- 1Stony Brook University School of Nursing, Stony Brook, New York, NY, United States
- 2Department of Clinical Nursing, Faculty of Health Sciences, Wroclaw Medical University, Wroclaw, Poland
- 3Department of Psychology, WSB University in Torun, Torun, Poland
- 4Department of Psychology, Faculty of Applied Studies, University of Lower Silesia, Wroclaw, Poland
- 5Department of Public Health, Faculty of Health Sciences, Wroclaw Medical University, Wroclaw, Poland
Background/Aim: Pathological processes associated with aging increase the risk of cognitive deficits. Frailty syndrome may significantly accelerate these pathological processes in elderly patients with heart failure. The objective of this review was to better understand the association between frailty syndrome and co-occurring cognitive decline in patients with heart failure.
Methods: We conducted a systematic review based on PubMed/MEDLINE, Scopus, EMBASE, and CINAHL as databases. The search followed the method described by Webb and Roe. For inclusions, the studies were selected employing cross-sectional and longitudinal designs. The included studies had to evaluate frailty syndrome and cognitive impairments among participants with heart failure. As we were interested in older adults, the search was limited to individuals >65 years of age. The search was limited to primary research articles written in English published since the year 2000.
Results: Of the 1,245 studies retrieved by the systematic review, 8 relevant studies were enclosed for the full-text review. Our review revealed that most studies of patients with HF demonstrated evidence of an association between greater frailty and cognitive impairment. In particular, six studies reported evidence for the significant association between higher levels of frailty and cognitive impairment in patients with heart failure. The remaining two studies failed to find an association between frailty and cognitive impairment.
Conclusions: The development of frailty and cognitive impairment in heart failure is particularly important because this cardiovascular disease is a common cause of both morbidity and mortality in the world. The results of this review fill the existing gap in the literature related to the identification of clinical factors linked with frailty syndrome that contribute to cognitive impairment in patients with a diagnosis of heart failure. The prevalence of overlapping frailty and cognitive impairment in patients with heart failure, therefore, necessitates a routine assessment of these components in the care of patients with cardiovascular disease.
Introduction
Heart failure (HF) is a common age-related disease that affects as many as 64.3 million people worldwide (1–3). It is the final stage of many cardiovascular diseases (CVDs), in which there is a progressive impairment of global cardiac function associated with dysfunctional hypertrophy and apoptosis of terminally differentiated cardiac myocytes (4). The number of patients living with heart failure is increasing as a result of an aging population, global population growth, and improved survival from diagnosis (5). Among older adults, both cardiovascular disease (CVD) and frailty syndrome are prevalent and often coexist (6, 7).
Frailty syndrome often coexists with HF. The prevalence of frailty in patients with HF is as high as 50% (7). Frailty increases the risk of HF and, in patients already diagnosed with HF, contributes to increased mortality, re-hospitalization, and reduced quality of life (8). Frailty often co-occurs with advanced HF, which is also accompanied by general muscle weakness and contributes to the development of cardiac cachexia at later stages of the disease.
Frailty is not consistently defined in the literature (9). Originally, frailty was synonymous with older age (9), however it was observed that a patient's response to disease, functional status, and survival are not determined solely by age. For instance, advanced HF is a clinical example of frailty independent of the patient's age (10). Further, although frailty is correlated with age, frailty does not affect only the elderly (11). Frailty also has been equated with disability and high comorbid disease burden (9). More recently, frailty has been defined as a syndrome of weakness or reserve depletion (9).
Sarcopenia is common in frailty and is associated with high disease burden, accelerated functional decline and repeated hospitalizations (12). Thus, frailty and HF, occurring together, are associated with poorer patient-reported outcomes as well as clinical outcomes (13–15). Although frailty is associated with poor outcomes, research suggests that frailty is a dynamic process and reversible. Therefore, there may be ways to prevent, modify, and control the adverse health consequences that occur through frailty. Early identification of frailty may enable the implementation of appropriate preventive health interventions that are inexpensive and easy to incorporate yet beneficial at improving clinical and patient-reported outcomes (16).
There are many tools for measuring frailty, and the number of validated tools has been increasing significantly over recent years (17). In the absence of a universal definition, measurement of frailty has been challenging. Fried et al. (9) proposed measuring frailty based on five phenotypic criteria: (1) low grip strength, (2) low energy, (3) slowness, (4) low physical activity, and/or (5) unintentional weight loss (9). According to these criteria, a person is considered frail if the patient meets at least three of the criteria listed above, and as precariously frail if one or two of the criteria are met (9). It is worth noting that Fried's criteria, while appearing to be objective and widely used by researchers and clinical practitioners around the world, refer only to physical weakness and do not include other important domains of this condition, such as cognitive weakness, psychological weakness, or social weakness (9, 18).
Although muscle strength and walking speed are impaired in frailty, cognitive function also is often affected (19). Cognitive function includes many separate domains responsible for different aspects of cognition and include memory, attention, language, psychomotor function, and executive function (20). Memory appears to be affected in frailty syndrome and is associated with increased risk of mortality (19).
Some studies have begun to include assessment of cognitive function as part of frailty diagnosis (21, 22). Remarkably, cross-sectional studies show an association between frailty and cognitive performance (9). Furthermore, longitudinal studies show an association between frailty and the onset of cognitive change, cognitive impairment, and dementia (23, 24).
Although frailty and cognitive impairment have been associated in the general population, the specific association in the HF population has not been established. People with HF are at increased risk for frailty, which can worsen symptoms, impede self-management and reduce compliance with therapy (25). Cognitive impairment is similarly prevalent in HF and leads to poorer HF outcomes (26). As both frailty and cognitive impairment are common in HF, and as both conditions are associated with poor clinical outcomes, It is important to determine if the two conditions are interrelated. If an association between frailty and cognitive impairment is identified, clinicians should be aware of the potential negative effects on clinical outcomes. Furthermore, researchers can begin to develop interventions designed to improve frailty and cognitive impairment and determine if improvement is associated with better clinical outcomes (27).
Although several studies on the association between frailty and cognitive impairment in HF have been completed, a summary of the existing evidence on the association between frailty and cognitive impairment in HF has not been completed. Therefore, the aim of this study was to complete a comprehensive review of the literature to describe the overall association between frailty and cognitive impairment in elderly patients with HF and to identify areas in need of research to better understand the association.
Methods
This review followed the method described by Webb and Roe (28). Two teams searched four databases for studies evaluating the association between frailty and cognitive function in patients with heart failure: one team (KF, IU, ML) searched PubMed/MEDLINE and Scopus while the other (EC, TC, RS) searched Excerpta Medica database (EMBASE) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL). Keywords included “heart failure,” “frail* AND “cognit*.” The “wild card” was used to ensure that words that included the stem “frail” (frail, frailty, etc.) and “cognit” (cognitive, cognition, etc.) would be included in the search. As we were interested in older adults, the search was limited to persons >65 years of age. The search was limited to primary research articles written in English published since the year 2000. Studies evaluating the association between frailty and dementia were excluded.
The titles of the studies retrieved during the initial search were screened to determine relevance. Following initial screening, the full text of the remaining articles were read to determine if they should be included. Reviews, letters to the editor, study protocols, commentaries, clinical guidelines, and duplicates were excluded. Studies also were excluded if they did not evaluate the association between frailty and cognitive function or if they evaluated the association between frailty and cognitive function in a population other than heart failure patients. Questions about whether an article should be included were resolved by asking the advice of other members of the team. Final decisions about inclusion were determined by consensus of the team.
Once articles were identified, data were extracted and entered into a table of evidence for ease of review. Extracted data included sample size and socio-demographic characteristics, study design, measure of frailty, measure of cognitive function, and findings regarding the association between frailty and cognitive function. Questions about the information to be included in the table of evidence were resolved by discussing the matter with other members of the team and coming to consensus.
The table of evidence was reviewed to identify trends across studies. The effect of frailty on cognitive function was reviewed first. Then, trends in sample characteristics, study design, and methods of measuring frailty and cognitive function were considered. Findings and implications were discussed at weekly meetings attended by all six co-authors via Microsoft Teams. All procedures and methods performed in this review were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Results
A flowchart demonstrating the literature review is presented in Figure 1 (29). After including all search terms and applying limitations, 1,245 articles were retrieved from the four databases: 126 articles from Pubmed, 939 from Scopus, 144 from Embase, and 36 from CINAHL. Following removal of 911 articles during title screening, 334 articles remained for full text review. After reading these articles, another 326 were excluded as the data had been reported in other articles or they did not report on the variables or associations of interest. Eight studies remained for inclusion in this review (30–37).
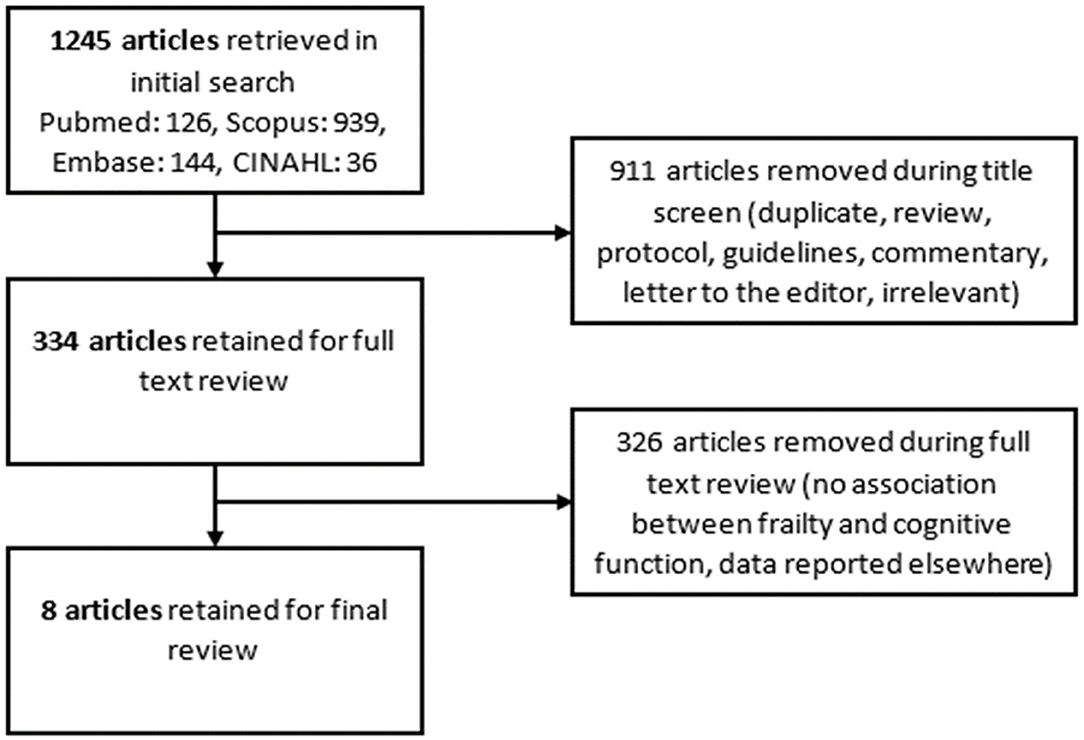
Figure 1. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flowchart outlining the search for relevant literature for this integrative review.
Frailty and Cognitive Impairment
Six studies reported an association between higher levels of frailty and cognitive impairment (30–35, 37). In a small (n = 49) study of patients with HF, Denfeld et al. reported that cognitive impairment was more prevalent among patients with frailty (58%) than among those without frailty (8%, p < 0.001) (31). In a larger study (n = 120), Cacciatore and colleagues reported that greater frailty was associated with lower scores on the Mini Mental State Examination (MMSE) (30).
Two studies failed to find an association between frailty and cognitive impairment (33, 36). Gunton and colleagues reported no difference in cognitive function among those with high or low levels of frailty (p = 0.12) (33). González-Moneo et al. identified an initial association between frailty and greater odds of cognitive impairment (OR 1.58, 95% CI [1.02, 2.46], p = 0.04), however this association lost significance after controlling for socio-demographics and clinical measures (OR 1.58, 95% CI [0.99, 2.50], p = 0.05).
Measures of Frailty
Five studies used Fried's frailty criteria as a measure of frailty (31, 34–37). Fried's frailty criteria is a well-validated indicator of physical frailty (9). Five physical criteria (shrinking, weakness, slowness, physical exhaustion, and low physical activity) are measured and then scored based on whether the measure falls above or below a given criterion. Four of the five studies using Fried's frailty criteria provided evidence of an association between frailty and cognitive impairment (31, 34, 35, 37). Joyce and colleagues reported that 15.2% of patients with frailty defined by Fried's frailty criteria also had cognitive impairment (34). Maurer et al. failed to find an association between Fried's frailty criteria and cognitive function, however this research team looked specifically at one cognitive domain rather than global cognitive function, which may explain the discrepancy (36).
Cacciatore et al. used the Frailty Staging System to rank participants based on degree of frailty (30, 38). Similar to the Fried criteria, the Frailty Staging System evaluates seven physical domains. Participants are awarded a point if the domain is intact. Higher scores indicate better function. There was evidence of an association between higher frailty stage and cognitive impairment in this study (p < 0.001)(30).
Gonzalez-Moneo evaluated frailty using the Barber questionnaire (32, 39). Whereas the Fried criteria and Frailty Staging System focus on physical frailty, the Barber questionnaire covers physical, psychological and social frailty (39). Although initial analysis revealed a significant association between cognitive impairment and frailty as indicated by the Barber questionnaire, the association lost significance in fully adjusted models (32). The lack of focus on the physical domain of frailty may explain the difference in the findings.
One study used a researcher-developed doorbell test as a proxy for frailty (33). Participants whose doorbell answering time fell above the median were considered more frail and those whose doorbell answering time fell below the mean were considered less frail (33). No significant difference in the proportion of people with cognitive impairment (Montreal Cognitive Impairment score <24) was observed between the two groups (p = 0.12), however these researchers used a researcher-developed indicator of frailty that has not been validated (33).
Measures of Cognitive Function
Screening measures, such as the Montreal Cognitive Assessment (MoCA) and Mini Mental State Examination (MMSE) were most commonly used to measure cognitive impairment. Three studies used the MoCA to evaluate cognitive function (31, 33, 37). The MoCA is a measure that evaluates several cognitive domains and takes ~10 min to complete. Scores range from 0 to 30 with higher scores indicating better cognitive function. One point is added to the score to correct for low levels of education (12 years or fewer). Scores <26 are considered indicative of cognitive impairment (40). Two of the research teams that evaluated cognitive function using the MoCA reported an association between greater frailty and cognitive impairment (31, 37). Gunton and colleagues reported that there was no association between frailty and MoCA scores (33). Gunton did not use a validated measure of frailty, however, which may explain the lack of association.
Two studies measured cognitive function using the Mini Mental State exam (MMSE) (30, 32). The MMSE is a measure of global cognitive function (41). The MMSE takes 5–10 min to complete (41). Scores range from 0 to 30 with higher scores indicating better global cognitive functioning function (41). Scores <24 indicate global cognitive impairment (42). Both studies that used the MMSE to measure cognitive function revealed an association between frailty and cognitive impairment. Greater frailty classification was associated with lower MMSE scores (p < 0.001) in the study by Cacciatore and colleagues (30).
Two studies used the Mini-Cog to evaluate cognitive function (34, 35). On the Mini-Cog, participants are given three words and asked to repeat them. Then the participants are asked to draw a clock given specific guidelines and then are asked to recall the three items they were given at the beginning of the evaluation (43, 44). Scores range from 0 to 5 with scores <2 indicating cognitive impairment (43, 44). Both of the studies that evaluated cognitive function with the MiniCog provided evidence of an association between cognitive impairment and frailty, although the proportion of frail persons who had cognitive impairment was small. Of the participants with frailty in the large (n = 1,180) study by Matsue and colleagues, 8% also had cognitive impairment (35).
One study used the Trail-Making Test Part B to measure executive function (20). Executive function is goal-directed behavior—the ability to identify a goal and perform what is needed to achieve that goal (20, 45). On the Trail-Making Test Part B, participants are presented with a series of circles, each of which contains a letter or number. Participants are asked to connect the circles in numerical and alphabetical sequence by alternating between numbers and letters (1-A-2-B-3-C). No association between frailty and Trail-Making Test score was identified by Maurer and colleagues (p = 0.61) (36). It is possible that frailty is not as closely associated with executive function as with other cognitive domains, such as memory or attention, however further research is needed to evaluate the association between frailty and specific cognitive domains.
Research Design
Seven studies used a cross-sectional research design (30–35, 37). Five of these demonstrated an association between frailty and cognitive function (30, 31, 34, 35, 37). Screening measures of global cognitive function and validated measures of frailty were used in each of these studies. The two cross-sectional studies that failed to provide evidence of an association between frailty and cognitive function included measures of frailty that have not been validated or did not focus on physical frailty specifically (32, 33).
Only one study evaluated change in frailty and cognitive function over time (36). Maurer and colleagues reported that improvements in frailty following implantation of a ventricular assist device were not associated with improvements in cognitive function (p = 0.61) (36). As discussed earlier, this research team focused on one, specific cognitive domain, which may have influenced the findings. Further, the sample in the final analysis of frailty and cognitive function was very small (n = 13). This study should be replicated on a larger sample to better understand the association between frailty and executive function following implantation of a ventricular assist device.
Discussion
Our review revealed that most studies of patients with HF demonstrated evidence of an association between greater frailty and cognitive impairment. Recently, the aging process has received increasing attention, with a focus on interactions between cognitive impairment and frailty syndrome necessitating this review. The elderly are a heterogeneous population, which means that with age, one could expect an increasing diversity of cognitive function, physical functioning, and aspects of social involvement (46). The coexistence of frailty and cognitive impairment has important implications in the clinical evaluation of the elderly. It is worth recalling that both frailty and cognitive impairment are known predictors of negative outcomes, such as risk of hospitalization, institutionalization, falls, and mortality (47–49). As both are known to occur in patients with HF, evaluation of both should occur on a regular basis to prevent poor clinical outcomes.
Presently, the reasons behind the association between frailty and cognitive function is unknown. The International Academy on Nutrition and Aging and the International Association of Gerontology and Geriatrics has proposed a concept known as “cognitive frailty” that represents the coexistence of physical frailty and cognitive impairment, yet no mechanism behind the association was proposed (50). It is possible that one condition may contribute to the development of the other or that both conditions share a pathophysiologic mechanism, however the lack of longitudinal research in this area makes it difficult to make any conclusions about shared biological mechanisms or causative associations.
Cognitive function includes several cognitive domains, each of which has a different purpose. Memory is the system of recording, storing, and reproducing information for future use. Attention involves selecting one perceptual object, one source of stimulation, or one topic of thought from among many possible options (51, 52). Through selection, we can focus on one stimulus or source of stimulation at the expense of others. Attention applies equally to perceptual as well as to “higher” cognitive processes, such as thinking (53). Executive functions encompass a wide range of different cognitive processes, including working memory, reasoning processes, problem solving, and planning (54). Different models of executive functions are described in varying ways, but generally defined as information processing abilities that are associated with goal-directed behavior or control of complex perceptions, especially in non-routine situations (20).
Patients with HF demonstrate numerous cognitive deficits related to memory, attention, and executive functions (55–58) and working memory (59). Miller et al. (60) compared three distinct groups of HF patients: patients with global cognitive decline, patients with memory impairment, and individuals without cognitive deficits. Analysis of the measures included in the study suggested that HF patients experienced deficits in the domains of memory, attention, executive functions, and learning (60). Although it is known that HF patients demonstrate deficits in multiple cognitive domains, most studies included in this review used a screening measure to evaluate global cognitive function rather than specific cognitive domains. Therefore, to advance knowledge, it is particularly important to conduct research not only on global cognitive function but on single cognitive domains.
Each of the aforementioned cognitive processes manifests very different functional brain activity. Neuronal areas correlated with executive function include the prefrontal cortex (especially the dorsolateral prefrontal cortex, the frontal pole region, the medial and supraorbital parts of the prefrontal cortex) but also the inferior parietal, occipital, and temporal regions (61, 62). The anatomy of attention includes many areas of the brain, including the temporoparietal junction, posterior parietal, superior frontal cortex, ventral prefrontal, superior, colliculus, and pulvinar of thalamus [(63) p. 396]. For working memory, the prefrontal cortex plays a key role, but there are many alternative views regarding neural organization of working memory (64). In contrast, the medial temporal lobe areas (hippocampal region) are responsible for memory itself [(63) p. 396].
The data obtained from the studies in our review point to a possible vascular etiology of cognitive deficits among patients with heart failure but the factors that contribute to these vascular deficits are unknown. As cognitive impairment is prevalent in persons with cardiovascular disease, it is possible that the same mechanisms that causes cardiovascular disease (atherosclerosis, etc.) also cause cognitive impairment. Frailty also is highly prevalent in the cardiovascular population, so it is possible that a shared mechanism may be responsible for all conditions. However, the nature of these associations is not established.
Only one of the included studies evaluated changes in frailty and cognitive function over time and the study was limited by small sample size and confined to one cognitive domain (36). Longitudinal research is needed to begin evaluating causal relationships and to determine if one factor influences another over time. It would be interesting to evaluate how changes in frailty influence cognitive function. Similarly, it would be interesting to determine if changes in cognitive function over time influence frailty syndrome. Once associations are identified, interventions could be developed that target one phenomenon in hopes of improving the other. However, the association between the frailty and cognitive function over time first has to be established.
Most studies selected for this review used MMSE and MoCA which are screening measures of cognitive impairment. The original purpose of MMSE is assessing cognitive functioning in an elderly hospitalized population (65). The MMSE has been recommended as a dementia screen (66, 67) and MMSE cutoffs are even used for qualification for Alzheimer's Disease (AD) treatment (68). The MMSE has been used to assess dementia among various populations such as Parkinson disease (69), delirium and memory post-delirium impairments in the stroke population (70) and elderly hospital patients (71). MMSE is also employed to monitor cognitive change during treatment in patients with depression (72). However, employing MMSE in some populations (e.g., stroke, patients with diabetes, cancer, multiple sclerosis) is questionable (65).
The MMSE is intended to screen for general cognitive decline, not to assess specific cognitive domains. Indeed, the MMSE focuses primarily on orientation (10/30 points) and language (9/30 points) but has limited ability to evaluate other cognitive domains. For example, MMSE had limited ability to detect changes in attention and processing speed among patients with diabetes (73). The studies have also shown the lack of correlation of the final score with age, education, gender and ethnic differences (67, 74–76). The studies that used the MMSE to measure cognitive function revealed an association between frailty and cognitive impairment. Greater frailty classification was associated with lower MMSE scores (30) and frailty was significantly associated with higher odds of cognitive impairment (32). However, there is evidence that the MMSE is not sensitive to mild forms of cognitive impairment (40, 41, 77), therefore the prevalence of cognitive impairment in these studies may be underestimated. Thus, MMSE may be useful for assessing severity of global cognitive dysfunctions, but may be not a sensitive tool for assessing cognitive domains in patients with mild cognitive impairments and early dementia (78). Using MMSE in research on frailty does not allow researchers to make conclusions about which specific cognitive domains are associated with frailty.
The MoCA resolves some of the limitations of the MMSE but still has limitations (40). The MoCA has the ability to assess several cognitive domains: (1) short term memory; (2) Visuospatial abilities; (3) Executive functions; (4) Attention, concentration and working memory; (5) Language and (6) Orientation to time and place (40). However, the developers of the MoCA have not recommended cut-off scores that indicate impairment in each cognitive domain so it is difficult to evaluate impairment in specific cognitive domains using a screening measure. In our review we found that one of three studies that evaluated cognitive function using the MoCA reported no association between frailty and MoCA scores (33). However, as indicated earlier, Gunton did not use a validated measure of frailty which may explain the lack of association. Further, it is possible that the lack of association is due to the use of inappropriate cutoff scores to define cognitive impairment in the sample. Although Gunton et al. employed the cutoff score recommended by the creators of the measure (40), O'Driscoll and Shaikh suggest that cultural differences (diet, education, employment, activities, living arrangements, etc.] could influence scores and that cutoff scores should be individualized based on cultural background (79) Therefore, it is possible that lack of associations between frailty and cognition results from using cutoff scores that were not sensitive enough to detect cognitive impairment in the given sample.
Neurocognitive batteries are more comprehensive tests that evaluate each cognitive domain in great detail (80). Neurocognitive batteries might include separate tests that measure the domains of memory, attention, language, orientation and executive function, for example. Although they are more comprehensive and provide a good indication about dysfunction in a specific cognitive domain, neurocognitive batteries are time-consuming to complete with many of them taking an hour or more to complete (45, 80, 81). Although neurocognitive batteries are impractical in a clinical setting, they are appropriate for research studies and would provide evidence of the association between frailty and function in specific cognitive domains.
Among crucial factors for maintaining health, reducing disease complications, and improving quality of life in patients with chronic disease are behaviors relevant to self-care and adherence (82, 83). Self-care is commonly understood as a “naturalistic decision-making process in which persons engage to maintain health and manage acute and chronic illness” (83). This implies that undertaking activities directed at self-care requires patients' cognitive ability and resources.
Cognitive impairment is frequently observed in patients with HF and negatively affects cognitive function in most domains, especially memory, learning, attention, and executive function (84–90). Moreover, poor motivation related to symptoms of depression, which are common among patients with HF, can lead to poor self-care and non-compliance with healthcare guides (91). Thus, these cognitive and emotional—motivation problems of HF patients can lead to a patient's inability to engage in effective self-care behavior. Unfortunately, the healthcare system is commonly built on the assumption that patients comply with healthcare providers' recommendations (83). As cognitive impairment interferes with the ability to follow recommendations, the combination of CI and HF is associated with increased mortality, re-hospitalization [see (92)], and poor quality of life (88).
Likewise, frailty syndrome is associated with decreased ability to perform basic activities of daily living which leads to more frequent hospitalizations and death (9). Recently, frailty has also been associated with self-care behaviors in several chronic diseases (93). However, very little is known about association between frailty and self-care behaviors in HF (94). Recently, the social aspect of frailty syndrome was found to adversely affect the ability to perform self-care in Polish elderly patients with HF (25). On the other hand, hierarchical regression analysis carried out by Son et al. (93) showed that frailty is not a significant predictor of self-care when health literacy is introduced into the model.
There also is a lack of knowledge about the combined effect of frailty and cognitive impairment on self-care behaviors. Frailty is not a relevant predictor of self-care when health literacy is controlled (93). However, cognitive decline can lower health literacy which could contribute to difficulty in obtaining healthcare services (95, 96) and difficulty performing self-care (93). To date, however, it is unclear if self-care differs among frail HF patients with cognitive impairment when compared to those without cognitive impairment. It also is not clear if self-care and QOL differ among cognitively impaired HF patients depending on whether or not they have frailty. It is possible that cognitive dysfunctions moderate the relationships between frailty and self-care behaviors, but more research is needed to elucidate this.
Limitations
The original aim of this review was to explore the relationship between cognition and frailty in the elderly with heart failure. Even though the present work has some limitations, our scrutiny has mainly achieved this purpose in our review. The main finding from this review is that the higher levels of frailty are associated with more cognitive impairments. In addition, this review highlights the presence of limitations in the previous studies exploring the association between frailty and cognition decline.
We found that majority of studies employ the frailty measures based on the Fried criteria, which addresses only physical frailty. Only one study used a more comprehensive measure of frailty which ended up with evidence for no association between frailty and cognitive decline. This suggests the large impact of conceptual issues with frailty on measures being applied to the relationship between frailty and cognitive impairments. Our recommendation is to use a consistent, validated measure of frailty in future studies exploring the relations between cognition and frailty. Also, the measure should encompass all aspects of frailty, including physical frailty, psychological frailty, and social frailty. The standard measure of frailty may be essential for comparing findings from various studies on specific domains of cognition. Further research on the relationship between cognition and frailty should also employ culturally adapted frailty measurements to the population with heart failure cut-offs point.
Like other systematic reviews, the quality of our review depends on the number and quality of the studies included. Only four databases were searched in this review, however other relevant studies may exist in other databases. There also is a risk of publication bias, in which positive results have a better chance of being published in high-impact journals (97–99). The absence of clinical studies with negative results may affect systematic reviews and distort the picture of relationships between factors being investigated (100). We also restricted our search to studies published since the year 2000, however it is possible that studies evaluating the association between frailty and cognitive function had been published earlier (98). Overall, the quality of the included studies was low, primarily because of the small sample size. Another limitation is that most of the studies were observational; hence, causation cannot be established. Lastly, all studies included in the present review investigated populations with HF patients, which also limits the generalizability of the findings of this review to other diseases.
Implications for Research
Our work highlights several gaps in our present knowledge in terms of frailty and cognition. A major gap is that only one study has evaluated the association between frailty and a specific cognitive domain. Researchers should begin to explore the association between frailty and cognitive function more deeply and identify which cognitive domains are most affected by frailty. Once identified, clinicians will be able to address the deficits in an effort to improve self-care.
To evaluate cognitive function in specific cognitive domains, researchers will have to use specialized measures designed to evaluate each cognitive domain. Several measures exist, including the Delayed Word Recall Test to evaluate memory (101), the Digit Symbol Substitution Test to evaluate attention (102), Boston Naming Test to evaluate language (103) and Trail Making Test Part B to evaluate executive function (20, 104). Researchers interested in exploring the effect of frailty on individual cognitive domains should create a battery of valid and reliable tests that evaluate the cognitive domains without the battery being too burdensome for research participants to complete.
Another important direction is to assess causal relationships. Although there is evidence of an association between frailty and cognitive impairment, it is unclear if one disorder precedes the other or even contributes to the development of the other. Only one longitudinal studies was identified but that one study was limited by small sample size and restricted to one cognitive domain. Future studies should examine if frailty affects cognitive function or there is the opposite direction. This knowledge would help clinicians understand the relationship between the variables and anticipate changes in functional status.
There are also important questions regarding the effects of frailty and cognitive impairment on HF outcomes. For instance, researchers should compare cognitive function in HF patients with frailty and those without frailty. Relatedly, researchers also should compare the degree of frailty in HF patients who have cognitive impairment and those who do not. Finally, although both frailty and cognitive impairment are associated with poor clinical outcomes individually, it is unknown if the combined impact of frailty and cognitive impairment influences the trajectory of HF disease outcomes. We recommend further investigation on the combined impact of frailty and cognition on important outcomes such as quality of life, stress management, severity of HF's symptoms and mortality.
Conclusions
Our review revealed an association between frailty and cognitive impairment. Although the evidence supports the association between frailty and cognitive impairment, the nature of the association has not yet been elucidated. Much more research needs to be done to determine which cognitive domains are affected by frailty and if there is a causative association between the two variables. Research also should begin to explore the combined effect of cognitive function and frailty on outcomes, such as self-care and mortality.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
All procedures and methods performed in this review were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Author Contributions
One team KF, IU, and ML searched PubMed/MEDLINE and Scopus while the other EC, TC, RS searched Excerpta Medica database (EMBASE) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL). KF recorded data from included studies into a table of evidence for all authors to review. Each author was assigned a portion of the manuscript and wrote the draft for their assigned section. KF revised the draft for grammar and flow. All authors reviewed, approved the final version of the manuscript, and contributed to conception and design of the study.
Funding
This publication of this review paper was supported by the Wroclaw Medical University internal research fund to the Department of Clinical Nursing, Faculty of Health Sciences.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. (2018) 391:572–80. doi: 10.1016/S0140-6736(17)32520-5
2. James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, national incidence prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1789–858. doi: 10.1016/S0140-6736(18)32279-7
3. van Riet EE, Hoes AW, Wagenaar KP, Limburg A, Landman MA, Rutten FH. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail. (2016) 18:242–52. doi: 10.1002/ejhf.483
4. Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol. (2012) 21:365–71. doi: 10.1016/j.carpath.2011.11.007
5. Dunlay SM, Roger VL. Understanding the epidemic of heart failure: past, present, and future. Curr Heart Fail Rep. (2014) 11:404–15. doi: 10.1007/s11897-014-0220-x
6. Stewart R. Cardiovascular disease and frailty: what are the mechanistic links? Clin Chem. (2019) 65:80–6. doi: 10.1373/clinchem.2018.28731
7. Veronese N, Cereda E, Stubbs B, Solmi M, Luchini C, Manzato E, et al. Risk of cardiovascular disease morbidity and mortality in frail and pre-frail older adults: results from a meta-analysis and exploratory meta-regression analysis. Ageing Res Rev. (2017) 35:63–73. doi: 10.1016/j.arr.2017.01.003
8. Chen MA. Frailty and cardiovascular disease: potential role of gait speed in surgical risk stratification in older adults. J Geriatr Cardiol. (2015) 12:44–56. doi: 10.11909/j.issn.1671-5411.2015.01.006
9. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. (2001) 56:M146–56. doi: 10.1093/gerona/56.3.M146
10. Joyce E. Frailty in advanced heart failure. Heart Fail Clin. (2016) 12:363–74. doi: 10.1016/j.hfc.2016.03.006
11. Bagshaw SM, Stelfox HT, McDermid RC, Rolfson DB, Tsuyuki RT, Baig N, et al. Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ. (2014) 186:E95–102. doi: 10.1503/cmaj.130639
12. Landi F, Cruz-Jentoft AJ, Liperoti R, Russo A, Giovannini S, Tosato M, et al. Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing. (2013) 42:203–9. doi: 10.1093/ageing/afs194
13. Forman DE, Santanasto AJ, Boudreau R, Harris T, Kanaya AM, Satterfield S, et al. Impact of incident heart failure on body composition over time in the health, aging, and body composition study population. Circ Heart Fail. (2017) 10:e003915. doi: 10.1161/CIRCHEARTFAILURE.117.003915
14. Uchmanowicz I, Loboz-Rudnicka M, Szelag P, Jankowska-Polanska B, Loboz-Grudzien K. Frailty in heart failure. Curr Heart Fail Rep. (2014) 11:266–73. doi: 10.1007/s11897-014-0198-4
15. Vitale C, Spoletini I, Rosano GM. Frailty in heart failure: implications for management. Card Fail Rev. (2018) 4:104–6. doi: 10.15420/cfr.2018.22.2
16. Gwyther H, Shaw R, Jaime Dauden EA, D'Avanzo B, Kurpas D, Bujnowska-Fedak M, et al. Understanding frailty: a qualitative study of European healthcare policy-makers' approaches to frailty screening and management. BMJ Open. (2018) 8:e018653. doi: 10.1136/bmjopen-2017-018653
17. Cesari M, Calvani R, Marzetti E. Frailty in older persons. Clin Geriatr Med. (2017) 33:293–303. doi: 10.1016/j.cger.2017.02.002
18. Uchmanowicz I. Oxidative stress, frailty and cardiovascular diseases: current evidence. Adv Exp Med Biol. (2020) 1216:65–77. doi: 10.1007/978-3-030-33330-0_8
19. Brigola AG, Rossetti ES, Dos Santos BR, Neri AL, Zazzetta MS, Inouye K, et al. Relationship between cognition and frailty in elderly: a systematic review. Dement Neuropsychol. (2015) 9:110–9. doi: 10.1590/1980-57642015DN92000005
20. Lezak MD, Howieson DB, Loring DW, Fischer JS. Neuropsychological Assessment. Oxford: Oxford University Press (2004).
21. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. (2005) 173:489–95. doi: 10.1503/cmaj.050051
22. Sternberg SA, Wershof Schwartz A, Karunananthan S, Bergman H, Mark Clarfield A. The identification of frailty: a systematic literature review. J Am Geriatr Soc. (2011) 59:2129–38. doi: 10.1111/j.1532-5415.2011.03597.x
23. Avila-Funes JA, Amieva H, Barberger-Gateau P, Le Goff M, Raoux N, Ritchie K, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc. (2009) 57:453–61. doi: 10.1111/j.1532-5415.2008.02136.x
24. Buchman AS, Schneider JA, Leurgans S, Bennett DA. Physical frailty in older persons is associated with Alzheimer disease pathology. Neurology. (2008) 71:499–504. doi: 10.1212/01.wnl.0000324864.81179.6a
25. Uchmanowicz I, Wleklik M, Gobbens RJ. Frailty syndrome and self-care ability in elderly patients with heart failure. Clin Interv Aging. (2015) 10:871–7. doi: 10.2147/CIA.S83414
26. Dodson JA, Truong, TT, Towle VR, Kerins G, Chaudhry SI. Cognitive impairment in older adults with heart failure: prevalence, documentation, and impact on outcomes. Am J Med. (2013) 126:120–6. doi: 10.1016/j.amjmed.2012.05.029
27. Butts B, Gary R. Coexisting frailty, cognitive impairment, and heart failure: implications for clinical care. J Clin Outcomes Manag. (2015) 22:38–46.
28. Webb C, Roe B. Reviewing Research Evidence for Nursing Practice: Systematic Reviews. Oxford: John Wiley and Sons (2008). doi: 10.1002/9780470692127
29. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. (2009) 62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005
30. Cacciatore F, Abete P, Mazzella F, Viati L, Della Morte D, D'Ambrosio D, et al. Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest. (2005) 35:723–30. doi: 10.1111/j.1365-2362.2005.01572.x
31. Denfeld QE, Winters-Stone K, Mudd JO, Hiatt SO, Chien CV, Lee CS. Frequency of and significance of physical frailty in patients with heart failure. Am J Cardiol. (2017) 119:1243–9. doi: 10.1016/j.amjcard.2016.12.024
32. Gonzalez-Moneo MJ, Sanchez-Benavides G, Verdu-Rotellar JM, Cladellas M, Bruguera J, Quinones-Ubeda S, et al. Ischemic aetiology, self-reported frailty, and gender with respect to cognitive impairment in chronic heart failure patients. BMC Cardiovasc Disord. (2016) 16:163. doi: 10.1186/s12872-016-0349-5
33. Gunton JE, Nandal S, Jones J, Chew DP, Marwick TH, De Pasquale CG. Validation of the “Doorbell Test”: a novel functional test of frailty and clinical status after acute decompensated heart failure. Heart Lung Circ. (2020) 29:1054–62. doi: 10.1016/j.hlc.2019.08.010
34. Joyce E, Howell EH, Senapati A, Starling RC, Gorodeski EZ. Prospective assessment of combined handgrip strength and Mini-Cog identifies hospitalized heart failure patients at increased post-hospitalization risk. ESC Heart Fail. (2018) 5:948–52. doi: 10.1002/ehf2.12300
35. Matsue Y, Kamiya K, Saito H, Saito K, Ogasahara Y, Maekawa E, et al. Prevalence and prognostic impact of the coexistence of multiple frailty domains in elderly patients with heart failure: the FRAGILE-HF cohort study. Eur J Heart Fail. (2020) 22:2112–9. doi: 10.1002/ejhf.1926
36. Maurer MS, Horn E, Reyentovich A, Dickson VV, Pinney S, Goldwater D, et al. Can a left ventricular assist device in individuals with advanced systolic heart failure improve or reverse frailty? J Am Geriatr Soc. (2017) 65:2383–90. doi: 10.1111/jgs.15124
37. Pandey A, Kitzman D, Whellan DJ, Duncan PW, Mentz RJ, Pastva AM, et al. Frailty among older decompensated heart failure patients: prevalence, association with patient-centered outcomes, and efficient detection methods. JACC Heart Fail. (2019) 7:1079–88. doi: 10.1016/j.jchf.2019.10.003
38. Lachs MS, Feinstein AR, Cooney LM Jr, Drickamer MA, Marottoli RA, Pannill FC, et al. A simple procedure for general screening for functional disability in elderly patients. Ann Intern Med. (1990) 112:699–706. doi: 10.7326/0003-4819-112-9-699
39. Barber JH, Wallis JB, McKeating E. A postal screening questionnaire in preventive geriatric care. J R Coll Gen Pract. (1980) 30:49–51.
40. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
41. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
42. Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. (1992) 40:922–35. doi: 10.1111/j.1532-5415.1992.tb01992.x
43. Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive 'vital signs' measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. (2000) 15:1021–7. doi: 10.1002/1099-1166(200011)15:11andlt;1021::AID-GPS234andgt;3.0.CO;2-6.
44. Borson S, Scanlan JM, Watanabe J, Tu SP, Lessig M. Simplifying detection of cognitive impairment: comparison of the Mini-Cog and Mini-Mental State Examination in a multiethnic sample. J Am Geriatr Soc. (2005) 53:871–4. doi: 10.1111/j.1532-5415.2005.53269.x
45. Bauer LC, Johnson JK, Pozehl BJ. Cognition in heart failure: an overview of the concepts and their measures. J Am Acad Nurse Pract. (2011) 23:577–85. doi: 10.1111/j.1745-7599.2011.00668.x
46. Santoni G, Angleman S, Welmer AK, Mangialasche F, Marengoni A, Fratiglioni L. Age-related variation in health status after age 60. PLoS ONE. (2015) 10:e0120077. doi: 10.1371/journal.pone.0120077
47. Ampadu J, Morley JE. Heart failure and cognitive dysfunction. Int J Cardiol. (2015) 178:12–23. doi: 10.1016/j.ijcard.2014.10.087
48. Murad K, Goff DC Jr, Morgan TM, Burke GL, Bartz TM, Kizer JR, et al. Burden of comorbidities and functional and cognitive impairments in elderly patients at the initial diagnosis of heart failure and their impact on total mortality: the cardiovascular health study. JACC Heart Fail. (2015) 3:542–50. doi: 10.1016/j.jchf.2015.03.004
49. Zucchelli A, Vetrano DL, Marengoni A, Grande G, Romanelli G, Calderon-Larranaga A, et al. Frailty predicts short-term survival even in older adults without multimorbidity. Eur J Intern Med. (2018) 56:53–6. doi: 10.1016/j.ejim.2018.06.012
50. Kelaiditi E, Cesari M, Canevelli M, van Kan GA, Ousset PJ, Gillette-Guyonnet S, et al. Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J Nutr Health Aging. (2013) 17:726–34. doi: 10.1007/s12603-013-0367-2
51. Broadbent DE. A mechanical model for human attention and immediate memory. Psychol Rev. (1957) 64:205–15. doi: 10.1037/h0047313
53. Necka E, Orzechowski J, Szymura B, Wichary S. Psychologia Poznawcza [Cognitive Psychology]. PWN SA (2020).
54. Collins A, Koechlin E. Reasoning, learning, and creativity: frontal lobe function and human decision-making. PLoS Biol. (2012) 10:e1001293. doi: 10.1371/journal.pbio.1001293
55. Hoth KF, Poppas A, Moser DJ, Paul RH, Cohen RA. Cardiac dysfunction and cognition in older adults with heart failure. Cogn Behav Neurol. (2008) 21:65–72. doi: 10.1097/WNN.0b013e3181799dc8
56. Incalzi RA, Trojano L, Acanfora D, Crisci C, Tarantino F, Abete P, et al. Verbal memory impairment in congestive heart failure. J Clin Exp Neuropsychol. (2003) 25:14–23. doi: 10.1076/jcen.25.1.14.13635
57. Vogels RL, Oosterman JM, van Harten B, Scheltens P, van der Flier WM, Schroeder-Tanka JM, et al. Profile of cognitive impairment in chronic heart failure. J Am Geriatr Soc. (2007) 55:1764–70. doi: 10.1111/j.1532-5415.2007.01395.x
58. Wolfe R, Worrall-Carter L, Foister K, Keks N, Howe V. Assessment of cognitive function in heart failure patients. Eur J Cardiovasc Nurs. (2006) 5:158–64. doi: 10.1016/j.ejcnurse.2005.10.005
59. Harkness K, Demers C, Heckman GA, McKelvie RS. Screening for cognitive deficits using the Montreal cognitive assessment tool in outpatients >/=65 years of age with heart failure. Am J Cardiol. (2011) 107:1203–7. doi: 10.1016/j.amjcard.2010.12.021
60. Miller LA, Spitznagel MB, Alosco ML, Cohen RA, Raz N, Sweet LH, et al. Cognitive profiles in heart failure: a cluster analytic approach. J Clin Exp Neuropsychol. (2012) 34:509–20. doi: 10.1080/13803395.2012.663344
61. Ragland JD, Glahn DC, Gur RC, Censits DM, Smith RJ, Mozley PD, et al. PET regional cerebral blood flow during working and declarative memory: relationship with task performance. Neuropsychology. (1997) 11:222–31. doi: 10.1037/0894-4105.11.2.222
62. Berman K. Physiological activation of a cortical network during performance of the Wisconsin Card Sorting Test: a positron emission tomography study. Neuropsychologia. (1995) 33:1027–46. doi: 10.1016/0028-3932(95)00035-2
63. Gazzaniga MS, Ivry RB, Mangun GR. Cognitive Neuroscience: The Biology of the Mind. Norton WW and Company (2018).
64. Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience. (2006) 139:23–38. doi: 10.1016/j.neuroscience.2005.06.005
65. Nieuwenhuis-Mark RE. The death knoll for the MMSE: has it outlived its purpose? J Geriatr Psychiatry Neurol. (2010) 23:151–7. doi: 10.1177/0891988710363714
66. Boustani M, Peterson B, Hanson L, Harris R, Lohr KN, et al. SPST screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. (2003) 138:927–37. doi: 10.7326/0003-4819-138-11-200306030-00015
67. Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. (2001) 56:1133–42. doi: 10.1212/WNL.56.9.1133
68. National Institute for Health and Care Excellence. Overview: Donepezil, Galantamine, Rivastigmine and Memantine for the Treatment of Alzheimer's Disease: Guidance. NICE (2011). Available online at: https://www.nice.org.uk/guidance/ta217
69. Harvey PD, Ferris SH, Cummings JL, Wesnes KA, Hsu C, Lane RM, et al. Evaluation of dementia rating scales in Parkinson's disease dementia. Am J Alzheimers Dis Other Demen. (2010) 25:142–8. doi: 10.1177/1533317509333904
70. McManus J, Pathansali R, Hassan H, Ouldred E, Cooper D, Stewart R, et al. The course of delirium in acute stroke. Age Ageing. (2009) 38:385–9. doi: 10.1093/ageing/afp038
71. O'Keeffe ST, Mulkerrin EC, Nayeem K, Varughese M, Pillay I. Use of serial Mini-Mental State Examinations to diagnose and monitor delirium in elderly hospital patients. J Am Geriatr Soc. (2005) 53:867–70. doi: 10.1111/j.1532-5415.2005.53266.x
72. Han L, McCusker J, Cole M, Abrahamowicz M, Capek R. 12-month cognitive outcomes of major and minor depression in older medical patients. Am J Geriatr Psychiatry. (2008) 16:742–51. doi: 10.1097/JGP.0b013e31817c6ad7
73. Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes–systematic overview of prospective observational studies. Diabetologia. (2005) 48:2460–9. doi: 10.1007/s00125-005-0023-4
74. Butler SM, Ashford JW, Snowdon DA. Age, education, and changes in the Mini-Mental State Exam scores of older women: findings from the Nun Study. J Am Geriatr Soc. (1996) 44:675–81. doi: 10.1111/j.1532-5415.1996.tb01831.x
75. Espino DV, Lichtenstein MJ, Palmer RF, Hazuda HP. Ethnic differences in mini-mental state examination (MMSE) scores: where you live makes a difference. J Am Geriatr Soc. (2001) 49:538–48. doi: 10.1046/j.1532-5415.2001.49111.x
76. Grigoletto F, Zappala G, Anderson DW, Lebowitz BD. Norms for the Mini-Mental State Examination in a healthy population. Neurology. (1999) 53:315–20. doi: 10.1212/WNL.53.2.315
78. Mitchell AJ. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res. (2009) 43:411–31. doi: 10.1016/j.jpsychires.2008.04.014
79. O'Driscoll C, Shaikh M. Cross-cultural applicability of the montreal cognitive assessment (MoCA): a systematic review. J Alzheimers Dis. (2017) 58:789–801. doi: 10.3233/JAD-161042
80. Pressler SJ. Cognitive functioning and chronic heart failure: a review of the literature (2002-July 2007). J Cardiovasc Nurs. (2008) 23:239–49. doi: 10.1097/01.JCN.0000305096.09710.ec
81. Bauer L, Pozehl B, Hertzog M, Johnson J, Zimmerman L, Filipi M. A brief neuropsychological battery for use in the chronic heart failure population. Eur J Cardiovasc Nurs. (2012) 11:223–30. doi: 10.1016/j.ejcnurse.2011.03.007
82. Osokpo O, Riegel B. Cultural factors influencing self-care by persons with cardiovascular disease: an integrative review. Int J Nurs Stud. (2019) 103383. doi: 10.1016/j.ijnurstu.2019.06.014
83. Riegel B, Moser DK, Buck HG, Dickson VV, Dunbar SB, Lee CS, et al. Self-Care for the prevention and management of cardiovascular disease and stroke: a scientific statement for healthcare professionals from the American Heart Association. J Am Heart Assoc. (2017) 6:e006997. doi: 10.1161/JAHA.117.006997
84. Dardiotis E, Giamouzis G, Mastrogiannis D, Vogiatzi C, Skoularigis J, Triposkiadis F, et al. Cognitive impairment in heart failure. Cardiol Res Pract. (2012) 2012:595821. doi: 10.1155/2012/595821
85. Gottesman RF, Grega MA, Bailey MM, Zeger SL, Baumgartner WA, McKhann GM, et al. Association between hypotension, low ejection fraction and cognitive performance in cardiac patients. Behav Neurol. (2010) 22:63–71. doi: 10.1155/2010/725353
86. Hanon O, Vidal JS, de Groote P, Galinier M, Isnard R, Logeart D, et al. Prevalence of memory disorders in ambulatory patients aged >/=70 years with chronic heart failure (from the EFICARE study). Am J Cardiol. (2014) 113:1205–10. doi: 10.1016/j.amjcard.2013.12.032
87. Harkness K, Heckman GA, Akhtar-Danesh N, Demers C, Gunn E, McKelvie RS. Cognitive function and self-care management in older patients with heart failure. Eur J Cardiovasc Nurs. (2014) 13:277–84. doi: 10.1177/1474515113492603
88. Pressler SJ, Subramanian U, Kareken D, Perkins SM, Gradus-Pizlo I, Sauve MJ, et al. Cognitive deficits and health-related quality of life in chronic heart failure. J Cardiovasc Nurs. (2010) 25:189–98. doi: 10.1097/JCN.0b013e3181ca36fe
89. Pressler SJ, Subramanian U, Kareken D, Perkins SM, Gradus-Pizlo I, Sauve MJ, et al. Cognitive deficits in chronic heart failure. Nurs Res. (2010) 59:127–39. doi: 10.1097/NNR.0b013e3181d1a747
90. Vogels RL, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: a systematic review of the literature. Eur J Heart Fail. (2007) 9:440–9. doi: 10.1016/j.ejheart.2006.11.001
91. Bauer LK, Caro MA, Beach SR, Mastromauro CA, Lenihan E, Januzzi JL, et al. Effects of depression and anxiety improvement on adherence to medication and health behaviors in recently hospitalized cardiac patients. Am J Cardiol. (2012) 109:1266–71. doi: 10.1016/j.amjcard.2011.12.017
92. Kewcharoen J, Prasitlumkum N, Kanitsoraphan C, Charoenpoonsiri N, Angsubhakorn N, Putthapiban P, et al. Cognitive impairment associated with increased mortality rate in patients with heart failure: a systematic review and meta-analysis. J Saudi Heart Assoc. (2019) 31:170–8. doi: 10.1016/j.jsha.2019.06.001
93. Son YJ, Shim DK, Seo EK, Seo EJ. Health literacy but not frailty predict self-care behaviors in patients with heart failure. Int J Environ Res Public Health. (2018) 15:2474. doi: 10.3390/ijerph15112474
94. Sedlar N, Lainscak M, Martensson J, Stromberg A, Jaarsma T, Farkas J. Factors related to self-care behaviours in heart failure: a systematic review of European Heart Failure Self-Care Behaviour Scale studies. Eur J Cardiovasc Nurs. (2017) 16:272–82. doi: 10.1177/1474515117691644
95. Cajita MI, Cajita TR, Han HR. Health literacy and heart failure: a systematic review. J Cardiovasc Nurs. (2016) 31:121–30. doi: 10.1097/JCN.0000000000000229
96. Wolf MS, Gazmararian JA, Baker DW. Health literacy and functional health status among older adults. Arch Intern Med. (2005) 165:1946–52. doi: 10.1001/archinte.165.17.1946
97. Chalmers I. Underreporting research is scientific misconduct. JAMA. (1990) 263:1405–08. doi: 10.1001/jama.1990.03440100121018
98. Dubben HH, Beck-Bornholdt HP. Systematic review of publication bias in studies on publication bias. BMJ. (2005) 331:433–4. doi: 10.1136/bmj.38478.497164.F7
99. Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. (1991) 337:867–72. doi: 10.1016/0140-6736(91)90201-Y
100. Ioannidis JP. Effect of the statistical significance of results on the time to completion and publication of randomized efficacy trials. JAMA. (1998) 279:281–6. doi: 10.1001/jama.279.4.281
101. Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol. (1989) 46:141–5. doi: 10.1001/archneur.1989.00520380041011
102. Hartman DE. Wechsler Adult Intelligence Scale IV (WAIS IV): return of the gold standard. Appl Neuropsychol. (2009) 16:85–7. doi: 10.1080/09084280802644466
Keywords: cognition, frailty, heart failure, review, cognitive impairment
Citation: Faulkner KM, Uchmanowicz I, Lisiak M, Cichoń E, Cyrkot T and Szczepanowski R (2021) Cognition and Frailty in Patients With Heart Failure: A Systematic Review of the Association Between Frailty and Cognitive Impairment. Front. Psychiatry 12:713386. doi: 10.3389/fpsyt.2021.713386
Received: 22 May 2021; Accepted: 08 June 2021;
Published: 02 July 2021.
Edited by:
Mateusz Cybulski, Medical University of Bialystok, PolandReviewed by:
Kinga Mastalerz, Karolinska University Hospital, SwedenJacek Migaj, Poznan University of Medical Sciences, Poland
Copyright © 2021 Faulkner, Uchmanowicz, Lisiak, Cichoń, Cyrkot and Szczepanowski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Remigiusz Szczepanowski, cmVtaWdpdXN6LnN6Y3plcGFub3dza2lAdW1lZC53cm9jLnBs
†These authors have contributed equally to this work and share first authorship
 Kenneth M. Faulkner
Kenneth M. Faulkner Izabella Uchmanowicz
Izabella Uchmanowicz Magdalena Lisiak
Magdalena Lisiak Ewelina Cichoń
Ewelina Cichoń Tomasz Cyrkot
Tomasz Cyrkot Remigiusz Szczepanowski
Remigiusz Szczepanowski