- 1Department of Health Promotion and Human Behavior, School of Public Health in the Graduate School of Medicine, Kyoto University, Kyoto, Japan
- 2Hospital Care Research Unit, Hyogo Prefectural Amagasaki General Medical Center, Hyogo, Japan
- 3Department of Health Sciences, Università degli Studi di Milano, Milan, Italy
- 4Department of Psychiatry, Warneford Hospital, University of Oxford, Oxford, United Kingdom
Few studies have delineated the real-world, long-term trends of prescription patterns of antidepressants for patients with major depressive disorder (MDD). This study aims to describe their vicissitudes in the nationally representative sample of the US from 1996 to 2015 and explore their characteristics. We used the Medical Expenditure Panel Survey, a nationally representative database of the US population, between 1996 and 2015. We estimated the prevalence of MDD among adults, calculated the proportions of those on antidepressant treatment as well as those on specific drugs through the two decades, and determined their dosages in 2015. We conducted multivariable regression to find possible factors related to their suboptimal prescriptions. The prevalence of adults diagnosed with MDD increased from 6.1% (95% CI, 5.7–6.6%) in 1996 to 10.4% (9.7–11.1%) in 2015. The proportion of patients without any antidepressant therapy decreased but still accounted for 30.6% (28.3–33.1%) in 2015. Sertraline and fluoxetine were among the most frequently prescribed antidepressants throughout the 20 years, while the trend for some new drugs changed dramatically. 16.1% (12.5–20.2%) of patients of MDD on antidepressant monotherapy were prescribed with suboptimal doses in 2015; the risk was lower for those who had higher Body Mass Index (OR 0.94 [0.90–0.99]), longer-term prescriptions (OR 0.92 [0.87–0.97]), and the risk was higher for those who were prescribed with tricyclic antidepressants (OR 11.21 [2.12–59.34], compared with serotonin reuptake inhibitors (SSRIs)), and antidepressants other than SSRIs and serotonin and norepinephrine reuptake inhibitors (OR 4.12 [1.95, 8.73], compared with SSRIs). This study confirmed the growing numbers of patients with MDD and the increase in the antidepressant prescriptions among them. However, the existence of patients without any antidepressant prescriptions or with suboptimal prescriptions and the variable prescription patterns through the decades might suggest some unresolved gaps between evidence and practice.
Introduction
Depression is one of the most common mental disorders, with high prevalence in the population, resulting in impaired functions of affected individuals, then leading to great burden to the individuals and the society. Approximately 4.4% of the population (equivalent to more than 300 million people) in the world are estimated to suffer from depression in 2015, and the number is still increasing (1). In the United States, according to the National Survey on Drug Use and Health, about 7.1% of the adults had experienced at least one episode of major depressive disorder (MDD) in 2017, among which 63.8% had severe impairment (2, 3). In 2015, depressive disorders caused 7.5% of all Years Lived with Disability (YLD) globally, which ranked as the largest single contributor to non-fatal health loss world-wide (1). In the US, the incremental economic burden of individuals with MDD was $210.5 billion in 2010, which had increased by 21.5% since 2005 (4, 5).
Antidepressants play a key role in the treatment of MDD due to their demonstrated efficacy (6, 7) and wide availability. Monotherapy is recommended as the first-line initial treatment, while combination of antidepressants could also be considered if the initial monotherapy fails. In 1990s, as the effectiveness of different types of antidepressant appeared comparable, no specific recommendations were proposed by guidelines (8, 9). As many new generation antidepressants ushered into the market and as more and more evidence from randomized controlled trials (RCTs) have accumulated in the past three decades, practice guidelines in recent years started to give more specific recommendations regarding the classes or even within-class types of medications (10–12).
The details of actual prescriptions of antidepressants in the real world could then be very informative for the practitioners and the health policy makers in benchmarking their performances in depression treatment. Unfortunately, however, such details have not been well known, especially the population-based prescriptions of specific antidepressants targeting MDD and their changes over the time. Optimizing the doses of antidepressant should be equally crucial. A recent meta-analysis found a positive dose-response up to the lower end of licensed dose ranges of various antidepressants, beyond which there was no further increase in efficacy but only sharp increase in side effects (13). The average doses for particular antidepressants prescribed as monotherapy in treating MDD in the US and the potential factors related to their under-prescription remain unclear.
This study therefore aims to describe the national trends in the numbers of patients diagnosed with MDD, the characteristics of those who received antidepressant monotherapy, and the prescription patterns of individual antidepressants in treating MDD, through the past two decades between 1996 and 2015 based on a nationally representative survey database. We further estimated the daily average doses of frequently used antidepressants and explored the possible factors related to their suboptimal prescriptions.
Materials and Methods
The protocol for this study has been published and is freely available (14). This study did not require institutional review board approval since only deidentified data were used. It was registered at UMIN Clinical Trials Registry (identifier: UMIN000031898).
Sources of Data
We used the household components of the Medical Expenditure Panel Survey (MEPS) database (15). MEPS is a database sponsored by the Agency for Healthcare Research and Quality (AHRQ) and composed of yearly large-scale surveys of a representative sample of families and individuals and their medical providers, collecting data on the use of specific health services, the cost, and the health insurance in the United States since 1996. The participants were drawn from a subsample of households that participated in the prior year's National Health Interview Survey (NHIS). The sampling frame in MEPS gives a nationally representative sample of the non-institutionalized population in the US. Every year about 9,000 to 15,000 households, equivalent to 20,000 to 40,000 individuals are included. Data are collected using computer-assisted personal interview questionnaires, and every participant in one MEPS panel is interviewed by well-trained interviewers for five consecutive rounds within 2 years. Each participant is given a weight adjusting for nonresponse over time and some poststratification variables (region, race/ethnicity, sex, age, poverty status, etc.), in order to produce national estimates. (Further details of the MEPS surveys can be found in their webpage (15).
Diagnosis of Depression
The MEPS collects information of diagnosis for each participant and codes them into 5-digit International Classification of Diseases, Ninth Revision (ICD-9) categories. The target population in this study were patients diagnosed with major depression, which had the corresponding ICD-9 code as 296.2 (major depressive disorder, single episode, 296.20–296.26), 296.3 (major depressive disorder, recurrent episode, 296.30–296.36), 311 (depressive disorder, not elsewhere classified). Patients with bipolar disorder were excluded. In order to use the detailed diagnostic information, this study has been approved by the AHRQ data center.
Medications
In the MEPS database, each participant provided prescriptions of specific drugs, which were then confirmed by pharmacy providers when written permissions were provided. This study focused on the prescriptions of antidepressants, which have been approved for depression by the US Food and Drug Administration (FDA) and grouped them into 4 categories according to National Drug Code Directory (16): 1) Tricyclic Antidepressants (TCAs): amitriptyline, amoxapine, clomipramine, desipramine, doxepin, imipramine, nortriptyline, protriptyline, trimipramine; 2) Serotonin Reuptake Inhibitor (SSRIs): citalopram, escitalopram, fluoxetine, nefazodone, paroxetine, sertraline, trazodone; 3) Serotonin and Norepinephrine Reuptake Inhibitor (SNRIs): desvenlafaxine, duloxetine, venlafaxine, levomilnacipran; 4) No Pharm Class: bupropion, mirtazapine, vilazodone, vortioxetine. As described above, each participant received two or three rounds of interview within one year; in each interview the prescriptions only within that round were obtained. We defined patients on monotherapy as those who were prescribed with the same one antidepressant in all the rounds within that year, while those who were prescribed with different antidepressants within the same round or in different rounds within the same year were regarded as “patients receiving multiple antidepressants”. Dosages, including dose strength, quantity of prescribed medicine, and days of supplies in 2015 were also extracted, for the purpose of calculating the daily doses. A suboptimal prescription for each drug was defined as a dose lower than therapeutic range, which was according to the approved treatment doses for MDD by FDA (Supplementary Table S1).
Concomitant use of benzodiazepines, mood stabilizers and antipsychotics were extracted as well, for they were commonly used by major depressive patients. Based on FDA National Drug Code Directory, benzodiazepines included: alprazolam, chlordiazepoxide, clobazam, clonazepam, clorazepate, diazepam, estazolam, flurazepam, halazepam, lorazepam, midazolam, oxazepam, quazepam, temazepam, triazolam, zaleplon, and zolpidem; mood stabilizers included: carbamazepine, divalproex, lamotrigine, lithium, valproate and valproic acid. Antipsychotics included aripiprazole, asenapine, brexipiprazole, cariprazine, chlorpromazine, clozapine, fluphenazine, haloperidol, iloperidone, loxapine, lurasidone, molindone, olanzapine, paliperidone, perphenazine, pimavanserin, quetiapine, risperidone, thioridazine, thiothixene, and ziprasidone.
Sociodemographic and Other Health-Related Characteristics
Sociodemographic information was collected for each participant, including age, sex, race/ethnicity, education level, marital status, family income level, health insurance. Body Mass Index (BMI) was also calculated in 2015. In this study, the target population was adults, aged 18 years or older.
Mental health status information was also available in the MEPS in 2015, as measured by Patient Health Questionnaire-2 (PHQ-2). Each participant was asked to complete the questionnaire during one interview in that year. The total score ranged from 0 to 6, and a cut-off of 3 was suggested by previous studies to be used as depression screening (17). The Kessler-6 Index (K6) was used to assess general psychological distress, with scores ranging between 0 and 24 and higher scores indicating higher level of distress in the past 30 days. Scores at 13 or more has been shown to indicate serious psychological stress (18, 19).
Statistical Analyses
Data were extracted from the MEPS every five years from 1996 to 2015, i.e. in 1996, 2000, 2005, 2010 and 2015, since we considered that data at 5-year interval would be sufficiently fine-grained to show the trends in diagnoses and prescriptions. All the analyses were based on national estimates using sampling weights. The prevalence of major depressive disorder among adults was calculated for each year. The absolute numbers and percentages of depression patients who were receiving different kinds of treatment (no antidepressant treatment, antidepressant monotherapy or multiple antidepressants treatment) were presented for each year. For major depressive patients on antidepressant monotherapy, the trend of changes in sociodemographic characteristics, together with other health-related status, and the concurrent psychotropic treatments, were summarized over time. The prescription pattern of antidepressants as monotherapy was indicated by the number of patients being prescribed a specific drug and the proportion of patients on that drug among all the patients on monotherapy in each year. Since the survey methodology such as sampling and weighting and the measured items were being constantly improved over the years, directly comparing datasets from different times needs caution. Hence for this trend analysis, instead of employing statistical methods to give a P value, we opted rather to present the trends in a descriptive way.
We analyzed the doses of antidepressants prescribed as monotherapy for patients with MDD. The average daily doses for frequently prescribed antidepressants were estimated when the observed cases using a certain drug in the sample were more than 10. Crude odds ratios (ORs) with their 95% confidence intervals (CIs) were estimated for all the factors that may be associated with suboptimal use. We then used a model which adjusted age, sex and BMI for each variable to explore if the variable was potentially related to suboptimal prescriptions. Finally, we used a multivariable regression model to discover the factors that were strongly related to suboptimal prescriptions independently with each other based on available data.
We used STATA Version 13 (StataCorp) for data extraction and all the analyses including estimation for the national populations from samples and multivariable logistic regression. We provided the STATA commands for the year 2015 in the Supplementary Materials.
Results
Numbers of Patients With MDD and Their Antidepressant Treatment Over the Years
Estimated numbers of patients diagnosed with MDD showed constant increase (Figure 1). Prevalence of MDD among the adult population was 6.1% (95% CI, 5.7 to 6.6%) in 1996, which has increased steadily up to 2015, when it reached 10.4% (95% CI, 9.7 to 11.1%). Patients with a diagnosis of MDD who were not on any antidepressant treatment accounted for 47.8% (95% CI, 44.3 to 51.3%) of all patients in 1996, but the proportion gradually decreased to 25.1% (95% CI, 23.0 to 27.4%) in 2010 or 30.6% (95% CI, 28.3 to 33.1%) in 2015 (Figure 1).
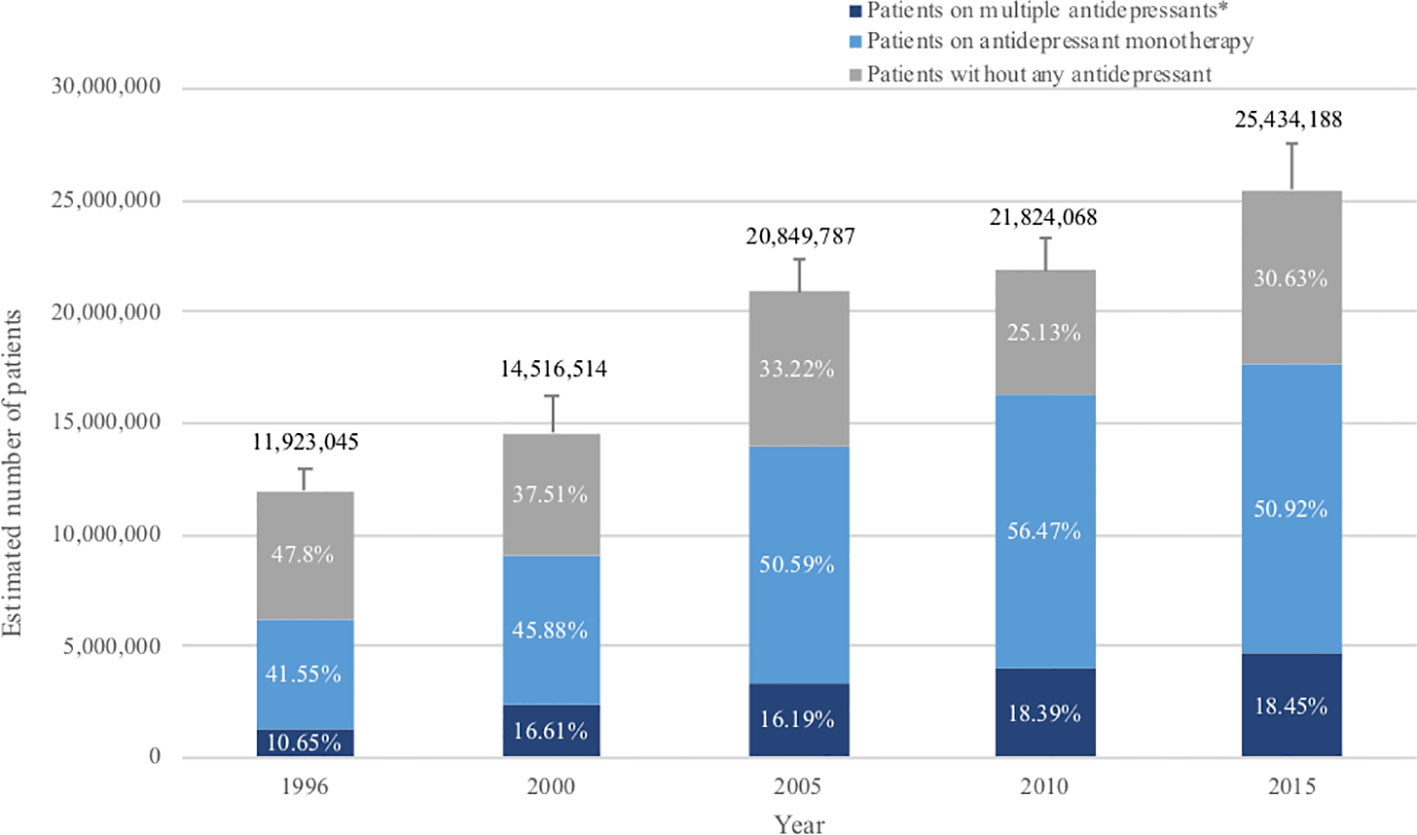
Figure 1 Antidepressant treatment for patients with major depression over the past 20 years. The standard error (SE) of number of adults with MDD is shown by the error bar. *Patients with multiple antidepressants: referring to patients who were prescribed with more than one antidepressant during that year, i.e. both patients with combination therapy and patients who changed previous monotherapy into a new drug in that year.
Characteristics of Patients With MDD Who Are on Antidepressant Monotherapy and Their Changes Over the Years
Table 1 and Supplementary Table S2 show characteristics of MDD patients who were prescribed with antidepressant monotherapy in the past 20 years. The mean age of these patients increased by about 10 years through the two decades, mainly due to the obvious increase of patients over 60 years. The sex ratio was roughly steady, with approximately 70% being women. The prescription numbers for male and female patients were shown separately in Supplementary Figure S1. The concomitant use of benzodiazepines was stable during the years at around 25%, whereas the use of mood stabilizers and antipsychotics increased from 3.1 to 5.6% and from 3.3 to 9.0%, respectively. More patients had long-term prescriptions of antidepressants in 2015, with 43.9% on antidepressants for more than 5 years, compared with only 13.4% in 1996. We further analyzed the proportion of long-term prescription of frequently prescribed drugs over the years (Supplementary Figure S3). In general, long-term prescription increased at approximately equal proportions for all the examined drugs. In 2015, for drugs known to cause discontinuation effect such as venlafaxine and paroxetine, almost 50% of their prescriptions (45.9 and 48.2%, respectively) were long-term uses. However, drugs less likely to cause withdrawal symptoms, for instance, fluoxetine, sertraline and bupropion, also had 46.4, 45.1 and 44.3% of prescriptions that have been used for more than 5 years respectively.
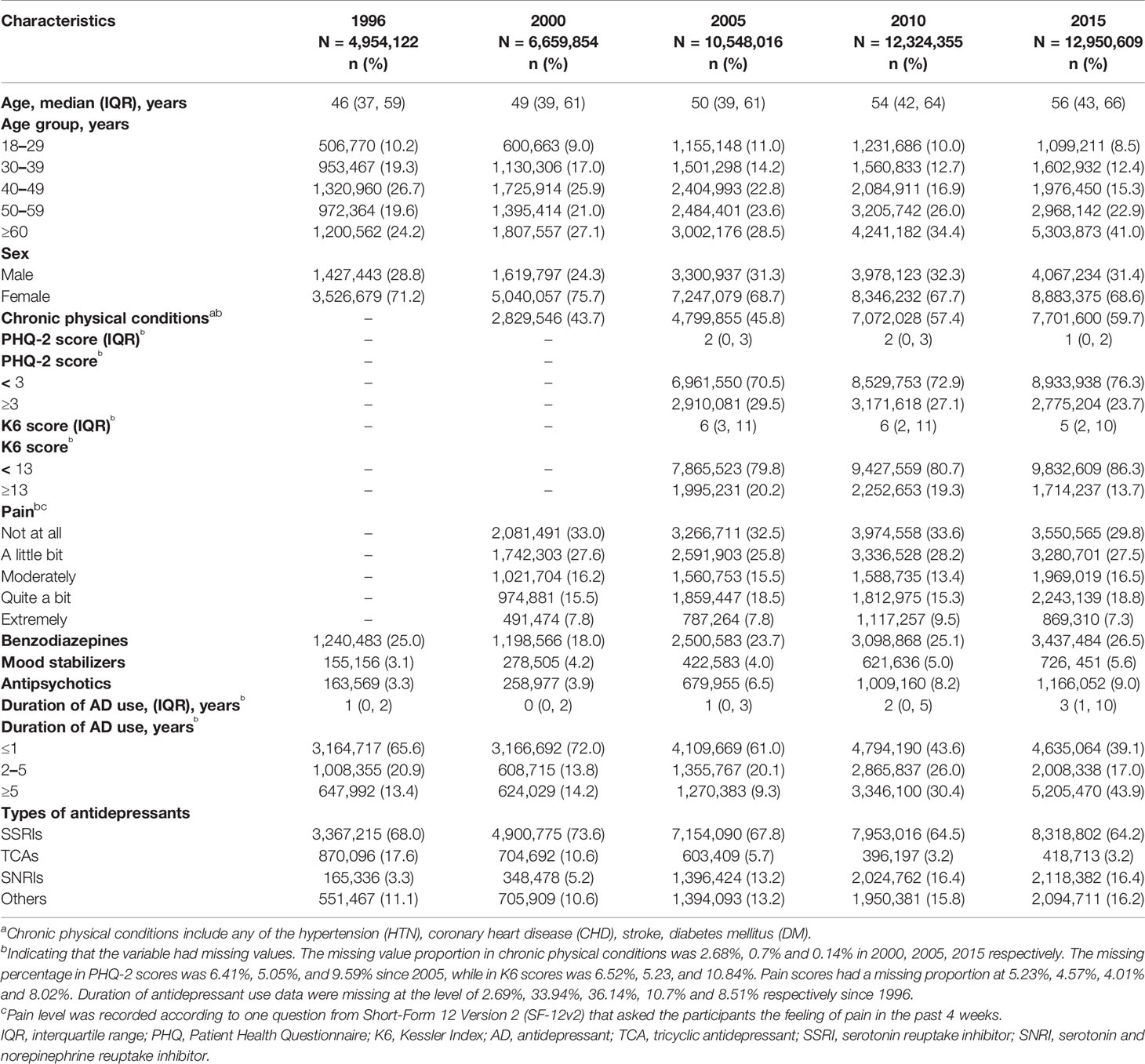
Table 1 Characteristics of depression patients on antidepressant monotherapy over the past 20 years.
Prescription Patterns of Antidepressant Monotherapy Among Patients With MDD Over the Years
Figure 2 shows the prescription patterns of individual antidepressants based on the proportion of patients being prescribed each drug among all patients on monotherapy. Supplementary Table S3 shows the absolute numbers of patients on each drug estimated with 95% CI and Supplementary Figure S2 depicts their trends over the years. Sertraline and fluoxetine were among the most prescribed antidepressants throughout the whole 20 years, with the absolute prescriptions increasing but prescription percentages decreasing, perhaps due to the introduction of more and more new drugs into the market in these years. Some relatively old antidepressants showed decrease both in absolute and relative numbers, such as paroxetine (from ranking the 3rd with 14.6% to ranking the 8th with 5.4%) and amitriptyline (from ranking the 4th with 8.8% to ranking the 10th with 2.0%), while some appeared be consistently prescribed although relatively infrequently (for example, trazodone). New drugs usually showed gradual increase, such as bupropion, venlafaxine and duloxetine, whereas several achieved surprisingly high prescription numbers upon their first appearance, such as escitalopram (dominating 18.7% and ranking the 2nd upon first appearance) and citalopram (occupying 12.4% and ranking the 4th upon first appearance).
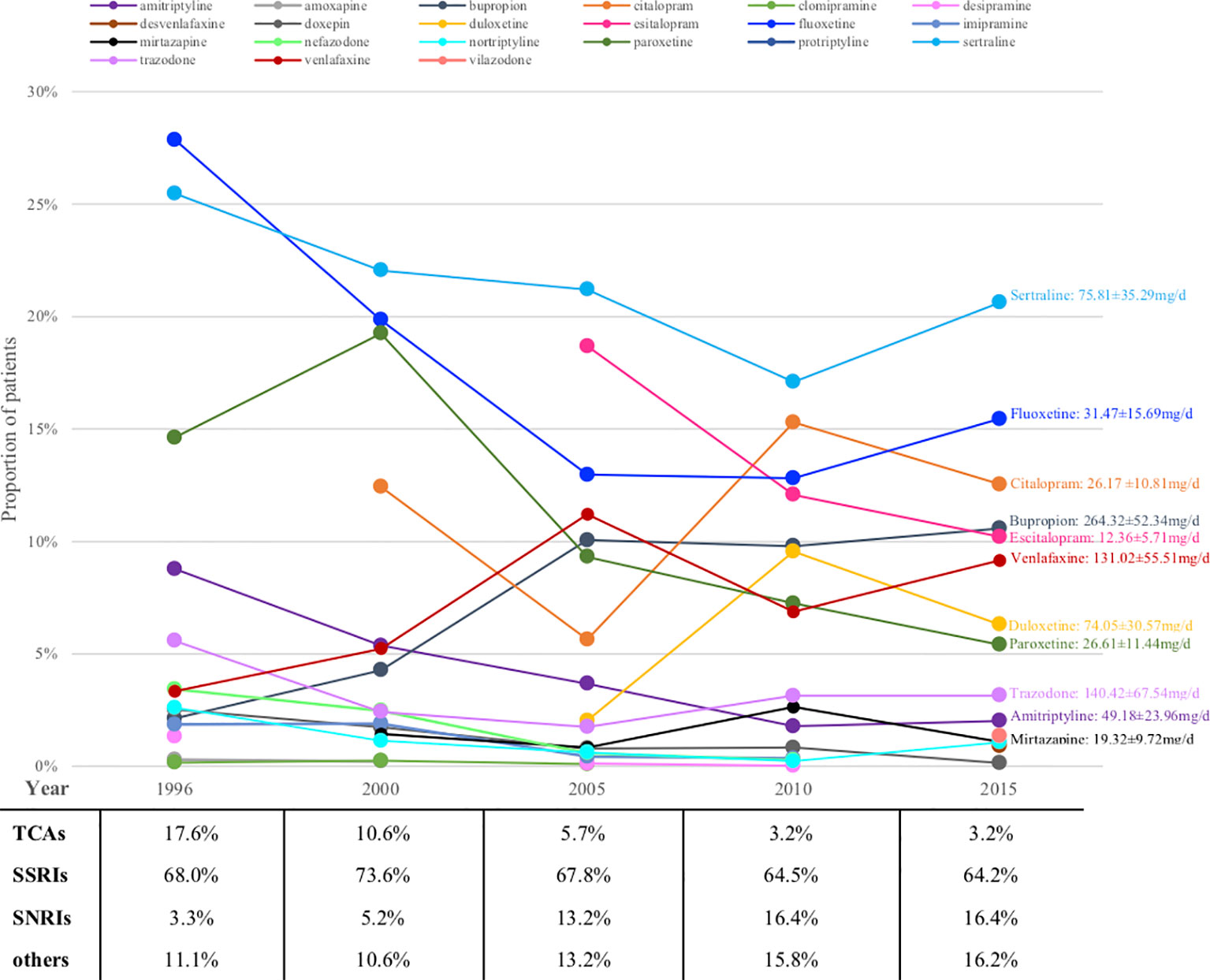
Figure 2 Prescriptions of antidepressants monotherapy for major depression patients over the years (proportions). TCA, tricyclic antidepressant; SSRI, serotonin reuptake inhibitor; SNRI, serotonin and norepinephrine reuptake inhibitor.
Looking at antidepressant classes, SSRIs remained steady at around 70% for the whole 20 years, whereas TCAs were declining and SNRIs were growing all along (Figure 2).
Average Dosages for Commonly Prescribed Antidepressant Monotherapy in 2015
Figure 2 also shows the average daily dose of prescription for patients with MDD based on available data in 2015. The average doses of bupropion, trazodone and amitriptyline were lower than the therapeutic dose range approved by FDA.
Factors Related to Suboptimal Prescriptions of Antidepressant Monotherapy in 2015
Data required for dose analysis were not complete in 43.1% of the major depressive patients on antidepressant monotherapy. Among the patients with sufficient data, 16.1% (95% CI, 12.5 to 20.2%) were prescribed with a dose lower than the approved range. After adjusting for age, sex and BMI, we discovered that patients being separated, widowed or divorced, or being prescribed with TCAs or any other antidepressants than SSRIs and SNRIs, tended to have higher risk to be prescribed with inadequate doses, while patients with higher BMI, or having long-term antidepressant treatment, had lower risk to receive inadequate prescriptions (Table 2). A multivariable regression implied that BMI, duration of antidepressant use and antidepressant type were the strongest factors related to suboptimal prescriptions (Table 2).
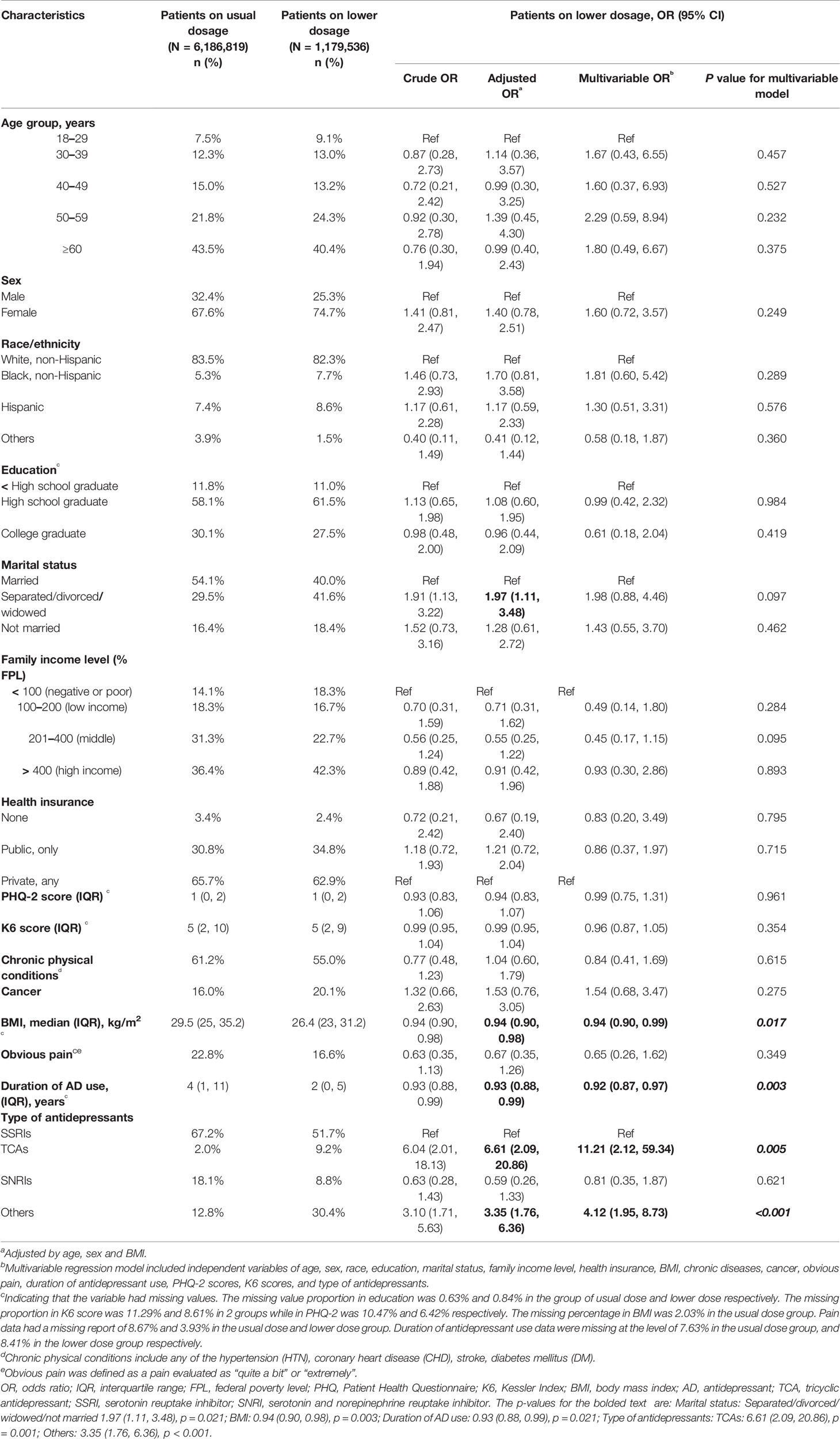
Table 2 Characteristics of patients prescribed with antidepressant monotherapy of suboptimal dose in 2015.
Discussion
We found that the absolute and relative numbers of adult patients diagnosed with MDD increased over the past 20 years, as well as the proportion of those on antidepressant treatment among those so diagnosed. There were approximately 30% of such patients who were not on any antidepressants in 2015. Among those who were on antidepressant monotherapy, there was substantial increase in long-term prescriptions and some increase in concurrent use of mood stabilizer or antipsychotics. The prescription patterns of specific drugs changed over the years as new antidepressants came into the market continuously. Sertraline and fluoxetine were among the most frequently prescribed antidepressants throughout these 20 years, while new drugs such as citalopram and escitalopram were prescribed by a dramatically large amount soon after their entry into the market. On the other hand, 16.1% of patients were using antidepressants below the licensed doses, especially when the patients had lower BMI, had shorter length of treatment, and were prescribed antidepressants other than SSRIs and SNRIs.
The prevalence of adult major depression estimated in our study was between 6.1 and 10.4% from 1996 to 2015, which was in line with the epidemiological studies from the same periods (20–23). The constantly growing total number of patients with MDD calls for more attention on how to implement effective interventions and care for the patients.
Antidepressants are one of the principal treatments for MDD, but still quite a few were not prescribed with any antidepressants. An antidepressant was originally recommended as the initial treatment for patients with moderate to severe depression by several guidelines (8, 11, 12, 24), whereas APA guideline recommends antidepressant as the first-line treatment also for mild patients (10, 25). Two individual participant data meta-analysis (26, 27) indicated that patients with lower baseline severity would achieve smaller improvement compared to placebo. However, a more recent study (28) found that the differential response of patients with different severity was due to larger improvement on non-core symptoms, and that baseline severity did not affect the efficacy for core depression symptoms. Besides, as we did not have the data for baseline severity or the treatment course for individual patients, we could not judge appropriateness of prescribing or not prescribing an antidepressant in individual cases or further explore the factors related to not receiving antidepressant treatment.
Our data suggested that there was dramatic increase in long-term prescriptions of antidepressant monotherapy, which was also observed by some other studies (29, 30). This phenomenon might be due to the increased prescriptions as appropriate maintenance treatment for patients with recurrent episodes, or due to improperly elongated use related to withdrawal symptoms, or both. Our results suggested that frequently prescribed drugs tended to have large proportion of prescriptions to be long-term, apparently regardless of the risk to cause withdrawal symptoms. It may imply that discontinuation syndrome might not be the only reason that caused significant increase in long-term prescriptions. The current observational study could not provide any further conclusions for this phenomenon, thus future studies are required. Although long-term maintenance treatment is recommended for patients with recurrent episodes (10, 12), future studies are required to explore the appropriateness of actual prolonged prescriptions (31, 32).
In the US an old study (33) based on office-based physician survey depicted the trend of antidepressant prescriptions for depression up to 2001, by which time SSRIs had clearly outnumbered TCAs. In most countries in Europe, SSRIs were the class being prescribed most frequently in 2004–2005, especially in France and the UK, whereas in Germany TCAs dominated (34). In Asia, though SSRIs dominated in almost every country, the particular prescription preferences were different from country to country (35–37). These various prescription patterns might be attributable to the perception that no single antidepressant appears much better than another, which in turn might suggest that particular marketing conditions and regulations, adverse effect spectrum and patients' preferences might impact greatly on the actual prescription patterns. As evidence accrues, we need to rigorously summarize it which then should guide us in actual prescriptions and should no longer let individual experiences or marketing efforts to distort it.
Several studies have pointed to the suboptimal prescription of antidepressants. A few studies suggested that older antidepressants such as TCAs were more susceptible to be prescribed in low doses (34, 38, 39), which was consistent with our study. Some studies further revealed that low dose prescriptions were especially related to primary care physicians, perhaps due to their concerns about the side effects related to TCAs, or the lack of confidence of those general practitioners (34, 38, 40). Besides, some antidepressants might be prescribed for their hypnotic effect rather than for depressive symptoms, such as amitriptyline or trazodone, which could lead to prescriptions with smaller doses. In our study, patients with longer-term prescription were less likely to receive inadequate doses, which might be ascribed to the fact that most long-term users were clinically severe or refractory so that sufficient doses were indispensable. Lower BMI was also associated of suboptimal prescription. This may be clinically understandable, because patients with less body weight may need lower dose, or they may be more likely to show adverse effects. The clinicians may also take advantage of placebo effect when it presents before the licensed dose range is achieved.
This study has some limitations. First, prescription of antidepressants is actually different from their real consumption. Although the MEPS is a large survey database with rigorous methodology based on representative samples in the US, some important information was not recorded, such as depression severity or treatment responses, the specialty of the doctor who prescribers a certain drug, among others. Second, even when recorded, some variables such as quantity of prescribed medications were often missing, thus prohibiting the calculation of average daily doses for some patients. Also, some of the available information was not very precise. For instance, the diagnosis in the household component of the MEPS is mainly dependent on patients' report. Although the sensitivity of a broader diagnosis in mental health disorders reported is above 90%, the specific diagnosis might be less uncertain, as addressed in a case study (41). However, there are several previous studies using the MEPS and their results are comparable with results generated from other sources (42–44). In addition, the combination antidepressant treatment was difficult to define. It was also not possible to distinguish incident patients from chronic patients therefore both acute phase and maintenance treatment were included in our analyses. These limitations may make some inferential statements of the observations challenging, and future studies focusing on these points are warranted.
In the literature, most population-based prescription studies are based on claims databases where the diagnoses were uncertain, while small cohort studies of patients with established diagnoses were usually institution-based with short-term follow-up and therefore had problems in generalizability. Our study represents the first detailed descriptions of population-based, long-term trends of antidepressant prescription patterns for patients diagnosed with MDD in the US. It has once again pointed to the increasing numbers of patients with MDD and also the increase in the antidepressant prescriptions among them. At the same time, it has revealed some unresolved gaps between evidence and practice, most notably existence of substantial minorities without any antidepressant prescriptions or with only subtherapeutic prescriptions among those diagnosed with MDD, dramatic increase in the number of patients with extremely long-term antidepressant prescriptions, and variable patterns in choices of individual antidepressants. These gaps need be filled in by independently funded future research.
Data Availability Statement
The datasets used in this study are publicly available in the Medical Expenditure Panel Survey (MEPS), https://meps.ahrq.gov/mepsweb/.
Ethics Statement
Ethical approval was not required as publicly available datasets were analysed.
Author Contributions
YL and TF designed the study. YL collected data and conducted statistical analyses. TF, YK, and EO gave suggestions for analytical plans. All the authors participated in interpretation of the results. YL drafted the manuscript and all authors critically revised the manuscript and approved the final version.
Funding
This study was supported in part by JSPS Grant-in-Aid for Scientific Research (Grant Number 17k19808) to TF. The funder has no role in study design, data collection, data analysis, data interpretation, writing of the report, or in the decision to submit for publication.
Conflict of Interest
TF reports personal fees from Meiji, Mitsubishi-Tanabe, MSD and Pfizer and a grant from Mitsubishi-Tanabe, outside the submitted work; TF has a patent 2018-177688. AC is supported by the National Institute for Health Research (NIHR) Oxford Cognitive Health Clinical Research Facility, by an NIHR Research Professorship (grant RP-2017-08-ST2-006) and by the NIHR Oxford Health Biomedical Research Centre (grant BRC-1215-20005). The views expressed are those of the authors and not necessarily those of the UK National Health Service, the NIHR, or the UK Department of Health.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The research in this paper was conducted at the CFACT Data Center, and the support of AHRQ is acknowledged. The results and conclusions in this paper are those of the authors and do not indicate concurrence by AHRQ or the Department of Health and Human Services. We would like to thank all the members from the meta-epidemiological study group in the School of Public Health, Kyoto University, for the constructive comments and advice.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2020.00035/full#supplementary-material
References
1. WHO. Depression and other common mental disorders: global health estimates. World Health Organization: World Health Organization. (2017). Available from: http://www.who.int/iris/handle/10665/254610. [Accessed April, 2019]
2. NIMH. 2017 National Survey on Drug Use and Health (NSDUH) on Major Depression Statistics. National Institute of Mental Health. (2019). Available from: https://www.nimh.nih.gov/health/statistics/major-depression.shtml#part_155033
3. Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry (2018) 75(4):336–46. doi: 10.1001/jamapsychiatry.2017.4602
4. Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry (2015) 76(2):155–62. doi: 10.4088/JCP.14m09298
5. Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PloS Med (2013) 10(11):e1001547. doi: 10.1371/journal.pmed.1001547
6. Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet (9665)(2009), 746–58. doi: 10.1016/S0140-6736(09)60046-5
7. Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet (2018) 391(10128):1357–66. doi: 10.1016/S0140-6736(17)32802-7
8. American Psychiatric Association. Practice guideline for major depressive disorder in adults. Am J Psychiatry (1993) 150(4 Suppl):1–26. doi: 10.1176/ajp.150.4.1
9. AHCPR Clinical Practice Guidelines N. Depression Guideline Panel. Depression in Primary Care: Volume 2. Treatment of Major Depression. Clinical Practice Guideline, Number 5. Rockville, MD. U.S.: Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research. AHCPR Publication. (1993).
10. American Psychiatric Association. (2010). Work Group on Major Depressive Disorder. Practice Guideline for the Treatment of Patients with Major Depressive Disorder (Third Edition). (2010). Available from: http://www.psychiatryonline.com/pracGuide/pracGuideTopic_7.aspx
11. National Institute for Health and Care Excellence. Depression in adults: recognition and management NICE Guideline. (2009). Available from: www.nice.org.uk/guidance/cg90
12. British Association for Psychopharmacology. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 british association for psychopharmacology guidelines. J Psychopharmacol (2015) 29(5):459–525. doi: 10.1177/0269881115581093
13. Furukawa TA, Cipriani A, Cowen PJ, Leucht S, Egger M, Salanti G. Optimal dose of selective serotonin reuptake inhibitors, venlafaxine, and mirtazapine in major depression: a systematic review and dose-response meta-analysis. Lancet Psychiatry (2019) 6(7):601–9. doi: 10.1016/S2215-0366(19)30217-2
14. Luo Y, Chaimani A, Kataoka Y, Ostinelli EG, Ogawa Y, Cipriani A, et al. Evidence synthesis, practice guidelines and real-world prescriptions of new generation antidepressants in the treatment of depression: a protocol for cumulative network meta-analyses and meta-epidemiological study. BMJ Open (2018) 8(12):e023222. doi: 10.1136/bmjopen-2018-023222
15. Medical Expenditure Panel Survey (MEPS). Available from: https://meps.ahrq.gov/mepsweb/
16. U.S. Food and Drug Administration. National drug code directory. (2018). Available from: https://www.fda.gov/drugs/informationondrugs/ucm142438.htm
17. Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care (2003) 41(11):1284–92. doi: 10.1097/01.MLR.0000093487.78664.3C
18. Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SL, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med (2002) 32(6):959–76. doi: 10.1017/S0033291702006074
19. Kessler RC, Barker PR, Colpe LJ, Epstein JF, Gfroerer JC, Hiripi E, et al. Screening for serious mental illness in the general population. Arch Gen Psychiatry (2003) 60(2):184–9. doi: 10.1001/archpsyc.60.2.184
20. Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA (2003) 289(23):3095–105. doi: 10.1001/jama.289.23.3095
21. Compton WM, Conway KP, Stinson FS, Grant BF. Changes in the prevalence of major depression and comorbid substance use disorders in the United States between 1991-1992 and 2001-2002. Am J Psychiatry (2006) 163(12):2141–7. doi: 10.1176/ajp.2006.163.12.2141
22. Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the national epidemiologic survey on alcoholism and related conditions. Arch Gen Psychiatry (2005) 62(10):1097–106. doi: 10.1001/archpsyc.62.10.1097
23. Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health (2013) 34:119–38. doi: 10.1146/annurev-publhealth-031912-114409
24. British Association for Psychopharmacology. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2000 British association for psychopharmacology guidelines. J Psychopharmacol (2008) 22(4):343–96. doi: 10.1177/0269881107088441
25. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry (2000) 157(4 Suppl):1–45.
26. Stone MK, Shamir K, Richardville K, Miller B. Components and Trends in Treatment Effects in Randomized Placebo-controlled Trials in Major Depressive Disorder from 1979-2016. Miami: American Society of Clinical Psychopharmacology (2018).
27. Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA (2010) 303(1):47–53. doi: 10.1001/jama.2009.1943
28. Hieronymus F, Lisinski A, Nilsson S, Eriksson E. Impact of baseline severity on the effects of selective serotonin reuptake inhibitors in depression: an item-based patient-level post hoc analysis. Lancet Psychiatry (2019) 6(9):745–52. doi: 10.1016/S2215-0366(19)30216-0
29. Moore M, Yuen HM, Dunn N, Mullee MA, Maskell J, Kendrick T. Explaining the rise in antidepressant prescribing: a descriptive study using the general practice research database. BMJ (2009) 339:b3999. doi: 10.1136/bmj.b3999
30. Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the U.S. National Health and Nutrition Examination Survey. J Clin Psychiatry (2014) 75(2):169–77. doi: 10.4088/JCP.13m08443
31. Petty DR, House A, Knapp P, Raynor T, Zermansky A. Prevalence, duration and indications for prescribing of antidepressants in primary care. Age Ageing (2006) 35(5):523–6. doi: 10.1093/ageing/afl023
32. Cruickshank G, Macgillivray S, Bruce D, Mather A, Matthews K, Williams B. Cross-sectional survey of patients in receipt of long-term repeat prescriptions for antidepressant drugs in primary care. Ment Health Fam Med (2008) 5(2):105–9.
33. Stafford RS, MacDonald EA, Finkelstein SN. National patterns of medication treatment for depression, 1987 to 2001. Prim Care Companion J Clin Psychiatry (2001) 3(6):232–5. doi: 10.4088/PCC.v03n0611
34. Bauer M, Monz BU, Montejo AL, Quail D, Dantchev N, Demyttenaere K, et al. Prescribing patterns of antidepressants in Europe: results from the factors influencing depression endpoints research (FINDER) study. Eur Psychiatry (2008) 23(1):66–73. doi: 10.1016/j.eurpsy.2007.11.001
35. Chee KY, Tripathi A, Avasthi A, Chong MY, Sim K, Yang SY, et al. International study on antidepressant prescription pattern at 40 major psychiatric institutions and hospitals in Asia: A 10-year comparison study. Asia Pac Psychiatry (2015) 7(4):366–74. doi: 10.1111/appy.12176
36. Uchida N, Chong MY, Tan CH, Nagai H, Tanaka M, Lee MS, et al. International study on antidepressant prescription pattern at 20 teaching hospitals and major psychiatric institutions in East Asia: analysis of 1898 cases from China, Japan, Korea, Singapore and Taiwan. Psychiatry Clin Neurosci (2007) 61(5):522–8. doi: 10.1111/j.1440-1819.2007.01702.x
37. Grover S, Avasth A, Kalita K, Dalal PK, Rao GP, Chadda RK, et al. IPS multicentric study: Antidepressant prescription patterns. Indian J Psychiatry (2013) 55(1):41–5. doi: 10.4103/0019-5545.105503
38. Donoghue JM, Tylee A. The treatment of depression: prescribing patterns of antidepressants in primary care in the UK. Br J Psychiatry (1996) 168(2):164–8. doi: 10.1192/bjp.168.2.164
39. Rosholm JU, Hallas J, Gram LF. Outpatient utilization of antidepressants: a prescription database analysis. J Affect Disord (1993) 27(1):21–8. doi: 10.1016/0165-0327(93)90092-X
40. Mojtabai R, Olfson M. National patterns in antidepressant treatment by psychiatrists and general medical providers: results from the national comorbidity survey replication. J Clin Psychiatry (2008) 69(7):1064–74. doi: 10.4088/JCP.v69n0704
41. Machlin S, Cohen J, Elixhauser A, Beauregard K, Steiner C. Sensitivity of household reported medical conditions in the medical expenditure panel survey. Med Care (2009) 47(6):618–25. doi: 10.1097/MLR.0b013e318195fa79
42. Hockenberry JM, Joski P, Yarbrough C, Druss BG. Trends in treatment and spending for patients receiving outpatient treatment of depression in the United States, 1998-2015. JAMA Psychiatry (2019) 76(8):810–7. doi: 10.1001/jamapsychiatry.2019.0633
43. Marcus SC, Olfson M. National trends in the treatment for depression from 1998 to 2007. Arch Gen Psychiatry (2010) 67(12):1265–73. doi: 10.1001/archgenpsychiatry.2010.151
Keywords: major depressive disorder, antidepressant, prescription, trend, suboptimal dose
Citation: Luo Y, Kataoka Y, Ostinelli EG, Cipriani A and Furukawa TA (2020) National Prescription Patterns of Antidepressants in the Treatment of Adults With Major Depression in the US Between 1996 and 2015: A Population Representative Survey Based Analysis. Front. Psychiatry 11:35. doi: 10.3389/fpsyt.2020.00035
Received: 18 October 2019; Accepted: 13 January 2020;
Published: 14 February 2020.
Edited by:
Amit Anand, Case Western Reserve University, United StatesReviewed by:
Philip Ninan, East Carolina University, United StatesCasimiro Cabrera Abreu, Queens University, Canada
Copyright © 2020 Luo, Kataoka, Ostinelli, Cipriani and Furukawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Toshi A. Furukawa, ZnVydWthd2FAa3VocC5reW90by11LmFjLmpw
 Yan Luo
Yan Luo Yuki Kataoka2
Yuki Kataoka2 Toshi A. Furukawa
Toshi A. Furukawa