- 1Mental Health Center, West China Hospital of Sichuan University, Chengdu, China
- 2Department of Medicine, Sinai Hospital of Baltimore, Baltimore, MD, United States
- 3Department of Cardiology, West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, China
Background and aims: Several pharmacological interventions, such as nicotine replacement therapy (NRT), varenicline, and bupropion, have been approved for clinical use of smoking cessation. E-cigarettes (EC) are increasingly explored by many RCTs for their potentiality in smoking cessation. In addition, some RCTs are attempting to explore new drugs for smoking cessation, such as cytisine. This network meta-analysis (NMA) aims to investigate how these drugs and e-cigarettes compare regarding their efficacy and acceptability.
Materials and methods: This systematic review and NMA searched all clinical studies on smoking cessation using pharmacological monotherapies or e-cigarettes published from January 2011 to May 2022 using MEDLINE, COCHRANE Library, and PsychINFO databases. NRTs were divided into transdermal (TDN) and oronasal nicotine (ONN) by administrative routes, thus 7 network nodes were set up for direct and indirect comparison. Two different indicators measured the efficacy: prevalent and continuous smoking abstinence. The drop-out rates measured the acceptability.
Results: The final 40 clinical studies included in this study comprised 77 study cohorts and 25,889 participants. Varenicline is more effective intervention to assist in smoking cessation during 16–32 weeks follow-up, and is very likely to prompt dropout. Cytisine shows more effectiveness in continuous smoking cessation but may also lead to dropout. E-cigarettes and oronasal nicotine are more effective than no treatment in encouraging prevalent abstinence, but least likely to prompt dropout. Finally, transdermal nicotine delivery is more effective than no treatment in continuous abstinence, with neither significant effect on prevalent abstinence nor dropout rate.
Conclusion: This review suggested and agreed that Varenicline, Cytisine and transdermal nicotine delivery, as smoking cessation intervention, have advantages and disadvantages. However, we had to have reservations about e-cigarettes as a way to quit smoking in adolescents.
1 Introduction
Unquestionably, tobacco smoking is one of the modifiable factors that heavily contribute to the global health burden. According to a global burden of disease study, there will be an increasing number of 7.69 million deaths and 200 million disability-adjusted life-years attributable to tobacco smoking within this decade if interventions are abscent1. Multiple behavioral and pharmacologic interventions, both in combination and individually, were proved effective and applied in practice (1). Even though previous randomized controlled trials (RCTs) and meta-analyses showed evidence supporting the effectiveness of behavioral interventions in smoking cessation (1, 2), their effectiveness is relatively modest compared with approved pharmacological interventions (1).
Current to the date when this study was performed, there were 7 pharmacological interventions widely approved by most countries: nicotine replacement therapy (NRT, including nicotine mouth spray, inhaler, gum, patch, and lozenge), varenicline, and bupropion. However, inconsistent effectiveness reported by RCTs and meta-analysis of the above pharmacological therapies is not excellent enough, and the relapse rate remains high (3). Besides, the relatively high cost of NRT and varenicline also prevent patients who are in low-income classes from approaching such smoking cessation aids (4). It is still important to innovate novel pharmacological interventions for more cost-effective and acceptable aids in assisting smoking cessation.
As a new product with the potential in assisting smoking cessation, the e-cigarette has already shown evidence of effectiveness and non-inferiority to NRTs in assisting smoking cessation from the previous meta-analysis of both RCTs and observational studies (5, 6). Cytisine, due to its similar mechanism with varenicline as a selective partial agonist of nicotinic acetylcholine receptors and low cost of production, has also been previously investigated and proved to be effective and globally affordable in assisting smoking cessation (4, 7).
This network meta-analysis aims to systemically and quantitatively evaluate and compare the overall effectiveness and acceptance of all above-mentioned interventions.
2 Method
2.1 Search strategies and literature resources
We searched MEDLINE, COCHRANE Library & PsychINFO for RCTs reporting pharmacological monotherapies and/or e-cigarettes (and equivalents) on smoking cessation. Due to the purpose of comparability and consistency of study cohorts, the time of publication was restricted to be from 2011 Jan 1st [in which the first RCT reporting e-cigarette was released (5)] to 2022 May 31st (in which this network meta-analysis was firstly proposed) during searching. Additionally, references to already-published reviews and meta-analyses with a similar topic were also screened for consideration of inclusion.
2.2 Eligibility criteria and study selection
Eligibility criteria were proposed before we perform this network meta-analysis. Inclusion of studies was considered if the study met the following: (1) RCTs; (2) reported in English; (3) study cohorts were recruited in a community-based setting; (4) study cohorts had a persistent smoking history; (5) pharmacological monotherapies or e-cigarette (or its equivalent) were used as an intervention in ≥1 study cohort. Furtherly, studies were excluded if: (1) duplicate records; (2) the study cohort was with a major health condition (e.g., cancer, chronic respiratory diseases, heart and vascular diseases, and schizophrenia or bipolar disorder); (3) follow-up of study endpoint was less than 4 weeks; (4) study outcomes (smoking abstinence, prevalent and/or continuous) were not supported by objective evidence (e.g., saliva cotinine, exhaled CO, serum cotinine, urine cotinine).
Two reviewers (L. Qu and S. Xiang) independently searched and selected studies according to the above strategies and criteria, with disagreement resolved by discussion. All citations retrieved from the database were firstly screened for eligibility at Title/Abstract level, and identified studies were furtherly acquired and examined in full text. Forty studies were eligible and included in the final analysis (8–47).
2.3 Data identification and extraction
We identified three study outcomes for this meta-analysis due to our study interest, which are defined and listed as the following: (1) Prevalent smoking abstinence (PSA): the percentages of the population who currently quit or reduced cigarette use during the follow-up investigation in between 16 and 32 weeks; (2) Continuous smoking abstinence (CSA) : the percentages of the population who consistently maintain smoke quitting or reduction from the first to the last follow up the investigation; (3) Treatment drop-out rates (TDR) : the percentages of the population who dropped out from the study or lost to follow-up during the treatment period.
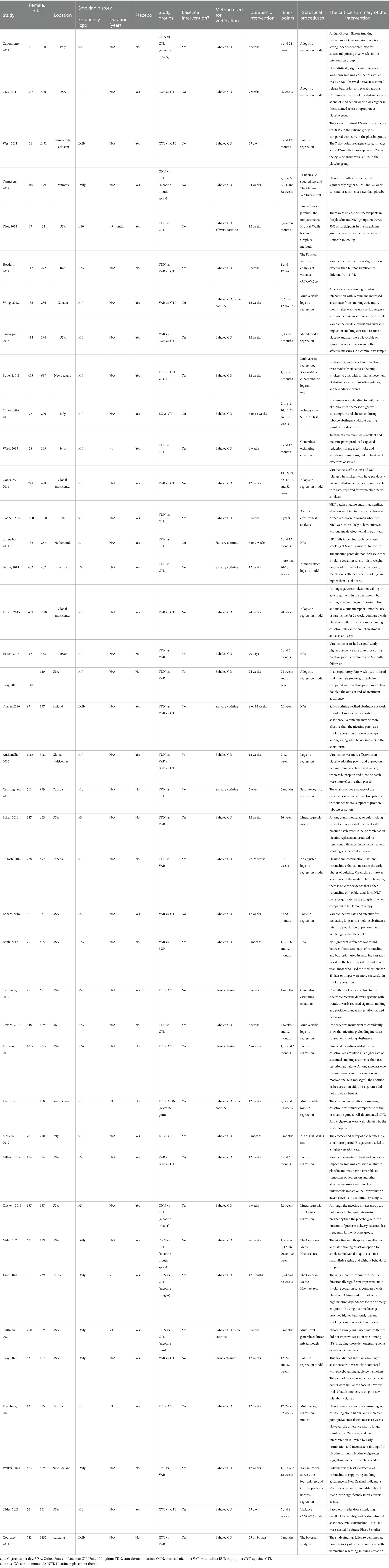
Data identification and extraction were performed by 2 reviewers (L. Qu and S. Xiang) independently. Additional to direct data and indirect data used for the calculation of study outcomes, the baseline characteristics of each study were evaluated and extracted: sampling population, age, location, sex, recruitment setting, smoking history, comparisons, pharmacological dosage, duration of exposure, length of follow up, and lab methods measuring smoking abstinence (see as Table 1).
2.4 Data analysis
2.4.1 Comparative arms
All considered interventions were classified into 6 arms for comparison: (1) Varenicline (VAR); (2) Bupropion (BUP); (3) Transdermal nicotine delivery (TND) (nicotine patch); (4) Oronasal nicotine delivery (ONN) (nicotine gum, nicotine nasal spray, nicotine inhaler, nicotine tablet/lozenge); (5) Cytisine (CYT); (6) Electronic cigarette (EC) (or its equivalents).
2.4.2 Data analysis
All outcomes were dichotomous variables measured as n/N (%). The odds ratio (OR) of each outcome was pooled for network meta-analysis (NMA). The NMA used the Bayesian method for multiple-treatment to pool the OR, under the assumption: the heterogeneity is independent of the comparative arms being used (48). We calculated the Bayesian 95% confidence interval (which is known as credible interval) to estimate the range of the OR results (49). As proposed by the previous study (50), the statistical models for NMA were chosen based on a model comparison criterion called the Deviance Information Criterion (DIC), which is the sum of the posterior expectation of the overall residual deviance and the posterior mean of the parameter of interest (50, 51). We firstly applied both random and fixed models for each outcome and calculated the DIC of both models, then the model with a lower DIC is chosen if the difference of DIC in each model is considerable (>5), otherwise the fixed model is chosen if the between-study difference of DIC in each model is insignificant (<5). The absolute value of the between-study variance in the random effect model was assessed by Tau2; the heterogeneity of variation across studies was estimated through I2 statistics. Additionally, we used the node-splitting method to evaluate the local consistency by separating direct evidence from indirect evidence (50, 52). To rank the interventions for each outcome, we estimated the posterior distribution of the ranking probability and their corresponding estimated surface under the cumulative ranking curve (SUCRA) (50, 52). The SUCRA is an estimated index to show the cumulative rank probabilities for each intervention and simplifies the entire information about treatment ranking into a single number.
As guided by the Cochrane Handbook for Systematic Reviews of Interventions, the internal validity and quality of this systemic review and NMA were evaluated through the aspects of randomization, blinding of intervention allocation and outcome assessment, and incomplete outcome data (51). All data synthesis and statistical analysis were performed in R with the gemtc package: https://github.com/gertvv/gemtc
3 Results
3.1 Baseline characteristics
The baseline characteristics of each study were summarized in Table 1. Forty studies included a total of 77 study cohorts and 25,889 participants, with an average age of 43.2 years old and 46.7% female (12,096) participants, and nearly half of the studies were performed in North America (19/40, 47.5%). Among the all 40 identified studies, three studies [Scherphof et al. (22), Berlin et al. (19), and Oncken et al. (39)] included pregnant patients only (18, 21, 38), seven studies (7/40, 17.5%) included more than 2 treatment arms for the NMA, 11 studies (11/40, 27.5%) had no placebo-controlled group, 15 studied (15/40, 37.5%) had no baseline intervention between each study’s comparative arms. For studies with multiple cohorts that used the same intervention with different dosages, only cohorts with higher nicotine dosage [7.2 mg nicotine EC cohort in Caponnetto et al. (17), 15 mg/16 h nicotine patches cohort in Tuisku et al. (30), 4 mg nicotine lozenge cohort in Xiao et al. (46), 3 mg cytisine three times per day cohort in Nides et al. (48)] were selected for the analysis (16, 29, 45, 47). Methods used for verification of smoking abstinence included CO concentration of exhaled air (29/40, 72.5%), salivary cotinine concentration (4/40, 10%), urine cotinine (3/40, 7.5%), and the combination of the above (4/40, 10%) (Figure 1).
3.2 Pooled effect
3.2.1 Prevalent smoking abstinence (PSA)
Figures 2A, 3A described the network used for the main analyses of PSA , comprising 60 study cohorts and 13,818 participants. We analyzed the pooled network effect of PSA for all interventions compared with the control group, using both random and fixed effect models initially. The random-effect model was selected for the final report due to significantly lower DIC (112.84 in the random model, 140.56 in the fixed model), indicating a better efficient result. As presented in Table 2, the confidential presentation of results in terms of mean OR with 95% credible intervals (Crl) compared with the control group was summarized. The pooled effects of all nicotine-containing products ( ONN , TDN, EC ) as well as buspirone did not exhibit significant superiority over the control group in terms of prevalent smoking abstinence. The CYT and VAR, both demonstrating significant superiority, exhibited similar odds of prevalent smoking abstinence, approximately twice that of the control group. Despite CYT and VAR showing significant superiority over the control group compared to other intervention groups, these two interventions mostly did not exhibit a significantly different odds ratio for PSA relative to other active intervention groups. The only notable significance observed among active intervention groups was in VAR , with approximately 50% higher odds compared to TDN (Table 2 and Figure 2 are shown here).
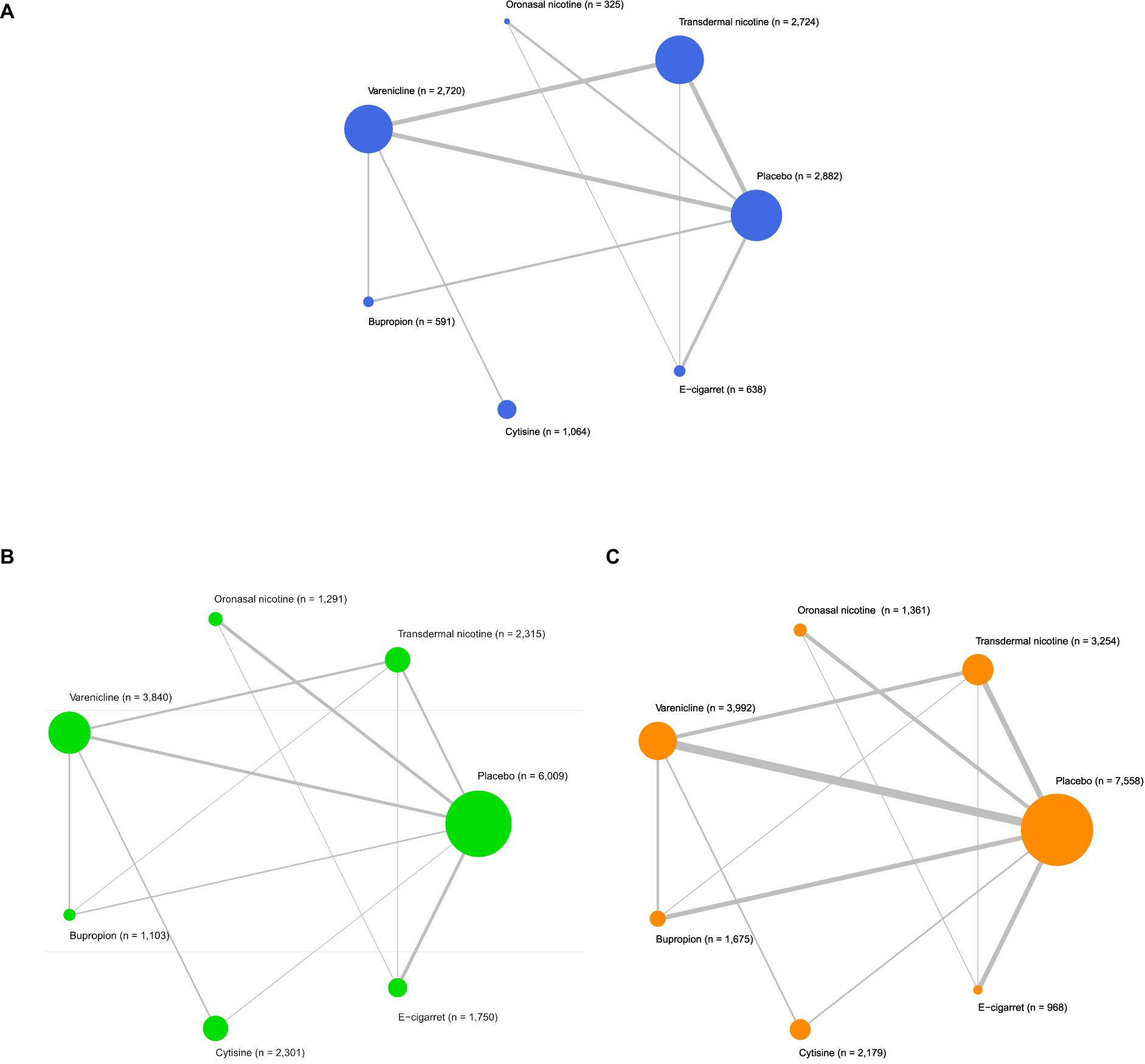
Figure 2. Network meta-analysis of eligible comparisons. (A) Network meta-analysis for comparisons of prevalent smoking abstinence (blue). (B) Network meta-analysis for comparisons of continuous smoking abstinence (green) and (C) Network meta-analysis for comparisons of treatment drop-out rates (orange). n indicates the number of total participants.
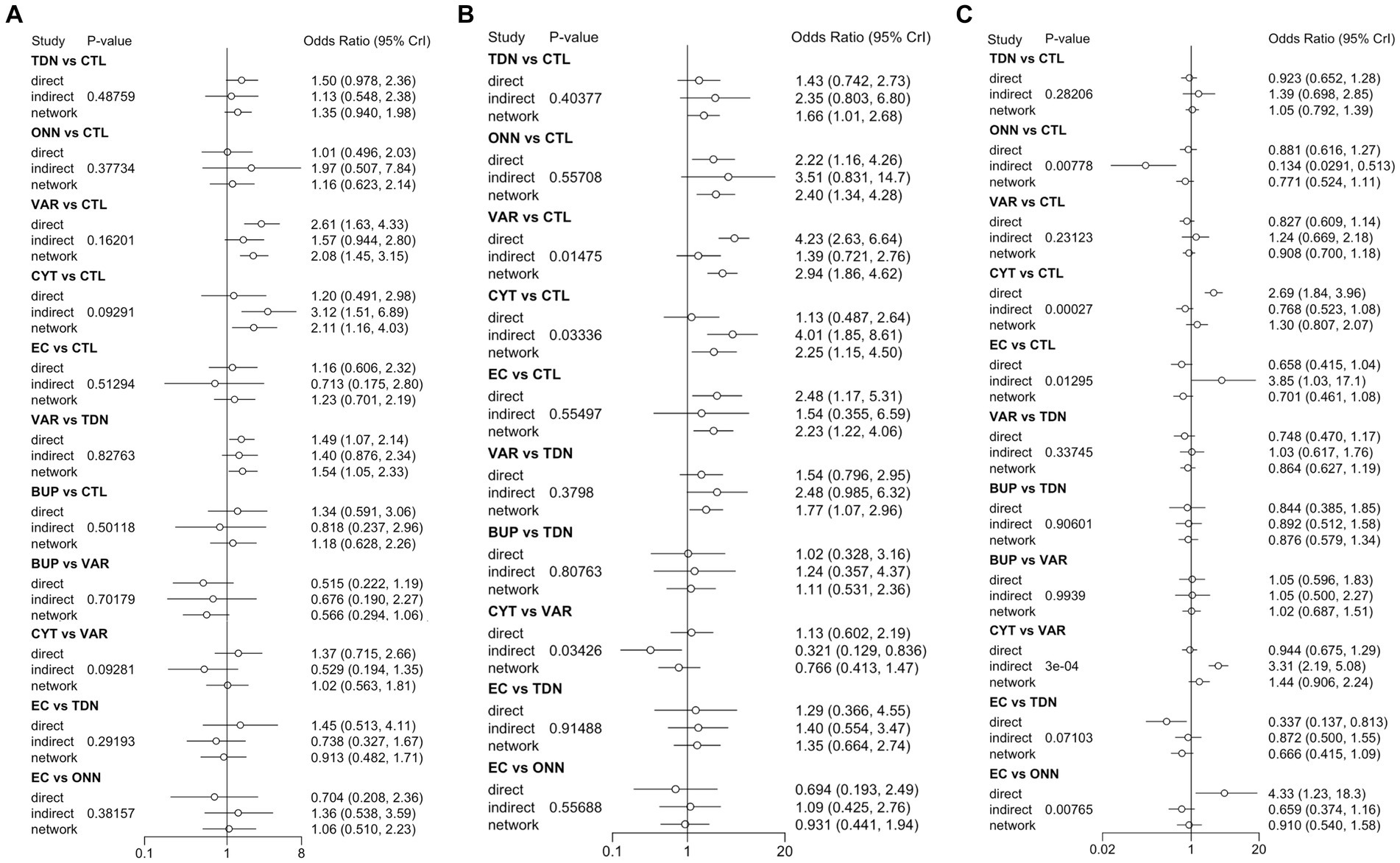
Figure 3. Inconsistency check between direct and indirect evidence in the network meta-analysis of PSA (A), CSA (B), and TDR (C). PSA, prevalent smoking abstinence; CSA, continuous smoking abstinence; DOR, drop-out rate; TDN, transdermal nicotine; ONN, oronasal nicotine; VAR, varenicline; BUP, bupropion; CYT, cytisine; CTL, controls; 95% Crl, Credible interval.
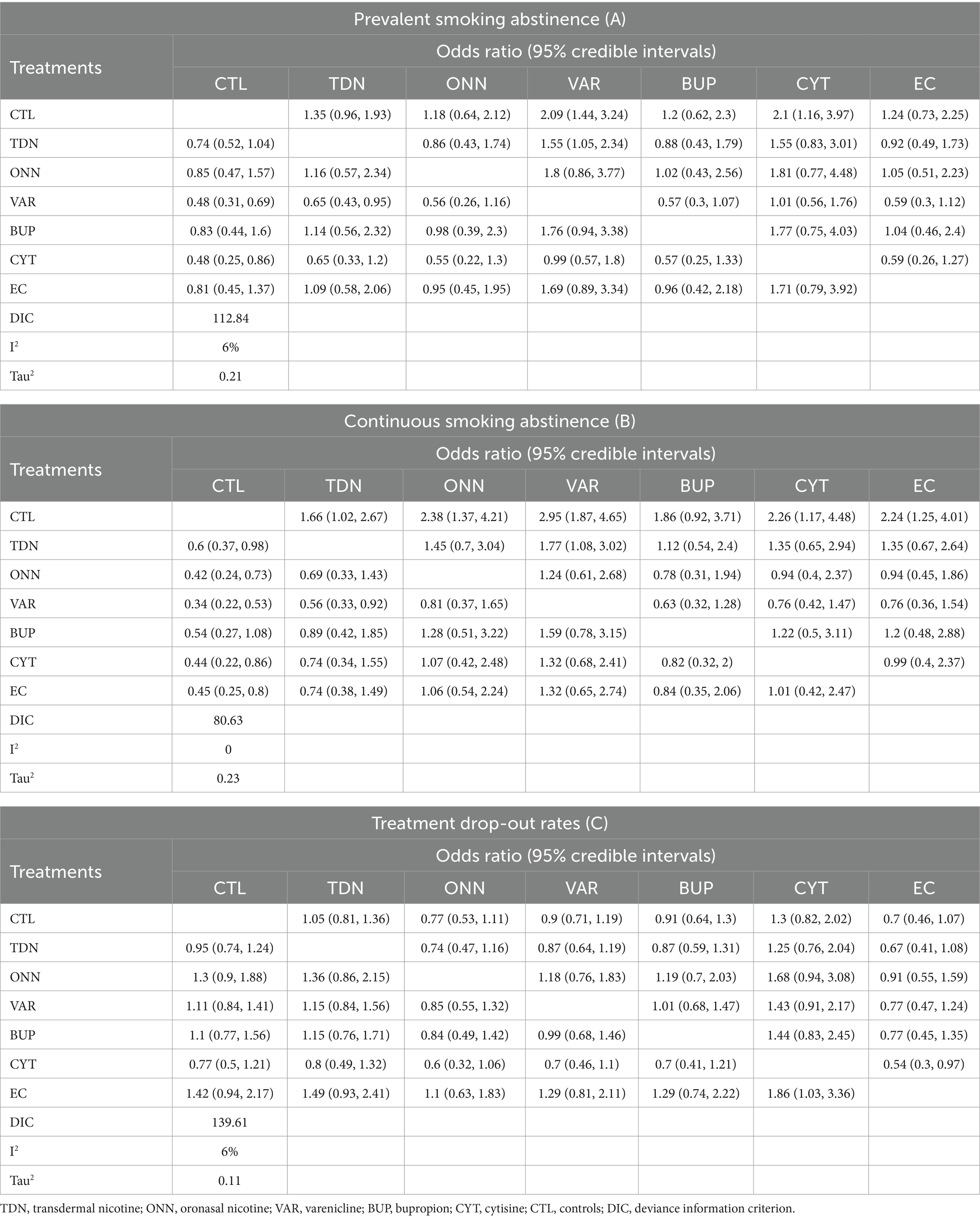
Table 2. Posterior distributions of odds ratios for random effect consistency model of each intervention and control group.
3.2.2 Continuous smoking abstinence (CSA)
Figures 2B, 3B described the network of CSA , comprising 42 study cohorts and 18,609 participants. Using the same algorithm described before, the random effect model was selected for the final report due to significantly lower DIC (77.67 in random, 140.62 in fixed model). As presented in Table 2, all comparative interventions except for BUP were associated with significant efficacy for the outcome of CSA compared with CTL . Similar to the absolute values of OR in analyses of PSA , VAR (OR 3.02, 95% Crl 1.9–4.81) and TDN (OR 1.83, 95% Crl 1.09–3.17) demonstrated the highest and lowest OR, respectively. Tau2 in the analyses of CSA was estimated to be 0.24, indicating a moderate variance; and the I2 was estimated to be 0.00%, indicating that heterogeneity was minimally considerable. As presented in Figure 3B, inconsistency between direct and indirect evidence was observed in the comparison of VAR/ CTL , CYT / CTL , and VAR / CYT . Among those inconsistent results, the direct evidence of VAR / CTL (OR 4.23, 95% Crl 2.57–6.77) yielded a positive CSA reduction on Varenicline use, but the direct evidence of CYT / CTL (OR 1.13, 95% Crl 0.508–2.52) and CYT / VAR (OR 1.13, 95% Crl 0.618–2.15) were ambiguous compared with their combined evidence. Comparative loops with e-cigarette ( EC/CTL , EC/TDN, and EC/ONN ) were exclusively consistent between direct and indirect evidence, and neither superiority nor inferiority was significant in EC/TDN (OR 1.25, 95% Crl 0.59–2.61) and EC/ONN (OR 0.96, 95% Crl 0.45–1.99) comparisons.
3.2.3 Treatment drop-out rates (TDR)
Figures 2C, 3C described the network of CSA , comprising 42 study cohorts and 18,609 participants. Following similar principles as before, we opted for a random-effects model as significantly lower DIC (80.63 in random, 140.62 in fixed model). As presented in Table 2, With the exception of BUP, all intervention groups exhibited significant superiority over the control group in terms of continuous abstinence rates. Among these, VAR, CYT , EC , and ONN showed odds approximately 2–3 times higher than the control group. Noneligible Tau2 in the analyses of CSA was estimated to be 0.24, indicating a moderate variance; and the I2 was estimated to be 0.00%, indicating that heterogeneity was minimally considerable. For the outcome measure of CSA , comparisons among active intervention groups mirrored those of PSA , with only VAR demonstrating significant superiority over TDN .
3.3 Treatment ranking
As presented in Table 3, we estimated the posterior distribution of the ranking probability and their corresponding SUCRA for all outcomes. Briefly, CYT is quite likely to encourage both prevalent and continuous smoking abstinence but may lead to dropout. VAR is quite likely to encourage prevalent abstinence, is not particularly effective with continuous abstinence, and is very likely to prompt dropout. In contrast, ONN and EC are least likely to prompt dropout and both are more effective than no treatment in encouraging prevalent abstinence. However, TDN is more effective than no treatment in continuous abstinence, with neither significant effect on prevalent abstinence nor dropout rate (More details seen in Table 3).”
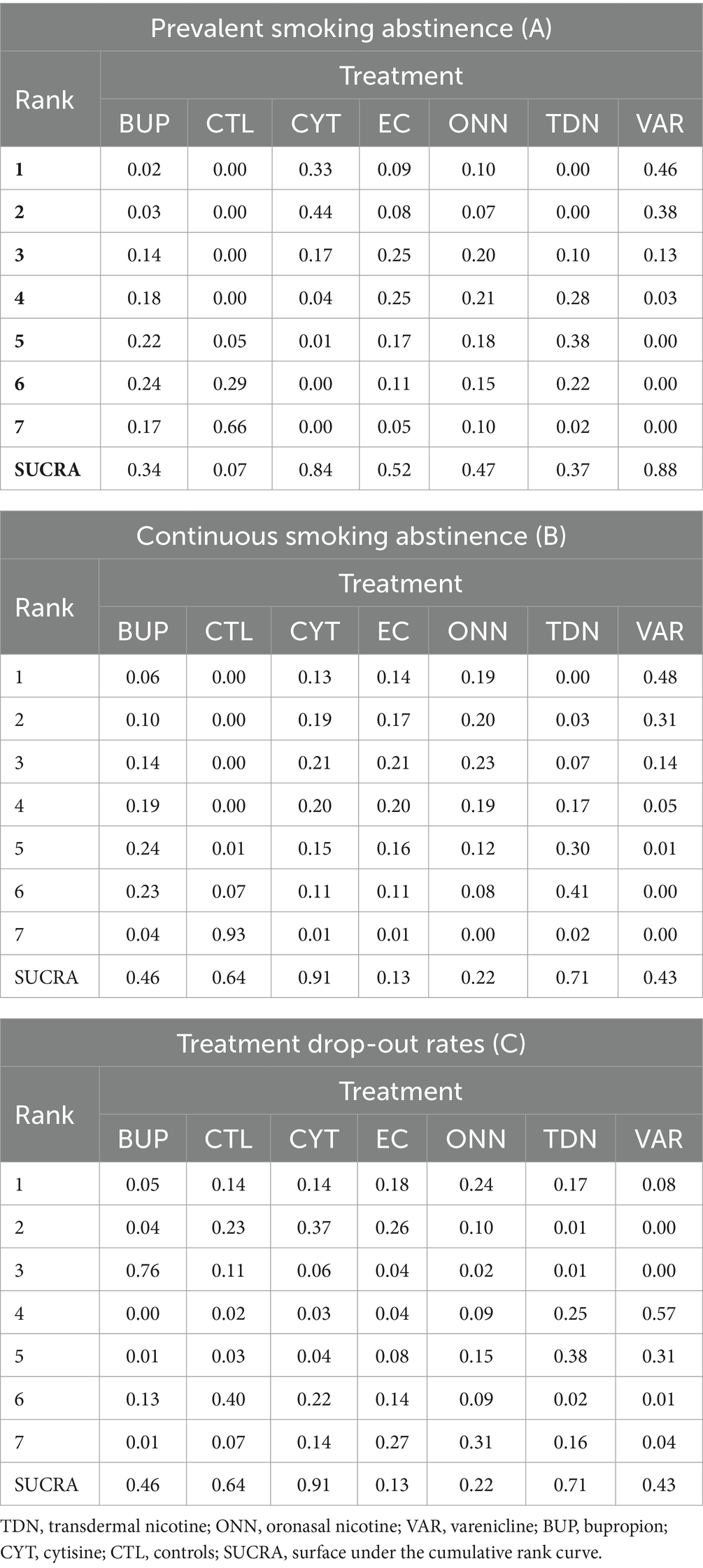
Table 3. Posterior distribution of the ranking probability and the surface under the cumulative rank curve (SUCRA) for each treatment in network meta-analysis.
4 Discussion
As far as we know, our study is the first to report the efficacy and acceptability of five major pharmacological monotherapies and e-cigarette on smoking cessation through network meta-analysis including RCT studies. And this NMA including 40 studies found that (1) Varenicline is more effective intervention to assist in smoking cessation during mid- to long-term (16–32 weeks) follow-up, but is not particularly effective with continuous abstinence, and is very likely to prompt dropout. (2) Cytisine shows more effectiveness in continuous smoking cessation but may lead to dropout. (3) E-cigarettes and oronasal nicotine are least likely to prompt dropout and both are more effective than no treatment in encouraging prevalent abstinence. Finally, transdermal nicotine delivery is more effective than no treatment in continuous abstinence, with neither significant effect on prevalent abstinence nor dropout rate.
Our findings are consistent with the approach recommended by current mainstream clinical smoking cessation guidelines, such as the use of Varenicline as a first-line pharmacological intervention to assist in smoking cessation by the 2020 American Thoracic Society guidelines (53) and the recommendation of NRT, varenicline and bupropion as first-line pharmacological interventions for smoking cessation in the 2018 ACC Expert Consensus (54). Since e-cigarettes (or equivalent products) have a pharmacological mechanism for distributing nicotine to the body, their potential cessation effect has also gained the attention of manufacturers. This study also showed their similar effects to NRT treatment in terms of smoking cessation effectiveness.
However, our findings should be cautiously interpreted. The ethnic distribution of overall participants involved in this NMA is considerably uneven since most of the included RCTs were performed in Europe and North America. Thus, the results of this NMA may not be generalized to other ethnical groups due to the differences in tobacco dependence and cessation in acculturation and nicotine metabolism levels described in previous studies (55, 56). Gender differences in pharmacotherapies of smoking cessation are also non-negligible since it is clear that certain medication shows different efficacy between male and female participants desiring smoking cessation (56). The selection criteria of smoking intensity and duration in each included study can vary considerably, ranging from light-intermittent to heavy-daily smoking, and such differences in smoking intensity may indirectly affect patients’ confidence in quitting smoking (57). A more specifically stratified discussion in participants with different smoking intensities should be investigated in further studies.
Overall, there is a moderate level of variance among all included studies. Such variance may result from several possible aspects. Firstly, interventions were artificially classified, and the oronasal nicotine replacement therapy includes four different FDA-approved pharmacotherapies (nicotine nasal spray, nicotine inhaler, nicotine gum, and nicotine lozenge) with possibly variant effectiveness due to different nicotine delivering dosages and delivering routes. Secondly, the overcall control group also has the potential of being part of the variance. We summarized all placebo groups and control groups without blind settings from each trial as one single group, and there may also be differences in the effect on treatment outcomes between the different placebo and the unblinded control settings. Additionally, it has been described by Chan 2021, etc. that the diversity of e-cigarette products may also be problematic to generalize the results to newly-created e-cigarette products (5). And last, of all, the method chosen for verification of outcome measurement is also concerning. Though serum and urine cotinine are used for smoking cessation verification and quantitative measurement in some trials, exhaled CO is the mostly applied biochemical method for the same purpose and has revealed several shortages, including short half-life (58), and false-positive results with other smoking products (e.g., cannabis) (59).
It has been proved by several previous meta-analysis and RCTs and has been validated by this NMA that e-cigarette is effective in assisting smoking cessation. Compared to PSA , E-cig had a higher probability of superior ranking in smoking cessation effectiveness as measured by CSA , and this similar finding is also observed in the oronasal nicotine group. Based on this finding, we hypothesized that patients who use e-cigarettes and oronasal nicotine products would have higher adherence due to their similar nicotine delivery pattern to conventional tobacco cigarettes. This hypothesis is also validated by the NMA of treatment drop-out rates which demonstrates the highest acceptance of e-cigarettes and oronasal nicotine treatment among all interventions.
However, we should still be cautious to approve e-cigarettes as a therapeutical intervention for smoking cessation. E-cigarette or vaping product use-associated lung injury (EVALI), a novel entity including a broad spectrum of pulmonary diseases and may lead to respiratory failure, has continuously been reported (60–66). There is also growing evidence indicating generalized pulmonary toxicity may be caused by inhaling electronic cigarette vapor (67). Additionally, the psychoactive substances and special flavors of vapor have led to a surge in usage, especially among adolescents (68). A study from the U.S. indicates that more than 40% of high school students have tried e-cigarettes in the past year in 2020 (69). What is more alarming is that studies have proved that initial e-cigarette use is also associated with subsequent cigarette smoking initiation among adolescents and young adults (70, 71). Further discussion on whether e-cigarettes can be used as a pros-outweigh-cons intervention in assisting smoking cessation should follow more investigations on their long-term safety. On balance, we have reservations about e-cigarettes as a way to quit smoking.
5 Conclusion
Our study reported the efficacy and acceptability of five major pharmacological monotherapies and e-cigarette on smoking cessation through network meta-analysis including 40 RCT studies. We recommended that Varenicline, Cytisine and transdermal nicotine delivery, as smoking cessation intervention, have advantages and disadvantages. However, we had to have reservations about e-cigarettes as a way to quit smoking in adolescents.
Author contributions
YM: Writing – original draft, Writing – review & editing. SX: Writing – review & editing. LQ: Writing – original draft. YL: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was partly supported by the National Nature Science Foundation of China (YM, 81501174), and the Department of Civil Affairs of the provincial government of Sichuan (“Analysis of the current situation of mental (psychological) health of campus adolescents and research on countermeasures and suggestions”). Sichuan University - Dazhou City Cooperative Special Fund (2022CDDZ-22 to YM).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Reitsma, MB, Flor, LS, Mullany, EC, Gupta, V, Hay, SI, and Gakidou, E. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and initiation among young people in 204 countries and territories, 1990-2019. Lancet Public Health. (2021) 6:e472–81. doi: 10.1016/S2468-2667(21)00102-X
2. Patnode, CD, Henderson, JT, Coppola, EL, Melnikow, J, Durbin, S, and Thomas, RG. Interventions for tobacco cessation in adults, including pregnant persons: updated evidence report and systematic review for the US preventive services task force. JAMA. (2021) 325:280–98. doi: 10.1001/jama.2020.23541
3. Mottillo, S, Filion, KB, Bélisle, P, Joseph, L, Gervais, A, O'Loughlin, J, et al. Behavioural interventions for smoking cessation: a meta-analysis of randomized controlled trials. Eur Heart J. (2009) 30:718–30. doi: 10.1093/eurheartj/ehn552
4. Livingstone-Banks, J, Norris, E, Hartmann-Boyce, J, West, R, Jarvis, M, and Hajek, P. Relapse prevention interventions for smoking cessation. Cochrane Database Syst Rev. (2019) 2:CD003999. doi: 10.1002/14651858.CD003999.pub5
5. West, R, Raw, M, McNeill, A, Stead, L, Aveyard, P, Bitton, J, et al. Health-care interventions to promote and assist tobacco cessation: a review of efficacy, effectiveness and affordability for use in national guideline development. Addiction. (2015) 110:1388–403. doi: 10.1111/add.12998
6. Chan, GCK, Stjepanović, D, Lim, C, Sun, T, Shanmuga Anandan, A, Connor, JP, et al. A systematic review of randomized controlled trials and network meta-analysis of e-cigarettes for smoking cessation. Addict Behav. (2021) 119:106912. doi: 10.1016/j.addbeh.2021.106912
7. Malas, M, van der Tempel, J, Schwartz, R, Minichiello, A, Lightfoot, C, Noormohamed, A, et al. Electronic cigarettes for smoking cessation: a systematic review. Nicotine Tob Res. (2016) 18:1926–36. doi: 10.1093/ntr/ntw119
8. West, R, Zatonski, W, Cedzynska, M, Lewandowska, D, Pazik, J, Aveyard, P, et al. Placebo-controlled trial of cytisine for smoking cessation. N Engl J Med. (2011) 365:1193–200. doi: 10.1056/NEJMoa1102035
9. Caponnetto, P, Cibella, F, Mancuso, S, Campagna, D, Arcidiacono, G, and Polosa, R. Effect of a nicotine-free inhalator as part of a smoking-cessation programme. Eur Respir J. (2011) 38:1005–11. doi: 10.1183/09031936.00109610
10. Ward, K, Asfar, T, al Ali, R, Rastam, S, Weg, MWV, Eissenberg, T, et al. Randomized trial of the effectiveness of combined behavioral/pharmacological smoking cessation treatment in Syrian primary care clinics. Addiction. (2013) 108:394–403. doi: 10.1111/j.1360-0443.2012.04048.x
11. Cox, LS, Nollen, NL, Mayo, MS, Choi, WS, Faseru, B, Benowitz, NL, et al. Bupropion for smoking cessation in African American light smokers: a randomized controlled trial. J Natl Cancer Inst. (2012) 104:290–8. doi: 10.1093/jnci/djr513
12. de Dios, MA, Anderson, BJ, Stanton, C, Audet, DA, and Stein, M. Project impact: a pharmacotherapy pilot trial investigating the abstinence and treatment adherence of Latino light smokers. J Subst Abus Treat. (2012) 43:322–30. doi: 10.1016/j.jsat.2012.01.004
13. Heydari, G, Talischi, F, Tafti, SF, and Masjedi, MR. Quitting smoking with varenicline: parallel, randomised efficacy trial in Iran. Int J Tuberc Lung Dis. (2012) 16:268–72. doi: 10.5588/ijtld.11.0183
14. Tønnesen, P, Lauri, H, Perfekt, R, Mann, K, and Batra, A. Efficacy of a nicotine mouth spray in smoking cessation: a randomised, double-blind trial. Eur Respir J. (2012) 40:548–54. doi: 10.1183/09031936.00155811
15. Wong, J, Abrishami, A, Yang, Y, Zaki, A, Friedman, Z, Selby, P, et al. A perioperative smoking cessation intervention with varenicline: a double-blind, randomized, placebo-controlled trial. Anesthesiology. (2012) 117:755–64. doi: 10.1097/ALN.0b013e3182698b42
16. Bullen, C, Howe, C, Laugesen, M, McRobbie, H, Parag, V, Williman, J, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. (2013) 382:1629–37. doi: 10.1016/s0140-6736(13)61842-5
17. Caponnetto, P, Campagna, D, Cibella, F, Morjaria, JB, Caruso, M, Russo, C, et al. EffiCiency and safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. (2013) 8:e66317. doi: 10.1371/journal.pone.0066317
18. Cinciripini, PM, Robinson, JD, Karam-Hage, M, Minnix, JA, Lam, C, Versace, F, et al. Effects of varenicline and bupropion sustained-release use plus intensive smoking cessation counseling on prolonged abstinence from smoking and on depression, negative affect, and other symptoms of nicotine withdrawal. JAMA Psychiatry. (2013) 70:522–33. doi: 10.1001/jamapsychiatry.2013.678
19. Berlin, I, Grangé, G, Jacob, N, and Tanguy, ML. Nicotine patches in pregnant smokers: randomised, placebo controlled, multicentre trial of efficacy. BMJ. (2014) 348:g1622. doi: 10.1136/bmj.g1622
20. Cooper, S, Lewis, S, Thornton, JG, Marlow, N, Watts, K, Britton, J, et al. The SNAP trial: a randomised placebo-controlled trial of nicotine replacement therapy in pregnancy--clinical effectiveness and safety until 2 years after delivery, with economic evaluation. Health Technol Assess. (2014) 18:1–128. doi: 10.3310/hta18540
21. Gonzales, D, Hajek, P, Pliamm, L, Nackaerts, K, Tseng, LJ, McRae, TD, et al. Retreatment with varenicline for smoking cessation in smokers who have previously taken varenicline: a randomized, placebo-controlled trial. Clin Pharmacol Ther. (2014) 96:390–6. doi: 10.1038/clpt.2014.124
22. Scherphof, CS, van den Eijnden, RJ, Engels, RC, and Vollebergh, WA. Long-term efficacy of nicotine replacement therapy for smoking cessation in adolescents: a randomized controlled trial. Drug Alcohol Depend. (2014) 140:217–20. doi: 10.1016/j.drugalcdep.2014.04.007
23. Ebbert, JO, Hughes, JR, West, RJ, Rennard, SI, Russ, C, McRae, TD, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. (2015) 313:687–94. doi: 10.1001/jama.2015.280
24. Gray, KM, McClure, EA, Baker, NL, Hartwell, KJ, Carpenter, MJ, and Saladin, ME. An exploratory short-term double-blind randomized trial of varenicline versus nicotine patch for smoking cessation in women. Addiction. (2015) 110:1027–34. doi: 10.1111/add.12895
25. Hsueh, SC, Hsueh, KC, Chou, MY, and Tu, MS. A comparison of the effectiveness of varenicline and transdermal nicotine patch in outpatients following a standardized smoking cessation program in southern Taiwan. Eval Health Prof. (2015) 38:115–25. doi: 10.1177/0163278712466868
26. Anthenelli, RM, Benowitz, NL, West, R, St Aubin, L, McRae, T, Lawrence, D, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. (2016) 387:2507–20. doi: 10.1016/s0140-6736(16)30272-0
27. Baker, TB, Piper, ME, Stein, JH, Smith, SS, Bolt, DM, Fraser, DL, et al. Effects of nicotine patch vs Varenicline vs combination nicotine replacement therapy on smoking cessation at 26 weeks: a randomized clinical trial. JAMA. (2016) 315:371–9. doi: 10.1001/jama.2015.19284
28. Cunningham, JA, Kushnir, V, Selby, P, Tyndale, RF, Zawertailo, L, and Leatherdale, ST. Effect of mailing nicotine patches on tobacco cessation among adult smokers: a randomized clinical trial. JAMA Intern Med. (2016) 176:184–90. doi: 10.1001/jamainternmed.2015.7792
29. Ebbert, JO, Croghan, IT, Hurt, RT, Schroeder, DR, and Hays, JT. Varenicline for smoking cessation in light smokers. Nicotine Tob Res. (2016) 18:2031–5. doi: 10.1093/ntr/ntw123
30. Tuisku, A, Salmela, M, Nieminen, P, and Toljamo, T. Varenicline and nicotine patch therapies in young adults motivated to quit smoking: a randomized, placebo-controlled, prospective study. Basic Clin Pharmacol Toxicol. (2016) 119:78–84. doi: 10.1111/bcpt.12548
31. Tulloch, HE, Pipe, AL, Els, C, Clyde, MJ, and Reid, RD. Flexible, dual-form nicotine replacement therapy or varenicline in comparison with nicotine patch for smoking cessation: a randomized controlled trial. BMC Med. (2016) 14:80. doi: 10.1186/s12916-016-0626-2
32. Benli, AR, Erturhan, S, Oruc, MA, Kalpakci, P, Sunay, D, and Demirel, Y. A comparison of the efficacy of varenicline and bupropion and an evaluation of the effect of the medications in the context of the smoking cessation programme. Tob Induc Dis. (2017) 15:10. doi: 10.1186/s12971-017-0116-0
33. Carpenter, MJ, Heckman, BW, Wahlquist, AE, Wagener, TL, Goniewicz, ML, Gray, KM, et al. A naturalistic, randomized pilot trial of E-cigarettes: uptake, exposure, and Behavioral effects. Cancer Epidemiol Biomarkers Prev. (2017) 26:1795–803. doi: 10.1158/1055-9965.Epi-17-0460
34. Halpern, SD, Harhay, MO, Saulsgiver, K, Brophy, C, Troxel, AB, and Volpp, KG. A pragmatic trial of E-cigarettes, incentives, and drugs for smoking cessation. N Engl J Med. (2018) 378:2302–10. doi: 10.1056/NEJMsa1715757
35. Preloading, I. Effects on abstinence of nicotine patch treatment before quitting smoking: parallel, two arm, pragmatic randomised trial. BMJ. (2018) 361:k2164. doi: 10.1136/bmj.k2164
36. Gilbert, DG, Rabinovich, NE, Gilbert-Matuskowitz, EA, Klein, KP, and Pergadia, ML. Smoking abstinence symptoms across 67 days compared with randomized controls-moderation by nicotine replacement therapy, bupropion, and negative-affect traits. Exp Clin Psychopharmacol. (2019) 27:536–51. doi: 10.1037/pha0000278
37. Lee, SH, Ahn, SH, and Cheong, YS. Effect of electronic cigarettes on smoking reduction and cessation in Korean male smokers: a randomized controlled study. J Am Board Fam Med. (2019) 32:567–74. doi: 10.3122/jabfm.2019.04.180384
38. Masiero, M, Lucchiari, C, Mazzocco, K, Veronesi, G, Maisonneuve, P, Jemos, C, et al. E-cigarettes may support smokers with high smoking-related risk awareness to stop smoking in the short run: preliminary results by randomized controlled trial. Nicotine Tob Res. (2019) 21:119–26. doi: 10.1093/ntr/nty047
39. Oncken, C, Dornelas, EA, Kuo, CL, Sankey, HZ, Kranzler, HR, Mead, EL, et al. Randomized trial of nicotine inhaler for pregnant smokers. Am J Obstet Gynecol MFM. (2019) 1:10–8. doi: 10.1016/j.ajogmf.2019.03.006
40. Dogar, O, Keding, A, Gabe, R, Marshall, AM, Huque, R, Barua, D, et al. Cytisine for smoking cessation in patients with tuberculosis: a multicentre, randomised, double-blind, placebo-controlled phase 3 trial. Lancet Glob Health. (2020) 8:e1408–17. doi: 10.1016/S2214-109X(20)30312-0
41. Eisenberg, MJ, Hébert-Losier, A, Windle, SB, Greenspoon, T, Brandys, T, Fülöp, T, et al. Effect of e-cigarettes plus Counseling vs Counseling alone on smoking cessation: a randomized clinical trial. JAMA. (2020) 324:1844–54. doi: 10.1001/jama.2020.18889
42. Gray, KM, Rubinstein, ML, Prochaska, JJ, DuBrava, SJ, Holstein, AR, Samuels, L, et al. High-dose and low-dose varenicline for smoking cessation in adolescents: a randomised, placebo-controlled trial. Lancet Child Adolesc Health. (2020) 4:837–45. doi: 10.1016/s2352-4642(20)30243-1
43. Nides, M, Danielsson, T, Saunders, F, Perfekt, R, Kapikian, R, Solla, J, et al. Efficacy and safety of a nicotine mouth spray for smoking cessation: a randomized, Multicenter, controlled study in a naturalistic setting. Nicotine Tob Res. (2020) 22:339–45. doi: 10.1093/ntr/nty246
44. Shiffman, S, Scholl, SM, Mao, J, Ferguson, SG, Hedeker, D, Primack, B, et al. Using nicotine gum to assist nondaily smokers in quitting: a randomized clinical trial. Nicotine Tob Res. (2020) 22:390–7. doi: 10.1093/ntr/ntz090
45. Walker, N, Parag, V, Verbiest, M, Laking, G, Laugesen, M, and Bullen, C. Nicotine patches used in combination with e-cigarettes (with and without nicotine) for smoking cessation: a pragmatic, randomised trial. Lancet Respir Med. (2020) 8:54–64. doi: 10.1016/s2213-2600(19)30269-3
46. Xiao, D, Kotler, M, Kang, J, and Wang, C. A Multicenter, randomized, double-blind, parallel, placebo-controlled clinical study to evaluate the efficacy and safety of a nicotine mint lozenge (2 and 4mg) in smoking cessation. J Addict Med. (2020) 14:69–77. doi: 10.1097/adm.0000000000000547
47. Courtney, RJ, McRobbie, H, Tutka, P, Weaver, NA, Petrie, D, Mendelsohn, CP, et al. Effect of Cytisine vs Varenicline on smoking cessation: a randomized clinical trial. JAMA. (2021) 326:56–64. doi: 10.1001/jama.2021.7621
48. Nides, M, Rigotti, NA, Benowitz, N, Clarke, A, and Jacobs, C. A Multicenter, double-blind, randomized, placebo-controlled phase 2b trial of Cytisinicline in adult smokers (the ORCA-1 trial). Nicotine Tob Res. (2021) 23:1656–63. doi: 10.1093/ntr/ntab073
49. Hespanhol, L, Vallio, CS, Costa, LM, and Saragiotto, BT. Understanding and interpreting confidence and credible intervals around effect estimates. Braz J Phys Ther. (2019) 23:290–301. doi: 10.1016/j.bjpt.2018.12.006
50. Greco, T, Landoni, G, Biondi-Zoccai, G, D'Ascenzo, F, and Zangrillo, A. A Bayesian network meta-analysis for binary outcome: how to do it. Stat Methods Med Res. (2016) 25:1757–73. doi: 10.1177/0962280213500185
51. Spiegelhalter, DJ, Best, NG, Carlin, BP, and Van der Linde, A. Bayesian deviance, the effective number of parameters, and the comparison of arbitrarily complex models. Research Report, (1998) 98–009.
52. Salanti, G, Higgins, JP, Ades, AE, and Ioannidis, JP. Evaluation of networks of randomized trials. Stat Methods Med Res. (2008) 17:279–301. doi: 10.1177/0962280207080643
53. Galiatsatos, P, Garfield, J, Melzer, AC, Leone, FT, Farber, HJ, Ruminjo, JK, et al. Summary for clinicians: an ATS clinical practice guideline for initiating pharmacologic treatment in tobacco-dependent adults. Ann Am Thorac Soc. (2021) 18:187–90. doi: 10.1513/AnnalsATS.202008-971CME
54. Barua, RS, Rigotti, NA, Benowitz, NL, Cummings, KM, Jazayeri, MA, Morris, PB, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of Cardiology Task Force on clinical expert consensus documents. J Am Coll Cardiol. (2018) 72:3332–65. doi: 10.1016/j.jacc.2018.10.027
55. Kim, SS, Ziedonis, D, and Chen, KW. Tobacco use and dependence in Asian Americans: a review of the literature. Nicotine Tob Res. (2007) 9:169–84. doi: 10.1080/14622200601080323
56. Kulak, JA, Cornelius, ME, Fong, GT, and Giovino, GA. Differences in quit attempts and cigarette smoking abstinence between whites and African Americans in the United States: literature review and results from the international tobacco control US survey. Nicotine Tob Res. (2016) 18:S79–87. doi: 10.1093/ntr/ntv228
57. Flower, M, Nandakumar, L, Singh, M, Wyld, D, Windsor, M, and Fielding, D. Respiratory bronchiolitis-associated interstitial lung disease secondary to electronic nicotine delivery system use confirmed with open lung biopsy. Respirol Case Rep. (2017) 5:e00230. doi: 10.1002/rcr2.230
58. McCauley, L, Markin, C, and Hosmer, D. An unexpected consequence of electronic cigarette use. Chest. (2012) 141:1110–3. doi: 10.1378/chest.11-1334
59. Arter, ZL, Wiggins, A, Hudspath, C, Kisling, A, Hostler, DC, and Hostler, JM. Acute eosinophilic pneumonia following electronic cigarette use. Respir Med Case Rep. (2019) 27:100825. doi: 10.1016/j.rmcr.2019.100825
60. Sommerfeld, CG, Weiner, DJ, Nowalk, A, and Larkin, A. Hypersensitivity pneumonitis and acute respiratory distress syndrome from E-cigarette use. Pediatrics. (2018) 141:e20163927. doi: 10.1542/peds.2016-3927
61. Edmonds, PJ, Copeland, C, Conger, A, and Richmond, BW. Vaping-induced diffuse alveolar hemorrhage. Respir Med Case Rep. (2020) 29:100996. doi: 10.1016/j.rmcr.2020.100996
62. Agustin, M, Yamamoto, M, Cabrera, F, and Eusebio, R. Diffuse alveolar Hemorrhage induced by vaping. Case Rep Pulmonol. (2018) 2018:9724530–3. doi: 10.1155/2018/9724530
63. Layden, JE, Ghinai, I, Pray, I, Kimball, A, Layer, M, Tenforde, MW, et al. Pulmonary illness related to E-cigarette use in Illinois and Wisconsin - final report. N Engl J Med. (2020) 382:903–16. doi: 10.1056/NEJMoa1911614
64. Fadus, MC, Smith, TT, and Squeglia, LM. The rise of e-cigarettes, pod mod devices, and JUUL among youth: factors influencing use, health implications, and downstream effects. Drug Alcohol Depend. (2019) 201:85–93. doi: 10.1016/j.drugalcdep.2019.04.011
65. Overbeek, DL, Kass, AP, Chiel, LE, Boyer, EW, and Casey, AMH. A review of toxic effects of electronic cigarettes/vaping in adolescents and young adults. Crit Rev Toxicol. (2020) 50:531–8. doi: 10.1080/10408444.2020.1794443
66. Soneji, S, Barrington-Trimis, JL, Wills, TA, Leventhal, AM, Unger, JB, Gibson, LA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. (2017) 171:788–97. doi: 10.1001/jamapediatrics.2017.1488
67. O'Brien, D, Long, J, Quigley, J, Lee, C, McCarthy, A, and Kavanagh, P. Association between electronic cigarette use and tobacco cigarette smoking initiation in adolescents: a systematic review and meta-analysis. BMC Public Health. (2021) 21:954. doi: 10.1186/s12889-021-10935-1
68. Higgins, JP, Jackson, D, Barrett, JK, Lu, G, Ades, AE, and White, IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. (2012) 3:98–110. doi: 10.1002/jrsm.1044
69. Dias, S, Welton, NJ, Caldwell, DM, and Ades, AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. (2010) 29:932–44. doi: 10.1002/sim.3767
70. Salanti, G, Ades, AE, and Ioannidis, JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. (2011) 64:163–71. doi: 10.1016/j.jclinepi.2010.03.016
71. Salanti, G, Del Giovane, C, Chaimani, A, Caldwell, DM, and Higgins, JP. Evaluating the quality of evidence from a network meta-analysis. PLoS One. (2014) 9:e99682. doi: 10.1371/journal.pone.0099682
72. Ni, K, Wang, B, Link, AR, and Sherman, SE. Does smoking intensity predict cessation rates? A study of light-intermittent, light-daily, and heavy smokers enrolled in two telephone-based Counseling interventions. Nicotine Tob Res. (2020) 22:423–30. doi: 10.1093/ntr/nty257
73. Benowitz, NL, Bernert, JT, Foulds, J, Hecht, SS, Jacob, P III, Jarvis, MJ, et al. Biochemical verification of tobacco use and abstinence: 2019 update. Nicotine Tob Res. (2020) 22:1086–97. doi: 10.1093/ntr/ntz132
74. Moolchan, ET, Zimmerman, D, Sehnert, SS, Zimmerman, D, Huestis, MA, and Epstein, DH. Recent marijuana blunt smoking impacts carbon monoxide as a measure of adolescent tobacco abstinence. Subst Use Misuse. (2005) 40:231–40. doi: 10.1081/ja-200048461
Keywords: smoking cessation, e-cigarette, nicotine replacement therapy, cytisine, varenicline, bupropion
Citation: Meng Y, Xiang S, Qu L and Li Y (2024) The efficacy and acceptability of pharmacological monotherapies and e-cigarette on smoking cessation: a systemic review and network meta-analysis. Front. Public Health. 12:1361186. doi: 10.3389/fpubh.2024.1361186
Edited by:
Jean Lud Cadet, National Institute on Drug Abuse (NIH), United StatesReviewed by:
Robert J. Wellman, UMass Chan Medical School, United StatesFrancis Kalemeera, Independent Researcher, Kampala, Uganda
Copyright © 2024 Meng, Xiang, Qu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lang Qu, lqu@lifebridgehealth.org; Ying Li, yingli.huaxi@foxmail.com
 Yajing Meng
Yajing Meng Sike Xiang1
Sike Xiang1 Lang Qu
Lang Qu