-X
148. Wise RA, Bozarth MA. Brain substrates for reinforcement and drug self-administration. Prog Neuropsychopharmacol (1981) 5:467–74. doi:10.1016/0364-7722(81)90028-X
149. Corcoran ME, Amit Z. Reluctance of rats to drink hashish suspensions: free-choice and forced consumption, and the effects of hypothalamic stimulation. Psychopharmacologia (1974) 35:129–47. doi:10.1007/BF00429580
150. Harris RT, Waters W, Mclendon D. Evaluation of reinforcing capability of delta-9-tetrahydrocannabinol in rhesus monkeys. Psychopharmacologia (1974) 37:23–9. doi:10.1007/BF00426679
151. Leite JR, Carlini EA. Failure to obtain “cannabis-directed behavior” and abstinence syndrome in rats chronically treated with cannabis sativa extracts. Psychopharmacologia (1974) 36:133–45. doi:10.1007/BF00421785
152. Carney JM, Uwaydah IM, Balster RL. Evaluation of a suspension system for intravenous self-administration studies of water-insoluble compounds in the rhesus monkey. Pharmacol Biochem Behav (1977) 7:357–64. doi:10.1016/0091-3057(77)90232-5
153. van Ree JM, Slangen JL, De Wied D. Intravenous self-administration of drugs in rats. J Pharmacol Exp Ther (1978) 204:547–57.
154. Young AM, Katz JL, Woods JH. Behavioral effects of levonantradol and nantradol in the rhesus monkey. J Clin Pharmacol (1981) 21:348S–60S. doi:10.1002/j.1552-4604.1981.tb02614.x
155. Mansbach RS, Nicholson KL, Martin BR, Balster RL. Failure of Delta(9)-tetrahydrocannabinol and CP 55,940 to maintain intravenous self-administration under a fixed-interval schedule in rhesus monkeys. Behav Pharmacol (1994) 5:219–25. doi:10.1097/00008877-199404000-00014
156. Deneau G, Kaymakcalan S. Physiological and psychological dependence to synthetic δ9-tetrahydrocannabinol (THC) in rhesus monkeys. Pharmacologist (1971) 13:246–8.
158. Takahashi RN, Singer G. Self-administration of delta 9-tetrahydrocannabinol by rats. Pharmacol Biochem Behav (1979) 11:737–40. doi:10.1016/0091-3057(79)90274-0
159. Takahashi RN, Singer G. Effects of body weight levels on cannabis self-injection self-administration of delta 9-tetrahydrocannabinol by rats. Pharmacol Biochem Behav (1980) 13:877–81. doi:10.1016/0091-3057(80)90222-1
160. de la Garza R, Johanson CE. The effects of food deprivation on the self-administration of psychoactive drugs. Drug Alcohol Depend (1987) 19:17–27. doi:10.1016/0376-8716(87)90083-4
161. Pickens R, Thompson T, Muchow DC. Cannabis and phencyclidine self-administration by animals. In: Goldfarb L, Hoffmeister F, editors. Psychic Dependence [Bayer Symposium IV]. Berlin Heidelberg New York: Springer (1973). p. 78–86.
162. Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci (2000) 3:1073–4. doi:10.1038/80577
163. Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci (2011) 34:411–20. doi:10.1016/j.tins.2011.06.001
164. Martellotta MC, Cossu G, Fattore L, Gessa GL, Fratta W. Self-administration of the cannabinoid receptor agonist WIN 55,212-2 in drug-naive mice. Neuroscience (1998) 85:327–30. doi:10.1016/S0306-4522(98)00052-9
165. Fattore L, Cossu G, Martellotta CM, Fratta W. Intravenous self-administration of the cannabinoid CB1 receptor agonist WIN 55,212-2 in rats. Psychopharmacology (Berl) (2001) 156:410–6. doi:10.1007/s002130100734
166. Justinova Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology (Berl) (2003) 169:135–40. doi:10.1007/s00213-003-1484-0
167. Justinova Z, Tanda G, Munzar P, Goldberg SR. The opioid antagonist naltrexone reduces the reinforcing effects of Delta 9 tetrahydrocannabinol (THC) in squirrel monkeys. Psychopharmacology (Berl) (2004) 173:186–94. doi:10.1007/s00213-003-1693-6
168. Drews E, Schneider M, Koch M. Effects of the cannabinoid receptor agonist WIN 55,212-2 on operant behavior and locomotor activity in rats. Pharmacol Biochem Behav (2005) 80:145–50. doi:10.1016/j.pbb.2004.10.023
169. Justinova Z, Solinas M, Tanda G, Redhi GH, Goldberg SR. The endogenous cannabinoid anandamide and its synthetic analog R(+)-methanandamide are intravenously self-administered by squirrel monkeys. J Neurosci (2005) 25:5645–50. doi:10.1523/JNEUROSCI.0951-05.2005
170. Zangen A, Solinas M, Ikemoto S, Goldberg SR, Wise RA. Two brain sites for cannabinoid reward. J Neurosci (2006) 26:4901–7. doi:10.1523/JNEUROSCI.3554-05.2006
171. Mendizabal V, Zimmer A, Maldonado R. Involvement of kappa/dynorphin system in WIN 55,212-2 self-administration in mice. Neuropsychopharmacology (2006) 31:1957–66. doi:10.1038/sj.npp.1300957
172. Lecca D, Cacciapaglia F, Valentini V, Di Chiara G. Monitoring extracellular dopamine in the rat nucleus accumbens shell and core during acquisition and maintenance of intravenous WIN 55,212-2 self-administration. Psychopharmacology (Berl) (2006) 188:63–74. doi:10.1007/s00213-006-0475-3
173. Fadda P, Scherma M, Spano MS, Salis P, Melis V, Fattore L, et al. Cannabinoid self-administration increases dopamine release in the nucleus accumbens. Neuroreport (2006) 17:1629–32. doi:10.1097/01.wnr.0000236853.40221.8e
174. Fattore L, Vigano D, Fadda P, Rubino T, Fratta W, Parolaro D. Bidirectional regulation of mu-opioid and CB1-cannabinoid receptor in rats self-administering heroin or WIN 55,212-2. Eur J Neurosci (2007) 25:2191–200. doi:10.1111/j.1460-9568.2007.05470.x
175. Justinova Z, Yasar S, Redhi GH, Goldberg SR. The endogenous cannabinoid 2-arachidonoylglycerol is intravenously self-administered by squirrel monkeys. J Neurosci (2011) 31:7043–8. doi:10.1523/JNEUROSCI.6058-10.2011
176. Li JX, Koek W, France CP. Interactions between Delta(9)-tetrahydrocannabinol and heroin: self-administration in rhesus monkeys. Behav Pharmacol (2012) 23:754–61. doi:10.1097/FBP.0b013e32835a3907
177. Justinova Z, Mascia P, Wu HQ, Secci ME, Redhi GH, Panlilio LV, et al. Reducing cannabinoid abuse and preventing relapse by enhancing endogenous brain levels of kynurenic acid. Nat Neurosci (2013) 16:1652–61. doi:10.1038/nn.3540
178. Flores A, Maldonado R, Berrendero F. The hypocretin/orexin receptor-1 as a novel target to modulate cannabinoid reward. Biol Psychiatry (2014) 75:499–507. doi:10.1016/j.biopsych.2013.06.012
179. Lefever TW, Marusich JA, Antonazzo KR, Wiley JL. Evaluation of WIN 55,212-2 self-administration in rats as a potential cannabinoid abuse liability model. Pharmacol Biochem Behav (2014) 118:30–5. doi:10.1016/j.pbb.2014.01.002
180. Justinova Z, Redhi GH, Goldberg SR, Ferre S. Differential effects of presynaptic versus postsynaptic adenosine A2A receptor blockade on Delta9-tetrahydrocannabinol (THC) self-administration in squirrel monkeys. J Neurosci (2014) 34:6480–4. doi:10.1523/JNEUROSCI.5073-13.2014
181. Bergman J, Johanson CE. The reinforcing properties of diazepam under several conditions in the rhesus monkey. Psychopharmacology (Berl) (1985) 86:108–13.
182. Carroll ME, France CP, Meisch RA. Food deprivation increases oral and intravenous drug intake in rats. Science (1979) 205:319–21. doi:10.1126/science.36665
183. Cabeza de Vaca S, Carr KD. Food restriction enhances the central rewarding effect of abused drugs. J Neurosci (1998) 18:7502–10.
184. Campbell UC, Carroll ME. Reduction of drug self-administration by an alternative non-drug reinforcer in rhesus monkeys: magnitude and temporal effects. Psychopharmacology (Berl) (2000) 147:418–25. doi:10.1007/s002130050011
185. Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav (2002) 76:353–64. doi:10.1016/S0031-9384(02)00759-X
186. Justinova Z, Munzar P, Panlilio LV, Yasar S, Redhi GH, Tanda G, et al. Blockade of THC-seeking behavior and relapse in monkeys by the cannabinoid CB(1)-receptor antagonist rimonabant. Neuropsychopharmacology (2008) 33:2870–7. doi:10.1038/npp.2008.21
187. Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science (1999) 283:401–4.
188. Navarro M, Carrera MR, Fratta W, Valverde O, Cossu G, Fattore L, et al. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J Neurosci (2001) 21(14):5344–50.
189. Justinova Z, Mangieri RA, Bortolato M, Chefer SI, Mukhin AG, Clapper JR, et al. Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biol Psychiatry (2008) 64:930–7. doi:10.1016/j.biopsych.2008.08.008
190. Gardner EL. Addictive potential of cannabinoids: the underlying neurobiology. Chem Phys Lipids (2002) 121:267–90. doi:10.1016/S0009-3084(02)00162-7
191. Fattore L, Cossu G, Spano MS, Deiana S, Fadda P, Scherma M, et al. Cannabinoids and reward: interactions with the opioid system. Crit Rev Neurobiol (2004) 16:147–58. doi:10.1615/CritRevNeurobiol.v16.i12.160
192. Yamamoto T, Anggadiredja K, Hiranita T. New perspectives in the studies on endocannabinoid and cannabis: a role for the endocannabinoid-arachidonic acid pathway in drug reward and long-lasting relapse to drug taking. J Pharmacol Sci (2004) 96:382–8. doi:10.1254/jphs.FMJ04003X5
193. Arnold JC. The role of endocannabinoid transmission in cocaine addiction. Pharmacol Biochem Behav (2005) 81:396–406. doi:10.1016/j.pbb.2005.02.015
194. Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav (2005) 81(2):263–84. doi:10.1016/j.pbb.2005.01.032
195. Le Foll B, Goldberg SR. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J Pharmacol Exp Ther (2005) 312:875–83. doi:10.1124/jpet.104.077974
196. Maccioni P, Colombo G, Carai MA. Blockade of the cannabinoid CB1 receptor and alcohol dependence: preclinical evidence and preliminary clinical data. CNS Neurol Disord Drug Targets (2010) 9:55–9. doi:10.2174/187152710790966623
197. Schenk S, Partridge B. Cocaine-seeking produced by experimenter-administered drug injections: dose-effect relationships in rats. Psychopharmacology (Berl) (1999) 147:285–90. doi:10.1007/s002130051169
198. De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, et al. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med (2001) 7:1151–4. doi:10.1038/nm1001-1151
199. De Vries TJ, Homberg JR, Binnekade R, Raaso H, Schoffelmeer AN. Cannabinoid modulation of the reinforcing and motivational properties of heroin and heroin-associated cues in rats. Psychopharmacology (Berl) (2003) 168:164–9. doi:10.1007/s00213-003-1422-1
200. Fattore L, Spano MS, Cossu G, Deiana S, Fratta W. Cannabinoid mechanism in reinstatement of heroin-seeking after a long period of abstinence in rats. Eur J Neurosci (2003) 17:1723–6. doi:10.1046/j.1460-9568.2003.02607.x
201. Anggadiredja K, Nakamichi M, Hiranita T, Tanaka H, Shoyama Y, Watanabe S, et al. Endocannabinoid system modulates relapse to methamphetamine seeking: possible mediation by the arachidonic acid cascade. Neuropsychopharmacology (2004) 29:1470–8. doi:10.1038/sj.npp.1300454
202. Spano MS, Fattore L, Cossu G, Deiana S, Fadda P, Fratta W. CB1 receptor agonist and heroin, but not cocaine, reinstate cannabinoid-seeking behaviour in the rat. Br J Pharmacol (2004) 143:343–50. doi:10.1038/sj.bjp.0705932
203. McGregor IS, Dam KD, Mallet PE, Gallate JE. Delta9-THC reinstates beer- and sucrose-seeking behaviour in abstinent rats: comparison with midazolam, food deprivation and predator odour. Alcohol Alcohol (2005) 40:35–45. doi:10.1093/alcalc/agh113
204. Fattore L, Spano S, Cossu G, Deiana S, Fadda P, Fratta W. Cannabinoid CB(1) antagonist SR 141716A attenuates reinstatement of heroin self-administration in heroin-abstinent rats. Neuropharmacology (2005) 48:1097–104. doi:10.1016/j.neuropharm.2005.01.022
205. Boctor SY, Martinez JL Jr., Koek W, France CP. The cannabinoid CB1 receptor antagonist AM251 does not modify methamphetamine reinstatement of responding. Eur J Pharmacol (2007) 571(1):39–43. doi:10.1016/j.ejphar.2007.06.004
206. Hiranita T, Nawata Y, Sakimura K, Yamamoto T. Methamphetamine-seeking behavior is due to inhibition of nicotinic cholinergic transmission by activation of cannabinoid CB1 receptors. Neuropharmacology (2008) 55:1300–6. doi:10.1016/j.neuropharm.2008.08.012
207. Shoaib M. The cannabinoid antagonist AM251 attenuates nicotine self-administration and nicotine-seeking behaviour in rats. Neuropharmacology (2008) 54(2):438–44.
208. Fattore L, Spano MS, Altea S, Fadda P, Fratta W. Drug- and cue-induced reinstatement of cannabinoid-seeking behaviour in male and female rats: influence of ovarian hormones. Br J Pharmacol (2010) 160:724–35. doi:10.1111/j.1476-5381.2010.00734.x
209. Fattore L, Spano M, Melis V, Fadda P, Fratta W. Differential effect of opioid and cannabinoid receptor blockade on heroin-seeking reinstatement and cannabinoid substitution in heroin-abstinent rats. Br J Pharmacol (2011) 163:1550–62. doi:10.1111/j.1476-5381.2011.01459.x
210. Justinova Z, Ferre S, Redhi GH, Mascia P, Stroik J, Quarta D, et al. Reinforcing and neurochemical effects of cannabinoid CB1 receptor agonists, but not cocaine, are altered by an adenosine A2A receptor antagonist. Addict Biol (2011) 16:405–15. doi:10.1111/j.1369-1600.2010.00258.x
211. Gonzalez-Cuevas G, Aujla H, Martin-Fardon R, Lopez-Moreno JA, Navarro M, Weiss F. Subchronic cannabinoid agonist (WIN 55,212-2) treatment during cocaine abstinence alters subsequent cocaine seeking behavior. Neuropsychopharmacology (2007) 32:2260–6. doi:10.1038/sj.npp.1301365
212. Economidou D, Mattioli L, Ubaldi M, Lourdusamy A, Soverchia L, Hardiman G, et al. Role of cannabinoidergic mechanisms in ethanol self-administration and ethanol seeking in rat adult offspring following perinatal exposure to Delta9-tetrahydrocannabinol. Toxicol Appl Pharmacol (2007) 223(1):73–85.
213. Cippitelli A, Bilbao A, Gorriti MA, Navarro M, Massi M, Piomelli D, et al. The anandamide transport inhibitor AM404 reduces ethanol self-administration. Eur J Neurosci (2007) 26:476–86. doi:10.1111/j.1460-9568.2007.05665.x
Keywords: cannabinoids, endocannabinoid system, brain-reward system, intracranial self-stimulation, self-administration, conditioned place preference, reinstatement of drug-seeking behavior, CB1 receptors
Citation: Panagis G, Mackey B and Vlachou S (2014) Cannabinoid regulation of brain reward processing with an emphasis on the role of CB1 receptors: a step back into the future. Front. Psychiatry 5:92. doi: 10.3389/fpsyt.2014.00092
Received: 20 May 2014; Accepted: 16 July 2014;
Published online: 31 July 2014.
Edited by:
Steven R. Laviolette, University of Western Ontario, CanadaReviewed by:
Joseph F. Cheer, University of Maryland School of Medicine, USASteven R. Laviolette, University of Western Ontario, Canada
Copyright: © 2014 Panagis, Mackey and Vlachou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Styliani Vlachou, Laboratory of Behavioural Neuroscience, School of Nursing and Human Sciences, Faculty of Science and Health, Dublin City University, Collins Avenue, Glasnevin, Dublin, Dublin 9, Ireland e-mail:c3RlbGxhLnZsYWNob3VAZGN1Lmll
 George Panagis
George Panagis Brian Mackey
Brian Mackey Styliani Vlachou
Styliani Vlachou