- 1Department of Clinical Psychology, Utrecht University, Utrecht, Netherlands
- 2Department of Psychiatry, Amsterdam University Medical Centres, Location AMC, University of Amsterdam, Amsterdam, Netherlands
- 3Departments of Psychiatry and Child and Adolescent Psychiatry/Psychology, Erasmus University Medical Centre Rotterdam, Rotterdam, Netherlands
- 4Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 5Department of General Practice, University Medical Centre Groningen, University of Groningen, Groningen, Netherlands
- 6Faculty of Science, School of Psychology, The University of New South Wales, Sydney, NSW, Australia
- 7Heymans Institute for Psychological Research, University of Groningen, Groningen, Netherlands
- 8Institute for Advanced Study, Netherlands Institute for Advanced Study in the Humanities and Social Sciences, Royal Netherlands Academy of Arts and Sciences, University of Amsterdam, Amsterdam, Netherlands
- 9Centre for Urban Mental Health, University of Amsterdam, Amsterdam, Netherlands
Background: Previous studies indicated that affect fluctuations, the use of antidepressant medication (ADM), as well as depression during pregnancy might have adverse effects on offspring outcomes. The aim of the current proof-of-principle study is to explore the effect of tapering ADM while receiving online preventive cognitive therapy (PCT) on pregnant women and the offspring as compared to pregnant women continuing ADM.
Objectives: We sought to compare positive and negative affect fluctuations in pregnant women receiving online PCT while tapering ADM vs. pregnant women continuing ADM, and to investigate if affect fluctuations in early pregnancy were related to offspring birth weight.
Method: An experience sampling methodology (ESM)-trial ran alongside a Dutch randomized controlled trial (RCT) and prospective observational cohort of women using ADM at the start of pregnancy. In the ESM-trial fluctuations of positive and negative affect were assessed in the first 8 weeks after inclusion. Recurrences of depression were assessed up to 12 weeks post-partum, and birth records were used to assess offspring birth weight. The RCT has been registered at the Netherlands Trial Register (NTR4694, https://www.trialregister.nl/trial/4551).
Results: In total, 19 pregnant women using ADM at start of their pregnancy participated in the ESM-trial. There were no significant differences in positive and negative affect fluctuations, nor recurrence rates between women receiving PCT while tapering ADM vs. women continuing ADM. We found no association between affect fluctuations, pre-natal depressive symptoms, and birth weight (all p > 0.05).
Conclusion: This explorative study showed that tapering ADM while receiving online PCT may protect pregnant women against recurrences of depression and affect fluctuations, without affecting birth weight. There is a high need for more controlled studies focusing on tapering ADM with (online) psychological interventions during pregnancy.
Introduction
Major depressive disorder (MDD) is a highly disabling and recurrent disorder that affects people worldwide, including pregnant women. Women with a history of mental disorders are at increased risk of perinatal depression (1, 2). Reported recurrence rates of MDD during pregnancy range widely from 2.5 to 68%, depending on the population, treatment, and follow-up period (3–7). MDD and recurrences in the perinatal period can place women and their offspring at risk of short- and long-term psychological and somatic problems, including low birth weight, pre-term birth, and shorter gestational age, which in turn are associated with psychopathology at later ages (8–10), development of MDD, anxiety, and developmental disorders (11–15). Birthweight and gestational age can hence be perceived as first indications of infant's future (mental) health. It is presumed that psychological and pharmacological treatment of pre-natal common mental disorders can mitigate associated adverse effects in offspring, yet strong evidence for the prophylactic benefits of (preventive) treatment is limited (16, 17).
Current treatments to prevent recurrences of MDD during pregnancy include the use of antidepressants medication (ADM). ADM use during pregnancy is estimated to be around 2–8% for various psychiatric disorders (18–21), however, reviews have linked pre-natal ADM use to negative offspring outcomes, including lower birth weight, shorter gestation, development of psychopathology, and cardiovascular problems (22–24). Alternatively, sequentially offering a specific psychological relapse prevention treatment after (partial) remission (i.e., mindfulness-based cognitive therapy [MBCT], preventive cognitive therapy [PCT], or well-being therapy [WBT]) protects against recurrences of MDD, when compared to active (e.g., ADM or treatment as usual [TAU]) or non-active (waitlist) control groups (25–35). Psychological prevention treatments have furthermore been demonstrated to be effective in lowering depressive symptoms during pregnancy (17, 36), and may lower the risk of recurrence of MDD (37). In a recent randomized controlled trial (RCT) (38–41), we investigated the efficacy of PCT with gradual, guided discontinuation of ADM compared to ADM continuation among formerly depressed women taking ADM at the start of their pregnancy. The women (n = 44) were followed throughout pregnancy and up to 3 months post-partum to evaluate recurrences of MDD and the potential impact of the interventions on mother and offspring. There was no evidence that PCT with gradual discontinuation of ADM during pregnancy altered the risk of recurrence of MDD as compared to continuation of ADM (41). This study provides first evidence that preventive therapy while tapering ADM may be a viable alternative to ADM usage for both the formerly depressed pregnant women and their offspring.
Early identification of women at risk and subsequent prevention of relapse or recurrence of MDD in the perinatal period are hence of importance. Previous research showed that certain patterns of variability of affect within individuals may indicate that the individual is prone to depressive relapse or recurrence (42–44). Some studies showed that low positive affect and high negative affect were related to increased depressive symptoms, and increased risk of recurrence of MDD (45–47). On the other hand, a case study and a study in depressed patients (n = 93) showed that the inertia of affect, i.e., the lack of variability, were indicators that a person relapsed into depression (43, 48), although this was not confirmed in a recurrently depressed patient sample (n = 42) (49). The variability of affect is nevertheless believed to be clinically relevant and warrants further investigation.
Affect and affect fluctuations are indeed important to consider in the context of pregnant women and their offspring since previous studies showed that increased positive affect may prevent pre-term birth, although without changing birth weight (50), and that positive and negative affect fluctuations are associated with poorer offspring outcomes such as disturbed fetal physiology [e.g., decreased fetal heart rate and intrauterine artery flow (51)]. At the same time, symptoms of depression and anxiety, and stress levels commonly vary throughout pregnancy (52–54), which in turn are associated with negative affect (reported by the mother) in 1–7 year old children (55), and delayed child development (54). These studies highlight that fluctuations in positive and negative affect and offspring outcomes need to be monitored, especially during relapse prevention interventions.
Experience Sampling Methodology (ESM) (56), which has been successfully used in previous studies (44, 47, 49), provides the opportunity to monitor affect fluctuations and the impact on offspring by multiple semi-random assessments through a mobile telephone application during the day. ESM-trials allow researchers to profoundly investigate day-to-day affect and mood (changes) and early warning signs of depressive relapse in pregnant women. ESM surpasses the problem of traditional self-report questionnaires, where there is an increased risk of a recall bias. For example, self-report questionnaires such as the Edinburgh post-natal depression scale, include questions regarding mood across 1 full week, while it is known that mood symptoms fluctuate throughout the week, or even 1 day (57). In addition, the use of ESM permits researchers to answer research questions by investigating less participants while gathering more information than in traditional designs, which enables including difficult-to-reach populations in research, like the population in the previously described RCT (41).
The question remains whether pregnant women receiving PCT while tapering ADM have more affect fluctuations as compared to pregnant women continuing ADM. Furthermore, it is currently unknown whether affect fluctuations can predict the return of depressive symptoms or recurrence of MDD in pregnant women, and predict offspring health (as measured in terms of birth weight, with and without correction for gestational age). Offspring birth weight is important to investigate due to its relation with future developmental, mental, and somatic disorders. Using an ESM-design alongside the previously described RCT and observational cohort (38–41), the aims of the current study are to (1) explore whether there are more fluctuations in negative and positive affect in pregnant women receiving PCT while tapering ADM vs. continuing ADM; (2) explore whether affect fluctuations predict subsequent recurrences and/or pre-natal depressive symptoms; and (3) explore whether affect fluctuations predict lower offspring birth weight.
Materials and Methods
Design
This article focuses on the ESM-trial run alongside an RCT and an observational cohort. Participants for the ESM-trial were drawn from an RCT [“Stop or Go study” (39)] and a prospective, longitudinal, observational cohort (38, 40), to investigate the effects of PCT while tapering ADM vs. the continuation of ADM. All studies were approved by the Medical Ethical Committee of the Erasmus Medical Center Rotterdam, the Netherlands (MEC-2014-505). All participants provided written informed consent prior to participation.
The RCT protocol has been pre-registered (Netherlands Trial Register: NTR4694) and published (39). The observational cohort has been described in other publications (38, 40) and the protocol has not been pre-registered, as this group of participants was a reference group for the RCT. The ESM-trial started at a later stage of the studies due to the development and coordination of the mobile application, and is therefore not reported in the protocol article.
Participants
ESM-Trial
Participants from the RCT and observational cohort were invited to participate in the ESM-trial after providing written informed consent for the RCT or observational cohort. Participant in-and exclusion criteria and procedures for the RCT and observational cohort are described below. There were no additional criteria to participate in the ESM-trial.
RCT and Observational Cohort
To participate in the RCT or cohort, women needed to (1) be <16 weeks pregnant; (2) use an ADM at the start of the pregnancy, such as selective serotonin reuptake inhibitors (SSRI), or selective noradrenaline reuptake inhibitor (SNRI); and (3) be proficient in Dutch and/or English. Additionally, to participate in the RCT, pregnant women needed to (1) have a history of at least one MDD episode according to the DSM-IV Axis-I (58); (2) be in remission or recovery, i.e., not have a diagnosis of MDD according to the DSM-IV Axis-I criteria since at least 4 months before participation; (3) be willing to be randomized to either PCT with tapering of AMD (“Stop”) or continuation of ADM (“Go”); and (4) have a singleton pregnancy.
Procedures
ESM-Trial
All participants in the RCT and observational cohort were invited to participate in the ESM-trial. Inclusion for the ESM-trial was from August 2016 to February 2018. The ESM-trial started immediately after the baseline assessments and (when applicable) randomization. As a result, the assessments were conducted at the same time as when the participants received PCT and tapered ADM, or continued ADM.
The ESM-trial consisted of questions that were sent in the first 8 weeks of study participation through the mobile telephone with the use of a pre-installed study application using the Tempest platform (59). This application was programmed on the participants' own phone, or on a study-smartphone that was available during study participation. It was programmed to send pre-defined questions 5 days a week, 5 times a day, for 8 consecutive weeks. The triggers were set semi-randomly between 9:00 a.m. and 7:00 p.m., triggering once every 100 min, including a pause of 10 min minimum between triggers. Time to complete the questions was 1–2 min. Participants were instructed to respond to the trigger as soon as possible. To monitor reasons for non-response, a research assistant contacted the participants to discuss problems or questions regarding the application.
RCT and Observational Cohort
Participants were recruited between April 2015 and February 2018 during their first pre-natal visits at the midwifery practices or hospitals in the Netherlands, through general practitioners, psychiatrists, or through advertisements in (social) media. After study researchers received contact information of potential eligible pregnant women, the study researchers counseled the women about the RCT and the observational cohort. After the counseling and a waiting period to think about participation, pregnant women decided to either (1) not participate; (2) participate in the RCT; or, (3) participate in the observational cohort. After this decision and written informed consent, there was a baseline assessment by means of a structured clinical interview through telephone [SCID-I DSM-IV (58)], and a self-report questionnaire. If participants met inclusion criteria and were willing to participate in the RCT, they were randomized into one of the two groups: PCT with tapering of ADM (“Stop”) or continuation of ADM (“Go”). Alternatively, the pregnant woman started participating in the observational cohort, where women decided themselves which relapse prevention strategy they wanted to use. Hereafter, women were assessed with questionnaires and interviewed at 24 and 36 weeks of pregnancy, and 4 and 12 weeks after the due-date. See Figure 1 for a flowchart of all studies.
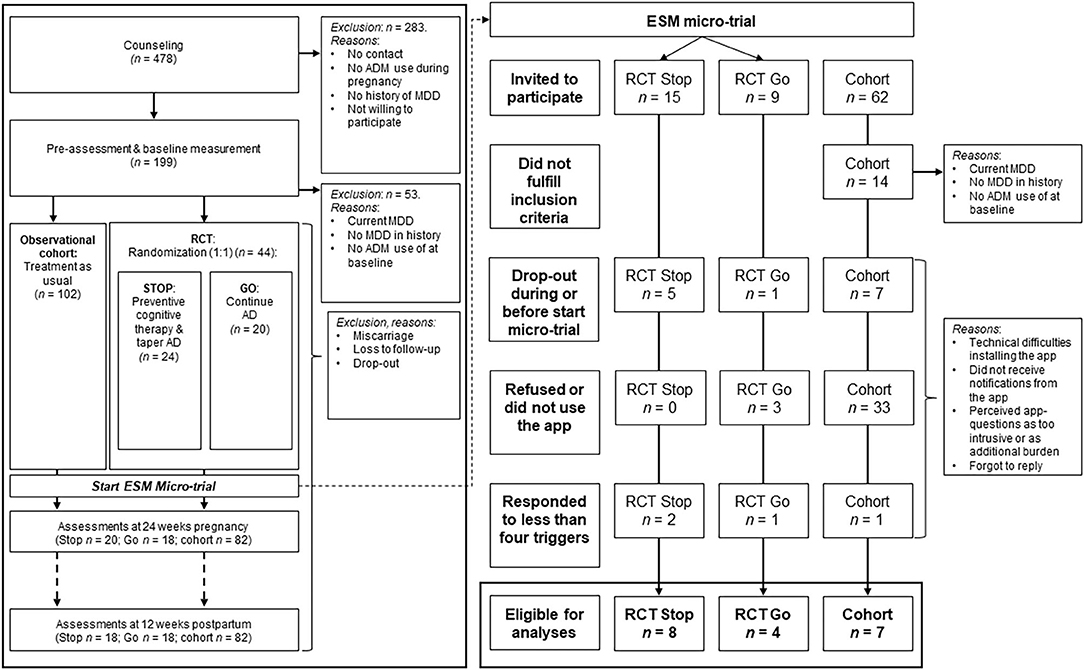
Figure 1. Flow chart of study participation and exclusion (reasons). RCT, Randomized controlled trial; RCT Stop, RCT group receiving preventive cognitive therapy while tapering ADM; RCT Go, RCT group continuing ADM; ADM, antidepressant medication; ESM, experience sampling methodology.
Interventions
PCT and Tapering ADM
Women assigned to the “Stop” -group received online PCT and tapered ADM. The PCT was provided by trained psychologists and consisted of 8 weekly individual sessions using an online telehealth app. In short, PCT uses techniques focused on dysfunctional beliefs and schema using cognitive challenging techniques, including phantasy (activation of positive network), enhance the recall of specific memories of positive experiences, positive feelings and thoughts, and formulating relapse prevention strategies (60). To taper the ADM, the woman was referred to a trained psychiatrist or general practitioner who then guided the tapering. Tapering schedule was based upon an expert-based discontinuation protocol, individual preferences, and drug characteristics. Participants were not allowed to receive another form of psychotherapy on a regular basis during PCT, i.e., first 8 weeks (during the ESM-trial).
Continuation of ADM
Pregnant women who were assigned to the “Go” -group continued the use of ADM as instructed by their prescribing doctor (care as usual).
Observational Cohort
Women in the observational cohort decided themselves which prevention strategy they used (care as usual). This therefore includes women who tapered ADM without PCT.
Measurements
A full overview of this schedule and all assessments can be found in the protocol article (39).
Clinical Diagnosis
Absence and history of MDD at the start of, and relapse and recurrence of MDD throughout study participation was assessed with the SCID-I for DSM-IV disorders (58) up to 12 weeks post-partum. Comorbidities on the Axis-I scale were assessed with the SCID-I as well.
Experience Sampling Methodology
The questions consisted of 25 items, including questions regarding positive and negative affect. The questions were derived from previous research (49) and are based upon the positive and negative affect schedule (PANAS) (61).1
Assessments
Participants' characteristics were assessed at baseline, including age, parity, and ADM usage. At baseline, and 24- and 36-weeks of pregnancy, the Edinburgh post-natal depression scale [EPDS (62)] for depressive symptoms, and the state-trait anxiety inventory [STAI (63)] for anxiety symptoms were assessed. The positive and negative affect scale [PANAS (61)] was used to assess different affective states of the participants at baseline, and 24- and 36-weeks of pregnancy. In addition, birth weight, gestational age and pregnancy-related complications were collected. In the RCT, birth outcomes were obtained from the reports from midwives and gynecologists. Participants in the cohort provided this information themselves during the 4 weeks post-partum assessment.
Statistical Plan
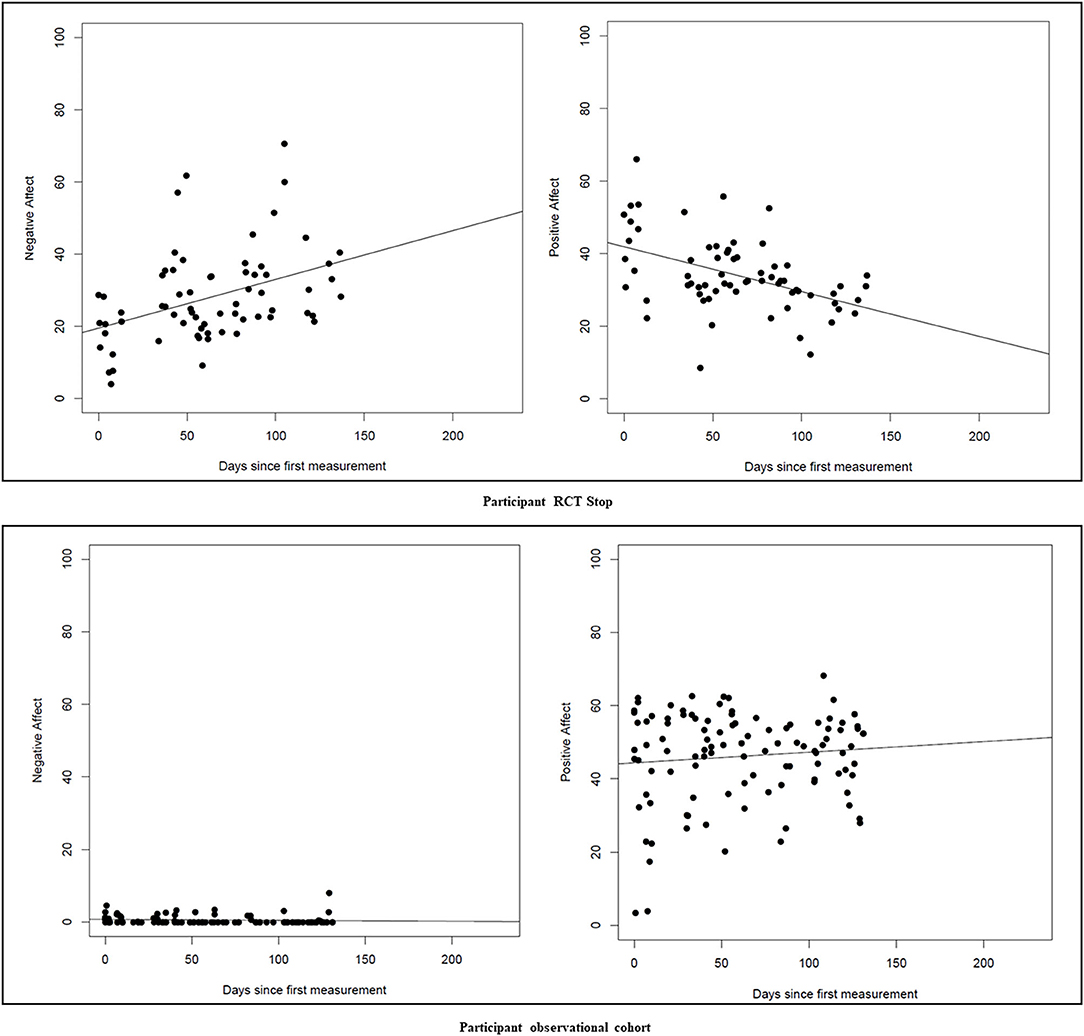
To exploratively analyze differences in affect fluctuations during the first 8 weeks of study participation between the PCT while tapering ADM group and the continuation of ADM group, the ESM-trial data was used. An average positive affect and negative affect score was calculated for each reply to the trigger, minimizing the number of separate variables. That way, there was one score for each trigger for both positive and negative affect. Participants were excluded if they responded to <4 triggers in total, but were allowed to have missing data. Missing data was not imputed. To estimate individual affect fluctuations, an individual linear regression equation was calculated using participants' average scores on the positive and negative affect items. For this individual linear regression equation, the dependent variable was the mean affect score for each trigger, and elapsed time was the independent variable. The individual beta coefficient, the coefficient of determination (R2), and one minus R2 (as a measure of fluctuations) for both positive and negative affect were saved for each participant and used in subsequent analyses. The responses and individual regression lines were plotted for each individual, to visually inspect patterns of positive and negative affect fluctuations.
For the analyses, the group differences in positive and negative affect fluctuations (ESM-trial data), with and without correction for depressive symptoms (assessed with the EPDS), and number of recurrences were analyzed if feasible. Second, three separate linear regression analyses were conducted; to (1) predict depressive symptoms with ESM positive and negative affect fluctuations; (2) predict recurrences with ESM positive and negative affect fluctuations; and (3) predict birth weight with ESM positive and negative affect fluctuations corrected for pre-natal depressive symptoms. All regression analyses were corrected for number of previous episodes, as this was used to stratify the randomization in the RCT sample and is a known predictor of depressive recurrences (64).
Results
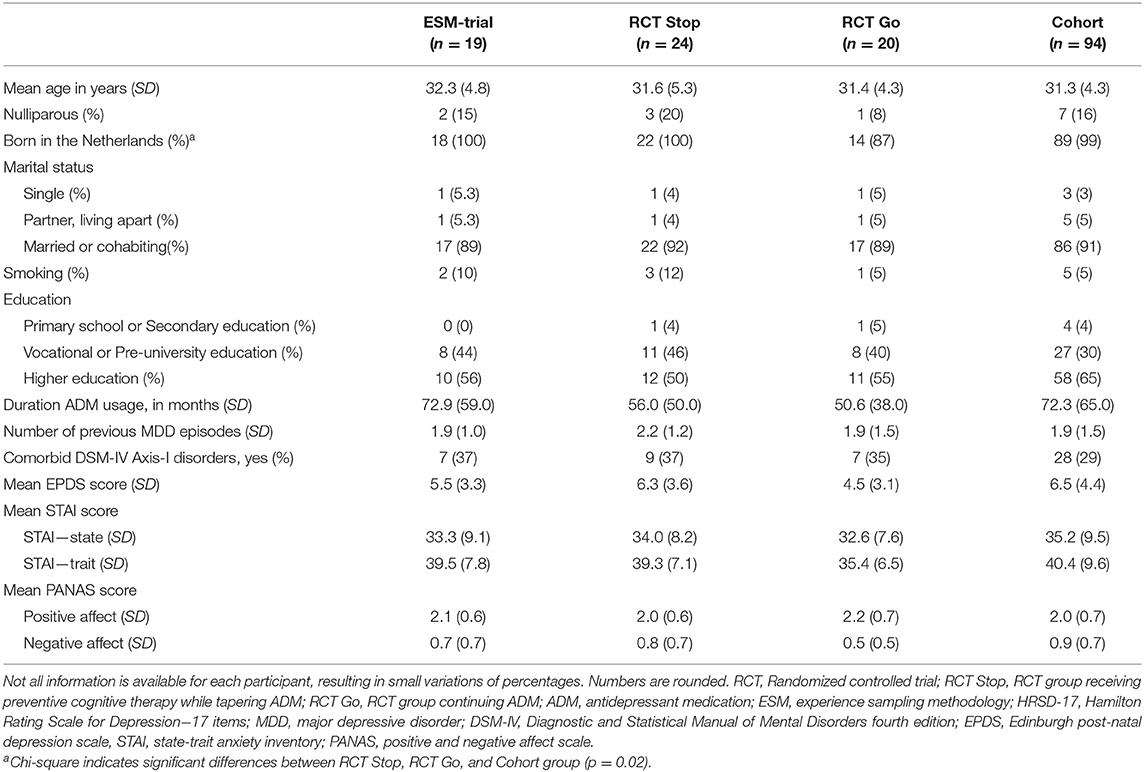
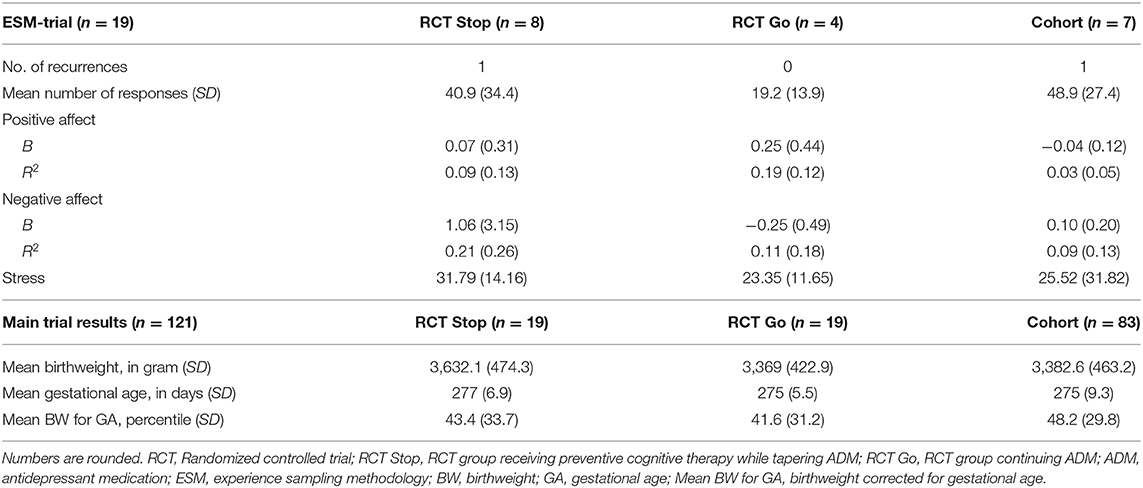
Overall, 478 pregnant women were referred to the studies for counseling, of whom 146 were eligible to participate (n = 44 participated for the RCT; n = 102 for the observational cohort). Among these participants, 24 women agreed to participate in the ESM-trial. See Figure 1 for a flow-chart. Sufficient data from 19 women were available for the analyses, including 12 RCT participants of whom eight received PCT and tapered the ADM, and four continued ADM usage. Seven participants where from the observational cohort, of whom six continued the use of ADM, and one tapered without additional relapse prevention intervention. An overview of baseline characteristics is displayed in Table 1, and the study flow-chart including reasons for drop-out is shown in Figure 1. The results of the follow-up measurements are reported in Table 2.
ESM-Trial Results (n = 19)
In total, 2 out of 19 women participating in the ESM-trial had a recurrence of MDD during the study period. Mean positive affect score across groups was 48.56 (SD = 16.61), and mean negative affect was 20.51 (SD = 13.35). There was no significant group effect (RCT women tapering ADM vs. RCT women continuing ADM) on positive or negative affect fluctuations (positive affect: F(1, 9) = 1.85, p = 0.20; negative affect: F(1, 9) = 0.44, p = 0.52). When including women from the observational cohort into the ESM-trial analyses, this effect between tapering and continuing ADM remained non-significant (positive affect: F(1, 15) = 0.16, p = 0.69; negative affect: F(1, 15) = 3.86, p = 0.07). Correcting the analyses for depressive symptoms at baseline did not change the main results (positive affect: F(1, 14) = 0.21, p = 0.65; negative affect: F(1, 14) = 3.45, p = 0.08). As an example of the ESM-trial outcomes, Figure 2 displays the positive and negative affect scores for a pregnant woman who received PCT and tapered ADM (RCT participant), and a pregnant woman who continued ADM (cohort participant).
Linear regression analyses indicated that positive or negative affect fluctuations did not significantly predict depressive symptoms at 36 weeks pregnancy (positive affect β = 0.22, 95% CI = −15.84, 37.20, p = 0.40; negative affect β = −0.26, 95% CI = −22.55, 7.96, p = 0.32). Furthermore, there was no significant relationship between affect fluctuations and birth weight (positive affect β = −0.08, 95% CI = −3,083.36, 2,310.35, p = 0.76; negative affect β = 0.18, 95% CI = −1,081.73, 2,052.82, p = 0.51) or corrected birth weight for gestational age (positive affect β = 0.17, 95% CI = −120.28, 217.74, p = 0.54; negative affect β = 0.18, 95% CI = −67.96, 128.48, p = 0.52). There was an insufficient number of recurrences in the ESM-trial to investigate and test the relationship between affect fluctuations and recurrences of MDD.
Main RCT and Cohort Results
In the main trials (RCT and observational cohort), 13 women had a recurrence of MDD during the study period (8.9%). The number of recurrences did not significantly differ between the groups in the main RCT (PCT with tapering ADM n = 3 vs. ADM continuation n = 3), but did in the full group (taper ADM with or without PCT n = 8 vs. ADM continuation n = 5; X2(1) = 8.60, p = 0.003).
Discussion
The aim of the current study was to explore the effect of tapering ADM in pregnant women while receiving PCT as compared to pregnant women continuing ADM. In this proof-of-principle study, there were no indications that pregnant women who received PCT and tapered ADM showed more affect fluctuations or recurrences than women continuing ADM. To our knowledge this is the first study to show individual fluctuations of positive and negative affect and depression recurrence rates in an RCT and observational cohort of pregnant women using ADM at the start of their pregnancy.
Although the study had an explorative nature with a relatively small group of women, there was a large amount of individual responses (ranging from 6 to 105 responses per individual). The results of the ESM-trial indicate that women receiving PCT while tapering ADM show similar patterns of fluctuations in positive and negative affect throughout pregnancy, as women continuing ADM. This may be due to the effects of PCT which targets cognitive vulnerabilities by challenging dysfunctional beliefs and schema's, activating positive networks, and enhancing the memory of positive experiences (28). PCT therefore partially targets emotion regulation (28), by changing the way a person processes and regulates emotion-related information (65). In addition, a previous study showed that people who received PCT were less affected by daily hassles, which may have led to a reduced risk of recurrence in comparison to care as usual (66). Potentially, women receiving this preventive psychotherapy therefore did not show increased fluctuations in their affect or inertia, as they were able to regulate their emotions and/or process daily hassles better, thereby potentially lowering the risk of recurrence of MDD.
This is in contrast to a previous study among formerly depressed remitted participants that showed that people receiving PCT while tapering ADM had a slightly higher risk of recurrence in the first 4 months of tapering, indicating there may have been a withdrawal effect in these participants due to various reasons [e.g., imbalance due to withdrawal symptoms, or fear of recurrence; (27)]. In the current study, there was no difference in the number of recurrences, as well as no difference in positive and negative affect fluctuations in the group of women receiving PCT while tapering ADM. This might imply that there was none or less emotional withdrawal or discontinuation symptoms (67, 68), or fear of recurrences, in this group. An explanation for this difference may be that women learned to regulate emotions with the help of PCT (28, 65). The ESM-trial was conducted during the time that women tapered ADM and received PCT. Alternatively, unknown factors caused by the pregnancy itself might have protected the women from fluctuating more in affect while tapering ADM vs. continuation of ADM. This alternative explanation is supported by the fact that the few recurrences of depression in the main RCT primarily occurred in the first 3 months post-partum.
Another potential explanation for the absence of significant differences in affect fluctuations between PCT while tapering ADM vs. continuation of ADM, may be the low recurrence rates among the participants, with no significant differences in recurrence rates between women receiving PCT while tapering ADM and women continuing ADM. Previous studies investigating recurrence rates in pregnant women discontinuing ADM are scarce and have produced conflicting results. Although previous research found even lower recurrence rates in a group of pregnant women with a history of MDD [2.5% (69)], another study showed an increased risk of recurrence after pre-natally tapering ADM compared to continuation of ADM [68% (3)]. Yet another study found comparable recurrence rates in both groups [16% (6)]. One of the main differences between these studies is the selection of specific populations using different prevention strategies. For example, the pregnant women from previous studies had a history of more severe MDD and/or more or severe comorbid psychopathology, tapered ADM without guidance or complementary psychotherapy, or were actively seeking help and therefore may have had subsyndromal depressive symptoms for which they needed help. In contrast, the current study focused on the results of the RCT and cohort with stable remitted previously depressed pregnant women.
There were also no differences in the birth weight of offspring from women tapering or continuing ADM. Birth weight may already be affected by ADM use in early pregnancy, as previous reviews indicated (23). On the other hand, psychotherapy may likewise positively or negatively influence birth weight. A recent meta-analysis found that birth weight could be negatively or positively affected when pregnant women with common mental disorders received psychotherapy, depending on the disorder and type of treatment (16). The absence of a direct influence of affect fluctuations on birth weight does not necessarily mean that these fluctuations do not affect the offspring. It may be the case that adverse effects are expressed later in life. For example, one study showed that levels of maternal mood symptoms during pregnancy predicted negative affect in the offspring at age one, two, and seven (55), and another study demonstrated that elevated anxiety symptoms increased the risk of offspring having delayed development at the age of 3 years (54). Alternatively, the absence of a link between affect fluctuations and birth weight can be explained by the small sample size.
Despite several strengths of the current study, being the first proof-of-principle ESM RCT to investigate tapering ADM in pregnant women while receiving a psychotherapeutic intervention as compared to continuation of ADM, there were several limitations that need to be addressed. First, the small sample size in the ESM-trial prevented us from drawing firm conclusions about the potential role of affect fluctuations. However, 800 datapoints have been gathered enabling us to analyse continuous fluctuations in affect with a minimal risk of recall bias [e.g., (57)], and more details regarding the effect of tapering ADM with PCT among pregnant women. Second, the low recurrence rates and low variation of depressive symptoms throughout pregnancy may have minimized the likelihood of detecting clinically meaningful associations. However, low recurrence rates while tapering ADM might also indicate that tapering ADM in pregnancy has limited risks while receiving PCT. Lastly, the participant group may have comprised relatively healthy women with low levels or residual depressive symptoms, which may have put them at low risk of recurrence. Although, our sample at baseline on average had two previous MDD episodes, comparable to other depression-related study samples. Due to these limitations, the results of this proof-of-principle study must be interpreted with caution, and a replication of the study is needed.
Overall, the current study and explorative analyses provide no first indications that pregnant women show more fluctuations in positive/negative affect, nor recurrences, when they taper ADM and receive PCT compared to when women continue ADM. This is supported by the results of the main trial, where the pregnant women in both groups had a comparable risk of relapse (41). Pregnant women may not be at increased risk of recurrence when they taper ADM, or put their child at risk of negative outcomes such as lower birth weight. Future research should explore recurrence risk in larger (international) samples and individual pathways of affect fluctuations and MDD recurrence in pregnant women with a history of MDD who use ADM. Moreover, the effects and effectiveness of relapse prevention treatments for both mother and child need to be further investigated. More research is needed to investigate whether indeed the PCT led to the current results.
To conclude, the current proof of principle study provided a first indication that tapering ADM with the guidance of PCT may indeed protect pregnant women against recurrence of depression and affect fluctuations, without evident negative effects on birth weight of the offspring.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Medical Ethical Committee of the Erasmus Medical Center Rotterdam, the Netherlands. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MB, NM, CB, ML, and HB: design, protocol, and data acquisition. CA, MB, CB, and HB: analyses. MB, NM, CB, HB, AW, ML, and CA: interpretation of data, drafting of the manuscript, and/or revisions. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant from the Netherlands Organization for Health Research and Development (836021011).
Conflict of Interest
CB was co-developer of the Dutch multidisciplinary clinical guideline for anxiety and depression, for which she received no remuneration. CB was furthermore a member of the scientific advisory board of the National Insurance Institute, for which she received an honorarium. This role had no direct relation to this study. CB received royalties from her books and co-edited books, and she developed Preventive Cognitive Therapy on the basis of the Cognitive Model of A. T. Beck.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
1. ^A full overview of the ESM micro-study questions is available upon request.
References
1. Biaggi A, Conroy S, Pawlby S, Pariante CM. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J Affect Disord. (2016) 191:62–77. doi: 10.1016/j.jad.2015.11.014
2. Stuart-Parrigon K, Stuart S. Perinatal depression: an update and overview. Curr Psychiatry Rep. (2014) 16:468. doi: 10.1007/s11920-014-0468-6
3. Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera A, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. (2006) 295:499. doi: 10.1001/jama.295.5.499
4. Ornoy A, Koren G. Selective serotonin reuptake inhibitors in human pregnancy: on the way to resolving the controversy. Semin Fetal Neonatal Med. (2014) 19:188–94. doi: 10.1016/j.siny.2013.11.007
5. Patton GC, Romaniuk H, Spry E, Coffey C, Olsson C, Doyle LW, et al. Prediction of perinatal depression from adolescence and before conception (VIHCS): 20-year prospective cohort study. Lancet. (2015) 386:875–83. doi: 10.1016/S0140-6736(14)62248-0
6. Yonkers KA, Vigod S, Ross LE. Diagnosis, pathophysiology, and management of mood disorders in pregnant and postpartum women. Obstet Gynecol. (2011) 117:961–77. doi: 10.1097/AOG.0b013e31821187a7
7. Stevens AWMM, Goossens PJJ, Knoppert-van der Klein EAM, Draisma S, Honig A, Kupka RW. Risk of recurrence of mood disorders during pregnancy and the impact of medication: a systematic review. J Affect Disord. (2019) 249:96–103. doi: 10.1016/j.jad.2019.02.018
8. Madigan S, Oatley H, Racine N, Fearon RMMP, Schumacher L, Akbari E, et al. A meta-analysis of maternal prenatal depression and anxiety on child socioemotional development. J Am Acad Child Adolesc Psychiatry. (2018) 57:645–57. doi: 10.1016/j.jaac.2018.06.012
9. Gentile S. Untreated depression during pregnancy: short- and long-term effects in offspring. A systematic review. Neuroscience. (2015) 342:154–66. doi: 10.1016/j.neuroscience.2015.09.001
10. Dadi AF, Miller ER, Bisetegn TA, Mwanri L. Global burden of antenatal depression and its association with adverse birth outcomes: an umbrella review. BMC Public Health. (2020) 20:173. doi: 10.1186/s12889-020-8293-9
11. Lahti M, Savolainen K, Tuovinen S, Pesonen A-K, Lahti J, Heinonen K, et al. Maternal depressive symptoms during and after pregnancy and psychiatric problems in children. J Am Acad Child Adolesc Psychiatry. (2017) 56:30–9. doi: 10.1016/j.jaac.2016.10.007
12. National Institute for Health and Clinical Excellence. Antenatal and Postnatal Mental Health: Clinical Management and Service Guidance: Updated Edition. NICE Clinical Guideline 192. London: NICE (2014).
13. Newman L, Judd F, Olsson CA, Castle D, Bousman C, Sheehan P, et al. Early origins of mental disorder - risk factors in the perinatal and infant period. BMC Psychiatry. (2016) 16:270. doi: 10.1186/s12888-016-0982-7
14. O'Donnell KJ, Glover V, Barker ED, O'Connor TG. The persisting effect of maternal mood in pregnancy on childhood psychopathology. Dev Psychopathol. (2014) 26:393–403. doi: 10.1017/S0954579414000029
15. Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, et al. Effects of perinatal mental disorders on the fetus and child. Lancet. (2014) 384:1800–19. doi: 10.1016/S0140-6736(14)61277-0
16. Brouwer ME, Williams AD, van Grinsven SE, Cuijpers P, Lambregtse-van den Berg MP, Burger H, et al. Offspring outcomes after prenatal treatment for common mental disorders: a meta-analysis. BMC Med. (2018) 16:22819749. doi: 10.1186/s12916-018-1192-6
17. Goodman SH, Cullum KA, Dimidjian S, River LM, Kim CY. Opening windows of opportunities: evidence for interventions to prevent or treat depression in pregnant women being associated with changes in offspring's developmental trajectories of psychopathology risk. Dev Psychopathol. (2018) 30:1179–96. doi: 10.1017/S0954579418000536
18. Andrade SE, Raebel MA, Brown J, Lane K, Livingston J, Boudreau D, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. (2008) 198:194.e1–194.e5. doi: 10.1016/j.ajog.2007.07.036
19. Bakker MK, Kölling P, van den Berg PB, de Walle HEK, de Jong van den Berg LTW. Increase in use of selective serotonin reuptake inhibitors in pregnancy during the last decade, a population-based cohort study from the Netherlands. Br J Clin Pharmacol. (2008) 65:600–6. doi: 10.1111/j.1365-2125.2007.03048.x
20. Charlton R, Jordan S, Pierini A, Garne E, Neville A, Hansen A, et al. Selective serotonin reuptake inhibitor prescribing before, during and after pregnancy: a population-based study in six European regions. BJOG An Int J Obstet Gynaecol. (2015) 122:1010–20. doi: 10.1111/1471-0528.13143
21. Ververs T, Kaasenbrood H, Visser G, Schobben F, de Jong-van den Berg L, Egberts T. Prevalence and patterns of antidepressant drug use during pregnancy. Eur J Clin Pharmacol. (2006) 62:863–70. doi: 10.1007/s00228-006-0177-0
22. Grigoriadis S, VonderPorten EH, Mamisashvili L, Roerecke M, Rehm J, Dennis C-L, et al. Antidepressant exposure during pregnancy and congenital malformations: is there an association? J Clin Psychiatry. (2013) 74:293–308. doi: 10.4088/JCP.12r07966
23. Lupattelli A, Wood M, Ystrom E, Skurtveit S, Handal M, Nordeng H. Effect of time-dependent selective serotonin reuptake inhibitor antidepressants during pregnancy on behavioral, emotional, and social development in preschool-aged children. J Am Acad Child Adolesc Psychiatry. (2018) 57:200–8. doi: 10.1016/j.jaac.2017.12.010
24. Ross LE, Grigoriadis S, Mamisashvili L, Vonderporten EH, Roerecke M, Rehm J, et al. Selected pregnancy and delivery outcomes after exposure to antidepressant medication: a systematic review and meta-analysis. JAMA Psychiatry. (2013) 70:436–43. doi: 10.1001/jamapsychiatry.2013.684
25. Beshai S, Dobson KS, Bockting CLH, Quigley L. Relapse and recurrence prevention in depression: current research and future prospects. Clin Psychol Rev. (2011) 31:1349–60. doi: 10.1016/j.cpr.2011.09.003
26. Biesheuvel-Leliefeld KEM, Kok GD, Bockting CLH, Cuijpers P, Hollon SD, van Marwijk HWJ, et al. Effectiveness of psychological interventions in preventing recurrence of depressive disorder: meta-analysis and meta-regression. J Affect Disord. (2015) 174:400–10. doi: 10.1016/j.jad.2014.12.016
27. Bockting CLH, Klein NS, Elgersma HJ, van Rijsbergen GD, Slofstra C, Ormel J, et al. Effectiveness of preventive cognitive therapy while tapering antidepressants versus maintenance antidepressant treatment versus their combination in prevention of depressive relapse or recurrence (DRD study): a three-group, multicentre, randomised control. Lancet Psychiatry. (2018) 5:401–10. doi: 10.1016/S2215-0366(18)30100-7
28. Bockting CL, Hollon SD, Jarrett RB, Kuyken W, Dobson K. A lifetime approach to major depressive disorder: the contributions of psychological interventions in preventing relapse and recurrence. Clin Psychol Rev. (2015) 41:16–26. doi: 10.1016/j.cpr.2015.02.003
29. Clarke K, Mayo-Wilson E, Kenny J, Pilling S. Can non-pharmacological interventions prevent relapse in adults who have recovered from depression? A systematic review and meta-analysis of randomised controlled trials. Clin Psychol Rev. (2015) 39:58–70. doi: 10.1016/j.cpr.2015.04.002
30. Guidi J, Tomba E, Fava GA. The sequential integration of pharmacotherapy and psychotherapy in the treatment of major depressive disorder: a meta-analysis of the sequential model and a critical review of the literature. Am J Psychiatry. (2016) 173:128–37. doi: 10.1176/appi.ajp.2015.15040476
31. Kuyken W, Warren FC, Taylor RS, Whalley B, Crane C, Bondolfi G, et al. Efficacy of mindfulness-based cognitive therapy in prevention of depressive relapse. JAMA Psychiatry. (2016) 73:565. doi: 10.1001/jamapsychiatry.2016.0076
32. Vittengl JR, Clark LA, Dunn TW, Jarrett RB. Reducing relapse and recurrence in unipolar depression: a comparative meta-analysis of cognitive-behavioral therapy's effects. J Consult Clin Psychol. (2007) 75:475–88. doi: 10.1037/0022-006X.75.3.475
33. Piet J, Hougaard E. The effect of mindfulness-based cognitive therapy for prevention of relapse in recurrent major depressive disorder: a systematic review and meta-analysis. Clin Psychol Rev. (2011) 31:1032–40. doi: 10.1016/j.cpr.2011.05.002
34. Guidi J, Fava GA, Fava M, Papakostas GI. Efficacy of the sequential integration of psychotherapy and pharmacotherapy in major depressive disorder: a preliminary meta-analysis. Psychol Med. (2011) 41:321–31. doi: 10.1017/S0033291710000826
35. Vittengl JR, Jarrett RB. Cognitive therapy to prevent depressive relapse in adults. Curr Opin Psychol. (2015) 4:26–31. doi: 10.1016/j.copsyc.2015.01.016
36. Sockol LE. A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. J Affect Disord. (2015) 177:7–21. doi: 10.1016/j.jad.2015.01.052
37. Dimidjian S, Goodman SH, Felder JN, Gallop R, Brown AP, Beck A. Staying well during pregnancy and the postpartum: a pilot randomized trial of mindfulness-based cognitive therapy for the prevention of depressive relapse/recurrence. J Consult Clin Psychol. (2016) 84:134–45. doi: 10.1037/ccp0000068
38. Molenaar NM, Brouwer ME, Kamperman AM, Burger H, Williams AD, Hoogendijk WJG, et al. Recurrence of depression in the perinatal period: clinical features and associated vulnerability markers in an observational cohort. PLoS ONE. (2019) 14:e0212964. doi: 10.1371/journal.pone.0212964
39. Molenaar NM, Brouwer ME, Bockting CLH, Bonsel GJ, van der Veere CN, Torij HW, et al. Stop or go? Preventive cognitive therapy with guided tapering of antidepressants during pregnancy: study protocol of a pragmatic multicentre non-inferiority randomized controlled trial. BMC Psychiatry. (2016) 16:72. doi: 10.1186/s12888-016-0752-6
40. Molenaar NM, Houtman D, Bijma HH, Brouwer ME, Burger H, Hoogendijk WJG, et al. Dose-effect of maternal serotonin reuptake inhibitor use during pregnancy on birth outcomes: a prospective cohort study. J Affect Disord. (2020) 267:57–62. doi: 10.1016/j.jad.2020.02.003
41. Molenaar NM, Brouwer ME, Burger H, Kamperman AM, Bergink V, Hoogendijk WJG, et al. Preventive cognitive therapy with antidepressant discontinuation during pregnancy: results from a randomized controlled trial. J Clin Psychiatry. (2020). 81:19l13099. doi: 10.4088/JCP.19l13099
42. Boumparis N, Karyotaki E, Kleiboer A, Hofmann SG, Cuijpers P. The effect of psychotherapeutic interventions on positive and negative affect in depression: a systematic review and meta-analysis. J Affect Disord. (2016) 202:153–62. doi: 10.1016/j.jad.2016.05.019
43. Wichers M, Groot PC, Psychosystems ESM Group EWS Group. Critical slowing down as a personalized early warning signal for depression. Psychother Psychosom. (2016) 85:114–6. doi: 10.1159/000441458
44. Wichers M, Peeters F, Geschwind N, Jacobs N, Simons CJP, Derom C, et al. Unveiling patterns of affective responses in daily life may improve outcome prediction in depression: a momentary assessment study. J Affect Disord. (2010) 124:191–5. doi: 10.1016/j.jad.2009.11.010
45. de Jonge M, Dekker JJM, Kikkert MJ, Peen J, van Rijsbergen GD, Bockting CLH. The role of affect in predicting depressive symptomatology in remitted recurrently depressed patients. J Affect Disord. (2017) 210:66–71. doi: 10.1016/j.jad.2016.12.015
46. Dunkley DM, Lewkowski M, Lee IA, Preacher KJ, Zuroff DC, Berg JL, et al. Daily stress, coping, and negative and positive affect in depression: complex trigger and maintenance patterns. Behav Ther. (2017) 48:349–65. doi: 10.1016/j.beth.2016.06.001
47. Höhn P, Menne-Lothmann C, Peeters F, Nicolson NA, Jacobs N, Derom C, et al. Moment-to-moment transfer of positive emotions in daily life predicts future course of depression in both general population and patient samples. PLoS ONE. (2013) 8:e0075655. doi: 10.1371/journal.pone.0075655
48. van de Leemput IA, Wichers M, Cramer AOJ, Borsboom D, Tuerlinckx F, Kuppens P, et al. Critical slowing down as early warning for the onset and termination of depression. Proc Natl Acad Sci USA. (2014) 111:87–92. doi: 10.1073/pnas.1312114110
49. Slofstra C, Nauta MH, Bringmann LF, Klein NS, Albers CJ, Batalas N, et al. Individual negative affective trajectories can be detected during different depressive relapse prevention strategies. Psychother Psychosom. (2018) 87:243–5. doi: 10.1159/000489044
50. Pesonen AK, Lahti M, Kuusinen T, Tuovinen S, Villa P, Hämäläinen E, et al. Maternal prenatal positive affect, depressive and anxiety symptoms and birth outcomes: the PREDO study. PLoS ONE. (2016) 11:e0150058. doi: 10.1371/journal.pone.0150058
51. Hanley GE, Rurak D, Lim K, Brain U, Oberlander TF. The impact of maternal positive and negative affect on fetal physiology and diurnal patterns. Isr J Psychiatry Relat Sci. (2014) 51:109–17.
52. Rallis S, Skouteris H, McCabe M, Milgrom J. A prospective examination of depression, anxiety and stress throughout pregnancy. Women Birth. (2014) 27:36–42. doi: 10.1016/j.wombi.2014.08.002
53. Bennett HA, Einarson A, Taddio A, Koren G, Einarson TRT. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol. (2004) 103:698–709. doi: 10.1097/01.AOG.0000116689.75396.5f
54. Mughal MK, Giallo R, Arnold P, Benzies K, Kehler H, Bright K, et al. Trajectories of maternal stress and anxiety from pregnancy to three years and child development at 3 years of age: findings from the All Our Families (AOF) pregnancy cohort. J Affect Disord. (2018) 234:318–26. doi: 10.1016/j.jad.2018.02.095
55. Glynn LM, Howland MA, Sandman CA, Davis EP, Phelan M, Baram TZ, et al. Prenatal maternal mood patterns predict child temperament and adolescent mental health. J Affect Disord. (2018) 228:83–90. doi: 10.1016/j.jad.2017.11.065
56. Sensky T, Cosci F, Brakemeier E-L, Jarrett RB, Rief W, Fava GA, et al. Methodological recommendations for trials of psychological interventions. Psychother Psychosom. (2018) 87:276–84. doi: 10.1159/000490574
57. Solhan MB, Trull TJ, Jahng S, Wood PK. Clinical assessment of affective instability: comparing EMA indices, questionnaire reports, and retrospective recall. Psychol Assess. (2009) 21:425–36. doi: 10.1037/a0016869
58. First MB, Spitzer RL, Gibbon M, Williams JBW. Structural Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition with Psychotic Screen. New York City, NY: Biometrics Research, New York State Psychiatric Institute (2002).
59. Batalas N, Markopoulos P. Introducing tempest, a modular platform for in situ data collection. In: Proceedings of the 7th Nordic Conference on Human-Computer Interaction Making Sense Through Design - NordiCHI'12. New York, NY: ACM Press (2012). p. 781.
60. Bockting C. Preventie Cognitieve Training bij terugkerende Depressie. Houten: Bohn Stafleu Van Loghum: Springer (2009). doi: 10.1007/978-90-313-7958-3
61. Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. (1988) 54:1063–70. doi: 10.1037/0022-3514.54.6.1063
62. Bergink V, Kooistra L, Lambregtse-van den Berg MP, Wijnen H, Bunevicius R, van Baar A, et al. Validation of the Edinburgh Depression Scale during pregnancy. J Psychosom Res. (2011) 70:385–9. doi: 10.1016/j.jpsychores.2010.07.008
63. Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br J Clin Psychol. (1992) 31:301–6. doi: 10.1111/j.2044-8260.1992.tb00997.x
64. Buckman JEJ, Underwood A, Clarke K, Saunders R, Hollon SD, Fearon P, et al. Risk factors for relapse and recurrence of depression in adults and how they operate: a four-phase systematic review and meta-synthesis. Clin Psychol Rev. (2018) 64:13–38. doi: 10.1016/j.cpr.2018.07.005
65. DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat Rev Neurosci. (2008) 9:788–96. doi: 10.1038/nrn2345
66. Bockting CLH, Spinhoven P, Koeter MWJ, Wouters LF, Visser I, Schene AH. Differential predictors of response to preventive cognitive therapy in recurrent depression: a 2-year prospective study. Psychother Psychosom. (2006) 75:229–36. doi: 10.1159/000092893
67. Fava GA, Belaise C. Discontinuing antidepressant drugs: lesson from a failed trial and extensive clinical experience. Psychother Psychosom. (2018) 87:257–67. doi: 10.1159/000492693
68. Cosci F, Chouinard G, Chouinard VA, Fava GA. The Diagnostic clinical Interview for Drug Withdrawal 1 (DID-W1) – New Symptoms of Selective Serotonin Reuptake Inhibitors (SSRI) or Serotonin Norepinephrine Reuptake Inhibitors (SNRI): inter-rater reliability. Dep Heal Sci. (2018) 6:285–495. doi: 10.1708/2891.29158
69. Banti S, Mauri M, Oppo A, Borri C, Rambelli C, Ramacciotti D, et al. From the third month of pregnancy to 1 year postpartum. Prevalence, incidence, recurrence, and new onset of depression. Results from the Perinatal Depression-Research & Screening Unit study. Compr Psychiatry. (2011) 52:343–51. doi: 10.1016/j.comppsych.2010.08.003
Keywords: pregnancy, preventive cognitive therapy, antidepressants, tapering and discontinuation, offspring, experience sampling methodology (ESM), proof-of-principle
Citation: Brouwer ME, Molenaar NM, Burger H, Williams AD, Albers CJ, Lambregtse-van den Berg MP and Bockting CLH (2020) Tapering Antidepressants While Receiving Digital Preventive Cognitive Therapy During Pregnancy: An Experience Sampling Methodology Trial. Front. Psychiatry 11:574357. doi: 10.3389/fpsyt.2020.574357
Received: 19 June 2020; Accepted: 25 September 2020;
Published: 22 October 2020.
Edited by:
Gayatri Saraf, University of British Columbia, CanadaReviewed by:
Paulomi Sudhir, National Institute of Mental Health and Neurosciences, IndiaCasimiro Cabrera Abreu, Queens University, Canada
Copyright © 2020 Brouwer, Molenaar, Burger, Williams, Albers, Lambregtse-van den Berg and Bockting. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudi L. H. Bockting, Yy5sLmJvY2t0aW5nQGFtc3RlcmRhbXVtYy5ubA==
†These authors share first authorship
 Marlies E. Brouwer
Marlies E. Brouwer Nina M. Molenaar3,4†
Nina M. Molenaar3,4† Huibert Burger
Huibert Burger Casper J. Albers
Casper J. Albers Mijke P. Lambregtse-van den Berg
Mijke P. Lambregtse-van den Berg