- 1Department of Nursing, Zhuhai Campus of Zunyi Medical University, Guangdong, China
- 2Department of Pediatrics, The Fifth Affiliated Hospital of Zunyi Medical University, Guangdong, China
- 3Department of Obstetrics, Zhuhai Maternity and Child Health Care Hospital, Guangdong, China
Background: Postpartum depression (PPD) is considered the most widespread puerperium complication. The associations of major depressive disorder with certain types of cerebrovascular diseases and cognitive function have been proposed, but the potential causal effects of PPD on these phenotypes are still unknown.
Methods: A Mendelian randomization (MR) research design with various methods (e.g., inverse-variance weighted method and MR pleiotropy residual sum and outlier test) was adopted to establish a causal relationship between PPD with cerebrovascular diseases and cognitive impairment.
Results: No causal relationship between PPD with carotid intima media thickness and cerebrovascular diseases (i.e., stroke, ischemic stroke, and cerebral aneurysm) was found. However, MR analyses indicated a causal association between PPD and decreased cognitive function (P = 3.55 × 10−3), which remained significant even after multiple comparison corrections using the Bonferroni method. Sensitivity analyses using weighted median and MR-Egger methods indicated a consistent direction of the association.
Conclusion: The causal association between PPD and cognitive impairment indicates that cognitive impairment is a critical aspect of PPD and thus cannot be regarded as an epiphenomenon. Addressing cognitive impairment and lessening the symptoms associated with PPD independently play significant roles in the treatment of PPD.
Introduction
Postpartum depression (PPD), also called postnatal depression, is a type of mental disorder related to childbirth that can affect both sexes (1). The Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) defines PPD as an episode of major depression, with its onset happening within the first month of delivery (2). However, for practical purposes, the duration of onset can be extended to around the first year after delivery (3). PPD is considered the most widespread puerperium complication (4). Furthermore, fathers tend to experience PPD at similar rates compared with maternal depression (5). Various risk factors increase susceptibility to PPD, such as life and infant care stress, insomnia, prenatal depression, and inadequate social support, but PPD remains relatively underdiagnosed despite its high prevalence rates (6). The effects of PPD on infants have been well studied. For instance, PPD tends to make children more likely to develop emotional issues, such as depressive disorders and anxiety disorders (7). Furthermore, infant studies have reported that the negative effects of PPD, such as cognitive deficits, may transfer into childhood (8, 9). However, the effects of PPD on parents themselves are less investigated.
Several studies have aimed to investigate the effect of baseline depression on the late onset of vascular diseases. Through a meta-analysis, it was revealed that the risk of stroke was significantly higher among patients with depression, which is independent of other comorbidities, such as diabetes and hypertension (10). The existence of a dose–response relationship between depression and stroke has also been reported (11). In addition to cerebrovascular diseases, the status of cognitive impairment worsened with accumulating depressive episodes (12, 13). Information from available psychosocial literature has been insufficient to designate PPD as a distinct syndrome or as a sub-category of a major depressive disorder (MDD) (14). However, the clinical expertise and psychosocial data support the idea that a distinction of symptomatic patterns exists between MDD and PPD (15). Thus, based on the evidence, we hypothesized that PPD led to cerebrovascular diseases and cognitive impairment, which was tested by Mendelian randomization (MR) analyses. The MR method circumvents the issues of residual confounding and reverse causality in traditional epidemiological studies (16) and can be used to infer the causal relationship between exposure (i.e., PPD) and outcomes (i.e., cerebrovascular diseases and cognitive impairment).
Methods
Study design
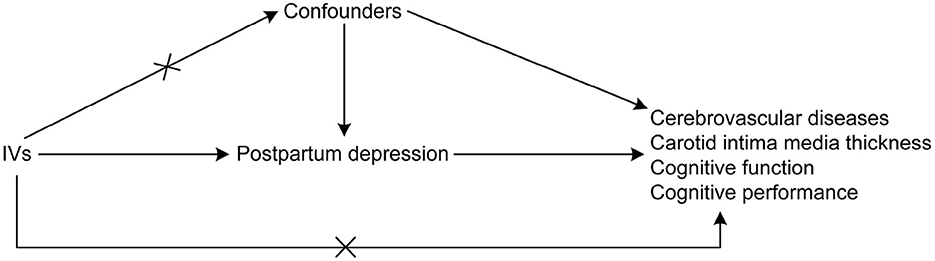
An MR research design was adopted to establish a causal relationship between PPD with cerebrovascular diseases and cognitive impairment. MR studies are based on the idea that alleles are randomly assigned during the meiotic phase of cell division, with conception as the basis of the natural experiment (17). In this design, the instrumental variables (IVs) used were genetic variations needed to derive the causal association of exposure with the outcome. Three assumptions were suggested for MR analyses (17) as follows: (1) there is a direct correlation between the single nucleotide polymorphisms (SNPs) utilized for PPD as IVs to the exposure; (2) the confounding variables cannot confound the IVs; and (3) the only causal pathway that links the IVs to the outcomes (i.e., cerebrovascular diseases and cognitive impairment) is through the PPD. The directed acyclic graph showing the assumptions of MR analyses is presented in Figure 1. In the MR analysis, the threshold of independent SNPs linked to the PPD as IVs was at a P-value of < 1 × 10−5. The F-statistics were also calculated to estimate the efficacy of the selected IVs.
Data sources
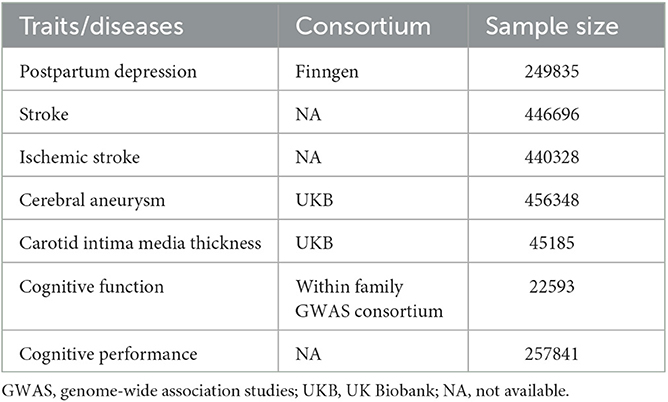
The GWAS summary statistics data of PPD were obtained from FinnGen, where the status of delivery and International Classification of Diseases, Tenth Revision (ICD-10) code F32, F33, and F53.0 were used to define PPD (18). As very few SNPs were left if a genome-wide significance level of P-value (P < 5 × 10−8) was used to prepare the IVs for PPD, we used a P threshold of 1 × 10−5. The independence of the included SNPs as IVs was ensured by using a clumping method with a linkage disequilibrium threshold of r2 > 0.001 in a 10,000 kb window. The F-statistics of all the IVs, representing their ability to predict PPD, were calculated and found to be higher than 10 (Supplementary Table 1). The GWAS summary statistics data of stroke and ischemic stroke were obtained from a genome-wide association meta-analysis, focusing on stroke and its subtypes (19). The GWAS summary data of cerebral aneurysm were obtained from the analyses of UK Biobank data using a generalized linear mixed model-based genome-wide association tool, in which cerebral aneurysm was defined by the PheCode 433.5 (20). Summary statistics of carotid intima media thickness (cIMT) were also generated using data from UK Biobank (21). Cognitive function was estimated via a general cognitive function score, with a higher score representing better cognitive function and vice versa. This score was used in the GWAS analysis to generate the summary data (22). The GWAS summary statistics of cognitive performance were created by combining the analyses with general cognitive ability by the Cognitive Genomics Consortium (COGENT) and the analyses of cognitive performance by UK Biobank (23). More detailed information is summarized in Table 1.
Statistical analysis
The summary statistics collected were aligned to the same single allele. We applied the inverse-variance weighted (IVW) MR method as the primary method to identify the potential associations between PDD and various phenotypes, such as cerebrovascular diseases (i.e., stroke, ischemic stroke, and cerebral aneurysm), cIMT (a measure for the diagnosis of carotid atherosclerotic vascular disease), cognitive function, and cognitive performance. The MR methods of MR-Egger, weighted median (WM), and MR pleiotropy residual sum and outlier (MR-PRESSO) were also applied for sensitivity analyses. The results of different MR analyses were shown on scatter plots and funnel plots, and the leave-one-out method was used to test the effect of individual SNPs by removing one SNP in turn. The MR-Egger regression was used to examine the genetic instruments for directional pleiotropy. Furthermore, the identification of heterogeneous results was accomplished through a Cochran-Q statistic test. A leave-one-out study with systematic elimination of one SNP at one time was also performed to examine the influence of the pleiotropic and/or marginal SNPs. Finally, the SNP outliers were identified using the MR-PRESSO program. The effects of PDD on binary phenotypes, such as cerebrovascular diseases, were presented as odds ratios (ORs) and 95% confidence intervals (CIs), while its effects on continuous variables, including cIMT, cognitive function, and cognitive performance, were presented as beta and 95% CIs. The Bonferroni correction was applied for multiple testing corrections. R software (version 4.2.1), together with its TwoSampleMR package, was applied in the current study (24).
Results
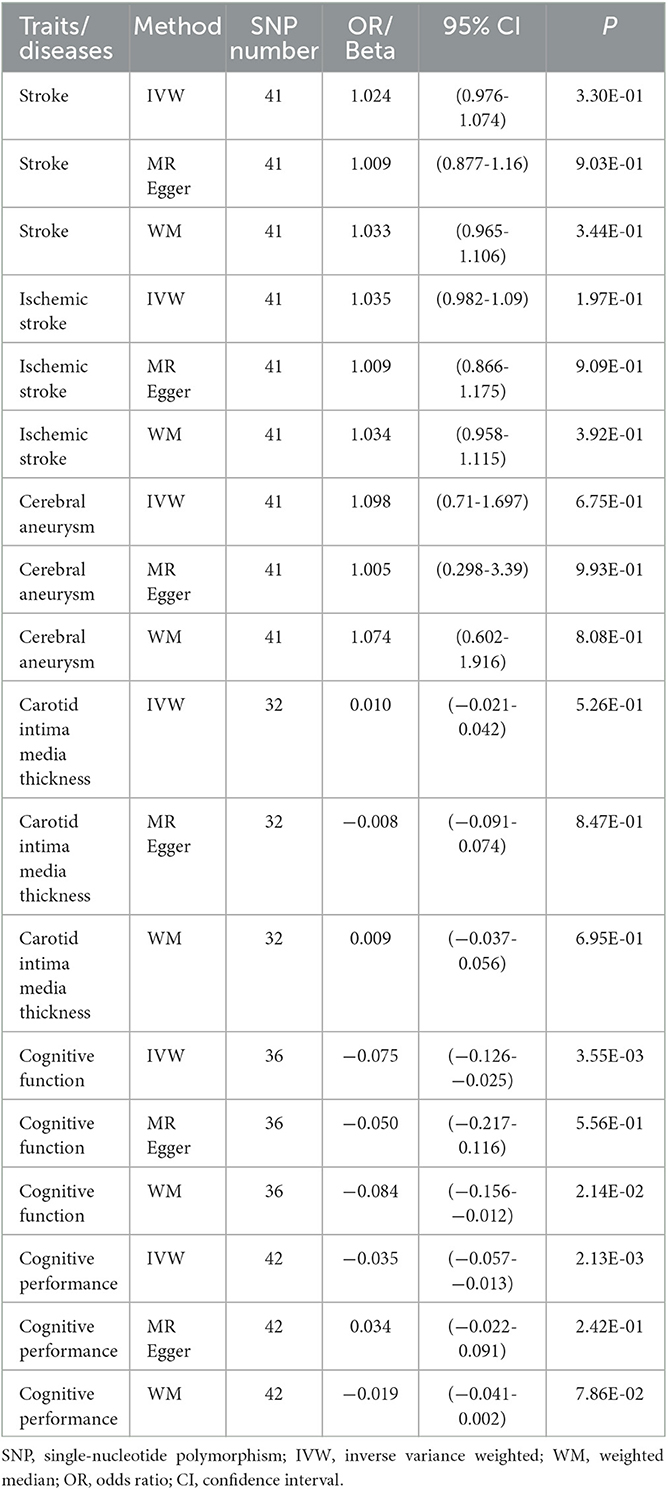
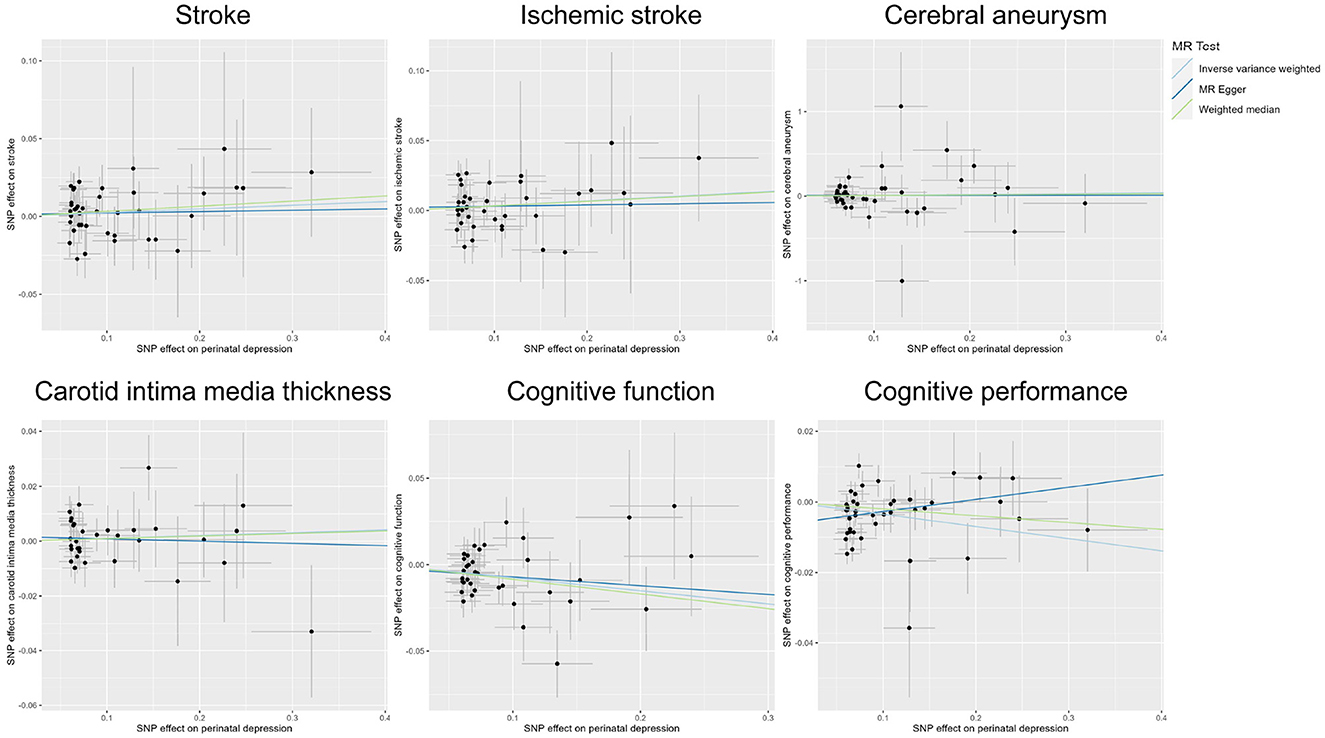
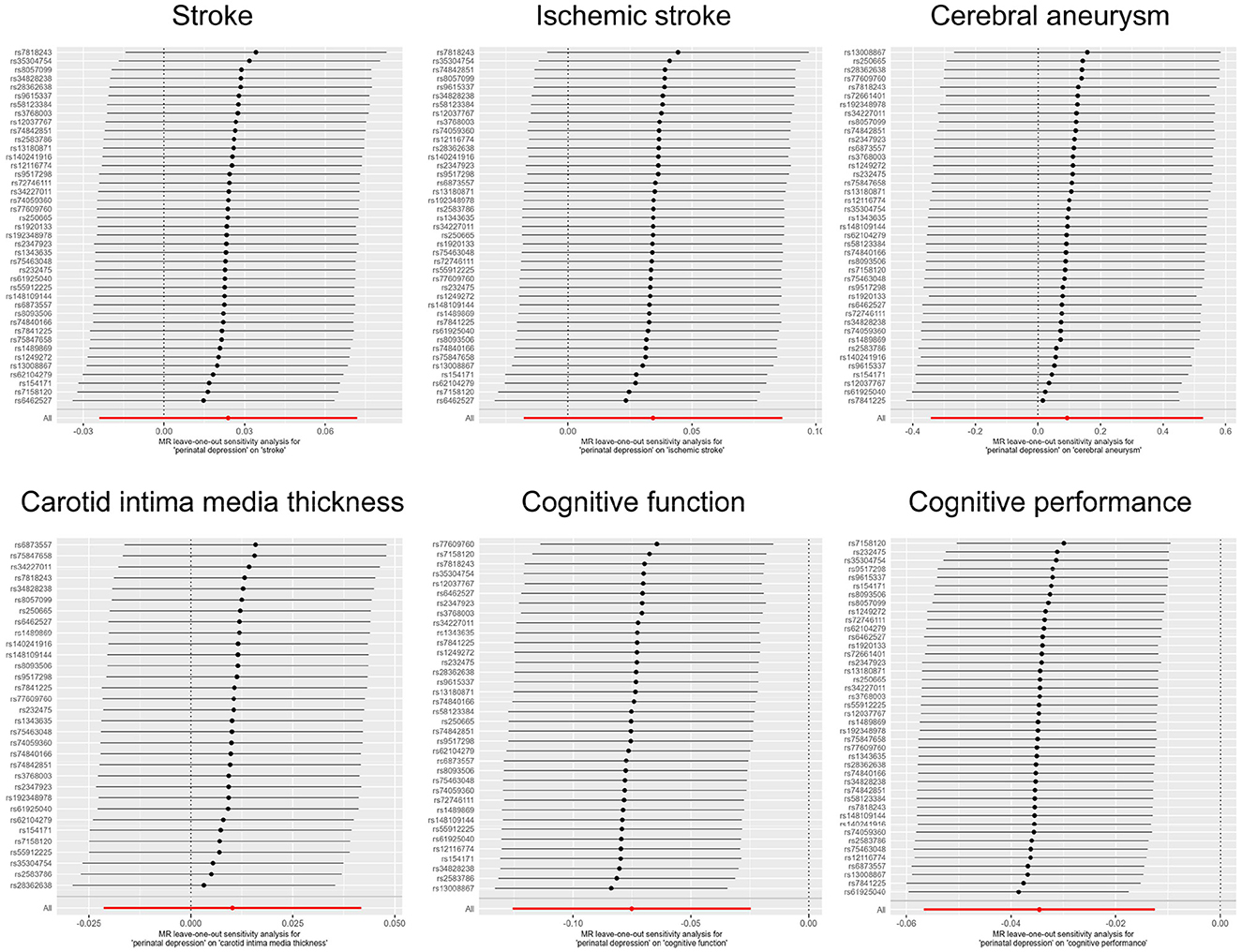
Publicly available GWAS summary statistics data were collected (Table 1), and MR analyses were performed to test the potential causal association between PPD and a variety of phenotypes, including cerebrovascular diseases (i.e., stroke, ischemic stroke, and cerebral aneurysm), cIMT, cognitive function, and cognitive performance. The results of the MR analyses indicated that PPD has no causal relationship with cIMT and the cerebrovascular diseases included in this study (Table 2, Figure 2, Supplementary Figure 1, and Supplementary Table 2). However, MR analyses using the IVW method indicated that PPD was causally associated with both cognitive function (P = 3.55 × 10−3) and cognitive performance (P = 2.13 × 10−3), and these associations were still significant after multiple comparison corrections using the Bonferroni method (P threshold of 0.05/6 = 8.33 × 10−3). Thus, the result revealed that PPD led to a decreased level of cognitive function and cognitive performance, with the beta value of −0.075 (95% CI, −0.126-−0.025) and −0.035 (95% CI, −0.057-−0.013), respectively (Table 2, Figure 2, Supplementary Figure 1, and Supplementary Table 2). The IVs used in this study were good to predict PPD, as reflected by their F-statistics, with a mean of 22.4 and a range of 19.7–40.5 (Supplementary Table 1). Sensitivity analyses using the WM methods indicated a consistent direction of the association, but the direction of association analyzed using the MR-Egger method was consistent only for cognitive function but not cognitive performance (Table 2). Sensitivity analysis using the leave-one-out method indicated that the observed associations of PDD with the six phenotypes were not driven by single SNPs (Figure 3). A heterogeneity test revealed no significant heterogeneity, except for the analysis of the association between PDD and cognitive performance (Supplementary Table 3). Direct pleiotropy was also observed in the causal association of PDD with cognitive performance but not with cognitive function, as examined by the intercept of MR-Egger (Supplementary Table 4). In contrast, MR-PRESSO indicated a consistent result after removing outlier SNPs in the analysis (Supplementary Table 5).
Discussion
This study tested the potential causal relationship of PPD with various cerebrovascular diseases and cognitive impairment using GWAS summary statistics and MR designs. The analyzed results indicated that PPD was negatively associated with cognitive function, which was also supported by the MR sensitivity analyses using the MR-Egger and WM methods. Furthermore, the causal association between PPD and cognitive function was not caused by direct pleiotropy as determined by the MR-Egger intercept test. Thus, cognitive impairment is an essential aspect of PPD, and our study highlights the significance of recognizing the impact of PPD on cognitive function and addressing it when treating the condition.
Major depressive disorder (MDD) has been linked to vascular illnesses, as revealed by epidemiological studies, and several mechanisms have been established to show how depression and vascular illnesses exacerbate each other (25). Notable increments in the risk of coronary artery disease in patients presenting with baseline depressive disorders have been established (26). A dose–response relationship between the severity of depression and late-onset vascular disease has also been reported (25). There are several related theories through which depression reasonably heightens one's susceptibility to developing vascular problems. For instance, depression contributes to the changes in platelet function as well as the mechanism of clotting, which links to the atherogenic process (27). Furthermore, patients with depression were observed to have elevated levels of cortisol, which are intrinsically linked to the severity of coronary atherosclerosis (28). The administration of steroids has been shown to augment the levels of triglycerides and cholesterol, thereby contributing to the development of cardiovascular diseases (29). Moreover, depression is also linked to unhealthy lifestyles which exacerbate vascular diseases (25). However, our MR analyses indicated no causal association between PDD and a variety of cerebrovascular diseases, including stroke, ischemic stroke, and cerebral aneurysm, as well as cIMT, the indicator for carotid atherosclerotic vascular disease. This observation may highlight the differences between MDDs and PPD. Owing to the significant psychological, physiological, and hormonal changes during postpartum, it is reasonable to presume that depression would be exhibited in a distinctive manner (15). PPD can be initiated by sudden changes in sex hormones (30), and studies also revealed that women experience unique disorders with more diverse symptoms during PPD compared with the mental disorders that occurred in non-puerperal instances (15). Nonetheless, this research area requires further exploration because the main reason why PPD is often underdiagnosed is the insufficient knowledge of primary care specialists regarding the postpartum period.
MDD has been linked to a dysfunction in cognition, as reported by several epidemiological studies. Compromised cognition occurs in more than half of the patients diagnosed with depression (31). The DSM-IV-TR has a diagnostic criteria of an impaired ability for decision-making, concentration, or thought processing for major depressive episodes (31). For patients with late-life depression (LLD), cognitive impairment is usually diagnosed, and LLD may promote the development of dementia (32). Recently, a link between depression and issues with planning, problem-solving, and cognitive functioning has been reported. For instance, while patients with MDD have expected automatic processing performance, they are largely limited when executing tasks that require extended attention (33). Treatment with anti-depressants helped to improve cognitive outcomes in patients with MDD, although overall performance was still lower than that of healthy individuals (34). During the postpartum period, the cognitive functioning of women might become dysfunctional, and the decline in cognitive performance is often linked to the total time spent in labor and the use of labor analgesia (35). However, the association between PPD and cognitive functioning is far from being understood. Our study inferred the causal relationship of PPD with cognitive function and revealed that PDD could cause a decreased level of cognitive function.
Alteration of cognitive functions in patients with PDD could be caused by various endocrine factors such as the dysregulation of the hypothalamus–pituitary–gonadal axis, and an increase and decrease in progesterone and estradiol during pregnancy and after delivery, respectively, could be observed (36). In addition, a direct correlation exists between PPD symptoms and the severity of insomnia, a link that may also contribute to the changes in cognitive abilities (37). Decreases in cognitive function induced by PPD may also share common mechanisms by which MDD is associated with cognitive dysfunction. According to the global-diffuse hypothesis, people with depression normally tend to have a lower cognitive profile, representing an indication of a global-diffuse impairment in several cognitive domains (38). Since brain-derived neurotrophic factor plays a mediatory role in the dorsolateral prefrontal cortex plasticity and hippocampal forms such as the long-term potentiation, a reduction in its expression, as witnessed in chronic stress, could be attributed to depression-induced cognitive deficits (39). The involvement of limbic dopaminergic signaling has also been reported as a reason for dismal performance such as low level of sustained effort and reduced reward (40). Moreover, it was established that the decrease in white matter in LLD diminished emotion processing (41). The cognitive effort hypothesis indicates that patients with depression function normally in automatic tasks but struggle in effortful tasks (13). An analysis of cognitive function changes in patients with depression indicated that mood improvements were linked to developments in psychomotor speed, verbal fluency, and memory, whereas both executive and attentive functions remained unaltered during treatment (42). How PDD affects different aspects of cognitive functioning requires further investigation. Considering that genetics plays a controlling role in both cognitive ability and MDD (43), the usage of genome-wide association investigations to isolate the common genetic variants strongly linked with PPD and cognition is recommended. As a key feature of depression, cognitive dysfunction may interact with the onset of depression (44). Thus, it is also possible that cognitive impairment can reversely cause PPD, which is beyond the scope of this study.
In this study, the causal associations between PDD and cerebrovascular diseases and between PDD and cognitive impairment were investigated for the first time using an MR design with reduced changes in residual confounding and reverse causality, which represents the major strength of our study. Various MR methods were also applied in this study for sensitivity analyses, and potential direct pleiotropy and heterogeneity were systematically examined. Several limitations were also present in our study. First, we mainly used the GWAS summary statistics from Europe, resulting in a conclusion that is not generalizable to other races and ethnicities. Second, as a drawback of any MR study, the effect of potential direct pleiotropy could not be fully removed.
Conclusion
This MR study revealed a causal association between PPD and cognitive impairment, indicating that cognitive impairment is a critical aspect of PPD and thus cannot be regarded as an epiphenomenon. Addressing cognitive impairment and lessening the symptoms associated with PPD independently play significant roles in the treatment of PPD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JL designed the study. JL, JQL, and LS carried out the statistical analyses, and drafted the manuscript. HW, TZ, YH, and XXL reviewed the manuscript. All authors have read and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1196055/full#supplementary-material
Abbreviations
PPD, postpartum depression; DSM-IV, the Diagnostic and Statistical Manual of Mental Disorders; MDD, major depressive disorder; cIMT, carotid intima-media thickness; COGENT, the Cognitive Genomics Consortium; LLD, late-life depression; SNPs, single nucleotide polymorphisms; MR, Mendelian Randomization; IVW, inverse-variance weighted; WM, weighted median; MR-PRESSO, Mendelian Randomization Pleiotropy RESidual Sum and Outlier; IVs, Instrumental variable; ORs, odds ratios; CIs, confidence intervals; GWAS, genome-wide association studies.
References
1. Escribà-Agüir V, Artazcoz L. Gender differences in postpartum depression: a longitudinal cohort study. J. Epidemiol. Commun. Health. (2011) 65:320–6. doi: 10.1136/jech.2008.085894
2. Sharma V, Sharma P. Postpartum depression: diagnostic and treatment issues. J Obstet Gynaecol Can. (2012) 34:436–42. doi: 10.1016/S1701-2163(16)35240-9
3. Maimburg RD, Vaeth M. Postpartum depression among first-time mothers - results from a parallel randomised trial. Sex Reprod Healthc. (2015) 6:95–100. doi: 10.1016/j.srhc.2015.01.003
4. Guintivano J, Manuck T, Meltzer-Brody S. Predictors of postpartum depression: a comprehensive review of the last decade of evidence. Clin Obstet Gynecol. (2018) 61:591. doi: 10.1097/GRF.0000000000000368
5. Veskrna L. Peripartum depression – does it occur in fathers and does it matter? J. Men's Health. (2010) 7:420–30. doi: 10.1016/j.jomh.2010.10.004
6. Heh SS. Relationship between social support and postnatal depression. Kaohsiung J Med Sci. (2003) 19:491–5. doi: 10.1016/S1607-551X(09)70496-6
7. Kassier SM, Madlala SS. Antenatal and postpartum depression: effects on infant and young child health and feeding practices. South African J Clin Nutrition. (2018) 31:17–22. doi: 10.1080/16070658.2017.1333753
8. Bernard-Bonnin AC. Canadian paediatric society, mental health and developmental disabilities committee. Mat Dep Child Dev Paediatr Child Health. (2004) 9:575–83. doi: 10.1093/pch/9.8.575
9. Smith-Nielsen J, Tharner A, Steele H, Cordes K, Mehlhase H, Vaever MS. Postpartum depression and infant-mother attachment security at one year: the impact of co-morbid maternal personality disorders. Infant Behav Dev. (2016) 44:148–58. doi: 10.1016/j.infbeh.2016.06.002
10. Dong JY, Zhang YH, Tong J, Qin LQ. Depression and risk of stroke: a meta-analysis of prospective studies. Stroke. (2012) 43:32–7. doi: 10.1161/STROKEAHA.111.630871
11. Sun J, Ma H, Yu C, Lv J, Guo Y, Bian Z, et al. Association of major depressive episodes with stroke risk in a prospective study of 0. 5 million. Chinese adults Stroke. (2016) 47:2203–8. doi: 10.1161/STROKEAHA.116.013512
12. Kessing LV. Cognitive impairment in the euthymic phase of affective disorder. Psychol Med. (1998) 28:1027–38. doi: 10.1017/S0033291798006862
13. Hammar Å, Årdal G. Cognitive functioning in major depression-a summary. Front Hum Neurosci. (2009) 3:26. doi: 10.3389/neuro.09.026.2009
14. Di Florio A, Meltzer-Brody S. Is postpartum depression a distinct disorder? Curr Psychiatry Rep. (2015) 17:1–6. doi: 10.1007/s11920-015-0617-6
15. Jolley SN, Betrus P. Comparing postpartum depression and major depressive disorder: issues in assessment. Issues Ment Health Nurs. (2007) 28:765–80. doi: 10.1080/01612840701413590
16. Gage SH, Smith GD, Zammit S, Hickman M, Munafò MR. Using Mendelian randomisation to infer causality in depression and anxiety research. Depress Anxiety. (2013) 30:1185–93. doi: 10.1002/da.22150
17. Davies NM, Holmes MV, Smith GD. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. (2018) 362: k601. doi: 10.1136/bmj.k601
18. Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. (2023) 613:508–18. doi: 10.1038/s41586-022-05473-8
19. Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. (2018) 50:524–37. doi: 10.1038/s41588-018-0058-3
20. Jiang L, Zheng Z, Fang H, Yang J, A. generalized linear mixed model association tool for biobank-scale data. Nat Genet. (2021) 53:1616–21. doi: 10.1038/s41588-021-00954-4
21. Yeung MW, Wang S, van de Vegte YJ, Borisov O, van Setten J, Snieder H, et al. Twenty-five novel loci for carotid intima-media thickness: a genome-wide association study in> 45 000 individuals and meta-analysis of> 100 000 individuals. Arterioscler Thromb Vasc Biol. (2022) 42:484–501. doi: 10.1161/ATVBAHA.121.317007
22. Howe LJ, Nivard MG, Morris TT, Hansen AF, Rasheed H, Cho Y, et al. Within-sibship genome-wide association analyses decrease bias in estimates of direct genetic effects. Nat Genet. (2022) 54:581–92. doi: 10.1038/s41588-022-01062-7
23. Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet. (2018) 50:1112–21. doi: 10.1038/s41588-018-0147-3
24. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. (2018) 7:e34408. doi: 10.7554/eLife.34408
25. Thomas AJ, Kalaria RN, O'Brien J. Depression and vascular disease: what is the relationship? J Aff Disorders. (2004) 79:81–95. doi: 10.1016/S0165-0327(02)00349-X
26. Parissis JT, Fountoulaki K, Filippatos G, Adamopoulos S, Paraskevaidis I, Kremastinos D. Depression in coronary artery disease: novel pathophysiologic mechanisms and therapeutic implications. Int J Cardiol. (2007) 116:153–60. doi: 10.1016/j.ijcard.2006.03.038
27. Nemeroff CB, Musselman DL. Are platelets the link between depression and ischemic heart disease? Am Heart J. (2000) 140:S57–62. doi: 10.1067/mhj.2000.109978
28. Vieweg WV, Julius DA, Fernandez A, Wulsin LR, Mohanty PK, Beatty-Brooks M, et al. Treatment of depression in patients with coronary heart disease. Am J Med. (2006) 119:567–73. doi: 10.1016/j.amjmed.2006.02.037
29. Vogelzangs N, Beekman AT, Dik MG, Bremmer MA, Comijs HC, Hoogendijk WJ, et al. Late-life depression, cortisol, and the metabolic syndrome. Am J Geriatr Psychiatry. (2009) 17:716–21. doi: 10.1097/JGP.0b013e3181aad5d7
30. Schiller CE, Meltzer-Brody S, Rubinow DR. The role of reproductive hormones in postpartum depression. CNS Spectr. (2015) 20:48–59. doi: 10.1017/S1092852914000480
31. Rock PL, Roiser JP, Riedel WJ, Blackwell A. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. (2014) 44:2029–40. doi: 10.1017/S0033291713002535
32. Weisenbach SL, Boore LA, Kales HC. Depression and cognitive impairment in older adults. Curr Psychiatry Rep. (2012) 14:280–8. doi: 10.1007/s11920-012-0278-7
33. Hammar A. Automatic and effortful information processing in unipolar major depression. Scand J Psychol. (2003) 44:409–13. doi: 10.1046/j.1467-9450.2003.00361.x
34. Gualtieri CT, Johnson LG, Benedict KB. Neurocognition in depression: patients on and off medication versus healthy comparison subjects. J Neuropsychiatry Clin Neurosci. (2006) 18:217–25. doi: 10.1176/jnp.2006.18.2.217
35. Qiu T, Wen H, Liu ZX, Pan XP, Zeng T. Investigation regarding early cognitive function of women in the postpartum period and the analysis of influencing factors. Risk Manag Healthc Policy. (2021) 10:3747–54. doi: 10.2147/RMHP.S309553
36. Henry JF, Sherwin BB. Hormones and cognitive functioning during late pregnancy and postpartum: a longitudinal study. Behav Neurosci. (2012) 126:73. doi: 10.1037/a0025540
37. Park EM, Meltzer-Brody S, Stickgold R. Poor sleep maintenance and subjective sleep quality are associated with postpartum maternal depression symptom severity. Archives Women Mental Health. (2013) 16:539–47. doi: 10.1007/s00737-013-0356-9
38. Kriesche D, Woll CF, Tschentscher N, Engel RR, Karch S. Neurocognitive deficits in depression: a systematic review of cognitive impairment in the acute and remitted state. Eur Arch Psychiatry Clin Neurosci. (2022) 1:1–24. doi: 10.1007/s00406-022-01479-5
39. Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. (2016) 22:238–49. doi: 10.1038/nm.4050
40. Millan MJ, Agid Y, Brüne M, Bullmore ET, Carter CS, Clayton NS, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nature Rev Drug Dis. (2012) 11:141–68. doi: 10.1038/nrd3628
41. Wu Y, Zhang F, Makris N, Ning Y, Norton I, She S, et al. Investigation into local white matter abnormality in emotional processing and sensorimotor areas using an automatically annotated fiber clustering in major depressive disorder. Neuroimage. (2018) 181:16–29. doi: 10.1016/j.neuroimage.2018.06.019
42. Douglas KM, Porter RJ. Longitudinal assessment of neuropsychological function in major depression. J Psychiatry. (2009) 43:1105–17. doi: 10.3109/00048670903279887
43. Halahakoon DC, Lewis G, Roiser JP. Cognitive impairment and depression—cause, consequence, or coincidence? JAMA psychiatry. (2019) 76:239–40. doi: 10.1001/jamapsychiatry.2018.3631
Keywords: postpartum depression, cerebrovascular diseases, cognitive impairment, Mendelian randomization, causal association
Citation: Li J, Li J, Shen L, Wang H, Zheng T, Hui Y and Li X (2023) Investigating the causal association of postpartum depression with cerebrovascular diseases and cognitive impairment: a Mendelian randomization study. Front. Psychiatry 14:1196055. doi: 10.3389/fpsyt.2023.1196055
Received: 29 March 2023; Accepted: 18 May 2023;
Published: 22 June 2023.
Edited by:
Sen Li, Beijing University of Chinese Medicine, ChinaReviewed by:
Yueshu Zhao, Third Affiliated Hospital of Zhengzhou University, ChinaWen-Wang Rao, McGill University, Canada
Copyright © 2023 Li, Li, Shen, Wang, Zheng, Hui and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Li, bGlqaWEwNTA4QHptdS5lZHUuY24=
†These authors have contributed equally to this work
 Jia Li
Jia Li Jinqiu Li
Jinqiu Li Lan Shen1†
Lan Shen1†