- 1Department of Psychiatry, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran
- 2Sleep Disorders Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 3Institute of Medical Science and Technology, Shahid Beheshti University, Tehran, Iran
- 4Psychosis Research Center, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran
Insomnia disorder (ID) is a common illness associated with mood and cognitive impairments. Subtyping ID is an ongoing debate in sleep medicine, but the underlying mechanisms of each subtype is poorly understood. Growing evidence suggests that subcortical brain structures play the key roles in pathophysiology of ID and its subtypes. Here, we aimed to investigate structural alteration of subcortical regions in patients with two common ID subtypes i.e., paradoxical and psychophysiological insomnia. Fifty-five patients and 49 healthy controls were recruited for this study and T1-weighted images and subjective and objective sleep parameters (i.e., Pittsburgh Sleep Quality Index and polysomnography) were collected from participants. Subcortical structures including the hippocampus, amygdala, caudate, putamen, globus pallidus, nucleus accumbens, and thalamus were automatically segmented in FSL. Volume and shape (using surface vertices) of each structure were compared between the groups, controlled for covariates, and corrected for multiple comparisons. In addition, correlations of sleep parameters and surface vertices or volumes were calculated. The caudate's volume was smaller in patients than controls. Compared with controls, we found regional shrinkage in the caudate, nucleus accumbens, posterior putamen, hippocampus, thalamus, and amygdala in paradoxical insomnia and shrinkage in the amygdala, caudate, hippocampus, and putamen in psychophysiological insomnia. Interestingly, comparing two patients groups, shape alteration in the caudate, putamen, and nucleus accumbens in paradoxical insomnia and shrinkage in the thalamus, amygdala, and hippocampus in psychophysiological insomnia were observed. Both subjective and objective sleep parameters were associated with these regional shape alterations in patients. Our results support the differential role of subcortical brain structures in pathophysiology of paradoxical and psychophysiological insomnia.
Introduction
Insomnia disorder (ID) is characterized by problems in initiating or maintaining sleep, or early morning awakening. The daytime consequences are fatigue, mood disturbance, and cognitive impairment (1, 2). Definition of chronic ID, based on the third edition of International Classification of Sleep Disorders (ICSD-3) criteria (3, 4), requires insomnia symptoms to occur for at least three times per week and lasts for more than 3 months. Rising prevalence of ID (3.9–22.1%) is probably due to genetic and psychosocial factors including aging population, high level of stress, and increasing rate of depression and anxiety in the modern societies (1). In addition to a significant economic burden (5), ID is associated with elevated body-mass index (BMI), higher rate of cardiovascular diseases, increased amount of motor vehicle accidents, and various psychiatric comorbidities such as depression (6–11). Despite all the severe medical and mental consequences of ID, its pathophysiology is poorly understood.
Several neuroimaging studies revealed widespread structural and functional cortical changes (12–14) including gray matter atrophy in the orbitofrontal cortex, dorsolateral prefrontal, pericentral cortices, temporal cortex, and precuneus, but increased gray matter volume in the anterior cingulate cortex (ACC). For review see (13, 14). The role of subcortical brain regions in pathophysiology of ID was previously examined, although the results were inconsistent and difficult to replicate. For review see (13, 14). A surface-based shape analysis of 27 patients with ID revealed that poor sleep quality and higher arousal are associated with subcortical atrophy including the hippocampus, amygdala, basal ganglia, and thalamus that was linked with impaired cognitive functions (15). Gong et al. also found regional atrophy in the amygdala, which was related to the severity of insomnia and anxiety in ID patients (16). Moreover, the critical role of amygdala toward negative sleep-related stimuli (17), the role of hippocampus on sleep-related maladaptive rumination (18, 19), and the role of caudate on hyperarousal state of patients (20) have been observed in ID previously. These studies collectively point to an important role of subcortical brain regions in pathophysiology of ID. Recently, we performed a neuroimaging meta-analysis on 19 ID studies, but failed to identify convergent regional abnormality (21). This indicates that ID heterogeneity is not only due to the variant neuroimaging data acquisition and analysis methods, but also related to clinical variability of the patients e.g., including different ID subtypes (22, 23). Thus, there is a clear need for more detailed investigations of the brain structures on the well-characterized subtypes of ID.
The second version of ICSD introduced several ID subtypes such as paradoxical insomnia and psychophysiological insomnia (24). Paradoxical insomnia is characterized by subjective sleep loss and daily insomnia symptoms, but normal objective sleep profile [e.g., using polysomnography (PSG)], indicating a discrepancy between subjective and objective sleep patterns (25). On the other hand, psychophysiological insomnia is characterized by the “learned sleep-preventing association,” which indicates that pre-sleep condition appears to be classically conditioned to the bedroom environment and prevents sleep (26). Hence, paradoxical insomnia is defined by misperception of sleep, while psychophysiological insomnia is characterized by fear of sleep and bedroom environment. The ICSD-3 highlights that physiological abnormalities in sleep tracing are present in various subtypes, but they are often subtle and could not be detected by available routine sleep recording methods and hence, subtyping ID should be ignored in clinical practice (3). However, several studies support subtyping ID, using various neurophysiological, cognitive and psychological methods (22, 25, 27, 28). Recently, Blanken et al. applied a data-driven approach on a multidimensional set of biologically based traits in a large-scale population and identified five new ID subtypes (29). This study further suggests that ID should be considered as a heterogenic disorder (22) and subtyping may resolve inconsistencies to identify differential etiologies of ID (29).
In the present study, we explored possible structural alterations in the subcortical gray matter structure (i.e., the caudate, putamen, pallidum, nucleus accumbens, thalamus, amygdala, and hippocampus) using volume and shape analyses based on surface vertices. Our main question was whether there is any structural difference between two main ID subtypes and whether these changes are associated with insomnia symptoms. It was assumed that there are more structural alterations in the areas responsible for sleep perception and regulation of sleep-wake patterns in patients with paradoxical insomnia, while there are more changes in the regions responsible for sleep-related anxiety and hyperarousal in patients with psychophysiological insomnia.
Methods
Subjects
We recruited 116 participants in the study. Chronic ID patients were recruited from Sleep Disorders Research Center, Kermanshah University of Medical Sciences. All patients were interviewed by a sleep specialist (H.K.) and met diagnostic criteria of ID according to ICSD and psychiatric interview, overnight PSG, and Pittsburgh Sleep Quality Index (PSQI) before brain MRI acquisition. Healthy subjects were recruited through local advertisement and were defined as those with no neurological or psychiatric illness at present or past and total PSQI score <5. Our exclusion criteria included taking any neuropsychiatric medications, pregnancy, any other medical, neurological, or psychiatric conditions, as well as contraindications to MR imaging. The study was approved by Ethics Committee of Kermanshah University of Medical Sciences and written informed consent was obtained from all participants. Two patients with comorbid periodic leg movement, five patients with mild/moderate obstructive sleep apnea, one patient with hydrocephaly, two patients with brain mass, and two subjects with too much movements in the scanner (which caused distortion in the images) were excluded from the study. Finally, analyses were performed on 55 chronic ID patients (including 29 individuals with paradoxical insomnia and 26 patients with psychophysiological insomnia), as well as 49 healthy subjects.
Insomnia Assessments
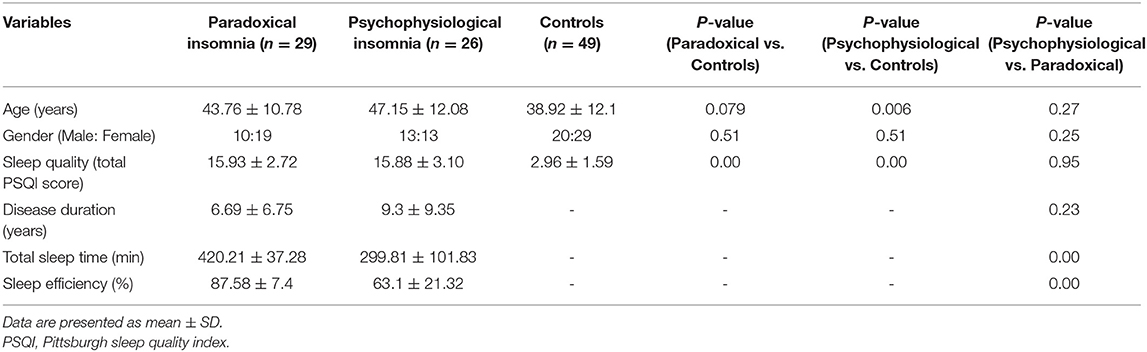
The patients were asked to avoid taking coffee, tea, heavy diet, and smoking during the day of the experiment. Our imaging assessment was carried out when the patients stopped taking any hypnotic medication for at least a week before imaging assessment. We excluded any patient, who was addicted to hypnotic medications such as benzodiazepines. The subjects arrived to the sleep center at 9 pm and completed the demographic and PSQI questionnaires. PSG measurements using SOMNOscreen™ plus model (Somnomedics, Germany) were performed at least 7 h based on the usual sleeping habits of subjects. Sleeping room was standardized for any noise and visual stimulus based on the international protocols (30). Diagnosis of ID subtypes was mainly based on ICSD-2 (24). All patients met diagnostic criteria for chronic ID based on ICSD-3 as well (3, 4), which are largely congruent with ICSD-2. They include a subjective report of sleep initiation or maintenance problems, adequate opportunity to sleep, as well as daytime consequences. In addition, insomnia symptoms were presented for at least three times per week and lasts for more than 3 months in our chronic ID groups. The insomnia symptoms were not associated with substance abuse and other psychiatric or sleep disorders. Paradoxical insomnia was diagnosed by the complaints of short sleep duration and poor sleep quality despite near-normal objective sleep patterns in PSG i.e., the misperception index ≥ 0.9 (31). Detailed criteria for paradoxical insomnia diagnosis include (i) subjective insomnia symptoms, but total sleep time (TST) > 6 h and 30 min and sleep efficiency (SE) > 85% using overnight PSG; (ii) discrepancy between objective (PSG) and subjective (self-report) sleep measures (i.e., a difference of 60 min or more for TST, or a difference of at least 15% for SE) (32). Psychophysiological insomnia were defined based on psychiatric interview, subjective insomnia symptoms, as well TST <6 h and 30 min and SE <85% (24), indicating that subjective and objective sleep assessment parameters are congruent in patients with psychophysiological insomnia. There is no difference on sleep quality, assessed by PSQI questionnaires, between the patients groups (p > 0.05) (Table 1).
MRI Acquisition and Quality Control
MRI was performed using a whole-body 1.5T Siemens Magnetom Avanto scanner with an 8- channel head coil. Structural images were acquired with a high-resolution, T1-weighted MPRAGE (TR = 1,950 ms, TE = 3.1 ms, flip angle = 15°, FOV = 256 × 256 mm2, matrix = 256 × 256 mm2, voxel size = 1 × 1 × 1 mm3, 176 sagittal slices). All images were visually checked by a radiologist to rule out any gross brain pathology. Quality control of data was carried out using the University of Southern California quality assurance pipeline (https://qc.loni.usc.edu/).
Segmentation of Subcortical Structures and Shape and Volume Analyses
The FIRST tool (part of FMRIB Software Library) was used to automatically segment seven subcortical brain structures including the caudate, putamen, pallidum, nucleus accumbens, thalamus, amygdala, and hippocampus in each hemisphere (33, 34). In brief, the FIRST is a probabilistic adaptation of the active appearance model. The method is informed by the shape and intensity variations of a structure from a training set for the purpose of automatically segmenting the structures. Surface of each structure is modeled by a deformable mesh, composed of a set of triangles and vertices. There are a fixed number of vertices with arbitrary positions for each structure. A multivariate Gaussian model of vertex location and intensity variation is used and is based on having point correspondence across subjects (same number and labeling of vertices across subjects). The necessary correspondence is imposed during the parameterization of the labeled images with a deformable model. The model is fit to new images by maximizing the posterior probability of shape given the observed intensities (33).
Between groups shape comparisons were carried out by comparing coordinates of each corresponding vertex, i.e., vertex-wise analysis, described in the FIRST tool (33, 34). We used the Randomize (part of FSL), which is a non-parametric permutation testing (10,000 permutations in our study) and allows modeling and inference using standard general linear model (GLM) design (35). We also compared the mean volume of subcortical regions between groups using GLM design in the FIRST. For both shape and volume analyses, we controlled for age, gender, and total brain volume as covariates of no-interest. For shape analysis, we used threshold-free cluster enhancement (TFCE) correction (36) to avoid arbitrary thresholds selection. Correction for multiple comparisons was applied using false discovery rate (FDR) correction (37). In patients, association between vertices and total PSQI/sleep efficiency was assessed using Spearman's rho two-tailed tests, with correction for covariates of no-interest (i.e., age, gender, and total brain volume) and multiple comparisons using FDR correction. Finally, for volume comparison, we used Least Significant Difference adjustment for multiple comparisons.
Results
Clinical Findings
Demographic data are presented in Table 1. No statistical differences were found for age (p = 0.079) or gender (p = 0.51) between healthy controls and paradoxical insomnia patients, while there were significant differences in age (p = 0.006), but not in gender (p = 0.51) between healthy controls and psychophysiological insomnia patients. The paradoxical and psychophysiological insomnia groups had no significant differences regarding age (p = 0.27) and gender (p = 0.25). In addition, paradoxical (p = 0.000) and psychophysiological (p = 0.000) insomnia patients had higher PSQI scores (poor sleep quality) than healthy controls, without any significant difference between two ID groups (p = 0.95). As expected, TST (p = 0.000) and SE (p = 0.000) were significantly better in paradoxical vs. psychophysiological insomnia patients. No statistical differences were found for duration of disease (p = 0.23) between two ID subtypes. Regarding total brain volume, healthy control subjects (1569.4 ± 22.5 cm3) had significantly larger brains than paradoxical (1447.3 ± 30.8 cm3) or psychophysiological insomnia groups (1485.7 ± 33.0 cm3) with p-values of 0.001 and 0.04, respectively. There was no significant difference between total brain volume of paradoxical and psychophysiological insomnia patients (p = 0.3).
Volumetric Findings of Subcortical Structures
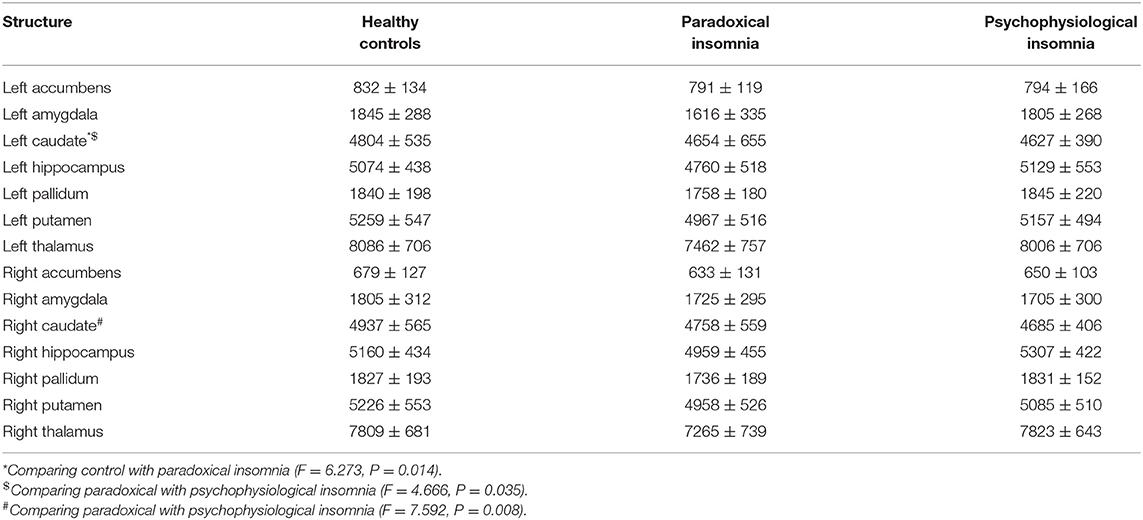
Mean volume of the left caudate was significantly smaller in the patient with paradoxical insomnia compared to psychophysiological insomnia, as well as to the control group. In addition, volume of bilateral caudate was different between paradoxical insomnia and psychophysiological insomnia (Table 2).
Vertex-Wise Shape Analysis Findings
Comparing paradoxical insomnia with healthy subjects, we found alterations in the anterior dorsal caudate, tail of left hippocampus, dorsal anterior thalamus, superior part of right amygdala, and anterolateral part of left putamen (p < 0.05, FDR corrected) (Figure 1A). Comparing psychophysiological insomnia with control individuals, we observed shrinkage in the anterior amygdala, dorsal part of right caudate, head and body of hippocampus (left tail > right tail), lateral part of putamen (right tail > left tail) (p < 0.05, FDR correction) (Figure 1B). Moreover, comparing two ID groups showed that bilateral hippocampus (head, ventral aspect of the body and dorsal aspect of the tail), dorsal thalamus, and anterior amygdala were shrunk in psychophysiological insomnia compared to paradoxical insomnia. In contrast, the caudate, putamen and nucleus accumbens (mainly the right side) showed relative shrinkage in paradoxical insomnia than psychophysiological insomnia (p < 0.05, FDR correction) (Figure 1C).
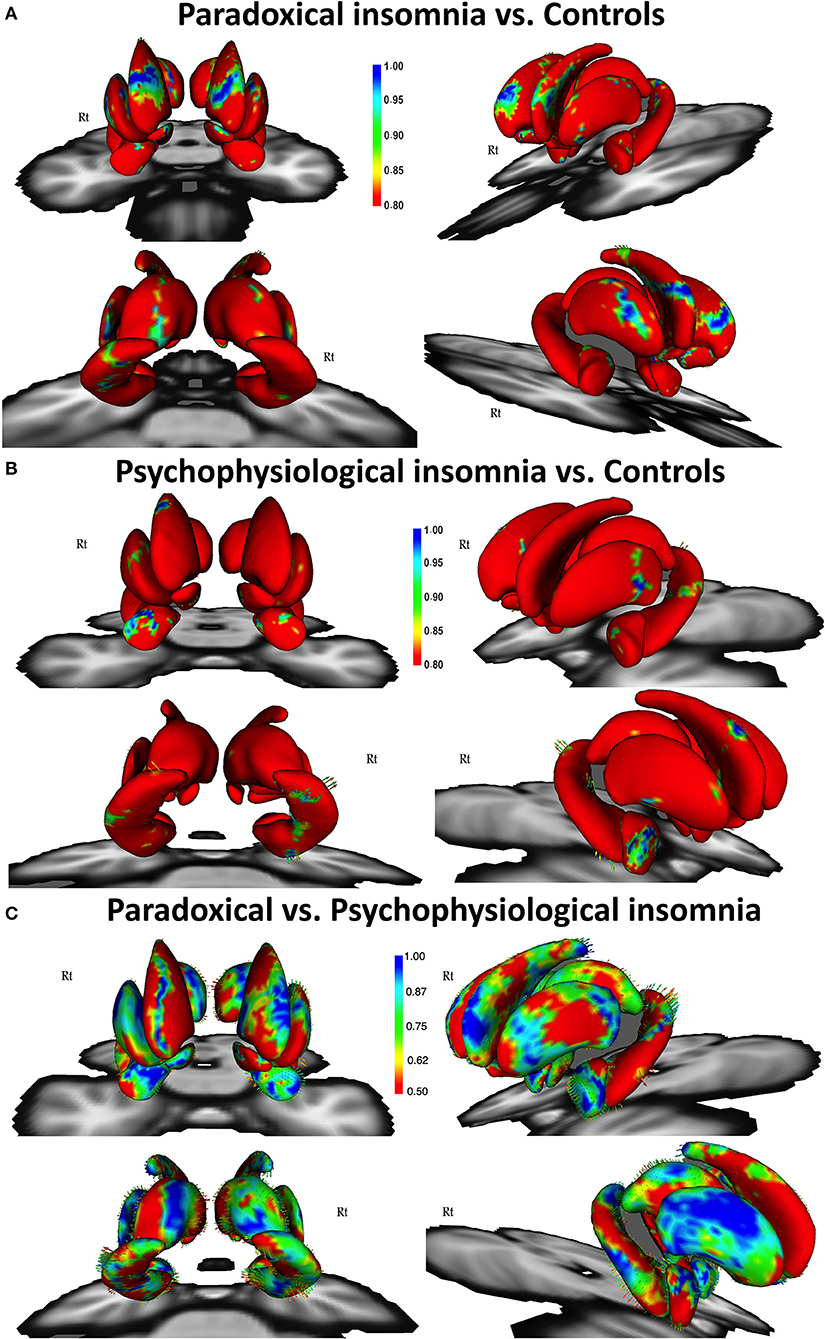
Figure 1. Vertex-wise surface analysis comparing patients with paradoxical insomnia vs. control group (A), patients with psychophysiological insomnia vs. control group (B), patients with paradoxical insomnia vs. psychophysiological insomnia (C) including covariates of no-interest (i.e., age, gender, and total brain volume). Color bar shows false discovery rate (FDR) corrected p-values.
Association Between Shape Abnormalities and Sleep Parameters
Patients with paradoxical insomnia, showed positive correlations between PSQI and shape changes in the putamen, nucleus accumbens, head and body of hippocampus (right tail > left tail), and anterior and posterior extremes of the caudate, but negative correlation with body of right caudate (Figure 2A). SE negatively correlated with dorsal and ventral part of the caudate, but positively correlated with head and tail of the caudate (right > left), nucleus accumbens, and posterior putamen (Figure 2B).
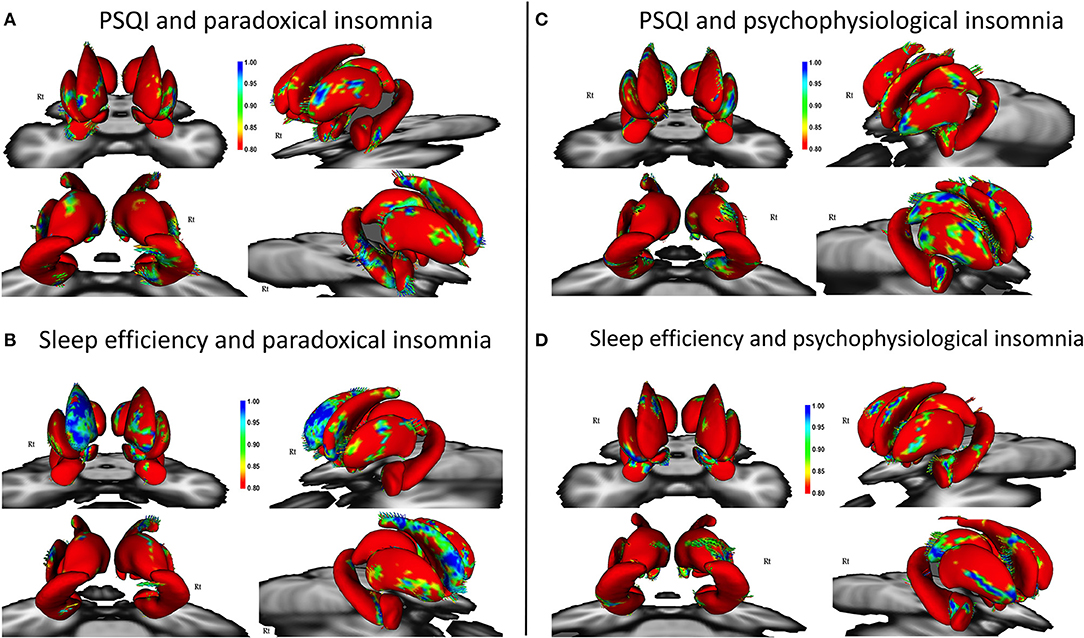
Figure 2. Correlation of Pittsburgh Sleep Quality Index (PSQI) and sleep efficiency with surface changes in patients with paradoxical insomnia (A,B); Correlation of PSQI and sleep efficiency with surface changes in patients with psychophysiological insomnia (C,D) including covariates of no-interest (i.e., age, gender, and total brain volume). Color bar shows false discovery rate (FDR) corrected p-values.
In patients with psychophysiological insomnia, PSQI scores were negatively correlated with the anterior amygdala, head and tail of caudate, head and body of hippocampus, and posterior dorsal thalamus, but positively correlated with the lateral part of putamen (Figure 2C). In addition, SE was positively correlated with shape changes in the anterior amygdala, right dorsal putamen and caudate, as well as inferior part of the hippocampus and posterior dorsal left thalamus, but negatively correlated with lateral part of the putamen (Figure 2D).
Discussion
ID is clinically a heterogeneous disorder and current ID literature indicates remarkable inconsistencies in terms of clinical features and treatment response (22, 29), which suggests that a bottom-up classification of ID should be reconsidered in sleep medicine (22, 25). Recently, a data-driven approach using multivariate profiles of affect, personality, and life history of 2,224 participants with ID and 2,098 controls was performed (29). Their result identified five new ID subtypes including highly distressed, moderately distressed but reward sensitive, moderately distressed and reward insensitive, slightly distressed with high reactivity, and slightly distressed with low reactivity (29). Previously, Turcotte et al. using event-related potentials measures demonstrated that psychophysiological insomnia had inability to inhibit information processing during sleep onset, but paradoxical insomnia showed enhanced attentional processing, which results in a higher need for inhibition (38). The present study suggests structural difference, not only between ID and healthy subjects, but also between paradoxical and psychophysiological insomnia, indicating importance of previously suggested subtypes in ICSD-2 for ID. In particular, we demonstrated that subcortical brain areas of patients with two ID subtypes undergo different structural alterations, and these changes are associated with both subjective and objective sleep-related measures.
Given the fact that the caudate nucleus showed atrophy and shrinkage in paradoxical insomnia (and not in psychophysiological insomnia), our shape and volumetric findings indicate a critical role of caudate in subjective-objective sleep discrepancy in paradoxical insomnia (25, 27, 39). A typical patient with paradoxical insomnia often feels that s/he has not slept enough, but polysomnographic data shows normal sleep duration. Our notion is in support of a crucial function of the caudate in pathophysiology of ID (20), particularly those with paradoxical insomnia who have sleep-state misperception (39). There is considerable evidence that the striatum (including caudate and putamen) plays an important role in sleep behavior. The caudate receives input from the orbitofrontal and parietal cortices, and the putamen receives input from the somatosensory, primary motor, and premotor cortices (40). Structural alterations in the cortically connected area such as the orbitofrontal cortex to the caudate has been reported previously in ID (13). The basal ganglia are recognized for disparate functions not only regulating movements, cognitive, affective and somatosensory functions, but also regulating sleep-wake patterns (40, 41). Indeed, dorsal striatum augments wakefulness and nucleus accumbens regulates sleep-wake pattern by promoting sleep (41). Sleep disturbances contribute to the striatal dopamine levels too. Adenosine and dopamine receptors in the ventral striatum promote wakefulness by motivational behavior, but locomotor and arousal systems are inhibited during sleep (41). Stoffers et al. demonstrated an impaired recruitment of the head of left caudate nucleus during executive functioning, which was associated with hyperarousal severity in ID patients (20). This may be predispose and perpetuate hyperarousal and insomnia. However, cognitive behavioral therapy for insomnia (CBT-I) did not normalize the observed hypoactivation of the caudate during the executive task performance (20). Literature is lacking for the specific role of the putamen in ID, but shrinkage in the putamen in obstructive sleep apnea and rapid eye movement (REM) sleep behavior disorder, and also increased synaptic dopamine in putamen in restless legs syndrome have been reported earlier (42–44). These findings collectively point out to the critical role of the striatum including the caudate and putamen in pathophysiology of ID, particularly paradoxical insomnia.
On the other hand, patients with psychophysiological insomnia revealed that poor subjective sleep quality (i.e., higher total PSQI score) was associated with regional shrinkage of thalamus, amygdala, hippocampus, putamen and caudate. In addition, low sleep efficiency was linked with shrinkage in the thalamus, amygdala, hippocampus, caudate, and dorsal putamen, as well as hypertrophy in the right posterior thalamus. Comparing two patients groups showed that although various subcortical regions undergo structural changes in psychophysiological insomnia, it is mainly associated with shrinkage in thalamus, amygdala, and hippocampus. This is in line with the hyperarousal model of insomnia and emotional memory impairment in ID (14, 45). This model suggests that ID is characterized by increased arousal at the physiological, endocrine, cognitive or emotional levels and increased amygdala activity (17). Importantly, poor subjective and objective sleep quality is linked with enlargement of lateral putamen, which might be a compensatory mechanism to dysfunction of the limbic circuits. Similar to our findings, Koo et al. found an association between subcortical atrophic shape abnormalities in the thalamus, amygdala, hippocampus, and basal ganglia in a group of ID patients and such patterns was associated with cognitive decline in those patients (15).
The amygdala plays a key role in processing negative emotional arousal and fear-inducing stimuli and therefore is involved in the hyperarousal model of ID (14, 45). Gong et al. also reported atrophy in the left superficial and right basolateral nucleus of amygdala, the association of insomnia severity with shape of the right centromedial nucleus, and the link between anxiety and shape of the basolateral nucleus of amygdala (16). Beside structural changes, functional imaging studies demonstrated that the amygdala response to insomnia-related stimuli is more robust in ID than healthy controls with lower habituation (17). Furthermore, decreased functional connectivity between amygdala and insula, striatum, and thalamus, as well as increased functional connectivity of amygdala with premotor and sensorimotor cortex in ID have been reported (46). Impaired connectivity and dysfunction of the amygdala due to emotional processing is a shared phenomenon in major depressive disorder (MDD) and ID (9, 17, 46). It has been demonstrated that amygdala volume is larger in MDD patients with insomnia symptoms compared to MDD patients without insomnia, regardless of the depression subtype (e.g., melancholic or psychotic) (9, 47).
Similar to the current study, hippocampal atrophy was previously reported in patients with ID (13). It has been revealed that distinct connectivity patterns of anterior and posterior hippocampus involve in memory processing and encoding success (48, 49). A multimodal parcellations and behavioral decoding of hippocampal sub-regions demonstrated a head–body and tail partition, subdivided along the anterior–posterior and medial–lateral axis and behavioral analyses suggested an emotion–cognition gradient along the anterior–posterior axis (50). According to our results, major hippocampal abnormality was observed within the head and body of the hippocampus. Previously, Joo et al. found that patients with ID had bilateral atrophy in the body and tail of hippocampus (i.e., CA2 and DG), which was associated with impaired cognitive functions, as well as in the head of hippocampus (i.e., CA1), which is associated with poor sleep quality (51). Some studies identified disruption of hippocampal functional connectivity within the default mode network (DMN) in ID (52). In particular, enhanced functional connectivity between the retrosplenial cortex/hippocampus and different hubs of the DMN is reported in ID previously (52). CBT-I normalized DMN hyperactivity and improved symptoms and quality of life of patients with psychophysiological insomnia (53). A meta-analysis found that patients with ID consistently have poor daily performance in several cognitive functions including working memory, episodic memory, and problem solving, which may be related to hippocampal dysfunction in ID (54).
The figure of thalamus in sleep regulation is well-established as well (55, 56). The anterior and dorsomedial nuclei of thalamus are responsible for organization of wake-sleep pattern, and affect pineal melatonin production and secretion (57). Thalamic lesions cause severe and persistent insomnia in the animal models (57). Severe impairment in mitochondrial function, protein synthesis, and neuronal loss in mediodorsal thalamus has been observed in fatal familial insomnia (58). Few studies on ID demonstrated structural and functional abnormalities in the thalamus. For example, patients with ID showed thalamic atrophy, as well as disruption of thalamus's functional connectivity with the ACC, orbitofrontal cortex, hippocampus, caudate, and putamen which were negatively correlated with PSQI score (59). Kim et al. observed cortical and thalamic hyperactivity in response to sleep-related tasks in psychophysiological insomnia (60). These studies indicate maladaptive role of amygdala, hippocampus, and thalamus in pathophysiology of ID, mainly in psychophysiological subtype.
Limitations
The results of this study should be interpreted with cautious, due to several potential limitations. Small sample size together with potential variability in shape analysis may over-estimate some structural alterations or miss some others. A stronger magnetic field e.g., 3T or 7T would increase signal to noise of the images and therefore increase the accuracy of subcortical segmentations. Moreover, in the current study, mean age of patients were higher than healthy subjects and it has been demonstrated that age has an important effect in gray matter structures in subjects with sleep disturbances (61, 62). Smaller total brain size could be argued to contribute in the group difference reported in this work. However, the effect of any total brain atrophy associated with normal aging would be diminished when age was taken as a covariate of no-interest, as applied in our analysis. In fact, total brain volume was lower in paradoxical than in psychophysiological insomnia patients. This argues that perhaps there is biological effect in paradoxical insomnia, which is associated with brain atrophy beyond what is normally seen in aging. Thus, although we included age as covariates of no-interest in all analyses, careful matching of the groups and controlling for the effects of comorbid anxiety and depression should be considered in the future studies. Combination of structural assessment with functional MRI and/or positron emission tomography (PET) using a multimodal approach could further enlighten the role of subcortical structures in pathophysiology of ID and its subtypes. Unfortunately, such tools were not available in our center at the time of study. Further longitudinal studies with high magnetic fields and molecular imaging techniques in a larger cohort are needed to endorse our findings.
Conclusion
The present work demonstrated structural alterations in the amygdala, hippocampus, corpus striatum, and thalamus in ID. Shape alterations were prominent in the caudate, putamen, and nucleus accumbens in paradoxical insomnia and were noticeable in the thalamus, amygdala, and hippocampus in psychophysiological insomnia. The volume of caudate was different between ID patients and controls, as well as between ID subtypes. The structural changes are associated with subjective and objective sleep symptoms and support different neurobiological mechanisms between paradoxical and psychophysiological insomnia. This study highlights the need for classification of ID and may have a great impact on clinical trials and developing better treatment for ID in future. Clearly, we need global sharing of multimodal imaging-genetic data using world-wide initiatives like ENIGMA-Sleep (http://enigma.ini.usc.edu/ongoing/enigma-sleep/) in sleep medicine (63).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Kermanshah University of Medical Sciences. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
FE, MT, MK-A, SM, HK, and MZ contributed to design and conceptualization of the idea. FE, KN, and MR collected data. MM and MZ analyzed data. All authors drafted the manuscript and revised the manuscript for intellectual content.
Funding
This work was supported by the Sleep Disorders Research Center of the Kermanshah University of Medical Sciences and the University of Social Welfare and Rehabilitation Sciences.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are thankful to the participants of the study and the staff of the Sleep Disorders Research Center for their help in recruitment and data collection.
References
1. Morin CM, Drake CL, Harvey AG, Krystal AD, Manber R, Riemann D, et al. Insomnia disorder. Nat Rev Dis Primers. (2015) 1:15026. doi: 10.1038/nrdp.2015.26
2. Riemann D, Nissen C, Palagini L, Otte A, Perlis ML, Spiegelhalder K. The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol. (2015) 14:547–58. doi: 10.1016/S1474-4422(15)00021-6
3. Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. (2014) 146:1387–94. doi: 10.1378/chest.14-0970
4. Aaos M. International Classification of Sleep Disorders—Third Edition (ICSD-3). Westchester, IL: AASM Resource Library. (2014).
5. Daley M, Morin CM, Leblanc M, Grégoire JP, Savard J. The economic burden of insomnia: direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep. (2009) 32:55–64. doi: 10.5665/sleep/32.1.55
6. Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. (2007) 3:S7–10. doi: 10.5664/jcsm.26929
7. Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Preventiv Cardiol. (2014) 21:57–64. doi: 10.1177/2047487312460020
8. Garbarino S, Magnavita N, Guglielmi O, Maestri M, Dini G, Bersi FM, et al. Insomnia is associated with road accidents. Further evidence from a study on truck drivers. PLoS ONE. (2017) 12:e0187256. doi: 10.1371/journal.pone.0187256
9. Bagherzadeh-Azbari S, Khazaie H, Zarei M, Spiegelhalder K, Walter M, Leerssen J, et al. Neuroimaging insights into the link between depression and Insomnia: a systematic review. J Affect Disord. (2019) 258:133–43. doi: 10.1016/j.jad.2019.07.089
10. Emamian F, Khazaie H, Okun ML, Tahmasian M, Sepehry AA. Link between insomnia and perinatal depressive symptoms: a meta-analysis. J Sleep Res. (2019). 28:e12858. doi: 10.1111/jsr.12858
11. Tahmasian M, Samea F, Khazaie H, Zarei M, Kharabian Masouleh S, Hoffstaedter F, et al. The interrelation of sleep and mental and physical health is anchored in grey-matter neuroanatomy and under genetic control. Commun Biol. (2020) 3:171. doi: 10.1038/s42003-020-0892-6
12. Riemann D, Kloepfer C, Berger M. Functional and structural brain alterations in insomnia: implications for pathophysiology. Eur J Neurosci. (2009) 29:1754–60. doi: 10.1111/j.1460-9568.2009.06721.x
13. Spiegelhalder K, Regen W, Baglioni C, Nissen C, Riemann D, Kyle SD. Neuroimaging insights into insomnia. Curr Neurol Neurosci Rep. (2015) 15:9. doi: 10.1007/s11910-015-0527-3
14. Schiel JE, Holub F, Petri R, Leerssen J, Tamm S, Tahmasian M, et al. Affect and arousal in insomnia: through a lens of neuroimaging studies. Curr Psychiatry Rep. (2020) 22:44. doi: 10.1007/s11920-020-01173-0
15. Koo DL, Shin JH, Lim JS, Seong JK, Joo EY. Changes in subcortical shape and cognitive function in patients with chronic insomnia. Sleep Med. (2017) 35:23–6. doi: 10.1016/j.sleep.2017.04.002
16. Gong L, Liao T, Liu D, Luo Q, Xu R, Huang Q, et al. Amygdala changes in chronic insomnia and their association with sleep and anxiety symptoms: insight from shape analysis. Neural Plasticity. (2019) 2019:8549237. doi: 10.1155/2019/8549237
17. Baglioni C, Spiegelhalder K, Regen W, Feige B, Nissen C, Lombardo C, et al. Insomnia disorder is associated with increased amygdala reactivity to insomnia-related stimuli. Sleep. (2014) 37:1907–17. doi: 10.5665/sleep.4240
18. Riemann D, Voderholzer U, Spiegelhalder K, Hornyak M, Buysse DJ, Nissen C, et al. Chronic insomnia and MRI-measured hippocampal volumes: a pilot study. Sleep. (2007) 30:955–8. doi: 10.1093/sleep/30.8.955
19. Leerssen J, Wassing R, Ramautar JR, Stoffers D, Lakbila-Kamal O, Perrier J, et al. Increased hippocampal-prefrontal functional connectivity in insomnia. Neurobiol Learn Mem. (2018) 160:144–50. doi: 10.1016/j.nlm.2018.02.006
20. Stoffers D, Altena E, Van Der Werf YD, Sanz-Arigita EJ, Voorn TA, Astill RG, et al. The caudate: a key node in the neuronal network imbalance of insomnia? Brain. (2014) 137:610–20. doi: 10.1093/brain/awt329
21. Tahmasian M, Noori K, Samea F, Zarei M, Spiegelhalder K, Eickhoff SB, et al. A lack of consistent brain alterations in insomnia disorder: an activation likelihood estimation meta-analysis. Sleep Med Rev. (2018) 42:111–8. doi: 10.1016/j.smrv.2018.07.004
22. Benjamins JS, Migliorati F, Dekker K, Wassing R, Moens S, Blanken TF, et al. Insomnia heterogeneity: characteristics to consider for data-driven multivariate subtyping. Sleep Med Rev. (2017) 36:71–81. doi: 10.1016/j.smrv.2016.10.005
23. Tahmasian M, Zarei M, Noori K, Khazaie H, Samea F, Spiegelhalder K, et al. Reply to Hua Liu, HaiCun Shi, and PingLei Pan: coordinate based meta-analyses in a medium sized literature: considerations, limitations and road ahead. Sleep Med Rev. (2018) 42:236–8. doi: 10.1016/j.smrv.2018.08.004
24. American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual. Westchester, NY: American Academy of Sleep Medicine (2005). Available online at: https://ci.nii.ac.jp/naid/20001061569/
25. Rezaie L, Fobian AD, Mccall WV, Khazaie H. Paradoxical insomnia and subjective-objective sleep discrepancy: a review. Sleep Med Rev. (2018) 40:196–202. doi: 10.1016/j.smrv.2018.01.002
26. Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. (1997) 6:179–88. doi: 10.1046/j.1365-2869.1997.00045.x
27. Castelnovo A, Ferri R, Punjabi NM, Castronovo V, Garbazza C, Zucconi M, et al. The paradox of paradoxical insomnia: a theoretical review towards a unifying evidence-based definition. Sleep Med Rev. (2019) 44:70–82. doi: 10.1016/j.smrv.2018.12.007
28. Bjorøy I, Jørgensen VA, Pallesen S, Bjorvatn B. The prevalence of insomnia subtypes in relation to demographic characteristics, anxiety, depression, alcohol consumption and use of hypnotics. Front Psychol. (2020) 11:527. doi: 10.3389/fpsyg.2020.00527
29. Blanken TF, Benjamins JS, Borsboom D, Vermunt JK, Paquola C, Ramautar J, et al. Insomnia disorder subtypes derived from life history and traits of affect and personality. Lancet Psychiatry. (2019) 6:151–63. doi: 10.1016/S2215-0366(18)30464-4
30. Keenan SA. Chapter 3 an overview of polysomnography. In: C. Guilleminault, editors, Handbook of Clinical Neurophysiology. Palo Alto, CA: Elsevier (2005). p. 33−50. doi: 10.1016/S1567-4231(09)70028-0
31. Manconi M, Ferri R, Sagrada C, Punjabi NM, Tettamanzi E, Zucconi M, et al. Measuring the error in sleep estimation in normal subjects and in patients with insomnia. J Sleep Res. (2010) 19:478–86. doi: 10.1111/j.1365-2869.2009.00801.x
32. Mohammadi H, Rezaei M, Faghihi F, Khazaie H. Hypothalamic-pituitary-gonadal activity in paradoxical and psychophysiological insomnia. J Med Signals Sens. (2019) 9:59–67. doi: 10.4103/jmss.JMSS_31_18
33. Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. (2011) 56:907–22. doi: 10.1016/j.neuroimage.2011.02.046
34. Zarei M, Mataix-Cols D, Heyman I, Hough M, Doherty J, Burge L, et al. Changes in gray matter volume and white matter microstructure in adolescents with obsessive-compulsive disorder. Biol Psychiatry. (2011) 70:1083–90. doi: 10.1016/j.biopsych.2011.06.032
35. Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. (2014) 92:381–97. doi: 10.1016/j.neuroimage.2014.01.060
36. Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. (2009) 44:83–98. doi: 10.1016/j.neuroimage.2008.03.061
37. Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. (2002) 15:870–8. doi: 10.1006/nimg.2001.1037
38. Turcotte I, St-Jean G, Bastien CH. Are individuals with paradoxical insomnia more hyperaroused than individuals with psychophysiological insomnia? Event-related potentials measures at the peri-onset of sleep. Int J Psychophysiol. (2011) 81:177–90. doi: 10.1016/j.ijpsycho.2011.06.008
39. Harvey AG, Tang NK. (Mis)perception of sleep in insomnia: a puzzle and a resolution. Psychol Bull. (2012) 138:77–101. doi: 10.1037/a0025730
40. Arsalidou M, Duerden EG, Taylor MJ. The centre of the brain: topographical model of motor, cognitive, affective, and somatosensory functions of the basal ganglia. Hum Brain Mapp. (2013) 34:3031–54. doi: 10.1002/hbm.22124
41. Lazarus M, Chen JF, Urade Y, Huang ZL. Role of the basal ganglia in the control of sleep and wakefulness. Curr Opin Neurobiol. (2013) 23:780–5. doi: 10.1016/j.conb.2013.02.001
42. Ellmore TM, Hood AJ, Castriotta RJ, Stimming EF, Bick RJ, Schiess MC. Reduced volume of the putamen in REM sleep behavior disorder patients. Parkinsonism Relat Disord. (2010) 16:645–9. doi: 10.1016/j.parkreldis.2010.08.014
43. Earley CJ, Kuwabara H, Wong DF, Gamaldo C, Salas RE, Brašić JR, et al. Increased synaptic dopamine in the putamen in restless legs syndrome. Sleep. (2013) 36:51–7. doi: 10.5665/sleep.2300
44. Kumar R, Farahvar S, Ogren JA, Macey PM, Thompson PM, Woo MA, et al. Brain putamen volume changes in newly-diagnosed patients with obstructive sleep apnea. Neuroimage Clin. (2014) 4:383–91. doi: 10.1016/j.nicl.2014.01.009
45. Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. (2010) 14:19–31. doi: 10.1016/j.smrv.2009.04.002
46. Khazaie H, Veronese M, Noori K, Emamian F, Zarei M, Ashkan K, et al. Functional reorganization in obstructive sleep apnoea and insomnia: a systematic review of the resting-state fMRI. Neurosci Biobehav Rev. (2017) 77:219–31. doi: 10.1016/j.neubiorev.2017.03.013
47. Vassilopoulou K, Papathanasiou M, Michopoulos I, Boufidou F, Oulis P, Kelekis N, et al. A magnetic resonance imaging study of hippocampal, amygdala and subgenual prefrontal cortex volumes in major depression subtypes: melancholic versus psychotic depression. J Affect Disord. (2013) 146:197–204. doi: 10.1016/j.jad.2012.09.003
48. Lee JK, Fandakova Y, Johnson EG, Cohen NJ, Bunge SA, Ghetti S. Changes in anterior and posterior hippocampus differentially predict item-space, item-time, and item-item memory improvement. Dev Cogni Neurosci. (2020) 41:100741. doi: 10.1016/j.dcn.2019.100741
49. Tang L, Pruitt PJ, Yu Q, Homayouni R, Daugherty AM, Damoiseaux JS, et al. Differential functional connectivity in anterior and posterior hippocampus supporting the development of memory formation. Front Hum Neurosci. (2020) 14:204. doi: 10.3389/fnhum.2020.00204
50. Plachti A, Eickhoff SB, Hoffstaedter F, Patil KR, Laird AR, Fox PT, et al. Multimodal parcellations and extensive behavioral profiling tackling the hippocampus gradient. Cerebral Cortex. (2019) 29:4595–612. doi: 10.1093/cercor/bhy336
51. Joo EY, Kim H, Suh S, Hong SB. Hippocampal substructural vulnerability to sleep disturbance and cognitive impairment in patients with chronic primary insomnia: magnetic resonance imaging morphometry. Sleep. (2014) 37:1189–98. doi: 10.5665/sleep.3836
52. Marques DR, Gomes AA, Caetano G, Castelo-Branco M. Insomnia disorder and brain's default-mode network. Curr Neurol Neurosci Rep. (2018) 18:45. doi: 10.1007/s11910-018-0861-3
53. Kim SJ, Lee YJ, Kim N, Kim S, Choi J.-W., Park J, et al. Exploration of changes in the brain response to sleep-related pictures after cognitive–behavioral therapy for psychophysiological insomnia. Sci Rep. (2017) 7:12528. doi: 10.1038/s41598-017-13065-0
54. Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, Morin CM. Insomnia and daytime cognitive performance: a meta-analysis. Sleep Med Rev. (2012) 16:83–94. doi: 10.1016/j.smrv.2011.03.008
55. Coulon P, Budde T, Pape H.-C. The sleep relay—the role of the thalamus in central and decentral sleep regulation. Pflügers Archiv-Eur J Physiol. (2012) 463:53–71. doi: 10.1007/s00424-011-1014-6
56. Gent TC, Bassetti CL, Adamantidis AR. Sleep-wake control and the thalamus. Curr Opin Neurobiol. (2018) 52:188–97. doi: 10.1016/j.conb.2018.08.002
57. Jan JE, Reiter RJ, Wasdell MB, Bax M. The role of the thalamus in sleep, pineal melatonin production, and circadian rhythm sleep disorders. J Pineal Res. (2009) 46:1–7. doi: 10.1111/j.1600-079X.2008.00628.x
58. Frau-Mendez MA, Fernandez-Vega I, Ansoleaga B, Blanco Tech R, Carmona Tech M, Antonio Del Rio J, et al. Fatal familial insomnia: mitochondrial and protein synthesis machinery decline in the mediodorsal thalamus. Brain Pathol. (2017) 27:95–106. doi: 10.1111/bpa.12408
59. Li M, Wang R, Zhao M, Zhai J, Liu B, Yu D, et al. Abnormalities of thalamus volume and resting state functional connectivity in primary insomnia patients. Brain Imaging Behav. (2019) 13:1193–201. doi: 10.1007/s11682-018-9932-y
60. Kim N, Kang SG, Lee YJ, Kim SJ, Kim S, Choi JW, et al. Decreased regional brain activity in response to sleep-related sounds after cognitive behavioral therapy for psychophysiological insomnia. Psychiatry Clin Neurosci. (2019) 73:254–61. doi: 10.1111/pcn.12822
61. Åkerstedt T, Lekander M, Nilsonne G, Tamm S, D'onofrio P, Kecklund G, et al. Gray matter volume correlates of sleepiness: a voxel-based morphometry study in younger and older adults. Nat Sci Sleep. (2020) 12:289–98. doi: 10.2147/NSS.S240493
62. Long Z, Zhao J, Chen D, Lei X. Age-related abnormalities of thalamic shape and dynamic functional connectivity after 3 h of sleep restriction. PeerJ. (2021) 9:e10751. doi: 10.7717/peerj.10751
Keywords: insomnia disorder, paradoxical insomnia, psychophysiological insomnia, shape analysis, gray matter volume, subcortical brain structures
Citation: Emamian F, Mahdipour M, Noori K, Rostampour M, Mousavi SB, Khazaie H, Khodaie-Ardakani M, Tahmasian M and Zarei M (2021) Alterations of Subcortical Brain Structures in Paradoxical and Psychophysiological Insomnia Disorder. Front. Psychiatry 12:661286. doi: 10.3389/fpsyt.2021.661286
Received: 30 January 2021; Accepted: 07 April 2021;
Published: 07 May 2021.
Edited by:
Liborio Parrino, University of Parma, ItalyReviewed by:
Carlotta Mutti, University Hospital of Parma, ItalyMarkku Partinen, University of Helsinki, Finland
Copyright © 2021 Emamian, Mahdipour, Noori, Rostampour, Mousavi, Khazaie, Khodaie-Ardakani, Tahmasian and Zarei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masoud Tahmasian, bWFzb3VkdGFobWFzaWFuQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Farnoosh Emamian
Farnoosh Emamian Mostafa Mahdipour
Mostafa Mahdipour Khadijeh Noori2
Khadijeh Noori2 Masoumeh Rostampour
Masoumeh Rostampour Habibolah Khazaie
Habibolah Khazaie Mohammadreza Khodaie-Ardakani
Mohammadreza Khodaie-Ardakani Masoud Tahmasian
Masoud Tahmasian Mojtaba Zarei
Mojtaba Zarei