- 1GoodHope Ehlers Danlos Syndrome Clinic, Toronto General Hospital, University Health Network, Toronto, ON, Canada
- 2Division of Physical Medicine and Rehabilitation, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada
- 3Faculty of Kinesiology and Physical Education, University of Toronto, Toronto, ON, Canada
- 4Department of Anesthesiology and Pain Management, Toronto General Hospital, University Health Network, Toronto, ON, Canada
- 5Department of Psychology, Faculty of Health, York University, Toronto, ON, Canada
- 6Department of Anesthesiology and Pain Medicine, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada
- 7Division of Respirology, Temerty Faculty of Medicine, Toronto General Hospital Research Institute, University Health Network, Toronto, ON, Canada
Introduction: The Ehlers-Danlos Syndromes (EDS) and Generalized Hypermobility Spectrum Disorders (G-HSD) comprise a heterogeneous group of genetic disorders of abnormal synthesis and/or maturation of collagen and other matricellular proteins. EDS is commonly characterized by manifestations such as multi joint hypermobility that can lead to musculoskeletal pains, subluxations and dislocations, fragile skin, organ dysfunction, and chronic significant diffuse pain with fatigue, deconditioning eventuating to poor quality of life. Evidence suggests exercise and rehabilitation interventions may ameliorate symptoms of unstable joints, recurrent subluxations/dislocations, and chronic widespread musculoskeletal pain. To date, there have only been a few reports describing exercise and rehabilitation care strategies for people with EDS.
Methods: In this manuscript, we describe the GoodHope Exercise and Rehabilitation (GEAR) program, its overarching principles, as well as the program development and delivery model. The GEAR program aims to decrease functional impairment, reduce pain, increase confidence in symptom self-management, and provide a community of support for people with EDS/G-HSD. To achieve these goals, we detail the model of care that includes exercise and rehabilitation therapy, education for self-management, and support accessing relevant community resources.
Strengths and Limitations of the Study: GEAR represents a novel exercise and rehabilitation care model for people with G-HSD and various clinical EDS subtypes, beyond the commonly included hEDS subtype. Systematic collection of data via validated measurements is ongoing and will guide the refinement of GEAR and support the development of emerging exercise and rehabilitation programs for people with EDS.
Introduction
The Ehlers-Danlos Syndromes (EDS) and Generalized Hypermobility Spectrum Disorders (G-HSD) are overarching terms for a heterogeneous group of genetic connective tissue disorders (1, 2). EDS/G-HSD predominantly affect the musculoskeletal, gastrointestinal, and cardiovascular systems and are characterized by the abnormal synthesis and/or maturation of collagen and matricellular proteins in the body (3, 4). The symptoms typically manifest as fragile skin, organ dysfunction, significant diffuse pain (2), and hypermobility that can lead to recurrent joint dislocations and other injuries (5–7). Of the 13 identified types of EDS, 12 can be confirmed by molecular analyses allowing for precise diagnosis and clinical direction on inheritance patterns, and can guide approaches to management. The most common EDS subtype, known as hypermobile EDS (hEDS), has an unidentifiable genetic profile. Thus, identification of this subtype relies on clinical presentation using objective 2017 EDS criteria including specific systematic manifestations of connective tissue disorder and joint hypermobility (1). The spectrum of joint hypermobility extends from asymptomatic joint laxity to generalized joint hypermobility. Individuals that exhibit generalized joint hypermobility (>4 or 5 joints [based on age]) and do not have a clinical presentation consistent with other heritable disease—including hEDS—are diagnosed with G-HSD (2). For consistency in this paper, all references to EDS or G-HSD will collectively be referred to as EDS/G-HSD.
Individuals with EDS/G-HSD can experience a unique set of physical sequelae, most commonly painful and/or unstable joints, chronic widespread musculoskeletal pain, and a history of recurrent joint subluxations and dislocations (8). For example, De Coster et al. (9) observed that dysfunction of the temporomandibular joint is reported in >70% of hEDS patients. Stanitski et al. (6) observed that 72% of hEDS patients reported joint dislocation −26% of which were hip dislocations. Additionally, Ainsworth et al. (10) observed dislocation of the shoulder and knee joints in 63 and 57% of hEDS patients, respectively. In light of the chronic pain and high risk of musculoskeletal injury, patients with EDS/G-HSD are prone to kinesiophobia [fear of movement and (re) injury] and behavioral responses that limit their involvement in essential and recreational activities (11). The resultant sedentary lifestyle may further exacerbate impairments, deconditioning, and compromise quality of life, while increasing the risk of cardiometabolic disease (12).
An often-proposed management strategy for clinical and functional manifestations of EDS/G-HSD is therapeutic exercise (13, 14). Therapeutic exercise is defined as a planned performance of physical movement, postures, or activities intended to provide patients with the means to remediate or prevent impairments of functions and structures; and in doing so, optimize overall health, fitness, or sense of well-being (15). Exercise in people with EDS/G-HSD has generally employed locoregional exercises that target isolated instability or impairment, such as knee proprioception (16), inspiratory muscle strength (17), and spinal stabilization (18). General conditioning-based exercise to ameliorate poor cardiorespiratory fitness and musculoskeletal strength that occur secondary to physical inactivity has also been highlighted in the literature as an important self-management strategy for symptom management (19, 20). Accordingly, exercise and rehabilitation are recommended in comprehensive EDS/G-HSD care models (18–20).
In this manuscript, we describe the GoodHope Exercise and Rehabilitation (GEAR) program. The mission of GEAR is to improve the physical function and psychosocial well-being of people with EDS/G-HSD through exercise and rehabilitation, education for self-management, and supported engagement in additional community resources. Opportunities for related research are also discussed in an effort to advance this nascent literature.
Methods
GEAR is a part of the GoodHope EDS Clinic that is situated in the Toronto General Hospital in Toronto, Ontario, Canada (details of the GoodHope EDS clinic are provided elsewhere) (21). GEAR is delivered in an exercise and rehabilitation facility of ~1,200 square feet where assessments, exercise and rehabilitation therapy, and consultations related to education and community resources are conducted. The facility includes exercise and rehabilitation equipment, clinician workstations, and a patient waiting and changing area. GEAR is delivered by physiotherapists and kinesiologists (hereafter referred to as GEAR clinicians). This protocol was informed by relevant literature (15, 19, 20, 22) and designed by an inter-professional healthcare team, including kinesiologists, nurses, physicians, physiotherapists, and psychologists—each with experience specific to EDS/G-HSD or related injuries and chronic conditions. The program design was guided by expertise in the management of functional manifestations of EDS, implementation science, integrated research, home-based exercise, and psychosocial influences on treatment.
Eligibility and Referral to GEAR
GoodHope EDS Clinic patients requiring exercise and rehabilitation care are directly referred to GEAR. Eligibility criteria for GEAR is determined in consultation with the patient and related to their overall function and symptom burden, interest and willingness to participate in exercise and rehabilitation, and ability to attend GEAR appointments. All referrals include a GoodHope EDS Impairment and Interference Scale (GEIIS) rating (23). The GEIIS is a brief, check-list style, medical screening tool that provides a general indication of a patient's physical function and impairment to advise appointment planning. Patients with a GEIIS rating of 4 (“Unable to perform all self-care activities; not ambulatory”) are not eligible for GEAR.
GEAR Programming/Interventions
GEAR is delivered via three integrated service components: (i) individually tailored, home-based exercise and rehabilitation therapy; (ii) education on self-management for localized and systemic EDS/G-HSD symptoms; and (iii) connections with community-based services. Patient care plans are divided into two streams, an intervention stream and a consultation stream, summarized in Figure 1.
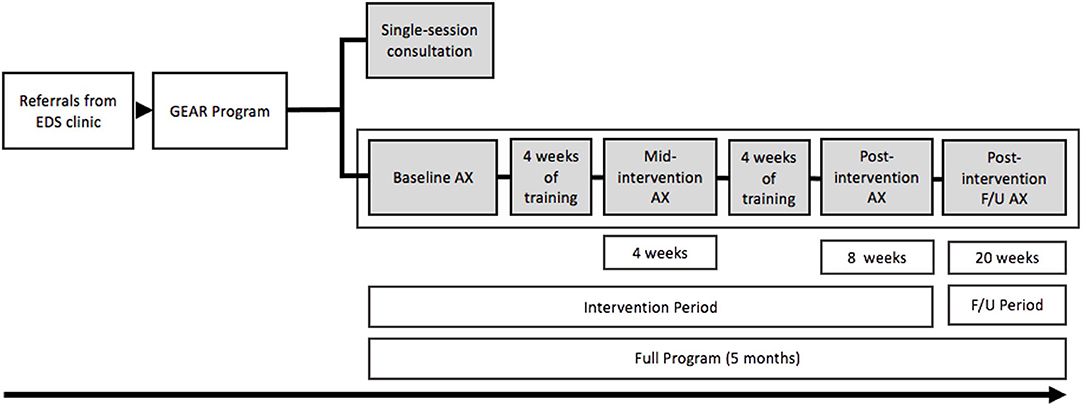
Figure 1. Depiction of the GEAR program and the full program time line of the intervention, assessments (AX), and follow up (F/U).
Intervention Stream
The GEAR intervention is initiated with a 2-h intake assessment with a GEAR clinician and includes a series of postural and functional tests to help identify symptomatic body regions and aberrant or dysfunctional movement patterns. It also includes a comprehensive patient interview focused on functional capacity, activities of daily living, pain (instigators and relievers), occupational and recreational physical activity, and general goals for physical and psychosocial wellness. Physical measures of function and pain are employed as needed and include range of motion, strength and muscle activation, proprioception, neurodynamic tension, balance, and aerobic endurance which collectively support the development of an 8-week care plan. Although exercise interventions have been observed to be effective on physical outcomes in as little as 4 to 6 weeks for individuals with EDS/G-HSD (16, 20, 22), To and Alexander (24) observed that the relationship between the length of the intervention and strength-gains is linear. Therefore, to effect greater change on physical outcomes, GEAR programming is delivered over an 8-week time frame, and to further these gains, has incorporated patient self-management education to enhance long-term adherence (20).
The intake assessment informs the provision of an 8-week individualized conditioning and rehabilitation exercise prescription that can be completed at home or at an available community-based facility that the patient has access to. GEAR intervention participants are expected to return at week-4 (mid-point) and week-8 (post-intervention) for re-assessment and programming adaptation (as required; see Figure 1 for patient flow). Patients return at 12 weeks post-intervention to assess the maintenance of symptom management goals and behaviors and are subsequently discharged from GEAR. If patients require further assistance with their GEAR activities or need assistance with transitioning care to a community health professional, they may book additional follow-up appointments (via telephone or in-person) for up to six months post-discharge. If patients require assistance beyond the six-month post-discharge timeframe, they require a re-referral from the GoodHope EDS Clinic. Patients may not complete the GEAR intervention multiple times; however, at the discretion of GoodHope EDS clinic staff, patients can be re-referred for a GEAR consultation (described below) to address new or evolved exercise and rehabilitation needs. The following sections detail the therapeutic objectives and strategies within each component of GEAR.
Exercise and Rehabilitation Therapy
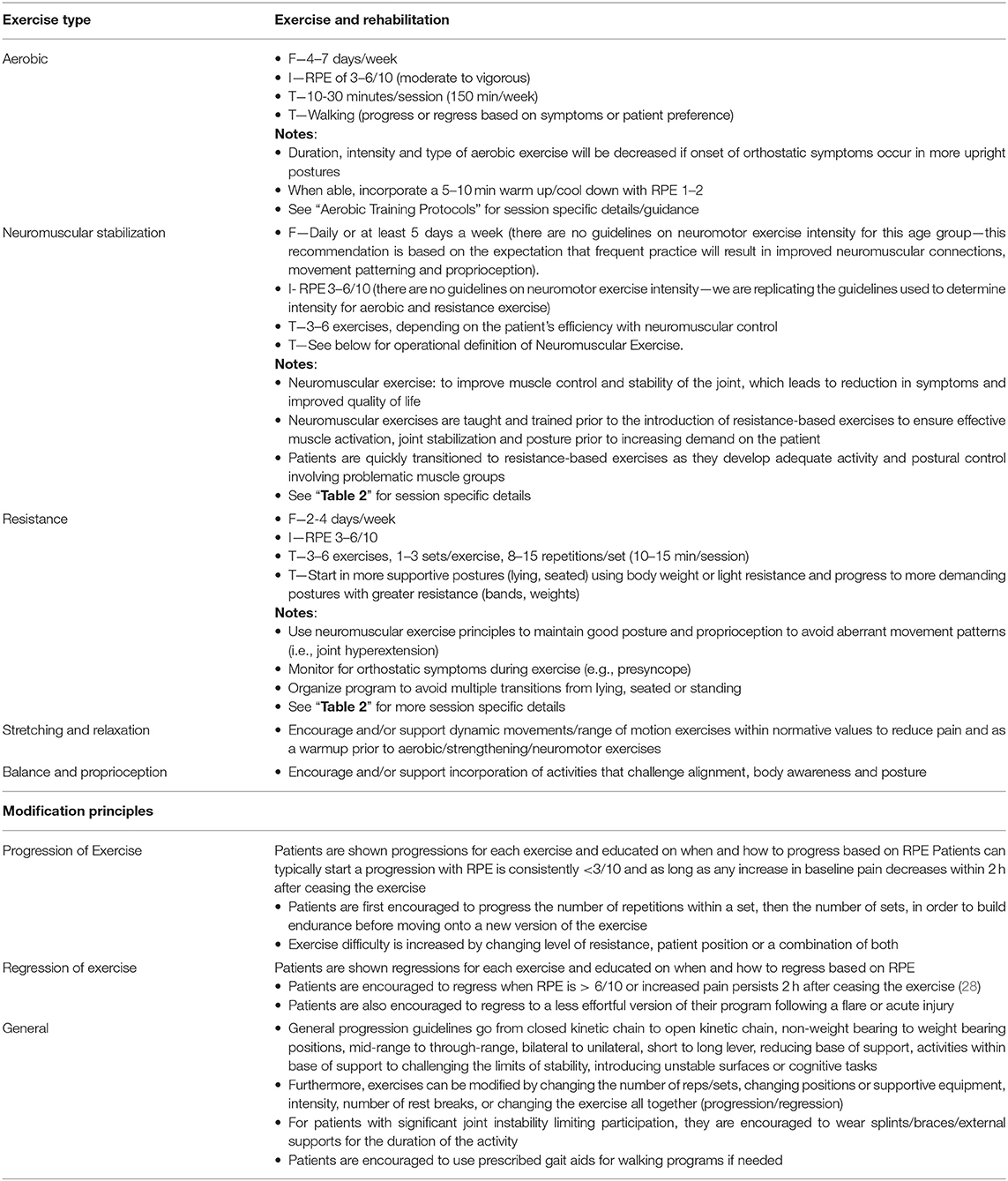
Informed by the intake assessment, therapeutic and conditioning exercises are prescribed to reduce symptom burden and improve functional capacity. Initial exercise prescriptions consist of general aerobic exercise recommendations and 3–5 neuromuscular and/or resistance training exercises. Aerobic exercises are typically focused on increasing endurance and overall physical activity volume to meet the Canada's physical activity guidelines for adults (25). To ensure patient safety, patients undergo cardio-pulmonary screening with resting (sitting and standing) blood pressure, resting heart rate, and arterial oxygen saturation level before engaging in or receiving a prescription for aerobic activity. The resistance and neuromuscular exercises follow general guidelines such as: progressions from closed kinetic chain to open kinetic chain, proximal to distal, non-weight bearing to weight bearing positions, mid-range isometrics to through-range activation, bilateral to unilateral, and short to long lever. Additionally, these exercises work to challenge the limits of stability by reducing base of support, and introducing unstable surfaces or cognitive tasks. Patients are encouraged to participate in gentle dynamic movements and range of motion exercises as warmup activities prior to proprioceptive and strengthening exercises in order to reduce pain and risk of injury (26). These exercises are reviewed and adapted or expanded upon as tolerated (i.e., additional exercises added to the exercise prescription) during their 4- and 8-week assessments. Each prescription is tailored to the patient's EDS/G-HSD symptoms and may involve adapted exercises to accommodate joint hypermobility, orthostatic intolerance, gastrointestinal symptoms, multiple joint/organ symptom involvement, and localized or diffuse pain.
Exercise prescriptions are provided using the conventional frequency, intensity, time, and type (FITT) parameters for exercise volume using the modalities described above. Patients have an opportunity to practice the exercises before they leave their appointments and are educated on how to use a modified Rating of Perceived Exertion (RPE) Scale to monitor aerobic, neuromuscular, and resistance-based exercises (27). GEAR uses the modified RPE scale measuring 0–10, in which easy measures at 0–2, moderate at 3, somewhat hard at 4, hard at 5–6, and extremely hard at 7–10. Neuromotor exercises are typically prescribed in the 4–6/10 range (somewhat hard to hard), and resistance-based and aerobic exercises are prescribed in the 3–6/10 range (moderate to hard), and all prescriptions are progressed as tolerated. In addition to RPE, additional indicators of exertion are instructed, such as the breath-sound check and talk test. Additional factors to RPE are considered when prescribing exercise including mood states, baseline pain, environmental conditions (i.e., access to equipment or positional preferences), exercise mode, and age. Table 1 provides the GEAR program guidelines for exercise prescriptions and sample exercises are provided in Table 2.
Self-Management Education
Basset's group found that patients' remissions and beliefs about their injuries or disorders can influence their level of rehabilitation adherence (23). Thus, individuals living with conditions such as EDS/G-HSD may perceive common symptoms such as chronic pain or reoccurring pain flare up episodes as barriers to exercise. GEAR patients receive self-management education (SME) in order to remain adherent to their prescribed exercise and rehabilitation program and engage in an active lifestyle for general health promotion, cope with episodic flare-ups of their pain, and reduce kinesiophobia. Progressive self-management strategies are discussed to support patient autonomy in establishing and adapting their treatment and health-related goals, as well as enhancing self-motivation and self-efficacy for long-term health behaviors (23).
GEAR SME focuses on the behavioral response to an acute pain flare-up which can include: employing pain-relieving positions; managing acute and recurrent subluxations; using mobility aids, braces or splints for joint support; using hot or cold modalities (e.g., compresses and packs); and modifying exercises to minimize or relieve pain. Second, energy conservation strategies are discussed in order to manage fatigue and to improve capacity for and adherence to physical activity. Finally, topics such as counter pressure maneuvers to manage orthostatic intolerance and long-term health behavior strategies, such as SMART goal setting (Specific, Measurable, Achievable, Realistic and Time-limited) approach (29) are used identify goals and monitor effectiveness of treatment.
Community Resource Engagement
Given the variable and recurrent nature of pain and impairment experienced by individuals with EDS/G-HSD, patients require hands-on therapy for acute symptom management, modification of a program, or aid in addressing the onset of a new symptom. As such, connecting with a community-based healthcare professional is key in providing ongoing exercise and rehabilitative care. Community connectedness is defined as belonging to a larger collective with whom an individual can establish mutually influential relationships, satisfy their needs, be rewarded through their affiliation, and build shared emotional connections (30, 31). It has been shown that community connectedness can lead to a positive impact on physical health outcomes with higher levels of connectedness being related to less pain, more energy, better physical functioning, and fewer limitations in those with chronic disease (32, 33). Further, maintenance of exercise regimes, especially in chronic disease populations, has been shown to significantly contribute to the preservation of functional benefits gained through short-term exercise programs (34, 35). Recognizing the impact of connectedness on health, the contribution of exercise maintenance, and the limited duration of the GEAR program (20 weeks including all standard follow-ups), the community resource engagement component aims to build a network of support for patients following their participation in the program. Patients are supported in this process through encouragement to develop relationships with a community exercise and rehabilitation professionals through the following supportive aids:
(i) Finding a local exercise and rehabilitation professional: Patients are guided through the process of finding a local clinician that is best suited for their individual rehabilitation and exercise-related conditions and/or goals. Patients are encouraged to request a clinician who has previous experiences treating people with EDS/G-HSD or an interest in learning about the sequelae that people with EDS/G-HSD commonly present with. Patients are also encouraged to seek clinicians that have specialization in specific areas of need common to the EDS/G-HSD population such as pelvic floor or TMJ dysfunction, acupuncture, or sport specific rehabilitation. Exercise and rehabilitation professionals that may be involved in community-based care include: physiotherapists, occupational therapists, orthotists, acupuncturists, massage therapists, kinesiologists, aquatic rehabilitation professionals, and personal trainers. These health professionals may be accessible at local fitness, community, wellness centers or private clinics. As (36) have observed, patients' experiences during treatment can significantly influence the outcomes of rehabilitation therapy, and thus patients are recommended to seek support from those who share similar interests and/or treatment philosophies.
(ii) Best practices while working with a community health professional: Patients are encouraged to employ several recommended practices when working with a community health professional, including setting SMART goals, which should be regularly revisited and measured in order to track changes. Patients are also encouraged to set a specific timeline and open discussion on plan of care (frequency and nature of sessions) to avoid early exhaustion of insurance benefits. Lastly, upon request, a summary of GEAR clinical notes and/or patient-specific recommendations can be shared with the local health professional.
Consultation Stream
Some GoodHope EDS Clinic patients who are appropriate but unable to participate in GEAR due to significant barriers to program participation, such as distance from the facility resulting in significant time or monetary costs associated with attending the program appointments or competing priorities that preclude engagement in routine exercise and rehabilitation. These patients may be referred for a single consultation session comprising generalized assessment and SME similar to those described in the baseline assessment in the GEAR intervention but is distilled to address only the top priorities of the patient's needs that can be supported within a single, two-h consultation appointment. Similar to the GEAR intervention, patients in this stream requiring further assistance are able to book additional follow-up telephone appointments with a GEAR clinician for up-to six months in efforts to help network-building with community-based services.
Anticipated Results and Data Collection
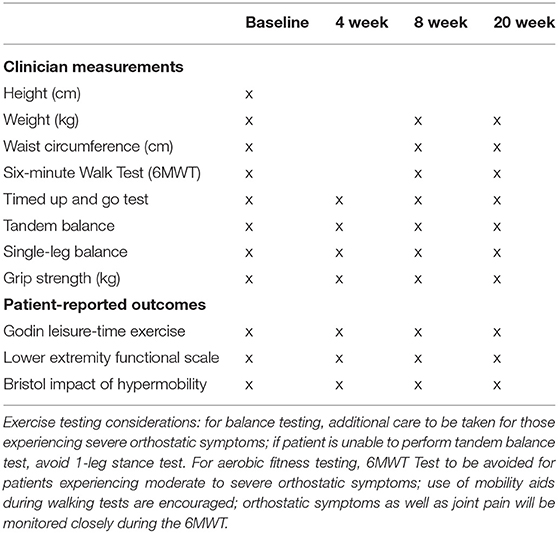
Given the dearth of evidence to guide exercise and rehabilitation therapy for people with EDS/G-HSD, a core function of GEAR is to systematically collect data relevant to patient outcomes and program participation. Within GEAR, standardized data collection occurs at each assessment which are intended to provide insight for patient care as well as contribute to a data repository for potential research use in cross-sectional and longitudinal analyses. It is important to make the distinction that GEAR itself is not a study but a program that supports current and future studies. An institutional ethics board clearance for data collection has been approved for all studies pertaining to GEAR, as will all future studies. All the patients willing to participate in the research arm of GEAR are contacted by a research assistant to explain the study in detail and for consent. Only consenting individual's data will be used in comprehensive analysis for publications purposes. The specific data collected at each time point are described in Table 3. The functional tests outlined in Table 3 were selected based on the following characteristics: (i) requirement of minimal equipment and easily replicable, (ii) a short test duration to limit patient burden, (iii) accessible given the physical sequelae common to individuals with EDS/G-HSD; (iv) and together capture changes in upper extremity strength, lower extremity strength, cardiovascular capacity, and postural control and balance. Although these are robust as a collection of tests, it is recognized that additional tests are needed to capture the full potential effects of GEAR interventions. Additional measures may be employed to further inform care but may not be routinely collected for all patients, such as five times sit-to-stand test (37), gait assessment, and stair climb test. Beyond the functional test results, comprehensive charting is also recorded which describes each patient's physical assessment, their response to previous sessions, maintenance therapy, and any adverse effects. This systematic collection of validated measurements and charting will guide the refinement of GEAR and support the development of emerging exercise and rehabilitation programs for this underserved population.
Discussion
In this manuscript, we describe a model of exercise and rehabilitation care for people with EDS/G-HSD—an area of clinical care that remains largely underreported. This is likely due to the limited evidence describing the use and benefit of such interventions in ameliorating EDS/G-HSD related symptoms. In a recent systematic review, Palmer et al. (38) identified only 11 studies that explored the effect of exercise interventions in people with EDS/G-HSD showing that they were beneficial for various physical and psychological outcomes, such as improved muscle strengthening, proprioceptive acuity, and quality of life (38). In light of the emerging evidence and clinical rationale for symptom-management and health promoting therapy, the GEAR program was developed as a core component of comprehensive EDS/G-HSD care in the GoodHope EDS clinic.
GEAR has several strengths to highlight: (i) program components have been developed based on an integrated research design, (ii) the program is delivered by an interprofessional clinical team including physiotherapists, kinesiologists, and physicians—each holding experience specific to EDS/G-HSD, (iii) it is a multi-pronged program including exercise and rehabilitation treatment, SME, and community resource engagement support; (iv) the program is offered to people with G-HSD and various clinical EDS subtypes—beyond the commonly included hEDS subtype, (v) treatment plans are individually tailored, (vi) focus is given to both acute and long-term outcomes; (vii) programming is delivered in a dedicated fully-equipped facility, and (viii) program participation is free of charge.
We also note challenges that GEAR has faced, namely its location within an urban hospital that poses attendance related barriers (e.g., commute time, cost of parking, and public transit can be a burden for some). GEAR is also only available to patients during regular business hours, which provides a barrier to those who have limited time available throughout the working day. Related to its setting, we also note that our program and its development may not be generalizable to community hospitals or other health care systems with different organizational barriers and resource constraints. Nevertheless, iterations of this program are likely possible in academic hospitals with a strong research program and sufficient financial support. An important aim of the GEAR program is to establish connections with community partners, to improve accessibility, and in turn, increase program reach. Importantly, the absence of high-quality research in this field is also noteworthy. This gap in the literature justifies an investment in research to optimally support evidence-based care. Through the combination of available evidence and interdisciplinary expert contributions, this initial iteration of GEAR has been established and will remain responsive to evolutions in the research literature.
Future growth of GEAR is multifaceted and involves both improving on the delivery of the program, as well as extending care to additional subgroups. In terms of program structure, GEAR will be implementing electronic data capture for both clinical and research data entry with the aim of improving the speed of certain processes, and the turnaround time for accessibility and updating data in real time. The program is also exploring social support interventions, such as group exercise classes to enhance participant adherence to positive behavioral changes. Lastly, with increasing access to the Internet and the ongoing development of technological platforms, remotely delivered Internet-based treatment approaches are being explored as a future alternative and/or complimentary support for the delivery of GEAR interventions. GEAR also aims to extend care as a potential management strategy beyond EDS/G-HSD, to include leading co-morbidities of EDS/G-HSD including Postural Orthostatic Tachycardia Syndrome (POTS) and Craniocervical Junction Instability (CCJI). Autonomic dysfunction, also known as dysautonomia, is a long-recognized complication of EDS/G-HSD and has symptoms reported at high rates (up to 78%) (39, 40), with POTS as the most prevalent autonomic profile (41). Aerobic reconditioning therapy through exercise has not yet been employed as treatment for POTS in patients with EDS/G-HSD, despite its known efficacy in improving or resolving POTS in most patients without EDS/G-HSD (28, 42). For example, George et al. demonstrated that in an international POTS patient registry, the vast majority of patients (71%) who completed 3 months of exercise training no longer qualified for POTS criteria and were thus effectively in remission (42). GEAR aims to extend these findings to the EDS/G-HSD population in an effort to reduce POTS-related symptoms, and thus potentially improve functional capacity and quality of life of those with EDS/G-HSD.
Additionally, individuals with EDS/G-HSD frequently present with headache, neck pain, and decreased cervical muscle strength. These symptoms have been hypothesized to be related to a potential CCJI (43). The craniocervical junction, a component of the spine, is made up exclusively of synovial joints and ligaments that allow for wide head and neck movements. It is especially vulnerable to the laxity of ligaments experienced by individuals with connective tissue disorders, such as EDS/G-HSD (44). Management of the symptoms related to CCJI are not well-established, and often when instability cannot be detected using current radiographic measures, the treatment of such symptoms remains limited. It has been suggested that non-surgical treatment should be employed with the goal of decreasing stresses on the involved spinal segments and to enhance the function of spinal stabilizing subsystems (45). Strengthening exercises have been shown to enhance the function of these subsystems (46). While the most effective exercises for this purpose have yet to be identified, exercises that focus on controlled motion and proprioception could address elements of stabilization, and if attained, prevent degenerative changes and the need for surgery (45). Further, exercise and rehabilitation therapy has been shown to improve pain and function of lumbar spine in people with EDS/G-HSD (18). It is hypothesized that such therapy has the potential to produce similar results for CCJI, and as such, GEAR aims to offer it as a form of treatment for the EDS/G-HSD-related complication of CCJI (45).
Conclusions
Amid the plethora of exercise and rehabilitation intervention studies for chronic diseases, there remains a significant gap in its effect on people with EDS/G-HSD. The goal of GEAR is to transform the management of EDS/G-HSD through a multi-pronged exercise and rehabilitation treatment approach that has been designed using integrated research and interdisciplinary expert contributions. Through this approach, GEAR aims to decrease functional impairment, reduce pain, increase confidence in symptom self-management, and provide a community of support. Future clinical and research initiatives of GEAR will support the development, improvement, and accessibility of exercise and rehabilitation therapy programs for the greater EDS/G-HSD population.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
NM, DSM, and HC designed the protocol and coordinated the project. NM, AF, LH, LL-H, and LMcG informed program design and delivery. DSM and SB-I informed data collection design. DSM, SB-I, and AF drafted the manuscript. HC is the guarantor. All authors critically reviewed and approved the manuscript.
Funding
The GEAR Program is supported by the GoodHope Foundation and by the UHN Foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
6MWT, Six-minute Walk Test; CCJI, craniocervical junction instability; EDS, Ehlers Danlos Syndromes; GEAR, GoodHope exercise and rehabilitation; G-HSD, generalized hypermobility spectrum disorder; HCP, health care professionals; hEDS, hypermobile EDS; POTS, Postural Orthostatic Tachycardia Syndrome; PT, physiotherapist; RPE, Rating of Perceived Exertion; SMART, Specific, Measurable, Achievable, Realistic and Time-limited; SME, self-management education.
References
1. Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, et al. The 2017 international classi fi cation of the Ehlers – Danlos syndromes. Am J Med Genet C Semin Med Genet. (2017) 26:8–26. doi: 10.1002/ajmg.c.31552
2. Castori M, Tinkle B, Levy H, Grahame R, Malfait F, Hakim A, et al. A framework for the classification of joint hypermobility and related conditions. Am J Med Genet C Semin Med Genet. (2017) 175:148–57. doi: 10.1002/ajmg.c.31539
3. Superti-Furga A, Steinmann B, Ramirez FBP. Molecular defects of type III procollagen in Ehlers-Danlos syndrome type IV. Hum Genet. (1989) 82:104–8. doi: 10.1007/BF00284038
4. Michael Pope F, Martin GR, Lichtenstein JR, Penttinen R, Gerson B, Rowe DW, et al. Patients with Ehlers-Danlos syndrome type IV lack type III collagen. Proc Natl Acad Sci U S A. (1975) 72:1314–6. doi: 10.1073/pnas.72.4.1314
5. Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Am J Med Genet. (1998) 77:31–7. doi: 10.1002/(sici)1096-8628(19980428)77:1<31::aid-ajmg8>3.0.co;2-o
6. Stanitski DF, Nadjarian R. Orthopaedic manifestations of Ehlers-Danlos syndrome. Clin Orthop Relat Res. (2000) 376:213–21. doi: 10.1097/00003086-200007000-00029
7. Rombaut L, Malfait F, Cools A, De Paepe A, Calders P. Musculoskeletal complaints, physical activity and health-related quality of life among patients with the Ehlers-Danlos syndrome hypermobility type. Disabil Rehabil. (2010) 32:1339–45. doi: 10.3109/09638280903514739
8. Tobias JH, Deere K, Palmer S, Clark EM, Clinch J. Joint hypermobility is a risk factor for musculoskeletal pain during adolescence findings of a prospective cohort study. Arthritis Rheum. (2013) 65:1107–15. doi: 10.1002/art.37836
9. De Coster P, Van den Berghe L, Martens L. Generalized joint hypermobility and temporomandibular disorders: inherited connective tissue disease as a model with maximum expression. J Orofac Pain. (2005) 19:47–57.
10. Ainsworth SR, Aulicino PL. A survey of patients with Ehlers-Danlos syndrome. Clin Orthop Relat Res. (1993) 286:250–6. doi: 10.1097/00003086-199301000-00037
11. Castori M. Ehlers-Danlos syndrome, hypermobility type: an underdiagnosed hereditary connective tissue disorder with mucocutaneous, articular, and systemic manifestations. Int Sch Res Netw ISRN Dermatol. (2012) 2012:22. doi: 10.5402/2012/751768
12. Tremblay MSTS, Colley RCCC, Saunders TJSJ, Healy GNHN, Owen NO. Physiological and health implications of a sedentary lifestyle. Appl Physiol Nutr Metab. (2010) 35:725–40. doi: 10.1139/H10-079
13. Fentem PH. ABC of sports medicine benefits of exercise in health and disease. BMJ. (1994) 308:1291–95. doi: 10.1136/bmj.308.6939.1291
14. Warby SA, Pizzari T, Ford JJ, Hahne AJ, Watson L. Exercise-based management versus surgery for multidirectional instability of the glenohumeral joint: a systematic review. Br J Sports Med. (2016) 50:1115–23. doi: 10.1136/bjsports-2015-094970
15. Kisner C, Colby LA, Borstad J. Therapeutic Exercise: Foundations and Techniques. Philadelphia, PA: Fa Davis. (2017).
16. Daman M, Shiravani F, Hemmati L, Taghizadeh S. The effect of combined exercise therapy on knee proprioception, pain intensity and quality of life in patients with hypermobility syndrome: a randomized clinical trial. J Bodyw Mov Ther. (2019) 23:202–5. doi: 10.1016/j.jbmt.2017.12.012
17. Sahin N, Baskent A, Cakmak A, Salli A, Ugurlu H, Berker E. Evaluation of knee proprioception and effects of proprioception exercise in patients with benign joint hypermobility syndrome. Rheumatol Int. (2008) 28:995–1000. doi: 10.1007/s00296-008-0566-z
18. Toprak Celenay S, Ozer Kaya D. Effects of spinal stabilization exercises in women with benign joint hypermobility syndrome: a randomized controlled trial. Rheumatol Int. (2017) 37:1461–8. doi: 10.1007/s00296-017-3713-6
19. Scheper MC, Engelbert RHH, Rameckers EAA, Verbunt J, Remvig L, Juul-Kristensen B. Children with generalised joint hypermobility and musculoskeletal complaints: state of the art on diagnostics, clinical characteristics, and treatment. Biomed Res Int. (2013) 2013:121054. doi: 10.1155/2013/121054
20. Palmer S, Bailey S, Barker L, Barney L, Elliott A. The effectiveness of therapeutic exercise for joint hypermobility syndrome: a systematic review. Physiotherapy (United Kingdom). (2014) 100:220–7. doi: 10.1016/j.physio.2013.09.002
21. Mittal N, Mina DS, McGillis L, Weinrib A, Slepian PM, Rachinsky M, et al. The goodhope Ehlers Danlos syndrome clinic: development and implementation of the first interdisciplinary program for multi-system issues in connective tissue disorders at the Toronto General Hospital. Orphanet J Rare Dis. (2021) 16:1–9. doi: 10.1186/s13023-021-01962-7
22. Corrado B, Ciardi G. Hypermobile Ehlers-Danlos syndrome and rehabilitation: taking stock of evidence based medicine: a systematic review of the literature. J Phys Ther Sci. (2018) 30:843–7. doi: 10.1589/jpts.30.847
23. Bassett SF. Bridging the intention-behaviour gap with behaviour change strategies for physiotherapy rehabilitation non-adherence. N Z J Physiother. (2015) 43:105–11. doi: 10.15619/NZJP/43.3.05
24. To M, Alexander MC. Are people with joint hypermobility syndrome slow to strengthen? Arch Phys Med Rehabil. (2018) 100:1243–50. doi: 10.1016/j.apmr.2018.11.021
25. Ross R, Chaput JP, Giangregorio LM, Janssen I, Saunders TJ, Kho ME, et al. Canadian 24-Hour Movement Guidelines for Adults aged 18–64 years and Adults aged 65 years or older: an integration of physical activity, sedentary behaviour, and sleep. Applied Physiology, Nutrition, and Metabolism. (2020) 45:S57–102.
26. Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. (2011) 43:1334–59. doi: 10.1249/MSS.0b013e318213fefb
27. Borg G. Borg's Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics. Available online at: https://psycnet.apa.org/record/1998-07179-000 (accessed August 30, 2021).
28. Winker R, Barth A, Bidmon D, Ponocny I, Weber M, Mayr O, et al. Endurance exercise training in orthostatic intolerance. Hypertension. (2005) 45:391–8. doi: 10.1161/01.HYP.0000156540.25707.af
29. Siegert RJ, Taylor WJ. Disability and rehabilitation theoretical aspects of goal-setting and motivation in rehabilitation. Disabil Rehabil. (2009) 26:1–8. doi: 10.1080/09638280410001644932
30. McMillan DW, Chavis DM. Sense of community: a definition and theory. J Commun Psychol. (1986) 14:6–23.
31. Whitlock J. The role of adults, public space, and power in adolescent community connectedness. J Commun Psychol. (2007) 35:499–518. doi: 10.1002/jcop.20161
32. Mitchinson AR, Kim HM, Geisser M, Rosenberg JM, Hinshaw DB. Social Connectedness and patient recovery after major operations. J Am Coll Surg. (2008) 206:292–300. doi: 10.1016/j.jamcollsurg.2007.08.017
33. Sawyer SM, Drew S, Yeo MS, Britto MT. Adolescents with a chronic condition: challenges living, challenges treating. Lancet. (2007) 369:1481–9. doi: 10.1016/S0140-6736(07)60370-5
34. Ries AL, Kaplan RM, Myers R, Prewitt LM. Maintenance after pulmonary rehabilitation in chronic lung disease. Am J Respir Crit Care Med. (2012) 167:880–8. doi: 10.1164/rccm.200204-318OC
35. Brooks D, Krip B, Mangovski-Alzamora S, Goldstein RS. The effect of postrehabilitation programmes among individuals with chronic obstructive pulmonary disease. Eur Respir J. (2002) 20:20–9. doi: 10.1183/09031936.02.01852001
36. Ohman A 2005. Qualitative methodology for rehabilitation research. J. Rehabil. Med. 37:273–80. doi: 10.1080/16501970510040056
37. Csuka M, McCarty DJ. Simple method for measurement of lower extremity muscle strength. Am J Med. (1985) 78:77–81. doi: 10.1016/0002-9343(85)90465-6
38. Palmer S, Davey I, Oliver L, Preece A, Sowerby L, House S. The effectiveness of conservative interventions for the management of syndromic hypermobility: a systematic literature review. Clin Rheumatol. (2020) 40:1113–29. doi: 10.1007/s10067-020-05284-0
39. Gazit Y, Nahir AM, Grahame R, Jacob G. Dysautonomia in the joint hypermobility syndrome. Am J Med. (2003) 115:33–40. doi: 10.1016/S0002-9343(03)00235-3
40. Hakim A, O'callaghan C, De Wandele I, Stiles L, Pocinki A, Rowe P. Cardiovascular autonomic dysfunction in Ehlers-Danlos syndrome-hypermobile type. Am J Med Genet C Sem Med Genet. (2017) 175:168–74. doi: 10.1002/ajmg.c.31543
41. De Wandele I, Rombaut L, Leybaert L, Van de Borne P, De Backer T, Malfait F, et al. Dysautonomia and its underlying mechanisms in the hypermobility type of Ehlers–Danlos syndrome. Semin Arthritis Rheum. (2014) 44:93–100. doi: 10.1016/j.semarthrit.2013.12.006
42. George SA, Bivens TB, Howden EJ, Saleem Y, Galbreath MM, Hendrickson D, et al. The international POTS registry: Evaluating the efficacy of an exercise training intervention in a community setting. Hear Rhythm. (2016) 13:943–50. doi: 10.1016/j.hrthm.2015.12.012
43. Henderson FC, Austin C, Benzel E, Bolognese P, Ellenbogen R, Francomano CA, et al. Neurological and spinal manifestations of the Ehlers–Danlos syndromes. Am J Med Genet Part C Semin Med Genet. (2017) 175:195–211. doi: 10.1002/ajmg.c.31549
44. Klinge PM, McElroy A, Donahue JE, Brinker T, Gokaslan ZL, Beland MD. Abnormal spinal cord motion at the craniocervical junction in hypermobile Ehlers-Danlos patients. J Neurosurg Spine. (2021) 35:18–24. doi: 10.3171/2020.10.SPINE201765
45. Olson KA, Joder D. Diagnosis and treatment of cervical spine clinical instability. J Orthop Sports Phys Ther. (2001) 31:194–206. doi: 10.2519/jospt.2001.31.4.194
Keywords: Ehlers-Danlos Syndrome, exercise, rehabilitation, models of care, Generalized Hypermobility Spectrum Disorder
Citation: Mittal N, Santa Mina D, Buryk-Iggers S, Lopez-Hernandez L, Hussey L, Franzese A, Katz J, Laflamme C, McGillis L, McLean L, Rachinsky M, Rozenberg D, Slepian M, Weinrib A and Clarke H (2021) The GoodHope Exercise and Rehabilitation (GEAR) Program for People With Ehlers-Danlos Syndromes and Generalized Hypermobility Spectrum Disorders. Front. Rehabilit. Sci. 2:769792. doi: 10.3389/fresc.2021.769792
Received: 02 September 2021; Accepted: 12 October 2021;
Published: 08 November 2021.
Edited by:
Nachiappan Chockalingam, Staffordshire University, United KingdomReviewed by:
Maciej Płaszewski, Józef Piłsudski University of Physical Education in Warsaw, PolandJean Claude De Mauroy, Independent Researcher, St Didier de la Tour, France
Copyright © 2021 Mittal, Santa Mina, Buryk-Iggers, Lopez-Hernandez, Hussey, Franzese, Katz, Laflamme, McGillis, McLean, Rachinsky, Rozenberg, Slepian, Weinrib and Clarke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nimish Mittal, bmltaXNoLm1pdHRhbEB1aG4uY2E=
 Nimish Mittal
Nimish Mittal Daniel Santa Mina
Daniel Santa Mina Stephanie Buryk-Iggers
Stephanie Buryk-Iggers Laura Lopez-Hernandez1
Laura Lopez-Hernandez1 Dmitry Rozenberg
Dmitry Rozenberg Maxwell Slepian
Maxwell Slepian Hance Clarke
Hance Clarke