- 1Department of Food Science, Faculty of Home Economics, Otsuma Women’s University, Tokyo, Japan
- 2Center for Maternal-Fetal, Neonatal and Reproductive Medicine, National Center for Child Health and Development, Tokyo, Japan
- 3Department of Social Medicine, National Research Institute for Child Health and Development, National Center for Child Health and Development, Tokyo, Japan
- 4Department of Global Health Promotion, Tokyo Medical and Dental University (TMDU), Tokyo, Japan
- 5Japan Society for the Promotion of Science, Tokyo, Japan
- 6Division of Endocrinology and Metabolism, National Center for Child Health and Development, Tokyo, Japan
Background: The use of n-3 polyunsaturated fatty acids (n-3PUFA) in preventive or therapeutic modalities for postpartum depression, especially long-chain types such as eicosapentaenoic acid (EPA) and a docosahexaenoic acid (DHA), is of considerable interest. High n-3PUFA consumption has been reported among pregnant Japanese women. Therefore, analysis of this group could provide important insights into the relationship between postpartum depression and dietary n-3PUFA consumption. To further examine the relationship between the risk of postpartum depression and n-3PUFA consumption, we conducted a prospective hospital-based birth cohort study in Japan.
Design and methods: Our prospective birth cohort study was performed at the National Center for Child Health and Development (NCCHD) in suburban Tokyo, Japan. Dietary n-3PUFA intake during late pregnancy was assessed by a semi-quantitative food questionnaire and participants were categorized by quintile distributions of n-3PUFA intake. A Japanese translation of the Edinburgh Postnatal Depression Scale was used to screen women for postpartum depression at 1 month after delivery (967 women) and at 6 months after delivery (710 women). We performed logistic regression analysis to examine the relationship between the risk of postpartum depression and n-3PUFA consumption after adjusting for confounding factors.
Results: Significant associations between EPA, DHA, and n-3PUFA intakes in late pregnancy and postpartum depression at both 1 and 6 months after delivery were not observed.
Conclusion: This prospective study indicated that EPA, DHA, and n-3PUFA intake during late pregnancy was not associated with the risk of postpartum depression.
Introduction
Postpartum depression is one of the most common psychopathologies with a reported prevalence between 10 and 15% (1). The condition is associated with long-term risks for women’s mental health and significant negative effects on the cognitive, social, and physical development of their children (2). Considerable interest has been shown in the potentiality of n-3 polyunsaturated fatty acids (n-3PUFA), especially long-chain ones such as an eicosapentaenoic acid (EPA) and a docosahexaenoic acid (DHA), to prevent depression (3–11). Moreover, these fatty acids could provide preventive or therapeutic modalities for postpartum depression by limiting the production of pro-inflammatory eicosanoids and cytokines and may be desirable because they provide an alternative to major tranquilizers, which may be toxic to the fetus (12). n-3PUFAs also help regulate the production, function, and metabolism of serotonergic neurotransmitters (13–15). However, reports of epidemiological studies on the relationship between postpartum depression and n-3PUFA consumption during pregnancy estimated from dietary surveys (16, 17) or n-3PUFA levels of biological information (18, 19) showed no associations. Furthermore, although several randomized control studies examined the relationship between postpartum depression and n-3PUFA supplementation during pregnancy, no evidence was reported to support the routine use of n-3 supplementation during pregnancy (20–23).
It is well known that pregnant Japanese women eat a wide range of n-3PUFA sources (24, 25). Therefore, pregnant Japanese women can be regarded as suitable participants for analyzing the relationship between postpartum depression and n-3PUFA consumption during pregnancy. Although one study on this relationship has already been conducted in Japan, the dietary survey used in the study to collect information on fish and PUFA intakes did not specify gestational week and the timeframe for the Edinburgh Postnatal Depression Scale (EPDS) was quite wide (16). To further examine the relationship between the risk of postpartum depression and n-3PUFA consumption, we conducted a prospective cohort study in Japan.
Materials and Methods
Study Population
Our prospective birth cohort study was performed at the National Center for Child Health and Development (NCCHD) in suburban Tokyo, Japan. Enrollment of pregnant women in the cohort occurred over a period of 3 years and 6 months, from May 13, 2010 until November 28, 2013. Participants were recruited during their first antenatal visits, which usually take place in gestational weeks’ 6–14. Medical records and anthropometric measurements for pregnant women and children were retrieved from hospital charts for all deliveries. A questionnaire including demographic data such as socioeconomic and lifestyle factors and a semi-quantitative food frequency questionnaire (sFFQ) was administered in gestational weeks’ 26–40. Psychiatric data were obtained at 1 and 6 months after delivery using a Japanese translation of the EPDS.
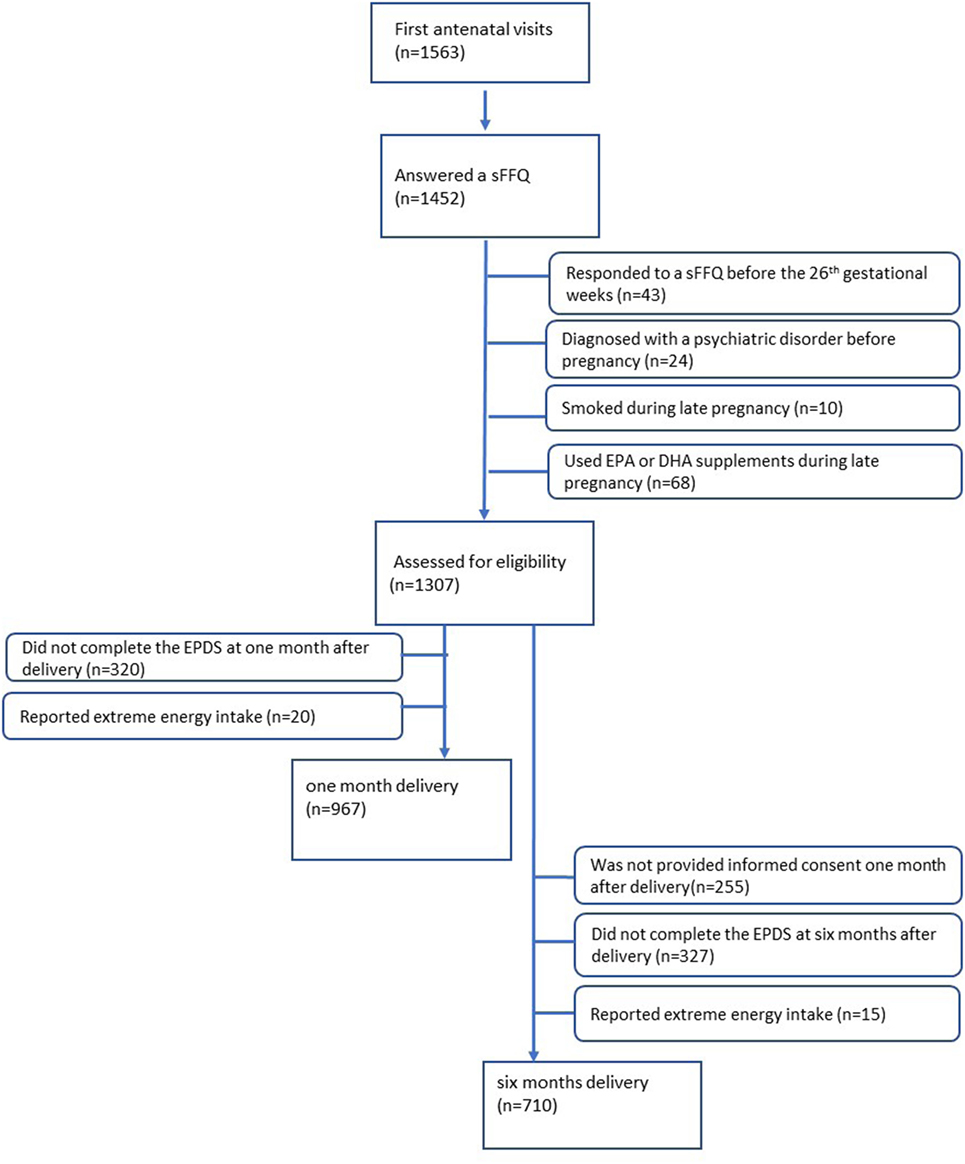
Of the 1,563 women who provided informed consent at their first antenatal visits, 1,452 women answered the sFFQ (response rate: 92.9%) and indicated consumption of fatty acids during mid- to late pregnancy. The exclusion criteria are as follows: women who responded to a sFFQ before the 26th gestational week (n = 43), women who were diagnosed with a psychiatric disorder before pregnancy (n = 24), women who smoked during late pregnancy (n = 10), and women who used EPA or DHA supplements during late pregnancy (n = 68). Of the 1,307 women eligible for assessment, 320 women did not complete the EPDS at 1 month after delivery, and 20 women reported extreme energy intake (top and bottom 1.0%). Therefore, 967 women were included in the analysis at 1 month after delivery. At 6 months after delivery, of the 1,307 women eligible for assessment, 255 women did not provide informed consent 1 month after delivery, 327 who did not complete a questionnaire at 6 months after delivery, and 15 reported extreme energy intake (top and bottom 1.0%) were excluded. Finally, 710 women were included in the analysis at 6 months after delivery (Figure 1). Although the same 642 women were included in the analysis both at 1 and 6 months after delivery, we used the sample of 710 women to increase statistical power. All procedures performed in this study were conducted in accordance with the ethical research standards on the institutional and national research ethics committee and with the 1964 Helsinki declaration of 1964. The study protocol was approved by the Ethics Committee at the National Center for Child Health and Development on August 2, 2010 (project number 417).
Assessment of Exposure
The sFFQ included 165 food items and nine frequency categories and asked participants about the habitual consumption of listed foods within the past 2 months (26). We calculated fish consumption based on the amount of 20 fish items consumed per day and then computed the total energy intake, and calculated each fatty acid intake per day using a food composition table developed for the sFFQ based on the Standardized Tables of Food Composition in Japan (2010 edition) (27). Fish and fatty acid intakes were adjusted by total energy intake with a residual model.
Validation of the sFFQ in computing total energy and n-3PUFA was assessed using the 3-day dietary records of 188 participants and their serum phospholipid levels. Spearman rank correlation coefficients between the sFFQ and the dietary records were 0.38 for EPA and 0.40 for DHA, and those between the sFFQ and serum phospholipid levels were 0.41 for EPA and 0.35 for DHA (26, 28).
Measurement of Postpartum Depression
A version of the EPDS translated into Japanese was used to screen women for postnatal depression, at 1 and 6 months after delivery. The EPDS is a 10-item self-report screening tool for postnatal depression. Each item is scored on a 4-point scale ranging from 0 to 3. As total scores can range from 0 to 30 and the cutoff value of ≥9 has been shown to indicate good sensitivity and specificity for postpartum Japanese women (29), we defined postpartum depression as an EPDS score of 9 or over.
Statistical Analysis
Initially, data were expressed as mean ± SD for continuous variables and a percentage for categorical variables. Differences in continuous variables among postnatal depression cases and controls were tested using the t-test whenever the variables had a normal distribution, while the Mann–Whitney was used for those with a skewed distribution. Differences in categorical variables among postnatal depression cases and controls were tested using the chi-square test whenever the expected values in any of the cells of a contingency table were above five, while Fisher’s exact test was used for those cells below five.
Eicosapentaenoic acid, DHA, and total n-3PUFA consumption were assessed by categories of quintile distribution. We performed logistic regression analysis to examine the relationship between risk of postpartum depression and n-3PUFA consumption using EPDS scores with a cutoff of nine points as the dependent variable, and EPA, DHA, total n-3PUFA, total n-6PUFA consumption and the ratio of n-3PUFA to n-6PUFA at 1 and 6 months after delivery as the independent variable. Finally, adjustments were made for possible confounding factors, including age (<30, 30–35; 35–40, and ≥40 years), pre-pregnancy BMI (<18.5, 18.5–25, and ≥25), marital status (yes, no), normal spontaneous delivery (yes, no), multiple fetuses (yes, no), parity (0 or ≥1), gestation period (before 37 weeks, after 37 weeks), baby’s sex (male or female), maternal educational background (high school or less, graduated college, and graduated university), annual income (<4 million, 4–8 million, and ≥8 million yen), and psychological distress during mid-pregnancy diagnosed with a Kessler-6 score (cut-off value of ≥9, <9) (30). These variables are either known or suspected from previous studies as risk factors for postpartum depression (31, 32). All analyses were conducted using the statistical software package Stata 14 (STATA Corp., College Station, TX, USA), and p-value <0.05 was considered as statistically significant when performing hypothesis tests.
Results
At 1 month after delivery, 19.8% (191/967) of participants reported significant depression (EPDS ≥9). The respective prevalence at 6 months after delivery was 12.8% (91/710). Lifestyle and background characteristics of cases and controls at 1 month after delivery and 6 months after delivery are shown in Tables 1 and 2, respectively. Although women with postpartum depression at 6 months had a higher energy intake (p = 0.011), differences were not observed for fish intake and PUFA consumption both at 1 and 6 months. Women with postpartum depression were more likely to experience psychological distress (Kessler-6 score ≥9) both at 1 month (p < 0.001) and 6 months (p < 0.001) postpartum. Women with a singleton pregnancy and multiparous women had a lower risk of postpartum depression only at 1 month after delivery. Women with a higher household income had a lower risk of postpartum depression both at 1 and 6 months.
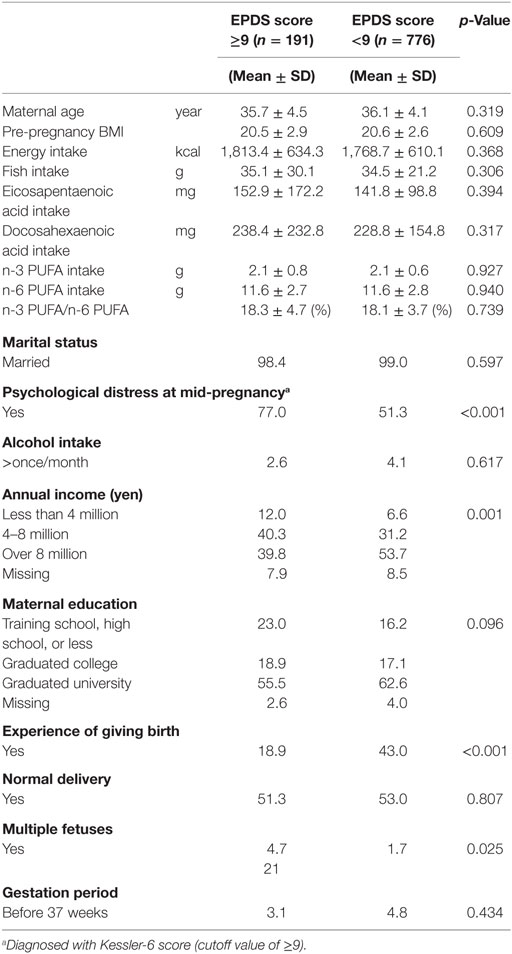
Table 1. Characteristics of participants with and without depression [Edinburgh Postnatal Depression Scale (EPDS) score ≥9] at 1 month after childbirth.
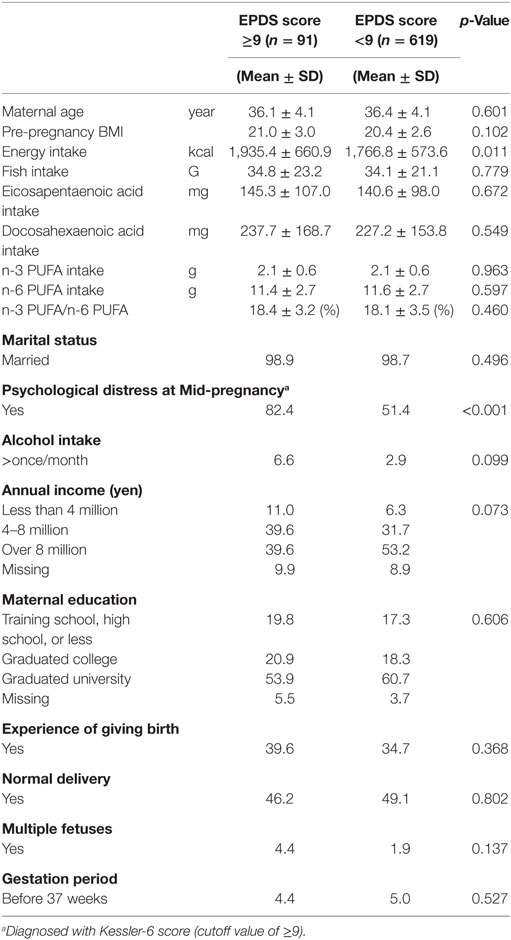
Table 2. Characteristics of participants with and without depression [Edinburgh Postnatal Depression Scale (EPDS) score ≥9] at 6 months after childbirth.
Crude and multivariate odds ratios (17) [95% confidence interval (CI)] for postpartum depression at 1 month after delivery across the quintile for fish and PUFA consumption are shown in Table 3. Median fish intake in the highest quintile (62.5 g per day) was about six times that of the lowest quintile (11.2 g per day) and median intake of EPA or DHA in the highest quintile (265.6 and 421.7 mg, respectively) was about eight times that of the lowest quintile (31.9 and 54.7 mg, respectively). There were no statistically significant associations between the risk of postpartum depression and the quintile of either fish or n-3 PUFA and n-6 PUFA intake.
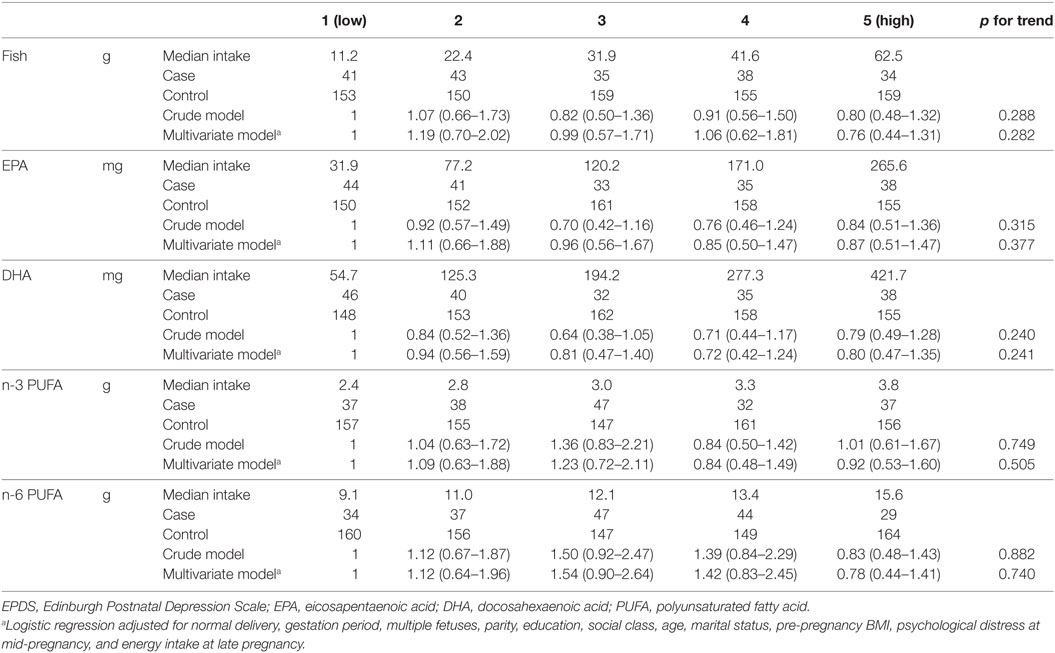
Table 3. Odds ratios [95% confidence interval (CI)] for depression (EPDS score ≥9) at 1 month after childbirth according to quintile of fish and PUFA intake in late pregnancy.
Crude and multivariate ORs (95% CI) for postpartum depression at 6 months after delivery across the quintile for fish and PUFA consumption are shown in Table 4. Median fish intake and PUFA intake at 6 months after delivery did not differ to those at 1 month after delivery. Similar to the results at 1 month after delivery, there were no significant relationships between fish and PUFA intake and postpartum depression at 6 months after delivery.
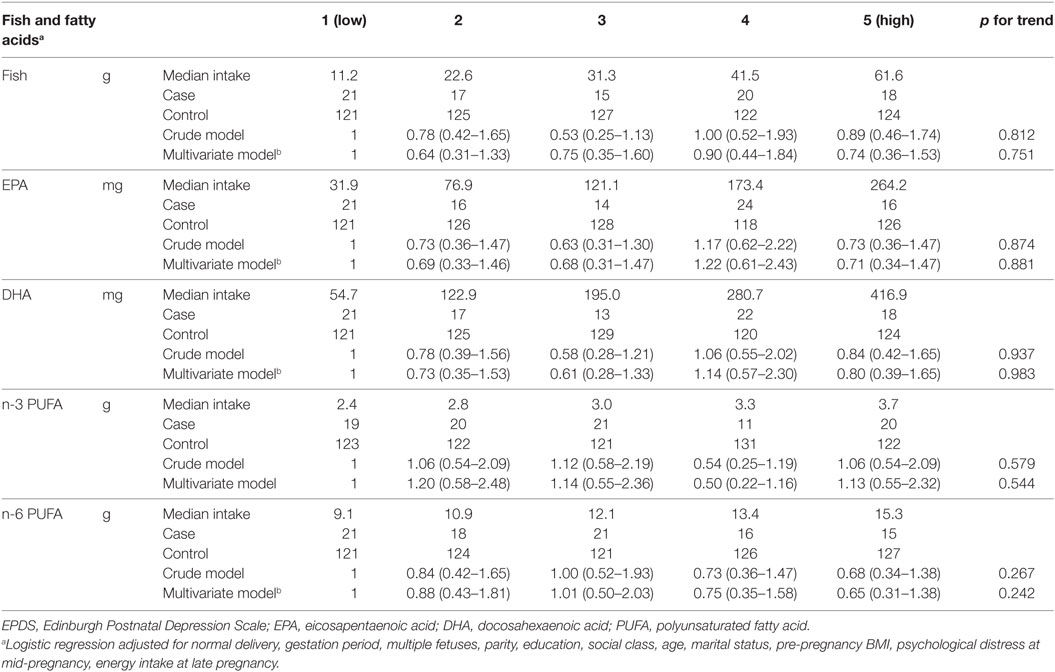
Table 4. Odds ratios [95% confidence interval (CI)] for depression (EPDS score ≥9) at 6 months after childbirth according to quintile of fish and PUFA intake in late pregnancy.
Discussion
Our study found that EPA and DHA intakes during late pregnancy did not significantly reduce the risk of postpartum depression both at 1 month after delivery and at 6 months after delivery among postpartum Japanese women, whose average dietary fish intake was higher than those of women in other countries (33, 34). As far as we know, this is the first prospective cohort study that evaluated the relationship between dietary intake of n-3PUFA at late pregnancy and measures of depressive symptoms with a focus on the time-points of 1 and 6 months after delivery.
Although several studies have attempted to clarify the relationship between n-3PUFA and prenatal depression (14, 35), only two prospective cohort studies have previously reported on dietary n-3PUFA consumption and postpartum depression (16, 17). Our findings were consistent with these two studies in that there was no association; however, in our study, we were able to be more specific regarding the time interval between effect of interest (fish and PUFA intake) and measurement of outcome (postpartum depression).
In these previous studies, the Japanese study collected information on dietary fish and n-3 PUFA intake throughout pregnancy, but not in specific gestational weeks (16), while in the other study a dietary survey was mailed to participants before 25 weeks’ gestation, but it did not include intake for specific gestational weeks (17). Middle or late gestation in pregnancy is an optimal time period to measure n-3 PUFA consumption, as intake is not affected with emesis at this time. Framing a shorter time interval for administration of the dietary survey helps to better clarify the relationship between n-3 PUFA intake and postpartum depression. Thus, in the present study, we limited the administration of sFFQ to gestational weeks’ 26–40.
Furthermore, the time-points used to conduct the EPDS survey in these two previous studies were markedly wide: from 2 to 9 months postpartum in Miyake’s study (16) and up to 1 year postpartum in Strom’s study (17). Postpartum depression is defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition as depressive symptoms experienced 1 month after delivery (36). In Japan, the EPDS for postpartum depression was validated 1 month after delivery (37). On the other hand, it is reported that postpartum depression can develop as late as 3–7 months postpartum (38). In the present study, we were able to show a difference between the percentage of participants with a depression score at 1 month (19.8%) and at 6 months (12.8%) after delivery, and examine the association between n-3 PUFA and depression at both of these time-points.
In addition to the observational studies, several double-blind randomized controlled trials (RCTs) of n-3PUFA supplementation for postpartum depression have shown inconsistent results. Although one study reported that DHA supplementation of 300 mg from 24 to 40 weeks’ gestation prevented postpartum depression, study participants were limited to only 42 women (23). Two other RCTs with high-dose supplementation of EPA for 8 weeks suggested beneficial effects (39, 40). On the other hand, DHA supplementation of 220 mg/day from 16 weeks of pregnancy until 3 months postpartum did not prevent postpartum depression among 119 women at 6 months after delivery (20). As well, EPA+DHA supplementation of 800 mg/day at 26–36 weeks’ gestation showed no beneficial effect for postpartum depression among 2,339 women at 6 weeks and 6 months after delivery (21). Another recent RCT showed that EPA+DHA supplementation of more than 1,000 mg/day did not prevent postpartum depression from 6 to 8 weeks after delivery for women with a high risk of depression (≥9 of EPDS score in early pregnancy) (22).
Docosahexaenoic acid deficiency has been reported to be associated with serotonin, norepinephrine, and dopamine transmission dysfunction as well as those of neuronal membrane stability, leading to both mood disorders as well as cognitive dysfunction of depression (41). Furthermore, EPA reduces membrane arachidonic acid (an n-6PUFA) and prostaglandin E2 synthesis, both which are vital for the immune system to function and to maintain physical health, leading to somatic manifestations and physical comorbidity in depression when deficient (3). The role of n-3 PUFAs in immunity and mood function supports the promising hypothesis of the psychoneuroimmunology of depression (40). However, recent reports from epidemiological studies on the relationship between n-3PUFA levels of biological information and postpartum depression were inconsistent (18, 19, 42, 43). A study from Norway found that low EPA+DHA concentration in red blood cells in late pregnancy was associated with higher depression at 3 months after delivery (43) and a study from Australia reported that EPA and total n-3PUFA concentration of erythrocytes in late pregnancy were associated with postpartum depression (42). However, these studies did not conduct multivariate analysis for risk assessment.
Our sFFQ included 20 kinds of fish items and successfully captured a large variation of EPA and DHA intakes, despite being a cohort study with a reasonably large sample size. As there was a low percentage of participants taking EPA or DHA supplements in this study, our sample size did not decrease notably after exclusion of EPA or DHA supplement users. On the other hand, a high percentage of participants in this study had high levels of maternal education, high incomes, and were more health conscious with a low rate of smoking. Therefore, the response rate was relatively high and the sample size was sufficiently large, even after excluding current smokers and those respondents who gave incomplete or unclear answers. On the other hand, the dietary habits of the participants may not be representative of the average pregnant Japanese woman. Therefore, n-3PUFA may not deficient even in the group with the lowest intake. However, the median fish consumption of the lowest quintile was 11.2 g and that of the highest quintile was 62.5 g, compared to 23.1 g for the lowest quartile and 72.9 g for the highest quartile in Miyake’s study (16). It is possible that a ceiling effect may be in play when fish consumption is high among participants; however, as we included participants with a lower fish intake, our analysis provided more robust results. In addition, it is also possible that low-dose omega-3 PUFA was not effective for the prevention of postpartum depression.
This study also had several additional limitations. First, the EPDS that we used for screening postpartum depression is a widely used screening tool for postpartum depression around the world. Although the cut-off point may differ among countries or races, the reliability and validity of the cut-off point of nine for Japanese populations has been confirmed and the prevalence of postpartum depression was found to be comparable to Western countries (29). Study bias still remains because women with depression may not wish to complete the questionnaire. Second, at the beginning of the follow-up, 1,452 women answered a sFFQ, but the number of subjects included in the analysis was less than 1,000. In particular, there were 320 women who did not complete the EPDS. However, there was no difference in fish intake of at least subjects who completed EPDS and those who did not complete. Third, EPA and DHA intakes may relate to other healthy dietary habits, lifestyle, or residual factors. These potential biases might have confounded the results. Although we excluded participants who were diagnosed with psychiatric disorders before pregnancy, the diagnoses for these participants were made by medical doctors. However, we took into account that depressed mood during pregnancy was diagnosed with a Kessler-6 score (cutoff value of ≥9). Fourth, sFFQ is known to commonly over- or underestimate dietary intake, and these are most likely to bias true associations toward the null. Nevertheless, sFFQ used in this study was validated for the assessment of fish and n-3 PUFA comparing with both the dietary records and serum phospholipid. Fifth, in Japan, there is no recommended dietary allowance for n-3 and n-6 PUFA intake, and a Japanese sample might be biased due to high intake of fish oil, although not as high as those who are taking EPA or DHA supplement. Therefore, n-3 PUFA, as both dietary intake and as a supplement, might have protective effect for postpartum depression even among Japanese. Finally, fish and n-3PUFA intakes were only measured at one point during pregnancy, and the measurement time varied, taking place between gestational weeks 26 and 40, which limited our ability to clarify the effective period of n-3PUFA supplementation.
This prospective study found EPA and DHA intakes were not associated with the risk of postpartum depression at 1 month after delivery. Our findings suggest that dietary EPA and DHA without use of supplementation might not have a protective effect on postpartum depression. Future studies are required to further evaluate the relationship between n-3PUFA consumption and postpartum depression across other characteristics or other gestational periods of n-3 PUFA consumption.
Ethics Statement
Ethics Committee at the National Center for Child Health and Development on August 2, 2010 (project number 417).
Author Contributions
MK conceived of the research idea. RH, TF, KO, NM, and YT conducted the research. MK analyzed the data, wrote the paper, and had primary responsibility for the final content. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the staff and participants of this work for their important contributions. We also thank Ms. Emma Barber for her editorial support.
Funding
This study was supported by grants from the Japan Agency for Medical Research and Development (AMED-6013), the Research Development Grant for Child Health and Development from the National Center for Child Health and Development (25-4), the Ministry of Health, Labour and Welfare (H24-jisedai-shitei-007), and Research Institute of Science and Technology for Society, Japan Science and Technology Agency. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
1. Couto TC, Brancaglion MY, Alvim-Soares A, Moreira L, Garcia FD, Nicolato R, et al. Postpartum depression: a systematic review of the genetics involved. World J Psychiatry (2015) 5(1):103–11. doi:10.5498/wjp.v5.i1.103
2. Raposa E, Hammen C, Brennan P, Najman J. The long-term effects of maternal depression: early childhood physical health as a pathway to offspring depression. J Adolesc Health (2014) 54(1):88–93. doi:10.1016/j.jadohealth.2013.07.038
3. Grosso G, Galvano F, Marventano S, Malaguarnera M, Bucolo C, Drago F, et al. Omega-3 fatty acids and depression: scientific evidence and biological mechanisms. Oxid Med Cell Longev (2014) 2014:313570. doi:10.1155/2014/313570
4. McNamara RK, Vannest JJ, Valentine CJ. Role of perinatal long-chain omega-3 fatty acids in cortical circuit maturation: mechanisms and implications for psychopathology. World J Psychiatry (2015) 5(1):15–34. doi:10.5498/wjp.v5.i1.15
5. Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J Am Coll Nutr (2009) 28(5):525–42. doi:10.1080/07315724.2009.10719785
6. Grosso G, Micek A, Marventano S, Castellano S, Mistretta A, Pajak A, et al. Dietary n-3 PUFA, fish consumption and depression: a systematic review and meta-analysis of observational studies. J Affect Disord (2016) 205:269–81. doi:10.1016/j.jad.2016.08.011
7. Chang YW, Assari S, Prossin AR, Stertz L, McInnis MG, Evans SJ. Bipolar disorder moderates associations between linoleic acid and markers of inflammation. J Psychiatr Res (2017) 85:29–36. doi:10.1016/j.jpsychires.2016.10.021
8. Evans SJ, Assari S, Harrington GJ, Chang YW, Burant CF, McInnis MG. Plasma linoleic acid partially mediates the association of bipolar disorder on self-reported mental health scales. J Psychiatr Res (2015) 68:61–7. doi:10.1016/j.jpsychires.2015.06.001
9. Evans SJ, Kamali M, Prossin AR, Harrington GJ, Ellingrod VL, McInnis MG, et al. Association of plasma omega-3 and omega-6 lipids with burden of disease measures in bipolar subjects. J Psychiatr Res (2012) 46(11):1435–41. doi:10.1016/j.jpsychires.2012.07.016
10. Evans SJ, Prossin AR, Harrington GJ, Kamali M, Ellingrod VL, Burant CF, et al. Fats and factors: lipid profiles associate with personality factors and suicidal history in bipolar subjects. PLoS One (2012) 7(1):e29297. doi:10.1371/journal.pone.0029297
11. Evans SJ, Ringrose RN, Harrington GJ, Mancuso P, Burant CF, McInnis MG. Dietary intake and plasma metabolomic analysis of polyunsaturated fatty acids in bipolar subjects reveal dysregulation of linoleic acid metabolism. J Psychiatr Res (2014) 57:58–64. doi:10.1016/j.jpsychires.2014.06.001
12. Borja-Hart NL, Marino J. Role of omega-3 Fatty acids for prevention or treatment of perinatal depression. Pharmacotherapy (2010) 30(2):210–6. doi:10.1592/phco.30.2.210
13. De Vriese SR, Christophe AB, Maes M. Lowered serum n-3 polyunsaturated fatty acid (PUFA) levels predict the occurrence of postpartum depression: further evidence that lowered n-PUFAs are related to major depression. Life Sci (2003) 73(25):3181–7. doi:10.1016/j.lfs.2003.02.001
14. Kendall-Tackett K. Long-chain omega-3 fatty acids and women’s mental health in the perinatal period and beyond. J Midwifery Womens Health (2010) 55(6):561–7. doi:10.1016/j.jmwh.2010.02.014
15. Levant B. N-3 (omega-3) fatty acids in postpartum depression: implications for prevention and treatment. Depress Res Treat (2011) 2011:467349. doi:10.1155/2011/467349
16. Miyake Y, Sasaki S, Yokoyama T, Tanaka K, Ohya Y, Fukushima W, et al. Risk of postpartum depression in relation to dietary fish and fat intake in Japan: the Osaka Maternal and Child Health Study. Psychol Med (2006) 36(12):1727–35. doi:10.1017/S0033291706008701
17. Strom M, Mortensen EL, Halldorsson TI, Thorsdottir I, Olsen SF. Fish and long-chain n-3 polyunsaturated fatty acid intakes during pregnancy and risk of postpartum depression: a prospective study based on a large national birth cohort. Am J Clin Nutr (2009) 90(1):149–55. doi:10.3945/ajcn.2009.27552
18. Chong MF, Ong YL, Calder PC, Colega M, Wong JX, Tan CS, et al. Long-chain polyunsaturated fatty acid status during pregnancy and maternal mental health in pregnancy and the postpartum period: results from the GUSTO study. J Clin Psychiatry (2015) 76(7):e848–56. doi:10.4088/JCP.14m09191
19. Sallis H, Steer C, Paternoster L, Davey Smith G, Evans J. Perinatal depression and omega-3 fatty acids: a Mendelian randomisation study. J Affect Disord (2014) 166:124–31. doi:10.1016/j.jad.2014.04.077
20. Doornbos B, van Goor SA, Dijck-Brouwer DA, Schaafsma A, Korf J, Muskiet FA. Supplementation of a low dose of DHA or DHA+AA does not prevent peripartum depressive symptoms in a small population based sample. Prog Neuropsychopharmacol Biol Psychiatry (2009) 33(1):49–52. doi:10.1016/j.pnpbp.2008.10.003
21. Makrides M, Gibson RA, McPhee AJ, Yelland L, Quinlivan J, Ryan P. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: a randomized controlled trial. JAMA (2010) 304(15):1675–83. doi:10.1001/jama.2010.1507
22. Mozurkewich EL, Clinton CM, Chilimigras JL, Hamilton SE, Allbaugh LJ, Berman DR, et al. The mothers, omega-3, and mental health study: a double-blind, randomized controlled trial. Am J Obstet Gynecol (2013) 208(4):313.e1–9. doi:10.1016/j.ajog.2012.10.203
23. Judge MP, Beck CT, Durham H, McKelvey MM, Lammi-Keefe CJ. Pilot trial evaluating maternal docosahexaenoic acid consumption during pregnancy: decreased postpartum depressive symptomatology. Int J Nurs Sci (2014) 1(4):339–45. doi:10.1016/j.ijnss.2014.10.005
24. Miyamoto S, Miyake Y, Sasaki S, Tanaka K, Ohya Y, Matsunaga I, et al. Fat and fish intake and asthma in Japanese women: baseline data from the Osaka Maternal and Child Health Study. Int J Tuberc Lung Dis (2007) 11(1):103–9.
25. Miyake Y, Tanaka K, Okubo H, Sasaki S, Arakawa M. Fish and fat intake and prevalence of depressive symptoms during pregnancy in Japan: baseline data from the Kyushu Okinawa Maternal and Child Health Study. J Psychiatr Res (2013) 47(5):572–8. doi:10.1016/j.jpsychires.2013.01.012
26. Ogawa K, Jwa SC, Kobayashi M, Morisaki N, Sago H, Fujiwara T. Validation of a food frequency questionnaire for Japanese pregnant women with and without nausea and vomiting in early pregnancy. J Epidemiol (2017) 27(5):201–8. doi:10.1016/j.je.2016.06.004
27. The Council for Science and Technology, Ministry of Education, Culture, Sports, Science and Technology. Standard Tables of Food Composition in Japan. Tokyo: Printing Bureau of the Ministry of Finance (2010). (in Japanese).
28. Kobayashi M, Jwa SC, Ogawa K, Morisaki N, Fujiwara T. Validity of a food frequency questionnaire to estimate long-chain polyunsaturated fatty acid intake among Japanese women in early and late pregnancy. J Epidemiol (2017) 27(1):30–5. doi:10.1016/j.je.2016.07.001
29. Okano TMM, Masuji F, Tamaki R, Nomura J, Miyaoko H. Validation and reliability of Japanese version of the EPDS. Arch Psychiatr Diag Clin Evaluat (1996) 7:525–33.
30. Hamazaki K, Harauma A, Otaka Y, Moriguchi T, Inadera H. Serum n-3 polyunsaturated fatty acids and psychological distress in early pregnancy: adjunct Study of Japan Environment and Children’s Study. Transl Psychiatry (2016) 6:e737. doi:10.1038/tp.2016.2
31. Evagorou O, Arvaniti A, Samakouri M. Cross-cultural approach of postpartum depression: manifestation, practices applied, risk factors and therapeutic interventions. Psychiatr Q (2016) 87(1):129–54. doi:10.1007/s11126-015-9367-1
32. Vliegen N, Casalin S, Luyten P. The course of postpartum depression: a review of longitudinal studies. Harv Rev Psychiatry (2014) 22(1):1–22. doi:10.1097/HRP.0000000000000013
33. Zhang GQ, Liu B, Li J, Luo CQ, Zhang Q, Chen JL, et al. Fish intake during pregnancy or infancy and allergic outcomes in children: a systematic review and meta-analysis. Pediatr Allergy Immunol (2017) 28(2):152–61. doi:10.1111/pai.12648.
34. Best KP, Gold M, Kennedy D, Martin J, Makrides M. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: a systematic review and meta-analysis of observational studies and randomized controlled trials. Am J Clin Nutr (2016) 103(1):128–43. doi:10.3945/ajcn.115.111104
35. Jans LA, Giltay EJ, Van der Does AJ. The efficacy of n-3 fatty acids DHA and EPA (fish oil) for perinatal depression. Br J Nutr (2010) 104(11):1577–85. doi:10.1017/S0007114510004125
36. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM. 5th ed. Washington, DC: American Psychiatric Association (2013).
37. Okano T, Murata M, Masuji F, Tamaki R, Nomura J, Miyaoka H, et al. Validation and reliability of Japanese version of the EPDS. Arch Psychiatr Diag Clin Evaluat (1996) 74:525–33.
38. Andrews-Fike C. A review of postpartum depression. Prim Care Companion J Clin Psychiatry (1999) 1(1):9–14. doi:10.4088/PCC.v01n0103
39. Freeman MP, Davis M, Sinha P, Wisner KL, Hibbeln JR, Gelenberg AJ. Omega-3 fatty acids and supportive psychotherapy for perinatal depression: a randomized placebo-controlled study. J Affect Disord (2008) 110(1–2):142–8. doi:10.1016/j.jad.2007.12.228
40. Su KP. Mind-body interface: the role of n-3 fatty acids in psychoneuroimmunology, somatic presentation, and medical illness comorbidity of depression. Asia Pac J Clin Nutr (2008) 17(Suppl 1):151–7.
41. Su KP. Biological mechanism of antidepressant effect of omega-3 fatty acids: how does fish oil act as a ’mind-body interface’? Neurosignals (2009) 17(2):144–52. doi:10.1159/000198167
42. Parker G, Hegarty B, Granville-Smith I, Ho J, Paterson A, Gokiert A, et al. Is essential fatty acid status in late pregnancy predictive of post-natal depression? Acta Psychiatr Scand (2015) 131(2):148–56. doi:10.1111/acps.12321
Keywords: fish intake, dietary n-3 polyunsaturated fatty acids, eicosapentaenoic acid, docosahexaenoic acid, postpartum depression, Japanese pregnant women
Citation: Kobayashi M, Ogawa K, Morisaki N, Tani Y, Horikawa R and Fujiwara T (2017) Dietary n-3 Polyunsaturated Fatty Acids in Late Pregnancy and Postpartum Depressive Symptom among Japanese Women. Front. Psychiatry 8:241. doi: 10.3389/fpsyt.2017.00241
Received: 16 August 2017; Accepted: 06 November 2017;
Published: 23 November 2017
Edited by:
Shervin Assari, University of Michigan, United StatesReviewed by:
Leandro Da Costa Lane Valiengo, University of São Paulo, BrazilAndreas Hoell, Zentralinstitut für Seelische Gesundheit, Germany
Copyright: © 2017 Kobayashi, Ogawa, Morisaki, Tani, Horikawa and Fujiwara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takeo Fujiwara, ZnVqaXdhcmEuaGx0aEB0bWQuYWMuanA=
 Minatsu Kobayashi
Minatsu Kobayashi Kohei Ogawa
Kohei Ogawa Naho Morisaki
Naho Morisaki Yukako Tani4,5
Yukako Tani4,5 Takeo Fujiwara
Takeo Fujiwara