- 1Alice Lee Centre for Nursing Studies, National University of Singapore, Singapore, Singapore
- 2Institute of Mental Health, Singapore, Singapore
Background: The Illness Management and Recovery (IMR) program has been established in response to the challenges faced by people with severe mental illnesses (SMIs). The program emphasizes the self-management of mental health conditions and the achievement of personally meaningful goals. However, reviews on its efficacy remain scarce, especially in recent years.
Objective: This review aimed to examine the efficacy of IMR in improving personal-recovery outcomes among people with SMIs.
Methods: A search was conducted on seven databases (CINAHL, Embase, ProQuest, PsycINFO, PubMed, Scopus, and Web of Science) from inception to February 2022, without limits on the dates and types of publications. Studies were included if they had examined the efficacy of IMR in one or more outcomes, investigated at least one group of participants, and been published in English. The participants were adults (at least 16 years of age) with a formal diagnosis of at least one SMI.
Results: Fourteen studies were included in this review, and eight outcomes were examined: personal recovery, global functioning, social functioning, hope, perceived social support, quality of life, substance abuse, and knowledge of mental illness. There is limited evidence on the superiority of IMR to existing treatment plans or other interventions in improving the outcomes of interest among people with SMIs. However, the low attendance rates in many included studies suggest the presence of a threshold of exposure to IMR beyond which its treatment effects could be observed. Suggestions for future IMR implementation are discussed.
Conclusions: The IMR program may serve as an alternative or complementary intervention for people with SMIs, especially with enhanced program exposure and access to resource materials.
Systematic review registration: https://inplasy.com/inplasy-2022-10-0005/.
Introduction
Various definitions for severe mental illnesses (SMIs) have been proposed over the years (1–3). Nonetheless, an established consensus is that the following clinical criteria have to be fulfilled in defining SMIs: (i) a diagnosis of non-organic psychosis or personality disorder; (ii) at least a two-year history of mental illness or service contact (including treatment); and (iii) disability, moderate impairment in work and non-work activities, and mild impairment in basic needs. People with SMIs experience substantial functional impediments to their ability to fully participate in society and major life activities (4). Additionally, besides barriers such as stigma (5) and employment problems (6), they face difficulties in accessing and navigating through a healthcare system (7). Collectively, such difficulties have been posited to lead to the elevated prevalence of somatic medical conditions among people with SMIs, potentially predisposing them to premature mortality (8).
Progressive decentralization of mental healthcare over recent decades might have partly been responsible for a fragmented medical system. This system not only relies on coordination between healthcare organizations, but also obliges individuals to establish and maintain contact with a multiplicity of such organizations (7, 9). The fragmentation is further confounded by existing challenges in the treatment for people with psychiatric-somatic comorbidities (10) and by difficulties faced by people with SMIs in establishing a point of contact with medical professionals (11). Against this background, people with SMIs continue to be disproportionately disadvantaged as compared with the general population.
In response to these challenges, the Illness Management and Recovery (IMR) program has been conceived to help people with SMIs acquire information and skills in managing their conditions and develop and attain personally meaningful goals (12). IMR has been developed under the National Implementing Evidence-Based Project, which focuses on the development of implementation and training materials for interventions to increase access for people with SMI (13). IMR revolves around the principles of recovery, viewing people with SMIs as individuals who can pursue meaningful goals and aspirations beyond the limitations of their conditions (14). Underpinned by the trans-theoretical and stress-vulnerability models, the IMR program, developed between 2000 and 2002, aims to improve personal and clinical recovery: it considers a given individual's stages of change and interrupts the cycle of stress and vulnerability responsible for relapses and functional impairments (15). IMR is curriculum-based and standardized, incorporating motivation-based, educational, and cognitive-behavioral strategies. As of 2011, the program comprises 11 modules: recovery strategies; facts of mental illnesses; stress-vulnerability model; social support; medication use; substance use; relapse prevention; coping with stress; coping with persistent symptoms; getting needs met in the mental healthcare system; and healthy lifestyles (16). The program had an earlier edition in 2006 (15), consisting of nine modules (excluding substance use and healthy lifestyles). IMR typically spans 6–12 months and may be conducted individually or in groups (16). Online resources are also available, such as educational handouts and practitioner guidelines on the Substance Abuse and Mental Health Services Administration (SAMHSA) website (17), thus making IMR accessible to practitioners globally.
To our knowledge, there has hitherto been one review published in 2014 on the IMR program that included studies published before June 2011 (18), a systematic review on self-management interventions which included IMR (19), and a review protocol (20) with similar outcomes as this paper. Another review published in 2002 compared 40 randomized controlled trials (RCTs) in individual components of IMR, such as knowledge of mental illness, medication adherence, symptom relapses and rates of re-hospitalization, and severity and distress of persistent symptoms (21). Additionally, McGuire, Kukla (18) explored client and implementation outcomes based on a combination of RCTs, quasi-controlled, and pre-post trials. In this context, with no reviews on this field of research for over a decade, an updated systematic review is critical as it examines the effectiveness of the IMR program in modern recovery interventions.
Aims
This review aimed to explore the effectiveness of IMR in improving personal-recovery outcomes among people with SMIs. Our specific research question was: compared with the standard care or other interventions, how effective are IMR programs in improving personal-recovery outcomes among people with SMIs?
Methods
To ensure methodological rigor (22), this review was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement (23). In addition, a review protocol has been registered on INPLASY2022100005.
Search strategy
To ensure a comprehensive and updated search for studies on IMR programs for people with SMIs, specific keywords and Medical Subject Headings (MeSH) terms were formulated with the help from the University librarian. These included “illness management and recovery”, “IMR”, “mental disorders” [MeSH], “mental illness”, “schizophrenia”, “bipolar”, and “psychosis”. Based on Boolean operators, seven databases were searched from inception to February 2022, with coverage across multiple disciplines (Scopus and Web of Science) and specific disciplines, including biomedicine (Embase, ProQuest, and Pubmed), nursing and allied health (CINAHL), and psychology (PsycINFO). To avoid omission of relevant materials, no limits were applied to the types and years of publications, and studies citing previous reviews (18, 19) were also retrieved. Given the lack of access to interpreters, only publications in English were included.
Eligibility criteria
Population
Participants in the included studies were adults (at least 18 years of age) diagnosed with schizophrenia, schizophreniform, schizoaffective, bipolar, or mood disorders. The diagnoses were based on a psychiatrist's clinical judgement or standardized criteria such as the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) or the International Classification of Diseases (ICD-10). Participants in the included studies were inpatients or individuals recruited from outpatient programs.
Intervention
The included studies were RCTs examining the efficacy of IMR among people with SMIs. The studies were required to have adhered to the standardized, curriculum-based IMR program based on the principles of recovery, with a focus on the following aspects: psycho-education for mental illnesses; cognitive-behavioral approaches to medication adherence; developing plans for relapse prevention; training for social skills; and skills to cope and manage symptoms (15). These five strategies may be implemented through the eleven or nine modules from the IMR manuals (15, 16) or adapted according to the population's needs.
Comparator
Studies with no comparators (participants receiving no interventions), a passive comparator (usual-care or wait-list control groups), or an active comparator (other interventions) were included.
Outcomes
The primary outcomes of this review included changes in global functioning and personal recovery for people with SMIs. Secondary outcomes included specific areas of functioning (such as social functioning), specific areas of personal recovery (such as hope and perceived social support), substance abuse, quality of life, and knowledge of the illness.
Selection of articles
All retrieved studies from the database and end-reference list search of included studies were uploaded into EndNote X9, where duplicates were electronically removed. The titles and abstracts of the remaining studies were screened independently by two reviewers (Authors 1 and 3); those not meeting the inclusion and exclusion criteria were removed at this stage. Studies deemed potentially suitable by at least one author were then downloaded and reviewed independently by Authors 1 and 3. Any disagreements between them were resolved by means of consensus through discussions. Protocols, abstracts, and publications not in English were excluded.
Data extraction
Study-related data (authors, locations, years, research designs, and sample sizes) and outcome measures were extracted by Authors 1 and 3. For studies with missing data on numerical outcomes, their authors were contacted for clarification. A pilot review was conducted by the two reviewers based on a data-extraction form adapted from the Cochrane Handbook for Systematic Reviews of Interventions to ensure consensus during data extraction (24). Any disagreements were resolved through discussions.
Risk of bias
The risk of bias (ROB) of the included studies was evaluated independently by Authors 1 and 3 based on the Cochrane ROB assessment tool (24). Relevant aspects included allocation concealment, blinding of outcome assessments, blinding of personnel, incomplete outcome data, random sequence generation, and selective reporting. For each study, the domains of bias were individually rated “low risk,” “high risk,” or “unclear risk,” and any disagreements were resolved through discussions. A ROB summary graph was then generated by the Review Manager 5.4 software (25).
Data analysis
For comparative analysis, post-program measurements were extracted from each of the studies. For each continuous outcome, the mean difference (MD) and its 95% confidence intervals (CIs) were computed as measures of treatment effects. Statistical heterogeneity between the studies was examined through the Chi-square test and I2 statistics: a statistically significant Chi-square P value (P < 0.10), accompanied by an I2 statistic of at least 50%, was interpreted as evidence of significant heterogeneity. A fixed-effect model was adopted for homogeneous studies; otherwise, a restricted maximum likelihood random-effects model was used (26). Aggregation of effect sizes was chosen over other methods for studies with the problem of effect-size multiplicity, given the limited number of studies examined in each meta-analysis in this review (27). The effect sizes in all meta-analyses were measured through Hedges' g statistic (28). All data were analyzed through the RStudio software (29).
Results
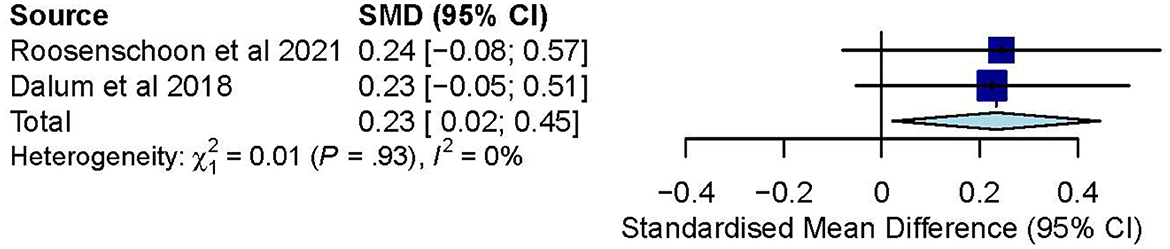
Publications were retrieved not only from databases but also from citations- and hand-searching. Of the 1,093 publications retrieved from the databases, 925 duplicates were removed. Upon title- and abstract screening, another 142 were removed. Full-text evaluation of the remaining 26 publications led to removing 13 (with reasons), leaving behind only 13. Additionally, of the 476 publications retrieved from citation- and hand-searching, four remained after duplicate removal and title- and abstract screening. Upon full-text evaluation, another three publications were removed (with reasons), leaving behind only one. These thus led to the final inclusion of 14 studies in this review (Figure 1).
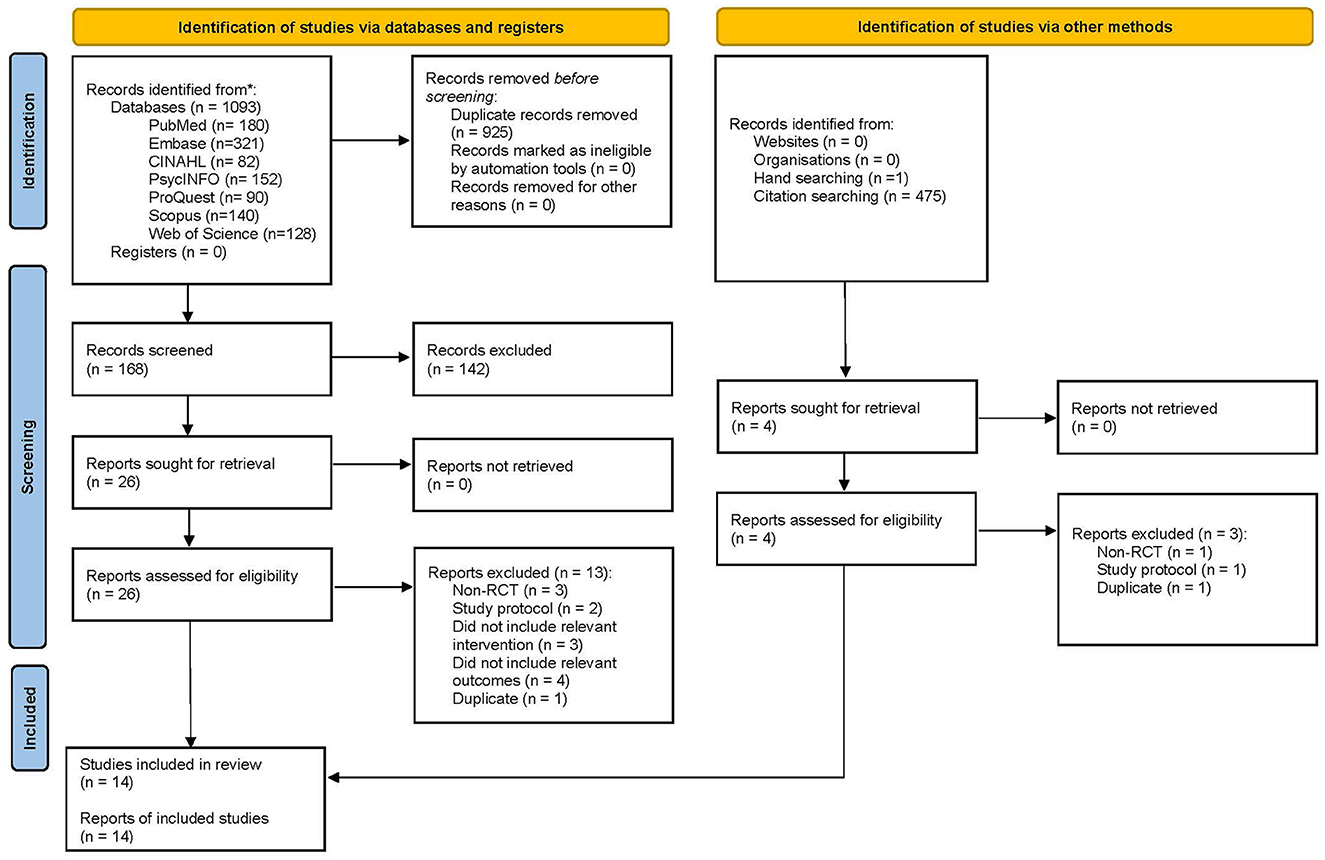
Figure 1. PRISMA 2020 flow diagram (23).
Characteristics of the included studies
The included studies were published between 2007 and 2021 across various countries: Turkey (30); the Netherlands (31); Singapore (32); Denmark (33–35); the United States of America (10, 36–39); Sweden (40); Taiwan (41); and Israel (42). Their sample sizes ranged from 34 (39) to 324 (38), with a median of 152 (Table 1).
Of the 14 studies, six compared the IMR intervention group with a treatment-as-usual (TAU) group (30, 32, 39–42). Another four studies compared a group receiving IMR and TAU with another group receiving only TAU (31, 33–35). One study compared a group receiving IMR and TAU with a wait-list (WL) group (37). Lastly, three studies compared a group receiving IMR with another group receiving only other interventions (10, 36, 38).
Risk of bias in the studies
Most of the 14 included studies (Figure 2) were assessed to have a low overall ROB. Over half of them registered an unclear risk originating chiefly from allocation concealment, on which insufficient information had been provided in the studies. Several studies also registered a high risk of bias from outcome blinding due to the use of participant-reported outcomes.
Primary outcomes personal recovery
A meta-analysis was conducted on 11 studies (10, 30–33, 35–40) for post-test complete-cases (CC) analysis and eight studies (10, 30, 31, 35–38, 40) for follow-up CC analysis. One study (42) could not be included in the post-test CC meta-analysis due to missing data. Intention-to-treat (ITT) analyses were also conducted for two studies (31, 33) for post-test data and another two studies (31, 35) for follow-ups.
The post-test CC meta-analysis included 1,016 participants and registered a combined standardized mean difference (SMD) of 0.89 (95% CI −0.03 to 1.81), with a heterogeneity of 91% (P < 0.10) (Figure 3A). A leave-one-out sensitivity analysis that excluded Tan, Ishak (32) yielded a statistically significant combined SMD of 0.39 (95% CI 0.12 to 0.65), with the heterogeneity diminishing to 70% (P < 0.10) (Figure 3B). Additionally, the follow-up CC meta-analysis included 743 participants and registered a combined SMD of 0.39 (95% CI 0.03 to 0.76), with a heterogeneity of 78% (P < 0.10) (Figure 3C). Similarly, a leave-one-out sensitivity analysis that excluded Polat and Kutlu (30) yielded a statistically significant combined SMD of 0.23 (95% CI 0.04 to 0.42), with heterogeneity diminishing to 43% (P > 0.10) (Figure 4A). Further subgroup analysis revealed no significant differences between studies with different comparators (P > 0.05) for both post-test and follow-up CC meta-analyses.
Figure 3. (A) Effectiveness of IMR program on personal recovery (post-test CC). (B) Effectiveness of IMR program on personal recovery (post-test CC)—after removal. (C) Effectiveness of IMR program on personal recovery (follow-up CC).
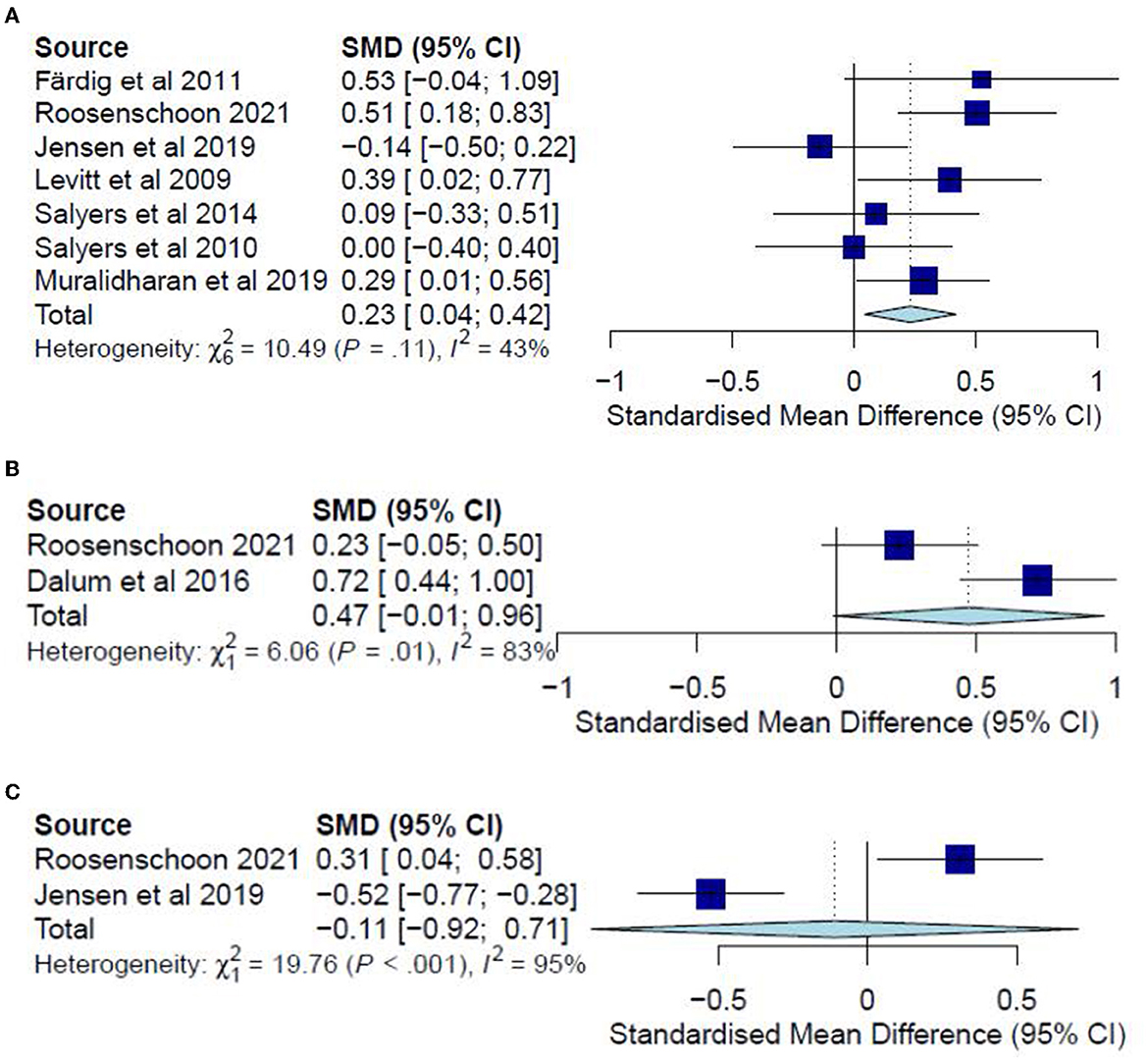
Figure 4. (A) Effectiveness of IMR program on personal recovery (follow-up CC)—after removal. (B) Effectiveness of IMR program on personal recovery (post-test ITT). (C) Effectiveness of IMR program on personal recovery (follow-up ITT).
The post-test ITT analysis included 358 participants and registered a non-significant combined SMD of 0.47 (95% CI−0.01 to 0.96), with a heterogeneity of 83% (P < 0.10) (Figure 4B). The follow-up ITT analysis included 360 participants and registered a combined SMD of−0.11 (95% CI−0.92 to 0.71), with a heterogeneity of 95% (P < 0.10) (Figure 4C). Accordingly, such results suggest a modest improvement in personal-recovery scores among participants who completed the program in the IMR group during the post-program periods and follow-ups compared to those in the non-IMR groups. However, the caveat is that these results should be interpreted judiciously since no significant differences were found in the more conservative ITT analyses.
Global functioning
A meta-analysis was conducted on three studies (32, 34, 35) for post-test CC analysis and two studies (32, 35) for follow-up CC analysis. However, no meta-analyses were conducted for post-test and follow-up ITT analyses since there was only one study for each.
The post-test CC meta-analysis included 324 participants and registered a combined SMD of 1.07 (95% CI −0.78 to 2.92), with a heterogeneity of 95% (P < 0.001) (Figure 5A). A leave-one-out sensitivity analysis that excluded Tan, Ishak (32) yielded a non-significant combined SMD of 0.14 (95% CI −0.10 to 0.38) and heterogeneity of 0%. However, no follow-up CC meta-analysis could be conducted since the results could not be pooled due to a high heterogeneity (I2 = 98%, P < 0.001) arising possibly from differences in sample sizes or in measures for global functioning. In this regard, Tan, Ishak (32) included 50 participants and adopted the Global Assessment Scale, reporting significant improvements (P < 0.001) for the IMR group (M = 81.12) as compared with the TAU group (M = 52.2). Conversely, Jensen, Dalum (35) included 128 participants and adopted the Global Assessment of Functioning, reporting no significant differences (P = 0.63) between the treatment group (M = 50.4) and the TAU group (M = 50.2).
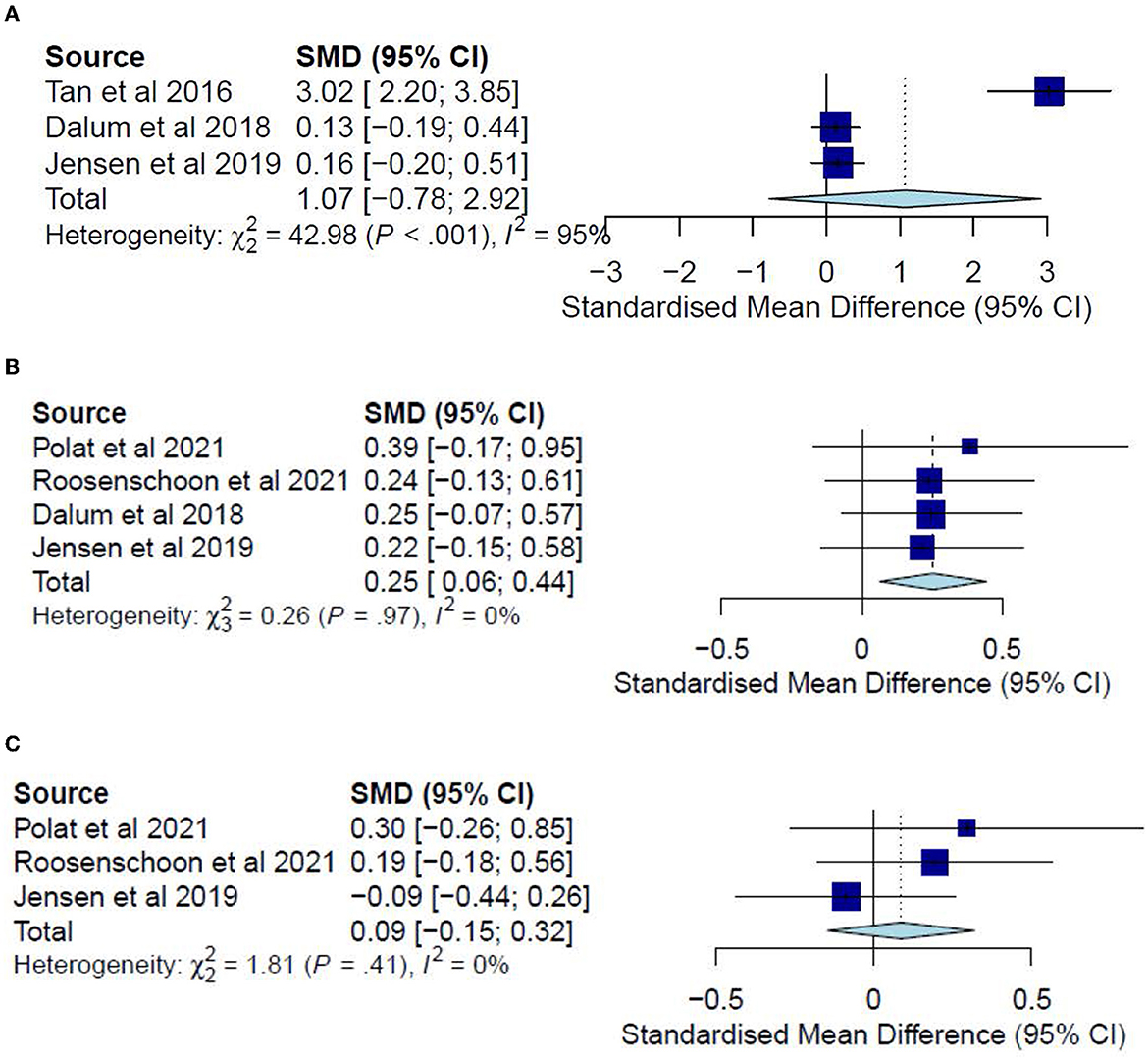
Figure 5. (A) Effectiveness of IMR program on global functioning (post-test CC). (B) Effectiveness of IMR program on social functioning (post-test CC). (C) Effectiveness of IMR program on global functioning (follow-up CC).
Only one study (34) measured global functioning in a post-test ITT analysis and reported non-significant differences (P = 0.21) between the treatment group (M = 46.4) and the TAU group (M = 44.0). Likewise, only one study (35) measured it in a follow-up ITT analysis and reported non-significant differences (P = 0.67) between the treatment group (M = 50.6) and the TAU group (M = 49.8). Accordingly, such results suggest only limited effectiveness of the IMR program in improving global-functioning outcomes compared with usual care.
Secondary outcomes
Social functioning
A meta-analysis was conducted on four studies (30, 31, 34, 35) for post-test CC analysis and three studies (30, 31, 35) for follow-up CC analysis. In addition, ITT analyses were also conducted for two post-test studies (31, 34) and follow-ups (31, 35).
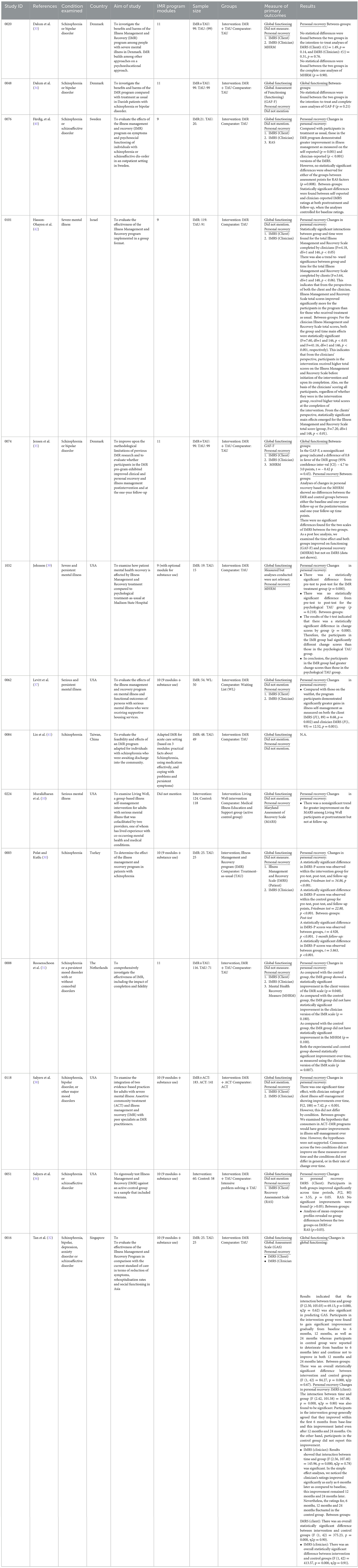
The post-test CC meta-analysis included 429 participants and registered a statistically significant combined SMD of 0.25 (95% CI 0.06 to 0.44), with a heterogeneity of 0% (P > 0.10) (Figure 5B). No significant subgroup differences were found (P = 0.62). This effect was not observed for the follow-up CC meta-analysis, which included 289 participants and registered a non-significant combined SMD of 0.09 (95% CI −0.15 to 0.32), with a heterogeneity of 0% (P > 0.10) (Figure 5C).
The post-test ITT meta-analysis included 355 participants and registered a statistically significant combined SMD of 0.23 (95% CI 0.02 to 0.45), with a heterogeneity of 0% (P > 0.10) (Figure 6). However, no follow-up ITT meta-analysis could not be conducted due to a high heterogeneity (I2 = 94%, P < 0.10) possibly arising from differences in social functioning measurements. In this regard, Roosenschoon, van Weeghel (31) used the Social Functioning Scale and reported a significant MD of 1.90 (95% CI −0.85 to 4.65). In contrast, Jensen, Dalum (35) used the Personal and Social Performance Scale and reported non-significant differences (P = 0.63) between the IMR group (M = 52.1) and the TAU group (M = 53.1). Thus, such results collectively suggest that the IMR program might have been modestly better at improving social functioning than usual care, though these positive effects might not be sustained.
Hope
A meta-analysis was conducted on four studies (33, 35, 36, 38) for post-test CC analysis and three studies (35, 36, 38) for follow-up CC analysis. No meta-analyses were conducted for ITT analyses since no studies examined hope in post-test ITT analysis, and only one study did so in the follow-up analysis. The post-test CC meta-analysis included 403 participants and registered a non-significant combined SMD of 0.05 (95% CI −0.15 to 0.25), with a heterogeneity of 0% (P > 0.10) (Figure 7A). The follow-up CC meta-analysis included 213 participants and registered a non-significant combined SMD of 0.03 (95% CI −0.25 to 0.30), with a heterogeneity of 0% (P > 0.10) (Figure 7B). In addition, Jensen, Dalum (35) examined hope in a follow-up ITT analysis, reporting non-significant differences (P = 0.62) between the treatment group (M = 34.1) and the TAU group (M = 34.9). Accordingly, such results suggest that the IMR program has not significantly differed from usual care or other interventions in increasing hope among the participants.
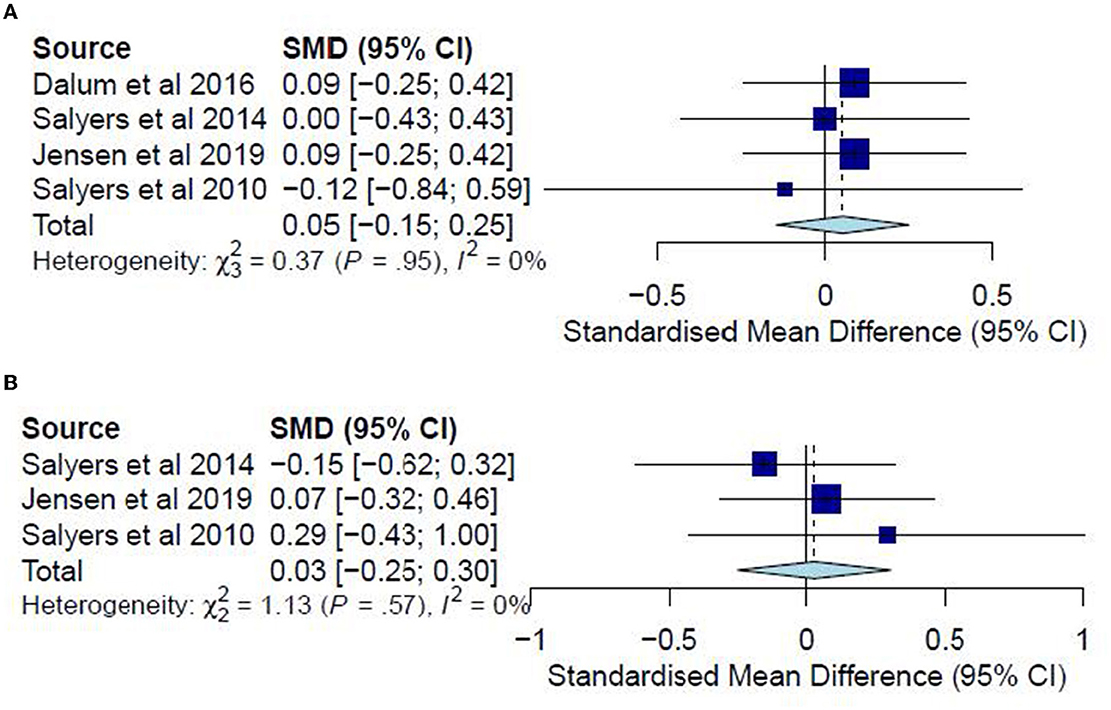
Figure 7. (A) Effectiveness of IMR program on hope (post-test CC). (B) Effectiveness of IMR program on hope (follow-up CC).
Perceived social support
Of the two studies examining perceived social support in a post-test CC analysis, one could not be included in the post-test CC meta-analysis due to missing data (42). Hence, no meta-analysis could be conducted for this outcome. Roosenschoon, van Weeghel (31) reported a significant MD for the post-test CC analysis (0.56, 95% CI 0.08 to 1.05) but found non-significant MDs for the follow-up CC (0.43, 95% CI −0.05 to 0.91), post-test ITT (0.41, 95% CI −0.05 to 0.88), and follow-up ITT analyses (0.28, 95% CI −0.16 to 0.72).
Quality of life
A meta-analysis was conducted on three studies (36, 37, 40) for post-test and follow-up CC analyses. No ITT meta-analyses were conducted since none of the included studies examined the quality of life in an ITT analysis. The post-test CC meta-analysis included 224 participants and registered a non-significant combined SMD of 0.15 (95% CI −0.11 to 0.41), with a heterogeneity of 0% (P > 0.10) (Figure 8A). Likewise, the follow-up CC meta-analysis included 196 participants and registered a non-significant combined SMD of 0.26 (95% CI −0.02 to 0.54), with a heterogeneity of 0% (P > 0.10) (Figure 8B). Accordingly, such results indicate that the IMR program has been similar to usual care, WL, and other interventions in improving the quality of life among the participants.
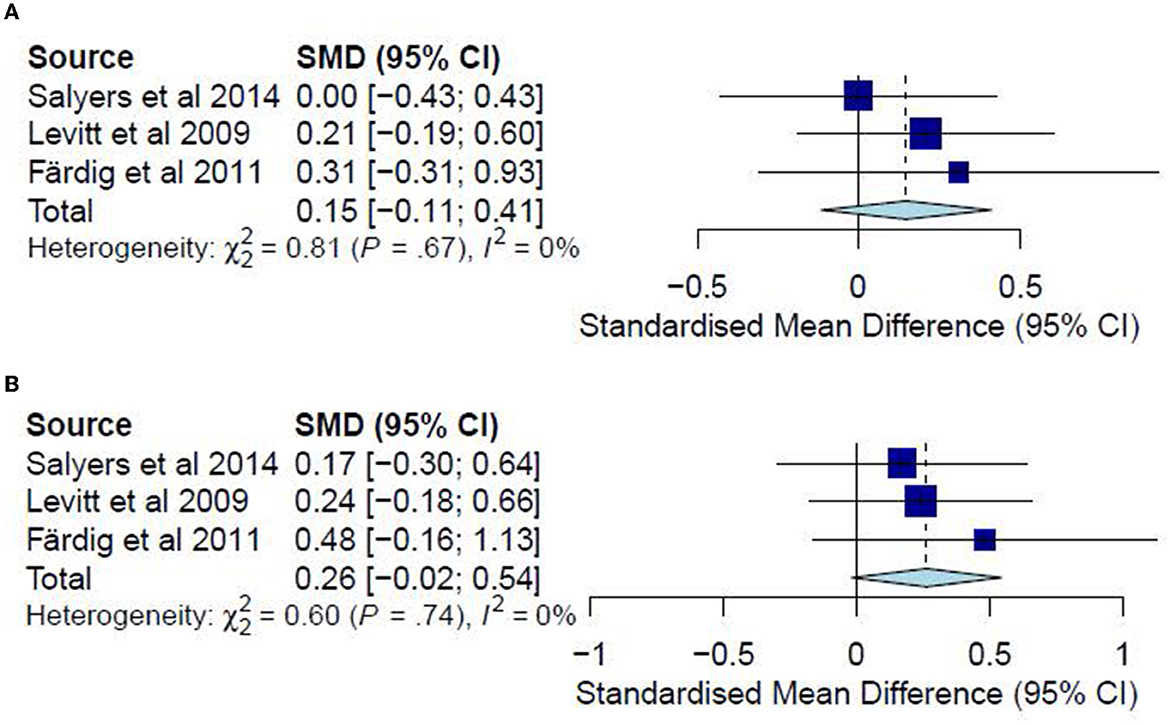
Figure 8. (A) Effectiveness of IMR program on quality of life (post-test CC). (B) Effectiveness of IMR program on quality of life (follow-up CC).
Substance abuse
A meta-analysis was conducted on three studies (31, 34, 37) for post-test CC analysis and two studies (31, 37) for follow-up CC analysis. No ITT meta-analyses were conducted since only one study examined substance abuse in an ITT analysis. The post-test CC analysis included 352 participants and registered a non-significant combined SMD of −0.11 (95% CI −0.31 to 0.09), with a heterogeneity of 0% (P > 0.10) (Figure 9A). Similarly, the follow-up CC analysis included 202 participants and registered a non-significant combined SMD of −0.07 (95% CI −0.33 to 0.19), with a heterogeneity of 0% (P > 0.10) (Figure 9B). Furthermore, in examining substance abuse, Roosenschoon, van Weeghel (31) reported non-significant MDs between the IMR and TAU groups for both post-test (−0.09, 95% CI −0.34 to 0.17) and follow-up analyses (−0.03, 95% CI −0.38 to 0.31). Thus, such results collectively indicate that the IMR program has not significantly reduced substance abuse among the participants compared to usual care or WL conditions.
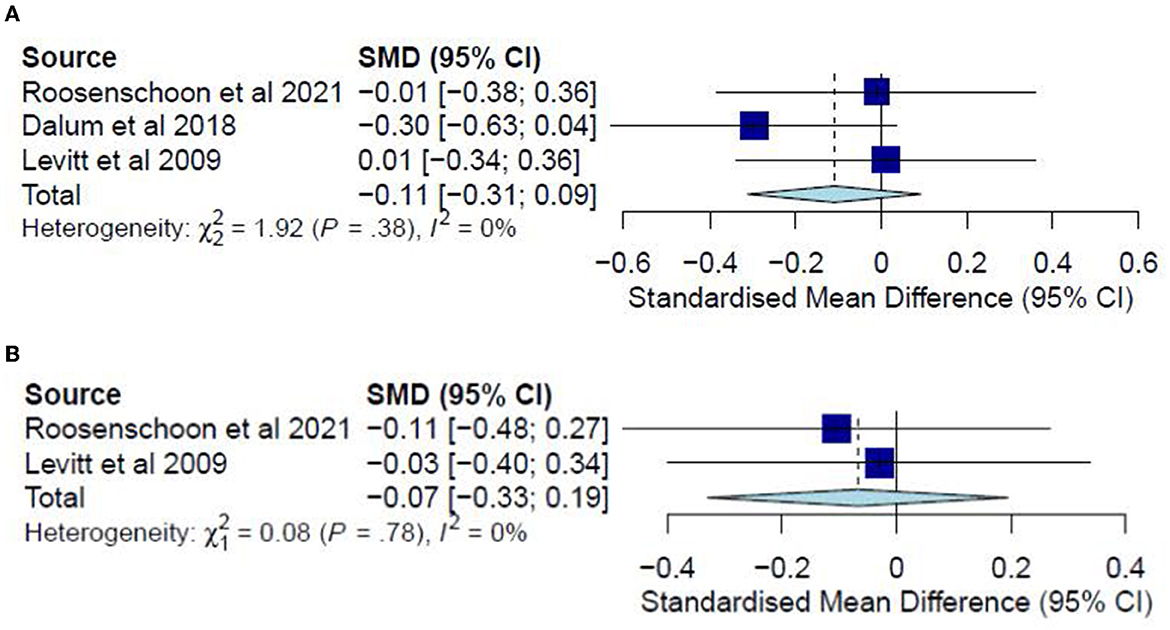
Figure 9. (A) Effectiveness of IMR program on substance abuse (post-test CC). (B) Effectiveness of IMR program on substance abuse (follow-up CC).
Knowledge of mental illness
Only one study examined knowledge of mental illness (41) and reported statistically significant improvements (P = 0.003) for both the post-test CC analysis for the IMR group (M = 51.6) compared with the TAU group (M = 49.2). Similar results were reported in the follow-up CC analysis for the IMR group (M = 54.5) and TAU group (M = 47.1).
Discussion
This review has examined the effectiveness of IMR programs in improving health-related outcomes among people with SMIs. Fourteen studies comparing IMR programs with passive and active comparators were included in the systematic review. However, one study (42) could not be included in any meta-analyses because of missing data. For the outcome of personal recovery among people with SMIs, the small to medium effect sizes observed in the post-program periods and at follow-ups suggest a modest advantage of IMR over TAU, WL, and other interventions. This is consistent with findings from a literature review (18).
Additionally, the absence of substantial subgroup differences in this review implied that IMR could improve personal recovery compared to active and passive intervention groups. The caveat, however, is that this finding should be judiciously interpreted since the more conservative ITT analyses have found no significant differences. For the other seven outcomes (global functioning, social functioning, hope, perceived social support, quality of life, substance abuse, and knowledge of the mental illness), IMR appeared to confer an advantage over TAU, WL, and other interventions in improving only social functioning. No benefits were discerned for the other six outcomes.
Participants' exposure to IMR (reflected by their program attendance or completion) represented a recurring determinant across the studies, of which only four achieved close to full completion rates (30, 32, 39, 40). Notably, in these four studies, the participants in the IMR group registered substantial improvements in personal-recovery scores. This finding thus suggests the presence of a threshold of exposure to IMR, beyond which its treatment effects are observable (31, 36). Moreover, further supporting evidence came from another finding in this review: improvements in personal recovery were found among those participants who had completed the IMR program in post-test and follow-up analyses but not in the ITT analyses. Furthermore, given the substantial non-adherence in the included studies, the conservative ITT analysis may have undervalued the treatment effects of IMR for those participants who were sufficiently exposed to it (43). Likewise, the absence of significant improvements in the secondary outcomes examined in this review may be due to the participants' partial exposure to the program. Nonetheless, the possibility remains that the effectiveness of IMR is not superior to other interventions or TAU. Hence, practical considerations such as the costs of implementing the program and its training should be weighed against those of other interventions.
Despite that, numerous insights for future IMR implementation emerge from this review. Firstly, while high attendance rates among people with SMIs for IMR programs may not be feasible in all intervention centers, research may be conducted to improve retention rates. This may include post-intervention interviews for participants to explore reasons for dropout and feedback on the program experience. For instance, in their qualitative interviews with subjects with low participation rates, Levitt, Mueser (37) found that those with better pre-existing knowledge of mental illness tended to drop out because of their perceived lack of benefits from the curriculum. Accordingly, pre-emptive steps should be taken to mitigate dropouts and enhance program outreach, such as stratifying participants based on their baseline knowledge (37) and tailoring the curriculum to their specific needs.
Secondly, given the severity of SMIs, some participants may require a lengthened time to complete the IMR curriculum (18), while others may tend to withdraw from the program (44). Thus, to augment the participants' exposure to the program, flexibility should be exercised, such as providing one-to-one remedial sessions for those who have missed classes or require extra assistance in specific modules in the curriculum. Additionally, such flexibility should extend to the class size of the IMR program. While individualized attention may lessen attrition among participants (44), it remains uncertain whether group sessions foster peer support and accountability that promote group cohesion and attendance (31). Most studies have adopted group sessions in this review, while only one has employed individualized home visits (32). Accordingly, future studies may compare the benefits of different IMR modalities (group-based, individualized, or mixed-model) on people with SMIs.
Lastly, the involvement of family, significant others (33, 35), and other treatment providers (36) appeared to be lacking in the IMR programs. However, evidence has demonstrated the importance of social networks in supporting the recovery of people with SMIs (45), as aided predominantly by their family members and care professionals (46). Therefore, it is unsurprising that many people with SMIs wish for greater family involvement in their care (47) and that such social support networks may mitigate the dropout rates from treatment (44). Against this background, it has been recommended that the IMR curriculum be better communicated to family members of people with SMIs and that the program be integrated with other services they use. For instance, family members can be invited to attend IMR sessions together. In addition, meetings may be held with the person with SMI, their family member, and other treatment providers to discuss and align recovery goals (48). Such recommendations may reinforce the skills and knowledge learned from IMR and enhance program attendance (36). In addition, the involvement of family and significant others would provide an important social network supporting the recovery of people with SMIs. However, the involvement of family members for people with SMI in such community interventions is often complex and involves multiple barriers (49) and may warrant the need for further research.
Aside from the possible implementation issues discussed, the effectiveness of the IMR program may have been affected by the content delivered within the modules. Given that the latest edition of the program was published in 2011, a possibility exists that the content covered may no longer be relevant in the modern context or that additional content may be required. Hence, an updated review of the program content may be timely to address the current needs of people with SMIs.
Strengths and limitations
This review contributes to the literature on the recovery of people with SMIs by providing an updated examination of the effectiveness of the IMR program in improving various outcomes among them. However, some limitations are noteworthy, one of which is the possible omission of relevant studies, given the inclusion of only publications in English. Others include the substantial heterogeneity in some analyses, the lack of studies examining specific outcomes such as knowledge of mental illnesses, and the limited number of studies comparing IMR with other active interventions. Overall, such shortcomings may limit the conclusions that could be drawn.
Conclusions
The IMR program incorporates motivation-based, educational, and cognitive-behavioral strategies encompassing multiple modules to enhance recovery among people with SMIs. However, the limited evidence presented in this review suggests that the program may not be significantly superior to existing treatment plans or other interventions. Nonetheless, the small to medium treatment effects observed in this paper suggest that the IMR program may benefit from further review and research, especially with regard to the implementation of the program.
Relevance to clinical practice
Although this review did not show a superiority of the illness management and recovery program over existing treatment programs, small to medium effect sizes were observed on all personal–recovery outcomes in the post-program periods and at follow-ups, suggesting it has an advantage over other interventions. As suggested by this review, the exposure to the IMR program showed a difference between treatment effects among the participants. Therefore, mental health nurses should note this and consider providing the IMR program on a platform that can have better exposure. With the advancement of technologies, the program can be provided online or within a mobile application, which can help ensure a more sustainable exposure threshold. Having the program over such a delivery modality would give autonomy and flexibility to the participants when self-managing their recovery journey. Finally, mental health nurses should consider involving family members and significant others in the IMR program when the client starts their participation. This would help reinforce the skills and knowledge the client learned from IMR and enhance their program attendance.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. National Institute of Mental Health. Towards a Model for a Comprehensive Community-Based Mental Health System. Washington, DC: National Institute of Mental Health (1987).
2. Ruggeri M, Leese M, Thornicroft G, Bisoffi G, Tansella M. Definition and prevalence of severe and persistent mental illness. Br J Psychiatry. (2000) 177:149–55. doi: 10.1192/bjp.177.2.149
3. Schinnar AP, Rothbard AB, Kanter R, Jung YS. An empirical literature review of definitions of severe and persistent mental illness. Am J Psychiatry. (1990). 147:1602–8. doi: 10.1176/ajp.147.12.1602
4. National Institute of Mental Health. Mental Illness. (2022). Available online at: https://www.nimh.nih.gov/health/statistics/mental-illness#part_2538 (accessed June 30, 2022).
5. Rivera-Segarra E, Varas-Díaz N, Santos-Figueroa A. “That's all fake”: health professionals stigma and physical healthcare of people living with serious mental illness. PLoS ONE. (2019) 14:e0226401. doi: 10.1371/journal.pone.0226401
6. Staiger T, Waldmann T, Oexle N, Wigand M, Rüsch N. Intersections of discrimination due to unemployment and mental health problems: the role of double stigma for job- and help-seeking behaviors. Soc Psychiatry Psychiatr Epidemiol. (2018) 53:1091–8. doi: 10.1007/s00127-018-1535-9
7. Björk Brämberg E, Torgerson J, Norman Kjellström A, Welin P, Rusner M, Sahlgrenska a, et al. Access to primary and specialized somatic health care for persons with severe mental illness: a qualitative study of perceived barriers and facilitators in swedish health care. BMC Family Pract. (2018) 19:12. doi: 10.1186/s12875-017-0687-0
8. Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS. Premature mortality among adults with Schizophrenia in the United States. JAMA Psychiatry. (2015) 72:1172–81. doi: 10.1001/jamapsychiatry.2015.1737
9. Goh Y-S, Ow Yong QY, Soo SC, Wan PCJ, Ng VCK. Experiences and challenges faced by community mental health workers when providing care to people with mental health conditions: a qualitative descriptive study. Int J Ment Health Nurs. (2022) 33:591–600. doi: 10.1111/inm.12977
10. Muralidharan A, Brown CH, Peer JE, Klingaman EA, Hack SM, Li L, et al. Living well: an intervention to improve medical illness self-management among individuals with serious mental illness. Psychiatr Serv. (2019) 70:19–25. doi: 10.1176/appi.ps.201800162
11. Ross LE, Vigod S, Wishart J, Waese M, Spence JD, Oliver J, et al. Barriers and facilitators to primary care for people with mental health and/or substance use issues: a qualitative study. BMC Family Pract. (2015) 16:135. doi: 10.1186/s12875-015-0353-3
12. Mueser KT, Torrey WC, Lynde D, Singer P, Drake RE. Implementing evidence-based practices for people with severe mental illness. Behav Modif. (2003) 27:387–411. doi: 10.1177/0145445503027003007
13. Drake RE, Goldman HH, Leff HS, Lehman AF, Dixon L, Mueser KT, et al. Implementing evidence-based practices in routine mental health service settings. Psychiatr Serv. (2001) 52:179–82. doi: 10.1176/appi.ps.52.2.179
14. Anthony WA. Recovery from mental illness: the guiding vision of the mental health service system in the (1990s). Psychosocial Rehabilitat J. (1993) 16:11. doi: 10.1037/h0095655
15. Mueser KT, Meyer PS, Penn DL, Clancy R, Clancy DM, Salyers MP. The illness management and recovery program: rationale, development, and preliminary findings. (2006). Oxford: Oxford University Press. doi: 10.1093/schbul/sbl022
16. Mueser KT, Gingerich S. Illness Management and Recovery. SAMHSA's Gains Center for Behavioral Health and Justice Transformation. Rockville, MD: Center for Mental Health Services, Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services (2013).
17. Substance Abuse and Mental Health Services Administration. Illness Management and Recovery: Practitioner Guides and Handouts. Rockville, MD: Center for Mental Health Services, Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services. (2009).
18. McGuire AB, Kukla M, Green A, Gilbride D, Mueser KT, Salyers MP. Illness management and recovery: a review of the literature. Psychiatr Serv. (2014) 65:171–9. doi: 10.1176/appi.ps.201200274
19. Whiteman KL, Naslund JA, DiNapoli EA, Bruce ML, Bartels SJ. Systematic review of integrated general medical and psychiatric self-management interventions for adults with serious mental illness. Psychiatr Serv. (2016) 67:1213–25. doi: 10.1176/appi.ps.201500521
20. Korsbek L, Dalum HS, Lindschou J, Eplov LF. Illness management and recovery programme for people with severe mental illness. In: Cochrane Database of Systematic Reviews. (2014). doi: 10.1002/14651858.CD011071. [Epub ahead of print].
21. Mueser KT, Corrigan PW, Hilton DW, Tanzman B, Schaub A, Gingerich S, et al. Illness management and recovery: a review of the research. Psychiatr Serv. (2002) 53:1272–84. doi: 10.1176/appi.ps.53.10.1272
22. Tam WSW, Tang A, Woo B, Goh Y-S. Perception of the preferred reporting items for systematic reviews and meta-analyses (Prisma) statement of authors publishing reviews in nursing journals: a cross-sectional online survey. BMJ Open. (2019) 9:e026271. doi: 10.1136/bmjopen-2018-026271
23. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The Prisma (2020) statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
24. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (Updated September 2020): The Cochrane Collaboration. (2020). Available online at: www.training.cochrane.org/handbook (accessed June 30, 2022).
25. RevMan. Review Manager (Revman). 5.4.1 ed. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration (2020).
26. Viechtbauer W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J Educ Behav Stat. (2005) 30:261–93. doi: 10.3102/10769986030003261
27. Tanner-Smith EE, Tipton E. Robust variance estimation with dependent effect sizes: practical considerations including a software tutorial in Stata and Spss. Res Synth Methods. (2014) 5:13–30. doi: 10.1002/jrsm.1091
28. Hedges LV. Distribution theory for glass's estimator of effect size and related estimators. J Educ Behav Stat. (1981) 6:107–28. doi: 10.3102/10769986006002107
30. Polat S, Kutlu Y. The effectiveness of illness management and recovery program in patients with Schizophrenia. Arch Psychiatr Nurs. (2021) 35:162–7. doi: 10.1016/j.apnu.2021.01.004
31. Roosenschoon BJ, van Weeghel J, Deen ML, van Esveld EW, Kamperman AM, Mulder CL. Effects of illness management and recovery: a multicenter randomized controlled trial. Front Psychiatry. (2021) 12:723435. doi: 10.3389/fpsyt.2021.723435
32. Tan CHS, Ishak RB, Lim TXG, Marimuthusamy P, Kaurss K, Leong JJ. Illness management and recovery program for mental health problems: reducing symptoms and increasing social functioning. J Clin Nurs. (2016) 26:3471–85. doi: 10.1111/jocn.13712
33. Dalum HS, Waldemar AK, Korsbek L, Hjorthøj C, Mikkelsen JH, Thomsen K, et al. Participants' and staffs' evaluation of the illness management and recovery program: a randomized clinical trial. J Ment Health. (2016) 27:30–7. doi: 10.1080/09638237.2016.1244716
34. Dalum HS, Waldemar AK, Korsbek L, Hjorthøj C, John Hagel M, Thomsen K, et al. Illness management and recovery: clinical outcomes of a randomized clinical trial in community mental health centers. PLoS ONE. (2018) 13:e0194027. doi: 10.1371/journal.pone.0194027
35. Jensen SB, Dalum HS, Korsbek L, Hjorthøj C, Mikkelsen JH, Thomsen K, et al. Illness management and recovery: one-year follow-up of a randomized controlled trial in danish community mental health centers: long-term effects on clinical and personal recovery. BMC Psychiatry. (2019) 19:65. doi: 10.1186/s12888-019-2048-0
36. Salyers MP, McGuire AB, Kukla M, Fukui S, Lysaker PH, Mueser KT, et al. Randomized controlled trial of illness management and recovery with an active control group. Psychiatr Serv. (2014) 65:1005–11. doi: 10.1176/appi.ps.201300354
37. Levitt AJ, Mueser KT, Degenova J, Lorenzo J, Bradford-Watt D, Barbosa A, et al. Randomized controlled trial of illness management and recovery in multiple-unit supportive housing. Psychiatr Serv. (2009) 60:1629–36. doi: 10.1176/ps.2009.60.12.1629
38. Salyers MP, McGuire AB, Rollins AL, Bond GR, Mueser KT, Macy VR. Integrating assertive community treatment and illness management and recovery for consumers with severe mental illness. Community Ment Health J. (2010) 46:319–29. doi: 10.1007/s10597-009-9284-6
39. Johnson BA. Comparison of Illness Management and Recovery Treatment and Psychological Treatment-as-Usual in a State Psychiatric Hospital. Dissertation Abstracts International: Section B: The Sciences and Engineering. (2007) p. 8400.
40. Färdig R, Lewander T, Melin L, Folke F, Fredriksson A. A randomized controlled trial of the illness management and recovery program for persons with Schizophrenia. Psychiatr Serv. (2011) 62:606–12. doi: 10.1176/ps.62.6.pss6206_0606
41. Lin EC, Chan CH, Shao WC, Lin MF, Shiau S, Mueser KT, et al. A randomized controlled trial of an adapted illness management and recovery program for people with schizophrenia awaiting discharge from a psychiatric hospital. Psychiatr Rehabil J. (2013) 36:243–9. doi: 10.1037/prj0000013
42. Hasson-Ohayon I, Roe D, Kravetz S. A randomized controlled trial of the effectiveness of the illness management and recovery program. Psychiatr Serv. (2007) 58:1461–6. doi: 10.1176/ps.2007.58.11.1461
43. McCoy CE. Understanding the intention-to-treat principle in randomized controlled trials. West J Emerg Med. (2017) 18:1075–8. doi: 10.5811/westjem.2017.8.35985
44. Harding B, Torres-Harding S, Bond GR, Salyers MP, Rollins AL, Hardin T. Factors associated with early attrition from psychosocial rehabilitation programs. Community Ment Health J. (2008) 44:283–8.doi: 10.1007/s10597-008-9128-9
45. Salehi A, Ehrlich C, Kendall E, Sav A. Bonding and bridging social capital in the recovery of severe mental illness: a synthesis of qualitative research. J Mental Health (Abingdon, England). (2019) 28:331–9. doi: 10.1080/09638237.2018.1466033
46. Nicaise P, Garin H, Smith P, d'Oreye de Lantremange S, Leleux L, Wyngaerden F, et al. Implementation of a computer-assisted face-to-face intervention for mapping the social support networks of patients with severe mental illness in routine clinical practice: analysis of the appropriateness and acceptability of the intervention. Int J Social Psychiat. (2021) 2021:207640211058977. doi: 10.1177/00207640211058977
47. Amy N, Cohen D, Amy L, Drapalski, Glynn SM, Medoff D, et al. Preferences for Family Involvement in Care among Consumers with Serious Mental Illness. Psychiatric Services. (2013) 64:257–63. doi: 10.1176/appi.ps.201200176
48. L. Perocier V. Severe Mental Illness and Family Involvement During Treatment. Los Angeles, CA: SAGE Publications (2023). p. 231–6. doi: 10.1177/10664807221123549
49. Hansson KM, Romøren M, Pedersen R, Weimand B, Hestmark L, Norheim I, et al. Barriers and facilitators when implementing family involvement for persons with psychotic disorders in community mental health centres – a nested qualitative study. BMC Health Serv Res. (2022) 22:1–1153. doi: 10.1186/s12913-022-08489-y
Keywords: Illness Management and Recovery, severe mental illness, personal-recovery, systematic review, meta-analysis
Citation: Goh YSS, Ow Yong JQY and Li AZ (2023) Effectiveness of Illness Management and Recovery program on people with severe mental illnesses: a systematic review and meta-analysis. Front. Psychiatry 14:1162288. doi: 10.3389/fpsyt.2023.1162288
Received: 09 February 2023; Accepted: 19 April 2023;
Published: 15 May 2023.
Edited by:
Emre Umucu, Michigan State University, United StatesReviewed by:
Julie Williams, King's College London, United KingdomPadmavati Ramachandran, Schizophrenia Research Foundation, India
Copyright © 2023 Goh, Ow Yong and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Shian Shawn Goh, bnVyZ3lzQG51cy5lZHUuc2c=
 Yong Shian Shawn Goh
Yong Shian Shawn Goh Jenna Qing Yun Ow Yong1
Jenna Qing Yun Ow Yong1