- 1Department of Orthopedics, Wuhan Hospital of Traditional Chinese Medicine, Wuhan, Hubei, China
- 2Department of Pain, Hubei Maternal and Child Health Hospital Affiliated to Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
Background: Persistent pain is a common complication following percutaneous transforaminal endoscopic discectomy (PTED) for lumbar disc herniation. Identifying associated risk factors and developing a predictive model are crucial for guiding clinical decisions. This study aims to utilize machine learning models to predict persistent pain, identify key influencing factors, and construct a risk model to assess the likelihood of persistent pain.
Methods: We first compared baseline characteristics and pathological indicators between patients who developed persistent pain and those who did not after PTED. Significant factors were used as input features in four machine learning models: Logistic Regression (LR), Support Vector Machine (SVM), XGBoost, and Multilayer Perceptron (MLP). Each model was optimized through grid search and 10-fold cross-validation. Performance was evaluated using ROC curves, F1 score, accuracy, recall, and precision. Models with AUC values exceeding 0.9, specifically XGBoost and MLP, were selected for SHAP visualization and risk prediction model construction.
Results: Among the four machine learning models, XGBoost and MLP achieved the best performance, with AUC values of 0.907 and 0.916, respectively. SHAP analysis identified a history of lumbar spine trauma and herniation calcification as key features positively influencing persistent pain risk. Elevated inflammatory markers (e.g., CRP, ESR, and WBC) and older age also significantly impacted predictions. Using the most important features from XGBoost and MLP, a risk prediction model was constructed and externally validated, achieving an AUC of 0.798, indicating good predictive accuracy.
Conclusion: History of lumbar spine trauma, herniation calcification, and inflammatory markers are important predictors of persistent pain after PTED. The risk prediction model based on XGBoost and MLP shows high predictive accuracy and can serve as a valuable tool for clinical decision-making.
1 Introduction
Lumbar disc herniation is a leading cause of lower back and leg pain, significantly affecting patients' quality of life and imposing substantial burdens on both society and families (1–3). With advancements in minimally invasive techniques, percutaneous transforaminal endoscopic discectomy (PTED) has emerged as a key treatment for lumbar disc herniation (4–6). This approach minimizes surgical incisions and reduces damage to surrounding tissues, offering benefits such as faster recovery and fewer complications, which has led to its widespread adoption in clinical practice (7). However, despite the overall success of the procedure, some patients continue to experience persistent postoperative pain, which extends the recovery period and adversely impacts their quality of life (8). The mechanisms behind this persistent pain are not yet fully understood, and accurately identifying high-risk patients before surgery remains challenging, complicating clinical management.
In recent years, machine learning has become increasingly prominent in medical research and clinical applications (9). Through training and optimization with large datasets, machine learning models can identify hidden patterns within complex data, significantly improving the accuracy and stability of disease prediction, especially for complex phenomena such as postoperative persistent pain (10). In this study, we applied four machine learning models—Logistic Regression (LR), Support Vector Machine (SVM) (11), XGBoost (12), and Multilayer Perceptron (MLP) (13)—to explore the effects of various features on persistent pain following PTED. By comparing the performance of these models, we selected the optimal model and used SHAP (Shapley Additive Explanations) values to interpret the impact of each feature on the prediction outcome, clarifying the role of key risk factors in persistent pain.
Through this research, we aim to identify the key factors contributing to persistent pain following lumbar disc herniation surgery and develop an effective risk prediction model. This model is intended to support clinical decision-making by helping clinicians identify high-risk patients prior to surgery, tailor individualized rehabilitation plans, and ultimately improve postoperative quality of life. We hope that the findings from this study will not only deepen the understanding of persistent pain after PTED but also provide valuable insights for managing other types of postoperative pain.
2 Materials and methods
2.1 Patient selection
Patients with lumbar disc herniation who underwent percutaneous transforaminal endoscopic discectomy at our hospital were retrospectively collected between May 2021 and May 2023 for the training cohort, and between June 2023 and June 2024 for the validation set. The former served as the training set, while the latter was used as the external validation set. Inclusion criteria were as follows: (1) age between 18 and 75; (2) grade of spinal spondylolisthesis ≤ Grade I; (3) Pfirrmann Grading of Grade II or Grade III; (4) lumbar segments involved were L3–L4, L4–L5, or L5–S1. Patients were divided into a persistent pain group and a non-persistent pain group based on the presence of persistent pain postoperatively. Exclusion criteria included: (1) presence of other diseases affecting the structure or function of the lumbar spine; (2) undergoing other interventions during follow-up that may influence lumbar pain (such as spinal fusion surgery or nerve block therapy); (3) severe hepatic or renal dysfunction; (4) serious systemic diseases such as malignancies; (5) severe mental or psychological disorders (e.g., major depression or severe anxiety) or poor treatment compliance; and (6) missing key data. Persistent pain was defined as a VAS score ≥ 4 and SF-36 scores < 50 in the pain and physical function domains at the 1-month postoperative follow-up. In the training set of 450 cases, 54 experienced postoperative persistent pain.
2.2 Data collection
We collected baseline characteristics and pathological indicators for each patient, including age, gender, BMI, history of lumbar spine trauma, duration of disease (whether more than 6 months), herniation calcification, spondylolisthesis grade, Pfirrmann Grading (14), lumbar segments (L3–L4, L4–L5, L5–S1), sagittal range of motion (sROM), and inflammatory indicators [C-Reactive Protein [CRP], Erythrocyte Sedimentation Rate [ESR], and White Blood Cell Count [WBC]]. These data were obtained from patient medical records and preoperative imaging results.
2.3 Machine learning model construction
To predict the occurrence of persistent postoperative pain, we selected four commonly used machine learning models for analysis: Logistic Regression (LR), Support Vector Machine (SVM), XGBoost, and Multilayer Perceptron (MLP). All input features were normalized before training to ensure consistency across different feature scales during model training. The DeLong test was used to perform pairwise comparisons of AUC values between models, with a P-value < 0.05 indicating a statistically significant difference in AUC.
2.4 Model tuning and validation
To optimize model performance, we employed grid search combined with 10-fold cross-validation to fine-tune the parameters of each model. In this process, the dataset was split into 10 subsets, with 9 subsets used for training and 1 subset for validation in each iteration. After completing all 10 iterations, the average performance metrics were calculated. The evaluation metrics included the area under the ROC curve (AUC), F1 Score, Accuracy, Recall, and Precision.
2.5 SHAP value analysis
To understand the prediction mechanism of the machine learning models, we used SHAP (Shapley Additive Explanations) values to explain the contribution of each feature to the prediction results (15). SHAP values quantify both the positive and negative impacts, as well as the importance of each feature on the prediction outcome, by assessing each feature's influence on the model output across different scenarios. We used SHAP values to analyze the direction and magnitude of each feature's effect on predicting persistent pain.
2.6 Risk model construction and validation
Based on the most important features identified through SHAP analysis, we constructed a simplified risk model to predict the risk of persistent postoperative pain. The primary formula was:
The SHAP values represent the average SHAP values from our chosen machine learning model. The top three important features were selected as input variables, and the model performance was validated on an external validation set. Model performance was evaluated using the AUC to assess its predictive capability.
2.7 Statistical analysis
All statistical analyses were conducted using R software, primarily utilizing the caret (16), iml, and shapviz packages. A P-value less than 0.05 was considered statistically significant. Continuous variables were presented as median (range) and compared using the Mann–Whitney U-test, while categorical variables were expressed as frequency (percentage) and analyzed with the Chi-square test or Fisher's exact test, as appropriate.
3 Results
3.1 Differences in baseline characteristics and pathological indicators between patients with and without persistent pain
The results showed that patients with persistent pain were older, had a higher proportion of lumbar spine trauma history, and a significantly higher percentage of disease duration exceeding 6 months. These patients also had higher rates of herniation calcification, mild spondylolisthesis (Grade I), and Pfirrmann Grade III, with a significant increase in L4-L5 segment involvement. Additionally, levels of inflammatory markers, including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and white blood cell count (WBC), were higher in patients with persistent pain compared to those without (Table 1). The training set (n = 316) and external validation set (n = 134) showed a balanced distribution across multiple demographic characteristics such as age and BMI, as well as clinical indicators including disease duration and lumbar spondylolisthesis. There were no significant statistical differences between the two cohorts, indicating good comparability (Supplementary Table S1).
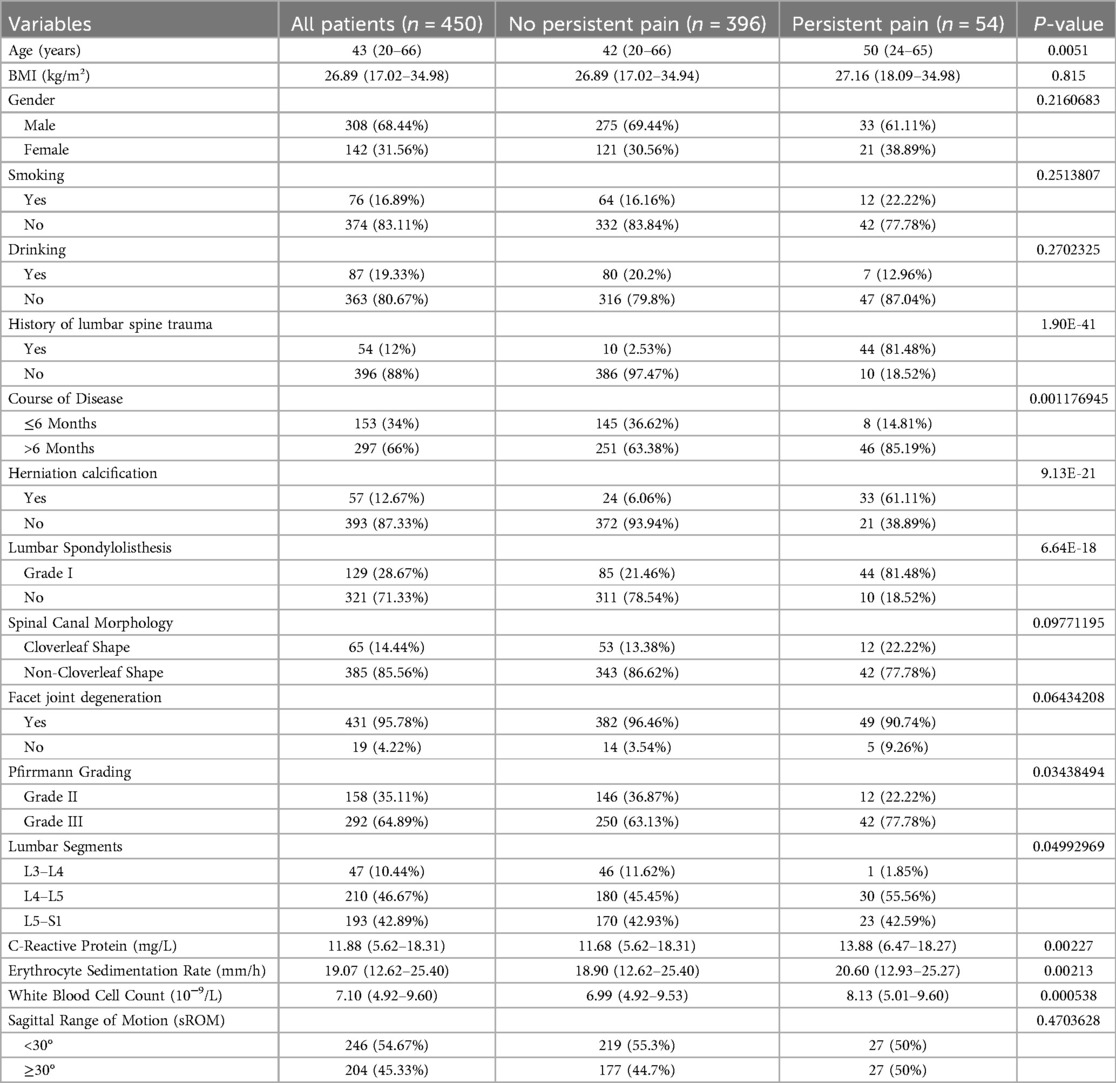
Table 1. Differences in basic characteristics and pathological indicators between patients with persistent postoperative pain and those without persistent postoperative pain.
3.2 Selection of machine learning models
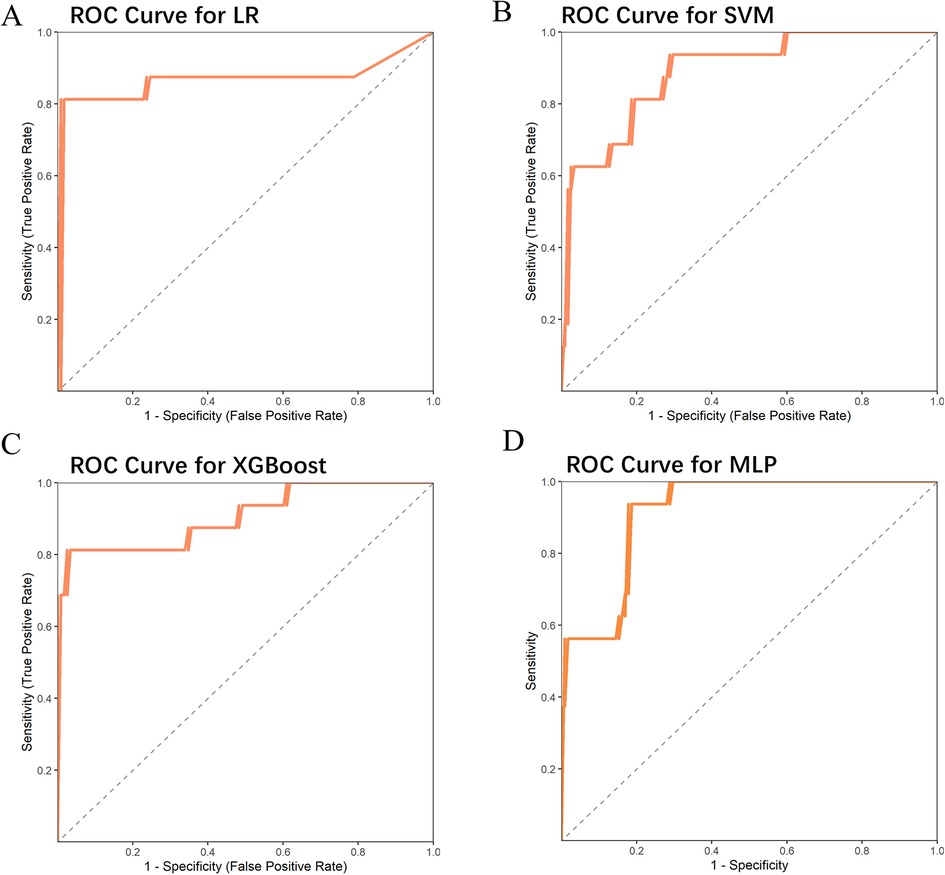
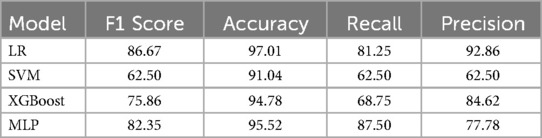
These significant factors were then input as independent variables into four machine learning models. The results showed that the ROC values for these four models were 0.867, 0.888, 0.907, and 0.916, respectively (Table 2) (Figure 1). Among them, the AUC values of the XGBoost and MLP models were both above 0.9, and these models also performed excellently in terms of F1 Score, Accuracy, Recall, and Precision. The DeLong test showed that the AUC values of the XGBoost and MLP models were significantly higher than those of the LR and SVM models (P < 0.05). Therefore, XGBoost and MLP were selected for visualization analysis (Table 3).
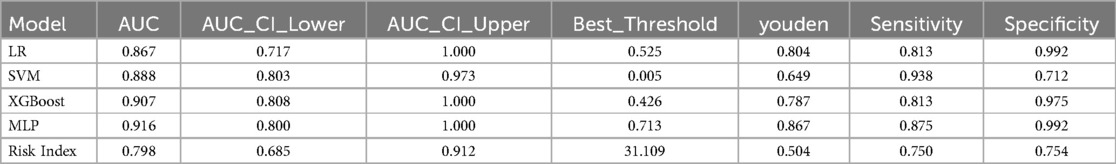
Table 2. ROC curve parameters of four machine learning models and risk indicators in the test Set and external validation Set.
3.3 SHAP value explanation of model results
For each machine learning model, a sample with persistent pain and a sample without persistent pain were randomly selected for analysis. The x-axis represents the direction of the change in the prediction value, with movement to the left indicating a negative influence and movement to the right indicating a positive influence. In Figure 2A, an age of 46 and CRP (C-Reactive Protein) level of 6.91 had a strong negative impact on the likelihood of persistent pain (−2.3 and −1.88, respectively), while certain lumbar segments and joint degeneration had a positive impact. The final expected prediction value was −2.99, indicating that the model predicts this patient is more likely not to experience persistent pain. In Figure 2B, a history of lumbar spine trauma (value of 1) and herniation calcification (value of 1) had a large positive impact on the prediction, significantly increasing the likelihood of persistent pain, with contributions of +4.68 and +3.3, respectively. Additionally, higher ESR (18.8) and WBC (7.56) levels had a negative impact on the prediction, reducing the risk of persistent pain.
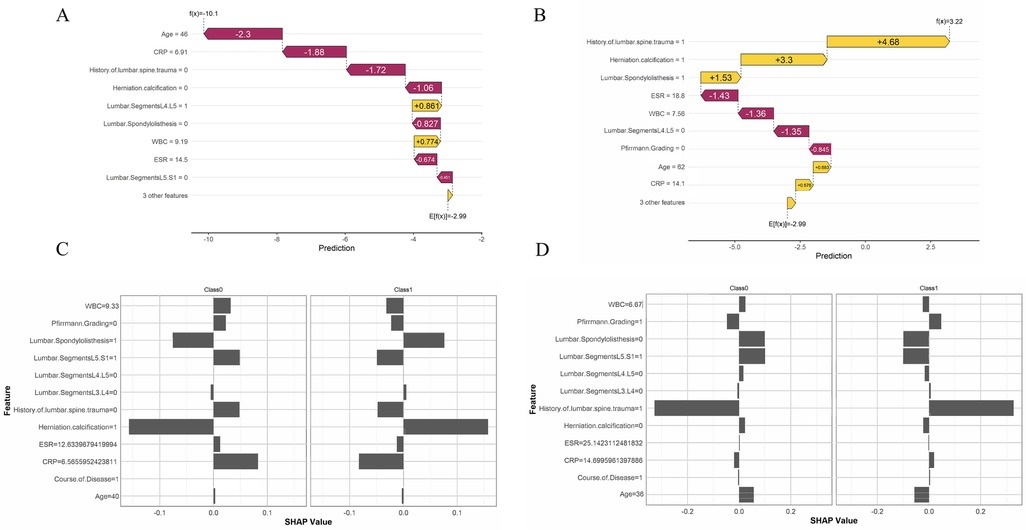
Figure 2. Model interpretation based on SHAP values. (A) A sample predicted by the XGBoost model not to experience persistent pain. (B) A sample predicted by the XGBoost model to experience persistent pain. (C) A sample predicted by the MLP model not to experience persistent pain. (D) A sample predicted by the MLP model to experience persistent pain.
In the MLP visualization results, in Figure 2C, for patients without persistent pain, herniation calcification and no history of lumbar spine trauma had a strong negative impact on the prediction. For patients with persistent pain, lumbar segment L5.S1 and herniation calcification had a significant positive impact on the prediction. In Figure 2D, a history of lumbar spine trauma had a strong positive impact on the prediction of persistent pain, while it showed a negative impact on the prediction of non-persistent pain, indicating that patients with a history of lumbar spine trauma are more likely to be predicted to experience persistent pain. Additionally, younger age also played a negative role in predicting the absence of persistent pain.
The feature value analysis for all samples in Figure 3A shows that the x-axis represents SHAP values, indicating the direction and magnitude of each feature's impact on the prediction. The color gradient from purple to yellow represents the change in feature values from low to high. In the figure, a history of lumbar spine trauma (yellow for high values) and older age have a significant positive impact on the risk of persistent pain, indicating that higher values for these features increase the risk of persistent pain. High values for inflammatory indicators (e.g., CRP, ESR, and WBC) also have varying degrees of impact on the prediction. The MLP model results indicate that a history of lumbar spine trauma and herniation calcification are the most important features affecting the prediction outcome, contributing the most to the prediction of persistent pain, followed by spondylolisthesis, ESR, Pfirrmann Grading, and CRP (Figure 3B). We summarized the SHAP visualization results of the XGBoost and MLP models in Supplementary Table S2 to enhance clarity and interpretability. We also conducted visualization analysis in the external validation cohort, and the results showed that the feature rankings in the external validation set were similar to those in the training set, especially the top three most important features remained consistent. This further supports the stability and generalizability of the model (Supplementary Figure S1).
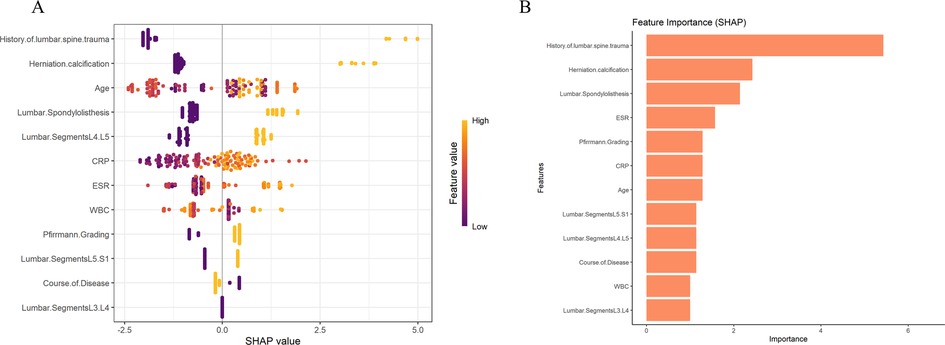
Figure 3. Visualization of feature importance and contribution to persistent pain risk based on (A) XGBoost and (B) MLP models.
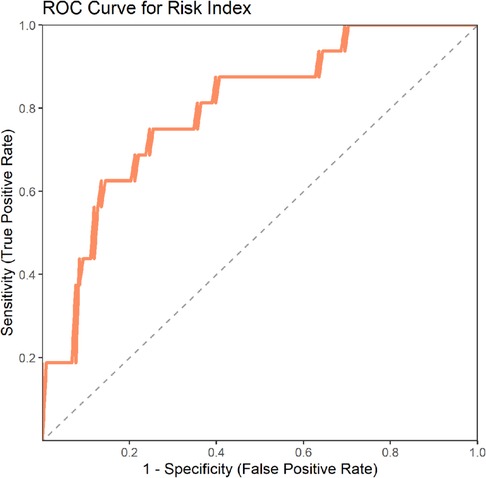
3.4 Construction and validation of the risk model
We constructed a risk model to predict the likelihood of persistent pain based on the top three important features from these two machine learning models, and validated it in an external validation set. The results showed an AUC value of 0.798, indicating that the model has good predictive capability (Figure 4) (Table 2).
4 Discussion
This study is the first to apply machine learning techniques to identify multiple key risk factors for persistent pain following PTED surgery and successfully develop a machine learning-based risk prediction model to estimate the likelihood of persistent pain in patients. The findings highlight that a history of lumbar spine trauma and herniation calcification were the most influential features in both the XGBoost and MLP models, exhibiting significant positive effects on the risk prediction for persistent pain. These results suggest that anatomical changes and prior injury history may play crucial roles in the persistence of postoperative pain, while age and inflammatory status also appear to be important predictors.
We employed four machine learning models—Logistic Regression, Support Vector Machine, XGBoost, and Multilayer Perceptron—to compare their performance in predicting postoperative persistent pain and to identify the optimal model. These models captured the multi-level relationships between features and pain risk, with nonlinear models such as XGBoost and MLP demonstrating particular strength in uncovering complex feature interactions. Moreover, utilizing multiple models enhanced the stability and robustness of the predictions, minimizing potential biases inherent to any single model. Through SHAP value analysis of feature importance, this study offers clinicians valuable data-driven insights into the key factors influencing pain risk, thereby supporting more personalized treatment decisions.
Our study found a significant association between a history of lumbar spine trauma and the occurrence of persistent postoperative pain. Previous research has suggested that prior lumbar injuries may lead to structural and functional alterations in local nerves, muscles, and soft tissues, resulting in a sustained state of hypersensitivity (17). This heightened sensitivity makes these areas more susceptible to surgical stimulation and may lead to more pronounced postoperative pain responses (18, 19). Additionally, compensatory movement patterns and muscular imbalances developed after trauma may persist even after apparent recovery, potentially affecting spinal biomechanics and interfering with postoperative rehabilitation, thereby exacerbating the pain experience. Psychological studies have also indicated that individuals with a history of trauma are more prone to anxiety, depression, and other negative emotional states, which are closely linked to the development of postoperative pain (20, 21). These psychological factors may amplify pain perception through central sensitization mechanisms. Therefore, lumbar trauma may contribute to persistent postoperative pain through both physiological and psychological pathways.
Herniation calcification has a significant impact on postoperative pain, possibly because calcification hardens the protruding disc, increasing its pressure and friction on surrounding tissues (such as nerve roots, ligaments, and muscles) (22). Calcified tissue is challenging to remove during surgery, increasing the risk of damage to surrounding nerves and tissues, thereby intensifying postoperative pain (23). Calcification complicates surgical manipulation, particularly in PTED, as the hardness of the calcified disc limits the maneuverability of surgical instruments, making it difficult for surgeons to completely remove the herniated portion. Residual calcified tissue may continue to exert nerve compression postoperatively, resulting in persistent pain. Moreover, calcified disc tissue can provoke a more intense inflammatory response, which may delay tissue healing and contribute to chronic inflammation. This inflammation activates pain transmission pathways, heightening the patient's pain perception and prolonging the duration of postoperative pain. Elevated levels of CRP and ESR reflect a low-grade chronic inflammation within the patient's body (24). Intraoperative stimuli may promote the release of inflammatory mediators, thereby exacerbating tissue damage, inducing postoperative neural sensitization, and leading to postoperative edema, exudation, or adhesions, which cause persistent postoperative pain. Moreover, at higher CRP and ESR levels, the ability of soft tissue repair after surgery is reduced, making scar tissue formation more likely and aggravating postoperative discomfort.
The AUC value of our risk model was 0.798, indicating that this simplified model has good predictive ability and can be used as a quick assessment tool in clinical settings. In the future, it could be implemented as a mobile application, a risk prediction calculator, or integrated into electronic health record (EHR) systems, allowing clinicians to rapidly assess the risk of persistent postoperative pain before surgery. By identifying high-risk patients in advance, clinicians can optimize preoperative preparation and adjust treatment strategies accordingly, thereby improving surgical safety and patient outcomes.
However, this study has some limitations. The sample size is limited, which may restrict the model's generalizability. Future studies could expand the sample size to improve the model's stability and applicability across different populations. Persistent pain is a subjective experience, and there is currently a lack of a unified and widely accepted standard for its definition. Therefore, the generalizability of our study may be limited. Future research could incorporate more objective indicators to improve the assessment system for persistent pain and enhance the general applicability and clinical relevance of the findings.
5 Conclusion
This study identified several factors—including age, history of lumbar spine trauma, disease duration, and herniation calcification—that significantly increase the risk of persistent pain after PTED. The XGBoost and MLP models, built using these key factors, demonstrated excellent performance in predicting postoperative pain while offering strong interpretability. SHAP analysis highlighted lumbar spine trauma history and herniation calcification as the most influential predictors. The resulting risk prediction model aids in the preoperative identification of high-risk patients and supports the development of personalized intervention strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
This research project has been approved by Ethics Committee of Hubei Maternal and Child Health Hospital Affiliated to Tongji Medical College, Huazhong University of Science and Technology and operated in strict accordance with ethical standards. In this study, we respect and protect the rights and privacy of participants and ensure the confidentiality of their personal information.
1. Participants’ informed consent: We explained the purpose, process, risks and benefits of the study to all individuals involved in the study orally or in writing, and obtained their informed consent. Participants have the right to know that their participation is voluntary and can withdraw from the study at any time.
2. Data confidentiality and privacy protection: We have taken appropriate measures to protect the privacy of participants’ personal information. We will not disclose or disclose any personal information that may lead to the identification of participants. In the research report, we will treat the participants’ information anonymously.
3. Assessment and management of potential risks: We assessed the potential risks that may be involved in the study during the project design stage and took appropriate measures to reduce or manage these risks. We guarantee that participants will not suffer any physical or psychological harm because of participating in the study.
4. Research data use: We will strictly abide by the principles of legality and transparency in data use to ensure the correct use and interpretation of research data. We will try our best to avoid data misunderstanding and abuse, and only use the data for research purposes.
The purpose of this statement is to ensure the ethical compliance of research projects and protect the rights and privacy of participants. If you have any further questions or doubts, please feel free to contact us.
Author contributions
JY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Software, Writing – original draft. JF: Investigation, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2025.1631651/full#supplementary-material
Supplementary Figure S1 | Visualization of feature importance and contribution for persistent pain risk in the external validation cohort based on (A) XGBoost and (B) MLP models.
References
1. Pan M, Li Q, Li S, Mao H, Meng B, Zhou F, et al. Percutaneous endoscopic lumbar discectomy: indications and complications. Pain Physician. (2020) 23(1):49–56.32013278
2. Zhang AS, Xu A, Ansari K, Hardacker K, Anderson G, Alsoof D, et al. Lumbar disc herniation: diagnosis and management. Am J Med. (2023) 136(7):645–51. doi: 10.1016/j.amjmed.2023.03.024
3. Kreiner DS, Hwang SW, Easa JE, Resnick DK, Baisden JL, Bess S, et al. An evidence-based clinical guideline for the diagnosis and treatment of lumbar disc herniation with radiculopathy. Spine J. (2014) 14(1):180–91. doi: 10.1016/j.spinee.2013.08.003
4. Zhang J, Gao Y, Zhao B, Li H, Hou X, Yin L. Comparison of percutaneous transforaminal endoscopic discectomy and open lumbar discectomy for lumbar disc herniations: a systematic review and meta-analysis. Front Surg. (2022) 9:984868. doi: 10.3389/fsurg.2022.984868
5. Ahn Y. Endoscopic spine discectomy: indications and outcomes. Int Orthop. (2019) 43(4):909–16. doi: 10.1007/s00264-018-04283-w
6. Gadjradj PS, Harhangi BS, Amelink J, van Susante J, Kamper S, van Tulder M, et al. Percutaneous transforaminal endoscopic discectomy versus open microdiscectomy for lumbar disc herniation: a systematic review and meta-analysis. Spine. (2021) 46(8):538–49. doi: 10.1097/brs.0000000000003843
7. Yu P, Zan P, Zhang X, Qiang H, Samsonraj RM, Tang J, et al. Comparison of percutaneous transforaminal endoscopic discectomy and microendoscopic discectomy for the surgical management of symptomatic lumbar disc herniation: a multicenter retrospective cohort study with a minimum of 2 Years’ follow-up. Pain Physician. (2021) 24(1):E117–e125.33400445
8. Duan L, Zhang J-Y, Zhang J-H, Kang J-Y, Zhou H-C. Effect of intradiscal local anesthetic injection on intraoperative pain during percutaneous transforaminal endoscopic discectomy: a retrospective study. Asian J Surg. (2023) 46(7):2682–8. doi: 10.1016/j.asjsur.2022.09.153
9. Handelman GS, Kok HK, Chandra RV, Razavi AH, Lee MJ, Asadi H. Edoctor: machine learning and the future of medicine. J Intern Med. (2018) 284(6):603–19. doi: 10.1111/joim.12822
10. Pruneski JA, Pareek A, Kunze KN, Martin RK, Karlsson J, Oeding JF, et al. Supervised machine learning and associated algorithms: applications in orthopedic surgery. Knee Surg Sports Traumatol Arthrosc. (2023) 31(4):1196–202. doi: 10.1007/s00167-022-07181-2
11. Valkenborg D, Rousseau A-J, Geubbelmans M, Burzykowski T. Support vector machines. Am J Orthod Dentofacial Orthop. (2023) 164(5):754–7. doi: 10.1016/j.ajodo.2023.08.003
12. Schönnagel L, Caffard T, Vu-Han T-L, Zhu J, Nathoo I, Finos K, et al. Predicting postoperative outcomes in lumbar spinal fusion: development of a machine learning model. Spine J. (2024) 24(2):239–49. doi: 10.1016/j.spinee.2023.09.029
13. Alkadri S, Ledwos N, Mirchi N, Reich A, Yilmaz R, Driscoll M, et al. Utilizing a multilayer perceptron artificial neural network to assess a virtual reality surgical procedure. Comput Biol Med. (2021) 136:104770. doi: 10.1016/j.compbiomed.2021.104770
14. Wang YXJ. Several concerns on grading lumbar disc degeneration on MR image with Pfirrmann criteria. J Orthop Translat. (2022) 32:101–2. doi: 10.1016/j.jot.2021.12.003
15. Ali S, Akhlaq F, Imran AS, Kastrati Z, Daudpota SM, Moosa M. The enlightening role of explainable artificial intelligence in medical & healthcare domains: a systematic literature review. Comput Biol Med. (2023) 166:107555. doi: 10.1016/j.compbiomed.2023.107555
16. Zhang Z, Zhao Y, Canes A, Steinberg D, Lyashevska O. Predictive analytics with gradient boosting in clinical medicine. Ann Transl Med. (2019) 7(7):152. doi: 10.21037/atm.2019.03.29
17. Idlett-Ali S, Kloefkorn H, Goolsby W, Hochman S. Relating spinal injury-induced neuropathic pain and spontaneous afferent activity to sleep and respiratory dysfunction. J Neurotrauma. (2023) 40(23-24):2654–66. doi: 10.1089/neu.2022.0305
18. Liu P, Song C, Wang C, Li Y, Su L, Li J, et al. Spinal SNAP-25 regulates membrane trafficking of GluA1-containing AMPA receptors in spinal injury-induced neuropathic pain in rats. Neurosci Lett. (2020) 715:134616. doi: 10.1016/j.neulet.2019.134616
19. Rekand T, Hagen EM, Grønning M. Chronic pain following spinal cord injury. Tidsskr Nor Laegeforen. (2012) 132(8):974–9. doi: 10.4045/tidsskr.11.0794
20. Yang Y, Tang T-t, Chen M-R, Xiang M-Y, Li L-L, Hou X-L. Prevalence and association of anxiety and depression among orthopaedic trauma inpatients: a retrospective analysis of 1994 cases. J Orthop Surg Res. (2020) 15(1):587. doi: 10.1186/s13018-020-02132-4
21. Nicholson V, Cole E, Christie R. Factors associated with anxiety and depression one year after trauma critical care admission: a multi-centre study. Injury. (2025) 56(5):112080. doi: 10.1016/j.injury.2024.112080
22. Zhou G, Liang Z, Gao F, Zhao L, Gao H, Xiao G, et al. Percutaneous endoscopic lumbar discectomy for calcified lumbar disc herniation: a retrospective cohort study, systematic review and meta-analysis. Pain Physician. (2024) 27(1):E1–e15.38285024
23. Gao S, Wei J, Li W, Zhang L, Cao C, Zhai J, et al. Full-endoscopic transforaminal ventral decompression for symptomatic thoracic disc herniation with or without calcification: technical notes and case series. Pain Res Manag. (2021) 2021:6454760. doi: 10.1155/2021/6454760
Keywords: lumbar disc herniation, machine learning, percutaneous transforaminal endoscopic discectomy, persistent pain, risk prediction model
Citation: Yuan J and Fu J (2025) Develop and validate a machine learning model to predict the risk of persistent pain after percutaneous transforaminal endoscopic discectomy. Front. Surg. 12:1631651. doi: 10.3389/fsurg.2025.1631651
Received: 20 May 2025; Accepted: 23 June 2025;
Published: 23 July 2025.
Edited by:
Siying Song, Massachusetts General Hospital and Harvard Medical School, United StatesReviewed by:
Shaocheng Liu, Capital Medical University, ChinaHang He, University Hospital of Düsseldorf, Germany
Qihan Guo, Capital Medical University, China
Copyright: © 2025 Yuan and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Fu, ZnVqdW42NjM4NjJAMTYzLmNvbQ==
 Jun Yuan1
Jun Yuan1 Jun Fu
Jun Fu