- 1National Institute on Drug Abuse Intramural Program, Molecular Neuropsychiatry Research Branch, Baltimore, MD, USA
- 2Instituto de Investigaciones Farmacológicas (ININFA), Universidad de Buenos Aires-CONICET, Buenos Aires, Argentina
Heavy use of drugs impacts of the daily activities of individuals in these activities. Several groups of investigators have indeed documented changes in cognitive performance by individuals who have a long history of chronic drug use. In the case of marijuana, a wealth of information suggests that heavy long-term use of the drug may have neurobehavioral consequences in some individuals. In humans, heavy cocaine use is accompanied by neuropathological changes that might serve as substrates for cognitive dysfunctions. Similarly, methamphetamine users suffer from cognitive abnormalities that may be consequent to alterations in structures and functions. Here, we detail the evidence for these neuropsychological consequences. The review suggests that improving the care of our patients will necessarily depend on the better characterization of drug-induced cognitive phenotypes because they might inform the development of better pharmacological and behavioral interventions, with the goal of improving cognitive functions in these subsets of drug users.
Introduction
Substance use disorders continue to be a major health concern worldwide. Chronic use of various drugs can impact brain structures and functions (1, 2). Use of these drugs may also be associated with both acute and chronic neuropsychological abnormalities (3). The present review summarizes some of the evidence documenting cognitive changes reported in drug users [with a focus on marijuana, cocaine, and methamphetamine (METH)]. We also discuss potential biological substrates for these observations. The neuropathological changes associated with the use of larger quantities of some of these drugs have been recently reviewed (1). In addition to having differential abuse liability, the use of some of these substances is also associated with differential pathoanatomic changes in the brain (1). There is also evidence that a history of substance use may also exacerbate pre-existing neuropsychological deficits (4) and comorbid neurological or psychiatric disorders (3). It is also clear that substance-related changes in neuropsychological functions may negatively impact activities of daily living, including ability to manage finances and/or holding on to jobs (5). A meta-analysis of METH users and cognition revealed that these individuals exhibited small-to-medium effect sizes for an association between neurocognitive impairment and employment (6). Cognitive domains associated with employment status included executive function, learning and memory, attention, and general intellectual ability (6). In the present review, we will discuss alterations that are linked to psychological and neural mechanisms that detect error signals and generate suitable behavioral responses (7). Also discussed is the accumulated evidence of poor learning and memory, diminished executive functions, and risky decision-making in some individuals with a history of heavy drug use (8–11).
Marijuana Use
Marijuana is the most commonly used illicit substance (12). Investigations of cognitive functions in heavy marijuana users have recently documented poor performance in a number of cognitive subdomains. Some of these deficits appear to be related to frequency of drug use and can impact activities of daily living.
Neuropsychological Findings
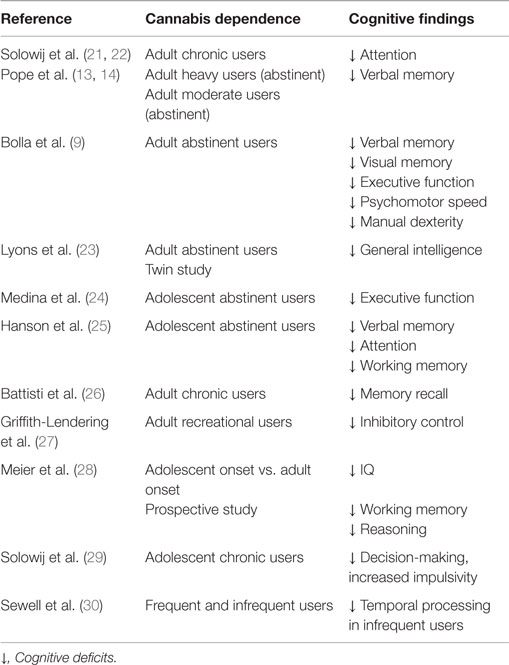
Adult marijuana users suffer from changes measured in broad cognitive domains (13, 14). These include memory (9, 13, 14, 15), attention (16), decision-making (17), and psychomotor speed (9, 18). Bolla et al. (9) reported that impairments observed in marijuana users could be measured in heavy users even after 28 days of forced abstinence during their participant stay on a closed research unit, with light use of marijuana not being associated with any significant decrements in performance (9). In a recent study, Colizzi et al. (19) studied whether functional variations in cannabinoid receptor 1 (CNR1) gene and marijuana exposure interact to modulate prefrontal functions and related behaviors. The authors suggested that deleterious effects of marijuana use may be more evident in individuals with specific genetic backgrounds that might impact receptor expression (19). Additionally, it is important to note that, even if marijuana use during early adulthood is associated with cognitive impairments in selected domains, prolonged abstinence may promote improvement in performance (13, 14, 20). These data are summarized in Table 1.
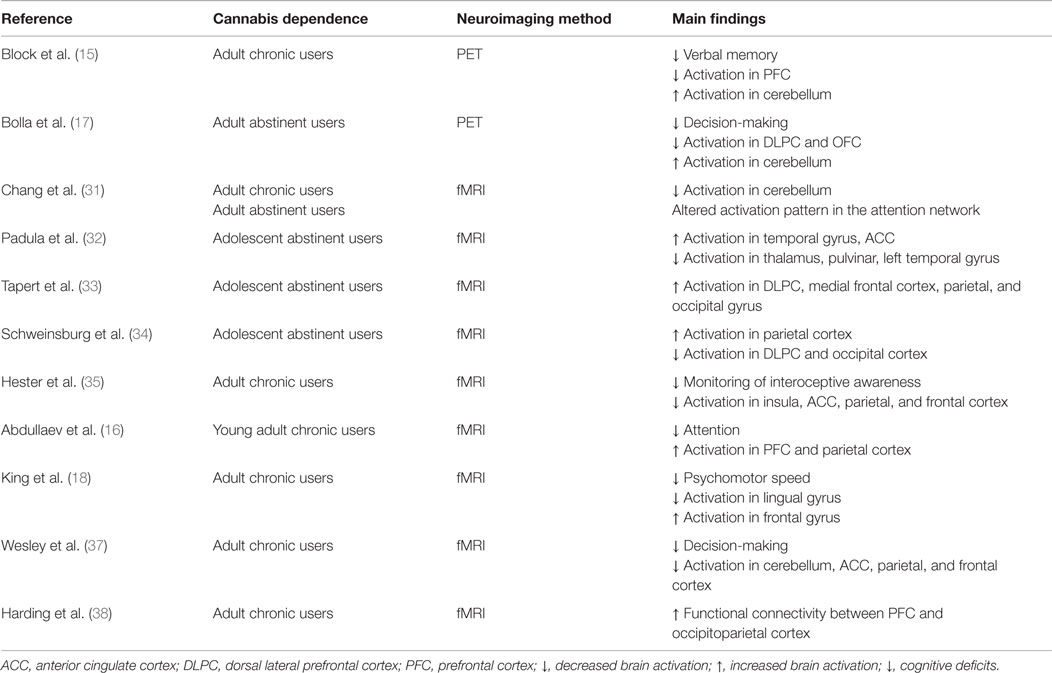
Functional imaging studies comparing activation in both adult and adolescent chronic marijuana users to healthy controls during the performance of different cognitive tasks have reported that chronic marijuana users showed altered patterns of brain activity [Ref. (31–38), see Table 2]. There is also evidence to suggest that heavy marijuana use may produce deficits on measures of decision-making and inhibitory control that persist for long periods of time (27). Among recreational marijuana users, lack of inhibitory control depends on contextual or situational factors, with loss of control being evident only when situations or tasks involve a motivational component (27). Also, poorer cognitive performance in areas of risk-taking, decision-making, and episodic memory may influence the degree to which marijuana users engage in risky behaviors with consequent negative health consequences (39). In addition, it has been reported that the main active ingredient in marijuana, delta-9 tetrahydrocannabinol (THC), can alter time perception by impairing time estimation and production in the seconds range (30). Temporal processing changes may have functional consequences because it is relevant to many everyday tasks, including driving (30).
Interestingly, although much more in-depth research remains to be done on this controversial issue, marijuana use during adolescence has been reported to increase the risk of developing psychotic disorders later in life (40). THC was also reported to induce acute psychotic symptoms in healthy individuals (41) and to increase the risk of psychotic disorders after long-term use (42). A recent study by Bhattacharyya et al. (43) reported a significant relationship between the effects of THC on striatal activation, its effects on task performance, and appearance of positive psychotic symptoms, suggesting that THC might induce psychosis by influencing the neural substrate of attentional salience processing (43). Although more research is needed on this subject, there are plausible biochemical pathways that marijuana can impact to induced psychotic responses in some individuals. Specifically, the endocannabinoid system consists of cannabinoids receptors and endogenous cannabinoid ligands that interact with these receptors to impact the release of several neurotransmitters, including GABA, glutamate, and dopamine (44, 45). Therefore, it seems possible that exposure to marijuana-based psychoactive substances during adolescence could negatively impact glutamatergic and GABAergic systems, with subsequent alterations of maturation processes of these systems, resulting in psychosis-like phenomena (46). The appearance of psychiatric disturbances might also depend on the exact dose, time windows during adolescence, and/or duration of drug exposure (24, 28, 40). Interestingly, hair analyses also revealed that marijuana users with high THC concentration were more likely to exhibit schizophrenia-like symptoms (47, 48). Some of the neuroimaging and cognitive changes reported in marijuana users appear to be moderated by gender (24, 49). These findings highlight potential THC-induced neuroadaptations in the adolescent brain and support the importance of prevention and treatment of adolescent users (28). Nevertheless, this topic needs to be further investigated before any firm conclusion can be reached concerning the relationship of THC to psychosis and other psychiatric diseases.
Cocaine Use
Although cocaine is a highly addictive agent, the vast majority of cocaine users do so recreationally over extended periods of time without developing dependence (50). Thus, documenting the potential cognitive effects of cocaine is an important public health issue because of its high prevalence in the general population. Recent neurobehavioral studies have shown that cocaine heavy users show a number of cognitive decrements that may be secondary to cocaine-induced changes in brain structure and function (1). These cognitive deficits are detailed below.
Neuropsychological Findings
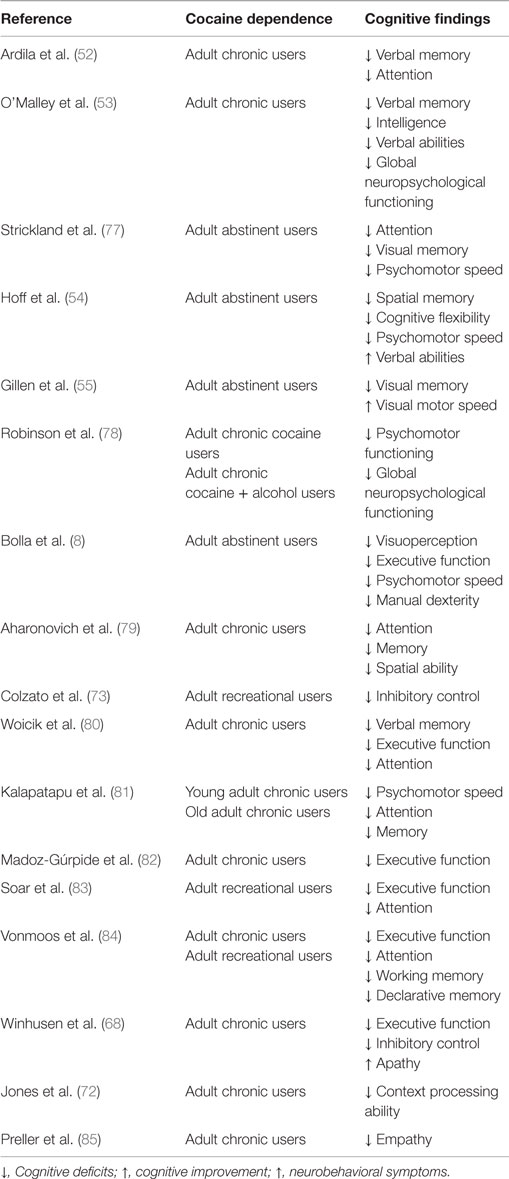
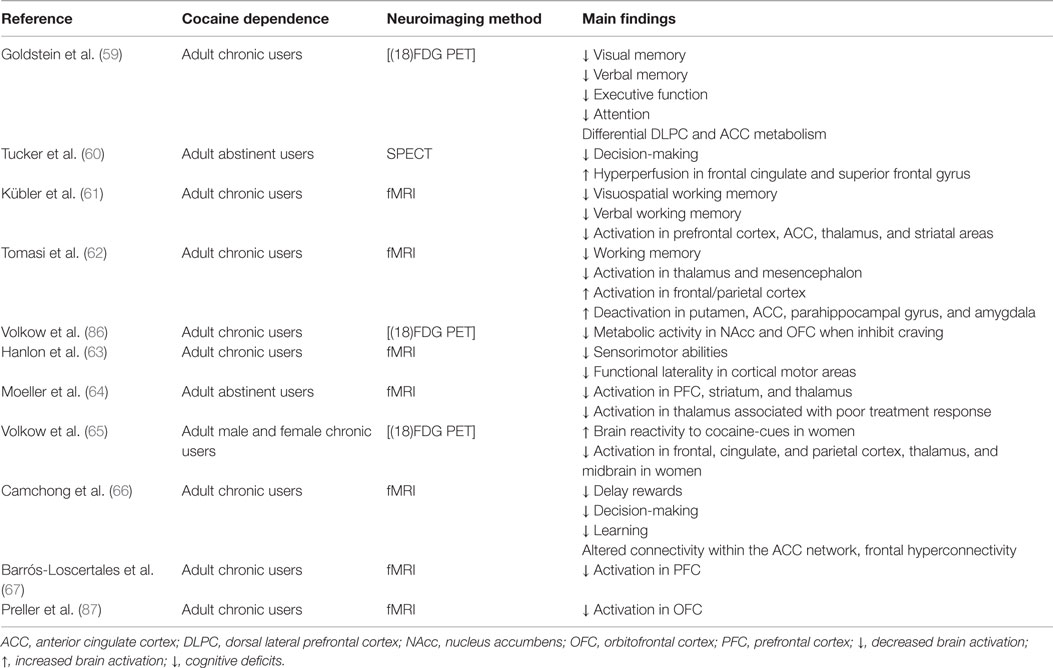
Heavy cocaine use is associated with decrements in performance in several cognitive domains [Ref. (51), detailed in Table 3]. These include problems in executive function, decision-making, increased impulsivity, abnormal visuoperception, abnormal psychomotor speed, impaired manual dexterity, poor verbal learning, and decrements in memory functions (8, 52–58). Additionally, cocaine users showed different patterns of brain activation while performing cognitive tasks [Ref. (59–67), see Table 4]. Chronic cocaine users show poor insight and judgment, lack foresight, and are also disinhibited (68). These cognitive changes are probably related to functional dysfunctions in the prefrontal cortex (69) since patients who suffer damage in this brain region manifest similar cognitive problems (70). This suggestion is supported by neuroimaging studies demonstrating hypofrontality in cocaine users performing tasks of attention and executive function (62, 71). From this perspective, the possibility that a core deficit in executive functions, such as context processing, might contribute to the well-documented impairments in top-down control that are commonly associated with heavy cocaine use (72). In addition to those observations in chronic heavy cocaine users, subtle cognitive deficits have been reported in non-dependent, recreational cocaine users (50, 73–76).
There is a compelling evidence to suggest that cocaine-associated impairments in cognitive functioning might be secondary to cocaine-induced dysfunctions in dopaminergic systems (88–93). Cerebral hypoperfusion observed in the frontal and temporo-parietal cortical areas of cocaine users (77, 94) may also subserve some of the observed cognitive deficits in these patients. These suggestions are consistent with the report of increased cerebral vascular resistance in cocaine users, abnormalities that lasted for, at least, 1 month of monitored abstinence (95).
In addition to specific deficits observed in cocaine users, these individuals may also suffer from psychosocial impairments. For example, a recent study by Preller et al. (87) suggests a relationship between social cognition test outcomes in cocaine-dependent patients and real-life social functioning. Specifically, participants showing more empathy and better mental processing abilities had a larger social network. In addition, social network size was correlated with duration and amount of cocaine use. This suggests that cocaine use and the associated altered empathy and insight may have consequences in everyday life, including fewer social contacts and deprivation of emotional support (87). Additionally, Preller et al. (85) also reported that individuals with cocaine dependence have blunted reward responses to social interactions as well as having reduced orbitofrontal cortex signals while performing a social cognition test. Taken together, these observations suggest that the treatment armamentarium may need to include interventions that boost more interactions of patients with other individuals in various social networks. This argument may explain, in part, why the affiliation-promoting peptide, oxytocin, may have beneficial effects in substance use treatment (96, 97). The possibility that social reward deficits might precede or be consequent to cocaine use needs to be investigated further (96).
In summary, although these cocaine-associated changes in cognitive functions have been well documented, their biological substrates have yet to be understood. Recent functional and structural imaging data provide ample support for impaired connectivity in frontostriatal (4, 98) and striatal-insular (99) connections that serve as neuroanatomical and functional substrates for some of the cognitive deficits reported in cocaine using individuals. A clinical approach that takes into consideration the fact that some patients may actually suffer from cognitive impairments should stimulate investigations in order to provide more details on the basic substrates of cocaine use by humans (74).
Methamphetamine Use
Methamphetamine use is a serious public health problem (100). Long-term exposure to the drug has been shown to cause severe neurotoxic and neuropathological effects with consequent disturbances in several cognitive domains (1). These neuropsychological impairments that can impact the daily lives of METH users are detailed below.
Neuropsychological Findings
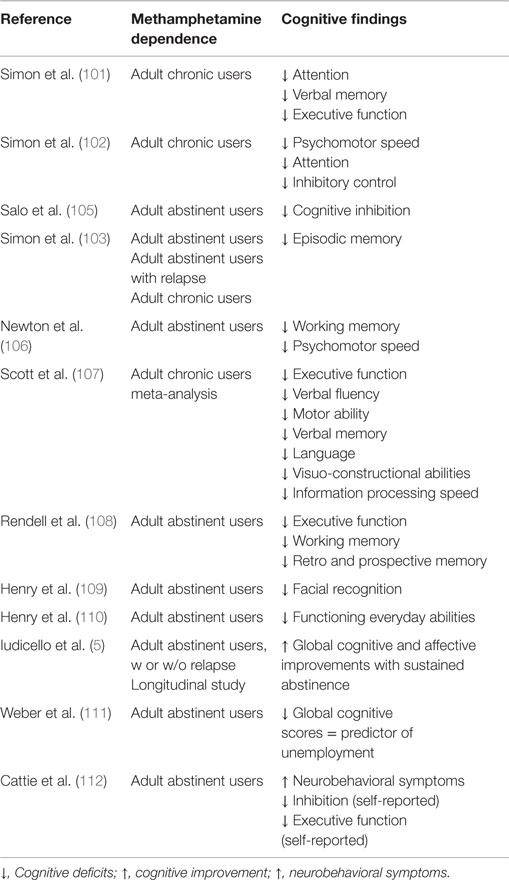
Chronic METH users show mild signs of cognitive decline (10) affecting a broad range of cognitive functions [Ref. (5, 6, 101–112), see details in Table 5; but see also Ref. (113) for a counterargument]. A meta-analysis study by Scott et al. (107) identified significant deficits of a medium magnitude in several different cognitive processes that are dependent on the functions of frontostriatal and limbic circuits. The affected domains include episodic memory, executive functions, complex information processing speed, and psychomotor functions (107). Additionally, METH use often results in irritability, agitation, and numerous other forms of psychiatric distress probably related to the myriad of interpersonal problems experienced by these patients (114, 115). METH dependence is also associated with complaints of cognitive dysfunctions including memory problems and self-reported deficits in everyday functioning (110). Additionally, impulsive behaviors may exacerbate their psychosocial difficulties and promote maintenance of drug-seeking behaviors, especially, by those who use large amounts of the drug (116, 117). The nature and magnitude of cognitive deficits associated with chronic METH use increase the risk of poorer health outcomes, high-risk behaviors, treatment non-adherence, and repeated relapses (110, 118). These adverse consequences might be secondary to poor executive function and memory deficits that may contribute to continuous drug-seeking behaviors (70). It needs to be noted that partial recovery of neuropsychological functioning and improvement in affective distress can be achieved after a period of sustained abstinence from METH (5). Hart et al. (113) have reviewed the literature and suggested that the deficits reported may be statistically but not clinically significant. In a follow-up analysis of similar data, Dean et al. (10) came to a different conclusion. These issues are important to clinicians who are responsible for the daily and/or long-term care of patients because small deficits may be of substantial importance when it comes to patients being able to follow instructions that would help them to participate in their own care, given the high rate of recidivism in that patient population (119, 120). Therefore, identifying patients with neuropsychological deficits would allow for the development of specific cognitive or pharmacological approaches that would benefit them.
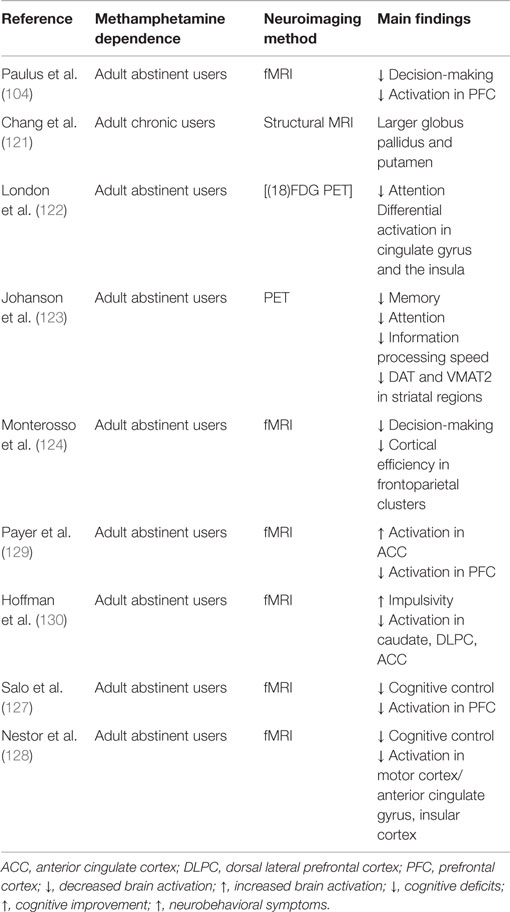
Neuroimaging studies have documented several alterations in brain activation patterns induced by METH [Ref. (104, 121–128), see Table 6]. These studies reported decreased frontal activation associated with impaired decision-making (104) and cognitive control (127). Other brain regions sensitive to METH effects include the cingulate gyrus and insula (122, 128). METH users who showed impaired attention (122) and impaired cognitive control (128) exhibited abnormalities in these brain regions (see Table 6). It is worth mentioning that, in some cases, stimulant-dependent patients report clinically significant neuropsychological abnormalities prior to lifetime initiation of psychostimulant use (68).
Recovery of Neurocognitive Functioning and Treatment Implications
Chronic use of several illicit drugs is associated with variable degrees of impaired cognitive functioning that shows different levels of improvement during sustained abstinence (3). Recovery from METH dependence is associated with improved performance in tests of mental flexibility, attention, processing speed, verbal memory, fine motor functioning, and verbal fluency (5). Improvements in performance are also seen in abstinent marijuana users (13, 14). Moreover, Brewer et al. (131) found that activation in corticostriatal regions, linked to cognitive control, correlated with abstinence and cocaine-free urine toxicology (131). There was also an inverse correlation between prefrontal cortex activation and treatment retention (131), thus supporting the notion that identification of patients with cognitive deficits are important for the long-term care of these patients (3, 132). This suggestion is supported by the results of a very recent report that strength of craving for METH can be reduced by cognitive strategies (133). In addition, patients who participated in computer-assisted cognitive behavioral therapy showed improved task performance and reduced task-related signal changes in several regions implicated in cognitive control, impulse control, and motivational salience, including the anterior cingulate and midbrain (134).
Conclusion
Chronic use of illicit substances, including marijuana, cocaine, and METH, is associated with abnormal goal-directed behaviors that are thought to be the manifestations of altered cortico-striatal-limbic circuits (2, 135). Nevertheless, the wealth of clinical presentations, neuroimaging studies, and some pathological findings suggest that the biochemical and structural effects of chronic heavy use of drugs may reach beyond the boundaries of these reward circuits (1). The data reviewed here indicate that chronic use of illicit drugs is accompanied by moderate cognitive impairments in some patients. These observations may be related to functional and structural changes in various brain regions, including both cortical and subcortical regions of the human brain (1, 98, 136). In addition, it has been reported frontal deficits in psychostimulant-dependent patients reporting current clinically neurobehavioral abnormalities may be linked to pre-existing abnormalities (68). Because drug dependence develop over many months, it is likely that drug-related changes of behaviors may be modulated by some of these pathological phenomena in such a way as to significantly impact the clinical course of chronic use of these substances. Thus, impaired learning and memory functions might negatively impact the ability of a specific subset of patients to benefit from general treatment approaches. This inability may explain, in part, the high rate of recidivism in this patient population. This argument suggests that approaches to these individuals should take into consideration the diversity of patterns of substance use and clinical presentations. This argument suggests that thorough neuropsychological and neuroimaging assessments should be undertaken to identify these subsets of drug users. This approach should help to dichotomize patients as being unimpaired or impaired, with specific cognitive and pharmacological treatments targeting subgroups of patients. Present approaches that group all patients together need to be revamped to allow for more rational and data-driven approaches to treatment.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
The Intramural Research Program of the National Institute on Drug Abuse, NIH, and DHHS supports JC. VB is supported by a grant from ANPCyT, PICT 2012-0924, Argentina.
References
1. Cadet JL, Bisagno V, Milroy CM. Neuropathology of substance use disorders. Acta Neuropathol (2014) 127:91–107. doi: 10.1007/s00401-013-1221-7
2. Volkow ND, Wang G-J, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol (2012) 52:321–36. doi:10.1146/annurev-pharmtox-010611-134625
3. Schulte MH, Cousijn J, den Uyl TE, Goudriaan AE, van den Brink W, Veltman DJ, et al. Recovery of neurocognitive functions following sustained abstinence after substance dependence and implications for treatment. Clin Psychol Rev (2014) 34:531–50. doi:10.1016/j.cpr.2014.08.002
4. Ersche KD, Turton AJ, Chamberlain SR, Müller U, Bullmore ET, Robbins TW. Cognitive dysfunction and anxious-impulsive personality traits are endophenotypes for drug dependence. Am J Psychiatry (2012) 169:926–36. doi:10.1176/appi.ajp.2012.11091421
5. Iudicello JE, Woods SP, Vigil O, Scott JC, Cherner M, Heaton RK, et al. Longer term improvement in neurocognitive functioning and affective distress among methamphetamine users who achieve stable abstinence. J Clin Exp Neuropsychol (2010) 32:704–18. doi:10.1080/13803390903512637
6. Kalechstein AD, Newton TF, Green M. Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. J Neuropsychiatry Clin Neurosci (2003) 15:215–20. doi:10.1176/jnp.15.2.215
7. Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends Cogn Sci (2005) 9:195–201. doi:10.1016/j.tics.2005.02.008
8. Bolla KI, Rothman R, Cadet JL. Dose-related neurobehavioral effects of chronic cocaine use. J Neuropsychiatry Clin Neurosci (1999) 11:361–9. doi:10.1176/jnp.11.3.361
9. Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology (2002) 59:1337–43. doi:10.1212/01.WNL.0000031422.66442.49
10. Dean AC, Groman SM, Morales AM, London ED. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology (2013) 38:259–74. doi:10.1038/npp.2012.179
11. Weinborn M, Moyle J, Bucks RS, Stritzke W, Leighton A, Woods SP. Time-based prospective memory predicts engagement in risk behaviors among substance users: results from clinical and nonclinical samples. J Int Neuropsychol Soc (2013) 19:284–94. doi:10.1017/S1355617712001361
12. UNODC 2013 World Drug Report. (2013). Available from: https://www.unodc.org/unodc/secured/wdr/wdr2013/World_Drug_Report_2013.pdf
13. Pope HG, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry (2001) 58:909–15. doi:10.1001/archpsyc.58.10.909
14. Pope HG Jr, Gruber AJ, Yurgelun-Todd D. Residual neuropsychologic effects of cannabis. Curr Psychiatry Rep (2001) 3:507–12. doi:10.1007/s11920-001-0045-7
15. Block RI, O’Leary DS, Hichwa RD, Augustinack JC, Boles Ponto LL, Ghoneim MM, et al. Effects of frequent marijuana use on memory-related regional cerebral blood flow. Pharmacol Biochem Behav (2002) 72:237–50. doi:10.1016/S0091-3057(01)00771-7
16. Abdullaev Y, Posner MI, Nunnally R, Dishion TJ. Functional MRI evidence for inefficient attentional control in adolescent chronic cannabis abuse. Behav Brain Res (2010) 215:45–57. doi:10.1016/j.bbr.2010.06.023
17. Bolla KI, Eldreth D, Matochik J, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage (2005) 26:480–92. doi:10.1016/j.neuroimage.2005.02.012
18. King GR, Ernst T, Deng W, Stenger A, Gonzales RM, Nakama H, et al. Altered brain activation during visuomotor integration in chronic active cannabis users: relationship to cortisol levels. J Neurosci (2011) 31:17923–31. doi:10.1523/JNEUROSCI.4148-11.2011
19. Colizzi M, Fazio L, Ferranti L, Porcelli A, Masellis R, Marvulli D, et al. Functional genetic variation of the cannabinoid receptor 1 and cannabis use interact on prefrontal connectivity and related working memory behavior. Neuropsychopharmacology (2015) 40:640–9. doi:10.1038/npp.2014.213
20. Fried PA, Watkinson B, Gray R. Neurocognitive consequences of marihuana – a comparison with pre-drug performance. Neurotoxicol Teratol (2005) 27:231–9. doi:10.1016/j.ntt.2004.11.003
21. Solowij N, Michie PT, Fox AM. Differential impairments of selective attention due to frequency and duration of cannabis use. Biol Psychiatry (1995) 37:731–9. doi:10.1016/0006-3223(94)00178-6
22. Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA (2002) 287(13):1123–31. Erratum in: JAMA (2002) 287:1651.
23. Lyons MJ, Bar JL, Panizzon MS, Toomey R, Eisen S, Xian H, et al. Neuropsychological consequences of regular marijuana use: a twin study. Psychol Med (2004) 34:1239–50. doi:10.1017/S0033291704002260
24. Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang TT, Tapert SF. Prefrontal cortex morphometry in abstinent adolescent marijuana users: subtle gender effects. Addict Biol (2009) 14:457–68. doi:10.1111/j.1369-1600.2009.00166.x
25. Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, Tapert SF. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav (2010) 35:970–6. doi:10.1016/j.addbeh.2010.06.012
26. Battisti RA, Roodenrys S, Johnstone SJ, Respondek C, Hermens DF, Solowij N. Chronic use of cannabis and poor neural efficiency in verbal memory ability. Psychopharmacology (2010) 209:319–30. doi:10.1007/s00213-010-1800-4
27. Griffith-Lendering MFH, Huijbregts SCJ, Vollebergh WAM, Swaab H. Motivational and cognitive inhibitory control in recreational cannabis users. J Clin Exp Neuropsychol (2012) 34:688–97. doi:10.1080/13803395.2012.668874
28. Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A (2012) 109:E2657–64. doi:10.1073/pnas.1206820109
29. Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, et al. Reflection impulsivity in adolescent cannabis users: a comparison with alcohol-using and non-substance-using adolescents. Psychopharmacology (2012) 219:575–86. doi:10.1007/s00213-011-2486-y
30. Sewell RA, Schnakenberg A, Elander J, Radhakrishnan R, Williams A, Skosnik PD, et al. Acute effects of THC on time perception in frequent and infrequent cannabis users. Psychopharmacology (Berl) (2013) 226:401–13. doi:10.1007/s00213-012-2915-6
31. Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain (2006) 129:1096–112. doi:10.1093/brain/awl064
32. Padula CB, Schweinsburg AD, Tapert SF. Spatial working memory performance and fMRI activation interaction in abstinent adolescent marijuana users. Psychol Addict Behav (2007) 21:478–87. doi:10.1037/0893-164X.21.4.478
33. Tapert SF, Schweinsburg AD, Drummond SPA, Paulus MP, Brown SA, Yang TT. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (2007) 194:173–83. doi:10.1007/s00213-007-0823-y
34. Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Res (2008) 163:40–51. doi:10.1016/j.pscychresns.2007.04.018
35. Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology (2009) 34:2450–8. doi:10.1038/npp.2009.67
36. Hanlon CA, Dufault DL, Wesley MJ, Porrino LJ. Elevated gray and white matter densities in cocaine abstainers compared to current users. Psychopharmacology (2011) 218:681–92. doi:10.1007/s00213-011-2360-y
37. Wesley MJ, Hanlon CA, Porrino LJ. Poor decision-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiatry Res (2011) 191:51–9. doi:10.1016/j.pscychresns.2010.10.002
38. Harding IH, Solowij N, Harrison BJ, Takagi M, Lorenzetti V, Lubman DI, et al. Functional connectivity in brain networks underlying cognitive control in chronic cannabis users. Neuropsychopharmacology (2012) 37:1923–33. doi:10.1038/npp.2012.39
39. Schuster RM, Crane NA, Mermelstein R, Gonzalez R. The influence of inhibitory control and episodic memory on the risky sexual behavior of young adult cannabis users. J Int Neuropsychol Soc (2012) 18:827–33. doi:10.1016/j.biopsych.2008
40. Bossong MG, Niesink RJM. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog Neurobiol (2010) 92:370–85. doi:10.1016/j.pneurobio.2010.06.010
41. Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet (2009) 374:1383–91. doi:10.1016/S0140-6736(09)61037-0
42. Moore THM, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet (2007) 370:319–28. doi:10.1016/S0140-6736(07)61162-3
43. Bhattacharyya S, Crippa JA, Allen P, Martin-Santos R, Borgwardt S, Fusar-Poli P, et al. Induction of psychosis by ∆9-tetrahydrocannabinol reflects modulation of prefrontal and striatal function during attentional salience processing. Arch Gen Psychiatry (2012) 69:27–36. doi:10.1001/archgenpsychiatry.2011.161
44. Pertwee RG. Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict Biol (2008) 13:147–59. doi:10.1111/j.1369-1600.2008.00108.x
45. Parsons LH, Hurd YL. Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci (2015) 16:579–94. doi:10.1038/nrn4004
46. Chadwick B, Miller ML, Hurd YL. Cannabis use during adolescent development: susceptibility to psychiatric illness. Front Psychiatry (2013) 4:129. doi:10.3389/fpsyt.2013.00129
47. Di Forti M, Morgan C, Dazzan P, Pariante C, Mondelli V, Marques TR, et al. High-potency cannabis and the risk of psychosis. Br J Psychiatry (2009) 195:488–91. doi:10.1192/bjp.bp.109.064220
48. Morgan CJA, Curran HV. Effects of cannabidiol on schizophrenia-like symptoms in people who use cannabis. Br J Psychiatry (2008) 192:306–7. doi:10.1192/bjp.bp.107.046649
49. McQueeny T, Padula CB, Price J, Medina KL, Logan P, Tapert SF. Gender effects on amygdala morphometry in adolescent marijuana users. Behav Brain Res (2011) 224:128–34. doi:10.1016/j.bbr.2011.05.031
50. Colzato LS, Hommel B. Recreational use of cocaine eliminates inhibition of return. Neuropsychology (2009) 23:125–9. doi:10.1037/a0013821
51. Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol (2012) 52:321–36. doi:10.1146/annurev-pharmtox-010611-134625
52. Ardila A, Rosselli M, Strumwasser S. Neuropsychological deficits in chronic cocaine abusers. Int J Neurosci (1991) 7:73–9. doi:10.3109/00207459109150348
53. O’Malley S, Adamse M, Heaton RK, Gawin FH. Neuropsychological impairment in chronic cocaine abusers. Am J Drug Alcohol Abuse (1992) 18:131–44. doi:10.3109/00952999208992826
54. Hoff AL, Riordan H, Morris L, Cestaro V, Wieneke M, Alpert R, et al. Effects of crack cocaine on neurocognitive function. Psychiatry Res (1996) 60:167–76. doi:10.1016/0165-1781(96)02758-8
55. Gillen RW, Kranzler HR, Bauer LO, Burleson JA, Samarel D, Morrison DJ. Neuropsychologic findings in cocaine-dependent outpatients. Prog Neuropsychopharmacol Biol Psychiatry (1998) 22:1061–76. doi:10.1016/S0278-5846(98)00057-8
56. Rosselli M, Ardila A, Lubomski M, Murray S, King K. Personality profile and neuropsychological test performance in chronic cocaine-abusers. Int J Neurosci (2001) 110:55–72. doi:10.3109/00207450108994221
57. Mittenberg W, Motta S. Effects of chronic cocaine abuse on memory and learning. Arch Clin Neuropsychol (1993) 8:477–83. doi:10.1016/0887-6177(93)90048-6
58. Smelson DA, Roy A, Santana S, Engelhart C. Neuropsychological deficits in withdrawn cocaine-dependent males. Am J Drug Alcohol Abuse (1999) 25:377–81. doi:10.1081/ADA-100101867
59. Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, et al. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia (2004) 42:1447–58. doi:10.1016/j.neuropsychologia.2004.04.002
60. Tucker KA, Potenza MN, Beauvais JE, Browndyke JN, Gottschalk PC, Kosten TR. Perfusion abnormalities and decision making in cocaine dependence. Biol Psychiatry (2004) 56:527–30. doi:10.1016/j.biopsych.2004.06.031
61. Kübler A, Murphy K, Garavan H. Cocaine dependence and attention switching within and between verbal and visuospatial working memory. Eur J Neurosci (2005) 21:1984–92. doi:10.1111/j.1460-9568.2005.04027.x
62. Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, et al. Widespread disruption in brain activation patterns to a working memory task during cocaine abstinence. Brain Res (2007) 1171:83–92. doi:10.1016/j.brainres.2007.06.102
63. Hanlon CA, Wesley MJ, Roth AJ, Miller MD, Porrino LJ. Loss of laterality in chronic cocaine users: an fMRI investigation of sensorimotor control. Psychiatry Res (2010) 181:15–23. doi:10.1016/j.pscychresns.2009.07.009
64. Moeller FG, Steinberg JL, Schmitz JM, Ma L, Liu S, Kjome KL. Working memory fMRI activation in cocaine-dependent subjects: association with treatment response. Psychiatry Res (2010) 181:174–82. doi:10.1016/j.pscychresns.2009.11.003
65. Volkow ND, Tomasi D, Wang G-J, Fowler JS, Telang F, Goldstein RZ, et al. Reduced metabolism in brain “control networks” following cocaine-cues exposure in female cocaine abusers. PLoS One (2011) 6:e16573. doi:10.1371/journal.pone.0016573
66. Camchong J, MacDonald AW, Nelson B, Bell C, Mueller BA, Specker S, et al. Frontal hyperconnectivity related to discounting and reversal learning in cocaine subjects. Biol Psychiatry (2011) 69:1117–23. doi:10.1016/j.biopsych.2011.01.008
67. Barrós-Loscertales A, Garavan H, Bustamante JC, Ventura-Campos N, Llopis JJ, Belloch V, et al. Reduced striatal volume in cocaine-dependent patients. Neuroimage (2011) 56:1021–6. doi:10.1016/j.neuroimage.2011.02.035
68. Winhusen TM, Somoza EC, Lewis DF, Kropp FB, Horigian VE, Adinoff B. Frontal systems deficits in stimulant-dependent patients: evidence of pre-illness dysfunction and relationship to treatment response. Drug Alcohol Depend (2013) 127:94–100. doi:10.1016/j.drugalcdep.2012.06.017
69. Bolla KI, Cadet JL, London ED. The neuropsychiatry of chronic cocaine abuse. J Neuropsychiatry Clin Neurosci (1998) 10:280–9. doi:10.1176/jnp.10.3.280
70. Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition (1994) 50:7–15. doi:10.1016/0010-0277(94)90018-3
71. Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci (2004) 16:456–64. doi:10.1176/jnp.16.4.456
72. Jones JA, Lim KO, Wozniak JR, Specker S, MacDonald AW III. Context-processing abilities in chronic cocaine users. Psychol Addict Behav (2013) 27:687–95. doi:10.1037/a0032237
73. Colzato LS, Van den Wildenberg WPM, Hommel B. Impaired inhibitory control in recreational cocaine users. PLoS One (2007) 2:e1143. doi:10.1371/journal.pone.0001143
74. Moreno-López L, Catena A, Fernández-Serrano MJ, Delgado-Rico E, Stamatakis EA, Pérez-García M, et al. Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug Alcohol Depend (2012) 125:208–14. doi:10.1016/j.drugalcdep.2012.02.012
75. Colzato LS, Huizinga M, Hommel B. Recreational cocaine polydrug use impairs cognitive flexibility but not working memory. Psychopharmacology (2009) 207:225–34. doi:10.1007/s00213-009-1650-0
76. Colzato LS, Van den Wildenberg WPM, Hommel B. Reduced attentional scope in cocaine polydrug users. PLoS One (2009) 4:e6043. doi:10.1371/journal.pone.0006043
77. Strickland TL, Mena I, Villanueva-Meyer J, Miller BL, Cummings J, Mehringer CM, et al. Cerebral perfusion and neuropsychological consequences of chronic cocaine use. J Neuropsychiatry Clin Neurosci (1993) 5:419–27. doi:10.1176/jnp.5.4.419
78. Robinson JE, Heaton RK, O’Malley SS. Neuropsychological functioning in cocaine abusers with and without alcohol dependence. J Int Soc (1999) 5:10–9.
79. Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend (2006) 81:313–22. doi:10.1016/j.drugalcdep.2005.08.003
80. Woicik PA, Moeller SJ, Alia-Klein N, Maloney T, Lukasik TM, Yeliosof O, et al. The neuropsychology of cocaine addiction: recent cocaine use masks impairment. Neuropsychopharmacology (2009) 34:1112–22. doi:10.1038/npp.2008.60
81. Kalapatapu RK, Vadhan NP, Rubin E, Bedi G, Cheng WY, Sullivan MA, et al. A pilot study of neurocognitive function in older and younger cocaine abusers and controls. Am J Addict (2011) 20:228–39. doi:10.1111/j.1521-0391.2011.00128.x
82. Madoz-Gúrpide A, Blasco-Fontecilla H, Baca-García E, Ochoa-Mangado E. Executive dysfunction in chronic cocaine users: an exploratory study. Drug Alcohol Depend (2011) 117:55–8. doi:10.1016/j.drugalcdep.2010.11.030
83. Soar K, Mason C, Potton A, Dawkins L. Neuropsychological effects associated with recreational cocaine use. Psychopharmacology (2012) 222:633–43. doi:10.1007/s00213-012-2666-4
84. Vonmoos M, Hulka LM, Preller KH, Jenni D, Baumgartner MR, Stohler R, et al. Cognitive dysfunctions in recreational and dependent cocaine users: role of attention-deficit hyperactivity disorder, craving and early age at onset. Br J Psychiatry (2013) 203:35–43. doi:10.1192/bjp.bp.112.118091
85. Preller KH, Hulka LM, Vonmoos M, Jenni D, Baumgartner MR, Seifritz E, et al. Impaired emotional empathy and related social network deficits in cocaine users. Addict Biol (2014) 19:452–66. doi:10.1111/adb.12070
86. Volkow ND, Fowler JS, Wang G-J, Telang F, Logan J, Jayne M, et al. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage (2010) 49:2536–43. doi:10.1016/j.neuroimage.2009.10.088
87. Preller KH, Herdener M, Schilbach L, Stämpfli P, Hulka LM, Vonmoos M, et al. Functional changes of the reward system underlie blunted response to social gaze in cocaine users. Proc Natl Acad Sci U S A (2014) 111:2842–7. doi:10.1073/pnas.1317090111
88. Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse (1993) 14:169–77. doi:10.1002/syn.890140210
89. Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, et al. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature (1997) 386:827–30. doi:10.1038/386827a0
90. Volkow ND, Fowler JS, Wang GJ. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. J Psychopharmacol (1999) 13:337–45. doi:10.1177/026988119901300406
91. Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, et al. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry (2007) 164:622–9. doi:10.1176/ajp.2007.164.4.622
92. Martinez D, Greene K, Broft A, Kumar D, Liu F, Narendran R, et al. Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion. Am J Psychiatry (2009) 166:1170–7. doi:10.1176/appi.ajp.2009.08121801. Erratum in: Am J Psychiatry (2009) 166:1299.
93. Tomasi D, Volkow ND, Wang R, Carrillo JH, Maloney T, Alia-Klein N, et al. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One (2010) 5:e10815. doi:10.1371/journal.pone.0010815
94. Ernst T, Chang L, Oropilla G, Gustavson A, Speck O. Cerebral perfusion abnormalities in abstinent cocaine abusers: a perfusion MRI and SPECT study. Psychiatry Res (2000) 99:63–74. doi:10.1016/S0925-4927(00)00056-1
95. Herning RI, King DE, Better WE, Cadet JL. Neurovascular deficits in cocaine abusers. Neuropsychopharmacology (1999) 21:110–8. doi:10.1016/S0893-133X(98)00141-9
96. Verdejo-Garcia A. Social cognition in cocaine addiction. Proc Natl Acad Sci U S A (2014) 111:2406–7. doi:10.1073/pnas.1324287111
97. Bisagno V, Cadet JL. Stress, sex, and addiction: potential roles of corticotropin-releasing factor, oxytocin, and arginine-vasopressin. Behav Pharmacol (2014) 25:445–57. doi:10.1097/FBP.0000000000000049
98. Hu Y, Salmeron BJ, Gu H, Stein EA, Yang Y. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA Psychiatry (2015) 72:584–92. doi:10.1001/jamapsychiatry.2015.1
99. McHugh MJ, Demers CH, Braud J, Briggs R, Adinoff B, Stein EA. Striatal-insula circuits in cocaine addiction: implications for impulsivity and relapse risk. Am J Drug Alcohol Abuse (2013) 39:424–32. doi:10.3109/00952990.2013.847446
100. Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet (2012) 379:55–70. doi:10.1016/S0140-6736(11)61138-0
101. Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. Cognitive impairment in individuals currently using methamphetamine. Am J Addict (2000) 9:222–31. doi:10.1080/10550490050148053
102. Simon SL, Domier CP, Sim T, Richardson K, Rawson RA, Ling W. Cognitive performance of current methamphetamine and cocaine abusers. J Addictive Dis (2002) 21:61–74. doi:10.1300/J069v21n01_06
103. Simon SL, Dacey J, Glynn S, Rawson R, Ling W. The effect of relapse on cognition in abstinent methamphetamine abusers. J Subst Abuse Treat (2004) 27:59–66. doi:10.1016/j.jsat.2004.03.011
104. Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology (2002) 26:53–63. doi:10.1016/S0893-133X(01)00334-7
105. Salo R, Nordahl TE, Possin K, Leamon M, Gibson DR, Galloway GP. Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiatry Res (2002) 111:65–74. doi:10.1016/S0165-1781(02)00111-7
106. Newton TF, Kalechstein AD, Hardy DJ, Cook IA, Nestor L, Ling W, et al. Association between quantitative EEG and neurocognition in methamphetamine-dependent volunteers. Clin Neurophysiol (2004) 115:194–8. doi:10.1016/S1388-2457(03)00314-6
107. Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev (2007) 17:275–97. doi:10.1007/s11065-007-9031-0
108. Rendell PG, Mazur M, Henry JD. Prospective memory impairment in former users of methamphetamine. Psychopharmacology (2009) 203:609–16. doi:10.1007/s00213-008-1408-0
109. Henry JD, Mazur M, Rendell PG. Social-cognitive difficulties in former users of methamphetamine. Br J Clin Psychol (2009) 48:323–7. doi:10.1348/000712609X435742
110. Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addict Behav (2010) 35:593–8. doi:10.1016/j.addbeh.2010.01.013
111. Weber E, Blackstone K, Iudicello JE, Morgan EE, Grant I, Moore DJ. Neurocognitive deficits are associated with unemployment in chronic methamphetamine users. Drug Alcohol Depend (2012) 125:146–53. doi:10.1016/j.drugalcdep.2012.04.002
112. Cattie JE, Woods SP, Iudicello JE, Posada C, Grant I; TMARC Group. Elevated neurobehavioral symptoms are associated with everyday functioning problems in chronic methamphetamine users. J Neuropsychiatry Clin Neurosci (2012) 24:331–9. doi:10.1176/appi.neuropsych.11080192
113. Hart CL, Marvin CB, Silver R, Smith EE. Is cognitive functioning impaired in methamphetamine users? A critical review. Neuropsychopharmacology (2012) 37:586–608. doi:10.1038/npp.2011.276
114. Cretzmeyer M, Sarrazin MV, Huber DL, Block RI, Hall JA. Treatment of methamphetamine abuse: research findings and clinical directions. J Subst Abuse Treat (2003) 24:267–77. doi:10.1016/S0740-5472(03)00028-X
115. Halkitis PN, Shrem MT. Psychological differences between binge and chronic methamphetamine using gay and bisexual men. Addict Behav (2006) 31:549–52. doi:10.1016/j.addbeh.2005.05.040
116. Semple SJ, Patterson TL, Grant I. A comparison of injection and non-injection methamphetamine-using HIV positive men who have sex with men. Drug Alcohol Depend (2004) 76:203–12. doi:10.1016/j.drugalcdep.2004.05.003
117. Semple SJ, Zians J, Grant I, Patterson TL. Impulsivity and methamphetamine use. J Subst Abuse Treat (2005) 29:85–93. doi:10.1016/j.jsat.2005.05.001
118. Hester R, Lee N, Pennay A, Nielsen S, Ferris J. The effects of modafinil treatment on neuropsychological and attentional bias performance during 7-day inpatient withdrawal from methamphetamine dependence. Exp Clin Psychopharmacol (2010) 18:489–97. doi:10.1037/a0021791
119. Elkashef A, Rawson RA, Smith E, Pearce V, Flammino F, Campbell J, et al. The NIDA methamphetamine clinical trials group: a strategy to increase clinical trials research capacity. Addiction (2007) 102(Suppl 1):107–13. doi:10.1111/j.1360-0443.2007.01779.x
120. Radfar SR, Rawson RA. Current research on methamphetamine: epidemiology, medical and psychiatric effects, treatment, and harm reduction efforts. Addict Health (2014) 6:146–54.
121. Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry (2005) 57:967–74. doi:10.1016/j.biopsych.2005.01.039
122. London ED, Berman SM, Voytek B, Simon SL, Mandelkern MA, Monterosso J, et al. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biol Psychiatry (2005) 58:770–8. doi:10.1016/j.biopsych.2005.04.039
123. Johanson C-E, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J, et al. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology (2006) 185:327–38. doi:10.1007/s00213-006-0330-6
124. Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp (2007) 28:383–93. doi:10.1002/hbm.20281
125. Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology (2006) 188:162–70. doi:10.1007/s00213-006-0494-0
126. Leland DS, Arce E, Miller DA, Paulus MP. Anterior cingulate cortex and benefit of predictive cueing on response inhibition in stimulant dependent individuals. Biol Psychiatry (2008) 63:184–90.
127. Salo R, Ursu S, Buonocore MH, Leamon MH, Carter C. Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphetamine abusers: a functional magnetic resonance imaging study. Biol Psychiatry (2009) 65:706–9. doi:10.1016/j.biopsych.2008.11.026
128. Nestor LJ, Ghahremani DG, Monterosso J, London ED. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res (2011) 194:287–95. doi:10.1016/j.pscychresns.2011.04.010
129. Payer DE, Lieberman MD, Monterosso JR, Xu J, Fong TW, London ED. Differences in cortical activity between methamphetamine-dependent and healthy individuals performing a facial affect matching task. Drug Alcohol Depend (2008) 93:93–102. doi:10.1016/j.drugalcdep.2007.09.009
130. Hoffman WF, Schwartz DL, Huckans MS, McFarland BH, Meiri G, Stevens AA, et al. Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology (2008) 201:183–93. doi:10.1007/s00213-008-1261-1
131. Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during Stroop task is associated with outcomes in cocaine-dependent patients. Biol Psychiatry (2008) 64:998–1004. doi:10.1016/j.biopsych.2008.05.024
132. Kober H, DeVito EE, DeLeone CM, Carroll KM, Potenza MN. Cannabis abstinence during treatment and one-year follow-up: relationship to neural activity in men. Neuropsychopharmacology (2014) 39:2288–98. doi:10.1038/npp.2014.82
133. Lopez RB, Onyemekwu C, Hart CL, Ochsner KN, Kober H. Boundary conditions of methamphetamine craving. Exp Clin Psychopharmacol (2015) 23:436–44. doi:10.1037/pha0000049
134. DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Kober H, Potenza MN. A preliminary study of the neural effects of behavioral therapy for substance use disorders. Drug Alcohol Depend (2012) 122:228–35. doi:10.1016/j.drugalcdep.2011.10.002
135. Everitt BJ, Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol (2016) 67:23–50. doi:10.1146/annurev-psych-122414-033457
Keywords: marijuana, cocaine, methamphetamine, frontal cortex, cognition
Citation: Cadet JL and Bisagno V (2016) Neuropsychological Consequences of Chronic Drug Use: Relevance to Treatment Approaches. Front. Psychiatry 6:189. doi: 10.3389/fpsyt.2015.00189
Received: 19 April 2015; Accepted: 27 December 2015;
Published: 15 January 2016
Edited by:
Vincent David, Centre National de la Recherche Scientifique, FranceCopyright: © 2016 Cadet and Bisagno. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jean Lud Cadet, amNhZGV0QGludHJhLm5pZGEubmloLmdvdg==;
Veronica Bisagno, dmJpc2Fnbm9AZmZ5Yi51YmEuYXI=
 Jean Lud Cadet
Jean Lud Cadet Veronica Bisagno
Veronica Bisagno