- 1School of Bio Sciences and Technology, Vellore Institute of Technology, Vellore, India
- 2Department of Periodontics, Saveetha Institute of Medical and Technical Sciences, Chennai, India
The oral cavity serves as a habitat for a diverse array of microorganisms, each performing distinct functions, thereby constituting a vibrant and intricate ecological community. The most common pathogenic bacteria in the oral ecosystem are the Red Complex group, which includes Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia. These bacteria have several ways to inflict damage, such as creating biofilms and secreting nano-sized vesicles from their outer membrane, called Outer Membrane Vesicles (OMV). OMVs are nano structures that carry proteins, lipids, and toxins from the outer membrane of Gram-negative bacteria. The OMVs of Red Complex bacteria play a role in the onset and development of oral pathological conditions such as gingivitis and periodontitis. Additionally, a substantial body of evidence supports the notion that these OMVs may exert influence on systemic pathologies, including atherosclerosis, alzheimer's, rheumatoid arthritis, and diabetes mellitus. This review will discuss the formation and composition of Red Complex bacterial OMVs, their impacts on the oral environment, the immune response, and their correlations with various systemic diseases. The suggested treatment approach by probiotics and bioactives focuses on the genetic elements that induce the production of OMVs by the Red Complex bacteria, offering a potent means to hinder the advancement of diseases propagated through these OMVs.
1 Introduction
The human oral cavity, a dynamic and diverse microbial ecosystem, hosts an ever-adapting community of microorganisms. It comprises more than 800 species, including approximately 700 bacterial species, 100 fungal species, and a few viruses and protozoa (1–3). The shift in oral microbiota from a balanced commensal state to a dysbiotic state results in the progression of oral infection. Subsequently, these oral infection often gets established from dynamic interactions between microorganisms, leading to microbial colonization and complex biofilm formation (dental plaque), ultimately resulting in the exacerbation of the disease severity (4). The continued accumulation of dental plaque due to inadequate oral hygiene initiates gingival inflammation. In this microenvironment, facultative anaerobic bacteria deplete the available oxygen within the plaque biofilm, creating favorable condition for the colonization and proliferation of strict anaerobes. The inflammatory response further supports the growth of these anaerobic species by increasing the availability of host derived nutrients, thereby sustaining the dysbiotic community. This results in alterations in the bacterial composition at subgingival tissues, with an increased presence of “Red Complex” bacterial species comprising Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola—the primary etiological agents of periodontitis (5). Other contributing pathogens include Gram-positive bacteria such as Filifactor alocis and Streptococcus spp., and Gram-negative species like Aggregatibacter actinomycetemcomitans and Fusobacterium nucleatum, as well as fungal species such as Candida spp (6). Among the Streptococcus species, S. mutans and S. sobrinus have long been recognized as primary cariogenic agents, whereas S. gordoni was considered an accessory pathogen due to its ability to potentiate P. gingivalis and A. actinomycetemcomitans pathogenesis. The accumulation of these oral pathogens contributes to dental plaque formation, which in turn causes gingival inflammation that can progress to periodontitis in some patients. This condition is characterized by significant inflammation, bleeding, tooth loss, and occasionally pain. Pain is rarely perceived during periodontitis due to altered periodontal nociception, which is influenced by bacterial virulence factors, host immune responses, and immune suppression. This blunted pain response hampers early detection, allows disease progression, and increases the risk of systemic complications (7).
A key virulence mechanism employed by Gram negative oral pathogenic bacteria is the release of outer membrane vesicles (OMVs) which are nanoscale, bilayered structures enriched with pathogenic cargo. These OMVs, due to their small size and complex composition, have garnered considerable scientific interest. They consist of a phospholipid bilayer embedded with lipopolysaccharides (LPS), membrane proteins, and receptors on the surface (8), while their luminal contents typically include peptidoglycan fragments, periplasmic proteins, and nucleic acids such as DNA and RNA (9). The production and molecular composition of OMVs vary significantly between and within bacterial species, reflecting the tightly regulated nature of vesiculation (10).
OMVs play multifaceted roles in bacterial survival and pathogenicity. These include the removal of toxins and metabolic waste, modulation of host immune responses, horizontal gene transfer, microbial communication, biofilm development, and enhanced resistance to antimicrobial agents (11). Such functions are largely mediated by virulence determinants like LPS and proteolytic enzymes, which help the pathogen to evade innate immune defenses and establish infection (12). Periodontal diseases may further facilitate the systemic translocation of these pathogens, potentially leading to multi-organ dysfunction. Emerging evidence links oral pathogens and their OMVs to a wide range of systemic diseases, including Alzheimer's disease (13, 14) cardiovascular disease (15), diabetes mellitus (16, 17), and rheumatoid arthritis (18). These associations will be explored in detail in this review.
Although several studies have examined P. gingivalis OMVs in systemic diseases, a holistic analysis of the Red Complex bacterial OMV-mediated virulence, systemic impact, and immunomodulatory roles remains lacking (19–22). This review aims to bridge that gap by presenting a comprehensive evaluation of OMVs secreted by Red Complex bacteria, their associated systemic pathologies, immune interactions, and potential therapeutic strategies involving commensal microbiota and plant based bioactive compounds.
A focused literature search was conducted using the PubMed database to identify relevant studies on OMVs produced by P. gingivalis, T. forsythia, and T. denticola, particularly in the context of systemic diseases. Two keyword strategies were employed to capture both general and disease-specific research involving vesicles. The first search strategy targeted broader vesicle-related literature: (“vesicles”) AND (“Porphyromonas gingivalis” OR “Tannerella forsythia” OR “Treponema denticola”). The second, more refined search focused on vesicle types, virulence factors, and systemic disease relevance: (“outer membrane vesicles” OR OMVs OR “extracellular vesicles” OR “vesicles” OR gingipains OR “virulence factors”) AND (“Porphyromonas gingivalis” OR “Tannerella forsythia” OR “Treponema denticola”) AND (“systemic disease” OR “atherosclerosis” OR “diabetes” OR “rheumatoid arthritis” OR “alzheimer's disease” OR “neuroinflammation” OR “immune response”). Only articles published in English were considered. Peer-reviewed original research articles, reviews, and meta-analyses were included. Titles and abstracts were screened for relevance, and full texts were evaluated to ensure alignment with the study's focus on vesicle biogenesis, composition, virulence, and interactions with host systems in the context of systemic disease. Additional literature was cited to support the background, biological mechanisms, and proposed strategies of the study.
2 Biogenesis of OMVs from Red Complex Bacteria
P. gingivalis produces OMVs ranging in size from 50 to 250 nm (23). These vesicles are enriched with outer membrane proteins such as PG1823, PG2105, and PG2106, which are essential for OMV structure and function (24). Vesiculation in P. gingivalis is closely linked to the presence of outer membrane porins, including OmpA-like proteins (Pgm6/7) and galactose 4-epimerase (GalE). OmpA maintains membrane integrity and is involved in peptidoglycan turnover, with evidence showing its crosslinking to peptidoglycan (25). Notably, Pgm6/7 homologous to Escherichia coli OmpA, are critical for sustaining outer membrane architecture; their absence results in a distorted membrane and increased vesicle and gingipain production, exacerbating periodontal tissue damage and immune evasion (23). GalE mutants exhibit a reduction or complete loss of OMV production, while ompA mutants tend to overproduce OMVs, underscoring ompA's regulatory role.
The expression of the major fimbrial subunit FimA correlates positively with vesiculation. OMV production is significantly reduced in fimA and fimR mutants, suggesting that the Fim locus plays a regulatory role in vesicle release, adhesion, host invasion, and pathogenicity (26). P. gingivalis contains two types of O-antigens: negatively charged LPS (A-LPS) and neutral LPS (O-LPS) (24). A-LPS, composed of phosphorylated mannan repeats linked to the lipid A core, which is integral to OMV formation. Dephosphorylation of A-LPS by outer membrane protein PG0027 destabilizes the membrane, facilitating vesicle release (23), which also modulates gingipain production. Mutants lacking A-LPS exhibit reduced levels of OMV-associated gingipains (27), and instead show increased levels of envelope proteins like RagA and RagB, typically absent in wild-type OMVs. Silencing the rgpA gene, which encodes arginine-specific gingipain A, leads to a reduction in OMV production (23). Key virulence factors—including arg-gingipains (Rgp), lys-gingipains (Kgp), and hemagglutinins (Hag)—are secreted via the Por secretion system (PorSS). Their C-terminal domain (CTD) ensures proper localization to the bacterial surface and is glycosylated with A-LPS, reinforcing the centrality of A-LPS in OMV biogenesis and virulence. Charged LPS microdomains, highly concentrated on the P. gingivalis outer surface, influence OMV formation. Deacylation of anionic LPS promotes membrane curvature, enhancing vesiculation (24).
In T. forsythia, the S-layer proteins TfsA and TfsB mediate adhesion to gingival cells and hemagglutination. While the native S-layer maintains a 1:1 TfsA:TfsB ratio, OMVs display a 3:1 ratio due to irregular S-layer formation and protein loss. Given the complexity of forming curved S-layers, this shift may aid OMV production by enhancing curvature (28). Beyond structural components, environmental stressors such as heat, nutrient deprivation (e.g., lysine deficiency), sublethal antibiotic exposure, oxidative damage, hemin limitation, and serum addition can trigger hypervesiculation without causing cell lysis. These stimuli, including quorum sensing and envelope stress, induce stress-responsive vesicle overproduction.
While OMV biogenesis has been extensively studied in other Red Complex bacteria such as Porphyromonas gingivalis, such mechanistic insights are notably absent for T. denticola. Most available literature on T. denticola OMVs—focuses on structural, proteomic, and virulence-related characterization rather than the molecular process of biogenesis.
3 Components of Red Complex bacterial OMVs
Bacterial OMVs serve as multifunctional tools to enhance microbial survival and pathogenesis. They transport virulence factors, mediate immune modulation, and promote bacterial communication (29). Structurally, OMVs possess a lipid bilayer rich in outer membrane proteins and LPS, while the lumen contains periplasmic proteins, enzymes, lipids, and nucleic acids, including DNA and RNA (30).
Among the critical OMV components in P. gingivalis, gingipains represent major virulence factors. The gene PGN_1251 (gtfB), involved in gingipain processing, encodes a protein homologous to glycosyltransferase-1. gtfB mutants lacking both O-LPS and A-LPS, show diminished surface expression of gingipains and other key proteins, underscoring its role in maintaining OMV structural integrity (23). The C-terminal domain (CTD) domain, about 70 amino acids in length is essential for surface attachment and secretion of OMV-associated proteins, including gingipains. Notably, OMV composition varies among P. gingivalis strains. For instance, fimbriated strains contain FimC, FimD, and FimE in their OMVs, which are absent in non-fimbriated strains (23).
OMVs from T. forsythia are highly heterogeneous, containing at least 297 proteins. Approximately 31% are substrates of the type IX secretion system, with 54% localized to the membrane, 12% to the lumen, and the remainder of unknown localization. TonB-dependent receptors (TDRs) constitute 70% of vesicle membrane proteins. Key proteins include: N-protein (Tanf_02375), involved in DNA binding and helicase activity, PorP (Tanf_02355), a DNA-binding auxiliary protein, proteinases with CTD-like domains, S8 peptidase (Tanf_00440, mirolase) with serine endopeptidase activity and Karilysin (Tanf_06550), a metalloendopeptidase (28). Approximately 98 lipoproteins were predicted in T. forsythia OMVs, including membrane-bound enzymes such as glucosyl hydrolases, peptidyl-prolyl cis-trans isomerase, and HmuY. Vesicle lumens contain sialidases, glucosidases, and 12 predicted glycosyl hydrolases such as galactosidases, mannosidases, and a newly identified membrane-associated fucosidase (28, 31). These findings, largely based on in silico predictions, require further experimental validation.
Using label-free quantitative proteomics, 1,448 proteins were identified from T. denticola, including 90 outer membrane proteins such as lipoproteins and β-barrel proteins. Distinct signal cleavage patterns differentiated outer membrane from inner membrane lipoproteins. OMVs were enriched in virulence factors like dentilisin, dentisilin protease complex PrtP, its auxiliary lipoproteins PcrA and PcrB and FGE-sulfatase domain-containing lipoproteins. Native PAGE revealed large protein complexes, including major surface protein (Msp) and β-barrel proteins. Homologous analysis also uncovered new outer membrane proteins in T. pallidum, offering promising vaccine targets for both pathogens (28, 32). Additional components include LrrA (TDE2258), a leucine-rich repeat surface antigen, and BspA, a putative lipoprotein implicated in interkingdom communication, host colonization, and coaggregation with other oral bacteria (28).
4 Red Complex bacterial OMVs and periodontal disease
Migration of Red Complex bacteria and their byproducts including LPS and proinflammatory cytokines, into distal organs through the bloodstream results in systemic diseases, often originating from plaque accumulation in the oral cavity (33, 34). The resulting polymicrobial biofilm forms through coordinated interactions among diverse bacteria, mediated by adhesins and receptors (35). P. gingivalis, a key member of the Red Complex, is extensively studied in oral infectious diseases. Notably, infection with P. gingivalis alone is sufficient to induce periodontitis in non-human primates, highlighting its pathogenic potential (36). OMVs from P. gingivalis facilitate interspecies aggregation, such as between Staphylococcus aureus, Streptococcus spp., and Candida spp (37). OMVs also promote interactions between T. denticola and Lactobacillus laburnum, enabling motility for typically non-motile bacteria within the oral microenvironment (38). Two adhesins, FimA and Mfa, present on P. gingivalis OMVs, enhance bacterial attachment, while OMV-associated gingipains inhibit the biofilm formation of commensal species, favoring colonization by pathogenic bacteria (39). However, the specific mechanisms underlying P. gingivalis OMV-mediated biofilm formation remain unexplored (36).
T. denticola OMVs are enriched with dentilisin, a trypsin-like protease complex that degrades extracellular matrix components such as fibronectin and laminin, thereby weakening connective tissue architecture (40). Additionally, OMVs contain the Msp, which disrupts epithelial barrier function and alters cell adhesion, facilitating bacterial invasion into deeper gingival tissues (41). These vesicles also contribute to biofilm maturation and enhance the cooperative virulence of the Red Complex consortium. Additionally, T. denticola OMVs are involved in interspecies and host cell communication, contributing to robust biofilm development, a major factor in chronic periodontitis (42).
T. forsythia OMVs similarly carry potent proteases such as karilysin and mirolase, which break down ECM proteins and basement membrane structures, promoting detachment of the junctional epithelium and periodontal pocket formation (31). The OMV-associated S-layer glycoproteins further assist in colonization and persistence in the subgingival environment (43). The OMVs also promote sialic acid catabolism and associated biofilm formation, aggravating periodontal disease severity (44). By enabling deeper bacterial penetration and local tissue degradation, OMVs from these pathogens play a direct role in the structural breakdown of periodontal tissues characteristic of periodontitis.
5 Red Complex bacterial OMVs and host cell interactions
OMVs derived from P. gingivalis are internalized by gingival epithelial and endothelial cells through two primary mechanisms: actin-mediated endocytosis and lipid raft dependent pathways. The former involves OMV recognition of α5β1 integrin on host cells, triggering F-actin polymerization regulated by phosphatidylinositol 3-kinase (PI3K). The latter depends on fimbriae and engages PI3K, Rac1, and regulatory GTPases (45). The route of internalization is OMV size dependent. OMV uptake disrupts key signaling molecules, including transferrin receptor (TfR) and paxillin/focal adhesion kinase (FAK), impairing epithelial cell motility (46, 47). P. gingivalis OMVs also interfere with oral squamous cells via gingipains (24, 48) and inhibit fibroblast and endothelial cell proliferation, hindering angiogenesis and delaying wound healing in periodontal tissues (49). These vesicles reduce endothelial nitric oxide synthase (eNOS) expression, leading to oxidative stress. Moreover, OMVs trigger apoptosis, cellular activation, and cytokine secretion by activating host pattern recognition receptors (PRRs) (50). The peptidoglycan components of P. gingivalis OMVs stimulate autophagy (51), while macrophages exposed to OMVs release pro- and anti-inflammatory cytokines, including interleukins IL-6, IL-10, IL-12p70, interferon-β (IFN-β), and tumor necrosis factor-α (TNF-α), promoting glycolysis-mediated apoptosis (52). Nuclear factor kappa B (NF-κB) activation further modulates monocyte and macrophage responses. Furthermore, P. gingivalis OMVs, through LPS and Toll-like receptor 2 (TLR2) interactions, promote osteoclast differentiation, contributing to alveolar bone resorption. Intriguingly, these vesicles can also stimulate the invasion and migration of oral squamous cell carcinoma, suggesting a role in tumor progression (53, 54).
T. denticola OMVs, rich in the protease dentilisin, degrade intercellular adhesion proteins, enhancing bacterial invasion. Additional components such as Msp and chymotrypsin-like proteases contribute to cytotoxicity by forming pores in epithelial cells (44). T. denticola OMVs also contain LPS, which is cytotoxic to gingival epithelium. These effects collectively lead to extracellular matrix degradation and chronic periodontitis (55).
Recent studies have demonstrated that OMVs of T. forsythia play a significant role in host–pathogen interactions within the oral cavity. These vesicles are capable of adhering to and being internalized by human oral epithelial cells through mechanisms mediated by surface virulence factors such as the BspA protein (43). Upon internalization, T. forsythia OMVs have been shown to stimulate pro-inflammatory responses, including the secretion of interleukin-8 (IL-8) and TNF-α, via TLR2-dependent signaling pathways (56). Proteomic analyses of T. forsythia OMVs further support their involvement in host modulation, revealing the presence of multiple glycosidases, proteases, and autotransporters capable of degrading host extracellular matrix components and altering immune responses (56). These findings suggest that T. forsythia OMVs contribute actively to periodontal pathogenesis by mediating epithelial cell responses and facilitating tissue destruction.
6 OMVs from Red Complex bacteria and systemic diseases
6.1 Atherosclerosis
Atherosclerosis, a condition characterized by arterial plaque buildup and reduced vessel elasticity, significantly increases the risk of cardiovascular events. There is accumulating evidence linking oral bacterial infections, particularly those involving biofilm-forming pathogens, with atherosclerosis. P. gingivalis is frequently detected in individuals with vascular disease, yet the precise mechanisms remain under investigation (57–59). OMVs are nanostructures capable of traversing vascular membranes more efficiently than whole bacteria (60, 61). They can influence key factors such as blood coagulation, endothelial integrity, calcium deposition, and lipoprotein metabolism. Among oral pathogens, P. gingivalis, T. forsythia, and T. denticola have been implicated in cardiovascular pathology (62, 63).
P. gingivalis OMVs in the bloodstream have been shown to promote platelet aggregation, a crucial step in thrombus and plaque formation (64). They also enhance foam cell development by increasing low-density lipoprotein (LDL) accumulation in macrophages, fueling atherosclerotic progression (65). Additionally, OMVs suppress eNOS, a key vasoprotective enzyme, via Rho-associated kinase (ROCK) activity. This suppression is mediated by the extracellular signal-regulated kinase (ERK1/2) and mitogen-activated protein kinase (MAPK) pathways and can be reversed with ROCK inhibitors like Y-27632 (66). In vivo studies using zebrafish larvae models have shown increased vascular damage upon P. gingivalis OMV exposure (67). Gingipains on OMV surfaces degrade platelet endothelial cell adhesion molecule-1 (PECAM-1 or CD31), a protein essential for endothelial junction integrity. Reduced PECAM-1 levels increase vascular permeability, exacerbating vascular inflammation and lesion development. OMVs also activate the ERK1/2 and runt-related transcription factor 2 (RUNX2) signaling pathways in vascular smooth muscle cells, promoting calcification and accelerating atherosclerosis (68).
T. forsythia OMVs and other bacterial components, were shown to stimulate foam cell formation in macrophages and accelerate lesion development in ApoE−/− mice via its BspA protein and OMV associated molecules. Chronic infection in these models also caused dyslipidemia, elevated serum amyloid A, and reduced nitric oxide, hallmark indicators of atherosclerotic risk. In human studies, T. forsythia DNA and virulence genes, including those associated with OMV cargo, have been detected in atherosclerotic plaques, alongside T. denticola and P. gingivalis, reinforcing a mechanistic link. T. denticola can activate endothelial cells by inducing the expression of IL8 and monocyte chemoattractant protein-1 (MCP1) which can facilitate chemotaxis and aggregation of monocytes which might cause atherosclerosis (69). The detailed summary were represented in Figure 1.
6.2 Alzheimer's disease
P. gingivalis and its toxic protease, gingipain, have been identified in the brains of patients with Alzheimer's disease (AD) (45). A growing body of evidence suggests that bacterial OMVs can enhance blood–brain barrier (BBB) permeability, although the exact mechanisms remain unclear (45). Despite the brain being an inhospitable environment for anaerobic bacteria such as P. gingivalis, its DNA and LPS have been detected in AD patient brain tissue, likely due to the nano-scale size of OMVs, which can more readily cross the BBB (70, 71). The “Gingipains Hypothesis” and Pg-induced iron dyshomeostasis, alongside reduced salivary lactoferrin and cholinergic dysfunction, suggest a broader pathogenic role. Pg's Type IX secretion system enables the release of toxin-rich OMVs, offering a more plausible explanation for widespread toxin distribution in the brain. This model unifies microbial and neurodegenerative hypotheses, providing a comprehensive framework for understanding and managing sporadic AD (72). Gingipains present in P. gingivalis OMVs have been shown to degrade tight junction proteins such as zonula occludens-1 (ZO-1) and occludin in human cerebral microvascular endothelial cells (hCMEC/D3), suggesting a possible route of entry into the brain (73, 74). Moreover, OMVs stimulate the production and maturation of IL-1β, a pro-inflammatory cytokine, via cathepsin B- and L-independent phagocytic mechanisms. These immune responses have been linked to cognitive decline and Alzheimer-like symptoms in murine models (75).
One study reported that repeated abdominal injections of P. gingivalis OMVs over 12 weeks led to their translocation into mouse brains (76). Another investigation found that OMVs were detectable in cortical and hippocampal regions within just three days of oral administration (77). Their presence was associated with astrocyte and microglial activation, increased phosphorylation of tau protein, and elevated hippocampal IL-1β levels. These OMVs also reduced expression of tight junction proteins, including ZO-1, occludin, and claudin-5 factors crucial for maintaining BBB integrity and cognitive function (77). Tau protein, predominantly found in neurons, plays a vital role in stabilizing microtubules. Gingipains in OMVs are highly neurotoxic, both in vitro and in vivo, and disrupt tau homeostasis, contributing to the pathogenesis of AD (78). Additionally, gingival exposure to P. gingivalis or its OMVs induces periodontitis, systemic inflammation, and neuroinflammatory responses in murine models, leading to memory impairment-like behaviors. OMVs were detected in the trigeminal ganglia and hippocampus, indicating a potential neural route of brain entry and subsequent impact on AD pathology (79). These mechanisms are summarized in Figure 1.
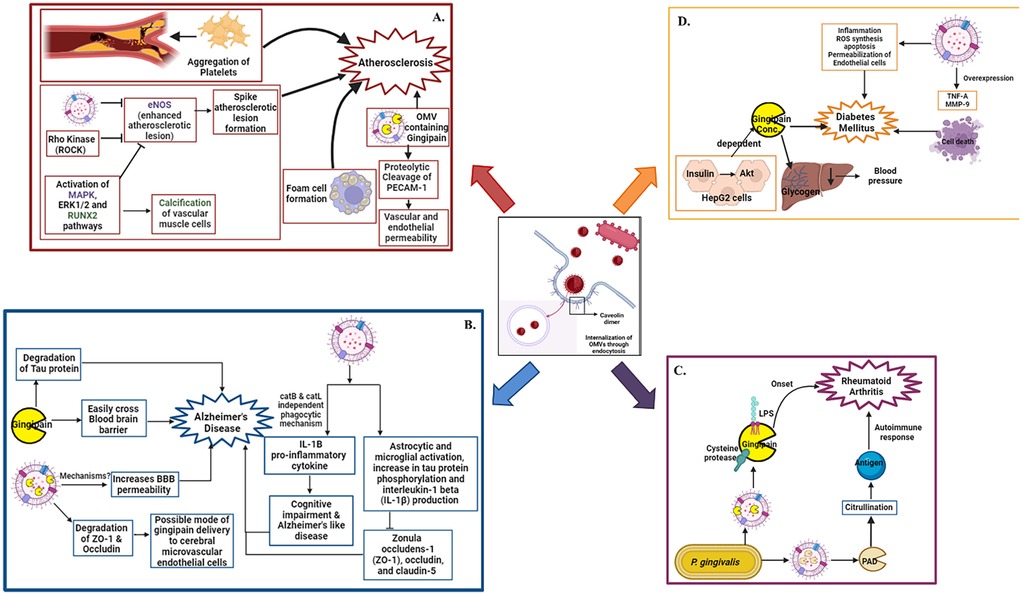
Figure 1. Oral pathogen OMV mediated systemic diseases: internalization of OMVs in host tissues through endocytosis. After internalization OMVs interact with various pathways and leads to emergence of various systemic diseases. The disease onset and its interlinking pathways are represented in panel (A). Atherosclerosis, (B) Alzheimer's disease, (C) Rheumatoid arthritis, (D) Diabetes mellitus respectively. Figure created with BioRender.com.
6.3 Rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by systemic inflammation, primarily targeting the joints. P. gingivalis contributes to RA pathogenesis not only by affecting the periodontium but also by initiating protein citrullination in synovial tissues and neurons. The bacterium facilitates citrullination via proteolytic cleavage of arginine-containing peptides, a process central to autoantigen formation in RA (80). Inactivation of P. gingivalis arginine-specific gingipains significantly reduces citrullination. OMVs are believed to be a major delivery system for gingipains, directly linking them to RA progression. Citrullination occurs after gingipain-mediated cleavage, through the action of peptidyl arginine deiminase (PAD), an enzyme enriched in P. gingivalis OMVs. Deletion of the PAD gene abolishes citrullination activity entirely (81).
When incubated with host proteins such as fibrinogen and α-enolase, P. gingivalis induces both protein degradation and citrullination, leading to the formation of neoantigens. These altered proteins provoke autoimmune responses, including the generation of anti-citrullinated protein antibodies (ACPAs), which are present in approximately 75% of RA patients (82, 83). ACPAs can be detected up to a decade before clinical onset, and their presence is strongly correlated with periodontitis (84, 85) Thus, OMVs from P. gingivalis, enriched with LPS, gingipains, and PAD, play a key role in driving citrullination and the subsequent autoimmune cascade in RA. The underlying mechanisms are illustrated in Figure 1.
6.4 Diabetes mellitus
Emerging research implicates OMVs from oral pathogens in the pathogenesis of diabetes mellitus, a metabolic disorder characterized by chronic hyperglycemia and insulin resistance (86). This relationship appears to be bidirectional: diabetes increases susceptibility to periodontal infections and impairs wound healing, while OMVs from pathogens like P. gingivalis exacerbate systemic inflammation and metabolic dysfunction (87). In murine models, P. gingivalis OMVs deliver gingipains to the liver, impairing insulin-stimulated glycogen synthesis and consequently elevating blood glucose levels. In hepatic HepG2 cells, insulin activates the Akt/glycogen synthase kinase-3β pathway, which is disrupted in a gingipain concentration–dependent manner (86). Moreover, OMVs contribute to diabetic complications such as retinopathy. In vitro studies have shown that P. gingivalis OMVs compromise the integrity of the retinal blood barrier by inducing inflammation, reactive oxygen species (ROS) production, apoptosis, and endothelial permeability. They also upregulate TNF-α and matrix metalloproteinase-9 (MMP-9), leading to retinal endothelial cell damage and death (88). The summary were represented in Figure 1.
7 Immune responses mediated by OMVs of Red Complex bacteria
Immunoglobulin G (IgG) antibodies from P. gingivalis seropositive patients interact strongly with wild-type P. gingivalis, but only modestly with OMV-deficient mutants. OMVs from P. gingivalis serve as reservoirs of immunogenic antigens and active proteases that contribute to tissue degradation in periodontal disease (60). These vesicles induce cellular activation, cytokine production, and apoptotic cell death by enhancing the activity of PRRs in gingival epithelial cells. Additionally, OMVs engage with macrophages via PRRs, triggering the secretion of both pro- and anti-inflammatory cytokines, thereby sustaining chronic inflammation (89).
Periodontitis is characterized by the activation of various immune cells including neutrophils, B and T lymphocytes, natural killer cells, macrophages, and osteoclasts, primarily driven by pro-inflammatory cytokines. When exposed to T. forsythia OMVs, periodontal ligament fibroblasts express significantly higher levels of IL-6, IL-8, and MCP-1 than when exposed to the bacteria themselves. These chemokines, particularly IL-8 and MCP-1, recruit neutrophils to the site of inflammation (31). OMVs from P. gingivalis also induce foam cell formation, which is associated with the release of inducible nitric oxide synthase (iNOS) and nitric oxide (NO). Moreover, P. gingivalis OMVs stimulate IL-8 secretion, while lipooligosaccharide (LOS) and T. denticola OMVs elicits strong inflammatory responses in human gingival fibroblasts via IL-6, IL-8, MCP-1, prostaglandin C, and NO production (23). Persistent overproduction of these mediators can result in significant tissue destruction. P. gingivalis OMVs contribute to periodontitis by delivering sRNA45033, which targets CBX5 in human periodontal ligament cells, leading to apoptosis and inflammatory cytokine release. CBX5 modulates p53 DNA methylation, revealing a novel OMV-mediated host–pathogen interaction in periodontal disease (90).
Gingipains present on P. gingivalis OMVs degrade IgG, immunoglobulin M (IgM), and complement component C3, compromising the protective function of serum (48, 91) OMVs from P. gingivalis, T. forsythia, and T. denticola interact with PRRs on monocytes and macrophages, leading to hypersecretion of NF-κB, TNF-α, IL-1β, and IL-8 (55). Specifically, P. gingivalis OMVs trigger strong TLR2 and TLR4 responses, along with moderate activation of nucleotide-binding oligomerization domain-containing proteins NOD1 and NOD2, and other TLRs (TLR4, TLR7, and TLR8), whereas OMVs from the other two Red Complex members provoke much weaker responses (92).
Furthermore, OMVs from P. gingivalis impair the expression of leukocyte surface markers on human umbilical vascular endothelial cells, thereby disrupting major histocompatibility complex class II (MHC II)-mediated adaptive immune responses (93). These OMVs also activate human neutrophils through a surface-coating mechanism that enhances degranulation and inhibits neutrophil apoptosis. Notably, gingipain proteases within OMVs degrade antimicrobial peptides such as LL-37 and enzymes like myeloperoxidase from neutrophil granules (94). This immune evasion strategy is similarly employed by A. actinomycetemcomitans, reinforcing the role of OMVs in counteracting neutrophil-mediated antimicrobial activity.
OMVs from P. gingivalis and T. forsythia promote osteoclast differentiation by activating TLR2 signaling in osteoclast precursors. Their osteoclastogenic effects are mediated by lipoproteins and LPS, as shown by reduced activity following treatment with lipoprotein lipase and polymyxin B (95). Infection of macrophages with T. forsythia results in the release of two distinct EV populations: macrophage-derived EVs enriched with pro-inflammatory mediators and T. forsythia-derived OMVs carrying virulence factors like BspA and GroEL. These OMVs trigger TLR2-mediated inflammatory responses and are actively released in response to macrophage-derived signals, highlighting their role in periodontitis progression (56).
Moreover, P. gingivalis OMVs modulate cellular metabolism in human trophoblast cells by disrupting glycolysis and suppressing ROS production. While overall cell viability remains unaffected, cellular migration and invasion are diminished, leading to a quiescent state. In murine models, OMV exposure results in reduced expression of glucose transporter 1 (GLUT1) and glycolytic activity, ultimately decreasing fetal weight gain (96). The overall representations were given in Figure 2.
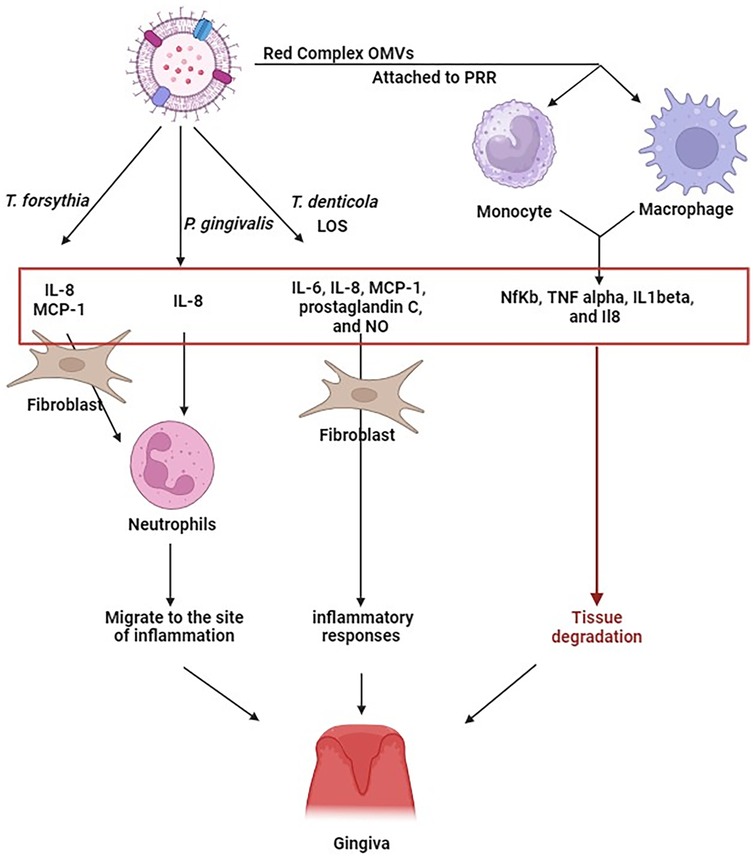
Figure 2. Exposure of periodontal ligament fibroblasts to T. forsythia OMV, leads to an increased production of IL-6, IL-8, and MCP-1. OMVs from P. gingivalis can induce the secretion of IL-8. Similarly, OMVs from T. denticola carrying lipooligosaccharides, produced IL-6, IL-8, monocyte chemoattractant protein-1 (MCP-1), prostaglandin E2 (PGE2), and nitric oxide (NO) in human gingival fibroblasts. The presence of IL-8 and MCP-1, in particular, triggers the migration of neutrophils towards the inflammation site. OMVs from P. gingivalis, T. forsythia, and T. denticola, upon interacting with monocyte and macrophage receptors, stimulate hypersecretion of NfKb, TNF alpha, IL1beta, and IL8. The continuous generation of these cytokines may potentially exacerbate deterioration of tissue. This indicates that the immune response to Red Complex OMVs might be more intense than to the bacteria themselves. Figure created with BioRender.com.
8 Strategies for the prevention of Red Complex bacterial OMV-mediated systemic diseases
The oral microbiota is a diverse and dynamic ecosystem comprising bacteria, fungi, viruses, and protozoa that colonize various niches of the oral cavity, including the teeth, tongue, gingiva, and mucosa. This complex microbial community plays a vital role in maintaining oral health and protecting against pathogenic infections (97). A key function of the commensal oral microbiota is “colonization resistance”, the prevention of pathogen adherence and proliferation through competition for binding sites and essential nutrients, or through direct microbial antagonism (98). Moreover, the normal oral flora can modulate host immune responses by inducing antibody and cytokine production, thereby enhancing host defense mechanisms against pathogens. Probiotic and prebiotic therapies have been proposed as promising strategies to restore or enhance this protective microbial balance (99).
Species of Lactobacillus, including L. acidophilus, L. casei, L. paracasei, L. plantarum, L. rhamnosus, and L. salivarius, have demonstrated significant antibacterial activity against P. gingivalis, a keystone member of the Red Complex consortium (100–103) Other species such as L. salicinius, L. rhamnosus, and L. paracasei exhibit antimicrobial effects against multiple oral pathogens—including Streptococcus mutans, P. gingivalis, Fusobacterium nucleatum, and A. actinomycetemcomitans—even in heat-killed forms, suggesting the presence of heat-stable antimicrobial compounds (104).
Supernatants from several Lactobacillus species, such as L. acidophilus, L. casei, L. crispatus, L. fermentum, L. plantarum, L. rhamnosus, L. salivarius, and L. vaginalis, have shown broad-spectrum antimicrobial activity against Gram-negative A. actinomycetemcomitans and Gram-positive Actinomyces naeslundii (105–109). Notably, the supernatant of L. kefiranofaciens inhibited S. mutans biofilm formation both phenotypically and at the transcriptomic level (110). These effects suggest that Lactobacillus species exert their protective role through competitive exclusion, nutrient limitation, and secretion of extracellular antimicrobial metabolites.
As previously discussed, P. gingivalis OMV production can be downregulated through mutations in key genes such as fimA, fimR, and rgpA. Targeting these genes presents a viable strategy for mitigating OMV-mediated pathogenesis. Supporting this hypothesis, supernatants from L. rhamnosus, L. acidophilus, and L. reuteri have been shown to downregulate fimA and rgpA expression in P. gingivalis (111). A study by Yang KM et al. further confirmed the inhibition of rgpA by L. reuteri supernatant (106). These findings suggest that Lactobacillus-derived probiotics, and potentially their secreted metabolites or vesicles, may be developed as therapeutic agents targeting OMV biogenesis in Red Complex pathogens. However, robust in vitro and in vivo studies are necessary to validate these approaches and fully explore their clinical potential (97).
In addition to probiotics, natural bioactive compounds derived from plants have shown promise in promoting oral health and targeting the pathogenic mechanisms of Red Complex bacteria (112). Among these, curcumin, a polyphenolic compound extensively studied for its anti-inflammatory and antimicrobial properties, has demonstrated significant inhibitory effects on the proliferation of P. gingivalis, T. forsythia, and T. denticola (113).
Curcumin reduces the cytotoxic effects of P. gingivalis OMVs by inhibiting OMV-induced epithelial cell apoptosis and preventing bacterial attachment. It also downregulates pro-inflammatory genes such as IL-6, IL-1β, and TNF-α in human epithelial cells (114). Importantly, curcumin has been shown to suppress the expression of key OMV-associated genes, fimA and rgpA, thereby reducing OMV production and virulence (115). Other plant-derived bioactives exhibit similar mechanisms. Resveratrol, a natural phenolic compound, significantly inhibits fimA and rgpA expression in P. gingivalis (116). Epigallocatechin-3-gallate (EGCG), a major catechin in green tea, also downregulates these genes (117). Rhein, an anthraquinone compound, markedly reduces their expression as well (118). Coumarin has been shown not only to suppress rgpA expression but also to reduce overall pathogenicity of P. gingivalis (119).
Further, omega-3 fatty acids such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) significantly inhibit rgpA gene expression, as demonstrated by Sun et al. (120). Eugenol, a phenolic compound commonly found in clove oil, also suppresses both fimA and rgpA expression (121). Collectively, these natural compounds offer a novel therapeutic avenue by attenuating OMV production at the genetic level, thereby weakening the virulence and disease-promoting capacity of Red Complex pathogens. The therapeutic strategies were represented in Figure 3.
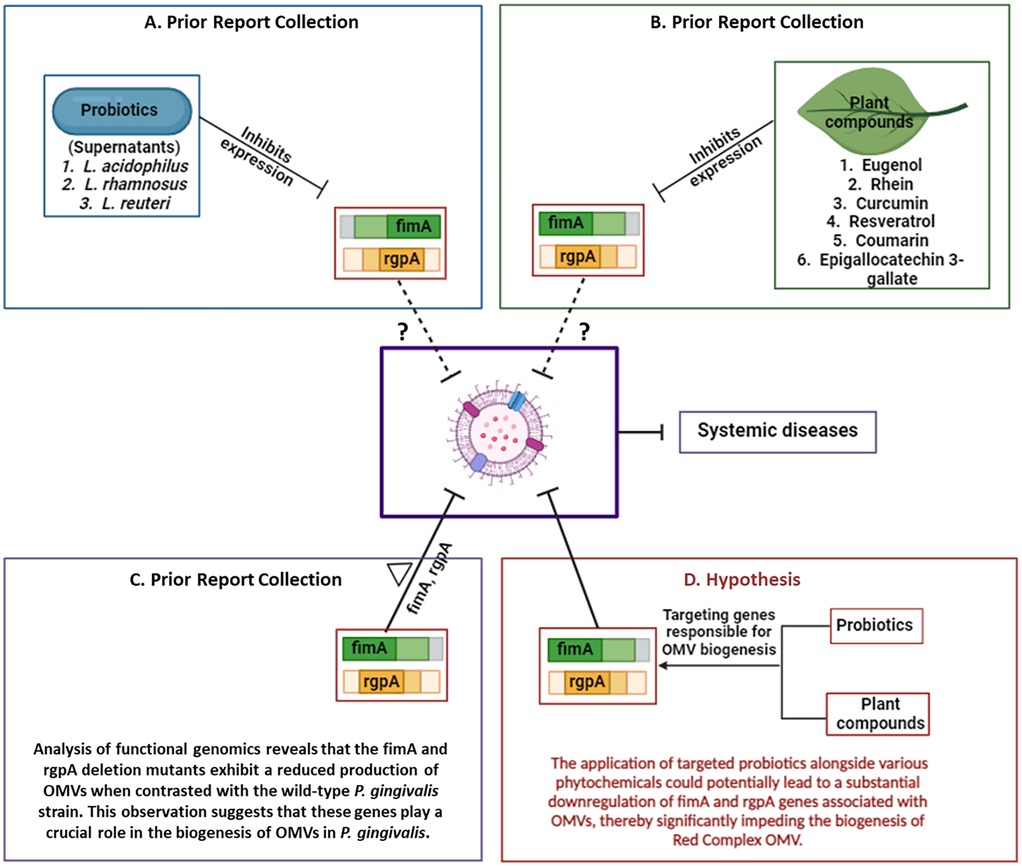
Figure 3. The proposed therapeutic strategy combining Lactobacillus and natural products against the OMVs of Red complex pathogens. (A) Probiotic strains and (B) plant-derived compounds have been shown to inhibit the expression of fimA and rgpA genes. (C) Deletion of these genes significantly impairs OMV production in P. gingivalis. (D) Based on these findings, we hypothesize that targeting fimA and rgpA using probiotics and phytochemicals may reduce OMV release and consequently mitigate the progression of OMV associated systemic diseases. Figure created with BioRender.com.
9 Discussion
OMVs released by red complex bacteria emerge as pivotal agents in both localized periodontal pathology and systemic inflammatory diseases (30). This review consolidates evidence that substantiates OMVs as not merely byproducts of microbial metabolism, but as sophisticated vectors of virulence capable of modulation of host immunity, alteration of epithelial integrity, and facilitating interspecies microbial synergy. The multifactorial composition of OMVs, including gingipains, LPS, fimbrial proteins, and various secretion system substrates, reflects the evolved mechanisms these bacteria employ to subvert host defenses (20). The presence of gingipains within OMVs has been particularly emphasized for their proteolytic degradation of immunoglobulins (IgG, IgM), complement proteins (C3), and junctional proteins such as PECAM-1 and tight junction proteins (ZO-1, occludin), all of which are critical for maintaining immune and endothelial barrier functions (74).
The internalization of OMVs by host cells through actin-mediated or lipid raft–dependent endocytosis initiates a cascade of detrimental effects, including impaired wound healing, oxidative stress, pro-inflammatory cytokine secretion, and even the promotion of oncogenic behavior in oral squamous cells. In P. gingivalis, for instance, gingipains contained in OMVs not only contribute to periodontal tissue destruction but also promote osteoclastogenesis through TLR2 signaling, thereby accelerating alveolar bone loss (20). In atherosclerosis, OMVs from P. gingivalis have been shown to induce foam cell formation (65), promote platelet aggregation, suppress eNOS, and activate RUNX2-mediated vascular calcification, through well-characterized ROCK and MAPK signaling pathways (66). Similarly, OMVs have been implicated in the pathogenesis of Alzheimer's disease by breaching the BBB, degrading tight junction proteins, and triggering neuroinflammation and tau phosphorylation. The contribution of OMVs to rheumatoid arthritis is likewise strongly supported wherein P. gingivalis OMVs, enriched with gingipains and PAD, mediate citrullination of host proteins, generating neoantigens and driving the production of ACPAs, a hallmark of RA pathogenesis (81). In diabetes mellitus, OMVs impair insulin signaling and hepatic glycogen synthesis through gingipain-mediated disruption of the Akt/GSK-3β pathway (86). These vesicles further exacerbate diabetic complications such as retinopathy by promoting oxidative stress, apoptosis, and increased endothelial permeability.
A recurring theme across these disease models is the heightened immunostimulatory potential of OMVs compared to whole bacteria. Periodontal ligament fibroblasts and macrophages exhibit exaggerated inflammatory responses, including elevated IL-6, IL-8, TNF-α, and NO production, upon exposure to OMVs (52). This hyperactivation suggests that OMVs may act as amplifiers of immune-mediated tissue damage. Importantly, therapeutic modulation of OMV biogenesis and function represents a promising avenue. Several Lactobacillus strains, including L. rhamnosus, L. acidophilus, and L. reuteri, have been demonstrated to downregulate OMV-associated genes such as fimA and rgpA in P. gingivalis, indicating a role in mitigating vesicle-mediated pathogenesis. Natural bioactives like curcumin, resveratrol, and EGCG also exhibit gene-suppressive effects on these virulence factors, further supporting the potential of probiotic and phytotherapeutic strategies. Collectively, the data presented support a paradigm in which Red Complex bacterial OMVs function as highly effective virulence delivery systems, influencing not only periodontal disease progression but also distant systemic pathologies. Future studies should prioritize in vivo validation of OMV-targeting therapies and elucidate the full spectrum of OMV-host interactions, including their epigenetic and metabolic effects.
10 Conclusion
Red Complex bacteria are key contributors to periodontal diseases and are strongly associated with systemic conditions such as atherosclerosis, alzheimer's disease, rheumatoid arthritis, and diabetes mellitus. As highlighted in this review, OMVs are critical virulence factors of these pathogens. Probiotic approaches, particularly involving Lactobacillus species and their secreted products, have shown potential in downregulating OMV-associated genes, including fimA and rgpA in these pathogens. Similarly, plant-derived bioactive compounds such as curcumin, resveratrol, and EGCG have demonstrated promising effects in suppressing the genetic machinery responsible for OMV biogenesis. Despite these encouraging findings, there remains a significant gap in the direct study of OMV biogenesis inhibition in the Red Complex bacteria. Future research focused on identifying and targeting genes involved in vesicle production could provide innovative strategies for the prevention and treatment of Red Complex bacterial oral and systemic diseases.
Author contributions
VM: Conceptualization, Data curation, Formal analysis, Writing – original draft. HL: Conceptualization, Data curation, Formal analysis, Writing – original draft. GM: Formal analysis, Writing – review & editing. SV: Formal analysis, Writing – review & editing. NE: Formal analysis, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors are thankful to the Indian Council of Medical Research for the sanctioned grant upon which the manuscript was hypothesized and written by the authors (Project no: 5/4/2-6/Oral Health/2022-NCD-II).
Acknowledgments
The authors thank Vellore University of Technology, Vellore, for providing sophisticated infrastructure facilities to promote research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Caselli E, Fabbri C, D’Accolti M, Soffritti I, Bassi C, Mazzacane S, et al. Defining the oral microbiome by whole-genome sequencing and resistome analysis: the complexity of the healthy picture. BMC Microbiol. (2020) 20(1):1–19. doi: 10.1186/s12866-020-01801-y
2. Deo PN, Deshmukh R. Oral microbiome: unveiling the fundamentals. J Oral Maxillofac Pathol. (2019) 23(1):122–8. doi: 10.4103/jomfp.JOMFP_304_18
3. Radaic A, Kapila YL. The oralome and its dysbiosis: new insights into oral microbiome-host interactions. Comput Struct Biotechnol J. (2021) 19:1335–60. doi: 10.1016/j.csbj.2021.02.010
4. Bowen WH, Burne RA, Wu H, Koo H. Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. (2018) 26(3):229–42. doi: 10.1016/j.tim.2017.09.008
5. Mohanty R, Asopa SJ, Joseph MD, Singh B, Rajguru JP, Saidath K, et al. Red complex: polymicrobial conglomerate in oral flora: a review. J Fam Med Prim Care. (2019) 8(11):3480–6. doi: 10.4103/jfmpc.jfmpc_759_19
6. Suzuki N, Yoneda M, Hirofuji T. Mixed red-complex bacterial infection in periodontitis. Int J Dent. (2013) 2013:587279. doi: 10.1155/2013/587279
7. Bhuyan R, Bhuyan SK, Mohanty JN, Das S, Juliana N, Juliana IF. Periodontitis and its inflammatory changes linked to Various systemic diseases: a review of its underlying mechanisms. Biomedicines. (2022) 10(10):2659. doi: 10.3390/biomedicines10102659
8. Avila-Calderón ED, Ruiz-Palma MS, Aguilera-Arreola MG, Velázquez-Guadarrama N, Ruiz EA, Gomez-Lunar Z, et al. Outer membrane vesicles of gram-negative Bacteria: an outlook on biogenesis. Front Microbiol. (2021) 12:557902. doi: 10.3389/fmicb.2021.557902
9. Furuyama N, Sircili MP. Outer membrane vesicles (OMVs) produced by Gram-negative bacteria: structure, functions, biogenesis, and vaccine application. Biomed Res Int. (2021) 2021:1490732. doi: 10.1155/2021/1490732
10. Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. (2010) 64:163–84. doi: 10.1146/annurev.micro.091208.073413
11. Balhuizen MD, Veldhuizen EJA, Haagsman HP. Outer membrane vesicle induction and isolation for vaccine development. Front Microbiol. (2021) 12:629090. doi: 10.3389/fmicb.2021.629090
12. Rueter C, Bielaszewska M. Secretion and delivery of intestinal pathogenic Escherichia coli virulence factors via outer membrane vesicles. Front Cell Infect Microbiol. (2020) 10(March):1–11. doi: 10.3389/fcimb.2020.00091
13. Leblhuber F, Huemer J, Steiner K, Gostner JM, Fuchs D. Knock-on effect of periodontitis to the pathogenesis of Alzheimer’s disease? Wien Klin Wochenschr. (2020) 132(17–18):493–8. doi: 10.1007/s00508-020-01638-5
14. Sansores-España D, Carrillo-Avila A, Melgar-Rodriguez S, Díaz-Zuñiga J, Martínez-Aguilar V. Periodontitis and alzheimers disease. Med Oral Patol Oral y Cir Bucal. (2021) 26(1):e43–8. doi: 10.4317/medoral.23940
15. Carrizales-Sepúlveda EF, Ordaz-Farías A, Vera-Pineda R, Flores-Ramírez R. Periodontal disease, systemic inflammation and the risk of cardiovascular disease. Hear Lung Circ. (2018) 27(11):1327–34. doi: 10.1016/j.hlc.2018.05.102
16. Casanova L, Hughes FJ, Preshaw PM. Diabetes and periodontal disease: a two-way relationship. Br Dent J. (2014) 217(8):433–7. doi: 10.1038/sj.bdj.2014.907
17. Preshaw PM, Bissett SM. Periodontitis and diabetes. Br Dent J. (2019) 227(7):577–84. doi: 10.1038/s41415-019-0794-5
18. Rodríguez-Lozano B, González-Febles J, Garnier-Rodríguez JL, Dadlani S, Bustabad-Reyes S, Sanz M, et al. Association between severity of periodontitis and clinical activity in rheumatoid arthritis patients: a case-control study. Arthritis Res Ther. (2019) 21(1):1–12. doi: 10.1186/s13075-019-1808-z
19. Uemura Y, Hiroshima Y, Tada A, Murakami K, Yoshida K, Inagaki Y, et al. Porphyromonas gingivalis outer membrane vesicles stimulate gingival epithelial cells to induce pro-inflammatory cytokines via the MAPK and STING pathways. Biomedicines. (2022) 10(10):2643. doi: 10.3390/biomedicines10102643
20. Wu Z, Long W, Yin Y, Tan B, Liu C, Li H, et al. Outer membrane vesicles of Porphyromonas gingivalis: recent advances in pathogenicity and associated mechanisms. Front Microbiol. (2025) 16:1555868. doi: 10.3389/fmicb.2025.1555868
21. Nonaka S, Okamoto R, Katsuta Y, Kanetsuki S, Nakanishi H. Gingipain-carrying outer membrane vesicles from Porphyromonas gingivalis cause barrier dysfunction of caco-2 cells by releasing gingipain into the cytosol. Biochem Biophys Res Commun. (2024) 707:149783. doi: 10.1016/j.bbrc.2024.149783
22. Zhang Z, Liu D, Liu S, Zhang S, Pan Y. The role of Porphyromonas gingivalis outer membrane vesicles in periodontal disease and related systemic diseases. Front Cell Infect Microbiol. (2021) 10(January):1–12. doi: 10.3389/fcimb.2020.585917
23. Okamura H, Hirota K, Yoshida K, Weng Y, He Y, Shiotsu N, et al. Outer membrane vesicles of Porphyromonas gingivalis: novel communication tool and strategy. Jpn Dent Sci Rev. (2021) 57:138–46. doi: 10.1016/j.jdsr.2021.07.003
24. Gui MJ, Dashper SG, Slakeski N, Chen YY, Reynolds EC. Spheres of influence: porphyromonas gingivalis outer membrane vesicles. Mol Oral Microbiol. (2016) 31(5):365–78. doi: 10.1111/omi.12134
25. Xie H. Biogenesis and function of Porphyromonas gingivalis outer membrane vesicles. Future Microbiol. (2015) 10(9):1517–27. doi: 10.2217/fmb.15.63
26. Mantri CK, Chen C-H, Dong X, Goodwin JS, Pratap S, Paromov V, et al. Fimbriae-mediated outer membrane vesicle production and invasion of Porphyromonas gingivalis. Microbiologyopen. (2015) 4(1):53–65. doi: 10.1002/mbo3.221
27. Yamaguchi M, Sato K, Yukitake H, Noiri Y, Ebisu S, Nakayama K. A Porphyromonas gingivalis mutant defective in a putative glycosyltransferase exhibits defective biosynthesis of the polysaccharide portions of lipopolysaccharide, decreased gingipain activities, strong autoaggregation, and increased biofilm formation. Infect Immun. (2010) 78(9):3801–12. doi: 10.1128/IAI.00071-10
28. Veith PD, Chen YY, Chen D, O’Brien-Simpson NM, Cecil JD, Holden JA, et al. Tannerella forsythia outer membrane vesicles are enriched with substrates of the type IX secretion system and TonB-dependent receptors. J Proteome Res. (2015) 14(12):5355–66. doi: 10.1021/acs.jproteome.5b00878
29. Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol. (2015) 13(10):605–19. doi: 10.1038/nrmicro3525
30. Magaña G, Harvey C, Taggart CC, Rodgers AM. Bacterial outer membrane vesicles: role in pathogenesis and host-cell interactions. Antibiot (Basel, Switzerland). (2023) 13(1):32. doi: 10.3390/antibiotics13010032
31. Friedrich V, Gruber C, Nimeth I, Pabinger S, Sekot G, Posch G, et al. Outer membrane vesicles of Tannerella forsythia: biogenesis, composition, and virulence. Mol Oral Microbiol. (2015) 30(6):451–73. doi: 10.1111/omi.12104
32. Veith PD, Glew MD, Gorasia DG, Chen D, O’Brien-Simpson NM, Reynolds EC. Localization of outer membrane proteins in Treponema denticola by quantitative proteome analyses of outer membrane vesicles and cellular fractions. J Proteome Res. (2019) 18(4):1567–81. doi: 10.1021/acs.jproteome.8b00860
33. Valm AM. The structure of dental plaque microbial communities in the transition from health to dental caries and periodontal disease. J Mol Biol. (2019) 431(16):2957–69. doi: 10.1016/j.jmb.2019.05.016
34. Mahendra J, Palathingal P, Mahendra L, Alzahrani KJ, Banjer HJ, Alsharif KF, et al. Impact of red Complex Bacteria and TNF-α levels on the diabetic and renal Status of chronic kidney disease patients in the presence and absence of periodontitis. Biology (Basel). (2022) 11(3):451. doi: 10.3390/biology11030451
35. Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Prim. (2017) 3:1–14. doi: 10.1038/nrdp.2017.38
36. Gibson F III, Genco C. Porphyromonas gingivalis mediated periodontal disease and atherosclerosis:disparate diseases with commonalities in pathogenesis through TLRs. Curr Pharm Des. (2007) 13(36):3665–75. doi: 10.2174/138161207783018554
37. Kamaguchi A, Nakayama K, Ichiyama S, Nakamura R, Watanabe T, Ohta M, et al. Effect of Porphyromonas gingivalis vesicles on coaggregation of Staphylococcus aureus to oral microorganisms. Curr Microbiol. (2003) 47(6):485–91. doi: 10.1007/s00284-003-4069-6
38. Grenier D. Porphyromonas gingivalis outer membrane vesicles mediate coaggregation and piggybacking of Treponema denticola and Lachnoanaerobaculum saburreum. Int J Dent. (2013) 2013:305476. doi: 10.1155/2013/305476
39. Hasegawa Y, Nagano K. Porphyromonas gingivalis FimA and Mfa1 fimbriae: current insights on localization, function, biogenesis, and genotype. Jpn Dent Sci Rev. (2021) 57:190–200. doi: 10.1016/j.jdsr.2021.09.003
40. Miao D, Fenno JC, Timm JC, Joo NE, Kapila YL. The Treponema denticola chymotrypsin-like protease dentilisin induces matrix metalloproteinase-2-dependent fibronectin fragmentation in periodontal ligament cells. Infect Immun. (2011) 79(2):806–11. doi: 10.1128/IAI.01001-10
41. Zhao Y, Chen J, Tian Y, Huang H, Zhao F, Deng X. Treponema denticola major surface protein (msp): a key player in periodontal pathogenicity and immune evasion. Arch Microbiol. (2025) 207(2):36. doi: 10.1007/s00203-024-04223-w
42. Dashper SG, Seers CA, Tan KH, Reynolds EC. Virulence factors of the oral spirochete Treponema denticola. J Dent Res. (2011) 90(6):691–703. doi: 10.1177/0022034510385242
43. Schäffer C, Andrukhov O. The intriguing strategies of Tannerella forsythia’s host interaction. Front Oral Heal. (2024) 5:1434217. doi: 10.3389/froh.2024.1434217
44. Shin J, Choi Y. The fate of Treponema denticola within human gingival epithelial cells. Mol Oral Microbiol. (2012) 27(6):471–82. doi: 10.1111/j.2041-1014.2012.00660.x
45. Liu S, Butler CA, Ayton S, Reynolds EC, Dashper SG. Porphyromonas gingivalis and the pathogenesis of Alzheimer’s disease. Crit Rev Microbiol. (2023) 50(2):127–37. doi: 10.1080/1040841X.2022.2163613
46. Tsuda K, Amano A, Umebayashi K, Inaba H, Nakagawa I, Nakanishi Y, et al. Molecular dissection of internalization of Porphyromonas gingivalis by cells using fluorescent beads coated with bacterial membrane vesicle. Cell Struct Funct. (2005) 30(2):81–91. doi: 10.1247/csf.30.81
47. Nakao R, Takashiba S, Kosono S, Yoshida M, Watanabe H, Ohnishi M, et al. Effect of Porphyromonas gingivalis outer membrane vesicles on gingipain-mediated detachment of cultured oral epithelial cells and immune responses. Microbes Infect. (2014) 16(1):6–16. doi: 10.1016/j.micinf.2013.10.005
48. Furuta N, Tsuda K, Omori H, Yoshimori T, Yoshimura F, Amano A. Porphyromonas gingivalis outer membrane vesicles enter human epithelial cells via an endocytic pathway and are sorted to lysosomal compartments. Infect Immun. (2009) 77(10):4187–96. doi: 10.1128/IAI.00009-09
49. Song LT, Tada H, Nishioka T, Nemoto E, Imamura T, Potempa J, et al. Porphyromonas gingivalis gingipains-mediated degradation of plasminogen activator inhibitor-1 leads to delayed wound healing responses in human endothelial cells. J Innate Immun. (2022) 14(4):306–19. doi: 10.1159/000519737
50. Chen S, Lei Q, Zou X, Ma D. The role and mechanisms of gram-negative bacterial outer membrane vesicles in inflammatory diseases. Front Immunol. (2023) 14:1157813. doi: 10.3389/fimmu.2023.1157813
51. Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. (2011) 469(7330):323–35. doi: 10.1038/nature09782
52. Cecil JD, O’Brien-Simpson NM, Lenzo JC, Holden JA, Singleton W, Perez-Gonzalez A, et al. Outer membrane vesicles prime and activate macrophage inflammasomes and cytokine secretion in vitro and in vivo. Front Immunol. (2017) 8(AUG):1–22. doi: 10.3389/fimmu.2017.01017
53. Zou JK, Cao YM, Tian Y, Li X, Wu RX, Tian BM, et al. Porphyromonas gingivalis outer membrane vesicles activate toll-like receptor 2 to promote osteoclast differentiation by carrying lipopolysaccharide. Chinese J Stomatol. (2024) 59(3):237–46. doi: 10.3760/cma.j.cn112144-20231026-00222
54. Liu D, Liu S, Liu J, Miao L, Zhang S, Pan Y. sRNA23392 packaged by Porphyromonas gingivalis outer membrane vesicles promotes oral squamous cell carcinomas migration and invasion by targeting desmocollin-2. Mol Oral Microbiol. (2021) 36(3):182–91. doi: 10.1111/omi.12334
55. Cecil JD, Sirisaengtaksin N, O’brien-Simpson NM, Krachler AM. Outer membrane vesicle-host cell interactions. Protein Secret Bact. (2019) 7(1):201–14. doi: 10.1128/9781683670285.ch17
56. Lim Y, Kim HY, Han D, Choi B-K. Proteome and immune responses of extracellular vesicles derived from macrophages infected with the periodontal pathogen Tannerella forsythia. J Extracell Vesicles. (2023) 12(12):e12381. doi: 10.1002/jev2.12381
57. Mughal MM, Khan MK, Demarco JK, Majid A, Shamoun F, Abela GS. Symptomatic and asymptomatic carotid artery plaque. Expert Rev Cardiovasc Ther. (2011) 9(10):1315–30. doi: 10.1586/erc.11.120
59. Cecoro G, Annunziata M, Iuorio MT, Nastri L, Guida L. Periodontitis, low-grade inflammation and systemic health: a scoping review. Medicina. (2020) 56(6):272. doi: 10.3390/medicina56060272
60. Zhang J, Xie M, Huang X, Chen G, Yin Y, Lu X, et al. The effects of Porphyromonas gingivalis on atherosclerosis-related cells. Front Immunol. (2021) 12(December):1–20. doi: 10.3389/fimmu.2021.766560
61. Bui FQ, Almeida-da-Silva CLC, Huynh B, Trinh A, Liu J, Woodward J, et al. Association between periodontal pathogens and systemic disease. Biomed J. (2019) 42(1):27–35. doi: 10.1016/j.bj.2018.12.001
62. Aarabi G, Heydecke G, Seedorf U. Roles of oral infections in the pathomechanism of atherosclerosis. Int J Mol Sci. (2018) 19(7):1978. doi: 10.3390/ijms19071978
63. Wang N, Zhou F, Chen C, Luo H, Guo J, Wang W, et al. Role of outer membrane vesicles from Helicobacter pylori in atherosclerosis. Front Cell Dev Biol. (2021) 9:673993. doi: 10.3389/fcell.2021.673993
64. Sharma A, Novak EK, Sojar HT, Swank RT, Kuramitsu HK, Genco RJ. Porphyromonas gingivalis platelet aggregation activity: outer membrane vesicles are potent activators of murine platelets. Oral Microbiol Immunol. (2000) 15(6):393–6. doi: 10.1034/j.1399-302x.2000.150610.x
65. Qi M, Miyakawa H, Kuramitsu HK. Porphyromonas gingivalis induces murine macrophage foam cell formation. Microb Pathog. (2003) 35(6):259–67. doi: 10.1016/j.micpath.2003.07.002
66. Jia Y, Guo B, Yang W, Zhao Q, Jia W, Wu Y. Rho kinase mediates Porphyromonas gingivalis outer membrane vesicle-induced suppression of endothelial nitric oxide synthase through ERK1/2 and p38 MAPK. Arch Oral Biol. (2015) 60(3):488–95. doi: 10.1016/j.archoralbio.2014.12.009
67. Farrugia C, Stafford GP, Murdoch C. Porphyromonas gingivalis outer membrane vesicles increase vascular permeability. J Dent Res. (2020) 99(13):1494–501. doi: 10.1177/0022034520943187
68. Yang WW, Guo B, Jia WY, Jia Y. Porphyromonas gingivalis-derived outer membrane vesicles promote calcification of vascular smooth muscle cells through ERK1/2-RUNX2. FEBS Open Bio. (2016) 6(12):1310–9. doi: 10.1002/2211-5463.12151
69. Huang X, Xie M, Lu X, Mei F, Song W, Liu Y, et al. The roles of periodontal Bacteria in atherosclerosis. Int J Mol Sci. (2023) 24(16):12861. doi: 10.3390/ijms241612861
70. Kanagasingam S, Chukkapalli SS, Welbury R, Singhrao SK. Porphyromonas gingivalis is a strong risk factor for Alzheimer’s disease. J Alzheimer’s Dis Reports. (2020) 4(1):501–11. doi: 10.3233/ADR-200250
71. Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, et al. Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. (2019) 5(1):eaau3333. doi: 10.1126/sciadv.aau3333
72. Nara PL, Sindelar D, Penn MS, Potempa J, Griffin WST. Porphyromonas gingivalis outer membrane vesicles as the Major driver of and explanation for neuropathogenesis, the cholinergic hypothesis, iron dyshomeostasis, and salivary lactoferrin in Alzheimer’s disease. J Alzheimers Dis. (2021) 82(4):1417–50. doi: 10.3233/JAD-210448
73. Nonaka S, Kadowaki T, Nakanishi H. Secreted gingipains from Porphyromonas gingivalis increase permeability in human cerebral microvascular endothelial cells through intracellular degradation of tight junction proteins. Neurochem Int. (2022) 154:105282. doi: 10.1016/j.neuint.2022.105282
74. Pritchard AB, Fabian Z, Lawrence CL, Morton G, Crean S, Alder JE. An investigation into the effects of outer membrane vesicles and lipopolysaccharide of Porphyromonas gingivalis on blood-brain barrier integrity, permeability, and disruption of scaffolding proteins in a human in vitro model. J Alzheimers Dis. (2022) 86(1):343–64. doi: 10.3233/JAD-215054
75. Inoue E, Minatozaki S, Shimizu S, Miyamoto S, Jo M, Ni J, et al. Human β-defensin 3 inhibition of P. gingivalis LPS-induced IL-1β production by BV-2 microglia through suppression of cathepsins B and L. Cells. (2024) 13(3):283. doi: 10.3390/cells13030283
76. Yoshida K, Yoshida K, Seyama M, Hiroshima Y, Mekata M, Fujiwara N, et al. Porphyromonas gingivalis outer membrane vesicles in cerebral ventricles activate microglia in mice. Oral Dis. (2023) 29(8):3688–97. doi: 10.1111/odi.14413
77. Gong T, Chen Q, Mao H, Zhang Y, Ren H, Xu M, et al. Outer membrane vesicles of Porphyromonas gingivalis trigger NLRP3 inflammasome and induce neuroinflammation, tau phosphorylation, and memory dysfunction in mice. Front Cell Infect Microbiol. (2022) 12:925435. doi: 10.3389/fcimb.2022.925435
78. Guo T, Noble W, Hanger DP. Roles of tau protein in health and disease. Acta Neuropathol. (2017) 133(5):665–704. doi: 10.1007/s00401-017-1707-9
79. Ma X, Shin Y-J, Yoo J-W, Park H-S, Kim D-H. Extracellular vesicles derived from Porphyromonas gingivalis induce trigeminal nerve-mediated cognitive impairment. J Adv Res. (2023) 54:293–303. doi: 10.1016/j.jare.2023.02.006
80. Krutyhołowa A, Strzelec K, Dziedzic A, Bereta GP, Łazarz-Bartyzel K, Potempa J, et al. Host and bacterial factors linking periodontitis and rheumatoid arthritis. Front Immunol. (2022) 13(August):1–17. doi: 10.3389/fimmu.2022.980805
81. Koziel J, Mydel P, Potempa J. The link between periodontal disease and rheumatoid arthritis: an updated review. Curr Rheumatol Rep. (2014) 16(3):408. doi: 10.1007/s11926-014-0408-9
82. Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. (2018) 6:15. doi: 10.1038/s41413-018-0016-9
83. Chow YC, Yam HC, Gunasekaran B, Lai WY, Wo WY, Agarwal T, et al. Implications of Porphyromonas gingivalis peptidyl arginine deiminase and gingipain R in human health and diseases. Front Cell Infect Microbiol. (2022) 12:987683. doi: 10.3389/fcimb.2022.987683
84. van Beers JJBC, Willemze A, Jansen JJ, Engbers GHM, Salden M, Raats J, et al. ACPA fine-specificity profiles in early rheumatoid arthritis patients do not correlate with clinical features at baseline or with disease progression. Arthritis Res Ther. (2013) 15(5):R140. doi: 10.1186/ar4322
85. Nik-Azis NM, Mohd N, Baharin B, Said MSM, Fadzilah FM, Haflah NHM. Periodontal disease in seropositive rheumatoid arthritis: scoping review of the epidemiological evidence. Germs. (2021) 11(2):266–86. doi: 10.18683/germs.2021.1263
86. Seyama M, Yoshida K, Yoshida K, Fujiwara N, Ono K, Eguchi T, et al. Outer membrane vesicles of Porphyromonas gingivalis attenuate insulin sensitivity by delivering gingipains to the liver. Biochim Biophys Acta - Mol Basis Dis. (2020) 1866(6):165731. doi: 10.1016/j.bbadis.2020.165731
87. Rohani B. Oral manifestations in patients with diabetes mellitus. World J Diabetes. (2019) 10(9):485–9. doi: 10.4239/wjd.v10.i9.485
88. Huang S, Cao G, Dai D, Xu Q, Ruiz S, Shindo S, et al. Porphyromonas gingivalis outer membrane vesicles exacerbate retinal microvascular endothelial cell dysfunction in diabetic retinopathy. Front Microbiol. (2023) 14:1167160. doi: 10.3389/fmicb.2023.1167160
89. Ramadan DE, Hariyani N, Indrawati R, Ridwan RD, Diyatri I. Cytokines and chemokines in periodontitis. Eur J Dent. (2020) 14(3):483–95. doi: 10.1055/s-0040-1712718
90. Fan R, Zhou Y, Chen X, Zhong X, He F, Peng W, et al. Porphyromonas gingivalis outer membrane vesicles promote apoptosis via msRNA-regulated DNA methylation in periodontitis. Microbiol Spectr. (2023) 11(1):e0328822. doi: 10.1128/spectrum.03288-22
91. Hajishengallis G, Lambris JD. Complement and dysbiosis in periodontal disease. Immunobiology. (2012) 217(11):1111–6. doi: 10.1016/j.imbio.2012.07.007
92. Cecil JD, O’Brien-Simpson NM, Lenzo JC, Holden JA, Chen YY, Singleton W, et al. Differential responses of pattern recognition receptors to outer membrane vesicles of three periodontal pathogens. PLoS One. (2016) 11(4):1–20. doi: 10.1371/journal.pone.0151967
93. Srisatjaluk R, Doyle RJ, Justus DE. Outer membrane vesicles of Porphyromonas gingivalis inhibit IFN-gamma-mediated MHC class II expression by human vascular endothelial cells. Microb Pathog. (1999) 27(2):81–91. doi: 10.1006/mpat.1999.0287
94. du Teil Espina M, Fu Y, van der Horst D, Hirschfeld C, López-Álvarez M, Mulder LM, et al. Coating and corruption of human neutrophils by bacterial outer membrane vesicles. Microbiol Spectr. (2022) 10(5):e0075322. doi: 10.1128/spectrum.00753-22
95. Kim HY, Song M-K, Lim Y, Jang JS, An S-J, Kim H-H, et al. Effects of extracellular vesicles derived from oral bacteria on osteoclast differentiation and activation. Sci Rep. (2022) 12(1):14239. doi: 10.1038/s41598-022-18412-4
96. Lara B, Loureiro I, Gliosca L, Castagnola L, Merech F, Gallino L, et al. Porphyromonas gingivalis outer membrane vesicles shape trophoblast cell metabolism impairing functions associated to adverse pregnancy outcome. J Cell Physiol. (2023) 238(11):2679–91. doi: 10.1002/jcp.31138
97. Inchingolo AD, Malcangi G, Semjonova A, Inchingolo AM, Patano A, Coloccia G, et al. Oralbiotica/oralbiotics: the impact of oral Microbiota on dental health and demineralization: a systematic review of the literature. Children. (2022) 9(7):1014. doi: 10.3390/children9071014
98. Sedghi L, DiMassa V, Harrington A, Lynch S V, Kapila YL. The oral microbiome: role of key organisms and complex networks in oral health and disease. Periodontol 2000. (2021) 87(1):107–31. doi: 10.1111/prd.12393
99. Araujo LDC, Furlaneto FAC, da Silva LAB, Kapila YL. Use of the probiotic Bifidobacterium animalis subsp. lactis HN019 in oral diseases. Int J Mol Sci. (2022) 23(16):9334. doi: 10.3390/ijms23169334
100. Mendi A, Köse S, Uçkan D, Akca G, Yilmaz D, Aral L, et al. Lactobacillus rhamnosus could inhibit porphyromonas gingivalis derived CXCl8 attenuation. J Appl Oral Sci. (2016) 24(1):67–75. doi: 10.1590/1678-775720150145
101. Babadi F, Amin M, Behbahani FA. Evaluation of the antibacterial properties of Lactobacillus acidophilus metabolites against oral plaque streptococci: an in vitro study. J Res Med Dent Sci. (2018) 6(5):198–202.
102. Khalaf H, Nakka SS, Sandén C, Svärd A, Hultenby K, Scherbak N, et al. Antibacterial effects of Lactobacillus and bacteriocin PLNC8 αβ on the periodontal pathogen Porphyromonas gingivalis. BMC Microbiol. (2016) 16(1):1–11. doi: 10.1186/s12866-016-0810-8
103. Chuang LC, Huang CS, Ou-Yang LW, Lin SY. Probiotic Lactobacillus paracasei effect on cariogenic bacterial flora. Clin Oral Investig. (2011) 15(4):471–6. doi: 10.1007/s00784-010-0423-9
104. Lin CW, Chen YT, Ho HH, Kuo YW, Lin WY, Chen JF, et al. Impact of the food grade heat-killed probiotic and postbiotic oral lozenges in oral hygiene. Aging (Albany NY). (2022) 14(5):2221–38. doi: 10.18632/aging.203923
105. Baker JL, Edlund A. Exploiting the oral microbiome to prevent tooth decay: has evolution already provided the best tools? Front Microbiol. (2019) 9:3323. doi: 10.3389/fmicb.2018.03323
106. Yang KM, Kim JS, Kim HS, Kim YY, Oh JK, Jung HW, et al. Lactobacillus reuteri AN417 cell-free culture supernatant as a novel antibacterial agent targeting oral pathogenic bacteria. Sci Rep. (2021) 11(1):1–16. doi: 10.1038/s41598-020-80921-x
107. Bueno MR, Ishikawa KH, Almeida-Santos G, Ando-Suguimoto ES, Shimabukuro N, Kawamoto D, et al. Lactobacilli attenuate the effect of Aggregatibacter actinomycetemcomitans infection in gingival epithelial cells. Front Microbiol. (2022) 13(May):1–11. doi: 10.3389/fmicb.2022.846192
108. Kameya M, Onaka H, Asano Y. Selective tryptophan determination using tryptophan oxidases involved in bis-indole antibiotic biosynthesis. Anal Biochem. (2013) 438(2):124–32. doi: 10.1016/j.ab.2013.03.024
109. Kawai T, Ohshima T, Tanaka T, Ikawa S, Tani A, Inazumi N, et al. Limosilactobacillus (Lactobacillus) fermentum ALAL020, a probiotic candidate bacterium, produces a cyclic dipeptide that suppresses the periodontal pathogens Porphyromonas gingivalis and Prevotella intermedia. Front Cell Infect Microbiol. (2022) 12(March):1–11. doi: 10.3389/fcimb.2022.804334
110. Jeong D, Kim DH, Song KY, Seo KH. Antimicrobial and anti-biofilm activities of Lactobacillus kefiranofaciens DD2 against oral pathogens. J Oral Microbiol. (2018) 10(1):1472985. doi: 10.1080/20002297.2018.1472985
111. Ishikawa KH, Mita D, Kawamoto D, Nicoli JR, Albuquerque-Souza E, Lorenzetti Simionato MR, et al. Probiotics alter biofilm formation and the transcription of Porphyromonas gingivalis virulence-associated genes. J Oral Microbiol. (2020) 12(1):1805553. doi: 10.1080/20002297.2020.1805553
112. Salehi B, Jornet PL, López EPF, Calina D, Sharifi-Rad M, Ramírez-Alarcón K, et al. Plant-derived bioactives in oral mucosal lesions: a key emphasis to curcumin, lycopene, chamomile, aloe Vera, green tea and coffee properties. Biomolecules. (2019) 9(3):106. doi: 10.3390/biom9030106
113. Sha AM, Garib BT. Antibacterial effect of curcumin against clinically isolated Porphyromonas gingivalis and connective tissue reactions to curcumin gel in the subcutaneous tissue of rats. Biomed Res Int. (2019) 2019:6810936. doi: 10.1155/2019/6810936
114. Izui S, Sekine S, Murai H, Takeuchi H, Amano A. Inhibitory effects of curcumin against cytotoxicity of Porphyromonas gingivalis outer membrane vesicles. Arch Oral Biol. (2021) 124:105058. doi: 10.1016/j.archoralbio.2021.105058
115. Kumbar VM, Peram MR, Kugaji MS, Shah T, Patil SP, Muddapur UM, et al. Effect of curcumin on growth, biofilm formation and virulence factor gene expression of Porphyromonas gingivalis. Odontology. (2021) 109(1):18–28. doi: 10.1007/s10266-020-00514-y
116. Kugaji MS, Kumbar VM, Peram MR, Patil S, Bhat KG, Diwan PV. Effect of resveratrol on biofilm formation and virulence factor gene expression of Porphyromonas gingivalis in periodontal disease. APMIS. (2019) 127(4):187–95. doi: 10.1111/apm.12930
117. Kong C, Zhang H, Li L, Liu Z. Effects of green tea extract epigallocatechin-3-gallate (EGCG) on oral disease-associated microbes: a review. J Oral Microbiol. (2022) 14(1):2131117. doi: 10.1080/20002297.2022.2131117
118. Azelmat J, Larente JF, Grenier D. The anthraquinone rhein exhibits synergistic antibacterial activity in association with metronidazole or natural compounds and attenuates virulence gene expression in Porphyromonas gingivalis. Arch Oral Biol. (2015) 60(2):342–6. doi: 10.1016/j.archoralbio.2014.11.006
119. He Z, Jiang W, Jiang Y, Dong J, Song Z, Xu J, et al. Anti-biofilm activities of coumarin as quorum sensing inhibitor for Porphyromonas gingivalis. J Oral Microbiol. (2022) 14(1):2055523. doi: 10.1080/20002297.2022.2055523
120. Sun M, Zhou Z, Dong J, Zhang J, Xia Y, Shu R. Antibacterial and antibiofilm activities of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) against periodontopathic bacteria. Microb Pathog. (2016) 99:196–203. doi: 10.1016/j.micpath.2016.08.025
121. Zhang Y, Wang Y, Zhu X, Cao P, Wei S, Lu Y. Antibacterial and antibiofilm activities of eugenol from essential oil of syzygium aromaticum (L.) merr. & L. M. Perry (clove) leaf against periodontal pathogen Porphyromonas gingivalis. Microb Pathog. (2017) 113:396–402. doi: 10.1016/j.micpath.2017.10.054
Keywords: Red Complex bacteria, outer membrane vesicles (OMVs), systemic diseases, probiotics, phytobioactives
Citation: Mahendrarajan V, Lazarus HPS, Muthukaliannan GK, Varghese S and Easwaran N (2025) Membrane vesicles from Red Complex bacteria: key players in oral pathogenesis, immune disruption, systemic diseases, and therapeutic insights. Front. Oral Health 6:1607931. doi: 10.3389/froh.2025.1607931
Received: 8 April 2025; Accepted: 10 July 2025;
Published: 28 July 2025.
Edited by:
Mohammad Alrashdan, University of Sharjah, United Arab EmiratesReviewed by:
Marta Katkowska, Medical University of Gdansk, PolandAnnica Almståhl, University of Gothenburg, Sweden
Copyright: © 2025 Mahendrarajan, Lazarus, Muthukaliannan, Varghese and Easwaran. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nalini Easwaran, bmFsaW5pLmVAdml0LmFjLmlu
 Venkatramanan Mahendrarajan
Venkatramanan Mahendrarajan Huldah Pearlin Sarah Lazarus
Huldah Pearlin Sarah Lazarus Gothandam Kodiveri Muthukaliannan1
Gothandam Kodiveri Muthukaliannan1 Nalini Easwaran
Nalini Easwaran