- 1Freudenthal Institute and Utrecht Institute for Pharmaceutical Sciences (UIPS), Faculty of Science, Utrecht University, Utrecht, Netherlands
- 2Department of Adolescent Psychiatry and Addiction Prevention, Brijder-Jeugd, The Hague, Netherlands
- 3Instituto de Medicina Social, Rio de Janeiro State University, Rio de Janeiro, Brazil
Objective: Adolescent depression is a heterogeneous disorder, with a wide variety of symptoms and inconsistent treatment response, and is not completely understood. A dysregulated stress system is a consistent finding, however, and exhaustion is a consistent trait in adolescent patients. The aim of this paper is to critically assess current hypotheses in adolescent depression research and reframe causes and treatment approaches.
Methods: A mixed-method approach involved a review based on publications from PubMed, Embase and PsycInfo, and two exemplary adolescent cases.
Results: Both cases show a spiral of stress and exhaustion, but with a different profile of symptoms and coping mechanisms. Reframing both cases from the perspective of coping behavior, searching for the sources of experienced stress and exhaustion, showed coping similarities. This proved essential in the successful personalized treatment and recovery process. In combination with recent evidence, both cases support the functional reframing of depression as the outcome of a stress- and exhaustion-related spiralling mechanism.
Conclusions: We propose to open up a symptom-based, mood-centered view to a model in which adolescent depression is framed as a consecutive failure of stress coping mechanisms and chronic exhaustion. Addressing exhaustion and coping primarily as a treatment strategy in adolescents and young adults might work in synergy with existing treatments and improve overall outcomes. This perspective warrants further investigation.
Introduction
Major depressive disorder (MDD) is the leading cause of disability worldwide (1), with 10 to 15% of patients proceeding to suicide (2, 3), and a substantive disease burden for adolescents and young adults (4–6). Depression is a heterogeneous group of brain disorders with varied contextualized origins, complex genetics and a neurobiology that is not completely understood. The etiology is not elucidated, and particularly for adolescents there is an evidence gap (4, 7, 8). The serendipitous discovery of first the tri- and tetracyclic antidepressants (TCAs) and later the stress-modulating serotonin reuptake inhibitors (SSRIs) led successively to the catecholamine and monoamine hypotheses of depression (9). In later years, reduced adult neurogenesis and changes in structural and functional neuronal plasticity have been linked to the onset and treatment opportunities of major depression (10, 11). Genetic research has shown that there is not a single genetic cause for depression, and all known genetic factors combined only explain a limited percentage of the variance in clinical outcomes (12, 13). The estimated heritability of depression is 35%–40%, indicating 60%–65% is explained by other factors, such as adverse life experiences (11, 14). Researchers have turned to epigenetics to develop new forms of genetic and pharmacological modeling, in an effort to describe the etiology of depression better (15). Despite many years of research by numerous investigators both in academia and industry, psychoactive targeted therapeutics with controllable and specific effects on the brain microcircuitry and chemistry did not and probably will not materialize due to the complex nature of mental disorders (16). In order to open up our thinking about MDD we take up the challenge to reframe depression, specifically focusing on adolescents.
Symptom-Based Approach
We note that within the current framework depression is diagnosed based on the presence of a series of mood-related symptoms and their effect on daily functioning. The seven most commonly used interviews and self-report questionnaires together describe a heterogenous group of 52 symptoms, such as either high or low appetite, more or less sleep than usual, and a feeling of sadness (17). This causes differences in diagnosis based on which scale is used (18). The widely varying patterns in which these symptoms often present themselves (19, 20), and the high occurrence of several comorbidities, such as anxiety, psychosis, and autism spectrum disorder, indicate that depression is not a homogenous disease, but a continuous, heterogeneous group of disorders associated with a wide variety of different risk factors (4, 8, 21–24).
Aim
Combined with the lack of understanding of the etiology of adolescent depression, the large variation of presentation and treatment approaches is the main driver for us to try to reframe the concept of MDD in adolescent patients. We also aim to explain why responses to treatment vary substantially and why older age is a consistent and important risk factor for a poorer MDD course (25–28). We will take a new perspective toward MDD by focusing on stress and the depressive mood related to development in adolescence. This yields a promise for novel therapeutic approaches and potential breakthroughs in depression research, treatment and prevention.
Methods
A mixed method approach was used involving clinical investigation of adolescent case reports and a narrative review. PubMed, Embase, and PsychInfo were searched for relevant publications, with select additions of recent findings based on collective suggestions of the authors. To make sure the patient perspective is not lost when critically assessing the current framework and new possibilities, two case reports were included. Written informed consent was obtained from both subjects for the case reports.
Depression and Stress
Etiology
Many findings in depression research have failed the scientific test of replication. For example, the volume of the amygdala of depressed patients has been found to be increased (29) in some studies, and decreased in others (30). Patients with melancholic depression, a subtype based on symptoms, were thought to respond better to TCAs than atypical patients (hence the name) (20, 27, 31, 32), but other researchers could not replicate this finding (33–36). Plasma levels of leptin, which reduces appetite, has been found higher in melancholic patients (37), or higher in atypical patients (38). Finally, childhood trauma and/or abuse is more common in melancholic than in atypical patients (39), or vice versa (40).
Stress System
One consistent finding, however, is a dysregulated stress system in depressed patients (41–44). In approximately 70% of depressed patients a dysfunction of the hypothalamic–pituitary–adrenal (HPA) axis is detected, mainly hyperactivity (38, 45). Also a disruption of the diurnal variation of cortisol is commonly seen (46, 47). Unfortunately, after decades of research efforts this finding has not resulted in a stress-targeted treatment option or a clinical test to predict treatment response (48, 49), and it remains debated whether HPA-axis dysregulation is a cause or a consequence of depression.
This does provide an important insight: depression is at least partially the result of stress and a differential dysregulation in the stress system is an important trait (38). The stressor may be in the past (e.g. childhood maltreatment or trauma) (4, 50), or acutely (e.g. dealing with new life events). The initial response of people to stress is typically a coping mechanism aimed at exerting control over the stressor either by avoiding, reducing or predicting its occurrence. Examples of such efforts are the canceling of obligations or disengagement from social interaction (51). The HPA axis exerts a fundamental role regulating both internal as external stimuli, integrating the physiopathological and behavioral dimensions of stress. We postulate that depression is the result of a failure of coping mechanisms to control the stressors and a differential dysregulation in the stress system.
Coping Mechanisms and Exhaustion
The accumulation of stressful events, and the eventual failure of coping mechanisms to deal with the stress, can lead to exhaustion and depressive behavior. Preclinical experiments already hinted at a relation between the effectiveness of coping behavior, the effort involved and feedback on the development of gastric ulcers. Although coping efforts were effective, ulcers still developed when coping took more effort and less feedback was offered (52, 53). Preclinical evidence indicated that chronic exposure to relatively mild stressors which rats can adapt to relatively easily (e.g. tilting the cage at a slight angle, emptying a water bottle in the cage, introducing new bedding material), ultimately resulted in the development of anhedonia (50). The chronic character of having to cope with mild stressful events over and over again, and the lack of control over stressors, was sufficient for depressive symptoms to develop (54). In a forced swimming test, rats who were dosed with psilocybin developed the coping technique of floating faster than other rats, indicating a window of behavioral flexibility (55). Ketamine displayed the opposite effect, with more mobility (56). We hypothesize that depressive behavior, specifically anhedonia and withdrawal, and the consequent loss of interest and enjoyment in usual activities, is an evolutionary mechanism to guard the organism against the exhaustion that may results from excessive or chronic coping behavior. As such, depressive behavior is both an expression of psychological pressure and a physiological precaution. This substantiates the entanglement of psychological and physiological factors in MDD.
Stress response mechanisms can change the allocation of metabolic resources in a stressful situation, where that is needed. Similarly, depression could be the expression of a forced change in allocation of attention. Depressed patients are known to ruminate, or continually analyze their problems and relive their memories (24). Anhedonia can be interpreted as a way to secure mental resources, by reducing the interest in distractions (20, 57). Depression can be seen as an exaggerated social navigating coping mechanism, caused by an accumulation of stress and a spiral of unsuccessful adaptive behaviors which leads to exhaustion. By entering a depressive mode, the organism aims to guard itself from exhaustion. The challenge is to interfere with this mood-affecting spiralling mechanism (see Figure 1) to prevent depression from developing. Dealing with stress and potential exhaustion, as opposed to dealing with the symptoms of depression, could prove to be an effective treatment approach.
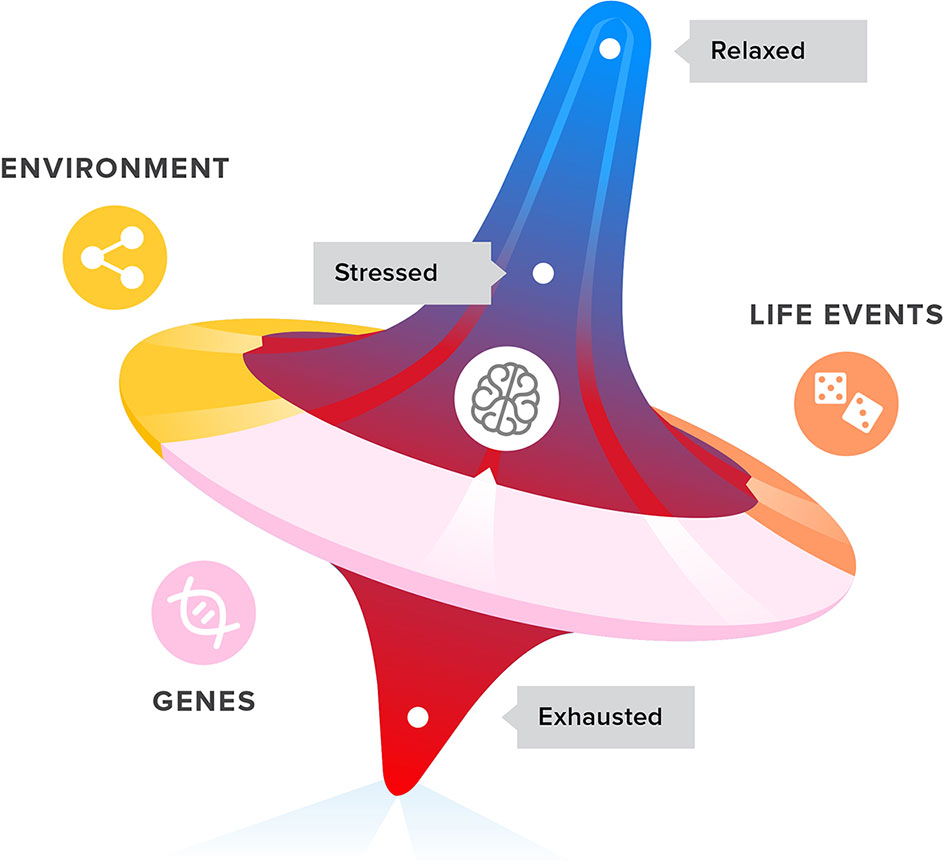
Figure 1 Life events, genetics, and environment all have an impact on the development of stress, coping with stress, and ultimately exhaustion and depression symptoms in adolescents and young adults.
Treatment
There is currently only limited evidence-based rationale for choosing one treatment over another for an individual patient (31, 58–60), with no differentiated approach for adolescents or adults (6, 61). Even defining depression subtypes based on symptoms has not helped (62). Despite guidelines and evidence-based interventions, treatment is still primarily based on trial and error (63, 64), and primarily aimed at improving mood. Yet, between one third (65) to half (20, 66) of adult patients show no response to weeks of first line treatment with antidepressant drugs, and are advised to try a different antidepressant. Further, one third of all patients never reach a response after four lines of antidepressant treatment (65). The current therapeutic shortcomings are the consequences of our lack of knowledge of causes, the underlying neurobiology and chemistry (67), and risk factors that contribute to the onset and maintenance of depression. As a consequence, the treatment paradigms are oversimplified with little attention for preventive measures (68).
Psychedelics
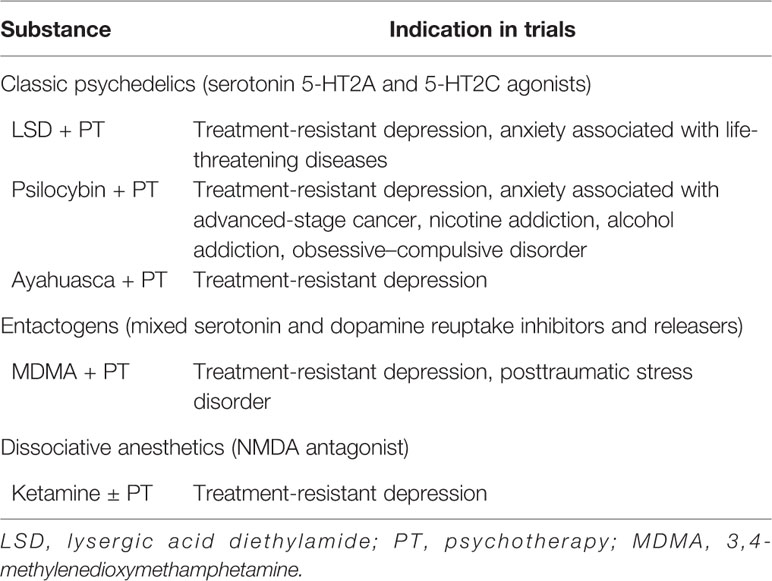
When Albert Hofmann, who first synthesized lysergic acid diethylamide (LSD), came in contact with it himself in 1943, he noted the hallucinogenic properties. In 1947 it was first marketed as a therapeutic drug. In the 1960s, Timothy Leary was the first to start experimenting with psilocybin combined with psychotherapy (69). In the 1950s and 1960s, LSD and psilocybin were tested in several small-scale clinical trials for anxiety, depression and addiction (70, 71). In response to increased recreational use, international legislation was introduced as part of the “war on drugs,” which brought an abrupt end to clinical research with these and similar substances in the 1970s (67, 69, 70, 72). In the last two decades, clinical trials with psychedelics have started to take place again (71). A brief overview of the indications these psychedelics have been investigated for post-2000 is indicated in Table 1 (69, 70, 72).
New insights in the complex etiology of depression might be offered by findings with the use of psychedelics for treatment-resistant depression (73, 74). Several psychedelics have shown to help depressive patients in a limited number of studies with small number of patients. The classic psychedelics, compounds such as LSD, psilocybin, and Ayahuasca, have diverse pharmacological profiles, including robust effects on the serotonergic system (67, 69, 72, 75). A psychedelic not acting on the serotonergic system is the dissociative anesthetic ketamine, which in subanesthetic doses acts as an antagonist on the N-methyl-D-aspartate (NMDA) receptor, a type of glutamate receptor (56, 69, 76, 77). Esketamine, the S-enantiomer of ketamine, has been approved by the Food and Drug Administration (FDA) for treatment-resistant depression (78), but ketamine has been and continues to be used off-label to treat depression too (79). This highlights that serotonergic activity, or even a mono-aminergic activity, is not required for the antidepressant effect of a psychedelic compound, further stressing the need for abandoning the old hypotheses. These hallucinogens, and the chemically related entactogen 3,4-methylenedioxy-methamphetamine (MDMA) may have a place in offering a positive experience to break the self-sustaining depressive state and allowing for introspection during psychotherapy to process stressful life-time experiences as a form of reverse medical engineering (80).
From a psychological point of view, psychedelics work through a different mechanism than classic antidepressants. Instead of the elevation of mood and the reduction of anxiety, psychedelic drugs induce a profound temporary positive experience (e.g. a mystical or religious sensation). This positive experience allows for the temporary disintegration of existing networks, which in turn facilitates reprocessing of past emotions and introspection (67). In turn, this improves the capacity to cope with stress (71). Also, the use of a psychedelic in combination with a psychotherapeutic process could have long-term effects, counteracting the effect of a negative experience and disrupting the negative and “downward spiralling” compulsive thinking (72).
Developmental Aspects
In this article we move away from mood improvement as a primary target (81, 82). We offer an alternative integrated approach for the treatment of adolescent and young adult depression by focusing on stress factors and exhaustion reduction, seeing anhedonia and withdrawal as an evolutionary coping mechanism. This integrates approaches such as the social navigation hypothesis of Watson and Andrews (83) with cognitive bias (84) and Selye's biological stress (85, 86). With this approach we take a functional perspective, and focus on the function the depressive state provides to the adolescent patient and how it develops. This perspective is instrumental for tailor-made treatment strategies.
We will discuss these insights on the basis of two adolescent patient reports. Mood disorders have been shown to be progressive, with patients developing more complex psychopathologies over time (87, 88). Approximately 50% of patients retrospectively state that their first depressive episode occurred before the age of 20 (88, 89); another report states 50% experience that before the age of 14 (90). This further highlights the progressive nature of depression and the need for early intervention.
Case Reports
Case 1
A 17-year-old Caucasian woman was referred by her own general practitioner to the department of adolescent psychiatry and addiction prevention for binge drinking and daily use of marijuana. The intake together with her parents showed that the patient already had a history of moderate depression and an eating disorder, anorectic of the purging type with moderate severity. No abnormalities were reported regarding appearance, behavior, eye contact, and rapport orientation and cognition [intelligence quotient (IQ) of 127]. However, she regularly suffered from suicidal thoughts and a low ability to experience pleasure. Though she had no concrete suicide plans, in gloomy periods she showed risky behavior, like crossing a busy road without looking. She usually performed well in school, despite occasional lags in attendance, which were compensated with short periods of active study. Her mother had a history of MDD.
At the department of adolescent psychiatry and addiction prevention, we classified the addiction behavior as mild. But we also established a comorbid psychiatric and substance-use disorder profile. Thus, we chose for an integrated treatment for comorbidity that has been found to be consistently superior (91). Effective treatment for comorbid conditions combines different therapeutic modalities, i.e. psychotherapy [e.g. motivational interviewing (MI), cognitive behavioral therapy (CBT)], pharmacotherapy (e.g. antidepressants), and family therapy. Using combinations of different modalities typically increases therapeutic effect by exerting a synergistic impact on symptoms (6).
With MI, the patient was motivated to choose a first education-related treatment goal. This was to prevent school dropout at all cost. We started CBT to control her marihuana and alcohol abuse and prevent school dropout. We added medication in order to try to stabilize her mood with fluoxetine, an SSRI, which might also modulate stress. The medication initially seemed to have some effect but after two months there was a sharp mood drop, increased suicidality and aggravation of eating disorder symptoms. Eventually she had a body mass index of 16 kg/m2. The eating problems were mapped and analysed by an eating disorder specialist. The latter used a problem-solving approach and focused on both directive counseling and emotional support. The eating disorder specialist also advised to choose a medication with low risks of weight gain. The psychiatrist changed the medication to citalopram.
Subsequently, the treatment team focused on teaching the patient how to cope with stressful situations and the associated anxiety. The stress appeared to be mainly caused by a feeling of lack of control. The patient turned out to have a high intelligence and learning ability, but also felt that she had no control over her learning process. She had not sufficiently developed social learning strategies in her early school years. In addition, there appeared to be an issue of individuation and separation problems. These problems got worse because it was almost impossible for her parents to let her develop in her own way due to the stress they had over her suicidal thoughts, drug use, and worsening physical condition due to bad eating habits. We decided on an additional family counseling approach to address these issues.
The integrated treatment modality approach proved effective. She developed a realistic idea of what caused her stress, how she reacted situationally and improved her awareness that she tends to have control over everything. Her parents were involved in helping her developing control coping skills and checking on achievements. Because of this insight, she succeeded in maintaining her diet less strictly and experimenting with behaving differently without alcohol or drugs. Her parents saw that she was doing better and were able to release her a bit more. This increased her sense of control and provided enough space to further discover what goals she wanted to achieve. In the process her mood and her ability to experience pleasure improved significantly. She successfully passed her school exams and proceeded to university.
Case 2
A 15-year old Caucasian girl was referred by her own general practitioner after a suicide attempt with symptoms of sadness, anxiety, and obsessive–compulsive behavior. The intake was together with her parents. She was struggling in school, despite her very supportive family. No drug abuse or other psychiatric symptoms were found. She told the counselor she tried hard, but felt that she could not keep up in school; it was never good enough, no matter how hard she tried. The counselor estimated that the school level was appropriate for the level of intelligence of the patient.
She had periods when her self-esteem was very low. During these periods she spent hours on her appearance, focusing on her hair and makeup. Her hair fell out as a result of these sessions. She could not stop herself, and always ended with self-harm. This in turn lowered her self-esteem and increased the experienced stress. She was locked in a downward spiral. Gradually her mood disorder worsened and made her passive. She no longer wanted to go to school and meet friends, but passed hours in front of the mirror. She attempted to end her life.
We hypothesized on the basis of the girl's stress complaints that she felt school, parents, and friends expected too much of her. After a neuropsychological assessment the testing showed that she had a disharmonic intelligence profile with an IQ of approximately 80 (using the Wechsler Intelligence Scale For Children-III (92)), inconsistent at all factor levels. We classified a mild intellectual developmental disorder in the conceptual and practical domain, which explained the structural struggle with the standard school curriculum instructions. We educated parents and school on how instructions might fit in better with her learning abilities and style. Her preferred method of learning new things was being shown how to do it, as opposed to having it explained to her. This led to significant stress reduction and positive school experiences. In the process her self-esteem improved, the experienced stress decreased, and her mood improved. CBT was adjusted to her learning style and was used to reduce her obsessive–compulsive behavior.
Discussion
Cases
Though both case reports show a different profile of symptoms and coping mechanisms, in both cases a downward spiral of stress, coping behavior, and exhaustion are central. Both patients described themselves as rarely feeling relaxed and as struggling to fulfill their daily tasks. After several years of chronic stress, a period followed in which they felt constantly exhausted.
The first patient coped with stressful situations through aberrant food intake behavior, suicidal thoughts, and mood swings. She overcompensated this restrictive behavior with recreational drug abuse. The second patient developed compulsory behavior, stress, and suicidal thoughts and overcompensated leading to self-harm. In the current framework both cases would be viewed as different and based on their symptomatology ask for different treatments. Reframing both cases, from the perspective of coping behavior, searching for the origin and sources of the experienced stress and exhaustion and coping with stressful situations, showed stress coping similarities between the two cases and proved an essential part of the personalized treatment and recovery process. Both cases support the added clinical value of the functional reframing of depression as the outcome of a mood-affecting stress and exhaustion related spiralling mechanism.
The adolescent cases presented here are good examples of how depression can be managed by relearning effective coping behavior. This prevents patients from reverting to a depressive state in order to cope with the life stressors. In more severe and chronic cases, patients suffering from difficult-to-treat or treatment resistant MDD, patients are in a deep depressive state and are not capable of learning new coping behaviors. We envision that in such situations more radical medical interventions are needed to first elevate patients from the depressive state into a state where learning new and effective coping strategies can take place. In these situations, psychedelics (e.g. ketamine) have proven to be effective to temporarily draw people out of a deep depressive state. With the support of follow-up medication and adequate psychological guidance, the patients may then develop effective coping strategies.
Methodological Notes
As this is a narrative review, and not a formal systematic review, caution needs to be used when interpreting this data. Select literature sources have been added based on informal searches, so contradicting studies may have been missed. Similarly, the two cases were selected for their exemplary stories, not because they are typical. As such, this paper aims to provide new insights and direction for future treatments, not a definitive answer on how to improve treatment.
Possible Implications for the Post-CoViD-19 Pandemic Period
After the Spanish flu of 1918, many infected patients developed post-viral fatigue in the following months and years. On top of that, the randomness of who was infected and who died as a result led to learned helplessness, which caused anhedonia and other depressive symptoms (93). The recent pandemic of the novel coronavirus, severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2, previously known as 2019-nCov (94)], has overwhelmed healthcare facilities with the high need for acute care. This puts a large psychological toll on the entire population, with high levels of stress (95). Next to the risk of the many life events that can be expected in these situations, coping behavior—such as avoiding conflict, or searching social support—is often limited due to confinements that are put in place to prevent further spread. Furthermore, the lockdown situation and mass isolation at home in many countries may increase the risk of domestic violence and divorces. This could all lead to a rise in trauma-related stress disorders in the months and years to come. Breaking a vicious cycle of stress, inadequate coping behavior and exhaustion with a holistic view, and possibly with psychedelic-supported psychotherapy, might help treat the many psychiatric patients that can be expected.
Conclusion
Reframing depression and shifting clinical practice to a more comprehensive and integrated look at the individual experience of a patient, including all causes for stress, pressure, and exhaustion, might be more helpful in developing promising treatment strategies. Also, treatment practices that take into account preventive mental health interventions, and that focus on stress, exhaustion, and coping strategies, could have a significant and lasting impact on many patients struggling with depression. The perspective of stress, coping, and exhaustion provides the therapist with another treatment approach that can work in synergy with the existing arsenal of therapeutic approaches, making the therapist more effective.
Increased focus is needed on support programs to help individuals develop functional coping mechanisms to deal with pressure, before more serious coping mechanisms develop in the form of withdrawal from stressful situations, compulsory behavior, or frequently occasional use of recreational drugs (96). Our intuition is that during successful treatment patients experience small successes of effective coping and re-live the rewarding properties of such experiences. Reliving experiences could repair the damaged reward mechanisms and diminishes the experienced anxiety and stress which then might subsequently drive and sustain further recovery (97). Psychedelics may offer help in breaking free from the existing cognitive bias, by facilitating introspection, re-living of past experiences, and development of new coping mechanisms.
Effective treatment strategies for adolescent and young adult depression should combine different therapeutic modalities and focus on exhaustion and sources of stress. Using a combination of treatment modalities could increase therapeutic effectiveness by improving the pace of learning new coping behaviors, exerting a synergistic impact on the developmental perspective, and breaking the downward spiral of stress and exhaustion, which eventually leads to a reduction of the depression symptoms. This might also help for other related mental disorders in adolescents and young adults where exhaustion and stress are central, such as burnout syndrome (98). But similarly, post-traumatic stress disorder, autism spectrum disorder, and generalized anxiety disorder are related to stress (24, 99–101). These disorders could also benefit from the reframing of the concept of mood and stress. We would like to offer this integrated and multidisciplinary perspective as a consideration for the development of new multimodal treatment approaches for MDD and other related psychiatric disorders.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
Written informed consent was obtained from both subjects for the case reports.
Author Contributions
Conception or design of the work: TP, TG, LL, RO, AH, RZ. Data collection cases: LL. Data analysis and interpretation: TP, TG, LL, AH. Drafting the manuscript: TG. Critical revision of the article: TP, LL, TG, RO, AH, RZ. Final approval of the version to be published: TP, LL, TG, RO, AH, RZ
Conflict of Interest
During the late stage development of this manuscript, TG accepted a position in oncology at AstraZeneca. AstraZeneca had no role in any aspect of this paper.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Prof Dr. Eric Vermetten, prof. Dr. Claudi Bockting, and the reviewers for their feedback on this paper, and the patients who consented to have their history described for the case reports. Furthermore, we would like to thank Cassandra Nemzoff for her English manuscript correction services. In addition, we would like to thank Frank-Jan van Lunteren for his comprehensive graphics and the U-Talent students Emma, Frits, Imke, Hannah, and Thijmen for their insightful LSD and psilocybin modeling contributions.
References
1. World Health Organisation. Fact sheet Depression. Media Cent (2016). Available at: http://www.who.int/mediacentre/factsheets/fs369/en/index.html.
2. Sharpley CF, Bitsika V. Differences in neurobiological pathways of four “clinical content” subtypes of depression. Behav Brain Res (2013) 256:368–76. doi: 10.1016/j.bbr.2013.08.030
3. Leadholm AKK, Rothschild AJ, Nielsen J, Bech P, Ostergaard SD. Risk factors for suicide among 34,671 patients with psychotic and non-psychotic severe depression. J Affect Disord (2014) 156:119–25. doi: 10.1016/j.jad.2013.12.003
4. Vibhakar V, Allen LR, Gee B, Meiser-Stedman R. A systematic review and meta-analysis on the prevalence of depression in children and adolescents after exposure to trauma. J Affect Disord (2019) 255:77–89. doi: 10.1016/j.jad.2019.05.005
5. Liu J-W, Tu Y-K, Lai Y-F, Lee H-C, Tsai P-S, Chen T-J, et al. Associations between sleep disturbances and suicidal ideation, plans, and attempts in adolescents: a systematic review and meta-analysis. Sleep (2019) 42(6):zsz054. doi: 10.1093/sleep/zsz054
6. Mullen S. Major depressive disorder in children and adolescents. Ment Heal Clin (2018) 8:275–83. doi: 10.9740/mhc.2018.11.275
7. Pedersen GA, Zajkowska Z, Kieling C, Gautam K, Mondelli V, Fisher HL, et al. Protocol for a systematic review of the development of depression among adolescents and young adults: psychological, biological, and contextual perspectives around the world. Syst Rev (2019) 8:179. doi: 10.1186/s13643-019-1104-7
8. DeFilippis M. Depression in Children and Adolescents with Autism Spectrum Disorder. Children (2018) 5:112. doi: 10.3390/children5090112
9. Niciu MJ, Ionescu DF, Richards EM, Zarate CA. Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J Neural Transm (2014) 121:907–24. doi: 10.1007/s00702-013-1130-x
10. Miller BR, Hen R. The current state of the neurogenic theory of depression and anxiety. Curr Opin Neurobiol (2015) 30:51—58. doi: 10.1016/j.conb.2014.08.012
11. Akil H, Gordon J, Hen R, Javitch J, Mayberg H, McEwen B, et al. Treatment resistant depression: A multi-scale, systems biology approach. Neurosci Biobehav Rev (2018) 84:272–88. doi: 10.1016/j.neubiorev.2017.08.019
12. Fabbri C, Di Girolamo G, Serretti A. Pharmacogenetics of antidepressant drugs: An update after almost 20 years of research. Am J Med Genet Part B Neuropsychiatr Genet (2013) 162:487–520. doi: 10.1002/ajmg.b.32184
13. Antypa N, Drago A, Serretti A. The role of COMT gene variants in depression: Bridging neuropsychological, behavioral and clinical phenotypes. Neurosci Biobehav Rev (2013) 37:1597–610. doi: 10.1016/j.neubiorev.2013.06.006
14. Flint J, Kendler KS. The Genetics of Major Depression. Neuron (2014) 81:484–503. doi: 10.1016/j.neuron.2014.01.027
15. Pieters T. The antidepressant era revisited; Towards differentiation and patient-empowerment in diagnosis and treatment. 2017th. Eghigian G, editor. London, United Kingdom (2017). Available at: https://www.researchgate.net/publication/318852989_The_antidepressant_era_revisited_Towards_differentiation_and_patient-empowerment_in_diagnosis_and_treatment.
16. Pieters T, Snelders S. Psychotropic drug use: Between healing and enhancing the mind. Neuroethics (2009) 2:63–73. doi: 10.1007/s12152-009-9033-0
17. Fried EI. The 52 symptoms of major depression: Lack of content overlap among seven common depression scales. J Affect Disord (2017) 208:191–7. doi: 10.1016/j.jad.2016.10.019
18. Zimmerman M, Walsh E, Friedman M, Boerescu DA, Attiullah N. Identifying Remission From Depression on 3 Self-Report Scales. J Clin Psychiatry (2017) 78:177–83. doi: 10.4088/JCP.16m10641
19. van Loo HM, de Jonge P, Romeijn J-W, Kessler RC. Schoevers R a. Data-driven subtypes of major depressive disorder: a systematic review. BMC Med (2012) 10:156. doi: 10.1186/1741-7015-10-156
20. Gold PW, Machado-Vieira R, Pavlatou MG. Clinical and biochemical manifestations of depression: Relation to the neurobiology of stress. Neural Plast (2015) 2015:7–9. doi: 10.1155/2015/581976
21. Ginsburg AD, Stadem PS, Takala CR, Croarkin PE, Mattson AB, Billings ML, et al. An Examination of Screening Tools for Collaborative Care of Adolescent Depression. J Clin Psychiatry (2018) 79(4):17m11543. doi: 10.4088/JCP.17m11543
22. Malas N, Plioplys S, Pao M. Depression in Medically Ill Children and Adolescents. Child Adolesc Psychiatr Clin N Am (2019) 28:421–45. doi: 10.1016/j.chc.2019.02.005
23. Dell'Osso L, Lorenzi P, Carpita B. Autistic Traits and Illness Trajectories. Clin Pract Epidemiol Ment Heal (2019) 15:94–8. doi: 10.2174/1745017901915010094
24. Dell'Osso L, Muti D, Carpita B, Cremone I, Bui E, Gesi C, et al. The Adult Autism Subthreshold Spectrum (AdAS) model: A neurodevelopmental approach to mental disorders. J Psychopathol (2018) 24:118–24.
25. Van Loo HM, Cai T, Gruber MJ, Li J, De Jonge P, Petukhova M, et al. Major depressive disorder subtypes to predict long-term course. Depress Anxiety (2014) 31:765–77. doi: 10.1002/da.22233
26. Fitzgerald PJ. Black bile: Are elevated monoamines an etiological factor in some cases of major depression? Med Hypotheses (2013) 80:823–6. doi: 10.1016/j.mehy.2013.03.023
27. Harald B, Gordon P. Meta-review of depressive subtyping models. J Affect Disord (2012) 139:126–40. doi: 10.1016/j.jad.2011.07.015
28. Schaakxs R, Comijs HC, Lamers F, Kok RM, Beekman ATF, Penninx BWJH. Associations between age and the course of major depressive disorder: a 2-year longitudinal cohort study. Lancet Psychiatry (2018) 256:368–76. doi: 10.1016/S2215-0366(18)30166-4
29. Vassilopoulou K, Papathanasiou M, Michopoulos I, Boufidou F, Oulis P, Kelekis N, et al. A magnetic resonance imaging study of hippocampal, amygdala and subgenual prefrontal cortex volumes in major depression subtypes: Melancholic versus psychotic depression. J Affect Disord (2013) 146:197–204. doi: 10.1016/j.jad.2012.09.003
30. Jentsch MC, Van Buel EM, Bosker FJ, Gladkevich AV, Klein HC, Oude Voshaar RC, et al. Biomarker approaches in major depressive disorder evaluated in the context of current hypotheses. Biomark Med (2015) 9:277–97. doi: 10.2217/bmm.14.114
31. Gili M, Roca M, Armengol S, Asensio D, Garcia-Campayo J, Parker G. Clinical Patterns and Treatment Outcome in Patients with Melancholic, Atypical and Non-Melancholic Depressions. PloS One (2012) 7(10):e48200. doi: 10.1371/journal.pone.0048200
32. Baldwin DS, Bolognesi F. On predicting the response to antidepressant treatment. Hum Psychopharmacol (2012) 27:343–4. doi: 10.1002/hup.2232
33. Arnow BA, Blasey C, Williams LM, Palmer DM, Rekshan W, Schatzberg AF, et al. Depression Subtypes in Predicting Antidepressant Response: A Report From the iSPOT-D Trial. Am J Psychiatry (2015) 172:743–50. doi: 10.1176/appi.ajp.2015.14020181
34. Uher R, Dernovsek MZ, Mors O, Hauser J, Souery D, Zobel A, et al. Melancholic, atypical and anxious depression subtypes and outcome of treatment with escitalopram and nortriptyline. J Affect Disord (2011) 132:112–20. doi: 10.1016/j.jad.2011.02.014
35. Yang SJ, Stewart R, Kang HJ, Kim SY, Bae KY, Kim JM, et al. Response to antidepressants in major depressive disorder with melancholic features: The CRESCEND study. J Affect Disord (2013) 144:42–50. doi: 10.1016/j.jad.2012.06.004
36. Baumeister H, Parker G. A second thought on subtyping major depression. Psychother Psychosom (2010) 79:388–9. doi: 10.1159/000320896
37. Cizza G, Ronsaville DS, Kleitz H, Eskandari F, Mistry S, Torvik S, et al. Clinical subtypes of depression are associated with specific metabolic parameters and circadian endocrine profiles in women: The power study. PloS One (2012) 7(1):e28912. doi: 10.1371/journal.pone.0028912
38. Baune B, Stuart M, Gilmour A, Wersching H, Heindel W, Arolt V, et al. The relationship between subtypes of depression and cardiovascular disease: a systematic review of biological models. Transl Psychiatry (2012) 2:e92. doi: 10.1038/tp.2012.18
39. Lamers F, Rhebergen D, Merikangas KR, de Jonge P, Beekman ATF, Penninx BWJH. Stability and transitions of depressive subtypes over a 2-year follow-up. Psychol Med (2012) 42:2083–93. doi: 10.1017/S0033291712000141
40. Li Y, Aggen S, Shi S, Gao J, Li Y, Tao M, et al. Subtypes of major depression: latent class analysis in depressed Han Chinese women. Psychol Med (2014) 44:3275–88. doi: 10.1017/S0033291714000749
41. Fischer S, Strawbridge R, Vives AH, Cleare AJ. Cortisol as a predictor of psychological therapy response in depressive disorders: systematic review and meta-analysis. Br J Psychiatry (2017) 210:105–9. doi: 10.1192/bjp.bp.115.180653
42. Belvederi Murri M, Pariante C, Mondelli V, Masotti M, Atti AR, Mellacqua Z, et al. HPA axis and aging in depression: systematic review and meta-analysis. Psychoneuroendocrinology (2014) 41:46–62. doi: 10.1016/j.psyneuen.2013.12.004
43. Zorn JV, Schür RR, Boks MP, Kahn RS, Joëls M, Vinkers CH. Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology (2017) 77:25–36. doi: 10.1016/j.psyneuen.2016.11.036
44. Teismann H, Wersching H, Nagel M, Arolt V, Heindel W, Baune BT, et al. Establishing the bidirectional relationship between depression and subclinical arteriosclerosis–rationale, design, and characteristics of the BiDirect Study. BMC Psychiatry (2014) 14:174. doi: 10.1186/1471-244X-14-174
45. Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology (2000) 23:477–501. doi: 10.1016/S0893-133X(00)00159-7
46. Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep (2011) 13:467–75. doi: 10.1007/s11920-011-0232-0
47. Fu CHY, Steiner H, Costafreda SG. Predictive neural biomarkers of clinical response in depression: A meta-analysis of functional and structural neuroimaging studies of pharmacological and psychological therapies. Neurobiol Dis (2013) 52:75–83. doi: 10.1016/j.nbd.2012.05.008
48. Horstmann S, Binder EB. Glucocorticoids as Predictors of Treatment Response in Depression. Harv Rev Psychiatry (2011) 19:125–43. doi: 10.3109/10673229.2011.586550
49. Herbert J. Cortisol and depression: three questions for psychiatry. Psychol Med (2013) 43:449–69. doi: 10.1017/S0033291712000955
50. Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev (1992) 16:525–34. doi: 10.1176/appi.ajp.2011.11020335
51. Orzechowska A, Zajączkowska M, Talarowska M, Gałecki P. Depression and ways of coping with stress: a preliminary study. Med Sci Monit (2013) 19:1050–6. doi: 10.12659/MSM.889778
52. Weiss JM. Somatic effects of predictable and unpredictable shock. Psychosom Med (1970) 32:397–408. doi: 10.1097/00006842-197007000-00008
53. Weiss JM. Psychological factors in stress and disease. Sci Am (1972) 226:104–13. doi: 10.1038/scientificamerican0672-104
54. Weiss JM. “Stress-induced depression: critical neurochemical and electrophysiological changes,”. In: Madden J, editor. Neurobiology of learning, emotion, and affect. New York: Raven Press, Ltd. (1991) p. 123–54. doi: 10.1002/depr.3050010211
55. Hibicke M, Landry AN, Kramer HM, Talman ZK, Nichols CD. Psychedelics, but Not Ketamine, Produce Persistent Antidepressant-like Effects in a Rodent Experimental System for the Study of Depression. ACS Chem Neurosci (2020) 11:864–71. doi: 10.1021/acschemneuro.9b00493
56. Carreno FR, Lodge DJ, Frazer A. Ketamine: Leading us into the future for development of antidepressants. Behav Brain Res (2020) 383:112532. doi: 10.1016/j.bbr.2020.112532
57. Andrews PW, Thomson JA. The bright side of being blue: depression as an adaptation for analyzing complex problems. Psychol Rev (2009) 116:620–54. doi: 10.1037/a0016242
58. Lopresti AL, Maker GL, Hood SD. Drummond PD. A review of peripheral biomarkers in major depression: The potential of inflammatory and oxidative stress biomarkers. Prog Neuropsychopharmacol Biol Psychiatry (2014) 48:102–11. doi: 10.1016/j.pnpbp.2013.09.017
59. Cipriani A, Zhou X, Del Giovane C, Hetrick SE, Qin B, Whittington C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet (2016) 388:881–90. doi: 10.1016/S0140-6736(16)30385-3
60. Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet (2018) 391:1357–66. doi: 10.1016/S0140-6736(17)32802-7
61. Bernaras E, Jaureguizar J, Garaigordobil M. Child and Adolescent Depression: A Review of Theories, Evaluation Instruments, Prevention Programs, and Treatments. Front Psychol (2019) 10:543. doi: 10.3389/fpsyg.2019.00543
62. Insel TR, Cuthbert BN. Brain disorders? Precisely. Sci (80-) (2015) 348:499–500. doi: 10.1126/science.aab2358
63. Miller DB, O'Callaghan JP. Personalized medicine in major depressive disorder - Opportunities and pitfalls. Metabolism (2013) 62:S34–9. doi: 10.1016/j.metabol.2012.08.021
64. Martin C, Tansey KE, Schalkwyk LC, Powell TR. The inflammatory cytokines: Molecular biomarkers for major depressive disorder? Biomark Med (2015) 9:169–80. doi: 10.2217/BMM.14.29
65. Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry (2006) 163:1905–17. doi: 10.1176/ajp.2006.163.11.1905
66. Lichtblau N, Schmidt FM, Schumann R, Kirkby KC, Himmerich H. Cytokines as biomarkers in depressive disorder: Current standing and prospects. Int Rev Psychiatry (2013) 25:592–603. doi: 10.3109/09540261.2013.813442
67. Nichols D, Johnson M, Nichols C. Psychedelics as Medicines: An Emerging New Paradigm. Clin Pharmacol Ther (2017) 101:209–19. doi: 10.1002/cpt.557
68. Bockting CLH, Klein NS, Elgersma HJ, van Rijsbergen GD, Slofstra C, Ormel J, et al. Effectiveness of preventive cognitive therapy while tapering antidepressants versus maintenance antidepressant treatment versus their combination in prevention of depressive relapse or recurrence (DRD study): a three-group, multicentre, randomised control. Lancet Psychiatry (2018) 5:401–10. doi: 10.1016/S2215-0366(18)30100-7
69. Reiff CM, Richman EE, Nemeroff CB, Carpenter LL, Widge AS, Rodriguez CI, et al. Psychedelics and Psychedelic-Assisted Psychotherapy. Am J Psychiatry (2020), appiajp201919010035. doi: 10.1176/appi.ajp.2019.19010035
70. Kvam T-M, Stewart LH, Andreassen OA. Psychedelic drugs in the treatment of anxiety, depression and addiction. Tidsskr Den Nor Legeforen (2018) 18. doi: 10.4045/tidsskr.17.1110
71. Reiche S, Hermle L, Gutwinski S, Jungaberle H, Gasser P, Majić T. Serotonergic hallucinogens in the treatment of anxiety and depression in patients suffering from a life-threatening disease: A systematic review. Prog Neuropsychopharmacol Biol Psychiatry (2018) 81:1–10. doi: 10.1016/j.pnpbp.2017.09.012
72. Dos Santos RG, Osório FL, Crippa JAS, Riba J, Zuardi AW, Hallak JEC. Antidepressive, anxiolytic, and antiaddictive effects of ayahuasca, psilocybin and lysergic acid diethylamide (LSD): a systematic review of clinical trials published in the last 25 years. Ther Adv Psychopharmacol (2016) 6:193–213. doi: 10.1177/2045125316638008
73. Carhart-Harris RL, Roseman L, Bolstridge M, Demetriou L, Pannekoek JN, Wall MB, et al. Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Sci Rep (2017) 7:13187. doi: 10.1038/s41598-017-13282-7
74. Roseman L, Nutt DJ, Carhart-Harris RL. Quality of Acute Psychedelic Experience Predicts Therapeutic Efficacy of Psilocybin for Treatment-Resistant Depression. Front Pharmacol (2017) 8:974. doi: 10.3389/fphar.2017.00974
75. Carhart-Harris RL, Bolstridge M, Rucker J, Day CMJ, Erritzoe D, Kaelen M, et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry (2016) 3:619–27. doi: 10.1016/S2215-0366(16)30065-7
76. Haile CN, Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Foulkes A, et al. Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int J Neuropsychopharmacol (2014) 17:331–6. doi: 10.1017/S1461145713001119
77. Monteggia LM, Zarate C. Antidepressant actions of ketamine: from molecular mechanisms to clinical practice. Curr Opin Neurobiol (2015) 30:139–43. doi: 10.1016/j.conb.2014.12.004
78. Kalmoe MC, Janski AM, Zorumski CF, Nagele P, Palanca BJ, Conway CR. Ketamine and nitrous oxide: The evolution of NMDA receptor antagonists as antidepressant agents. J Neurol Sci (2020) 412:116778. doi: 10.1016/j.jns.2020.116778
79. Gastaldon C, Papola D, Ostuzzi G, Barbui C. Esketamine for treatment resistant depression: a trick of smoke and mirrors? Epidemiol Psychiatr Sci (2020) 29:e79. doi: 10.1017/S2045796019000751
80. Wagner MT, Mithoefer MC, Mithoefer AT, MacAulay RK, Jerome L, Yazar-Klosinski B, et al. Therapeutic effect of increased openness: Investigating mechanism of action in MDMA-assisted psychotherapy. J Psychopharmacol (2017) 31:967–74. doi: 10.1177/0269881117711712
81. Valkanova V, Ebmeier KP, Allan CL. CRP. IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. J Affect Disord (2013) 150:736–44. doi: 10.1016/j.jad.2013.06.004
82. Müller N, Myint AM, Schwarz MJ. Inflammatory biomarkers and depression. Neurotox Res (2011) 19:308–18. doi: 10.1007/s12640-010-9210-2
83. Watson PJ, Andrews PW. Toward a revised evolutionary adaptationist analysis of depression: the social navigation hypothesis. J Affect Disord (2002) 72:1–14. doi: 10.1016/S0165-0327(01)00459-1
84. Beard C. Cognitive bias modification for anxiety: current evidence and future directions. Expert Rev Neurother (2011) 11:299–311. doi: 10.1586/ern.10.194
85. Taché Y, Brunnhuber S. From Hans Selye's discovery of biological stress to the identification of corticotropin-releasing factor signaling pathways: implication in stress-related functional bowel diseases. Ann N Y Acad Sci (2008) 1148:29–41. doi: 10.1196/annals.1410.007
86. Kopp M, Skrabski A. What does the legacy of Hans Selye and Franz Alexander mean today? (The psychophysiological approach in medical practice). Int J Psychophysiol (1989) 8:99–105. doi: 10.1016/0167-8760(89)90001-9
87. Hillegers MH, Reichart CG, Wals M, Verhulst FC, Ormel J, Nolen WA. Five-year prospective outcome of psychopathology in the adolescent offspring of bipolar parents. Bipolar Disord (2005) 7:344–50. doi: 10.1111/j.1399-5618.2005.00215.x
88. Fleisher WP, Katz LY. Early onset major depressive disorder. Paediatr Child Health (2001) 6:444–8. doi: 10.1093/pch/6.7.444
89. Hawkins E. Screen teenagers annually for depression, say US doctors. Guard (2018). Available at: https://www.theguardian.com/society/2018/mar/03/screen-teenagers-annually-for-depression-say-us-doctors?CMP=Share_iOSApp_Other.
90. García-Carrión R, Villarejo-Carballido B, Villardón-Gallego L. Children and Adolescents Mental Health: A Systematic Review of Interaction-Based Interventions in Schools and Communities. Front Psychol (2019) 10:918. doi: 10.3389/fpsyg.2019.00918
91. Kelly TM, Daley DC. Integrated treatment of substance use and psychiatric disorders. Soc Work Public Health (2013) 28:388–406. doi: 10.1080/19371918.2013.774673
92. Woolger C. “Wechsler Intelligence Scale for Children-Third Edition (wisc-iii),”. In: Understanding Psychological Assessment. (Boston, MA: Springer US) (2001) p. 219–33. doi: 10.1007/978-1-4615-1185-4_11
93. Spinney L. Pale Rider: The Spanish Flu of 1918 and How It Changed the World. New York: PublicAffairs (2017).
94. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol (2020) 5:536–44. doi: 10.1038/s41564-020-0695-z
95. Galea S, Merchant RM, Lurie N. The Mental Health Consequences of COVID-19 and Physical Distancing: The Need for Prevention and Early Intervention. JAMA Intern Med (2020). doi: 10.1001/jamainternmed.2020.1562
96. Hinckley JD, Riggs P. Integrated Treatment of Adolescents with Co-occurring Depression and Substance Use Disorder. Child Adolesc Psychiatr Clin N Am (2019) 28:461–72. doi: 10.1016/j.chc.2019.02.006
97. Carhart-Harris RL, Leech R, Hellyer PJ, Shanahan M, Feilding A, Tagliazucchi E, et al. The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci (2014) 8:20. doi: 10.3389/fnhum.2014.00020
99. Okech D, Hansen N, Howard W, Anarfi JK, Burns AC. Social Support, Dysfunctional Coping, and Community Reintegration as Predictors of PTSD Among Human Trafficking Survivors. Behav Med (2018) 44:209–18. doi: 10.1080/08964289.2018.1432553
100. Saxon L, Makhashvili N, Chikovani I, Seguin M, McKee M, Patel V, et al. Coping strategies and mental health outcomes of conflict-affected persons in the Republic of Georgia. Epidemiol Psychiatr Sci (2017) 26:276–86. doi: 10.1017/S2045796016000019
Keywords: depression, adolescents, stress, exhaustion, treatment
Citation: van der Gronde T, Los L, Herremans A, Oosting R, Zorzanelli R and Pieters T (2020) Toward a New Model of Understanding, Preventing, and Treating Adolescent Depression Focusing on Exhaustion and Stress. Front. Psychiatry 11:412. doi: 10.3389/fpsyt.2020.00412
Received: 05 November 2019; Accepted: 22 April 2020;
Published: 06 May 2020.
Edited by:
Xenia Gonda, Semmelweis University, HungaryReviewed by:
Liliana Dell'Osso, University of Pisa, ItalyMassimo Pasquini, Sapienza University of Rome, Italy
Copyright © 2020 van der Gronde, Los, Herremans, Oosting, Zorzanelli and Pieters. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Toine Pieters, dC5waWV0ZXJzQHV1Lm5s
†ORCID: Toon van der Gronde, orcid.org/0000-0002-8688-8180
Arnoud Herremans, orcid.org/0000-0003-2650-3610
Rafaela Zorzanelli, orcid.org/0000-0001-7531-8492
Toine Pieters, orcid.org/0000-0002-8156-8436
 Toon van der Gronde
Toon van der Gronde Leontien Los2
Leontien Los2 Toine Pieters
Toine Pieters