- Institute of Medical Psychology and Behavioral Immunobiology, University Hospital Essen, University of Duisburg-Essen, Essen, Germany
Visceroception is a complex phenomenon comprising the sensation, interpretation, and integration of sensations along the gut-brain axis, including pain or defecatory urgency. Stress is considered a crucial risk factor for the development and maintenance of disorders of gut-brain signaling, which are characterized by altered visceroception. Although the broad role of stress and stress mediators in disturbed visceroception is widely acknowledged, the putative contribution of chronic stress to variations in normal visceroception remains incompletely understood. We aimed to elucidate the role of chronic stress in shaping different facets of visceroception. From a well-characterized, large sample of healthy men and women (N = 180, 50% female), volunteers presenting with low (n = 57) and elevated (n = 61) perceived chronic stress were identified based on the validated Trier Inventory for Chronic Stress (TICS). Visceral sensitivity together with perceived and recalled intensity and defecatory urgency induced by repeated rectal distensions was experimentally assessed, and compared between low and elevated stress groups. Subgroups were compared regarding state anxiety and salivary cortisol concentrations across experimental phases and with respect to psychological measures. Finally, in the full sample and in chronic stress subgroups, a recall bias in terms of a discrepancy between the perception of experimentally-induced symptoms and their recall was tested. Participants with elevated chronic stress presented with increased state anxiety and higher cortisol concentrations throughout the experimental phases compared to the group with low chronic stress. Group differences in visceral sensitivity were not evident. The elevated stress group perceived significantly higher urgency during the stimulation phase, and recalled substantially higher feelings of urgency induced by rectal distensions, while perceived and recalled intensity were comparable between groups. Volunteers with elevated stress exhibited a recall bias in terms of a higher recall relative to mean perception of urgency, whereas no such bias was observed for the intensity of experimental visceral stimulation. Our findings in healthy men and women provide first evidence that the troublesome symptom of urgency might be particularly modifiable by chronic stress and support the relevance of memory biases in visceroception. These results may help to disentangle the impact of chronic stress on altered visceroception in disturbances of gut-brain communication.
Introduction
Visceroception is defined as the perception and processing of interoceptive signals arising from visceral organs (1, 2). Importantly, visceroception is not fully captured by visceral sensitivity alone, which primarily reflects sensory-discriminative aspects of perception. It is rather conceptualized more broadly as a specific facet of interoception, involving the sensation, interpretation, and integration of visceral signals (2) along the gut-brain axis. The complex affective-motivational and cognitive dimensions of visceroception shape gastrointestinal (GI) symptom perception, including visceral pain and defecatory urgency, in healthy individuals as well as in patients with chronic GI symptoms (3). The clinical relevance of disturbed visceroception is particularly evident in the context of disorders of disturbed gut-brain interactions, like irritable bowel syndrome (IBS) and functional dyspepsia. Altered GI symptoms perception, involving visceral hyperalgesia and hypervigilance, plays a pivotal role in their pathophysiology and treatment. However, the complex mechanisms underlying altered visceroception remain incompletely understood, especially with respect to psychological modulation.
As a crucial psychological factor, stress plays a broad role in disorders of disturbed gut-brain interactions (4–6). This has most clearly been underscored by evidence that acute stress or stress mediators of the hypothalamus-pituitary-adrenal (HPA) axis increase visceral sensitivity and neural processing of visceral stimuli in patients (7) but also in healthy volunteers (8). Chronic stress burden has been identified as an important risk factor for disease onset (9), and for the exacerbation of GI symptoms, particularly of visceral pain in patients with IBS (10, 11). Importantly, symptom burden in patients is often not limited to pain, but also involves defecatory urgency as a highly troublesome symptom in a broad range of GI conditions (12–14). Psychological modulation of defecatory urgency has previously been proposed (15), and our own recent data suggested that acute stress amplified nocebo effects especially for the symptom of distension-induced urgency more so then the symptom of pain in healthy volunteers (16). While together these findings clearly support a role of acute as well as chronic stress in different dimensions of visceroception, experimental work particularly on effects of chronic stress remains scarce.
Building on our earlier work on the modulation of visceroception by acute stress and stress mediators (7, 8, 16), we herein aimed to elucidate the putative role of chronic stress in different clinically-relevant facets of normal visceroception. From a large sample of well-characterized healthy men and women that underwent rectal sensitivity testing and repeated painful rectal distensions as part of a larger study (16, 17), we compared individuals with elevated and low perceived chronic stress with respect to sensory and pain thresholds and rectal distension-induced symptom reports of pain and urgency. We hypothesized that individuals with elevated levels of stress would reveal increased sensitivity, as reflected by lower thresholds for graded distensions of the rectum as well as higher pain and urgency ratings in response to individually-calibrated repeated distensions. In addition to analyses of symptom reports based on individual distensions, we also elucidated overall symptom recall based on a retrospective symptom rating. This was accomplished given evidence that retrospective overall symptom ratings may be more susceptible to psychological modulation, especially in patients with IBS (18). Given our interest in visceral pain-related memory effects (8, 19–22), together with evidence supporting the role of reporting bias in IBS (23), we introduced a new “memory bias” measure. This measure was based on the difference between perceptual ratings of individual distensions and retrospective overall ratings, the former being presumably more reflective of sensory-discriminative facts of visceroception, the latter possibly more prone to psychological modulation, both with relevance to collective symptom reporting in experimental and clinical trials as well as clinical practice.
Methods
Participants
From a well-characterized large sample of young healthy men and women (N = 180; 90 women), tertiles based on the validated Trier Inventory for Chronic Stress (TICS) were identified. The top and bottom tertile were chosen to define participants with low and elevated perceived chronic stress, as detailed below, and included in the current analysis. Participants had been recruited through local advertisements for the primary study on the modulation of placebo and nocebo effects by acute experimental stress (16) or relaxation (17) in visceral pain. They had been informed that the aim of the study would be to investigate psychological mechanisms underlying effects of different drugs on experimentally-induced visceral symptoms. Of note, all measures included in the current analyses were assessed prior to randomization of participants for subsequent experimental manipulations. They served as baseline measures in the primary studies, and observations reported herein were therefore independent of subsequent manipulations. Participants were excluded according to the following criteria: age < 18 or > 65 years, body mass index (BMI) < 18 or > 30, a history of or acute medical and psychiatric conditions and current medication use except for hormonal contraceptives, thyroid medication, and occasional use of over-the-counter pain or allergy medication. Moreover, subclinical gastrointestinal (GI) symptoms experienced during a 3 month period preceding study participation were measured using a standardized in-house questionnaire (24). As in our prior studies (19, 21, 24) a cut-off score of 11 was used as an indicator of a putative undiagnosed gastrointestinal condition, which led to exclusion from study participation. Only women using hormonal contraception were included and pregnancy was ruled out on the study day with a commercially available urinary test. All participants underwent a physical examination to exclude perianal tissue damage (e.g., fissures or painful hemorrhoids), which might interfere with the experimental procedure. Participants gave written informed consent and received 200€ for their participation. The study protocol was approved by the local ethics committee (protocol number 13-5565-BO) and followed the provisions of the Declaration of Helsinki.
Experimental Design and Study Procedures
An overview over the experimental design and the study procedures relevant to the current analyses is given in Figure 1. All experimental procedures were conducted between 12:00 and 18:00 h to account for effects of the circadian rhythm. Initially, an inflatable balloon attached to a pressure-controlled barostat system (modified ISOBAR 3 device; G & J Electronics, ON, Toronto, Canada) was placed 5 cm from the anal verge, for the application of rectal distensions. Rectal sensory and pain thresholds were determined using a double-random staircase distension protocol with random pressure increments between 2 and 6 mmHg and a maximal distension pressure of 55 mmHg. Participants rated each sensation on a Likert-type scale labeled 1 = no perception, 2 = doubtful perception, 3 = sure perception, 4 = little discomfort, 5 = severe discomfort, and 6 = pain, not tolerable distension. The sensory threshold was defined as a pressure when ratings changed from 2 to 3 and the pain threshold was determined at the change from 5 to 6. The individual pain threshold was used as an anchor for a subsequent pressure calibration to identify a moderately painful intensity for the repeated application of rectal distensions. Specifically, a pressure corresponding to a pain intensity rating not higher than 80 on a visual analog scale (VAS) with endpoints labeled 0 = none at all and 100 = very much was identified, as previously described (16). This intensity was used for the subsequent stimulation phase, during which six rectal distensions with a duration of 30 s and a rest interval of 30 s following each stimulus were applied. Salivary cortisol concentrations and state anxiety as measures of acute stress and arousal were collected at different time points across the experimental phases and ratings of stimulus intensity and urgency perception and recall as different facets of visceroception were assessed, as detailed below.
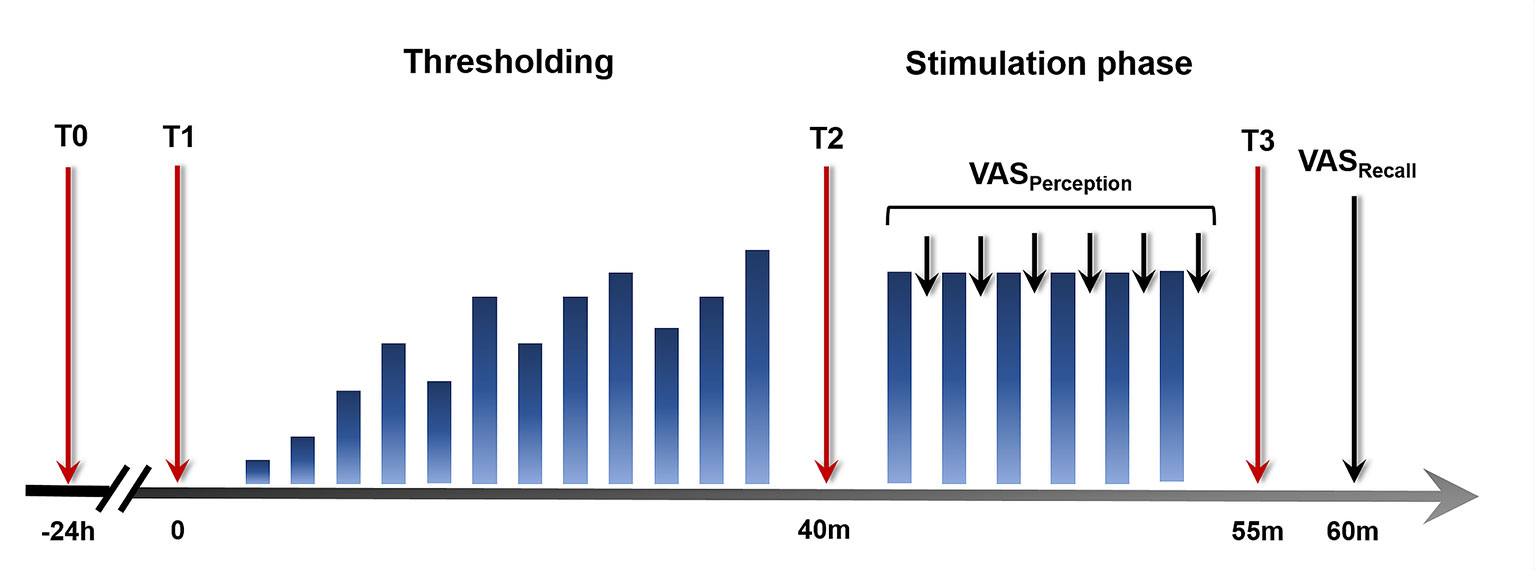
Figure 1 Study design and experimental procedures. Twenty-four hours prior to study participation (T0), a salivary cortisol sample was collected as a baseline measure unaffected by the experimental procedure. On the study day, salivary cortisol and state anxiety were assessed upon arrival (T1), before (T2), and after (T3) the stimulation phase. Visual analog scale (VAS) ratings of intensity and urgency perception were acquired during the stimulation phase. At the conclusion of the experimental phase, VAS ratings assessing intensity and urgency recall were accomplished.
Measures of Visceroception
In addition to sensory and pain thresholds, mean scores of trial-by-trial VAS ratings of stimulus intensity and urgency perception and ratings of overall recalled intensity and urgency induced by the experienced rectal distensions during the stimulation phase were assessed as measures of visceroception. Specifically, during the stimulation phase, participants were prompted to rate the intensity of each distension and the urgency it induces on separate VAS with endpoints labeled “none” (0) and “very much” (100) for intensity and “none (0) and “very high” (100) for urgency. Following the stimulation phase, participants were asked to recall the overall intensity of and urgency induced by the experienced distensions using separate VAS. In order to elucidate a potential memory bias in visceroception in terms of a deviation of recalled from mean perceived visceral sensations, delta values between perceived and recalled intensity and urgency were calculated, respectively, to test for recall biases in measures of visceroception.
Assessment of State Anxiety and Salivary Cortisol
Twenty-four hours prior to the study appointment (time point T0), a baseline salivary cortisol sample was collected by participants in their home environment, using Salivettes (Sarstedt, Nürnbrecht, Germany), and stored at 4°C until transport to the laboratory on the study day. On the study day, salivary cortisol as a marker of acute stress and HPA axis activation was collected upon arrival (T1), following the thresholding procedure before the stimulation phase (T2), and after the stimulation phase (T3). As a self-report measure of acute arousal, state anxiety was assessed at time points T1–T3 along with cortisol sample collection, using the state version of the validated State Trait Anxiety Inventory (STAI-S) (25, 26). Saliva samples were centrifuged (2,000 rpm, 2 min, 4°C) and stored at −20°C until analysis. Cortisol concentrations were measured using an enzyme-linked immunosorbent assay (ELISA; IBL International, Hamburg, Germany) in accordance with the manufacturer’s protocol with a detection limit at 0.138 nmol/L.
Assessment of Chronic Stress and Identification of Stress Subgroups
Following informed consent, participants completed the validated Trier Inventory for Chronic Stress (TICS) screening scale (27). The self-assessment instrument allows an evaluation of individual experiences with chronic stressors in everyday life, providing a reliable global measure of perceived stress during the previous 3 months with a Cronbach’s α of .91 (28). Each of the 12 items is scored on a five-point Likert-scale as “never” (0), “rarely” (1), “sometimes” (2), “often” (3), and “very often” (4 points). The total score ranges from 0 to 48 points, expressing the subjectively perceived presence and frequency of chronic stressors. Norm values from healthy volunteers are available (22), with a mean TICS score of 13 corresponding to T = 50. TICS sum scores were used herein to evaluate overall perceived chronic stress and to allocate participants to a subgroup with low or elevated chronic stress. This was accomplished by subdividing participants into tertile subgroups based on TICS scores. Participants in the top tertile were defined as an elevated stress subgroup, the bottom tertile as a group with low chronic stress.
Questionnaires
In addition to TICS for the assessment of chronic stress, participants completed the following comprehensive questionnaire battery for a characterization with respect to psychological factors of putative relevance to both, stress, and visceroception: The trait version of the State Trait Anxiety Inventory (STAI-T) (25, 26) for the assessment of trait anxiety (sum scores between 20 and 80), the Pain-Related Self Statements Scale (PRSS) (29) to measure pain-related cognitions in terms of maladaptive pain catastrophizing and adaptive pain coping (sum scores ranging from 0 to 45, respectively), and the Generalized Self-efficacy (GSE) Scale (30) to assess optimistic self-beliefs to cope with life demands (total scores 10–40) as a marker of resilience to stressors.
Statistical Analyses
All statistical analyses were performed using IBM SPSS version 25 (IBM Corporation, Armonk, NY, USA). As described above, participants were stratified based on the level of perceived chronic stress, allowing to define and compare groups with low and elevated perceived chronic stress. Notably, due to this stratification strategy, the investigated samples displayed non-normal distribution in some of the relevant outcome measures, as evidenced by significant Kolmogorov-Smirnov tests. However, no outliers were detected in either sample. Given sufficient sample sizes and the robustness of parametric statistical approaches under these circumstances, parametric tests were performed. Accordingly, stress subgroups were compared with respect to sociodemographic and psychological characteristics using two sample t-tests or chi square test where appropriate. Group comparisons of sensory and pain thresholds as measures of visceral sensitivity, as well as baseline cortisol (T0) were accomplished using two sample t-tests. Repeated measures ANOVA with the within-group factor time and the between-group factor stress subgroup were applied to analyze state anxiety and salivary cortisol concentrations on the study day (T1–T3). Independent sample t-tests were further conducted for group comparisons of mean perceived and recalled measures of visceroception, as assessed with VAS. In addition, bias scores based on the difference between perceptual and retrospective ratings were entered into one sample t-tests for effects in the full sample and into two sample t-tests for stress subgroup comparisons. To account for a possible impact of acute stress and arousal on effects of chronic stress on visceroception, analyses of covariance (ANCOVA) with mean cortisol concentrations and mean state anxiety scores as covariates were additionally conducted for measures of visceroception. Further, to address possibly divergent effects of chronic stress on measures of visceroception in men and women, interactions between the factors sex and stress subgroup were explored using ANOVA. Finally, to confirm the specificity of findings to chronic stress, stepwise multiple regression analyses (probability to enter ≤.05, probability to remove ≥.10) were performed in the full sample, entering TICS scores as a measure of perceived chronic stress together with closely related psychological traits, such as trait anxiety, pain coping, and self-efficacy, as predictors of variance in visceroceptive markers. Results from ANOVA and ANCOVA are reported with Greenhouse-Geisser correction to account for a possible violation of the sphericity assumption and results from post hoc t-tests were Bonferroni corrected for multiple comparisons where appropriate. Alpha level was set at p < .05, exact two-tailed p values are reported and ηp2, Cohen’s d, or Cramer’s V are provided as indicators of effect size, respectively. All descriptive statistics are reported as mean ± standard error of the mean (SEM), unless indicated otherwise.
Results
Sample Characterization
A characterization of the full sample and comparisons of stress subgroups with respect to sociodemographic and psychological measures are summarized in Table 1. TICS scores in the full sample indicated an average level of perceived chronic stress according to available norm values (28). The mean score in the low stress group corresponded to an average level of chronic stress within a lower range in a healthy population. The elevated stress group presented with mean TICS scores above average, confirming the stratification strategy and the identification of healthy volunteers with low and elevated levels of perceived chronic stress. Accordingly, chronic stress scores were substantially higher in the elevated chronic stress group. Subgroups were comparable regarding age, BMI, and distribution of men and women. Participants with elevated stress presented with increased trait anxiety, lower self-efficacy, and reported more catastrophizing cognitions when coping with pain, whereas groups did not differ regarding the use of active pain coping.
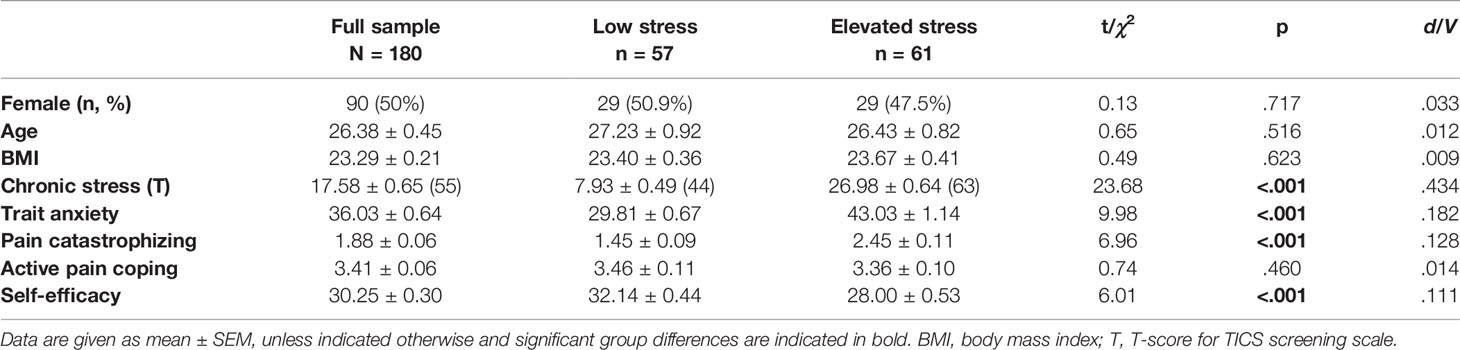
Table 1 Characterization of the full sample and chronic stress groups with respect to sociodemographic and psychological variables.
Salivary Cortisol and State Anxiety
Baseline cortisol concentrations 24 h prior to the study appointment (T0) were significantly increased in the group with elevated chronic stress (13.29 ± 1.24 nmol/l) relative to individuals with low perceived chronic stress (10.09 ± 0.72 nmol/l; t = 2.24; p = .027; d = .042). Analysis of cortisol concentrations on the study day (T1–T3) revealed a significant effect of stress subgroup (F = 6.60; p = .011; ηp2 = .054), which was attributable to higher cortisol levels across the experimental phases in participants with elevated perceived chronic stress (Figure 2A). No effect of time was evident (p = .372). Analysis of state anxiety also demonstrated a significant effect of stress subgroup (F = 19.76; p < .001; ηp2 = .146), with higher state anxiety in the elevated stress compared to the low stress group (Figure 2B). No effect of time was observed (p = .257).
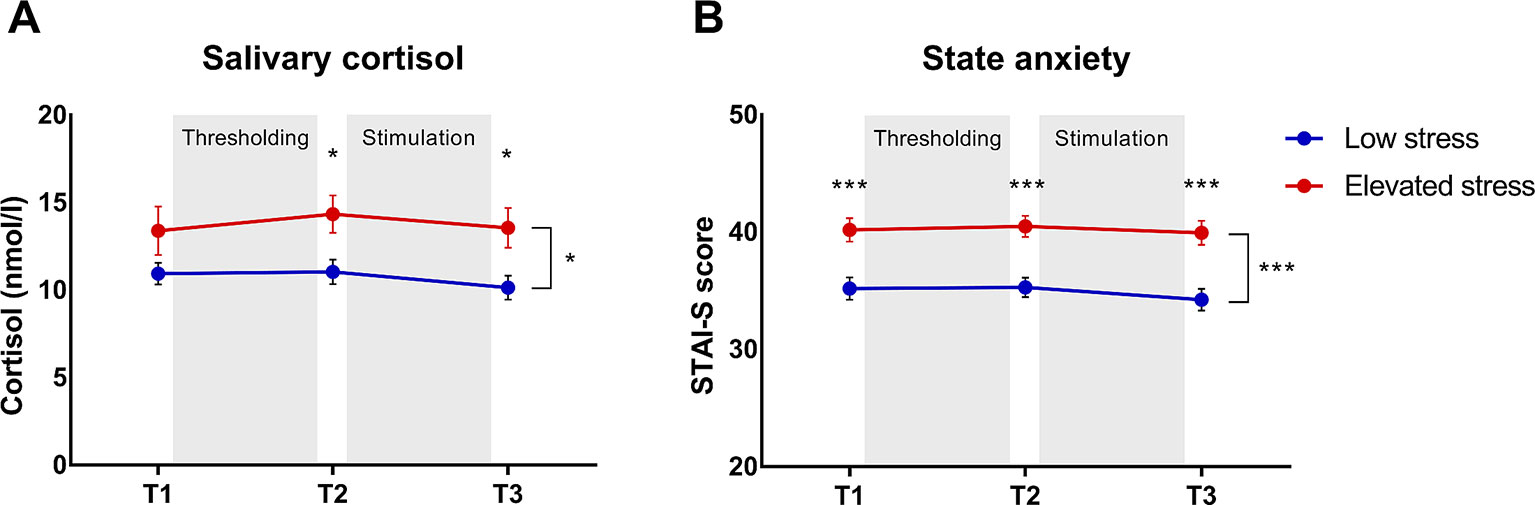
Figure 2 Group comparisons of (A) salivary cortisol concentrations and (B) state anxiety across the experimental phases (T1–T3) in subjects with elevated (n = 61) versus those with low chronic stress (n = 57). Data are given as mean ± SEM. *p < .05 ***p < .001.
Visceroception in Subgroups With Low and Elevated Chronic Stress
Sensory and Pain Thresholds
Analyses of sensory and pain thresholds in individuals with elevated and low perceived chronic stress revealed comparable thresholds for both, first sensation (p = .789; Figures 3A, C) and pain (p = .794; Figures 3B, D). Controlling for state anxiety and salivary cortisol concentrations did not affect these results (data not shown).
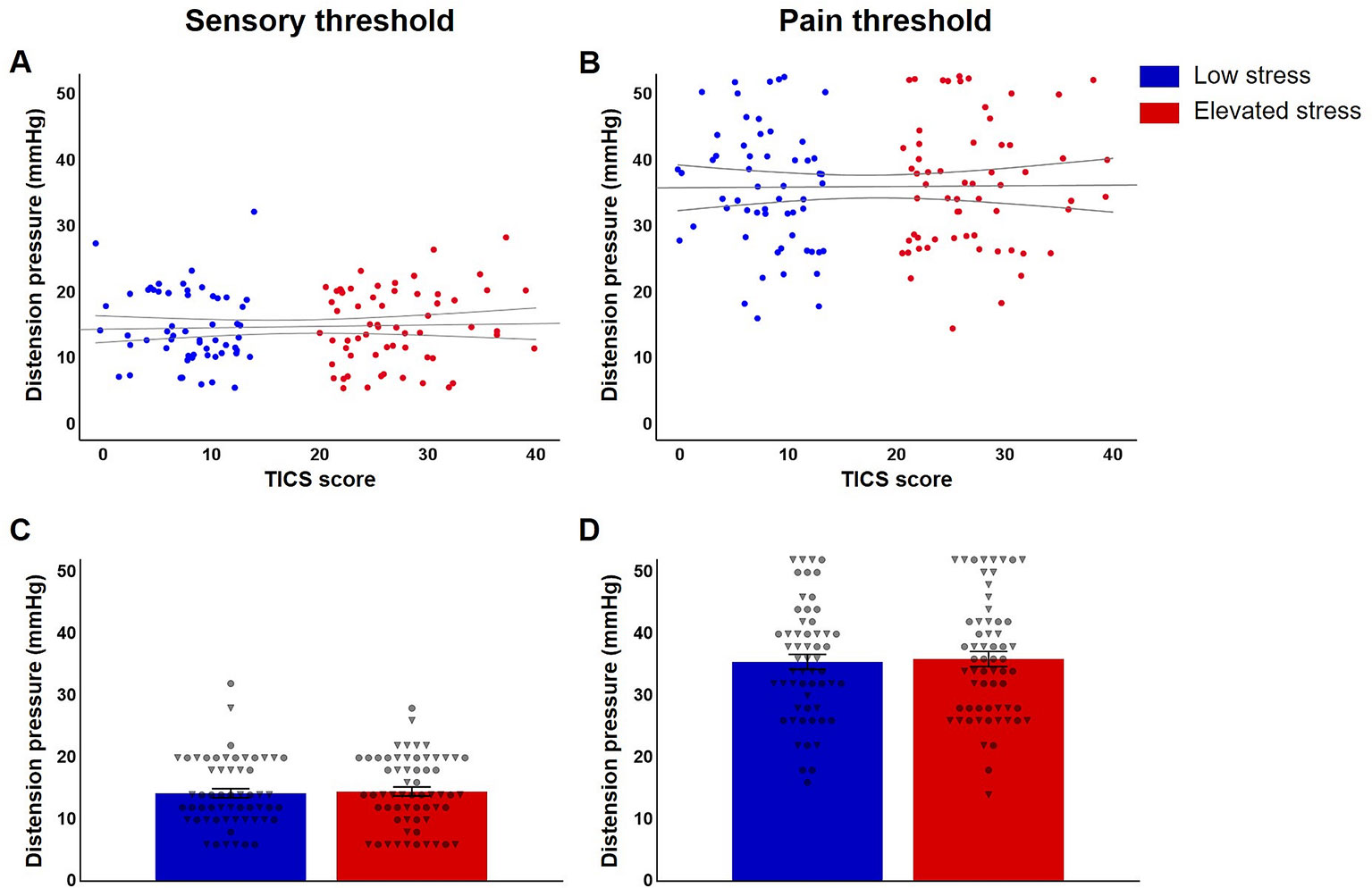
Figure 3 Jittered scatterplots with regression curves and 95% confidence intervals of individual (A) sensory and (B) pain thresholds in participants with low (n = 57, blue) and elevated chronic stress (n = 61, red) and group comparisons regarding mean thresholds (C, D), provided ± SEM and depicted with individual data points for women (indicated as circles) and men (shown as triangles).
Perception and Recall of Visceroceptive Stimulation
Subgroup comparisons of mean perceived urgency based on trial-by-trial ratings following each visceral sensation during the stimulation phase revealed significantly higher urgency in the group with elevated stress (t = 2.04; p = .043; d = 0.37; Figure 4A), whereas groups did not differ regarding mean perceived intensity (p = .507; Figure 4C). Similarly, participants with elevated perceived chronic stress recalled significantly higher overall urgency experienced during the stimulation phase (t = 3.57; p = .001; d = 0.66; Figure 4B), but no group difference in recalled intensity was observed (p = .517; Figure 4D). In covariance analyses, group differences in mean perceived urgency failed statistical significance (p = .095) when including mean state anxiety and mean cortisol concentrations, while differences in urgency recall remained widely unaffected (F = 9.44; p = .003; ηp2 = .076) and no changes were evident regarding perceived or recalled intensity (data not shown).
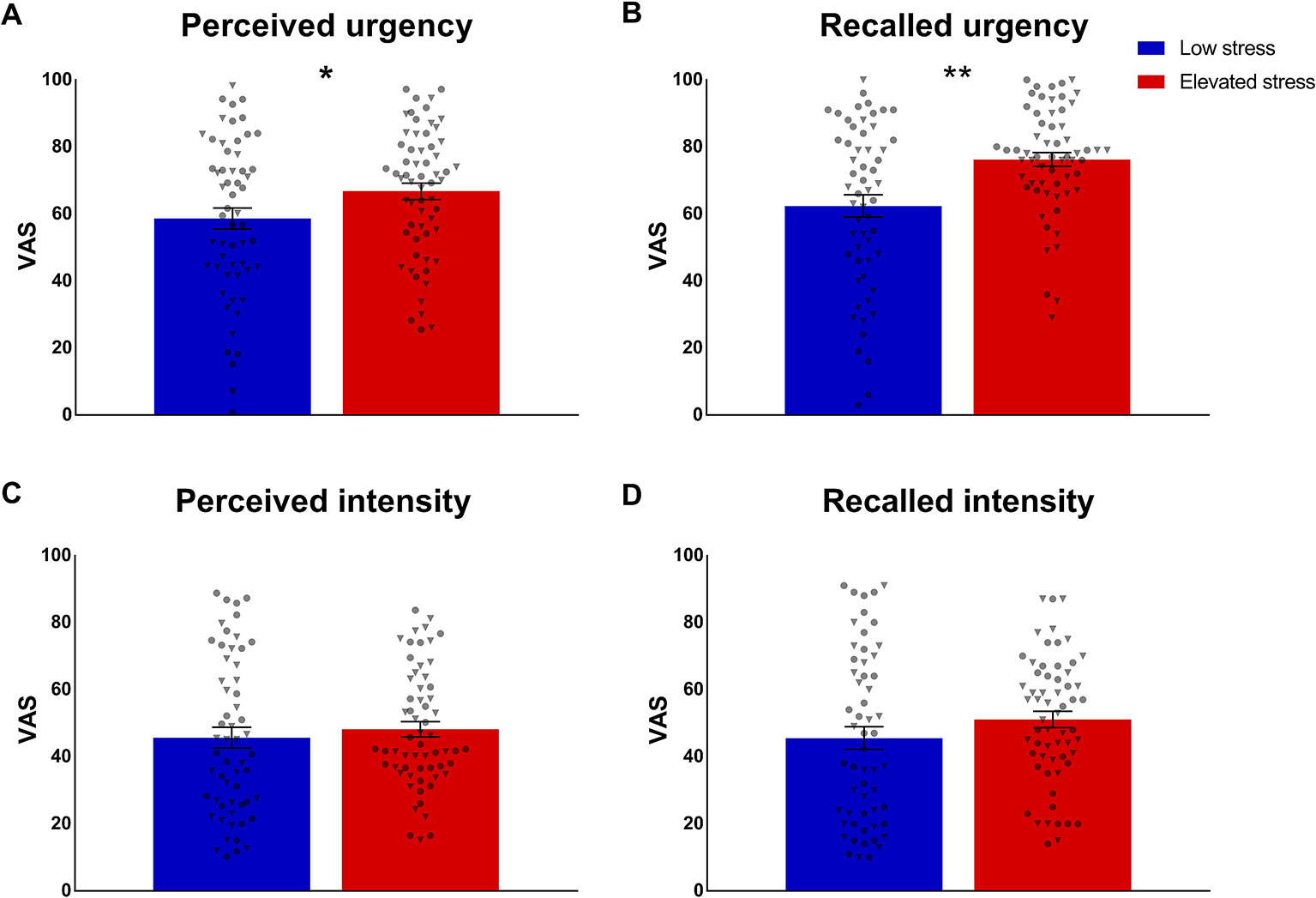
Figure 4 Group comparisons of (A) mean perception and (B) recall of urgency and (C, D) intensity of repeated rectal distensions during the stimulation phase in participants with low (n = 57) and elevated (n = 61) chronic stress. Data are given as mean ± SEM and individual data points for women (circles) and men (triangles) are provided. *p < .05; **p < .01.
Recall Bias
To elucidate a putative exaggeration of intensity or urgency recall, the full sample and stress subgroups were tested for a recall bias in visceroception, operationalized as the differences between mean reported perception during the stimulation phase and overall recall, respectively. One sample t-tests revealed significant effects for both, intensity (t = 2.48; p = .014; d = 0.18) and, more pronounced, for urgency (t = 8.38; p < .001; d = 0.62), indicating higher recall relative to mean perception in the full sample. Individuals with elevated chronic stress exhibited a significant bias for recalled defecatory urgency, i.e., recalled more intense feelings of urgency relative to their mean perception (t = 2.96; p = .004; d = 0.55; Figure 5A). The recall bias for intensity was comparable between stress subgroups (p = .132; Figure 5B). ANCOVA including mean state anxiety and cortisol did not affect these finding (urgency recall bias: F = 6.68; p = .011; ηp2 = .055; intensity recall bias: p = .305).
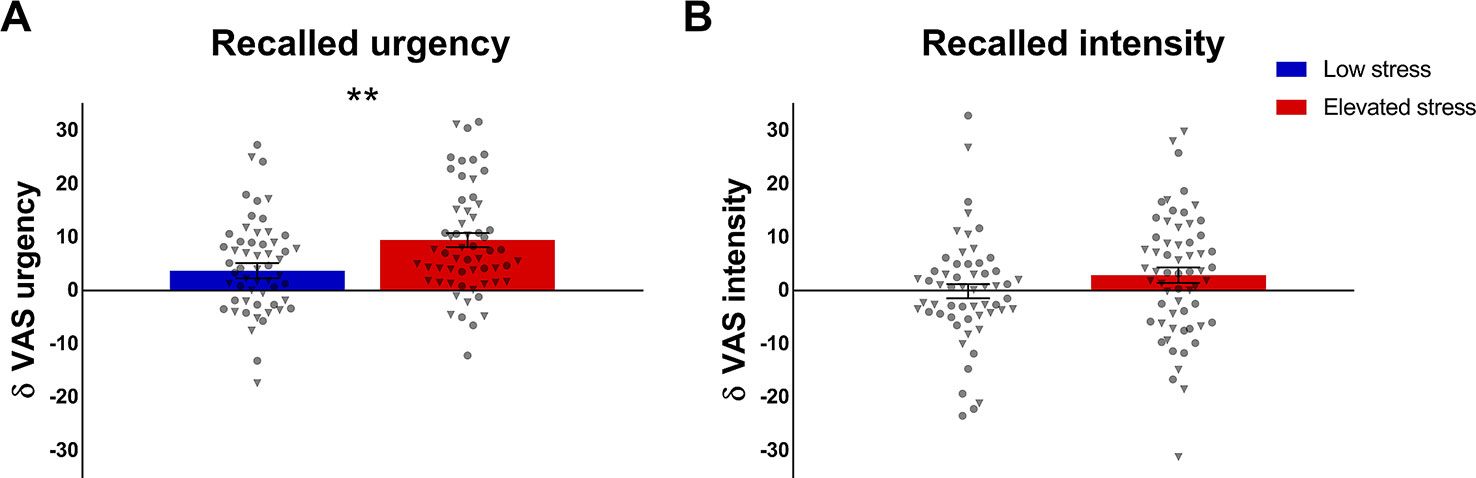
Figure 5 Group comparisons in recall bias of (A) defecatory urgency and (B) intensity of rectal distensions during the stimulation phase, operationalized as the difference between mean perceived and recalled symptoms. Data from individuals with low (n = 57) and elevated chronic stress levels (n = 61) are given as mean ± SEM and individual data points are illustrated as circles for women and as triangles for men. **p < .01.
Interactions Between Chronic Stress and Sex
Possible sex differences in the effects of perceived chronic stress on visceroception were addressed in exploratory analyses. For thresholds, results revealed no interaction between stress level and sex for either first sensation (p = .950; Figure 3C) or pain (p = .451; Figure 3D). No evidence of sex-specific effects of chronic stress emerged for perceived (p = .503; Figure 4A) and recalled urgency (p = .824; Figure 4B) or intensity (perceived: p = .143; Figure 4C; recalled: p = .222; Figure 4D). Finally, neither urgency (p = .352; Figure 5A) nor intensity recall bias (p = .793; Figure 5B) indicated sex-specific effects of perceived chronic stress.
Specificity to Chronic Stress
Stepwise multiple regression analyses were performed in the full sample of N = 180 participants to evaluate whether the observed effects were specific to chronic stress or could also be attributed to effects of other psychological traits, including trait anxiety, pain coping strategies, and self-efficacy. This exploratory approach focused on significant findings from subgroup analyses, and was therefore conducted on perceived and recalled urgency as well as the urgency recall bias. These analyses confirmed chronic stress to be the main predictor of visceroception. Specifically, TICS scores were a single significant predictor of mean perceived (F = 4.49; p = 0.035; adj. R2 = 0.018; ß = 0.157) and, more pronounced, recalled urgency (F = 12.84; p < .001; adj. R2 = 0.062; ß = 0.259). Further, perceived chronic stress was identified as a single predictor of variance in the urgency recall bias (F = 8.59; p = .004; adj. R2 = 0.041; ß = 0.215). Trait anxiety, pain coping, and self-efficacy did not contribute additionally to explaining variance in visceroception.
Discussion
The relevance of interoception for both health and disease is increasingly acknowledged (1, 2, 31), especially in the context of visceral hypersensitivity in disorders of gut-brain interactions. Although the broad role of stress and stress mediators in disturbed visceroception is widely appreciated (32–34), the putative contribution of chronic stress to variations in normal visceroception remains incompletely understood. To fill this research gap, we herein assessed the impact of chronic stress on different dimensions of visceroception induced by rectal distensions by comparing stress subgroups classified based on a validated chronic stress questionnaire. While individuals with elevated versus low levels of perceived chronic stress did not differ in rectal sensory or pain thresholds, both the perception as well as the recall of rectal urgency were significantly enhanced in individuals with elevated chronic stress. Furthermore, a recall bias for previously experienced distension-induced urgency was more pronounced in the group with elevated stress. Together, these findings support that the sensation of urgency might be particularly modifiable by chronic stress in healthy young men and women, with implications for the pathophysiology of chronic GI symptoms.
A link between chronic stress and the perception and recall of urgency complements our previous result that the symptom of urgency was demonstrably highly modifiable by acute psychosocial stress in a placebo/nocebo paradigm (16). Psychological modulation of urgency is interesting from a clinical perspective for a broad range of conditions characterized by chronic gastrointestinal symptoms, which are often not limited to the experience of visceral pain. For example, defecatory urgency is a symptom frequently reported by patients with IBS (12), which has recently been identified as the most troublesome symptom in diarrhea-predominant IBS (13). Urgency has also emerged as an independent predictor of quality of life not only in IBS and other disturbances of gut-brain communication (35, 36), but also in the general population (37).
In light of the fact that symptom reports guide diagnosis and treatment in many conditions involving the gut-brain axis, our findings suggesting a putative role of chronic stress in GI symptom recall are noteworthy and deserve more attention. The recall of defecatory urgency induced by previously experienced visceral sensations was enhanced in participants with elevated chronic stress. Further, individuals who reported more chronic stress also demonstrated a more pronounced recall bias for urgency, herein quantified as the difference between the individual distension ratings and the overall urgency recall. The role of reporting bias and its possible contribution to findings of visceral hypersensitivity in IBS has previously been elegantly demonstrated (23). Our results expand on these data using a somewhat simpler yet clinically-relevant method, following a line of research on memory processes in visceroception (8, 19–22), with a particular focus on interoceptive hypervigilance. It is indeed intriguing to speculate that chronic stress may contribute to interoceptive hypervigilance, either indirectly involving a reporting bias or more directly by biasing specific memory processes, including immediate recall, toward more “negative” memories of symptoms. Future studies should therefore test the hypothesis that altered visceroceptive recall may constitute a nocebo mechanism in the pathophysiology of altered gut-brain interactions. Support for this assumption is provided by experimental findings from the field of associative visceral pain-related conditioning (19, 21, 22), with documented alterations in pain-related learning and memory processes in patients with IBS (20, 38). Stress and stress mediators might play a key role in these alterations, as evidenced by findings that antagonizing corticotropin releasing factor, one of the main signaling peptides of the HPA axis released in response to stress, normalized aberrant neural and psychophysiological correlates of abdominal pain-related learning and memory in women with IBS (38). In healthy individuals, we recently observed pharmacologically increased cortisol levels to induce a reduction in visceral pain thresholds and to affect the formation of pain-related emotional memories (8). Importantly, these effects appeared to be specific to the visceral domain and were not observed for somatic stimuli of identical intensities, in line with prior research on distinct mechanisms underlying the processing of visceral and somatic pain (39–42), and suggesting that visceroception might be particularly vulnerable to stress and stress mediators. Our findings expand this evidence to the dimension of chronic stress, with putative clinical implications for vulnerability and resilience in health and in disorders of gut-brain communication.
On a critical note, some of the mechanisms underlying our findings remain difficult to discern. We observed significantly elevated state anxiety and cortisol concentrations in our cohort of individuals with higher perceived chronic stress across experimental time points. Hence, higher perceived chronic stress was clearly associated with differences in “state” measures, which reportedly modulate visceral pain processing (43), but also with psychological traits, such as increased trait anxiety, maladaptive pain coping, and lower self-efficacy. While our explorative covariance and regression analyses widely supported the observed effects to be distinctly attributable to chronic stress, our study design and the present results do not allow conclusive answers. Clearly, there exists a large overlap between chronic stress and trait anxiety, including its underlying neurobiology (44) and maladaptive coping is likely to further increase not only acute stress responsivity but also the burden arising from physical or psychological stressors (45). These psychological factors might therefore further increase detrimental effects of chronic stress in patients with disorders of gut-brain interactions. Future studies may consider including patients with disturbed visceroception with and without a comorbidity with anxiety. This could shed more light on additive or interactive relations between stress and anxiety in visceroception, which cannot be fully captured in our sample of young, healthy individuals with overall low anxiety symptom burden and adaptive coping skills. Likewise, although we identified a group of healthy individuals in our sample reporting levels of chronic stress above average, the stress burden was not substantially increased to clinically-relevant levels (20, 46). While posing a limitation regarding the generalizability of our findings to the impact of severe chronic stress in patients with disturbed visceroception, our data support that even subtle increases in perceived chronic stress might modulate visceroception. Finally, we addressed our research questions regarding the role of chronic stress in visceroception in a mixed sample of men and women. A recent analysis conducted in a large pooled sample including the current study cohort confirmed no differences between healthy male and female participants with respect to visceral sensitivity, yet did not test other aspects of visceroception (47). Stress subgroups in the current sample did not differ in the relation between male and female participants, suggesting that men and women suffered from elevated chronic stress to comparable extents. Further, exploratory analyses indicated no sex- or gender-specific effects of perceived chronic stress on the tested dimensions of visceroception, suggesting that, at least in young healthy men and women, chronic stress and sex/gender do not interact in altering visceroception. On the other hand, pharmacologically increased cortisol was recently shown to affect visceral sensitivity distinctly in women (8), in support of a role of sex/gender in the impact of the stress mediator on visceroception. Importantly, such putative sex-dependent effects of stress or stress markers might be mediated by interactions with gonadal hormone status, which in women is subject to substantial variations across the menstrual cycle, reportedly impacts visceroception (48), and appears to affect the vulnerability and responsivity to stressors (49, 50). To control for confounding effects of menstrual cycle phase, all women in the sample under investigation were on hormonal contraception. However, this selection does not allow a generalization to women with a natural menstrual cycle, calling for future research including female participants in different mentrual cycle phases.
Taken together, our findings support that elevated perceived chronic stress affects visceroception in healthy individuals, particularly the perception and recall of defecatory urgency as a highly disturbing and clinically-relevant marker in patients with disturbances of gut-brain interactions (35, 36). As two major pathways in the communication along the gut-brain axis, the descending stress system and the ascending visceroceptive system are tightly interacting (32) and their dysfunction may have profound detrimental effects on the communication pathways connecting the brain and the gut. Therefore, investigating chronic stress also in otherwise healthy individuals may aid to gain further insights into mechanisms contributing to long-lasting disturbances along the gut-brain axis. Importantly, the relevance of a dysfunctional gut-brain interaction is increasingly acknowledged beyond disorders primarily characterized by GI symptoms. These developments are not least owed to a growing appreciation of the crucial role of gut microbiota in health and disease (51–53). Particularly, tremendous advances have been made in understanding the impact of pre- and postnatal microbial composition on responsivity to stress later in life (54). These transdisciplinary findings strongly suggest a key role of the microbiota-gut-brain axis and its neural, humoral, endocrine, and immunological communication pathways in stress-related disturbances. This has implications for both the pathophysiology, and also the therapy of diseases affecting visceroception (6, 55), as well as highly comorbid stress-related central nervous system (CNS) disorders (56), such as anxiety (57), depression (58), and posttraumatic stress disorder (PTSD) (59). Interdisciplinary research on the complex communication pathways along the gut-brain axis bridging neurogastroenterology, psychiatry, and the neurosciences therefore promises important new insights into pathophysiological processes and may inspire new treatments of diseases characterized by altered stress responsivity, including those with visceroceptive malfunction.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Ethics Review Board of the Medical Faculty of the University of Duisburg-Essen. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SE and SB designed the research study. TR performed the research. AI and FL analyzed the data. AI, SB, and SE wrote the paper. All authors revised the manuscript for critical intellectual content and approved the final version of the manuscript.
Funding
This project was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) as part of the DFG Research Unit FOR 1328 (grant number: EL 236/8-2) and project number 316803389 – SFB 1280. The funding agency had no role in the conception, analysis, or interpretation of the data.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Ceunen E, Vlaeyen JWSS, Van Diest I. On the origin of interoception. Front Psychol (2016) 7:743. doi: 10.3389/fpsyg.2016.00743
2. Khalsa SS, Adolphs R, Cameron OG, Critchley HD, Davenport PW, Feinstein JS, et al. Interoception and Mental Health : A Roadmap. Biol Psychiatry Cogn Neurosci Neuroimaging (2018) 3:501–13. doi: 10.1016/j.bpsc.2017.12.004
3. Elsenbruch S. Abdominal pain in irritable bowel syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain Behav Immun (2011) 25:386–94. doi: 10.1016/j.bbi.2010.11.010
4. Meerveld BG-V, Johnson AC. Mechanisms of Stress-induced Visceral Pain. J Neurogastroenterol Motil (2018) 24:7–18. doi: 10.5056/jnm17137
5. Moloney RD, O'Mahony SM, Dinan TG, Cryan JF. Stress-induced visceral pain: Toward animal models of irritable-bowel syndrome and associated comorbidities. Front Psychiatry (2015) 6:15. doi: 10.3389/fpsyt.2015.00015
6. Moloney RD, Johnson AC, O'Mahony SM, Dinan TG, Greenwood-Van Meerveld B, Cryan JF. Stress and the microbiota–gut–brain axis in visceral pain: relevance to irritable bowel syndrome. CNS Neurosci Ther (2016) 22:102–17. doi: 10.1111/cns.12490
7. Elsenbruch S, Rosenberger C, Bingel U, Forsting M, Schedlowski M, Gizewski ER. Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology (2010) 139:1310–9. doi: 10.1053/j.gastro.2010.06.054
8. Benson S, Siebert C, Koenen LR, Engler H, Kleine-Borgmann J, Bingel U, et al. Cortisol affects pain sensitivity and pain-related emotional learning in experimental visceral but not somatic pain: a randomized-controlled study in healthy men and women. Pain (2019) 160:1719–28. doi: 10.1097/j.pain.0000000000001579
9. Creed F. Review article: the incidence and risk factors for irritable bowel syndrome in population-based studies. Aliment Pharmacol Ther (2019) 50:507–16. doi: 10.1111/apt.15396
10. Fukudo S. Stress and visceral pain: focusing on irritable bowel syndrome. Pain (2013) 154 Suppl1:S63–S70. doi: 10.1016/j.pain.2013.09.008
11. Boeckxstaens GE, Wouters MM. Neuroimmune factors in functional gastrointestinal disorders: a focus on irritable bowel syndrome. Neurogastroenterol Motil (2017) 29:e13007. doi: 10.1111/nmo.13007
12. Polster A, Van Oudenhove L, Jones M, Ohman L, Tornblom H, Simren M. Mixture model analysis identifies irritable bowel syndrome subgroups characterised by specific profiles of gastrointestinal, extraintestinal somatic and psychological symptoms. Aliment Pharmacol Ther (2017) 46:529–39. doi: 10.1111/apt.14207
13. Tornblom H, Goosey R, Wiseman G, Baker S, Emmanuel A. Understanding symptom burden and attitudes to irritable bowel syndrome with diarrhoea: Results from patient and healthcare professional surveys. U Eur Gastroenterol J (2018) 6:1417–27. doi: 10.1177/2050640618787648
14. Spiegel BMR, Khanna D, Bolus R, Agarwal N, Khanna P, Chang L. Understanding gastrointestinal distress: a framework for clinical practice. Am J Gastroenterol (2011) 106:380–5. doi: 10.1038/ajg.2010.383
15. Posserud I, Agerforz P, Ekman R, Bjornsson ES, Abrahamsson H, Simren M. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut (2004) 53:1102–8. doi: 10.1136/gut.2003.017962
16. Roderigo T, Benson S, Schöls M, Hetkamp M, Schedlowski M, Enck P, et al. Effects of acute psychological stress on placebo and nocebo responses in a clinically relevant model of visceroception. Pain (2017) 158:1489–98. doi: 10.1097/j.pain.0000000000000940
17. Elsenbruch S, Roderigo T, Enck P, Benson S. Can a brief relaxation exercise modulate placebo or nocebo effects in a visceral pain model? Front Psychiatry (2019) 10:144. doi: 10.3389/fpsyt.2019.00144
18. Elsenbruch S, Rosenberger C, Enck P, Forsting M, Schedlowski M, Gizewski ER. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut (2010) 59:489–95. doi: 10.1136/gut.2008.175000
19. Icenhour A, Labrenz F, Ritter C, Theysohn N, Forsting M, Bingel U, et al. Learning by experience? Visceral pain-related neural and behavioral responses in a classical conditioning paradigm. Neurogastroenterol Motil (2017) 29:e13026–n/a. doi: 10.1111/nmo.13026
20. Icenhour A, Langhorst J, Benson S, Schlamann M, Hampel S, Engler H, et al. Neural circuitry of abdominal pain-related fear learning and reinstatement in irritable bowel syndrome. Neurogastroenterol Motil (2015) 27:114–27. doi: 10.1111/nmo.12489
21. Icenhour A, Kattoor J, Benson S, Boekstegers A, Schlamann M, Merz CJ, et al. Neural circuitry underlying effects of context on human pain-related fear extinction in a renewal paradigm. Hum Brain Mapp (2015) 36:3179–93. doi: 10.1002/hbm.22837
22. Labrenz F, Icenhour A, Schlamann M, Forsting M, Bingel U, Elsenbruch S. From Pavlov to pain: How predictability affects the anticipation and processing of visceral pain in a fear conditioning paradigm. Neuroimage (2016) 130:104–14. doi: 10.1016/j.neuroimage.2016.01.064
23. Dorn SD, Palsson OS, Thiwan SIM, Kanazawa M, Clark WC, van Tilburg MAL, et al. Increased colonic pain sensitivity in irritable bowel syndrome is the result of an increased tendency to report pain rather than increased neurosensory sensitivity. Gut (2007) 56:1202–9. doi: 10.1136/gut.2006.117390
24. Lacourt TE, Houtveen JH, Doornen LJP, Benson S, Grigoleit J-S, Cesko E, et al. Biological and psychological predictors of visceral pain sensitivity in healthy premenopausal women. Eur J Pain (2014) 18:567–74. doi: 10.1002/j.1532-2149.2013.00397.x
25. Laux L, Glanzmann P, Schaffner P, Spielberger C. Das State-Trait-Angstinventar. Theoretische Grundlagen und Handanweisung. Weinheim: Beltz (1981).
26. Spielberger C. State-Trait Anxiety Inventory: Bibliography. 2nd ed. Palo Alto, CA: Consulting Psychologists Press (1989).
27. Petrowski K, Paul S, Albani C, Brähler E. Factor structure and psychometric properties of the trier inventory for chronic stress (TICS) in a representative German sample. BMC Med Res Methodol (2012) 12:42. doi: 10.1186/1471-2288-12-42
28. Schulz P, Schlotz W. The Trier Inventory for the Assessment of Chronic Stress (TICS): scale construction, statistical testing, and validation of the scale work overload. Diagnostica (1999) 45:8–19. doi: 10.1026//0012-1924.45.1.8
29. Flor H, Behle DJ, Birbaumer N. Assessment of pain-related cognitions in chronic pain patients. Behav Res Ther (1993) 31:63–73. doi: 10.1016/0005-7967(93)90044-U
30. Scholz U, Doña BG, Sud S, Schwarzer R. Is general self-efficacy a universal construct? Psychometric findings from 25 countries. Eur J Psychol Assess (2002) 18:242–51. doi: 10.1027//1015-5759.18.3.242
31. Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci (2002) 3:655–66. doi: 10.1038/nrn894
32. Schulz A, Vögele C. Interoception and stress. Front Psychol (2015) 6:993. doi: 10.3389/fpsyg.2015.00993
33. Critchley HD, Garfinkel SN. Interoception and emotion. Curr Opin Psychol (2017) 17:7–14. doi: 10.1016/j.copsyc.2017.04.020
34. Pace-Schott EF, Amole MC, Aue T, Balconi M, Bylsma LM, Critchley H, et al. Physiological feelings. Neurosci Biobehav Rev (2019) 103:267–304. doi: 10.1016/j.neubiorev.2019.05.002
35. Zhu L, Huang D, Shi L, Liang L, Xu T, Chang M, et al. Intestinal symptoms and psychological factors jointly affect quality of life of patients with irritable bowel syndrome with diarrhea. Health Qual Life Outcomes (2015) 13:49. doi: 10.1186/s12955-015-0243-3
36. Mayer EA, Bradesi S, Chang L, Spiegel BMR, Bueller JA, Naliboff BD. Functional GI disorders: from animal models to drug development. Gut (2008) 57:384–404. doi: 10.1136/gut.2006.101675
37. Bharucha AE, Zinsmeister AR, Locke GR, Seide BM, McKeon K, Schleck CD, et al. Prevalence and burden of fecal incontinence: a population-based study in women. Gastroenterology (2005) 129:42–9. doi: 10.1053/j.gastro.2005.04.006
38. Labus JS, Hubbard CS, Bueller J, Ebrat B, Tillisch K, Chen M, et al. Impaired emotional learning and involvement of the corticotropin-releasing factor signaling system in patients with irritable bowel syndrome. Gastroenterology (2013) 145:1253. doi: 10.1053/j.gastro.2013.08.016
39. Dunckley P, Wise RG, Aziz Q, Painter D, Brooks J, Tracey I, et al. Cortical processing of visceral and somatic stimulation: differentiating pain intensity from unpleasantness. Neuroscience (2005) 133:533–42. doi: 10.1016/j.neuroscience.2005.02.041
40. Strigo IA, Bushnell MC, Boivin M, Duncan GH. Psychophysical analysis of visceral and cutaneous pain in human subjects. Pain (2002) 97:235–46. doi: 10.1016/S0304-3959(02)00023-4
41. Koenen LR, Icenhour A, Forkmann K, Pasler A, Theysohn N, Forsting M, et al. Greater fear of visceral pain contributes to differences between visceral and somatic pain in healthy women. Pain (2017) 158:1599–608. doi: 10.1097/j.pain.0000000000000924
42. Koenen LR, Icenhour A, Forkmann K, Theysohn N, Forsting M, Bingel U, et al. From anticipation to the experience of pain: the importance of visceral versus somatic pain modality in neural and behavioral responses to pain-predictive cues. Psychosom Med (2018) 80:826–35. doi: 10.1097/PSY.0000000000000612
43. Elsenbruch S, Enck P. The stress concept in gastroenterology: from Selye to today. F1000Research (2017) 6:2149. doi: 10.12688/f1000research.12435.1
44. Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology (2010) 35:169–91. doi: 10.1038/npp.2009.83
45. Hannibal KE, Bishop MD. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys Ther (2014) 94:1816–25. doi: 10.2522/ptj.20130597
46. Engler H, Elsenbruch S, Rebernik L, Kocke J, Cramer H, Schols M, et al. Stress burden and neuroendocrine regulation of cytokine production in patients with ulcerative colitis in remission. Psychoneuroendocrinology (2018) 98:101–7. doi: 10.1016/j.psyneuen.2018.08.009
47. Icenhour A, Labrenz F, Roderigo T, Siebert C, Elsenbruch S, Benson S. Are there sex differences in visceral sensitivity in young healthy men and women ? Neurogastroenterol Motil (2019) 31:e13664. doi: 10.1111/nmo.13664
48. Mulak A, Tache Y, Larauche M. Sex hormones in the modulation of irritable bowel syndrome. World J Gastroenterol (2014) 20:2433–48. doi: 10.3748/wjg.v20.i10.2433
49. Oyola MG, Handa RJ. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress (2017) 20:476–94. doi: 10.1080/10253890.2017.1369523
50. Li SH, Graham BM. Why are women so vulnerable to anxiety, trauma-related and stress-related disorders? The potential role of sex hormones. Lancet Psychiatry (2017) 4:73–82. doi: 10.1016/S2215-0366(16)30358-3
51. Kåhrström CT, Pariente N, Weiss U. Intestinal microbiota in health and disease. Nature (2016) 535:47. doi: 10.1038/535047a
52. Rieder R, Wisniewski PJ, Alderman BL, Campbell SC. Microbes and mental health: a review. Brain Behav Immun (2017) 66:9–17. doi: 10.1016/j.bbi.2017.01.016
53. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci (2012) 13:701–12. doi: 10.1038/nrn3346
54. Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The Microbiota-Gut-Brain Axis. Physiol Rev (2019) 99:1877–2013. doi: 10.1152/physrev.00018.2018
55. Jeffery IB, Quigley EMM, Öhman L, Simrén M, O'Toole PW. The microbiota link to irritable bowel syndrome an emerging story. Gut Microbes (2012) 3:572–6. doi: 10.4161/gmic.21772
56. Moloney RD, Desbonnet L, Clarke G, Dinan TG, Cryan JF. The microbiome: stress, health and disease. Mamm Genome (2014) 25:49–74. doi: 10.1007/s00335-013-9488-5
57. Yang B, Wei J, Ju P, Chen J. Effects of regulating intestinal microbiota on anxiety symptoms: a systematic review. Gen Psychiatry (2019) 32:e100056. doi: 10.1136/gpsych-2019-100056
58. Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol (2019) 4:623–32. doi: 10.1038/s41564-018-0337-x
Keywords: chronic stress, visceroception, gut-brain axis, visceral pain, urgency, recall bias, memory
Citation: Icenhour A, Labrenz F, Roderigo T, Benson S and Elsenbruch S (2020) The Role of Chronic Stress in Normal Visceroception: Insights From an Experimental Visceral Pain Study in Healthy Volunteers. Front. Psychiatry 11:107. doi: 10.3389/fpsyt.2020.00107
Received: 26 September 2019; Accepted: 07 February 2020;
Published: 03 March 2020.
Edited by:
Andreas Stengel, Charité Medical University of Berlin, GermanyReviewed by:
Katja Weimer, University of Ulm, GermanyMuriel Larauche, University of California, Los Angeles, United States
Copyright © 2020 Icenhour, Labrenz, Roderigo, Benson and Elsenbruch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adriane Icenhour, YWRyaWFuZS5pY2VuaG91ckB1ay1lc3Nlbi5kZQ==
†These authors have contributed equally to this work
 Adriane Icenhour
Adriane Icenhour Franziska Labrenz
Franziska Labrenz Till Roderigo
Till Roderigo Sven Benson
Sven Benson Sigrid Elsenbruch
Sigrid Elsenbruch