- 1Department of Psychiatry, Okitama Public General Hospital, Yamagata, Japan
- 2Department of Neurology, Okitama Public General Hospital, Yamagata, Japan
- 3Department of Psychiatry, Yamagata University School of Medicine, Yamagata, Japan
- 4Department of Radiology, Okitama Public General Hospital, Yamagata, Japan
Recoverin is a neuron-specific calcium-binding protein that is mainly located in the retina and pineal gland. Few reports have described patients with anti-recoverin antibody-positive encephalitis, and no cases of psychosis associated with this encephalitis have been reported. We report a patient with anti-recoverin antibody-positive encephalitis with Cotard and Capgras delusions who was successfully treated with electroconvulsive therapy (ECT). The patient was a 25-year-old woman. She exhibited disorientation, executive function deficits, tremors in the upper limbs, generalized athetoid-like involuntary movements, hallucinations, incontinence, and fever, which led to her admission to our hospital. Upon admission, she complained of Cotard delusions. Various diagnostic tests, including cerebrospinal fluid analysis, antibody screening, and brain imaging, were unremarkable, except for positivity for serum anti-recoverin antibodies, non-specific general slowing on electroencephalography and decreased regional cerebral blood flow (rCBF) in the frontal and occipital lobes, and increased rCBF in the basal ganglia and pons on single-photon emission computed tomography. She was eventually diagnosed with encephalitis positive for anti-recoverin antibodies and treated with immunoglobulins and steroids. Her neurological symptoms improved temporarily, but three months later, psychiatric symptoms, i.e., suicidal thoughts and Cotard and Capgras delusions, were exaggerated. After ECT, her condition significantly improved. In conclusion, the present report suggests that pineal gland dysfunction due to anti-recoverin antibody or its cross-reactivity with neuron-specific calcium-binding proteins may contribute to the neuropsychiatric symptoms observed in anti-recoverin antibody-positive encephalitis and that ECT can be a viable treatment option if immunotherapy proves ineffective. Additionally, decreased rCBF in the prefrontal cortex may be associated with the clinical features of Capgras and Cotard delusions.
1 Introduction
Autoimmune encephalitis is a form of autoimmune-mediated disease affecting the central nervous system. Clinical features of autoimmune encephalitis include various neuropsychiatric symptoms, including altered consciousness, seizures, memory deficits, mood changes, delusions, hallucinations, and catatonia (1, 2). Although the specific brain regions or neural circuits associated with these psychiatric symptoms remain unclear, abnormalities in the right hemisphere, insular cortex, prefrontal cortex, frontoparietal circuits, and midline structures have been implicated in certain types of delusions, particularly Cotard and Capgras delusions (3, 4). Autoimmune encephalitis can be triggered by antibodies against neuronal cell-surface antigens, such as the N-methyl-D-aspartate (NMDA) receptor, leucine-rich glioma-inactivated 1 (LGI1), contactin-associated protein-like 2 (Caspr2), alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, gamma-aminobutyric acid-B (GABA-B) receptor, and dipeptidyl-peptidase-like protein 6 (DPPX), as well as antibodies against intracellular antigens, such as Hu, Ma2, and glutamic acid decarboxylase (1).
Recoverin is a neuron-specific calcium-binding protein that is mainly located in the retina and pineal gland (5). Antibodies against recoverin are found in the serum of patients with cancer-associated retinopathy (6). There have been few reports describing patients with anti-recoverin antibody-positive encephalitis exhibiting neuropsychiatric symptoms such as ataxia, seizures, altered consciousness, agitation, and depressive mood (7–12). Notably, there have been no cases of psychosis associated with this encephalitis. Multiple abnormal examination findings have been reported in relation to this condition. These include pleocytosis observed in cerebrospinal fluid tests, leukoaraiosis and hyperintense regions at the basal ganglia as observed in magnetic resonance imaging, reduced radiotracer uptake at the basal ganglia as seen in dopamine transporter single emission computed tomography, and generalized slow waves observed in electroencephalography. It is worth noting that the results have been inconsistent across different studies (7–12). Regarding treatment, immunotherapies involving steroids, immunoglobulins, and rituximab have shown promising results in improving symptoms in certain cases (8, 10, 11). Here, we report the case of a patient with anti-recoverin antibody-positive encephalitis exhibiting Cotard and Capgras delusions, who was successfully treated with electroconvulsive therapy (ECT).
2 Case report
The patient was a 25-year-old woman with no significant medical or family history. The patient provided written informed consent to report her clinical course when she was in a state of clear consciousness after being discharged from our hospital. This report was approved by the Ethical Review Committee of Yamagata University Faculty of Medicine.
Nine days before admission to our hospital, she displayed a lack of response to her mother’s calls, impaired executive function, such as an inability to open and close a pencil case, disorientation, tremors in the upper limbs, visual hallucinations of suicide victims, incontinence, and wandering. Seven days prior to admission, she developed a slight fever and was admitted to our hospital.
Upon admission, the patient exhibited agitation, restlessness, incoherent thoughts, disorientation, and poor conversational fluency. She also displayed generalized athetoid-like involuntary movements and weakness in the lower limbs. She showed a fever of 39.5 °C and an increased heart rate of 156/min. Tendon reflexes and pupillary responses were unremarkable, and no nuchal rigidity or pathological reflexes were observed. She complained of Cotard delusions such as “I am dead,” “I have no uterus,” and “my heart is not beating.” Her laboratory blood tests showed increased levels of white blood cells at 18,000/μL, total bilirubin at 1.70 mg/dL, lactate dehydrogenase at 410 U/L, and creatine kinase at 1,705 U/L. C-reactive protein levels were measured at 0.01 mg/dL. Thyroid function (free T3, free T4, and thyroid stimulating hormone levels) was normal. A toxicological screening test and blood psychotropic level measurements were not performed because her parents, who lived with her, claimed that she did not consume alcohol, take prescribed psychotropics, or use illegal drugs. She underwent a lumbar puncture, and the results showed an opening pressure of 15 cm H2O, no pleocytosis with 2 counts/μL, and a normal IgG index of 0.43, along with normal protein and glucose levels. PCR results for herpes simplex virus DNA in the cerebrospinal fluid was negative. There were no IgG or IgM antibodies found against herpes simplex virus, varicella-zoster virus, and cytomegalovirus in the cerebrospinal fluid. The screening for antibodies against the NMDA receptor, LGI1, Caspr2, AMPA receptor, GABA-B receptor, and DPPX in the cerebrospinal fluid all yielded negative results. Serum antibodies against nuclear, NH2 terminal of alpha-enolase (NAE), thyroglobulin, and thyroid peroxidase were also negative. However, she tested slightly positive for serum anti-recoverin antibodies (BML Inc., Tokyo, Japan). The contrast-enhanced magnetic resonance imaging of the head was unremarkable (Figure 1A). Her electroencephalogram showed diffuse nonspecific general slowing without any signs of epileptic discharge. Other examinations, such as whole-body computed tomography and magnetic resonance imaging of the spine, pelvic region, and ovaries, did not reveal any remarkable abnormalities, including tumors. Tumor markers, including CEA, AFP, CA125, CA19-9, SLX, SCC antigen, SYFRA21-1, ProGRP, NSE, CA15-3, sIL-2L, and BCA225, all yielded negative results. An ophthalmological examination revealed no signs of retinopathy.
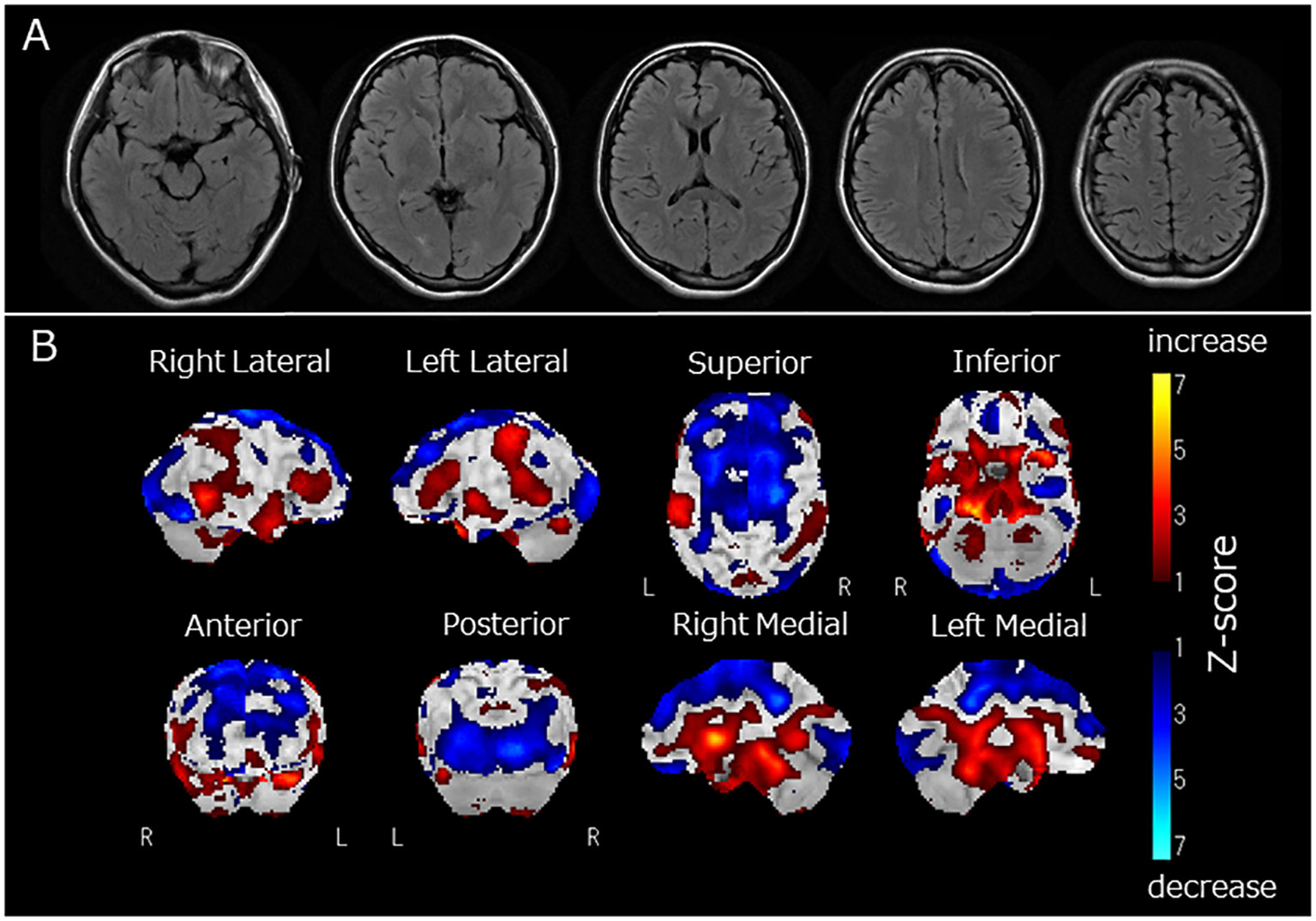
Figure 1 (A) Fluid Attenuated Inversion Recovery magnetic resonance imaging of the brain, (B) regional cerebral blood flow (rCBF) using single-photon emission computed tomography (SPECT) with 123I-iodoamphetamine. Fluid-attenuated inversion recovery magnetic resonance imaging revealed no apparent brain atrophy or high-intensity areas (A). SPECT using a 123I-iodoamphetamine was performed, and SPECT data were analyzed by 3D stereotactic surface projections (3D-SSP) included in “medi+FALCON version 1.4” (Nihon Medi-Physics Co. Ltd., Tokyo, Japan). Z-score maps of regional cerebral blood flow (rCBF) were created based using 3D-SSP. The SPECT images showed decreased rCBF in the occipital and frontal lobes (B). In contrast, rCBF increased in the basal ganglia and pons (B).
She was suspected of having autoimmune encephalitis and was treated with two cycles of intravenous immunoglobulins 20,000 mg/day for 5 days, and three cycles of steroid pulse therapy using methylprednisolone 1,000 mg/day for 3 days. Levetiracetam 1,000 mg/day and valproate (800 mg/day) were administered to prevent epileptic seizures. Approximately one month after admission, her neurological symptoms, such as disorientation and poor conversational fluency, improved. The dose of prednisolone was tapered to 17.5 mg/day. For psychiatric symptoms, olanzapine (up to 20 mg/day), risperidone (up to 6 mg/day), lorazepam (up to 6 mg/day), quazepam (20 mg/day), and lemborexant (5 mg/day) were used, but these treatments were discontinued due to a lack of apparent improvement.
Two months after admission, she displayed pessimistic thoughts, suicidal ideation, Cotard delusions, and Capgras delusions such as “Other persons, who have the same facial features as my parents but who have been replaced, live in my home. My real parents are dead.” She attempted suicide by jumping or hanging because she believed that her entire family was dead and that there was no reason to live. Regional cerebral blood flow (rCBF) was evaluated using single-photon emission computed tomography (SPECT) with 123I-iodoamphetamine. rCBF was decreased in the bilateral frontal and occipital lobes and increased in the bilateral basal ganglia and pons (Figure 1B). Fourteen sessions of bilateral ECT were conducted. The decision to employ ECT was based on the urgent need to address frequent suicidal attempts. ECT has been recognized for its efficacy in preventing such attempts (13). Additionally, it has proven to be effective in treating autoimmune encephalitis (2). Two months and three months after admission, two serum examinations were conducted and both showed a strong positive result for anti-recoverin antibodies. After ECT, her psychiatric symptoms, including Cotard delusions, Capgras delusions, and suicidal thoughts, markedly improved. At 8 months, steroid therapy was discontinued, and the patient was discharged from the hospital after physical and verbal rehabilitation. One year after discharge, the patient remained in remission.
3 Discussion
In the present case, there was an acute onset and rapid progression within 9 days of neuropsychiatric symptoms, which included delusions, hallucinations, altered consciousness, and focal neurological findings such as tremors, athetoid-like involuntary movements, weakness, and impaired executive function (Table 1). The patient tested positive for serum anti-recoverin antibodies. Diffuse, nonspecific general slowing was observed on electroencephalography, along with decreased rCBF in the frontal and occipital lobes and increased rCBF in the basal ganglia and pons on SPECT. Treatment with immunotherapy and ECT led to the remission of the patient’s neuropsychiatric symptoms. Viral and other autoimmune encephalitides were ruled out, as there was no detection of herpes simplex virus DNA or various antibodies associated with autoimmune encephalitis (1). While Cotard and Capgras delusions have been rarely reported in patients with schizophrenia (14), the diagnosis of schizophrenia was considered unlikely in this case due to the presence of autonomic symptoms such as fever and increased heart rate, neurological symptoms, and abnormal findings on electroencephalography. Additionally, complete remission persisted for 1 year after discontinuation of antipsychotic treatment. Therefore, it was considered that the present case had possible autoimmune encephalitis caused by anti-recoverin antibodies, according to the diagnostic criteria proposed by Graus et al. (1).
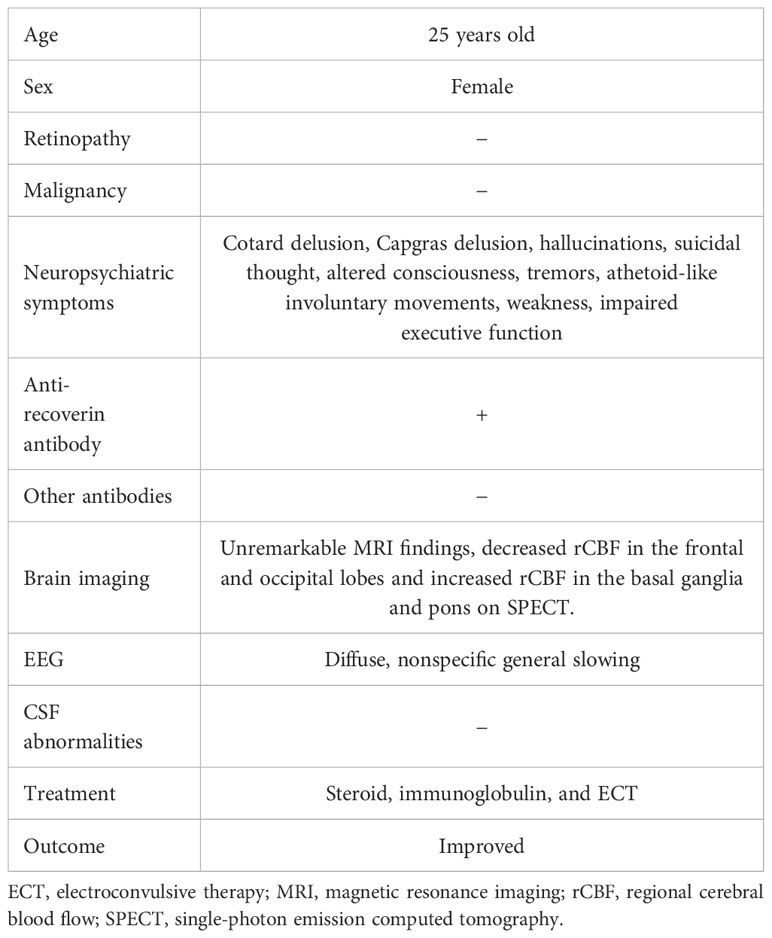
Table 1 Characteristics, clinical symptoms, examination findings, and treatments in the patient with anti-recoverin antibody-positive encephalitis.
The effects of anti-recoverin antibodies on the central nervous system are not fully understood. Recoverin is expressed in the pineal gland (6) as well as the retina. The pineal gland, which secretes melatonin, is involved in the modulation of circadian rhythms, regulation of sleep, reproductive physiology, and immunological regulation (15). It has been shown that patients with psychosis and mood disorders have a smaller pineal gland volume, lower blood levels of melatonin, and aberrant patterns of melatonin secretion (15), whereas the administration of melatonin to hospitalized patients is related to a reduction in delirium incidence (16). These studies suggest that pineal gland function is involved in psychosis, mood disorders, and delirium. Thus, it is possible that altered dysfunction of the pineal gland caused by anti-recoverin antibodies may have been associated with the neuropsychiatric symptoms observed in this case. Meanwhile, recoverin has 40–55% sequence identity with other neuron-specific calcium-binding proteins, i.e., hippocalcin, neuronal calcium sensor-1, and visinin-like protein (17). These neuron-specific calcium-binding proteins are widely expressed in the brain, including the neocortex, caudate, brain stem, hippocampus, amygdala, putamen, forebrain, and cerebellum (5), and are reportedly associated with various neuropsychiatric diseases, such as intellectual disability, autism, attention deficit disorder, dystonia, and Alzheimer disease (18). Therefore, as suggested by Kitazaki et al. (11), the cross-reactivity of the anti-recoverin antibody with these neuron-specific calcium-binding proteins may induce autoimmune encephalitis.
ECT is effective in treating anti-NMDA receptor encephalitis, particularly in patients with catatonia (2), presumably because of its effects on glutamate, GABA, serotonin, and dopamine neurotransmission (19). In this case, immunotherapy (immunoglobulins or steroids) and antipsychotics were insufficient to manage intense and severe psychiatric symptoms such as Cotard delusions, Capgras delusions, and suicidal ideation, whereas ECT treatment yielded excellent outcomes for these psychiatric symptoms. Previous reports on anti-recoverin antibody-positive encephalitis have shown that immunotherapy improved symptoms in some cases (8, 10, 11), but not in others (9, 10). Thus, the present case suggests that ECT may be the next treatment option when immunotherapy is ineffective for this type of encephalitis.
In this case, we observed Cotard and Capgras delusions. Cotard and Capgras delusions belong to delusional misidentification syndrome, caused by various organic brain diseases such as dementia, cerebrovascular disease, and encephalitis, as well as psychiatric disorders such as schizophrenia and depression (20, 21). These delusions are reportedly developed by two factors (20): abnormal perception leading to sensations of derealization or depersonalization, which is associated with dysfunction of the insular cortex, and abnormal rationalization towards external causes (Capgras delusion) and internal causes (Cotard delusion), which are related to dysfunction of the prefrontal cortex (3). The present case displayed decreased rCBF in the frontal and occipital lobes and increased rCBF in the basal ganglia and pons. Therefore, dysfunction in the prefrontal cortex, reflected as decreased rCBF, might be associated with the clinical features of Capgras and Cotard delusions observed in this case.
One limitation of the report is that the pathological significance of anti-recoverin is not well characterized. Consequently, the possibility that other undetected antibodies might be responsible for the neuropsychiatric symptoms in this case and that anti-recoverin antibodies co-existed by chance, cannot be ruled out. Secondly, this report presents a single case, highlighting the need to accumulate more cases in order to draw conclusive findings for clinical practice in anti-recoverin antibody-positive encephalitis.
In conclusion, the present case report suggests the importance for clinicians to assess the presence of anti-recoverin antibodies and to consider this encephalitis in the differential diagnosis when confronted with acute onset neuropsychiatric symptoms, including Cotard and Capgras delusions. In cases where immunotherapy is not effective, ECT may be the next treatment option for this encephalitis. Altered dysfunction of the pineal gland caused by anti-recoverin antibodies or cross-reactivity of anti-recoverin antibodies with neuron-specific calcium-binding proteins may be associated with neuropsychiatric symptoms observed in anti-recoverin antibody-positive encephalitis. This report also suggests that decreased rCBF in the prefrontal cortex may be associated with the clinical features of Capgras and Cotard delusions. It is important to gather more cases to confirm these findings.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Ethical Review Committee of Yamagata University Faculty of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
TA: Conceptualization, Investigation, Writing – original draft. NT: Conceptualization, Investigation, Writing – original draft. RK: Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – review & editing. KoN: Conceptualization, Data curation, Investigation, Writing – original draft. MA: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. YS: Conceptualization, Investigation, Supervision, Writing – review & editing. KeN: Data curation, Formal Analysis, Writing – review & editing. AS: Conceptualization, Formal Analysis, Investigation, Methodology, Supervision, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank Dr. Keiko Tanaka at the Department of Animal Model Development, Brain Research Institute, Niigata University and the Department of Multiple Sclerosis Therapeutics, School of Medicine, Fukushima Medical University, for kindly examining the antibodies in the serum and cerebrospinal fluid of the patient. The authors are grateful to the patient for providing informed consent for the publication of this case report. We would like to thank Editage (www.editage.jp) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol (2016) 15:391–404. doi: 10.1016/S1474-4422(15)00401-9
2. Dalmau J, Armangué T, Planagumà J, Radosevic M, Mannara F, Leypoldt F, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: Mechanisms and models. Lancet Neurol (2019) 18:1045–57. doi: 10.1016/S1474-4422(19)30244-3
3. Restrepo-Martínez M, Espinola-Nadurille M, Bayliss L, Díaz-Meneses I, Kerik NE, Mendez MF, et al. FDG-PET in Cotard syndrome before and after treatment: Can functional brain imaging support a two-factor hypothesis of nihilistic delusions? Cognit Neuropsychiatry (2019) 24:470–80. doi: 10.1080/13546805.2019.1676710
4. Lozano-Cuervo R, Espinola-Nadurille M, Restrepo-Martinez M, Rotenberg NK, Pollak TA, Ramirez-Bermudez J. Capgras delusion in anti-NMDAR encephalitis: A case of autoimmune psychosis. Asian J Psychiatr (2020) 54:102208. doi: 10.1016/j.ajp.2020.102208
5. Paterlini M, Revilla V, Grant AL, Wisden W. Expression of the neuronal calcium sensor protein family in the rat brain. Neuroscience (2000) 99:205–16. doi: 10.1016/s0306-4522(00)00201-3
6. Bazhin AV, SChadendorf D, Philippov PP, Eichmüller SB. Recoverin as a cancer-retina antigen. Cancer Immunol Immunother (2007) 56:110–6. doi: 10.1007/s00262-006-0132-z
7. Saraya AW, Worachotsueptrakun K, Vutipongsatorn K, Sonpee C, Hemachudha T. Differences and diversity of autoimmune encephalitis in 77 cases from a single tertiary care center. BMC Neurol (2019) 19:273. doi: 10.1186/s12883-019-1501-5
8. Herzog R, Brüggemann N, Sprenger A, Münte TF. Recoverin antibody-associated late-onset ataxia without retinopathy. BMJ Case Rep (2020) 13:e237479. doi: 10.1136/bcr-2020-237479
9. Ryu HS, Lee SY, Park DH, Lee JM. A case of paraneoplastic neurological syndrome expressing dual antineuronal antibodies: Anti-Hu and recoverin. Ann Indian Acad Neurol (2020) 23:133–5. doi: 10.4103/aian.AIAN_185_19
10. Ávila GM, Escamilla EE, González AP, Corral JAM, Fernández CP, Marcos AR. Antineuronal antibodies: Anti-recoverin in neurological syndromes without retinopathy. SARS-CoV2 infection as trigger. Neurol (Engl Ed) (2022) 37:409–10. doi: 10.1016/j.nrleng.2021.07.003
11. Kitazaki Y, Shirafuji N, Takaku N, Yamaguchi T, Enomoto S, Ikawa M, et al. Autoimmune basal ganglia encephalitis associated with anti-recoverin antibodies: A case report. eNeurologicalSci (2021) 25:100382. doi: 10.1016/j.ensci.2021.100382
12. Hansen N, Bartels C, Rentzsch K, Stöcker W, Fitzner D. Dysfunctional learning and verbal memory in patients with elevated Tau protein levels and serum recoverin autoantibodies-case series and review. Brain Sci (2021) 12:15. doi: 10.3390/brainsci12010015
13. Gonda X, Dome P, Serafini G, Pompili M. How to save a life: From neurobiological underpinnings to psychopharmacotherapies in the prevention of suicide. Pharmacol Ther (2023) 244:108390. doi: 10.1016/j.pharmthera.2023.108390
14. Revilla J, Aliaga S, Lozano-Vargas A. Cotard and capgras syndrome in a patient with treatment-resistant schizophrenia. Case Rep Psychiatry (2021) 2021:6652336. doi: 10.1155/2021/6652336
15. Chauhan S, Barbanta A, Ettinger U, Kumari V. Pineal abnormalities in psychosis and mood disorders: A systematic review. Brain Sci (2023) 13:827. doi: 10.3390/brainsci13050827
16. Khaing K, Nair BR. Melatonin for delirium prevention in hospitalized patients: A systematic review and meta-analysis. J Psychiatr Res (2021) 133:181–90. doi: 10.1016/j.jpsychires.2020.12.020
17. Burgoyne RD, Weiss JL. he neuronal calcium sensor family of Ca2+-binding proteins. Biochem J (2001) 353:1–12. doi: 10.1042/bj3530001
18. Burgoyne RD, Helassa N, McCue HV, Haynes LP. Calcium sensors in neuronal function and dysfunction. Cold Spring Harb Perspect Biol (2019) 11:a035154. doi: 10.1101/cshperspect.a035154
19. Subramanian S, Lopez R, Zorumski CF, Cristancho P. Electroconvulsive therapy in treatment resistant depression. J Neurol Sci (2022) 434:120095. doi: 10.1016/j.jns.2021.120095
20. Tomasetti C, Valchera A, Fornaro M, Vellante F, Orsolini L, Carano A, et al. The “dead man walking” disorder: An update on Cotard’s syndrome. Int Rev Psychiatry (2020) 32:500–9. doi: 10.1080/09540261.2020.1769881
Keywords: anti-recoverin antibody, encephalitis, ECT, case report, Cotard delusion, Capgras delusion, rCBF
Citation: Akahane T, Takahashi N, Kobayashi R, Nomura K, Akiho M, Shikama Y, Noto K and Suzuki A (2024) Case report: A case of anti-recoverin antibody-positive encephalitis exhibiting Cotard and Capgras delusions that was successfully treated with electroconvulsive therapy. Front. Psychiatry 15:1330745. doi: 10.3389/fpsyt.2024.1330745
Received: 31 October 2023; Accepted: 08 January 2024;
Published: 25 January 2024.
Edited by:
Helge Frieling, Hannover Medical School, GermanyReviewed by:
Takashi Kanbayashi, University of Tsukuba, JapanAlexandra Neyazi, University Hospital Magdeburg, Germany
Copyright © 2024 Akahane, Takahashi, Kobayashi, Nomura, Akiho, Shikama, Noto and Suzuki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ryota Kobayashi, cnlvLmtvYmF5YXNoaUBtZWQuaWQueWFtYWdhdGEtdS5hYy5qcA==
 Takaki Akahane1
Takaki Akahane1 Naomi Takahashi
Naomi Takahashi Ryota Kobayashi
Ryota Kobayashi Konoka Nomura
Konoka Nomura Akihito Suzuki
Akihito Suzuki