- 1Department of Internal Medicine, National Hospital Organization Awara Hospital, Awara, Japan
- 2Division of Infection Control and Prevention, University of Fukui Hospital, Fukui, Japan
1. Introduction
The annual prevalence of major depressive disorder (MDD) in the US is 6.7% (1), and an estimated 35 million US adults will be affected by MMD during their lifetime (1–3). However, MDD is a global problem with an economic burden estimated at 83.1 billion USD in 2000 (2, 4). Furthermore, the prevalence of psychiatric disorders, including MDD, has increased during the COVID-19 pandemic (5). MDD is considered to be a multifactorial disorder caused by both environmental and genetic factors, but the mechanism underlying its pathogenesis is not fully understood (6). It is likely that there are multiple underlying mechanisms of pathogenesis (2, 6), given the existence of multiple patient subgroups with different characteristics. Current treatments for MDD, including pharmacotherapy, have not yet achieved satisfactory results (7–9). We hypothesize that ganciclovir may be a potential therapeutic candidate for MDD based on not only its antiviral action, but also its modulation of innate immune pathways in the brain that are activated in response to stress.
2. Mechanisms of disease for the various human herpesviruses/their relationship with depression
2.1. HHV as a risk for developing MDD
2.1.1. Herpes simplex virus
Few studies have examined the association between depression and herpes simplex virus (HSV) infection and reactivation (10). In a study of US adults, HSV-1 was not associated with an increased risk of depression, but HSV-2 was associated with an increased risk of depression (11). In a study of Finnish adults, HSV-1 infection was not associated with new-onset depression (12). In studies of adolescents and adults, depressed patients are more likely to engage in risky sexual behaviors and consequently develop HSV-2 infection (13–15). Sexually transmitted diseases (STDs) are associated with depression, and depressive symptoms tend to be more severe in patients with STDs (14, 15). The odds ratio for depression was higher in HSV-2 patients (OR 2.1, 95% CI 1.5–2.9), and HSV-2 may be both a cause and a consequence of depression, including the fact that STDs are associated with depression (13). Persistent psychological stress is a risk for activation of HSV-2 (16).
2.1.2. Varicella-zoster virus
A cross-sectional cohort study of 104 older adults aged 60 years and older found that indices of varicella-zoster virus (VZV) cell-mediated immunity were significantly lower in a group of subjects with MDD than in an age- and sex-matched control group without a history of depression or psychiatric disorders (17). In a matched case-control study of subjects aged 50 years and older, 389 herpes zoster (HZ) cases and 511 matched controls were enrolled, and the adjusted odds ratio for depression was 3.81 higher in the HZ group than in the control group, and stress was a risk factor for HZ (aOR 2.80) (18). In a study conducted in Taiwan of HZ patients aged 18 years and older, 1,888 HZ patients were compared with 7,552 age- and sex-matched controls, and HZ patients were significantly more likely to develop MDD, and HZ was an independent risk factor for MDD (hazard ratio 1.49, 95% confidence interval 1.04–2.13) (19). Postherpetic neuralgia is associated with the development of depression (20).
2.1.3. Epstein-Barr virus
In the study of Finns over 30 years of age cited above, Epstein-Barr virus (EBV) seropositivity was not associated with the risk of developing depression (12). On the other hand, several studies have suggested an association with depression. In a study of 87 patients with MDD and 312 controls, in which antibodies to EBV were measured by solid phase immunoassay and Western blotting, low levels of antibodies to EBNA-1 and high levels of antibodies to EBV virions increased the likelihood that the individuals would be diagnosed with MDD (21). These findings suggest that altered immunity to EBV may be associated with the immunopathology of MDD (21). In adolescent females, increased depressive symptoms are significantly associated with salivary shedding of EBV DNA (22). In studies of pregnant women, EBV reactivation has been associated with increased rates of depression (23, 24). Infectious mononucleosis is most commonly caused by EBV infection (25), but a study in Denmark found that infectious mononucleosis was associated with a higher risk of depression compared with unaffected individuals (HR 1.40, 95% CI 1.26–1.56) (26).
2.1.4. Cytomegalovirus
In a study of 137 older adults in the United Kingdom, among the cytomegalovirus (CMV) seropositive group, the higher the CMV IgG, the more likely they were to be anxious and depressed (27). In a study in US adults, higher CMV antibody levels were associated with depression in CMV antibody-positive individuals (11). A study analyzing data from older US Latinos aged 60 years and older found that CMV seropositivity was significantly associated with an increased likelihood of developing depression (OR 1.38, 95% CI 1.00–1.90) (28).
2.1.5. Human herpesvirus 6
Human herpesvirus 6 (HHV-6) is reactivated from latent infection in the cerebellum of patients with MDD (29). HHV-6B is widespread around the world, including in Europe, the US, and Japan, with primary infection occurring between the ages of 6–12 months, followed by latency in the human body (30). This latent HHV-6B infection has been reported to produce a small latent protein, encoded by the intermediate stage transcript of HHV-6-1 (SITH-1), in olfactory bulb astrocytes. SITH-1 forms a complex with calcium-regulated cyclophilin ligand to cause calcium influx into the cell and induce apoptosis. In SITH-1 model mice, SITH-1 is produced by olfactory bulb sheath cells, a type of olfactory astrocyte, leading to apoptosis in the olfactory bulb and the expression of depressive symptoms. Patients with MDD have been found to show significantly higher detection of SITH-1-specific antibodies compared with healthy controls, with an odds ratio of 12.2 (31). Overexertion leads to increased HHV-6B in saliva, which can increase the number of SITH-1-producing cells (32). Among patients with MDD, late proteins indicative of HHV-6B activity and viral DNA have been detected in the cerebellum 80 and 53% more frequently than in controls, respectively (29).
2.2. HHV as a risk for worsening MDD
In a study of antibody titers to HSV, CMV, and EBV in 65 patients with coronary artery disease, the greater the severity of depression, the higher the rate of seropositivity to latent viruses (33). Cytomegalovirus infection is associated with decreased volume of gray matter in the brain in patients with MDD. This result suggests that cytomegalovirus infection may be a treatable cause of structural brain abnormalities in depressed patients (34). SITH-1-induced olfactory bulb apoptosis may also facilitate HHV-6B or other HHVs invasion into the brain, which is associated with worsening depressive symptoms (31). In a study of 11- to 18-year-olds in Turkey, depressed patients with suicidal ideation had significantly higher levels of HHV-6 antibodies, suggesting that persistent HHV-6 infection may be a risk factor for suicidal ideation (35).
The adult prevalence of HHV in the general population is that HSV-1 infects about 70% of adults, HSV-2 about 30%, VZV more than 90%, EBV also more than 90%, CMV about 70%, and more than 95% of those 2 years and older are infected with either HHV-6A or HHV-6B or both (36). In contrast, in the depressed population, a study examining serologic testing in Turkish adolescents found HSV-1 in 71.4%, EBV in 82.9%, CMV in 94.2%, and HHV6 in 91.4%, and no statistically significant difference in healthy controls in the same study (35). No significant differences in HHV morbidity are expected between the general population and the depressed population, and it is likely that some infected individuals are more susceptible to depression than others. Therefore, HHV infection alone cannot be considered a risk factor for depression.
3. Antiviral mechanism of intervention and previous studies on efficacy for the various viruses
Although HHV-6 has no established treatment, anti-cytomegalovirus agents including ganciclovir are known to be effective (37, 38). Ganciclovir is also effective against herpesviruses such as HHV-1, herpes zoster virus, and Epstein–Barr virus (38–40). In an open label study of valganciclovir (a prodrug of ganciclovir), 75% of patients with high immunoglobulin G antibody titers to HHV-6 and Epstein–Barr virus and four or more of the following symptoms for at least 1 year, cognitive dysfunction, slow processing speed, sleep disturbance, short-term memory impairment, fatigue, and symptoms consistent with depression, achieved almost complete resolution of symptoms, and all returned to work or full-time activities (41). A phase I clinical trial of valganciclovir treatment for CMV positive patients with MDD is currently underway (ClinicalTrials.gov Identifier: NCT04724447). Based on these findings, it is conceivable that the antiviral effect of ganciclovir on herpesviruses is beneficial in the treatment of MDD.
4. Effects of ganciclovir on neuroinflammation with STING pathway activity
The relationship between stress and MDD is well-known, with stress causing activation of the brain's innate immune response pathways (42). Stimulator of interferon genes (STING), an adaptor protein expressed in microglia, plays an important role in regulating innate immune signaling processes in the central nervous system by detecting abnormal cytoplasmic DNA (43). Cyclic guanosine monophosphate (GMP)-adenosine monophosphate (AMP) synthase (cGAS) catalyzes the generation of 2′3′-cyclic-GMP-AMP (cGAMP), a second messenger that binds and activates STING. STING then recruits and activates TANK-binding kinase 1 and the transcription factor interferon regulatory factor 3 to produce interferon-β (IFN-β) (43). In an experimental mouse model of chronic restraint stress, decreased levels of STING and activation of its downstream molecules were observed in the hippocampus and prefrontal cortex (44). In addition, the mice exhibited depression-like behavior and elevated levels of the inflammatory cytokines tumor necrosis factor α, interleukin (IL)-6, and IL-1β in the brain (44). Activation of STING by the agonist cGAMP was shown to enhance phagocytosis of microglia in the brains of the mice, suppress the release of inflammatory cytokines, and exert antidepressant effects (44). Ganciclovir inhibited neuroinflammation by stimulating INF-β production in microglia depending on the STING pathway activation level (45). These findings suggest that the second point of action of ganciclovir is to promote phagocytosis of microglia by increasing INF-β production through activation of the STING pathway, which may lead to the improvement of MDD symptoms by suppressing neuroinflammation.
5. FDA data on ganciclovir adverse events
FDA data have reported depressive symptoms were observed in 27 (0.59%) of the 4,593 people, especially 40–49-year-old women, treated with ganciclovir from 1997 to 2022 (46). However, many of these patients were infected with cytomegalovirus, human immunodeficiency virus, or had acute lymphocytic leukemia (hematological malignancy), and many were also steroid users (46). It is possible that these patient characteristics were highly associated with depressive symptoms, and the mechanism of the association with ganciclovir is not clarified. Therefore, whether ganciclovir treatment causes depression requires careful interpretation. Side effects other than depression reported in the FDA data included cytomegalovirus infection, febrile neutropenia, pancreatitis, stress and anxiety, decreased hemoglobin, decreased weight, decreased hematocrit, thrombocytopenia, nosebleed, and urinary tract infection (46). Ganciclovir is primarily indicated for the treatment of CMV, so its administration to depressed patients is not indicated (47). In addition, it is administered with caution to patients with psychiatric disorders, and informed consent should be obtained prior to use in a clinical trial. Drug label information does not specifically list interactions with antidepressants, but warnings generally list hematologic toxicity, reproductive impairment, fetotoxicity, mutagenicity, and carcinogenicity (47).
6. Limitations
Neuroinflammation alone is not enough to explain MDD; psychological, social, environmental and cultural factors are also involved (48–51). Several clinical trials of anticytokine therapy for the neuroinflammatory hypothesis have been reported. Two clinical trials of the TNF-α inhibitor infliximab in depression showed no overall significant efficacy (52, 53). In addition, a meta-analysis of anticytokine therapy found a significant antidepressant effect, but the subjects were patients with chronic inflammatory diseases such as psoriasis and Crohn's disease, and depressive symptoms were measured only as a secondary outcome, so the results cannot be generalized as a therapeutic effect of anticytokine therapy for depressed patients (54). Toll-like receptor (TLR) 2/4 has been shown to be an important mediator of microglial activation in the medial prefrontal cortex by repeated social defeat stress, leading to neuronal and behavioral changes through inflammatory cytokines (55). The antimicrobial agent minocycline has been shown to inhibit inflammatory cytokine production by blocking phosphorylation of downstream molecules in the TLR 4 pathway (56). A pilot study of adjunctive minocycline treatment in patients with treatment-resistant depression showed an improvement in depressive symptoms in the minocycline group compared to placebo (57), but a randomized controlled trial of the efficacy of minocycline and celecoxib in combination or as monotherapy in bipolar depression found no significant difference in either group compared to placebo (58). Thus, at this time, clinical trials have not demonstrated sufficient power to support the neuroinflammation hypothesis. In addition, as noted above, HHV infection does not explain all causes of MDD.
7. Conclusion
We reviewed data from basic (in vitro and in vivo) and observational studies on MDD and HHV, as well as basic neuroinflammation experiments (in vitro and in vivo) on MDD and the STING pathway. In addition, we presented an early-stage study on the potential therapeutic use of ganciclovir for MDD. Ganciclovir may be a potential therapeutic candidate for MDD from two different perspectives: antiviral activity against herpesviruses and inhibition of neuroinflammation through activation of the STING pathway, as shown in Figure 1. If we can measure the presence or absence of viral infection and antibody titers or the degree of neuroinflammation in patients with MDD as future work, we may be able to select subjects with MDD for whom ganciclovir is effective. We hope that validation by further clinical research can expand the range of treatment options because many patients with MDD still show a poor response to treatment.
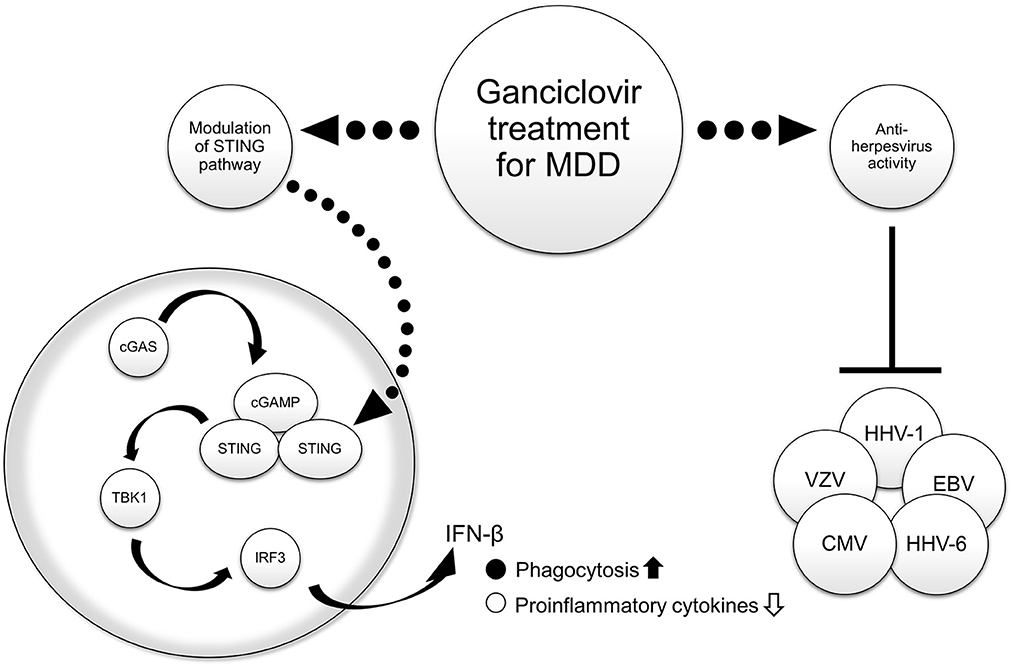
Figure 1. Hypothesized mechanisms of the therapeutic effect of ganciclovir in major depressive disorder (MDD). The right side of the figure shows antiviral activity, and the left side shows that activation of the STING pathway in central nervous system cells leads to the suppression of neuroinflammation by promoting phagocytosis and inhibiting inflammatory cytokine production. Source: Figure made by the authors with reference to Poole et al. (38) and Duan et al. (44). cGAS, cyclic guanosine monophosphate (GMP)-adenosine monophosphate (AMP) synthase; cGAMP, 2′ 3′ -cyclic-GMP-AMP; CMV, cytomegalovirus; EBV, Epstein–Barr virus; HHV, human herpesvirus; IFN-β, interferon beta; IRF3, interferon regulatory factor 3; MDD, major depressive disorder; STING, stimulator of interferon genes; TBK1, TANK-binding kinase 1; VZV, varicella-zoster virus.
Author contributions
KI, HT, YM, and HI contributed to conception and methodology of the manuscript and wrote sections of the manuscript. KI performed data curation. KI and HI wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. (2005) 62:617–27. doi: 10.1001/archpsyc.62.6.617
2. Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: insights from human and rodent studies. Neuroscience. (2016) 321:138–62. doi: 10.1016/j.neuroscience.2015.05.053
3. Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. (2003) 289:3095–105. doi: 10.1001/jama.289.23.3095
4. Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. (2003) 64:1465–75. doi: 10.4088/JCP.v64n1211
5. Nochaiwong S, Ruengorn C, Thavorn K, Hutton B, Awiphan R, Phosuya C, et al. Global prevalence of mental health issues among the general population during the coronavirus disease-2019 pandemic: a systematic review and meta-analysis. Sci Rep. (2021) 11:10173. doi: 10.1038/s41598-021-89700-8
6. Miyata S, Ishino Y, Shimizu S, Tohyama M. Involvement of inflammatory responses in the brain to the onset of major depressive disorder due to stress exposure. Front Aging Neurosci. (2022) 14:934346. doi: 10.3389/fnagi.2022.934346
7. Hieronymus F, Emilsson JF, Nilsson S, Eriksson E. Consistent superiority of selective serotonin reuptake inhibitors over placebo in reducing depressed mood in patients with major depression. Mol Psychiatry. (2016) 21:523–30. doi: 10.1038/mp.2015.53
8. Zhao X, Karkare S, Nash AI, Sheehan JJ, Aboumrad M, Near AM, et al. Characteristics and current standard of care among veterans with major depressive disorder in the United States: a real-world data analysis. J Affect Disord. (2022) 307:184–90. doi: 10.1016/j.jad.2022.03.058
9. Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. (2006) 163:1905–17. doi: 10.1176/ajp.2006.163.11.1905
10. Coughlin SS. Anxiety and depression: linkages with viral diseases. Public Health Rev. (2012) 34:7. doi: 10.1007/BF03391675
11. Gale SD, Berrett AN, Erickson LD, Brown BL, Hedges DW. Association between virus exposure and depression in US adults. Psychiatry Res. (2018) 261:73–9. doi: 10.1016/j.psychres.2017.12.037
12. Markkula N, Lindgren M, Yolken RH, Suvisaari J. Association of exposure to Toxoplasma gondii, Epstein-Barr Virus, Herpes Simplex virus Type 1 and Cytomegalovirus with new-onset depressive and anxiety disorders: an 11-year follow-up study. Brain Behav Immun. (2020) 87:238–42. doi: 10.1016/j.bbi.2019.12.001
13. Pratt LA, Xu F, McQuillan GM, Robitz R. The association of depression, risky sexual behaviours and herpes simplex virus type 2 in adults in NHANES, 2005-2008. Sex Transm Infect. (2012) 88:40–4. doi: 10.1136/sextrans-2011-050138
14. Khan MR, Kaufman JS, Pence BW, Gaynes BN, Adimora AA, Weir SS, et al. Depression, sexually transmitted infection, and sexual risk behavior among young adults in the United States. Arch Pediatr Adolesc Med. (2009) 163:644–52. doi: 10.1001/archpediatrics.2009.95
15. Salazar LF, DiClemente RJ, Wingood GM, Crosby RA, Lang DL, Harrington K. Biologically confirmed sexually transmitted infection and depressive symptomatology among African-American female adolescents. Sex Transm Infect. (2006) 82:55–60. doi: 10.1136/sti.2005.015289
16. Cohen F, Kemeny ME, Kearney KA, Zegans LS, Neuhaus JM, Conant MA. Persistent stress as a predictor of genital herpes recurrence. Arch Intern Med. (1999) 159:2430–6. doi: 10.1001/archinte.159.20.2430
17. Irwin MR, Levin MJ, Carrillo C, Olmstead R, Lucko A, Lang N, et al. Major depressive disorder and immunity to varicella-zoster virus in the elderly. Brain Behav Immun. (2011) 25:759–66. doi: 10.1016/j.bbi.2011.02.001
18. Marin M, Harpaz R, Zhang J, Wollan PC, Bialek SR, Yawn BP. Risk factors for herpes zoster among adults. Open Forum Infect Dis. (2016) 3:ofw119. doi: 10.1093/ofid/ofw119
19. Chen M-H, Wei H-T, Su T-P, Li C-T, Lin W-C, Chang W-H, et al. Risk of depressive disorder among patients with herpes zoster: a nationwide population-based prospective study. Psychosom Med. (2014) 76:285–91. doi: 10.1097/PSY.0000000000000051
20. Baron R. Post-herpetic neuralgia case study: optimizing pain control. Eur J Neurol. (2004) 11(Suppl 1):3–11. doi: 10.1111/j.1471-0552.2004.00794.x
21. Jones-Brando L, Dickerson F, Ford G, Stallings C, Origoni A, Katsafanas E, et al. Atypical immune response to Epstein-Barr virus in major depressive disorder. J Affect Disord. (2020) 264:221–6. doi: 10.1016/j.jad.2019.11.150
22. Ford JL, Stowe RP. Depressive symptoms are associated with salivary shedding of Epstein-Barr virus in female adolescents: the role of sex differences. Psychoneuroendocrinology. (2017) 86:128–33. doi: 10.1016/j.psyneuen.2017.09.009
23. Haeri S, Johnson N, Baker AM, Stuebe AM, Raines C, Barrow DA, et al. Maternal depression and Epstein-Barr virus reactivation in early pregnancy. Obstet Gynecol. (2011) 117:862–6. doi: 10.1097/AOG.0b013e31820f3a30
24. Zhu P, Chen Y-J, Hao J-H, Ge J-F, Huang K, Tao R-X, et al. Maternal depressive symptoms related to Epstein-Barr virus reactivation in late pregnancy. Sci Rep. (2013) 3:3096. doi: 10.1038/srep03096
25. Hurt C, Tammaro D. Diagnostic evaluation of mononucleosis-like illnesses. Am J Med. (2007) 120:911.e1–8. doi: 10.1016/j.amjmed.2006.12.011
26. Vindegaard N, Petersen LV, Lyng-Rasmussen BI, Dalsgaard S, Benros ME. Infectious mononucleosis as a risk factor for depression: a nationwide cohort study. Brain Behav Immun. (2021) 94:259–65. doi: 10.1016/j.bbi.2021.01.035
27. Phillips AC, Carroll D, Khan N, Moss P. Cytomegalovirus is associated with depression and anxiety in older adults. Brain Behav Immun. (2008) 22:52–5. doi: 10.1016/j.bbi.2007.06.012
28. Simanek AM, Zheng C, Yolken R, Haan M, Aiello AE. A longitudinal study of the association between persistent pathogens and incident depression among older US Latinos. J Gerontol A Biol Sci Med Sci. (2019) 74:634–41. doi: 10.1093/gerona/gly172
29. Prusty BK, Gulve N, Govind S, Krueger GRF, Feichtinger J, Larcombe L, et al. Active HHV-6 infection of cerebellar purkinje cells in mood disorders. Front Microbiol. (2018) 9:1955. doi: 10.3389/fmicb.2018.01955
30. Ablashi D, Agut H, Alvarez-Lafuente R, Clark DA, Dewhurst S, DiLuca D, et al. Classification of HHV-6A and HHV-6B as distinct viruses. Arch Virol. (2014) 159:863–70. doi: 10.1007/s00705-013-1902-5
31. Kobayashi N, Oka N, Takahashi M, Shimada K, Ishii A, Tatebayashi Y, et al. Human herpesvirus 6B greatly increases risk of depression by activating hypothalamic-pituitary -adrenal axis during latent phase of infection. iScience. (2020) 23:101187. doi: 10.1016/j.isci.2020.101187
32. Aoki R, Kobayashi N, Suzuki G, Kuratsune H, Shimada K, Oka N, et al. Human herpesvirus 6 and 7 are biomarkers for fatigue, which distinguish between physiological fatigue and pathological fatigue. Biochem Biophys Res Commun. (2016) 478:424–30. doi: 10.1016/j.bbrc.2016.07.010
33. Miller GE, Freedland KE, Duntley S, Carney RM. Relation of depressive symptoms to C-reactive protein and pathogen burden (cytomegalovirus, herpes simplex virus, Epstein-Barr virus) in patients with earlier acute coronary syndromes. Am J Cardiol. (2005) 95:317–21. doi: 10.1016/j.amjcard.2004.09.026
34. Zheng H, Ford BN, Bergamino M, Kuplicki R, Tulsa 1000 Investigators, Hunt PW, et al. A hidden menace? Cytomegalovirus infection is associated with reduced cortical gray matter volume in major depressive disorder. Mol Psychiatry. (2021) 26:4234–44. doi: 10.1038/s41380-020-00932-y
35. Bayturan S, Sapmaz SY, Uzun AD, Kandemir H, Ecemiş T. Relationship of herpesvirus (HSV1, EBV, CMV, HHV6) seropositivity with depressive disorder and its clinical aspects: the first study in children. J Med Virol. (2022) 94:5484–91. doi: 10.1002/jmv.27995
36. Braun DK, Dominguez G, Pellett PE. Human herpesvirus 6. Clin Microbiol Rev. (1997) 10:521–67. doi: 10.1128/CMR.10.3.521
37. Agut H, Bonnafous P, Gautheret-Dejean A. Human herpesviruses 6A, 6B, and 7. Microbiol Spectr. (2016) 4:1–18. doi: 10.1128/microbiolspec.DMIH2-0007-2015
38. Poole CL, James SH. Antiviral therapies for herpesviruses: current agents and new directions. Clin Ther. (2018) 40:1282–98. doi: 10.1016/j.clinthera.2018.07.006
39. Pagano JS, Whitehurst CB, Andrei G. Antiviral drugs for EBV. Cancers. (2018) 10:197. doi: 10.3390/cancers10060197
40. Meng Q, Hagemeier SR, Fingeroth JD, Gershburg E, Pagano JS, Kenney SC. The Epstein-Barr virus (EBV)-encoded protein kinase, EBV-PK, but not the thymidine kinase (EBV-TK), is required for ganciclovir and acyclovir inhibition of lytic viral production. J Virol. (2010) 84:4534–42. doi: 10.1128/JVI.02487-09
41. Kogelnik AM, Loomis K, Hoegh-Petersen M, Rosso F, Hischier C, Montoya JG. Use of valganciclovir in patients with elevated antibody titers against Human Herpesvirus-6 (HHV-6) and Epstein-Barr Virus (EBV) who were experiencing central nervous system dysfunction including long-standing fatigue. J Clin Virol. (2006) 37(Suppl 1):S33–38. doi: 10.1016/S1386-6532(06)70009-9
42. Ménard C, Pfau ML, Hodes GE, Russo SJ. Immune and neuroendocrine mechanisms of stress vulnerability and resilience. Neuropsychopharmacology. (2017) 42:62–80. doi: 10.1038/npp.2016.90
43. Paul BD, Snyder SH, Bohr VA. Signaling by cGAS-STING in neurodegeneration, neuroinflammation, and aging. Trends Neurosci. (2021) 44:83–96. doi: 10.1016/j.tins.2020.10.008
44. Duan N, Zhang Y, Tan S, Sun J, Ye M, Gao H, et al. Therapeutic targeting of STING-TBK1-IRF3 signalling ameliorates chronic stress induced depression-like behaviours by modulating neuroinflammation and microglia phagocytosis. Neurobiol Dis. (2022) 169:105739. doi: 10.1016/j.nbd.2022.105739
45. Mathur V, Burai R, Vest RT, Bonanno LN, Lehallier B, Zardeneta ME, et al. Activation of the STING-dependent type I interferon response reduces microglial reactivity and neuroinflammation. Neuron. (2017) 96:1290–302.e6. doi: 10.1016/j.neuron.2017.11.032
46. eHealthMe. Ganciclovir and Depression – A Phase IV Clinical Study of FDA Data. (2022). Available online at: https://www.ehealthme.com/ds/ganciclovir/depression/ (accessed November 24, 2022).
47. National Library of Medicine. DailyMed - GANCICLOVIR- Ganciclovir Sodium Injection, Solution. Available online at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3059c2da-d4a7-4aea-828c-20e671cb875c (accessed March 28, 2023).
48. Chiriţă AL, Gheorman V, Bondari D, Rogoveanu I. Current understanding of the neurobiology of major depressive disorder. Rom J Morphol Embryol. (2015) 56:651–8. Available online at: https://rjme.ro/RJME/resources/files/561215651658.pdf
49. Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. (2013) 34:119–38. doi: 10.1146/annurev-publhealth-031912-114409
50. Koenig HG. Research on religion, spirituality, and mental health: a review. Can J Psychiatry. (2009) 54:283–91. doi: 10.1177/070674370905400502
51. Weightman MJ, Air TM, Baune BT. A review of the role of social cognition in major depressive disorder. Front Psychiatry. (2014) 5:179. doi: 10.3389/fpsyt.2014.00179
52. Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. (2013) 70:31–41. doi: 10.1001/2013.jamapsychiatry.4
53. McIntyre RS, Subramaniapillai M, Lee Y, Pan Z, Carmona NE, Shekotikhina M, et al. Efficacy of adjunctive infliximab vs placebo in the treatment of adults with bipolar I/II depression: a randomized clinical trial. JAMA Psychiatry. (2019) 76:783–90. doi: 10.1001/jamapsychiatry.2019.0779
54. Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry. (2018) 23:335–43. doi: 10.1038/mp.2016.167
55. Nie X, Kitaoka S, Tanaka K, Segi-Nishida E, Imoto Y, Ogawa A, et al. The innate immune receptors TLR2/4 mediate repeated social defeat stress-induced social avoidance through prefrontal microglial activation. Neuron. (2018) 99:464–79.e7. doi: 10.1016/j.neuron.2018.06.035
56. Tai K, Iwasaki H, Ikegaya S, Ueda T. Minocycline modulates cytokine and chemokine production in lipopolysaccharide-stimulated THP-1 monocytic cells by inhibiting IκB kinase α/β phosphorylation. Transl Res. (2013) 161:99–109. doi: 10.1016/j.trsl.2012.10.001
57. Husain MI, Chaudhry IB, Husain N, Khoso AB, Rahman RR, Hamirani MM, et al. Minocycline as an adjunct for treatment-resistant depressive symptoms: a pilot randomised placebo-controlled trial. J Psychopharmacol. (2017) 31:1166–75. doi: 10.1177/0269881117724352
Keywords: ganciclovir, major depressive disorder, human herpesvirus 6B, interferon beta, neuroinflammation, interferon stimulated genes
Citation: Itoh K, Tsutani H, Mitsuke Y and Iwasaki H (2023) Two possible mechanisms of ganciclovir for treatment of major depressive disorder. Front. Psychiatry 14:1109723. doi: 10.3389/fpsyt.2023.1109723
Received: 28 November 2022; Accepted: 04 April 2023;
Published: 25 April 2023.
Edited by:
Shinsuke Hidese, Teikyo University, JapanReviewed by:
Maurizio Simmaco, Sapienza University of Rome, ItalyMaria Susanne Simon, LMU Munich University Hospital, Germany
Mani Yavi, National Institutes of Health (NIH), United States
Copyright © 2023 Itoh, Tsutani, Mitsuke and Iwasaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kazuhiro Itoh, a2l0b2hAdS1mdWt1aS5hYy5qcA==
†ORCID: Kazuhiro Itoh orcid.org/0000000155747118
 Kazuhiro Itoh
Kazuhiro Itoh Hiroshi Tsutani1
Hiroshi Tsutani1