- 1Faculty of Dentistry, The University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 2Division of Oral & Maxillofacial Surgery, Faculty of Dentistry, The University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 3Division of Restorative Dental Sciences, Faculty of Dentistry, The University of Hong Kong, Hong Kong, Hong Kong SAR, China
Introduction: The aim of this systematic review is to provide a clinical update of the current knowledge on COVID-19 and oral mucosal lesions, to analyze the types and prevalence of oral mucosal lesions in patients with COVID-19, and to clarify the potential association between COVID-19 and oral mucosal lesions.
Methods: The literature search was conducted using PubMed, Web of Science, Scopus and the Cochrane Library, as well as literatures via manual searches of the reference lists of included studies. Studies published in English that mentioned oral mucosal lesions in patients with COVID-19 were included, resulting in a total of 31 studies.
Results: Most of the included studies were considered to have a moderate to high risk of bias according to the Joanna Briggs Institute bias assessment tools. Based on COVID-19 severity, the characteristics and patterns of oral mucosal lesions in COVID-19 patients were described, analyzed and synthesized. Overall, ulcers without specific diagnosis had the highest prevalence in COVID-19 patients, followed by traumatic ulcers, candidiasis, petechiae and aphthous-like lesions. Homogeneity of data cannot be achieved in statical analysis, indicating randomness of outcome (ulcers without specific diagnosis, 95% CI: 28%–96%, I2 = 98.7%).
Discussion: Given the limited evidence from currently available studies, the association between COVID-19 and oral mucosal lesions remains difficult to clarify. Healthcare professionals should be aware of the possible association between COVID-19 and oral mucosal lesions, and we hereby discuss our findings.
1. Introduction
COVID-19, a pandemic that emerged in December 2020, has since affected more than 600 million people and caused more than 6 million deaths. Fever, cough, fatigue and loss of smell or taste have been reported as the most common symptoms of COVID-19. In severe cases, critical illness with pneumonia or even respiratory failure has been demonstrated (1).
Current research suggests that Angiotensin-converting enzyme 2 (ACE2), a functional receptor on cell surfaces, is the entry point of SARS-CoV-2 into the host cell (2). It is highly expressed in oral mucosa, especially in tongue epithelial cells, allowing virus invasion via the oral cavity. Thus, these previous findings indicate that oral mucosa may be a target for the SARS-CoV-2.
Therefore, COVID-19 may have potential manifestations in the oral cavity. Oral signs and symptoms of COVID-19 have been reported (3–24). Among these signs and symptoms, lesions of the oral mucosa have been commonly demonstrated. These include ulceration, desquamative gingivitis, petechiae and co-infections such as candidiasis and recurrent HSV. The mechanisms underlying the relationship between oral mucosal lesions and COVID-19 are not yet clear and well understood. It is still controversial whether they are a consequence of direct viral infection, systemic deterioration, adverse effects of medical treatments, or coincidence (5, 6, 10–12, 15, 24–27).
The mechanisms underlying the relationship between oral mucosal lesions and COVID-19 are not yet clear and well understood. It is still controversial whether they are a consequence of direct viral infection, systemic deterioration, adverse effects of medical treatments, or coincidence (5, 6, 10–12, 15, 24–27). The effect of COVID-19 on the oral cavity has been noted in previous review articles (3, 16, 18–21, 23, 24, 28–30). However, only a few of them shed light on oral mucosal lesions. As such, clinical update is needed to obtain a better understanding of oral mucosal lesions presented in COVID-19 cases.
In this context, a systematic review has been conducted. The objective of this review is to perform a clinical update of the current knowledge about COVID-19 and oral mucosal lesions, analyze the types and prevalence of oral mucosal lesions in patients with COVID-19, and clarify the potential association between COVID-19 and oral mucosal lesions.
2. Method
2.1. Study protocol
PRISMA-P (PRISMA for protocols) guideline (31) was followed for a systematic and comprehensive approach. The systemic review was registered at the International Prospective Register of Systematic Reviews (PROSPERO) database under registration code CRD42022377790.
2.2. Eligibility criteria
Inclusion and exclusion criteria were defined as follows, consisting of observational studies investigating the prevalence of oral mucosal lesions in patients with COVID-19.
The inclusion criteria were: (1) case reports, case series, cross-sectional studies and cohort retrospective studies; (2) report oral mucosal findings in PCR-confirmed cases of COVID-19; and (3) publish between January 2020 and August 2022.
Exclusion criteria were: (1) non-English studies; (2) lack of description of oral mucosal findings; and (3) cannot access to the full article.
2.3. Information source and search strategy
Electronic searches were performed in PubMed, Web of Science, Scopus and Cochrane Library databases. The following combination of MeSH and non-MeSH keywords were used: “COVID-19”, “SARS-CoV-2”, “2019 NCOV”, “oral mucosal lesions”, “oral lesions”, “oral ulcer”.
An additional search of the literature was performed by manual search across reference lists of included studies. EndNote 20 was used to collect references and remove duplicate articles. Covidence.org was used to select studies and retrieve data.
2.4. Study selection
The selection was conducted in two phases. In phase 1, two authors (W.Y.K and L.W.Z) independently reviewed the titles and abstracts of all relevant references. In phase 2, the same selection criteria were applied to the full-text articles independently by the same 2 authors, ensuring that all the eligibility criteria were met. The final selection was always based on the full text of the publication.
2.5. Data collection
Initially, the necessary information from the selected articles was collected by two authors (W.Y.K and L.W.Z). Any differences of opinion were resolved through discussion and mutual agreement between the two authors.
2.6. Risk of bias assessment
The Joanna Briggs Institute's Critical Appraisal Checklist (32–35) was used by two authors to check the risk-of-bias of all included case reports, case series, cross-sectional studies and cohort retrospective studies. A study was classified as having a high risk of bias when it reached a “yes” score of up to 49%, moderate when 50%–69%, and low when >70%.
2.7. Summary measures
As the severity of COVID-19 progresses, the immune system of the infected patient may be altered, which may contribute to the different presentation patterns of oral mucosal lesions in COVID-19 patients. To explore the possible association between COVID-19 severity and oral mucosal lesions, the results were divided into 3 groups based on COVID-19 severity: self-monitoring patients as mild cases, hospitalized patients as moderate cases, and intensive care unit (ICU) patients as severe cases. For all three groups, secondary outcomes included the clinical presentation and prevalence of oral mucosal lesions.
2.8. Synthesis result
Qualitative and quantitative synthesis was performed by sorting and comparing data extracted from the included studies on primary and secondary outcomes. Analyses of the proportions of oral mucosal lesions at different COVID-19 severity levels were calculated using Microsoft Excel software. The prevalence of non-specific diagnosed ulcers in studies with more than one case was expressed through relative or absolute frequencies and 95% CI, and a statistical analysis was performed using Stata. The I2 test was used to calculate statistical heterogeneity, which defined whether a fixed (I2 < 50%) or random (I2 ≥ 50%) effect model should be applied.
3. Result
3.1. Study selection and characteristics
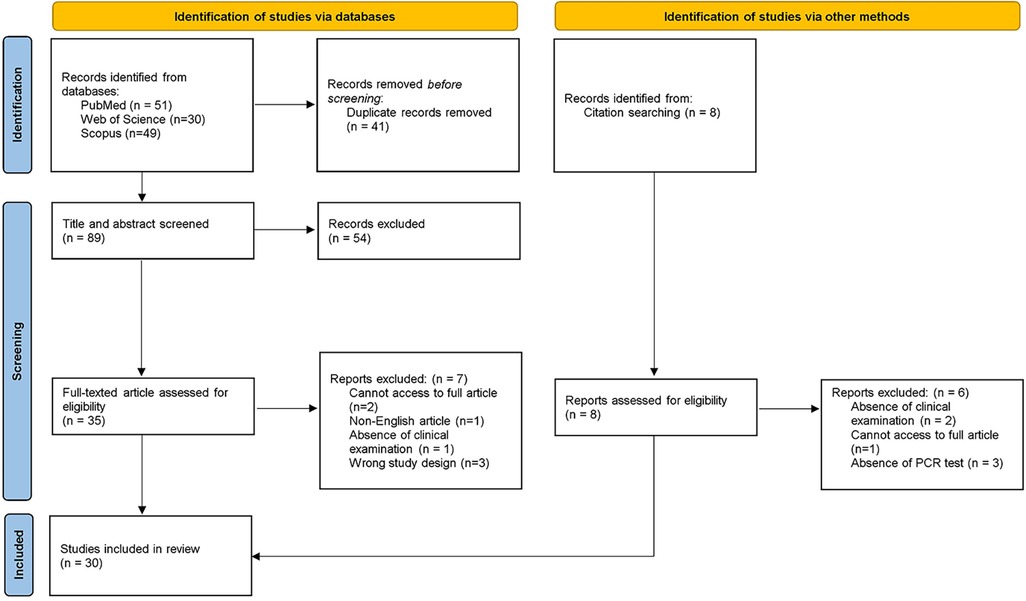
In the first phase, 130 studies were identified from databases. After the removal of duplicates, 89 references remained for title and abstract screening. After the evaluation of all records, 35 articles remained for the second phase. A full-text reading was conducted, and 7 studies were excluded according to pre-defined eligibility criteria. The reference lists of all included articles were assessed and 8 were selected for full-text analysis. 6 articles were excluded according to the eligibility criteria. Thereafter, 30 studies were selected for result synthesis and statistical analysis. A flowchart describing the process is illustrated in Figure 1.
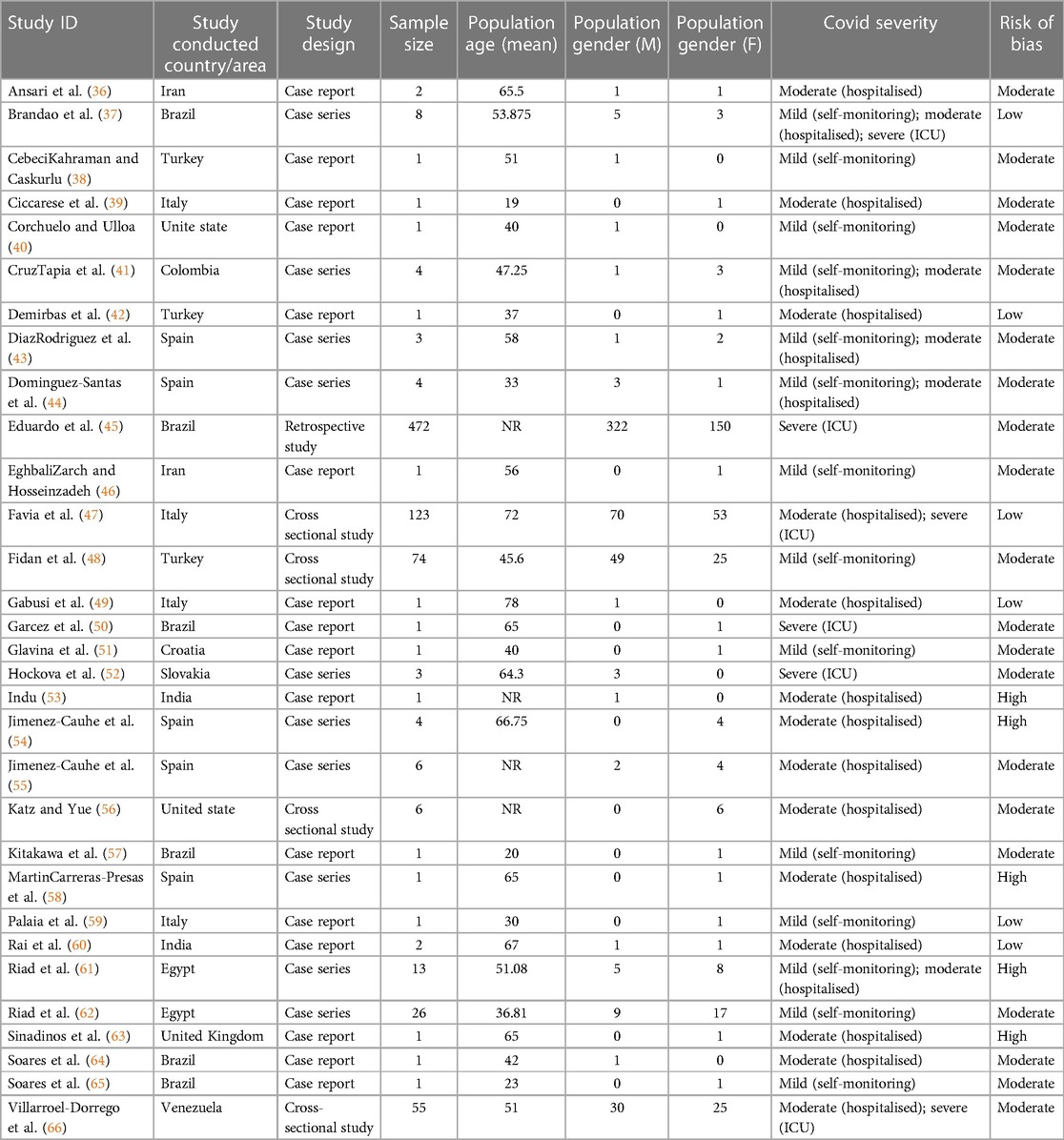
The included studies were distributed worldwide, with a higher prevalence in Europe. 11 studies were conducted in Europe, 7 in Asia, 2 in North America, 2 in Africa, and 8 in Latin America (Table 1). Within the included studies, 15 were case reports, 10 were case series, 4 were cross-sectional studies, and 1 was a retrospective study.
3.2. Risk of bias in studies
Risk-of-bias assessment in individual studies is summarized in Table 1. Case reports, case series, cross-sectional studies and retrospective cohort studies were evaluated using the Joanna Briggs Institute Critical Appraisal Checklist for each study design (32–35). Most of the included studies were case reports (16/31) or case series (10/30) with moderate (20/30) to high (5/30) risk of bias. Only 6 studies were at low risk of bias.
3.3. Result of syntheses
A total of 820 patients with oral mucosal lesions were included in this systemic review. A higher proportion of males was observed in the study, of which 507 were male, 313 were female. The mean age of the patients ranged from 19 to 78 years. All patients had obtained positive PCR tests for SARS-COV-2 virus. 14 studies reported mild COVID-19 cases, 19 studies were included for moderate COVID-19 cases, and 6 studies demonstrated severe COVID-19 cases. Demographic characteristics of included patients are provided in Table 1.
3.3.1. Characteristics of oral mucosal lesions
Considering that the definition of COVID-19 severity was variable among included studies, we re-grouped patients according to categorization terms reported by authors. The characteristics of oral mucosal lesions were first classified according to the COVID-19 severity of the patient, and then further subclassified according to the diagnosis/clinical presentation of the lesion.
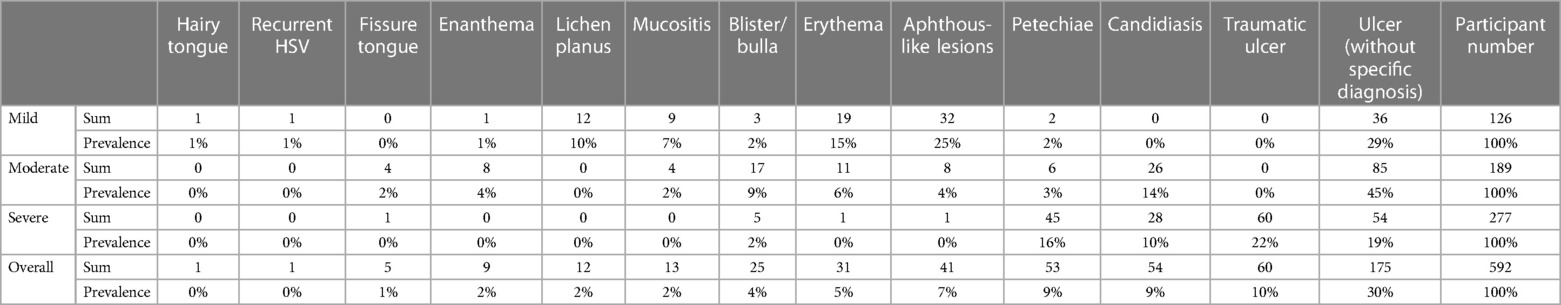
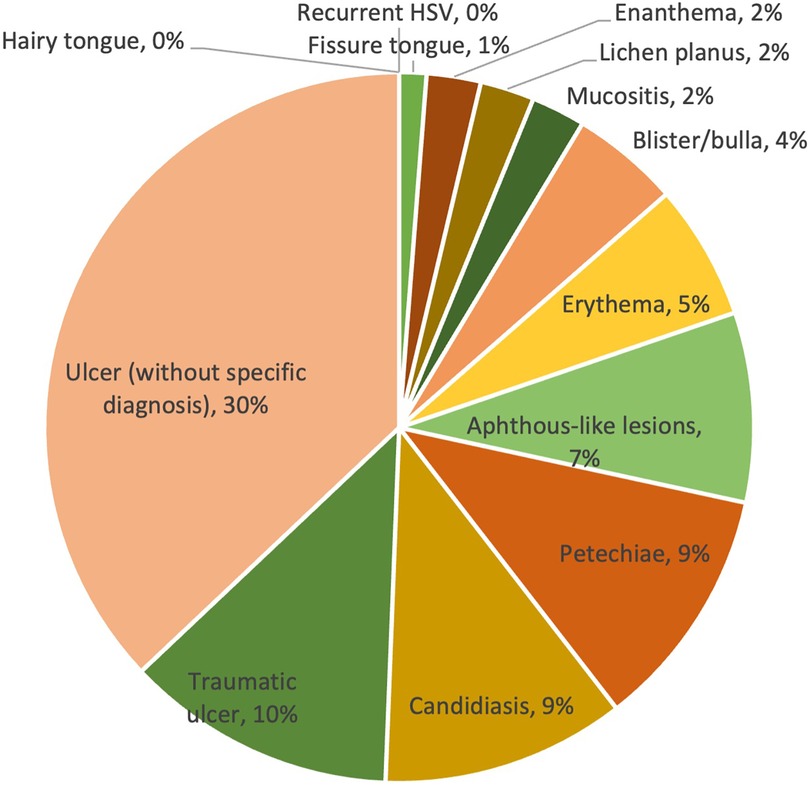
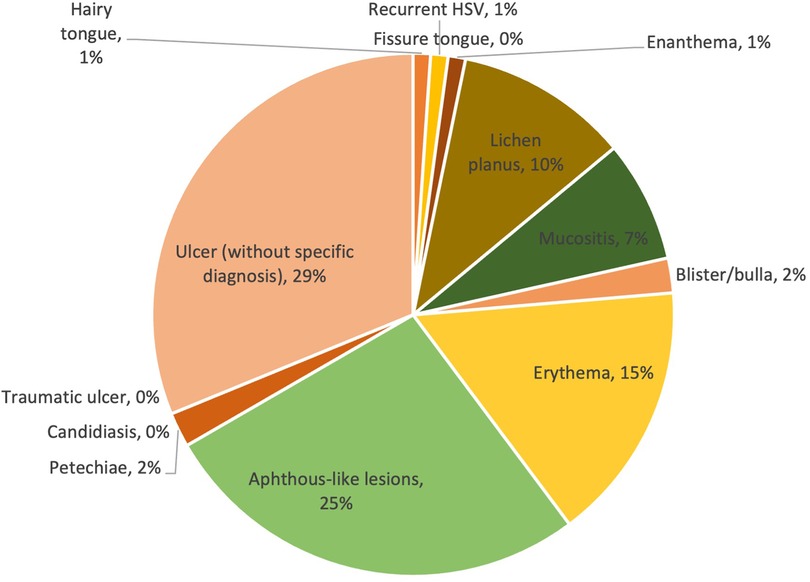
Overall, the prevalence of lesions ranged from 0% to 30%. The majority of lesions were ulcers without a specific diagnosis, reported in 30% of cases. Traumatic ulcers (10%) were the second most frequent lesions observed in patients with COVID-19, followed by petechiae (9%), candidiasis (9%) and aphthous-like lesions (7%). Other less commonly reported lesion diagnosis/clinical presentations were erythema (5%), blister/bulla (4%), enanthema (2%), lichen planus (2%), mucositis (2%) and fissure tongue (1%). The prevalence of lesion diagnosis/clinical presentation is shown in Table 2 and Figure 2.
3.3.2. Mild COVID-19 cases
Of the 125 mild COVID-19 cases, the prevalence of lesion diagnosis/clinical presentation ranged from 0% to 29%. With a prevalence of 29% and 25% respectively, ulcers without specific diagnosis and aphthous-like lesions were two of the most prevalently reported oral mucosal lesions. The other types of lesions had a relatively low prevalence in comparison with the first two, with a prevalence equal to or less than 15%. The prevalence of lesion diagnosis/clinical presentation is shown in Figure 3.
3.3.3. Moderate COVID-19 cases
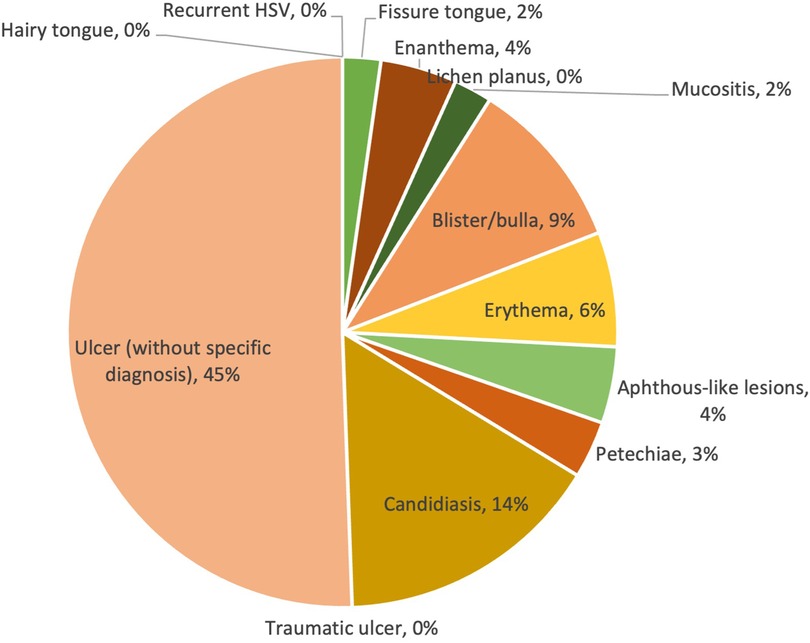
Similar to the mild COVID-19 group, ulcers without specific diagnosis were still the most commonly reported lesion type. However, it showed a much higher prevalence in the moderate group (45%) than in the mild group (29%). It should be noted that although no cases of candidiasis were reported in the mild group, 26 cases (14%) were reported in the moderate group, being the second most common type of lesion in this group; whereas the aphthous-like lesions decreased dramatically from 25% (mild) to 4% (moderate). Other types of lesions had a relatively low prevalence compared to the first two, reaching a prevalence of less than 10%. The prevalence of lesion diagnosis/clinical presentation is shown in Figure 4.
3.3.4. Severe COVID-19 cases
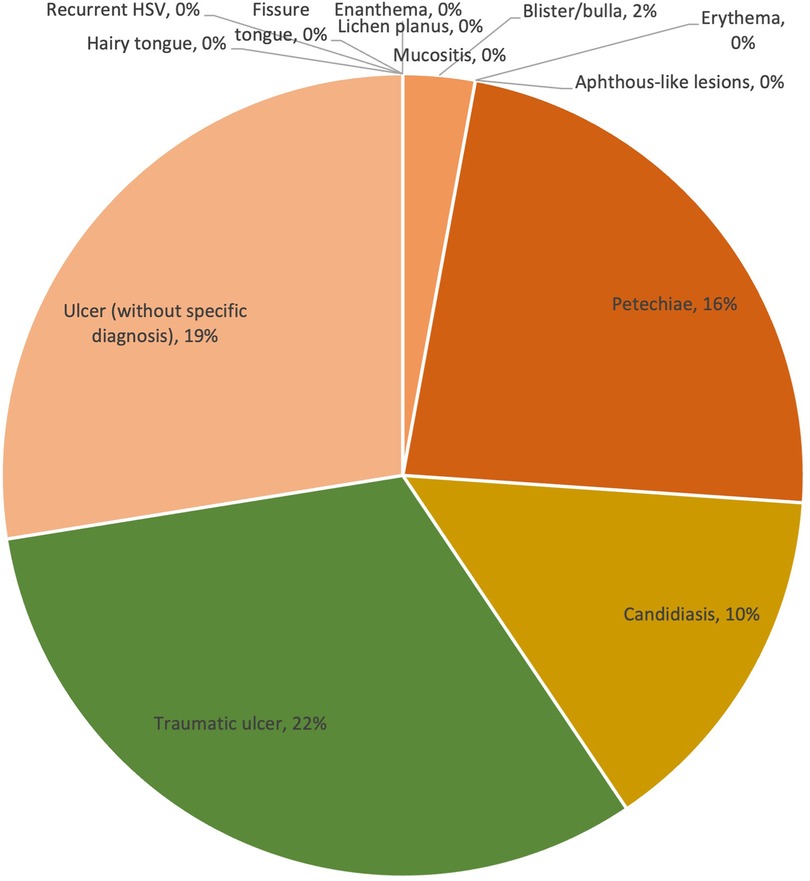
Within the severe COVID-19 group, ulcers without specific diagnosis remained to be one of the most common lesion types (19%). However, traumatic ulcers (22%), which were not observed in mild or moderate cases, became the most common lesion type in severe COVID-19 cases. Similar to traumatic ulcers, the prevalence of petechiae increased significantly from 2% to 3% (mild and moderate) to 16% (severe). Candidiasis (10%) and blister/bulla (2%) were also presented in severe cases, other types were not reported. The prevalence of lesion diagnosis/clinical presentation is shown in Figure 5.
3.3.5. Statistical analysis of ulcers without specific diagnosis
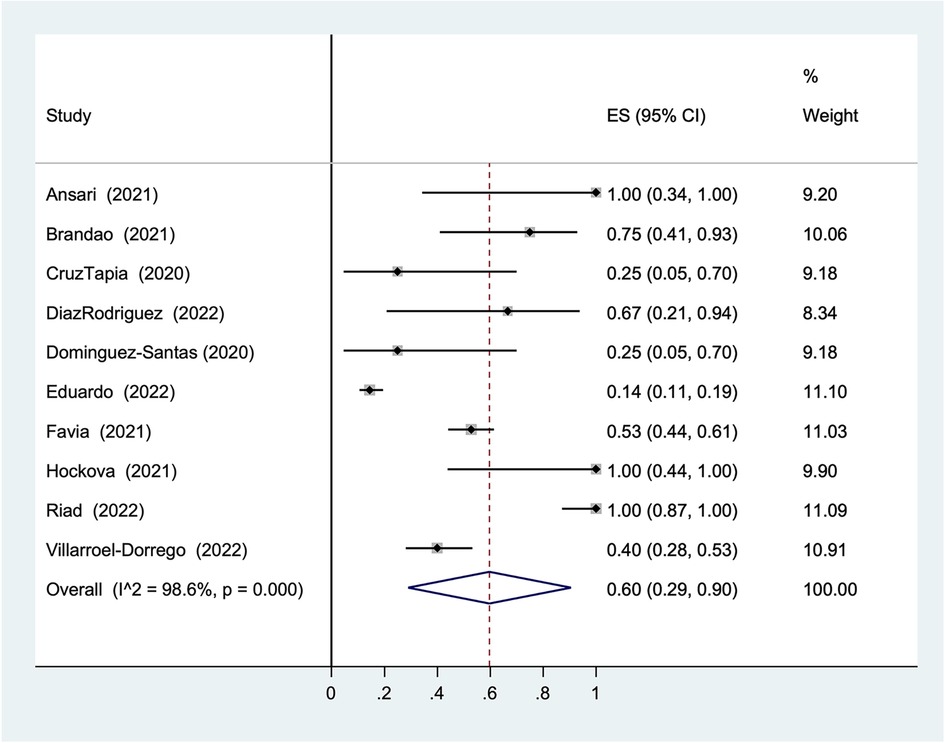
Considering the distinguishing high proportion of ulcers without specific diagnosis in the result (30%), a statistical analysis was performed to investigate its overall prevalence in COVID-19 patients. The overall prevalence was 60% (95% CI: 29%–90%, I2 = 98.6%, Figure 6).
4. Discussion
4.1. Ulcers without specific diagnosis
Ulcers are defined as the breaks in the continuity of the epithelial covering of the body, followed by inflammatory changes in the exposed connective tissue (67). They are commonly caused by infectious or non-infectious conditions, and also act as an oral manifestation of systemic diseases or an indication of internal malignancy (68).
Although the overall result of the statistical analysis is still statistically significant (60%), the result does not show homogeneity of the data. Given the range of prevalence from 28% to 96% in 95% CI with 98.6 I2, the randomness of the results is indicated. Therefore, the results cannot support the hypothesis of an association between COVID-19 and ulcers without specific diagnosis.
4.2. Traumatic ulcers, candidiasis, petechiae and aphthous-like lesions
In the collected results, traumatic ulcers (10%), petechiae (9%), candidiasis (9%) and aphthous-like lesions (7%) have a similar prevalence in patients with COVID-19. As the severity of COVID-19 increased, aphthous-like lesions decreased substantially from 25% to 0%, whereas traumatic ulcers, petechiae and candidiasis all followed the increasing trend.
4.2.1. Traumatic ulcers
Traumatic ulcers are superficial ulcerations that are associated with acute or chronic injury to the oral mucosa (67). The source of irritation is often found adjacent to the ulceration, indicating the cause of the lesion. Once the source of irritation is removed, the ulcer usually resolves within days.
According to the synthesized result, traumatic ulcers were only recognized in severe COVID-19 cases (60/277, 22%) when patients were in intensive care (ICU). It is common for patients in ICU to face life-threatening situations, such as severe pneumonia or even respiratory failure. As such, they are likely to be intubated for oxygenation, thereby resulting in traumatic ulcers at the intubation sites. Therefore, intubation in the course of treatment may be associated with the occurrence of traumatic ulcers, thus explaining their high prevalence in severe COVID-19 cases. Interestingly, in line with our findings, a recent study (69) also demonstrated a possible association between the intubation process in COVID-19 patients and traumatic ulcers. The cohort study analyzed COVID-19 patients with oral mucosal changes associated with hospitalization and found a statistical association between intubation and oral lesions. Taken together, a positive association between the incubation process and traumatic ulcers in COVID-19 patients can be considered.
4.2.2. Aphthous-like lesions
Aphthous lesions are a common type of recurrent ulcer of infectious or non-infectious nature (68). It is believed that the mucosal destruction is the result of the production of tumor necrosis factor-alpha (TNF-α), which is caused by a T cell–mediated immunologic reaction (67). Its causative agents vary in different subgroups of patients, making it difficult to trace its etiology.
According to the synthesized result, aphthous-like lesions are mainly reported in mild COVID-19 cases (32/126, 25%) and less frequently in moderate and severe cases, with only 8 and 1 cases reported respectively. However, as aphthous-like lesions can be caused by a wide range of causative agents and it is difficult to associate the exact causative agent with COVID-19, it is difficult to substantiate the possible association between COVID-19 and aphthous-like lesions.
4.2.3. Petechiae
Petechiae are classified as small hemorrhages in the skin, mucosa or serosa (67). It can result from repeated or prolonged elevation of intrathoracic pressure associated with many activities, such as repeated coughing or vomiting, or from non-traumatic causes, such as anticoagulant therapy or thrombocytopenia.
According to the synthesized result, petechiae are observed in mild (2%), moderate (3%) and severe (16%) COVID-19 cases, with a significantly higher prevalence in the severe group. Interestingly, a recent review (12) suggested that petechiae in COVID-19 patients with thromboembolic phenomena could be a consequence of anticoagulant therapy. It should be noted that thromboembolic phenomena are common complications in intensive care, so our finding of higher prevalence in severe cases (ICU patients) is consistent with the suggested association. However, this view remained a hypothesis because the reports included in the review did not indicate whether the patients were receiving anticoagulant therapy. Therefore, the hypothesis is not supported by evidence and more research is needed before a conclusion can be drawn.
Furthermore, it should not be neglected that petechiae only describe the clinical feature of the lesions observed, with an enormous variety of possible causes. Without differential diagnosis, the causative agents cannot be determined and therefore the correlation between petechiae and COVID-19 is still unclear.
4.2.4. Candidiasis
Candidiasis is classified as a fungal infection caused by Candida species (67). Innocuous Candida carriage is thought to be associated with the yeast form, and only the invasive hyphal form causes disease. As Candida is part of the commensal oral microflora, candidiasis is recognized as a “disease of the diseased”. This means that the disease is the result of an interaction between the host and the organism, and that the switch of the organism into a pathogenic form is rather related to the immune status and the oral mucosal environment of the host.
According to the synthesized result, candidiasis is only detected in patients with moderate (14%) and severe (10%) COVID-19 cases. As the patient was hospitalized (moderate) or in intensive care (severe) and showed more advanced symptoms of COVID-19 infection, it is more likely that the patient's immune system is altered. In support of this hypothesis, a recent study (27) found that COVID-19 patients had reduced upregulation of CD80 on monocytes and abrogated release of IL-6, TNF, IL-1a and IL-1b in response to Candida albicans. Therefore, the observation of the prevalence of candidiasis is consistent with their histopathological findings, suggesting that the occurrence of candidiasis in COVID-19 patients may be due to the impaired host immune system.
4.3. The others
Similar to petechiae, erythema (5%), blister (4%), mucositis (2%) and enanthema (1%) only describe the clinical feature of the oral mucosal lesion, which may have numerous causes in different clinical situations. Thus, without a differential diagnosis, the possible association between them and COVID-19 infection is difficult to investigate.
Lichen planus is a common T cell-mediated immune reaction to an unknown trigger that results in cutanemucosal lesions (67). With a prevalence of 2% in the synthesized result, which is similar to the prevalence of oral lichen planus in the population (70), it is difficult to relate lichen planus to COVID-19.
Fissure tongue, which is thought to be strongly associated with heredity, is a condition characterized by the presence of grooves or fissures on the dorsal surface of the tongue (67). Due to its congenital nature, it is unlikely that COVID-19 is associated with its occurrence.
Hairy tongue is defined as a hair-like appearance on the dorsal tongue caused by the accumulation of keratin on the filiform papillae. It may be associated with smoking, general debilitation, poor oral hygiene, xerostomia-inducing medications, and a history of head and neck radiation therapy (67). As it is more associated with the medical history and behavior of the host, similar to fissure tongue, it is difficult to make an association with COVID-19.
4.4. Limitation of evidence
As shown in Table 1, most of the included studies were case reports (15/30) or case series (10/30), which lack control and exposure groups for scientific analysis, and most of them have a moderate (20/30) to high (5/30) risk of bias. Consequently, the source of these studies may be inaccurate or incomplete, resulting in heterogeneity of data. Hence, it is of low possibility to conclude the association between COVID-19 and oral mucosal lesions based on the available studies.
The previous review either only included systematic reviews which were not the primary article (71) or only summarised the present of oral lesions among all oral manifestations of COVID-19 (3, 7, 11). Therefore, the present review provides a detailed update on the prevalence of oral mucosal lesions presented in COVID-19 cases, especially an analysis of the oral mucosal lesion presentation pattern in different COVID-19 severity cases, identifying the possible causes of some oral mucosal lesions presented in COVID-19 patients.
The included case reports and case series, despite having a low level of evidence, clearly identified and reported the oral mucosal features presented in COVID-19 cases. Although lacking higher level studies, such as clinical trials, the summarised result can still provide a clinically relevant presentation on the types of lesions that are presented in COVID-19 patients and their possible associations, therefore providing a reference for clinicians.
Previous reviews have also demonstrated the common oral features of oral mucosal lesions in COVID-19-positive patients, and their corresponding hypothesis on the association between COVID and oral mucosal lesions. Some proposed that oral mucosal lesions may act as a secondary manifestation of COVID-19 (6, 10, 12, 17), or that COVID-19 disrupts the local microbiome or impairs oral mucosal immunity, causing oral mucosal lesions (12, 27, 72). Others pointed out that it may be caused by poor oral hygiene practice or intubation process during hospitalization, or adverse effects of COVID-19 medication (15, 69). However, similar to the findings of this systemic review, most of them (3, 5, 16, 20, 21, 23, 24) still cannot conclude that COVID-19 has an association with oral mucosal lesions due to the lack of detailed reports.
Therefore, combining the information from previous reviews and our results, further well-designed case-control studies are needed to analyze the association between COVID-19 and oral mucosal lesions and its possible mechanism.
5. Conclusion
This systematic review has updated the current knowledge on COVID-19 and oral mucosal lesions by identifying and summarising the common oral mucosal lesions and their prevalence in COVID-19 patients. Ulcers without specific diagnosis and aphthous lesions are unlikely to be associated with COVID-19, whereas traumatic ulcers, petechiae and candidiasis may be associated with COVID-19 due to the process of COVID-19 treatment or immune system impairment caused by COVID-19 infection. However, given the limited evidence from currently available studies, the association between COVID-19 and oral mucosal lesions remains difficult to clarify. The need for further studies has been demonstrated and emphasized. Health professionals should raise awareness of oral mucosal lesions in COVID-19 patients and provide appropriate treatment when necessary.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
WK: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. JL: Conceptualization, Methodology, Writing – review & editing. AC: Writing – review & editing. LZ: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Organization WH. Coronavirus (2022). Available at: https://www.who.int/health-topics/coronavirus#tab=tab_3
2. Beyerstedt S, Casaro EB, Rangel EB. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis. (2021) 40(5):905–19. doi: 10.1007/s10096-020-04138-6
3. Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, Acevedo AC, De Luca Canto G, Sugaya N, et al. Oral manifestations in patients with COVID-19: a living systematic review. J Dent Res. (2021) 100(2):141–54. doi: 10.1177/0022034520957289
4. Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, Acevedo AC, De Luca Canto G, Sugaya N, et al. Oral manifestations in patients with COVID-19: a 6-month update. J Dent Res. (2021) 100(12):1321–9. doi: 10.1177/00220345211029637
5. Bhujel N, Zaheer K, Singh RP. Oral mucosal lesions in patients with COVID-19: a systematic review. Br J Oral Maxillofac Surg. (2021) 59(9):1024–30. doi: 10.1016/j.bjoms.2021.06.011
6. Brandini DA, Takamiya AS, Thakkar P, Schaller S, Rahat R, Naqvi AR. COVID-19 and oral diseases: crosstalk, synergy or association? Rev Med Virol. (2021) 31(6):e2226. doi: 10.1002/rmv.2226
7. Cuevas-Gonzalez MV, Espinosa-Cristóbal LF, Donohue-Cornejo A, Tovar-Carrillo KL, Saucedo-Acuña RA, García-Calderón AG, et al. COVID-19 and its manifestations in the oral cavity: a systematic review. Medicine (Baltimore). (2021) 100(51):e28327. doi: 10.1097/MD.0000000000028327
8. Doceda MV, Gavriiloglou M, Petit C, Huck O. Oral health implications of SARS-CoV-2/COVID-19: a systematic review. Oral Health Prev Dent. (2022) 20(1):207–18. doi: 10.3290/j.ohpd.b2960801
9. Drago F, Ciccarese G, Merlo G, Trave I, Javor S, Rebora A, et al. Oral and cutaneous manifestations of viral and bacterial infections: not only COVID-19 disease. Clin Dermatol. (2021) 39(3):384–404. doi: 10.1016/j.clindermatol.2021.01.021
10. Egido-Moreno S, Valls-Roca-Umbert J, Jané-Salas E, López-López J, Estrugo-Devesa A. COVID-19 and oral lesions, short communication and review. J Clin Exp Dent. (2021) 13(3):e287–e94. doi: 10.4317/jced.57981
11. Erbas GS, Botsali A, Erden N, Ari C, Taskin B, Alper S, et al. COVID-19-related oral mucosa lesions among confirmed SARS-CoV-2 patients: a systematic review. Int J Dermatol. (2022) 61(1):20–32. doi: 10.1111/ijd.15889
12. Fakhruddin KS, Samaranayake LP, Buranawat B, Ngo H. Oro-facial mucocutaneous manifestations of coronavirus disease-2019 (COVID-19): a systematic review. PLoS One. (2022) 17(6):e0265531. doi: 10.1371/journal.pone.0265531
13. Farid H, Khah M, Jamal S, Ghafoor R. Oral manifestations of COVID-19-A literature review. Rev Med Virol. (2022) 32(1):e2248. doi: 10.1002/rmv.2248
14. Halboub E, Al-Maweri SA, Alanazi RH, Qaid NM, Abdulrab S. Orofacial manifestations of COVID-19: a brief review of the published literature. Braz Oral Res. (2020) 34:e124. doi: 10.1590/1807-3107bor-2020.vol34.0124
15. Hupsch-Marzec H, Dziedzic A, Skaba D, Tanasiewicz M. The spectrum of non-characteristic oral manifestations in COVID-19—a scoping brief commentary. Med Pr. (2021) 72(6):685–92. doi: 10.13075/mp.5893.01135
16. Iranmanesh B, Khalili M, Amiri R, Zartab H, Aflatoonian M. Oral manifestations of COVID-19 disease: a review article. Dermatol Ther. (2021) 34(1):e14578. doi: 10.1111/dth.14578
17. Kusiak A, Cichońska D, Tubaja M, Skorek A, Jereczek-Fossa BA, Corrao G, et al. COVID-19 manifestation in the oral cavity—a narrative literature review. Acta Otorhinolaryngol Ital. (2021) 41(5):395–400. doi: 10.14639/0392-100X-N1584
18. Mortazavi H, Rezaeifar K, Nasrabadi N. Oral manifestations of coronavirus disease-19: a mini-review. Open Access Maced J Med Sci. (2020) 8(T1):286–9. doi: 10.3889/oamjms.2020.4999
19. Nijakowski K, Wyzga S, Singh N, Podgórski F, Surdacka A. Oral manifestations in SARS-CoV-2 positive patients: a systematic review. J Clin Med. (2022) 11(8):2202–204. doi: 10.3390/jcm11082202
20. Reis VP, Bezerra AR, Maia ABP, Marques LC, Conde DC. An integrative review of oral manifestations in patients with COVID-19: signs directly related to SARS-CoV-2 infection or secondary findings? Int J Dermatol. (2022) 61(3):278–90. doi: 10.1111/ijd.15881
21. Rusu LC, Ardelean LC, Tigmeanu CV, Matichescu A, Sauciur I, Bratu EA. COVID-19 and its repercussions on oral health: a review. Medicina (Kaunas). (2021) 57(11):1189–209. doi: 10.3390/medicina57111189
22. Toader MP, Branisteanu DC, Glod M, Esanu IM, Branisteanu CI, Capsa MS, et al. Mucocutaneous lesions associated with SARS-CoV-2 infection (review). Exp Ther Med. (2022) 23(4):258. doi: 10.3892/etm.2022.11183
23. Tsuchiya H. Oral symptoms associated with COVID-19 and their pathogenic mechanisms: a literature review. Dent J (Basel). (2021) 9(3):32–59. doi: 10.3390/dj9030032
24. Uzêda ESVD, de Sá IB, Martins J, Pedreira N, Vieira VPS, Silva BHM. Oral lesions associated with COVID-19: a systematic review. Stomatologija. (2021) 23(1):3–8.
25. Atukorallaya DS, Ratnayake RK. Oral Mucosa, Saliva, and COVID-19 infection in oral health care. Front Med. (2021) 8:9. doi: 10.3389/fmed.2021.656926
26. Gutierrez-Camacho JR, Avila-Carrasco L, Martinez-Vazquez MC, Garza-Veloz I, Zorrilla-Alfaro SM, Gutierrez-Camacho V, et al. Oral lesions associated with COVID-19 and the participation of the buccal cavity as a key player for establishment of immunity against SARS-CoV-2. Int J Environ Res Public Health. (2022) 19(18):11383–404. doi: 10.3390/ijerph191811383
27. Moser D, Biere K, Han B, Hoerl M, Schelling G, Chouker A, et al. COVID-19 impairs immune response to Candida albicans. Front Immunol. (2021) 12:640644. doi: 10.3389/fimmu.2021.640644
28. Elangovan S. Taste disorders and xerostomia are highly prevalent in patients with COVID-19. J Evid-Based Dent Pract. (2022) 22(1):2. doi: 10.1016/j.jebdp.2021.101687
29. Etemad-Moghadam S, Alaeddini M. Is SARS-CoV-2 an etiologic agent or predisposing factor for oral lesions in COVID-19 patients? A concise review of reported cases in the literature. Int J Dent. (2021) 2021. doi: 10.1155/2021/6648082
30. Grover N, Prabhat KB, Singh S, Verma N. COVID-19 aoosciated with oral mucosal manifestation: a review article. J Pharm Negat Results. (2022) 13:849–53.
31. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol (Engl Ed). (2021) 74(9):790–9. doi: 10.1016/j.recesp.2021.06.016
32. Institute TJB. Checklist for Case Series (2017). Available at: https://jbi.global/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Case_Series2017_0.pdf
33. Institute TJB. Checklist for Case Reports (2017). Available at: https://jbi.global/sites/default/files/2020-08/Checklist_for_Case_Reports.pdf
34. Institute TJB. Checklist for Analytical Cross Sectional Studies (2017). Available at: https://jbi.global/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Analytical_Cross_Sectional_Studies2017_0.pdf
35. Institute TJB. Checklist for Cohort Studies (2017). Available at: https://jbi.global/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Cohort_Studies2017_0.pdf
36. Ansari R, Gheitani M, Heidari F, Heidari F. Oral cavity lesions as a manifestation of the novel virus (COVID-19). Oral Dis. (2021) 27(Suppl. 3):771–2. doi: 10.1111/odi.13465
37. Brandao TB, Gueiros LA, Melo TS, Prado-Ribeiro AC, Nesrallah A, Prado GVB, et al. Oral lesions in patients with SARS-CoV-2 infection: could the oral cavity be a target organ? Oral Surg Oral Med Oral Pathol Oral Radiol. (2021) 131(2):e45–51. doi: 10.1016/j.oooo.2020.07.014
38. Cebeci Kahraman F, Caskurlu H. Mucosal involvement in a COVID-19-positive patient: a case report. Dermatol Ther. (2020) 33(4):e13797. doi: 10.1111/dth.13797
39. Ciccarese G, Drago F, Boatti M, Porro A, Muzic SI, Parodi A. Oral erosions and petechiae during SARS-CoV-2 infection. J Med Virol. (2021) 93(1):129–32. doi: 10.1002/jmv.26221
40. Corchuelo J, Ulloa FC. Oral manifestations in a patient with a history of asymptomatic COVID-19: case report. Int J Infect Dis. (2020) 100:154–7. doi: 10.1016/j.ijid.2020.08.071
41. Cruz Tapia RO, Peraza Labrador AJ, Guimaraes DM, Matos Valdez LH. Oral mucosal lesions in patients with SARS-CoV-2 infection. Report of four cases. Are they a true sign of COVID-19 disease? Spec Care Dentist. (2020) 40(6):555–60. doi: 10.1111/scd.12520
42. Demirbas A, Elmas OF, Atasoy M, Tursen U, Lotti T. A case of erythema multiforme major in a patient with COVID 19: the role of corticosteroid treatment. Dermatol Ther. (2020) 33(6):e13899. doi: 10.1111/dth.13899
43. Diaz Rodriguez M, Jimenez Romera A, Villarroel M. Oral manifestations associated with COVID-19. Oral Dis. (2022) 28(Suppl. 1):960–2. doi: 10.1111/odi.13555
44. Dominguez-Santas M, Diaz-Guimaraens B, Fernandez-Nieto D, Jimenez-Cauhe J, Ortega-Quijano D, Suarez-Valle A. Minor aphthae associated with SARS-CoV-2 infection. Int J Dermatol. (2020) 59(8):1022–3. doi: 10.1111/ijd.15004
45. Eduardo FP, Bezinelli LM, Gobbi MF, Bergamin LG, de Carvalho DLC, Corrêa L. Oral lesions and saliva alterations of COVID-19 patients in an intensive care unit: a retrospective study. Spec Care Dentist. (2022) 42(5):494–502. doi: 10.1111/scd.12705
46. Eghbali Zarch R, Hosseinzadeh P. COVID-19 from the perspective of dentists: a case report and brief review of more than 170 cases. Dermatol Ther. (2021) 34(1):e14717. doi: 10.1111/dth.14717
47. Favia G, Tempesta A, Barile G, Brienza N, Capodiferro S, Vestito MC, et al. COVID-19 symptomatic patients with oral lesions: clinical and histopathological study on 123 cases of the university hospital policlinic of Bari with a purpose of a new classification. J Clin Med. (2021) 10(4):757–69. doi: 10.3390/jcm10040757
48. Fidan V, Koyuncu H, Akin O. Oral lesions in COVID 19 positive patients. Am J Otolaryngol. (2021) 42(3):102905. doi: 10.1016/j.amjoto.2021.102905
49. Gabusi A, Gissi DB, Rossi R, Foschini MP, Montebugnoli L. Persistent lesions in oral cavity after SARS-CoV-2 infection. Oral Dis. (2021) 28(Suppl 2):2577–8. doi: 10.1111/odi.13805
50. Garcez AS, Delgado MGT, Sperandio M, Dantas ESFT, de Assis JSR, Suzuki SS. Photodynamic therapy and photobiomodulation on oral lesion in patient with coronavirus disease 2019: a case report. Photobiomodul Photomed Laser Surg. (2021) 39(6):386–9. doi: 10.1089/photob.2020.4977
51. Glavina A, Biocina-Lukenda D, Mravak-Stipetic M, Markeljevic J. Oral symptoms and lesions in SARS-CoV-2-positive patient. Oral Dis. (2022) 28(Suppl. 1):979–80. doi: 10.1111/odi.13596
52. Hockova B, Riad A, Valky J, Sulajova Z, Stebel A, Slavik R, et al. Oral complications of ICU patients with COVID-19: case-series and review of two hundred ten cases. J Clin Med. (2021) 10(4):581–94. doi: 10.3390/jcm10040581
53. Indu S. Multiple oral ulcerations—an initial manifestation of COVID 19 infection: a personal experience!!. J Oral Maxillofac Pathol. (2020) 24(2):227–9. doi: 10.4103/jomfp.JOMFP_324_20
54. Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, Suarez-Valle A, Saceda-Corralo D, Moreno-Garcia Del Real C, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Clin Exp Dermatol. (2020) 45(7):892–5. doi: 10.1111/ced.14281
55. Jimenez-Cauhe J, Ortega-Quijano D, de Perosanz-Lobo D, Burgos-Blasco P, Vañó-Galván S, Fernandez-Guarino M, et al. Enanthem in patients with COVID-19 and skin rash. JAMA Dermatol. (2020) 156(10):1134–6. doi: 10.1001/jamadermatol.2020.2550
56. Katz J, Yue S. Increased odds ratio for COVID-19 in patients with recurrent aphthous stomatitis. J Oral Pathol Med. (2021) 50(1):114–7. doi: 10.1111/jop.13114
57. Kitakawa D, Oliveira FE, Neves de Castro P, Carvalho L. Short report—herpes simplex lesion in the lip semimucosa in a COVID-19 patient. Eur Rev Med Pharmacol Sci. (2020) 24(17):9151–3. doi: 10.26355/eurrev_202009_22863
58. Martin Carreras-Presas C, Amaro Sanchez J, Lopez-Sanchez AF, Jane-Salas E, Somacarrera Perez ML. Oral vesiculobullous lesions associated with SARS-CoV-2 infection. Oral Dis. (2021) 27(Suppl. 3):710–2. doi: 10.1111/odi.13382
59. Palaia G, Pernice E, Pergolini D, Impellizzeri A, Migliau G, Gambarini G, et al. Erythema Multiforme as early manifestation of COVID-19: a case report. Pathogens. (2022) 11(6):654–62. doi: 10.3390/pathogens11060654
60. Rai A, Kumar A, Rana A, Singh S, Sybil D. Oral findings in patients with COVID-19 infection: report of two cases and review of literature. J Pharm Res Int. (2021) 33(47B):265–73. doi: 10.9734/jpri/2021/v33i47B33123
61. Riad A, Kassem I, Badrah M, Klugar M. The manifestation of oral mucositis in COVID-19 patients: a case-series. Dermatol Ther. (2020) 33(6):e14479. doi: 10.1111/dth.14479
62. Riad A, Kassem I, Hockova B, Badrah M, Klugar M. Tongue ulcers associated with SARS-CoV-2 infection: a case series. Oral Dis. (2022) 28(Suppl. 1):988–90. doi: 10.1111/odi.13635
63. Sinadinos A, Shelswell J. Oral ulceration and blistering in patients with COVID-19. Evid Based Dent. (2020) 21(2):49. doi: 10.1038/s41432-020-0100-z
64. Soares CD, de Carvalho RA, de Carvalho KA, de Carvalho MG, de Almeida OP. Letter to editor: oral lesions in a patient with COVID-19. Med Oral Patol Oral Cir Bucal. (2020) 25(4):563–4. doi: 10.4317/medoral.24044
65. Soares CD, Mosqueda-Taylor A, De Carvalho MG, De Almeida OP. Oral vesiculobullous lesions as an early sign of COVID-19: immunohistochemical detection of SARS-CoV-2 spike protein. Br J Dermatol. (2021) 184(1):6. doi: 10.1111/bjd.19569
66. Villarroel-Dorrego M, Chacón L, Rosas R, Barrios V, Pernía Y, Vélez H. Oral findings in patients with COVID-19. Actas Dermosifiliogr. (2022) 113(2):183–6. doi: 10.1016/j.ad.2021.08.007
67. Neville BW, Damm DD, Allen CM, Chi AC. Oral and maxillofacial pathology. 4th ed. St. Louis, MO: Elsevier (2016).
68. Odell EW. Cawson’s essentials of oral pathology and oral medicine. 9th ed. Edinburgh: Elsevier (2017).
69. Schwab G, Palmieri M, Zerbinati RM, Sarmento DJS, Reis T, Ortega KL, et al. Lack of direct association between oral mucosal lesions and SARS-CoV-2 in a cohort of patients hospitalised with COVID-19. J Oral Microbiol. (2022) 14(1):2047491. doi: 10.1080/20002297.2022.2047491
70. González-Moles MÁ, Warnakulasuriya S, González-Ruiz I, González-Ruiz L, Ayén Á, Lenouvel D, et al. Worldwide prevalence of oral lichen planus: a systematic review and meta-analysis. Oral Dis. (2021) 27(4):813–28. doi: 10.1111/odi.13323
71. Di Spirito F, Iandolo A, Amato A, Caggiano M, Raimondo A, Lembo S, et al. Prevalence, features and degree of association of oral lesions in COVID-19: a systematic review of systematic reviews. Int J Environ Res Public Health. (2022) 19(12):7486–500. doi: 10.3390/ijerph19127486
Keywords: oral mucosal lesions, oral lesions, COVID-19, SARS-CoV-2, oral manifestations, systemic review
Citation: Kot WY, Li JW, Chan AKY and Zheng LW (2023) A reflection on COVID-19 and oral mucosal lesion: a systematic review. Front. Oral. Health 4:1322458. doi: 10.3389/froh.2023.1322458
Received: 18 October 2023; Accepted: 30 November 2023;
Published: 18 December 2023.
Edited by:
Roger Junges, University of Oslo, NorwayReviewed by:
Wagner VP, University of São Paulo, BrazilFelipe Silveira, University of the Republic, Uruguay
© 2023 Kot, Li, Chan and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Wu Zheng bHd6aGVuZ0Boa3UuaGs=
 Wai Ying Kot
Wai Ying Kot Jing Wen Li
Jing Wen Li Alice Kit Ying Chan
Alice Kit Ying Chan Li Wu Zheng
Li Wu Zheng