- 1Department of Neuroscience “Rita Levi Montalcini”, Azienda Ospedaliera Universitaria (AOU) Città della Salute e della Scienza, University of Turin, Turin, Italy
- 2Department of Mental Health, Azianda Sanitaria Locale (ASL) TO3 & Azienda Ospedaliera Universitaria (AOU) San Luigi Gonzaga, Orbassano, Italy
We aimed to evaluate in a sample of outpatients with schizophrenia (SCZ) the effectiveness of a cognitive remediation (CR) program (through the use of the Cogpack software) [computer-assisted CR (CACR)] in addition to standard therapy on cognitive outcomes as compared with that in a control active group (CAG) and to highlight a possible effect on social cognition (SC), metacognition, symptomatology, and real-world functioning. Of the 66 subjects enrolled, 33 were allocated to CACR and 33 to the CAG. Twenty-three patients in the CACR group and 25 subjects in the CAG completed at least 80% of the 48 prescribed CACR sessions, performed twice a week, for a total of 24 weeks of treatment. A significant time × group interaction was evident, suggesting that patients undergoing CACR intervention improved in specific metacognitive sub-functions (understanding others' mind and mastery), some cognitive domains (verbal learning processing speed, visual learning, reasoning, and problem solving) (h2 = 0.126), depressive symptoms, SC, awareness of symptoms, and real-world functioning domains (community activities and interpersonal relationships) more significantly than did patients undergoing CAG. The most noticeable differential improvement between the two groups was detected in two metacognitive sub-functions (understanding others' mind and mastery), in verbal learning, in interpersonal relationship, and in depressive symptomatology, achieving large effect sizes. These are encouraging findings in support of the possible integration of CACR in rehabilitation practice in the Italian mental health services.
Introduction
Cognitive deficits represent one of schizophrenia's (SCZ's) core features since they affect patients' functioning and are related to high levels of functional disability (1–4).
Moreover, cognitive impairment attenuates the potential benefit of rehabilitation programs, such as supported employment and social skills training-, even when high-quality rehabilitation is provided (5–7).
Since cognitive deficits of patients with SCZ respond only moderately to pharmacotherapy (8), cognitive remediation (CR) techniques have received an increasing development in the last 15 years.
At the Cognitive Remediation Experts Workshop (Florence, Italy, April 2010), CR has been defined as “a behavioral training based intervention that aims to improve cognitive processes (attention, memory, executive function, social cognition, or metacognition) with the goal of durability and generalization” (9).
It has proved to be an evidence-based approach for ameliorating cognitive impairment in SCZ as confirmed in different meta-analytic studies (9–12), which show significant effect-size impact on cognition (0.45) and daily functioning (0.36) (9, 10).
More specifically, Wykes et al. (9) found that cognitive outcomes improve with both drill-and-practice and strategic training; McGurk et al. (10) corroborated that drill or practice training alone has a larger effect on memory and verbal learning, when associated with strategic training. Lastly, Grynszpan et al. (11) proved smaller effect sizes on verbal memory, working memory, attention/vigilance, and speed of processing and a significant medium effect size for social cognition (SC), while general cognition provides positive results. Interestingly, it appears that effects of CR programs are “non-specific”; targeted cognitive domains did not reach higher improvement than those that were not specifically targeted.
Computer-assisted CR (CACR) effects on cognition have been widely analyzed in two recent meta-analyses. The first one (13) includes 24 studies specifically centered on computerized drill-and-practice training. This study demonstrated that, compared with a control condition, specific training obtains better results on attention, working memory, and positive and depressive symptoms. Furthermore, even if only marginally significant, small-to-moderate effects were noticed for verbal fluency, processing speed, memory, and verbal and visual learning. No evidence was found sustaining improvement in general condition, reasoning and problem solving, SC, and functional outcomes. The second one (14) evaluates CR treatment effect on cognitive, functional, and clinical outcomes in patients with SCZ from 67 studies and found significant improvements with small-to-moderate effect sizes in CR treatment on all of the three outcome domains and suggests that cognitive gains trigger improvement in both clinical symptoms and psychosocial functioning.
Thus, some discrepancies across studies about SCZ (15) were noticed regarding the improvement in community functioning and SC following CR, while an improvement in cognition seems to be commonly reported.
Therefore, in light of these considerations, the objective of the present study was to evaluate, in a sample of outpatients with SCZ, the effectiveness of a CR program (through the use of the Cogpack software) in addition to standard therapy on cognitive outcomes compared with a control active group (CAG) and to evaluate if there is any impact on SC, metacognition, symptomatology, and real-world functioning.
Materials and Methods
Subjects
The present study was conducted at the Department of Neuroscience “Rita Levi Montalcini,” University of Turin; Department of Neuroscience and Mental Health, Psychiatry Unit, AOU Città della Salute e della Scienza, Turin, Italy' and the Department of Mental Health, ASL TO3, Italy, between October 2017 and December 2019.
The trial was carried out in accordance with the Declaration of Helsinki of 1995 (as revised in Edinburgh in 2000) and was approved by the Local Ethical Committee on September 27, 2018 (Prot. N 00958893).
Inclusion Criteria
Patients, initially evaluated by a psychiatrist if they met the DSM-5 criteria for the diagnosis of SCZ, were subsequently visited by our research group. The patients examined were aged between 18 and 65 years. The study was conducted on a sample of outpatients with diagnosis of SCZ in a stable phase of illness.
Exclusion Criteria
The exclusion criteria were the following: (a) co-presence of a diagnosis of intellectual disability and learning disabilities; and (b) hospitalization in psychiatric facilities in the 6 months prior to evaluation and significant change of antipsychotic medications during the previous 3 months (according to clinical judgment). Participants were recruited through referrals from attending psychiatrists or clinical staff at the psychiatric medical facilities where the study was conducted.
Participants
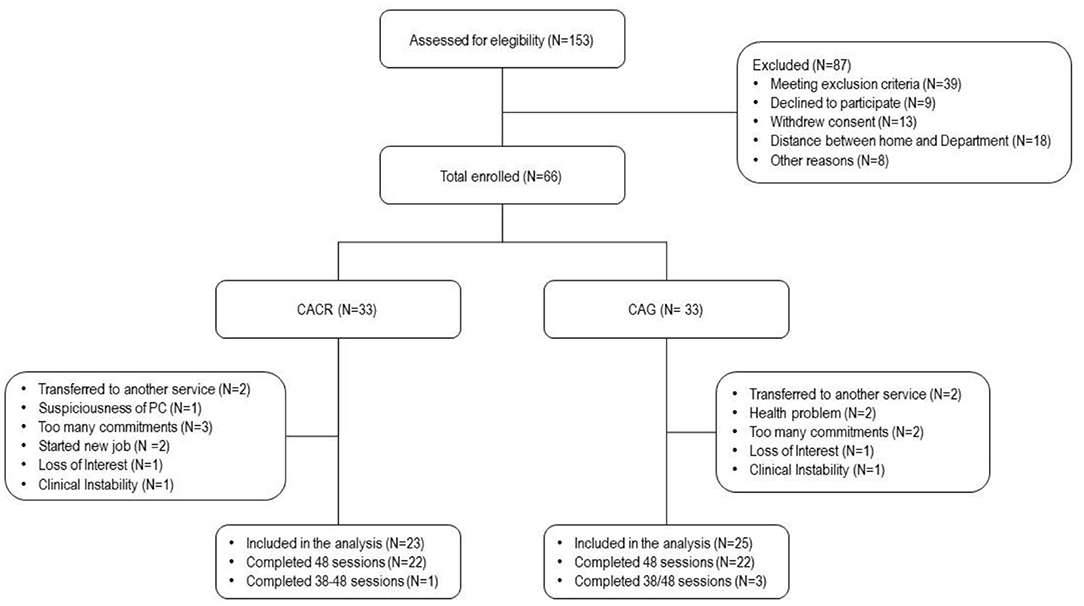
Of the 153 patients with SCZ attending the two sites, only a minority was approached for the study. Finally, 66 patients were invited to participate. Details of factors contributing to reduction of the number of potential participants are given in Figure 1.
Written informed consent was obtained from all subjects after a complete description of the study.
All patients were submitted to standard care provided in community mental health centers in Italy (pharmacological treatment, clinical monitoring at least on a monthly basis, and home care when required).
Interventions
All patients recruited in the trial were allocated to one of the two interventions, CACR (Cogpack), and CAG. In this trial, patients were considered completers if they attended at least 80% of the planned sessions.
When a participant decided not to continue or stopped the program session for at least 2 weeks or presented clinical relapse, the participant was considered to have dropped out. Reasons for discontinuation were registered.
Control Active Group
This condition was designed to control for non-specific treatment effects. It specified an equal number of one-on-one computer sessions with the same trainers who conducted the CACR sessions, using the same schedule as the treatment arm: 2-h biweekly sessions. It offered supportive trainer interactions and matched experience with computers and varied computer activities. Control activities were selected for game-like properties and low cognitive demand. Participants in this condition did not receive problem-solving training or guided practice on the exercises used in the remediation condition.
Cognitive Remediation Group
The CACR group received 48 sessions computerized rehabilitation using Cogpack software, performed twice a week, for a total of 24 weeks of treatment, in addition to standard therapy. CACR characteristics are described in another paper (16).
Patients were evaluated using a semi-structured interview to assess demographic and clinical features.
Psychiatric Assessment
All subjects were evaluated at baseline (T0), after 3 months (T1), and after 6 months (T2).
Patients were assessed by two psychiatrists (ED and CR). Neuropsychological tests were administered by a trained psychologist (LC) who was unaware of the patients' clinical characteristics or the results of the psychiatric rating scales. The battery was administered and scored according to the established procedures for each test the day after the psychiatric assessment. The raters were all masked with regard to the patient's treatment assignment, and patients were instructed not to reveal their treatment to the investigators. The interviewing psychiatrists were never members of the patients' treating team.
Clinical Assessment
The Positive and Negative Syndrome Scale (PANSS) (17) contains 30 items rated on 1 (absent) to 7 (extreme) scales. Three symptom domains are included in the scale: positive symptoms (seven items), negative symptoms (seven items), and general psychopathology (16 items). Symptoms are typically rated for the week immediately preceding the interview.
Depressive symptoms were evaluated using the Calgary Depression Scale for Schizophrenia (CDSS) (18).
The Scale to assess the Unawareness of Mental Disorder (SUMD) was used to assess illness insight (19). For the purpose of the present research, data analysis was primarily focused on current awareness and attribution of symptoms. All the scores are located in a Likert-type scale from 1 (good insight) to 5 (no insight). The insight of the patients was categorized as generally “preserved” or “impaired” on a threshold mean score of ≤ 3.0.
Cognitive Assessment
The MATRICS Consensus Cognitive Battery (MCCB) (20) consists of 10 measurement tools for recording the six neurocognitive areas and the social–cognitive domain of emotion perception. The six neurocognitive measures derive from scores of 10 cognitive measures: processing speed (Trail Making Test Part A; Brief Assessment of Cognition in Schizophrenia: symbol coding; Category Fluency Test: animal naming), attention/vigilance (Continuous Performance Test: identical pairs), working memory (Wechsler Memory Scale: spatial span subset; Letter Number Span Test), verbal learning (refers to immediate verbal memory, Hopkins Verbal Learning Test (HVLT)-Revised, immediate recall), visual learning (refers to immediate visual memory, Brief Visuospatial Memory Test-Revised), reasoning, and problem solving (Neuropsychological Assessment Battery (NAB), mazes subtest). The social–cognitive domain is assessed by the Mayer–Salovey–Caruso Emotional Intelligence Test (MSCEIT) (21), managing emotion section.
The Metacognition Assessment Scale (MAS) (22) was developed to quantitatively assess synthetic aspects of metacognition. MAS-A is a modification of the original scale made in 2004 that contains four scales that pertain to different foci of metacognitive acts: Self Reflectivity, or the comprehension of one's own mental states; Understanding of others' minds, or the comprehension of other individuals' mental states; Decentration, which is the ability to see the world as existing with others having independent motives; and Mastery, which is the ability to use one's mental states to implement effective action strategies in order to accomplish cognitive tasks or cope with psychological distress.
Real-World Functioning Assessment
The Specific Levels of Functioning Scale (SLOF) (23–25) assesses the patient's current functioning and behavior across the following six domains: physical functioning, personal care skills, interpersonal relationships, social acceptability, activities of community living, and work skills. The rate of each questions is included between 0 (the lowest level of functioning evaluated) and 5 (the maximum level of functioning evaluated) on a 5-point Likert scale, and the exact time frame that the survey attempts to assess is unspecified.
Research Design and Data Analysis
Analyses were planned in two stages.
In stage 1, we performed chi-square tests for categorical variables and the univariate analysis of variance (one-way ANOVA) for continuous variables, to examine whether the two groups differed in baseline demographic, clinical, neuropsychological, and functional variables. p-Values < 0.0083 (0.05/6) were considered significant.
In stage 2, pre-to-post treatment changes were analyzed with repeated-measures ANOVA entering neurocognitive, metacognitive, and functioning score subtests as the dependent variables; time as the fixed factor (with the three levels T0, T1, and T2); and treatment group as the independent variable. The significance of F test for group × time interaction will indicate if there are significant differences in outcomes between the two groups.
Size effect was assessed with partial η2. Values ranging between 0.01 and 0.06 were considered as small effect, values ranging between 0.07 and 0.14 were considered as medium effect, and values higher than 0.14 were considered as large effect (26).
The researchers ensured that there were no significant outliers in the related groups.
Statistical analyses were conducted using Statistical Package for the Social Sciences, SPSS, version 25 for Windows (SPSS, Chicago, IL, USA).
Results
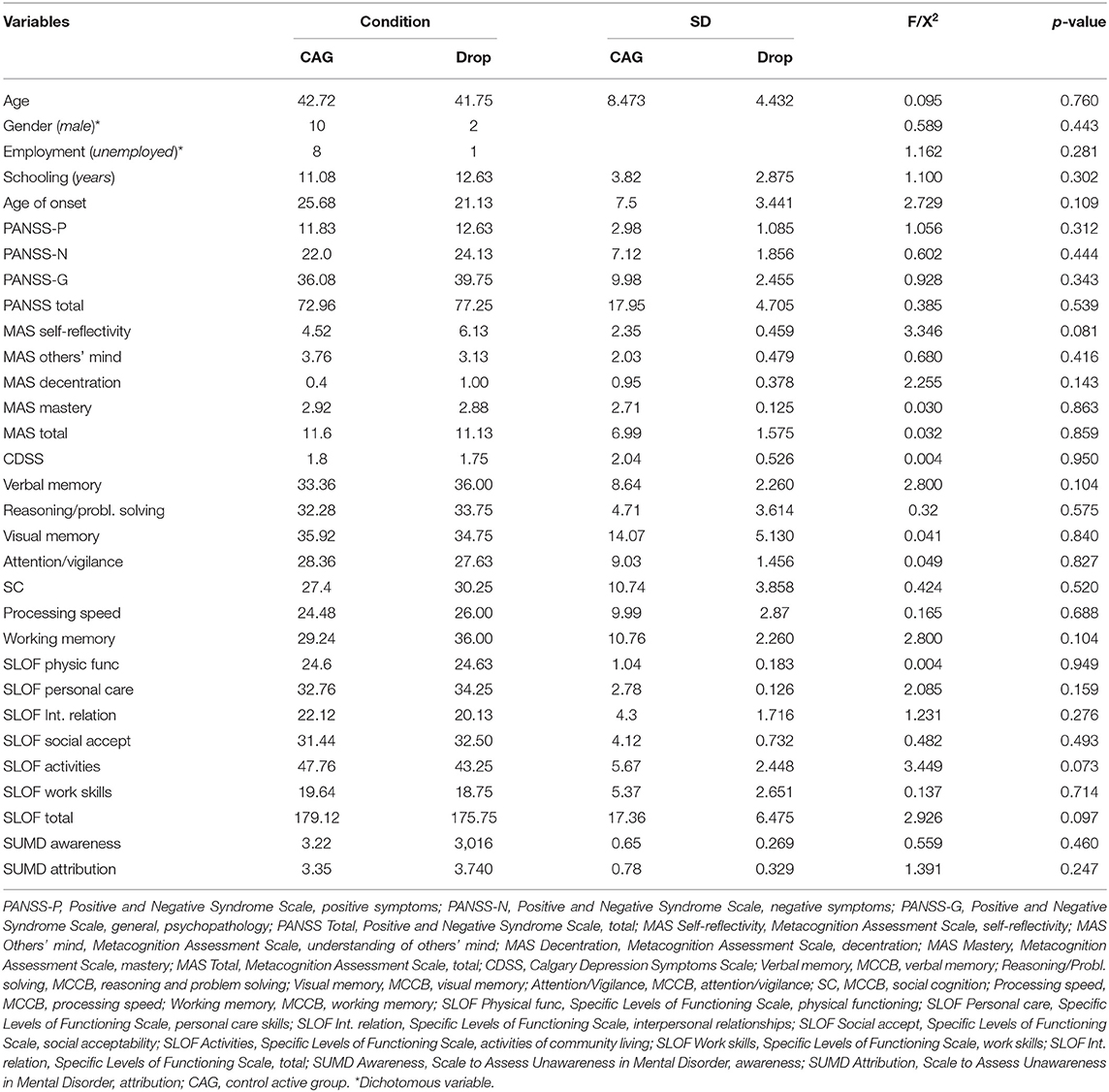
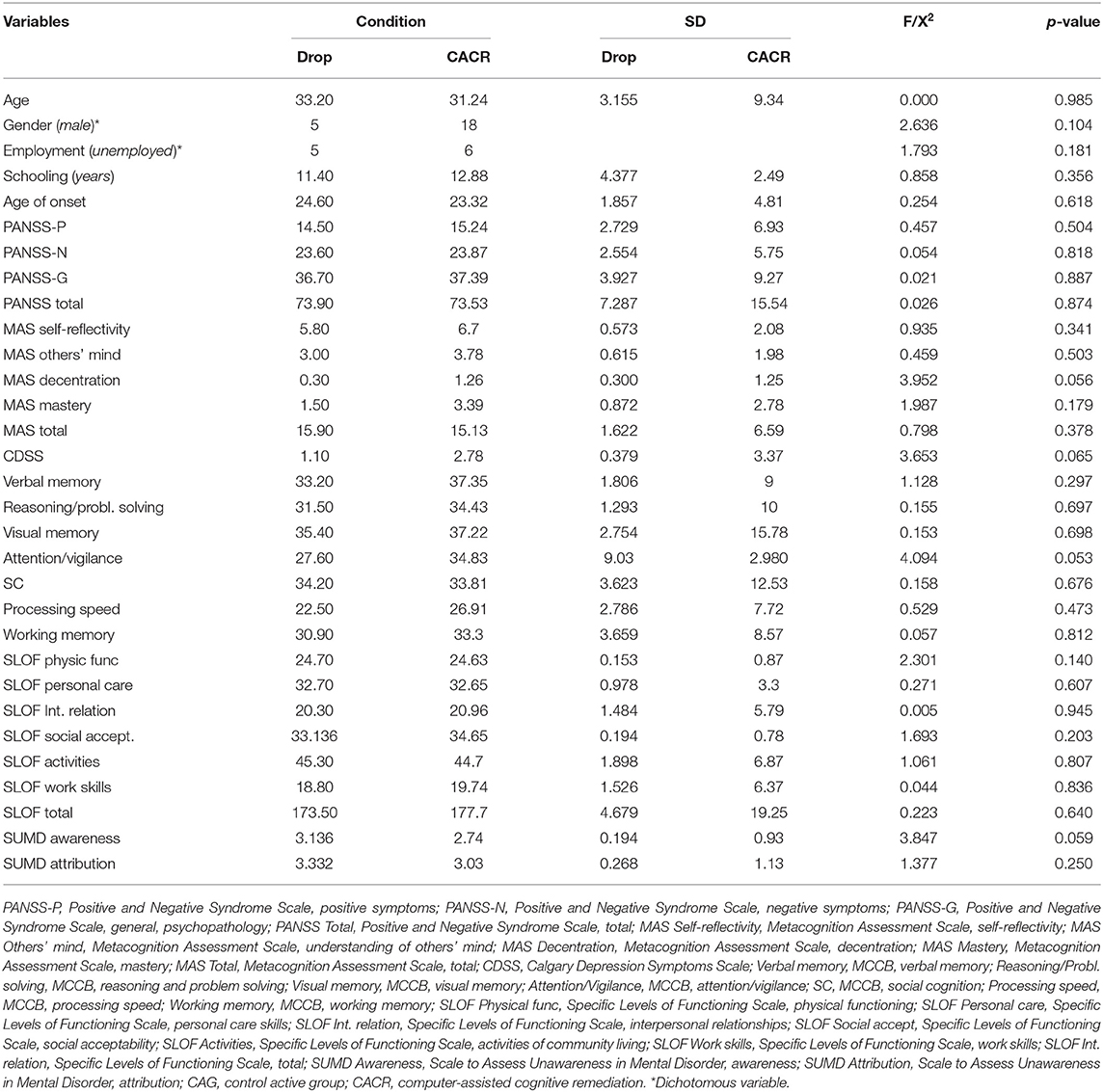
A total of 66 subjects were enrolled and provided consent to participate in the study. Thirty-three of these patients were allocated to CACR and 33 to the CAG. Ten patients dropped out from the CACR group and eight from the CAG. Therefore, the final sample included 23 patients in the CACR group and 25 subjects in the CAG. No statistical differences were found between dropouts and completers in both groups (Tables 1, 2).
The flow diagram in Figure 1 shows dropout reasons in the two groups.
Statistical analyses were performed on patients who completed the sessions of treatment.
All the demographical, clinical, neuropsychological, metacognitive, and functioning characteristics of the sample are reported in Table 3.
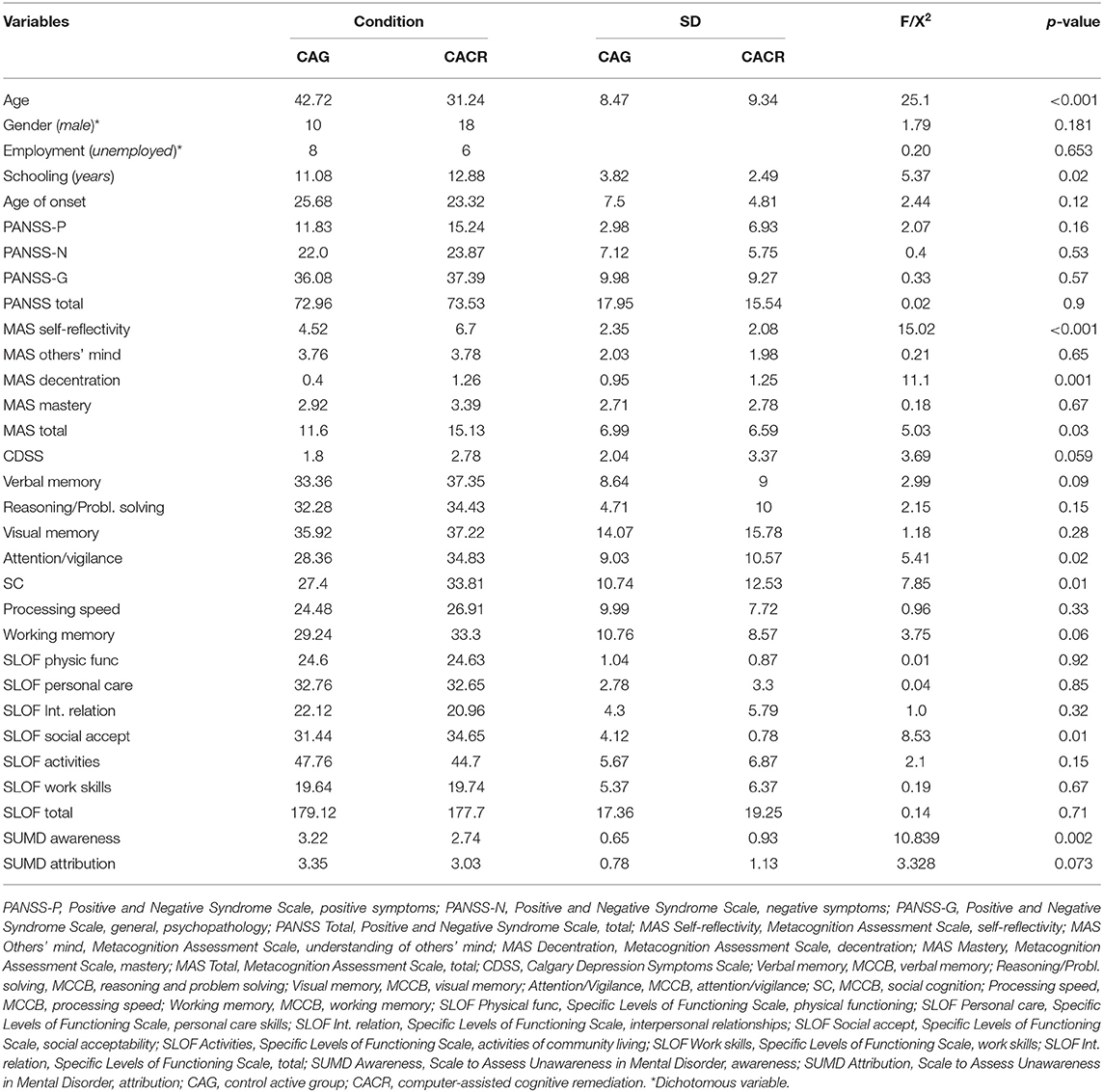
Table 3. Demographical, clinical, neuropsychological, metacognitive and functioning characteristics of the sample.
No statistically differences in gender distribution, clinical, and cognitive variables, and real-world functioning emerged between the two groups, except for age, MAS self-reflectivity, MAS decentration, as patients in the CACR group were younger, and had a higher level of metacognition (self-reflectivity and decentration) than those in the control group.
A significant improvement over trial duration (within-group effect) was observed for both treatments in verbal learning, MAS self-reflectivity, and MAS total score.
Moreover, patients in the CACR group demonstrated significantly greater improvements over 6 months than the control group (time × treatment interaction), with effect sizes ranging from medium to high levels for MAS others' mind (h2 = 0.436), verbal learning (h2 = 0.321), SLOF interpersonal relationship (h2 = 0.224), depressive symptoms (h2 = 0.215), SC (h2 = 0.183), MAS mastery (η2 = 0.178), processing speed (η2 = 0.143), visual learning (h2 = 0.131), reasoning and problem solving (h2 = 0.126), SLOF activities (h2 = 0.105), and awareness of symptoms (h2 = 0.063).
Table 4 presents scores for the neuropsychological, clinical, and functional outcomes at baseline and post-intervention.
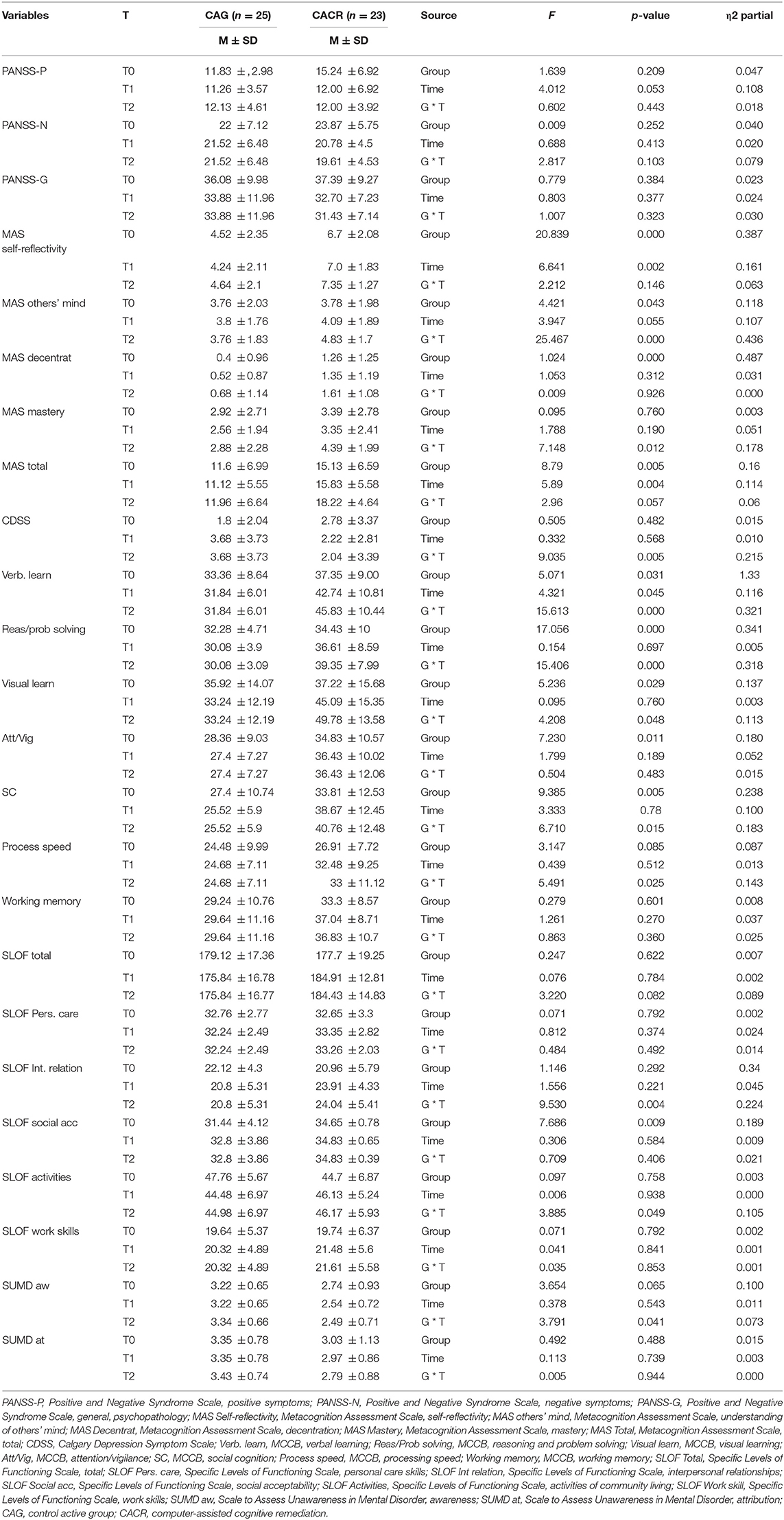
Table 4. Scores for the neuropsychological, clinical, and functional outcomes at baseline and post-intervention.
Discussion
Our study was aimed to assess the effectiveness of CACR vs. a CAG on specific cognitive, clinical, and functional domains in a sample of outpatients with SCZ. The CACR group was compared with an active experimental condition centered on non-specific elements of the remediation training, such as supportive therapist interactions and exposure to interesting computer activities. Outcomes were evaluated at three levels: proximally, on the remediation training exercises; intermediately, on neuropsychological measures not involved in the training; and more distally, on proxy measures of everyday functioning. Both groups were well-matched at baseline evaluation on demographic (except for age) and clinical confounding variables (i.e., metacognitive abilities) that might negatively impact outcome measures and might create a bias in study results. Furthermore, as indicated in a recent and relevant study, age does not appear to represent a significant moderator of effect (27).
There are several key observations from the study.
First, results showed a satisfactory adherence rate, with almost 70% of the participants completing the entire course of the protocol and adhering to over 80% of the prescribed sessions and outcome measures, suggesting that subjects did not seem to find the time commitment, the assessments, or the training burdensome. These completion rates are comparable with some studies (15, 28–30) and in contrast with other ones conducted so far (31), showing a higher attrition rate. Nevertheless, the present study adopted several measures to sustain adherence, mostly by strengthening the relationship with the therapist that has been shown to have a fundamental role in ensuring participants' completion of the training modules (32) and providing social cues to improve patients' self-esteem and motivation, supporting the use of strategies, motivation, or reinforcement and helping to develop metacognition, which is thought to be a key component for improving transfer from cognitive change to functional development (33, 34). Indeed, supportive, adaptive, and instructive trainers are thought to be a key ingredient of CACR programs and ultimately what makes them a therapeutic tool rather than a common “brain training,” being instrumental in achieving better training outcomes in CR (34, 35).
Second, while verbal learning and metacognitive sub-functions (understanding one's own mind and MAS total score) improved over assessment occasions, a significant time × group interaction was evident, suggesting that patients undergoing CACR intervention improved in domains related to specific cognitive domains (reasoning and problem solving, visual learning, verbal learning, and processing speed), understanding others' mind, mastery, SC, depressive symptoms, awareness of symptoms, and real-world functioning domains (community activities and interpersonal relationships) more significantly than did patients undergoing control active intervention.
Third, overall, the most noticeable differential improvement between the two groups were detected in two metacognitive sub-functions (understanding others' mind and mastery), in two cognitive domains (verbal learning and processing speed), in SLOF interpersonal relationship, in depressive symptomatology, and in SC, with effect sizes ranging from medium to high levels (see Table 1).
The positive findings regarding the amelioration of verbal and visual learning and reasoning and problem solving reported in the present study are consistent with the results of prior studies (13). The advantage of CR for verbal and visual learning support the possibility that at least some additional neurocognitive benefit may derive from an accurate titration of task difficulty of cognitive exercises to guarantee appropriate cognitive challenge, the rapid repetition of exacting exercises, and the frequency of reinforcement associated with achievement of intermediate and overall task goals characteristic of this condition. The hierarchical nature of the training program, starting with training in elementary attention skills and then gradually shifting to considerably more complex episodic and verbal memory tasks, might play a role in the advantage of this condition (36).
Thus, it is a paradox that there is no finding supporting an advantage of CACR on working memory (a skill related to holding information in mind and manipulating that information), while there is extensive evidence of CACR advantage in reasoning/executive-function and problem-solving domains. This result would not be expected because several studies have shown a close link between elementary working-memory functions and higher-level reasoning and problem-solving skills (37). Our results could be explained by the teaching strategies used by the therapist during and after CACR training sessions. Indeed, the one-to-one interaction with a therapist who can explicitly promote “bridging” strategies, as well as provide non-specific support and motivational coaching, represents important elements of the CACR therapy.
As for SC, we found a significant effect for this domain in the CACR group compared with the CAG. SC is the set of mental functions that allow individuals to interact with each other, i.e., the ability to understand, predict, and respond appropriately to the thoughts, feelings, and behavior of others in different and often unfamiliar social contexts (38, 39). The integrity of basic neuropsychological functions constitutes an essential prerequisite for the development of an adequate SC. Almost all published CACR studies agree that when coupled with rehabilitative programs focused on emotional intelligence, the benefits on recognition of emotions and the ability to interpret the feelings and behavior of others are improved (40, 41). However, the three studies included in the meta-analysis of Prikken et al. (13) did not detect a connection between CACR treatment and an improvement in emotional intelligence.
Alongside the improvement in neurocognitive functioning and SC obtained in the CACR group, we found an improvement in the metacognition (understanding others' mind and mastery) and insight (awareness of symptoms).
“Metacognition” identifies the main skills currently used to understand mental state and to properly attribute it to oneself or others. These skills include the ability to decipher the emotion expressions, to reason about mental states, and to use the obtained information to decide and solve problems and/or interpersonal conflicts. Finally, all these abilities also allow everyone to manage the subjective sufferance (42–44). It has been described as the memory processes that are related to the insights people have about their own and others' cognitive processes.
In order to develop an appropriate insight, metacognitive processes are fundamental because they allow individuals to notice changes in mental state over time and to produce plausible explanations for them, becoming more aware of their psychotic illness (45). Unfortunately, even if it is known that insight and metacognition are two integrative cognitive abilities, the exact neural and cognitive processes that support them are still unclear.
However, the great impairments in neurocognition (verbal, working, and visual memory; executive function; and overall intelligence) showed by patients with SCZ may be related to the severe deficits in metacognition (45–48). This impairment may lead to a lower ability to form complex mental representations of themselves and others (45, 46). In fact, SCZ patients with poor verbal memory might show more difficulties in remembering and integrating life experiences, even losing sense of previous experiences, with consequences in understanding the context complexity and their proper and others role on it (49).
Thus, the observed improvements in metacognitive skills and awareness of symptoms may be related to changes in neurocognitive functioning due to CACR in our study. Another possible explanation may be that the CACR group provided treatments in which patients' ability to reflect and to think about themselves was fostered to a greater extent than during a control active task. Indeed, all CACR sessions were facilitated by trained therapists who actively interacted with all participants to support their progression with the exercises, did troubleshooting, and spent time with participants on difficult exercises.
Moreover, participants showed significant improvements in two real-world functioning domains (interpersonal relationships and community activities) from baseline to 6-month follow-up with large effect sizes. The impact of CACR on psychosocial functioning is still controversial. A meta-analysis of Prikken et al. (13) found small-to-moderate but only marginally significant effects on functional outcomes. This finding is in contrast with other studies, also including strategy training, which showed larger and significant effects (9–11). It is plausible that CACR training alone might not be enough to improve daily functioning. Moreover, it has been observed that 6 months of treatment period might be too short to evaluate more differences in functioning between the two groups; probably, some changes in real-world functioning need a longer time to become evident, such as 1 year or more.
A plausible explanation of our results may be found on the fact that beneficial effects on verbal and visual memory may mediate the effect of CACR on social functioning; i.e., verbal memory has been considered to be associated with social competence (50) and daily functioning (51, 52); better visual–spatial memory after CACR may favor better skills in recognizing visual cues in social interactions and hence favor better social response and adaptation after CACR (53). Second, on the other hand, it appears that learning strategies could be a prerequisite for generalization of CACR treatment effects, explaining the reason why improvement in neurocognition does not automatically translate into improvement in social functioning (54, 55). Indeed, it was demonstrated that CACR can better influence functional outcomes when we give patients the opportunity to train cognitive skills in a context of a social learning environment through transferring skills from laboratory to real world (10, 55–57). According to this knowledge, our protocol study included a 10-min discussion with the therapist at the end of each CACR session in order to help in transferring learned abilities to the real world. Moreover, the differences in literature might depend on the different scales used to evaluate patients.
This study presents some limitations. First, the sample size is relatively small. Second, only patients in stable phase of disease were included, limiting generalization of the results toward the whole SCZ population. Third, there was no independent randomization. This clearly reduces methodological quality, although, current empirical evidence seems to indicate that non-randomized allocation does not influence effect sizes of cognitive training interventions (9). Fourth, the choice of the control intervention might represent another factor that theoretically has the potential to influence whether CACR is found to be effective or not (9). The CAG we employed engaged cognitive functions, not of a drill-and-practice type. A task with minimal demands on executive function was used, and while it does require memory skills, in this case, it involved procedural memory that is universally considered to be dissociable from episodic memory and trained in CACR (58). Furthermore, our control intervention therapeutic is unlikely to be considered because we found significant differences at the end of the treatment between the CACR and the CAG on some neuropsychological measures. Fifth, a factor that might have impacted the results of our study is that it was conducted in open units, where patients had the opportunity to go out to the community; this could have introduced uncontrolled variable, i.e., time spent in the community. Sixth, it could be argued that our study lacks enough power to detect differences between CACR and control group. It is a common issue in CACR trials, because detection of small effects requires large samples, and the recruitment of large group of patients is a great challenge, especially when trials are conducted in a single unit (59). Lastly, this study has not done a follow-up evaluation; for this reason, it lacks information about the duration of the outcomes.
Some strengths of this study deserve to be noticed, such as the well-matched baseline clinical, demographic, and cognitive characteristics of the two groups; the accurate assessment of cognitive functions, performed through the MCCB, and of real-world functioning, evaluated through the SLOF that is considered as the best scale to estimate psychosocial functioning of SCZ patients among those included in the VALERO program. Moreover, diagnoses were based on structured clinical interviews, and all patients were evaluated by trained raters. A further strength of this study is that the control group had the same number of hours of computer exposure as the treatment group. Lastly, another advantage is that there was no changing in pharmacological treatment during the study, allowing to specifically explain the effect of interventions.
In conclusion, if confirmed, our data point out the strength of CACR in implementing cognitive skills, SC, insight, metacognitive skills, and functioning in daily life in patients with SCZ. These are encouraging findings in support of the possible integration of CACR in rehabilitation practice in the Italian mental health services.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Comitato Etico Interaziendale, AOU Città della Salute e della Scienza, Torino. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CM: conceptualization, methodology, investigation, and writing – original draft. ED and CR: conceptualization, investigation, and writing – original draft. LC, MT, and EZ: conceptualization and validation. PR: conceptualization, methodology, investigation, and formal analysis. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Allott K, Liu P, Proffitt TM, Killackey E. Cognition at illness onset as a predictor of later functional outcome in early psychosis: systematic review and methodological critique. Schizophr Res. (2011) 125:221–35. doi: 10.1016/j.schres.2010.11.001
2. Bowie CR, Leung WW, Reichenberg A, McClure MM, Patterson TL, Heaton RK, et al. Predicting schizophrenia patients' real-world behavior with specific neuropsychological and functional capacity measures. Biol Psychiatry. (2008) 63:505–11. doi: 10.1016/j.biopsych.2007.05.022
3. Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. (2000) 26:119–36. doi: 10.1093/oxfordjournals.schbul.a033430
4. Seccomandi B, Tsapekos D, Newbery K, Wykes T, Cella M. A systematic review of moderators of cognitive remediation response for people with schizophrenia. Schizophr Res Cogn. (2019) 19:100160. doi: 10.1016/j.scog.2019.100160
5. Bell MD, Bryson G. Work rehabilitation in schizophrenia: does cognitive impairment limit improvement? Schizophr Bull. (2001) 27:269–79. doi: 10.1093/oxfordjournals.schbul.a006873
6. McGurk SR, Meltzer HY. The role of cognition in vocational functioning in schizophrenia. Schizophr Res. (2000) 45:175–84. doi: 10.1016/S0920-9964(99)00198-X
7. Wykes T. Predicting symptomatic and behavioural outcomes of community care. Br J Psychiatry. (1994) 165:486–92. doi: 10.1192/bjp.165.4.486
8. Choi KH, Wykes T, Kurtz MM. Adjunctive pharmacotherapy for cognitive deficits in schizophrenia: meta-analytical investigation of efficacy. Br J Psychiatry. (2013) 203:172–8. doi: 10.1192/bjp.bp.111.107359
9. Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. (2011) 168:472–85. doi: 10.1176/appi.ajp.2010.10060855
10. McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. (2007) 164:1791–802. doi: 10.1176/appi.ajp.2007.07060906
11. Grynszpan O, Perbal S, Pelissolo A, Fossati P, Jouvent R, Dubal S, et al. Efficacy and specificity of computer-assisted cognitive remediation in schizophrenia: a meta-analytical study. Psychol Med. (2011) 41:163–73. doi: 10.1017/S0033291710000607
12. Revell ER, Neill JC, Harte M, Khan Z, Drake RJ. A systematic review and meta-analysis of cognitive remediation in early schizophrenia. Schizophr Res. (2015) 168:213–22. doi: 10.1016/j.schres.2015.08.017
13. Prikken M, Konings MJ, Lei WU, Begemann MJH, Sommer IEC. The efficacy of computerized cognitive drill and practice training for patients with a schizophrenia-spectrum disorder: a meta-analysis. Schizophr Res. (2019) 204:368–74. doi: 10.1016/j.schres.2018.07.034
14. Kambeitz-Ilankovic L, Betz LT, Dominke C, Haas SS, Subramaniam K, Fisher M, et al. Multi-outcome meta-analysis (MOMA) of cognitive remediation in schizophrenia: revisiting the relevance of human coaching and elucidating interplay between multiple outcomes. Neurosci Biobehav Rev. (2019) 107:828–45. doi: 10.1016/j.neubiorev.2019.09.031
15. Bowie CR. Cognitive remediation for severe mental illness: state of the field and future directions. World Psychiatry. (2019) 18:274–5. doi: 10.1002/wps.20660
16. Montemagni C, Bracale N, Bellino S, Bozzatello P, Enrico Z, Toye M, et al. Cognitive deficits and psychosocial functioning in schizophrenia: role of computer-assisted cognitive remediation. Evid Based Psychiatr Care. (2019) 5:1–9.
17. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261
18. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. (1990) 3:247–51. doi: 10.1016/0920-9964(90)90005-R
19. Amador XF, Strauss DH. The Scale to assess Unawareness of Mental Disorder. New York, NY: Columbia University and New York Psychiatric Institute (1990).
20. Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am J Psychiatry. (2008) 165:203–13 doi: 10.1176/appi.ajp.2007.07010042
21. Mayer JD, Salovey P, Caruso D. Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT) User's Manual. Toronto, ON: Multi-Health Systems. Inc. (2002).
22. Semerari A, Carcione A, Dimaggio G, Falcone M, Nicolò G, Procacci M, et al. How to evaluate metacognitive functioning in psychotherapy? The metacognition assessment scale and its applications. Clin Psychol Psychother. (2003) 10:238–61. doi: 10.1002/cpp.362
23. Schneider LC, Struening EL. SLOF: a behavioral rating scale for assessing the mentally ill. Soc Work Res Abstr. (1983) 19:9–21. doi: 10.1093/swra/19.3.9
24. Mucci A, Rucci P, Rocca P, Bucci P, Gibertoni D, Merlotti E, et al. Italian network for research on psychoses. The specific level of functioning scale: construct validity, internal consistency and factor structure in a large Italian sample of people with schizophrenia living in the community. Schizophr Res. (2014) 159:144–50. doi: 10.1016/j.schres.2014.07.044
25. Montemagni C, Rocca P, Mucci A, Galderisi S, Maj M. Italian version of the “specific level of functioning”. Versione italiana della “specific level of functioning”. J Psychopath. (2015) 21:287–96.
26. Richardson JTE. Eta squared and partial eta squared as measures of effect size in educational research. Educ Res Rev. (2011) 6:135–47. doi: 10.1016/j.edurev.2010.12.001
27. Seccomandi B, Agbedjro D, Bell M, Keefe RSE, Keshavan M, Galderisi S, et al. Exploring the role of age as a moderator of cognitive remediation for people with schizophrenia. Schizophr Res. (2021) 228:29–35. doi: 10.1016/j.schres.2020.11.060
28. Klingberg S, Wölwer W, Engel C, Wittorf A, Herrlich J, Meisner C, et al. Negative symptoms of schizophrenia as primary target of cognitive behavioral therapy: results of the randomized clinical TONES study. Schizophr Bull. (2011) 37(Suppl 2):S98–110. doi: 10.1093/schbul/sbr073
29. Wykes T, Spaulding WD. Thinking about the future cognitive remediation therapy–what works and could we do better? Schizophr Bull. (2011) 37(Suppl 2):S80–90. doi: 10.1093/schbul/sbr064
30. Siu AMH, Ng RSH, Poon MYC, Chong CSY, Siu CMW, Lau SPK. Evaluation of a computer-assisted cognitive remediation program for young people with psychosis: a pilot study. Schizophr Res Cogn. (2020) 23:100188. doi: 10.1016/j.scog.2020.100188
31. Dillon R, Hargreaves A, Anderson-Schmidt H, Castorina M, Corvin A, Fitzmaurice B, et al. Adherence to a low-support cognitive remediation training program for psychosis. J Nerv Ment Dis. (2016) 204:741–5. doi: 10.1097/NMD.0000000000000557
32. Garrido G, Barrios M, Penadés R, Enríquez M, Garolera M, Aragay N, et al. Computer-assisted cognitive remediation therapy: cognition, self-esteem and quality of life in schizophrenia. Schizophr Res. (2013) 150:563–9. doi: 10.1016/j.schres.2013.08.025
33. Cella M, Reeder C, Wykes T. Cognitive remediation in schizophrenia—Now it is really getting personal. Curr Opin Behav Sci. (2015) 4:147–51. doi: 10.1016/j.cobeha.2015.05.005
34. Contreras NA, Lee S, Tan EJ, Castle DJ, Rossell SL. How is cognitive remediation training perceived by people with schizophrenia? A qualitative study examining personal experiences. J Ment Health. (2016) 25:260–6. doi: 10.3109/09638237.2016.1167856
35. Moura BM, Avila A, Chendo I, Frade P, Barandas R, Vian J, et al. Facilitating the delivery of cognitive remediation in first-episode psychosis: pilot study of a home-delivered web-based intervention. J Nerv Ment Dis. (2019) 207:951–7. doi: 10.1097/NMD.0000000000001055
36. Kurtz MM, Seltzer JC, Shagan DS, Thime WR, Wexler BE. Computer-assisted cognitive remediation in schizophrenia: what is the active ingredient? Schizophr Res. (2007) 89:251–60. doi: 10.1016/j.schres.2006.09.001
37. Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry. (1997) 54:159–65. doi: 10.1001/archpsyc.1997.01830140071013
38. Hogarty G, Flesher S. Developmental theory for a cognitive enhancement therapy of schizophrenia. Schizophr Res. (1999) 25:677–92. doi: 10.1093/oxfordjournals.schbul.a033410
39. Frith CD, Frith U. Social cognition in humans. Curr Biol. (2007) 17:R724–32. doi: 10.1016/j.cub.2007.05.068
40. Wölwer W, Frommann N, Halfmann S, Piaszek A, Streit M, Gaebel W. Remediation of impairments in facial affect recognition in schizophrenia: efficacy and specificity of a new training program. Schizophr Res. (2005) 80:295–303. doi: 10.1016/j.schres.2005.07.018
41. Lindenmayer JP, McGurk SR, Khan A, Kaushik S, Thanju A, Hoffman L, et al. Improving social cognition in schizophrenia: a pilot intervention combining computerized social cognition training with cognitive remediation. Schizophr Bull. (2013) 39:507–17. doi: 10.1093/schbul/sbs120
42. Carcione A, Nicolò G, Pedone R, Popolo R, Conti L, Fiore D, et al. Metacognitive mastery dysfunctions in personality disorder psychotherapy. Psychiatry Res. (2011) 190:60–71. (2010) 12.032. doi: 10.1016/j.psychres.2010.12.032
43. Brüne M, Abdel-Hamid M, Lehmkämper C, Sonntag C. Mental state attribution, neurocognitive functioning, and psychopathology: what predicts poor social competence in schizophrenia best? Schizophr Res. (2007) 92:151–9. doi: 10.1016/j.schres.2007.01.006
44. Tas C, Brown EC, Aydemir O, Brüne M, Lysaker PH. Metacognition in psychosis: comparison of schizophrenia with bipolar disorder. Psychiatry Res. (2014) 219:464–9. doi: 10.1016/j.psychres.2014.06.040
45. Lysaker PH, Carcione A, Dimaggio G, Johannesen JK, Nicolò G, Procacci M, et al. Metacognition amidst narratives of self and illness in schizophrenia: associations with neurocognition, symptoms, insight and quality of life. Acta Psychiatr Scand. (2005) 112:64–71. doi: 10.1111/j.1600-0447.2005.00514.x
46. Nicolò G, Dimaggio G, Popolo R, Carcione A, Procacci M, Hamm J, et al. Associations of metacognition with symptoms, insight, and neurocognition in clinically stable outpatients with schizophrenia. J Nerv Ment Dis. (2012) 200:644–7. doi: 10.1097/NMD.0b013e31825bfb10
47. Brune M. Theory of mind and the role of IQ in chronic disorganized schizophrenia. Schizophr Res. (2003) 60:57–64. doi: 10.1016/S0920-9964(02)00162-7
48. Doody GA, Götz M, Johnstone EC, Frith CD, Owens DG. Theory of mind and psychoses. Psychol Med. (1998) 28:397–405. doi: 10.1017/S003329179700648X
49. Leonhardt BL, Vohs JL, Bartolomeo LA, Visco A, Hetrick WP, Bolbecker AR, et al. Relationship of metacognition and insight to neural synchronization and cognitive function in early phase psychosis. Clin EEG Neurosci. (2020) 51:259–66. doi: 10.1177/1550059419857971
50. McClure MM, Bowie CR, Patterson TL, Heaton RK, Weaver C, Anderson H, et al. Correlations of functional capacity and neuropsychological performance in older patients with schizophrenia: evidence for specificity of relationships? Schizophr Res. (2007) 89:330–8 doi: 10.1016/j.schres.2006.07.024
51. Penadés R, Catalán R, Puig O, Masana G, Pujol N, Navarro V, et al. Executive function needs to be targeted to improve social functioning with Cognitive Remediation Therapy (CRT) in schizophrenia. Psychiatry Res. (2010) 177:41–5. doi: 10.1016/j.psychres.2009.01.032
52. Lanfredi M, Deste G, Ferrari C, Barlati S, Magni LR, Rossi R, et al. Effects of cognitive remediation therapy on neurocognition and negative symptoms in schizophrenia: an Italian naturalistic study. Cogn Neuropsychiatry. (2017) 22:53–68. doi: 10.1080/13546805.2016.1260537
53. Lindenmayer JP, Fregenti S, Kang G, Ozog V, Ljuri I, Khan A, et al. The relationship of cognitive improvement after cognitive remediation with social functioning in patients with schizophrenia and severe cognitive deficits. Schizophr Res. (2017) 185:154–60. doi: 10.1016/j.schres.2017.01.007
54. Lu H, Li Y, Li F, Jiao X, Shi W, Guo K, et al. Randomized controlled trial on adjunctive cognitive remediation therapy for chronically hospitalized patients with schizophrenia. Shanghai Arch Psychiatry. (2012) 24:149–54. doi: 10.3969/j.issn.1002-0829.2012.03.004
55. Medalia A, Saperstein AM. Does cognitive remediation for schizophrenia improve functional outcomes? Curr Opin Psychiatry. (2013) 26:151–7. doi: 10.1097/YCO.0b013e32835dcbd4
56. Nibbio G, Barlati S, Cacciani P, Corsini P, Mosca A, Ceraso A, et al. Evidence-based integrated intervention in patients with schizophrenia: a pilot study of feasibility and effectiveness in a real-world rehabilitation setting. Int J Environ Res Public Health. (2020) 17:3352. doi: 10.3390/ijerph17103352
57. Van Duin D, de Winter L, Oud M, Kroon H, Veling W, van Weeghel J. The effect of rehabilitation combined with cognitive remediation on functioning in persons with severe mental illness: systematic review and meta-analysis. Psychol Med. (2019) 49:1414–25. doi: 10.1017/S003329171800418X
58. Gomar JJ, Valls E, Radua J, Mareca C, Tristany J, del Olmo F, et al. Cognitive rehabilitation study group. A multisite, randomized controlled clinical trial of computerized cognitive remediation therapy for schizophrenia. Schizophr Bull. (2015) 41:1387–96. doi: 10.1093/schbul/sbv059
Keywords: schizophrenia, cognitive remediation, metacognition, social cognition, cognition
Citation: Montemagni C, Del Favero E, Riccardi C, Canta L, Toye M, Zanalda E and Rocca P (2021) Effects of Cognitive Remediation on Cognition, Metacognition, and Social Cognition in Patients With Schizophrenia. Front. Psychiatry 12:649737. doi: 10.3389/fpsyt.2021.649737
Received: 05 January 2021; Accepted: 09 June 2021;
Published: 09 July 2021.
Edited by:
Błażej Misiak, Wroclaw Medical University, PolandReviewed by:
Stefano Barlati, University of Brescia, ItalyGiacomo Deste, Civil Hospital of Brescia, Italy
Roberto Cavallaro, Vita-Salute San Raffaele University, Italy
Copyright © 2021 Montemagni, Del Favero, Riccardi, Canta, Toye, Zanalda and Rocca. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paola Rocca, cGFvbGEucm9jY2FAdW5pdG8uaXQ=
 Cristiana Montemagni
Cristiana Montemagni Elisa Del Favero
Elisa Del Favero Cecilia Riccardi1
Cecilia Riccardi1 Paola Rocca
Paola Rocca