- 1Fengxian Hospital, Southern Medical University, Shanghai, China
- 2Fengxian Hospital, Jinzhou Medical University Graduate Base, Shanghai, China
About 30% of diabetes patients suffer from varying degrees of depression. Diabetes itself is associated with abnormal carbohydrate and energy metabolism. Whether chronic stress-induced depression-like behavior impacts the metabolome of blood plasma and urine in diabetes is not clear. This study aimed to investigate the effect of chronic stress on metabolome of plasma and urine in spontaneously diabetic Goto-Kakizaki (GK) rats. The GK rats were subjected to 8 weeks' chronic unpredictable mild stress (CUMS) to induce depression-like behavior. Metabolome analysis of blood plasma and urine using liquid chromatography mass spectrometry (LC/MS) was performed. Multivariate data analysis was used to evaluate the data. Behavior and biochemical assay confirmed the successful establishment of CUMS-induced depression-like behavior model in rats. Disturbance of 20 plasma metabolites and 16 urine metabolites were altered in CUMS-induced depression GK rats as compared to control ones. These disturbed metabolites were involved in fatty acid metabolism, sphingolipid metabolism, phenylalanine metabolism, citrate cycle, glycolysis, glutathione metabolism, and nicotinate and nicotinamide metabolism. This study suggest that chronic stress-induced depression-like behavior may further disturb diabetes-itself energy metabolome. The plasma and urine lipid metabolites monitoring may be useful for early detection of depression in patients with diabetes mellitus.
Introduction
Lifelong medication, risk of various complications, and heavy care burdens result in psychosocial stress in patients with diabetes mellitus, which is detrimental to mental health and often lead to depression (1). About 30% of diabetes patients experience varying degree of depression (2, 3), and therefore have worse outcomes (4, 5). The pathological mechanisms underlying depression are complex and still not fully understood. Abnormity of catecholamine neurotransmitters in the brain have been considered the primary cause of depression, Levels of 5-hydroxytryptamine (5-HT), noradrenaline (NE), and dopamine (DA) are generally reduced in patients with depression (6). Neuroinflammation is also associated with the occurrence and development of depression (7). Recently, evidences suggested that depression might be related to carbohydrate and energy metabolism abnormity (8, 9).
Since diabetes mellitus itself is a chronic metabolic disease (10), depression co-morbid is speculated to affect the abnormal metabolome in diabetics, and to increase cardiovascular complication risk (11, 12). Accordingly, early diagnosis of depression comorbidity is of importance in diabetes management. However, early identification of depression is difficult in clinical practice due to its complex etiology (13).
Metabolomics is a top-down systems biology approach for studying the metabolic responses of living systems to genetic or environmental stimuli (14, 15). It has been increasingly used to diagnose diseases, in pharmacology/toxicology studies, and for the identification of biomarkers (16). Combined metabolomics and proteomics analysis was conducted to identify potential biomarkers in an animal model of depression-like behavior (17, 18).
Among the analytical platforms of metabolomics, LC-MS is recognized as one of the best powerful tools in sensitivity, selectivity and reproducibility (19, 20). Meanwhile, LC-MS has increased the number of lipid classes that can be analyzed, it can separation and identification trace components of complex mixtures (21). So now LC-MS has become a mainstay of metabolomics for metabolite identification.
To better understand the complex metabolome changes that occur in diabetes with depression comorbidity, plasma and urine metabolome was investigated in spontaneous diabetic GK rats with depression-like behavior. This study aimed to provide a method for early identification of depression in diabetes based on changes in metabolites in plasma and urine.
Materials and Methods
Animals
Five weeks old male spontaneous diabetic GK rats were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Animal Quality Certificate: 2007000562918). The rats were housed in the Laboratory Animal Center, East China Normal University, Shanghai (Animal Experiment License: SYXK 2010-0094) for 7 weeks in a SPF-grade lab until they developed diabetes (with postprandial blood glucose level ≥ 11 mmol/L). Then they were randomly divided into the normal control group and CUMS model group (12 rats per group). Animal procedures were performed in accordance with the guidelines of the Institutional Ethics Committee.
Chemicals
LC/MS-grade acetonitrile and HPLC-grade methanol were purchased from Merck (Darmstadt, Germany). Methanoic acid was purchased from CNW Company (Germany). All other chemicals were of analytical grade and were purchased from Sigma (St. Louis, MO, USA). Leucine-enkephalin (LE) standard was obtained from Sigma Corporation (St. Louis, MO, USA). Watson's distilled water was used.
Behavior Testing–Open-Field Test
The open-field test was conducted in a quiet room (22). Briefly, the Open-field consisted of an opaque plastic box (100 × 100 × 40 cm) divided equally into 33 × 33 cm2 squares. Rats were placed in the center of this field, and the number of squares rats moved and standing times were monitored using a ZS-ZFT Video Analysis System (ZSDC Science and technology Co., Ltd., China) as indexes of locomotion activity and exploratory behavior, respectively. Animals with abnormal behavior scores >120 or < 30 were removed from the experiment prior to chronic stress. 10 rats in control group and 11 in model group ultimately completed the experiment. The test lasted 5 min and was conducted before and after 8 weeks of chronic stress.
CUMS-Induced Depression-Like Behavior Model
The CUMS-induced depression-like behavior model was established in GK rats (23, 24). Rats in the CUMS model group were individually housed in cages for 8 weeks with food and water ad libitum. Each rat was exposed to the following stressors in random order: restraint (activity restriction in a bottle, 1 h), hot water swimming (45°C, 5 min), cold water swimming (4°C, 5 min), clip tail (1cm from the end of the tail, 1min), cage tilting (45°, 24h), horizontal shaking (10 min), damp padding (24 h), noise interference (10 min), and day/night inversion (24 h). The same stress was not used twice continuously in a row to avoid prediction. After using 6–7 times of each stress treatment randomly, the rats were relocated to their cages. Control rats were fed normally without any stress during this period.
Biochemical Assay
Before and after model establishment, blood was collected from eye can thus, kept in heparinized tubes, and centrifuged at 3,000 × g at 4°C for 5 min to obtain plasma. Plasma serotonin and dopamine levels were measured by enzyme-linked immunosorbent assay (CUSABIO, USA) according to instruction.
Metabolome Analysis Using LC/MS
After model establishment, blood was collected as above, and the supernatant plasma was stored at −80°C. Rats were placed in metabolism cages and urine samples were collected every 24 h, aliquoted and stored at −80°C. All samples were then thawed for 15 min and vortexed for 5s prior to analysis. A total of 100 μL of plasma/urine sample was mixed with 300 μL of methanol, vortexed for 30 s, and left undisturbed for 20 min. After centrifugation at 12,000 × g for 15 min at 4°C, 200 μL supernatant was extracted for LC/MS assay.
LC/MS Analysis
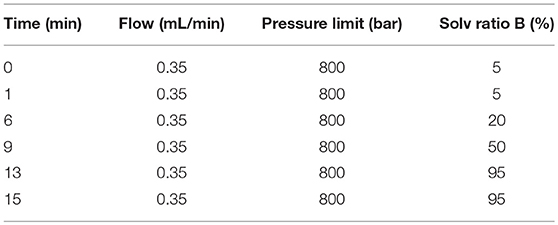
LC/MS (Agilent, 1290 Infinity LC, 6530 UHD and Accurate-Mass Q-TOF/MS) were used to analyze the samples. The chromatographic separation conditions are as follows. Each sample (4 μL) was injected into a C18 column (Agilent, USA, 100 × 2.1 mm, 1.8 μm) at 4°C, and the following mobile phases were used at a flow-rate of 0.4 mL/min at 40°C: H2O with 0.1% methane acid (A) and acetonitrile with 0.1% methane acid (B). Gradient elution values are shown in Table 1. Mass spectrometry analysis was performed in V flight tube detection mode with nitrogen as the atomization cone gas in both positive and negative ion modes. The source temperature was set at 100°C, the extraction cone was at 4v, and a cone gas flow of 50 L/h was used in both modes. Capillary voltage was set at 4.0 and 3.5 kV, the sampling cone was 35 and 50 kV, the desolvation temperature was 350°C and 300°C, respectively, and the desolvation gas flow was set at 600 and 700 L/h, respectively. Mass spectrometric data was collected in centroid mode from 50 to 1,200 m/z.
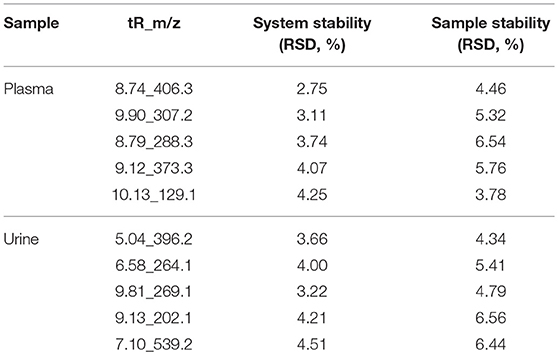
The peak height for the internal standard was continuously monitored during analysis to ensure signal stability. Quality control (QC) procedures were used to validate the methods and to ensure stability. 50 μL QC samples prepared by pooling identical volumes of individual plasma sample. To ensure that the system was suitable for use, 5 pooled QC samples were run prior to analysis in each ion mode. Five ions (min_m/z) were selected to evaluate the relative standard deviation (RSD) of retention time, m/z, and peak area. The peak area RSD values of reproducibility for most of the compounds were <10%.
Identification of Metabolites
OPLS-DA and variable importance for projection statistics (VIP > 1) were used to select significant variables driving group separation. Metabolites were represented by chromatographic peaks in total ion current chromatograms (TICs), and identified by comparing their chromatographic retention times and MS spectra with the available references and the METLIN database. In addition, the Kyoto encyclopedia of genes and genomes (KEGG, http://www.genome.jp/kegg/) biochemical database was used to interpret possible pathways involving the identified metabolites.
Data Processing and Statistical Analysis
Data from the behavioral and 5-HT/DA level tests are expressed as means ± standard errors of the mean. Student's t-tests with two tails and unequal variance were performed with GraphPad software; significant differences were indicated by p < 0.05. Mass Profile software was used for peak extraction, retention time (RT) alignment, peak alignment, and deconvolution analysis. Finally, data were imported into SIMCA-P software (v13.0, Umetric, Umea, Sweden) for PCA, PLS-DA, and OPLS-DA. VIP > 1 and p < 0.05 were considered statistically significant.
Results
Behavioral Testing-Open-Field Test
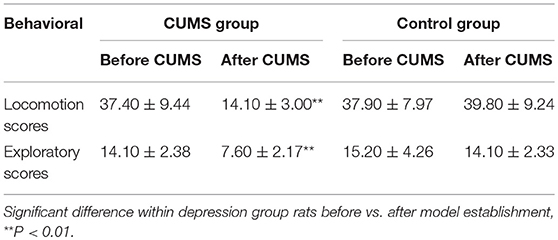
An open-field behavioral test revealed significant differences between 2 groups after 8 weeks of chronic stress treatment. At the end of 8 weeks CUMS, the locomotion and exploratory scores are decreased in CUMS model group rats compared to baseline (14.1 vs. 37.4, p < 0.01, and 7.6 vs. 14.2, p < 0.01, respectively). No different change occurred in control group rats (Table 2).
Biochemical Assay
Biochemical test was done to show that chronic stress treatment also reduces serotonin (5-HT) and dopamine (DA) levels in CUMS model group rats [from 62.09 to 22.17 ng/mL (p < 0.01) and from 0.55 to 0.21 ng/mL (p < 0.01), respectively]. Neither serotonin nor dopamine levels change over time in normal control group rats (Table 3). These results confirm that CUMS successfully induce depression-like behavior in GK rats.
Metabolome Analysis Using LC/MS
Total ion chromatograms (TICs) were visually inspected to examine the stability and reproducibility of the instrument. The results indicate that it was functioning optimally (Figures 1A–D). Typical LC/MS TICs for plasma and urine samples from the two groups (Figures 2A–H) indicate that the analysis was highly reproducible. Quality control tests of LC/MS TICs were performed for plasma and urine samples. As shown in Table 4, RSD values for the quality control samples were all <10%, indicate that the analysis system was stable.
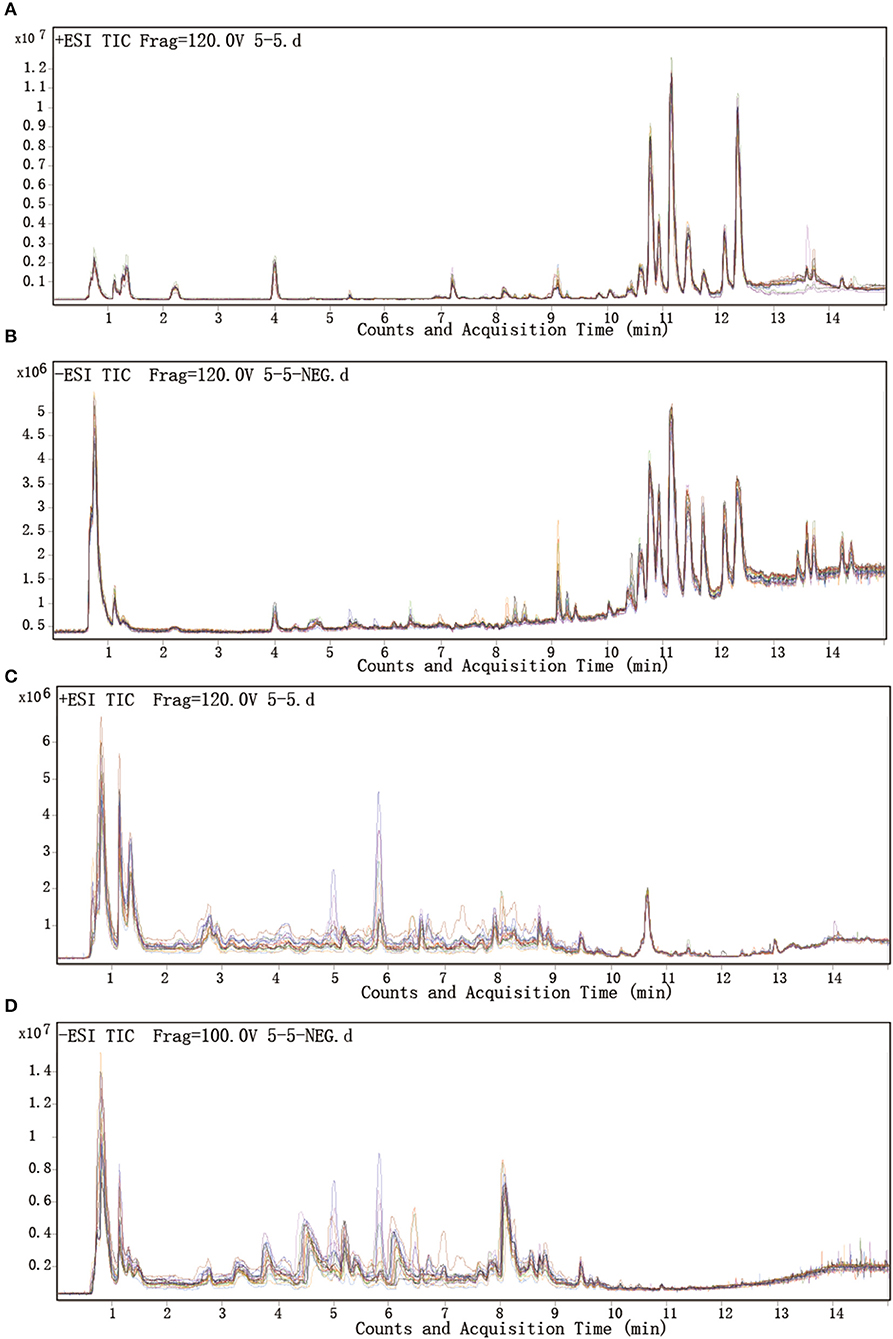
Figure 1. Representative LC-Q/TOF-MS analysis of total ion chromatograms for plasma and urine. (A,B) Plasma samples in positive and negative mode. (C,D) Urine samples in positive and negative mode.
Figure 2. Typical LC/MS TICs of plasma and urine samples from the two groups. (A,B) Plasma samples from the model group in positive and negative mode. (C,D) Plasma samples from the control group in positive and negative mode. (E,F) Urine samples from the model group in positive and negative mode. (G,H) Urine samples from the control group in positive and negative mode.
Unsupervised principal component analysis (PCA) was performed to provide an overview of the LC-MS data. PCA score plots are shown in Figure 3. Significant differences are observed between the normal control and CUMS model groups in both ion modes for plasma and urine samples.
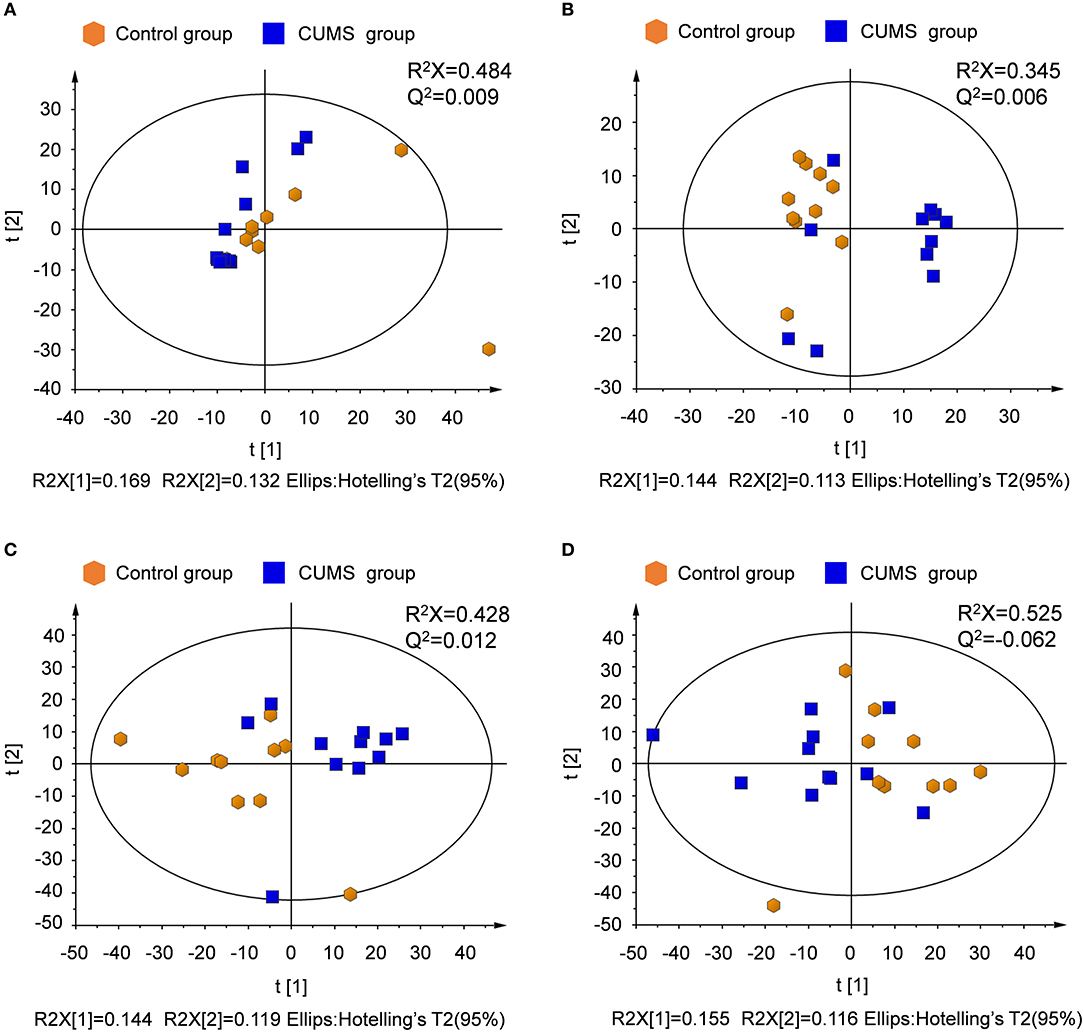
Figure 3. PCA score plots derived from LC-MS analysis of plasma and urine samples from model and control group rats. (A) Plasma in positive ion mode, (B) plasma in negative ion mode, (C) urine in positive ion mode, (D) urine in negative ion mode.
Supervised partial least squares-discriminate analysis (PLS-DA) was used to examine metabolic patterns and to detect changes in the concentration of individual metabolites in the model. Measures of the quality of the resulting discrimination, including the values of R2X, R2Y, and Q2, are shown in Figure 4. The values of R2 and Q2 were > 0.5, indicating good fitness and prediction.
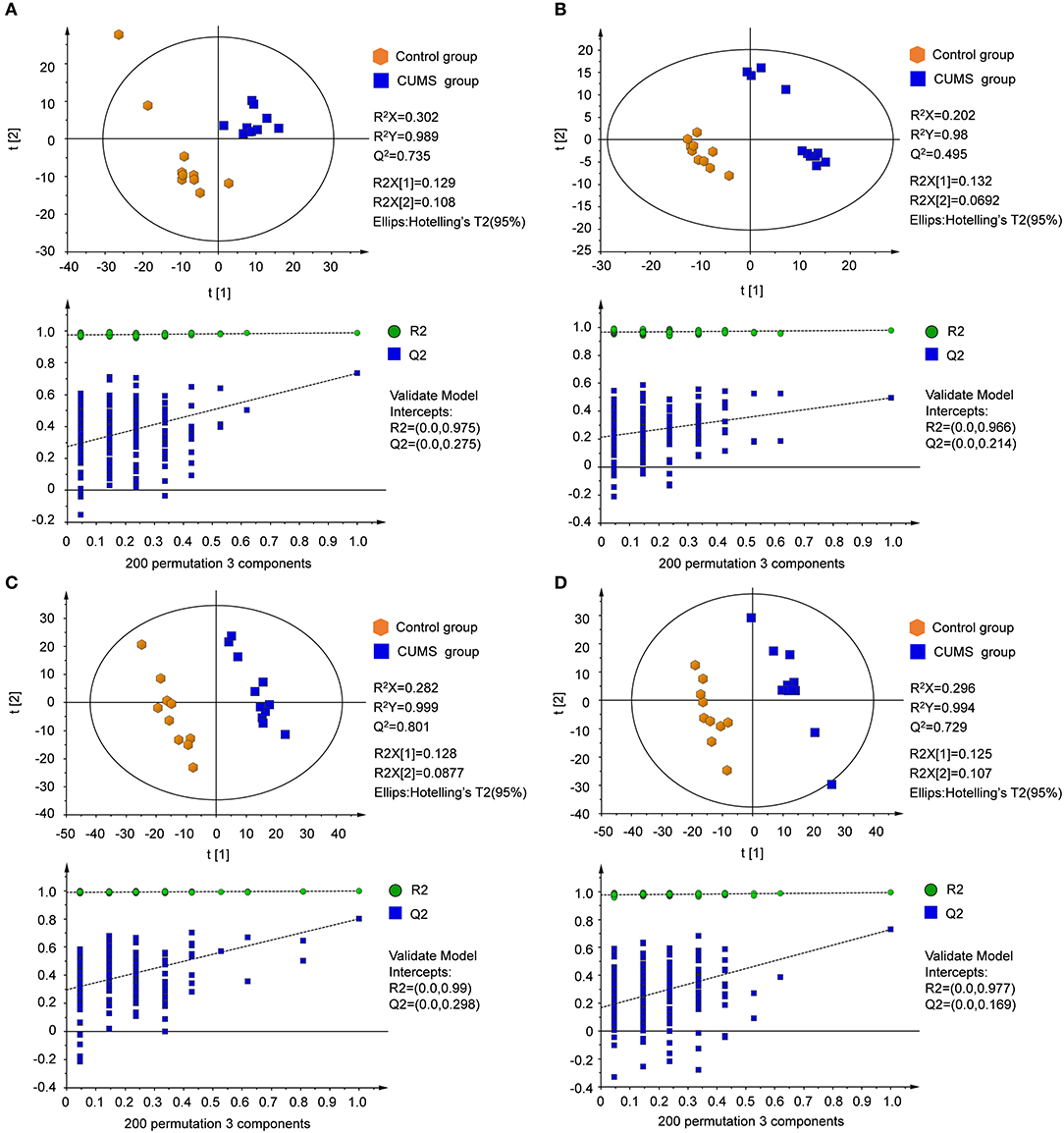
Figure 4. PLS-DA score plots and sorting validation figures derived from LC-MS analysis of plasma and urine samples from model and control groups in different ion modes. (A) PLS-DA score plot and sorting validation of plasma in ESI+, (B) PLS-DA score plot and sorting validation of plasma in ESI–, (C) PLS-DA score plot and sorting validation of urine in ESI+, (D) PLS-DA score plot and sorting validation of urine in ESI–.
The orthogonal partial least squares-discriminate analysis (OPLS-DA) model was constructed after the PLS-DA analysis. The key model parameters are summarized in Figure 5. Results for different groups were visualized as score plots to show group clusters. S-plots were used to identify variables that contributed to the classification. Good separation between the groups was observed.
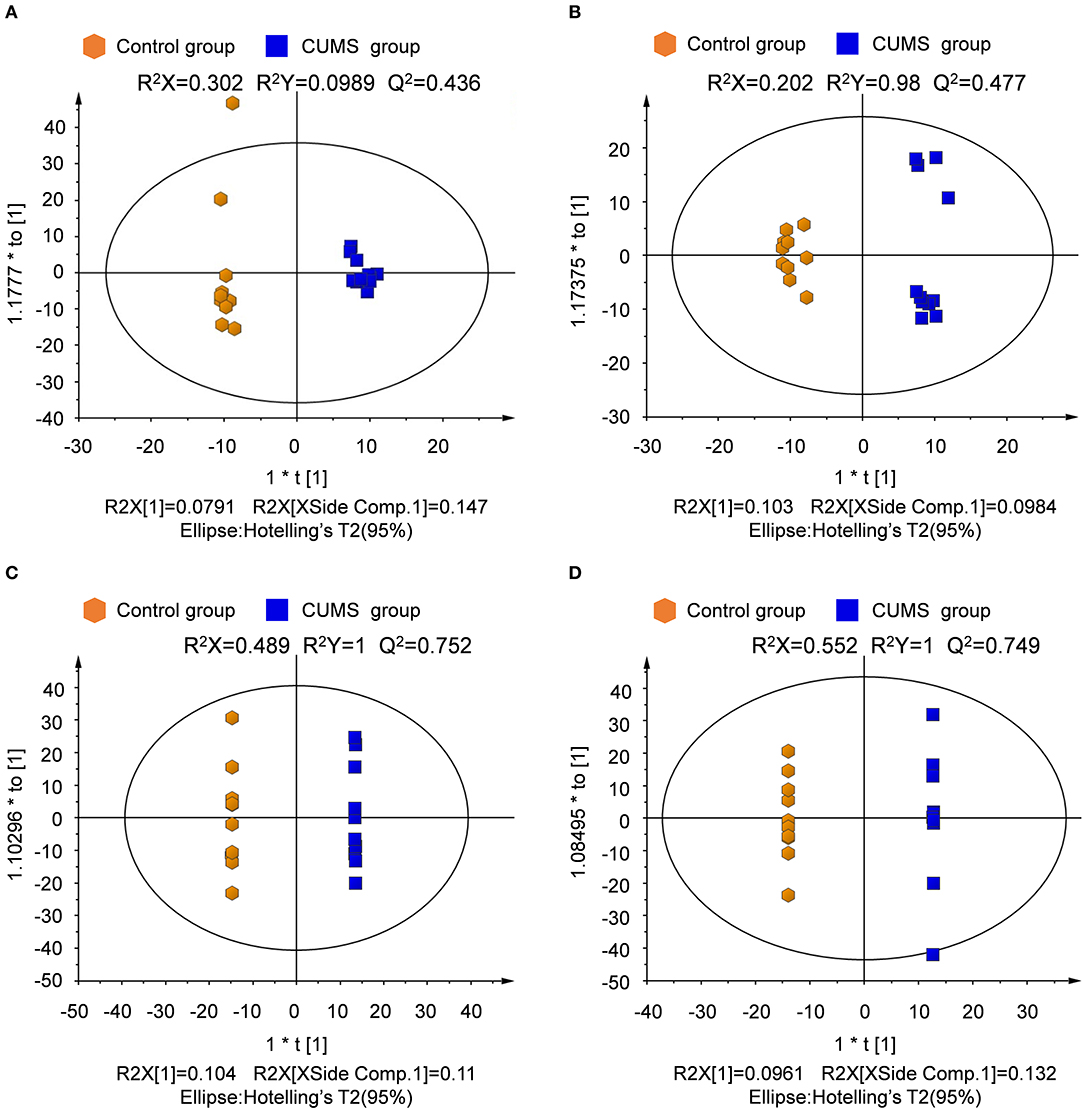
Figure 5. Score plot derived from OPLS-DA model classification. (A) Plasma in positive ion mode, (B) plasma in negative ion mode, (C) urine in positive ion mode, (D) urine in negative ion mode.
Chronic Stress Disturbs Metabolome
Differentially-expressed metabolites were identified based on the variable VIP with a threshold of 1.0 from the OPLS-DA model and p-value < 0.05 in the t-test. Metabolites were identified based on mass assignment and Rt-m/z and were then compared with authentic standards or database resources, such as KEGG (http://www.genome.jp/kegg/) and METLIN (http://metlin.scripps.edu).
Among thousands of metabolites, 20 molecules in plasma and 16 in urine were significantly correlated with CUMS-induced depression-like behavior. Specifically, in the plasma of CUMS group, levels of 2-methylhippuric acid, bilirubin, sphinganine, and sphingosine were increased, meanwhile levels of oleamide, oleic acid, L-tryptophan, L-tyrosine, lysophosphatidylcholine (lysoPC), lysophosphatidylethanolamine (lysoPE), and α-D-glucose were decreased. In the urine, 2-methylhippuric acid, hydrocinnamic acid, phenyllactic, 4-hydroxy-L-glutamic acid, pyroglutamic acid, and cholic acid were significantly up-regulated., whereas the levels of oleamide, cortisone, cytosine, hypoxanthine, malic acid, and α-linolenic acid were down-regulated. The plasma and urinary metabolites responsible for the observed difference between CUMS and normal control rats were summarized in Table 5, together with retention time, mass-to-charge ratios, possible pathways, and KEGG IDs (obtained using the KEGG pathway database). These data suggest that changes occurred in the following 7 metabolic pathways in CUMS group (Figures 6A,B): fatty acid metabolism, sphingolipid metabolism, phenylalanine metabolism, citrate cycle, glycolysis, glutathione metabolism, nicotinate and nicotinamide metabolism.
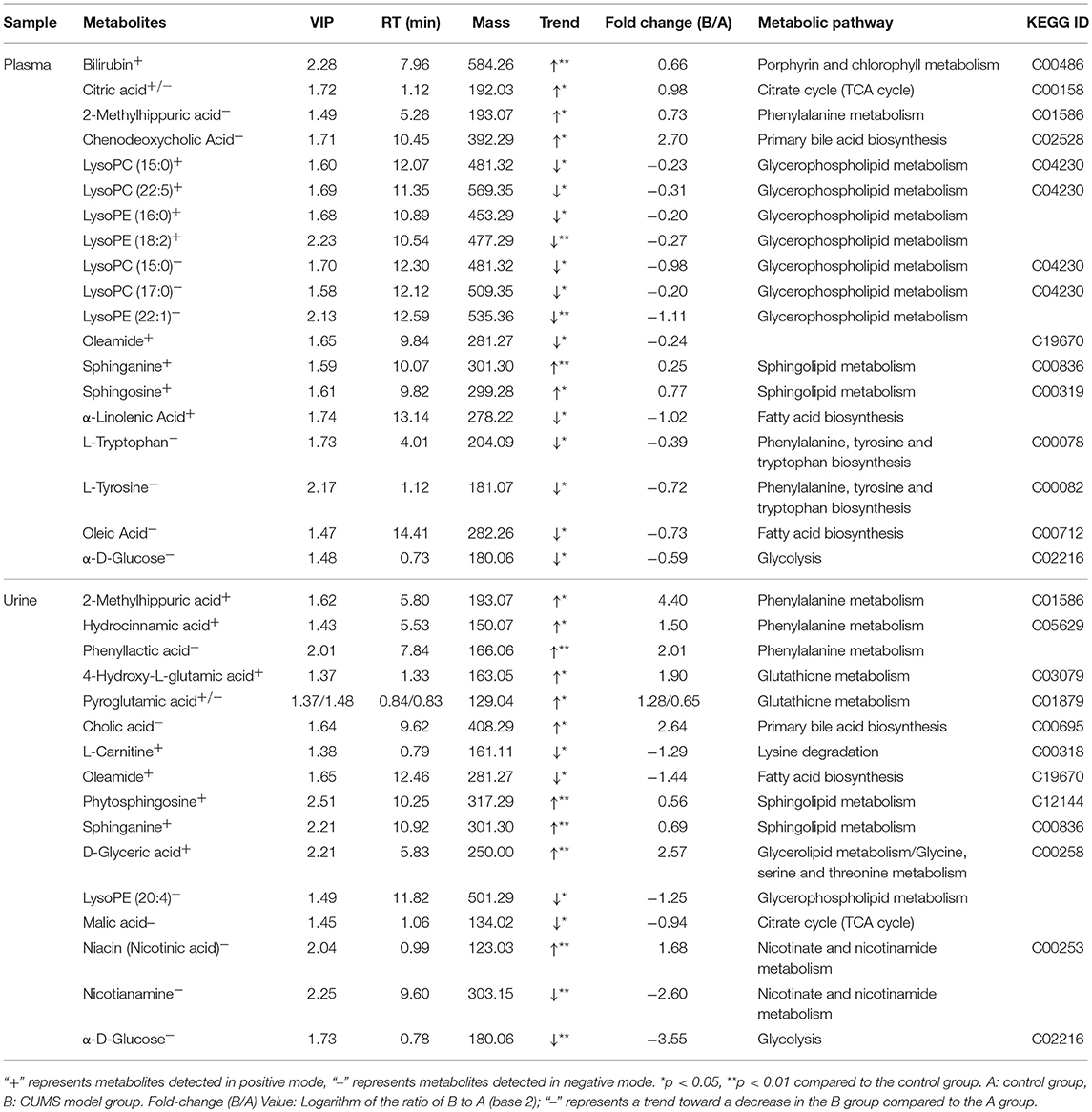
Table 5. Metabolites in plasma/urine samples that differed between the control and CUMS-induced depression groups.
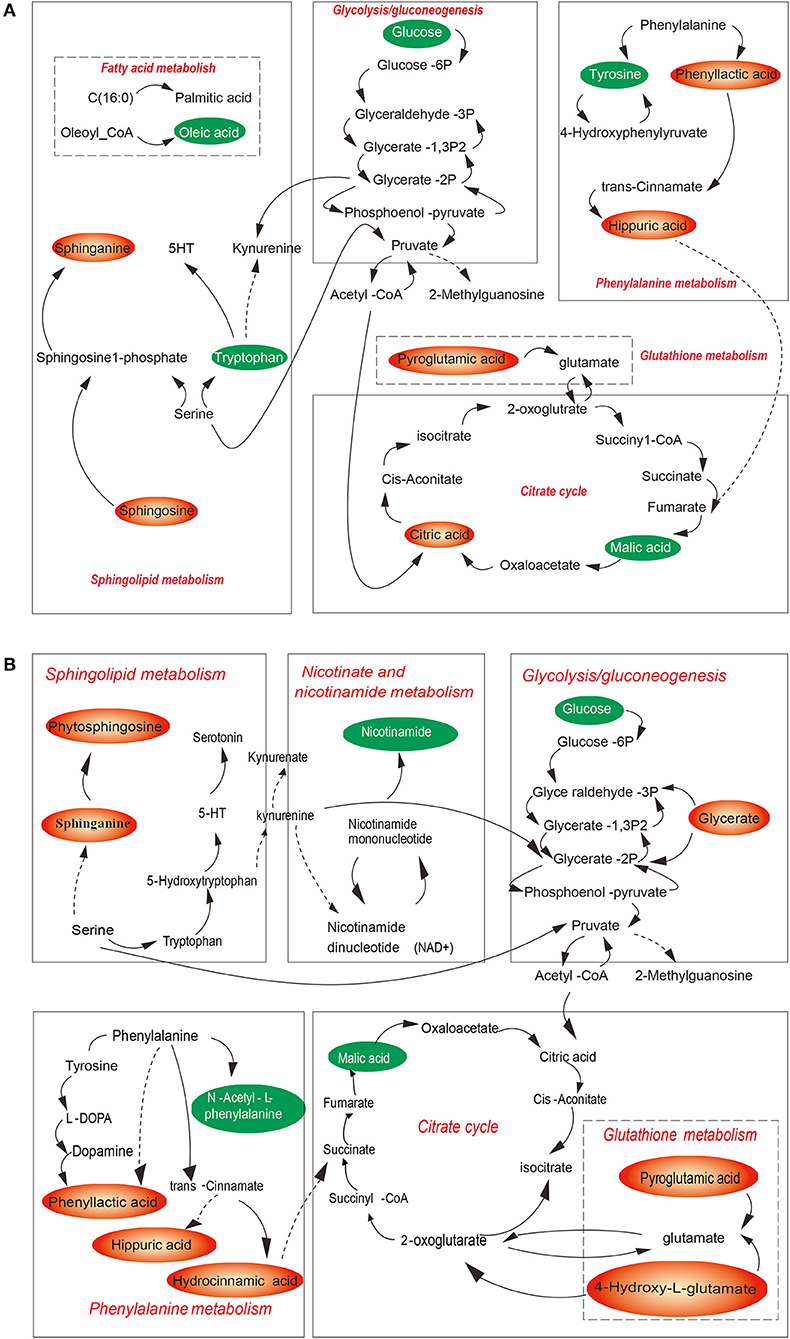
Figure 6. Overview of the metabolic pathways associated with CUMS-induced depression for plasma (A) and of urine (B) samples. Red metabolites were increased in the model group, green metabolites were decreased in model group. Red pathways are associated with CUMS-induced depression like bebavior.
Discussion
Up to now, few researches focused on the impact of chronic stress-induced depression-like behavior on blood plasma and urine metabolome in diabetes patients or in experimental diabetic rats, although metabolome analysis has been proposed for screening for depression (25, 26). In this study, we found that the disturbance in lipid/amino acid/glucose metabolism occurred in diabetes rats with depression comorbidity. We identified 20 plasma metabolites and 16 urine metabolites of GK rats with depression comorbidity. In CUMS model group, we noted with interest that lipid metabolite changes occurred primarily in blood plasma, meanwhile amino acid metabolite changes occurred primarily in urine. The different metabolites identified between plasma and urine due to the different content they have.
In this study we found that lysoPC/lysoPE was decreased in blood plasma in CUMS model rats. PC and PE are considered to be related with normal cognition function and memory (27). Therefore, chronic stress-induced depression-like behavior might be contributed by decrease of lysoPC/lysoPE. Besides, plasma sphingosine and urine phytosphingosine, plasma sphinganine and urine sphinganine were increased in CUMS model rats, which might resulted in ceramide increase. High level of ceramide expression in the brain induces depression (28, 29).
It is worth mentioning that among these altered metabolites, 2-methylhippuric acid was increased meanwhile oleamide was decreased both in blood plasma and in urine, respectivelty, although the biological mechanism is not clear right now. Oleamide is thought to be beneficial to enhance the conductivity of 5-HT receptors and neurons (30), meanwhile 2-methylhippuric acid is thought to be detrimental for it induces nervous system damage in vivo and leads to depression (31, 32).
In this study, chronic stress decrease plasma/urine α-D-glucose levels in diabetic rats, which was different from other reports (12, 33). This might be due to different experimental animal model. In this work only male rats were used for study. Gender also influences blood glucose levels in depression patients. Additional research is needed to examine these contradictory results.
The application of LC-MS coupled with PCA makes it possible to show the fact that the biochemical changes of CUMS induced depression-like behavior rats are obviously different from those of the normal control ones, but the mechanism is not clear. Chronic stress was known to disturb of homeostasis, and lead to psychological diseases such as depression. HPA axis with the endpoint release of corticosterone into the circulation plays an important role in mediating the neuroendocrine response to stress (34), so we supposed that the possible mechanism is that changes of metabolic profile resulted from chronic stress via the HPA axis, lead to the metabolic disorders in GK rats.
Clinical practice indicates that glycemic control in diabetes with depression could be improved by anti-depressants co-administration (35). Therefore, diagnosis and interventions as early as possible for depression comorbidity are of importance for successful diabetes management. Blood plasma and urine metabolome detection might be useful for this purpose.
Conclusion
We conclude that chronic stress-induced depression-like behavior may further disturb diabetes-itself energy metabolome. The plasma and urine lipid metabolites monitor may be useful for early detection of depression in patients with diabetes mellitus.
Author Contributions
TZ and FX conceived and designed the projects. YR, CY, ZL, ZY, ZX, and JD performed the experiments and carried out data analysis. YR and CY drafted the article, TZ and FX finalized the paper and provided suggestions to improve it.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81402995). Shanghai Science and Technology Commission (No. 16411973200), Shanghai Fengxian Science and Technology Research Commission (No. 20161007, No. 20161008), and Shanghai University of Medicine and Health Science (No. SFP-18-20-15-002).
References
1. Egede LE, Ellis C. Diabetes and depression: global perspectives. Diabetes Res Clin Pract. (2010) 87:302–12. doi: 10.1016/j.diabres.2010.01.024
2. Singer S, Das-Munshi J, Brahler E. Prevalence of mental health conditions in cancer patients in acute care–a meta-analysis. Ann Oncol. (2010) 21:925–30. doi: 10.1093/annonc/mdp515
3. Al-Asadi AM, Klein B, Meyer D. Multiple comorbidities of 21 psychological disorders and relationships with psychosocial variables: a study of the online assessment and diagnostic system within a web-based population. J Med Internet Res. (2015) 17:e55. doi: 10.2196/jmir.4143
4. Semenkovich K, Brown ME, Svrakic DM, Lustman PJ. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs (2015) 75:577–87. doi: 10.1007/s40265-015-0347-4
5. Katon WJ, Rutter C, Simon G, Lin EH, Ludman E, Ciechanowski P, et al. The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care (2005) 28:2668–72. doi: 10.2337/diacare.28.11.2668
6. Du H, Wang K, Su L, Zhao H, Gao S, Lin Q, et al. Metabonomic identification of the effects of the Zhimu-Baihe saponins on a chronic unpredictable mild stress-induced rat model of depression. J Pharm Biomed Anal. (2016) 128:469–79. doi: 10.1016/j.jpba.2016.06.019
7. Dale E, Bang-Andersen B, Sanchez C. Emerging mechanisms and treatments for depression beyond SSRIs and SNRIs. Biochem Pharmacol. (2015) 95:81–97. doi: 10.1016/j.bcp.2015.03.011
8. Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care (2001) 24:1069–78. doi: 10.2337/diacare.24.6.1069
9. Reus GZ, Dos Santos MAB, Strassi AP, Abelaira HM, Ceretta LB, Quevedo J. Pathophysiological mechanisms involved in the relationship between diabetes and major depressive disorder. Life Sci. (2017) 183:78–82. doi: 10.1016/j.lfs.2017.06.025
10. Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. (2011) 17:448–53. doi: 10.1038/nm.2307
11. Katon WJ, Lin EH, Russo J, Von Korff M, Ciechanowski P, Simon G, et al. Cardiac risk factors in patients with diabetes mellitus and major depression. J Gen Intern Med. (2004) 19:1192–9. doi: 10.1111/j.1525-1497.2004.30405.x
12. Zhang X, Norris SL, Gregg EW, Cheng YJ, Beckles G, Kahn HS. Depressive symptoms and mortality among persons with and without diabetes. Am J Epidemiol. (2005) 161:652–60. doi: 10.1093/aje/kwi089
14. Hong M, Jin Y, Mai YQ, Boersma A, Han KK, Vantyghem MC, et al. The decline of atrial natriuretic peptide (ANP) gene expression in older rats and the effects of ginsenoside on ANP gene expression. Comp Biochem Physiol B. (1992) 101:35–9. doi: 10.1016/0305-0491(92)90155-K
15. Jia HM, Li Q, Zhou C, Yu M, Yang Y, Zhang HW, et al. Chronic unpredictive mild stress leads to altered hepatic metabolic profile and gene expression. Sci Rep. (2016) 6:23441. doi: 10.1038/srep23441
16. Zhao L, Xiong Z, Lu X, Zheng S, Wang F, Ge L, et al. Metabonomic evaluation of chronic unpredictable mild stress-induced changes in rats by intervention of fluoxetine by HILIC-UHPLC/MS. PLoS ONE (2015) 10:e0129146. doi: 10.1371/journal.pone.0129146
17. Shao WH, Chen JJ, Fan SH, Lei Y, Xu HB, Zhou J, et al. Combined metabolomics and proteomics analysis of major depression in an animal model: perturbed energy metabolism in the chronic mild stressed rat cerebellum. OMICS (2015) 19:383–92. doi: 10.1089/omi.2014.0164
18. Wu H, Wang P, Liu M, Tang L, Fang J, Zhao Y, et al. A 1H-NMR-Based metabonomic study on the anti-depressive effect of the total alkaloid of corydalis rhizoma. Molecules (2015) 20:10047–64. doi: 10.3390/molecules200610047
19. Chen H, Cao G, Chen DQ, Wang M, Vaziri ND, Zhang ZH, et al. Metabolomics insights into activated redox signaling and lipid metabolism dysfunction in chronic kidney disease progression. Redox Biol. (2016) 10:168–78. doi: 10.1016/j.redox.2016.09.014
20. Zhao YY, Wu SP, Liu S, Zhang Y, Lin RC. Ultra-performance liquid chromatography-mass spectrometry as a sensitive and powerful technology in lipidomic applications. Chem Biol Interact. (2014) 220:181–92. doi: 10.1016/j.cbi.2014.06.029
21. Zhao YY, Liu J, Cheng XL, Bai X, Lin RC. Urinary metabonomics study on biochemical changes in an experimental model of chronic renal failure by adenine based on UPLC Q-TOF/MS. Clin Chim Acta (2012) 413:642–9. doi: 10.1016/j.cca.2011.12.014
22. Roth KA, Katz RJ. Further studies on a novel animal model of depression: therapeutic effects of a tricyclic antidepressant. Neurosci Biobehav Rev. (1981) 5:253–8. doi: 10.1016/0149-7634(81)90006-3
23. Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl). (1997) 134:319–29. doi: 10.1007/s002130050473
24. Zeng Y, Xie X, Duan J, Zhou T, Zhang Y, Yang M, et al. Perturbation of mitiglinide metabolism by chronic unpredicted mild stress in rats. Sci Rep. (2014) 4:3794. doi: 10.1038/srep03794
25. Zheng P, Wang Y, Chen L, Yang D, Meng H, Zhou D, et al. Identification and validation of urinary metabolite biomarkers for major depressive disorder. Mol Cell Proteomics (2013) 12:207–14. doi: 10.1074/mcp.M112.021816
26. Jia HM, Feng YF, Liu YT, Chang X, Chen L, Zhang HW, et al. Integration of (1)H NMR and UPLC-Q-TOF/MS for a comprehensive urinary metabonomics study on a rat model of depression induced by chronic unpredictable mild stress. PLoS ONE (2013) 8:e63624. doi: 10.1371/journal.pone.0063624
27. Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA, et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes (2009) 58:337–43. doi: 10.2337/db08-1228
28. Gulbins E, Palmada M, Reichel M, Luth A, Bohmer C, Amato D, et al. Acid sphingomyelinase-ceramide system mediates effects of antidepressant drugs. Nat Med. (2013) 19:934–8. doi: 10.1038/nm.3214
29. Kornhuber J, Muller CP, Becker KA, Reichel M, Gulbins E. The ceramide system as a novel antidepressant target. Trends Pharmacol Sci. (2014) 35:293–304. doi: 10.1016/j.tips.2014.04.003
30. Boger DL, Patterson JE, Jin Q. Structural requirements for 5-HT2A and 5-HT1A serotonin receptor potentiation by the biologically active lipid oleamide. Proc Natl Acad Sci USA. (1998) 95:4102–7. doi: 10.1073/pnas.95.8.4102
31. Xu T, Liu S, Zhao J, Feng G, Pi Z, Song F, et al. A study on the effective substance of the Wu-tou formula based on the metabonomic method using UPLC-Q-TOF-HDMS. Mol Biosyst. (2015) 11:3081–91. doi: 10.1039/C5MB00454C
32. Suzuki M, Suzuki M, Kitamura Y, Mori S, Sato K, Dohi S, et al. Beta-hydroxybutyrate, a cerebral function improving agent, protects rat brain against ischemic damage caused by permanent and transient focal cerebral ischemia. JPN J Pharmacol. (2002) 89:36–43. doi: 10.1254/jjp.89.36
33. Marano CM, Workman CI, Lyman CH, Kramer E, Hermann CR, Ma Y, et al. The relationship between fasting serum glucose and cerebral glucose metabolism in late-life depression and normal aging. Psychiatry Res. (2014) 222:84–90. doi: 10.1016/j.pscychresns.2014.01.009
34. Franco AJ, Chen C, Scullen T, Zsombok A, Salahudeen AA, Di S, et al. Sensitization of the hypothalamic-pituitary-adrenal axis in a male rat chronic stress model. Endocrinology (2016) 157:2346–55. doi: 10.1210/en.2015-1641
Keywords: chronic unpredictable mild stress, depression, diabetes, metabolome, rats
Citation: Ren Y, Yang C, Li Z, Yang Z, Xiao Z, Duan J, Zhou T and Xu F (2018) Chronic Stress Disturbs Metabolome of Blood Plasma and Urine in Diabetic Rats. Front. Psychiatry 9:525. doi: 10.3389/fpsyt.2018.00525
Received: 25 July 2018; Accepted: 03 October 2018;
Published: 23 October 2018.
Edited by:
Gianluca Serafini, Ospedale San Martino (IRCCS), ItalyCopyright © 2018 Ren, Yang, Li, Yang, Xiao, Duan, Zhou and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Zhou, d293aW5uZXIyMDExQDE2My5jb20=
Feng Xu, eHVmQHNtdS5lZHUuY24=
†These authors have contributed equally to this work
 Yu Ren
Yu Ren Cheng-hua Yang2†
Cheng-hua Yang2† Zhen Yang
Zhen Yang Zhi-jun Xiao
Zhi-jun Xiao Ting Zhou
Ting Zhou Feng Xu
Feng Xu