- 1Center on Ageing Psychology, CAS Key Laboratory of Mental Health, Institute of Psychology, Beijing, China
- 2Department of Psychology, University of Chinese Academy of Sciences, Beijing, China
- 3Magnetic Resonance Imaging Research Center, Institute of Psychology, Chinese Academy of Sciences, Beijing, China
- 4State Key Laboratory of Brain and Cognitive Science, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China
- 5Institute of Psychology, Tianjin Medical University, Tianjin, China
- 6Clinic for Hearing-, Speech- and Voice Disorders, Medical University of Innsbruck, Innsbruck, Austria
Background: Depressed populations demonstrate a greater tendency to have negative interpretations on ambiguous situations. Cognitive theories concerning depression proposed that such a negative bias plays an important role in developing and maintaining depression. There is now fairly consistent evidence arising from different stimuli and assessment methods that depression is featured by such a bias. The current study aimed to explore the neural signatures associated with the interpretation bias in the elderly with depressive symptoms confronted with different facial expressions using event-related brain potentials (ERPs).
Methods: Participants were 14 community-dwelling older adults with depressive symptoms assessed by the Center for Epidemiologic Studies Depression scale scores. We collected event-related potentials of their brain compared to that of 14 healthy aged-matched adults. The late positive potential (LPP) was used to examine cognitive-affective processes associated with judgment of emotional facial expressions between the two groups.
Results: Old adults with depressive symptoms have much smaller amplitude than healthy older adults irrespective of the prime types. When processing the targets, the two groups showed different patterns regarding the LPP. The healthy control group revealed no differences between ambiguous and happy primes, irrespective of whether the targets were sad or happy facial expressions. However, significant differences were found between happy and sad and between ambiguous and sad primes. Such a pattern indicates a positive bias in healthy elderly adults. Regarding the elderly with depressive symptoms, there were no significant differences between ambiguous versus happy, ambiguous versus sad primes, and happy versus sad primes. Concerning reaction times, there was no group difference. Thus, the findings provide some support for cognitive theories of depression.
Conclusion: The current study shows that there is an association between interpretative biases and depressive symptoms in the elderly by using the neuroscientific method of ERPs. The results suggest that ERPs are sensitive to explore the interpretation bias in depressed populations.
Introduction
The predominant symptoms of depression include negative beliefs about the world, the self, the future, as well as periodical and unmanageable negative thoughts which frequently linger around the self. Cognitive theories of depression proposed that depressed populations show a greater tendency to have negative interpretations on ambiguous stimuli, situations, and events (1). According to cognitive theories of depression, such a negative interpretation bias is assumed to play a central role for both development and maintenance of depression (1, 2). There is now fairly consistent scientific evidence concerning the interpretative biases in depression. Previous studies have employed various methods to explore this issue, the most prevalent being self-reports of subjects’ interpretations of scenarios and stories with ambiguity (3–5). Such methods substantially contributed to the establishment of cognitive theories of depression. Even so, as Lawson and MacLeod (6) put forward, self-reporting methodologies are vulnerable to effects caused by response bias. For instance, depressed individuals probably process the negative and neutral interpretations of ambiguous information in a similar way to non-depressed ones but show a greater inclination to give the more negative interpretations.
In order to circumvent this problem, many studies switched to the usage of performance-based measures, such as priming tasks, but failed to discover interpretation biases in populations with depression (6–8). Some researchers proposed that the failure in finding interpretive biases through priming methods possibly owning to the application of reaction times (RTs) measures to evaluate the cognitive processing of depressed individuals (9). Distinctively, severity of depression is correlated with both the retardation and increased variability of the response latencies to carry out voluntary reactions. Thus, response latencies are not sensitive enough to detect interpretive biases in depressed individuals. To resolve the problems related to response latencies in priming studies, Lawson et al. (9) used physiological indices, like the magnitude of the human startle reflex. They found that depressed people showed a similar eye blink reaction elicited by negative and ambiguous words, but the blink reflexes magnitudes to ambiguous words was much smaller than those to negative words in healthy controls. Previous studies have found that magnitude of the startle reflex was increased after the presentation of negative stimuli (10, 11). Therefore, such findings are consistent with the hypothesis of a negative interpretation bias in depressed individuals.
The heterogeneity of results probably, at least was partially caused by methodological difficulties in evaluating the biased information processing in depression. Given this issue, it is of great necessity to adopt an alternative method, which should not use measurement reflecting speed to execute voluntary responses, and also must avoid the potential impact from response bias effects. As a neurophysiological measure assessing online brain processing mechanisms, event-related brain potentials (ERPs) bear the potential to overcome the limitations mentioned above, providing a unique chance to explore how depressed populations preliminarily process incoming information. Furthermore, evidence from ERPs might be of essential importance to cognitive processing theories of depression (12–14). In addition, featured by high temporal resolution, ERPs can directly measure the neural activity occurred just before the elicitation of behavioral response. A positive ERP component beginning about 300 ms after the stimulus onset has been consistently associated with arousal and emotion (15). Put forward by Kissler et al. (16), this component has been named as the late positive potential (LPP). Studies have demonstrated that the LPP tend to be augmented for emotional stimuli (17, 18). Furthermore, this potential was also found to be correlated with subjective ratings of emotion intensity (19). Amusingly, some researchers have also found that LPP can differentiate negative stimuli from positive ones (20).
The majority of previous studies try to explore the interpretation bias in individuals with depression by using words, sentences, scenarios, and events with ambiguity as experimental stimuli. Such stimuli are loaded with low levels of emotion, thus have poor sensitivity and ecological validity. Recently, considerable empirical researches have used faces with emotional expressions as experimental materials (21, 22). Emotional faces have the following advantages when compared with those used in the majority of previous studies. First, emotional faces are less influenced by different cultural background and people come from different ethnic group incline to interpret basic facial expressions in a similar way (23). Second, facial expressions, as one of the most important message sources in the ongoing stream of various social cues during social interactions, can pass on a wide-spreading of information among social partners (24), about 60% of information (25). Third, interpersonal theories of depression proposed that depressed individuals elicit rejection from others in their social interaction, which in turn exacerbates their future risk of suffering from depression (26). Fourth, faces with emotional expressions are closely allied to the estimations of social approval or disapproval (27) hence it is essential to precisely interpret and react to them for effective social functioning (28). Last but not least, as individuals usually attempt to control their emotional expression in daily interaction, it is quite common to see mild or ambiguous facial expressions. Therefore, it is very difficult to aware and interprets these ambiguous social signals. As a result, it would be more sensitive to use ambiguous facial expressions to explore the interpretive bias in depressed populations. By using facial expressions, some previous studies have detected the interpretation bias in depressed individuals (21, 27, 29).
Individuals with depressive symptoms reside in a stage during which psychometrically identified depression is above the average. However, they do not fulfill the diagnostic criteria of clinical depression overall. Thus, they lie in the middle of a continuum between normal mood and clinical depression. As for preclinical depression, adults with depressive symptoms probably then develop into clinical depression (30). Investigating populations with depressive symptoms allows studying both susceptibility and compensation mechanisms of depression (31). As a consequence, exploration of such mechanisms would enhance our comprehension of the underlying processes bring about to clinical relevant depression. Detection of ERPs biomarkers of individuals with depressive symptoms will have important illuminations for early diagnosis of risk populations thus possibly preventing depression onset.
Studies concerning adults and adolescents have revealed that depression and negative interpretation bias is closely correlated, however, its emergence in the elderly still remained unknown due to the discrepancy between young and older adults in depression manifestation and emotion processing (32, 33). First, the elderly with depression showed more sleep disorder and loss of appetite when compared with depressed adults and adolescents [National Institutes of Health (34)]. Second, proposed by the socioemotional selectivity theory, healthy old adults would show a positivity bias in emotion processing compared with the younger ones (33, 35).
In summary, the present study aimed to assess the interpretation bias in the elderly with depressive symptoms when making judgments of facial expressions via a priming paradigm. For this purpose, ERPs will be used to evaluate the biased information processing in the elderly. According to the socioemotional selectivity theory proposed by Carstensen (33, 35), we hypothesized that older adult without depressive symptoms would exhibit a positive bias in processing emotional faces. Based on cognitive theories of depression, we hypothesized that the elderly with depressive symptoms would show a negative interpretation bias compared with healthy controls. Measured by RTs, previous studies failed to find interpretation bias in depressed individuals. We hypothesized that the ERP component LPP would reflect an interpretation bias in the elderly with depressive symptoms.
Method
Participants
All subjects in the current study were chose from the participants’ database of our previous study (36). There are 61 elderly with depressive symptoms and 245 healthy normal controls (NCs) in the database. First, 14 subjects were randomly selected from those 61 elderly with depressive symptoms. A control group was then randomly selected from the 245 normal old adults. The two groups were matched in their demographic variables.
The average age of the healthy NC group was 65.64 years. There are five males and nine females in this group. The mean age of the older adults with depressive symptoms (ADs) was 66.36 years. There are also five males and nine females in this group. The two groups have no difference in their mean age (p = 0.71) or education (p = 0.78). The Center for Epidemiologic Studies Depression Scale (CES-D) (37) was applied to obtain each participant’s depression scores. There are 20 items in the CES-D, and participants are asked to self-rate the presence of their depressive symptoms during the past week on a 4-point Likert-type scale. The total score of the CES-D is 60. Participants with a score of 16 or greater were considered to have depressive symptoms. Studies have shown that the CES-D possess well-established psychometric attributes with older adults (38). The mean CES-D score for the ADs group was 20.21 (SD = 5.65) and that for the NC group was 3.5 (SD = 3.3). The ADs participants had a Mini-Mental State Examination (MMSE) cutoff of ≥24, and they did not meet the DSM-IV clinical criteria for major depressive disorder (MDD). The NC participants had a CES-D cutoff of ≤5 and a MMSE score ≥24.
Written informed consent was obtained from all participants to study participation. They received a compensation for their participation. They all declared that they had no neurological and psychiatric diseases. All participants were right-handed and had normal or corrected-to-normal vision. The clinical and demographic characteristics of these participants are shown in Table 1.
Stimuli
Digitalized photographs of affective expressions with both male and female model identities were taken from the Chinese Facial Affective Picture System. The photographs included 870 emotional faces (39). By using the morphing software (Morpheus v. 1.95), affective expressions of each model identity were then blended into each other to create a 50% happy and 50% sad intensity level of ambiguity. Emotional faces of five male and five females with happy and sad expressions, as well as five 50% happy–50% sad ambiguous expressions were applied as primes in the current study. Therefore, there were three kinds of primes, namely happy, sad, and ambiguous facial expressions. Moreover, another series of emotional faces of five male and female with happy and sad expressions were selected from the same Chinese Facial Affective Picture System and used as targets, which resulted in two kinds of targets, happy and sad faces. Hence, the stimulus material for the experiment consisted of 300 distinct images, with 50 happy–happy pairs, 50 happy–sad pairs, 50 sad–happy pairs, 50 sad–sad pairs, 50 ambiguous–happy pairs, and 50 ambiguous–sad pairs. These stimuli were split into five blocks, with 60 trails in each block. A separate set of stimuli was created in the same manner for the practice trials. All pictures were situated within an area of 184 × 198 pixels. Picture size was 7.9 × 7.3 cm2, and they were shown in the center of the computer screen.
Procedure
Participants with informed consent were given instructions on how to prepare for the ERP recording. In the formal experiment, participants were stayed in a room with dim lighting, sound insulation and electric shield. In each trial, a fixation cross of 400 ms was presented first. Then, a prime face appeared for 200 ms, which was followed by a 100 ms interval. Finally, the target face was presented for 2,000 ms. Following the target face, a blank screen was presented for 1,500 ms, during which the participants were allowed to blink. Participants were required to classify the target face as either “happy” or “sad” as fast and accurately as possible by pushing one of the two horizontally arranged buttons with their index fingers. The face-to-hand assignment was counterbalanced across participants.
Electrophysiological Recordings
The electroencephalogram (EEG) was recorded with a 64 Ag/AgCl cap placed in referring to the extended 10–20 positioning system (http://www.neuroscan.com/). EEG signal was recorded with 500 Hz sampling rate and referenced to the right mastoid (M2) online. Impedances were kept below 5 kΩ. During recording, a 30 Hz low-pass filter was used. The eye blinks of participants were corrected mathematically. The remaining artifacts were rejected manually. For the LPP component, segments of 1,000 ms beginning 200 ms pretarget and ending 1,000 ms after target onsets were obtained. A 200 ms pretarget baseline was used to average the segments in order to acquire ERPs. Signals exceeding ±80 µV in any given epoch were discarded automatically.
Behavioral Data Analysis
The judgment RTs were analyzed, while accuracy rates were ignored because of the high accuracy (>95%). The analysis of variance (ANOVA) performed on RTs revealed a significant main effect of prime, F (1, 26) = 4.48, p < 0.05, η2 = 0.26, with judgment latencies of ambiguous primes (617.15 ± 17.91 ms) being faster than that of both happy (633.97 ± 15.98 ms) and sad primes (639.77 ± 18.83 ms), but the happy and sad primes did not differ from each other. Furthermore, no other main effect or interactions approached significance.
ERP Data Analysis
The mean amplitudes in the time range 350–650 ms (LPP) were then exported. According to previous studies and on account of visual inspection of the present data, we focused the analysis of the LPP in this time range over three regions of interest, i.e., frontocentral: FC1, FC3, FCz, FC2, and FC4; centroparietal: CP1, CP3, CPz, CP2, and CP4; parietooccipital: PO3, PO5, POz, PO4, and PO6.
We calculated an ANOVA with the factors prime (happy, sad, and ambiguous), target (happy, sad), and topographical region (frontocentral, centroparietal, and parietooccipital) as a within-subject factor, and group (NC, ADs) as a between-subject factor.
Results showed that there is a significant main effect of group, F (1, 27) = 16.8, p < 0.001, η2 = 0.17; target, F (1, 82) = 13.66, p < 0.001, η2 = 0.14 and topographical region, F (1, 81) = 39.35, p < 0.001, η2 = 0.49. Moreover, the interaction between prime and target also approached significance, F (1, 81) = 51.59, p < 0.001, η2 = 0.56. Simple effect analysis showed that no matter the target was happy or sad faces, there were no significant differences between happy and ambiguous primes, but significant differences were present between happy versus sad and ambiguous versus sad primes, with amplitudes of happy primes larger than that of sad primes (p < 0.05), and also larger amplitudes for ambiguous compared to sad primes (p < 0.05). ERP waveforms and topographic maps (time range: 350–650 ms) of both sad targets and happy targets for two groups of participants are shown in Figures 1 and 2.
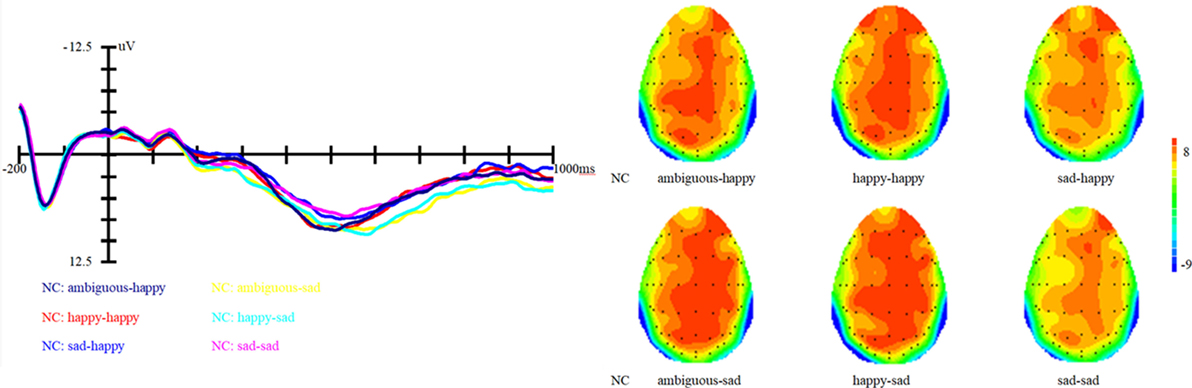
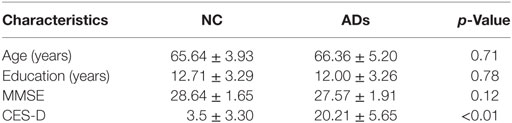
Figure 1. Grand average event-related brain potentials at the electrode CPz of the analyzed topography to ambiguous, happy, and sad facial expressions for both the happy and sad target face for healthy old adults.
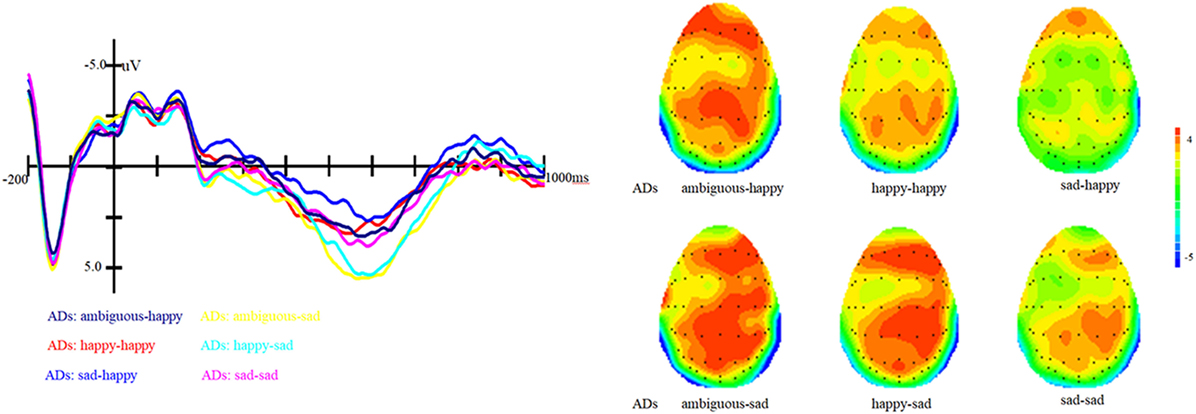
Figure 2. Grand average event-related brain potentials at the electrode CPz of the analyzed topography to ambiguous, happy, and sad facial expressions for both the happy and sad target face for old adults with depressive symptoms.
In addition, the interaction between prime and group is marginally significant, F (1, 81) = 2.62, p = 0.07, η2 = 0.06. Simple effect analysis showed that ADs have much smaller amplitude than healthy older adults, irrespective of the prime types (all three ps < 0.01). For healthy older adults, happy primes did not differ from ambiguous primes (p > 0.05), but significant differences were present between happy versus sad and ambiguous versus sad primes, with amplitudes of happy primes larger than that of sad primes (p < 0.05), and also larger amplitudes for ambiguous compared to sad primes (p < 0.05). For ADs, there were no significant differences between happy versus sad, ambiguous versus sad, and ambiguous versus happy primes (all three ps > 0.05). Grand-average ERP waveforms and topographic maps (time range: 350–650 ms) for both groups of participants are shown in Figures 3 and 4.
Figure 3. Grand average event-related brain potentials at the electrode CPz of the analyzed topography to ambiguous, happy, and sad facial expressions when the target was a happy face for both the healthy old adults and old adults with depressive symptoms.
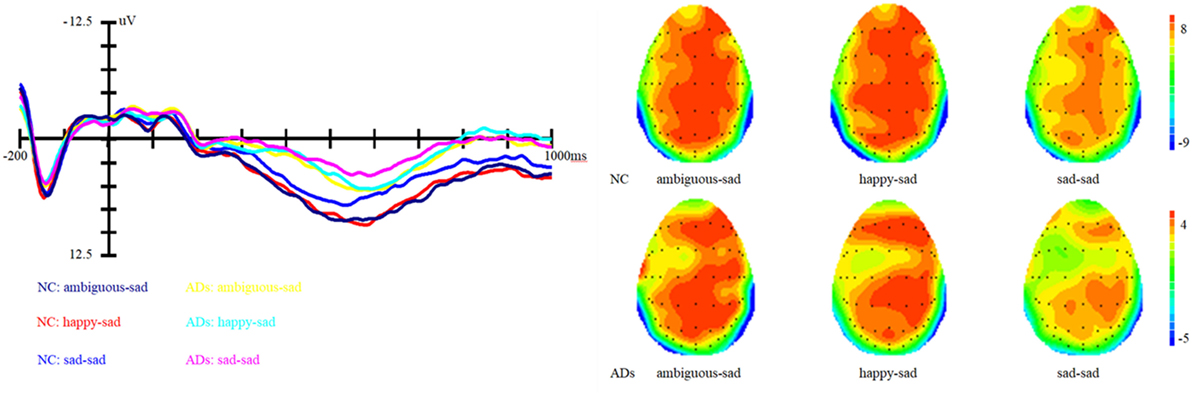
Figure 4. Grand average event-related brain potentials at the electrode CPz of the analyzed topography to ambiguous, happy, and sad facial expressions when the target was a sad face for both the healthy old adults and old adults with depressive symptoms.
Discussion
By using ERPs, the current study aimed to assess the interpretive bias in the elderly with depressive symptoms when making judgments of facial expressions. We hypothesized that the elderly with depressive symptoms would show a negative interpretation bias compared with healthy controls. Results of the current study demonstrated that elderly adults with depressive symptoms have much smaller amplitude than healthy older adults irrespective of the prime types. When processing the targets, the two groups showed different ERP patterns concerning the LPP. The healthy control group revealed no differences between ambiguous and happy primes, irrespective of whether the targets were sad or happy facial expressions. However, significant differences were found between happy and sad and between ambiguous and sad primes. Regarding the elderly with depressive symptoms, there were no significant differences between ambiguous versus happy, ambiguous versus sad primes, and happy versus sad primes. Again, these modulations occurred in a similar strength irrespective of whether a sad or happy target was presented. Concerning reactions times, there was no difference between the two groups.
The LPP reflects a late and controlled attentional evaluation of emotion. Results of the present study with respect to the LPP showed differences between healthy old adults and elderly with depressive symptoms. The LPP findings reveal that normal old adults were more possibly to identify ambiguous facial expressions as happy ones. Such a pattern indicated that there was a positive bias in the normal old adults. This result provided evidence for Carstensen’s socioemotional selectivity theory (33, 35), which proposed that normal older adults showed positive preferences for positive over negative emotional information (33, 40). Nevertheless, these characteristics might not apply to the elderly with depressive symptoms. Perhaps, elderly adults with depressive symptoms cannot make a distinction between ambiguous, happy and sad faces, which might suggest an elimination of the positive bias.
Compared to the positive bias observed in healthy controls, the elimination of such a bias in elderly adults with depressive symptoms could also be considered as a kind of negative trend. Therefore, the present study further confirms that there is a depression-linked interpretive bias in depressed individuals and provides further evidence for the cognitive theories of depression (1, 2). However, result of the current study is different from that of Lawson et al. (9). They used the human eye blink reflex to explore the negative interpretation bias in depressed college students and found that depression is associated with a negative interpretation bias. The current study, similar to Lawson et al. (9), used a method which does not depend on subjects’ self-reports and can also evade the troubles related to response latency measurement of cognitive processing in individuals with depression, only found an elimination of the positive bias but not a negative bias. It is essential to notice that the difference between our findings and that of Lawson et al. (9) might have been potentially caused by factors such as differences in demographic characteristics of the participants and the kind of depression measures. In Lawson et al. (9), undergraduate students were selected as participants. While in our study, old adults were recruited as participants. Actually, Wood and Kisley (41) had found that old adults and young adults are quite different in processing emotional information. Such differences in participant samples may contribute to the inconsistent findings. Concerning the use of different measurement tools to assess depression, Lawson et al. (9) selected participants with scores on the Beck Depression Inventory. In contrast, we used CES-D to define the depressive symptoms in the elderly. Such differences in measuring depression may also contribute to the inconsistent results. The inconsistency between our study and Lawson et al. (9) also implicated the pressing need for more studies on interpretation bias associated with depression. The current study just catch a glimpse of the neural mechanisms underlying depression, thus future research should explore other related aspects so as to obtain a more integrated understanding of the potential neural underpinnings of the interpretation bias in depression.
The current study demonstrated that the LPP amplitude of elderly adults with depressive symptoms is significantly smaller than that of the healthy older adults. Although the interaction between group and target is marginally significant, simple effect analysis showed that the two groups of participants showed different LPP patterns irrespective of whether a sad or happy target was presented. Concerning such findings, the possible reason is that the sample size of the current study is relatively modest. However, one thing for sure is that depressive symptoms really have some influences on the LPP amplitude of old adults. Therefore, a large sample would be employed in the future to validate the findings of the current study.
Conclusion
In summary, the present study found that there exists a positive bias in the healthy elderly. However, depressive symptoms in the elderly not only caused a reduction of LPP amplitude but also an elimination of the positive biases. Such findings highlighted a relation between interpretative biases and depressive symptoms in the elderly by using the neuroscientific method of event-related potentials. The results suggest that ERPs are sensitive to explore the interpretation bias in depressed populations, which enhanced our understanding of the underlying processes bring about to clinical relevant depression and also have important illuminations for early diagnosis of at risk populations. In addition, the present study expanded research concerning interpretation bias to the elderly.
Ethics Approval and Consent to Participate
The current study was approved by the Ethics Committee of the Institute of Psychology, Chinese Academy of Sciences. Written informed consent of participants was obtained for participation after a detailed explanation of the study procedure.
Availability of Data and Materials
The data and materials supporting this study cannot be made publicly available because it will be in conflict with patients’ privacy. All interested parties can request the data from bGlqdWFuQHBzeWNoLmFjLmNu.
Author Contributions
JL, HZ, and BD initiated the design for this article together. HZ undertook the statistical analysis and wrote the manuscript. BD participated in the data collection and also made some critical comments on the manuscript. SR made critical comments and revision on the manuscript. As the principal investigator of this project, JL supervised the statistical analysis and the manuscript writing and revision. All authors read and have approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to extend our appreciations to all the elderly adults who took part in the current study.
Funding
This work was supported by the National Natural Science Foundation of China (31470998, 31271108, and 31671157), Beijing Municipal Science & Technology Commission (Z171100000117006), the Pioneer Initiative of the Chinese Academy of Sciences, Feature Institutes Program (TSS-2015-06), National Key Research and Development Program of China (2016YFC1305904) and the Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences (KLMH2014ZK02) granted to JL and also the China Postdoctoral Science Foundation (2015M570164), and the Scientific Foundation of Institute of Psychology, Chinese Academy of Sciences (Y6CX242007) granted to HZ.
Abbreviations
ERPs, event-related brain potentials; LPP, late positive potential; NC, normal control; ADs, older adults with depressive symptoms; CES-D, Center for Epidemiologic Studies Depression scale; MMSE, Mini-Mental State Examination; MDD, major depressive disorders; EEG, electroencephalogram; ANOVA, analysis of variance; RTs, reaction times.
References
2. Williams JMG, Watts FN, MacLeod CM, Mathews A. Cognitive Psychology and Emotional Disorders. 2nd ed. Chichester, England: Wiley (1997).
3. Gupta R, Kar BR. Interpretative bias: indicators of cognitive vulnerability to depression. Ger J Psychiatry (2008) 11(3):98–102.
4. Pury CLS. Information-processing predictors of emotional response to stress. Cognit Emotion (2002) 16(5):667–83. doi:10.1080/02699930143000400
5. Butler G, Mathews A. Cognitive processes in anxiety. Adv Behav Res Ther (1983) 5:51–62. doi:10.1016/0146-6402(83)90015-2
6. Lawson C, MacLeod C. Depression and the interpretation of ambiguity. Behav Res Ther (1999) 37(5):463–74. doi:10.1016/S0005-7967(98)00131-4
7. Bisson MAS, Sears CR. The effect of depressed mood on the interpretation of ambiguity, with and without negative mood induction. Cognit Emotion (2007) 21(3):614–45. doi:10.1080/02699930600750715
8. Mogg K, Bradbury KE, Bradley BP. Interpretation of ambiguous information in clinical depression. Behav Res Ther (2006) 44(10):1411–9. doi:10.1016/j.brat.2005.10.008
9. Lawson C, MacLeod C, Hammond G. Interpretation revealed in the blink of an eye: depressive bias in the resolution of ambiguity. J Abnorm Psychol (2002) 111(2):321–8. doi:10.1037/0021-843X.111.2.321
10. Bradley MM, Cuthbert BN, Lang PJ. Startle reflex modulation: emotion or attention? Psychophysiology (1990) 27:513–22. doi:10.1111/j.1469-8986.1990.tb01966.x
11. Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychol Rev (1990) 97:377–95. doi:10.1037/0033-295X.97.3.377
12. Clark DM, Wells A. A cognitive model of social phobia. In: Heimberg RG, Liebowitz MR, Hope DA, Schneider FR, editors. Social Phobia: Diagnosis, Assessment, and Treatment. New York: Guilford Press (1995). p. 69–93.
13. Huppert JD, Foa EB. Maintenance mechanisms in social anxiety: an integration of cognitive biases and emotional processing theory. In: Yiend J, editor. Cognition, Emotion and Psychopathology: Theoretical, Empirical and Clinical Directions. New York: Cambridge University Press (2004). p. 213–31.
14. Mathews A, Mackintosh B. A cognitive model of selective processing in anxiety. Cognit Ther Res (1998) 22:539–60. doi:10.1023/A:1018738019346
15. Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: an integrative review of ERP findings. Biol Psychol (2008) 77:247–65. doi:10.1016/j.biopsycho.2007.11.006
16. Kissler J, Herbert C, Junghofer M. Emotion and attention in visual word processing – an ERP study. Biol Psychol (2009) 80:75–83. doi:10.1016/j.biopsycho.2008.03.004
17. Hajcak G, Macnamara A, Olvet DM. Event-related potentials, emotion, and emotion regulation: an integrative review. Dev Neuropsychol (2010) 35:129–55. doi:10.1080/87565640903526504
18. Kaestner EJ, Polich J. Affective recognition memory processing and event-related brain potentials. Cognit Affect Behav Neurosci (2011) 11:186–98. doi:10.3758/s13415-011-0023-4
19. Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol Psychol (2000) 52:95–111. doi:10.1016/S0301-0511(99)00044-7
20. Schacht A, Adler N, Chen PY, Guo TM, Sommer W. Association with positive outcome induces early effects in event-related brain potentials. Biol Psychol (2012) 89:130–6. doi:10.1016/j.biopsycho.2011.10.001
21. Beevers CG, Wells TT, Ellis AJ, Fischer K. Identification of emotionally ambiguous interpersonal stimuli among dysphoric and nondysphoric individuals. Cognit Ther Res (2009) 33(3):283–90. doi:10.1007/s10608-008-9198-6
22. Jusyte A, Schönenberg M. Threat processing in generalized social phobia: an investigation of interpretation biases in ambiguous facial affect. Psychiatry Res (2014) 217(1–2):100–6. doi:10.1016/j.psychres.2013.12.031
23. Ekman P, Friesen WV. Constants across cultures in the face and emotion. J Pers Soc Psychol (1971) 17(2):124–9. doi:10.1037/h0030377
24. Mayer JD, DiPaolo M, Salovey P. Perceiving affective content in ambiguous visual stimuli: a component of emotional intelligence. J Pers Assess (1990) 54(3–4):772–81. doi:10.1080/00223891.1990.9674037
25. Burgoon JK. Nonverbal signals. In: Knapp ML, Miller GR, editors. Handbook of Interpersonal Communication. Beverly Hills, CA: SAGE (1985). p. 344–90.
26. Hames JL, Hagan CR, Joiner TE. Interpersonal processes in depression. Annu Rev Clin Psychol (2013) 9:355–77. doi:10.1146/annurev-clinpsy-050212-185553
27. Gilboa-Schechtman E, Foa E, Vaknin Y, Marom S, Hermesh H. Interpersonal sensitivity and response bias in social phobia and depression: labeling emotional expressions. Cognit Ther Res (2008) 32(5):605–18. doi:10.1007/s10608-008-9208-8
28. Montagne B, Schutters S, Westenberg HGM, van Honk J, Kessels RPC, de Haan EHF. Reduced sensitivity in the recognition of anger and disgust in social anxiety disorder. Cogn Neuropsychiatry (2006) 11:389–401. doi:10.1080/13546800444000254
29. Joormann J, Gotlib IH. Is this happiness I see? Biases in the identification of emotional facial expressions in depression and social phobia? J Abnorm Psychol (2006) 115(4):705–14. doi:10.1037/0021-843X.115.4.705
30. Murphy JM, Nierenberg AA, Laird NM, Monson RR, Sobol AM, Leighton AH. Incidence of major depression: prediction from subthreshold categories in the Stirling County study. J Affect Disord (2002) 68:251–9. doi:10.1016/S0165-0327(00)00334-7
31. Gruzelier JH. Theory, methods and new directions in the psychophysiology of the schizophrenic process and schizotypy. Int J Psychophysiol (2003) 48(3):221–45. doi:10.1016/S0167-8760(03)00055-2
32. Bucks RS, Garner M, Tarrant L, Bradley BP, Mogg K. Interpretation of emotionally ambiguous faces in older adults. J Gerontol B Psychol Sci Soc Sci (2008) 63(6):337–43. doi:10.1093/geronb/63.6.P337
33. Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously: a theory of socioemotional selectivity. Am Psychol (1999) 54(3):165–81. doi:10.1037/0003-066X.54.3.165
34. National Institutes of Health. Diagnosis and treatment of depression in late life. Natl Inst Health Consen Stat (1991) 9:1–27.
35. Carstensen LL, Mikels JA. At the intersection of emotion and cognition: aging and the positivity effect. Curr Dir Psychol Sci (2005) 14(3):117–21. doi:10.1111/j.0963-7214.2005.00348.x
36. Dai BB, Peng YS, Li J. A cross-sectional study of depressive symptom and emotion regulation in older adults. Chin Ment Health J (2014) 28(3):192–6. doi:10.3969/j.issn.1000-6729.2014.03.006
37. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas (1977) 1(3):385–401. doi:10.1177/014662167700100306
38. Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for epidemiologic studies depression scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging (1997) 12(2):277–87. doi:10.1037/0882-7974.12.2.277
39. Gong X, Huang YX, Wang Y, Luo YJ. Revision of the Chinese facial affective picture system. Chin Ment Health J (2011) 25(1):40–6. doi:10.3969/j.issn.1000-6729.2011.01.011
40. Murphy NA, Isaacowitz DM. Preferences for emotional information in older and younger adults: a meta-analysis of memory and attention tasks. Psychol Aging (2008) 23(2):263–86. doi:10.1037/0882-7974.23.2.263
Keywords: elderly with depressive symptoms, cognitive theories of depression, positive bias, event-related brain potentials, late positive potential
Citation: Zhou H, Dai B, Rossi S and Li J (2018) Electrophysiological Evidence for Elimination of the Positive Bias in Elderly Adults with Depressive Symptoms. Front. Psychiatry 9:62. doi: 10.3389/fpsyt.2018.00062
Received: 24 November 2017; Accepted: 13 February 2018;
Published: 05 March 2018
Edited by:
Wenbin Guo, Central South University, ChinaReviewed by:
Xuhai Chen, Shaanxi Normal University, ChinaWenguang He, Qufu Normal University, China
Copyright: © 2018 Zhou, Dai, Rossi and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Juan, bGlqdWFuQHBzeWNoLmFjLmNu
 Huixia Zhou
Huixia Zhou Bibing Dai
Bibing Dai Sonja Rossi
Sonja Rossi Juan Li
Juan Li