- 1Research Center for Child Mental Development, University of Fukui, Fukui, Japan
- 2Department of Child Development, United Graduate School of Child Development, Osaka University, Kanazawa University, Hamamatsu University School of Medicine, Chiba University, and University of Fukui, Fukui, Japan
- 3Biomedical Imaging Research Center, University of Fukui, Fukui, Japan
- 4Department of Child and Adolescent Psychological Medicine, University of Fukui Hospital, Fukui, Japan
The purpose of this study was to examine whether the beneficial effects of behavioral parent training (BPT), as an indirect type of psychosocial treatment, are extended to cognitive manifestations beyond behavioral symptoms of attention-deficit/hyperactivity disorder (ADHD). Although previous studies of community families have shown an association between parenting quality and a child’s cognitive functions, little is known about the effects of BPT on cognitive manifestations in children with ADHD. In this study, we focused on inhibitory control among cognitive domains, which is considered to be the most malleable to direct types of psychosocial treatment for ADHD. We hypothesized that inhibitory control is affected by BPT, which uses parents as the primary agents of change to help their children. Thirty school-age children (6–12 years old) with ADHD and their parents (mothers) participated and were randomly assigned to either the standard BPT or waitlist control group. Using two objective laboratory-based tasks of inhibitory control (i.e., go/no-go and single response selection tasks), we assessed baseline and post-treatment response inhibition to suppress task-irrelevant responses and response selection to select task-relevant responses. In addition to decreased ADHD symptoms and negative parenting, the BPT group exhibited significantly improved performance in the single response selection task, but not in the go/no-go task, compared with the waitlist control group. Although tentative, these findings partially support our hypothesis that BPT has beneficial effects on the cognitive inhibitory control of ADHD, highlighting the potential for supportive environmental modifications to advance cognitive development in children with ADHD.
Introduction
Behavioral parent training (BPT) is commonly used as an indirect type of psychosocial treatment for attention-deficit/hyperactivity disorder (ADHD), which is the most common neurodevelopmental disorder in childhood (1, 2). In contrast to child-centered treatment, BPT is indirect because it encourages parents to increase positive parent–child contact and teaches them specific management strategies to cope with children’s behavioral problems (3). In BPT, parents are acknowledged to be the primary agents of change for managing behavioral problems in their children (4). Although there is a discrepancy between parental and independently rated assessments of ADHD symptoms, recent meta-analysis studies (5, 6) have reported that BPT decreases ADHD symptoms, as subjectively reported by parents, who are the most ecologically valid assessors of children’s symptom expression (7).
Given that a transactional model of ADHD and family functioning has suggested that suboptimal parenting practices exacerbate ADHD symptoms and their associated difficulties (8), improved parenting practices via BPT may improve behavioral symptoms and the underlying cognitive mechanisms of ADHD. ADHD’s behavioral symptoms are characterized by deficits in cognitive (neuropsychological) functions, including executive function (EF) implemented mainly in the prefrontal cortex (PFC) (9, 10). Previously, a preventive intervention study of community families demonstrated that a child’s inhibitory control of EF, measured subjectively by parents, improved when supportive and responsive parenting increased (11). Similarly, there are also findings of observational studies showing an association between parenting quality and a child’s cognitive functions by using cross-sectional (12, 13) and longitudinal study designs (14, 15). Here, the current study aimed to investigate the hypothetical effects of BPT on cognitive manifestations in children with ADHD.
To date, improvements in the cognitive domains of ADHD have been examined by several studies using direct types of non-pharmacological psychosocial treatments (16–20). Direct psychosocial treatments target ADHD-related behaviors and cognition with the child as the primary agent of change (e.g., cognitive behavioral therapy, cognitive training, neurofeedback, physical exercise). However, BPT is considered to be an indirect type of psychosocial treatment, as adults are recognized as agents of change (3). As reported by several meta-analyses (16, 18–20), cognitive training is likely to be restricted to near transfer (but not far transfer) effects on cognitive outcomes. A more recent meta-analysis (17) has suggested that of the cognitive domains (e.g., working memory, inhibitory control, flexibility, attention), the inhibitory control component of EF was most affected by a wide range of direct psychosocial treatments and thus could be considered to be the most malleable. Conversely, it is not known whether BPT, as an indirect approach, has beneficial effects on ADHD’s cognitive domains. Although objective cognitive tasks have not been included as outcomes measures in most BPT studies (17), a few BPT studies have examined EF improvements, including inhibitory control and working memory (21–24). However, the results of these studies are limited because they lack control group comparisons. A randomized controlled trial (RCT) using objective cognitive measures is thus needed to produce extended evidence that BPT has beneficial effects on the cognitive domains of children with ADHD.
The current study aims to provide extended RCT evidence of BPT as a current evidence-based standard psychosocial treatment for children with ADHD (4, 25). From the transactional model of ADHD and family functioning as well as the related studies reviewed above, we hypothesized that BPT improves parenting practices and ADHD behavioral symptoms as well as cognitive manifestations in children with ADHD. Of the cognitive functions, we focused on inhibitory control, which is considered to be the most malleable to psychosocial treatments (17). Inhibitory control is assumed to have at least two distinct cognitive operations (26, 27): response inhibition to suppress task-irrelevant information/responses (go/no-go task) and response selection to select task-relevant information/responses (single response selection task). If our hypothesis were supported, the BPT group would exhibit more improvements in behavioral symptoms, inhibitory control, and parenting practices than the waitlist control group.
Materials and Methods
Participants
Parents (mothers) and their children aged 6–12 years were recruited in three cohorts through advertisements at the University of Fukui Hospital outpatient clinic. All children were diagnosed with ADHD by child psychiatrists in the outpatient clinic according to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) interview (28), based on school reports, observations of the child, and clinical interviews with the family. All children had normal or corrected-to-normal vision. Most (88%) were right handed. All parents had completed at least 12years of education (non-compulsory secondary-level or university-level education) and were thus categorized as having a relatively high education level. Most (84%) were living above the relative poverty line, which was set at 50% of the country’s median household income. Informed consent/assent was obtained from all participants included in this study. The study protocol was approved by the Research Ethics Committee of the University of Fukui and was conducted in accordance with the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects of Japan.
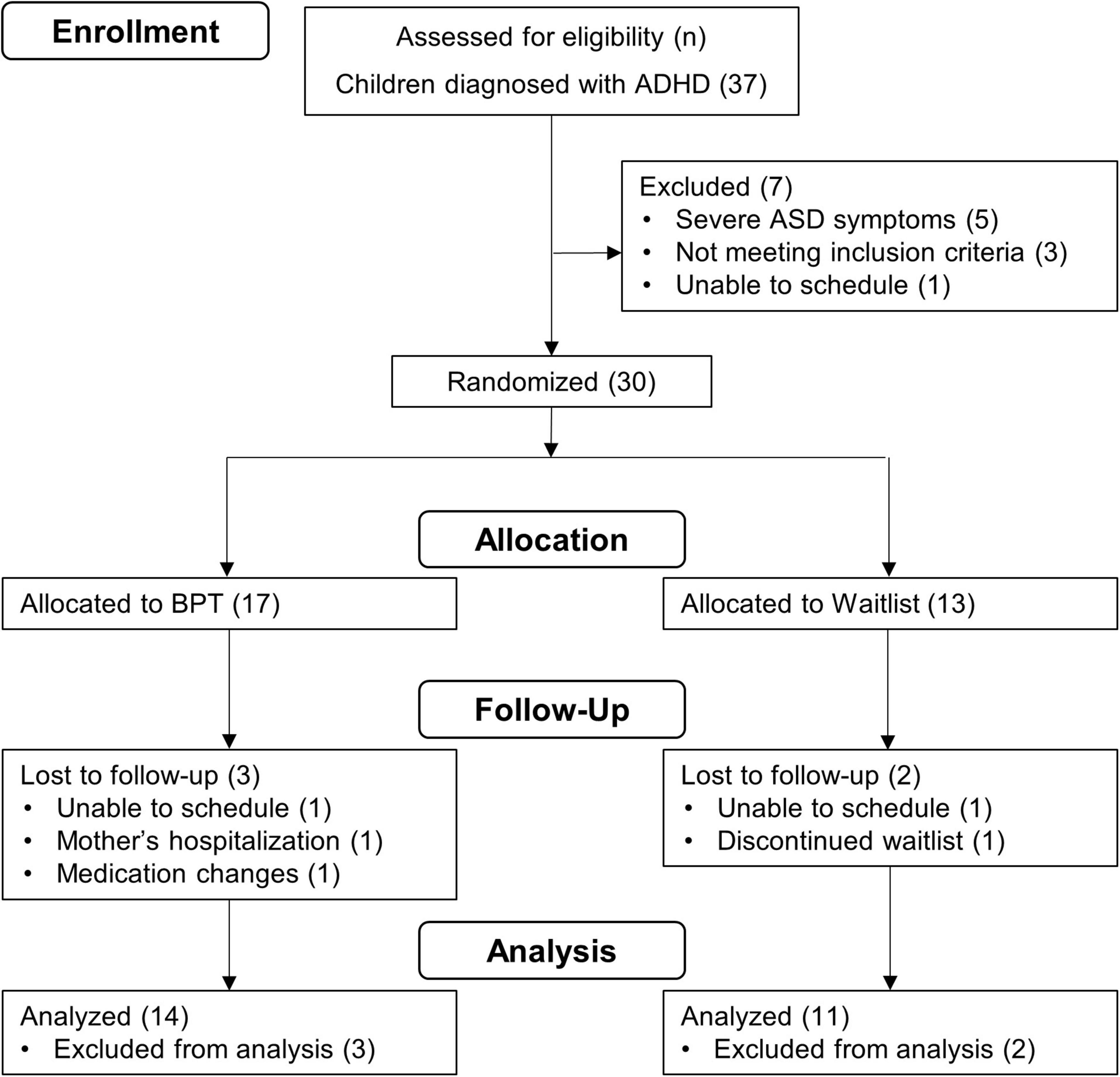
The inclusion criteria were as follows: Japanese as a first language spoken at home; parenting an elementary school-age child (6–12 years old) with ADHD diagnosis per the DSM-5 (28); and more than mild symptoms of inattention and/or hyperactivity/impulsivity on the Swanson, Nolan, and Pelham rating scale (SNAP) (29, 30). Exclusion criteria were as follows: intellectual disability (IQ < 70) measured by the Wechsler Intelligence Scale for Children (WISC); presence of moderate to severe symptoms of autism spectrum disorder (ASD) per the DSM-5 (28); self-reported psychiatric symptomatology in the parent; and participation in another BPT program within two months of screening. For children taking medication (e.g., osmotic-release oral system formulation of methylphenidate) for ADHD symptoms, parents were asked to maintain their medication status throughout this study. Based on these criteria, 30 eligible participants were selected and randomized into either the BPT or the waitlist control group (Figure 1).
Procedure
Participants were randomly assigned to either the BPT (n = 17) or the waitlist control (n = 13) group using a permuted-block randomization with varying block/cohort size, after sufficient participants meeting the criteria were recruited. Randomization was conducted by an independent research coordinator using a computer-generated random number sequence. The time between baseline (T1) and post-treatment (T2) assessments for the two groups was approximately 13 weeks. The BPT group participated in a 13-week BPT program (see section “Behavioral Parent Training”) between T1 and T2, whereas the waitlist control group received no training. Additionally, they did not have any form of social contact with the researchers between T1 and T2. Because of ethical considerations, participants in the waitlist control group were promised the same BPT program in the future. Both groups were assessed using behavioral and cognitive (neuropsychological) measures at baseline and post-treatment. Parents answered questionnaires to assess children’s ADHD symptoms and related behavioral problems, and parents’ parenting practices and mental health. Children performed laboratory cognitive measures of inhibitory control (go/no-go and single response selection tasks) and working memory storage (digit span task).
Behavioral Parent Training
Behavioral parent training (BPT) was conducted in person in a group format across 13 consecutive, 2-h weekly sessions for each cohort. Each group session was facilitated by two behaviorally trained female clinical psychologists. To maintain treatment fidelity, the psychologists covered the BPT content by conducting sessions with the BPT program guidebook in all sessions (31). They reviewed the topics to be covered before each session and checked the coverage after the session. Any topic omitted from a session was presented in the following session. The BPT was based on a culturally adapted version of the BPT program (31), which originated from the UCLA and Barkley BPT programs for families of children with a wide range of disorders/disabilities, including ADHD (32, 33). The BPT content aimed to increase positive parent–child interactions and parental communication skills, while reducing parent–child conflicts and child oppositional defiant behaviors through lectures, group discussions, modeling, and role playing. Weekly homework assignments facilitated BPT skill implementation at home.
There were 13 weekly sessions of the BPT program, and each session lasted approximately 2 h (31–33). Psychological education for ADHD and parent’s stress managements were taught in sessions 1–3. Parenting skills for observing the child’s behaviors and paying positive attention to the child’s adaptive behaviors were taught in sessions 4–6. Parenting skills for providing clear rule, clear instruction and more structure in time and space were taught in sessions 7–9. Techniques for planned ignoring of child’s non-adaptive behaviors were taught in sessions 10–12. Session 13 was a wrap-up. Parents were asked to complete homework, specific to each session.
Subjective Questionnaire Measures
The Parenting Scale (PS) (34, 35) was used to assess parenting style/discipline practices, including laxness and over-reactivity. Parents rated their discipline strategies such as spanking and yelling, as forms of harsh physical and verbal discipline, on a seven-point Likert scale. The spanking ratings ranged from “I spank, slap, grab, or hit my child” (7) to “Never or rarely” (1) in the discipline situation “when my child misbehaves,” and the yelling ratings ranged from “I raise my voice or yell” (7) to “I speak to my child calmly” (1). The Parenting Stress Index (PSI) (36, 37) evaluated parenting stress (child and parent domains).
Attention-deficit/hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD) symptoms were assessed using parent-reported ratings on the SNAP (29, 30). Parents rated their children’s inattentive (items 1–9), hyperactive/impulsive (items 10–18), and defiant (items 19–26) behaviors using a four-point Likert scale (0 = not at all, 1 = just a little, 2 = quite a bit, 3 = very much). Higher scores in the SNAP indicates more severe ADHD and ODD symptoms. The subscale scores of > 9.0 for inattention and hyperactivity/impulsivity are clinically suggestive of ADHD (30, 38). To assess children’s problems with regulating affect, behavior, and cognition, parents rated the Child Behavior Checklist (CBCL/6-18) (39, 40), which included the Anxious/Depressed, Aggressive Behavior, and Attention Problems scales (41).
Objective Cognitive Measures
Response inhibition and selection were assessed by two objective, computer-based tasks of inhibitory control (go/no-go and single response selection tasks) (26, 27, 42). In each task, the children were required to discriminate a target stimulus from non-target stimuli. Each child determined the target stimulus from five characters of a popular video game (Super Mario Brothers) to facilitate motivation for task engagement. In the go/no-go task, participants were instructed to press a response key on a computer keyboard with the right hand as soon as the target (go) character stimulus (e.g., Mario) appeared in the center of the screen, but to withhold the response if one of the non-target (no-go) character stimuli (e.g., Luigi, Princess Peach) appeared. The stimulus duration was 500 ms and the interstimulus interval was randomly varied between 1100 and 1900 ms. After short practice trials, participants performed 100 trials of the task (80 go and 20 no-go trials). Commission error (CE) reflected failed inhibitions (i.e., incorrect go response to no-go trials) as the primary index of the task. The secondary indexes were omission error (OE), response time (RT), and RT variability (RTV).
In the single response selection task, participants were instructed to press one of two response keys on a computer keyboard with the right hand as soon as the target character stimulus (e.g., Mario) appeared in the center of the screen and to press a different key with the left hand if one of the non-target object stimuli (e.g., Coin, Flower) appeared. The stimulus duration was 500 ms and the interstimulus interval was randomly varied between 1500 and 2400 ms. After short practice trials, participants performed 80 trials of the task (10 target and 70 non-target selection trials). RT to target trials reflected fluent selection as the primary index of the task. The secondary indexes were target trial errors (i.e., incorrect non-target responses to target trials) and non-target trial errors (i.e., incorrect target response to non-target trials).
In addition, the forward digit span task was used to assess working memory storage. A sequence of digits appeared in the center of the screen at a rate of one digit every 1000 ms, and participants were instructed to immediately recall each sequence in the same order. The task began with a sequence length of two digits and increased by one digit after two trials. The task ended when the recalled sequence was incorrect in two trials of the same length. The longest correctly recalled sequences were used as the key measure of working memory storage.
Data Analysis
All analyses were based on per-protocol analysis where participants who received the BPT program or the waitlist schedule were included. Participants who dropped out of the BPT program or the waitlist schedule were excluded from the analyses. Owing to the small-scale nature of the trial, we decided that intention-to-treat analysis was not viable and could be misleading (43). The BPT and waitlist control groups were compared on all outcome measures at baseline (T1) and post-treatment (T2). For the questionnaire measures, missing item responses were imputed using the individual mean imputation method, which imputes the calculated mean of a given participant’s complete responses to other items of the same scale. The missing response rates were 0.13% in the BPT group and 0.08% in the waitlist control group, which were not significantly different (p = 0.555). For the cognitive measures, because of technical problems, data from two participants in the go/no-go task and five participants in the single response selection task were not recorded, and were not included in data analyses. A two-way analysis of variance (ANOVA) was conducted with group (BPT, waitlist control) as a between-subjects factor and time (T1, T2) as a within-subjects factor. An interaction between these two factors indicated a change in the treatment effects between T1 and T2. Effect size (Cohen’s d) calculations were based on Morris’s formula (44), where the mean baseline to post-treatment change in the BPT group minus the mean baseline to post-treatment change in the waitlist control group was divided by the pooled baseline standard deviation. Statistical analyses were conducted using IBM SPSS Statistics version 26 (IBM Japan, Tokyo, Japan). All p-values below 5% were considered to be statistically significant.
Results
Allocation, Dropout, and Engagement
Thirty participants were randomized (Figure 1). Seventeen were allocated to the BPT and 14 of these received the treatment program from T1 to T2. Participants attended most of the BPT program sessions (88.17% of all 13 sessions), indicating higher attendance rates (fidelity of treatment receipt) relative to the 71% in previous group-format BPT studies (45). For the waitlist control group, 13 participants were allocated and 11 of these received a waiting schedule from T1 to T2. Post-treatment (T2) data were obtained for 14 participants in the BPT group and 11 in the waitlist control group.
Baseline Characteristics
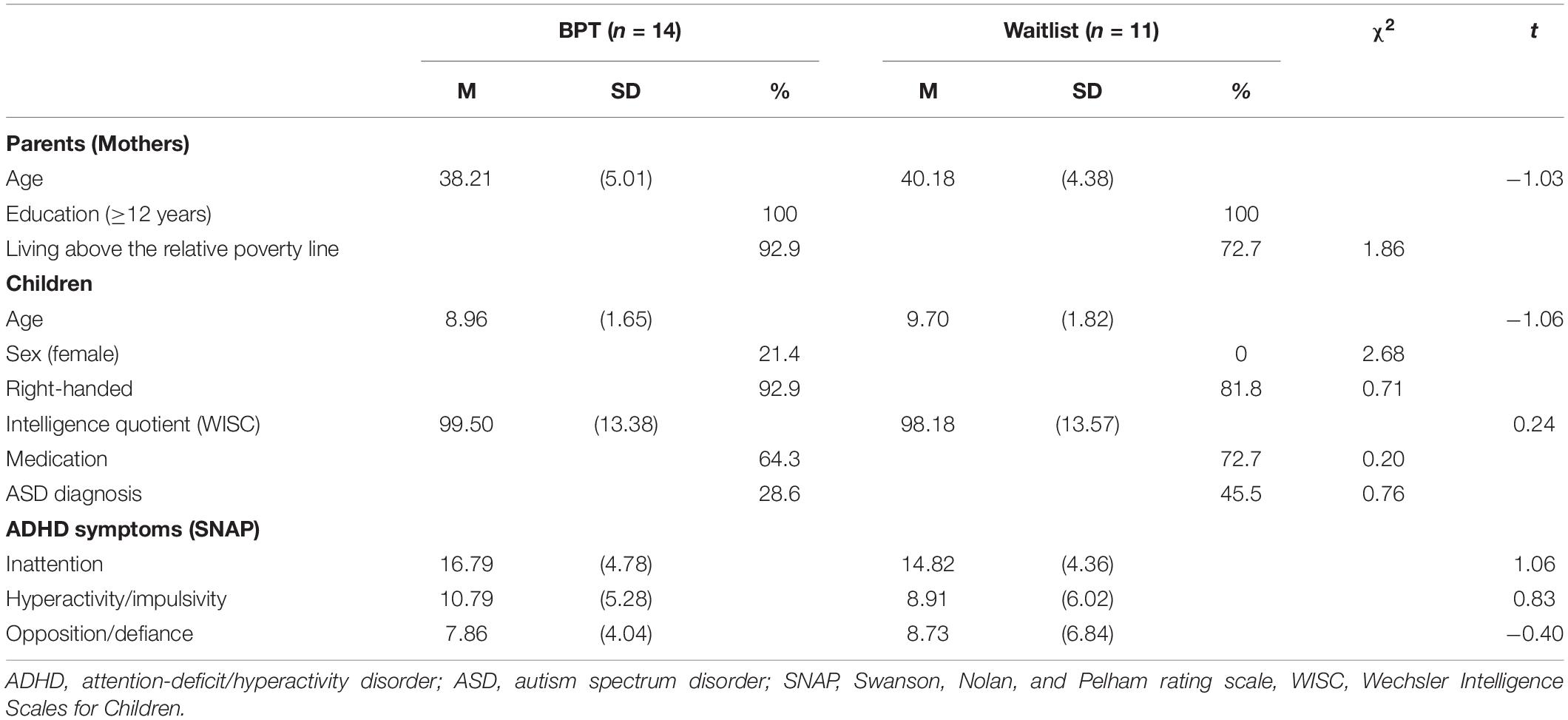
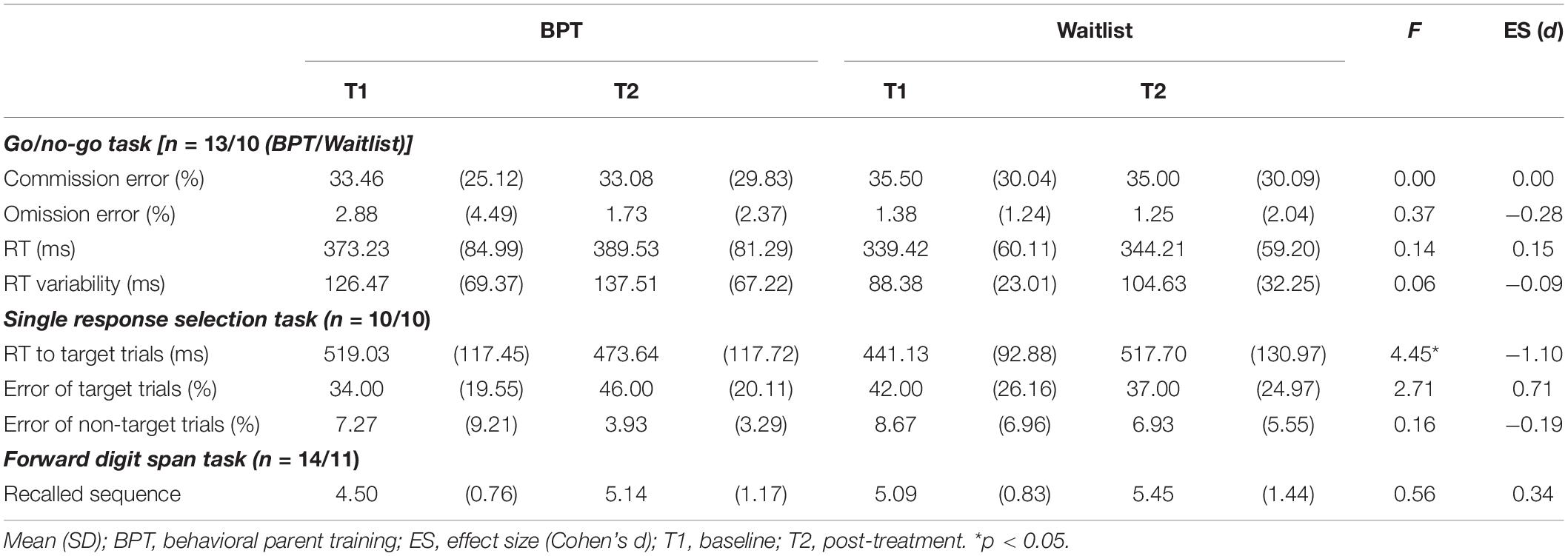
Table 1 presents the demographic characteristics of the parents and children in the BPT and waitlist control groups. There were no significant between-group differences for any assessed parent (age, education, relative poverty) or child (age, sex, handedness, IQ, medication status, ASD diagnosis, ADHD symptoms) characteristics (ps > 0.102). For subjective questionnaire outcomes at baseline (Table 2), the BPT and waitlist control groups were equivalent in terms of the PS, PSI, SNAP, and CBCL (ps > 0.299). Furthermore, for objective cognitive outcomes (Table 3), these two groups did not significantly differ on any baseline measures (ps > 0.077).
Table 2. Subjective (parent-reported) questionnaire outcomes for the BPT and waitlist control groups.
Subjective Questionnaire Outcomes
Table 2 presents the descriptive data and effect sizes of the questionnaire outcomes. There were significant interactions between group (BPT, waitlist control) and time (T1, T2) on four measures: yelling discipline rating [F(1,23) = 12.01, p = 0.002, d = −1.31], PSI child domain score [F(1,23) = 5.33, p = 0.030, d = −0.76], PSI parent domain score [F(1,23) = 7.94, p = 0.010, d = −0.72], and SNAP Inattention score [F(1,23) = 7.07, p = 0.014, d = −0.80]. These interactions showed that the BPT group had significant reductions from T1 to T2 in the questionnaire measures compared with the waitlist control group. The remaining scores showed no significant interactions (ps > 0.063).
Objective Cognitive Outcomes
Table 3 presents the descriptive data and effect sizes for the cognitive outcomes. For the go/no-go task, there were no significant interactions between group and time on the primary (CE) and secondary indexes (OE, RT, RTV) (ps > 0.548). For the single response selection task, significant interactions were found in the primary index, i.e., the RT to target trials [F(1, 18) = 4.45, p = 0.049, d = −1.10], but not in the secondary indexes, i.e., target trial and non-target trial errors (ps > 0.117). The BPT group exhibited significant reductions from T1 to T2 on the response selection task index compared with the waitlist control group (-45.39 ms vs. 76.56 ms). Furthermore, the forward digit span task showed no significant interactions in the recalled sequence (p = 0.462).
Discussion
The current study examined whether BPT effects extend to cognitive manifestations beyond the behavioral symptoms in children with ADHD. The objective cognitive measures from our small-scale RCT study showed that compared with the waitlist control group, the BPT group performed better in the single response selection task (response selection), but not in the go/no-go (response inhibition) or digit span tasks (working memory storage), partially supporting the hypothetical effects of BPT on the cognitive manifestations of ADHD. Moreover, in line with recent meta-analyses (5, 6), the BPT group showed decreased ADHD symptoms and decreased negative parenting and parental stress based on parent-reported assessments. From a view of the transactional model of ADHD and family functioning that suboptimal parenting practices exacerbate ADHD symptoms and their associated difficulties (8), improved parenting practices in the BPT group may improve behavioral symptoms and cognitive manifestations of ADHD and parental stress. However, suboptimal parenting practices in the waitlist control group are likely to negatively influence these outcomes.
To the best of our knowledge, this small-scale RCT study is the first to investigate the effects of BPT on inhibitory control, including response selection and inhibition, in children with ADHD, although previous ADHD studies have examined changes in response inhibition through BPT without employing a control group comparison (22). The BPT group in the current study displayed a more fluent response selection to specific external stimuli (or the mapping of sensory input to a motor response) than the waitlist control group. In the BPT program, parents learned to scaffold (support) their child through routines across the day and other cue-based reminders (e.g., lists of tasks to be completed), and feedback and contingencies to reinforce the successful implementation of daily activities and tasks (31, 32). The parents are encouraged via the BPT program to reward alternative adaptive behaviors to the non-adaptive (unwanted) behaviors in their child. Through such parental scaffolding, the child is supported in making effective selection between the non-adaptive and adaptive behaviors in real-world (information-rich) situations, rather than simply suppressing the non-adaptive behaviors. Consistent with the BPT program’s content and practice, the child’s hyperactive/impulsive behaviors did not decrease statistically but the inattentive behaviors decreased, which may be dependent on different inhibitory control components (response inhibition and selection) of EF. Correspondingly, children’s EF can be improved through parental scaffolding (supporting) (46). As reported by a recent meta-analysis study (17), in addition to this indirect type of psychosocial treatment (i.e., BPT) for children with ADHD, their EF, specifically inhibitory control, was affected by a wide range of direct psychosocial treatments. Regarding cognitive training, the effects on cognitive outcomes were significantly beneficial but were restricted to near transfer cognitive functions (16, 18–20). In particular, significant, small magnitude effects were evident among the 11 studies that included objective cognitive performance, such as inhibitory control tasks (20). For example, a previous RCT study using cognitive training found that treatment group children with ADHD exhibited faster performance in response selection, measured by a Stroop interference task, than the comparison group (47). The direction of RT changes for the task across treatment was consistent with that of developmental trajectories in inhibitory control (48), which indicated faster performance with age (6 to 18 years of age) despite high and low symptoms of ADHD.
Previous studies have suggested that inhibitory control is more malleable to psychosocial ADHD treatments than other cognitive functions such as basic working memory (17). However, it is important to understand why BPT’s beneficial effects in this study were found only for response selection, but not for response inhibition, even though these two cognitive operations are included within inhibitory control (26, 27). A possible reason for this may be associated with the predominantly right-lateralized, particularly PFC deficits in ADHD (49–51), suggesting that the right hemisphere PFC functions in ADHD have less potential for malleability to experience and treatment than the left hemisphere PFC functions. There is evidence of a partially dissociated lateralization of response selection and inhibition in inhibitory control (26, 42, 52–54): the left hemisphere PFC is predominantly involved in response selection, while the right hemisphere PFC is responsible for response inhibition. Regarding inhibitory control in ADHD, as summarized by meta-analysis studies of neuroimaging (50, 55), the right PFC activation during response inhibition (e.g., go/no-go task) is prominently reduced in children with ADHD compared with those without ADHD, supporting the theory of right-lateralized deficits in ADHD (51). Such hypoactivation in the right PFC during response inhibition in ADHD is known to be increased through direct psychosocial treatment (i.e., neurofeedback training) for children with ADHD (56). More direct treatments of the underlying neurocognitive causes of ADHD may be needed to improve the response inhibition anchored in the right PFC. Combined with the most prominent delayed maturation of the right PFC surface area in ADHD (57), our findings on the different effects of BPT on inhibitory control in children with ADHD may result from the different potential for malleability (plasticity) in the left and right PFC lateralization of response selection and inhibition. Further studies with functional neuroimaging techniques are necessary to better elucidate the neural mechanisms for the different effects of BPT on different inhibitory control components.
Although children’s cognitive functions (e.g., EF) have not previously been examined in most BPT studies (17), it is not surprising that BPT effects were found to be partially associated with cognitive manifestations beyond behavioral symptoms in children with ADHD. Parental negative discipline (e.g., verbal punishment) in response to a child’s misbehavior was decreased via BPT in our study, which may contribute a more supportive home environment that improves children’s EF (e.g., response selection). This is consistent with the traditional view (58) that children’s cognitive skills are socially constructed through interactions with supportive, responsive adults. A transactional model of ADHD and family functioning has also suggested that suboptimal parenting practices exacerbate children’s ADHD symptoms and their associated difficulties (8). Previous studies including a community sample have found an association between parenting behaviors and children’s EF using cross-sectional (12, 13) and longitudinal observational study designs (14, 15). There is also evidence that negative parenting (e.g., hostility) may have a larger impact on children’s EF than positive parenting (e.g., warmth) (59). Executive function is likely to be influenced by a wide range of circumstances and experiences, specifically during periods of relative plasticity in EF-related neural systems (e.g., PFC), including the preschool years, transition to adolescence, and late adolescence (46). Furthermore, improved parenting behaviors (e.g., using praise) via BPT may enhance children’s motivation by reinforcing their efforts in changing their own behaviors at home (60), which may then affect EF. Regarding the relationship between motivation and cognitive control (EF), a recent framework (61, 62) has argued that higher motivation can offset the higher cognitive control costs in shaping goal-directed selection and behaviors that are likely implemented by a motivationally triggered dopamine release in the PFC. Thus, BPT’s effects on children’s EF are also likely to be associated with their motivation, which is enhanced by improved parenting behaviors via BPT.
Finally, the limitations of the current study should be taken into consideration in future studies. First, given the relatively small sample size, this study was slightly underpowered. Thus, other potentially significant findings may have been neglected. The effects of BPT on other subjective (i.e., PS over-reactivity, CBCL attention problems) and objective measures (i.e., error of target trials in single response selection task) did not reach statistical significance, but showed medium to large effect sizes. The small sample size may diminish the statistical power of the effects. Studies involving a larger number of participants are essential to replicate and generalize our results. Second, most children in this study were taking concurrent medication treatment, which may limit the generalizability of our results. However, there were no differences for medication status between the BPT and waitlist control groups. Third, for generalization of our results, the participant’s characteristics (e.g., socioeconomic status, parental education) and the treatment properties (e.g., content, practice) may need to be taken into consideration. For example, it has been previously reported that the participants with lower socioeconomic status profited less from the treatment (63). Moreover, the treatment is more likely to be effective when the content most closely approximates the behavior targeted in daily life and the duration of practice and feedback is high (25). Fourth, regarding the subjective questionnaire outcomes, the beneficial effects of BPT may be overestimated due to treatment expectancy biases of unmasked parents (5), although they are the most ecologically valid assessors of children’s symptom expression (7). Further studies with independent masked assessments are required to avoid an overestimation of the beneficial effects of BPT on subjective outcomes. Fifth, the objective cognitive measures used in this study were limited to one EF component (i.e., inhibitory control) in children with ADHD. Three partially separable EF components are assumed to be inhibitory control, working memory updating, and set shifting (flexibility) (46). Although inhibitory control—the focus of this study—is more malleable to psychosocial treatments for ADHD than other cognitive functions (17), neurocognitive heterogeneity has been increasingly recognized as a valid ADHD phenomenon (10, 64–66). In addition to assessing inhibitory control (EF) and working memory storage (non-EF), further studies assessing multiple neurocognitive constructs (e.g., a hot-cool EF continuum) with multiple tasks would help better understand ADHD’s neurocognitive heterogeneity, allowing us to tailor psychosocial ADHD treatments according to neurocognitive subtypes.
Conclusion
In this study, we found that in addition to decreased ADHD symptoms and decreased negative parenting, the BPT group exhibited significantly improved performance in the single response selection task, but not in the go/no-go or digit span tasks, compared with the waitlist control group. Improvements in cognitive inhibitory control have previously been demonstrated by direct, child-centered psychosocial ADHD treatments (17). Although tentative, the current study provides partial evidence that BPT, as an indirect type of psychosocial treatment, has beneficial effects on cognitive inhibitory control (specifically response selection) beyond ADHD’s behavioral symptoms, highlighting the potential for supportive environmental modifications for cognitive development in children with ADHD.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Research Ethics Committee of the University of Fukui, Japan. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
AY, KS, RK, and AT conceptualized and designed the study. AY, KS, and RK collected and analyzed the data. AY and KS wrote the first draft of the manuscript. All authors edited and revised subsequent drafts of the manuscript, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work.
Funding
This study was supported, in part, by JSPS KAKENHI (grant numbers JP16K16622, JP19K14174, JP19K21755, and JP19H00617). This study was also partially supported by JST/RISTEX “Creating a Safe and Secure Living Environment in the Changing Public and Private Spheres,” the Center of Developmental Education and Research, and the Mayekawa Foundation. The funders had no role in the study design, data collection, analysis, interpretation, writing up, or the decision to submit the manuscript for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank all the parents and children who participated in our study as well as the staff at the Research Center for Child Mental Development and at the Department of Child and Adolescent Psychological Medicine, University of Fukui Hospital for their cooperation.
References
1. National Institute for Health and Care Excellence. Attention Deficit Hyperactivity Disorder: Diagnosis and Management (NG87). London: National Institute for Health and Care Excellence (2018).
2. Wolraich ML, Hagan JF, Allan C, Chan E, Davison D, Earls M, et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. (2019) 144:e20192528.
3. Young S, Amarasinghe JM. Practitioner review: non-pharmacological treatments for ADHD: a lifespan approach. J Child Psychol Psychiatry. (2010) 51:116–33. doi: 10.1111/j.1469-7610.2009.02191.x
4. Daley D, Van Der Oord S, Ferrin M, Cortese S, Danckaerts M, Doepfner M, et al. Practitioner review: current best practice in the use of parent training and other behavioural interventions in the treatment of children and adolescents with attention deficit hyperactivity disorder. J Child Psychol Psychiatry. (2018) 59:932–47. doi: 10.1111/jcpp.12825
5. Daley D, van der Oord S, Ferrin M, Danckaerts M, Doepfner M, Cortese S, et al. Behavioral interventions in attention-deficit/hyperactivity disorder: a meta-analysis of randomized controlled trials across multiple outcome domains. J Am Acad Child Adolesc Psychiatry. (2014) 53:835–47. doi: 10.1016/j.jaac.2014.05.013
6. Sonuga-Barke EJ, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, et al. Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry. (2013) 170:275–89. doi: 10.1176/appi.ajp.2012.12070991
7. Rimestad ML, Lambek R, Zacher Christiansen H, Hougaard E. Short- and long-term effects of parent training for preschool children with or at risk of ADHD: a systematic review and meta-analysis. J Atten Disord. (2019) 23:423–34. doi: 10.1177/1087054716648775
8. Johnston C, Mash EJ. Families of children with attention-deficit/hyperactivity disorder: review and recommendations for future research. Clin Child Fam Psychol Rev. (2001) 4:183–207. doi: 10.1023/a:1017592030434
9. Coghill D, Nigg J, Rothenberger A, Sonuga-Barke E, Tannock R. Whither causal models in the neuroscience of ADHD. Dev Sci. (2005) 8:105–14. doi: 10.1111/j.1467-7687.2005.00397.x
10. Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes. Biol Psychiatry. (2005) 57:1224–30. doi: 10.1016/j.biopsych.2004.08.025
11. Brody GH, McBride Murry V, McNair L, Chen YF, Gibbons FX, Gerrard M, et al. Linking changes in parenting to parent–child relationship quality and youth self-control: the strong African American families program. J Res Adolesc. (2005) 15:47–69. doi: 10.1111/j.1532-7795.2005.00086.x
12. Schroeder VM, Kelley ML. Family environment and parent-child relationships as related to executive functioning in children. Early Child Dev Care. (2010) 180:1285–98. doi: 10.1080/03004430902981512
13. Sosic-Vasic Z, Kröner J, Schneider S, Vasic N, Spitzer M, Streb J. The association between parenting behavior and executive functioning in children and young adolescents. Front Psychol. (2017) 8:472. doi: 10.3389/fpsyg.2017.00472
14. Belsky J, Pasco Fearon RM, Bell B. Parenting, attention and externalizing problems: testing mediation longitudinally, repeatedly and reciprocally. J Child Psychol Psychiatry. (2007) 48:1233–42. doi: 10.1111/j.1469-7610.2007.01807.x
15. Eisenberg N, Zhou Q, Spinrad TL, Valiente C, Fabes RA, Liew J. Relations among positive parenting, children’s effortful control, and externalizing problems: a three-wave longitudinal study. Child Dev. (2005) 76:1055–71. doi: 10.1111/j.1467-8624.2005.00897.x
16. Cortese S, Ferrin M, Brandeis D, Buitelaar J, Daley D, Dittmann RW, et al. Cognitive training for attention-deficit/hyperactivity disorder: meta-analysis of clinical and neuropsychological outcomes from randomized controlled trials. J Am Acad Child Adolesc Psychiatry. (2015) 54:164–74. doi: 10.1016/j.jaac.2014.12.010
17. Lambez B, Harwood-Gross A, Golumbic EZ, Rassovsky Y. Non-pharmacological interventions for cognitive difficulties in ADHD: a systematic review and meta-analysis. J Psychiatr Res. (2020) 120:40–55. doi: 10.1016/j.jpsychires.2019.10.007
18. Melby-Lervåg M, Hulme C. Is working memory training effective? A meta-analytic review. Dev Psychol. (2013) 49:270–91. doi: 10.1037/a0028228
19. Melby-Lervåg M, Redick TS, Hulme C. Working memory training does not improve performance on measures of intelligence or other measures of “far transfer”: evidence from a meta-analytic review. Perspect Psychol Sci. (2016) 11:512–34. doi: 10.1177/1745691616635612
20. Rapport MD, Orban SA, Kofler MJ, Friedman LM. Do programs designed to train working memory, other executive functions, and attention benefit children with ADHD? A meta-analytic review of cognitive, academic, and behavioral outcomes. Clin Psychol Rev. (2013) 33:1237–52. doi: 10.1016/j.cpr.2013.08.005
21. Chacko A, Bedard AV, Marks D, Gopalan G, Feirsen N, Uderman J, et al. Sequenced neurocognitive and behavioral parent training for the treatment of ADHD in school-age children. Child Neuropsychol. (2018) 24:427–50. doi: 10.1080/09297049.2017.1282450
22. Hannesdottir DK, Ingvarsdottir E, Bjornsson A. The OutSMARTers Program for Children With ADHD. J Atten Disord. (2017) 21:353–64. doi: 10.1177/1087054713520617
23. Kofler MJ, Sarver DE, Austin KE, Schaefer HS, Holland E, Aduen PA, et al. Can working memory training work for ADHD? Development of central executive training and comparison with behavioral parent training. J Consult Clin Psychol. (2018) 86:964–79. doi: 10.1037/ccp0000308
24. Steeger CM, Gondoli DM, Gibson BS, Morrissey RA. Combined cognitive and parent training interventions for adolescents with ADHD and their mothers: a randomized controlled trial. Child Neuropsychol. (2016) 22:394–419. doi: 10.1080/09297049.2014.994485
25. Evans SW, Owens JS, Wymbs BT, Ray AR. Evidence-based psychosocial treatments for children and adolescents with attention deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. (2018) 47:157–98.
26. Bender AD, Filmer HL, Garner KG, Naughtin CK, Dux PE. On the relationship between response selection and response inhibition: an individual differences approach. Atten Percept Psychophys. (2016) 78:2420–32. doi: 10.3758/s13414-016-1158-8
27. Bender AD, Filmer HL, Naughtin CK, Dux PE. Dynamic, continuous multitasking training leads to task-specific improvements but does not transfer across action selection tasks. NPJ Sci Learn. (2017) 2:14. doi: 10.1038/s41539-017-0015-4
28. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association (2013).
29. Inoue Y, Ito K, Kita Y, Inagaki M, Kaga M, Swanson JM. Psychometric properties of Japanese version of the Swanson, Nolan, and Pelham, version-IV scale-teacher form: a study of school children in community samples. Brain Dev. (2014) 36:700–6. doi: 10.1016/j.braindev.2013.09.003
30. Swanson JM, Kraemer HC, Hinshaw SP, Arnold LE, Conners CK, Abikoff HB, et al. Clinical relevance of the primary findings of the MTA: success rates based on severity of ADHD and ODD symptoms at the end of treatment. J Am Acad Child Adolesc Psychiatry. (2001) 40:168–79. doi: 10.1097/00004583-200102000-00011
32. Barkley RA. Defiant Children: A Clinician’s Manual for Parent Training. 3rd ed. New York, NY: The Guilford Press (2013).
33. Whitham C. Win the Whining War and Other Skirmishes: A Family Peace Plan. Los Angeles, CA: Perspective Publishing (1991).
34. Arnold DS, O’Leary SG, Wolff LS, Acker MM. The parenting scale: a measure of dysfunctional parenting in discipline situations. Psychol Assess. (1993) 5:137. doi: 10.1037/1040-3590.5.2.137
35. Itani T. [The Japanese version of the parenting scale: factor structure and psychometric properties]. Shinrigaku Kenkyu. (2010) 81:446–52. doi: 10.4992/jjpsy.81.446
36. Abidin RR. Parenting Stress Index: Professional Manual. Lutz, FL: Psychological Assessment Resources (1995).
37. Narama M, Kanematsu Y, Araki A, Maru M, Nakamura N, Takeda J, et al. Validity and reliability of the Japanese version of the parenting stress index. J Child Health. (1999) 58:610–6.
38. Coghill D, Seth S. Effective management of attention-deficit/hyperactivity disorder (ADHD) through structured re-assessment: the Dundee ADHD clinical care pathway. Child Adolesc Psychiatry Ment Health. (2015) 9:52. doi: 10.1186/s13034-015-0083-2
39. Achenbach TM, Rescorla L. Manual for the ASEBA School-age Forms & Profiles: An Integrated System of Multi-Informant Assessment. Burlington, NJ: University of Vermont (2001).
40. Funabiki Y, Murai T. Standardization of the Japanese version of the child behavior checklist/6-18. Japan J Child Adol Psychol. (2017) 58:175–84.
41. Deutz MH, Geeraerts SB, van Baar AL, Dekoviæ M, Prinzie P. The Dysregulation Profile in middle childhood and adolescence across reporters: factor structure, measurement invariance, and links with self-harm and suicidal ideation. Eur Child Adolesc Psychiatry. (2016) 25:431–42. doi: 10.1007/s00787-015-0745-x
42. Filmer HL, Mattingley JB, Marois R, Dux PE. Disrupting prefrontal cortex prevents performance gains from sensory-motor training. J Neurosci. (2013) 33:18654–60. doi: 10.1523/JNEUROSCI.2019-13.2013
43. Thompson MJ, Laver-Bradbury C, Ayres M, Le Poidevin E, Mead S, Dodds C, et al. A small-scale randomized controlled trial of the revised new forest parenting programme for preschoolers with attention deficit hyperactivity disorder. Eur Child Adolesc Psychiatry. (2009) 18:605–16. doi: 10.1007/s00787-009-0020-0
44. Morris SB. Estimating effect sizes from pretest-posttest-control group designs. Organ Res Methods. (2008) 11:364–86. doi: 10.1037/a0014699
45. Chacko A, Jensen SA, Lowry LS, Cornwell M, Chimklis A, Chan E, et al. Engagement in behavioral parent training: review of the literature and implications for practice. Clin Child Fam Psychol Rev. (2016) 19:204–15. doi: 10.1007/s10567-016-0205-2
46. Zelazo PD. Executive function and psychopathology: a neurodevelopmental perspective. Annu Rev Clin Psychol. (2020) 16:431–54. doi: 10.1146/annurev-clinpsy-072319-024242
47. Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlström K, et al. Computerized training of working memory in children with ADHD: a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry. (2005) 44:177–86. doi: 10.1097/00004583-200502000-00010
48. Crosbie J, Arnold P, Paterson A, Swanson J, Dupuis A, Li X, et al. Response inhibition and ADHD traits: correlates and heritability in a community sample. J Abnorm Child Psychol. (2013) 41:497–507. doi: 10.1007/s10802-012-9693-9
49. Doi H, Shinohara K. fNIRS studies on hemispheric asymmetry in atypical neural function in developmental disorders. Front Hum Neurosci. (2017) 11:137. doi: 10.3389/fnhum.2017.0013
50. Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. (2013) 70:185–98. doi: 10.1001/jamapsychiatry.2013.277
51. Stefanatos GA, Wasserstein J. Attention deficit/hyperactivity disorder as a right hemisphere syndrome: selective literature review and detailed neuropsychological case studies. Ann NY Acad Sci. (2001) 931:172–95. doi: 10.1111/j.1749-6632.2001.tb05779.x
52. Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci. (2014) 18:177–85. doi: 10.1016/j.tics.2013.12.003
53. Dux PE, Tombu MN, Harrison S, Rogers BP, Tong F, Marois R. Training improves multitasking performance by increasing the speed of information processing in human prefrontal cortex. Neuron. (2009) 63:127–38. doi: 10.1016/j.neuron.2009.06.005
54. Levy BJ, Wagner AD. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann N Y Acad Sci. (2011) 1224:40–62. doi: 10.1111/j.1749-6632.2011.05958.x
55. Lukito S, Norman L, Carlisi C, Radua J, Hart H, Simonoff E, et al. Comparative meta-analyses of brain structural and functional abnormalities during cognitive control in attention-deficit/hyperactivity disorder and autism spectrum disorder. Psychol Med. (2020) 50:894–919. doi: 10.1017/S0033291720000574
56. Alegria AA, Wulff M, Brinson H, Barker GJ, Norman LJ, Brandeis D, et al. Real-time fMRI neurofeedback in adolescents with attention deficit hyperactivity disorder. Hum Brain Mapp. (2017) 38:3190–209. doi: 10.1002/hbm.23584
57. Shaw P, Malek M, Watson B, Sharp W, Evans A, Greenstein D. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biol Psychiatry. (2012) 72:191–7. doi: 10.1016/j.biopsych.2012.01.031
58. Vygotsky LS. Mind in Society: The Development of Higher Psychological Processes. Cambridge, MA: Harvard University Press (1978).
59. Lam CB, Chung KKH, Li X. Parental warmth and hostility and child executive function problems: a longitudinal study of Chinese families. Front Psychol. (2018) 9:1063. doi: 10.3389/fpsyg.2018.01063
60. van der Oord S, Tripp G. How to improve behavioral parent and teacher training for children with ADHD: integrating empirical research on learning and motivation into treatment. Clin Child Fam Psychol Rev. (2020) 23:577–604. doi: 10.1007/s10567-020-00327-z
61. Botvinick M, Braver T. Motivation and cognitive control: from behavior to neural mechanism. Annu Rev Psychol. (2015) 66:83–113. doi: 10.1146/annurev-psych-010814-015044
62. Yee DM, Braver TS. Interactions of motivation and cognitive control. Curr Opin Behav Sci. (2018) 19:83–90.
63. Lundahl B, Risser HJ, Lovejoy MC. A meta-analysis of parent training: moderators and follow-up effects. Clin Psychol Rev. (2006) 26:86–104. doi: 10.1016/j.cpr.2005.07.004
64. Fair DA, Bathula D, Nikolas MA, Nigg JT. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc Natl Acad Sci USA. (2012) 109:6769–74. doi: 10.1073/pnas.1115365109
65. Kofler MJ, Irwin LN, Soto EF, Groves NB, Harmon SL, Sarver DE. Executive functioning heterogeneity in pediatric ADHD. J Abnorm Child Psychol. (2019) 47:273–86. doi: 10.1007/s10802-018-0438-2
66. Shimada K, Fujisawa TX, Takiguchi S, Naruse H, Kosaka H, Okazawa H, et al. Ethnic differences in COMT genetic effects on striatal grey matter alterations associated with childhood ADHD: a voxel-based morphometry study in a Japanese sample. World J Biol Psychiatry. (2017) 18:322–8. doi: 10.3109/15622975.2015.1102325
Keywords: ADHD, behavioral parent training, inhibitory control, response selection, response inhibition
Citation: Yao A, Shimada K, Kasaba R and Tomoda A (2022) Beneficial Effects of Behavioral Parent Training on Inhibitory Control in Children With Attention-Deficit/Hyperactivity Disorder: A Small-Scale Randomized Controlled Trial. Front. Psychiatry 13:859249. doi: 10.3389/fpsyt.2022.859249
Received: 21 January 2022; Accepted: 17 March 2022;
Published: 27 April 2022.
Edited by:
Veit Roessner, Technical University Dresden, GermanyReviewed by:
Heather Joseph, University of Pittsburgh, United StatesHarshini Manohar, National Institute of Mental Health and Neurosciences (NIMHANS), India
Copyright © 2022 Yao, Shimada, Kasaba and Tomoda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Koji Shimada, a3NoaW1hZGFAdS1mdWt1aS5hYy5qcA==; Akemi Tomoda, YXRvbW9kYUB1LWZ1a3VpLmFjLmpw
 Akiko Yao
Akiko Yao Koji Shimada
Koji Shimada Ryoko Kasaba
Ryoko Kasaba Akemi Tomoda
Akemi Tomoda