- 1Department of Ultrasound, The First Affiliated Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, China
- 2Department of Anesthesiology, The First Affiliated Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, China
- 3Neuroscience Program, Department of Physiology, Michigan State University, East Lansing, MI, United States
- 4Department of Radiology, Michigan State University, East Lansing, MI, United States
Insomnia, affecting up to 30% of adults (typically 18–65 years), is characterized by GABAergic dysfunction and hyperarousal. This mini-review establishes three pivotal advances in insomnia therapeutics: Firstly, it is demonstrated that microbiota-gut-brain axis (MGBA) dysregulation is mechanistically central to insomnia, directly linking gut dysbiosis to vagal, hypothalamic-pituitary-adrenal (HPA), and γ-aminobutyric acid (GABA) axis dysfunction and neuroinflammation. Secondly, the present study documents the unique multitarget effects of electroacupuncture (EA), which have been shown to simultaneously normalize HPA axis activity, enrich GABA-producing microbiota, improve the vagal tone, and suppress neuroimmune activation. The aforementioned effects collectively resolve insomnia’s multifactorial etiology. Thirdly, clinical evidence confirms the sustained efficacy of EA to be comparable to that of hypnotics, yet with superior safety and durability. EA redefines therapeutic frameworks by integrating biological and neural interventions that are inaccessible to single-target approaches.
1 Introduction
Insomnia is the second most prevalent mental disorder on a global scale (1), affecting up to 30% of adults (typically between the ages of 18 and 65) with severe consequences for public health, occupational functioning, and economic productivity (2–4). Notwithstanding its clinical significance, contemporary therapeutic strategies are encumbered by critical limitations. Hypnotics are associated with the risks of dependency and residual daytime impairment, while cognitive behavioral therapy for insomnia (CBT-I) remains underutilized due to accessibility barriers (1, 5, 6). This unmet need underscores the urgency to elucidate novel pathophysiological mechanisms.
Review evidence primarily from human studies supports the hyperactivation of corticolimbic circuits during both sleep and wake states as a neural substrate of insomnia pathophysiology (7). Concurrently, preclinical research in mice has demonstrated that antibiotic-induced dysbiosis can impact cortical interneuron dendritic morphology (8). Reviews synthesizing this preclinical evidence further suggest that MGBA dysregulation may contribute to insomnia pathophysiology through mechanisms involving GABAergic signaling and HPA axis hyperactivity (9). Neuroimaging studies reveal that insomnia patients exhibit reduced cortical GABA concentrations measured by magnetic resonance spectroscopy (10–12). These observations may be linked to gut microbiota (GM) alterations in chronic insomnia, suggesting GM modulation as a novel therapeutic target to restore GABAergic function in chronic insomnia patients (13). Crucially, gut dysbiosis in these patients depletes GABA-producing bacteria (e.g., Bacteroides, Bifidobacterium) (14), establishing a bidirectional link between microbial ecology and the central nervous system (CNS) hyperarousal. Recent findings have indicated that insomnia patients exhibit impaired peripheral GABAergic inhibition, which is associated with reduced GABAA receptor α1/α2 mRNA expression. Reduced α1 levels have been shown to predict poorer sleep metrics (sleep quality and sleep time), while diminished α2 has been linked to daytime dysfunction (15). Beyond GABAA receptors, research now implicates GABAB and ρ‐containing GABAA (GABAC) receptors in sleep-wake regulation (16).
A recent review suggests traditional Chinese medicine and acupuncture may offer a mechanism-based strategy for treating insomnia guided by the MGBA theory (17). Conventional pharmacotherapies that target specific neurotransmitter systems, electroacupuncture (EA), however, has been shown to modulate the HPA axis, the gut microbiota, and neuroimmune interactions, thereby addressing the multifactorial etiology of insomnia (18–21). This multifaceted regulation of neurochemical, inflammatory, and gut-barrier pathways has the potential to overcome the limitations of current treatments.
2 Pathophysiological basis of insomnia: GABAergic dysregulation and gut-brain crosstalk
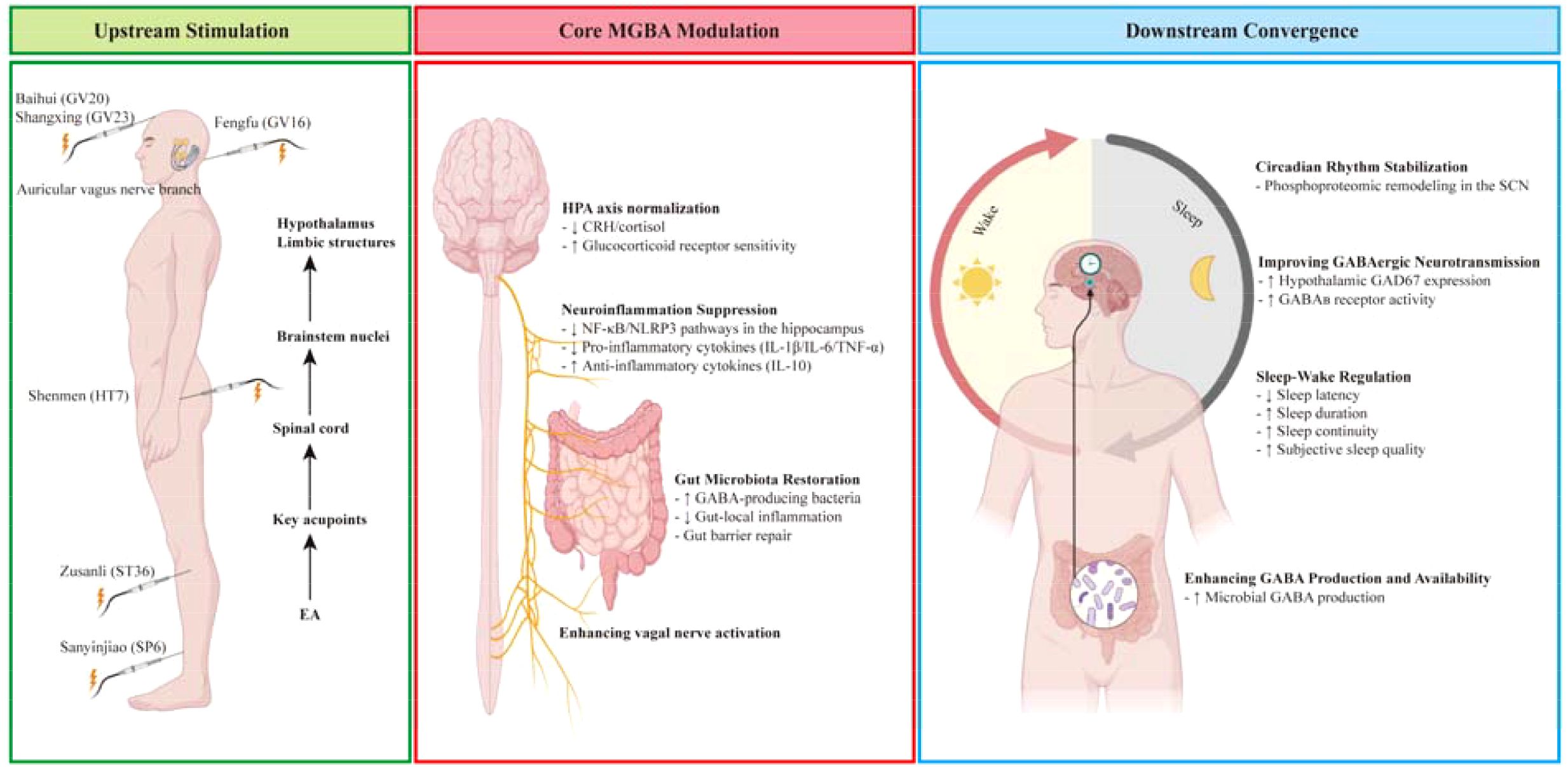
The etiology of insomnia is multifactorial, with the pathophysiology being driven by gut microbial dysbiosis. The MGBA has been identified as a central regulator, exerting its influence via neuroendocrine (HPA axis), neural (vagal signaling), and neuroimmune (microbial metabolite-mediated) pathways (8, 14, 17, 22, 23). As demonstrated in the extant literature, these pathways collectively disrupt central nervous system function and sleep regulation via GABAergic dysfunction, systemic inflammation, and circadian rhythm disruption (24, 25).
2.1 HPA axis dyshomeostasis: a neuroendocrine bridge between stress and sleep disruption
Chronic stress triggers bidirectional gut-brain disturbances involving microbial dysbiosis and intestinal hyperpermeability (26, 27). Based on in vivo findings, commensal microbiota calibrates HPA axis responsiveness in mice, as evidenced by amplified stress reactivity in germ-free models compared to specific pathogen-free and gnotobiotic counterparts (28). Several reviews have confirmed that insomnia pathophysiology involves sleep deprivation-induced sympatho-adrenal activation, driving HPA axis hyperactivity characterized by elevated cortisol, corticotropin-releasing hormone (CRH) hypersecretion, and impaired glucocorticoid receptor feedback (27, 29). This initiates a self-perpetuating cycle: stress-induced intestinal permeability facilitates bacterial translocation, activating TLR4/NF-κB pathways that exacerbate systemic inflammation and HPA axis activation (26).
The influence of gut microbes on the HPA axis is a complex process involving the release of neuroactive metabolites, neurotransmitters, and direct neural stimulation. Experimental evidence in gnotobiotic mice demonstrates that L. rhamnosus (JB-1) modulates HPA axis activity via vagus nerve-mediated pathways, modulating the GABAergic system and reducing stress-induced corticosterone (30). Clinical reviews have identified a further association between gut microbiota-derived neuroactive metabolites and HPA regulation across neurological disorders, highlighting vagal neurotransmission as a conserved mechanism in mammals (31). The influence of short-chain fatty acids (SCFAs) on clock gene expression and sleep patterns suggests a potential role for gut microbiota in propagating the circadian rhythm at the molecular level (32). As demonstrated by preclinical studies, probiotic-treated animals exhibit an attenuated HPA axis response to stress, as indicated by reduced corticosterone elevation (26). In contrast, a double-blind, randomized controlled trial (RCT) in humans undergoing academic stress revealed that Lactobacillus casei strain Shirota (LcS) maintained sleep quality, suggesting the potential for microbiota-targeted interventions to have clinical relevance (33). As demonstrated in the relevant literature, probiotics have been shown to reverse host metabolic alterations or modulate immune responses associated with gut dysbiosis (Bifidobacterium in human irritable bowel syndrome; Lactobacillus/Bifidobacterium in maternal separation of rats) (34–36). It is noteworthy that their capacity to modulate corticosterone secretion and reverse HPA axis dysfunction in rodents furnishes a mechanistic framework for investigating the potential of probiotic interventions in the context of insomnia (9, 36).
2.2 Vagus nerve mediation in insomnia pathophysiology
The vagus nerve serves as the primary neural conduit for gut-brain communication in insomnia, with 90% of fibers transmitting afferent gut-derived signals—including microbial GABA, serotonin, and norepinephrine—to the CNS (14, 22, 23). The vagal functional mechanism was initiated by the activation and regulation of the HPA axis, which resulted in the generation of CRH. This hormone plays a pivotal role in coordinating the organism’s adaptive stress reaction and maintaining physiological homeostasis (37). Experimental evidence demonstrated that probiotics like Lactobacillus and Bifidobacterium modulate CNS function via vagus nerve-dependent GABA signaling and systemic neuroactive metabolite circulation (9, 30). Simultaneously, oral Lactobacillus administration upregulates GABA receptors in sleep-related brain regions (prefrontal cortex, hypothalamus). This phenomenon is counteracted by vagotomy, as evidenced by the findings of the study (30). Vagal neurotransmission has been demonstrated to enhance emotional regulation through GABAergic inhibition of amygdala CRH neurons, thereby establishing a link between microbial modulation and insomnia-related anxiety (9).
2.3 Microbial metabolite crosstalk: neuroimmune axis regulation in insomnia pathogenesis
Gut microbiota orchestrates insomnia through neuroactive metabolite production, immune modulation, and neurotransmitter regulation. The mediation of sleep-wake homeostasis by microbial GABA, serotonin, and SCFAs occurs via bidirectional gut-brain communication (32, 38, 39).
GABA, the predominant inhibitory neurotransmitter, has been demonstrated to modulate thalamocortical synchronization and hypothalamic sleep-promoting circuits (8). Beyond the suppression of neuronal activity, GABA exerts anxiolytic, autonomic-stabilizing, and neuroprotective effects that are critical for the maintenance of sleep. A clinical study has demonstrated that the ingestion of fermented rice germ extract containing GABA has a positive effect on sleep latency and subjective sleep quality in patients suffering from insomnia (40). From a mechanistic perspective, the ingestion of GABA-rich fermented milk has been demonstrated to reduce sleep latency and prolong sleep duration in sub-threshold sodium pentobarbital dose-induced sleep experiments utilizing ICB mice, in comparison to control groups (41). This finding validates conserved physiological pathways. Additionally, GABAergic dysregulation may mediate the bidirectional link between Parkinson’s disease pathogenesis and comorbid sleep disorders (16). In recent neuroimaging studies, the concentrations of glutamate and GABA (in addition to the density and activity of neurotransmitter receptors and transporters) have been associated with mood disorders (13). Neuroimaging reveals insomnia severity correlates with reduced cortical GABA concentrations (measured via magnetic resonance spectroscopy) rather than peripheral levels (16, 41, 42), emphasizing central GABAergic tone’s clinical relevance.
Dysbiosis-induced GABA depletion has been demonstrated to trigger glutamatergic excitotoxicity and neuroinflammation, which are recognized as key drivers of sleep-wake dysregulation (3, 43). The regulation of GABA metabolism by gut microbiota is achieved through the synthesis of GABA by Lactobacillus and Bifidobacterium via glutamate decarboxylase (14, 44) and GABA utilization by Bacteroides and Parabacteroides as a nitrogen source, creating bidirectional host-microbe metabolic crosstalk (45). GABA receptor agonists and uptake inhibitors have been demonstrated to effectively regulate sleep (16, 46), while GABA supplementation is promising for both sleep initiation and maintenance (40, 41, 46, 47). It is hypothesized that these effects may involve interactions with SCFAs, such as butyrate, which are critical gut-brain mediators (38, 41, 48). While exogenous GABA’s BBB permeability remains debated, it exerts indirect neural effects via gut pathways, including modulating microbiota and stimulating GABAB receptors expressing on intestinal/vagal afferents (41). Given these multifaceted interactions, therapeutic strategies targeting GABAergic-microbiome interactions hold promise for insomnia treatment (39), warranting further mechanistic studies
3 EA’s possible multimodal regulation of GABAergic MGBA in insomnia and coexisting diseases therapeutics
EA modulates the MGBA to address central GABAergic dysfunction and gut dysbiosis (18, 19, 48, 49), thereby demonstrating superior anti-insomnia effects compared to conventional therapies.
HPA Axis Modulation: The anti-insomnia effects of EA appear particularly robust in regulating HPA axis hyperactivity, a well-established feature of insomnia pathophysiology (50). By modulating HPA axis hyperactivity, enhancing vagal tone, and restoring microbial production of sleep-regulating metabolites, EA targets the multifactorial etiology of insomnia. In a mouse model of depression, EA synergizes electrical stimulation with acupuncture to uniquely regulate central GABAergic neurotransmission and gut microbial ecology, specifically modulating the abundance of Lactobacillus and staphylococci (19). In SPF Sprague-Dawley rats under cage-change-induced insomnia, EA at Ganshu (BL18) and Zusanli (ST36) reduced wakefulness and increased non-rapid eye movement (NREM) sleep. Mechanistically, EA regulated hypothalamic dopamine (DA) and DA receptors (D1R/D2R) within the HPA axis, counteracting stress-induced neurotransmitter alterations to normalize sleep-wake cycles (50). In addition, in the context of the maternal separation rats model, the efficacy of acupuncture in reducing corticosterone (CORT) and ACTH levels in plasma, as well as the hypothalamic immunoreactivity (IR) of arginine vasopressin (AVP) in the hypothalamic paraventricular nucleus, has been well-documented (51, 52).
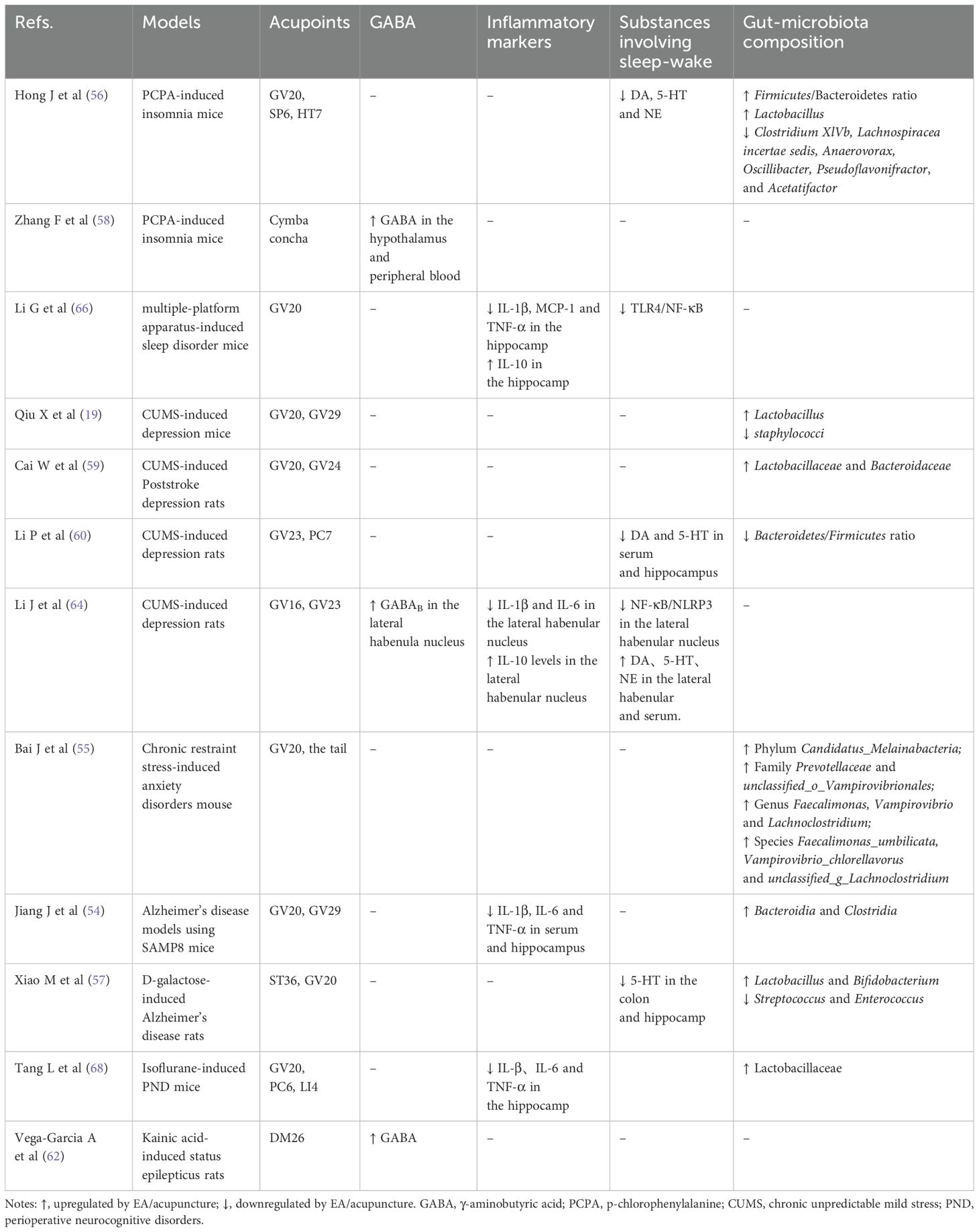
Gut Microbial Regulation: Under physiological and pathological conditions, gut microorganisms can influence the functions and behaviors of the brain through bidirectional regulation of various immune, endocrine, and vagus nerve pathways via the gut-brain axis (53). EA inhibits peripheral inflammation by balancing gut microbiota, the attenuating hippocampal neuroinflammation (54) (Table 1). In chronic restraint stress-induced anxiety disorders mouse, EA at Baihui (GV20) partially alleviated anxiety-like behavior and mitigated gut microbiome dysbiosis (55). In C57BL/6 male mice with p-chlorophenylalanine (PCPA)-induced insomnia, acupuncture at Baihui (GV20), Sanyinjiao (SP6), and Shenmen (HT7) ameliorated sleep disturbances through regulating the gut flora to modulate the host immune response (56). In addition, longitudinal metagenomic analyses demonstrate that a standardized 8-week EA regimen induces significant ecological shifts in D-galactose-induced Alzheimer’s disease (AD) model rats, marked by significant enrichment of GABA-producing Lactobacillus and Bifidobacterium alongside depletion of Streptococcus and Enterococcus (57). These microbial changes have been shown to correlate with measurable neurochemical alterations, including elevated GABA and glutamate levels in the hypothalamus and peripheral blood (58). It is noteworthy that EA has been observed to alleviate symptoms of depression-like behaviors by regulating Lactobacillaceae and Bacteroidaceae (59, 60), a finding that aligns with the insomnia model. However, while these mechanisms have been established in models of central nervous system disorders, rigorous validation in insomnia-specific paradigms remains essential.
Neural Mechanisms: The neural mechanisms underlying EA’s effects involve multiple complementary pathways that enhance central GABAergic tone. The modulation of sleep patterns is contingent upon the activity of discrete populations of GABAergic neurons. It has been demonstrated that elevations in GABA levels facilitate both sleep initiation and maintenance (61). This is achieved through EA-induced stimulation of the auricular vagus nerve branch of PCPA-induced insomnia models in mice, which increases hypothalamic and peripheral blood GABA concentrations (58). The hypothesis that EA stimulates hypothalamic GABAergic neurons has been posited. These neurons have been shown to inhibit hyperactive neural circuits in the limbic system and prefrontal cortex. Presynaptically, in status epilepticus models induced by kainic acid in Sprague-Dawley rats, EA has been shown to enhance GABA synthesis capacity by upregulating glutamic acid decarboxylase (GAD67) expression (62). Notably, by modulating the release of neurotransmitters like serotonin (5-HT), DA, and norepinephrine (NE), GABAB receptors thereby influence essential neural mechanisms encompassing synaptic transmission, plasticity, precursor cell proliferation, and survival pathways in neurons (63). Postsynaptically, in models of chronic unpredictable mild stress-induced depression in Sprague-Dawley rats, EA at Shangxing (GV23) and Fengfu (GV16) elevated GABAB receptor expression, promoting synaptic plasticity while suppressing NF-κB/NLRP3-driven neuroinflammation (64). In addition, EA exerts its neuroprotective effects primarily by suppressing inflammation in the hippocampus. IL-1β has been demonstrated to potentiate GABAergic neurotransmission in a bidirectional manner, with the capacity to enhance presynaptic GABA release in preoptic/anterior hypothalamic neurons and to amplify postsynaptic GABA responses across a range of experimental models (65).
Cytokines or Inflammatory Markers: EA has been demonstrated to attenuate sleep deprivation-induced upregulation of pro-inflammatory cytokines (IL-1β, MCP-1, TNF-α) while elevating anti-inflammatory IL-10 expression (66). Similarly, in models of acute colitis, EA intervention has been observed to induce parallel cytokine modulation in plasma (67). Concurrently, EA reduces circulating pro-inflammatory cytokines (such as IL-6 and TNF-α), creating an optimal microenvironment for GABAergic neurotransmission (68, 69). Integrated cytokine or inflammatory markers effects converge on wakefulness modulation through three principal aspects: spatially via vigilance-regulating brain regions (hypothalamus, basal forebrain, brainstem) where IL-1β alters neuronal discharge and IL-1β/TNF-α exhibit diurnal rhythms (65, 70–72); neurally through vagus nerve signaling where peripheral IL-1β binds paraganglia receptors projecting to brainstem solitary nucleus (73); and molecularly via biochemical cascades involving adenosine/NF-κB/PGD2, neurotransmitters (GABA/glutamate/NE), and somnogenic hormones (GHRH/CRH) (65). It is hypothesized that pro-inflammatory cytokines may also exert some of their sleep-modulating effects via GHRH (65).
Circadian Rhythm: It has been demonstrated that glutamatergic and GABAergic synapses exhibit significant molecular enrichment with regard to the regulation of the sleep-wake cycle. The regulation of cortical arousal and wakefulness is primarily governed by dual neurochemical systems. These systems consist of brainstem monoaminergic cell groups (noradrenergic cells, serotonergic cells, histaminergic neurons, and dopaminergic neurons) and basal forebrain (BF) neurons. The latter are predominantly cholinergic and GABAergic subtypes (65). The basal forebrain functions as a pivotal relay station where hypocretin (Hcrt) neurons from the lateral hypothalamus regulate arousal states (74), and the absence of Hcrt neurotransmission leads to frequent transitions between wakeful and sleep states (75). Acupuncture modulates sleep-wake regulation through convergent main areas of the brain’s structures as well as balancing wake-promoting neurotransmitters (NE, serotonin, histamine, dopamine, acetylcholine) and sleep-promoting substances (GABA, opioids) through comprehensive coordination of multiple targets, levels, links, and pathways (76, 77). Furthermore, the most significant enriched phosphor-proteins and phosphosites are involved in post-synapse and glutamatergic synapses. EA has been demonstrated to induce circadian resynchronization through phosphoproteomic remodeling in the suprachiasmatic nucleus (SCN), with phosphorylation events serving as the primary regulatory mechanism. The sleep-wake cycle is subject to modulation by glutamatergic and GABAergic synaptic pathways, which adjust the levels of glutamate and GABA within the SCN (78).
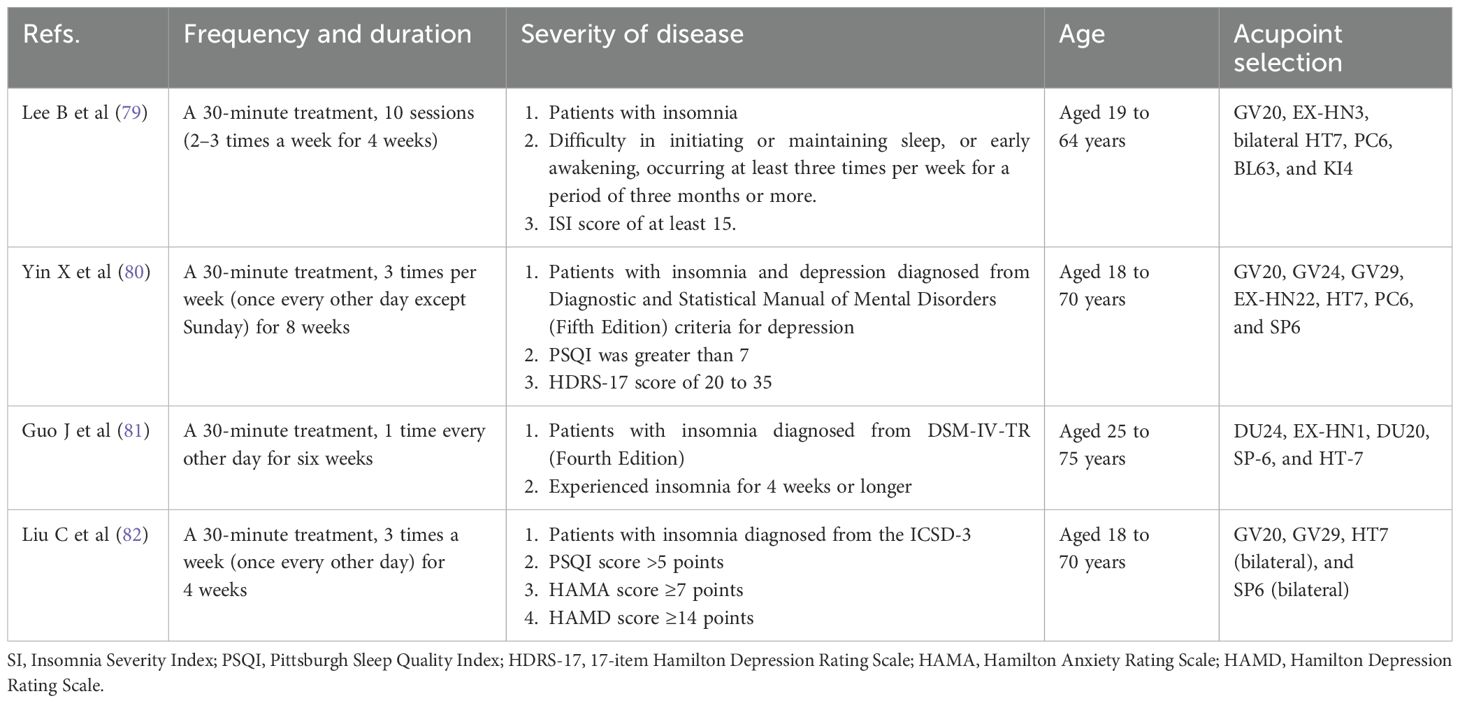
Clinical Evidence: Randomized controlled trials (RCTs) provide compelling clinical evidence for these mechanisms (Table 2). In a multicenter RCT, EA significantly reduced Insomnia Severity Index (ISI) scores compared to sham-EA and usual care, with effects sustained at 8-week and 12-week follow-ups (79). In a multicenter study of 270 patients with comorbid insomnia and depression, EA with standard care outperforms sham acupuncture with standard care and standard care alone in sleep quality (80). In a double-dummy, single-blinded RCT involving patients with primary insomnia, six-week acupuncture treatment demonstrated significantly greater improvements in sleep quality, total sleep time, sleep efficiency, and daytime functioning compared to sham acupuncture, effectively facilitating the reestablishment of normal sleep-wake cycles (81). In a single-blind RCT, patients diagnosed with chronic insomnia were administered acupuncture at Baihui (GV20), Yintang (GV29), bilateral Shenmen (HT7), and bilateral Sanyinjiao (SP6). The intervention was administered thrice weekly (every other day) for four weeks, and it was found to have a significant effect on the quality, efficiency, and latency of sleep (82). In addition, a systematic review and meta-analysis also demonstrated EA’s efficacy in managing cancer-related insomnia, evidenced by significant increases in total sleep duration and reductions in sleep disruptions (83). Although multicenter RCTs validate EA’s clinical efficacy, reproducibility remains limited by heterogeneous stimulation parameters and non-standardized acupoint selection protocols varied across studies.
4 Future perspectives
It is recommended that subsequent studies examine EA’s parameters in greater detail, with particular attention to stimulation intensity, frequency, duration, and repetition rate, to optimize intervention efficacy. Multi-omics integration (metagenomic/metabolomic/proteomic) to decode strain-specific microbial-GABA interactions and their neurocircuitry impacts should be considered, particularly in aging populations with metabolic dysfunction. Our recently published study protocol in Frontiers in Neurology (84) has provided a methodological foundation for such studies. Finally, it is necessary to explore synergistic interventions combining EA with probiotics to potentiate MGBA modulation and amplify therapeutic outcomes.
5 Conclusion
EA presents a transformative non-pharmacological intervention for insomnia management, offering superior safety through multilevel MGBA modulation. This modulation simultaneously normalizes HPA axis activity, enriches GABA-producing microbiota, enhances vagal tone, and suppresses neuroinflammation.
Author contributions
XW: Conceptualization, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing. LY: Conceptualization, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing, Funding acquisition. XZ: Conceptualization, Supervision, Writing – review & editing. XL: Conceptualization, Methodology, Project administration, Writing – review & editing. BY: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work is supported by the Natural Science Foundation of Xiamen, China (grant number: 3502Z20227345), Natural Science Foundation of Fujian Province (grant number:2022J011369), Xiamen Medical and Health Guiding Project (grant number:3502Z20224ZD1023), Fujian Research and Training Grants for Young and Middle-aged Leaders in Healthcare (B. Yang), and Scientific Research Special Grant Fund Project of Wu Jieping Medical Foundation (grant number: 320.6750.2024-05-50).
Acknowledgments
We thank BioRender.com (https://BioRender.com) for providing the graphical elements used in the Graphic Abstract.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Van Someren EJW. Brain mechanisms of insomnia: new perspectives on causes and consequences. Physiol Rev. (2021) 101:995–1046. doi: 10.1152/physrev.00046.2019
2. The L. Waking up to the importance of sleep. Lancet. (2022) 400:973. doi: 10.1016/S0140-6736(22)01774-3
3. Kang Y, Kang X, and Cai Y. The gut microbiome as a target for adjuvant therapy in insomnia disorder. Clin Res Hepatol Gastroenterol. (2022) 46:101834. doi: 10.1016/j.clinre.2021.101834
4. Souza Lopes CD, Rodrigues J, and Rotenberg L. Epidemiology of insomnia: prevalence and risk factors. In: Sahoo S, editor. Can’t Sleep? Issues of Being an Insomniac. IntechOpen, Rijeka (2012).
5. Henson J, Covenant A, Hall AP, Herring L, Rowlands AV, Yates T, et al. Waking up to the importance of sleep in type 2 diabetes management: A narrative review. Diabetes Care. (2024) 47:331–43. doi: 10.2337/dci23-0037
6. Li C, Chen S, Wang Y, and Su Q. Microbiome-based therapeutics for insomnia. Int J Mol Sci. (2024) 25:13208. doi: 10.3390/ijms252313208
7. Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. (2010) 14:19–31. doi: 10.1016/j.smrv.2009.04.002
8. Nakhal MM, Mydeen AB, Yassin LK, Almazrouei R, Alkamali R, Alsulaimi M, et al. Antibiotics-induced dysbiosis impacts dendritic morphology of adult mouse cortical interneurons. Front Neuroanat. (2025) 19:1557961. doi: 10.3389/fnana.2025.1557961
9. Tette FM, Kwofie SK, and Wilson MD. Therapeutic anti-depressant potential of microbial GABA produced by lactobacillus rhamnosus strains for GABAergic signaling restoration and inhibition of addiction-induced HPA axis hyperactivity. Curr Issues Mol Biol. (2022) 44:1434–51. doi: 10.3390/cimb44040096
10. Spiegelhalder K, Regen W, Baglioni C, Riemann D, and Winkelman JW. Neuroimaging studies in insomnia. Curr Psychiatry Rep. (2013) 15:405. doi: 10.1007/s11920-013-0405-0
11. Morgan PT, Pace-Schott EF, Mason GF, Forselius E, Fasula M, Valentine GW, et al. Cortical GABA levels in primary insomnia. Sleep. (2012) 35:807–14. doi: 10.5665/sleep.1880
12. Kay DB and Buysse DJ. Hyperarousal and beyond: new insights to the pathophysiology of insomnia disorder through functional neuroimaging studies. Brain Sci. (2017) 7:23. doi: 10.3390/brainsci7030023
13. Feng Y, Fu S, Li C, Ma X, Wu Y, Chen F, et al. Interaction of gut microbiota and brain function in patients with chronic insomnia: A regional homogeneity study. Front Neurosci. (2021) 15:804843. doi: 10.3389/fnins.2021.804843
14. Wang Z, Wang Z, Lu T, Chen W, Yan W, Yuan K, et al. The microbiota-gut-brain axis in sleep disorders. Sleep Med Rev. (2022) 65:101691. doi: 10.1016/j.smrv.2022.101691
15. Xiang T, Liao J, Cai Y, Fan M, Li C, Zhang X, et al. Impairment of GABA inhibition in insomnia disorders: Evidence from the peripheral blood system. Front Psychiatry. (2023) 14:1134434. doi: 10.3389/fpsyt.2023.1134434
16. Al-Kuraishy HM, Al-Gareeb AI, Albuhadily AK, Elewa YHA, Al-Farga A, Aqlan F, et al. Sleep disorders cause Parkinson’s disease or the reverse is true: Good GABA good night. CNS Neurosci Ther. (2024) 30:e14521. doi: 10.1111/cns.14521
17. Pan LM, Hong ZB, and Guan RQ. Research progress on insomnia treated by traditional Chinese medicine and acupuncture based on microbial-gut-brain axis theory. World J Clin Cases. (2024) 12:3314–20. doi: 10.12998/wjcc.v12.i18.3314
18. Zhao FY, Spencer SJ, Kennedy GA, Zheng Z, Conduit R, Zhang WJ, et al. Acupuncture for primary insomnia: Effectiveness, safety, mechanisms and recommendations for clinical practice. Sleep Med Rev. (2024) 74:101892. doi: 10.1016/j.smrv.2023.101892
19. Qiu X, Li Z, Huang S, Cai X, Qu S, Zheng Z, et al. Electroacupuncture improves depression-like behavior by regulating the abundance of lactobacillus and staphylococci in mice. J Integr Neurosci. (2023) 22:28. doi: 10.31083/j.jin2202028
20. Zhang H, Rong H, Xiong S, Chen X, and Lin Z. Intervention effect of electroacupuncture therapy on drug reducing and discontinuation of insomnia patients with long-term excessive use of zolpidem. Explore (NY). (2024) 20:328–33. doi: 10.1016/j.explore.2023.09.003
21. Zheng JY, Zhu J, Wang Y, and Tian ZZ. Effects of acupuncture on hypothalamic-pituitary-adrenal axis: Current status and future perspectives. J Integr Med. (2024) 22:445–58. doi: 10.1016/j.joim.2024.06.004
22. Feng W, Yang Z, Liu Y, Chen R, Song Z, Pan G, et al. Gut microbiota: A new target of traditional Chinese medicine for insomnia. BioMed Pharmacother. (2023) 160:114344. doi: 10.1016/j.biopha.2023.114344
23. Bonaz B, Bazin T, and Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. (2018) 12:49. doi: 10.3389/fnins.2018.00049
24. Li Y, Hao Y, Fan F, and Zhang B. The role of microbiome in insomnia, circadian disturbance and depression. Front Psychiatry. (2018) 9:669. doi: 10.3389/fpsyt.2018.00669
25. Dos Santos A and Galie S. The microbiota-gut-brain axis in metabolic syndrome and sleep disorders: A systematic review. Nutrients. (2024) 16:390. doi: 10.3390/nu16030390
26. Dinan TG and Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. (2012) 37:1369–78. doi: 10.1016/j.psyneuen.2012.03.007
27. Reynolds AC, Paterson JL, Ferguson SA, Stanley D, Wright KP Jr., and Dawson D. The shift work and health research agenda: Considering changes in gut microbiota as a pathway linking shift work, sleep loss and circadian misalignment, and metabolic disease. Sleep Med Rev. (2017) 34:3–9. doi: 10.1016/j.smrv.2016.06.009
28. Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. (2004) 558:263–75. doi: 10.1113/jphysiol.2004.063388
29. Meerlo P, Sgoifo A, and Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. (2008) 12:197–210. doi: 10.1016/j.smrv.2007.07.007
30. Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. (2011) 108:16050–5. doi: 10.1073/pnas.1102999108
31. Mitrea L, Nemes SA, Szabo K, Teleky BE, and Vodnar DC. Guts imbalance imbalances the brain: A review of gut microbiota association with neurological and psychiatric disorders. Front Med (Lausanne). (2022) 9:813204. doi: 10.3389/fmed.2022.813204
32. Matenchuk BA, Mandhane PJ, and Kozyrskyj AL. Sleep, circadian rhythm, and gut microbiota. Sleep Med Rev. (2020) 53:101340. doi: 10.1016/j.smrv.2020.101340
33. Takada M, Nishida K, Gondo Y, Kikuchi-Hayakawa H, Ishikawa H, Suda K, et al. Beneficial effects of Lactobacillus casei strain Shirota on academic stress-induced sleep disturbance in healthy adults: a double-blind, randomised, placebo-controlled trial. Benef Microbes. (2017) 8:153–62. doi: 10.3920/BM2016.0150
34. Cani PD and Delzenne NM. Interplay between obesity and associated metabolic disorders: new insights into the gut microbiota. Curr Opin Pharmacol. (2009) 9:737–43. doi: 10.1016/j.coph.2009.06.016
35. O’Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. (2005) 128:541–51. doi: 10.1053/j.gastro.2004.11.050
36. Gareau MG, Jury J, MacQueen G, Sherman PM, and Perdue MH. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. (2007) 56:1522–8. doi: 10.1136/gut.2006.117176
37. Du X and Pang TY. Is dysregulation of the HPA-axis a core pathophysiology mediating co-morbid depression in neurodegenerative diseases? Front Psychiatry. (2015) 6:32. doi: 10.3389/fpsyt.2015.00032
38. Han M, Yuan S, and Zhang J. The interplay between sleep and gut microbiota. Brain Res Bull. (2022) 180:131–46. doi: 10.1016/j.brainresbull.2021.12.016
39. Varinthra P, Anwar S, Shih SC, and Liu IY. The role of the GABAergic system on insomnia. Tzu Chi Med J. (2024) 36:103–9. doi: 10.4103/tcmj.tcmj_243_23
40. Byun JI, Shin YY, Chung SE, and Shin WC. Safety and efficacy of gamma-aminobutyric acid from fermented rice germ in patients with insomnia symptoms: A randomized, double-blind trial. J Clin Neurol. (2018) 14:291–5. doi: 10.3988/jcn.2018.14.3.291
41. Yu L, Han X, Cen S, Duan H, Feng S, Xue Y, et al. Beneficial effect of GABA-rich fermented milk on insomnia involving regulation of gut microbiota. Microbiol Res. (2020) 233:126409. doi: 10.1016/j.micres.2020.126409
42. Meyerhoff DJ, Mon A, Metzler T, and Neylan TC. Cortical gamma-aminobutyric acid and glutamate in posttraumatic stress disorder and their relationships to self-reported sleep quality. Sleep. (2014) 37:893–900. doi: 10.5665/sleep.3654
43. El-Ansary A and Al-Ayadhi L. GABAergic/glutamatergic imbalance relative to excessive neuroinflammation in autism spectrum disorders. J Neuroinflammation. (2014) 11:189. doi: 10.1186/s12974-014-0189-0
44. Higuchi T, Hayashi H, and Abe K. Exchange of glutamate and gamma-aminobutyrate in a Lactobacillus strain. J Bacteriol. (1997) 179:3362–4. doi: 10.1128/jb.179.10.3362-3364.1997
45. Strandwitz P, Kim KH, Terekhova D, Liu JK, Sharma A, Levering J, et al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol. (2019) 4:396–403. doi: 10.1038/s41564-018-0307-3
46. Mabunga DF, Gonzales EL, Kim HJ, and Choung SY. Treatment of GABA from fermented rice germ ameliorates caffeine-induced sleep disturbance in mice. Biomol Ther (Seoul). (2015) 23:268–74. doi: 10.4062/biomolther.2015.022
47. Yamatsu A, Yamashita Y, Pandharipande T, Maru I, and Kim M. Effect of oral gamma-aminobutyric acid (GABA) administration on sleep and its absorption in humans. Food Sci Biotechnol. (2016) 25:547–51. doi: 10.1007/s10068-016-0076-9
48. Wang Z, Wang Z, Lu T, Yuan G, Chen W, Jin J, et al. Gut microbiota regulate insomnia-like behaviors via gut-brain metabolic axis. Mol Psychiatry. (2025) 30:2597–611. doi: 10.1038/s41380-024-02867-0
49. Doenyas C, Clarke G, and Cserjesi R. Gut-brain axis and neuropsychiatric health: recent advances. Sci Rep. (2025) 15:3415. doi: 10.1038/s41598-025-86858-3
50. Xie C, Wang J, Zhao N, Yang W, Gao X, Liu Z, et al. Effects of Electroacupuncture on Sleep via the Dopamine System of the HPA Axis in Rats after Cage Change. Evid Based Complement Alternat Med. (2021) 2021:5527060. doi: 10.1155/2021/5527060
51. Park HJ, Park HJ, Chae Y, Kim JW, Lee H, and Chung JH. Effect of acupuncture on hypothalamic-pituitary-adrenal system in maternal separation rats. Cell Mol Neurobiol. (2011) 31:1123–7. doi: 10.1007/s10571-011-9718-x
52. Zheng Y, He J, Guo L, Yao L, Zheng X, Yang Z, et al. Transcriptome analysis on maternal separation rats with depression-related manifestations ameliorated by electroacupuncture. Front Neurosci. (2019) 13:314. doi: 10.3389/fnins.2019.00314
53. Carabotti M, Scirocco A, Maselli MA, and Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. (2015) 28:203–9.
54. Jiang J, Liu H, Wang Z, Tian H, Wang S, Yang J, et al. Electroacupuncture could balance the gut microbiota and improve the learning and memory abilities of Alzheimer’s disease animal model. PLoS One. (2021) 16:e0259530. doi: 10.1371/journal.pone.0259530
55. Bai J, Wei JQ, Tian Q, Xue F, Zhang W, and He H. The impact of electroacupuncture on anxiety-like behavior and gut microbiome in a mouse model of chronic restraint stress. Front Behav Neurosci. (2023) 17:1292835. doi: 10.3389/fnbeh.2023.1292835
56. Hong J, Chen J, Kan J, Liu M, and Yang D. Effects of acupuncture treatment in reducing sleep disorder and gut microbiota alterations in PCPA-induced insomnia mice. Evid Based Complement Alternat Med. (2020) 2020:3626120. doi: 10.1155/2020/3626120
57. Xiao M, Wang XS, He C, Huang ZS, Chen HR, and Kong LH. The gut-brain axis: Effect of electroacupuncture pretreatment on learning, memory, and JNK signaling in D-galactose-induced AD-like rats. Iran J Basic Med Sci. (2023) 26:532–9. doi: 10.22038/IJBMS.2023.66954.14683
58. Zhang F, Zhang X, Peng Q, and Tang L. Electroacupuncture of the cymba concha alleviates p-chlorophenylalanine-induced insomnia in mice. Acupunct Med. (2023) 41:345–53. doi: 10.1177/09645284231160193
59. Cai W, Wei XF, Hu C, Ji J, Cui HS, and Shen WD. Effects of electroacupuncture on gut microbiota and fecal metabolites in rats with poststroke depression. Neuropsychiatr Dis Treat. (2023) 19:1581–92. doi: 10.2147/NDT.S415098
60. Li P, Huang W, Yan YN, Cheng W, Liu S, Huang Y, et al. Acupuncture can play an antidepressant role by regulating the intestinal microbes and neurotransmitters in a rat model of depression. Med Sci Monit. (2021) 27:e929027. doi: 10.12659/MSM.929027
61. Wu Q, Wang J, Fan L, Qian L, Han D, Hu H, et al. Efficacy and safety of auricular acupressure on reduction of estazolam in patients with insomnia: a study protocol for a three-arm, blinded randomized controlled trial. BMC Complement Med Ther. (2024) 24:367. doi: 10.1186/s12906-024-04651-7
62. Vega-Garcia A, Neri-Gomez T, Buzoianu-Anguiano V, Guerra-Araiza C, Segura-Uribe J, Feria-Romero I, et al. Electroacupuncture reduces seizure activity and enhances GAD 67 and glutamate transporter expression in kainic acid induced status epilepticus in infant rats. Behav Sci (Basel). (2019) 9:68. doi: 10.3390/bs9070068
63. Guo W, Tang ZY, Cai ZY, Zhao WE, Yang J, Wang XP, et al. Iptakalim alleviates synaptic damages via targeting mitochondrial ATP-sensitive potassium channel in depression. FASEB J. (2021) 35:e21581. doi: 10.1096/fj.202100124RR
64. Li J, Yao D, Zhang T, Tong T, Shen J, Yan S, et al. GABA(B) modulate NF-kappaB/NLRP3 pathways in electroacupuncture prevention of depression in CUMS rats. Brain Res Bull. (2024) 218:111108. doi: 10.1016/j.brainresbull.2024.111108
65. Weschenfelder J, Sander C, Kluge M, Kirkby KC, and Himmerich H. The influence of cytokines on wakefulness regulation: clinical relevance, mechanisms and methodological problems. Psychiatr Danub. (2012) 24:112–26.
66. Li G, Liu J, and Zhang K. Electroacupuncture improves sleep deprivation-induced cognitive impairment by suppressing hippocampal inflammatory response in mice. Stress. (2025) 28:2502742. doi: 10.1080/10253890.2025.2502742
67. Song S, An J, Li Y, and Liu S. Electroacupuncture at ST-36 ameliorates DSS-induced acute colitis via regulating macrophage polarization induced by suppressing NLRP3/IL-1beta and promoting Nrf2/HO-1. Mol Immunol. (2019) 106:143–52. doi: 10.1016/j.molimm.2018.12.023
68. Tang L, Zhang X, Zhang B, Chen T, Du Z, Song W, et al. Electroacupuncture remodels gut microbiota and metabolites in mice with perioperative neurocognitive impairment. Exp Gerontol. (2024) 194:112507. doi: 10.1016/j.exger.2024.112507
69. Li N, Guo Y, Gong Y, Zhang Y, Fan W, Yao K, et al. The anti-inflammatory actions and mechanisms of acupuncture from acupoint to target organs via neuro-immune regulation. J Inflammation Res. (2021) 14:7191–224. doi: 10.2147/JIR.S341581
70. Alam MN, McGinty D, Bashir T, Kumar S, Imeri L, Opp MR, et al. Interleukin-1beta modulates state-dependent discharge activity of preoptic area and basal forebrain neurons: role in sleep regulation. Eur J Neurosci. (2004) 20:207–16. doi: 10.1111/j.1460-9568.2004.03469.x
71. Bredow S, Guha-Thakurta N, Taishi P, Obal F Jr., and Krueger JM. Diurnal variations of tumor necrosis factor alpha mRNA and alpha-tubulin mRNA in rat brain. Neuroimmunomodulation. (1997) 4:84–90. doi: 10.1159/000097325
72. Taishi P, Chen Z, Obal F Jr., Hansen MK, Zhang J, Fang J, et al. Sleep-associated changes in interleukin-1beta mRNA in the brain. J Interferon Cytokine Res. (1998) 18:793–8. doi: 10.1089/jir.1998.18.793
73. Goehler LE, Relton JK, Dripps D, Kiechle R, Tartaglia N, Maier SF, et al. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain Res Bull. (1997) 43:357–64. doi: 10.1016/s0361-9230(97)00020-8
74. Rosenwasser AM. Functional neuroanatomy of sleep and circadian rhythms. Brain Res Rev. (2009) 61:281–306. doi: 10.1016/j.brainresrev.2009.08.001
75. Arrigoni E, Mochizuki T, and Scammell TE. Activation of the basal forebrain by the orexin/hypocretin neurones. Acta Physiol. (2010) 198:223–35. doi: 10.1111/j.1748-1716.2009.02036.x
76. Kalavapalli R and Singareddy R. Role of acupuncture in the treatment of insomnia: a comprehensive review. Complement Ther Clin Pract. (2007) 13:184–93. doi: 10.1016/j.ctcp.2007.01.001
77. Luo M, Song B, and Zhu J. Electroacupuncture: A new approach for improved postoperative sleep quality after general anesthesia. Nat Sci Sleep. (2020) 12:583–92. doi: 10.2147/NSS.S261043
78. Lu X, Zhou M, Liu N, Zhang C, Zhao Z, and Cai D. Synaptic protein phosphorylation networks are associated with electroacupuncture-induced circadian control in the suprachiasmatic nucleus. Front Genet. (2021) 12:762557. doi: 10.3389/fgene.2021.762557
79. Lee B, Kim BK, Kim HJ, Jung IC, Kim AR, Park HJ, et al. Efficacy and safety of electroacupuncture for insomnia disorder: A multicenter, randomized, assessor-blinded, controlled trial. Nat Sci Sleep. (2020) 12:1145–59. doi: 10.2147/NSS.S281231
80. Yin X, Li W, Liang T, Lu B, Yue H, Li S, et al. Effect of electroacupuncture on insomnia in patients with depression: A randomized clinical trial. JAMA Netw Open. (2022) 5:e2220563. doi: 10.1001/jamanetworkopen.2022.20563
81. Guo J, Wang LP, Liu CZ, Zhang J, Wang GL, Yi JH, et al. Efficacy of acupuncture for primary insomnia: a randomized controlled clinical trial. Evid Based Complement Alternat Med. (2013) 2013:163850. doi: 10.1155/2013/163850
82. Liu C, Zhao Y, Qin S, Wang X, Jiang Y, and Wu W. Randomized controlled trial of acupuncture for anxiety and depression in patients with chronic insomnia. Ann Transl Med. (2021) 9:1426. doi: 10.21037/atm-21-3845
83. Liu X, Xu N, Wang S, and Jia Q. Efficacy of electroacupuncture for insomnia in cancer patients: a systematic review and meta-analysis. Front Neurol. (2025) 16:1512052. doi: 10.3389/fneur.2025.1512052
Keywords: insomnia, electroacupuncture, γ-aminobutyric acid, gut microbiota, microbiota-gut-brain axis
Citation: Wang X, Yan L, Zhang X, Liu X and Yang B (2025) Electroacupuncture as adjunctive therapy for insomnia via targeting the GABAergic microbiota-gut-brain axis: a mini review. Front. Psychiatry 16:1613408. doi: 10.3389/fpsyt.2025.1613408
Received: 17 April 2025; Accepted: 30 June 2025;
Published: 18 July 2025.
Edited by:
Yi-Hung Chen, China Medical University (Taiwan), TaiwanReviewed by:
Ingrid Y Liu, Tzu Chi University, TaiwanCopyright © 2025 Wang, Yan, Zhang, Liu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Yang, eWFuZ2JpbjQzMzJAb3V0bG9vay5jb20=; Xiang Liu, bGl1eGlhNzZAbXN1LmVkdQ==
†These authors have contributed equally to this work and share first authorship
‡ORCID: Xiao Wang, orcid.org/0000-0002-6539-9732
Lijuan Yan, orcid.org/0000-0003-1143-2733
Xiang Liu, orcid.org/0009-0005-1591-466X
Bin Yang, orcid.org/0000-0002-6604-5565
 Xiao Wang
Xiao Wang Lijuan Yan
Lijuan Yan Xiaodong Zhang
Xiaodong Zhang Xiang Liu
Xiang Liu Bin Yang
Bin Yang