Abstract
Sex differences are prevalent in multiple mental disorders. Internalizing disorders are more commonly diagnosed in women, whereas externalizing and neurodevelopmental disorders are more often diagnosed in men. Significant sex/gender differences are reported in prevalence, symptom profile, age of onset, comorbidities, functional impairment, prognosis, as well as in responses to various treatments. In this conceptual article, we discuss theories and empirical studies of sex- and gender-related influences in mental health, by focusing on three examples: autism spectrum disorder (ASD), acknowledged as a disorder whose roots are mainly biological; eating disorders, whose origins are considered to be mainly psychosocial, and posttraumatic stress disorder (PTSD), an environmentally caused disorder with both psychosocial and biological underpinnings. We examine the ways in which sex differences emerge, from conception through adulthood. We also examine how gender dichotomies in exposures, expectations, role assumptions, and cultural traditions impact the expression of our three selected mental illnesses. We are especially interested in how sex-based influences and gender-based influences interact with one another to affect mental illness. We suggest that sex and gender are multi-faceted and complex phenomena that result in variations, not only between men and women, but also within each sex and gender through alterations in genes, hormone levels, self-perceptions, trauma experiences, and interpersonal relationships. Finally, we propose a conceptual diatheses-stress model, depicting how sex and gender come together to result in multiple sex/gender differences across mental disorders. In our model, we categorize diatheses into several categories: biological, intrapersonal, interpersonal, and environmental. These diatheses interact with exposure to stressors, ranging from relatively minor to traumatic, which allows for the sometimes bidirectional influences of acute and long-term stress responses. Sex and gender are discussed at every level of the model, thereby providing a framework for understanding and predicting sex/gender differences in expression, prevalence and treatment response of mental disorders. We encourage more research into this important field of study.
Introduction
This hypothesis and theory paper is based on observations of a substantial effect of sex and gender on the expression of many mental disorders. We consider “sex” and “gender” to be two different, though overlapping, concepts. Both refer to the designation of “male/masculine” or “female/feminine,” but research has traditionally addressed the two terms differently. Individual disciplines have used different methodologies, and sometimes different terminologies to study sex and gender. Sex is generally defined as the biological distinction between males and females based on sex chromosomes XX or XY, established at conception. The male/female distinction is identified visually, even prior to birth, by the structure of external genitalia, and is later confirmed by its consistency with internal reproductive organs.
In contrast to sex, gender signifies one's relationship to the subjective perception of oneself as male or female. Gender is grounded in biological sex and strongly influenced by genotype and the prenatal hormonal environment, yet it is also subject to the perception and expectations of others and affected by parental rearing practices, by environmental exposures, by the development of sexual preferences, by cultural pressures, and by the process of identity formation. Research on the influence of sex has used biological tools, and has often been based on animal models. Research on gender has used anthropological or sociological terminology and methodology, with observations often viewed through a feminist lens. Though often treated as separate constructs, the two (sex and gender) are inseparable and neither can be fully understood in the absence of the other.
The worldwide prevalence of some mental disorders is reported as significantly more prevalent in girls and women whereas other disorders are more often diagnosed in boys and men. As a general rule of thumb, from puberty onward, internalizing disorders (those during which negative emotions are subjectively experienced) are more commonly seen in women (1–3). This group of disorders includes, among others, disordered eating and disorders and posttraumatic stress disorder (PTSD) (1, 4–7), which are characterized by subjective feelings of low self-esteem and insecurity, a tendency to self-blame, and a habit of inhibiting the outward expression of anger (8). In contrast, externalizing disorders, where distress is outwardly expressed through impulsive or aggressive behavior, are more commonly reported in men (8). Externalizing disorders are characterized by behaviors rather than by emotions, and by impaired ability to communicate feelings (1, 4, 6, 7, 9). Another general distinction with respect to prevalence, is that disorders that first manifest in childhood are usually more prevalent in boys. This is true for autism spectrum disorders (ASD) (10, 11). The rates of mental disorders more commonly seen in women rise after puberty and come to surpass those of men. In the elderly, male/female differences in prevalence tend to wane (11). For some DSM-5 disorders, the extent of prevalence differences by sex/gender is substantial (please see Table 1).
Table 1
| Diagnosis | OR | 95% CI | References |
|---|---|---|---|
| MDD | 2.0a | 1.9–2.2* | (12) |
| Binge-eating disorder | 2.3 | 1.8–2.9* | (13) |
| Bulimia nervosa | 3.5 | 2.6–4.7* | (13) |
| PTSD | 2.0a | 1.8–2.2* | (14) |
| GADd | 1.8 | 1.7–2.0* | (15) |
| ASD | 4.3b | 4.0–4.6 | (16) |
| Schizophrenia | 1.0c | 0.9–1.2 | (17) |
| Bipolar I disorder | 0.9 | 0.7–1.1 | (18) |
| ADHD | 0.4a | 0.4–0.5* | (19) |
| Alcohol use disorder | 0.5a | 0.5–0.5* | (20) |
| Drug use disorder | 0.6a | 0.5–0.6* | (21) |
| ASPD | 0.3a | 0.2–0.3 | (22) |
| Agoraphobia | 1.9d | 1.5–2.4 | (23) |
F:M sex differences across mental disorders.
PR, F:M prevalence ratio; MDD, major depressive disorder; PTSD, posttraumatic stress disorder; FAD, generalized anxiety disorder; ASD, autism spectrum disorder; ADHD, attention deficit hyperactivity disorder; ASPD, antisocial personality disorder.
Converted from source to represent odds ratio in women compared to men.
Sex ratio for ASD caseness among 8-year-old children.
Data are based on a Chinese population; other data are based on Western populations, mostly from the USA.
Data based on DSM-5 only diagnosis; similar results were found for those meeting both DSM-5 and DSM-IV criteria.
Sex difference significant at p < 0.05.
Whereas, most research on sex/gender differences in mental disorders has focused on prevalence and symptom severity, there also exist differences in the nature of symptoms, in age of onset, in frequency of episode recurrence, in chronicity, comorbidities, precursors, triggers, functional impairment, and treatment response (24, 25). In some cases, sex/gender differences are found in the psychiatric comorbidities associated with a predominant mental disorder -e.g., aggressive behavior and substance use disorder in men, contrasting with eating disorders and depression in women (26–30). The purpose of this hypothesis and theory paper is to apply new interpretations of recent data to the role of sex and gender in mental illness. Our aim is to highlight how sex and gender coalesce to result in differences between men's and women's mental health, the focus of this special Frontiers issue. Our three selected diagnoses each represent different poles of the sex/gender continuum, with ASD being more commonly diagnosed in boys, PTSD presenting in both sexes, and eating disorders seen far more frequently in women. Another important distinction among the three is that ASD begins early in life; eating disorders usually start at adolescence, and PTSD in adulthood. Finally, these three disorders were selected because they are differently influenced by diatheses (predispositions) and stressors (triggers): ASD more by biological diatheses, eating disorders more by societal diatheses, and PTSD requiring, by definition, a traumatic stressor. These three disorders thus fit into different niches of the new theoretical framework that we will present at the end of this paper.
Autism Spectrum Disorders
ASD is ~4 times more prevalent in boys than in girls (16, 31). The source of this marked difference remains unclear; its basis is likely to be multifactorial but is poorly understood. There is no doubt about the biological etiology of ASD—these disorders are highly heritable. But reasons for the sex ratio in prevalence is a mystery because few of the 100+ thus far identified risk genes reside on sex chromosomes. Baron-Cohen has proposed the “extreme male brain theory” of ASD, attributing sex differences to testosterone levels (32). This is plausible because multiple lines of evidence suggest dysregulated steroidogenesis (33, 34), but testosterone levels are unlikely to fully explain the male/female discrepancy. Rather than searching for the causes of heightened risk in males, one might look, instead, for female protective factors. Girls who are diagnosed with ASD carry significantly more genetic load than boys (35), suggesting adaptive mechanisms such as the ability to effectively mask ASD symptoms (36). If such mechanisms exist, they remain to be discovered. There are also several clinical differences between boys and girls with ASD: more externalizing behavior (e.g., aggressive outbursts, social impairment, hyperactivity, restricted behaviors and interests) in boys and more internalizing symptoms in girls (e.g., depression, anxiety, emotional symptoms) (37). Since diagnostic criteria are based on the much more prevalent male presentation, it is likely that girls, who present somewhat differently, are underdiagnosed (38).
Eating Disorders
If one omits neurodevelopmental disorders such as ASD, the male/female prevalence ratio is higher in eating disorders than in any other forms of mental illness. Anorexia nervosa affects 0.9–4% of women and roughly 0.3% of men (39). This major difference in prevalence rate is usually attributed not to biology but to contrasts in the gender roles assumed by women and men in adolescence and to the peer pressures to which they are exposed (40). Multiple interacting factors, however, probably play a part. Genetic and epigenetic causes, gonadal hormone levels, and constituents of the immune system have all been considered as potentially contributory to the prevalence ratio. In other words, socialization into gender-specific roles and societal expectations may be exaggerating innate proclivities (41). Because thinness in many cultures equates to female beauty, and beauty serves as a proxy for youth, health and reproductive fitness, eating disorders may, from an evolutionary perspective, reflect an ancient mating strategy—enhancing beauty to attract men who will ensure long term survival of offspring (42). In modern times, this strategy is made increasingly complex because media and the fashion industry define beauty standards.
Posttraumatic Stress Disorder
In most prevalence studies, approximately twice as many women as men are diagnosed with PTSD, and this ratio is consistent across diagnostic systems, measurement methods, ethnicities, cultural backgrounds, and study populations (43). The trauma preceding this disorder is considered to be an exposure to one or more extremely threatening events, such as sexual, physical, or severe emotional abuse, a death in the family, or a serious accident, natural or manmade disaster (44). Symptoms of PTSD are more severe, chronic, and recurrent in women than they are in men (45–47). Associated symptoms/behaviors also differ. Women with PTSD in addition to the core symptoms report dissociative phenomena, somatization, disordered eating, low self-esteem, guilt, and shame. Men, in contrast, present with problems relating to aggression, thrill-seeking, risk-taking, and impulse-control (7). Diagnosable comorbidities induced by trauma also differentiate men and women -e.g., depressive and anxiety disorders accompany PTSD in women while substance use disorders are prevalent in men (48).
We will now explore in greater detail some of the theories that have been proposed to explain sex and gender differences in mental illnesses, using our three selected disorders as examples. The emphasis in each of the theories is different, but they all acknowledge that they are, in themselves, incomplete, and that complementary perspectives are needed for a full explanation of the many factors that result in sex/gender differences.
Sex-Based Theories
Sex Chromosomes
At conception, most females possess two X chromosomes and most males have one X and one Y. Some male-specific genes are located on the Y chromosome, the most important one being the SRY or testis-determining gene. Once testes develop, the process of subsequent differentiation between the sexes begins (49). Male testes and female ovaries secrete different levels of hormones (estrogens, androgens, progestins, and anti-Müllerian hormone) that subsequently influence most body organs, including the brain. Because females have two X chromosomes, one in each body cell is automatically and randomly inactivated so that males and females end up with an approximately equal total of gene products (50). But ~25% of genes on the inactivated X chromosome escape full inactivation. This process, in addition to epigenetic effects on genes on all chromosomes (50, 51), results in sex/gender differences in the rates of many medical conditions. Proposed mechanisms include the unequal level of gene products expressed by sex biased genes. These are genes that are present in both sexes but that express different quantities of protein products in men and women due to the impact of either epigenetic factors or sex hormone levels (52).
Genes are important even in conditions such as eating disorders, which result from mainly social causes. Twin-based heritability for anorexia nervosa ranges from 48 to 74%, for bulimia nervosa from 55 to 62%, and for binge-eating disorder from 39 to 45% (53). A recent genome-wide association study (GWAS) of ~17,000 anorexia nervosa cases and 55,000 controls yielded 8 significant genetic loci that may contribute to etiology (54). Susceptibility genes, it should be noted, may lie dormant for long periods and only become expressed under specific dietary or perhaps hormonal conditions (55). Sex chromosomes have only recently begun to be included in GWAS screens. In addition, many of the databases used for transcriptome analysis are skewed toward data that has been generated predominantly in males, which means that more genes relevant to sex differences in medical conditions still await discovery (56).
Brain Structure
Sex biased genes are present in the central nervous system in great number and may be partially responsible for the neuroanatomical sex differences that have been reported in the human brain. Brain structure develops not only through the action of genes, but also in response to environmental influence so that postnatal experience, which usually differs significantly in girls and boys, affects the size of specialized brain regions. In spite of many reported sex/gender differences in both animal and human brains, these are subject to considerable heterogeneity, even within regions, so that the human brain is not male or female, but best described as a mosaic of relative masculinity and femininity (57). Nonetheless, the question remains as to whether early life programming by testosterone in male fetuses leads to enduring sex differences in brain and behavior (33). Girls who experience elevated androgens in utero tend to display prototypic male behaviors to a variable degree that does not necessarily correlate with structural changes in the genitalia (58). For instance, persons who are born completely insensitive to androgen can be chromosomally XY but phenotypically and psychosexually female (59). This is important because it means that in humans, as in experimental animals, the lack of androgens, rather than the presence of ovarian steroids, is what principally determines femininity.
The developmental trajectory of the brain differs by region and by sex such that the pace of maturation of large areas of the brain, such as the prefrontal cortex and the hippocampus, differs in boys and girls (60, 61). As a result, experiences of trauma or injury, though occurring at comparable ages, may manifest very differently in the two sexes/genders (62). In general, the pace of maturation and development of the nervous system proceeds at a faster pace in girls (63) and, at puberty, comes under the influence of estrogens, which exert protective effects on brain systems via reduction in neuro-inflammation, promotion of synaptic plasticity, and regulation of neurotransmitter signals (64). Growing scientific attention is also being given to sex-based differences in brain circuitry (65), which probably affect response to environmental insult. This means that, although differences in the brain are commonly considered the product of biological sex, gender-based influences may also play a strong, if not equal, role (66).
The Central Nervous System and HPA-Axis
The central nervous system (CNS) and the hypothalamic-pituitary-adrenal (HPA) axis are both central to the body's response to stress. The role of the CNS is to respond quickly and efficiently to perceived stressors and the role of the HPA axis is to maintain that response for as long as needed, and to return the body to homeostasis once the threat has subsided (67, 68). The HPA axis connects the CNS to the endocrine system while also impacting the sympathetic-adrenal medullary (SAM) axis, the immune system, and the cardiovascular system, as well as directly modulating affective and cognitive processes in the brain (69, 70). A dysregulated HPA axis is central to the trauma and stress response. Dysregulation can result in either an inadequate response to threat and or an excessive stress response that persists long after the threat has passed; both are potentially harmful (71). As a result, the HPA axis is hypothesized to strongly influence the development of stress- and trauma-related disorders such as PTSD. Furthermore, a dysregulated HPA axis can help to explain why people suffering from PTSD respond to traumatic triggers either with hyper-arousal (increased heart rate, respiration, and blood pressure), often resulting in a startle response and a feeling of panic, or with hypo-arousal (decreased heart rate, immobility), a frequent precursor to dissociation. Interestingly, the former response appears more common in men and the latter in women (71), for unknown reasons.
The development and functioning of the HPA axis are influenced by one's genome, the prenatal environment, and by life experiences. HPA functioning is especially sensitive to repeated early life trauma (72). As a result, there are multiple ways in which both sex and gender can affect the HPA axis. Both adrenocorticotropic hormone (ACTH) and free cortisol levels have been reported to increase twice as much in men as in women in response to a psychosocial stress task, though results may depend on the specific stressor studied (69). Some studies have suggested that women's monthly hormonal fluctuations sensitize the HPA-axis (73, 74). This may be one reason why women, more than men, risk developing stress-and-trauma-related disorders. In ASD, on the other hand, multiple lines of evidence suggest that dysregulated steroidogenesis during early development contributes to male preponderance (33, 34). With respect to eating disorders, an activated HPA axis has been closely associated with these conditions although the activation may be secondary, an effect of starvation (75).
Somatic Factors
Somatic factors outside the brain, such as immune processes, are capable of influencing the brain and resulting in psychiatric symptoms. In most animals, male sex is associated with a relatively lowered immune response and a relatively higher susceptibility to infection (76). The explanation may lie in the many immune-related genes that are encoded on the X chromosome. Although, as explained earlier, one of the two copies in females is silenced, immune-related genes may escape inactivation in some body cells. As well, immune cells such as T cells and B cells show varying sex-related rates of age-associated decline, with immunosenescence starting earlier in males than in females by ~6 years (76). Furthermore, female hormones play an important protective role in immunity. Estradiol, for instance, has been convincingly shown to reduce the levels of inflammatory cytokines (77). This accords with preclinical animal research, which finds both greater levels of inflammatory signaling molecules and activated immune cells in healthy males than in healthy females (78). From an evolutionary perspective, immune sex differences serve an important purpose because the female immune system needs to suppress an antibody response to male sperm during conception and to male fetuses during pregnancy (79).
Sex differences in the immune system affect the brain, and are seen most notably in the functions of astroglia and microglia, both brain cells that reportedly play a role in psychiatric disease (80). It is currently believed that a combination of genetic sex, plus the organizational and activational effects of gonadal hormones, plus levels of stress hormones contributes significantly to brain immune function (81).
Inflammation experienced either in utero or very early in life is a known risk factor for ASD (81–83), and transcriptome analysis of fetal human brain reveals high levels of gene expression consistent with increased baseline immune reactivity in PTSD (46, 47), which may be transmitted to offspring in a sex-specific manner through varying levels of maternal proinflammatory cytokines over the course of pregnancy (84). Animal research suggests that maternal immune activation or early life inflammation exert negative impact on the brains and subsequent behavior of males, but not females (85).
There are connections between the immune system, the brain, and the gut (86). The composition of intestinal microorganisms appears to affect brain functioning and sex/gender reportedly plays a part in that composition, although study results are inconsistent (87, 88). Men and women tend to eat different foods, take different medications, show different susceptibility to weight gain, and their colonic transit times, on average, differ. These are all factors that affect gut bacteria and potentially influence the expression of many psychiatric disorders (89–91), but this area of research is still in its infancy.
Gonadal Hormones
Gonadal hormones play important roles in differentiating the manifestations of mental disorders in men and women (92–94). Besides conferring protection against the effects of injury, inflammation, ischemia and apoptosis, estrogens also promote neuronal growth and enhance neuro-reparative processes such as remyelination. They also serve important regulatory functions in relation to neurotransmitter systems (94). There is evidence that gonadal hormones play a critical role in aiding the epigenetic expression of eating disorder genes (95–97). Gonadal hormones also contribute to our understanding of ASD (98) and PTSD (99).
Though findings are confounded by differences in measurement methods and ascertainment times, time periods associated with female reproduction, such as pregnancy, the postpartum, and menopause are often associated with the emergence or aggravation of psychiatric symptoms (100). For example, women exposed to traumatic events during the early follicular phase of their menstrual cycle show comparatively increased risk of developing PTSD (100). Naturally occurring hormonal fluctuations in women can also affect response to treatment with psychiatric medications (101). The efficacy of certain psychotropic medications appears to decrease during the luteal phase of the menstrual cycle (102) and the risk of drop-outs from trauma-focused cognitive behavior therapy varies according to the time of the month in which it is initiated (7).
An important limitation to note when discussing the influences of gonadal hormones on symptom development is that much of the data is based on animal studies and requires further study in humans (101).
Gender-Based Theories
Parental Expectations
Whereas, sex differentiation is initiated at conception, the influences of gender start to take effect after birth. Parental expectations of offspring, however, precede birth. Parents choose nursery decor, infant clothing, distinctive toys and given names to match cultural gender norms. For example, in many cultures, girls' names allude to beauty and grace while boys' names suggest strength and sturdiness (103). Differentiation along similar lines is widely accepted, as shown by results of a recent study from China. College students, when asked to rate forenames, characterized feminine names as reflecting warmth and masculine names as representing competence (104).
Gender norms are defined as the expectation that boys and girls, men and women, think, feel, talk, and generally behave in gendered ways. The content and rigidity of gender norms and their endorsement by parents and peers vary across cultures and sub-cultures, but the general rule is that boys are expected to be strong and to control their emotions (except anger, which is permitted) whereas girls throughout the world are expected to be amiable, relatively docile, and nurturing (8). Gender traits are often grouped together and referred to as femininity vs. masculinity.
Gender Socialization
Children learn to act and to think according to gender norms, with parents considered to be the primary channel through which these norms are transmitted (105). Parents interact differently with girl and boy children, encouraging more physical exploration and toughness in boys and more emotional communication, tenderness, and focus on appearance in girls (106). This has been seen as contributing to the high prevalence of eating disorders in girls and women. Parental expectations are reinforced by interpersonal interactions and by the media and can lead to the development, in girls, of vulnerability to body image concerns. Though this is a generality, gender norms frequently encourage girls and women to aim for an idealized body shape and boys and men to nourish a drive for muscularity, accomplishment and power (107, 108).
In general, children are rewarded for what the world around them judges to be desirable behavior and criticized for behaviors that are idiosyncratic (109). In terms of gender socialization, this means that society, including one's parents, teachers, and peers, have low tolerance for personality traits and characteristics traditionally assigned to the opposite gender. Over time, such socialization processes strongly impact the way children see themselves. This happens through mechanisms such as the Rosenthal (Pygmalion) effect (110). Phenomena such as the frequently impaired communicative abilities in boys and underdeveloped mathematical abilities in girls have been linked to this effect.
Stress arises when the environment poses threats to the gender norms that one's culture values. For this reason, men and women are to an extent stressed by different kinds of threats. Women, reportedly, are most threatened by relationship losses whereas threats to status and financial stability are especially stressful for men (111).
Gender Roles
Children internalize the gender norms to which they are exposed and, over time, impose these stereotypes on themselves, a phenomenon referred to as self-socialization (112). Children especially take on gender roles modeled by same-sex adults and peers whom they admire and this affects their actions, appearance, hobbies, dreams, educational pursuits, careers, intimate relationships, and eventual parenting styles (113). Structural theories suggest that different gender roles lead to exposure to different kinds of threats and stressors, each calling for different coping strategies, and resulting in gender differences in adaptability and mental health (114). One such theory, role restraint theory, states that, when men and women are allowed to take on similar roles and face similar stressors, differences in their responses disappear (115). Socialization theories address not only exposures but also the development of sex-specific vulnerabilities to stressors and sex-specific coping strategies, e.g., women tending to confide and share, men tending to deny and seek distraction (116).
Gender Identity
Though the term “gender identity” is sometimes used to refer to self-perceived gender, it is best defined as a person's deeply felt internal and personal experience of their own gender (117). This includes how one sees oneself, the possibilities and restraints attributable to one's gender, a sense of one's body, societal role, and sexual preferences. It also includes non-binary gender identification (118). Gender identity is most lastingly developed during adolescence, with body image, sexual orientation, and range of potential gender roles becoming central themes (119). A perceived incongruence between society's gender norms and one's gender identity can become a source of emotional problems and felt constraints. For example, masculine gender-role stress, defined as stress resulting from the struggle to live up to masculine ideals, has been associated with PTSD (1, 120). A striking example would be a male veteran who suffers from PTSD after a sexual assault and struggles with the perceived discrepancy between military masculine ideals and the role of victimhood and someone struggling with mental illness (120).
Diagnostic Bias
There are multiple sources of gender bias both in mental health research and in the clinical setting. Women have traditionally not been widely recruited for biologically-based mental health research studies because their hormonal fluctuations complicate the task of appropriate matching and because of the concern that an experimental intervention may potentially harm the fetus should an unexpected pregnancy occur. This means that clinical guidance is often still based on research involving mainly men (7). In contrast, many psychotherapeutic trials are conducted primarily on women, simply because more women than men seek help for mental health problems. Furthermore, the vast majority of PTSD research on men is conducted on military personnel or veterans and may not be generalizable to civilian men, let alone to women.
Even in a biologically based disorder, such as ASD, there is evidence of diagnostic bias. This is likely due to the fact that the earliest cases of these disorders were observed in boys, so that the diagnostic criteria were established based on characteristic male symptoms. As stated earlier, it is perhaps because of this that ASD remains often undetected in girls, especially when symptoms are mild (37, 121). Sources of data have substantial clinical impact on how diagnostic criteria are formulated, on screening tools, on diagnostic and clinical guidelines, and on the efficacy of the many different ways in which mental illnesses are treated. Most standardized tests, diagnostic questionnaires, and diagnostic criteria themselves are based on the assumption that men and women experience symptoms in the same way and that a specific question is equally well-suited for eliciting relevant symptoms in women and men, whereas this may not be the case (122). In contrast, in the absence of standardized measures, clinicians may preferentially ask questions of one sex/gender and not of the other. For example, women (but not men) are commonly asked during assessments about exposure to sexual trauma and perinatal loss, though men, too, are exposed to such experiences and their sequelae. The same is true for questions about spousal abuse. Women, on the other hand, are unlikely to be asked about ever engaging in acts of aggression. Similar to the Rosenthal effect mentioned previously, when men and women are repeatedly asked questions about specific experiences and/or symptoms, in time, they will become more likely to report such symptoms whereas, when these areas are left unprobed, they stay unreported, especially so if they are perceived as incongruent with gender norms (115). Thus, clinicians may be more likely to overlook the presence of certain disorders in one sex compared to the other.
Furthermore, using dichotomous categories to assess sex and gender forces both male and female study participants into highly heterogenous groups, with no allowance for intra-group variability. It may be time to rethink how we assess sex and gender and introduce continuous measures, allowing for a wider range of gender-normative variability.
The Coming Together of Sex and Gender
As can be gathered from the literature we have cited, it is essentially impossible to state with certainty which differences between male/female expression of illness are primarily based in biology and which are products of nurture, learning, or environment. For most mental health syndromes, there is evidence for the impact of both sex and gender, through the different factors summarized in Tables 2, 3. Some distinctions between the two influences may, however, be hypothesized. The fact, for instance, that sex/gender differences are found across cultures favors sex-related explanations, whereas cross-cultural variations in the prevalence of differences suggest the influence of gender. In most cases, including the three disorders we focus on here, though sex/gender differences are found universally, their strength varies somewhat across regional and cultural groups, indicating that both sex (innate vulnerability) and gender (specific exposures and expectations) play a part (123, 124). Because most published research has been conducted in Western cultures, relatively little is known of the extent to which results from studies on gender-based factors, such as gender identity and gender-role stress, generalize across cultures. For example, whereas the negative effects of masculine gender-role stress are relatively well-established within Western culture, it is possible that feminine gender-role stress may be clinically significant in parts of the world where women's roles are more restricted. Indeed, many culture-bound syndromes (125–127) can be considered as expressions of women's difficulties with their assigned gender roles.
Table 2
| • Responsible genes may present only on the X or Y chromosome or be expressed differently due to escape from X inactivation or epigenetic influences |
| • Sex differences may exist in brain anatomy, circuitry, function, or output, causing the brain to be viewed, not as dichotomous, but as a mosaic of relative maleness and femaleness |
| • Functional sex differences exist in the CNS, HPA-axis, SAM-axis |
| • Sex differences in the immune system, cardiovascular system, and gut exert influence on brain pathways |
| • Gonadal hormones influence the human experience throughout life, especially through puberty, the menstrual cycle, pregnancy, the postpartum period, lactation, and menopause and through use of hormone-based contraceptives, hormone-replacement therapy at menopause, and fertility hormones in women, and through hormones used for gender affirming care and treatment of transgender individuals |
| • Adrenal androgens and estrogen both exert protective effects against affective and anxiety disorders |
| • Both endogenous and exogenous gonadal hormones may influence treatment efficacy in women |
| • Hormonal instability may in itself pose a risk to the mental health of women by destabilizing homeostasis |
Summary of sex-based theories.
Table 3
| • Infants are often born into a world that has already been prepared for them in accordance with their sex |
| • Children grow up in the context of gender, as they are met with gender-based expectations, rules, norms, bias, and interpersonal behavior |
| • Children internalize cultural gender norms in terms of gender-based schemas, gender roles, and gender identity, which affect actions, hobbies, dreams, choice of education and career, intimate relationship behavior, etc. |
| • Whereas, femininity does not appear to influence mental health, low levels of masculinity, gender-role stress, and gender minority status are associated with mental disorders |
| • There are multiple sources of gender-based bias in both clinical practice and research that limit our ability to draw conclusions on the influences of sex and gender, generalize research findings, and properly treat mental disorders |
| • A first step toward illuminating the importance of sex and gender in research may be to properly assess the two in the first place and to begin measuring sex/gender in non-dichotomous ways |
Summary of gender-based theories.
In most cases, when looking for etiological strands leading to mental illnesses, it is impossible to disentangle the many interacting influences of sex vs. gender. For example. though sex/gender differences in “masculine” and “feminine” traits are rooted in biological sex, socialization processes prod girls to adopt traits assigned to femininity and boys to model traits generally assigned to masculinity (112). Furthermore, the sudden increase of sex/gender differences around the time of puberty in many mental disorders is often interpreted as support for the influence of gonadal hormones, but to what degree hormones directly contribute to this finding is unclear. Of probably equal importance is the increased exposure in adolescence to societal pressures to conform to gender-role expectations (72). Evidence for the impact of both sex and gender is present in almost all such attributions.
It is also important to note that sex- and gender-based influences often modulate each other. For example, prenatal gonadal hormones affect gender identity and sexuality, which, along with secondary sex characteristics and hormones, lead to different socialization experiences, which, in their turn, impact the body's gonadal hormone production (128, 129). As such, sex and gender appear to influence each other in multiple bidirectional ways. In general, both brain functions and HPA-axis reactivity are affected by genetic predisposition, biological development, and environmental exposures/expectations (72).
In the following section, we build on the observations presented above to suggest how sex and gender based influences coalesce.
Discussion
We have cited literature demonstrating multiple potential explanations for men's and women's different vulnerabilities to specific psychiatric disorders. On the basis of such explanations, several theoretical models have been constructed, of which mediation models form one subgroup. Mediation models are built on the premise of risk factors and protective factors. For example, in one such model (130), most of the variance of sex/gender in PTSD was attributed to pre-existing symptoms and traits, peri-traumatic reactions, and subsequent negative thoughts, on one hand, and absence of social support on the other (130). Such models, however, generally fail to explain why, as a group, men and women differ in the all-important risk and protective factors. We, thus, run the risk of tautological arguments (e.g., women have higher symptom levels after a traumatic event because they had higher symptom levels prior to the event). One way of arriving at more accurate models is to analyze the roots of sex- and gender-related mediators, be they risk or good fortune. For example, whereas the presence of substantial social support may distinguish women from men, we still need to understand why men and women elicit different levels of social support, use their supports for different purposes, and appraise their helpfulness in different ways. As stated by Kistner, naming mediators is not sufficient (11). Instead, we need to better understand how sex/gender differences in both distal and proximal risk factors arise.
In what follows, we examine how exactly sex and gender come to be associated with specific sex differences in mental disorders by proposing a model that combines multiple sex and gender based influences on mental health.
A Diathesis-Stress Model for Understanding Sex and Gender Differences in Mental Disorders
Diathesis-stress models have been used across disciplines to integrate multiple baseline vulnerabilities or predispositions, so-called diatheses, with situational stressors (triggers) (131). According to these models, whether or not a specific person develops a disorder depends upon the interaction between pre-existing vulnerabilities and the extent, number, timing, and severity of the stressors they experience.
We propose a diathesis-stress model for sex and gender differences across mental disorders (though we illustrate the model via our three selected disorders—ASD, eating disorders, and PTSD). As indicated in Figure 1 through the use of color gradations, the emphasis on diathesis vs stress varies according to disorder. A diagnostic category thought to originate mostly from diatheses is shown in a darker blue than one mostly caused by exposure to stressors and traumatic events. For example, ASD shows substantial heritability and results mainly from biological causes (132). Moreover, it emerges very early in life before many environmental stresses have had a chance to accumulate. However, in utero stressors, are also part of the genesis of ASD (133), thus a diathesis-stress model is appropriate. Furthermore, disruptions in everyday life and comorbidities elicit symptoms and, thus, are central to the expression and quality of life in ASD (134). In contrast, PTSD requires exposure to a traumatic event and cannot, by definition, develop in the absence of such an event. However, whereas highly resilient individuals may only develop PTSD after a particularly toxic traumatic event, extensive underlying diatheses can cause others to succumb in response to relatively minor stresses. Finally, though most investigators consider social pressures to be the main factors causing eating disorders, there is evidence for substantial individual predispositions (135), thus placing eating disorders in between ASD and PTSD. Please see Table 4.
Figure 1
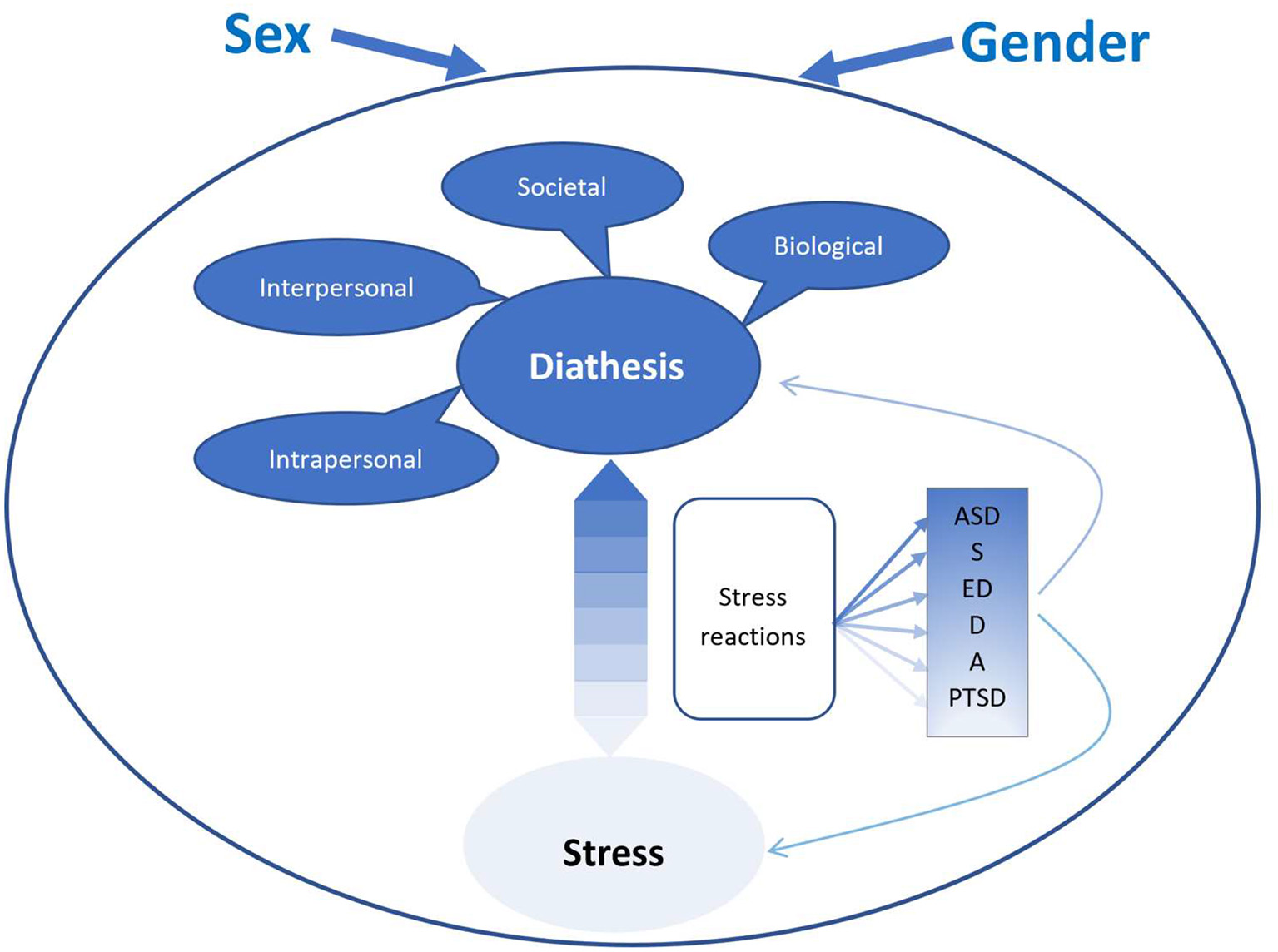
A diathesis stress model of sex and gender differences in mental disorders. ASD, autism spectrum disorder; S, schizophrenia; ED, eating disorders; D, depression; A, anxiety; PTSD, posttraumatic stress disorder. The differences in shading represents a shift in the diathesis vs. stress balance for the different disoders.
Table 4
| Autism spectrum disorders |
|---|
| Biological diatheses |
| • ASD has a strong biological basis with sex differences being attributed to dysregulated steroidogenesis during early development, testosterone levels, and brain differences (i.e., “Extreme Male Brain Theory”). |
| • ASD may require more genetic load in girls than boys |
| Intrapersonal diatheses |
| • Higher levels of aggression in boys may lead to more aggressive outbursts |
| • Girls/women may be more adept at masking, and possibly compensating for, ASD symptoms |
| Interpersonal diatheses |
| • More externalizing behavior in boys may increase social exclusion and bullying, increasing alienation and social withdrawal and reducing quality of life. More prosocial behavior in girls may reduce this. |
| Societal diatheses |
| • Diagnostic bias may cause ASD to be overlooked in girls/women—and possibly over-diagnosed in boys/men |
| Stressors |
| • Pre-natal or early postnatal inflammation in boys may serve as a stressor that may, in some cases, serve as a triggering stressor |
| • Smaller everyday life stressors may affect functioning and quality of life, though the impact on sex/gender differences in ASD is unknown. |
| Peri-/post-stressor factors |
| • Boys may be more likely to become outwardly aggressive in response to stressors |
| Eating disorders |
| Biological diatheses |
| • Though biological diatheses are believed to be less involved than in ASD and PTSD, genetic factors, female gonadal hormones, the immune system, and intestinal bacteria may help increase the risk for eating disorders in girls |
| • Epigenetic processes may be triggered by dieting behavior and the onset of puberty in girls and women |
| Intrapersonal diatheses |
| • Internalized beauty standards in young girls and women call for more strive for thinness and dieting behavior |
| • Negative affectivity and rumination, both more common in girls, are risk factors for eating disorders |
| Interpersonal diatheses |
| • Peer pressure and other group dynamics are believed to contribute more to problematic dieting behavior in girls than boys |
| Societal diatheses |
| • Gender roles and societal expectations, including beauty standards in many cultures, of young women are believed to be the largest contributor to eating disorders in girls and women |
| • Cultural changes in eating habits with increased intake in high caloric and sugary foods may contribute to increases in some cultures, though the specific influence on sex/gender differences is unknown |
| Stressors |
| • External pressures and stressors (e.g., early mealtime conflicts, bullying, body criticism) are believed to contribute substantially to the genesis of eating disorders. In most cases, it is believed that it is the accumulated effects of such stressors, that contribute to the development of eating disorders. Thus, most such stressors will contribute to symptom development as diatheses. |
| • External stressors may trigger specific episodes of binge-eating, purging, or fasting |
| • Exposure to traumatic events have been found to increase the risk for eating disorders. This may especially be the case for events associated with high levels of shame and disgust, such as sexual assault/abuse |
| Peri-/post-stressor factors |
| • Problematic eating behavior and dieting as a way of coping with stress are more common in girls and are often precursors to eating disorders |
| PTSD |
| Biological diatheses |
| • Women are believed to have an inherent preparedness to respond to stress with excessive fear and anxiety, making them more vulnerable to trauma |
| • The HPA-axis is believed to become more easily dysregulated in women |
| • Female gonadal hormones, and perhaps especially the constant fluctuations in these, are believed to contribute to their higher risk of developing PTSD |
| Intrapersonal diatheses |
| • Multiple intrapersonal risk factors (e.g., negative affectivity, rumination, low self-esteem, depression) are more common in girls |
| Interpersonal diatheses |
| • Though women receive more social support, they are also exposed to more negative social responses (e.g., victim blaming) than men |
| • Women are indirectly exposed to more traumatic events than men, as others share their trauma with them |
| Societal diatheses |
| • Boys are encouraged to confront their fears more than girls, which has been speculated to reduce avoidance behavior in boys but induce learned helplessness in girls |
| • PTSD may be under-diagnosed in men, likely partly due to men not seeking help and under-reporting symptoms, both of which may be related to gender norms (and to masculine gender-role stress) |
| • Certain potentially traumatic events (e.g., sexual assault/abuse, infant loss) are believed to be under-reported in men, causing PTSD to go undetected for longer |
| Stressors |
| • Women are exposed to more potentially traumatic events associated with high risk of PTSD, such as rape, childhood sexual abuse, and betrayal trauma |
| Peri-/post-stressor factors |
| • Women are more likely to than men to respond to potentially traumatic events with intense fear, horror, helplessness, dissociation, and negative appraisals, all of which are risk factors for PTSD |
| • Trauma-related guilt and shame are more common in women and known risk factors for PTSD |
Examples of how the diathesis-stress model may account for sex/gender differences in ASD, eating disorders, and PTSD.
Few models have addressed the potential effects of multiple diatheses in combination with an accumulation of situational stressors. Such a model (131), however, is truer to life given that the etiology of psychiatric disorders is multifactorial and complex. One complexity is the usual categorization of sex and gender either as a diathesis or as a risk factor (136), but this is an oversimplification because sex/gender exerts its combined effects through a relatively large number of mediating factors (136).
Hyde and colleagues (137) constructed an integrated model of the emergence of sex/gender differences in depression in which they hypothesize that affective, biological, and cognitive factors converge to result in a vulnerability that is only expressed when a severe stressor triggers symptoms, the so-called ABC model (137). Factors a, b, and c interact with each other and with sex/gender to form the diathesis. The presence of a particularly strong risk factor within one of these domains may be sufficient for symptoms to emerge. Equally possible is for relatively weak risk factors across two or three domains to combine and, together, trigger depression. Although Hyde et al.'s model focuses on depression, a similar model can be constructed for other disorders, including those that first emerge in childhood (e.g., ASD) and those that most commonly emerge in adolescence (eating disorders) or adulthood (PTSD).
We suggest that a diathesis-stress model can be created to account for the complex interaction between sex and gender and their combined involvement in the development and expression of multiple mental disorders. Whereas, some aspects of the model will be specific to certain disorders, many elements will be relevant across diagnoses. We will now introduce some of the key elements of such a model.
Diatheses
In accordance with a model presented by Parker and Brotchie (138), designed to account for sex/gender differences in depression, Christiansen (1) first suggested that the diathesis component of the model represents an inherent preparedness toward responding to a stressor with excessive fear and anxiety, which, when a person is exposed to trauma, significantly increases the risk of developing symptoms (1, 138). This inherent preparedness is, on average, more marked in women than in men and, thus, represents a biological sex difference in PTSD vulnerability (139). There is evidence that women, as a group, have a greater likelihood than men to be genetically predisposed toward fear (140). In line with other models (130, 131), we acknowledge that diatheses may vary in their degree of specificity, with some being disorder-specific (e.g., early childhood mealtime conflicts as a specific risk factor for eating disorder in adolescence), while others are shared among multiple disorders (e.g., negative affectivity or childhood trauma) (141, 142). Elwood and colleagues (136) suggest a possible hierarchy of diatheses that are more or less essential for the development of a specific disorder. Their model supports our earlier statement, that a single high-level diathesis may be sufficient for a person to develop a disorder when exposed to stress, whereas a lower-level (less specific) diathesis can only lead to symptoms when combined with other pre-existing vulnerabilities.
McKeever and Huff (131), in their diathesis-stress model of PTSD, suggest two types of diatheses: ecological and biological, which influence each other. Moreover, either one can elicit specific stressors from the environment. Ecological diatheses consist of risk factors linked to an individual's self (e.g., developmental history, coping mechanisms, social support). The distinction between ecological and biological diatheses is particularly relevant in a model of sex/gender differences, as gender would probably be considered an ecological diathesis and sex a biological diathesis, depending on the disorder. The distinction is further relevant to a model spanning multiple disorders, with some being biologically influenced (e.g., ASD) and others (e.g., PTSD) being more ecologically based. Our model distinguishes among different ecological diatheses by separating out intrapersonal factors, interpersonal/relational factors, and societal factors. Our model also differentiates between sex and gender because we believe that biological diatheses will mostly (but not exclusively) rely on sex, whereas societal diatheses will mostly rely on gender, and intra- and interpersonal factors will likely rely equally on both. A non-conclusive list of diatheses related to sex/gender differences in mental disorders can be found in Table 5.
Table 5
| Biological: |
|---|
| • Chromosomal, genetic, and epigenetic influences, including family genetic predispositions to certain disorders |
| • Brain anatomy, circuitry, function, or output, especially relating to the amygdala, hippocampus, or pre-frontal cortex |
| • Dysregulation of important systems/organs, including the CNS, HPA axis, SAM axis, immune system, gut, metabolism |
| • High/low levels of certain neurotransmitters, peptides, hormones, including gonadal hormones |
| • Exogenous influences such as food, substances, hormone-based contraceptives |
| • Biological and physical consequences of prior stressful and traumatic exposure (e.g., physical trauma, pain, dysregulated HPA axis) |
| • Overall health, including pre-existing injuries, diseases, and health complications |
| Intrapersonal: |
| • Affective factors, including personality style and temperament, negative affectivity/neuroticism, introversion, anxiety sensitivity |
| • Cognitive factors, including cognitive bias, schemas, appraisal, coping style, rumination |
| • Factors related to self and identity, including self-worth, self-confidence, bodily self-image |
| • Psychological defenses, such as dissociation, somatization, splitting |
| • Gender-related concepts, such as internalized gender roles, masculinity, femininity, gender identity, sexual preferences |
| • Intrapersonal consequences of prior stressful and traumatic exposure (e.g., self-blame, doubt, survivor's guilt) |
| • Pre-existing symptoms and disorders |
| Interpersonal: |
| • Attachment (e.g., attachment anxiety and avoidance) |
| • Social relations, including social networks, positive and negative social support |
| • Presence and quality of close interpersonal relations (romantic partners, family, friendships) |
| • Repetitive or prior negative social interactions (e.g., bullying, domestic abuse, ostracism, loneliness) |
| • Boundaries, forgiveness, diplomacy, social intelligence |
| • Affiliative, supportive, helpful, and help-seeking behavior |
| • Interpersonal consequences of prior stressful and traumatic exposures (e.g., diminished trust, isolation) |
| Societal: |
| • Minority status, socio-economic status, living situation, educational level, occupational status |
| • Degree of independence, marginalization, |
| • Access to financial resources, support, protection, medical and psychological treatment, police assistance, justice |
| • Societal attitudes toward exposure to distribution of work, minor stressors, violence |
| • Type and rigidity of gender norms and identities including wide-spread prejudice (e.g., homophobia, transphobia, misogyny, misandry) and related concepts (e.g., systemic racism) |
| • Prior stressful and traumatic environmental exposures, including upbringing, living in violence-prone areas, pollution, and poisonous agents |
Examples of diatheses relevant to sex differences.
Finally, whereas diathesis-stress models generally focus specifically on personal vulnerabilities to disorders, we think of the presence or absence of external resources also being diatheses. An example would be the presence of social capital serving as protective factor against symptom development (143).
Biological Diatheses
Biological vulnerabilities in any one disorder are often specific to that disorder, but some are more general. One example is the involvement of the same neurotransmitter in multiple disorders, which is why selective serotonin re-uptake inhibitors (SSRIs), for instance, are effective in several different categories of mental disorder. Biological vulnerabilities relevant to sex/gender differences are likely to include genes on X and Y chromosomes (but also on autosomes), impairments of brain structure and function, insufficient or excessive levels of gonadal and other hormones, disturbances of HPA axis reactivity, immune system, and/or microbiome irregularities, as addressed earlier. Biological diatheses are often present at birth and strongly impacted by sex. However, gender also affects biological factors through experience, particularly through the influence of socialization and gender-specific stressors that lead, for instance, to the activation of the HPA axis or to epigenetic arousal of dormant genes.
Intrapersonal Diatheses
Intrapersonal diatheses include affective and cognitive vulnerabilities that, along with pre-existing symptoms and disorders, serve as predispositions to new disorders. Affective factors consist of temperament, negative affectivity, sometimes referred to as neuroticism, and mood. Most research agrees that women score higher than men on measures such as sadness, grief, negative affectivity, and anxiety proneness, whereas men tend to score higher on measures of aggression, anger, and impulsivity. Common cognitive vulnerabilities include rumination, maladaptive coping styles, poor self-perceived coping efficacy, negative appraisals, and negative schemas. Cognitive schemas are best understood as internal working models of how individuals view themselves, other people, and the world. Understanding gender norms, gender roles and gender-related schemas, and one's expectations of oneself and others fall into this category.
In our model, coping strategies and appraisals are considered post-stressor factors in that they are often situationally specific and associated with a particular stressor and a person's response to that stressor, as well as with subsequent symptoms. However, there are overall general coping styles and attributional styles that remain relatively stable over a person's life, and, as such, should be considered diatheses. For instance, women have repeatedly been found to ruminate more than men, to appraise situations as more threatening/severe than men do, and to score higher on maladaptive aspects of emotion-focused coping (144). These are enduring traits that serve as diatheses for all internalizing disorders. Furthermore, they exacerbate existing disorders, including neurodevelopmental disorders such as ASD, by undermining levels of functioning and quality of life, making it more difficult for already vulnerable individuals to cope with their symptoms. By contrast, enduring tendencies toward anger, aggression, and lack of impulse control serve as diatheses for externalizing disorders. Finally, our model also places internalized gender roles, masculine and feminine traits, gender identification, and sexual preferences into the category of intrapersonal vulnerabilities.
Interpersonal Diatheses
Interpersonal aspects of our model include sex/gender differences related to insecure attachment, poor or negative social support (145), and victim blaming (146). Attachment is usually considered an intrapersonal factor, but we have included it in this category, as the quality of early attachments exerts a strong influence on subsequent relationships. Though women, as a group, are not less securely attached than men, they do score higher on the attachment anxiety dimension of attachment, which has been linked to later symptom development (147). With respect to social support, women have consistently been found to be recipients of both positive and negative social support, more so than men. Though positive support serves to buffer the impact of stressors, the effects of negative support (hurtful social interactions) may carry greater weight and undermine mental health.
Recurrent patterns of intimate interpersonal interactions with friends, family members, and lovers (e.g., abusive relationships, response to sexual intimacy, enjoyment of sex, relationship gender roles) fall into the category of interpersonal diatheses, whereas we categorize one off stressful interactions (e.g., fights, assaults) as stressors.
Societal Diatheses
The last group of diatheses relating to sex/gender differences in mental disorders are social and environmental: socio-economic status, caste, residential area, educational level, work status, degree of independence, marginalization, politics and other societal factors that set individuals apart. Social hierarchies affect men's and women's access to financial resources, support, protection, financial aid, medical and psychological treatment, police assistance, and legal justice and matter greatly in the aftermath of a traumatic exposure. They are strongly influenced by gender. Factors such as financial dependence, poor access to healthcare, and exposure to domestic abuse put women at a much higher risk than men for developing trauma-related disorders, even though men, as a group, are more exposed to industrial accidents, criminal victimization, and the trauma of war (7).
Gender-based socialization, gendered violence, misogyny (and, to a much lesser extent, misandry), homophobia, and transphobia, also belong in the social diathesis category, whereas the internalized version and enactment of such phenomena fit best under intrapersonal diatheses. Though we designated gender roles as intrapersonal (because they arguably result from internalized gender norms), we have called gender-role stress a social diathesis as it results from a mismatch between internalized gender identity and society's gender-based expectations.
We note in our model that sex and gender has the power to modulate societal diatheses, for example through the ability of secondary sexual characteristics, dress, hair styles, the application of cosmetics or scents, and plastic surgery to elicit and change the behavior of others, leading to changes in one's social status. Traits associated with masculinity, such as assertiveness and aggressiveness, are also able to alter established social hierarchies.
Stressors
The stress component of our model is composed of a range of events, from severe to minor. A non-exclusive list of such events shown previously to contribute to sex/gender differences in mental disorders is shown in Table 6. Aversive childhood events such as childhood sexual abuse are associated with multiple mental disorders later in life. But even a relatively minor break in routine upsets a child with ASD and can trigger a cascade of symptoms. As proposed elsewhere (1), the stress component of this model is much more likely to be affected by gender than by sex.
Table 6
| Traumatic stressors |
|---|
| • Sexual abuse and assault (women) |
| • Betrayal trauma (women) |
| • Combat, war-related traumatic events, imprisonment, non-sexual violence, severe accidents, injury, life-threatening illness, witnessing severe injury/death of |
| others (men) |
| Daily or minor stressors |
| • Childcare, elder-care, domestic chores, family responsibilities, support provider, caregiver (women) |
| • Financial responsibilities, physically demanding chores, role of protector (men) |
| • Loss of access to children associated with partnership break-down (men) |
| • Microaggressions and interpersonal conflict (women) |
| • Financial stressors and status loss (men) |
Examples of stressors relevant to sex differences.
Men, on average, experience a higher number of traumatic stressors than women. These is particularly the case for non-sexual torture, physical violence, severe accidents, gang violence, and witnessing severe injuries and death of others during both war and peacetime (46, 48, 144). Women, on the other hand, experience obstetrical trauma, sexual violence, and betrayal trauma, including intimate partner violence. With respect to everyday stressors, female gender role norms result in women being more embedded than men in the social networks of family and friends. As a result, women are exposed to more non-romantic interpersonal conflicts, losses, and indirect stressors [i.e., negative events that happen to others, such as the divorce or illness and death of close friends (148)]. Though slightly dated, one study of married heterosexual American couples found that wives reported more everyday stressors than husbands, especially in terms of household stressors, family demands, demands from others, and arguments with children (148). In turn, husbands reported more work stressors, stressors related to transportation, and arguments with non-family others. These differences may be accurate but may also result from reporting bias, as men and women differ, both in term of what stresses them, and how willingly they report such stressors. According to a classical paper, unmarried women live longer than married women, whereas married men live longer than single men (149). This may no longer apply because the division of labor between wife and husband has changed markedly over the years (at least in the West), but married women still take on additional caretaker tasks in families, as well as being more affected than men by partnership and family conflict, whereas men mostly benefit from the positive social support they receive through marriage (150).
Finally, sex/gender differences in the types of traumatic and non-traumatic events that men and women are exposed to may influence the type of symptoms they develop. For example, perhaps as a result of being more commonly exposed to betrayal trauma, women may be more likely develop self-doubts that lowers their self-esteem (151). Another example referred to earlier: whereas sexual assault often causes multiple problems with intimacy, sexuality, and self-worth in both men and women, male victims may, in addition, subsequently struggle with what they perceive to be a threat to their masculinity (120).
In accordance with other diathesis-stress models (e.g., 120), only immediate stressors are represented in the stressor component of our model. Once the stress has either subsided or become chronic, it may become part of the diathesis going forward, influencing the response to subsequent stressors. For example, a specific incident of racism, sexism, or homophobia may initially serve as a stressor and subsequently contribute to societal (e.g., perceived systemic racism or institutional sexism), intrapersonal (e.g., rumination), interpersonal (e.g., problems with intimacy), and biological diatheses (e.g., a more reactive HPA axis). The reverse is also true. Diatheses contribute not only to symptomatology but also to the types of stressors to which men and women are differentially exposed. Furthermore, an acute episode of severe mental illness may itself serve as a stressor, thus perpetuating the cycle of stress and distress. In this way, the diathesis and the stressor components of our model are impacted by each another.
Peri- and Post-stressors
Though peri- and post-stressors do not fit neatly into either the diathesis or the stressor part of the model, they play a very important role in the development of many psychiatric disorders. In the case of PTSD, two meta-analyses have found that pre-traumatic risk factors account for only a small percentage of variance in PTSD development compared to peritraumatic and posttraumatic risk factors (152). Thus, a full diathesis-stress model can fully explain sex/gender differences in PTSD only to the extent that it can find a way to include effects of risk factors occurring at the time of and following a trauma. Similarly, for other disorders, the manner in which an individual appraises, responds to, and copes with a given stressor is often central to severity and chronicity. Although very relevant to chronicity, few diathesis-stress models have explicitly included peri and post-stressor factors (153).
It could be argued that peri-stressor factors should be part of the stress component of the model, as it is not the event in itself, but rather the full experience of it, e.g., perception, appraisal, and response, that is central to symptom development. However, this solution does not accommodate post-stressor factors that are vital to the development, and especially the maintenance, of symptoms.
It is important to note that peri-and post-stressor risk factors are strongly linked to pre-stressor risk factors. How a person responds to an event is, in large part, dependent on personality, affectivity, neurobiology, and past experience. Thus, we argue here that, since peri- and post-stressor factors are simultaneously influenced by diatheses and by the type and severity of the stressor, while also contributing to the overall experience and effect of the stressor, they deserve separate representation in the model. We have linked them to the diathesis-stressor interaction by referring to them as “stress reactions,” which includes both acute and long-term responses. Acute responses are appraisals of the stressor plus the biological and psychological responses that occur during and shortly after stress exposure. More long-term responses are continued appraisal, situational coping, social support, and the reactions of others. While these stress reactions contribute to both the type and the severity of symptoms, they are also, in turn, affected by the symptoms that emerge. For example, an individual may appraise early symptom development as either a natural reaction to a traumatic event or, at the opposite extreme, as a sign of losing one's mind. This may, in turn, either decrease or increase the number and severity of symptoms, as well as affect re-appraisal of the traumatic event. Though this interaction is bidirectional during the early aftermath of the stressor, we have chosen in our illustration of the model to include only arrows going from stress reactions to disorders/symptoms in Figure 1, in order to acknowledge that, over time, this association goes mostly in one direction.
Stress reactions are particularly relevant to consider in a diathesis-stress model of sex/gender differences as men and women tend to differ widely in both their acute and their long-term reactions to stress. As previously discussed, sex/gender differences have been demonstrated in the immediate physiological and psychological stress response, e.g., in hypo- vs. hyperarousal, perception of social support, coping ability, and treatment-seeking. It is our belief that sex/gender differences in these early responses are central to the emergence of clinically relevant symptoms and to their maintenance.
Conclusions
Figure 1 is our diathesis stress model that focuses on the role of sex and gender in psychiatric disorders. We argue that sex/gender disparities in (a) biological, intrapersonal, interpersonal, and societal diatheses, (b) everyday stressors, major life events, and traumatic events, and (c) the physiological and psychological response to such stressors, when combined together, result in sex/gender differences in the epidemiology, clinical expression, and treatment response of most, if not all, mental disorders. Thus, though the model is exemplified here with ASD, eating disorders, and PTSD, it is our belief that it may equally be used with other disorders, including, but not limited to, schizophrenia, anxiety disorders, and depression, as shown in Figure 1. It is a major strength of this model that, in addition to highlighting differences between men and women, it also takes account of the many intra-sex variations (hormone levels, sexual identity, minority status) that are sometimes used in attempts to dismiss the influence of sex/gender.
The distinctions between different groups of diatheses is admittedly somewhat artificial because they are almost always intertwined. The concept of specificity within our model is compatible with the idea of multiple pathways, consisting of different combinations of diatheses and stressors, resulting in different categories of disorders, different symptom constellations, and different comorbidities (152). As an example, the high female/male prevalence ratio of trauma-related disorders, such as PTSD anxiety disorders, depression, somatization, and dissociative disorders, may to a large extent be accounted for by shared diatheses from each of the four domains: a more reactive HPA-axis, more negative affectivity, greater exposure to negative social support, and socialization processes that reinforce learned helplessness. It is important to emphasize that learned helplessness is, indeed, learned and not a function of biological sex. Preclinical research shows that female rodents are less likely than their male counterparts to show learned helplessness (154). Women may be vulnerable because of the types of traumatic events (e.g., rape) to which they are exposed (155). In contrast, men, for reasons not yet understood, are particularly vulnerable to in utero and early life adversity affecting brain maturation (156).
The combination of the relative specificity of diatheses and the complex interplay of sex and gender in our model may help to explain why certain disorders tend to aggregate together (internalizing vs. externalizing disorders) and to become, to some extent, sexually dimorphic in prevalence. We believe that this adds to the clinical relevance of our model.
Specificity in terms of high- vs. low-level diatheses for a disorder opens the possibility for the establishment of a hierarchy of diatheses, which may, in the future, help with the creation of reliable vulnerability models. This would serve the cause of prevention in persons at high risk of developing specific disorders. For example, such research could enable clinicians to target interventions at high-risk disaster survivors or military veterans to help prevent the development of PTSD.
Finally, it should be noted that the diathesis-stress model may itself be moderated by sex. The tendency for women to suffer more from trauma- and stress-related disorders and for men to suffer more from neurodevelopmental disorders suggests that the overall weight of stress on one hand and diathesis on the other, may shift according to sex. If it is true that males are particularly vulnerable to pre-natal or very early life stressors, whereas females show special vulnerability at times of hormonal flux, this may also help in structuring prevention programs. Another moderating effect on psychiatric symptoms may turn out to be coping style. Strategies that use emotional processing and emotional expression to manage adversity may engender protective social support, but may do so more effectively in one sex than the other. Cognitive therapies may gain in effectiveness if they take sex into account.
There are many ways in which both sex and gender affect mental health. It is our belief that, by systematically including analyses of sex and/or gender in research, and conducting moderation analyses of sex/gender when interpreting study results, we can improve our understanding of differences between men and women's expression of disorder, and make sense of previously inconsistent study results. Our recommendation is to include sex and gender in the design phase of clinical trials in order to ensure sufficient power to conduct meaningful analyses. However, even in the absence of a priori inclusion of sex/gender, consideration of how results may potentially be affected by sex/gender would represent an improvement in trial methodology.
Improving the meaningfulness of psychiatric research goes beyond detecting group differences between men and women. When differences are found, it is important to search for underlying causes and to search for heterogeneity within the same sex.
This paper does not constitute a systematic search of the vast literature on all aspects of sex/gender effects on mental illnesses. Instead, it proposes a model that we hope readers will critique and expand on. One day, we will have a model that more completely accounts for the accumulating knowledge in this area of research. Our hope is that researchers across disciplines will test the theories presented and examine the intersection of sex, gender, and mental illness, thus continuing to contribute new insights that will advance the prevention of and recovery from mental illnesses.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
DC, MM, and MS contributed to the article and approved of the final version. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
ChristiansenDM. Examining sex and gender differences in anxiety disorders. In: DurbanoF editor. A Fresh Look at Anxiety Disorders. Rijeka, Croatia: In Tech (2015). p. 17–49. 10.5772/60662
2.
ParkerGBrotchieH. Gender differences in depression. Int Rev Psychiatry. (2010) 22:429–36. 10.3109/09540261.2010.492391
3.
SchulzWMuschallaB. What predicts internal and external mental disorders in adolescent boys and girls? Results from a 10-year longitudinal study. Eur J Develop Psychol. (2021) 19:234–50. 10.1080/17405629.2021.189004
4.
OttenDTibubosANSchomerusGBrählerEBinderHKruseJet al. Similarities and differences of mental health in women and men: a systematic review of findings in three large German cohorts. Front Pub Health. (2021) 9:553071. 10.3389/fpubh.2021.553071
5.
Mackinaw-KoonsBVaseyMW. Considering sex differences in anxiety and its disorders across the life span: a construct-validation approach. Appl Prevent Psychol. (2000) 9:191–209. 10.1016/S0962-1849(05)80004-6
6.
EatonNRKeyesKMKruegerRFBalsisSSkodolAEMarkonKEet al. An invariant dimensional liability model of gender differences in mental disorder: evidence from a national sample. J Abnorm Psychol. (2012) 121:282–8. 10.1037/a0024780
7.
ChristiansenDM. Trauma and gender in primary care. In: LegatoMJ editor. Principles of Gender-Specific Medicine. 4th ed. In press.
8.
BekkerMHJvan AssenMALM. Autonomy-connectedness mediates sex differences in symptoms of psychopathology. PLoS ONE. (2017) 12:e0181626. 10.1371/journal.pone.0181626
9.
Schulte HolthausenBHabelU. Sex differences in personality disorders. Curr Psychiatry Rep. (2018) 20:107. 10.1007/s11920-018-0975-y
10.
KleinLCCorwinEJ. Seeing the unexpected: how sex differences in stress responses may provide a new perspective on the manifestation of psychiatric disorders. Curr Psychiatry Rep. (2002) 4:441–8. 10.1007/s11920-002-0072-z
11.
KistnerJA. Sex differences in child and adolescent psychopathology: an introduction to the special section. J Clinical Child Adolesc Psychol. (2009) 38:453–9. 10.1080/15374410902976387
12.
HasinDSSarvetALMeyersJLSahaTDRuanWJStohlMet al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. (2018) 75:336–46. 10.1001/jamapsychiatry.2017.4602
13.
KesslerRCHudsonJIShahlyVKiejnaACardosoG. Bulimia nervosa and binge-eating disorder. In: SteinDJScottKMde JongePKesslerRC editors. Mental Disorders Around the World: Facts and Figures From the WHO World Mental Health Surveys. Cambridge: Cambridge University Press (2018). p. 263–85. 10.1017/9781316336168.018
14.
GoldsteinRBSmithSMChouSPSahaTDJungJZhangHet al. The epidemiology of DSM-5 posttraumatic stress disorder in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Soc Psychiatry Psychiatr Epidemiol. (2016) 51:1137–48. 10.1007/s00127-016-1208-5
15.
RuscioAMHallionLSLimCCWAguilar-GaxiolaSAl-HamzawiAAlonsoJet al. Cross-sectional comparison of the epidemiology of DSM-5 generalized anxiety disorder across the globe. JAMA Psychiatry. (2017) 74:465–75. 10.1001/jamapsychiatry.2017.0056
16.
MaennerMJShawKABaioJWashingtonAPatrickMDiRienzoMet al. Prevalence of autism spectrum disorder among children aged 8 years. autism and developmental disabilities monitoring network, 11 sites, United States 2016. MMWR Surveill Summ. (2020) 69:1–12. 10.15585/mmwr.ss6904a1
17.
LongJHuangGLiangWLiangBChenQXieJet al. The prevalence of schizophrenia in mainland China: evidence from epidemiological surveys. Acta Psychiatrica Scand. (2014) 130:244–56. 10.1111/acps.12296
18.
BlancoCComptonWMSahaTDGoldsteinBIRuanWJHuangBet al. Epidemiology of DSM-5 bipolar I disorder: results from the national epidemiologic survey on alcohol and related conditions - III. J Psychiatr Res. (2017) 84:3. 10.1016/j.jpsychires.2016.10.003
19.
DanielsonMLBitskoRHGhandourRMHolbrookJRKoganMDBlumbergSJ. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents 2016. J Clin Child Adolesc Psychol. (2018) 47:199–212. 10.1080/15374416.2017.1417860
20.
GrantBFGoldsteinRBSahaTDChouSPJungJZhangHet al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. (2015) 72:757–66. 10.1001/jamapsychiatry.2015.0584
21.
GrantBFSahaTDRuanWJGoldsteinRBChouSPJungJet al. Epidemiology of DSM-5 drug use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. JAMA Psychiatry. (2016) 73:39–47. 10.1001/jamapsychiatry.2015.2132
22.
GoldsteinRBChouSPSahaTDSmithSMJungJZhangHet al. The epidemiology of antisocial behavioral syndromes in adulthood: results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. J Clin Psychiatry. (2017) 78:90–8. 10.4088/JCP.15m10358
23.
RoestAMde VriesYALimCCWWittchenHUSteinDJAdamowskiTet al. A comparison of DSM-5 and DSM-IV agoraphobia in the World Mental Health Surveys. Depress Anxiety. (2019) 36:499–5. 10.1002/da.22885
24.
MayneBTBianco-MiottoTBuckberrySBreenJCliftonVShoubridgeCet al. Large scale gene expression meta-analysis reveals tissue-specific, sex-biased gene expression in humans. Front Genetics. (2016) 7:183. 10.3389/fgene.2016.00183
25.
SmigielskiLJagannathVRösslerWWalitzaSGrünblattE. Epigenetic mechanisms in schizophrenia and other psychotic disorders: a systematic review of empirical human findings. Mol Psychiatry. (2020) 25:1718–48. 10.1038/s41380-019-0601-3
26.
Schultze-LutterFSchimmelmannBGFluckigerRMichelC. Effects of age and sex on clinical high-risk for psychosis in the community. World J Psychiatry. (2020) 10:101–24. 10.5498/wjp.v10.i5.101
27.
RosenMHaidlTKRuhrmannSVogeleyKSchultze-LutterF. Sex differences in symptomatology of psychosis-risk patients and in prediction of psychosis. Arch Womens Ment Health. (2020) 23:339–49. 10.1007/s00737-019-01000-3
28.
ComacchioCLasalviaABonettoCCristofaloDMigliettaEPetterliniSet al. Gender and 5-years course of psychosis patients: focus on clinical and social variables. Arch Womens Ment Health. (2020) 23:63–70. 10.1007/s00737-019-0945-3
29.
SeemanMV. Eating disorders and psychosis: seven hypotheses. World J Psychiatry. (2014) 4:112–9. 10.5498/wjp.v4.i4.112
30.
CullenAEHolmesSPollakTABlackmanGJoyceDWKemptonMJet al. Associations between non-neurological autoimmune disorders and psychosis: a meta-analysis. Biol Psychiatry. (2019) 85:35–48. 10.1016/j.biopsych.2018.06.016
31.
FerriSLAbelTBrodkinES. Sex differences in autism spectrum disorder: a review. Curr Psychiatry Rep. (2018) 20:9. 10.1007/s11920-018-0874-2
32.
Baron-CohenS. The extreme male brain theory of autism. Trends Cogn Sci. (2002) 6:248–54. 10.1016/S1364-6613(02)01904-6
33.
Baron-CohenSTsompanidisAAuyeungBNørgaard-PedersenBHougaardDMAbdallahMet al. Foetal oestrogens and autism. Mol Psychiatry. (2020) 25:2970–8. 10.1038/s41380-019-0454-9
34.
Baron-CohenSAuyeungBNørgaard-PedersenBHougaardDMAbdallahMWMelgaardLet al. Elevated fetal steroidogenic activity in autism. Mol Psychiatry. (2015) 20:369–76. 10.1038/mp.2014.48
35.
GockleyJWillseyAJDongSDoughertyJDConstantinoJNSandersSJ. The female protective effect in autism spectrum disorder is not mediated by a single genetic locus. Mol Autism. (2015) 6:25. 10.1186/s13229-015-0014-3
36.
DeanMHarwoodRKasariC. The art of camouflage: gender differences in the social behaviors of girls and boys with autism spectrum disorder. Autism. (2017) 21:678–89. 10.1177/1362361316671845
37.
WerlingDMGeschwindDH. Sex differences in autism spectrum disorders. Curr Opin Neurol. (2013) 26:146–53. 10.1097/WCO.0b013e32835ee548
38.
ReaHMØienRAShicFWebbSJRattoAB. Sex differences on the ADOS-2. J Autism Dev Disord. (2022). 10.1007/s10803-022-05566-3. [Epub ahead of print].
39.
PaolacciSKianiAKManaraEBeccariTCeccariniMRStuppiaLet al. Genetic contributions to the etiology of anorexia nervosa: new perspectives in molecular diagnosis and treatment. Mol Genet Genom Med. (2020) 8:e1244–e. 10.1002/mgg3.1244
40.
HartungCLeflerE. Sex and gender in psychopathology: DSM-5 and beyond. Psychol Bull. (2019) 145:390–409. 10.1037/bul0000183
41.
PolivyJHermanCP. Causes of eating disorders. Ann Rev Psychol. (2002) 53:187213. 10.1146/annurev.psych.53.100901.135103
42.
BussDMSchmittDP. Sexual strategies theory: an evolutionary perspective on human mating. Psychol Rev. (1993) 100:204–32. 10.1037/0033-295X.100.2.204
43.
World Health Organization. International Statistical Classification of Diseases and Related Health Problems 2019. Available online at: https://icd.who.int/ (accessed May 25, 2022).
44.
DavidBPayneLChoateMBarlowDHAllenLBChoateML. Toward a unified treatment for emotional disorders. Behav Ther. (2004) 35:205–30. 10.1016/S0005-7894(04)80036-4
45.
BreslauNKesslerRCChilcoatHDSchultzLRDavisGCAndreskiP. Trauma and posttraumatic stress disorder in the community. Arch Gen Psychiatry. (1998) 55:626–32. 10.1001/archpsyc.55.7.626
46.
KesslerRCSonnegaABrometEHughesMNelsonCB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. (1995) 52:1048–60. 10.1001/archpsyc.1995.03950240066012
47.
HapkeUSchumannARumpfH-JJohnUMeyerC. Post-traumatic stress disorder. Eur Arch Psychiatry Clin Neurosci. (2006) 256:29906. 10.1007/s00406-006-0654-6
48.
TolinDFFoaEB. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol Trauma. (2008) S:37–85. 10.1037/1942-9681.S.1.37
49.
EllegrenHParschJ. The evolution of sex-biased genes and sex-biased gene expression. Nat Rev Genet. (2007) 8:689–98. 10.1038/nrg2167
50.
CeraseAPintacudaGTattermuschAAvnerP. Xist localization and function: new insights from multiple levels. Genome Biol. (2015) 16:166. 10.1186/s13059-015-0733-y
51.
MaschiettoMBastosLCTahiraACBastosEPEuclydesVLBrentaniAet al. Sex differences in DNA methylation of the cord blood are related to sex-bias psychiatric diseases. Sci Rep. (2017) 7:44547. 10.1038/srep44547
52.
XiaYDaiRWangKJiaoCZhangCXuYet al. Sex-differential DNA methylation and associated regulation networks in human brain implicated in the sex-biased risks of psychiatric disorders. Mol Psychiatry. (2021) 26:1. 10.1038/s41380-019-0416-2
53.
YilmazZHardawayJABulikCM. Genetics and epigenetics of eating disorders. Adv Genomics Genet. (2015) 5:131–50. 10.2147/AGG.S55776
54.
WatsonHJYilmazZThorntonLMHübelCColemanJRIGasparHAet al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat Genet. (2019) 51:1207–14. 10.1038/s41588-019-0439-2
55.
HübelCMarziSJBreenGBulikCM. Epigenetics in eating disorders: a systematic review. Mol Psychiatry. (2019) 24:901–15. 10.1038/s41380-018-0254-7
56.
YehudaRHogeCWMcFarlaneACVermettenELaniusRANievergeltCMet al. Post-traumatic stress disorder. Nat Rev Dis Primers. (2015) 1:15057. 10.1038/nrdp.2015.57
57.
JoelDBermanZTavorIWexlerNGaberOSteinYet al. Sex beyond the genitalia: the human brain mosaic. Proc Natl Acad Sci USA. (2015) 112:15468–73. 10.1073/pnas.1509654112
58.
HinesM. Gender development and the human brain. Annu Rev Neurosci. (2011) 34:69–88. 10.1146/annurev-neuro-061010-113654
59.
HinesMAhmedSFHughesIA. Psychological outcomes and gender-related development in complete androgen insensitivity syndrome. Arch Sex Behav. (2003) 32:93–101. 10.1023/a:1022492106974
60.
RaznahanALeeYStiddRLongRGreensteinDClasenLet al. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc Natl Acad Sci USA. (2010) 107:16988–93. 10.1073/pnas.1006025107
61.
GieddJNRapoportJL. Structural MRI of pediatric brain development: what have we learned and where are we going?Neuron. (2010) 107:16988–93. 10.1016/j.neuron.2010.08.040
62.
WhiteJDKaffmanA. The moderating effects of sex on consequences of childhood maltreatment: from clinical studies to animal models. Front Neurosci. (2019) 13:1082. 10.3389/fnins.2019.01082
63.
DiPietroJAVoegtlineKM. The gestational foundation of sex differences in development and vulnerability. Neurosci. (2017) 342:4–20. 10.1016/j.neuroscience.2015.07.068
64.
GreenPSSimpkinsJW. Neuroprotective effects of estrogens: potential mechanisms of action. Int J Develop Neurosci. (2000) 18:347–58. 10.1016/S0736-5748(00)00017-4
65.
McCarthyMMArnoldAPBallGFBlausteinJDDeVriesGJ. Sex differences in the brain: the not so inconvenient truth. J Neurosci. (2012) 32:2241–7. 10.1523/JNEUROSCI.5372-11.2012
66.
TooleyUABassettDSMackeyAP. Environmental influences on the pace of brain development. Nat Rev Neurosci. (2021) 22:372–84. 10.1038/s41583-021-00457-5
67.
McEwenBSMilnerTA. Understanding the broad influence of sex hormones and sex differences in the brain. J Neurosci Res. (2017) 95:24–39. 10.1002/jnr.23809
68.
TiwariAGonzalezA. Biological alterations affecting risk of adult psychopathology following childhood trauma: a review of sex differences. Clin Psychol Rev. (2018) 66:69–79. 10.1016/j.cpr.2018.01.006
69.
KudielkaBMKirschbaumC. Sex differences in HPA axis responses to stress: a review. Biol Psychol. (2005) 69:113–32. 10.1016/j.biopsycho.2004.11.009
70.
ReynoldsRMHiiHLPennellCEMcKeagueIWKloetERdLyeSet al. Analysis of baseline hypothalamic-pituitary-adrenal activity in late adolescence reveals gender specific sensitivity of the stress axis. Psychoneuroendocrinol. (2012) 38:1271–80. 10.1016/j.psyneuen.2012.11.010
71.
StamR. PTSD and stress sensitisation: a tale of brain and body. part 1: human studies. Neurosci Biobehavior Rev. (2007) 31:530–7. 10.1016/j.neubiorev.2006.11.010
72.
ChristiansenDM. Mediation and moderation effects of sex and gender in PTSD: PhD dissertation. Aarhus: Aarhus University, Department of Psychology and Behavioural Sciences (2017).
73.
AboSSmithDStadtMLaytonA. Modelling female physiology from head to Toe: impact of sex hormones, menstrual cycle, and pregnancy. J Theor Biol. (2022) 540:1110.1016/j.jtbi.2022.111074
74.
MeewisseMLReitsmaJBde VriesGJGersonsBPOlffM. Cortisol and posttraumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry. (2007) 191:387–92. 10.1192/bjp.bp.106.024877
75.
KhalilRBSouaibyLFaresN. The importance of the hypothalamo-pituitary-adrenal axis as a therapeutic target in anorexia nervosa. Physiol Behav. (2017) 171:13–20. 10.1016/j.physbeh.2016.12.035
76.
TakahashiTIwasakiA. Sex differences in immune responses. Science. (2021) 371:347. 10.1126/science.abe7199
77.
CarlessiASBorbaLAZugnoAIQuevedoJRéusGZ. Gut microbiota–brain axis in depression: the role of neuroinflammation. Eur J Neurosci. (2021) 53:222–5. 10.1111/ejn.14631
78.
McCarthyMM. Sex differences in neuroimmunity as an inherent risk factor. Neuropsychopharmacology. (2019) 44:38–44. 10.1038/s41386-018-0138-1
79.
HuppertzB. The feto–maternal interface: setting the stage for potential immune interactions. Semin Immunopathol. (2007) 29:83–94. 10.1007/s00281-007-0070-7
80.
NakagawaYChibaK. Role of microglial m1/m2 polarization in relapse and remission of psychiatric disorders and diseases. Pharmaceuticals (Basel). (2014) 7:1028–48. 10.3390/ph7121028
81.
BreachMRLenzKM. Sex differences in neurodevelopmental disorders: a key role for the immune system. In: Current Topics in Behavioral Neurosciences.Berlin, Heidelberg: Springer (2022). 10.1007/7854_2022_308
82.
McCarthyMMWrightCL. Convergence of sex differences and the neuroimmune system in autism spectrum disorder. Biol Psychiatry. (2017) 81:402–10. 10.1016/j.biopsych.2016.10.004
83.
PattersonPH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. (2009) 204:313–21. 10.1016/j.bbr.2008.12.016
84.
GoldsteinJMCohenJEMareckovaKHolsenLWhitfield-GabrieliSGilmanSEet al. Impact of prenatal maternal cytokine exposure on sex differences in brain circuitry regulating stress in offspring 45 years later. Proc Natl Acad Sci USA. (2021) 118:e2014464118. 10.1073/pnas.2014464118
85.
HoffmanJFWrightCLMcCarthyMM. A critical period in Purkinje cell development is mediated by local estradiol synthesis, disrupted by inflammation and has enduring consequences only for males. J Neurosci. (2016) 36:10039–49. 10.1523/JNEUROSCI.1262-16.2016
86.
RizzettoLFavaFTuohyKMSelmiC. Connecting the immune system, systemic chronic inflammation and the gut microbiome: the role of sex. J Autoimmun. (2018) 92:12–34. 10.1016/j.jaut.2018.05.008
87.
KimYSUnnoTKimB-YParkM-S. Sex differences in gut microbiota. World J Mens Health. (2020) 38:48–60. 10.5534/wjmh.190009
88.
MargolisKGCryanJFMayerEA. The microbiota-gut-brain axis: from motility to mood. Gastroenterology. (2021) 160:1486–501. 10.1053/j.gastro.2020.10.066
89.
ButlerMJPerriniAAEckelLA. The role of the gut microbiome, immunity, and neuroinflammation in the pathophysiology of eating disorders. Nutrients. (2021) 13:500. 10.3390/nu13020500
90.
HemmingsSMJMalan-MüllerSvan den HeuvelLLDemmittBAStanislawskiMASmithDGet al. The microbiome in posttraumatic stress disorder and trauma-exposed controls: an exploratory study. Psychosom Med. (2017) 79:936–46. 10.1097/PSY.0000000000000512
91.
VuongHEHsiaoEY. Emerging roles for the gut microbiome in autism spectrum disorder. Biol Psychiatry. (2017) 81:411–23. 10.1016/j.biopsych.2016.08.024
92.
AzcoitiaIBarretoGEGarcia-SeguraLM. Molecular mechanisms and cellular events involved in the neuroprotective actions of estradiol. analysis of sex differences. Front Neuroendocrinol. (2019) 55:100787. 10.1016/j.yfrne.2019.100787
93.
CahillL. Why sex matters for neuroscience. Nat Rev Neurosci. (2006) 7:477–84. 10.1038/nrn1909
94.
GrumbachMM. To an understanding of the biology of sex and gender differences: “an idea whose time has come. J Men's Health Gender. (2004) 1:12–9. 10.1016/j.jmhg.2004.03.013
95.
HardinSLThorntonLMMunn-ChernoffMABakerJH. Premenstrual symptoms as a marker of ovarian hormone sensitivity in eating disorders. Int J Eating Disord. (2020) 53:296–301. 10.1002/eat.23213
96.
MikhailMECulbertKMSiskCLKlumpKL. Gonadal hormone contributions to individual differences in eating disorder risk. Curr Opin Psychiatry. (2019) 32:484–90. 10.1097/YCO.0000000000000543
97.
Steegers-TheunissenRPMWiegelREJansenPWLavenJSESinclairKD. Polycystic ovary syndrome: a brain disorder characterized by eating problems originating during puberty and adolescence. Int J Mol Sci. (2020) 21:8211. 10.3390/ijms21218211
98.
WilsonHACreightonCScharfmanHCholerisEMacLuskyNJ. Endocrine insights into the pathophysiology of autism spectrum disorder. Neuroscientist. (2021) 27:650–67. 10.1177/1073858420952046
99.
GogosANeyLJSeymourNVan RheenenTEFelminghamKL. Sex differences in schizophrenia, bipolar disorder, and post-traumatic stress disorder: are gonadal hormones the link?Br J Pharmacol. (2019) 176:4119–35. 10.1111/bph.14584
100.
NillniYIPinelesSLPattonSCRouseMHSawyerATRasmussonAM. Menstrual cycle effects on psychological symptoms in women with PTSD. J Trauma Stress. (2015) 28:1–7. 10.1002/jts.21984
101.
GilliesGEMcArthurS. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev. (2005) 14:609–16. 10.1124/pr.109.002071
102.
ThaseMEEntsuahRCantillonMKornsteinSG. Relative antidepressant efficacy of venlafaxine and SSRIs: sex-age interactions. J Womens Health. (2005) 14:609–16. 10.1089/jwh.2005.14.609
103.
ZwebnerYSellierALRosenfeldNGoldenbergJMayoR. We look like our names: the manifestation of name stereotypes in facial appearance. J Pers Soc Psychol. (2017) 112:527–54. 10.1037/pspa0000076
104.
ZuoBLiuCWenFTanXXieZ. The impact of gender orientation of names on individuals' evaluation of impressions and interpersonal interaction. Acta Psychol Sinica. (2021) 53:387–99. 10.3724/SP.J.1041.2021.00387
105.
KeelPKForneyKJ. Psychosocial risk factors for eating disorders. Int J Eating Disord. (2013) 46:433–9. 10.1002/eat.22094
106.
MesmanJGroeneveldMG. Gendered parenting in early childhood: subtle but unmistakable if you know where to look. Child Develop Persp. (2018) 12:22–7. 10.1111/cdep.12250
107.
MurraySBRiegerEKarlovLTouyzSW. Masculinity and femininity in the divergence of male body image concerns. J Eating Disord. (2013) 1:11. 10.1186/2050-2974-1-11
108.
YeanCBenauEDakanalisAHormesJPeroneJTimkoA. The relationship of sex and sexual orientation to self-esteem, body shape satisfaction, and eating disorder symptomatology. Front Psychol. (2013) 4:887. 10.3389/fpsyg.2013.00887
109.
BusseyKBanduraA. Social cognitive theory of gender development and differentiation. Psychol Rev. (1999) 106:676–713. 10.1037/0033-295X.106.4.676
110.
RosenthalR. On the social psychology of the psychological experiment: the experimenter's hypothesis as unintended determinant of experimental results. Am Sci. (1963) 51:268–83.
111.
VandelloJABossonJK. Hard won and easily lost: a review and synthesis of theory and research on precarious manhood. Psychol Men Masculinity. (2012) 14: 10.1037/a0029826
112.
HalimMLDWalshASTamis-LeMondaCSZosulsKMRubleDN. The roles of self-socialization and parent socialization in toddlers' gender-typed appearance. Arch Sex Behav. (2018) 47:2277–85. 10.1007/s10508-018-1263-y
113.
Solbes-CanalesIValverde-MontesinoSHerranz-HernándezP. Socialization of gender stereotypes related to attributes and professions among young Spanish school-aged children. Front Psychol. (2020) 11:609. 10.3389/fpsyg.2020.00609
114.
PtacekJTSmithREZanasJ. Gender, appraisal, and coping: a longitudinal analysis. J Personality. (1992) 60:747–70. 10.1111/j.1467-6494.1992.tb00272.x
115.
RosarioMShinnMMørchHHuckabeeCB. Gender differences in coping and social supports: testing socialization and role constraint theories. J Community Psychol. (1988) 16:55–69. 10.1002/1520-6629(198801)16:1<55::AID-JCOP2290160108>3.0.CO;2-U
116.
SousaCKuschelKBritoAGonçalvesG. Work-oriented men and women: similar levels of work-family conflict and guilt yet different coping strategies. Psychol Thought. (2018) 11:195–211. 10.23668/psycharchives.1885
117.
The Yogyakarta principles on the application of international human rights law in relation to sexual orientation gender identity. (2006). Available online at: https://yogyakartaprinciples.org/ (accessed May 25, 2022).
118.
ChewDTollitMAPoulakisZZwicklSCheungASPangKC. Youths with a nonbinary gender identity: a review of their sociodemographic and clinical profile. Lancet Child Adolesc Health. (2020) 4:322–30. 10.1016/S2352-4642(19)30403-1
119.
MousaviMSShahriariMSalehiMKohanS. Gender identity development in the shadow of socialization: a grounded theory approach. Arch Womens Ment Health. (2019) 22:245–51. 10.1007/s00737-018-0888-0
120.
ChristiansenDMBerkeET. Gender- and sex-based contributors to sex differences in PTSD. Curr Psychiatr Rep. (2020) 224:19. 10.1007/s11920-020-1140-y
121.
LaiMCLombardoMVAuyeungBChakrabartiBBaron-CohenS. Sex/gender differences and autism: setting the scene for future research. J Am Acad Child Adolesc Psychiatry. (2015) 54:11–24. 10.1016/j.jaac.2014.10.003
122.
ReynoldsCR. Need we measure anxiety differently for males and females?J Person Ass. (1998) 70:212–21. 10.1207/s15327752jpa7002_2
123.
NorrisFHPerillaJLIbanezGEMurphyAD. Sex differences in symptoms of posttraumatic stress: does culture play a role. J Trauma Stress. (2001) 14:7–28. 10.1023/A:1007851413867
124.
ChristiansenDMElklitA. Sex Differences in PTSD. In: LazinicaAOvugaE editors. InTech - Open Access Publisher (2012). 10.5772/28363
125.
CoreilJBarnes-JosiahDLAugustinACayemittesM. Arrested pregnancy syndrome in Haiti: findings from a national survey. Med Anthropol Q. (1996) 10:424–36. 10.1525/maq.1996.10.3.02a00080
126.
FarmerP. Bad blood, spoiled milk: bodily fluids as moral barometers in rural Haiti. Am Ethnologist. (1988) 15:62–83. 10.1525/ae.1988.15.1.02a00050
127.
WeaverLJ. Tension among women in North India: an idiom of distress and a cultural syndrome. Cult Med Psychiatry. (2017) 41:35–55. 10.1007/s11013-016-9516-5
128.
DavisMEmoryE. Sex differences in neonatal stress reactivity. Child Develop. (1995) 66:14–27. 10.1111/j.1467-8624.1995.tb00852.x
129.
ChristiansenDM. Sex and gender differences in trauma victims presenting for treatment. In: LegatoMJ editor. Principles of Gender-Specific Medicine:Academic Press (2017). p. 497–511. 10.1016/B978-0-12-803506-1.00043-7
130.
ChristiansenDMHansenM. Accounting for sex differences in PTSD: a multivariable mediation model. Eur J Psychotraumatol. (2015) 6:26068. 10.3402/ejpt.v6.26068
131.
McKeeverVMHuffME. A diathesis-stress model of posttraumatic stress disorder: ecological, biological, and residual stress pathways. Rev Gen Psychol. (2003) 7:237–50. 10.1037/1089-2680.7.3.237
132.
WerlingDMParikshakNNGeschwindDH. Gene expression in human brain implicates sexually dimorphic pathways in autism spectrum disorders. Nat Commun. (2016) 7:10717. 10.1038/ncomms10717
133.
BölteSGirdlerSMarschikPB. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell Mol Life Sci. (2019) 76:1275–97. 10.1007/s00018-018-2988-4
134.
FuldS. Autism spectrum disorder: the impact of stressful and traumatic life events and implications for clinical practice. Clin Soc Work J. (2018) 46:210–9. 10.1007/s10615-018-0649-6
135.
TascaGA. Attachment and eating disorders: a research update. Curr Opin Psychol. (2019) 25:59–64. 10.1016/j.copsyc.2018.03.003
136.
ElwoodLSHahnKSOlatunjiBOWilliamsNL. Cognitive vulnerabilities to the development of PTSD: a review of four vulnerabilities and the proposal of an integrative vulnerability model. Clin Psychol Rev. (2009) 29:87–100. 10.1016/j.cpr.2008.10.002
137.
HydeJSMezulisAHAbramsonLY. The ABCs of depression: integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychol Rev. (2008) 115:291–313. 10.1037/0033-295X.115.2.291
138.
ParkerGBBrotchieHL. From diathesis to dimorphism: the biology of gender differences in depression. J Nerv Ment Dis. (2004) 192:210–6. 10.1097/01.nmd.0000116464.60500.63
139.
DayHLLStevensonCW. The neurobiological basis of sex differences in learned fear and its inhibition. Eur J Neurosci. (2019) 52:2466–86. 10.1111/ejn.14602
140.
McLeanCPAndersonER. Brave men and timid women? A review of the gender differences in fear and anxiety. Clin Psychol Rev. (2009) 29:496–505. 10.1016/j.cpr.2009.05.003
141.
BarlowDH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. Am Psychol. (2000) 55:1247–63. 10.1037/0003-066X.55.11.1247
142.
KotlerLACohenPDaviesMPineDSWalshBT. Longitudinal relationships between childhood, adolescent, and adult eating disorders. J Am Acad Child Adolesc Psychiatry. (2001) 40:1434–40. 10.1097/00004583-200112000-00014
143.
McKenzieKWhitleyRWeichS. Social capital and mental health. Br J Psychiatry. (2018) 181:280–3. 10.1192/bjp.181.4.280
144.
ChristiansenDMOlffMElklitA. Parents bereaved by infant death: sex differences and moderation in PTSD, attachment, coping and social support. Gen Hosp Psychiatry. (2014) 36:655–61. 10.1016/j.genhosppsych.2014.07.012
145.
LincolnKD. Social support, negative social interactions, and psychological well-being. Soc Serv Rev. (2000) 74:231–52. 10.1086/514478
146.
JohnsonVENadalKLSissokoGKingR. “It's not in your head”: Gaslighting, ‘splaining, victim blaming, and other harmful reactions to microaggressions. Perspect Psychol Sci. (2021). 16:1024–36. 10.1177/17456916211011963
147.
GoldbergLRFreydJJ. Self-reports of potentially traumatic experiences in an adult community sample: gender differences and test-retest stabilities of the items in a brief betrayal trauma survey. J Trauma Dissoc. (2006) 7:39–63. 10.1300/J229v07n03_04
148.
AlmeidaDMKesslerRC. Everyday stressors and gender differences in daily distress. J Pers Soc Psychol. (1998) 75:670–80. 10.1037/0022-3514.75.3.670
149.
GoveWR. Sex, marital status, and mortality. Am J Sociol. (1973) 79:45–67. 10.1086/225505
150.
Kamp DushCMYavorskyJESchoppe-SullivanSJ. What are men doing while women perform extra unpaid labor? Leisure and specialization at the transitions to parenthood. Sex Roles. (2018) 78:715–30. 10.1007/s11199-017-0841-0
151.
TangSSSFreydJJ. Betrayal trauma and gender differences in posttraumatic stress. Psychol Trauma Theory Res Pract Policy. (2012) 4:469–78. 10.1037/a0025765
152.
BrewinCRAndrewsBValentineJD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consul Clin Psychol. (2000) 68:748–66. 10.1037/0022-006X.68.5.748
153.
KiddSAHowisonMPillingMRossLEMcKenzieK. Severe mental illness in LGBT populations: a scoping review. Psychiatr Serv. (2016) 67:779–83. 10.1176/appi.ps.201500209
154.
DallaCEdgecombCWhetstoneASShorsTJ. Females do not express learned helplessness like males do. Neuropsychopharmacol. (2008) 33:1559–69. 10.1038/sj.npp.1301533
155.
NothlingJAbrahamsNJewkesRMhlongoALombardCHemmingsSMJet al. Risk and protective factors affecting the symptom trajectory of posttraumatic stress disorder post-rape. J Affect Disord. (2022) 309:151–64. 10.1016/j.jad.2022.04.032
156.
Poggi DavisEPfaffD. Sexually dimorphic responses to early adversity: implications for affective problems and autism spectrum disorder. Psychoneuroendocrinology. (2014) 49:11–25. 10.1016/j.psyneuen.2014.06.014
Summary
Keywords
sex differences, gender differences, mental health, diathesis-stress model, posttraumatic stress disorder, autism spectrum disorders, eating disorders
Citation
Christiansen DM, McCarthy MM and Seeman MV (2022) Where Sex Meets Gender: How Sex and Gender Come Together to Cause Sex Differences in Mental Illness. Front. Psychiatry 13:856436. doi: 10.3389/fpsyt.2022.856436
Received
17 January 2022
Accepted
02 June 2022
Published
28 June 2022
Volume
13 - 2022
Edited by
Richard Adams, Kent State University, United States
Reviewed by
William Davies, Cardiff University, United Kingdom; Kathleen Brady, MUSC Health, United States
Updates
Copyright
© 2022 Christiansen, McCarthy and Seeman.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dorte M. Christiansen dochristiansen@health.sdu.dk
This article was submitted to Psychopathology, a section of the journal Frontiers in Psychiatry
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.