- 1Center for Affective, Stress and Sleep Disorders, Psychiatric Clinics, University of Basel, Basel, Switzerland
- 2Substance Abuse Prevention Research Center and Sleep Disorders Research Center, Department of Psychiatry, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 3Isfahan Neurosciences Research Center, Alzahra Research Institute, Isfahan University of Medical Sciences, Isfahan, Iran
- 4Kliniken Valens, Valens, Switzerland
- 5Sport Science Section, Department of Sport, Exercise and Health, University of Basel, Basel, Switzerland
- 6Department of Neurology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
Background: Multiple sclerosis (MS) patients suffer from various difficulties including sleep complaints, symptoms of depression and fatigue, paresthesia, and cognitive impairments. There is growing evidence that regular physical activity has a positive effect on both sleep and psychological functioning, though there is limited evidence of this kind for MS patients. The aim of the present study was therefore to investigate the impact on this patient group of a regular exercise program with respect to subjective and objective sleep, depression, paresthesia, fatigue, and cognitive performance.
Methods: A total of 46 patients [mean age: 50.74 years; Expanded Disability Status Scale (EDSS): mean: 5.3, 78.4% females] completed this 3-week intervention study. At baseline and 3 weeks later, they answered questionnaires covering sociodemographic information, subjective sleep, depression, fatigue, paresthesia, and subjective physical activity. Objective sleep [sleep electroencephalogram (EEG) recordings] and cognitive performance were also assessed at both time points. Patients participated in a regular exercise activity every weekday for about 60 min.
Results: Compared to the baseline, by the end of the study, objective sleep had significantly improved (sleep efficiency, sleep onset latency, and wake time after sleep onset), and symptoms of sleep complaints, depression, fatigue, and paresthesia were significantly reduced. Subjective physical activity (moderate and vigorous) and cognitive performance also increased over the course of the intervention.
Conclusions: In patients with MS, participation in regular exercise impacted positively on their objective and subjective sleep, depression, paresthesia, fatigue, and cognitive performance.
Introduction
Worldwide, about 2.5 million people suffer from multiple sclerosis (MS) (1). Multiple sclerosis (MS) is defined as a neurodegenerative disease involving both psychological and physical impairments (2). Typically, MS sufferers have sleep complaints, symptoms of depression, fatigue, and paresthesia (3–8), reduced physical activity levels (9–13), and cognitive impairments (14–17). Importantly, Carta et al. (18) and Fornaro et al. (19) underlined the importance of a thorough pharmacological treatment of psychiatric symptoms in individuals with MS. With regard to sleep, recent research has shown that at MS onset, sleep is not impaired (20). The same research group also found that sleep quality in these patients remained unchanged 2 years after disease onset (21). Not surprising, poor sleep, symptoms of depression, paresthesia, fatigue, and cognitive impairments have a negative impact on quality of life (22, 23). It follows therefore that their improvements should have a beneficial effect on patients’ quality of life (24–26). There are various treatment options for improving attributes such as sleep and cognitive performance and reducing depression and fatigue, among MS patients, and given that these psychological and physical characteristics appear to be highly interlinked, one might expect that their improvements or impairments would also be associated with improvements or impairments in the others (27–31).
With regard to sleep, numerous studies of otherwise healthy individuals have shown poor sleep to be associated with poor emotion regulation (32–34), lower cognitive performance (35, 36), and mood alterations (37, 38). Among MS patients, poor sleep has been linked to impaired cognitive performance (28, 31), higher fatigue scores, and depression (27, 39, 40). Several reviews also conclude that sleep is impaired in about 50% of MS patients (3, 4, 6–8, 29, 41–43). However, Sadeghi Bahmani et al. (20) showed that, among a small sample of MS patients aged 32.3 years, sleep quality indices at disease onset did not differ from the indices of nonclinical adolescents and young adults. It is notable that in the same group of patients, sleep quality indices were no different 2 years later (21), though sedentary behavior had decreased and levels of moderate physical activity increased, but vigorous physical activity had decreased.
There are several potential treatment options for improving the sleep of MS patients. Pharmacological interventions mainly consist of the prescription of benzodiazepines and selective serotonin reuptake inhibitors (SSRIs), although their long-term efficacy appears to be questionable. Furthermore, their side effects can be irritating and may even increase daytime sleepiness, depression, and fatigue, and worsen cognitive performance (3, 44, 45); for these reasons, they are not suitable for long-term treatment. Nonpharmacological treatment approaches include cognitive behavioral therapy (46, 47) and relaxation techniques (43, 48–50). Several studies of healthy individuals have also shown that exercise interventions can improve sleep (51–55). However, there are only a few studies on the benefits of interventions for MS patients. As one example, Siengsukon et al. (56) randomly allocated 28 MS patients either to moderate-intensive aerobic exercise or to low-intensive walking/stretching. Patients engaged in one or other of these activities for 30 min three times a week, and for consecutive 12 weeks. Outcome variables were sleep quality, daytime sleepiness, fatigue, and depression. Results showed that sleep quality improved descriptively from baseline to postintervention. Nevertheless, a significant improvement in sleep was only observed in the low-intensity walking/stretching condition, whereas daytime sleepiness improved only in the moderate-intensity aerobic exercise condition. Interestingly, improvements in sleep and daytime sleepiness were not associated with any changes in depression or fatigue. To conclude, given the paucity of research into physical activity interventions to improve sleep among patients with MS, the first aim of the present study was to examine whether an intervention involving regular exercise could improve both objective and subjective sleep.
Additionally, there is a growing body of research showing that regular physical activity has a positive impact on fatigue, depression, and, for example, cardiovascular fitness in MS patients (10–12, 24, 57–63). In this regard, De Berardis et al. (64), Marini et al. (65), and Lang and Borgwardt (66) showed associations between symptoms of major depressive disorder and inflammatory markers.
With regard to paresthesia, to our knowledge, Razazian et al. (67) were first to show that regular physical activity (yoga and aquatic exercise) could reduce paresthesia, although indirectly via reductions in depression. Therefore, the second aim of the present study was to determine whether regular exercise would also have a positive impact on depression, fatigue, and paresthesia. Accordingly, the endpoints were as follows: Changes in objective and subjective sleep, depression, fatigue, and paresthesia. Secondary endpoint variables were physical activity and cognitive function.
Four hypotheses were formulated. First, following others (51, 52, 54), we expected that the objective sleep of MS patients would improve over 3 weeks of regular exercise. Second, following Siengsukon et al. (56), we anticipated that 3 weeks of regular exercise would improve their subjective sleep. Third, following others (10, 24, 57, 58, 67, 68), we hypothesized that 3 weeks of regular exercise would have a positive impact on depression, fatigue, and paresthesia among such patients. Fourth, in line with previous research (69–71), we expected that a 3-week program of regular exercise would improve the cognitive performance of MS patients.
Methods
Procedure
In the present study, inpatients with MS of the Klinik Valens (a rehabilitation center located in the Southeastern part of Switzerland) were approached and asked to participate in the present 3-week intervention study (a 3-week stay was the fixed duration of hospitalization in the clinic based on the insurance system in Switzerland). All participants were informed about the voluntary character of their participation and were assured that all the data were gathered anonymously. Signed written informed consent was obtained from the participants. All assessments took place both at baseline and 3 weeks later at discharge. At both time points, participants completed a series of questionnaires covering sociodemographic data, sleep complaints, depression, fatigue, paresthesia, and physical activity (see Tools below). Completion of the questionnaire took 30–40 min. A trained expert assessed participants’ cognitive performance and applied the EEG device for sleep assessment during one night at baseline and study end.
The local ethics committees of Basel (Ethikkommission Nordwestschweiz; EKNZ: 2016-01347) and St. Gallen (EKOS; Switzerland) approved the study, which was carried out in accordance with the ethical principles laid down in the Declaration of Helsinki and its later amendments.
Participants
Fifty-one patients were consecutively selected. Details of their EDSS scores and disease duration data were extracted from the Klinik Valens registered data. Inclusion criteria were as follows: a) physician-determined diagnosis of MS (following the McDonalds’ diagnostic criteria for MS) (72) irrespective of subtype (relapse–remitting, primary progressive, and secondary progressive), b) neurologist-rated EDSS score below 6.0, c) age between 18 and 65 years, d) willing and able to comply with the study requirements as attested by signed written informed consent, e) able to read and write in German, and f) stable MS-related pharmacological treatment such as glatiramer acetate, interferons, fumarates and immune suppressiva, and monoclonal antibodies at the beginning of the study and throughout the whole study. Exclusion criteria were as follows: a) other neurological or severe psychiatric disorders and b) intake of medications apart from MS-related medications and antidepressants.
Measures
Objective Sleep
As in previous studies (73–77), we assessed objective sleep first for one night at baseline and then for one night at the end of the study using a one-channel, portable sleep-EEG recording device. Its validity has been repeatedly confirmed (77). The following sleep continuity parameters were measured: sleep onset latency, sleep period time, total sleep time, and number and time of awakenings after sleep onset. The following sleep architecture parameters were measured: awakenings after sleep onset (number and duration in minutes); (non-REM sleep) stages 1 and 2 (light sleep; min and %) and 3 and 4 (slow wave sleep/deep sleep; min and %); rapid eye movement sleep (REM; min and %); and REM latency (time between sleep onset and first appearance of REM sleep; min).
Subjective Sleep
In line with previous research (78, 79), we measured sleep disturbances using the Insomnia Severity Index (ISI) (78). This questionnaire is a seven-item screening measure for insomnia and an outcome measure for use in treatment research. The items, answered on a five-point Likert scale ranging from 0 (not at all) to 4 (very much), refer in part to the Diagnostic and Statistical Manual of Mental Disorders (80) criteria for insomnia by assessing difficulty falling asleep, difficulty remaining asleep, early morning awakenings, impaired daytime performance, low satisfaction with sleep, and worry about sleep. The higher the overall score, the more the participant is assumed to suffer from insomnia. Cronbach’s α for the present sample was 0.87.
Depressive Symptoms
To assess symptoms of depression, we used the Beck Depression Inventory–Fast Screen (BDI-FS) (81). The BDI-FS is a brief self-report inventory designed to evaluate depression in patients with medical illness. It consists of seven items, and every item has a set of four possible responses, representing different levels of symptom severity (e.g., or sadness: 0 = “I don’t feel sad”; 1 = “I feel sad”; 2 = “I’m sad all the time and I can’t snap out of it”; 3 = “I’m so sad/unhappy, that I can’t stand it”). Higher scores reflect a greater severity of depressive symptoms (range: 0–21). Cronbach’s α for the present sample was 0.90.
Fatigue
Participants completed the Fatigue Severity Scale (FSS) (82). The FSS consists of nine items, and answers are given on seven-point rating scales ranging from 1 (not at all) to 7 (definitively/almost always), with higher scores reflecting higher levels of fatigue. Cronbach’s α for the present sample was 0.91.
Paresthesia
Patients rated their degree of paresthesia on a 10-point visual analogue scale ranging from 0 (no sensations at all) to 10 (severe sensations) [see also Ref. (67)].
Physical Activity
Physical activity was assessed with the short form of the International Physical Activity Questionnaire (IPAQ-SF). The IPAQ-SF was developed by a working group initiated by the World Health Organization and the Centers for Disease Control and Prevention (83). Based on the results from 12 countries, reliability and validity of IPAQ are comparable to other self-reported measures of physical activity. The IPAQ-SF asks participants about time spent in physical activity over the last 7 days. Minutes of moderate- and vigorous-intensity activities were calculated for the previous week.
Physical Disability; the Expanded Disability Status Scale (EDSS)
The Expanded Disability Status Scale (EDSS) was employed by a trained neurologist to assess patients’ level of physical disability. The EDSS is an internationally accepted and widely used tool to provide an objective assessment of the disability levels of patients with MS (84, 85). The total score is on a scale from 0 (no impairment in neurological dimensions) to 10 (death due to MS), with increments of 0.5 to 1, and with higher scores reflecting higher levels of disability. Meyer-Moock et al. (86) reported in their systematic review the high validity and reliability of the EDSS. They further concluded that the EDSS is suitable to describe clinical status and physical disability and to monitor disease progression.
Cognitive Performance
Two instruments were used to measure cognitive performance, the Montreal Cognitive Assessment (MoCA) (87) and the Symbol Digit Modality Test (SDMT) (88). To avoid the learning effect, parallel versions of each tool were used.
Global Measurement of Cognitive Performance
The MoCA is a screening instrument that allows a global cognitive measurement to be made through the assessment of a wide range of cognitive functions, such as i) short-term memory; ii) executive functions; iii) visuospatial abilities; iv) language; v) attention, concentration, and working memory; and vi) temporal and spatial orientation. Its extensive validation, international recognition, and recommendation in various guidelines make the MoCA a useful brief cognitive screening tool in both clinical and research contexts (89) (see also https://www.mocatest.org). In the present study, we report the overall score, with higher scores reflecting better cognitive performance.
Attention, Concentration, and Information Processing Speed
The Symbol Digit Modality Test (SDMT) (88) is an instrument to assess attention, concentration, and information processing speed. The test consists of single digits paired with nine abstract symbols. Rows of the symbols are arranged pseudo-randomly. The patient must state the number that corresponds to each symbol. The SDMT can be completed within 5 min, including instructions, practice, and testing. Evidence indicating the satisfactory psychometric properties of the SDMT has been provided in previous research (90).
Exercise Intervention
Regular endurance exercise was undertaken in the course of a standardized 3-week inpatient rehabilitation program. Training consisted of physiologically defined heart-rate-controlled cycling at 60 rpm at the lactate threshold (75% of HRmax or 65% of VO2-peak). A training session lasted 30 min, with warm-up and cool-down for the first and last 2 min, respectively. A total of five training sessions were conducted per week, representing standard care at the rehabilitation center. A sport scientist expert in exercise interventions for patients with MS supervised the intervention sessions.
Regular exercise was performed in addition to the normal rehabilitation program that consisted of two physiotherapy interventions per day. Physiotherapy consisted of progressive resistance training (45 min) and a low-intensity physiotherapeutic session (30 min).
Statistical Analysis
Data were analyzed per protocol. Main outcome variables were changes in objective and subjective sleep, depression, fatigue, and paresthesia. Secondary outcome variables were physical activity and cognitive function.
To compare performances at baseline with performances at study end (3 weeks later), a series of paired t-tests was performed. Pearson’s correlations were computed between level of impairment (EDSS score) and sleep, psychological functioning, physical activity, and cognitive performance. The level of significance was set at α ≤ .05. All statistical analyses were performed with SPSS® 25.0 (IBM Corporation, Armonk, NY, USA) for Apple® Mac®.
Results
Sample Characteristics
Of the 51 patients enrolled at baseline, 46 completed the study (pre/post assessment, and intervention); 5 did not want to participate in the second assessment or left the clinic earlier than scheduled. The mean age of the sample at baseline was 50.74 years (SD = 11.28). In total, 78.4% of the participants were females. There was no difference in age between females (M = 51.89; SD = 11.70) and males [M = 46.00; SD = 8.20; t(44) = 1.42, p = .16], nor did they differ in EDSS scores [females: M = 5.49, SD = 1.11; males: M = 4.89, SD = 1.27; t(44) = 1.41, p = .17]. Next, not all participants completed all questionnaires; degrees of freedom therefore vary for the different outcomes. EEG values were available only for 26 patients (14 patients were not willing to participate in the sleep-EEG assessment, and for 6 patients, data were not recorded due to technical failures). EEG completers did not statistically significantly differ from noncompleters as regards age, EDSS, fatigue, paresthesia, cognitive dysfunction (all t values < 1.00; p values > .50), physical activity, and gender (χ2 < 0.5; p > .50).
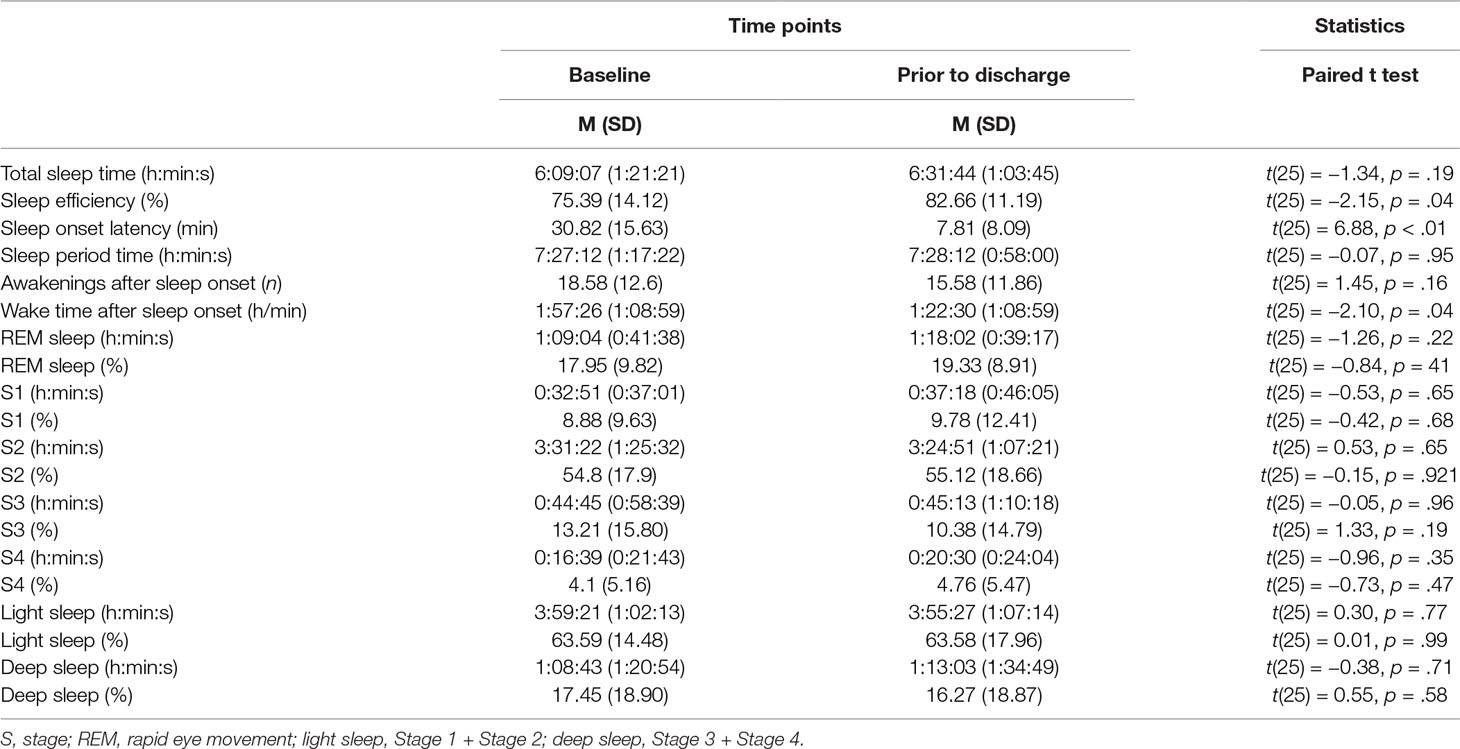
Objective Sleep
Table 1 reports the descriptive and inferential statistical indices of objective sleep parameters.
With regard to sleep continuity, sleep onset latency significantly decreased, sleep efficacy improved, and the time of awakenings after sleep onset diminished. For all other dimensions of sleep continuity (total sleep time, sleep period time, and numbers of awakenings after sleep onset), there were no significant differences between the baseline and the study end. With regard to sleep architecture (stages 1–4 and REM-sleep: min and %), there were no significant differences.
Subjective Sleep, Depression, Fatigue, and Paresthesia
Table 2 reports the descriptive and inferential statistical indices for subjective sleep, depression, fatigue, and paresthesia. These statistical indices are not therefore repeated in the text. From baseline to the end of the study, subjective sleep (complaints), depression, fatigue, and paresthesia decreased significantly.
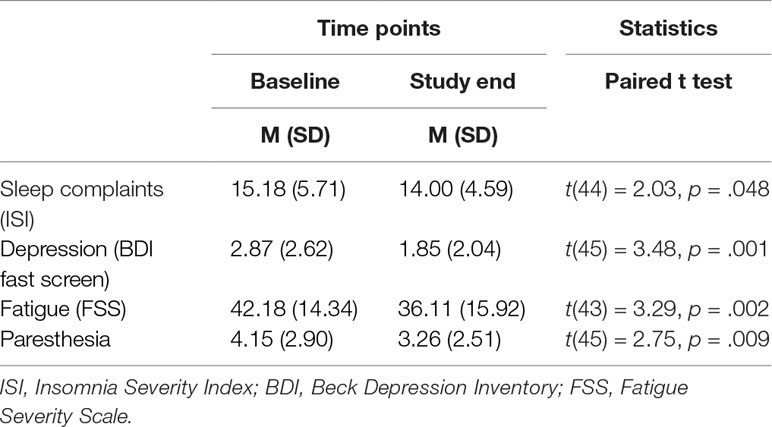
Table 2 Descriptive and inferential statistical overview of values for subjective sleep, depression, fatigue, and paresthesia at baseline and at the study end.
Subjective Physical Activity
Table 3 reports the descriptive and inferential statistical indices for subjective physical activity. From baseline to the study end, subjective vigorous and moderate physical activity increased significantly.
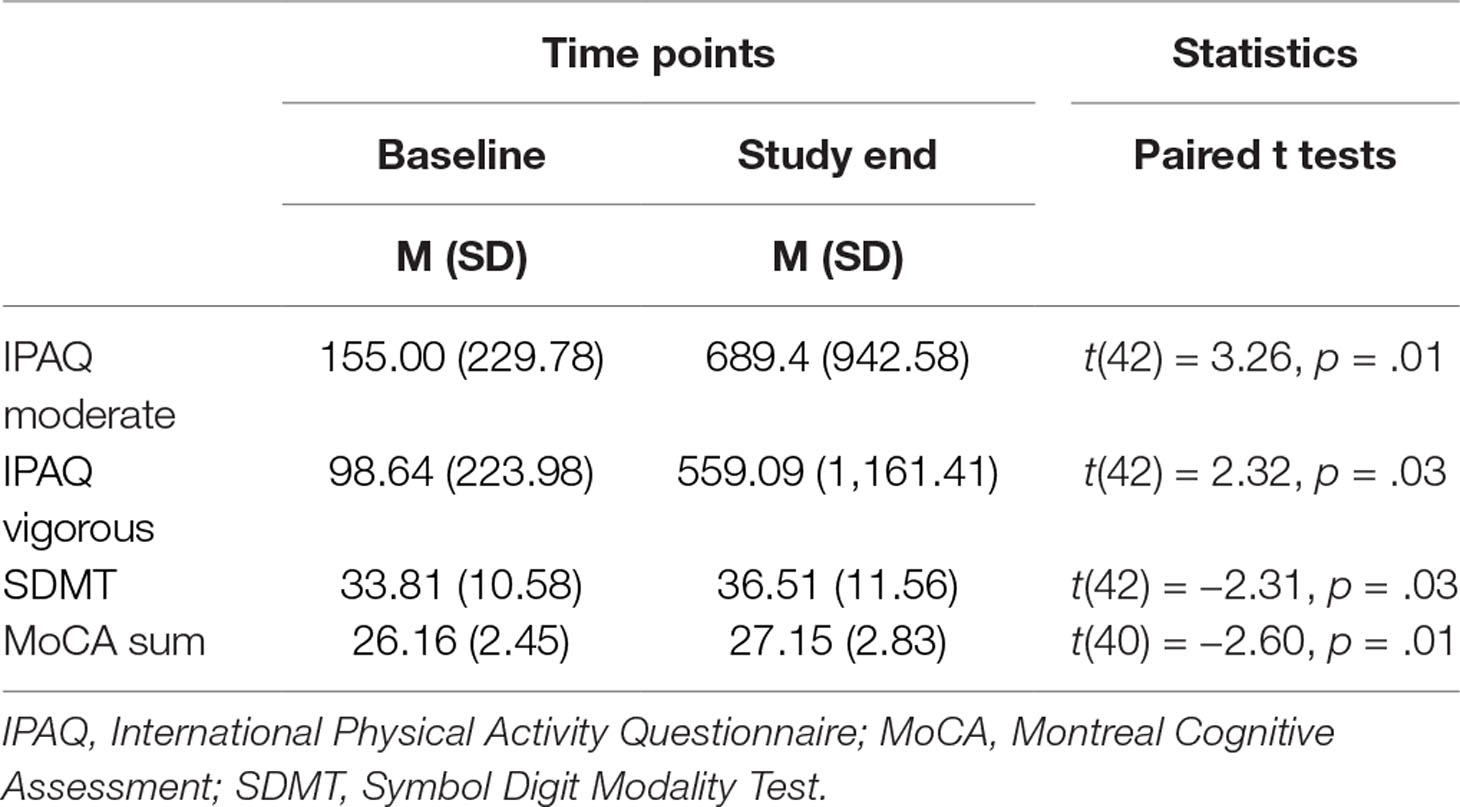
Table 3 Descriptive and inferential statistical overview of values for subjective physical activity and cognitive performance at baseline and at the study end.
Cognitive Performance
Table 3 also provides the descriptive and inferential statistical indices for cognitive performance. From baseline to the study end, attention, concentration, and information processing speed (SDMT), as well as global cognitive performance (MoCA), significantly improved.
Correlations Between Degree of Impairment (Expanded Disability Status Scale Scores) and Objective and Subjective Sleep, Depression, Fatigue, Paresthesia, Subjective Physical Activity, and Cognitive Performances
All correlation coefficients were small (all r values < .15) and nonsignificant (all p values > .30). In other words, degree of physical impairment (EDSS) was not related to objective or subjective sleep, depression, fatigue, paresthesia, subjective physical activity, or cognitive performances either at baseline or at study end.
Discussion
The key findings of the present study were that in a sample of patients with MS, a 3-week exercising program led to improvements in both objective and subjective sleep, depression, fatigue, paresthesia, and cognitive performance. The pattern of the result makes an important contribution to the current literature as, to the best of our knowledge, this is the first study to show that regular exercising has positive effects on both the objective and subjective sleep of MS patients.
Four hypotheses were formulated and these are considered in turn.
Following others (51, 52, 54), our first hypotheses was that a 3-week program of regular exercise would benefit objective sleep, and this hypothesis was confirmed. Specifically, sleep efficiency improved, sleep onset latency shortened, and the wake time after sleep onset decreased; thus, improvements in sleep continuity were observed, though we found no significant changes in sleep architecture. We believe that these findings add to the current literature in an important way as, to the best of our knowledge, these effects have not previously been investigated or observed among patients with MS.
We also note that, at baseline, indices of sleep quality such as sleep efficiency (75.4%) and sleep onset latency (about 31 min) had particularly low values. In our opinion, these findings are consistent with observations that sleep quality deteriorates with the progression of MS (3, 4, 6, 7, 29, 31, 42), while in studies by Sadeghi Bahmani and colleagues (20, 21), sleep complaints were not found at disease onset or 2 years later.
Our second hypothesis, following Siengsukon et al. (56), was that a 3-week program of regular exercise would improve subjective sleep, and this was confirmed. These findings therefore replicate those from the only previous study of which we are aware, that by Siengsukon et al. (56). However, we expanded upon their study, in finding significant changes in subjective sleep within a 3-week period.
Several studies have shown that physical activity has the potential to ameliorate sleep complaints both in healthy individuals and in patients with chronic diseases. Of various possible explanations, the most plausible are the consequences of reduced physical tiredness and rumination and enhanced mood in producing improvement in sleep complaints following a program of physical activity (51, 53).
Our third hypothesis was that a 3-week program of regular exercise would have a positive impact on the symptoms of depression, fatigue, and paresthesia of MS patients. This was confirmed for all three variables. Similar effects for depression have been reported in other studies. For example, Mota-Pereira et al. (91) showed that a program of physical activity involving regular walking for 12 consecutive weeks reduced symptoms of depression in a sample of treatment-resistant patients with major depressive disorders. In this respect, several meta-analyses have concluded that regular physical activity has potential benefits with respect to symptoms of depression both among patients with mental impairments (92–98) and among patients with MS (67). As regards fatigue, our results are consistent with findings from previous studies of MS patients (24, 57, 99–101), though we add to the current literature in demonstrating such improvements within a period of 3 weeks of regular exercising.
As regards paresthesia, so far only one study, Razazian et al. (67), has focused on improvements with respect to this characteristic following an intervention involving physical activity intervention. They showed that both a 2-month yoga and a 2-month aquatic exercise intervention reduced paresthesia in MS patients. A closer inspection of their findings reveals these improvements were due to reductions in depression.
Our fourth hypothesis was that a 3-week program of regular exercise would lead to improvements in cognitive function, and this was supported. These results therefore match the conclusions of a review by Sandroff et al. (102) analyzing the impact of physical activity on cognitive performance in MS patients across 26 studies. However, these authors also found that not all subtests significantly changed over time. Similarly, for results based on the MoCA assessment, we found significant effects with respect to memory and language, but not executive function, visuospatial ability, attention, or abstract thinking. That can be explained in terms of the type of cognitive skill, the timing of the intervention, the type of intervention, and the severity of the disease. Results equivalent to those for MoCA were obtained for SDMT. We were able to show first that, as in other studies, SDMT is a very precise tool for assessing cognitive performance in patients with MS. Second, changes in cognitive function were more related to the total score for cognitive performance than to specific skills. After all, all cognitive abilities are influenced by other skills, and what we need in coping with daily life is more related to the overall cognitive ability than to individual cognitive skills. Our findings indicate that a 3-week program of regular exercise is useful in enhancing general cognition, although the short period of this intervention may have been responsible for the lack of improvement in every aspect of cognition.
Despite the novelty of the present findings, several limitations warrant against their overgeneralization. The main limitation of this study is the absence of a control group. Given that the exercise program was part of a defined schedule for all patients hospitalized at the Valens Rehabilitation Center, including a control group without an exercise program was not an option. Nevertheless, we believe that the present pattern of results is robust, given that changes could be observed across a broad range of characteristics (objective and subjective sleep, psychological functioning, cognition). Second, it is possible that the pattern of results might have arisen because further unassessed variables biased two or more dimensions in the same or the opposite directions. Third, bias is also possible in the sample as, for reasons related to health insurance conditions, not all patients with MS are admitted to the rehabilitation center. Fourth, as regards depression, we relied on self-reports, while experts’ ratings would have further strengthened the present the pattern of the results. Fifth, a follow-up study could provide more insight into the longer-term effects of such interventions once patients have returned home and to their normal working and social environments.
Conclusions
A 3-week exercise program for patients with MS improved both objective and subjective sleep, along with improvements in psychological functioning and cognitive performance.
Ethics Statement
The local ethics committees of Basel (Ethikkommission Nordwestschweiz; EKNZ: 2016-01347) and St. Gallen (EKOS; Switzerland) approved the study.
Author Contributions
Study design: DSB, JK, JB, MP, UP, VS, MG, EHT, and SB. Data gathering: DSB, JK, JB, and MP. Statistics: DSB, MG, VS, EHT, and SB. Manuscript draft: DSB, JK, JB, MG, EHT, and SB. Approved final version: DSB, JK, JB, MP, UP, VS, MG, EHT, and SB.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Marielle Koenig, Vladimir Djurdjevic, and Daryl Niedermoser for the EEG analysis. Further, we thank Joao Ulyssea for helping us in data gathering, and we thank Edit Caric and Raphael Zihlmann for data entry. Finally, we thank Nick Emler (University of Surrey, Surrey UK) for proofreading the manuscript.
References
1. Pugliatti M, Sotgiu S, Rosati G. The worldwide prevalence of multiple sclerosis. Clin Neurol Neurosurg (2002) 104(3):182–91. doi: 10.1016/S0303-8467(02)00036-7
2. Alghwiri AA, Khalil H, Al-Sharman A, El-Salem K. Depression is a predictor for balance in people with multiple sclerosis. Mult Scler Relat Disord (2018) 24:28–31. doi: 10.1016/j.msard.2018.05.013
3. Bamer AM, Johnson KL, Amtmann D, Kraft GH. Prevalence of sleep problems in individuals with multiple sclerosis. Mult Scler (2008) 14(8):1127–30. doi: 10.1177/1352458508092807
4. Braley TJ, Boudreau EA. Sleep disorders in multiple sclerosis. Curr Neurol Neurosci Rep (2016) 16(5):50. doi: 10.1007/s11910-016-0649-2
5. Kallweit U, Baumann CR, Harzheim M, Hidalgo H, Pohlau D, Bassetti CL, et al. Fatigue and sleep-disordered breathing in multiple sclerosis: a clinically relevant association? Mult Scler Int (2013). doi: 10.1155/2013/286581
6. Veauthier C. Sleep disorders in multiple sclerosis. Curr Neurol Neurosci Rep (2015) 15(5):21. doi: 10.1007/s11910-015-0546-0
7. Veauthier C, Gaede G, Radbruch H, Wernecke KD, Paul F. Poor sleep in multiple sclerosis correlates with beck depression inventory values, but not with polysomnographic data. Sleep Disord (2016). doi: 10.1155/2016/8378423
8. Veauthier C, Paul F. Sleep disorders in multiple sclerosis and their relationship to fatigue. Sleep Med (2014) 15(1):5–14. doi: 10.1016/j.sleep.2013.08.791
9. Motl RW, Dlugonski D, Pilutti L, Sandroff B, McAuley E. Premorbid physical activity predicts disability progression in relapsing–remitting multiple sclerosis. J Neurol Sci (2012) 323(1–2):123–7. doi: 10.1016/j.jns.2012.08.033
10. Motl RW, Pilutti LA. Is physical exercise a multiple sclerosis disease modifying treatment? Expert Rev Neurother (2016) 16(8):951–60. doi: 10.1080/14737175.2016.1193008
11. Pilutti LA, Platta ME, Motl RW, Latimer-Cheung AE. The safety of exercise training in multiple sclerosis: a systematic review. J Neurol Sci (2014) 343(1–2):3–7. doi: 10.1016/j.jns.2014.05.016
12. Platta ME, Ensari I, Motl RW, Pilutti LA. Effect of exercise training on fitness in multiple sclerosis: a meta-analysis. Arch Phys Med Rehabil (2016) 97(9):1564–72. doi: 10.1016/j.apmr.2016.01.023
13. Veldhuijzen van Zanten JJ, Pilutti LA, Duda JL, Motl RW. Sedentary behaviour in people with multiple sclerosis: is it time to stand up against MS? Mult Scler (2016) 22(10):1250–6. doi: 10.1177/1352458516644340
14. Amato MP, Zipoli V, Portaccio E. Multiple sclerosis-related cognitive changes: a review of cross-sectional and longitudinal studies. J Neurol Sci (2006) 245(1–2):41–6. doi: 10.1016/j.jns.2005.08.019
15. Benedict RH, Zivadinov R. Risk factors for and management of cognitive dysfunction in multiple sclerosis. Nat Rev Neurol (2011) 7(6):332–42. doi: 10.1038/nrneurol.2011.61
16. Benedict RHB, DeLuca J, Enzinger C, Geurts JJG, Krupp LB, Rao SM. Neuropsychology of multiple sclerosis: looking back and moving forward. J Int Neuropsychol Soc (2017) 23(9–10):832–42. doi: 10.1017/S1355617717000959
17. Sumowski JF, Benedict R, Enzinger C, Filippi M, Geurts JJ, Hamalainen P, et al. Cognition in multiple sclerosis: state of the field and priorities for the future. Neurology (2018) 90(6):278–88. doi: 10.1212/WNL.0000000000004977
18. Carta MG, Paribello P, Anastasia A, De Berardis D, Nardi AE, Fornaro M. Pharmacological management of depression in patients with multiple sclerosis. Expert Opin Pharmacother (2018) 19(14):1533–40. doi: 10.1080/14656566.2018.1516207
19. Fornaro M, Solmi M, Veronese N, De Berardis D, Buonaguro EF, Tomasetti C, et al. The burden of mood-disorder/cerebrovascular disease comorbidity: essential neurobiology, psychopharmacology, and physical activity interventions. Int Rev Psychiatry (2017) 29(5):425–35. doi: 10.1080/09540261.2017.1299695
20. Sadeghi Bahmani D, Gerber M, Kalak N, Lemola S, Clough PJ, Calabrese P, et al. Mental toughness, sleep disturbances, and physical activity in patients with multiple sclerosis compared to healthy adolescents and young adults. Neuropsychiatr Dis Treat (2016) 12:1571–9. doi: 10.2147/NDT.S111208
21. Sadeghi Bahmani D, Esmaeili L, Shaygannejad V, Gerber M, Kesselring J, Lang UE, et al. Stability of mental toughness, sleep disturbances, and physical activity in patients with multiple sclerosis (MS)—a longitudinal and pilot study. Front Psychiatry (2018) 9:182. doi: 10.3389/fpsyt.2018.00182
22. Delle Fave A, Bassi M, Allegri B, Cilia S, Falautano M, Goretti B, et al. Beyond disease: happiness, goals, and meanings among persons with multiple sclerosis and their caregivers. Front Psychol (2017) 8:2216. doi: 10.3389/fpsyg.2017.02216
23. Kargiotis O, Paschali A, Messinis L, Papathanasopoulos P. Quality of life in multiple sclerosis: effects of current treatment options. Int Rev Psychiatry (2010) 22(1):67–82. doi: 10.3109/09540261003589521
24. Motl RW, McAuley E, Snook EM, Gliottoni RC. Physical activity and quality of life in multiple sclerosis: intermediary roles of disability, fatigue, mood, pain, self-efficacy and social support. Psychol Health Med (2009) 14(1):111–24. doi: 10.1080/13548500802241902
25. Salhofer-Polanyi S, Friedrich F, Loffler S, Rommer PS, Gleiss A, Engelmaier R, et al. Health-related quality of life in multiple sclerosis: temperament outweighs EDSS. BMC Psychiatry (2018) 18(1):143. doi: 10.1186/s12888-018-1719-6
26. Strober LB, Becker A, Randolph JJ. Role of positive lifestyle activities on mood, cognition, well-being, and disease characteristics in multiple sclerosis. Appl Neuropsychol Adult (2018) 25(4):304–11. doi: 10.1080/23279095.2018.1458518
27. Barzegar M, Badihian S, Mirmosayyeb O, Ashtari F, Jamadi M, Emami S, et al. Comparative study of quality of life, anxiety, depression, and fatigue among patients with neuromyelitis optica spectrum disorder and multiple sclerosis: the first report from Iran. Mult Scler Relat Disord (2018) 22:161–5. doi: 10.1016/j.msard.2018.04.009
28. Braley TJ, Kratz AL, Kaplish N, Chervin RD. Sleep and cognitive function in multiple sclerosis. Sleep (2016) 39(8):1525–33. doi: 10.5665/sleep.6012
29. Hughes AJ, Dunn KM, Chaffee T. Sleep disturbance and cognitive dysfunction in multiple sclerosis: a systematic review. Curr Neurol Neurosci Rep (2018) 18(1):2. doi: 10.1007/s11910-018-0809-7
30. Induruwa I, Constantinescu CS, Gran B. Fatigue in multiple sclerosis—a brief review. J Neurol Sci (2012) 323(1–2):9–15. doi: 10.1016/j.jns.2012.08.007
31. Sater RA, Gudesblatt M, Kresa-Reahl K, Brandes DW, Sater PA. The relationship between objective parameters of sleep and measures of fatigue, depression, and cognition in multiple sclerosis. Mult Scler J Exp Transl Clin (2015) 1:1–8. doi: 10.1177/2055217315577828
32. Brand S, Kirov R, Kalak N, Gerber M, Puhse U, Lemola S, et al. Perfectionism related to self-reported insomnia severity, but not when controlled for stress and emotion regulation. Neuropsychiatr Dis Treat (2015) 11:263–71. doi: 10.2147/NDT.S74905
33. Brand S, Kirov R, Kalak N, Gerber M, Schmidt NB, Lemola S, et al. Poor sleep is related to lower emotional competence among adolescents. Behav Sleep Med (2016) 14(6):602–14. doi: 10.1080/15402002.2015.1048450
34. Kirov R, Brand S, Kolev V, Yordanova J. The sleeping brain and the neural basis of emotions. Behav Brain Sci (2012) 35(3):155–6. doi: 10.1017/S0140525X11001531
35. Ahrberg K, Dresler M, Niedermaier S, Steiger A, Genzel L. The interaction between sleep quality and academic performance. J Psychiatr Res (2012) 46(12):1618–22. doi: 10.1016/j.jpsychires.2012.09.008
36. Curcio G, Ferrara M, De Gennaro L. Sleep loss, learning capacity and academic performance. Sleep Med Rev (2006) 10(5):323–37. doi: 10.1016/j.smrv.2005.11.001
37. Becker SP, Dvorsky MR, Holdaway AS, Luebbe AM. Sleep problems and suicidal behaviors in college students. J Psychiatr Res (2018) 99:122–8. doi: 10.1016/j.jpsychires.2018.01.009
38. Gangwisch JE, Babiss LA, Malaspina D, Turner JB, Zammit GK, Posner K. Earlier parental set bedtimes as a protective factor against depression and suicidal ideation. Sleep (2010) 33(1):97–106. doi: 10.1093/sleep/33.1.97
39. Amtmann D, Askew RL, Kim J, Chung H, Ehde DM, Bombardier CH, et al. Pain affects depression through anxiety, fatigue, and sleep in multiple sclerosis. Rehabil Psychol (2015) 60(1):81–90. doi: 10.1037/rep0000027
40. Hare CJ, Crangle CJ, Carney CE, Hart T. Insomnia symptoms, subjective appraisals, and fatigue: a multiple mediation model. Behav Sleep Med (2017)17(3):1–12. doi: 10.1080/15402002.2017.1342167
41. Brass SD, Duquette P, Proulx-Therrien J, Auerbach S. Sleep disorders in patients with multiple sclerosis. Sleep Med Rev (2010) 14(2):121–9. doi: 10.1016/j.smrv.2009.07.005
42. Caminero A, Bartolome M. Sleep disturbances in multiple sclerosis. J Neurol Sci (2011) 309(1–2):86–91. doi: 10.1016/j.jns.2011.07.015
43. Fleming WE, Pollak CP. Sleep disorders in multiple sclerosis. Semin Neurol (2005) 25(1):64–8. doi: 10.1055/s-2005-867075
44. Bamer AM, Johnson KL, Amtmann DA, Kraft GH. Beyond fatigue: assessing variables associated with sleep problems and use of sleep medications in multiple sclerosis. Clin Epidemiol (2010) 2010(2):99–106. doi: 10.2147/CLEP.S10425
45. Thelen JM, Lynch SG, Bruce AS, Hancock LM, Bruce JM. Polypharmacy in multiple sclerosis: relationship with fatigue, perceived cognition, and objective cognitive performance. J Psychosom Res (2014) 76(5):400–4. doi: 10.1016/j.jpsychores.2014.02.013
46. Clancy M, Drerup M, Sullivan AB. Outcomes of cognitive–behavioral treatment for insomnia on insomnia, depression, and fatigue for individuals with multiple sclerosis: a case series. Int J MS Care (2015) 17(6):261–7. doi: 10.7224/1537-2073.2014-071
47. Majendie CMA, Dysch L, Carrigan N. Cognitive behavioral therapy for insomnia (CBT-I) for an adult with multiple sclerosis. Clin Case Stud (2016) 16(2):115–31. doi: 10.1177/1534650116674594
48. Dayapoglu N, Tan M. Evaluation of the effect of progressive relaxation exercises on fatigue and sleep quality in patients with multiple sclerosis. J Altern Complement Med (2012) 18(10):983–7. doi: 10.1089/acm.2011.0390
49. Kraft GH. Rehabilitation principles for patients with multiple sclerosis. J Spinal Cord Med (1998) 21(2):117–20. doi: 10.1080/10790268.1998.11719518
50. Lisak D. Overview of symptomatic management of multiple sclerosis. J Neurosci Nurs (2001) 33(5):224–30. doi: 10.1097/01376517-200110000-00002
51. Chennaoui M, Arnal PJ, Sauvet F, Leger D. Sleep and exercise: a reciprocal issue? Sleep Med Rev (2015) 20:59–72. doi: 10.1016/j.smrv.2014.06.008
52. Kalak N, Gerber M, Kirov R, Mikoteit T, Yordanova J, Puhse U, et al. Daily morning running for 3 weeks improved sleep and psychological functioning in healthy adolescents compared with controls. J Adolesc Health (2012) 51(6):615–22. doi: 10.1016/j.jadohealth.2012.02.020
53. Kredlow MA, Capozzoli MC, Hearon BA, Calkins AW, Otto MW. The effects of physical activity on sleep: a meta-analytic review. J Behav Med (2015) 38(3):427–49. doi: 10.1007/s10865-015-9617-6
54. Lang C, Brand S, Feldmeth AK, Holsboer-Trachsler E, Puhse U, Gerber M. Increased self-reported and objectively assessed physical activity predict sleep quality among adolescents. Physiol Behav (2013) 120:46–53. doi: 10.1016/j.physbeh.2013.07.001
55. Lang C, Kalak N, Brand S, Holsboer-Trachsler E, Puhse U, Gerber M. The relationship between physical activity and sleep from mid adolescence to early adulthood. Sleep Med Rev (2016) 28:32–45. doi: 10.1016/j.smrv.2015.07.004
56. Siengsukon CF, Aldughmi M, Kahya M, Bruce J, Lynch S, Ness Norouzinia A, et al. Randomized controlled trial of exercise interventions to improve sleep quality and daytime sleepiness in individuals with multiple sclerosis: a pilot study. Mult Scler J Exp Transl Clin (2016) 2:1–9.doi: 10.1177/2055217316680639
57. Coote S, Uszynski M, Herring MP, Hayes S, Scarrott C, Newell J, et al. Effect of exercising at minimum recommendations of the multiple sclerosis exercise guideline combined with structured education or attention control education—secondary results of the step it up randomised controlled trial. BMC Neurol (2017) 17(1):119. doi: 10.1186/s12883-017-0898-y
58. Ensari I, Adamson BC, Motl RW. Longitudinal association between depressive symptoms and walking impairment in people with relapsing–remitting multiple sclerosis. J Health Psychol (2015) 21(11):2732–41. doi: 10.1177/1359105315584837
59. Ensari I, Motl RW, Pilutti LA. Exercise training improves depressive symptoms in people with multiple sclerosis: results of a meta-analysis. J Psychosom Res (2014) 76(6):465–71. doi: 10.1016/j.jpsychores.2014.03.014
60. Latimer-Cheung AE, Martin Ginis KA, Hicks AL, Motl RW, Pilutti LA, Duggan M, et al. Development of evidence-informed physical activity guidelines for adults with multiple sclerosis. Arch Phys Med Rehabil (2013) 94(9):1829–36.e1827. doi: 10.1016/j.apmr.2013.05.015
61. Latimer-Cheung AE, Pilutti LA, Hicks AL, Martin Ginis KA, Fenuta AM, MacKibbon KA, et al. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: a systematic review to inform guideline development. Arch Phys Med Rehabil (2013) 94(9):1800–28.e1803. doi: 10.1016/j.apmr.2013.04.020
62. Motl RW, Sandroff BM, Kwakkel G, Dalgas U, Feinstein A, Heesen C, et al. Exercise in patients with multiple sclerosis. Lancet Neurol (2017) 16(10):848–56. doi: 10.1016/S1474-4422(17)30281-8
63. Snook EM, Motl RW. Effect of exercise training on walking mobility in multiple sclerosis: a meta-analysis. Neurorehabil Neural Repair (2009) 23(2):108–16. doi: 10.1177/1545968308320641
64. De Berardis D, Fornaro M, Orsolini L, Iasevoli F, Tomasetti C, de Bartolomeis A, et al. Effect of agomelatine treatment on C-reactive protein levels in patients with major depressive disorder: an exploratory study in “real-world,” everyday clinical practice. CNS Spectr (2017) 22(4):342–7. doi: 10.1017/S1092852916000572
65. Marini S, Vellante F, Matarazzo I, De Berardis D, Serroni N, Gianfelice D, et al. Inflammatory markers and suicidal attempts in depressed patients: a review. Int J Immunopathol Pharmacol (2016) 29(4):583–94. doi: 10.1177/0394632015623793
66. Lang UE, Borgwardt S. Molecular mechanisms of depression: perspectives on new treatment strategies. Cell Physiol Biochem (2013) 31(6):761–77. doi: 10.1159/000350094
67. Razazian N, Yavari Z, Farnia V, Azizi A, Kordavani L, Bahmani DS, et al. Exercising impacts on fatigue, depression, and paresthesia in female patients with multiple sclerosis. Med Sci Sports Exerc (2016) 48(5):796–803. doi: 10.1249/MSS.0000000000000834
68. Brand S, Colledge F, Ludyga S, Emmenegger R, Kalak N, Sadeghi Bahmani D, et al. Acute bouts of exercising improved mood, rumination and social interaction in inpatients with mental disorders. Front Psychol (2018) 9(249). doi: 10.3389/fpsyg.2018.00249
69. Ludyga S, Gerber M, Brand S, Holsboer-Trachsler E, Puhse U. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: a meta-analysis. Psychophysiology (2016) 53(11):1611–26. doi: 10.1111/psyp.12736
70. Ludyga S, Gerber M, Mucke M, Brand S, Weber P, Brotzmann M, et al. The acute effects of aerobic exercise on cognitive flexibility and task-related heart rate variability in children with ADHD and healthy controls. J Atten Disord (2018). doi: 10.1177/1087054718757647
71. Prakash RS, Patterson B, Janssen A, Abduljalil A, Boster A. Physical activity associated with increased resting-state functional connectivity in multiple sclerosis. J Int Neuropsychol Soc (2011) 17(6):986–97. doi: 10.1017/S1355617711001093
72. Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol (2011) 69(2):292–302. doi: 10.1002/ana.22366
73. Brand S, Beck J, Gerber M, Hatzinger M, Holsboer-Trachsler E. Evidence of favorable sleep-EEG patterns in adolescent male vigorous football players compared to controls. World J Biol Psychiatry (2010) 11(2 Pt 2):465–75. doi: 10.1080/15622970903079820
74. Brand S, Gerber M, Beck J, Hatzinger M, Puhse U, Holsboer-Trachsler E. Exercising, sleep-EEG patterns, and psychological functioning are related among adolescents. World J Biol Psychiatry (2010) 11(2):129–40. doi: 10.3109/15622970903522501
75. Gerber M, Brand S, Herrmann C, Colledge F, Holsboer-Trachsler E, Puhse U. Increased objectively assessed vigorous-intensity exercise is associated with reduced stress, increased mental health and good objective and subjective sleep in young adults. Physiol Behav (2014) 135:17–24. doi: 10.1016/j.physbeh.2014.05.047
76. Gerber M, Colledge F, Puhse U, Holsboer-Trachsler E, Zimmerer S, Brand S. Sleep quality, sleep EEG pattern, mental well-being and cortisol secretion in patients with ruptured aneurysm post-treatment: a comparison with post-surgery meningioma patients and controls. Neuropsychobiology (2016) 73(3):148–59. doi: 10.1159/000444492
77. Hornung OP, Regen F, Warnstedt C, Anghelescu I, Danker-Hopfe H, Heuser I, et al. Declarative and procedural memory consolidation during sleep in patients with borderline personality disorder. J Psychiatr Res (2008) 42(8):653–8. doi: 10.1016/j.jpsychires.2007.07.001
78. Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med (2001) 2(4):297–307. doi: 10.1016/S1389-9457(00)00065-4
79. Gerber M, Lang C, Lemola S, Colledge F, Kalak N, Holsboer-Trachsler E, et al. Validation of the German version of the insomnia severity index in adolescents, young adults and adult workers: results from three cross-sectional studies. BMC Psychiatry (2016) 16:174. doi: 10.1186/s12888-016-0876-8
80. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 5th edition: DSM 5. Arlington VA: American Psychiatric Association (2013).
81. Benedict RH, Fishman I, McClellan MM, Bakshi R, Weinstock-Guttman B. Validity of the beck depression inventory-fast screen in multiple sclerosis. Mult Scler (2003) 9(4):393–6. doi: 10.1191/1352458503ms902oa
82. Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Arch Neurol (1989) 46(10):1121–3. doi: 10.1001/archneur.1989.00520460115022
83. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc (2003) 35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB
84. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology (1983) 33(11):1444–52. doi: 10.1212/WNL.33.11.1444
85. Kurtzke JF. On the origin of EDSS. Mult Scler Relat Disord (2015) 4(2):95–103. doi: 10.1016/j.msard.2015.02.003
86. Meyer-Moock S, Feng YS, Maeurer M, Dippel FW, Kohlmann T. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurol (2014) 14:58. doi: 10.1186/1471-2377-14-58
87. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc (2005) 53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x
88. Smith A. Symbol digits modalities test: Manual. Los Angeles: Western Psychological Services (1982).
89. Gauthier S, Patterson C, Gordon M, Soucy JP, Schubert F, Leuzy A. Commentary on “Recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease.” A Canadian perspective. Alzheimers Dement (2011) 7(3):330–2. doi: 10.1016/j.jalz.2011.03.006
90. Thomann AE, Goettel N, Monsch RJ, Berres M, Jahn T, Steiner LA, et al. The montreal cognitive assessment: normative data from a german-speaking cohort and comparison with international normative samples. J Alzheimers Dis (2018) 64(2):643–55. doi: 10.3233/JAD-180080
91. Mota-Pereira J, Carvalho S, Silverio J, Fonte D, Pizarro A, Teixeira J, et al. Moderate physical exercise and quality of life in patients with treatment-resistant major depressive disorder. J Psychiatr Res (2011) 45(12):1657–9. doi: 10.1016/j.jpsychires.2011.08.008
92. Bailey AP, Hetrick SE, Rosenbaum S, Purcell R, Parker AG. Treating depression with physical activity in adolescents and young adults: a systematic review and meta-analysis of randomised controlled trials. Psychol Med (2017) 48(7):1068–83. doi: 10.1017/S0033291717002653
93. Josefsson T, Lindwall M, Archer T. Physical exercise intervention in depressive disorders: meta-analysis and systematic review. Scand J Med Sci Sports (2014) 24(2):259–72. doi: 10.1111/sms.12050
94. Perez-Lopez FR, Martinez-Dominguez SJ, Lajusticia H, Chedraui P. Effects of programmed exercise on depressive symptoms in midlife and older women: a meta-analysis of randomized controlled trials. Maturitas (2017) 106:38–47. doi: 10.1016/j.maturitas.2017.09.001
95. Poyatos-Leon R, Garcia-Hermoso A, Sanabria-Martinez G, Alvarez-Bueno C, Cavero-Redondo I, Martinez-Vizcaino V. Effects of exercise-based interventions on postpartum depression: a meta-analysis of randomized controlled trials. Birth (2017) 44(3):200–8. doi: 10.1111/birt.12294
96. Schuch F, Vancampfort D, Firth J, Rosenbaum S, Ward P, Reichert T, et al. Physical activity and sedentary behavior in people with major depressive disorder: a systematic review and meta-analysis. J Affect Disord (2017) 210:139–50. doi: 10.1016/j.jad.2016.10.050
97. Sun M, Lanctot K, Herrmann N, Gallagher D. Exercise for cognitive symptoms in depression: a systematic review of interventional studies. Can J Psychiatry (2017) 63(2):115–28. doi: 10.1177/0706743717738493
98. Wu PL, Lee M, Huang TT. Effectiveness of physical activity on patients with depression and Parkinson’s disease: a systematic review. PLoS One (2017) 12(7):e0181515. doi: 10.1371/journal.pone.0181515
99. Backus D. Increasing physical activity and participation in people with multiple sclerosis: a review. Arch Phys Med Rehabil (2016) 97(9 Suppl):S210–17. doi: 10.1016/j.apmr.2015.09.027
100. Heine M, de Port I, Rietberg MB, van Wegen EE, Kwakkel G. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst Rev (2015) (9):Cd009956. doi: 10.1002/14651858.CD009956.pub2
101. Waschbisch A, Tallner A, Pfeifer K, Maurer M. Multiple sclerosis and exercise: effects of physical activity on the immune system. Nervenarzt (2009) 80(6):688–92. doi: 10.1007/s00115-008-2639-3
Keywords: cognitive performance, exercise, rehabilitation, multiple sclerosis, physical activity intervention, sleep-EEG, sleep
Citation: Sadeghi Bahmani D, Kesselring J, Papadimitriou M, Bansi J, Pühse U, Gerber M, Shaygannejad V, Holsboer-Trachsler E and Brand S (2019) In Patients With Multiple Sclerosis, Both Objective and Subjective Sleep, Depression, Fatigue, and Paresthesia Improved After 3 Weeks of Regular Exercise. Front. Psychiatry 10:265. doi: 10.3389/fpsyt.2019.00265
Received: 15 January 2019; Accepted: 08 April 2019;
Published: 03 May 2019.
Edited by:
Bahar Güntekin, Istanbul Medipol University, TurkeyReviewed by:
Domenico De Berardis, Azienda Usl Teramo, ItalyAxel Steiger, Ludwig Maximilian University of Munich, Germany
Copyright © 2019 Sadeghi Bahmani, Kesselring, Papadimitriou, Bansi, Pühse, Gerber, Shaygannejad, Holsboer-Trachsler and Brand. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dena Sadeghi Bahmani ZGVuYS5zYWRlZ2hpYmFobWFuaUB1cGsuY2g=
 Dena Sadeghi Bahmani
Dena Sadeghi Bahmani Juerg Kesselring
Juerg Kesselring Malamati Papadimitriou
Malamati Papadimitriou Jens Bansi
Jens Bansi Uwe Pühse
Uwe Pühse Markus Gerber
Markus Gerber Vahid Shaygannejad3,6
Vahid Shaygannejad3,6 Serge Brand
Serge Brand