- 1Department of Oral Pathology and Microbiology, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai, India
- 2College of Dentistry, Ajman University, Ajman, United Arab Emirates
- 3Centre of Medical and Bio-Allied Health Sciences Research, Ajman University, Ajman, United Arab Emirates
Background: Head and neck squamous cell carcinoma (HNSCC) is a group of heterogeneous malignancies and constitutes one of the most prevalent forms of cancer. Oral metronomic chemotherapy (OMCT) is a treatment in which low doses of anticancer drugs are given at regular intervals over a long time, with many advantages over conventional therapies, particularly in nations with high cancer burden. The present systematic review aimed to evaluate the efficacy of OMCT in the management of HNSCC in comparison to other standard chemotherapy regimens. Methodology: The review was registered in the Prospero database (CRD42023426000). An electronic search was conducted using PubMed, Scopus, Web of Science and Google Scholar databases. Articles in which OMCT was used to treat HNSCC were included for systematic review, and the survival and response rates were analyzed.
Results: Twenty-four eligible articles were included for evaluation, which revealed that administration of OMCT produced higher survival and response rates in subjects compared to standard chemotherapy.
Conclusion: The evidence from the included studies supports that oral metronomic chemotherapy is substantially more effective as compared to standard chemotherapy regimens in squamous cell carcinomas of the head and neck.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42023426000, identifier (CRD42023426000).
Introduction
Head and neck squamous cell carcinoma (HNSCC) is ranked as the seventh most common cancer in the world and has been frequently linked to tobacco and alcohol use, as well as human papillomavirus (HPV), Helicobacter pylori and Candida albicans infections (1–3). In cases of recurrent or metastatic cancer, conventional therapeutic approaches such as surgery, radiation and systemic chemotherapy frequently result in considerable morbidity and have poor efficacy (4, 5). Oral metronomic chemotherapy (OMCT) has become a viable option due to its ability to suppress tumor angiogenesis, modulate the immune system, and minimize toxicity (6). It entails frequently administering low-dose chemotherapy drugs without extended intervals.
Angiogenesis is a process by which new blood vessels proliferate to deliver nourishment to the tumor cells (7). Additionally, alternate mechanisms such as vasculogenic mimicry have been described in various human malignancies (8). Inhibiting angiogenesis is the main mechanism by which metronomic chemotherapy functions (9). The anti-angiogenic action of metronomic chemotherapy relies upon targeting the endothelial cells in the tumor vasculature, which are more susceptible to continual low-dose chemotherapy. Furthermore, it is believed that metronomic chemotherapy has immunomodulatory effects, specifically through the reduction of regulatory T-cells (Tregs), which are responsible for inhibiting the body's immune response against tumors (6). These processes differ from the classic cytotoxic effects of chemotherapy, which are designed to destroy rapidly dividing tumor cells.
Oral metronomic chemotherapy has been researched in several therapeutic contexts, especially for patients with recurrent or metastatic HNSCC who have few alternative options for treatment. OMCT could be a better alternative for patients who are not promising candidates for aggressive therapy, according to a phase II study assessing the use of low-dose oral cyclophosphamide in combination with celecoxib in patients with advanced HNSCC (10). The study found that a subset of patients experienced a significant reduction in tumor size with minimal toxicity. Patients receiving OMCT also experienced fewer adverse effects, such as neutropenia and mucositis, which are common in standard chemotherapy (11).
OMCT has also been explored as a maintenance therapy to prolong disease control following induction chemotherapy or chemoradiation (12). A study reported that patients with HNSCC who received maintenance OMCT with methotrexate and celecoxib had a longer median PFS compared to those who received no further treatment after completing standard therapy (13). This suggests that OMCT may help in sustaining the therapeutic response and delaying disease progression. An area of growing attention is the use of immune checkpoint inhibitors in conjunction with OMCT. Metronomic chemotherapy has been demonstrated in preclinical investigations to increase the effectiveness of immunotherapy by altering the tumor microenvironment and promoting the infiltration of cytotoxic T-cells (1). Many clinical trials are now underway to assess the synergistic effects of OMCT in HNSCC (14).
The present systematic review was thus designed to evaluate the efficacy of oral metronomic chemotherapy in the management of head and neck squamous cell carcinoma in comparison to other standard chemotherapy regimens.
Methodology
Protocol and registration
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA (15)] guidelines were used to design this systematic review (9). This review was registered at the International Prospective Register of Systematic Reviews database (CRD42023426000). The research question was, “Does oral metronomic chemotherapy play any role in the treatment of head and neck squamous cell carcinoma?” The PICO for the present systematic review was as follows:
• Population: Head and neck squamous cell carcinoma, including all the subsites
• Intervention: Oral metronomic chemotherapy or low-dose chemotherapy
• Control: Conventional treatment, including surgical intervention with or without adjuvant therapies
• Outcome: Comparison of the efficacy of OMCT with conventional therapy
Eligibility criteria
All relevant articles obtained from information sources (PubMed, SCOPUS, Web of Science and Google Scholar) were screened and included in the review only if the papers were original research studies, full-length text was available irrespective of the language, the studies were conducted only on human participants, and the studies included an analysis of oral metronomic chemotherapy in head and neck squamous cell carcinoma.
Information sources and search strategy
Two authors (ABSC and DP) independently searched PubMed, SCOPUS, Web of Science and Google Scholar for the keywords alone and in combination, followed by a manual search and assessment of cross references. The MeSH terminology formulated for each database for the literature search was (TITLE-ABS-KEY (metronomic AND chemotherapy) AND TITLE-ABS-KEY (head AND neck AND squamous AND cell AND carcinoma)).
Selection and data collection process
The same authors individually screened the titles and abstracts of all the articles. The papers that did not meet the eligibility criteria were excluded. Full-length texts were downloaded for all the eligible articles. The complete articles were read and evaluated for eligibility, and the reasons for exclusion were recorded. The third author (RPK) was involved in resolving the discordance if any discrepancy was noted. The following information was extracted from each included article: author(s), country of origin, year of publication, study design, number of cases, number of controls, drug or drug combination used for oral metronomic chemotherapy, and control treatment. The criteria for evaluation the efficacy of OMCT were divided into two main categories: (1) primary study end point (Overall survival) and (2) secondary end points. The secondary end points included progression-free survival (PFS), disease-free survival (DFS), distant metastasis-free survival (DMFS), disease-specific survival (DSS), complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), and clinical benefit rate (CR + PR + SD). For the assessment of overall survival, death due to any cause was considered as the event, while distant metastasis, death from any cause, were considered as events in the secondary end points. Any response other than disease progression was regarded as clinical response. All included studies were carefully assessed for evaluation and recording of the end points post-OMCT.
Statistical analysis
The quantitative data were tabulated and processed in Microsoft Excel 2021 (Microsoft Corporation, Redmond, Washington, United States) and analyzed descriptively. For estimating means, the data were processed using IBM SPSS Statistics for Windows, Version 26.0 (Released 2019; IBM Corp., Armonk, New York, United States), and means and standard deviations were estimated.
Risk of bias analysis
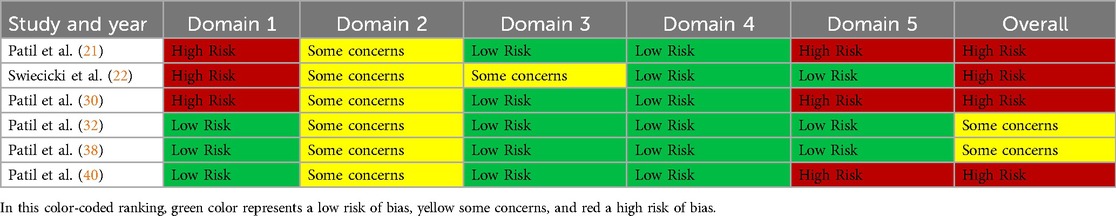
The revised Cochrane risk-of-bias tool for randomized trials (RoB 2) was used to assess the risk of bias for randomized controlled trials (16), and the Newcastle Ottawa scale was employed for the included cross-sectional and case-control studies (17). There are five domains in RoB 2 tool, including Domain 1: Risk of bias arising from the randomization process, Domain 2: Risk of bias due to deviations from the intended interventions (effect of assignment to intervention), Domain 3: Missing outcome data, Domain 4: Risk of bias in the measurement of the outcome and Domain 5: Risk of bias in the selection of the reported result. Each domain contains answerable questions, where the answer is provided as “yes”, “no”, “partial yes”, “partial no” or “not included”. The entire risk based on the domains is expressed as “low risk”, “high risk” or “some concerns”.
The Newcastle Ottawa scale for cross-sectional and case-control studies bears slight differences. Regarding cross-sectional studies, the three categories include selection, exposure, and comparability. For selection and exposure, a maximum of one star is assigned for each numbered parameter for each included study. While assessing comparability, a maximum of two stars could be given. Each category was judged by two authors (DP & RPK), and any discordance was resolved by discussion with the third observer (ABS). Studies that obtained ≥7 or more stars, 4–6 stars and ≤3 stars were respectively marked as having “low risk”, “high risk” and “very high” risk of bias. The scale is similar for case-control studies with slight modifications, where the third category is exposure instead of outcome.
Results
The literature search strategy revealed 147 articles published until 2024 in various electronic scientific databases. Out of these 147 articles, forty papers were excluded after reading the titles and abstracts for eligibility, along with 55 duplicate papers, yielding 52 articles for inclusion in the review. These 52 articles were further evaluated by reading the full text for eligibility. At this stage, three articles were excluded as the full texts could not be retrieved. From the 49 full-text articles that were retrieved, 25 articles were again excluded based on the eligibility criteria. Finally, 24 articles were included in the present systematic review (Figure 1) (18–41).
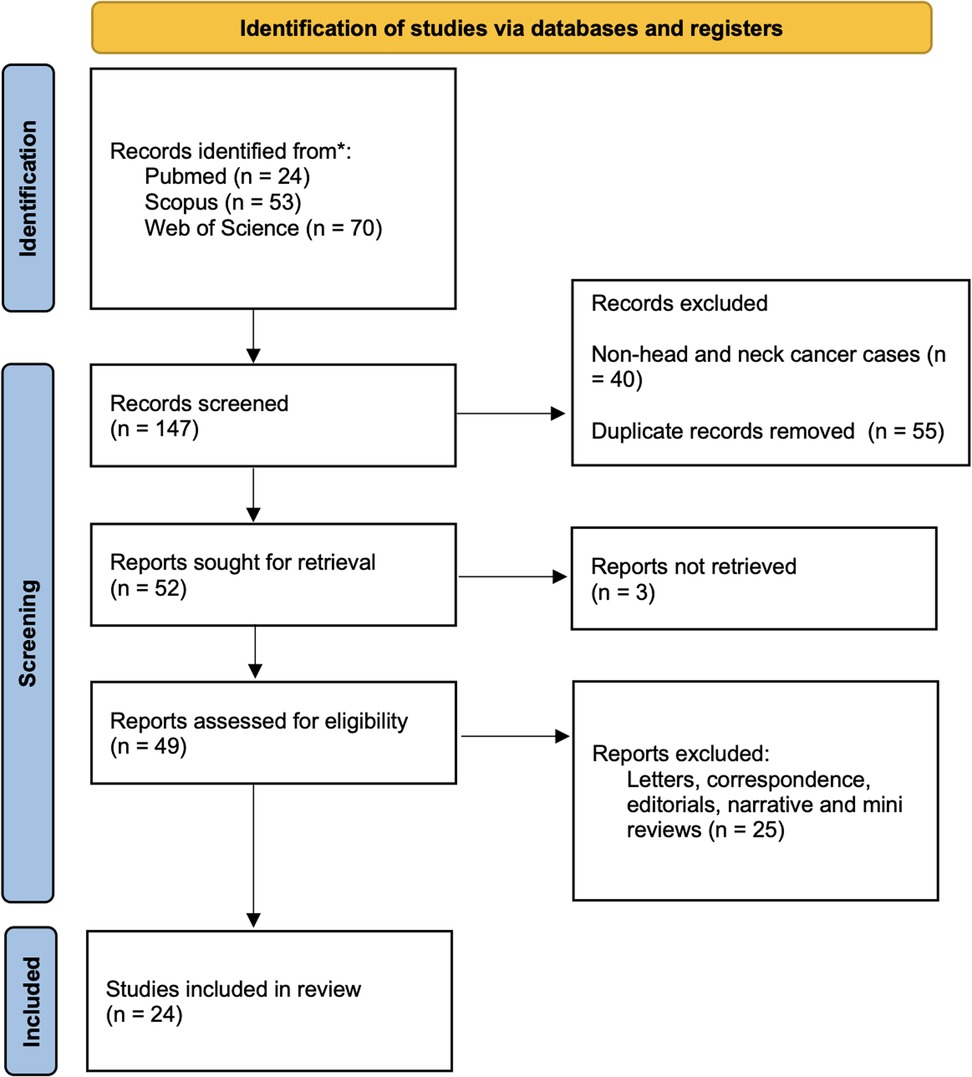
Figure 1. Flowchart showing the article selection process according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2020 guidelines; *deciphers reporting the number of records identified from each database or register searched (no records were found from other sources).
Characteristics of the included studies
All the included studies were published between 2009 and 2024. Out of the 24 included studies, 16 were from India (19–21, 23, 25, 28, 30, 32, 35, 38, 40), four studies were from Japan (24, 34, 39, 41),, two from Taiwan (29, 33), one from Spain (18) and one from the USA (22) (Table 1). A total of 1964 OMCT cases and 1,266 control cases were included. There were six randomized controlled trials (RCTs) (21, 22, 30, 32, 38, 40), nine cross-sectional studies (18, 19, 26, 28, 31, 35, 36, 37, 39), and nine case-control studies (20, 23–25, 27, 29, 33, 34, 41). The data regarding the measures of outcome were heterogeneous. While most studies analyzed the overall survival of the patients, others studied parameters, included progression-free survival, disease-free survival, distant metastasis-free survival, disease-specific survival, complete response/partial response, stable disease/progressive disease, and the clinical benefit rate. There were nineteen studies where OS was analyzed (19, 21–25, 27–35, 38–40), nine studies analyzed progression-free survival (PFS) (19, 21, 22, 26, 27, 35, 40), six studies evaluated DFS (20, 23, 24, 29, 33, 41), DMFS was reported in three studies (24, 33, 34), another study analyzed disease-specific survival (29), eight studies analyzed both OS and PFS (19, 21, 22, 27, 30, 31, 35, 40), and five studies analyzed both OS and DFS (23, 24, 29, 33, 41). Regarding a control group for comparison, no controls were included in 11/24 studies (18, 19, 25, 26, 28, 30, 31, 35–37, 39). Conventional surgical approaches with or without adjuvant therapy were instituted in the studies with controls.
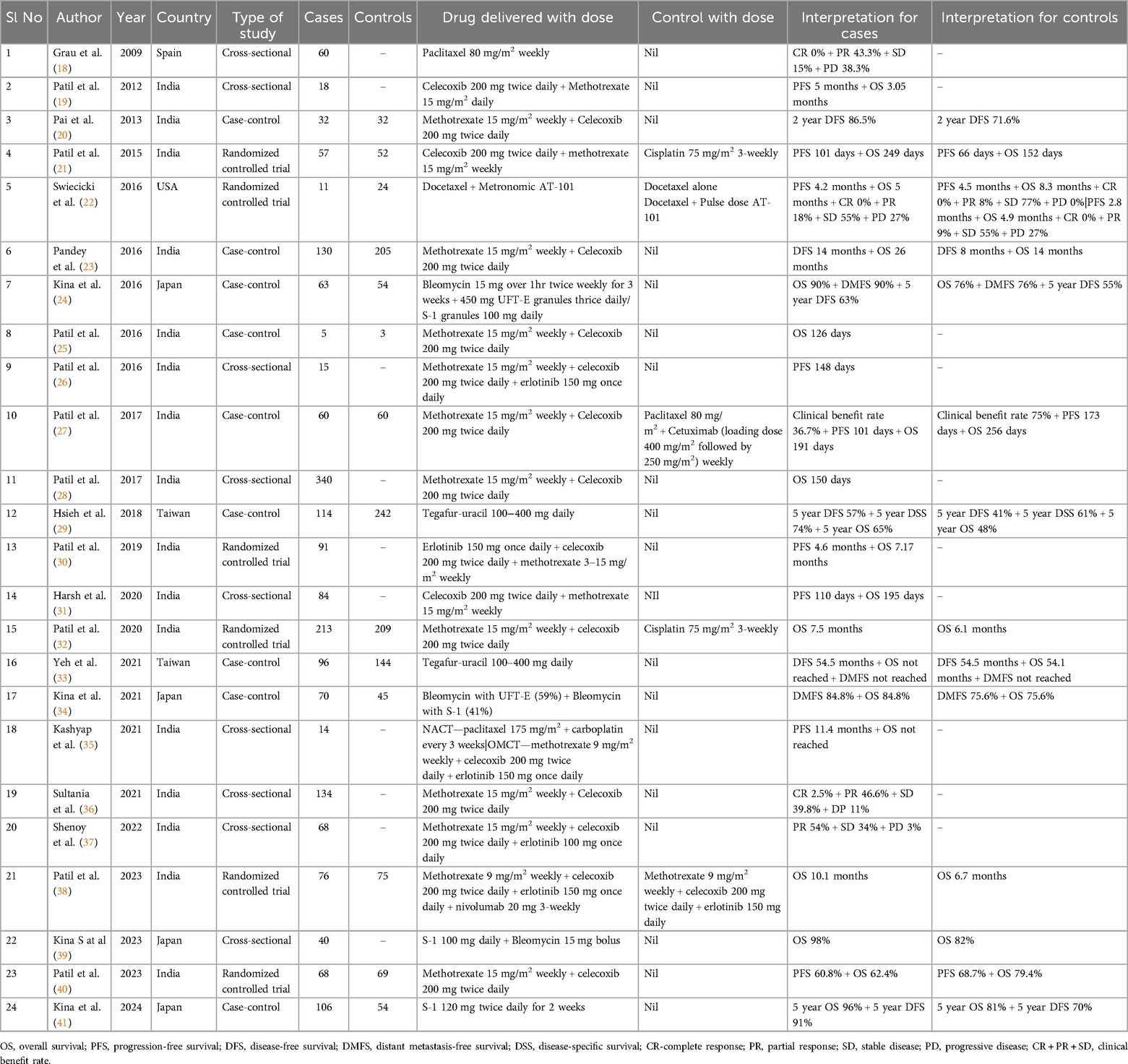
Table 1. Clinico-demographic details of the twenty-four included studies in the present systematic review.
Demographic data
The demographic data of the case and control patients were retrieved from all 24 studies. Out of the 1964 case and 1,269 control participants, 2,685 (83%) participants were male and 548 (17%) were female (M:F ratio of 4.89:1).
Overall survival (OS)
As aforementioned, nineteen (19/24; 79.2%) of the twenty-four studies examined the overall survival (OS) to assess the effectiveness of OMCT. Yet again, there was geneity in the presentation of values. 13/19 (68.4%) studies reported the length (in months) of OS for the participants (19, 21–23, 26–28, 30–33, 35, 37), while the remaining 6 (31.6%) studies presented survival in percentage (24, 29, 34, 39–41). Twelve (70.6%) studies compared the overall survival rates in OMCT and control treatments; 9/12 (75%) studies showed that the OMCT group had higher overall survival rates (21, 23, 24, 29, 32, 34, 38, 39, 41). The mean overall survival duration was calculated, which yielded a value of 6.85 months, whereas the mean overall survival as estimated in percentage was 82.7%.
Progression free survival (PFS)
Nine out of twenty-four studies reported the end point measurement as progression-free survival (PFS) (19, 21, 22, 26, 27, 30, 31, 35, 40). Similar to OS data, there was heterogeneity in the presentation of values. While most of the included studies presented values in months, Kina et al., showed the data values in percentages (41). Out of nine studies, 5 (55.6%) did not have any control group for comparison. In the studies, where a control group was available, it was found that the PFS was higher in control groups (22, 27, 40). The estimated mean progression-free survival duration was 5.07 months, and the mean progression-free survival percentage was 60.8%.
Disease free survival
Out of the 24 studies, 6 (25%) evaluated the efficacy of OMCT by analyzing the disease-free survival (DFS) of the participants (20, 23, 24, 29, 33, 41). The disease-free survival was estimated for two years, three years and 5 years, respectively, in one (20), three (24, 29, 41) and two studies (23, 33). Out of the six studies, Yeh et al., showed equal duration of disease-free survival in both the OMCT and control groups (33), while the remaining five showed that the DFS was higher when OMCT was administered (20, 23, 24, 29, 41). The estimated mean disease-free survival duration was 34.25 months, and the mean disease-free survival percentage was 74.38%.
Distant metastasis free and disease-specific survival
Out of the 24 studies, 3 (12.5%) studies (14, 23, 24) evaluated the efficacy of OMCT by analyzing the distant metastasis-free survival (DMFS) of the participants (24, 33, 34). It was shown that the patients who were treated with OMCT had longer distant metastasis-free survival rates; the mean distant metastasis-free survival percentage was 87.4%. In one of the included papers, the author estimated disease-specific survival and showed a higher disease-specific survival rate of 74% in the OMCT group compared to 61% in the control group.
Complete or partial response
Complete response was the parameter of assessment in three studies, and the mean complete response percentage was 0.83% (18, 22, 36), while the percentage of mean partial response was 40.48%, based on the four included studies (18, 22, 36, 37).
Progression of disease: stable disease/progressive disease
Out of the included twenty-four studies, the progression of the disease was analyzed in four papers (18, 22, 36, 37). However, only one group of authors included a control group for comparison, where the percentage of patients showing stability/progression of disease was found to be equal in the study group and controls (22). The mean stable disease and progressive disease percentages were estimated to be 35.95% and 19.83%, respectively.
Clinical benefit rate (CR + PR + SD)
The clinical benefit rate of the participants was evaluated by Patil VM et al., and this study reported a higher clinical benefit rate of 75% in the control group compared to 36.7% in the OMCT group (27).
Details of OMCT treatment regimens
In general, Methotrexate (9 mg/m2 weekly–15 mg/m2 weekly) was used in most studies as the primary regimen in combination with other drugs (20, 21, 23, 25–28, 32, 36, 38). In five included papers, a control group was used for comparison (21, 22, 27, 32, 38). Out of these five stuidies, in three papers, Methotrexate (9 mg/m2 weekly–15 mg/m2 weekly) was used in combination with other drugs like celecoxib, erlotinib and nivolumab, and it was found that in two of these three studies, the overall survival was better than the control group (21, 32, 38). A lower survival was noted where Paclitaxel was used in the control group (27). Other regimens used as OMCT were Docetaxel + Metronomic AT-101 (22), which was used in comparison with Docetaxel + Pulse dose AT-101 or Docetaxel alone as control. Interestingly, the survival for cases (5 months) in comparison with Docetaxel + Pulse dose AT-101 control group was similar (4.9 months), however, the Docetaxel alone group yielded a higher survival of 8.3 months. Other regimens used as OMCT were, Paclitaxel 80 mg/m2 weekly (18), Bleomycin (15 mg over 1hr twice weekly for 3 weeks + 450 mg UFT-E granules thrice daily/S-1 granules 100 mg daily (24), Tegafur-uracil 100–400 mg daily (29, 33), Erlotinib 150 mg once daily with celecoxib and methotrexate (30), and S-1 100 mg daily with 15 mg bolus of Bleomycin (30).
Risk of bias analysis
Four of the six included RCTs showed a high overall risk of bias (21, 22, 30, 40), and the remaining 2 had some concerns (32, 38) (Table 2). Regarding cross-sectional studies, only two studies showed a score of seven and were categorized as having low risk (18, 36), and the other seven had a high risk of bias (19, 26, 28, 31, 35, 37, 39) (Table 3). Out of the nine case-control studies, six studies had a score of seven or more and bore a low risk (24, 27, 29, 33, 24, 41), while the remaining three studies had a high risk of bias (20, 23, 25) (Table 4).
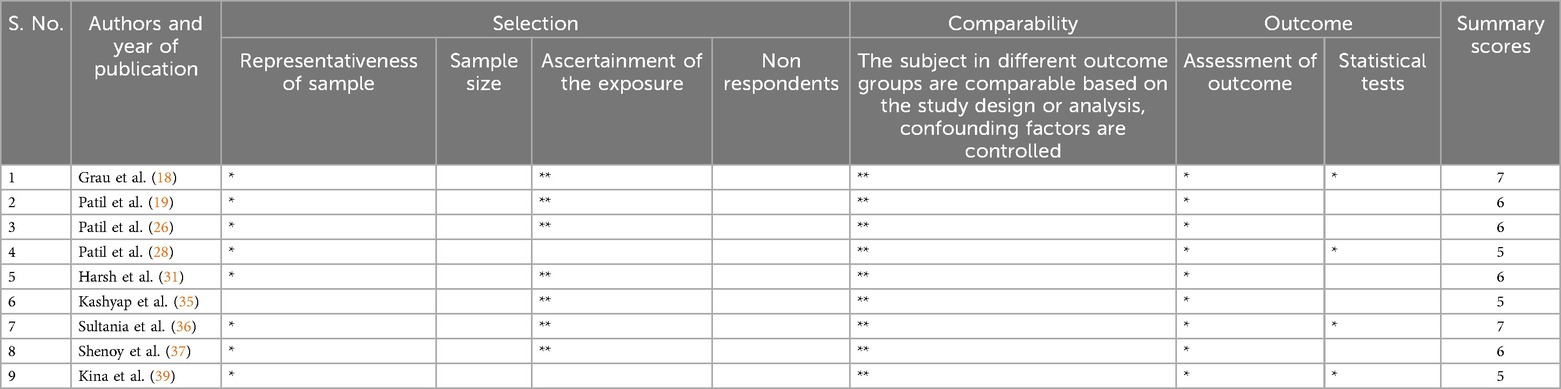
Table 3. Quality assessment tool for the included cross-sectional studies using Newcastle-Ottawa scale.
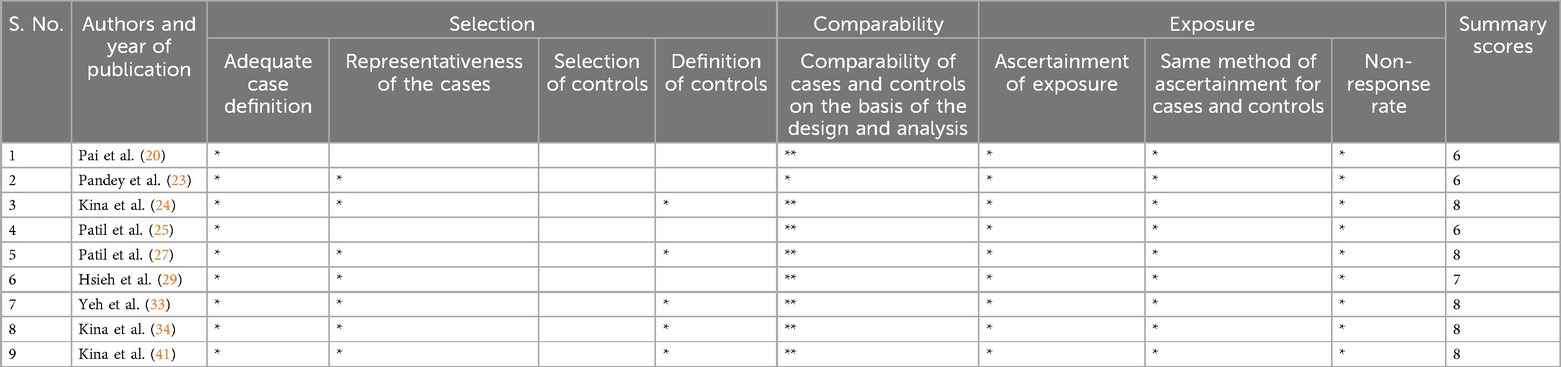
Table 4. Quality assessment tool for the included case-control studies using Newcastle-Ottawa scale.
Discussion
With few treatment options and a poor prognosis, head and neck squamous cell carcinoma (HNSCC) is still a serious health concern, particularly in underdeveloped and developing nations where the resources are limited, with added financial burden and diagnosis of the disease at an advanced stage (42–44). Although surgery, with adjuvant chemotherapy and radiotherapy (CTRT), remains the mainstay treatment for head and neck squamous cell carcinoma, the high dosage and repeated cycles of CTRT are usually linked to serious side effects, which makes them unsuitable for patients with advanced or recurrent disease (45, 46). This has raised interest in metronomic chemotherapy, a method of administering chemotherapeutics continuously at low doses to modify immune responses, lower angiogenesis, and target the tumor microenvironment without the significant toxicity of traditional regimens (47–49). OMCT is not only advantageous in the advanced clinical stage or recurrent diseases but could also be provided to patients where the waiting period is long or in large unresectable tumors with difficulty in obtaining free margins. This holds particularly true for the malignancies of the posterior part of the oral cavity. Preliminary studies suggest that OMCT may offer an effective and less toxic alternative for advanced HNSCC, though robust clinical evidence is still needed (18–22). The present systematic review was performed to evaluate the efficacy of OMCT in HNSCC, aiming to expand treatment options for this challenging patient population and to investigate its potential as a sustainable, less invasive therapeutic approach.
From the systematic review conducted, it was found that the studies where controls were included for comparison of the efficacy of OMCT in head and neck squamous cell carcinoma, most studies demonstrated that the overall survival with OMCT was better as compared to the control groups (20, 21, 23, 24, 29, 32, 34, 38, 39, 41). Few studies, however, showed a reverse trend (22, 27, 40). Owing to the fact that the values of end point determination were expressed variably in percentage, months, years or days, homogeneous data could not be generated to assess the significance and to perform a meta-analysis. The longest duration of overall survival was 26 months observed with the weekly administration of 15 mg/m2 of Methotrexate and twice-daily administration of 200 mg of Celecoxib (23). In contrast, the control group showed an overall survival of only 14 months. Similar results were obtained from other studies. The highest percentage of overall survival was 98% observed with the daily administration of 100–400 mg of Tegafur-Uracil, contrasting with the 82% OS in the control group (39). Similarly, the longest duration of progression-free survival was 11.4 months, which was observed with the combination of neoadjuvant chemotherapy (175 mg/m2 of Paclitaxel with Carboplatin every 3 weeks) and oral metronomic chemotherapy (9 mg/m2 of Methotrexate weekly with 200 mg of Celecoxib twice daily, and 150 mg of Erlotinib once daily) (35). The highest percentage of progression-free survival was 60.8% observed with the weekly administration of 15 mg/m2 of Methotrexate and twice-daily administration of 200 mg of Celecoxib (40). The longest duration of disease-free progression was 54.5 months observed with weekly administration of 15 mg/m2 of Methotrexate and twice-daily administration of 200 mg of Celecoxib (33). The highest percentage of disease-free survival was 91% observed with the twice-daily administration of 120 mg of S-1 for 2 weeks (41) (Table 1).
The highest degree of suitable results was obtained with the oral metronomic administration of the combination of Methotrexate and Celecoxib. The mechanism of action of Methotrexate is due to its ability to inhibit the enzymes responsible for nucleotide synthesis, including dihydrofolate reductase (DHFR), thymidylate synthase (TS), aminoimidazole carboxamide ribonucleotide transformylase (AICART), and amido phosphoribosyl transferase (APRT) (50). As a result, methotrexate prevents tumor cells from proliferating and also has additional anti-inflammatory effects through several mechanisms, including adenosine signaling, the generation of reactive oxygen species (ROS), the decrease of pro-inflammatory cytokine levels, and the enhancement of immune balance through the increase in Treg cells (51).
The usage of a combination of celecoxib with methotrexate was commonly administered as OMCT and showed better outcome measures, as evidenced in the present review (33). Celecoxib inhibits the 3-phosphoinositide-dependent kinase-1 (PDK-1) signaling pathway and binds to the cadherin-11 (CDH11) protein, which is believed to be involved in the development of cancers, to provide anticancer effects (52). It mainly regulates the proliferation, migration, and invasion of tumor cells by inhibiting the cyclooxygenase 2 (COX2)- prostaglandin E2 (PGE2) signal axis, thereby inhibiting the phosphorylation of nuclear factor-κ-gene binding and the expression of matrix metalloproteinases 2 (MMP2) and 9 (MMP9). Celecoxib also promotes the apoptosis of tumor cells by enhancing mitochondrial oxidation, thereby activating the mitochondrial apoptotic process. Celecoxib further reduces drug resistance by increasing the sensitivity of cancer cells to chemotherapy drugs (6). The above mechanisms of methotrexate and celecoxib show that these suppress tumor growth by inhibiting cell division and promoting apoptosis of tumor cells, respectively. These two mechanisms work hand in hand to provide the heightened anticancer effect.
Our compiled results are in line with earlier studies that demonstrated the possible advantages of OMCT in HNSCC (25–30). A better overall immunological response may result from the persistently low dosage, which may lessen immune cell suppression (47). Low-dose, continuous chemotherapy is given orally as part of OMCT, which makes administration simpler and increases patient compliance. According to earlier research, metronomic chemotherapy may be able to control tumor growth with fewer side effects than traditional high-dose regimens by targeting the tumor microenvironment, namely by preventing angiogenesis and regulating the immune response (48, 53).
The current review has, however, a few limitations, including a small sample size and limited follow-up, indicating the need for larger randomized trials. The reporting of survival results, such as the duration of survival in some studies and the percentage of surviving patients in other studies, makes it difficult to effectively compare the results of the studies and also hinders the conduct of a meta-analysis of the extracted data. 67% of the included studies were conducted in India, which may have affected the results, as previous research shows that pharmaco-ethnicity impacts the treatment outcomes of patients undergoing various forms of anti-cancer therapy, primarily through polymorphism within the genes responsible for metabolism (54).
Standardization of reporting criteria is the next step in the systematic analysis of studies into oral metronomic chemotherapy, as it facilitates the proper comparison of treatment outcomes and statistical analysis of the efficacy of various metronomic drug dosages and multidrug combinations. Future studies should also explore biomarkers to identify patients most likely to benefit from OMC and examine combinations with immunotherapies for enhanced efficacy (8). The evidence supporting the efficacy of OMCT in HNSCC is promising, particularly in the context of recurrent or metastatic disease, where traditional treatments often fail. The advantages of OMCT include its favorable toxicity profile, ease of administration, and potential to be combined with other therapeutic modalities, such as immunotherapy. However, challenges remain, including identifying the optimal dosing regimens, understanding the long-term effects, and determining the patient populations that would benefit the most from this approach.
Conclusion
Based on the data collected and analyzed, oral metronomic chemotherapy showed a comparatively better survival compared to the standard chemotherapy regimens, particularly in the case of squamous cell carcinomas of the head and neck. However, it must be noted that there was heterogeneity in the usage of drug regimen and dosages among various studies, necessitating large scale multicentric studies for affirmation of the findings. The anti-angiogenic and immunomodulatory effects of OMCT, combined with its low toxicity, make it an attractive alternative to conventional chemotherapy. The utilization of OMCT for anti-cancer treatment has the added advantage of producing decreased drug resistance, which thereby increases the efficacy of the administered treatment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
AS: Data curation, Investigation, Writing – original draft. DP: Conceptualization, Formal analysis, Methodology, Supervision, Writing – review & editing. RK: Validation, Writing – review & editing. DG: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article. The source of funding for publication is from Ajman University, United Arab Emirates.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Krishnan RP, Pandiar D, Ramani P, Jayaraman S. Necroptosis in human cancers with special emphasis on oral squamous cell carcinoma. J Stomatol Oral Maxillofac Surg. (2023) 124(6S):101565. doi: 10.1016/j.jormas.2023.101565
2. Pandiar D, Nayanar SK, Babu S, Babu S. Expression of p16INK4a in oropharyngeal squamous cell carcinoma from a tertiary cancer centre of South India: a preliminary study. Indian J Med Res. (2021) 154(3):497–503. doi: 10.4103/ijmr.IJMR_386_19
3. Kannan N, Pandiar D, Subramanian R, Krishnan RP. Helicobacter pylori positive oral squamous cell carcinoma demonstrate higher pathological tumor staging and poorer overall survival. J Stomatol Oral Maxillofac Surg. (2024) 125(4S):101952. doi: 10.1016/j.jormas.2024.101952
4. Chang JH, Wu CC, Yuan KS, Wu ATH, Wu SY. Locoregionally recurrent head and neck squamous cell carcinoma: incidence, survival, prognostic factors, and treatment outcomes. Oncotarget. (2017) 8(33):55600–12. doi: 10.18632/oncotarget.16340
5. Ionna F, Bossi P, Guida A, Alberti A, Muto P, Salzano G, et al. Recurrent/metastatic squamous cell carcinoma of the head and neck: a big and intriguing challenge which may be resolved by integrated treatments combining locoregional and systemic therapies. Cancers (Basel). (2021) 13(10):2371. doi: 10.3390/cancers13102371
6. Bravetti G, Falvo P, Talarico G, Orecchioni S, Bertolini F. Metronomic chemotherapy, dampening of immunosuppressive cells, antigen presenting cell activation, and T cells. A quartet against refractoriness and resistance to checkpoint inhibitors. Cancer Lett. (2023) 577:216441. doi: 10.1016/j.canlet.2023.216441
7. Saman H, Raza SS, Uddin S, Rasul K. Inducing angiogenesis, a key step in cancer vascularization, and treatment approaches. Cancers (Basel). (2020) 12(5):1172. doi: 10.3390/cancers12051172
8. Pandiar D, Smitha T, Krishnan RP. Vasculogenic mimicry. J Oral Maxillofac Pathol. (2023) 27(1):228–9. doi: 10.4103/jomfp.jomfp_532_22
9. Liu ZL, Chen HH, Zheng LL, Sun LP, Shi L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther. (2023) 8(1):198. doi: 10.1038/s41392-023-01460-1
10. Khan OA, Blann AD, Payne MJ, Middleton MR, Protheroe AS, Talbot DC, et al. Continuous low-dose cyclophosphamide and methotrexate combined with celecoxib for patients with advanced cancer. Br J Cancer. (2011) 104(12):1822–7. doi: 10.1038/bjc.2011.154
11. Kamal MV, Rao M, Damerla RR, Pai A, Sharan K, Palod A, et al. A mechanistic review of methotrexate and celecoxib as a potential metronomic chemotherapy for oral squamous cell carcinoma. Cancer Invest. (2023) 41(2):144–54. doi: 10.1080/07357907.2022.2139840
12. Kumar NAN, Dikhit PS, Jose A, Mehta V, Pai A, Kudva A, et al. Oral metronomic chemotherapy in advanced and metastatic oral squamous cell carcinoma: a need of the hour. J Maxillofac Oral Surg. (2024) 23(4):793–800. doi: 10.1007/s12663-023-01963-y
13. Simsek C, Esin E, Yalcin S. Metronomic chemotherapy: a systematic review of the literature and clinical experience. J Oncol. (2019) 2019:5483791. doi: 10.1155/2019/5483791
14. André N, Carré M, Pasquier E. Metronomics: towards personalized chemotherapy? Nat Rev Clin Oncol. (2014) 11(7):413–31. doi: 10.1038/nrclinonc.2014.89
15. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. (2021) 10(1):89. doi: 10.1186/s13643-021-01626-4
16. Nejadghaderi SA, Balibegloo M, Rezaei N. The cochrane risk of bias assessment tool 2 (RoB 2) versus the original RoB: a perspective on the pros and cons. Health Sci Rep. (2024) 7(6):e2165. doi: 10.1002/hsr2.2165
17. Luchini C, Stubbs B, Solmi M, Veronese N. Assessing the quality of studies in meta-analyses: advantages and limitations of the Newcastle Ottawa Scale. World J Metaanal. (2017) 5(4):80–4. doi: 10.13105/wjma.v5.i4.80
18. Grau JJ, Caballero M, Verger E, Monzó M, Blanch JL. Weekly paclitaxel for platin-resistant stage IV head and neck cancer patients. Acta Otolaryngol. (2009) 129(11):1294–9. doi: 10.3109/00016480802590451
19. Patil V, Noronha V, D'cruz AK, Banavali SD, Prabhash K. Metronomic chemotherapy in advanced oral cancers. J Cancer Res Ther. (2012) 8(Suppl 1):S106–10. doi: 10.4103/0973-1482.92223
20. Pai PS, Vaidya AD, Prabhash K, Banavali SD. Oral metronomic scheduling of anticancer therapy-based treatment compared to existing standard of care in locally advanced oral squamous cell cancers: a matched-pair analysis. Indian J Cancer. (2013) 50(2):135–41. doi: 10.4103/0019-509X.117024
21. Patil VM, Noronha V, Joshi A, Muddu VK, Dhumal S, Bhosale B, et al. A prospective randomized phase II study comparing metronomic chemotherapy with chemotherapy (single agent cisplatin), in patients with metastatic, relapsed or inoperable squamous cell carcinoma of head and neck. Oral Oncol. (2015) 51(3):279–86. doi: 10.1016/j.oraloncology.2014.12.002
22. Swiecicki PL, Bellile E, Sacco AG, Pearson AT, Taylor JM, Jackson TL, et al. A phase II trial of the BCL-2 homolog domain 3 mimetic AT-101 in combination with docetaxel for recurrent, locally advanced, or metastatic head and neck cancer. Invest New Drugs. (2016) 34(4):481–9. doi: 10.1007/s10637-016-0364-5
23. Pandey A, Desai A, Ostwal V, Patil V, Kulkarni A, Kulkarni R, et al. Outcome of operable oral cavity cancer and impact of maintenance metronomic chemotherapy: a retrospective study from rural India. South Asian J Cancer. (2016) 5(2):52–5. doi: 10.4103/2278-330X.181625
24. Kina S, Nakasone T, Kinjo T, Maruyama T, Kawano T, Arasaki A. Impact of metronomic neoadjuvant chemotherapy on early tongue cancer. Cancer Chemother Pharmacol. (2016) 78(4):833–40. doi: 10.1007/s00280-016-3141-4
25. Patil VM, Noronh V, Joshi A, Karpe A, Talreja V, Chandrasekharan A, et al. Metronomic palliative chemotherapy in maxillary sinus tumor. South Asian J Cancer. (2016) 5(2):56–8. doi: 10.4103/2278-330X.181626
26. Patil VM, Chakraborty S, Jithin TK, Sajith Babu TP, Babu S, Kumar S, et al. An audit of the results of a triplet metronomic chemotherapy regimen incorporating a tyrosine kinase inhibitor in recurrent/metastatic head and neck cancers patients. South Asian J Cancer. (2016) 5(2):48–51. doi: 10.4103/2278-330X.181624
27. Patil VM, Noronha V, Joshi A, Agarwala V, Muddu V, Ramaswamy A, et al. Comparison of paclitaxel-cetuximab chemotherapy versus metronomic chemotherapy consisting of methotrexate and celecoxib as palliative chemotherapy in head and neck cancers. Indian J Cancer. (2017) 54(1):20–4. doi: 10.4103/ijc.IJC_160_17
28. Patil VM, Noronha V, Joshi A, Nayak L, Pande N, Chandrashekharan A, et al. Retrospective analysis of palliative metronomic chemotherapy in head and neck cancer. Indian J Cancer. (2017) 54(1):25–9. doi: 10.4103/ijc.IJC_161_17
29. Hsieh MY, Chen G, Chang DC, Chien SY, Chen MK. The impact of metronomic adjuvant chemotherapy in patients with advanced oral cancer. Ann Surg Oncol. (2018) 25(7):2091–7. doi: 10.1245/s10434-018-6497-3
30. Patil VM, Noronha V, Joshi A, Dhumal S, Mahimkar M, Bhattacharjee A, et al. Phase I/II study of palliative triple metronomic chemotherapy in platinum-refractory/early-failure oral cancer. J Clin Oncol. (2019) 37(32):3032–41. doi: 10.1200/JCO.19.01076
31. Harsh KK, Maharia SR, Nirban RK, Khatri P, Beniwal S, Kumar HS, et al. Metronomic palliative chemotherapy in locally advanced, recurrent and metastatic head-and-neck cancer: a single-arm, retrospective study of a regional cancer center of north India (Asia). J Cancer Res Ther. (2020) 16(3):559–64. doi: 10.4103/jcrt.JCRT_702_18
32. Patil V, Noronha V, Dhumal SB, Joshi A, Menon N, Bhattacharjee A, et al. Low-cost oral metronomic chemotherapy versus intravenous cisplatin in patients with recurrent, metastatic, inoperable head and neck carcinoma: an open-label, parallel-group, non-inferiority, randomised, phase 3 trial. Lancet Glob Health. (2020) 8(9):e1213–22. doi: 10.1016/S2214-109X(20)30275-8
33. Yeh TJ, Chan LP, Tsai HT, Hsu CM, Cho SF, Pan MR, et al. The overall efficacy and outcomes of metronomic tegafur-uracil chemotherapy on locally advanced head and neck squamous cell carcinoma: a real-world cohort experience. Biology (Basel). (2021) 10(2):168. doi: 10.3390/biology10020168
34. Kina S, Kawabata-Iwakawa R, Miyamoto S, Arasaki A, Sunakawa H, Kinjo T. A molecular signature of well-differentiated oral squamous cell carcinoma reveals a resistance mechanism to metronomic chemotherapy and novel therapeutic candidates. J Drug Target. (2021) 29(10):1118–27. doi: 10.1080/1061186X.2021.1929256
35. Kashyap L, Patil V, Noronha V, Joshi A, Menon N, Jobanputra K, et al. Efficacy and safety of neoadjuvant chemotherapy (NACT) with paclitaxel plus carboplatin and oral metronomic chemotherapy (OMCT) in patients with technically unresectable oral squamous cell carcinoma (OSCC). Ecancermedicalscience. (2021) 15:1325. doi: 10.3332/ecancer.2021.1325
36. Sultania M, Imaduddin M, SV Deo S, Kar M, K Muduly D, Kumar S, et al. Role of metronomic therapy for advanced oral cancers and predictors of response: multi-institutional feasibility study. Head Neck. (2022) 44(1):104–12. doi: 10.1002/hed.26904
37. Shenoy VPPK, Manuprasad A, Babu S, Aravind S, Narayanan VN, Nayanar S, et al. Oral metronomic chemotherapy as a feasible preoperative therapy in advanced resectable oral cavity squamous cell carcinomas- a preliminary experience. Ecancermedicalscience. (2022) 16:1425. doi: 10.3332/ecancer.2022.1425
38. Patil VM, Noronha V, Menon N, Rai R, Bhattacharjee A, Singh A, et al. Low-Dose immunotherapy in head and neck cancer: a randomized study. J Clin Oncol. (2023) 41(2):222–32. doi: 10.1200/JCO.22.01015
39. Kina S, Kawabata-Iwakawa R, Miyamoto S, Kato T, Kina-Tanada M, Arasaki A. Epha4 signaling is involved in the phenotype of well-differentiated oral squamous cell carcinoma with decreased tumor immunity. Eur J Pharmacol. (2023) 945:175611. doi: 10.1016/j.ejphar.2023.175611
40. Patil V, Noronha V, Menon N, Mathrudev V, Bhattacharjee A, Nawale K, et al. Metronomic adjuvant chemotherapy evaluation in locally advanced head and neck cancers post radical chemoradiation—a randomised trial. Lancet Reg Health Southeast Asia. (2023) 12:100162. doi: 10.1016/j.lansea.2023.100162
41. Kina S, Miyamoto S, Kawabata-Iwakawa R, Kina-Tanada M, Ogawa M, Yokoo S. Higher overall survival rates of oral squamous cell carcinoma treated with metronomic neoadjuvant chemotherapy. Am J Cancer Res. (2024) 14(3):1033–51. doi: 10.62347/EYNT8387
42. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: gLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68(6):394–424. doi: 10.3322/caac.21492 Erratum in: CA Cancer J Clin. 2020;70(4):313. doi: 10.3322/caac.21609.30207593
43. Pereira-Prado V, Martins-Silveira F, Sicco E, Hochmann J, Isiordia-Espinoza MA, González RG, et al. Artificial intelligence for image analysis in oral squamous cell carcinoma: a review. Diagnostics (Basel). (2023) 13(14):2416. doi: 10.3390/diagnostics13142416
44. Anand R, Pandiar D, Kamboj M. Financial burden of oral squamous cell carcinoma in India. Oral Oncol. (2020) 103:104528. doi: 10.1016/j.oraloncology.2019.104528
45. Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. (2016) 91(3):386–96. doi: 10.1016/j.mayocp.2015.12.017
46. Thamilselvan S, Pandiar D, Krishnan RP, Chitra S. Cytokeratin 8 depicts nodal metastasis in head and neck squamous cell carcinoma. J Oral Maxillofac Pathol. (2024) 28(2):247–52. doi: 10.4103/jomfp.jomfp_168_23
47. Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. (2004) 4(6):423–36. doi: 10.1038/nrc1369
48. Browder T, Butterfield CE, Kräling BM, Shi B, Marshall B, O'Reilly MS, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. (2000) 60(7):1878–86.10766175
49. Sethuraman S, Ramalingam K. Metronomic chemotherapy in oral cancer: a review. Cureus. (2023) 15(12):e49825. doi: 10.7759/cureus.49825
50. Tian H, Cronstein BN. Understanding the mechanisms of action of methotrexate: implications for the treatment of rheumatoid arthritis. Bull NYU Hosp Jt Dis. (2007) 65(3):168–73.17922664
51. Chan ES, Cronstein BN. Molecular action of methotrexate in inflammatory diseases. Arthritis Res. (2002) 4(4):266–73. doi: 10.1186/ar419
52. Basu GD, Pathangey LB, Tinder TL, Gendler SJ, Mukherjee P. Mechanisms underlying the growth inhibitory effects of the cyclo-oxygenase-2 inhibitor celecoxib in human breast cancer cells. Breast Cancer Res. (2005) 7(4):R422–35. doi: 10.1186/bcr1019
53. Bocci G, Kerbel RS. Pharmacokinetics of metronomic chemotherapy: a neglected but crucial aspect. Nat Rev Clin Oncol. (2016) 13(11):659–73. doi: 10.1038/nrclinonc.2016.64
Keywords: oral metronomic chemotherapy, head and neck squamous cell carcinoma, survival rate, angiogenesis, OSCC (oral squamous cell carcinoma)
Citation: Segin Chandran AB, Pandiar D, Krishnan RP and Gopinath D (2025) Efficacy of oral metronomic chemotherapy in the management of head and neck squamous cell carcinoma—a systematic review. Front. Oral Health 6:1632316. doi: 10.3389/froh.2025.1632316
Received: 21 May 2025; Accepted: 15 July 2025;
Published: 30 July 2025.
Edited by:
Gabriela Anaya-Saavedra, Metropolitan Autonomous University, MexicoReviewed by:
Jessica Maldonado-Mendoza, Metropolitan Autonomous University, MexicoItzel Castillejos Garcia, Metropolitan Autonomous University, Mexico
Copyright: © 2025 Segin Chandran, Pandiar, Krishnan and Gopinath. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deepak Pandiar, ZGVlcGFrcGFuZGlhcjE5MjNAeWFob28uY29t; Divya Gopinath, ZHJkaXZ5YWttZW5vbkBnbWFpbC5jb20=
 Aardra Binithadas Segin Chandran1
Aardra Binithadas Segin Chandran1 Deepak Pandiar
Deepak Pandiar