- 1School of Philosophy and Sociology, Lanzhou University, Lanzhou, China
- 2Evidence Based Social Science Research Center, Lanzhou University, Lanzhou, China
- 3Suzanne Dworak-Peck School of Social Work, University of Southern California, Los Angeles, CA, United States
- 4Edward R. Roybal Institute on Aging, University of Southern California, Los Angeles, CA, United States
- 5School of Economics and Management, Hangzhou Normal University, Hangzhou, China
Background: Neurocognitive disorders, such as mild cognitive impairment (MCI), dementia, and Alzheimer's disease, not only harm people's cognitive function but also lead to negative emotions, poor quality of life (QOL), and unsatisfactory level of well-being. Resilience can be defined as a dynamic and amendable process, which maintains or improves life satisfaction and quick recovery from own dilemma. However, no meta-analysis of randomized controlled trials (RCTs) has thus far examined the effectiveness of resilience interventions among persons with neurocognitive disorders, and the results of RCTs were inconsistent. This systematic review aimed to assess the effectiveness of resilience interventions on psychosocial outcomes among persons with neurocognitive disorders.
Methods: Nine electronic Chinese and English databases (the Cochrane Library, PsycINFO, Web of Science, PubMed, Medline, Eric, JSTOR, CNKI, and WANGFANG) were searched through April 2021. Only RCTs were included, and the quality of the included studies was assessed by the Cochrane “Risk of Bias” tool. Meta-analysis was carried out on psychosocial outcomes, and heterogeneity was investigated by subgroup and sensitivity analysis. RevMan 5.4 was used for meta-analysis.
Results: Fourteen RCT studies were identified, representing a total of 2,442 participants with neurocognitive disorders. The risk of bias was high or unclear for most included studies in the domains of allocation concealment, blinding participants, and interventionists. Meta-analysis showed that heterogeneity was low or moderate. There were significant differences in favor of resilience interventions compared with control on the outcome of QOL, using the Quality of Life-Alzheimer Disease scale (QOL-AD) [I2 = 36%, standardized mean difference (SMD) = 0.14, 95% CI (0.02, 0.26), p = 0.02], and no significant differences on depression, using the Cornell Scale for Depression in Dementia (CSDD) [I2 = 41%, SMD = −0.14, 95% CI (−0.34, 0.05), p = 0.16], and neuropsychiatric symptoms using the Neuropsychiatric Inventory Questionnaire (NPI-Q) [I2 = 62%, SMD = −0.10, 95% CI (−0.37, −0.16), p ≤ 0.46].
Conclusions: Resilience interventions had a significant benefit on QOL but no significant benefit on depression and neuropsychiatric behavioral symptoms. More evidence is needed to answer questions about how to implement resilience interventions and how to evaluate their effectiveness.
Introduction
Mild cognitive impairment (MCI), dementia, or Alzheimer's disease (following abbreviation neurocognitive disorders) are chronic progressive syndrome. During the phase transition process, divergent sections of the brain are affected, and a persons' capability of adaptation to the disease and environment gradually decreases (1). Neurocognitive disorders not only impair the person's memory, orientation, thinking, cognitive functioning, and language (2), but also trigger emotions and psychological symptoms, such as depression and anxiety (3, 4). In addition, the impact of neurocognitive disorders is long-lasting; it is extremely exacting or nearly impossible to be cured completely (5, 6). With these detrimental outcomes, neurocognitive disorders further lead to an unsatisfactory level of well-being and quality of life (QOL), such as physical function, and financial instability of individuals and families; these results also undermine people's ability to fulfill family, social, and professional roles (7). Neurocognitive disorders currently affected tens of millions of people all over the world and caused enormous medical and economic burdens. For example, there were 50 million people with dementia worldwide (2), and the number of people with dementia worldwide is projected to be 152 million in 2050 (8). Dementia contributes significantly to the global burden of disease, costing an estimated $818 billion annually (9), expected to reach $2 trillion by 2030 (8, 10–12). Thus, neurocognitive disorders are regarded as one of the greatest social, health, and economic challenges of the twenty-first century (8, 11–13). It is progressively crucial to develop strategies that facilitate and help persons with neurocognitive disorders to maintain independence, well-functioning, and high QOL in the long run.
There were various approaches to coping with the challenges affiliated with neurocognitive disorders. Resilience-centered interventions can be seen as one important approach to adapting to stress and reducing the adverse impact of the stressors (7). Luthar and Cicchetti (14) defined resilience as “a dynamic and amendable process,” in which people use resources to acclimate to adversity (15). Meanwhile, Kunzler et al. (16) highlighted that resilience-centered interventions could be seen as the process of maintenance, withstanding, overcoming, adjustment, adaptation, posttraumatic growth, stress-related growth, rebound from a stressor, or rapid readjustment. As mentioned above, resilience can be defined as a process: after experiencing acute (short-run) or chronic (long-run) issues on health and stress, an individual can actively adapt, withstand, overcome, adjust, cope, and grow to maintain and improve his or her QOL with the support from multifaceted resources on individual and social levels.
The literature illustrated that protective factors of resilience were diverse, such as self-care, adherence to treatment programs, patient perceptions of pain and disease, adherence to physical activities, self-empowerment, health-related QOL, self-efficacy improvement, stress, depression, and anxiety reduction, optimism in viewpoints, and recovery acceleration (17–21). However, the process of resilience interventions focuses on reinforcing personal characteristics and exterior assets in response to a severe challenge to build an inclusive environment with multifaceted psychosocial supports (22). Similarly, other literature also suggested that resilience framework should include individual, family, community, and social components (23, 24). For example, Harris (24) stressed strengthening personal attributes in the process of resilience interventions, which could include self-acceptance of a person with neurocognitive disorders with the shifts in self, nurturing the individual's remaining competence, a positive perspective on diseases and dilemmas, and recognition of numerous means in which someone with dementia can contribute meaningly to their friends, family, and/or the community. Casey et al. (22) suggested five domains to implement resilience interventions: having a “fighting spirit” and personal control, maintaining solid family relationships, maintaining ties to communities, increasing awareness, addressing negative attitudes through dementia education, and engaging in physical activity. Kunzler et al. (16) suggested that resilience should include supportive doctors, linkages to helpful community groups and events, and sympathetic and supportive social surroundings. Overall, the literature mentioned above indicated that in the resilience process, multifaceted interventions should be taken, involving interactions between individuals and the external resources.
The outcomes of resilience-centered interventions are psychosocial, such as improving QOL, restoring normal performance, maintaining mental health, improving adaptability (25), better adjustment (26), enhancing mental well-being (27), reducing care dependence, good social relations, positive self-image (28), reducing burden or stress (27, 29), enhancing intent or meaning of life, and obtaining self-esteem, positive emotions, self-efficacy, boldness, active coping, optimism, social support, adaptation, and cognitive flexibility (including positive reassessment and acceptance) (16). However, one question arises regarding how to measure resilience-centered intervention outcomes in different resilience approaches. Windle et al. (30) studied tools for resilience interventions and concluded that the conceptual and theoretical adequacy of the scales was questionable, with no existing “gold standard” of resilience measures. Whelan et al. (15) indicated that key sets of outcomes for resilience in neurocognitive disorders have not been identified. For example, Ghanei Gheshlagh et al. (7) used three scales, which are Resilience Scale-25 (RS-25), Connor–Davidson Resilience Scale-10 (CDRISC-10), and Connor–Davidson Resilience Scale-25 (CDRISC-25), to assess the effectiveness of the resilience process for people with chronic physical diseases. Saint-Bryant et al. (26) used the Cornell Scale for Depression in Dementia (CSDD), Quality of Life Alzheimer's Disease scale (QOL-AD), and the Index of Relocation Adjustment Scale (IRA) to measure depression, QOL, and adjustment conditions as the outcomes of resilience process for older adults with dementia.
Another question is that the effect of resilience interventions is inconclusive. Thus far, there are only three systematic reviews (31–33) related to resilience interventions or outcome measures. Although Li et al. (31) claimed to contain resilience training, it was a mere strength training different from resilience. They also assessed cognitive outcomes, such as executive cognitive ability, global cognitive function, memory, and attention. Findings indicated positive effects on the executive cognitive capability and overall cognitive function, a weak-positive effect on memory, and no significance in attention. In the review of Carrion et al. (32), the resilience interventions focused on cognition-oriented caregiving approaches. The included 47 randomized controlled trials (RCTs) did not conduct a meta-analysis, and the results were inconclusive. In the review of Regan and Varanelli (33), the resilience interventions used modified cognitive behavior therapy (CBT) and problem-solving approach. They assessed three outcomes, including depression, anxiety, and adjustment in older adults with mild cognitive impairment and early dementia. The included seven RCTs and eight pre–post studies indicated positive effects in reducing depression in older persons with early dementia. However, Regan and Varanelli's (33) review did not conduct a meta-analysis, so it was unable to draw a clear conclusion about the intervention effect because of the divergent methods of the included studies. Additionally, the narrative review (15) identified five resilience interventions in three empirical studies of six papers, including Peer Support Network Services, Dementia Advisors, Memory Makers, Visual Arts Enrichment Activities, and Early-Stage and Beyond Community Activities. However, this narrative review included empirical studies, and the effectiveness of the resilience interventions could not be determined due to the study design.
Overall, there exist several dissimilarities regarding methodology and quality of studies among previous systematic reviews, which leads to inconsistent results regarding the effectiveness of resilience interventions. Currently, there is no systematic review that both includes RCT studies and conducts a meta-analysis. Therefore, this review aimed to identify RCT resilience interventions among persons with neurocognitive disorders to assess the effectiveness and provide further detailed evidence. This may contribute to enhancing existing resilience interventions and to facilitating the future development of such programs.
Methods
Criteria for Considering Studies for This Review
Participants
Participants were people of all ages with neurocognitive disorders, including dementia, mild cognitive impairment (MCI), and Alzheimer's disease. Participants' formal diagnoses on types and severity of those neurocognitive disorders were based on corresponding scales, including the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) (34); International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) (35); or other comparable diagnostic criteria. We included people living in diverse settings, such as the community, hospitals, and nursing homes. We did not use a criterion for age so as not to exclude studies in which some participants were below 60 years old. If a mixed sample of participants (e.g., people with dementia and their caregivers) were found and the data of persons with dementia were reported separately or were collected by contacting the author, these studies also were included.
Interventions
Any intervention that promotes a person's state of adaptation and adjustment with the help of personal attributes and external assets, regardless of content, duration, setting, or mode of delivery, was included. Resilience, for example, can include active coping (e.g., planning, problem solving), self-efficacy, optimism or positive attributional style, cognitive flexibility (e.g., positive reassessment and acceptance of negative emotions and conditions), religiosity and spirituality (e.g., frequent religious visits), positive emotions or positive affect, hardiness, self-esteem, intent or meaning of life, sense of coherency (internal), locus of control, coping flexibility, hope, humor, altruism (16), physical strength training, formal or informal care, social connection, and community or other external resource support.
Studies were excluded if they involved animal trials and non-psychological or non-social interventions of resilience, such as pharmacological interventions (e.g., treatment with antidepressants).
Comparators
Comparators included no treatment, treatment as usual (TAU) (e.g., routine medication and usual social activities), and wait-list control. If the control group adopted active control, such as music, physical, and cognitive–behavioral, rather than no treatment, TAU, or wait-list control, the literature was excluded. For studies with two or more controls, our meta-analysis was conducted only using the control group of no treatment, TAU, or wait-list control.
Outcome Measures
We defined outcomes as assessments of psychosocial adaptation. For these outcomes, QOL was a primary outcome, and others were secondary outcomes, such as social relations, positive self-image, self-efficacy, hardiness, anxiety, and depression. We accepted all psychosocial assessment tools used in the included studies. Outcomes were assessed before the treatment, upon completion of the treatment, and follow-up evaluations to assess long-term effects. We considered measures self-assessed and scored by observers or clinicians.
Studies were excluded if the studies contained non-psychosocial outcomes of resilience, such as brain structure, immediate memory, attention and calculation, deferred memory, time orientation, location orientation, language, visual space, or the geographical environment. This ensured that the review focused on the psychosocial outcomes of resilience interventions. The absence of the outcome values was an exclusion criterion for this review: if the values of mean and standard deviation (SD) were not reported in the description of outcome, mean and SD cannot be obtained by contacting the authors, or mean and SD cannot be calculated by the review manager software or calculator provided by Cochran, the original study was deleted (Review manager software or calculator: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-5-download; https://training.cochrane.org/resource/revman-calculator).
Types of Studies
Our review intended to include both published and unpublished RCTs in Chinese or English language. We also took into account cluster RCTs.
Electronic Searches
Nine electronic databases (the Cochrane Library, PsycINFO, Web of Science, PubMed, Medline, Eric, JSTOR, CNKI, and WANGFANG) were searched through April 2021. Gray literature was also searched from ProQuest Dissertations & Theses Database (PQDT) and DUXIU and reviewed. Authors of relevant conference abstracts were reached out for possible information sharing. The search terms used were the following: (a) dementia or Alzheimer or cognitive loss: MCI or dementia or Alzheimer or ADRD or “cognitive impai*” or “cognitive loss” or “cognitive decline”; (b) resilience or resiliency: resilien* OR adjust* OR adapt* OR “post-traumatic growth” OR “post-traumatic growth” OR “stress-related growth” OR withstand* OR overcom* OR resist* OR recover* OR thriv* OR adapt* OR adjust* OR bounc* back; and (c) RCT or random*. We used “subject OR title OR abstract OR keywords OR topic” to search. Search strategy was (MCI or dementia or Alzheimer or “cognitive impai*” or “cognitive loss” or “cognitive decline”) AND (resilien* OR adjust* OR adapt* OR “post-traumatic growth” OR “post-traumatic growth” OR “stress-related growth” OR withstand* OR overcom* OR resist* OR recover* OR thriv* OR adapt* OR adjust* OR bounc* back) AND (RCT or random*).
Assessment of Risk of Bias in Included Studies
We employed the Cochrane “Risk of Bias” tool (36) to identify any risk of bias with a judgment of low risk, high risk, or unclear risk of bias for each study of the following areas: (1) selection bias, (2) random sequence generation, (3) allocation concealment, (4) blinding of participants and personnel, (5) blinding of outcome assessment, (6) incomplete outcome data, and (7) selective reporting.
Data Collection and Analysis
Studies Screening
Three reviewers (YW, YZH, and WCH) screened articles according to inclusion and exclusion criteria. Three reviewers independently reviewed the studies' title and abstract, then screened the full paper, and independently evaluated methodological quality. Any uncertainties concerning suitability were discussed at weekly group meetings with all reviewers.
Data Extraction
Three reviewers independently extracted data using a predesigned form from the included studies. The following data were extracted: (1) basic study information, namely, authors, reference, and country/region; (2) participant characteristics, namely, illness/condition, total number, and number in each group, age, gender, and race/ethnicity; (3) intervention characteristics, namely, intervention content, individual or group format, in-person or virtual, setting, length (e.g., number of weeks), number of sessions, duration per session, and control; (4) intervention assessment information, namely, time point (e.g., pretest, posttest, follow-up), measures, outcomes with screenshots (including the mean, standard deviation, and number of participants in each group at each time point), and outcome raters (e.g., patients, caregivers, and staff); and (5) information on bias risk assessment (see Assessment of risk of bias in included studies). After comparing results, any uncertainties that could not be solved were discussed in weekly meetings with all reviewers.
Data Analysis
RevMan 5.4 was used for meta-analysis. Meta-analysis was performed if outcomes were measured by the same scales in at least two studies. Heterogeneity was assessed using an I2 statistic. To interpret heterogeneity, reviewers followed Cochrane guidance: 0–40% as not important, 30–60% as moderate heterogeneity, 50–90% as substantial heterogeneity, and 75–100% as considerable heterogeneity (37). A random-effects model was used if I2 statistics reports the value of 50% or above. A fixed-effects model was used if I2 statistics were lower than 50%. If one study used more than one instrument to measure the same outcome variable, the team employed the more commonly used instrument for the analysis.
Subgroup analyses were conducted with the following characteristics if applicable: outcome instrument, disease/conditions, country/region, rater, and follow-up. If the heterogeneity showed moderate or high, we performed subgroup and sensitivity analysis. Publication biases were assessed by a funnel plot if the number of studies used for meta-analysis was more than 10.
Results
Search Results
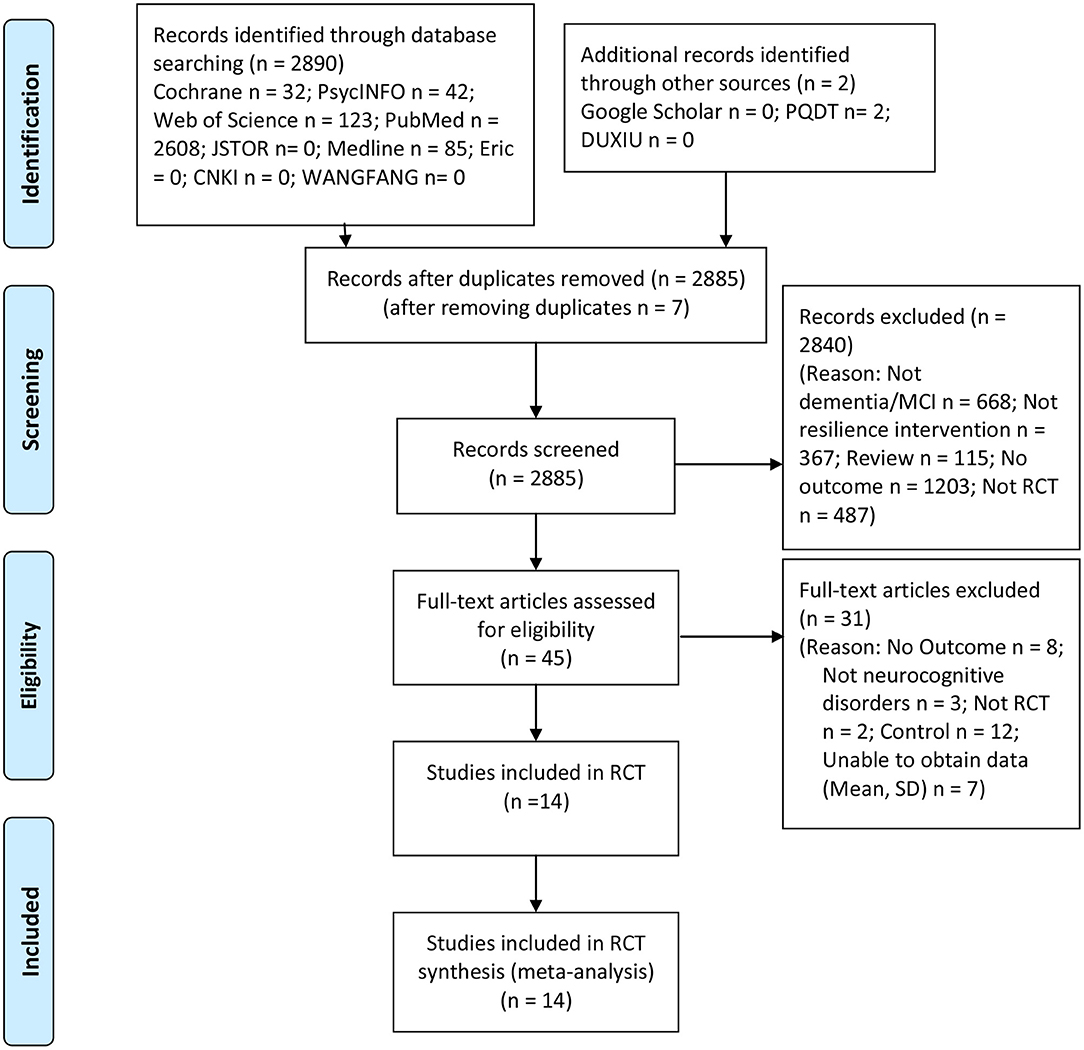
Figure 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow chart of the study review and selection process. A total of 2,890 studies were searched from electronic searches. After deduplication, we considered a total of 2,885 studies. The remaining studies were screened at the title and abstract level based on the pre-established inclusion and exclusion criteria, depending on the study type, population, intervention, control, and outcome. Forty-five full papers were then reviewed, from which 31 were excluded utilizing the same criteria. Fourteen RCT studies satisfied all the inclusion criteria. Thus, 14 RCT studies representing a total of 2,442 participants with neurocognitive disorders were included in the systematic review and meta-analysis.
Included Study Characteristics
Location
Among 14 studies, 7 were conducted in the UK, 2 in the USA, 1 in Germany, 1 in Denmark, 1 in France, 1 in Netherlands, and 1 in Norway (Table 1).
Participants
Participants in most studies (n = 10) were persons with dementia (26, 28, 29, 38, 41–44, 46, 47), one as persons with MCI (39), one as persons with MCI and early dementia (27), one as persons with dementia or cognitive impairment (45), and one was Alzheimer's (40). The number of participants ranged from 19 to 726, and seven studies had more than 100 participants. Participants were recruited from communities (n = 6) and nursing homes/residential care facilities (n = 8).
Interventions
We identified six resilience approaches based on content descriptions, including integrated approaches (n = 5), exercise regimen or activity programs (n = 4), psychological interventional technique (n = 2), a psychiatric intervention (n = 1), disease/case management (n = 1), and cognitive stimulation therapy (n = 1). Among five studies with integrated approaches, one was individualized person-centered care provided by standard procedures to diminish turmoil in care home residents with dementia (41), one was multimodal non-drug therapy on dementia's symptom and care need (38), one was multidimensional home-based care coordination provided by an interdisciplinary team to maximize independence for persons with MCI living home (39), one was a staff-led intervention that comprises four mandatory modules and one optional module to facilitate the adaptation of seniors with dementia after placement into residential care (26), and one was a psychosocial intervention including multifaceted and semi-tailored counseling, education, and support (40).
Four studies provided exercise regimens or activity programs (28, 43–45), such as adapted Tai Chi and cognition-action program, walking, aerobic, and strength exercise training. Two mental interventional techniques used mindfulness (29) and reminiscence (46). One study of recovery-orientated psychiatric intervention packages included prediagnostic well-being assessment and counseling, diagnostic consultation with written feedback, and postdiagnostic support (27). One disease management employed an internet-based care management software system for care planning and coordination (42). One individual cognitive stimulation therapy intervened and assessed adaptive functioning and QOL of participants with dementia (47). All interventions were conducted in groups of persons older than 40 years old.
Regarding intervention intensity, the length ranged from 6 weeks to 12 months. The duration of sessions included 30 min twice a week (n = 2), 40 min twice a week (n = 1), 30–45 min once a week (n = 1), 38 min once per 1–2 weeks (n = 1), 20–30 min at least five times per week (n = 1), 1 h at least once a week (n = 4), and 2 h, 6 days a week (n = 4).
Comparators
Control groups included usual care or TAU (n = 12), routine follow-up (n = 1) (40), and a wait-list control group (n = 1) (47).
Outcome Measurement
Table 1 shows that 14 studies assessed psychosocial outcomes, including QOL, well-being, mood state, neuropsychiatric symptom, positive self-image, adaption, goal attainment, and adjustment. Most studies evaluated QOL (n = 11). Specifically, QOL was rated by seven scales [Quality of Life in Alzheimer Disease (QOL-AD-participant), Quality of Life in A.D. for study partners (QOL-AD-proxy), quality of life of participants with dementia (DemQOL-Proxy), Dementia Quality of Life instrument (DQOL), the Alzheimer's Disease Rated Quality of Life-40 item scale (ADRQL-40), EUROQOL (EQ-5D), the Health Utilities Index Mark 3 (HUI3), and QOL-AD] in 11 studies. Mental well-being was rated by one scale [WHO Well-being Index (WHO-5)] in one study. Adaption was rated by three scales [Perceived Stress Scale (PSS-13), Zarit Burden Interview (ZBI), and Cornell depression scale (CDS)] in three studies. Goal attainment was rated by one scale [Goal Attainment Scale (GAS)] in one study. Adjustment was rated by one scale (IRA) in one study. Mood state was assessed by 11 scales, including depression [Geriatric Depression Scale (GDS), Cornell Scale for Depression in Dementia (CS), CDS, and CSDD] in seven studies, agitation [Agitated Behaviors in Dementia scale (ABID), the Brief Agitation Rating Scale (BARS), Cohen–Mansfield Agitation Inventory (CMAI), and the Pittsburgh Agitation Scale (PAS)] in three studies, and anxiety [the Rating Anxiety in Dementia Scale (RAID)] in one study. Neuropsychiatric symptom was rated by two scales [Neuropsychiatric Inventory (NPI) and the Neuropsychiatric Inventory questionnaire (NPI-Q)] in five studies. In addition, resilience was rated in two studies by two comprehensive scales [Qualidem and the Nurses' Observation Scale for Geriatric Patients (NOSGER)], including positive self-image, social relations, and mood.
All 14 studies had pre- and postintervention assessments. Eleven studies had follow-up (f/u) assessments, and the f/u assessments were conducted at different time points (e.g., 7, 12, and 21 weeks and 3, 6, 10, 12, 16, and 18 months).
Effects of Interventions
Overall, the effects of resilience interventions were diverse in various outcomes. In terms of social behavior, one study indicated significant differences in favor of resilience interventions compared with controls (38). Meanwhile, no significant differences were found in neuropsychiatric symptoms (39–41, 43, 44), adjustment (26), stress (29), and anxiety (29) in favor of resilience interventions compared with controls. Besides, the results of other outcomes' assessments were inconsistent. Specifically, regarding QOL, seven studies showed no statistical significance in favor of resilience interventions compared with controls (26, 27, 40–42, 44, 46), while two showed a significant effect (29, 47). One showed a significant enhancement in self-reported QOL but no significant improvement in proxy-rated QOL (39). One showed that activities of daily living (ADL) training had positively affected overall QOL, but no benefits were observed for exercise on QOL (28). As for depression, four included studies showed that the resilience interventions were not statistically different compared with controls (27, 29, 39, 40), while one showed statistically significant differences (45). In terms of well-being, one study showed no significance in favor of resilience interventions compared with controls (26), while the other study showed statistical significance (27). About the effects on agitation, one showed no significance in favor of resilience interventions compared with controls (41), while one showed statistical significance (45).
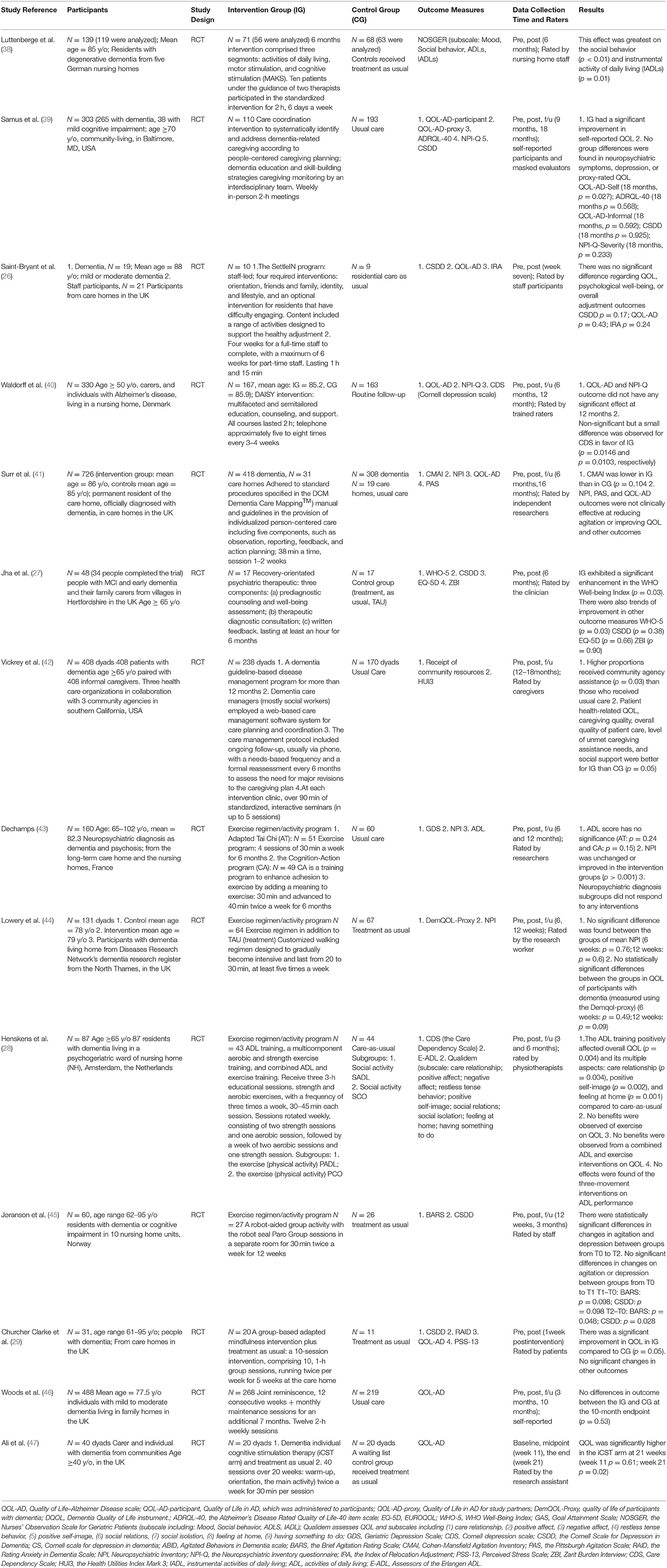
Risk of Bias
Risks of bias are summarized in Figure 2. The main flaws for risks of bias across 14 studies were in allocation concealment and blinding participants and interventionists. Regarding random sequence generation, 13 studies were judged to be at low risk, which used computer-generated random numbers, a block randomization method, a custom Excel program, or a web-based system.
For allocation concealment, five studies were rated as low risk, which used sealed envelopes, allocation numbers by a blind assigner, or emphasis on allocation concealment. Five studies were judged to be high risk, which reported no concealment in the allocation process. The remaining four studies did not report information on allocation concealment and were rated as unclear.
Regarding blinding participants and interventionists, 3 studies were rated as low risk, 10 studies as high risk, and 1 study as unclear. Meanwhile, most studies (n = 13) were judged as low risk for blinding outcome assessment, by using personnel not included in the intervention process, and one study was judged as high risk due to non-blinding outcome assessment.
All studies were judged to be at low risk for incomplete outcome data because the dropout rate was low (<30%) during the intervention, and they explained the numbers and reasons for dropout and the data analysis methods of dealing with missing values. Lastly, all studies were judged as low risk for selective reporting.
Meta-Analysis Results for Quality of Life
Meta-Analysis
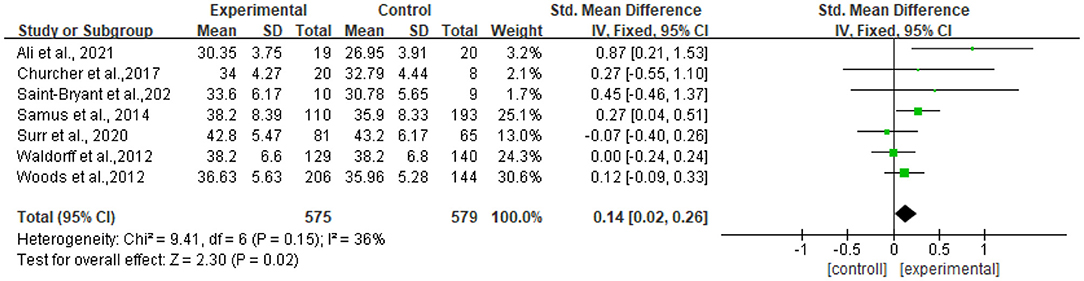
Seven studies reported data on quality of life, assessed by QOL-AD and were pooled for a meta-analysis using a fixed-effects model. Results illustrated that there were significant standardized mean differences in favor of resilience interventions compared with controls for QOL [SMD = 0.14, 95% CI (0.02, 0.32), p = 0.02] (Figure 3).
Subgroup Analyses
We further performed subgroup analyses with results shown in Figure 4. The subgroup result of the studies of the f/u assessments with 6 months showed significant standardized mean differences in favor of controls compared with resilience interventions [SMD = −0.21, 95% CI (−0.38, −0.03), p = 0.02] and no heterogeneity (I2 = 0%). The subgroup result of the studies of outcome rated by patients showed significant standardized mean differences in favor of resilience interventions compared with controls [SMD = 0.14, 95% CI (0.01, 0.27), p = 0.03] and no heterogeneity (I2 = 0%). Other subgroup result analyses showed no significant standardized mean differences in favor of resilience interventions compared with controls, and moderate heterogeneity (I2 = 36%−41%), which included the subgroup of the studies of persons with dementia [SMD = 0.14, 95% CI (−0.03, 0.30), p = 0.11], the subgroup of the studies of approaches using integrated approaches [SMD = 0.11, 95% CI (−0.04, 0.25), p = 0.16], and the subgroup of the studies conducted in the UK [SMD = 0.14, 95% CI (−0.03, 0.30), p = 0.11].
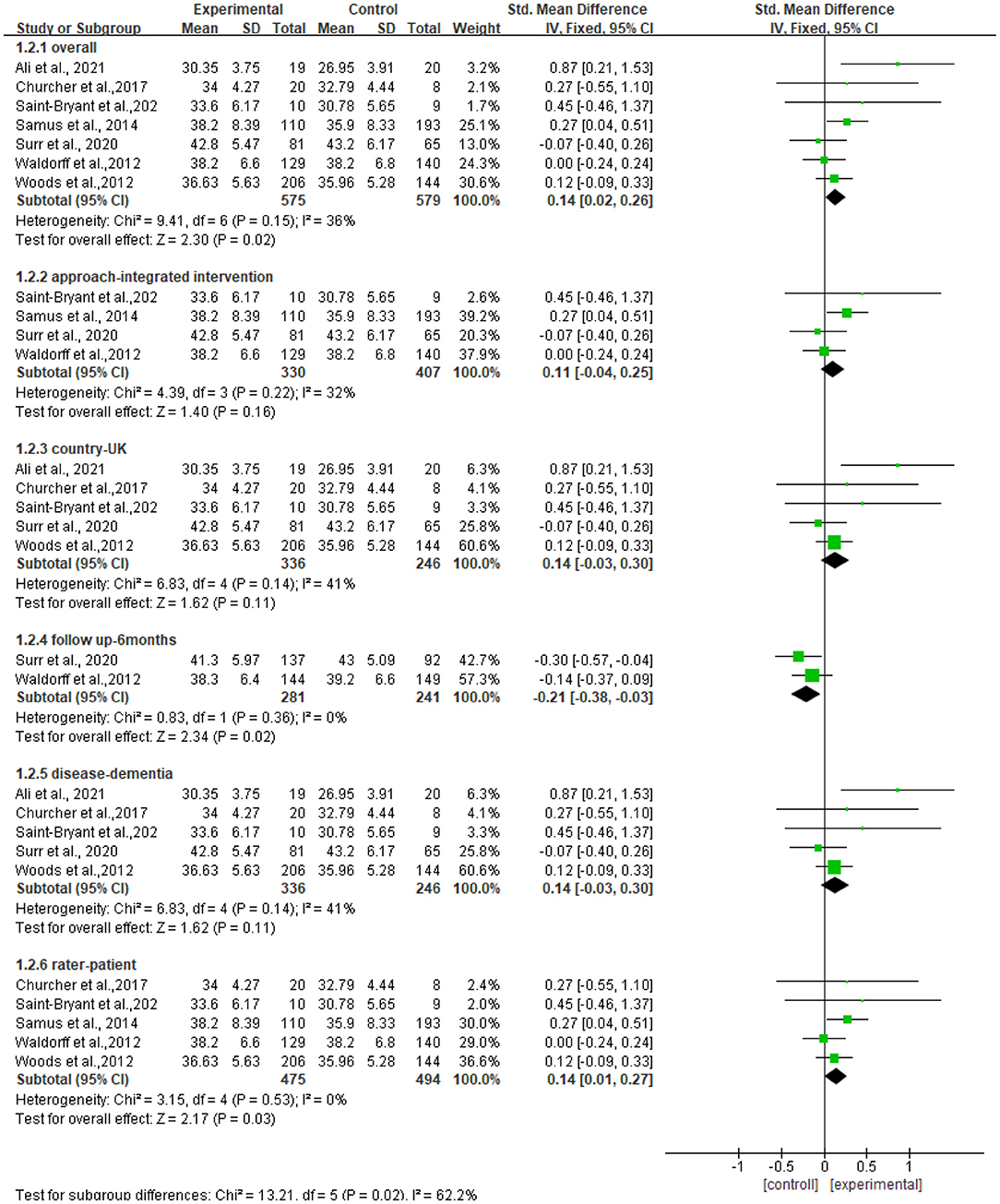
Figure 4. Subgroup analyses of the intervention group vs. the control group on QOL-AD rating scores.
Assessment of Sensitivity
The heterogeneity of the seven included studies was moderate (I2 = 36, chi2 = 9.41), which suggested that heterogeneity might not be important as explained in Cochrane guidance. The sensitivity analysis showed no heterogeneity (I2 = 0% chi2 = 4.55) after a small sample study (n = 40) (47) was deleted (see Figure 5).
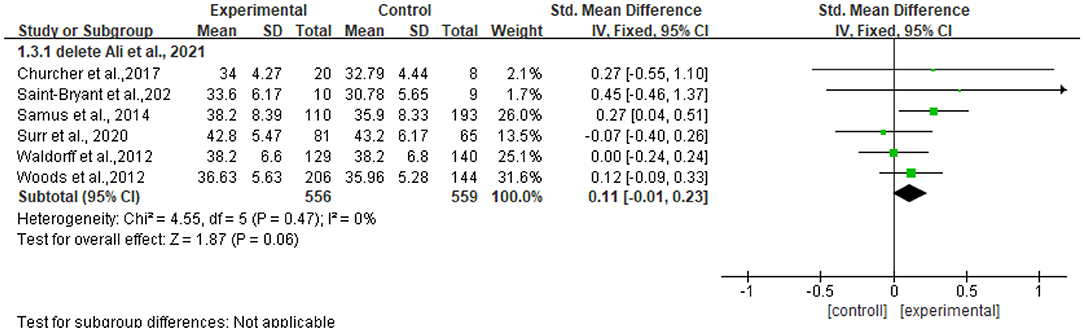
Figure 5. Sensitivity analyses of the intervention group vs. the control group on QOL-AD rating scores.
Analysis of Publication
Only seven RCTs were included, so the funnel plot was not made, but publication bias may exist.
Meta-Analysis Results for Depression
Meta-Analysis
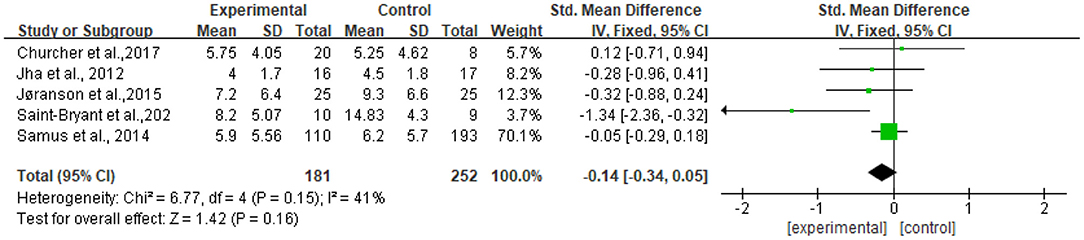
Five studies reported data on depression assessed by the CSDD and were pooled for a meta-analysis using a fixed-effects model. Results demonstrated that there were no significant standardized mean differences in favor of resilience interventions compared with controls for depression [SMD = −0.14, 95% CI (−0.34, 0.05), p = 0.16] (Figure 6).
Subgroup Analyses
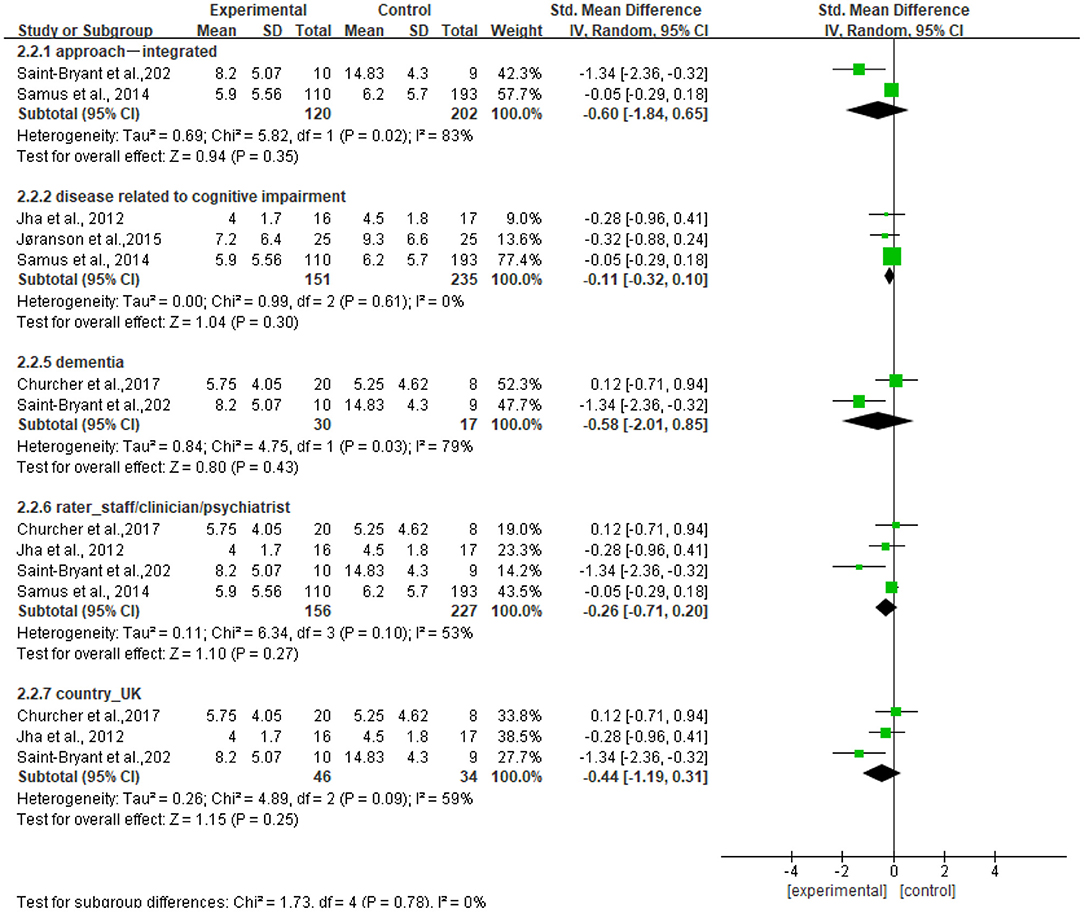
Figure 7 shows that no significant standardized mean differences in favor of resilience interventions compared with controls for CSDD were found in subgroup analyses. The heterogeneity ranged from 0 to 83%. Specifically, the subgroup result of participants with dementia showed no heterogeneity (I2 = 0%). Other subgroup results showed moderate or high heterogeneity (I2 = 53%−83%).
Assessment of Sensitivity
The heterogeneity of the five included studies was moderate (I2 = 41 chi2 = 6.77). The sensitivity analysis showed that the heterogeneity decreased (I2 = 0% chi2 = 1.26) after a small sample study (n = 19) (26) was deleted (see Figure 8).
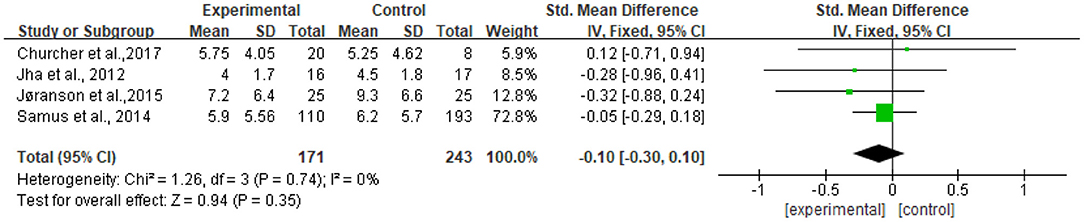
Figure 8. Sensitivity analyses of the intervention group vs. the control group on CSDD rating scores.
Analysis of Publication Bias
Only five RCTs were included, so the funnel plot was not made, but publication bias may exist.
Meta-Analysis Results for Neuropsychiatric Symptoms
Meta-Analysis
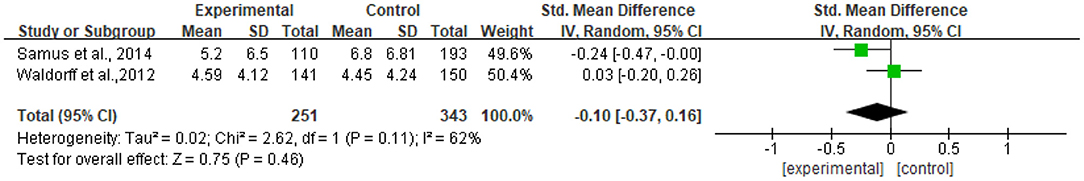
Two studies reported data on neuropsychiatric symptoms assessed by NPI-Q and were pooled for a meta-analysis using the random-effects model. Results revealed that there were no significant standardized mean differences in favor of resilience interventions compared with controls for neuropsychiatric symptoms [SMD = −0.10, 95% CI (−0.37, −0.16), p = 0.46] (Figure 9). Meanwhile, there tended to be substantial heterogeneity in the two included studies (I2 = 62%, chi2 = 2.62).
Analysis of Publication Bias
Only two RCTs were included, so the funnel plot was not made, but publication bias may exist.
Discussion
This systematic review and meta-analysis examined the effectiveness of resilience interventions among persons with neurocognitive disorders. A total of 14 RCT studies representing 2,442 participants were identified that fulfilled the inclusion criteria of this review. The risk of bias was either high or unclear for most studies in allocation concealment, blinding participants, and interventionists domains. Meta-analyses were conducted for a primary outcome of QOL and secondary outcomes of depression and neuropsychiatric symptoms. Our results indicated that resilience interventions had a significant positive effect on persons with neurocognitive disorders in enhancing QOL but might not be beneficial in decreasing depression and neuropsychiatric symptoms. Meanwhile, many other psychosocial outcomes were measured less frequently.
Our review identified target groups of neurocognitive disorders based on various conditions including symptoms and level of severities: mild or moderate dementia, cognitive impairment or MCI, and Alzheimer's disease. Similarly, specific target groups in the review of Regan and Varanelli (33) included mild cognitive impairment and early dementia. Inconsistent with our study, some other reviews (31, 32) focused on mixed target groups, including both healthy older adults and older adults at risk of dementia, MCI, and Alzheimer's disease, focusing on the early prevention and intervention of neurocognitive disorders. This might also be one reason why it was impossible to judge the effect of interventions due to sample heterogeneity.
Meanwhile, our review included RCTs and conducted meta-analyses to assess the effect of resilience interventions. Consistent with our study, two previous systematic reviews also contained RCTs, which included 12 RCTs (31) and 47 RCTs (32), respectively. However, they applied a narrative approach to synthesize the findings without conducting a meta-analysis. Inconsistent with our study, the review of Regan et al. (33) included other study designs, such as pre–post studies, besides RCTs, which might be one of the reasons why the findings were inconclusive. However, the quality of RCTs included in our review is not very high. Only 5 of the 14 studies were judged low risk in allocation concealment, and only 3 of the 14 studies were judged low risk in blinding of participants and interventionists domains. Although it is very difficult to implement allocation concealment and blinding of participants and interventionists domains in real-world RCT research, it is strongly recommended that more rigorous RCT research should be carried out in the future, with special attention to allocation concealment and blinding of participants and interventionists domains.
In addition, our review identified integrated resilience approaches. In contrast, the reviews of Regan and Varanelli (33) focused on psychotherapeutic approaches; the review of Li et al. (31) paid more attention to resistance training, strength, and exercise programs; and the review of Carrion et al. (32) solely focused on cognitive therapy. A broader range of resilience approaches was considered in our review, which rendered interventions diverse. Among them, integrated resilience approaches that were used by most included studies involved multiple components, such as caregiver's support, social connection, and resource support. Results indicated the possible advantages of the multiple-component interventions and multidisciplinary teamwork in active coping with complex symptoms and stress of persons with neurocognitive disorders. Thus, we call for more resilience research using integrated approaches for persons with dementia to better understand the effectiveness of integrated approaches and how to appropriately adopt and implement them.
Furthermore, our review focused on psychosocial outcomes (e.g., QOL, ADL, mental health, coping ability, adaption, adjustment). However, social outcomes were measured less frequently. The meta-analysis of psychological outcomes was conducted, so it is impossible to judge the effect of resilience interventions on social outcomes, such as improving social connection, social well-being, and resource support. Similarly, Regan and Varanelli (33) assessed depression, anxiety, and adjustment. Carrion et al. (32) rated depression. In contrast, Li et al. (31) largely focused on the effect of resistance training on cognitive function, such as executive cognitive ability, global cognitive function, attention, and memory. Since there is no “gold standard” for measuring the outcomes of resilience interventions in persons with neurocognitive disorders, we recommend that the measurement instruments can be further developed and validated to measure more effectively.
Additionally, regarding the effectiveness of resilience interventions, the previous systematic reviews' findings indicated positive effects (31, 33) or inconclusive (32) on different outcomes. However, due to the different design of the included original studies or the lack of meta-analysis of RCTs in these reviews, the statistical significance of the effect of resilience interventions could not be judged, and the level of evidence was not high. Therefore, these conclusions of intervention effects needed to be drawn cautiously. One synthesis of systematic reviews also indicated that due to the heterogeneity of the included studies, there was no sufficient evidence to determine whether resilience interventions may promote psychosocial outcomes (48). Our meta-analysis of the RCTs confirmed that resilience interventions had significant benefit to persons with neurocognitive disorders in enhancing QOL but might not be beneficial in decreasing depression and neuropsychiatric symptoms. It should be pointed out that several aspects of the original study limited the generalizability of our results: distinct approaches, diverse measurement tools and raters, and divergent settings and locations, and different data collection points during interventions and in f/u assessments. Therefore, further research is needed to address the development, implementation, and application of resilience interventions and conduct more rigorous and higher-quality RCT trials among persons with neurocognitive disorders.
This study has several limitations. First, due to the research team's language capacity, we only included English and Chinese literature, thus excluding potential useful information written in other languages. Second, although to some extent there was an accord on resilience as a dynamic process (14) and leading to psychosocial outcome (49, 50), and our review also identified various resilience interventions assessed by psychosocial outcomes, there was still no consensus about the definition of resilience and proper outcome measures. Thus, there may be some resilience studies that our study did not identify, and there could be other, equally valid, ways to define resilience that we did not consider. Last but not least, this study might be limited by the selected databases. Although the investigators included the most widely used English and Chinese databases, it remains possible that some works, particularly unpublished studies conducted in other countries, were not located and examined.
Conclusion
The study findings indicated significant benefits of resilience interventions on QOL but no significant benefits of resilience interventions on depression and neuropsychiatric behavioral symptoms among persons with neurocognitive disorders. There is an ongoing need for additional evidence to support the effectiveness of resilience interventions, how to further improve resilience interventions, how to implement them, and how to evaluate the effectiveness in persons with neurocognitive disorders. In addition, there is a need to strengthen methodological quality to assess and determine the effects of resilience interventions.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
YW led the conception, data extraction, risk bias assessment, data analysis, drafting, critical review, and revision of the manuscript. YZ and WC were responsible for data search, data screening, and extraction. TL helped to proofread and edit. IC critically revised the manuscript and eventually approved the upcoming version. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to acknowledge Professor Kehu Yang and Dr. Xiuying Li for their support and guidance on research from the Major Project of the National Social Science Fund of China: Research on the Theoretical System, International Experience, and Chinese Path of Evidence-based Social Science (Project No. 19ZDA142).
References
1. Streater A, Aguirre E, Spector A, Orrell M. Cognitive stimulation therapy for people with dementia in practice: a service evaluation. Br J Occup Ther. (2016) 79:574–80. doi: 10.1177/0308022616659886
2. World Health Organization. World Health Organization Dementia Factsheet. Available online at: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed January 11, 2021) (2019).
3. Porter VR, Buxton WG, Fairbanks LA, Strickland T, O'Connor SM, Rosenberg-Thompson S, et al. Frequency and characteristics of anxiety among patients with Alzheimer's disease and related dementias. JNP. (2003) 15:180–6. doi: 10.1176/jnp.15.2.180
4. Ganguli M. Depression, cognitive impairment and dementia: why should clinicians care about the web of causation? Indian J Psychiatry. (2009) 51:S29–34. Available online at: https://www.indianjpsychiatry.org/temp/IndianJPsychiatry51529-1416072_035600.pdf
5. Compas BE, Connor-Smith JK, Saltzman H, Thomsen AH, Wadsworth ME. Coping with stress during childhood and adolescence: problems, progress, and potential in theory and research. Psychol Bull. (2001) 127:87–127. doi: 10.1037/0033-2909.127.1.87
6. Eggenberger SK, Meirs SJ, Krumwiede N, Bliesmer M, Earle P. Reintegration within families in the context of chronic illness: a family health-promoting process: reintegration within families. J Nurs Healthc Chronic Illness. (2011) 3:283–92. doi: 10.1111/j.1752-9824.2011.01101.x
7. Ghanei Gheshlagh R, Sayehmiri K, Ebadi A, Dalvandi A, Dalvand S, Nourozi Tabrizi K. Resilience of patients with chronic physical diseases: a systematic review and meta-analysis. Iran Red Crescent Med J. (2016) 18:e38562. doi: 10.5812/ircmj.38562
8. World Health Organization. WHO | Global Action Plan on the Public Health Response to Dementia 2017–2025. Available online at: http://www.who.int/mental_health/neurology/dementia/action_plan_2017_2025/en/ (accessed January 11, 2021) (2017).
9. Prince M, Wimo A, Guerchet M, Ali GC, Prina M. World Alzheimer Report 2015. The Global Impact of Dementia. An Analysis of Prevalence, Incidence, Cost, and Trends. Alzheimer's Disease International (2015).
10. Frankish H, Horton R. Prevention and management of dementia: a priority for public health. Lancet. (2017) 390:2614–5. doi: 10.1016/S0140-6736(17)31756-7
11. Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet. (2017) 390:2673–734. doi: 10.1016/S0140-6736(17)31363-6
12. Edick C, Holland N, Ashbourne J, Elliott J, Stolee P. A review of Canadian and international dementia strategies. Healthc Manage Forum. (2017) 30:32–9. doi: 10.1177/0840470416664533
13. Carney P, O'Shea E. Philanthropy and dementia care in Ireland. Dementia. (2020) 19:951–64. doi: 10.1177/1471301218791847
14. Luthar SS, Cicchetti D. The construct of resilience: implications for interventions and social policies. Dev Psychopathol. (2000) 12:857–85. doi: 10.1017/S0954579400004156
15. Whelan S, Teahan Á, Casey D. Fostering the resilience of people with dementia: a narrative literature review. Front Med. (2020) 7:45. doi: 10.3389/fmed.2020.00045
16. Kunzler AM, Helmreich I, Chmitorz A, König J, Binder H, Wessa M, et al. Psychological interventions to foster resilience in healthcare professionals. Cochrane Database Syst Rev. (2020) 7:CD012527. doi: 10.1002/14651858.CD012527.pub2
17. Santos FRM, Bernardo V, Gabbay MAL, Dib SA, Sigulem D. The impact of knowledge about diabetes, resilience and depression on glycemic control: a cross-sectional study among adolescents and young adults with type 1 diabetes. Diabetol Metab Syndr. (2013) 5:55. doi: 10.1186/1758-5996-5-55
18. Steinhardt MA, Mamerow MM, Brown SA, Jolly CA. A Resilience intervention in african american adults with type 2 diabetes. Diabetes Educ. (2009) 35:274–84. doi: 10.1177/0145721708329698
19. Kilic SA, Dorstyn DS, Guiver NG. Examining factors that contribute to the process of resilience following spinal cord injury. Spinal Cord. (2013) 51:553–7. doi: 10.1038/sc.2013.25
20. Chan IWS, Lai JCL, Wong KWN. Resilience is associated with better recovery in Chinese people diagnosed with coronary heart disease. Psychol Health. (2006) 21:335–49. doi: 10.1080/14768320500215137
21. Robottom BJ, Gruber-Baldini AL, Anderson KE, Reich SG, Fishman PS, Weiner WJ, et al. What determines resilience in patients with Parkinson's disease? Parkinsonism Relat Disord. (2012) 18:174–7. doi: 10.1016/j.parkreldis.2011.09.021
22. Casey D, Gallagher N, Devane D, Woods B, Murphy K, Smyth S, et al. The feasibility of a Comprehensive Resilience-building psychosocial Intervention (CREST) for people with dementia in the community: protocol for a non-randomised feasibility study. Pilot Feasibil Stud. (2020) 6:177. doi: 10.1186/s40814-020-00701-2
23. Windle G. The contribution of resilience to healthy ageing. Perspect Public Health. (2012) 132:159–60. doi: 10.1177/1757913912449572
24. Harris P, editor. Is Resilience a Key to Living a Meaningful Life with Dementia? Factors That Contribute to the Resilience Process in EarlyStage Dementia. New Orleans, LA: Oxford University Press (2010).
25. Garroway A. Resilience in Parkinson's disease: an empirical examination of age-related components of the construct. [Dissertations & Theses]. Virginia Commonwealth University, Gradworks, Richmond, VA, United States (2015). Available online at: https://core.ac.uk/download/pdf/51290332.pdf
26. Saint-Bryant CA, Murrill J, Hayward JK, Nunez K-M, Spector A. SettleIN: using a manualised intervention to facilitate the adjustment of older adults with dementia following placement into residential care. IJERPH. (2020) 17:2606. doi: 10.3390/ijerph17072606
27. Jha A, Jan F, Gale T, Newman C. Effectiveness of a recovery-orientated psychiatric intervention package on the well-being of people with early dementia: a preliminary randomised controlled trial: effectiveness of a recovery-orientated intervention in early dementia. Int J Geriatr Psychiatry. (2013) 28:589–596. doi: 10.1002/gps.3863
28. Henskens M, Nauta I, Drost K, Scherder E. The effects of movement stimulation on activities of daily living performance and quality of life in nursing home residents with dementia: a randomized controlled trial. CIA. (2018) 13:805–17. doi: 10.2147/CIA.S160031
29. Churcher Clarke A, Chan JMY, Stott J, Royan L, Spector A. An adapted mindfulness intervention for people with dementia in care homes: feasibility pilot study: mindfulness for people with dementia in care homes. Int J Geriatr Psychiatry. (2017) 32:e123–31. doi: 10.1002/gps.4669
30. Windle G, Bennett KM, Noyes J. A methodological review of resilience measurement scales. Health Qual Life Outcomes. (2011) 9:8. doi: 10.1186/1477-7525-9-8
31. Li Z, Peng X, Xiang W, Han J, Li K. The effect of resistance training on cognitive function in the older adults: a systematic review of randomized clinical trials. Aging Clin Exp Res. (2018) 30:1259–73. doi: 10.1007/s40520-018-0998-6
32. Carrion C, Folkvord F, Anastasiadou D, Aymerich M. Cognitive therapy for dementia patients: a systematic review. Dement Geriatr Cogn Disord. (2018) 46:1–26. doi: 10.1159/000490851
33. Regan B, Varanelli L. Adjustment, depression, and anxiety in mild cognitive impairment and early dementia: a systematic review of psychological intervention studies. Int Psychogeriatr. (2013) 25:1963–84. doi: 10.1017/S104161021300152X
34. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association (2013).
35. World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders. Available online at: https://www.who.int/classifications/icd/en/bluebook.pdf
36. Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman DA, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
37. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1. (2020). Available online at:www.training.cochrane.org/handbook
38. Luttenberger K, Donath C, Uter W, Graessel E. Effects of multimodal nondrug therapy on dementia symptoms and need for care in nursing home residents with degenerative dementia: a randomized-controlled study with 6-month follow-up. J Am Geriatr Soc. (2012) 60:830–40. doi: 10.1111/j.1532-5415.2012.03938.x
39. Samus QM, Johnston D, Black BS, Hess E, Lyman C, Vavilikolanu A, et al. A multidimensional home-based care coordination intervention for elders with memory disorders: the Maximizing Independence at Home (MIND) pilot randomized trial. Am J Geriatr Psychiatry. (2014) 22:398–414. doi: 10.1016/j.jagp.2013.12.175
40. Waldorff FB, Buss DV, Eckermann A, Rasmussen MLH, Keiding N, Rishoj S, et al. Efficacy of psychosocial intervention in patients with mild Alzheimer's disease: the multicentre, rater blinded, randomised Danish Alzheimer Intervention Study (DAISY). BMJ. (2012) 345:e4693. doi: 10.1136/bmj.e4693
41. Surr CA, Holloway I, Walwyn REA, Griffiths AW, Meads D, Martin A, et al. Effectiveness of Dementia Care Mapping™ to reduce agitation in care home residents with dementia: an open-cohort cluster randomised controlled trial. Aging Mental Health. (2020) 24:1–72. doi: 10.1080/13607863.2020.1745144
42. Vickrey BG, Mittman BS, Connor KI, Pearson ML, Della Penna RD, Ganiats TG, et al. The effect of a disease management intervention on quality and outcomes of dementia care: a randomized, controlled trial. Ann Intern Med. (2006) 145:713. doi: 10.7326/0003-4819-145-10-200611210-00004
43. Dechamps A. Effects of exercise programs to prevent decline in health-related quality of life in highly deconditioned institutionalized elderly persons: a randomized controlled trial. Arch Intern Med. (2010) 170:162. doi: 10.1001/archinternmed.2009.489
44. Lowery D, Cerga-Pashoja A, Iliffe S, Thuné-Boyle I, Griffin M, Lee J, et al. The effect of exercise on behavioural and psychological symptoms of dementia: the EVIDEM-E randomised controlled clinical trial: exercise for behavioural symptoms of dementia. Int J Geriatr Psychiatry. (2014) 29:819–27. doi: 10.1002/gps.4062
45. Jøranson N, Pedersen I, Rokstad AMM, Ihlebæk C. Effects on symptoms of agitation and depression in persons with dementia participating in robot-assisted activity: a cluster-randomized controlled trial. J Am Med Direct Assoc. (2015) 16:867–73. doi: 10.1016/j.jamda.2015.05.002
46. Woods R, Bruce E, Edwards R, Hounsome B, Elvish R, Keady J, et al. REMCARE: reminiscence groups for people with dementia and their family caregivers – effectiveness and cost-effectiveness pragmatic multicentre randomised trial. Health Technol Assess. (2012) 16:1–116. doi: 10.3310/hta16480
47. Ali A, Brown E, Tsang W, Spector A, Hassiotis A. Individual cognitive stimulation therapy (iCST) for people with intellectual disability and dementia: a feasibility randomised controlled trial. Aging Mental Health. (2021) 9:1–11. doi: 10.1080/13607863.2020.1869180
48. McDermott O, Charlesworth G, Hogervorst E, Stoner C, Moniz-Cook E, Spector A, et al. Psychosocial interventions for people with dementia: a synthesis of systematic reviews. Aging Mental Health. (2019) 23:393–403. doi: 10.1080/13607863.2017.1423031
49. Bonanno GA, Romero SA, Klein SI. The temporal elements of psychological resilience: an integrative framework for the study of individuals, families, and communities. Psychol Inquiry. (2015) 26:139–69. doi: 10.1080/1047840X.2015.992677
Keywords: resilience, intervention, meta-analysis, psychosocial outcomes, neurocognitive disorders
Citation: Wang Y, Chi I, Zhan Y, Chen W and Li T (2021) Effectiveness of Resilience Interventions on Psychosocial Outcomes for Persons With Neurocognitive Disorders: A Systematic Review and Meta-Analysis. Front. Psychiatry 12:709860. doi: 10.3389/fpsyt.2021.709860
Received: 14 May 2021; Accepted: 12 July 2021;
Published: 19 August 2021.
Edited by:
Zeng-Jie Ye, Guangzhou University of Chinese Medicine, ChinaReviewed by:
Yingchun Zeng, Third Affiliated Hospital of Guangzhou Medical University, ChinaMohsen Khosravi, Zahedan University of Medical Sciences, Iran
Sheikh Shoib, Directorate of Health Services, India
Copyright © 2021 Wang, Chi, Zhan, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuning Zhan, aGFwcHl1bmluZ0Boem51LmVkdS5jbg==
 Ying Wang
Ying Wang Iris Chi
Iris Chi Yuning Zhan5*
Yuning Zhan5* Tongtong Li
Tongtong Li