- University Hospital Essen, Institute of Physiological Chemistry, Essen, Germany
Sigmund Freud´s drive theory, often (and erroneously) referred to as his theory of the instincts, was an early attempt to describe the motivations behind both healthy and mentally ill individuals. In his final formulation, Freud identified two main categories of drives: life drives (which he termed Eros), encompassing physiological drives, and a set of inherent impulses, including self-destructive tendencies (which he called “death drives”), with masochism – a form of auto-addictive disorder - as a prime example. Freud´s drive theory was developed with the framework of a 19th-century medical mind-set, but this model has since been up-dated in various publications using advanced data from neuroanatomy, (neuro)endocrinology and biochemistry. Modern research has shown that all physiological drives (i.e., hunger, thirst, sleep, sexual drive and attachment) are regulated in key brain regions, including the nucleus accumbens and lateral hypothalamus, by neurotransmitters like dopamine and 5-hydroxytryptamine. These, in turn, stimulate the release of β-endorphin, maintaining its levels within physiological norms. Importantly, nearly all forms of addiction are associated with altered β-endorphin levels, and one or more essential drives are disrupted. Since addictive behaviors often aim to restore β-endorphin levels to normal, addiction - whether behavioral or substance-related - acts, from the perspective of an up-dated Freudian drive theory, as a surrogate for malfunctioning drives. To restore all five healthy drives, particularly attachment, it is crucial to recognize and address the underlying causative factors, such as trauma, epigenetic changes, genetic predisposition, environmental stress, or co-occurring chronic illnesses.
1 Introduction
Adolescence is a critical period for the development of addictive behaviors. In the United States, the 2018 prevalence of lifetime substance use among adolescents was reported to be 26.3% for alcohol, 15.3% for cannabis, and 13.4% for tobacco (1). On one hand, substance use during adolescence can initiate the persistent non-medical use of opioids into adulthood (2). On the other hand, it can significantly disrupt brain development (3–5).
Furthermore, there is a strong comorbidity between mental health issues and substance use disorders among adolescents. For example, one study found that 40.8% of adolescents in public mental health treatment programs also met the criteria for a substance use disorder (6).
This raises the question of what drives addiction. Given that independent causal factors - such as childhood trauma and chronic illness (discussed below) - can lead to addiction, it becomes important to explore whether the driving force behind addiction is dependent or independent of these causal factors.
To address this question and optimize addiction treatment, a comprehensive theoretical construct of human motivation, strongly supported by clinical data, is needed. Such a framework is currently somewhat lacking. In this manuscript, an updated version of Freudian Drive Theory is proposed to describe all forms of addiction. This revised theory posits that all addictions stem from a deficit of a specific signaling molecule, and the addictive behavior is executed as an attempt to compensate for this deficit. Since the theory´s predictions are underpinned by experimental clinical data, the updated Freudian Drive Theory may serve as a useful tool for clinical therapists.
2 Materials and methods
Although this work is a narrative review, the manuscript is based on a structured and transparent literature selection process. Given the interdisciplinary nature of this review — drawing on sources from fields such as biochemistry, endocrinology, neuroanatomy, neurobiology, physiology, psychoanalysis, and psychiatry — a narrative format was chosen. This approach allows for the integration of heterogeneous concepts, theories, and data without the methodological constraints of a systematic review, which may be less suitable for synthesising foundational and conceptual material across disciplines.
In addition, the use of acronyms was deliberately minimised, as their meanings and conventions often vary significantly across the above-mentioned fields. Where acronyms were used, they were selected to align with psychiatric usage in order to maintain conceptual coherence and accessibility for clinicians and mental health professionals.
Prior to writing, a detailed chapter outline was developed to guide the organisation of the review. For each chapter, relevant literature was identified using general search engines (Google and Bing) and scientific databases (primarily Medline). When these methods did not yield sufficient or suitable sources, additional material was retrieved through manual searches in the holdings of the university library.
Artificial intelligence-based tools such as ChatGPT and Perplexity were also tested, but proved unsuitable for identifying original and citable sources in this context.
In total, approximately 1,500 references were identified. Of these, about 200 were selected and included in the final manuscript following critical reading and evaluation over a two-year period.
3 Freudian drive theory
3.1 Traditional Freudian drive theory
In his early work, Sigmund Freud proposed the existence of two primary motivational drives: the sexual drive (libido) and the self-preservation or ego drive (7). Later, Freud refined this theory to encompass auto-addictive conditions like masochism (8). He posited two main drive collections: life drives (called by Freud Eros), which included ego drives, the sexual drive and those that foster human connection) and “death drives”, which were less clearly defined but suggested as an inherent collection of impulses related to destruction, aggression, and self-harm (9, 10).
Freud´s efforts to understand the drives influencing human behavior emerged from his clinical experience with neurotic and psychotic patients. The development of his drive theory and its central theoretical postulates was shaped by both clinical observations and the scientific knowledge available to him at the time. The Freudian drive theory represents an early attempt to objectively explain human behavior using verified clinical and scientific data.
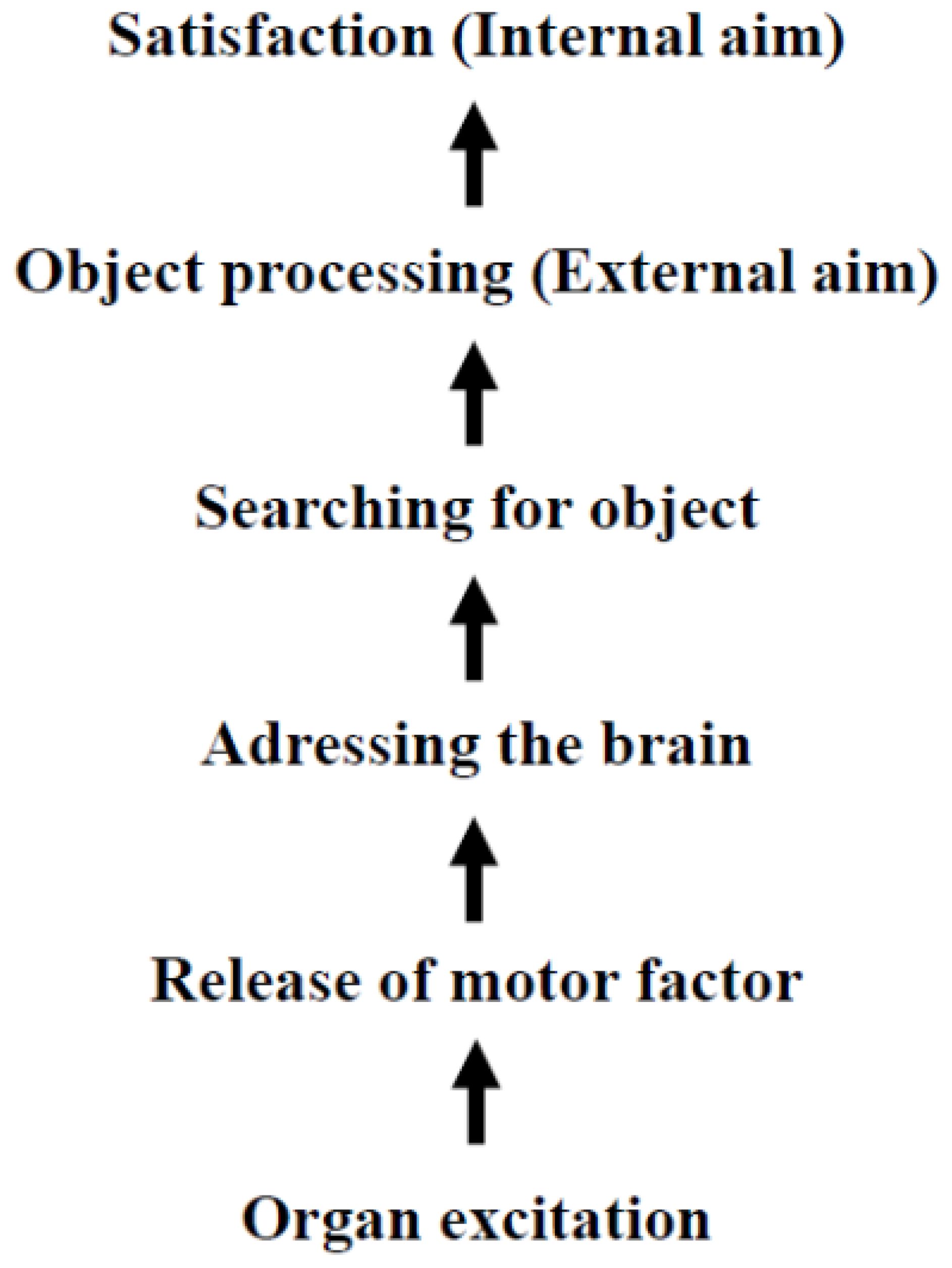
In 1905 Freud clarified the origins of drives: “The source of an instinct is a process of excitation occurring in an organ and…” [(7), p. 1492].1 He also described the motor component of the sexual drive: “It seems probable, then, that special chemical substances are produce in the interstitial portion of the sex-glands; these are then taken up in the blood stream and cause particular parts of the central nervous system to be charged with sexual tension.” [(7), p. 1530]. Freud suggested that a drive is initiated by the excitation of an organ, thereby activating the motor component of the drive, which acts as the executing entity. In 1915, Freud outlined the architecture of drives, identifying four components: pressure (or motor factor), aim, object and source ((12), p. 2960). He asserted that the “aim [Ziel] of an instinct is in every instance satisfaction” [((12),p. 2960]. Notably, Freud´s concept of the “aim” refers to a psychological goal (satisfaction), not necessarily the physical competition of an act – such as eating food in response to hunger. In 1933 Freud elaborated on the connection between aim and object: “The aim can be achieved in the subject´s own body: as a rule an external object is brought in, in regard to which the instinct achieves its external aim; its internal aim invariably remains the bodily change which is felt as satisfaction.” [(10), p. 96]. Based on these statements, the following Scheme can be constructed:
It is noteworthy that in 1905, Freud could not use the term “hormone” to describe the motor component of drives, as the term was only coined in the same year by Starling [(13), p. 340]. In subsequent years, Freud retained his original terminology to maintain consistency with his earlier writings. Since Scheme 1 intrinsically predicts that peripheral hormones (unknown in 1905)2 can cross the blood brain barrier and posits the existence of an unknown metabolite that produces satisfaction as a feedback signal for human well-being, these declarations were highly speculative at the time of their presentation.
3.2 Fortuitously support for Freudian drive theory
Surprisingly, even scientists who did not intend to support the Freudian Drive Theory have uncovered mechanisms that align the framework outlined in Scheme 1. The first major contribution came from Henry´s landmark study on the physiological role of endorphins in the central nervous system (15). Henry concluded: “Finally, when the endorphin system is hypoactive, and here perhaps duration of hypoactivity might also play an important role, an increased drive ensues to satisfy a deprived state, whether this is an appetite for food, water, social contact, sexual satisfaction, etc.:” [(15), p. 239]. This conclusion supports Freud´s assertion that all drives share a common internal aim.
Given that various β-endorphin derivatives maintain a general state of well-being and pleasure by binding to the corresponding µ-receptor (16, 17), Freud´s concept of an internal aim (i.e., human satisfaction) can be interpreted through the release of central β-endorphin. While other endorphin families may also be involved, they are currently less well understood than β-endorphin derivatives.
The second significant insight comes from the Incentive-Sensitization Theory developed by Berridge and Robinson (18). This theory highlights the role of dopaminergic systems, not only in the sensation of satisfaction (reward3) but also in the process by which incentives gain motivational significance over time. The theory posits that dopamine is more closely associated with ‘wanting’ rather than ‘liking’. In other words, dopamine intensifies the pursuit and desire for satisfaction rather than the actual pleasure derived from it, which is achieved through the release of β-endorphin (as discussed above).
The third key support came from Panksepp, the principal developer of the concept of emotional Command Systems. Panksepp (21–24) proposed seven distinct types of motivations that drive specific behaviors, such as seeking resources, lust, caregiving, panic, rage, fear and play. These behaviors are associated with characteristic subcortical regions of the brain, collectively termed as Command Systems. For example, the brain areas associated with the motivation to seek resources and satisfaction are labelled as the SEEKING system.
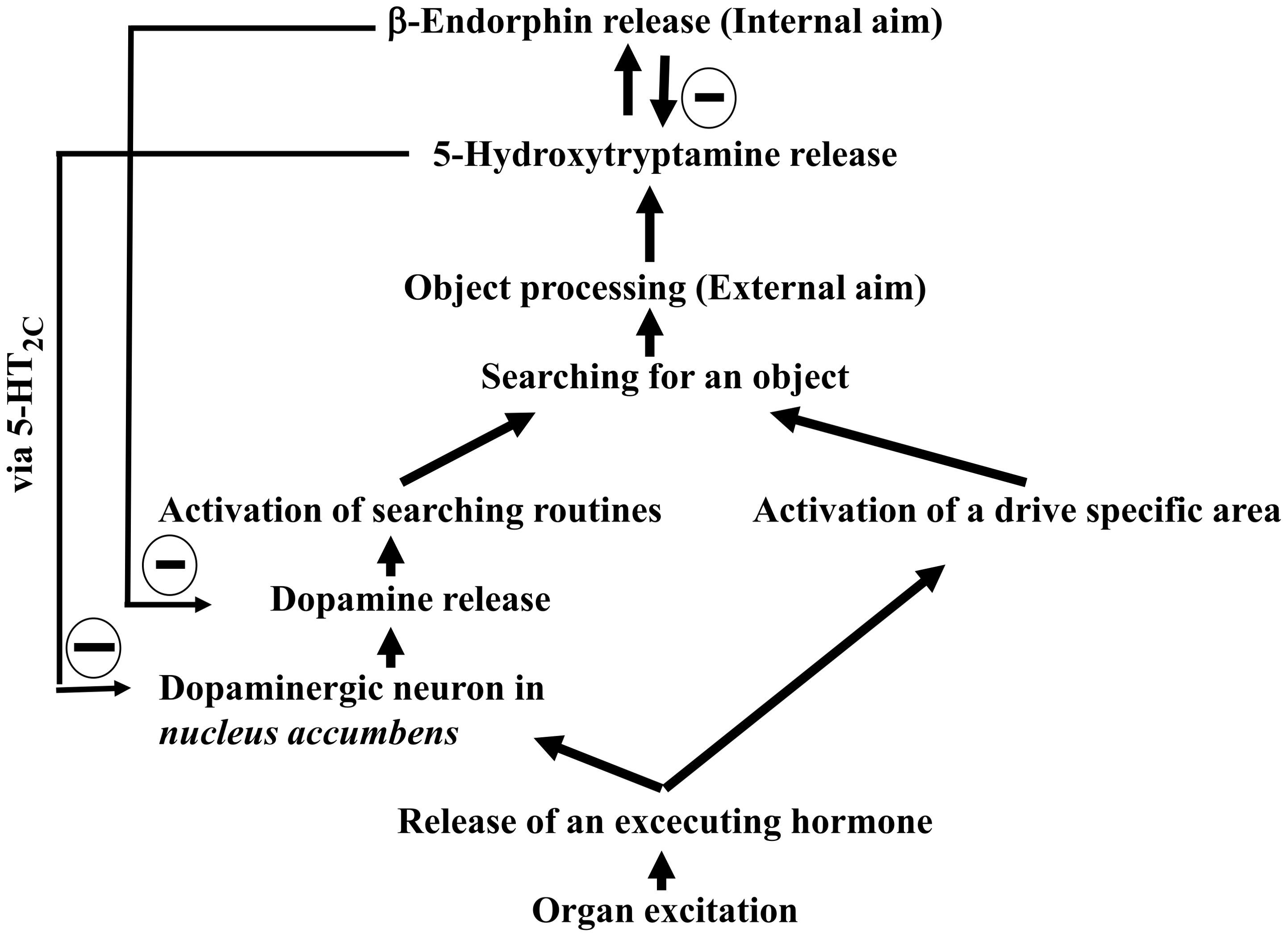
Of particular importance to the refinement of Freudian Drive Theory is Panksepp´s concept of SEEKING, which acknowledges drives as inputs (21). This model operates on the principle that drives act as independent impulse generators ((25), p. 18). For Freud´s drive construct (Scheme 1) to remain valid, the hormones executing a drive must simultaneously target the brain areas characteristic of SEEKING (e.g., nucleus accumbens and lateral hypothalamus) to induce dopamine release, as well as an additional brain region specific to the particular drive in question.
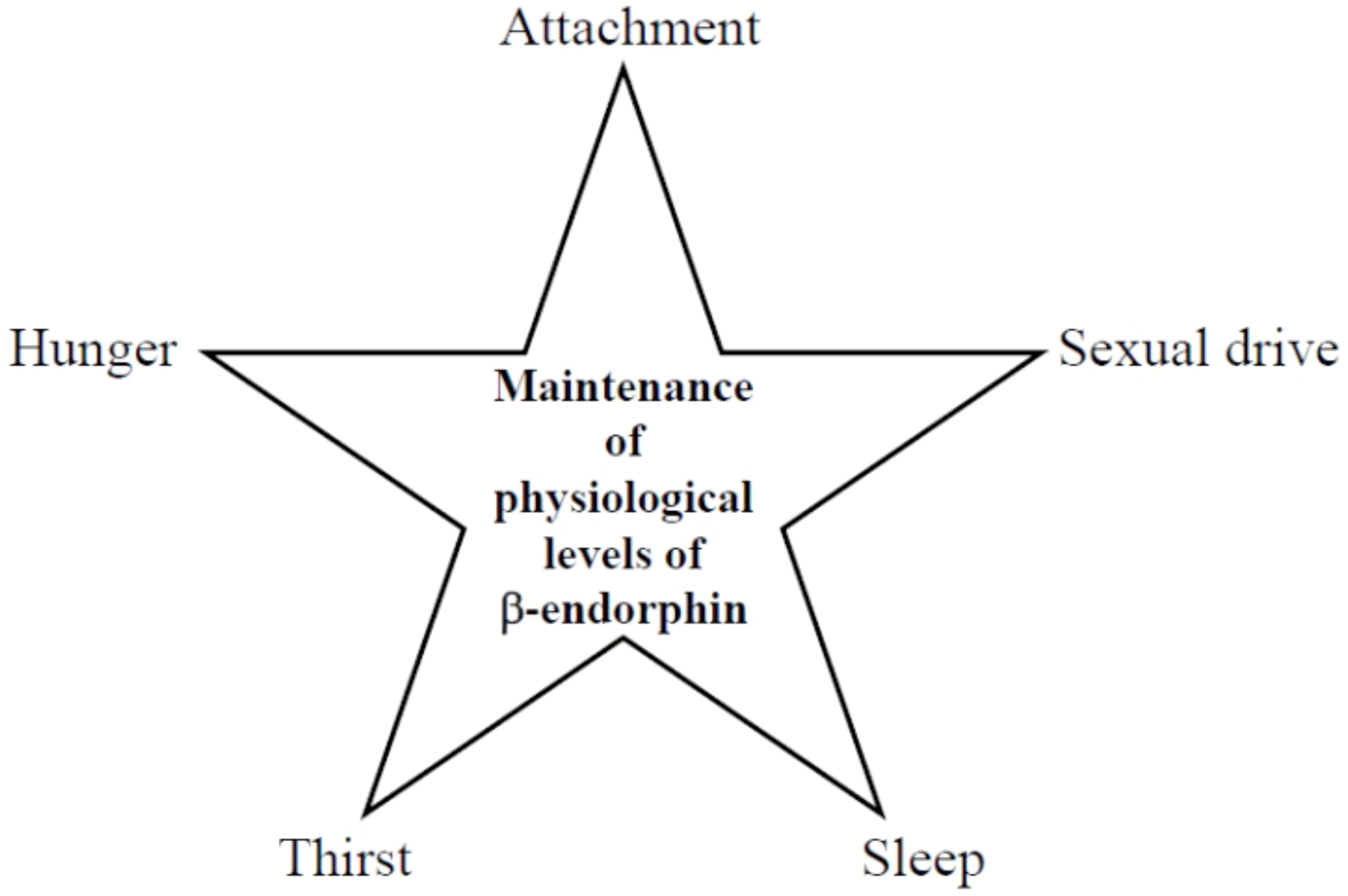
An additional advantage of Panksepp’s concept of Emotional Systems is its usefulness as a framework for distinguishing motivational drives from instincts (11). The terms drive and instinct have often been used interchangeably since their inception, leading to conceptual confusion. In principle, five core drives can be identified — attachment, hunger, thirst, sexuality, and sleep — alongside four basic instincts: fear, panic, rage, and play.
Instincts are triggered by sensory input or reflexive mechanisms and are regulated by the five primary drives. Thus, a drive can activate an instinct when needed through its corresponding effector hormones, whereas an instinct cannot, in turn, activate a drive (11).
3.3 Purposefully support for Freudian drive theory
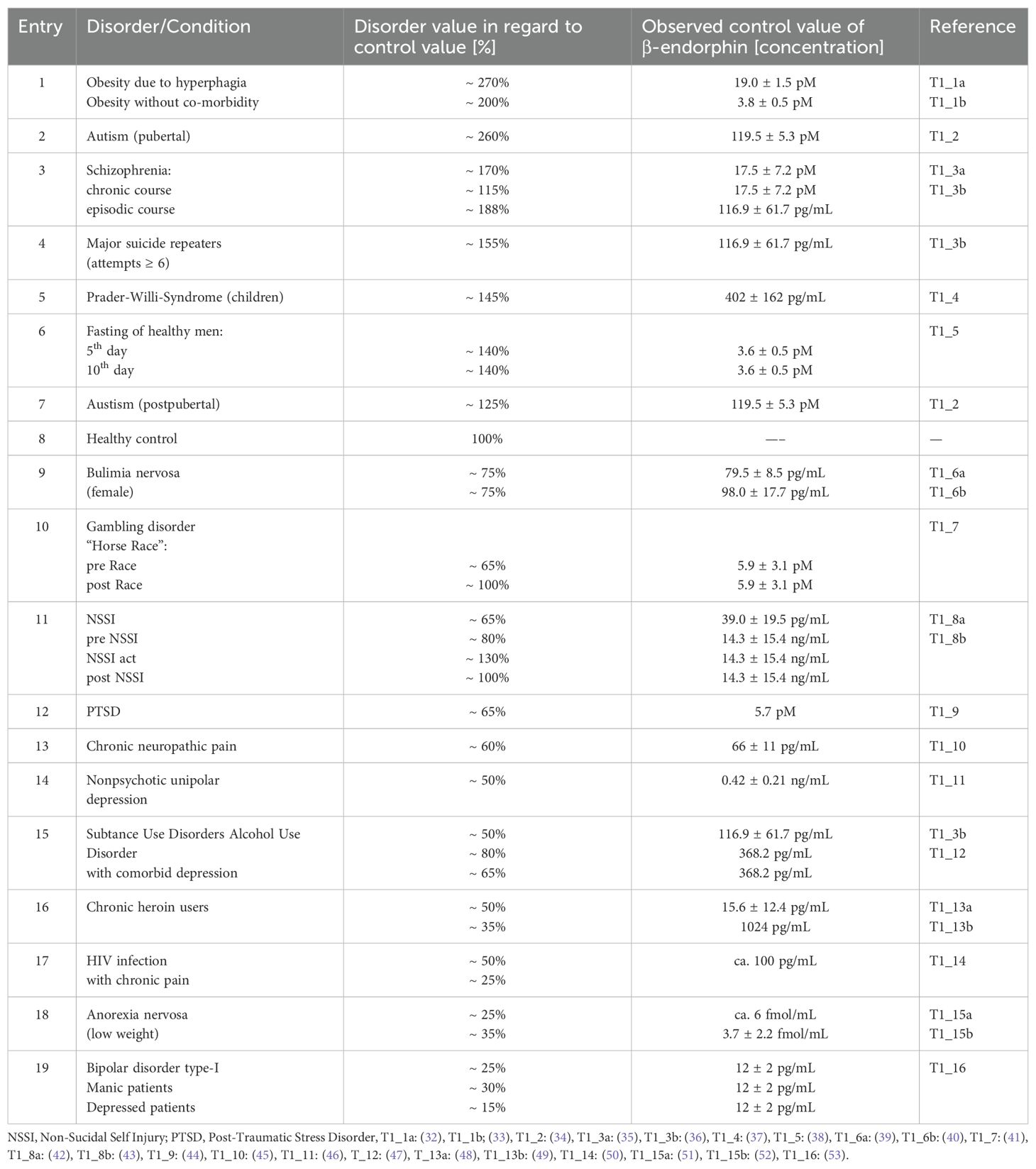
Over the past decade, Johnson and colleagues have provide significant evidence supporting Henry´s prediction by demonstrating that healthy individuals maintain opioids concentrations (with β-endorphin as the most effective µ-receptor activator) within a characteristic range in the central nervous system (26–31). Persistent opioid levels that are either abnormally high (as observed in certain forms of autism (26) or abnormally low (as seen in patients with anorexia nervosa) are indicative of pathophysiological conditions (Table 1).
3.4 Advances in Freudian drive theory
The researcher of this manuscript evaluated the previously overlooked drive-specific areas, hormones and the biochemical mechanisms to further refine Freudian Drive Theory (8, 54–56). These findings culminate in an updated model, presented as Scheme 2:
3.5 Mother-infant attachment as Bowlbyian drive
While Scheme 2 explains the onset of certain disorders, it lacks a framework for understanding complex social interactions. For instance, Harlow´s observation that attachment (e.g., in monkey infants) can be temporally more important than hunger (57) cannot be explained by Scheme 2. Recently, the architecture of the mother-infant tie was deconstructed from the perspective of the infant (55), and has been up-dated4 as shown in Scheme 3:
At first glance, Scheme 3 appears structurally similar to Scheme 2. Due to this resemblance, the mother-infant tie is classified here as a Bowlbyian drive, honoring John Bowlby´s seminal work on attachment theory (55). Paradigmatically, the other-infant tie serves as a foundational example of attachment. However, the broader spectrum of human attachment drives (e.g., pair bonding) is likely mediated by additional signaling molecules, making the mother-infant tie a basal but comprehensive model of attachment.
4 Surrogates mechanisms
Furthermore, consistent with Henry´s earlier predictions, the attachment drive also facilitates β-endorphin release (vide supra). This insight allows the construction of Scheme 4, which emphasizes the role of basal physiological β-endorphin levels in maintaining key drives:
Thus, if one of the β-endorphin providers reduce its activity for any reason, the basal physiological β-endorphin level can no longer be maintained (examples are provided in Table 1, vide supra), and the patient unconsciously begins to seek alternative sources (8). Patients with a hypoactive drive unconsciously utilize three different strategies to compensate for the endogenously decreased β-endorphin release:
1. Overstimulating another physiological drive(s);
2. Engaging in behaviors that become compulsive and persist despite harmful consequences (often referred to as non-substance addiction or auto-addictive diseases);
3. Using drugs (substance addiction).
4.1 Overstimulating a physiological drive
Overstimulating the hunger drive is an effective way to release β-endorphin (Table 1, Entry 1). As a result, a hyperactive hunger drive is often developed as a coping strategy to counteract the hypoactive one. Table 2 provides examples of obesity, as an excessive hunger drive, compensating for hypoactive attachment, sleep or sexual drives:
The relationship between obesity and hypoactive drives is complex, with both factors influencing each other. For instance, obesity can induce hormonal changes that affect sexual function (68), physical limitations (69), and psychological issues such as low self-esteem, (70)). These factors further suppress the sexual drive, reducing β-endorphin release, which in turn amplifies hunger drive activity and exacerbates obesity.
In summary, when patients adopt coping strategies to maintain physiological β-endorphins levels, two drives are disrupted: one becomes hypoactive, while another becomes hyperactive, creating a feedback loop. This cycle further suppresses the hypoactive drive while intensifying the hyperactive one, perpetuating the imbalance.
4.2 Non-substance addiction
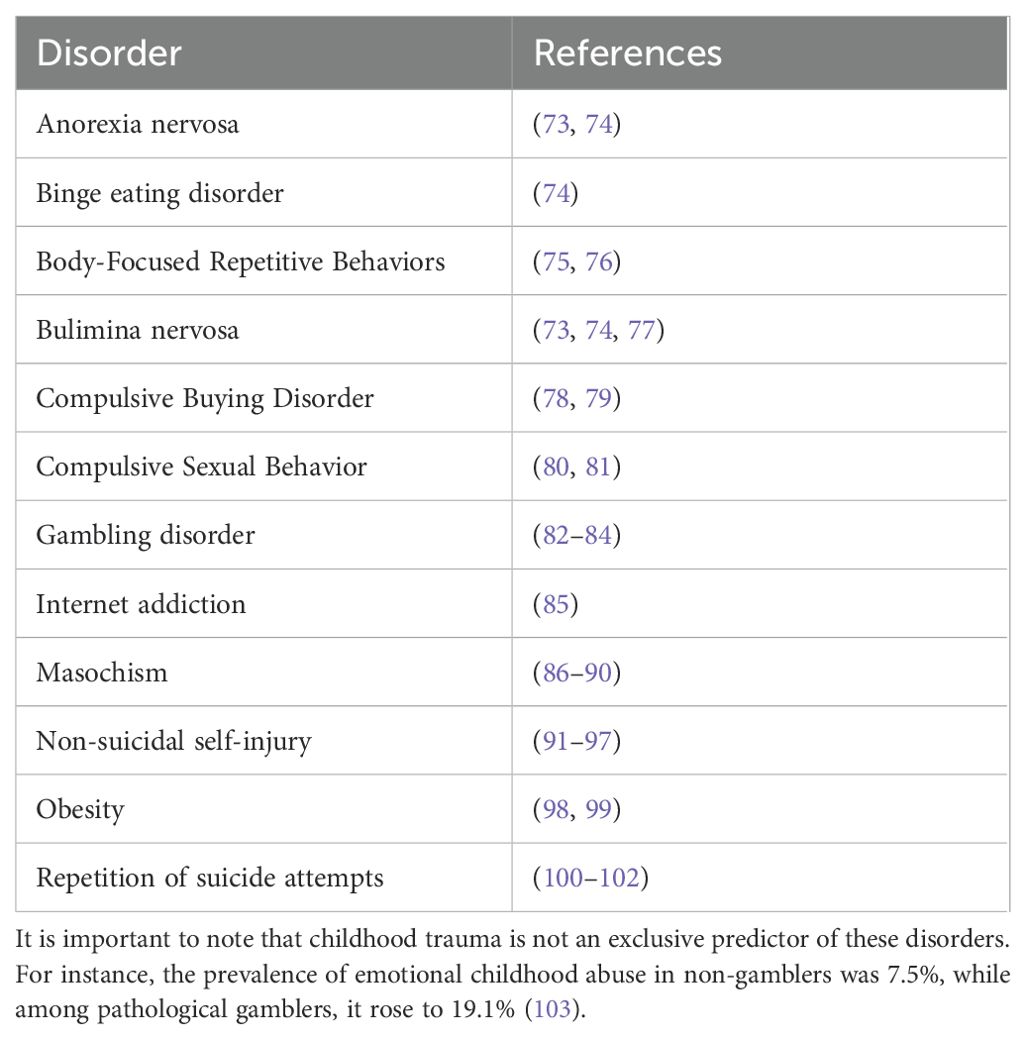
A childhood trauma is characterized as the experience of an extreme stressor that functions as a persistent threat to a child during early life (71). Typical examples include neglect and abuse, the death of a caregiver, exposure to violence, harrowing accidents, life-threatening illnesses, and war (72). Such early life stress can leave individuals at lifelong risk of developing non-substance addictions (Table 3).
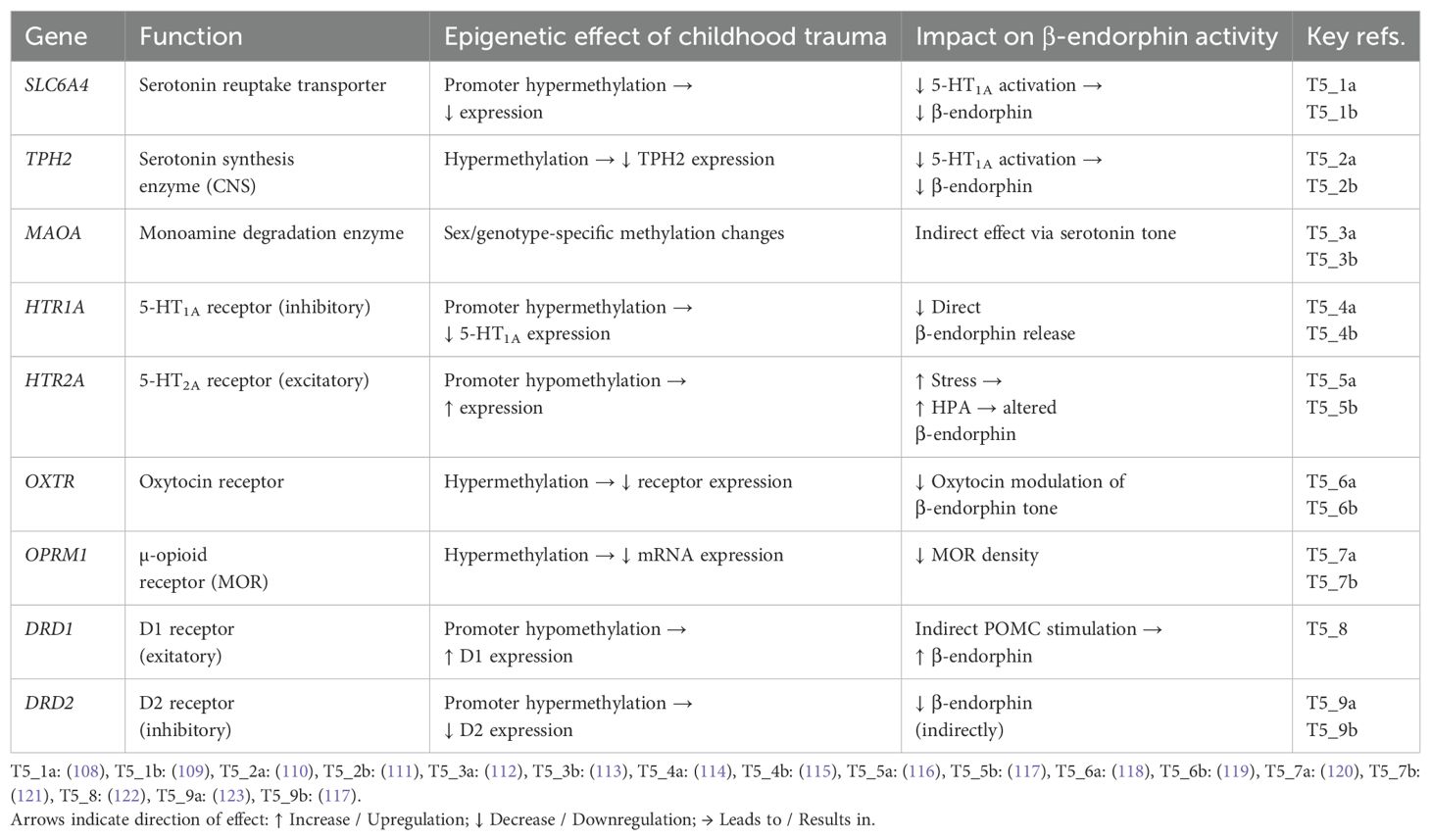
A childhood trauma can activate long-lasting epigenetic mechanisms, sometimes persisting into adulthood (104–106). These epigenetic changes involve modifications in DNA methylation, histone structure, or the levels of various non-coding RNAs (A. 107). Table 4 documented epigenetic changes associated with childhood maltreatment
These epigenetic modifications have two major consequences:
1. Neurodevelopmental and neuroanatomical alterations:
Serotonin contributes to brain development and healthy brain function (124, 125). Childhood trauma decreases serotonin activity leading neuroanatomical changes such as altered brain plasticity, (126–130).
2. Insecure attachment:
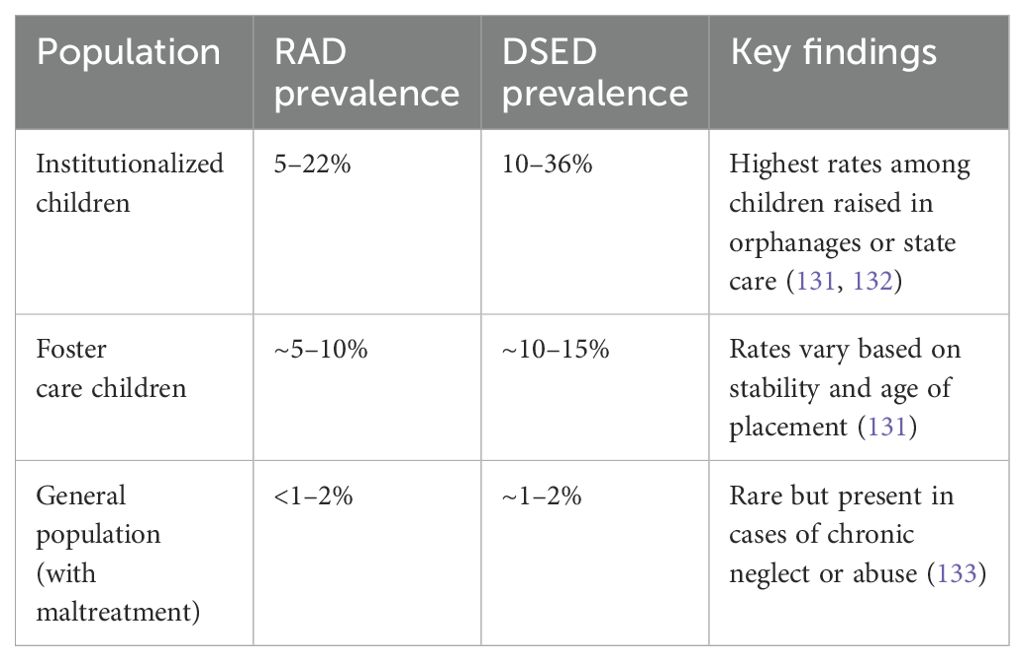
The development of disturbed attachment disorders is strongly associated with the severity and chronicity of early trauma. According to the DSM-5, two primary forms are recognized:
● Reactive Attachment Disorder (RAD): Characterized by emotionally withdrawn behavior toward caregivers, limited social responsiveness, and a lack of seeking comfort.
● Disinhibited Social Engagement Disorder (DSED): Involves indiscriminate sociability, reduced fear of strangers, and overly familiar behavior with unfamiliar adults.
Table 5 summarizes the prevalence and neurobiological correlates of disturbed attachment disorders in relation to childhood trauma.
Given that childhood trauma significantly disrupts the attachment system (as illustrated in Table 5), the model outlined in Scheme 3 (vide supra) becomes particularly relevant.
Notably, the attachment drive appears to be impaired in both gambling disorder (134, 135) and non-suicidal self-injury (136, 137), likely due to the documented epigenetic alterations outlined in Table 4, which may contribute to the persistent reduction in β-endorphin levels (see Table 1, entries 10 and 11).
For both gambling disorder and non-suicidal self-injury, experimental data are available regarding β-endorphin levels throughout the course of the illness (41, 43). These findings provide insight into the purpose of the repetitive, harmful behaviors. The evidence suggests that such behaviors act as surrogates for the malfunctioning attachment drive, as executing the addictive act restores the persistently decreased basal β-endorphin levels to normal (Table 1, Entries 10 and 11).
In conclusion, early-life stress can lead to a persistent decrease in 5-hydroxytryptamine levels through epigenetic modifications. Consequently, beyond impairing brain development, it results in hypoactivity of the attachment drive. These changes disrupt the ability to maintain physiological β-endorphin levels in adulthood, thereby accelerating the onset of an alternative β-endorphin source.
4.3 Substance addiction
There is substantial evidence that a significant proportion - typically as high as 80% to 95% - of individuals seeking treatment for drug use (e.g., alcohol, marijuana, cocaine, amphetamine, heroin) have experienced trauma (138–140). Examination of Table 1 (Entries 15 and 16, vide supra) reveals that basal β-endorphin levels are decreased in pathological drug users.
From the perspective of the up-dated Freudian drive theory, drug use can be interpreted as a surrogate mechanism for the malfunctioning attachment drive. Drugs act as alternative suppliers of β-endorphin, compensating for the deficiency caused by the impaired drive. This prediction is supported by experimental evidence, as summarized in Table 6.
This evidence highlights how various substances can act as β-endorphin modulators, effectively compensating for deficiencies linked to an impaired attachment drive. The addictive behavior thereby serves as a maladaptive coping mechanism, restoring temporarily decreased β-endorphin levels.
5 Causative factors
When one of the five drives outlined in Scheme 4 is disrupted, the physiological β-endorphin level can no longer be maintained. A causative factor can render a drive hypoactive, preventing the balance of physiological β-endorphin levels. While these causative factors are not fully defined, trauma (inclusive epigenetic changes), genetic predisposition, environmental stress, and chronic illnesses are proposed as independent contributors.
5.1 Trauma as causative factor
When trauma, such as childhood adversity or PTSD, serves as the causative factor, it may lead to attachment disorders (Table 5), likely by reducing the activity of key components of the attachment drive (see Scheme 3) through epigenetic modifications (Table 4). This chronic downregulation of the attachment drive prompts patients to seek surrogates to (over)compensate the decreased β-endorphin tone. Alongside coping strategies and non-substance addictions (vide supra), drug use becomes a viable option for these individuals. Research has shown that substance use addicts in treatment frequently exhibit insecure attachment patterns (138, 148).
5.2 Environmental stress as causative factor
Environmental stress is another independent causative factor for drive disruption. Stress is defined as the perception of a threat, real or imagined, to one’s well-being (149). In principle, the activation of various stress systems enhances the organism´s ability to improve its chances for survival (150). When an individual experiences stress, known as acute stress, the sympathetic-adrenal-medullary system is activated, resulting in the release of various catecholamines (151). In addition to the well-known effects of catecholamines – such as increased heart rate and reduced blood flow to digestive systems, kidneys and skin (151) –, the catecholamine noradrenaline suppresses food intake (152). Since β-endorphin is also released in response to stress (153), the underlying mechanism warrants investigation. Notably, fasting triggers β-endorphin release in healthy individuals (Table 1, Entry 6). It is therefore possible that the reduced food intake during acute stress contributes to an increased β-endorphin tone.
However, when acute stress persists for extended periods or its intensity increases, the hypothalamic–pituitary–adrenal (HPA) axis, which regulates numerous functions, including metabolic and immune responses, is activated to manage the stress-response (153, 154). The initial stimulation of this axis leads to β-endorphin release (153). Hyperactivation of the HPA axis is a hallmark of chronic stress and results in the release of various hormones, including corticotropin-releasing hormone (CRH) (151). Under non-chronic stress conditions, β-endorphinergic neurons are rapidly activated by corticotropin-releasing hormone, establishing a negative feedback loop that inhibits further CRH release. However, during chronic stress, this inhibitory feedback is disrupted, resulting in sustained CRH hyperactivity. As a consequence, β-endorphin’s modulatory capacity is diminished, allowing unopposed CRH-driven stress signaling. A hyperactive CRH system can act either as an anorexigenic agent by inhibiting the synthesis of neuropeptide Y (155), or as an orexigenic factor by promoting cortisol release (151).
The sustained release of cortisol impairs the ability to fall asleep and to stay asleep in adults (156) and especially in adolescents (157, 158) resulting in a hypoactive sleep drive under chronic stress (159). The increased vulnerability to addiction during chronic stress (160) may thus serve as a compensatory response to the hypoactivity for either the hunger or sleep drive.
5.3 Genetic predisposition as causative factor
Genetic factors can also persistently alter drive activity. For example, Prader-Willi syndrome (PWS), caused by the absence of paternally expressed imprinted genes in the 15q11.2-q13 region of chromosome 15 (161), is characterized by disrupted sleep drive activity. Excessive daytime sleepiness is reported in nearly all PWS patients (162, 163), and sleep-disordered breathing affects 44% to 100% of patients across studies (164), with one reporting a 95% prevalence based on polysomnography (165). PWS patients likely compensates for the decreased β-endorphin tone caused by a disrupted sleep drive through hyperphagic behavior. Research shows that ghrelin, the hormone governing the hunger drive (Table 7), is persistently elevated in PWS patients (170, 171). The complications arising from resulting obesity are the major causes of morbidity and mortality in this population (161). Notably, the hyperphagic hunger drive, which establishes high β-endorphin levels in PWS patients (Table 1, Entry 5), is often describe as addiction-like behavior (172).

Table 7. Supporting data for Scheme 2.
5.4 Chronic illness as causative factor
The forth independent causative factor for addiction is chronic illness, such as HIV. Chronic HIV infection reduces nutrient intake due to appetite loss (induced by co-occurring depression or systemic inflammation) and oral or gastrointestinal complications (e.g., candidiasis, esophagitis, cryptosporidiosis) (173–175). As a result, 59-84% of HIV-positive individuals experience significant weight loss (175). Likely due to the malfunctioning hunger drive, basal β-endorphin concentrations in HIV-positive patients are reduced to 50% of normal levels in pain-free individuals and 25% in those with chronic widespread pain (Table 1, Entry 17). This low β-endorphin tone may exacerbate the psychological stress and social stigma with HIV, driving individuals to seek satisfaction through addiction. For example, a study of 10,652 HIV-positive individuals reported a 48% prevalence of substance use disorder with specific prevalence rates of 31% for marijuana, 19% for alcohol, 13% for methamphetamine, 11% for cocaine, and 4% for opiates (176). Notably, all of these substances release β-endorphin in the nucleus accumbens (Table 6).
6 Discussion
6.1 Addiction and updated Freudian drive theory
Freud´s drive theory, developed been 1905 and 1933, lacked the benefit of later scientific discoveries, which limited his ability to pinpoint specific physiological underpinnings. For instance, Freud resorted to terms like “motor factor” and “satisfaction” because the concepts of “hormone” and “endorphins” were still in their infancy during his time. This absence of precise scientific terminology may have contributed to the undervaluation of Freudian principles in subsequent theoretical constructs. Notably, Freud´s concept of “satisfaction” as the intrinsic objective of a drive is often overlooked. He proposed that every drive ultimately seeks a uniform “internal aim” — satisfaction (Scheme 1).
Henry (15) suggested that various drives release endorphins. Given the association between β-endorphin and feelings of satisfaction (vide supra), Freud´s construct can be seen as a precursor to understanding the role of β-endorphin´s in motivation. Considering the original purpose of Freudian drive theory — to explain mental disorders — it is relevant to examine the significance of β-endorphin´s in such conditions. A review of Table 1 reveals that many addictive disorders exhibit altered β-endorphin levels.
While Freud´s original drive theory provides invaluable insights, it also present ambiguities when viewed through a modern lens. By integrating findings from Henry (15), Johnson (28), Berridge (18), and Panksepp (21); revisiting the concept of motor factors (54, 56); and introducing β-endorphin as a biochemical equivalent for satisfaction (8, 55), it is now possible to propose an updated Freudian drive theory (illustrated in Scheme 2, Table 7 and Schemes 3, 4).
What, then, is the precise connection between Freudian drives and addiction? Freud stated: “The libido has the task of making the destroying instinct innocuous…” ((177), p. 163). This original statement may confuse some readers, but replacing both “Eros/libido” with the essential drives outlined in Scheme 4 and “death drive or destroying instinct” (masochism being a prime example) with addiction clarifies the concept. Freud´s assertion implies that essential drives can either down-regulate or prevent the onset of addiction. Notably, in laboratory animals, exogenous β-endorphin has been shown to decrease cocaine-seeking behavior (178). Therefore, the regulation Freud suggested might be feasible if both essential drives and addictions share the same termination signal (8). Since all five drives depend on dopamine, which is decreased by β-endorphin release (Schemes 2, 3), addictions must also release β-endorphin to down-regulate addictive impulses for Freud´s speculative statement to hold true. Thus, it can be concluded that the addictive act, regardless of the type of addiction, releases β-endorphin. Evidence supports this conclusion for both non-substance additions (Table 1, Entries 10 and 11) and drug addictions (Table 6).
The downside of such a mechanism, where both essential drives and addictions share the same end-product inhibitor, is that addictive acts may down-regulate essential drives. Indeed, morphine and heroin users show reduced activity in both attachment and sexual drives (179). Similarly, in the sixteenth century, de la Vega observed that cocaine “satisfy the hungry” (180, 181). In line with this, the cocaine´s disturbance of the sexual drive is well documented, manifesting as impotence, gynecomastia, and difficulties achieving orgasm in both genders (182).
On the other hand, when a patient selects a hyperactive hunger drive as a surrogate for a malfunctioning drive, the β-endorphin deficit caused by the hypoactive drive is often overcompensated. This can result in excessively high β-endorphin levels, as observed in Prader-Willi Syndrome patients (Table 1, Entry 5).
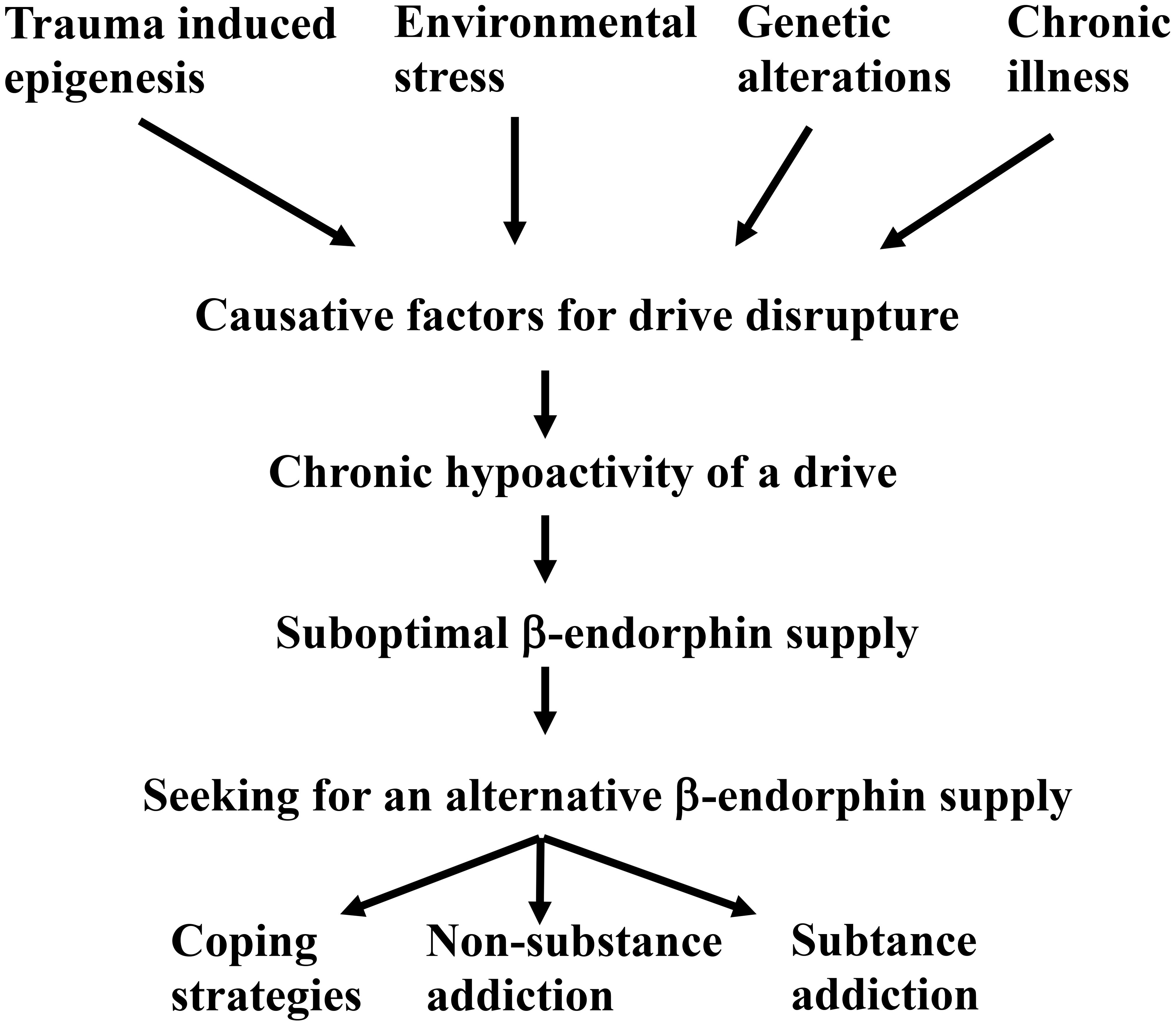
In summary, a causative factor can lead to the malfunctioning of an essential drive (Scheme 5).
As a result of the hypoactive drive, the patient is unable to maintain physiological β-endorphin levels and seeks an addiction – whether coping strategies, non-substance use or substance use addiction - to compensate for the deficit.
Addiction often co-occurs with major psychiatric disorders. Ricci et al. (183, 184) showed that users of synthetic cannabinoids experience more severe psychotic symptoms, higher aberrant salience, dissociation, and suicidal ideation than non-users or natural cannabis users.
These findings highlight the need to both view addiction as a causative factor for other disorders and to critically deconstruct the concept of addiction itself.
6.2 Possible therapy options
From Freud´s (somewhat hidden) assertion that the functioning of all five essential drives counteracts the resilience of addiction (vide supra), an important therapeutic goal for treating addiction should be the revitalization of the disrupted drive. Consistently, the author was unable to identify an instance of addition coexisting with all five drives functioning healthily.
Given that β-endorphin levels are decreased in various addictions (Table 1), addiction could be classified, like other conditions (e.g., diabetes mellitus, hypothyroidism, morbus Parkinson) as a deficiency disease of a signal molecule. For patients with such disorders, hormone replacement therapy is often the treatment of choice. Indeed, laboratory studies have demonstrated that cocaine addiction can be successfully treated with exogenous β-endorphin (178). However, clinical applications of exogenous β-endorphin in psychiatric patients have been limited due to ethical concerns, as β-endorphin, like heroin and morphine, is a μ-receptor agonist (142, 143) and thus carries a potential for addiction. Preliminary investigations into β-endorphin treatment were reported in four bipolar and two unipolar depressive female patients (185). Bipolar disorder with depression is associated with both very low β-endorphin levels (Table 1, Entry 19) and a 48% prevalence of addiction (non-substance and substance misuse) (186). While β-endorphin injections induced a noticeable improvement in depressive psychopathology within 20 - 30 minutes, the effect generally lasted only two hours, with four out of six patients relapsing spontaneously (185). As a result, the high addiction potential and short duration of exogenous β-endorphin´s therapeutic effect prelude its use as a reliable hormone replacement therapy.
Since hormone replacement therapy cannot be effectively implemented, the use of selective serotonin reuptake inhibitors might be considered. Serotonin (5-hydroxytryptamine) plays a role in inducing β-endorphin release (Schemes 2, 3), and studies have shown that fluoxetine significantly increases β-endorphin levels in the arcuate nucleus and nucleus accumbens in laboratory animals (187).
As fluoxetine, like the drugs listed in Table 6, releases β-endorphin in the nucleus accumbens, it may reduce the capacity of these drugs to induce satisfaction, thereby decreasing the patient´s need for their consumption. In fact, a meta-analysis of 64 randomized controlled trials involving 6128 participants demonstrated that fluoxetine facilitated abstinence from opioids, alcohol, cocaine, cannabis, and nicotine while also reducing depressive symptoms (188). However. it should be noted that serotonin acts as a termination signal for the Freudian drives outlined in Scheme 2 and Table 7. Prolonged use of serotonin reuptake inhibitors may suppress these drives, potentially rendering the quality of life for patients. Consistent with these findings, while fluoxetine is approved in the United States for treating depression in children and adolescents (189), there remains uncertainty about whether selective serotonin reuptake inhibitors are associated with an increased risk of suicidal ideation and behavior in individuals under 25 years of age (190). Given these uncertainties, the use of fluoxetine in patients under 25 years old should be considered a treatment of last resort. Since major suicide repeaters exhibit elevated β-endorphin levels (Table 1, Entry 4), and the role of this opioid during such life-threatening acts remains poorly understood, the SSRI-induced increase in synaptic serotonin activity may represent a contraindication in suicidal patients.
Among these, the HTR1A gene — encoding the 5-HT1A receptor — is of particular clinical importance. In psychiatric patients, epigenetic modification of HTR1A (see Table 4) can be identified by increased methylation, which is measurable in leukocyte DNA (114, 115). Such hypermethylation leads to reduced postsynaptic 5-HT1A receptor expression and diminished serotonin-induced β-endorphin release. Consequently, SSRIs, which rely on intact 5-HT1A signaling (see Scheme 3), may show limited therapeutic efficacy in such patients.
From the perspective of the updated Freudian drive theory, it may therefore be clinically prudent to evaluate the epigenetic status of the HTR1A gene before initiating SSRI treatment with agents such as fluoxetine.
In addition, psychological therapies may have the potential to revitalize the disrupted drive (Table 8).
However, many psychoanalysts would agree that persistent non-substance addictions (e.g., masochism, pathological gambling, anorexia nervosa, and particularly bipolar disorders) often show limited responsiveness to individual psychotherapy. Notably, nearly all of these disorders involve the corruption of the attachment drive. Thus, psychotherapeutic interventions should focus on revitalizing the impaired attachment drive. This can be achieved by emphasizing interpersonal orientation and incorporating group psychotherapy into treatment plans.
6.3 Limitations
1. To avoid delving into the labyrinth of biochemical possibilities, only well-analysed signal molecules, such as β-endorphin, were proposed as having key importance in the processing of both essential drives and addictions. While this approach aligns with current knowledge, my conclusions may require revision if presently underexplored metabolites or under-evaluated brain areas are later found to influence the suggested signal-molecules.
2. As noted by an anonymous reviewer (see above), the Bowlbyian drive (attachment) is less well supported by experimental data compared to the other drives. Although I have expanded the connection to experimental findings in the revised version — by introducing relevant receptors and discussing the potential impact of epigenetic changes on both attachment and β-endorphin activity (see Table 4, Scheme 3) — the deconstruction of this drive remains primarily limited to the mother-infant bond from the infant’s perspective. As such, this framework may help explain potential consequences of (early) childhood trauma but does not provide reliable predictions for psychological trauma occurring in adulthood.
3. Although I believe I have identified nearly all relevant studies on the role of β-endorphin in addiction, the number of available studies is limited, and randomized controlled trials are notably scarce. As a result, it can be confidently stated that Freudian drive disruption can induce addiction, but it cannot be definitively concluded that such a disruption is a necessary prerequisite for addiction.
6.4 General conclusion
Give the addicted patient five: five healthy drives!
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
MK: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. I acknowledge support by the Open Access Publication Fund of the University of Duisburg Essen.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ Freud distinguished in his work between drives (Triebe in German) and instincts (Instinke in German). As mentioned in the abstract, both terms were unfortunately translated as “instincts”, introducing unnecessary ambiguities. Recently, the precise distinctions between drives, instincts, and reflexes have been clarified (11).
- ^ For example, the motor factor of the hunger drive, i.e., the hormone ghrelin, was identified 60 years after Freud´s death. (14)
- ^ The term “reward” was introduced by Skinner (19) in psychology and was later applied to rat behaviour (20), whereas Freud´s overlooked yet compatible term, “satisfaction”, is used exclusively for human beings. Since rats cannot experience psychotic illness, the author considers “satisfaction” to be the more appropriate term.
- ^ Referring to the deconstruction of the attachment drive proposed by (55), an anonymous reviewer noted:“Although intriguing, the 'Bowlbyian drive' theory is not as well supported by empirical data as the other drives.” Unfortunately, this observation is valid; however, an updated version of the deconstruction is now possible, presenting key receptors that may be modified through childhood trauma–dependent epigenesis (vide infra).
References
1. Volkow ND, Han B, Einstein EB, and Compton WM. Prevalence of substance use disorders by time since first substance use among young people in the US. JAMA Pediatr. (2021) 175:640–3. doi: 10.1001/jamapediatrics.2020.6981
2. Arterberry BJ, Horbal SR, Buu A, and Lin HC. The effects of alcohol, cannabis, and cigarette use on the initiation, reinitiation and persistence of non-medical use of opioids, sedatives, and tranquilizers in adults. Drug Alcohol Depend. (2016) 159:86–92. doi: 10.1016/j.drugalcdep.2015.1011.1029
3. Ertl N, Freeman TP, Mokrysz C, Ofori S, Borissova A, Petrille K, et al. Acute effects of different types of cannabis on young adult and adolescent resting-state brain networks. Neuropsychopharmacology. (2024) 49:1640–51. doi: 10.1038/s41386-41024-01891-41386
4. Lees B, Meredith LR, Kirkland AE, Bryant BE, and Squeglia LM. Effect of alcohol use on the adolescent brain and behavior. Pharmacol Biochem Behav. (2020) 192:172906. doi: 10.171016/j.pbb.172020.172906
5. Lubman D, Cheetham A, and Yücel M. Cannabis and adolescent brain development. Pharmacol Ther. (2015) 148:1–16. doi: 10.1016/j.pharmthera.2014.1011.1009
6. Aarons GA, Brown SA, Hough RL, Garland AF, and Wood PA. Prevalence of adolescent substance use disorders across five sectors of care. J Am Acad Child Adolesc. Psychiatry. (2001) 40:419–26. doi: 10.1097/00004583-200104000-200100010
7. Freud S. Three essays on the theory of sexuality. In: Strachey J, Ed. & Trans., Freud complete works (Standard Edition, Vol. 7). London: Hogarth Press (1953). p. 1457–552. Available online at: https://www.valas.fr/IMG/pdf/Freud_Complete_Works.pdf. Ivan Smith (2010).
8. Kirsch M, Dimitrijevic A, and Buchholz M. Death drive” scientifically reconsidered: Not a drive but a collection of trauma-induced auto-addictive diseases. Front Psychol. (2022) 13:941328. doi: 10.943389/fpsyg.942022.941328
9. Freud S. “Freud complete works.” In: Strachey J., Ed. & Trans Beyond the pleasure pinciple. Standard Edition, Vol. 18. London: Hogarth Press. (1955) 18:3715–62. Available online at: https://www.valas.fr/IMG/pdf/Freud_Complete_Works.pdf. Ivan Smith (2010).
10. Freud S. New introductory lectures on psycho-analysis Vol. 22. Strachey J, editor. London: S.E (1933).
11. Johnson B, Brand D, Zimmerman E, and Kirsch M. Drive, instinct, reflex—Applications to treatment of anxiety, depressive and addictive disorders. Front Psychol. (2022) 13:870415. doi: 10.3389/fpsyg.2022.870415
12. Freud S. “Instincts and their vicissitudes”. In: Strachey J (Ed.), Freud Complete Works (1915). 7:2955–74. Available online at: https://www.valas.fr/IMG/pdf/Freud_Complete_Works.pdf. Ivan Smith (2010).
13. Starling EH. The Croonian Lectures. I. On the chemical correlation of the functions of the body. Lancet. (1905) 166:339–41. doi: 10.1016/S0140-6736(01)62437-1
14. Kojima M, Hosoda H, Date Y, Nakazato M, and Matsuo H. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. (1999) 402:656–60. doi: 10.1038/45230
15. Henry JL. Circulating opioids: possible physiological roles in central nervous function. Neurosci Biobehav Rev. (1982) 6:229–45. doi: 10.1016/0149-7634(82)90040-9
16. Bodnar RJ and Klein GE. Endogenous opiates and behavior. Peptides. (2005) 27:3391–478. doi: 10.1016/j.peptides.2006.07.011
17. Veening JG and Barendregt HP. The effects of Beta-Endorphin: state change modification. Fluids Barriers CNS. (2015) 12:3. Available online at: http://www.fluidsbarrierscns.com/content/12/11/13 (Accessed January 29, 2015).
18. Berridge KC and Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. (1998) 28:309–69. doi: 10.1016/s0165-0173(1098)00019-00018
19. Skinner BF. The Behavior of Organisms: An Experimental Analysis. 1st ed. New York: D. Appleton-Century Company (1938).
20. Olds J and Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. (1954) 47:419–27. doi: 10.1037/h0058775
21. Panksepp J. Affective neuroscience, The foundation of human and animal emotions. Oxford: Oxford University Press (1998).
22. Panksepp J. At primal levels, vast subcortical brain networks mediate instinctual emotional reactions that hepl program higher-order emotional-cognitive abilities in higher regions of the brain and mind. In: Fox AS, Lapate RC, Shackman AJ, and Davidson AJ, editors. The Nature of Emotion. Fundamental Questions. Oxford University Press, New York (2018). p. 99–104.
24. Solms M and Panksepp J. The “id” knows more than the “ego”admits: neuropsychoanalytic and primal consciousness perspectives on the interface between affective and cognitive neuroscience. Brain Sci. (2012) 2:147–75. doi: 10.3390/brainsci2020147
25. Wright JS and Panksepp J. An evolutionary framework to understand foraging, wanting, and desire: the neuropsychology of the SEEKING system. Neuropsychoanalysis. (2012) 14:5–39. doi: 10.1080/15294145.2012.10773683
26. Anugu V, Ringhisen J, and Johnson B. Case report: Cause and treatment of “High opioid tone” autism. Front Psychol. (2021) 12. doi: 10.3389/fpsyg.2021.657952
27. Jackson D, Singh S, Zhang-James Y, Faraone S, and Johnson B. The effects of low dose naltrexone on opioid induced hyperalgesia and fibromyalgia. Front Psychiatry. (2021) 12. doi: 10.3389/psyt.2021.593842
28. Johnson B. Engineering neurobiological systems: addiction. Psychiatr Clin North Am. (2018) 41:331–9. doi: 10.1016/j.psc.2018.01.011
29. Johnson B and Faraone S. Outpatient detoxification completion and one month outcomes for opioid dependence: A preliminary open label study of a neuropsychoanalytic treatment in pain patients and addicted patients. Neuropsychoanalysis. (2013) 15:145–60. doi: 10.1080/15294145.2013.10799827
30. Johnson B, Ulberg S, Shivale S, Donalson J, Milczarski B, and Faraone SV. Fibromyalgia, autism, and opioid addiction as natural and induced disorders of the endogenous opioid hormonal system. Discov Med. (2014) 18:209–20.
31. Tabi S, Heitner S, Shivale S, Minchenberg S, Faraone S, and Johnson B. Opioid addiction/pregnancy and neonatal abstinence syndrome: a preliminary open-label study of buprenorphine maintenance and drug use targeted psychotherapy (DUST) on cessation of addictive drug use. Front Psychiatry. (2020) 11:563409. doi: 10.3389/fpsyt.2020.563409
32. Donatelli M, Terrizzi C, Russo V, Bucalo ML, Scarpinato A, Verga S, et al. Plasma levels of beta-endorphin in obese subjects with normal glucose tolerance test and in diabetics. Recenti Progressi Medicina. (1991) 82:1–3.
33. Zelissen PM, Koppeschar HP, Erkelens DW, and Thijssen JH. beta-Endorphin and adrenocortical function in obesity. Clin Endocrinol (Oxf). (1991) 35:369–72. doi: 10.1111/j.1365-2265.1991.tb03550.x
34. Tordjman S, Anderson GM, Botbol M, Brailly-Tabard S, Perez-Diaz F, Graignic R, et al. Pain reactivity and plasma b-endorphin in children and adolescents with autistic disorder. PLoS One. (2009) 4:e5289. doi: 10.1371/journal.pone.0005289
35. Urban-Kowalczyk M, Kotlicka-Antczak M, Strzelecki D, Rudecka E, and Smigielski J. The relationship between course of illness and β-endorphin plasma levels in patients with schizophrenia. Neuropsychiatr Dis Treat. (2019) 15:3609–16. doi: 10.2147/NDT.S225321
36. Blasco-Fontecilla H, Herranz-Herrer J, Ponte-Lopez T, Gil-Benito E, Donoso-Navarro E, Hernandez-Aövarez E, et al. Serum β-endorphin levels are associated with addiction to suicidal behavior: A pilot study. Eur Neuropsychopharmacol. (2020) 40:38–51. doi: 10.1016/j.euroneuro.2020.1007.1010
37. Butler MG, Nelson TA, Driscoll DJ, and Manzardo AM. Evaluation of plasma substance p and beta-endorphin levels in children with prader-willi syndrome. J Rare Disord. (2015) 3. Available online at: www.journalofraredisorders.com/pub/IssuePDFs/Final.pdf (Accessed August 25, 2016).
38. Komaki G, Tamai H, Sumioki H, Mori T, Kobayashi N, Mori K, et al. Plasma beta-endorphin during fasting in man. Horm. Res Paediatr. (1993) 33:239–43. doi: 10.1159/000181525
39. Waller DA, Kiser RS, Hardy BW, Fuchs I, Feigenbaum LP, and Uauy R. Eating behavior and plasma beta-endorphin in bulimia. Am J Clin Nutr. (1986) 44:20–3. doi: 10.1093/ajcn/1044.1091.1020
40. Brewerton TD, Lydiard RB, Laraia MT, Shook JE, and Ballenger JC. CSF β-endorphin and dynorphin in bulimia nervosa. Am J Psychiatry. (1992) 149:1086–90. doi: 10.1176/ajp.149.8.1086
41. Blaszcynski AP, Winter SW, and McConaghy N. Plasma endorphin levels in pathological gambling. J Gambl. Behav. (1986) 2:3–14. doi: 10.1007/BF01019930
42. van der Venne P, Balint A, Drews E, Parzer P, Resch F, Koenig J, et al. Pain sensitivity and plasma beta-endorphin in adolescent non-suicidal selfinjury. J Affect. Disord. (2021) 278:199–208. doi: 10.1016/j.jad.2020.1009.1036
43. Störkel LM, Karabatsiakis A, Hepp J, Kolassa I-T, Schmahl C, and Niedtfeld I. Salivary beta-endorphin in nonsuicidal self-injury: an ambulatory assessment study. Neuropsychopharmacology. (2021) 46:1357–63. doi: 10.1038/s41386-41020-00914-41382
44. Hoffmann L, Watson PB, Wilson G, and Montgomery J. Low plasma p-endorphin in posttraumatic stress disorder. Aust N Z. J Psychiatry. (1989) 23:269–73.
45. Bäckryd E, Ghafouri B, Larsson B, and Gerdle B. Do low levels of beta-endorphin in the cerebrospinal fluid indicate defective top-down inhibition in patients with chronic neuropathic pain? A cross-sectional, comparative study. Pain Med. (2014) 15:111–9. doi: 10.1111/pme.12248
46. Kubryak OV, Umriukhin AE, Emeljanova IN, Antipova OS, Guseva AL, Pertsov SS, et al. Increased β-endorphin level in blood plasma as an indicator of positive response to depression treatment. Bull Exp Biol Med. (2012) 153:758–60. doi: 10.1007/s10517-012-1819-0
47. RosChina OV, Levchuk LA, Boiko AS, Michalitskaya EV, Epimakhova EV, Losenkov IS, et al. Beta-endorphin and oxytocin in patients with alcohol use disorder and comorbid depression. J Clin Med. (2021) 10:5696. doi: 10.3390/jcm10235696
48. Kosten TR, Morgan C, and Kreek M-J. Beta endorphin levels during heroin, methadone, buprenorphine, and naloxone challenges: preliminary findings. Biol Psychiatry. (1992) 32:523–8. doi: 10.1016/0006-3223(92)90220-T
49. Ho WKK, Wen HL, and Ling N. Beta-endorphin-like immunoactivity in the plasma of heroin addicts and normal subjects. Neuropharmacology. (1980) 19:117–20. doi: 10.1016/0028-3908(1080)90175-90176
50. Aggarwal S, DeBerry JJ, Ahmad I, Lynn P, Dewitte C, Malik S, et al. Heme attenuates beta-endorphin levels in leukocytes of HIV positive individuals with chronic widespread pain. Redox Biol. (2020) 36:101684. doi: 10.101016/j.redox.102020.101684
51. Kaye WH, Berrettini WH, Gwirtsman HE, Chretien M, Gold PW, George DT, et al. Reduced cerebrospinal fluid levels of immunoreactive proopiomelanocortin related peptides (including beta-endorphin in anorexia nervosa. Life Sci. (1987) 41:2147–55. doi: 10.1016/0024-3205(87)90533-9
52. Demitrack MA, Putnam FW, Rubinow DR, Pigott TA, Altemus M, Krahn DD, et al. Relation of dissociative phenomena to levels of cerebrospinal fluid monoamine metabolites and betaEndorphin in patients with eatingDisorders: A pilot study. Psychiatry Res. (1993) 49:1–10. doi: 10.1016/0165-1781(93)90026-D
53. Escelsior A, Sterlini B, Tardito S, Altosole T, Magnioncalda P, Martino M, et al. Evidence of alterations of Beta-endorphin levels and Mu-opioid receptor gene expression in bipolar disorder. Psychiatry Res. (2022) 316:114787. doi: 10.111016/j.psychres.112022.114787
54. Kirsch M. On the abilities of unconscious freudian motivational drives to evoke conscious emotion. Front.Psychol. (2019) 10:2019.00470. doi: 10.3389/fpsyg.2019.00470
55. Kirsch M and Buchholz MB. On the nature of the mother-infant tie and its interaction with Freudian drives. Front Psychol. (2020) 11:317. doi: 10.3389/fpsyg.2020.00317
56. Kirsch M and Mertens W. On the drive specificity of freudian drives for the generation of SEEKING activities: the importance of the underestimated imperative motor factor. Front Psychol. (2018) 9:616. doi: 10.3389/fpsyg.2018.00616
58. Hawkley LC and Cacioppo JT. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann Behav Med. (2010) 40:218–27. doi: 10.1007/s12160-12010-19210-12168
59. Hormes JM, Kearns B, and Timko CA. Craving chocolate and friendship: social exclusion evokes desire for sweets. Front Psychol. (2012) 3:441. doi: 10.3389/fpsyg.2012.00441
60. Patterson F, Neil-Sztramko S, and Singh G. A pilot study of social isolation and loneliness in post-operative bariatric surgery patients. Obes Surg. (2018) 28:3434–42. doi: 10.1007/s11695-11018-13419-11697
61. Stroud LR, Salovery P, and Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biol Psychiatry. (2002) 52:318–27. doi: 10.1016/s0006-3223(1002)01333-01331
62. Knutson KL and van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y. Acad Sci. (2008) 1129:287–304. doi: 10.1196/annals.1417.1033
63. Spiegel K, Leproult R, and van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. (1999) 354:1435–9. doi: 10.1016/S0140-6736(1499)01376-01378
64. Taheri S. The link between short sleep duration and obesity: we should recommend more sleep to prevent obesity. Arch Dis Child. (2006) 91:881–4. doi: 10.1136/adc.2005.093013
65. Dube SR, Anda RF, Whitfield CL, Brown DW, Felitti VJ, Dong M, et al. Long-term consequences of childhood sexual abuse by gender of victim. Am J Prev Med. (2005) 28:430–8. doi: 10.1016/j.amepre.2005.1001.1015
66. Faden J, Leonard D, O`Reardon J, and Hanson R. Obesity as a defense mechanism. Int J Surg Case Rep. (2013) 4:127–9. doi: 10.1016/j.ijscr.2012.1010.1011
67. Grillo CM and Masheb RM. Childhood psychological, physical, and sexual maltreatment in outpatients with binge eating disorder: frequency and associations with gender, obesity, and eating-related psychopathology. Obes Res. (2001) 9:320–5.
68. Finkelstein JS, Lee H, Burnett-Bowie SA, and Pallais JC. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese boys. J Clin Endocrinol Metab. (2012) 97:182–9. doi: 10.1210/jc.2011-1729
69. Deeks AA, Lombard CB, and Michelmore JF. Teasing, weight concerns and self-efficacy as predictors of dietary restraint in women. J Health Psychol. (2006) 11:551–63. doi: 10.1177/1359105306065049
70. Kolotkin RL, Zunker C, and Ostbye T. Sexual functioning and obesity. review. Obes. (2012) 20:2325–33. doi: 10.1038/oby.2012.104
71. Gladish N, Merrill SM, and Kobor MS. Childhood trauma and epigenetics: state of the science and future. Curr Environ Health Rep. (2022) 9:661–72. doi: 10.1007/s40572-40022-00381-40575
72. Bulut S. Classifcation of posttraumatic stress disorder and its evolution in Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria. Int J Psych. Counsell. (2020) 12:105–8.
73. Monteleone AM, Monteleone P, Serino I, Scognamiglio P, di Genio M, and Maj M. Childhood trauma and cortisol awakening response in symptomatic patients with anorexia nervosa and bulimia nervosa. Int J Eat. Disord. (2014) 48:615–21. doi: 10.1002/eat.22375
74. Rai T, Mainali P, Raza A, Rashid J, and Rutkofsky I. Exploring the link between emotional child abuse and anorexia nervosa: A psychopathological correlation. Cureus. (2019) 11:e5318. doi: 10.7759/cureus.5318
75. Ibiloglu AO, Atli A, Kaya MC, Demir S, and Bulut M. A case of skin picking disorder of a patient with a history of childhood abuse. Noro. Psikiyatr. Ars. (2016) 53:181–3. doi: 10.5152/npa.2015.10110
76. Özten E, Sayar GH, Eryilmaz G, Kagan G, Isik S, and Karamustafalioglu O. The relationship of psychological trauma with trichotillomania and skin picking. Neuropsychiatr Dis Treat. (2015) 11:1203–10. doi: 10.2147/NDT.S79554
77. Rorty M, Yager J, and Rossotto E. Childhood sexual, physical and psychological abuse in bulimia nervosa. Am J Psychiatry. (1994) 151:1122–6. doi: 10.1176/ajp.151.8.1122
78. David J, Kim HS, Hodgins DC, Dawson SJ, Tabri N, Will Shead N, et al. Emotional difficulties mediate the impact of adverse childhood experiences on compulsive buying-shopping problems. J Behav Addic. (2024) 13. doi: 10.1556/2006.2024.00056
79. Sansone RA, Chang J, Jewell B, and Rock R. Childhood trauma and compulsive buying. Int J Psychiatry Clin Pract. (2013) 17:73–6. doi: 10.3109/13651501.13652011.13653379
80. Efrati Y and Gola M. The effect of early life trauma on compulsive sexual behavior among members of a 12-step group. J Sex Med. (2019) 16:803–11. doi: 10.1016/j.jsxm.2019.1003.1272
81. Slavin MN, Scoglio AAJ, Blycker GR, Potenza MN, and Kraus SW. Child sexual abuse and compulsive sexual behavior: A systematic literature review. Curr Addict. Rep. (2020) 7:76–88. doi: 10.1007/s40429-40020-00298-40429
82. Felsher JR, Derevensky JL, and Gupta R. Young adults with gambling problems: The impact of childhood maltreatment. Int J Ment Health Addict. (2010) 8:545–56. doi: 10.1007/s11469-009-9230-4
83. Horak NS, Eagle G, Stein DJ, and Lochner C. Gambling disorder and childhood trauma: A complex association. J Gambl Stud. (2021) 37:515–28. doi: 10.1007/s10899-10020-09983-w
84. Schwaninger PV, Mueller SE, Dittmann R, Poespodihardjo R, Vogel M, Wiesbeck GA, et al. Patients with non-substance-related disorders report a similar profile of childhood trauma experiences compared to heroin-dependent patients. Am J Addict. (2017) 26:215–20. doi: 10.1111/ajad.12512
85. Tang H, Li Y, Dong W, Guo X, Wu S, Chen C, et al. The relationship between childhood trauma and internet addiction in adolescents: A meta-analysis. J Behav Addict. (2024) 13:36–50. doi: 10.1556/2006.2024.00001
86. Blum HP. Masochism and trauma. In: Holtzman D and Kulish N, editors. The clinical problem of masochism. Jason Aronson, Washington (2012). p. 145–59.
87. Gabriel J and Beratis S. Early trauma in the development of masochism and depression. Int Forum Psychoanal. (1997) 6:231–6. doi: 10.1080/08037069708405715
88. Gavin B. No pain, no gain Masochism as a response to early trauma and implications for therapy. Psychodyn. Pract. (2010) 16:183–200.
89. Glenn J. Psychic trauma and masochism. J Am Psychoanal. Assoc. (1984) 32:357–86. doi: 10.1177/000306518403200206
90. van der Kolk BA. The compulsion to repeat the trauma. Re-enactment, revictimization, and masochism. Psychiatr Clin North Am. (1989) 12:389–411. doi: 10.1016/S0193-953X(18)30439-8
91. Arens AM, Gaher RM, and Simons JS. Child maltreatment and deliberate self-harm among college students: testing mediation and moderation models for impulsivity. Am J Orthopsychiatry. (2012) 82:328–37. doi: 10.1111/j.1939-0025.2012.01165.x
92. Auerbach RP, Kim JC, Chango JM, Spiro WJ, Cha C, Gold J, et al. Adolescent nonsuicidal self-injury: examining the role of child abuse, comorbidity, and disinhibition. Psychiatry Res. (2014) 220:579–84. doi: 10.1016/j.psychres.2014.1007.1027
93. Cipriano A, Cella S, and Cotrufo P. Nonsuicidal self-injury: A systematic review. Front Psychol. (2017) 8:1946. doi: 10.3389/fpsyg.2017.01946
94. Gratz KL. Risk factors for deliberate self-harm among female college students: the role and interaction of childhood maltreatment, emotional inexpressivity, and affect intensity/reactivity. Am J Orthopsychiatry. (2006) 76:238–50. doi: 10.1037/0002-9432.1076.1032.1238
95. Paivio SC and McCulloch CR. Alexithymia as a mediator between childhood trauma and self-injurious behaviours. Child Abuse Negl. (2004) 28:339–54. doi: 10.1016/j.chiabu.2003.1011.1018
96. van der Kolk BA, Perry JC, and Herman JL. Childhood origins of selfdestructive behavior. Am J Psychiatry. (1991) 148:1665–71. doi: 10.1176/ajp.1148.1612.1665
97. Wan Y, Chen J, Sun Y, and Tao FB. Impact of childhood abuse on the risk of non-suicidal self-injury in mainland Chinese adolescents. PLoS One. (2015) 10:e0131239. doi: 10.0131371/journal.pone.0013123
98. Bentley T and Widom CS. A 30-year follow-up of the effects of child abuse and neglect on obesity in adulthood. Obesity. (2009) 17:1900–5. doi: 10.1038/oby.2009.160
99. Thomas C, Hypponen E, and Power C. Obesity and type 2 diabetes risk in midadult life: the role of childhood adversity. Pediatrics. (2008) 121:e1240–1249. doi: 10.1542/peds.2007-2403
100. Celik FGH and Hocaoglu C. Implications of childhood trauma on suicidal behavior and deliberate self-harm in patients with major depressive disorder. Psychiatr Danub. (2022) 34:57–63. doi: 10.24869/psyd.22022.24857
101. Mandelli L, Carli V, Roy A, Serretti A, and Sarchiapone M. The influence of childhood trauma on the onset and repetition of suicidal behavior: an investigation in a high risk sample of male prisoners. J Psychiatr Res. (2011) 45:742–7. doi: 10.1016/j.jpsychires.2010.1011.1005
102. Ryan AT, Daruwala SE, Perera KU, Lee-Tauler SY, Tucker J, Grammer G, et al. The relationship between trauma exposure and psychiatric hospitalization for suicide ideation or suicide attempt among patients admitted to a military treatment setting. Int J Environ Res Public Health. (2020) 17:2729. doi: 10.3390/ijerph17082729
103. Shama A and Sacco P. Adverse childhood experiences and gambling: results from a national survey. In: Wiechelt SA and Straussner SL, editors. Examining the Relationship between Trauma and Addiction. Routledge, Abingdon, Oxon UK, New York USA (2016). p. 25–43.
104. Bick J, Naumova O, Hunter S, Barbot B, Lee M, Luthar SS, et al. Childhood adversity and DNA methylation of genes involved in the hypothalamus-pituitary-adrenal axis and immune system: whole-genome and candidate-gene associations. Dev Psychopathol. (2012) 24:1417–25. doi: 10.1017/S0954579412000806
105. Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. (2013) 16:33–41. doi: 10.1038/nn.3275
106. Vaiserman AM and Koliada AK. Early-life adversity and long-term neurobehavioral outcomes: epigenome as a bridge? Hum Genomics. (2017) 11:34. doi: 10.1186/s40246-40017-40129-z
107. Hoffmann A and Spengler D. DNA memories of early social life. Neuroscience. (2014) 264:64–75. doi: 10.1016/j.neuroscience.2012.04.003
108. Beach SRH, Brody GH, Todorov AA, Gunter TD, and Philibert RA. Methylation at SLC6A4 is linked to family history of child abuse: An examination of the Iowa Adoptee sample. Am J Med Genet B Neuropsychiatr Genet. (2010) 153B:710–3. doi: 10.1002/ajmg.b.31028
109. McGowan PO, Sasaki A, D`Alessio AC, Dymov S, Labonte B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. (2009) 12:342–8. doi: 10.1038/nn.2270
110. Murgatroyd C and Spengler D. Epigenetic programming of the HPA axis: early life decides. Stress. (2011) 14:581–9. doi: 10.3109/10253890.10252011.10602146
111. Shen T, Li X, Chen L, Chen Z, Tan T, Hua T, et al. The relationship of tryptophan hydroxylase-2 methylation to early-life stress and its impact on short-term antidepressant treatment response. J Affect. Disord. (2020) 276:850–8. doi: 10.1016/j.jad.2020.1007.1111
112. Checknita D, Bendre M, Ekström TJ, Comasco E, Tiihonen J, Hodgins S, et al. Monoamine oxidase A genotype and methylation moderate the association of maltreatment and aggressive behaviour. Behav Brain Res. (2020) 382:112476. doi: 10.111016/j.bbr.112020.112476
113. Enoch MA, Steer CD, Newman TK, Gibson N, and Goldman D. Early life stress, MAOA, and gene-environment interactions predict behavioral disinhibition in children. Genes Brain Behav. (2010) 9:65–74. doi: 10.1111/j.1601-1183X.2009.00535.x
114. Carrard A, Salzmann A, Malafosse A, and Karege F. Increased DNA methylation status of the serotonin receptor 5HTR1A gene promoter in schizophrenia and bipolar disorder. J Affect. Disord. (2011) 132:450–3. doi: 10.1016/j.jad.2011.1003.1018
115. Fuchikami M, Morinobu S, Segawa M, Okamoto Y, Yamawaki S, Ozaki N, et al. DNA methylation profiles of the brain-derived neurotrophic factor (BDNF) gene as a potent diagnostic biomarker in major depression. PLoS One. (2011) 6:e23881. doi: 10.21371/journal.pone.0023881
116. Frodl T, Reinhold E, Koutsouleris N, Donohoe G, Bondy B, Reiser M, et al. Childhood stress, serotonin transporter gene and brain structures in major depression. Neuropsychopharmacology. (2010) 35:1383–90. doi: 10.1038/npp.2010.1388
117. Labonte B, Suderman M, Maussion G, Navaro L, Yerko V, Mahar I, et al. Genome-wide epigenetic regulation by early-life trauma. Arch Gen Psychiatry. (2012) 69:722–31. doi: 10.1001/archgenpsychiatry.2011.2287
118. Verhallen RJ, Bosten JM, Goodbourn PT, Lawrance-Owen AJ, Bargary G, and Mollon JD. The oxytocin receptor gene (OXTR) and face recognition. Psychol Sci. (2017) 28:47–55. doi: 10.1177/0956797616672269
119. Ziegler C, Dannlowski U, Bräuer D, Stevens S, Laeger I, Wittmann H, et al. Oxytocin receptor gene methylation: converging multilevel evidence for a role in social anxiety. Neuropsychopharmacology. (2015) 40:1528–38. doi: 10.1038/npp.2015.1522
120. Miller JP, Wolf EA, Stiffler SM, Kamens JL, Stitzel JR, Belsky JS, et al. OPRM1 DNA methylation mediates the association between early-life adversity and response to social distress. Dev Psychopathol. (2013) 25:909–21.
121. Nummenmaa L, Karjalainen T, Isojärvi J, Kantonen T, Tuisku J, Kaasinen V, et al. Lowered endogenous mu-opioid receptor availability in subclinical depression and anxiety. Neuropsychopharmacology. (2020) 45:1953–9. doi: 10.1038/s41386-41020-40725-41389
122. Pena CJ, Smith M, Ramakrishnan A, Cates HM, Bagot RC, Kronman HG, et al. Early life stress alters transcriptomic patterning across reward circuitry in male and female mice. Nat Commun. (2019) 10:5098. doi: 10.1038/s41467-41019-13085-41466
123. Groleau P, Joober R, Israel M, Zeramdini N, de Guzman R, and Steiger H. Methylation of the dopamine D2 receptor (DRD2) gene promoter in women with a bulimia-spectrum disorder: Associations with borderline personality disorder and exposure to childhood abuse. J Psychiatr Res. (2014) 48:121–7. doi: 10.1016/j.jpsychires.2013.1010.1003
124. Jiang S, Postovit L, Cattaneo A, Binder EB, and Aitchison KJ. Epigenetic modifications in stressResponse genes associated with childhood trauma. Front Psychiatry. (2019) 10:808. doi: 10.3389/fpsyt.2019.00808
125. Nordquist N and Oreland L. Serotonin, genetic variability, behaviour, and psychiatric disorders–a review. Ups. J Med Sci. (2010) 115:2–10. doi: 10.3109/03009730903573246
126. McCrory EJ, Gerin MI, and Viding E. Annual research review: Childhooh maltreatment, latent vulnerability and the shift to preventative psychiatry - the contribution of functional brain imaging. J Child Psychol Psychiatry. (2017) 58:338–57. doi: 10.1111/jcpp.12713
127. Teicher MH, Samson JA, Anderson CM, and Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. (2014) 17:652–66. doi: 10.1038/nrn.2014.1111
128. Thumfart KM, Jawaid A, Bright K, Flachsmann M, and Mansuy IM. Epigenetics of childhood trauma: Long term sequelae and potential for treatment. Neurosci Biobehav Rev. (2022) 132:1049–66. doi: 10.1016/j.neubiorev.2021.1010.1042
129. Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. (2010) 13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x
130. van Harmelen AL, van Tol MJ, Demenescu LR, van der Wee NJ, Veltman DJ, Aleman A, et al. Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Soc Cogn. Affect. Neurosci. (2010) 6:490–8. doi: 10.1093/scan/nsq1079
131. Seim AR, Jozefiak T, Wichstrom L, Lydersen S, and Kayed NS. Reactive attachment disorder and disinhibited social engagement disorder in adolescence: co-occurring psychopathology and psychosocial problems. Eur Child Adolesc. Psychiatry. (2020) 31:85–98. doi: 10.1007/s00787-00020-01673-00787
132. Zeanah CH, Scheeringa M, Boris NW, Heller SS, Smyke AT, and Trapani J. Reactive attachment disorder in maltreated toddlers. Child Abuse Negl. (2004) 28:877–88. doi: 10.1016/j.chiabu.2004.1001.1010
133. Minnis H, Macmillan S, Pritchett R, Young D, Wallace B, Butcher J, et al. Prevalence of reactive attachment disorder in a deprived population. Br J Psychiatry. (2013) 202:342–6. doi: 10.1192/bjp.bp.1112.114074
134. Magoon ME and Ingersoll GM. Parental modeling, attachment, and supervision as moderators of adolescent gambling. J Gambl. Stud. (2006) 22:1–22. doi: 10.1007/s10899-005-9000-6
135. Terrone G, Gori A, Topino E, Musetti A, Scarinci A, Guccione C, et al. The link between attachment and gambling in adolescence: A multiple mediation analysis with developmental perspective, theory of mind (Friend) and adaptive response. J Pers. Med. (2021) 11:228–39. doi: 10.3390/jpm11030228
136. Aghamohammadi S, Mazaheri MA, Fata L, and Mootabi F. The relationship between nonsuicidal self-injury and attachment: protocol for a systematic review and meta-analysis. JMIR Res Protoc. (2023) 12:e40808. doi: 10.42196/40808
137. Gander M, Fuchs M, Franz N, Jahnke-Majorkovits A-C, Buchheim A, Bock A, et al. Non-suicidal self-injury and attachment trauma in adolescent inpatients with psychiatric disorders. Compr Psychiatry. (2021) 111:152273. doi: 10.151016/j.comppsych.152021.152273
138. Carliner H, Keyes KM, McLaughlin KA, Meyers JL, Dunn EC, and Martins SS. Childhood trauma and illicit drug use in adolescence: A population-based national comorbidity survey replication–adolescent supplement study. J Am Acad Child Adolesc. Psychiatry. (2016) 55:701–8. doi: 10.1016/j.jaac.2016.1005.1010
139. Khoury L, Tang YL, Bradley B, Cubells JF, and Ressler KJ. Substance use, childhood traumatic experience, and Posttraumatic Stress Disorder in an urban civilian population. Depress. Anxiety. (2010) 27:1077–86. doi: 10.1002/da.20751
140. Wolf MR, Nochajski TH, and Farrell HMG. The effects of childhood sexual abuse and other trauma on drug court participants. In: Wiechelt SA and Straussner SL, editors. Examining the Relationship between Trauma and Addiction. Routledge, Abingdon, Oxon UK, New York USA (2016). p. 44–65.
141. Olive MF, Koenig HN, Nannini MA, and Hodge CW. Stimulation of endorphin neurotransmission in thze nucleus accumbens by ethanol, cocaine, and amphetamine. J Neurosci. (2001) 21:RC184. doi: 10.1523/JNEUROSCI.1521-1523-j0002.2001
142. Spanagel R, Herz A, Bals-Kubik R, and Shippenberg TS. Beta-endorphin-induced locomotor stimulation and reinforcement are associated with an increase in dopamine release in the nucleus accumbens. Psychopharmacol (Berl.). (1991) 104:51–6. doi: 10.1007/BF02244553
143. Stinus L, Koob GF, Ling N, Bloom FE, and Le Moal M. Locomotor activation induced by infusion of endorphins into the ventral tegmental area: evidence for opiate-dopamine interactions. Proc Natl Acad Sci USA. (1980) 77:2323–7. doi: 10.1073/pnas.2377.2324.2323
144. Roth-Deri I, Zangen A, Aleli M, Goelman RG, Pelled G, Nakash R, et al. Effect of experimenter-delivered and self-administered cocaine on extracellular beta-endorphin levels in the nucleus accumbens. J Neurochem. (2003) 84:930–8. doi: 10.1046/j.1471-4159.2003.01584.x
145. Marinelli PW, Quirion R, and Gianoulakis C. A microdialysis profile of beta-endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharmacol (Berl.). (2003) 169:60–7. doi: 10.1007/s00213-00003-01490-00212
146. Sim-Selley LJ, Selley DE, Vogt LJ, Childers SR, and Martin TJ. Chronic heroin self-administration desensitizes μ Opioid receptor-activated G-proteins in specific regions of rat brain. J Neurosci. (2000) 20:4555–62. doi: 10.1523/JNEUROSCI.4520-4512-04555.02000
147. Solinas M, Zangen A, Thiriet N, and Goldberg SR. β-Endorphin elevations in the ventral tegmental area regulate the discriminative effects of Δ-9-tetrahydrocannabinol. Eur J Neurosci. (2004) 19:3183–92. doi: 10.1111/j.0953-3816X.2004.03420.x
148. Fairbairn CE, Briley DA, Kang D, Fraley RC, Hankin BL, and Ariss T. A meta-analysis of longitudinal associations between substance use and interpersonal attachment security. Psychol Bull. (2018) 144:532–55. doi: 10.1037/bul0000141
149. Smith SM and Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. (2006) 8:383–95. doi: 10.31887/DCNS.32006.31888.31884/ssmith
150. Chrousos GP and Gold PW. The concepts of stress and stress system disorders. Overview Phys Behav homeostasis. (1992) 267:1244–52. doi: 10.1001/jama.1992.03480090092034
151. Ans AH, Anjum I, Satija V, Inayat A, Asghar Z, Akram Z, et al. Neurohormonal regulation of appetite and its relationship with stress: A mini literature review. Cureus. (2018) 10:e3032. doi: 10.7759/cureus.3032
152. Halford JC. Pharmacology of appetite suppression: implication for the treatment of obesity. Drug Targets. (2001) 2:353–70. doi: 10.2174/1389450013348209
153. Pilozzi A, Carro C, and Huang X. Roles of β-endorphin in stress, behavior, neuroinflammation, and brain energy metabolism. Int J Mol Sci. (2020) 22:338. doi: 10.3390/ijms22010338
154. Pariante CM and Lightman SL. The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. (2008) 31:464–8. doi: 10.1016/j.tins.2008.1006.1006
155. White JD. Neuropeptide Y: a central regulator of energy homeostasis. Regul Pept. (1993) 49:93–107. doi: 10.1016/0167-0115(1093)90431-90437
156. Irwin M, Thompson J, Miller C, Gillin JC, and Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J Clin Endocrinol Metab. (1999) 84:1979–85. doi: 10.1210/jcem.1984.1976.5788
157. Capaldi VF, Handwerger K, Richardson E, and Stroud LR. Associations between sleep and cortisol responses to stress in children and adolescents: a pilot study. Behav Sleep Med. (2005) 3:177–92. doi: 10.1207/s15402010bsm15400304_15402011
158. Kuhlman KR, Chiang JJ, Bower JE, Irwin MR, Seeman TE, McCreath HE, et al. Sleep problems in adolescence are prospectively linked to later depressive symptoms via the cortisol awakening response. Dev Psychopathol. (2020) 32:997–1006. doi: 10.1017/S0954579419000762
159. Kim E-J and Dimsdale JE. The effect of psychosocial stress on sleep: A review of polysomnographic evidence. Behav Sleep Med. (2014) 5:256–78. doi: 10.1080/15402000701557383
160. Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y. Acad Sci. (2008) 1141:105–30. doi: 10.1196/annals.1441.1030
161. Cassidy SB, Schwartz S, Miller JL, and Driscoll DJ. Prader-willi syndrome. Genet Med. (2012) 14:10–26. doi: 10.1038/gim.1030b1013e31822bead31820
162. Ingram DG, Arganbright JM, Paprocki E, and Halpin K. Sleep disorders in children with prader willi syndrome: current perspectives. Nat Sci Sleep. (2022) 14:2065–74. doi: 10.2147/NSS.S361518
163. Vela-Bueno A, Kales A, Soldatos CR, Dobladez-Blanco B, Campos-Castello J, Espino-Hurtado P, et al. Sleep in the prader-willi syndrome. Clinical and polygraphic findings. Arch Neurol. (1984) 41:294–6. doi: 10.1001/archneur.1984.04050150072020
164. Sedky K, Bennett DS, and Pumariega A. Prader willi syndrome and obstructive sleep apnea: co-occurrence in the pediatric population. J Clin Sleep Med. (2014) 10:403–10. doi: 10.5664/jcsm.3616
165. Abushahin A, Al-Naimi A, Abu-Hasan M, Arar R, Hayati ML, Belavendra A, et al. Prevalence of sleep-disordered breathing in prader–willi syndrome. Can Respir J. (2023). doi: 10.1155/2023/9992668
167. Gizowski C and Bourque CW. Neurons that drive and quench thirst. Science. (2017) 357:1092–3. doi: 10.1126/science.aao1095
168. Davidson JM. Activation of the male rat´s sexual behavior by intracerebral implantion of androgen. Endocrinology. (1966) 79:783–94. doi: 10.1210/endo-79-4-783
169. Oishi Y, Huang Z-L, Fredholm BB, Urade Y, and Hayaishi O. Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via A1 receptors and promotes non-rapid eye movement sleep. Proc Natl Acad Sci USA. (2008) 105:19992–7. doi: 10.1073/pnas.0810926105
170. Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS, et al. Elevated plasma ghrelin levels in Prader Wille syndrome. Nat Med. (2002) 8:643–4. doi: 10.1038/nm0702-1643
171. DelParigi A, Tschöp M, Heiman ML, Salbe AD, Vozarova B, Sell SM, et al. High circulating ghrelin: a potential cause for hyperphagia and obesity in prader-willi syndrome. J Clin Endocrinol Metab. (2002) 87:5461–4. doi: 10.1210/jc.2002-020871
172. Salles J, Lacassagne E, Eddiry S, Franchitto N, Salles JP, and Tauber M. What can we learn from PWS and SNORD116 genes about the pathophysiology of addictive disorders? Mol. Psychiatry. (2021) 26:51–9. doi: 10.1038/s41380-41020-00917-x
173. Green CJ. Nutritional support in HIV infection and AIDS. Clin Nutr. (1995) 14:197–212. doi: 10.1016/s0261-5614(1095)80001-80008
174. Roenn JH and Knopf K. Anorexia/Cachexia in patients with HIV: lessons for the oncologist. Oncol (Williston Park). (1996) 10:1062–56.
175. Scevola D, Giglio O, and Scevola S. Treatment of AIDS anorexia-cachexia syndrome and lipodystrophy. In: Cachexia and Wasting: A Modern Approach. Milano: Springer (2006). p. 429–56. doi: 10.1007/1978-1088-1470-0552-1005_1041
176. Hartzler B, Dombrowski JC, Crane HM, Eron JJ, Geng EH, Mathews WC, et al. Prevalence and predictors of substance use disorders among HIV care enrollees in the United States. AIDS Behav. (2017) 21:1138–48. doi: 10.1007/s10461-10016-11584-10466
178. Dikshtein Y, Barnea R, Kronfeld N, Lax E, Rotrh-Deri I, Friedman A, et al. β-endorphin via the delta opioid receptor is a major factor in the incubation of cocaine craving. Neuropsychopharmacology. (2013) 38:2508–14. doi: 10.1038/npp.2013.2155
179. Ringwood T, Cox L, Felldin B, Kirsch M, and Johnson B. Drive and instinct - how they produce relatedness and addiction. Front Psychol. (2021) 12:657944. doi: 10.3389/fpsyg.2021.657944
180. Ersche KD, Stochl J, Woodward JM, and Fletcher PC. The skinny on cocaine: Insight into eating behavior and body weight in cocaine-dependent men. Appetite. (2013) 71:75–80. doi: 10.1016/j.appet.2013.07.011
181. Johanson CE and Fischman MW. The pharmacology of cocaine related to its abuse. Pharmacol Rev. (1989) 41:3–52. doi: 10.1016/S0031-6997(25)00022-5
183. Ricci V, de Berardis D, Martinotti G, and Maina G. Effects of persistent cannabis use on depression, psychosis, and suicidality following cannabis-induced psychosis: A longitudinal study. Am J Addict. (2025). doi: 10.1111/ajad.70048
184. Ricci V, di Muzio I, Ceci F, di Carlo F, Mancusi G, Piro T, et al. Aberrant salience in cannabis-induced psychosis: a comparative study. Front Psychiatry. (2024) 14:1343884. doi: 10.1343389/fpsyt.1342023.1343884
185. Angst J, Autenrieth V, Brem F, Koukkou M, Meyer H, Stassen HH, et al. Preliminary results of treatment with β-endorphin in depression. In: Usdin E, Bunney WE, and Kline NS, editors. Endorphins in Mental Health Research. The MacMillan Press Ltd, London and Basinstoke (1979). p. 518–28.
186. Stokes PRA, Kalk NJ, and Young AH. Bipolar disorder and addictions: The elephant in the room. B J Psych. (2017) 211:132–4. doi: 10.1192/bjp.bp.1116.193912
187. Zangen A, Nakash R, and Yadid G. Serotonin-mediated increases in the extracellular levels of beta-endorphin in the arcuate nucleus and nucleus accumbens: a microdialysis study. J Neurochem. (1999) 73:2569–74. doi: 10.1046/j.1471-4159.1999.0732569.x
188. Fluyaz D, Mitra P, Jain A, Kailasam VK, and Pierre CG. Selective serotonin reuptake inhibitors in the treatment of depression, anxiety, and post-traumatic stress disorder in substance use disorders: a Bayesian meta-analysis. Eur J Clin Pharmacol. (2022) 78:931–42. doi: 10.1007/s00228-00022-03303-00224
189. Korczak DJ, Canadian Paediatric Society, and Mental Health and Developmental Disabilities Committee. Use of selective serotonin reuptake inhibitor medications for the treatment of child and adolescent mental illness. Paediatr Child Health. (2013) 18:487–91. doi: 10.1093/pch/1018.1099.1487
190. Hetrick S, Merry S, McKenzie J, Sindahl P, and Proctor M. Selective serotonin reuptake inhibitors (SSRIs) for depressive disorders in children and adolescents. Cochrane Database Syst Rev. (2007) 18:1465–1858. doi: 10.1002/14651858.CD14004851.pub14651852
191. Carrol KM and Kiluk BD. Cognitive behavioral interventions for alcohol and drug use disorders: Through the stage model and back again. Psychol Addict. Behav. (2017) 31:847–61. doi: 10.1037/adb0000311
192. Magill M and Ray LA. Cognitive-behavioral treatment with adult alcohol and illicit drug users: A meta-analysis of randomized controlled trials. J Stud Alcohol Drugs. (2009) 70:516–27. doi: 10.15288/jsad.12009.15270.15516
193. Lundahl B and Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Psychol. (2009) 65:1232–45. doi: 10.1002/jclp.20638
194. Project_Match_Research_Group. Matching Alcoholism Treatments to Client Heterogeneity: Project MATCH posttreatment drinking outcomes. J Stud Alcohol. (1997) 58:7–29. doi: 10.15288/jsa.11997.15258.15287
195. Larimer ME, Palmer RS, and Marlatt GA. Relapse prevention. An overview of Marlatt’s cognitive-behavioral model. Alcohol Res Health. (1999) 23:151–60.
196. Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, and Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. (2008) 165:179–87. doi: 10.1176/appi.ajp.2007.06111851
197. Meyers RJ, Miller WR, Hill DE, and Tonigan JS. Community reinforcement and family training (CRAFT): engaging unmotivated drug users in treatment. J Subst Abuse. (1998) 10:291–398. doi: 10.1016/s0899-3289(1099)00003-00006
198. A-Tjak JGL, Davis ML, Morina N, Powers MB, Smits JAJ, and Emmelkamp PMG. A meta-analysis of the efficacy of acceptance and commitment therapy for clinically relevant mental and physical health problems. Psychother. Psychosom. (2015) 84:30–6. doi: 10.1159/000365764
199. Garland EL, Hanley AW, Nakamura Y, Barrett JW, Baker AK, Reese SE, et al. Mindfulness-oriented recovery enhancement vs supportive group therapy for co-occurring opioid misuse and chronic pain in primary care: A randomized clinical trial. JAMA Intern Med. (2022) 182:407–17. doi: 10.1001/jamainternmed.2022.0033
Keywords: beta-endorphin, Freudian drive disruption, surrogates mechanisms, insecure attachment, fluoxetine
Citation: Kirsch M (2025) Give me five! A modern perspective on addiction from the up-dated Freudian drive theory. Front. Psychiatry 16:1562619. doi: 10.3389/fpsyt.2025.1562619
Received: 17 January 2025; Accepted: 04 July 2025;
Published: 05 September 2025.
Edited by:
Valerio Ricci, San Luigi Gonzaga University Hospital, ItalyReviewed by:
Domenico De Berardis, ASL 4, ItalyAmjed Ben Haouala, Hospital Fatuma Bourguiba Monastir, Tunisia
Copyright © 2025 Kirsch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Kirsch, bWljaGFlbC5raXJzY2hAdW5pLWR1ZS5kZQ==
 Michael Kirsch
Michael Kirsch